Abstract
Primary bladder diffuse large B-cell lymphoma (PB-DLBCL) is an exceedingly rare form of non-Hodgkin lymphoma, accounting for only 0.2% of all extranodal lymphomas. Here we report a case of PB-DLBCL in a woman presenting with hematuria. To determine the disease stage, an 18F-FDG PET/CT scan was performed after surgery. However, intense physiological 18F-FDG activity within the bladder lumen obscured potential lesions and concurrent CT imaging revealed suboptimal bladder distension limiting anatomical delineation. To address these limitations, 20 mg of intravenous furosemide was administered. Delayed imaging was performed 2 h after 18F-FDG injection (1 h after diuretic administration), revealing a focal hypermetabolic lesion in the left bladder wall. This metabolic pattern confirmed the diagnosis of PB-DLBCL, staged as IE according to the Ann Arbor classification. Following 4 cycles of R-CHOP chemotherapy, an 18F-FDG PET/CT scan was performed for treatment response assessment, confirming complete metabolic remission of the PB-DLBCL. This case highlights that dual-phase 18F-FDG PET/CT with intravenous diuretics can reduce bladder background activity and improve lesion visualization. Moreover, this approach holds significant clinical value in assisting with disease staging and evaluating treatment response.
Introduction
As an exceptionally rare extranodal malignancy, primary bladder lymphoma comprises just 0.2% of all non-Hodgkin lymphoma cases originating outside lymph nodes (1). Extranodal lymphoma diagnosis relies on comprehensive histopathological examination and immunohistochemistry (2). 18F-FDG PET/CT combining functional and anatomical imaging, forms the cornerstone of lymphoma patient management (3). PET/CT demonstrates superior accuracy and sensitivity to CT in identifying involved nodal sites (4). However, diagnostic challenges persist in regions with physiological 18F-FDG uptake or excretion, such as the brain and urinary tract. We present a rare case of primary bladder DLBCL. Initial 18F-FDG PET/CT imaging at 45 min post-intravenous tracer administration revealed that physiological tracer excretion limited lesion visualization. Accurate identification of bladder lesion sites presented significant challenges. To address this limitation, intravenous diuretic administration was implemented to mitigate physiological uptake within the bladder. Following intravenous diuretic administration, delayed imaging performed at 2-h post-radiotracer injection clearly delineated the bladder wall lesion. Compared with post-chemotherapy PET/CT images without diuretics, the diuretic-assisted protocol showed reduced urinary activity and clearer lesion visualization.
Case presentation
A 50-year-old woman presented to the Urology Department in November 2024 with a 1-week history of gross hematuria and nocturia. She had no associated symptoms such as urinary frequency, dysuria, voiding difficulty, flank pain, or fever. The patient had a 6-year history of chronic hepatitis B, with no documented family history of lymphoma or hematologic malignancies. The patient’s vital signs were stable and superficial lymph nodes were not palpably enlarged. Urinalysis revealed nitrite positivity, leukocyturia (2 cells/μL), and occult blood (3 cells/μL). Serological testing for hepatitis B demonstrated: Hepatitis B surface antigen (HBsAg): Positive (>250,000 IU/mL), Anti-hepatitis Be antibody (Anti-HBe): Negative (0.02 S/CO), Anti-hepatitis B core antibody (Anti-HBc): Positive (7.88 S/CO), HBV DNA: Negative (<500 IU/mL). Routine urological color ultrasound examination suggested that a low and weak echogenic mass measuring about 33 mm × 20 mm was seen in the left wall of the bladder. Uro-pelvic CT scan and enhancement showed a mass on the left posterior wall of the bladder, which was considered to be a high possibility of bladder cancer (Figure 1). Cystoscopy revealed a mucosal bulge extending from the 12 to 5 o’clock position, involving the anterior bladder wall and left wall near the bladder neck, measuring approximately 40 mm × 30 mm. After exclusion of contraindications to surgery, “transurethral cystectomy” was performed. Postoperative pathology confirmed DLBCL of the bladder, Hans classification: anaplastic B-cell subset. Immunohistochemistry showed positivity for CD20, PAX-5, BcL-6, MUM1, Ki-67 (90%), and wild-type P53; negative for CD10, BCL-2, CD21, CD3, CD5, CK, c-Myc, CyclinD1, and GATA3 (Figure 2). To further evaluate disease staging, a PET/CT scan was performed after the transurethral cystectomy. Whole-body imaging performed 45 min after intravenous injection of 18F-FDG (185 MBq) showed diffusely increased bladder activity, which interfered with lesion detection. To reduce urinary radioactivity and enhance lesion conspicuity, pelvic delayed imaging was performed 60 min following intravenous furosemide injection (20 mg) at 120-min post-radiotracer administration. This revealed focal wall thickening with intense hypermetabolic activity with a SUVmax of 22.7 in the left posterior bladder wall (Figure 3), while no abnormal 18F-FDG uptake was detected in other organs or lymph nodes. Based on the above findings, the diagnosis was primary stage I DLBCL of the bladder. The patient was referred to another hospital in the city for further treatment. Bone marrow aspiration and biopsy revealed no evidence of lymphoma cells. After completing 4 cycles of R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) chemotherapy, a follow-up 18F-FDG PET/CT scan demonstrated no abnormal increased uptake in the bladder wall (Figure 3), indicating favorable treatment response. During follow-up, the patient remained asymptomatic with no hematuria and in good clinical condition, continuing to receive regular hospital treatments.
Figure 1

Non-contrast abdominal CT scan demonstrated an irregular soft tissue density lesion along the left lateral wall of the urinary bladder (A, arrow). The mass exhibited contrast enhancement during both arterial and venous phases (B,C, arrow), with subsequent excretory phase imaging revealing a focal filling defect in the corresponding region of the left lateral bladder wall (D, arrow).
Figure 2
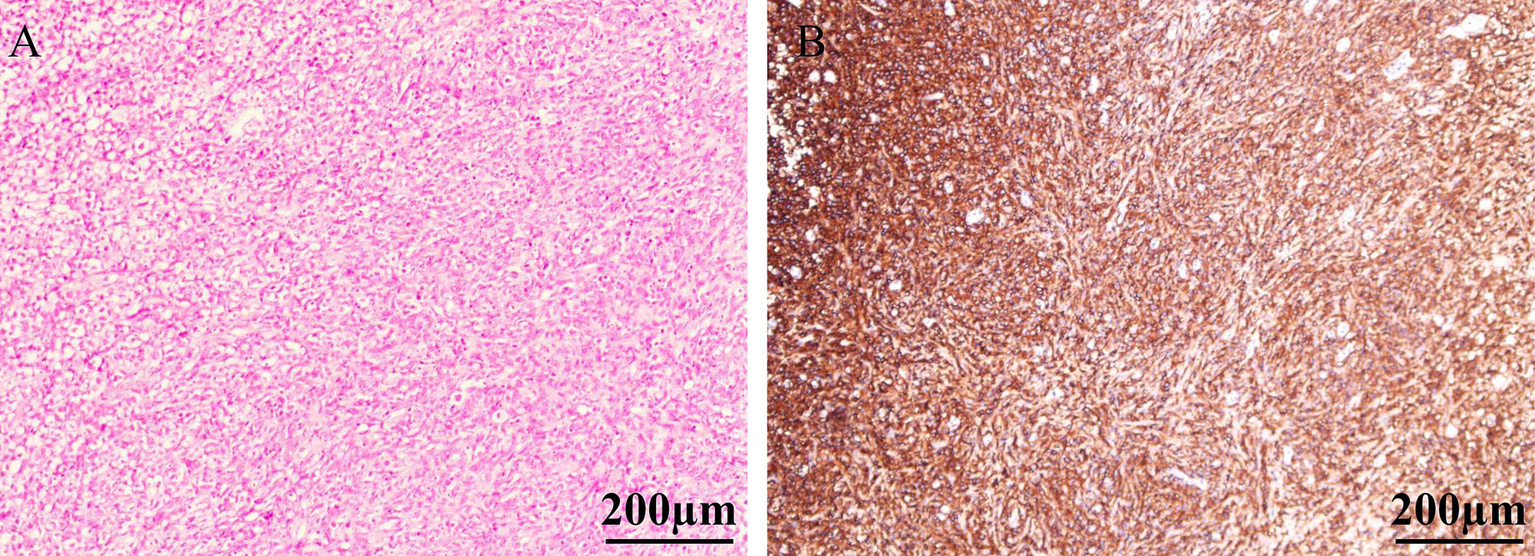
H&E staining revealed diffuse infiltration of the bladder lamina propria by a large number of atypical lymphoid cells, with near-complete replacement of the normal bladder tissue architecture (A). The tumor cells exhibited diffuse strong positivity with brown staining on the cell membrane, indicating widespread expression of CD20 antigen (B).
Figure 3

The whole-body 18F-FDG PET/CT. Maximum intensity projection (MIP) image prior to chemotherapy (A). Axial PET, PET/CT fusion and CT before furosemide administration (B–D) demonstrate the lesion. Following intravenous furosemide administration, post-diuretic axial PET (E), PET/CT fusion (F), and CT (G) images reveal prominent delineation of the left lateral bladder wall lesion. Post-therapy evaluation after 4 cycles of R-CHOP regimen chemotherapy displays axial PET/CT fusion images of the bladder during standard acquisition (H) and subsequent delayed imaging at 1 h (I), with comparison to pre-chemotherapy post-furosemide images demonstrating notably retained radiotracer activity within the bladder lumen. Corresponding post-chemotherapy CT image of the bladder is presented (J).
Literature review
A comprehensive literature search was conducted in the Web of Science database to identify case reports and case series on primary bladder lymphoma published in English up to March 31, 2025. After systematic search and rigorous full-text review, secondary cases and publications lacking accessible full-text were excluded. The following data were extracted from eligible studies: first author, publication year, patient demographics (gender and age), clinical presentation, imaging findings, and follow-up outcomes (Table 1).
Table 1
| Case no. | First author, year | Age (y)/sex | Symptoms | Location/bladder | US | CT/MRI | Cystoscopy | Bone marrow aspiration | Treatment | Recurrence |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 (13) | Jian-Hui Xin, 2024 | 83/F | Frequency urination | Posterior wall | Uneven hypoechoic lesion | CE: irregular enhancing nodule | Mild trigonal congestion, no neoplasia (post-treatment) | (−) | R-CHOP | (−) |
| 2 (12) | Hideshige Seki, 2024 | 77/M | Fever, anorexia, general malaise, pyuria, bacteremia | NA | NA | Bladder wall thickening with perivesical stranding | NA | (−) | R-CHOP | (+) |
| 3 (2) | Po-Sung Liang, 2024 | 79/F | Urinary frequency and urgency, suprapu-bic pain | Anterior wall | A suspicious 32 mm bladder tumor | CE: 39 mm polypoid enhancing lesion | Multiple bladder tumors | (−) | Radiation therapy | (−) |
| 4 (11) | Xi Tu, 2023 | 81/F | Frequent urination and painful urination | Right side and posterior wall | A hypoechoic mass | Long T1 signal/short T2 signal, homogeneous CE enhancement, DWI restriction | Two nodular lesions | NA | TURBT | (−) |
| 5 (10) | Atsuto Katano, 2022 | 65/F | Macroscopic hematuria, chronic cystitis | Bladder trigone | NA | Intermediate T2 signal intensity | A submucosal tumor | NA | TURBT, Radiation therapy | (−) |
| 6 (9) | Zhen-Zhen Jiang, 2021 | 48/F | Macroscopic hematuria | NA | A large hypoechoic mass | Bladder wall thickening; ill-defined low-density nodules | Multiple bulging bladder masses | (−) | R-CHOP | (−) |
| 7 (8) | Naoya Ishibashi, 2021 | 77/F | Hematuria and severe perineal pain | Bladder trigone | NA | Enlargement of an irregular elevated lesion | Non-papillary nodular tumor | NA | Radiation therapy | (−) |
| 8 (5) | Rodolfo Montironi MD, 2015 | 81/M | Macroscopic hematuria, pain on micturition | Anterior wall to the right lateral bladder wall | NA | A solitary mass lesion | A wide-based mass | (−) | TURBT | (−) |
| 9 (6) | Nischith D’Souza, 2017 | 56/M | Clot retention | Right lateral wall | A mass | NA | Polypoidal mass | NA | TURBT | (−) |
| 10 (7) | Hewei Xue, 2020 | 77/F | Frequent urination, urinary urgency, dysuria | NA | Roughness in the inner walls of the bladder | Multiple nodules | Bladder occupying tumor | (−) | TURBT | (−) |
Clinical and imaging features of the cases of primary bladder lymphoma from the literature review and current case.
US, ultrasound; CT, computed tomography; MRI, magnetic resonance imaging; CE, contrast-enhanced; NA, not applicable; F, female; M, male; T1, T1-weighted imaging; T2, T2-weighted imaging; DWI, diffusion-weighted imaging; TURBT, transurethral resection of bladder tumor; R-CHOP, rituximab + cyclophosphamide + hydroxydaunorubicin + oncovin + prednisone.
A total of 11 cases of primary bladder lymphoma, including the present case, were included in the analysis (2, 5–13). The cohort comprised 3 males (3/11) and 8 females (8/11), with a median age of 77 years (range 48–83). Mucosa-associated lymphoid tissue (MALT) lymphoma was the predominant histopathological subtype. Hematuria emerged as the most frequent clinical manifestation, followed by dysuria, urinary frequency, and recurrent urinary tract infections predominantly caused by Escherichia coli. Most lesions were localized to the lateral walls and trigone region of the bladder, presenting as single or multiple nodular lesions with occasional bladder wall thickening. Ultrasonographic evaluation revealed hypoechoic lesions demonstrating contrast enhancement and restricted diffusion on advanced imaging. Notably, none of the reviewed cases exhibited bone marrow infiltration. Treatment strategies encompassed chemotherapy, radiotherapy, and transurethral resection of bladder tumor (TURBT). In selected cases with localized disease, TURBT alone achieved therapeutic success. The majority of cases demonstrated favorable outcomes with standardized management, with only one case showing tumor recurrence during follow-up, which was associated with documented extravesical infiltration.
Discussion
Approximately 40% of DLBCL cases originate from extranodal sites and the gastrointestinal tract representing the most common extranodal involvement (14, 15). While DLBCL demonstrates men predominance and higher incidence in individuals over 60 years, primary bladder lymphoma exhibits women preponderance (16). Notably, secondary bladder lymphoma occurs more frequently than its primary counterpart (1). The pathogenesis of DLBCL involves multifactorial mechanisms, with established risk factors encompassing genetic predisposition, immune dysregulation, and viral/environmental/occupational exposures (17). Recurrent urinary tract infections (UTIs) have been identified as a predisposing factor for primary bladder lymphoma (16, 18). Although our patient reported no definitive UTI symptoms or medical history, urinalysis revealed leukocyturia. Characteristic clinical manifestations of bladder lymphoma include gross hematuria, dysuria, urinary frequency, nocturia, and abdominal/back pain (16). The present case presented with gross hematuria and increased nocturnal voiding frequency. These nonspecific symptoms necessitate thorough differentiation from common urological conditions such as urinary tract infection, urothelial carcinoma, and mechanical obstruction.
The diagnosis of DLBCL of the bladder relies on imaging techniques, cystoscopy, and complete pathologic evaluation of tissue biopsies. On CT, bladder lymphoma may appear as an intraluminal nodular mass or focal wall thickening with early enhancement within approximately 60 s of contrast injection (19). In our patient, non-contrast CT revealed a nodular soft-tissue density in the left bladder wall with minimal enhancement on contrast imaging. During the excretory phase, a filling defect was observed against the high-density contrast background (Figure 1). Tumors on both T1 and T2-weighted images (T1WI and T2WI) on MRI show intermediate signal, and T1WI are suitable for detecting extravesical infiltration, lymph nodes, and bone metastases, while T2WI are suitable for assessing the depth of the tumor and differentiating the tumor from fibrosis, as well as for detecting peripheral organ invasion and bone marrow metastases (19). However, the limitations of CT and MRI imaging are difficult for the detection of metastatic lesions in normal-sized lymph nodes (20). 18F-FDG PET/CT demonstrates high sensitivity for lesion detection, particularly in identifying foci with no or minimal anatomical abnormalities on CT (21). This modality enables more accurate disease assessment and frequently alters lymphoma staging, consequently modifying therapeutic strategies (22). For patients with baseline PET-positive disease, serial monitoring of SUV changes and detection of new hypermetabolic lesions facilitate treatment response evaluation (22). Metabolic tumor volume (MTV), total lesion glycolysis (TLG), and maximal distance between lesions (Dmax) serve as critical prognostic parameters (23–25). Based on these advantages, we perform 18F-FDG PET/CT for suspected bladder lymphoma cases, which rapidly delineates extravesical involvement and determines whether the disease is secondary to nodal lymphoma. Some studies have shown that diuretic use significantly increases the lesion-to-bladder activity ratio, improving the detection rate (26–28). Moreover, dual-phase 18F-FDG PET/CT is of great clinical value for the staging of bladder cancer (29). Therefore, in the case of this patient, where the bladder lesion did not show well on early visualization (45 min after intravenous administration of 18F-FDG), a delayed visualization (2-h after intravenous administration of 18F-FDG) of 1 h after intravenous furosemide injection was taken (Figure 3). Before the use of diuretics, diffuse physiological 18F-FDG uptake was seen in the bladder due to physiological elimination of the radiotracer, which made it difficult to distinguish the metabolic distribution of the lesion on PET images. Following diuretic administration, PET imaging revealed a focal area of markedly increased metabolic activity in the left lateral wall of the bladder with a SUVmax of 22.7. This value significantly exceeded the average SUVmax of bladder cancer (16.1 ± 6.2) (30). Comparative analysis with the patient’s 18F-FDG PET/CT scan of post-chemotherapy (performed without intravenous diuretic bolus dual-phase imaging) demonstrated reduced background activity within the bladder after diuretic administration (Figure 3).
Although imaging studies provide preliminary differentiation between benign and malignant bladder lesions, histopathological examination remains essential for definitive diagnosis. MALT lymphoma/marginal zone lymphoma (MZL) has been historically regarded as the most common primary bladder lymphoma, while cases with extravesical extension are predominantly DLBCL (12, 31). DLBCL, not otherwise specified (NOS), represents the most frequent category (80–85% of DLBCL cases) and is histologically characterized by diffuse proliferation of large neoplastic cells effacing normal tissue architecture. These tumors demonstrate positivity for B-cell markers (CD20, CD79α, PAX5, CD22, CD19) and CD45 (1). Molecularly, DLBCL can be classified into two major subtypes by gene expression profiling: the germinal center B-cell-like (GCB) subtype and the activated B-cell-like (ABC) subtype (32). However, given the limited clinical availability of gene expression profiling, the immunohistochemistry-based Hans algorithm is routinely employed to categorize cases into GCB and non-GCB groups (the latter encompassing ABC subtypes and most unclassifiable cases) (14). In the present case, Hans classification indicated a non-germinal center B-cell phenotype. Histological examination revealed diffuse infiltration of the bladder lamina propria by sheets of atypical lymphoid cells with near-total effacement of normal bladder architecture. Immunohistochemistry confirmed CD20(+) and PAX-5(+) expression, establishing a B-cell lineage. A high Ki-67 proliferation index indicated aggressive tumor biology. These findings are consistent with DLBCL, leading to a definitive diagnosis of primary bladder diffuse large B-cell lymphoma.
DLBCL is a group of highly heterogeneous malignancies in terms of clinical manifestations, histomorphology and prognosis (1). The R-CHOP regimen remains the first-line treatment for DLBCL (1, 14, 33). However, its efficacy is suboptimal in elderly patients or those with comorbidities (34). For relapsed or refractory DLBCL, novel therapies such as chimeric antigen receptor T-cell (CAR-T) therapy and hematopoietic stem cell transplantation have emerged (34). Other approaches, including localized radiotherapy, BTK inhibitors, and immunomodulators, are primarily used as monotherapy or in combination to alleviate symptoms (35). The majority of primary bladder DLBCL cases (56.56%) were diagnosed at Stage I. Advanced age was identified as a poor prognostic factor, with patients aged ≥75 years exhibiting 2–3 times higher mortality than younger patients. The 5-year overall survival rate was 27.10% for patients ≥75 years versus 64.29% for those under 60 years (1). The patient has completed 4 cycles of R-CHOP chemotherapy. A follow-up 18F-FDG PET/CT scan revealed no increased uptake in the bladder wall lesion. Mildly increased uptake was observed in the bone marrow cavity due to post-chemotherapy bone marrow hyperplasia, with no other lesions showing elevated 18F-FDG uptake. Clinical evaluation indicated complete remission, and the patient is currently in good condition under regular treatment.
Conclusion
Primary bladder DLBCL is a rare subtype of bladder lymphoma, predominantly affecting women. Dual-phase 18F-FDG PET/CT imaging significantly improves diagnostic accuracy for primary bladder lymphoma. When high bladder radiotracer activity causes imaging artifacts, a diuretic-enhanced washout protocol combined with controlled bladder distension can accelerate urine clearance and improve the target-to-background ratio.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Writing – original draft. XH: Conceptualization, Methodology, Project administration, Validation, Writing – original draft. JC: Investigation, Project administration, Supervision, Visualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Zanelli M Sanguedolce F Zizzo M Palicelli A Pellegrini D Farinacci S et al . Primary diffuse large B-cell lymphoma of the urinary bladder: update on a rare disease and potential diagnostic pitfalls. Curr Oncol. (2022) 29:956–68. doi: 10.3390/curroncol29020081
2.
Liang PS Shih HJ Huang SH Chen YZ . Primary lymphoma of mucosa associated lymphoid tissue (MALT lymphoma) in the urinary bladder mimicking recurrent urinary tract infection: a case report and literature review. BMC Urol. (2024) 24:223. doi: 10.1186/s12894-024-01616-3
3.
Trotman J Barrington SF . The role of PET in first-line treatment of Hodgkin lymphoma. Lancet Haematol. (2021) 8:e67–79. doi: 10.1016/s2352-3026(20)30357-4
4.
Cronin CG Swords R Truong MT Viswanathan C Rohren E Giles FJ et al . Clinical utility of PET/CT in lymphoma. AJR Am J Roentgenol. (2010) 194:W91–W103. doi: 10.2214/AJR.09.2637
5.
Montironi R Santoni M Goteri G Mazzucchelli R Lopez-Beltran A Cheng L et al . Pseudocarcinomatous hyperplasia associated with primary lymphoma in the urinary bladder: a case report. Hum Pathol. (2015) 46:1040–4. doi: 10.1016/j.humpath.2015.03.004
6.
D'Souza N Verma A Rai A . Two cases of non-Hodgkin lymphoma of urinary bladder: primary and secondary. J Cancer Res Ther. (2017) 13:1053–5. doi: 10.4103/0973-1482.179071
7.
Xu H Chen Z Shen B Wei Z . Primary bladder mucosa-associated lymphoid tissue lymphoma: a case report and literature review. Medicine (Baltimore). (2020) 99:e20825. doi: 10.1097/md.0000000000020825
8.
Ishibashi N Nakanishi Y Nishimaki H Maebayashi T Masuda S Okada M . Bladder mucosa-associated lymphoid tissue lymphoma progressed from chronic cystitis along with a comparative genetic analysis during long-term follow-up: a case report. Transl Androl Urol. (2021) 10:3899–906. doi: 10.21037/tau-21-602
9.
Jiang ZZ Zheng YY Hou CL Liu XT . Primary mucosal-associated lymphoid tissue extranodal marginal zone lymphoma of the bladder from an imaging perspective: a case report. World J Clin Cases. (2021) 9:10024–32. doi: 10.12998/wjcc.v9.i32.10024
10.
Katano A Yamashita H . Primary urinary bladder marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue. J Cancer Res Ther. (2022) 18:1814–6. doi: 10.4103/jcrt.jcrt_238_21
11.
Tu X Zhuang X Li F Huang C Qian Y . Rare primary bladder mucosa-associated lymphoid tissue lymphoma: a case report and review of literature. Front Oncol. (2022) 12:1042459. doi: 10.3389/fonc.2022.1042459
12.
Seki H Mizuno S Saigusa S Sugita Y Iida Y Shinohara S et al . Primary bladder lymphoma with Extravesical extension: a case report and literature review on prognosis and clinical characteristics. J Clin Med. (2024) 13:4340. doi: 10.3390/jcm13154340
13.
Xin JH Jiang B Yuan YH Zou XF . Primary diffuse large B-cell lymphoma of the urinary bladder: a case report. Asian J Surg. (2024) 47:1930–1. doi: 10.1016/j.asjsur.2023.12.050
14.
Sehn LH Salles G . Diffuse large B-cell lymphoma. N Engl J Med. (2021) 384:842–58. doi: 10.1056/NEJMra2027612
15.
Ilica AT Kocacelebi K Savas R Ayan A . Imaging of extranodal lymphoma with PET/CT. Clin Nucl Med. (2011) 36:e127–38. doi: 10.1097/RLU.0b013e31821c99cd
16.
Venyo AK . Lymphoma of the urinary bladder. Adv Urol. (2014) 2014:327917. doi: 10.1155/2014/327917
17.
Berhan A Almaw A Damtie S Solomon Y . Diffuse large B cell lymphoma (DLBCL): epidemiology, pathophysiology, risk stratification, advancement in diagnostic approaches and prospects: narrative review. Discov Oncol. (2025) 16:184. doi: 10.1007/s12672-025-01958-w
18.
Lontos K Tsagianni A Msaouel P Appleman LJ Nasioudis D . Primary urinary tract lymphoma: rare but aggressive. Anticancer Res. (2017) 37:6989–95. doi: 10.21873/anticanres.12167
19.
Wong-You-Cheong JJ Woodward PJ Manning MA Sesterhenn IA . From the archives of the AFIP: neoplasms of the urinary bladder: radiologic-pathologic correlation. Radiographics. (2006) 26:553–80. doi: 10.1148/rg.262055172
20.
Zhu M Zhuo Q Liu W Guan C Zuo Y . Imaging evaluation of Para-aortic lymph nodes in cervical cancer. Acta Radiol. (2023) 64:2611–7. doi: 10.1177/02841851231179178
21.
Musto A Grassetto G Marzola MC Chondrogiannis S Maffione AM Rampin L et al . Role of 18F-FDG PET/CT in the carcinoma of the uterus: a review of literature. Yonsei Med J. (2014) 55:1467–72. doi: 10.3349/ymj.2014.55.6.1467
22.
Society of Nuclear Medicine, Chinese Medical Association . Clinical application guideline for PET/CT and PET/MR imaging in lymphoma (2025 edition). Chin J Nucl Med Mol Imaging. (2025) 45:94–104. doi: 10.3760/cma.j.cn321828-20240924-00333
23.
Driessen J Zwezerijnen GJC Schöder H Drees EEE Kersten MJ Moskowitz AJ et al . The impact of semiautomatic segmentation methods on metabolic tumor volume, intensity, and dissemination Radiomics in (18)F-FDG PET scans of patients with classical Hodgkin lymphoma. J Nucl Med. (2022) 63:1424–30. doi: 10.2967/jnumed.121.263067
24.
Durmo R Donati B Rebaud L Cottereau AS Ruffini A Nizzoli ME et al . Prognostic value of lesion dissemination in doxorubicin, bleomycin, vinblastine, and dacarbazine-treated, interimPET-negative classical Hodgkin lymphoma patients: a radio-genomic study. Hematol Oncol. (2022) 40:645–57. doi: 10.1002/hon.3025
25.
Cottereau AS Meignan M Nioche C Capobianco N Clerc J Chartier L et al . Risk stratification in diffuse large B-cell lymphoma using lesion dissemination and metabolic tumor burden calculated from baseline PET/CT(dagger). Ann Oncol. (2021) 32:404–11. doi: 10.1016/j.annonc.2020.11.019
26.
Nayak B Dogra PN Naswa N Kumar R . Diuretic 18F-FDG PET/CT imaging for detection and locoregional staging of urinary bladder cancer: prospective evaluation of a novel technique. Eur J Nucl Med Mol Imaging. (2013) 40:386–93. doi: 10.1007/s00259-012-2294-6
27.
Hsieh YC Yao WJ Chiu NT Yang WH Huang HS . Pre-hydration and a forced diuresis protocol for (18)F-FDG PET/CT yielded an optimal effect on primary pelvic malignancies. J Clin Med. (2024) 13:6090. doi: 10.3390/jcm13206090
28.
Mantzarides M Papathanassiou D Bonardel G Soret M Gontier E Foehrenbach H . High-grade lymphoma of the bladder visualized on PET. Clin Nucl Med. (2005) 30:478–80. doi: 10.1097/01.rlu.0000167482.23562.ab
29.
Li H Wu H Wang Q Han Y Wang Q . Value of dual-phase (18)F-FDG PET/CT in preoperative staging of bladder cancer. Nan Fang Yi Ke Da Xue Xue Bao. (2014) 34:500–3.
30.
Ateş SG Demirel BB Başar H Uçmak G . The added-value of staging (18)F-FDG PET/CT in the prediction of overall survival in the patients with bladder Cancer. Mol Imaging Radionucl Ther. (2024) 33:11–8. doi: 10.4274/mirt.galenos.2023.65002
31.
Kempton CL Kurtin PJ Inwards DJ Wollan P Bostwick DG . Malignant lymphoma of the bladder: evidence from 36 cases that low-grade lymphoma of the MALT-type is the most common primary bladder lymphoma. Am J Surg Pathol. (1997) 21:1324–33. doi: 10.1097/00000478-199711000-00007
32.
Alizadeh AA Eisen MB Davis RE Ma C Lossos IS Rosenwald A et al . Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature. (2000) 403:503–11. doi: 10.1038/35000501
33.
Susanibar-Adaniya S Barta SK . 2021 update on diffuse large B cell lymphoma: a review of current data and potential applications on risk stratification and management. Am J Hematol. (2021) 96:617–29. doi: 10.1002/ajh.26151
34.
Lymphoid Disease Group CSoHCMA . Chinese expert consensus on the diagnosis and management of elderly patients with diffuse large B-cell lymphoma. Zhonghua Xue Ye Xue Za Zhi. (2024) 45:322–9. doi: 10.3760/cma.j.cn121090-20231228-00343
35.
Wedding U . Palliative care of patients with haematological malignancies: strategies to overcome difficulties via integrated care. Lancet Healthy Longev. (2021) 2:e746–53. doi: 10.1016/s2666-7568(21)00213-0
Summary
Keywords
primary bladder lymphoma, Extranodal lymphoma, diffuse large B-cell lymphoma (DLBCL), PET-CT, diuretic
Citation
Li Y, Hu X and Cai J (2025) Primary bladder diffuse large B-cell lymphoma: a rare case report and literature review. Front. Med. 12:1613673. doi: 10.3389/fmed.2025.1613673
Received
17 April 2025
Accepted
30 June 2025
Published
21 July 2025
Volume
12 - 2025
Edited by
Carmelo Caldarella, Fondazione Policlinico Universitario A. Gemelli IRCCS, Italy
Reviewed by
Susovan Jana, National Institute of Mental Health (NIH), United States
Yassir Benameur, Mohammed V Military Teaching Hospital, Morocco
Updates
Copyright
© 2025 Li, Hu and Cai.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiong Cai, jiong_cai@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.