- Medical Laboratory Department, Affiliated Dongtai Hospital of Nantong University, Dongtai, China
The gut microbiota plays a pivotal role in the pathogenesis and progression of various liver diseases, including viral hepatitis, alcoholic fatty liver disease, metabolic dysfunction-associated steatotic liver disease, drug-induced hepatitis, liver cirrhosis, hepatocellular carcinoma, and other hepatic disorders. Research indicates that dysbiosis of the gut microbiota can disrupt the integrity of the intestinal barrier and interfere with the immune functions of the gut-liver axis, thereby mediating the progression of liver diseases. Analysis of microbial composition and metabolites in fecal samples can assess the diversity of gut microbiota and the abundance of specific microbial populations, providing auxiliary diagnostic information for liver diseases. Furthermore, interventions such as fecal microbiota transplantation, probiotics, prebiotics, bacteriophages, and necessary antibiotic treatments offer multiple approaches to modulate the gut microbiota, presenting promising new strategies for the prevention and treatment of liver diseases. This review summarizes the latest research advances on the role of gut microbiota in liver diseases, offering novel theoretical foundations and practical directions for the diagnosis and treatment of hepatic disorders.
1 Introduction
Liver diseases have emerged as a significant global health challenge, encompassing various conditions such as viral hepatitis, metabolic dysfunction-associated steatotic liver disease (MASLD), alcoholic liver disease (ALD), and hepatocellular carcinoma (HCC) (1). According to GLOBOCAN 2023 statistics, over 800 million people worldwide are affected by liver diseases, with approximately 2 million deaths annually, accounting for 4% of global mortality (2, 3). The gut microbiota, the largest microbial community in the human body, comprises more than 50 phyla and approximately 1,500 different species, playing a crucial role in maintaining health and contributing to disease pathogenesis (4). In healthy individuals, the gut microbiota is predominantly composed of beneficial bacteria such as Bacteroidetes and Firmicutes, along with smaller proportions of Proteobacteria, Actinobacteria, and Verrucomicrobia (5). Recent studies have demonstrated that gut microbiota dysbiosis is closely associated with the development and progression of various liver diseases, including chronic hepatitis B (CHB), ALD, MASLD, liver cirrhosis (LC), and HCC (6). The underlying mechanisms involve gut-liver axis signaling, metabolite regulation, and immune microenvironment remodeling, among others (7–10). Despite advancements in diagnostic and therapeutic technologies for liver diseases, significant challenges remain in diagnostic methods, criteria, and therapeutic targets, highlighting the need for further improvements.
The gut microbiota and liver diseases are closely interconnected through the gut-liver axis. Nutrients, bacterial metabolites, and potential harmful substances in the gut can enter the liver via the portal venous system (11, 12). Simultaneously, the liver delivers metabolites such as bile acids (BA) to the intestinal lumen through the biliary tract, thereby regulating the composition and function of the gut microbiota (13). Additionally, liver diseases can disrupt intestinal barrier function, promote gut microbiota dysbiosis, and further impair the normal function of the gut-liver axis. Dysbiosis of the gut microbiota may exacerbate inflammatory and fibrotic processes in liver diseases, aggravating liver injury.
With advancing research, beyond traditional antiviral and immunomodulatory therapies, modulating the gut microbiota through fecal microbiota transplantation (FMT), probiotics, and specific dietary interventions has emerged as a novel adjunctive approach for liver disease treatment (14, 15). This review summarizes the role of the gut microbiota in the onset, progression, diagnosis, and pathogenesis of liver diseases, and explores the impact of therapeutic strategies aimed at restoring gut microbiota balance on disease progression, thereby providing new insights and methods for the diagnosis and treatment of liver diseases. Furthermore, this review analyzes the specific manifestations of the gut microbiota in different liver diseases, discusses its potential as a diagnostic biomarker in liver diseases, elucidates the mechanisms of microbiota in disease pathogenesis, and evaluates the potential of microbiota-targeted therapies in the management of liver diseases.
2 Expression characteristics, mechanistic research, and diagnostic-therapeutic applications of gut microbiota in viral hepatitis
In 2022, the World Health Organization (WHO) revised the 2030 strategy for the comprehensive elimination of viral hepatitis, initially adopted in 2016, setting forth more specific and quantifiable targets (16). Viral hepatitis, a significant global health burden, is primarily caused by five viruses: hepatitis A virus (HAV), hepatitis B virus (HBV), hepatitis C virus (HCV), hepatitis D virus (HDV), and hepatitis E virus (HEV) (17). The Global Hepatitis Report 2024 disclosed that the number of deaths from viral hepatitis increased from 1.1 million in 2019 to 1.3 million in 2022, with 83% attributable to hepatitis B and 17% to hepatitis C, resulting in approximately 3,500 deaths daily worldwide from these two types of hepatitis (18). Recent studies have indicated a correlation between gut microbiota dysbiosis and the occurrence of viral hepatitis, suggesting that hepatitis virus infections can alter the diversity of the gut microbiome (9, 10).
2.1 Alteration of gut microbiota in HBV
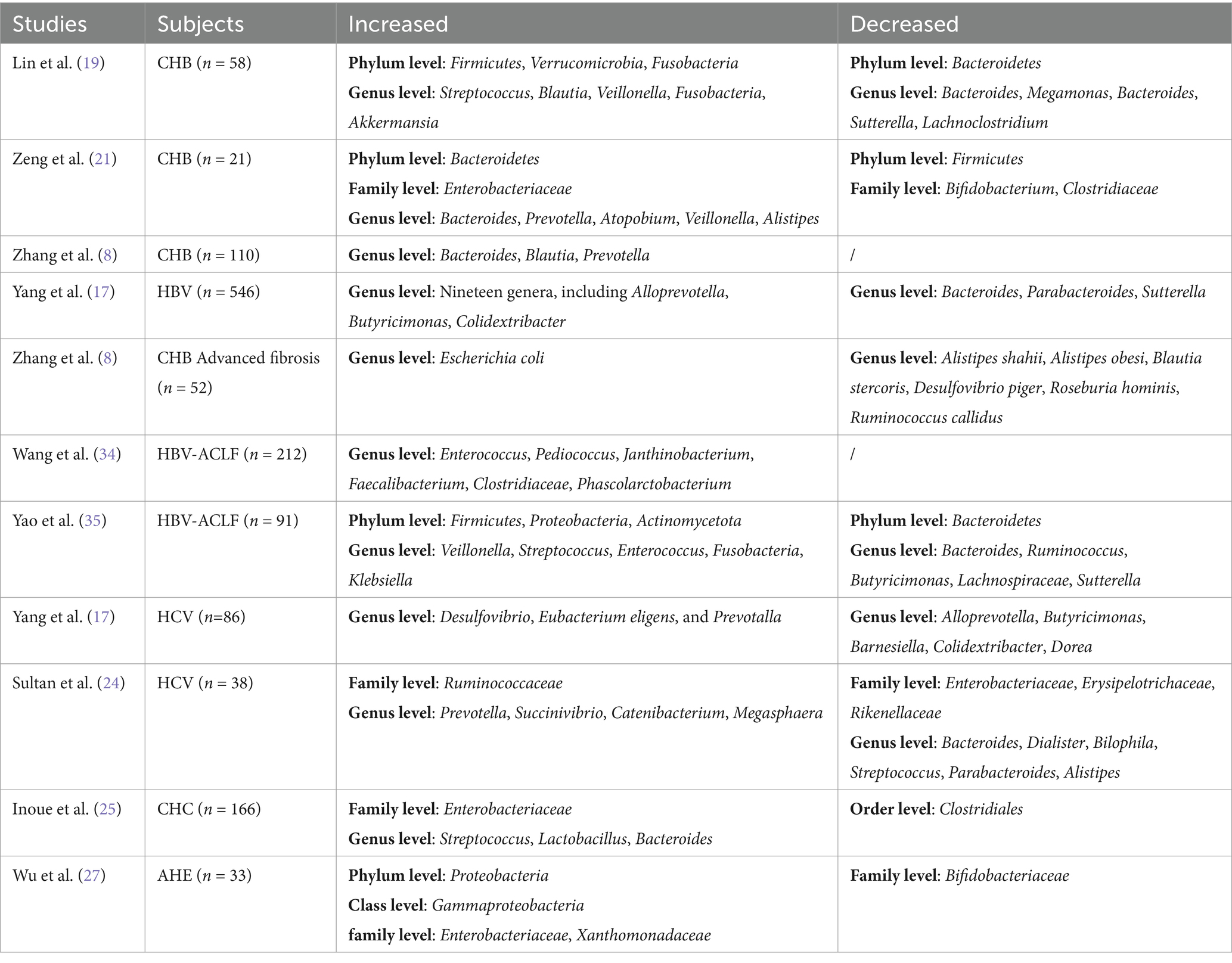
Chronic hepatitis B (CHB) patients exhibit characteristic alterations in gut microbiota: multiple 16S rRNA sequencing studies demonstrate a significant increase in Firmicutes abundance and a decrease in Bacteroidetes, resulting in an elevated Firmicutes/Bacteroidetes (F/B) ratio (19, 20). However, geographical variations may influence this trend, as one study observed reduced Firmicutes and increased Bacteroidetes in CHB patients (21). Further analysis reveals three distinct enterotypes in CHB patients, dominated by Bacteroides, Blautia, and Prevotella, respectively (8). Notably, fecal samples from occult HBV-infected individuals show a marked depletion of butyrate-producing Faecalibacterium coupled with an abnormal enrichment of opportunistic pathogen Subdoligranulum (22). LEfSe analysis confirms differential regulation of 19 genera in HBV-infected individuals, including upregulated Alloprevotella and downregulated Bacteroides (17). Most concerningly, HBV-related liver disease patients exhibit a pro-inflammatory microbiome signature characterized by opportunistic pathogens (e.g., Proteus, Klebsiella) enrichment and butyrate-producing bacteria (e.g., Ruminococcus) depletion (23).
2.2 Alteration of gut microbiota in HCV
In 86 patients with HCV infection, the abundance of 10 taxa, including Desulfovibrio, Eubacterium eligens, and Prevotella, was significantly higher than that in the HC group, while the abundance of 11 genera, such as Barnesiella, Colidextribacter, and Dorea, was significantly reduced (17). Additionally, treatment-naïve HCV patients exhibited increased gut microbiota diversity, with elevated abundances of Prevotella, Megasphaera, and Ruminococcaceae, and decreased abundances of Bacteroides, Streptococcus, and Enterobacteriaceae (24). 16S RNA sequencing analysis also revealed lower bacterial diversity in 166 Japanese patients with chronic hepatitis C (CHC), characterized by a reduction in the order Clostridiales and an increase in Streptococcus and Lactobacillus (25). Compared to the HC group, the total abundance of Lactobacillus and Lactobacillus acidophilus was significantly lower in patients with chronic HCV infection (26).
2.3 Alteration of gut microbiota in HEV, HAV, and HDV
In 33 patients with acute hepatitis E (AHE), the abundance of Proteobacteria, Gammaproteobacteria, and Enterobacteriaceae was significantly higher in the gut compared to the HC group (27). Furthermore, compared to the AHE group, the HEV-associated acute liver failure (ALF) group showed increased abundances of Gammaproteobacteria, Proteobacteria, Xanthomonadaceae, and Stenotrophomonas, and decreased abundances of Firmicutes, Streptococcus, Subdoligranulum, and Lactobacillus (28). HAV, an acute and self-limiting disease, has limited research on gut microbiota changes during infection. 16S rRNA analysis revealed gut microbiota dysbiosis in HIV patients co-infected with HAV, characterized by reduced Proteobacteria abundance and enrichment of Bifidobacterium and Bacteroides, with this dysbiosis persisting long after clinical recovery (29). As for HDV infection, no relevant studies on gut microbiota have been identified, likely because HDV is an incomplete virus requiring HBV for replication, making it challenging to obtain relevant data (17). These findings suggest that regional, dietary, and ethnic differences may contribute to the variability in gut microbiota expression in viral hepatitis-related liver diseases (Table 1). Therefore, long-term, multicenter studies are still needed to further explore the relationship between gut microbiota and viral hepatitis.
2.4 The diagnostic value of microbiota in viral hepatitis
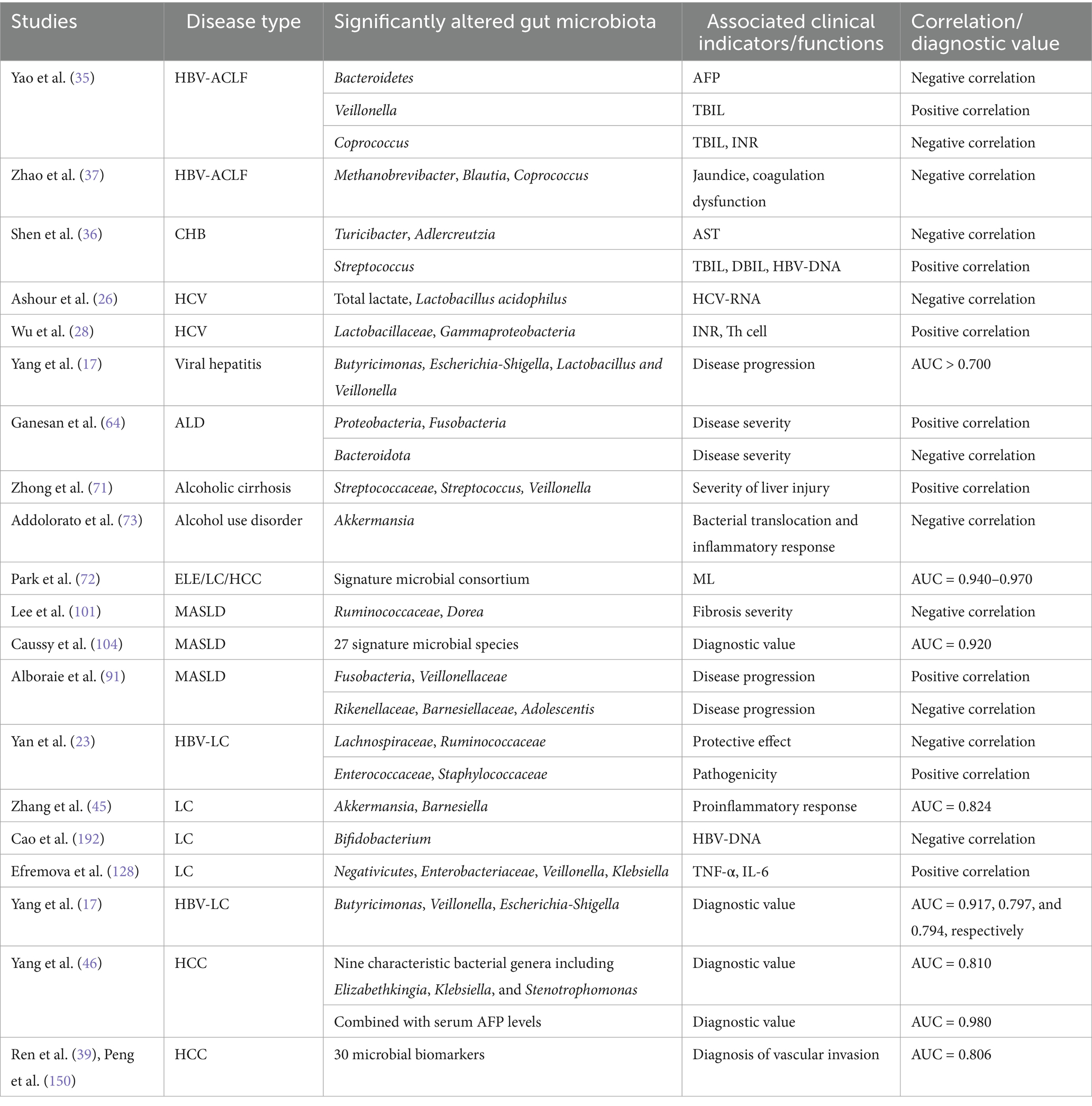
The gut microbiota plays a pivotal role in the progression, early detection, and diagnosis of HBV-related liver diseases, with compositional shifts serving as both prognostic indicators and diagnostic biomarkers (30). A case–control study utilizing Culturomics technology identified Enterocloster bolteae as a novel microbial signature in CHB patients, distinguishing them from healthy controls (31). In treatment-naïve CHB populations, HBeAg positivity correlates with specific taxonomic enrichments: HBeAg+ individuals exhibit elevated levels of Eubacterium coprostanoligenes, Christensenellaceae_R_7, Oscillospirales_UCG_010, and Haemophilus, paralleled by reduced Erysipelatoclostridium and Lachnoclostridium abundance compared to HBeAg- counterparts (32). Notably, these phyla-level differences remain statistically insignificant despite genus-level alterations. Antiviral therapy significantly reshapes microbial profiles. Tenofovir alafenamide-treated HBeAg+ patients demonstrate marked increases in Bacteroidetes, Prevotella, Alistipes, Oxalobacter, and Butyricicoccaceae_UCG_009, coupled with depletion of Proteobacteria, Actinobacteria, and Bifidobacterium species (29). These changes contrast with immune phase-specific biomarkers, where Ruminococcus gnavus and Akkermansia muciniphila differentiate immune-tolerant from immune-active CHB states (33). Microbial dynamics correlate with clinical outcomes across disease spectra. In HBV-ACLF, metagenomic analysis reveals Enterococcus enrichment correlates with disease progression, while Faecalibacterium dominance associates with recovery (34). Bacteroidetes abundance inversely correlates with serum AFP levels, whereas Veillonella shows positive association with total bilirubin (TBIL), and Coprococcus demonstrates dual correlations with TBIL/INR (negative) and prothrombin time (positive) (35). Antiviral intervention partially restores microbial balance, particularly in Blautia, Dorea, and Ruminococcaceae_UCG-013 populations (36). Complications such as portal hypertension and hepatic encephalopathy (HE) exhibit microbial predictors. TIPS therapy preserves microbial diversity in HBV-related portal hypertension, though HE-free patients show superior microbiota synergy compared to post-TIPS HE cases (37). In ACLF, Blautia, Coprococcus, and Methanobrevibacter abundance inversely correlates with coagulopathy and jaundice severity (37). Cross-viral comparisons reveal conserved and disease-specific patterns. HCV patients demonstrate post-treatment enrichment of Coriobacteriaceae and Staphylococcaceae with concurrent Morganellaceae reduction (38), while HEV severity correlates with Lactobacillaceae and Gammaproteobacteria abundance (28). ROC analysis identifies Butyricimonas, Escherichia-Shigella, and Veillonella as potential progression markers for viral hepatitis (AUC > 0.700) (17), with five HCV-specific OTUs achieving AUC > 0.710 (24) (Table 2). Despite these advances, the field lacks standardized biomarkers. Longitudinal multicenter studies remain essential to validate microbial signatures, clarify causal mechanisms, and establish microbiota-based diagnostics for viral hepatitis management.
2.5 Mechanisms of the gut microbiota in viral hepatitis
The gut microbiota plays a crucial role in metabolic processes, not only facilitating the digestion and absorption of food but also producing various metabolites that influence host metabolic functions. During HBV infection, bacteria from the Leptospiraceae family may exert a positive role in managing HBV infection by reducing bacterial translocation and lowering lipopolysaccharide (LPS) levels (39). Multi-omics analysis has demonstrated that electroacupuncture combined with tenofovir disoproxil fumarate can increase the abundance of gut microbiota such as Bacteroides and Blautia by modulating the PPAR signaling pathway, while enhancing the expression of tight junction proteins (ZO-1, Occludin, Claudin-4), thereby improving intestinal barrier integrity (40). Additionally, Enterocloster bolteae isolated from chronic HBV patients can produce ethanol, potentially promoting the progression of liver disease (31). Ruminococcus gnavus promotes cholic acid production by secreting bile salt hydrolase, which activates the farnesoid X receptor alpha (FXRα) signaling pathway. This process enhances the transcription of HBV core antigen (HBcAg), thereby prolonging the HBV immune tolerance phase. Conversely, Akkermansia muciniphila suppresses Ruminococcus gnavus growth and its bile acid-converting function through metabolite secretion, reduces CA levels, blocks the FXRα-HBcAg axis, and facilitates HBV clearance (33). The reduction of BA in viral hepatitis is associated with increased intestinal permeability, leading to elevated levels of LPS and other endotoxins, which promote the progression of liver disease (41). In an LPS-treated mouse model of HBV replication, gut microbiota dysbiosis triggers endotoxemia, inducing Kupffer cells to produce IL-10 and enhancing Kupffer cell-mediated T cell suppression, which plays a critical role in HBV persistence (42). Depletion of gut microbiota impairs systemic anti-HBV humoral and cellular immune responses, resulting in delayed clearance of HBV antigens (43). Furthermore, bacterial extracts derived from HBV-CLD patients stimulate peripheral blood mononuclear cells, potentially promoting fibrotic progression by altering peripheral immune responses (elevated Th17 and reduced Th1), which adversely affects patient prognosis (36).
HCV infection drives disease progression by inducing alterations in the intestinal bile acid profile and gut microbiota dysbiosis, which downregulate CYP8B1 expression (a key enzyme in cholic acid biosynthesis), thereby perpetuating pathogenesis through the gut-microbiome-liver axis (44). Increased circulating LPS levels in CHC patients indicate that microbial translocation is closely linked to hepatic inflammation and injury, thereby driving disease progression (45). Additionally, impaired intestinal barrier function in HCV patients is evidenced by elevated levels of zonulin-1, LPS, and calprotectin, suggesting that intestinal inflammation, microbial imbalance, and increased barrier permeability play significant roles in the pathophysiology of HCV infection (46). These studies demonstrate that the pathogenesis of viral hepatitis is closely related to intestinal barrier function, microbiota-derived metabolites, and BA metabolism. Viral infections can alter the diversity and composition of gut microbiota, leading to gut-liver axis dysregulation and exacerbating hepatic inflammation and injury. Therefore, modulating gut microbiota may emerge as a novel strategy to improve intestinal barrier function and mitigate liver disease progression. Metabolites and microbiota signatures may serve as potential biomarkers for disease diagnosis, though their clinical application requires further validation. Future research should focus on elucidating the specific mechanisms of gut microbiota in liver diseases to enhance clinical diagnosis and treatment efficacy.
2.6 Treatment of the gut microbiota in viral hepatitis
2.6.1 FMT
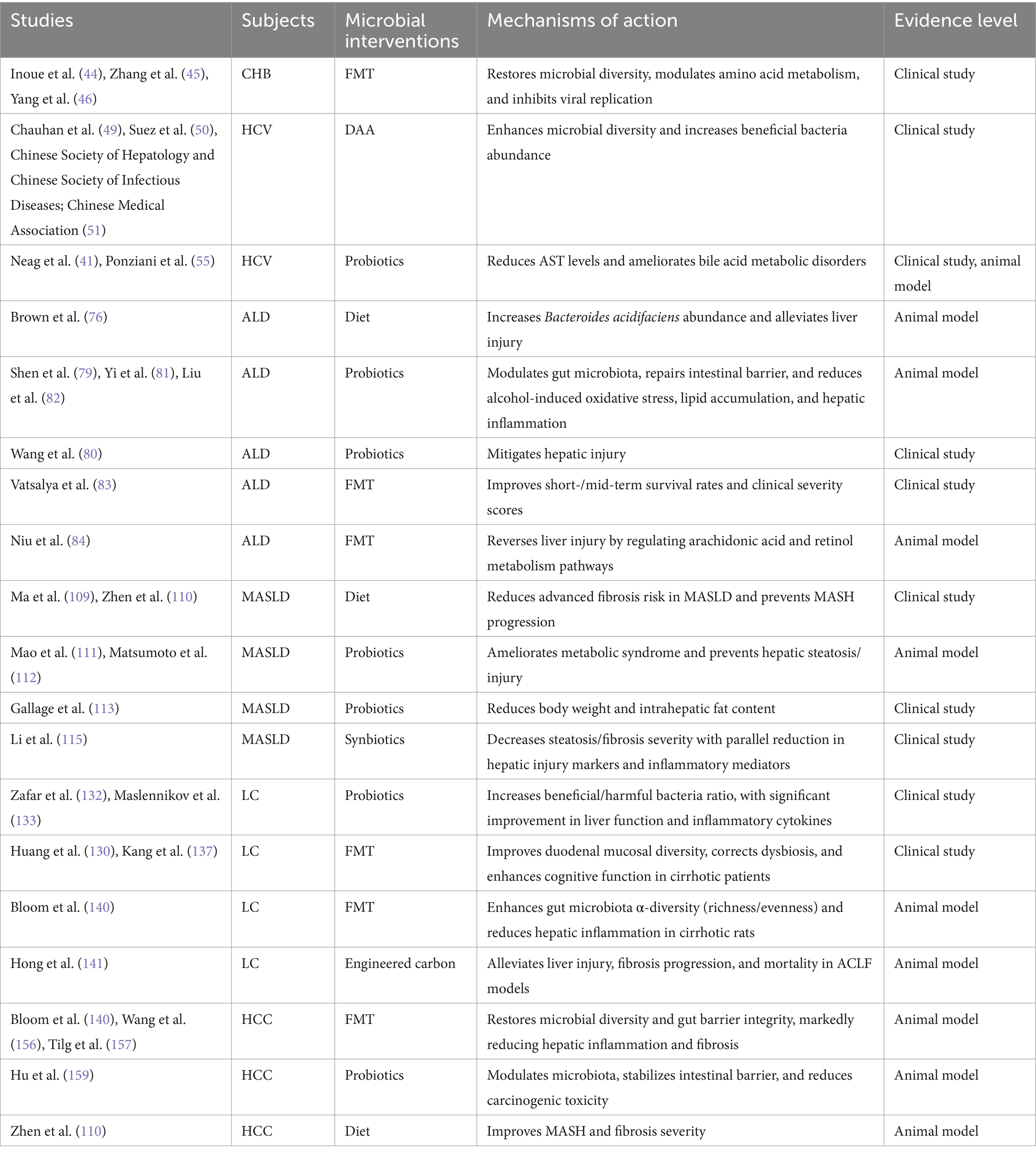
Currently, targeting the gut microbiota has emerged as a novel therapeutic approach for viral hepatitis infections and their complications. FMT as a method to restore and reconstruct the balance and diversity of gut microecology, has demonstrated promising outcomes. In a study involving 20 patients with liver disease related to CHB progression, FMT treatment significantly improved the Shannon and Simpson indices of gut microbiota, repaired the impaired abundance of gut microbiota, and subsequently promoted the improvement of amino acid metabolism (47). In a preliminary study in China, FMT induced HBeAg clearance in 18 HBeAg-positive patients who had undergone long-term antiviral therapy (48). Similarly, in a non-randomized pilot clinical trial involving 14 CHB patients in India, the potential safety and efficacy of FMT in achieving viral suppression and HBeAg clearance in HBeAg-positive CHB patients were observed (49). Furthermore, a study by Suez et al. found that the benefits of probiotics might be counteracted by the restoration of the intestinal mucosa following antibiotic use, whereas autologous FMT could rapidly and nearly completely restore the intestinal mucosa within days after administration (50). This suggests that autologous FMT or the development of personalized probiotic approaches may help achieve intestinal mucosal protection without interfering with the antibiotic-induced disruption of host microbiome recolonization (50).
2.6.2 Direct-acting antiviral
In studies targeting HCV, the goal of antiviral therapy is to eradicate HCV, mitigate associated liver damage, and ultimately achieve a cure (51). A 72-week DAA treatment study (n = 50) demonstrated significant recovery of microbial diversity, particularly enriching short-chain fatty acid (SCFA)-producing genera (Blautia, Bifidobacterium, Subdoligranulum, and Fusicatenibacter) while reducing microbial translocation markers like lipopolysaccharide-binding protein (LBP) (52). Other studies have also demonstrated that DAA treatment increases microbial diversity, alters bacterial abundance, and benefits intestinal health in patients with HCV-related chronic liver disease (53, 54). However, existing research findings exhibit some inconsistencies. A 12-week trial (n = 42) found quantitative but not qualitative microbiota alterations, providing important insights into the complex relationship between CHC and gut microbiota dysbiosis (38). In contrast, a study by Ponziani et al. found that 12 HCV-related cirrhosis patients exhibited significant changes in their overall gut microbial composition after 1 year of DAA treatment, characterized by a reduction in the abundance of potentially pathogenic bacteria such as Enterobacteriaceae, Enterococcus, and Staphylococcus (55). These discrepancies may be attributed to factors such as sample size and the timing of fecal sample collection. Additionally, studies on the impact of DAA treatment on gut microbiota diversity in CHC patients have yielded divergent results. A prospective study in Germany observed an increase in gut microbiota diversity among non-cirrhotic patients following treatment (56), whereas a prospective study in Taiwan found no significant differences between cirrhotic and non-cirrhotic patient subgroups (57). These inconsistencies may be attributed to confounding factors such as ethnicity and dietary habits, highlighting the need for larger-scale and more rigorously designed studies to further elucidate the impact and mechanisms of DAA treatment on gut microbiota.
2.6.3 Probiotics, farnesoid X receptor ligands, and bacteriophages
Several studies have also explored the potential roles of probiotics, FXR ligands, and bacteriophages in treating HCV infection and its complications. HCV-infected patients administered heat-treated Enterococcus faecalis strain FK-23 exhibited a significant reduction in serum AST levels without adverse side effects (58). CHC patients taking probiotics containing Lactobacillus acidophilus and Bifidobacterium showed a 25% improvement in response to interferon IFN-α and ribavirin therapy (59). DCA, an FXR ligand, may overcome BA metabolic disturbances and ameliorate CHC progression when supplemented directly or through probiotics that convert cholic acid to DCA (44). Additionally, correcting gut microbiota dysbiosis in HCV-infected individuals using specific bacteriophages targeting relevant bacteria has been proposed (60) (Table 3). Although these studies suggest that maintaining intestinal barrier integrity, correcting gut microbiota dysbiosis, preventing microbial translocation, and further reducing chronic inflammation may serve as novel strategies for treating HCV infection and its complications, current research remains limited by small sample sizes and a lack of randomized controlled trial designs. Therefore, the efficacy and safety of these approaches require further validation through large-scale clinical trials. Future research should focus on elucidating the underlying mechanisms and optimizing individualized treatment regimens to enhance clinical utility.
3 Expression characteristics, mechanistic research, and diagnostic-therapeutic applications of gut microbiota in ALD
3.1 Alteration of gut microbiota in ALD
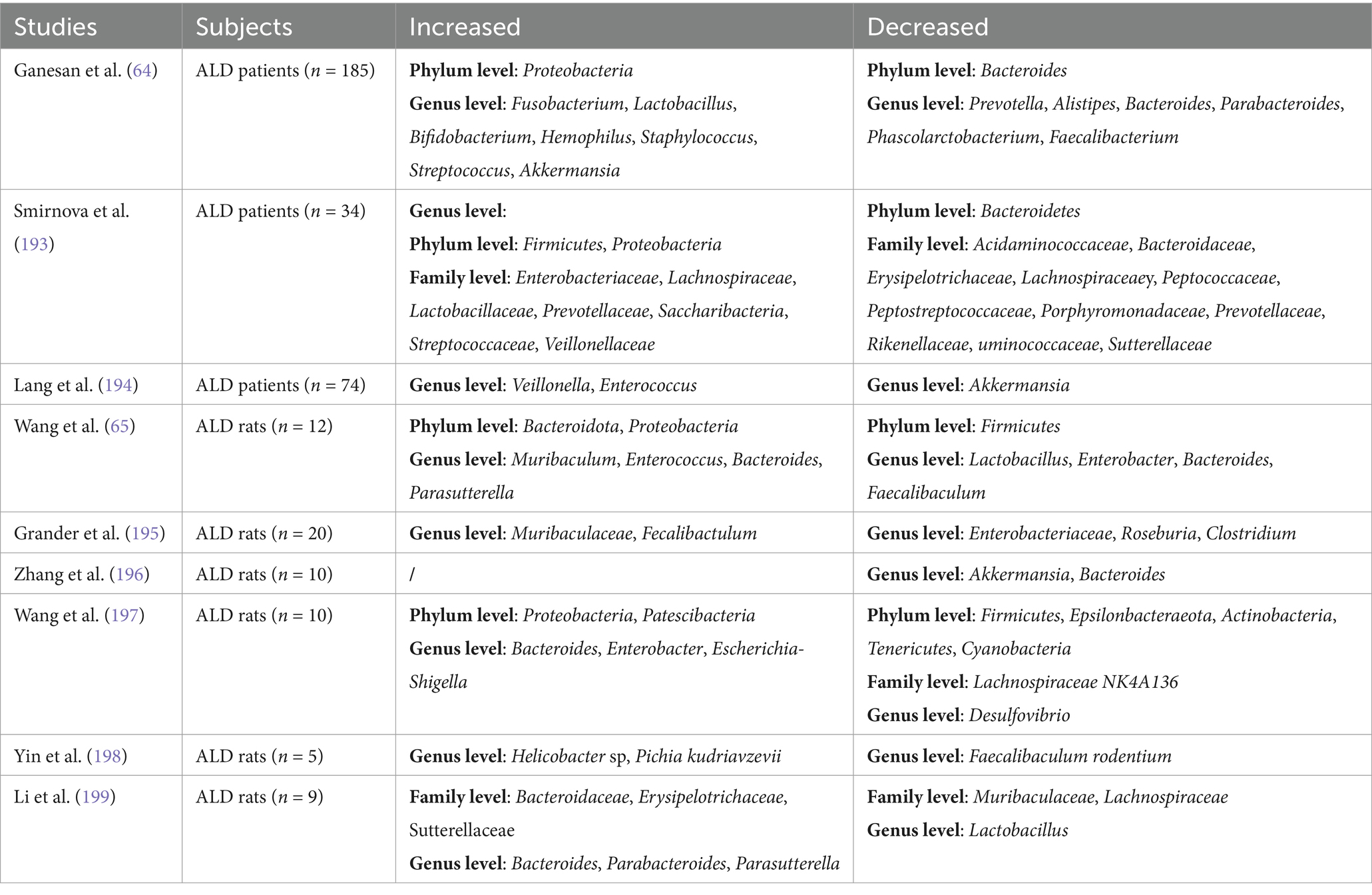
ALD, driven by chronic excessive alcohol intake, progresses from hepatic steatosis to fibrosis and cirrhosis via gut microbiota dysbiosis (61–63). Clinical and animal studies consistently show ALD-associated microbial shifts: increased Proteobacteria and decreased Bacteroidota in patients (64), while murine models reveal ethanol exposure duration-dependent changes—short-term (9 h) ethanol gavage elevates Bacteroidota/Parabacteroides (65), whereas prolonged intake (10–14 days) increases Firmicutes and Akkermansia (66, 67). Alcohol reduces anti-inflammatory taxa (Muribaculaceae, Bacteroides) (68), promotes pathogenic overgrowth (Enterococcus, Alistipes) (69), and disrupts intestinal permeability. Despite elevated Enterococcus faecalis in alcoholic hepatitis patients (n = 75), its abundance lacks correlation with disease severity or mortality (70) (Table 4). These findings highlight the need for larger human cohorts to reconcile interspecies discrepancies and validate therapeutic targets.
3.2 The diagnostic value of microbiota in ALD
Long-term alcohol consumption significantly alters the diversity and composition of gut microbiota, which greatly contributes to the progression of ALD. Studies have shown that the overall structure of gut microbial communities varies significantly across different stages of ALD. Advanced ALD is marked by Proteobacteria/Fusobacteria enrichment and Bacteroidota depletion, with Streptococcus dominance emerging as a potential biomarker for liver injury severity (71). Metagenomic analysis further confirms that the abundance of Proteobacteria increases while that of Bacteroidota decreases with the progression of ALD severity (64). Additionally, analysis of gut microbiota data from 263 ALD patients including elevated liver enzymes (ELE), cirrhosis, and HCC using a machine learning (ML) strategy revealed that the ML strategy achieved diagnostic AUC values of 0.940, 0.970, and 0.960 for ELE, LC, and HCC, respectively, indicating the significant diagnostic value of gut microbiota in ALD (72). In patients with alcohol use disorder, a notable feature of gut microbiota is the reduction of Akkermansia and the increase of Bacteroides. These changes are closely associated with bacterial translocation, inflammatory responses, and enhanced functions of the γ-aminobutyric acid metabolic pathway and energy metabolism, potentially further driving the progression of alcohol-related liver disease (73). Current research highlights the critical role of gut microbiota in the onset and progression of ALD, although the specific mechanisms remain to be further explored. Future studies should integrate multi-omics technologies (e.g., metagenomics, metabolomics) and advanced methods such as machine learning to delve deeper into the relationship between gut microbiota and ALD, and to validate its potential as a diagnostic biomarker.
3.3 Mechanisms of the gut microbiota in ALD
The pathogenesis of ALD has not been fully elucidated and is currently believed to involve multiple factors, including alcohol and its metabolites, gut microbiota dysbiosis, oxidative stress, and gut-liver axis dysfunction (74). Alcohol disrupts the gut-liver axis at multiple levels, including altering the composition of the gut microbiome, impairing mucus and epithelial barrier functions, and suppressing the production of antimicrobial peptides. These changes increase the translocation of microbes and their metabolites, thereby exacerbating the pro-inflammatory environment in the liver (75). Excessive alcohol consumption disrupts the balance of gut microbiota and affects the metabolism of intestinal contents, such as SCFAs, indoles, and BAs, which play critical roles in various physiological and pathological processes and directly influence the progression of ALD (76). Gut bacteria exacerbate hepatic inflammatory injury in ALD by disrupting the intestinal barrier, activating pattern recognition receptors in the liver, altering the metabolism of luminal contents such as BAs, indoleacetic acid, and SCFAs, and producing bacterial exotoxins (e.g., cytolysin) (74). Alcohol induces oxidative damage in hepatocytes by promoting reactive oxygen species generation and inhibiting antioxidant enzyme activity, while blocking Nrf2 nuclear translocation and the expression of its downstream antioxidant genes, thereby weakening hepatic antioxidant defenses; gut microbiota dysbiosis exacerbates hepatic oxidative stress, creating a vicious cycle that highlights the critical role of Nrf2-Keap1 signaling pathway inhibition in ALD progression (77, 78). Current research indicates that the pathogenesis of ALD involves complex interactions among multiple factors and pathways. Future studies should further focus on the interplay between gut microbiota and host metabolism, particularly the specific mechanisms of metabolites such as SCFAs, BAs, and indoles in ALD. Integrating multi-omics technologies and animal model studies will help comprehensively elucidate the pathogenesis of ALD and provide a theoretical basis for developing combined strategies based on gut microbiota modulation and antioxidant therapy.
3.4 Treatment of the gut microbiota in ALD
3.4.1 Diet
In recent years, interventions targeting gut microbiota, including dietary modifications, probiotic supplementation, FMT, and bacteriophage therapy, have demonstrated significant efficacy in improving ALD in numerous animal experiments and clinical trials. A diet rich in soluble dietary fiber increases the abundance of Bacteroides acidifaciens, thereby alleviating alcohol-induced liver injury in mice (79). Additionally, Solanum nigrum L. berry extract significantly ameliorates alcoholic liver injury by modulating gut microbiota, lipid metabolism, inflammation, and oxidative stress (80). Trilobatin, a novel natural food additive, exhibits potential for preventing and treating ALD by regulating the microbiota-gut-liver axis and the YAP/Nrf2 pathway (81).
3.4.2 Probiotics
Probiotics have garnered considerable attention for their role in improving ALD. Lactobacillus rhamnosus NKU FL1-8 reduces alcohol-induced oxidative stress, lipid accumulation, and hepatic inflammation by modulating gut microbiota and repairing the intestinal barrier (82). In clinical studies, a 6-month treatment with Lactobacillus rhamnosus GG significantly reduced liver injury and alcohol consumption in patients with moderate to severe alcoholic hepatitis (83). Furthermore, both viable and inactivated Lactobacillus paracasei CCFM1120 V and D effectively protect the liver from ethanol-induced damage by altering gut microbiota composition, strengthening the intestinal barrier, and enhancing hepatic antioxidant capacity (84). Lactic acid bacteria (LAB) strains have also been shown to directly alleviate ALD symptoms, including reducing inflammatory cytokines, inhibiting fatty liver, and restoring gut microbiota dysbiosis (85).
3.4.3 FMT
FMT, as an emerging therapy, has demonstrated significant efficacy in ALD treatment. Studies indicate that FMT improves short- and medium-term survival rates and clinical severity scores in patients with severe alcoholic hepatitis (86). In animal models, FMT reverses alcohol-induced liver injury by ameliorating gut microbiota dysbiosis and modulating metabolic pathways such as arachidonic acid and retinol metabolism (87). Bacteriophage therapy specifically targeting Enterococcus faecalis has shown promising results in mouse models, reducing cytolysin in the liver and significantly improving ALD (70) (Table 3). However, this therapy still requires validation in larger-scale prospective clinical trials to confirm its relevance in humans.
In a mouse model of acute alcohol exposure, Musculus senhousei peptide intervention exerts protective effects by mitigating gut-liver axis injury and reversing abnormalities in oxidative stress and inflammation-related biomarkers (88). Additionally, lentinan demonstrates significant hepatoprotective effects by reducing hepatic steatosis, alleviating oxidative stress and inflammatory responses, and promoting the proliferation of antioxidant probiotics (89). These findings suggest that interventions targeting gut microbiota hold broad application prospects in ALD treatment.
However, most current studies are limited to animal models or small-scale clinical trials. Future large-scale, multicenter prospective studies are needed to validate the efficacy and safety of these approaches.
4 Expression characteristics, mechanistic research, and diagnostic-therapeutic applications of gut microbiota in MASLD
4.1 Alteration of gut microbiota in MASLD
MASLD, formerly known as non-alcoholic fatty liver disease, is a chronic liver condition affecting approximately 30% of the global population (90). Characterized by abnormal lipid accumulation in hepatocytes, MASLD can progress from simple steatosis to metabolic dysfunction-associated steatohepatitis (MASH), and ultimately lead to hepatic fibrosis, cirrhosis, and even hepatocellular carcinoma (91). Studies have shown that the diversity and abundance of gut microbiota are significantly reduced in MASLD patients compared to HC (92). However, findings vary across regions and ethnicities. For example, a study in Indonesia involving 37 MASLD patients reported a predominance of Firmicutes and an elevated F/B ratio (93). In contrast, a study in Taiwan involving 50 biopsy-confirmed MASLD patients demonstrated a higher abundance of Bacteroidetes, a lower abundance ofFirmicutes, a reduced F/B ratio, and decreased levels of Ruminococcaceae, Clostridiales, and Clostridium compared to healthy individuals (94). A study in Korea involving 23 MASLD patients with elevated liver enzymes found an enrichment of Firmicutes, an increased F/B ratio, and a significant rise in the abundance of Veillonella, Dialister, Collinsella, Latilactobacillus, and Bifidobacterium (95). These inconsistencies in F/B ratios may be attributed to differences in dietary habits across countries. Furthermore, animal model studies support the role of gut microbiota in MASLD. In mouse models of MASLD induced by a high-fat diet (HFD) or Western diet, the F/B ratio was significantly elevated (96). In MASLD patients, the abundance of beneficial bacteria such as Akkermansia muciniphila, Faecalibacterium prausnitzii, and Bifidobacterium is significantly reduced (97). A study involving 100 adolescent MASLD patients found a notable decrease in the abundance of Lactobacillus and Escherichia coli and a significant increase in Prevotella (98). Metagenomic sequencing analysis further revealed an increased abundance of Bacteroidetes and a reduced abundance of 11 genera, including Alistipes, Barnesiella, and Eisenbergiella, in MASLD patients (99). These findings indicate that the gut microbiota of MASLD patients exhibits significant diversity, which may be closely related to factors such as ethnicity, dietary habits, and geographic environment.
4.2 The diagnostic value of microbiota in MASLD
Numerous studies have elucidated the critical role of the gut microbiome in the progression of MASLD (100). The gut microbiome critically influences MASLD progression through compositional and functional alterations. Non-obese MASLD patients exhibit reduced Ruminococcaceae abundance compared to obese counterparts, with its depletion correlating with fibrosis severity (101), while protective Dorea shows similar depletion patterns (102). Pathogenic Klebsiella pneumoniae (identified in 60% of Chinese MASLD patients) directly drives MASLD development via alcohol production, as validated by FMT experiments (103). Distinct microbial signatures differentiate disease stages: Streptococcus enrichment occurs in both MASLD and MASLD-cirrhosis groups, but Megasphaera is exclusive to cirrhosis (104). Diagnostic models integrating 27 bacterial features achieve high accuracy (AUC = 0.920) for cirrhosis detection (104). Metabolic shifts are evident in MASLD, characterized by ethanol-producing bacteria (Enterobacteriaceae, Megasphaera) dominance and SCFA-producing taxa (Ruminococcus, Eggerthellaceae) reduction (105). Advanced fibrosis associates with Methanobrevibacter depletion and Slackia enrichment (106). Machine learning identifies 12 MASLD-linked taxa, including Fusobacteria (positive correlation) and Rikenellaceae (negative correlation) (91) (Table 2). These findings underscore gut microbiota’s role in MASLD severity stratification and prognostic prediction.
4.3 Mechanisms of the gut microbiota in MASLD
The onset of MASLD is closely associated with dysfunction of the gut microbiome, primarily involving two mechanisms: abnormal elevation of intestinal barrier permeability and imbalance of microbiota-derived metabolites (107). Trimethylamine-N-oxide (TMAO), a microbiota-derived metabolite, disrupts tight junction proteins to impair intestinal barrier integrity while directly promoting hepatic lipid accumulation in both cellular (HepG2) and rodent models (108). This process is driven by gut bacterial conversion of trimethylamine (TMA) to TMAO via hepatic FMO3, with clinical studies confirming serum TMAO levels as a biomarker of steatosis severity (109, 110). Concurrently, pathogenic bacteria such as Klebsiella pneumoniae exacerbate MASLD through alcohol-mediated steatosis and inflammation (103), while Gram-negative overgrowth (e.g., Enterobacter cloacae B29) triggers TLR4/NF-κB signaling via endotoxin release (107). Contrastingly, protective microbiota like Ruminococcus attenuate lipid deposition through SCFA production and 7α-dehydroxylase regulation (102). Bile acid metabolism is further modulated by gut microbes, with ursodeoxycholic acid (UDCA) enhancing autophagy and mitochondrial function to restore host-microbiota equilibrium (111). These findings suggest that the role of gut microbiota in MASLD is dualistic, encompassing both disease-promoting factors and potential protective mechanisms (Figure 1).
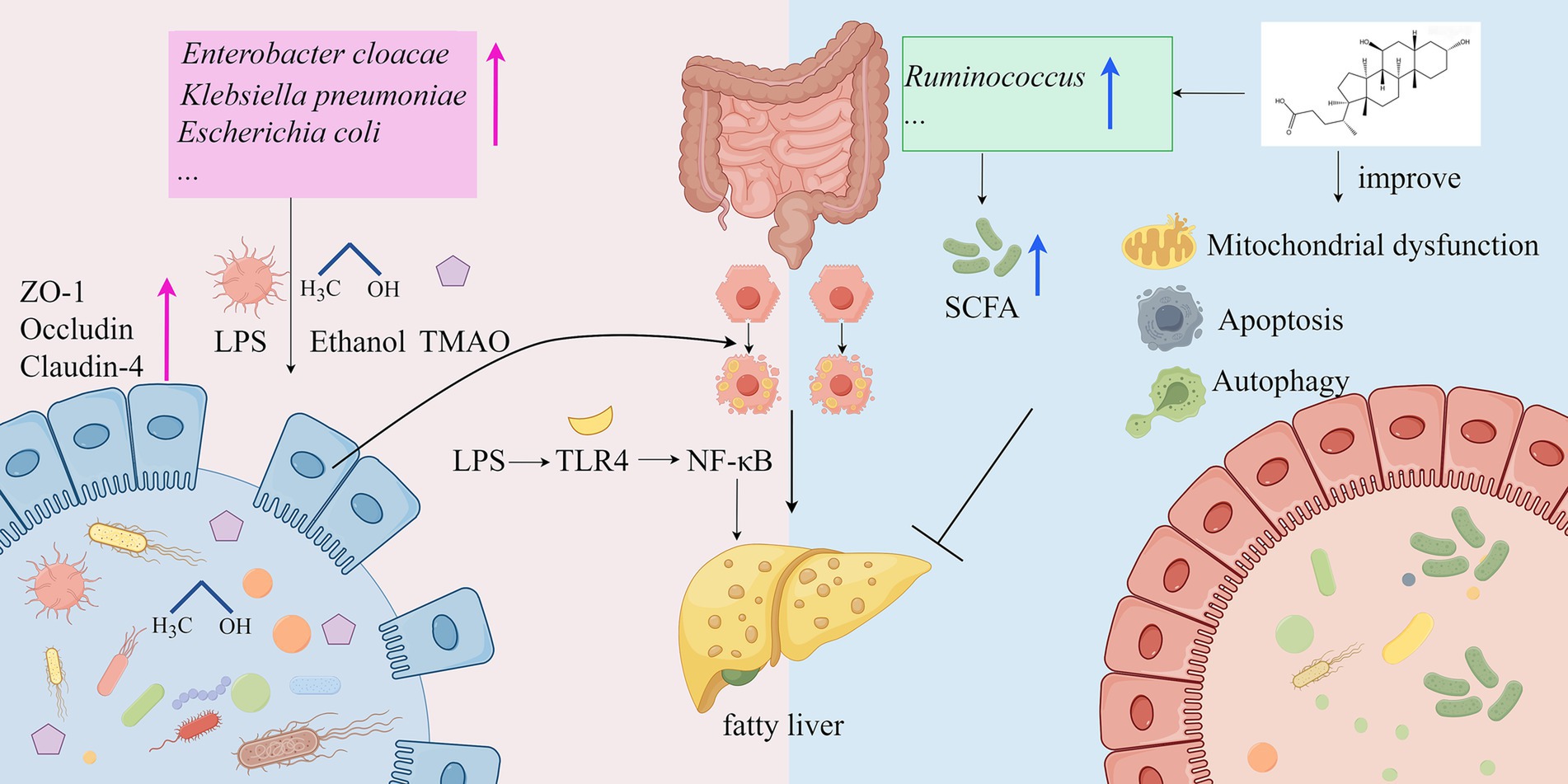
Figure 1. Mechanisms of the gut microbiota in the development of MASLD (by Figdraw). LPS, lipopolysaccharide; ZO-1, Zonula occludens-1; TMAO, Trimethylamine-N-oxide; TLR4: Toll-like Receptor 4; NF-KB, Nuclear Factor kappa-light-chain-enhancer of activated B cells; SCFA, short-chain fatty acid.
4.4 Treatment of the gut microbiota in MASLD
4.4.1 Diet
Currently, a balanced diet and healthy lifestyle are considered the best strategies for improving MASLD. The high intake of soy and its products, fish, shellfish, and seaweed in the Japanese diet is significantly associated with a reduced risk of advanced fibrosis in MASLD patients (112), likely due to the anti-inflammatory and antioxidant components abundant in these foods. Additionally, intermittent fasting (e.g., the 5:2 regimen) can effectively prevent the development of MASH and improve diagnosed MASH and fibrosis without affecting total caloric intake (113), suggesting that dietary pattern modulation may have unique intervention effects on MASLD.
4.4.2 Probiotic
In animal models, MASLD mice induced by HFD showed significant reductions in body weight, triglycerides, total cholesterol, and low-density lipoprotein cholesterol levels after supplementation with Lactiplantibacillus plantarum DSR33 (114). Furthermore, the abundance of Bacteroides thetaiotaomicron (B. theta) is positively correlated with the alleviation of metabolic syndrome in both early and late stages of MASLD. HFD-fed mice supplemented with B. theta for 12 weeks exhibited reduced body weight and fat accumulation, improved hyperlipidemia and insulin resistance, and prevention of hepatic steatohepatitis and liver injury (115). These findings suggest that specific bacterial strains may exert therapeutic effects by modulating host metabolism and inflammatory responses. A randomized double-blind study showed that 68 obese MASLD patients experienced significant reductions in body weight and intrahepatic fat after 12 weeks of supplementation with a probiotic mixture (including Lactobacillus acidophilus, Lactobacillus rhamnosus, Lactobacillus paracasei, Pediococcus pentosaceus, Bifidobacterium lactis, and Bacillus breve) (116). However, in another PROBILIVER clinical trial, 44 biopsy-confirmed MASH patients showed no significant improvement in serum liver enzymes, transient elastography, MASLD fibrosis score, or fatty liver index after 24 weeks of probiotic mixture supplementation (117). This discrepancy may be related to the selection of probiotic strains, dosage, treatment duration, and individual patient differences.
4.4.3 Synbiotics and Postbiotics
Additionally, synbiotics (a combination of probiotics and prebiotics) have shown promise in MASLD treatment. A randomized double-blind clinical trial demonstrated that 50 MASLD patients had significant reductions in steatosis and fibrosis, along with decreased levels of liver injury markers and inflammatory mediators, after 28 weeks of synbiotic supplementation (118). Notably, the probiotic Bifidobacterium adolescentis alleviates steatohepatitis by inhibiting lipid peroxidation and NF-κB activation and mitigates non-alcoholic fatty liver through the production of SCFAs (119). Moreover, postbiotics (bacterially derived functional compounds) exhibit significant hepatoprotective properties by enhancing intestinal barrier function, modulating gut microbiota composition, optimizing lipid metabolism, and reducing liver inflammation and steatosis (120) (Table 3).
These studies indicate that dietary adjustments, supplementation with probiotics, prebiotics, or synbiotics positively reshape gut microbiota composition and enhance its activity, thereby improving liver function damage in MASLD patients. However, current research findings remain inconsistent, and future large-scale, long-term clinical trials are needed to validate the efficacy of different intervention strategies and further explore their mechanisms of action.
5 Expression characteristics, mechanistic research, and diagnostic-therapeutic applications of gut microbiota in LC
5.1 Alteration of gut microbiota in LC
LC represents a severe stage of chronic liver disease characterized by extensive hepatocyte degeneration, fibrosis, and nodular regeneration, leading to significant morbidity and mortality (121, 122). In hepatitis B-related cirrhosis, a common subtype of LC, progressive liver damage is further aggravated by gut microbiota dysbiosis and associated metabolic dysfunction (123). Studies consistently demonstrate that LC patients exhibit markedly reduced gut microbial diversity compared to healthy individuals (39, 124), with notable depletion of beneficial bacteria such as Agathobacter and Prevotella_9 alongside overgrowth of opportunistic pathogens including Streptococcus (124, 125). This dysbiotic pattern becomes particularly pronounced in HBV-related cirrhosis patients progressing toward hepatocellular carcinoma, where protective bacterial families like Lachnospiraceae and Ruminococcaceae diminish while potentially harmful Enterobacteriaceae and Staphylococcaceae proliferate (23). Geographic variations in microbiota profiles have been observed, with Chinese LC patients showing elevated Bacteroidota/Firmicutes ratios and Proteobacteria abundance correlated with inflammatory responses (45), while North American cohorts demonstrate distinct associations between Enterobacteriaceae/Streptococcaceae dominance and clinical outcomes including extrahepatic organ failure (126). Emerging diagnostic approaches utilizing microbial signatures, such as machine learning models based on 14 differential bacterial genera, show promising accuracy for LC detection (AUC 0.824) (45). The clinical relevance of these microbial alterations is underscored by their correlations with disease complications, including the association between Akkermansia muciniphila depletion and sarcopenia development (127), as well as the close relationship between pathogenic bacterial overgrowth and systemic inflammatory markers like TNF-α and IL-6 (128) (Table 2). These findings collectively establish gut microbiota dysbiosis as a key contributor to LC progression through multiple interconnected pathways.
5.2 Mechanisms of the gut microbiota in LC
The gut microbiota plays a critical role in the pathogenesis of LC. In LC patients, gut microbiota dysbiosis, bacterial overgrowth, and increased intestinal permeability disrupt the protective mechanisms of the gut, leading to pathological bacterial translocation and increased endotoxin uptake. These endotoxins subsequently reach the liver and mesenteric lymph nodes, activating immune cells and triggering the release of pro-inflammatory cytokines such as TNF-α and IL-8 (129). Meta-analysis results indicate that endotoxin-producing Enterobacteriaceae and Enterococcus are significantly increased in LC patients, which may be related to the impaired intestinal mucosal barrier function caused by LC (130). Gut microbiota dysbiosis leads to intestinal barrier injury (DAO reduction, Claudin-3 dysfunction), bacterial toxin translocation (LPS elevation) triggers systemic inflammation (TNF-α), forming a vicious gut-liver axis cycle that exacerbates liver fibrosis and portal hypertension (125). In LC patients, reduced liver function and decreased BA secretion result in bacterial overgrowth and changes in microbiota composition, weakening the inhibition of potential pathogenic microorganisms and exacerbating intestinal inflammation and mucosal barrier damage (131). Small intestinal bacterial overgrowth (SIBO) can also trigger bacterial translocation and endotoxemia, activating chronic liver inflammation and promoting liver fibrosis (132). Moreover, SIBO is closely associated with hyperdynamic circulation and other hemodynamic changes in cirrhosis patients, potentially serving as a primary factor in inducing these changes through systemic inflammation (133). The impaired intestinal barrier and gut microbiota dysbiosis not only lead to bacterial translocation and endotoxemia but also exacerbate liver injury and fibrosis through abnormal related metabolites. In LC patients, dysbiosis of the ascending colon mucosa-associated microbiota, particularly the reduction of SCFA-producing bacteria, compromises intestinal barrier integrity and BA metabolism, thereby exacerbating liver fibrosis progression via the gut-liver axis. This microbial imbalance correlates with downregulated FGF19 expression and upregulated profibrogenic factors (e.g., TGF-β1), establishing a vicious cycle (134). Additionally, the overgrowth of Viridans streptococci may induce hyperammonemia in CHC and LC patients (25). The gut microbiota plays a pivotal role in the development of cirrhosis, with its dysbiosis not only directly affecting liver inflammation and fibrosis but also influencing systemic metabolism and immune responses through the gut-liver axis (Figure 2).
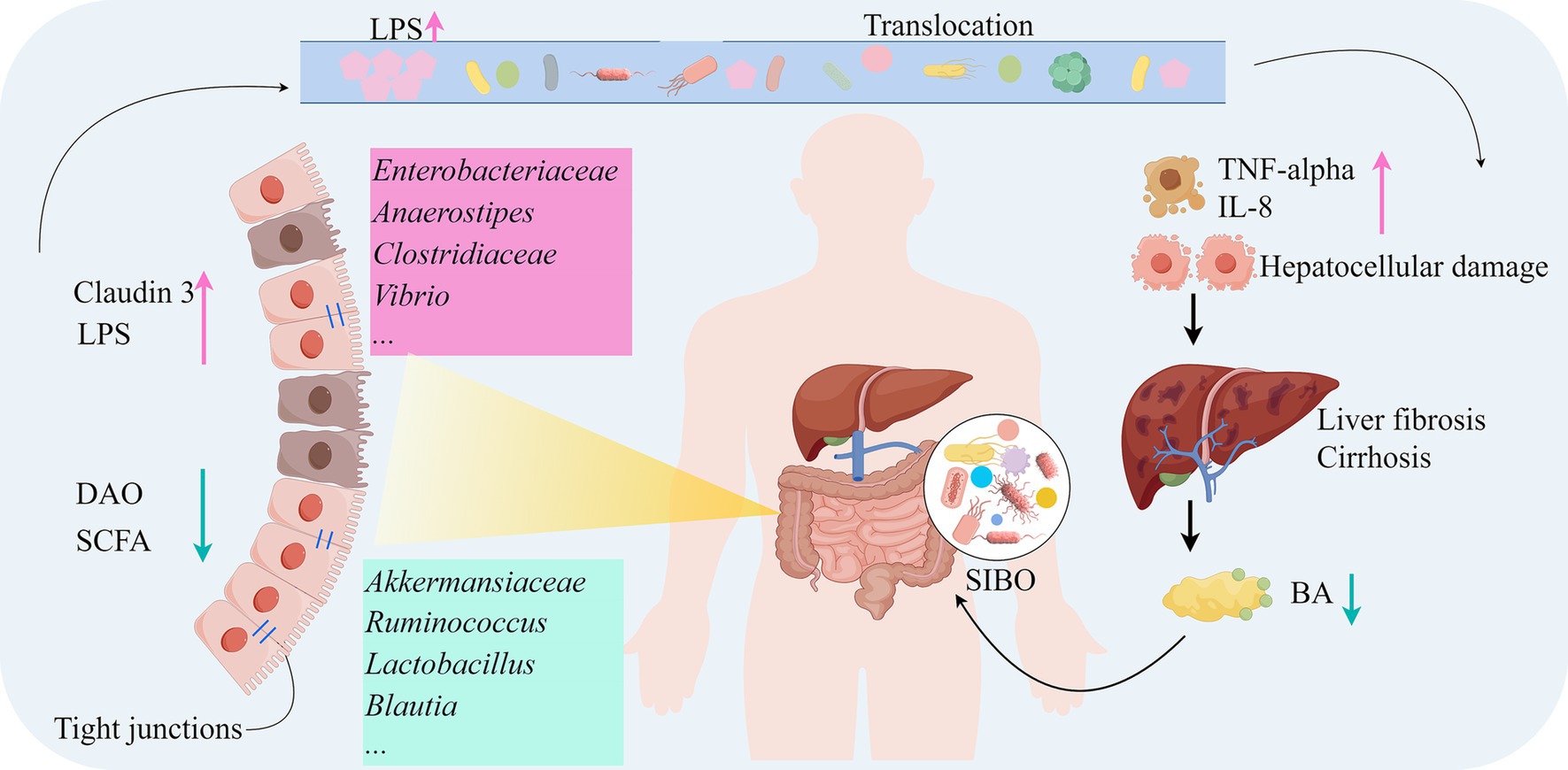
Figure 2. Mechanisms of the gut microbiota in the development of LC (by Figdraw). DAO, Diamine oxidase; BA, Bile acids; IL-8, Interleukin-8; SIBO, Small intestinal bacterial overgrowth; TNF-alpha, Tumor necrosis factor-alpha.
5.3 Treatment of the gut microbiota in LC
5.3.1 Probiotics
Targeting gut microbiota dysbiosis may serve as an effective strategy for preventing and slowing the progression of liver cirrhosis. In a study involving 160 patients with HBC, the administration of the ready-to-eat supplement Lactobacillus paracasei N1115 significantly increased gut microbiota diversity, elevated the proportion of beneficial bacteria such as Bacteroides and Bifidobacterium, and reduced the proportion of harmful bacteria such as Escherichia, Shigella, and Streptococcus. Concurrently, liver function indicators improved significantly, and levels of inflammatory factors decreased markedly (135). A meta-analysis further supported these findings, with 22 included randomized controlled trials demonstrating that probiotics can enhance intestinal permeability and liver toxin filtration capacity, significantly reducing gamma-glutamyl transferase, AST, blood ammonia, and endotoxin levels (136). Additionally, animal experiments and human tissue analyses confirmed that the probiotic Akkermansia muciniphila can restore levels of pro-inflammatory cytokines, endotoxins (LPS and LBP), serotonin-related cognitive function, and liver injury, making it a potential therapeutic candidate for alleviating liver fibrosis and cognitive impairment symptoms (137). Four single-arm trials (58 participants) and two randomized controlled trials (66 participants) showed that the use of probiotics significantly reduced hepatic venous pressure gradient (138). Another randomized controlled trial indicated that a multifactorial intervention consisting of multi-strain probiotics, home-based exercise, and branched-chain amino acids improved frailty in cirrhosis patients and reduced emergency visits and fall incidents (139).
5.3.2 FMT
Furthermore, after oral administration of FMT capsules, the abundance of SCFA-producing bacteria (Bifidobacterium adolescentis and Bifidobacterium angulatum) increased in LC patients, positively correlating with improvements in HE psychological scores, suggesting that FMT has a beneficial effect on cognitive function in LC patients (140). However, in decompensated cirrhosis patients, defects in immune status may reduce the therapeutic efficacy of FMT (141). Despite this, oral FMT capsules enriched with Lachnospiraceae and Ruminococcaceae demonstrated good safety and tolerability in LC patients and improved duodenal mucosal diversity and dysbiosis (142). Animal experiments also confirmed that FMT increased the abundance of beneficial bacteria such as Lactobacillaceae and Bacteroidaceae, significantly improving gut microbiota diversity, richness, and evenness in cirrhotic rats, reducing liver inflammation, and thereby ameliorating liver fibrosis and cirrhosis (143).
5.3.3 Engineered carbon
In a LC mouse model, the engineered carbon bead adsorbent Yaq-001 positively influenced microbial composition and metabolism by reducing intestinal permeability, significantly alleviating liver injury, fibrosis progression, and mortality in ACLF animals, and achieved primary endpoints for safety and tolerability in clinical trials, providing strong preclinical theoretical and safety support for LC patients (144) (Table 3).
Probiotics, FMT, and engineered carbon beads improve gut microbiota dysbiosis through different mechanisms, reduce inflammation, enhance liver function, and positively impact cognitive function, offering new approaches for the treatment of cirrhosis patients. However, these methods each have unique advantages and limitations. Future research could further explore the combined application and optimized integration of these methods to enhance treatment efficacy and patient quality of life.
6 Expression characteristics, mechanistic research, and diagnostic-therapeutic applications of gut microbiota in HCC
6.1 Alteration of gut microbiota in HCC
HCC is the fourth leading cause of cancer-related deaths globally, with its incidence primarily associated with hepatitis B (40%), hepatitis C (40%), alcohol (11%), and MASH (2). According to GLOBOCAN data, there were 905,677 new cases and 830,180 deaths from liver cancer worldwide in 2020 (145). Recent studies have shown that alterations in the gut microbiota are closely related to the occurrence and progression of HCC (146). In early-stage HCC patients, the species richness of fecal microbiota is increased compared to the LC group (39, 124). Analysis of published fecal datasets from four different regions in China revealed that the relative abundance of Firmicutes was significantly lower in HCC patients compared to HC, and further decreased with disease progression, while the relative abundance of Bacteroidetes and Proteobacteria significantly increased (45). However, Yan et al. reported inconsistent findings in a study conducted in Beijing, showing that the abundance of both Bacteroidetes and Firmicutes gradually decreased in HCC patients (23). Additionally, the abundance of Proteobacteria, Streptococcus, and Ruminococcus was significantly higher in the HCC group compared to controls, while the abundance of Subdoligranulum was significantly reduced (46). In early-stage HCC patients, the abundance of Actinobacteria increased, and 13 genera, including Gemmiger and Parabacteroides, were enriched in early HCC (39). The relative abundance of potentially beneficial bacteria, such as Lactobacillus, Bifidobacterium, and Bacteroides, was significantly reduced in HCC patients, while the relative abundance of potentially pathogenic bacteria, such as Escherichia-Shigella and Enterococcus, was significantly increased (147). Furthermore, Akkermansia was most enriched in LC patients, while its abundance was relatively lower in the HC group, CHB patients, and HCC patients (21). In conclusion, to gain a more comprehensive understanding of the specific role and expression characteristics of gut microbiota in HCC, multicenter, large-sample clinical trials are still needed for further exploration.
6.2 The occurrence and early warning value of metabolites in HCC
Gut microbiota-derived metabolites play a pivotal role in HCC progression and early detection. Comparative analyses reveal significant enrichment of Proteobacteria and Actinobacteria in advanced liver disease (HCC and cirrhosis) versus HC, with Escherichia-Shigella, Veillonella, and Streptococcus consistently elevated at the genus level (15). Viral-related HCC patients exhibit increased Faecalibacterium and Coprococcus, while non-hepatitis C-related cases show Bacteroides and Ruminococcus dominance (148). Early postoperative recurrence is associated with higher Dialister, Veillonella, and Bifidobacterium faecale abundance (149), and Streptococcus/Escherichia-Shigella levels correlate with disease severity (46). Macrovascular invasion (MVI) in HCC is marked by Firmicutes depletion and Proteobacteria/Bacteroidetes enrichment (150), particularly in HBV-related cases where Prevotella_9 and Megamonas increase (150). Notably, Bacteroides thetaiotaomicron depletion distinguishes recurrence-prone HCC patients (151). Mendelian randomization identifies Ruminococcaceae and Bacteroidetes as protective against HCC development (152), underscoring the potential of microbiota-targeted interventions in precancerous stages to mitigate progression.
6.3 The diagnostic value of microbiota in HCC
RF analysis revealed that nine gut microbial genera, including Elizabethkingia, Burkholderia_Caballeron-ia_Paraburkholderia, Klebsiella, Delftia, Faecalibaculum, Acetatifactor, Lactobacillus, Ruminococcaceae_UCG-010, and Stenotrophomonas, could significantly distinguish the HCC group from the control group, with an AUC of 0.810. When these genera were combined with serum AFP levels, the AUC further improved to 0.980, demonstrating higher diagnostic efficacy (46). Additionally, 14 and 10 cross-dataset reproducible differential genera were identified in LC and HCC patients, respectively. RF models constructed based on these genera achieved AUCs of 0.820 and 0.900 for distinguishing cirrhosis and HCC in the training dataset and successfully achieved cross-regional validation (45). Studies based on ML algorithms further demonstrated that gut microbial biomarkers have high diagnostic accuracy in HCC subgroup classification, with an AUC of up to 0.940 (148). Peng et al. established a gut microbial diagnostic model, validating the potential of gut microbiota as a non-invasive tool for preoperative diagnosis of MVI (150). Through five-fold cross-validation, the study identified the optimal 30 microbial biomarkers and achieved an AUC of 80.64% in 75 early HCC samples and 105 non-HCC samples, demonstrating strong diagnostic capabilities for both early and advanced HCC (39). More importantly, the study successfully achieved cross-regional validation of microbial biomarkers in HCC patients from northwest and central China (39) (Table 2). The combination of gut microbial biomarkers with existing diagnostic methods, such as serum AFP, can significantly improve the diagnostic accuracy of HCC. In the future, validating these microbial biomarkers in larger, multi-regional clinical cohorts will help further enhance the stability and reliability of HCC diagnosis.
6.4 Mechanisms of the gut microbiota in HCC
Recent studies have shown that gut microbiota dysbiosis plays a critical regulatory role in the pathological progression of HCC. The gut microbiota of patients with cirrhosis-related HCC exhibits characteristic changes, including a reduction in butyrate-producing bacteria (Clostridium, Ruminococcus, and Coprococcus) and an increase in LPS-producing bacteria (Neisseria, Peptostreptococcus, Enterobacteriaceae, and Veillonella). This imbalance in microbial metabolites may accelerate disease progression (153). LPS can activate TLR4 signaling in resident hepatocytes, stimulating the secretion of cytokines (IL-6 and TNF-α), leading to liver inflammation and oxidative damage (154, 155). Klebsiella pneumoniae promotes the development of precancerous lesions and HCC in mice by disrupting intestinal barrier integrity and translocating to the liver. Mechanistically, its PBP1B protein binds to TLR4 on HCC cells, thereby activating TLR4-mediated oncogenic signaling and driving tumorigenesis (156). Additionally, the gut microbiome transports microbial-associated molecular patterns and metabolites to the liver via the portal vein, thereby regulating HCC progression through the gut-liver axis (157) Akkermansia muciniphila enhances the efficacy of PD1 therapy by restoring gut barrier integrity to reduce LPS influx, suppressing the TLR2/NF-κB signaling pathway to diminish immunosuppressive m-MDSCs and M2 macrophages, while modulating cholesterol and BA metabolism (158). Notably, in viral hepatitis-related HCC patients, SCFA-producing bacteria are significantly enriched (148). Integrated analysis of the gut microbiome and tissue metabolome reveals that gut microbiota-derived acetate can be absorbed by the liver, providing energy support for tumor cell growth and proliferation, which may be an important mechanism for microbiota-mediated HCC recurrence (149). Particularly insightful is the finding that acetate derived from Bacteroides thetaiotaomicron can promote macrophage polarization toward a pro-inflammatory phenotype while enhancing T cell-mediated tumor cell killing, thereby inhibiting HCC progression (151). These groundbreaking findings collectively confirm that gut microbiota dysbiosis and the resulting barrier dysfunction and metabolite imbalance play a pivotal regulatory role in the pathological progression of liver cancer by promoting bacterial translocation and reshaping the immune microenvironment (Figure 3).
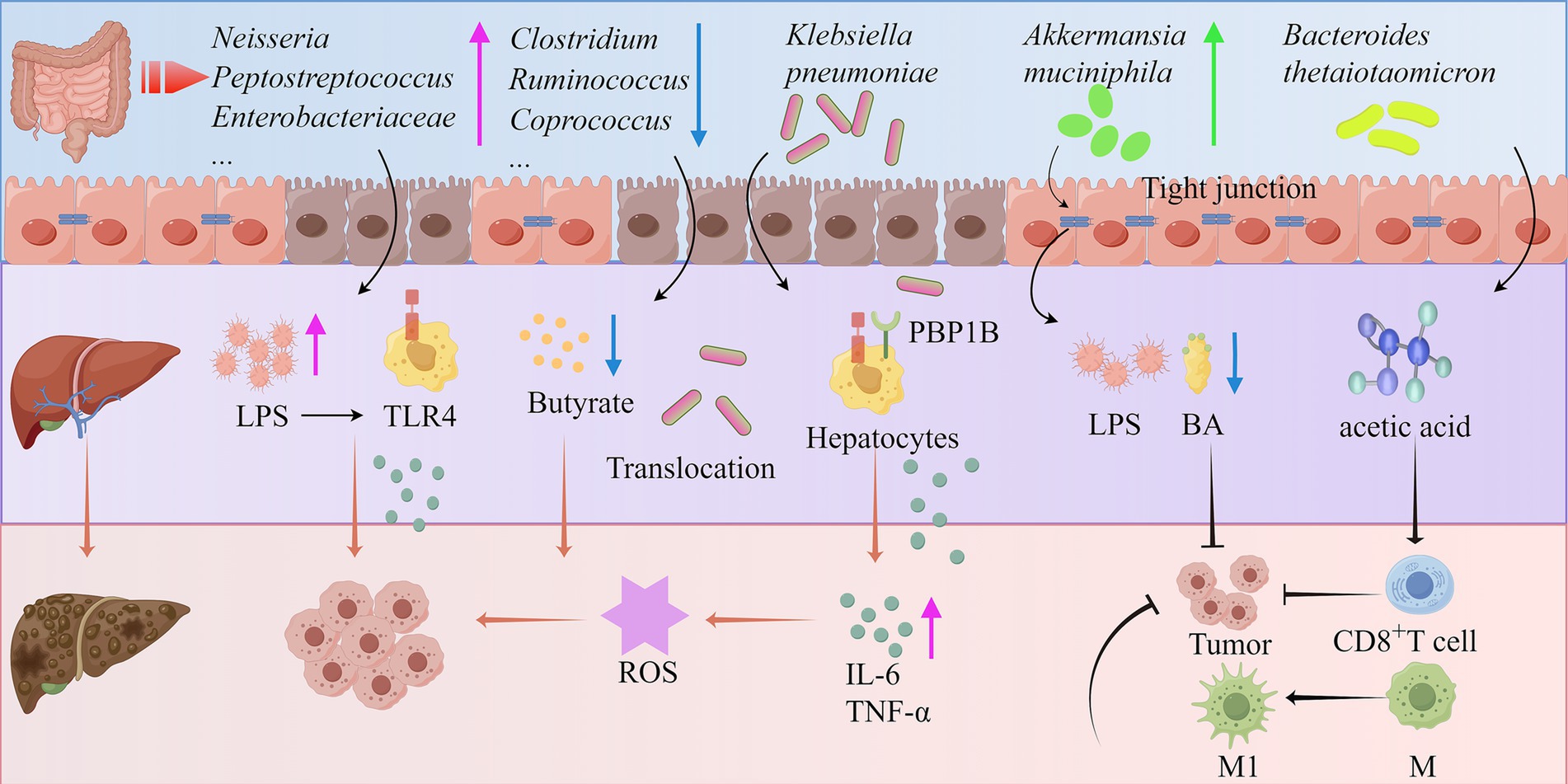
Figure 3. Mechanisms of the gut microbiota in the development of HCC (by Figdraw). ROS, Reactive oxygen species; IL-6, Interleukin-6; M1, Macrophage 1; M2, Macrophage 2; PBP1B, Penicillin binding protein 1B.
6.5 Treatment of the gut microbiota in HCC
6.5.1 FMT
Following FMT, the abundance of beneficial gut bacteria such as Lactobacillaceae, Bacilli, and Bacteroides significantly increased, improving the diversity, richness, and evenness of the gut microbiota in cirrhotic rats, thereby alleviating liver fibrosis (143). Transplantation of fecal bacteria from wild-type mice or Lactobacillus reuteri into HCC mice elevated acetate levels and reduced IL-17A secretion, enhancing the anticancer effects in HCC mice (159). The absence of Akkermansia muciniphila has been associated with increased abundance of hepatic monocytic myeloid-derived suppressor cells. Notably, FMT-mediated reintroduction of Akkermansia muciniphila in Nlrp6−/− mice restored intestinal barrier integrity and markedly attenuated hepatic inflammation and fibrosis (160).
6.5.2 Probiotics
Probiotics, as important microbial agents for maintaining gut microbiota stability, include LAB strains such as Lactobacillus and Bifidobacterium (161). They can modulate the gut microbiota, stabilize the intestinal barrier, and mitigate carcinogenic toxicity, thereby influencing the development and progression of liver cancer (162). Studies have found that after 48 h of LAB treatment, the abundance of Firmicutes, Bacteroidetes, and Actinobacteria significantly increased in HCC mice, while the abundance of Proteobacteria was significantly lower than in untreated HCC groups (147). Furthermore, LAB, particularly L. brevis SR52-2 and L. delbrueckii Q80, exhibit antiviral properties that help improve gastrointestinal health in HCC patients (147).
6.5.3 Diet
In a high-fructose diet-fed HCC mouse model, microbiota-derived acetate increased levels of glutamine and UDP-N-acetylglucosamine in HCC, enhancing protein O-GlcNAcylation and promoting HCC progression (163). Conversely, a 5:2 intermittent fasting regimen improved MASH and fibrosis and inhibited HCC development by activating hepatic PPARα and PCK1 (113) (Table 3).
These findings suggest that FMT, probiotics, and dietary interventions may exert beneficial effects on liver cirrhosis and HCC by modulating gut microbiota functionality. However, current research remains predominantly limited to animal studies, highlighting the need for further clinical validation to address existing gaps in translational applicability.
7 Expression characteristics, mechanistic research, and diagnostic-therapeutic applications of gut microbiota in drug-induced liver injury
7.1 Alteration of gut microbiota in drug-induced liver injury
Drug-induced liver injury (DILI), a leading cause of ALF and acute hepatitis globally (1), is a severe adverse drug reaction associated with medications such as anti-infectives, herbal products, and non-steroidal anti-inflammatory drugs (164, 165). Emerging evidence highlights the critical role of gut microbiota in DILI pathogenesis. Patients with DILI exhibit significant gut microbial dysbiosis, characterized by reduced richness and diversity (99), with distinct patterns across drug types. In acetaminophen (APAP)-induced models, APAP exposure increases Cyanobacteria and Deferribacteres while decreasing Firmicutes at the phylum level, and elevates Bacteroides/Enterococcus but depletes Bifidobacterium/Lactobacillus at the genus level (166, 167). Similar dysbiosis is observed in dietary supplement- or conventional drug-induced DILI, marked by reductions in Acetobacteroides, Blautia, and Coprococcus (99). Idiosyncratic DILI patients show Bacillota enrichment and Bacteroidota/Verrucomicrobiota depletion, with Alloprevotella dominance over Eubacterium eligens (168). Metronidazole (MNZ) exposure in mice reduces α-diversity and elevates F/B ratios alongside Lactobacillus reuteri abundance (169), though conflicting results on microbiota changes [e.g., Tulstrup et al. (170)] suggest dose- and duration-dependent effects. Mendelian randomization identifies Oscillospira, Blautia, and Prevotella_7 as risk-associated taxa (171), while cisplatin and methotrexate models link Proteobacteria, Enterococcus, and Collinsella to liver injury (172, 173). These findings collectively underscore gut microbiota as a pivotal mediator in DILI progression.
7.2 Mechanisms of the gut microbiota in DILI
Thousands of drugs can induce direct, indirect, or idiosyncratic liver injury, with mechanisms that are complex and not yet fully elucidated. Studies have shown that the loss of intestinal barrier integrity leading to increased intestinal permeability may be one of the important mechanisms of DILI. APAP administration can upregulate the colonic epithelial chemokine (C-C motif) ligand 7, thereby mediating intestinal barrier dysfunction, which may be a key factor in APAP-induced hepatotoxicity (174). Additionally, metronidazole can disrupt the structure and function of the intestinal barrier, leading to gut microbiota dysbiosis and subsequent intestinal and liver injury (169). Numerous studies have demonstrated that the composition of gut microbiota is altered in DILI patients and animal models, manifesting as increased intestinal permeability, elevated LPS translocation, reduced SCFA production, and disrupted BA metabolic homeostasis (175). In a clinical trial, antithyroid drugs were found to increase fecal and serum LPS levels in patients, activating LPS-related signaling pathways and thereby inducing liver injury (176). In an APAP-induced mouse model of acute liver injury, ampicillin exacerbated APAP-induced liver injury by inducing gut microbiota imbalance and reducing butyrate levels (177). However, some studies have also suggested that gut microbiota metabolites may have protective effects on the liver. For instance, daidzein released by β-galactosidase from Lactobacillus vaginalis can inhibit Fdps-mediated hepatocyte ferroptosis, thereby ameliorating APAP-induced liver injury in mice (178). These findings indicate that the mechanisms by which gut microbiota regulates drug-related liver injury may be associated with intestinal barrier disruption, alterations in gut microbiota composition, and its metabolites. Future research should further explore the specific mechanisms of gut microbiota and its metabolites in liver injury and develop intervention strategies based on gut microbiota modulation, providing new targets for the prevention and treatment of drug-induced liver injury.
7.3 Treatment of the gut microbiota in DILI
The occurrence of DILI is accompanied by structural changes in the gut microbiota, and modulating the gut microbiota can effectively alleviate DILI. Compared to donor feces, oral fecal gavage enriches Lachnospiraceae and butyrate in the gut, mitigating APAP-induced ferroptosis through the AMPK-ULK1-p62 signaling pathway while simultaneously inducing mitochondrial autophagy and the Nrf2 antioxidant response, thereby effectively alleviating ALI in mice (179). Additionally, oral magnesium reduces APAP-induced liver injury by increasing the abundance of Bifidobacterium and inhibiting the production of the gut microbiota metabolite CYP2E1 (180). Bacteroides vulgatus exhibits probiotic effects in vivo, inhibiting the colonization of pathogenic microorganisms and alleviating APAP-induced oxidative stress and liver injury (181). Studies have also found that triptolide (TP) significantly disrupts gut microbiota composition, particularly reducing the relative abundance of Lactobacillus rhamnosus GG (LGG). Supplementation with LGG can reverse TP-induced hepatotoxicity by increasing bile salt hydrolase activity and reducing elevated conjugated BAs (182). Magnesium isoglycyrrhizinate treatment increases the abundance of the probiotic Lactobacillus, restores the intestinal barrier, and ameliorates methotrexate-induced liver injury (200). Furthermore, a randomized clinical trial demonstrated that probiotics may help alleviate drug-induced liver dysfunction in patients with depression (183). These studies indicate that FMT and probiotic treatments can restore gut microbiota homeostasis, enhance intestinal barrier function, and improve liver function parameters, thereby effectively mitigating the onset and progression of DILI. These findings provide new insights and approaches for the treatment of DILI. At the same time, attention should be paid to individual differences and the complexity of gut microbiota changes to achieve more precise and personalized treatments.
8 Other liver diseases and gut microbiota
In recent years, studies have revealed that, in addition to common liver diseases, autoimmune and genetic liver diseases such as primary biliary cholangitis (PBC), primary sclerosing cholangitis (PSC), autoimmune hepatitis (AIH), and Wilson’s disease (WD) are also closely associated with gut microbiota dysbiosis. Among these, PBC, as a progressive autoimmune liver disease, often presents with subtle early clinical manifestations. Research indicates that the albumin-bilirubin (ALBI) score and its grading system can effectively assess disease progression and prognostic risk in PBC patients. Notably, ALBI grade 1 patients exhibit higher gut microbiota α-diversity and ecological balance, with a significant predominance of Clostridia and Lachnospira genera. ML models have identified Lachnospira as a key biomarker for distinguishing different ALBI grades (184). In terms of therapeutic interventions, UDCA treatment can induce gut microbiota remodeling in PBC patients, with a particularly prominent expansion of Bacteroides in high-Clostridia microbiota. This microbial modulation may enhance the clinical response to UDCA by restoring gut homeostasis (185).
PSC is a chronic cholestatic liver disease characterized by typical pathological alterations including abnormal liver enzymes, dysregulated bile acid metabolism, and altered gut microbiota composition. Patients with PSC exhibit significantly reduced α-diversity and markedly decreased species richness in their gut microbiota (186). An observational study of 43 Czech PSC patients demonstrated significant upregulation of fecal bacterial genera including Haemophilus, Rothia, Clostridium, Enterococcus, Streptococcus, and Veillonella compared to HC (187). Inverse variance weighted analysis further revealed that the relative abundance of Eubacterium hallii was positively associated with PSC risk, whereas Clostridiaceae1 and Lachnospiraceae families showed significant negative correlations with PSC susceptibility, suggesting their potential protective roles (188). In clinical interventions, single FMT administration in 10 PSC patients resulted in ≥50% reduction in alkaline phosphatase levels in 3 cases. Notably, FMT not only significantly enhanced microbial diversity but also induced clinical remission of comorbid ulcerative colitis with efficacy comparable to biologic agents (189).
For AIH, the chronic liver inflammation process is closely linked to gut microecological imbalance. AIH patients exhibit a significant reduction in gut microbiota diversity, which not only alters microbial metabolic profiles but may also exacerbate liver inflammation by disrupting intestinal barrier integrity and immune regulatory functions (190). In the context of inherited metabolic liver diseases, WD patients experience copper metabolism disorders due to mutations in the ATP7B gene. 16S rRNA sequencing has shown that the abundance of Selenomonaceae and Megamonas genera in the gut of WD patients is significantly higher than in healthy individuals. This microbial abnormality may be pathologically associated with copper deposition and toxic damage in hepatocytes caused by impaired biliary copper excretion (191). Gut microbiota dysbiosis plays a crucial role in the development and progression of autoimmune and genetic liver diseases. Modulating the gut microbiota may provide new insights for the diagnosis and treatment of these diseases. Future research should further explore the specific mechanisms of gut microbiota in these liver diseases and develop gut microbiota-based early diagnostic and therapeutic approaches, thereby offering more precise and personalized treatment strategies for patients.
9 Summary and perspectives
This review explores the role of gut microbiota in various liver diseases, analyzing its applications in disease diagnosis and its contributions to disease progression. Studies have shown that patients with liver diseases exhibit a reduction in beneficial bacteria and an increase in potentially pathogenic bacteria within the gut, leading to dysbiosis of the gut microbiota. This imbalance further disrupts the integrity of the intestinal mucosal barrier, facilitating the translocation of bacteria and their toxins to the liver, creating a vicious cycle that exacerbates liver disease progression. Interventions such as FMT, dietary modifications, and oral administration of probiotics or prebiotics can effectively modulate the structure of gut microbiota, increase the abundance of beneficial bacteria, inhibit the growth of harmful bacteria, improve the gut microenvironment, alleviate hepatic inflammation, and protect hepatocytes from damage.
Despite the growing attention and extensive research on the relationship between gut microbiota and liver diseases, the gut microbiota is influenced by multiple factors, including dietary habits, geographic environment, host genetic background, age, technical variations, and pharmacological interventions. This has led to inconsistencies and limited comparability across studies regarding gut microbiota alterations. Current research primarily focuses on changes in microbiota composition, while the functional roles of microbiota, their metabolites, and their interaction mechanisms with the liver require further in-depth investigation. Future studies should aim to elucidate the specific molecular targets of gut microbiota in the pathogenesis of liver diseases and clarify the unique interactions between gut microbiota and different types of liver diseases. In recent years, research on microbial communities beyond the gut, such as oral microbiota, has also demonstrated their significant relevance to the onset, progression, and prognosis of liver diseases. This discovery provides a new perspective for liver disease research and may offer important insights for developing precision diagnostic tools and personalized treatment strategies based on microbial community characteristics. Additionally, research on gut microbiota modulation strategies should be expanded, optimizing the use of probiotics, prebiotics, and exploring novel microbiota transplantation methods. These efforts will provide more effective approaches for the comprehensive prevention and treatment of liver diseases, ultimately improving patient prognosis and quality of life.
Author contributions
J-XY: Writing – review & editing, Funding acquisition, Writing – original draft. JW: Visualization, Resources, Writing – original draft. XC: Writing – original draft, Visualization, Resources. S-gZ: Writing – original draft, Visualization, Resources. X-bL: Writing – original draft, Resources, Visualization. L-PW: Writing – original draft, Writing – review & editing. S-hX: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Special Fund Project of Yancheng Science and Technology Bureau (No. YCBE202475), the Medical Research Project of Yancheng Health Commission (No. YK2023130), and the Special Scientific Research Fund for Clinical Medicine of Nantong University (No.2024LZ006).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang, Y, Wu, J, Xu, J, and Lin, S. Clinical significance of high expression of stanniocalcin-2 in hepatocellular carcinoma. Biosci Rep. (2019) 39:BSR20182057. doi: 10.1042/BSR20182057
2. Devarbhavi, H, Asrani, SK, Arab, JP, Nartey, YA, Pose, E, and Kamath, PS. Global burden ofliver disease: 2023 update. J Hepatol. (2023) 79:516–37. doi: 10.1016/j.jhep.2023.03.017
3. Li, SS, Lei, DL, Yu, HR, Xiang, S, Wang, YH, Wu, ZJ, et al. Diagnostic value and immune infiltration characterization of WTAP as a critical m6A regulator in liver transplantation. Hepatobiliary Pancreat Dis Int. (2025) 24:138–46. doi: 10.1016/j.hbpd.2024.12.004
4. Rana, D, Salave, S, Perla, A, Nadkarni, A, Kolhe, S, Jindal, AB, et al. Bugs as drugs: understanding the linkage between gut microbiota and cancer treatment. Curr Drug Targets. (2022) 23:869–88. doi: 10.2174/1389450123666220309101345
5. Wang, R, Tang, R, Li, B, Ma, X, Schnabl, B, and Tilg, H. Gut microbiome, liver immunology, and liver diseases. Cell Mol Immunol. (2021) 18:4–17. doi: 10.1038/s41423-020-00592-6
6. Li, J, Xuan, S, Dong, P, Xiang, Z, Gao, C, Li, M, et al. Immunotherapy of hepatocellular carcinoma: recent progress and new strategy. Front Immunol. (2023) 14:1192506. doi: 10.3389/fimmu.2023.1192506
7. Albhaisi, SAM, Bajaj, JS, and Sanyal, AJ. Role of gut microbiota in liver disease. Am J Physiol Gastrointest Liver Physiol. (2020) 318:G84–98. doi: 10.1152/ajpgi.00118.2019
8. Zhang, S, Chau, HT, Tun, HM, Huang, FY, Wong, DK, Mak, LY, et al. Virological response to nucleos(t)ide analogues treatment in chronic hepatitis B patients is associated with Bacteroides-dominant gut microbiome. EBioMedicine. (2024) 103:105101. doi: 10.1016/j.ebiom.2024.105101
9. Ali, RO, Quinn, GM, Umarova, R, Haddad, JA, Zhang, GY, Townsend, EC, et al. Longitudinal multi-omics analyses of the gut-liver axis reveals metabolic dysregulation in hepatitis C infection and cirrhosis. Nat Microbiol. (2023) 8:12–27. doi: 10.1038/s41564-022-01273-y
10. Chen, B, Huang, H, and Pan, CQ. The role of gut microbiota in hepatitis B disease progression and treatment. J Viral Hepat. (2022) 29:94–106. doi: 10.1111/jvh.13595
11. Hao, Y, Hao, Z, Zeng, X, and Lin, Y. Gut microbiota and metabolites of cirrhotic portal hypertension: a novel target on the therapeutic regulation. J Gastroenterol. (2024) 59:788–97. doi: 10.1007/s00535-024-02134-7
12. Siddiqui, ZR, Rahman, S, Verma, N, Srivastava, A, Jahan, N, Mohanty, S, et al. Gut–Oral microbial Dysbiosis: a correlated ecosystem In: Advanced Gut and Microbiome Research (2025)
13. Xie, XM, Feng, S, Liu, T, Feng, J, Xu, Y, Fan, ZJ, et al. Role of gut/liver metabolites and gut microbiota in liver fibrosis caused by cholestasis. Int Immunopharmacol. (2024) 139:112747. doi: 10.1016/j.intimp.2024.112747
14. Saeed, H, Díaz, LA, Gil-Gómez, A, Burton, J, Bajaj, JS, Romero-Gomez, M, et al. Microbiome-centered therapies for the management of metabolic dysfunction-associated steatotic liver disease. Clin Mol Hepatol. (2025) 31:S94–S111. doi: 10.3350/cmh.2024.0811
15. Rajapakse, J, Khatiwada, S, Akon, AC, Yu, KL, Shen, S, and Zekry, A. Unveiling the complex relationship between gut microbiota and liver cancer: opportunities for novel therapeutic interventions. Gut Microbes. (2023) 15:2240031. doi: 10.1080/19490976.2023.2240031
16. World Health Organization. Global health sector strategies on, respectively, HIV, viral hepatitis and sexually transmitted infections for the period 2022-2030. Available online at: https://cdn.who.int/media/docs/default-source/hq-hiv-hepatitis-and-stis-library/full-final-who-ghss-hiv-vh-sti_1-june2022.pdf?sfvrsn=7c074b36_9 (2022).
17. Yang, X, Mai, H, Zhou, J, Li, Z, Wang, Q, Lan, L, et al. Alterations of the gut microbiota associated with the occurrence and progression of viral hepatitis. Front Cell Infect Microbiol. (2023) 13:1119875. doi: 10.3389/fcimb.2023.1119875
18. World Health Organization. Global hepatitis report 2024: action for access in low- and middle-income countries. Available online at: https://www.who.int/publications/i/item/9789240091672 (2024).
19. Lin, MJ, Su, TH, Chen, CC, Wu, WK, Hsu, SJ, Tseng, TC, et al. Diversity and composition of gut microbiota in healthy individuals and patients at different stages of hepatitis B virus-related liver disease. Gut Pathog. (2023) 15:24. doi: 10.1186/s13099-023-00549-w
20. Long, J, Saw, M, Zhang, P, Wang, L, Li, L, Ren, H, et al. Role of tenofovir dipivoxil in gut microbiota recovery from HBV-infection induced dysbiosis. BMC Microbiol. (2024) 24:359. doi: 10.1186/s12866-024-03457-4
21. Zeng, Y, Chen, S, Fu, Y, Wu, W, Chen, T, Chen, J, et al. Gut microbiota dysbiosis in patients with hepatitis B virus-induced chronic liver disease covering chronic hepatitis, liver cirrhosis and hepatocellular carcinoma. J Viral Hepat. (2020) 27:143–55. doi: 10.1111/jvh.13216
22. Liu, B, Yang, H, Liao, Q, Wang, M, Huang, J, Xu, R, et al. Altered gut microbiota is associated with the formation of occult hepatitis B virus infection. Microbiol Spectr. (2024) 12:e0023924. doi: 10.1128/spectrum.00239-24
23. Yan, F, Zhang, Q, Shi, K, Zhang, Y, Zhu, B, Bi, Y, et al. Gut microbiota dysbiosis with hepatitis B virus liver disease and association with immune response. Front Cell Infect Microbiol. (2023) 13:1152987. doi: 10.3389/fcimb.2023.1152987
24. Sultan, S, El-Mowafy, M, Elgaml, A, El-Mesery, M, El Shabrawi, A, Elegezy, M, et al. Alterations of the treatment-naive gut microbiome in newly diagnosed hepatitis C virus infection. ACS Infect Dis. (2021) 7:1059–68. doi: 10.1021/acsinfecdis.0c00432
25. Inoue, T, Nakayama, J, Moriya, K, Kawaratani, H, Momoda, R, Ito, K, et al. Gut Dysbiosis associated with hepatitis C virus infection. Clin Infect Dis. (2018) 67:869–77. doi: 10.1093/cid/ciy205
26. Ashour, Z, Shahin, R, Ali-Eldin, Z, El-Shayeb, M, El-Tayeb, T, and Bakr, S. Potential impact of gut Lactobacillus acidophilus and Bifidobacterium bifidum on hepatic histopathological changes in non-cirrhotic hepatitis C virus patients with different viral load. Gut Pathog. (2022) 14:25. doi: 10.1186/s13099-022-00501-4
27. Wu, J, Bortolanza, M, Zhai, G, Shang, A, Ling, Z, Jiang, B, et al. Gut microbiota dysbiosis associated with plasma levels of interferon-γ and viral load in patients with acute hepatitis E infection. J Med Virol. (2022) 94:692–702. doi: 10.1002/jmv.27356
28. Wu, J, Huang, F, Ling, Z, Liu, S, Liu, J, Fan, J, et al. Altered faecal microbiota on the expression of Th cells responses in the exacerbation of patients with hepatitis E infection. J Viral Hepat. (2020) 27:1243–52. doi: 10.1111/jvh.13344
29. Ishizaka, A, Koga, M, Mizutani, T, Lim, LA, Adachi, E, Ikeuchi, K, et al. Prolonged gut Dysbiosis and fecal excretion of hepatitis a virus in patients infected with human immunodeficiency virus. Viruses. (2021) 13:2101. doi: 10.3390/v13102101
30. Dazıroğlu MEÇ, Y, and Yıldıran, H. Intestinal dysbiosis and probiotic use: its place in hepatic encephalopathy in cirrhosis. Ann Gastroenterol. (2023) 36:141–8. doi: 10.20524/aog.2023.0776
31. Magdy Wasfy, R, Mbaye, B, Borentain, P, Tidjani Alou, M, Murillo Ruiz, ML, Caputo, A, et al. Ethanol-producing Enterocloster bolteae is enriched in chronic hepatitis B-associated gut Dysbiosis: a case-control Culturomics study. Microorganisms. (2023) 11:2437. doi: 10.3390/microorganisms11102437
32. Long, J, Gong, J, Zhu, H, Liu, X, Li, L, Chen, B, et al. Difference of gut microbiota between patients with negative and positive HBeAg in chronic hepatitis B and the effect of tenofovir alafenamide on intestinal flora. Front Microbiol. (2023) 14:1232180. doi: 10.3389/fmicb.2023.1232180
33. Chua, HH, Chen, YH, Wu, LL, Yang, HC, Lin, CR, Chen, HL, et al. Antagonism between gut Ruminococcus gnavus and Akkermansia muciniphila modulates the progression of chronic hepatitis B. Cell Mol Gastroenterol Hepatol. (2024) 17:361–81. doi: 10.1016/j.jcmgh.2023.12.003
34. Wang, K, Zhang, Z, Mo, ZS, Yang, XH, Lin, BL, Peng, L, et al. Gut microbiota as prognosis markers for patients with HBV-related acute-on-chronic liver failure. Gut Microbes. (2021) 13:1–15. doi: 10.1080/19490976.2021.1921925
35. Yao, X, Yu, H, Fan, G, Xiang, H, Long, L, Xu, H, et al. Impact of the gut microbiome on the progression of hepatitis B virus related acute-on-chronic liver failure. Front Cell Infect Microbiol. (2021) 11:573923. doi: 10.3389/fcimb.2021.573923
36. Shen, Y, Wu, SD, Chen, Y, Li, XY, Zhu, Q, Nakayama, K, et al. Alterations in gut microbiome and metabolomics in chronic hepatitis B infection-associated liver disease and their impact on peripheral immune response. Gut Microbes. (2023) 15:2155018. doi: 10.1080/19490976.2022.2155018
37. Zhao, HW, Zhang, JL, Liu, FQ, Yue, ZD, Wang, L, Zhang, Y, et al. Alterations in the gut microbiome after transjugular intrahepatic portosystemic shunt in patients with hepatitis B virus-related portal hypertension. World J Gastroenterol. (2024) 30:3668–79. doi: 10.3748/wjg.v30.i31.3668
38. Hsu, YC, Chen, CC, Lee, WH, Chang, CY, Lee, FJ, Tseng, CH, et al. Compositions of gut microbiota before and shortly after hepatitis C viral eradication by direct antiviral agents. Sci Rep. (2022) 12:5481. doi: 10.1038/s41598-022-09534-w
39. Ren, Z, Li, A, Jiang, J, Zhou, L, Yu, Z, Lu, H, et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut. (2019) 68:1014–23. doi: 10.1136/gutjnl-2017-315084
40. Yang, Y, Ge, F, Luo, C, Chen, Y, Deng, J, Yang, Y, et al. Inhibition of hepatitis B virus through PPAR-JAK/STAT pathway modulation by electroacupuncture and tenofovir disoproxil fumarate combination therapy. Int Immunopharmacol. (2024) 143:113304. doi: 10.1016/j.intimp.2024.113304
41. Neag, MA, Mitre, AO, Catinean, A, and Buzoianu, AD. Overview of the microbiota in the gut-liver axis in viral B and C hepatitis. World J Gastroenterol. (2021) 27:7446–61. doi: 10.3748/wjg.v27.i43.7446
42. Zhou, W, Luo, J, Xie, X, Yang, S, Zhu, D, Huang, H, et al. Gut microbiota Dysbiosis strengthens Kupffer cell-mediated hepatitis B virus persistence through inducing Endotoxemia in mice. J Clin Transl Hepatol. (2022) 10:17–25. doi: 10.14218/JCTH.2020.00161
43. Li, Y, Zhong, S, Jin, Z, Ye, G, Zhang, T, Liu, Z, et al. Peyer's patch-involved gut microbiota facilitates anti-HBV immunity in mice. Virus Res. (2023) 331:199129. doi: 10.1016/j.virusres.2023.199129
44. Inoue, T, Funatsu, Y, Ohnishi, M, Isogawa, M, Kawashima, K, Tanaka, M, et al. Bile acid dysmetabolism in the gut-microbiota-liver axis under hepatitis C virus infection. Liver Int. (2022) 42:124–34. doi: 10.1111/liv.15041
45. Zhang, H, Wu, J, Liu, Y, Zeng, Y, Jiang, Z, Yan, H, et al. Identification reproducible microbiota biomarkers for the diagnosis of cirrhosis and hepatocellular carcinoma. AMB Express. (2023) 13:35. doi: 10.1186/s13568-023-01539-6
46. Yang, J, He, Q, Lu, F, Chen, K, Ni, Z, Wang, H, et al. A distinct microbiota signature precedes the clinical diagnosis of hepatocellular carcinoma. Gut Microbes. (2023) 15:2201159. doi: 10.1080/19490976.2023.2201159
47. Deng, L, Guo, X, Chen, J, Li, B, Liu, N, Xia, J, et al. Effect of intestinal microbiota transplantation on chronic hepatitis B virus infection associated liver disease. Front Microbiol. (2024) 15:1458754. doi: 10.3389/fmicb.2024.1458754
48. Ren, YD, Ye, ZS, Yang, LZ, Jin, LX, Wei, WJ, Deng, YY, et al. Fecal microbiota transplantation induces hepatitis B virus e-antigen (HBeAg) clearance in patients with positive HBeAg after long-term antiviral therapy. Hepatology. (2017) 65:1765–8. doi: 10.1002/hep.29008
49. Chauhan, A, Kumar, R, Sharma, S, Mahanta, M, Vayuuru, SK, Nayak, B, et al. Fecal microbiota transplantation in hepatitis B e antigen-positive chronic hepatitis B patients: a pilot study. Dig Dis Sci. (2021) 66:873–80. doi: 10.1007/s10620-020-06246-x
50. Suez, J, Zmora, N, Zilberman-Schapira, G, Mor, U, Dori-Bachash, M, Bashiardes, S, et al. Post-antibiotic gut mucosal microbiome reconstitution is impaired by probiotics and improved by autologous FMT. Cell. (2018) 174:1406–1423.e16. doi: 10.1016/j.cell.2018.08.047
51. Chinese Society of Hepatology and Chinese Society of Infectious Diseases; Chinese Medical Association. Guideline for the prevention and treatment of hepatitis C (2022 version). Zhonghua Gan Zang Bing Za Zhi. (2022) 30:1332–48. Chinese. doi: 10.3760/cma.j.cn501113-20221220-00605
52. Chuaypen, N, Jinato, T, Avihingsanon, A, Nookaew, I, Tanaka, Y, and Tangkijvanich, P. Long-term benefit of DAAs on gut dysbiosis and microbial translocation in HCV-infected patients with and without HIV coinfection. Sci Rep. (2023) 13:14413. doi: 10.1038/s41598-023-41664-7
53. Pinchera, B, Scotto, R, Zappulo, E, Buonomo, AR, Maraolo, AE, Schiano Moriello, N, et al. Impact of oral antiviral therapy against HCV on gut microbiota. A prospective study. New Microbiol. (2023) 46:196–201.
54. Pérez-Matute, P, Íñiguez, M, Villanueva-Millán, MJ, Recio-Fernández, E, Vázquez, AM, Sánchez, SC, et al. Short-term effects of direct-acting antiviral agents on inflammation and gut microbiota in hepatitis C-infected patients. Eur J Intern Med. (2019) 67:47–58. doi: 10.1016/j.ejim.2019.06.005
55. Ponziani, FR, Putignani, L, Paroni Sterbini, F, Petito, V, Picca, A, Del Chierico, F, et al. Influence of hepatitis C virus eradication with direct-acting antivirals on the gut microbiota in patients with cirrhosis. Aliment Pharmacol Ther. (2018) 48:1301–11. doi: 10.1111/apt.15004
56. Wellhöner, F, Döscher, N, Woelfl, F, Vital, M, Plumeier, I, Kahl, S, et al. Eradication of chronic HCV infection: improvement of dysbiosis only in patients without liver cirrhosis. Hepatology. (2021) 74:72–82. doi: 10.1002/hep.31700
57. Huang, PY, Chen, CH, Tsai, MJ, Yao, CC, Wang, HM, Kuo, YH, et al. Effects of direct anti-viral agents on the gut microbiota in patients with chronic hepatitis C. J Formos Med Assoc. (2023) 122:157–63. doi: 10.1016/j.jfma.2022.08.022
58. Oo, KM, Lwin, AA, Kyaw, YY, Tun, WM, Fukada, K, Goshima, A, et al. Safety and long-term effect of the probiotic FK-23 in patients with hepatitis C virus infection. Biosci Microbiota Food Health. (2016) 35:123–8. doi: 10.12938/bmfh.2015-024
59. Nanis, GA, Mohamed, LS, Hassan, E, and Maii, MN. Lactobacillus acidophilus and Bifidobacteria spp having antibacterial and antiviral effects on chronic HCV infection. Afr J Microbiol Res. (2019) 13:77–90. doi: 10.5897/AJMR2018.9028
60. Stern, J, Miller, G, Li, X, and Saxena, D. Virome and bacteriome: two sides of the same coin. Curr Opin Virol. (2019) 37:37–43. doi: 10.1016/j.coviro.2019.05.007
61. Liu, L, Zhao, Z, Liu, H, Xia, X, Ai, C, Song, S, et al. Haematococcus pluvialis polysaccharides improve microbiota-driven gut epithelial and vascular barrier and prevent alcoholic steatohepatitis development. Int J Biol Macromol. (2024) 274:133014. doi: 10.1016/j.ijbiomac.2024.133014
62. Zhang, D, Liu, Z, and Bai, F. Roles of gut microbiota in alcoholic liver disease. Int J Gen Med. (2023) 16:3735–46. doi: 10.2147/IJGM.S420195
63. Kim, HH, Shim, YR, Choi, SE, Falana, TE, Yoo, JK, Ahn, SH, et al. Exosome-based delivery of super-repressor IκBα alleviates alcohol-associated liver injury in mice. Pharmaceutics. (2023) 15:636. doi: 10.3390/pharmaceutics15020636
64. Ganesan, R, Gupta, H, Jeong, JJ, Sharma, SP, Won, SM, Oh, KK, et al. Characteristics of microbiome-derived metabolomics according to the progression of alcoholic liver disease. Hepatol Int. (2024) 18:486–99. doi: 10.1007/s12072-023-10518-9
65. Wang, H, Yan, J, Wang, K, Liu, Y, Liu, S, Wu, K, et al. The gut-liver axis perspective: exploring the protective potential of polysaccharides from Cistanche deserticola against alcoholic liver disease. Int J Biol Macromol. (2024) 256:128394. doi: 10.1016/j.ijbiomac.2023.128394
66. Zhang, T, Bao, L, Zhao, Q, Wu, ZE, Dai, M, Rao, Q, et al. Metabolomics reveals gut microbiota contribute to PPARα deficiency-induced alcoholic liver injury. J Proteome Res. (2023) 22:2327–38. doi: 10.1021/acs.jproteome.3c00093
67. Gao, R, Huang, Q, Zeng, Y, Chen, D, Jia, Z, Han, B, et al. Pueraria lobata-Prunus mume Complex alleviates alcoholic liver disease by regulating lipid metabolism and inhibiting inflammation: a transcriptome and gut microbiota analysis. Food Secur. (2024) 13:2431. doi: 10.3390/foods13152431
68. Zhu, H, Jiang, W, Liu, C, Wang, C, Hu, B, Guo, Y, et al. Ameliorative effects of chlorogenic acid on alcoholic liver injury in mice via gut microbiota informatics. Eur J Pharmacol. (2022) 928:175096. doi: 10.1016/j.ejphar.2022.175096
69. Gu, J, Chen, Y, Wang, J, Gao, Y, Gai, Z, Zhao, Y, et al. Lacticaseibacillus rhamnosus LRa05 alleviated liver injury in mice with alcoholic fatty liver disease by improving intestinal permeability and balancing gut microbiota. Benef Microbes. (2024) 15:481–93. doi: 10.1163/18762891-bja00022
70. Duan, Y, Llorente, C, Lang, S, Brandl, K, Chu, H, Jiang, L, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. (2019) 575:505–11. doi: 10.1038/s41586-019-1742-x
71. Zhong, X, Cui, P, Jiang, J, Ning, C, Liang, B, Zhou, J, et al. Streptococcus, the predominant bacterium to predict the severity of liver injury in alcoholic liver disease. Front Cell Infect Microbiol. (2021) 11:649060. doi: 10.3389/fcimb.2021.649060
72. Park, IG, Yoon, SJ, Won, SM, Oh, KK, Hyun, JY, Suk, KT, et al. Gut microbiota-based machine-learning signature for the diagnosis of alcohol-associated and metabolic dysfunction-associated steatotic liver disease. Sci Rep. (2024) 14:16122. doi: 10.1038/s41598-024-60768-2
73. Addolorato, G, Ponziani, FR, Dionisi, T, Mosoni, C, Vassallo, GA, Sestito, L, et al. Gut microbiota compositional and functional fingerprint in patients with alcohol use disorder and alcohol-associated liver disease. Liver Int. (2020) 40:878–88. doi: 10.1111/liv.14383
74. Cheng, Z, Yang, L, and Chu, H. The role of gut microbiota, exosomes, and their interaction in the pathogenesis of ALD. J Adv Res. (2024) 72:353–67. doi: 10.1016/j.jare.2024.07.002
75. Albillos, A, de Gottardi, A, and Rescigno, M. The gut-liver axis in liver disease: pathophysiological basis for therapy. J Hepatol. (2020) 72:558–77. doi: 10.1016/j.jhep.2019.10.003
76. Brown, JM, and Hazen, SL. The gut microbial endocrine organ: bacterially derived signals driving cardiometabolic diseases. Annu Rev Med. (2015) 66:343–59. doi: 10.1146/annurev-med-060513-093205
77. Xu, Q, Guo, M, Wang, H, Liu, H, Wei, Y, Wang, X, et al. A butyrate-yielding dietary supplement prevents acute alcoholic liver injury by modulating Nrf2-mediated hepatic oxidative stress and gut microbiota. Int J Mol Sci. (2024) 25:9420. doi: 10.3390/ijms25179420
78. Chen, Y, Ma, H, Liang, J, Sun, C, Wang, D, Chen, K, et al. Hepatoprotective potential of four fruit extracts rich in different structural flavonoids against alcohol-induced liver injury via gut microbiota-liver axis. Food Chem. (2024) 460:140460. doi: 10.1016/j.foodchem.2024.140460
79. Shen, H, Zhou, L, Zhang, H, Yang, Y, Jiang, L, Wu, D, et al. Dietary fiber alleviates alcoholic liver injury via Bacteroides acidifaciens and subsequent ammonia detoxification. Cell Host Microbe. (2024) 32:1331–1346.e6. doi: 10.1016/j.chom.2024.06.008
80. Wang, X, Sun, Z, Wang, X, Li, M, Zhou, B, and Zhang, X. Solanum nigrum L. berries extract ameliorated the alcoholic liver injury by regulating gut microbiota, lipid metabolism, inflammation, and oxidative stress. Food Res Int. (2024) 188:114489. doi: 10.1016/j.foodres.2024.114489
81. Yi, Y, Yan, Y, Zhan, G, Deng, W, Wei, Y, Zhang, Y, et al. Trilobatin, a novel naturally occurring food additive, ameliorates alcoholic liver disease in mice: involvement of microbiota-gut-liver Axis and yap/Nrf2 signaling pathway. J Agric Food Chem. (2024) 72:23819–31. doi: 10.1021/acs.jafc.4c04131
82. Liu, H, Fan, D, Wang, J, Wang, Y, Li, A, Wu, S, et al. Lactobacillus rhamnosus NKU FL1-8 isolated from infant feces ameliorates the alcoholic liver damage by regulating the gut microbiota and intestinal barrier in C57BL/6J mice. Nutrients. (2024) 16:2139. doi: 10.3390/nu16132139
83. Vatsalya, V, Feng, W, Kong, M, Hu, H, Szabo, G, McCullough, A, et al. The beneficial effects of Lactobacillus GG therapy on liver and drinking assessments in patients with moderate alcohol-associated hepatitis. Am J Gastroenterol. (2023) 118:1457–60. doi: 10.14309/ajg.0000000000002283
84. Niu, B, Feng, Y, Cheng, X, Xiao, Y, Zhao, J, Lu, W, et al. The alleviative effects of viable and inactive Lactobacillus paracasei CCFM1120 against alcoholic liver disease via modulation of gut microbiota and the Nrf2/HO-1 and TLR4/MyD88/NF-κB pathways. Food Funct. (2024) 15:8797–809. doi: 10.1039/d4fo02592j
85. Kim, J, Ahn, SW, Kim, JY, Whon, TW, Lim, SK, Ryu, BH, et al. Probiotic lactobacilli ameliorate alcohol-induced hepatic damage via gut microbial alteration. Front Microbiol. (2022) 13:869250. doi: 10.3389/fmicb.2022.869250
86. Sharma, A, Roy, A, Premkumar, M, Verma, N, Duseja, A, Taneja, S, et al. Fecal microbiota transplantation in alcohol-associated acute-on-chronic liver failure: an open-label clinical trial. Hepatol Int. (2022) 16:433–46. doi: 10.1007/s12072-022-10312-z
87. Zhang, Y, Li, P, Chen, B, and Zheng, R. Therapeutic effects of fecal microbial transplantation on alcoholic liver injury in rat models. Clin Res Hepatol Gastroenterol. (2024) 48:102478. doi: 10.1016/j.clinre.2024.102478
88. Xiao, C, Jia, R, Li, XG, Zhao, M, Liao, W, Zhao, S, et al. Musculus senhousei peptides alleviated alcoholic liver injury via the gut-liver axis. Food Funct. (2024) 15:7124–35. doi: 10.1039/d4fo01070a
89. Zhao, F, Li, M, Luo, M, Zhang, M, Yuan, Y, Niu, H, et al. The dose-dependent mechanism behind the protective effect of lentinan against acute alcoholic liver injury via proliferating intestinal probiotics. Food Funct. (2024) 15:10067–87. doi: 10.1039/d4fo02256d
90. Rinella, ME, and Sookoian, S. From NAFLD to MASLD: updated naming and diagnosis criteria for fatty liver disease. J Lipid Res. (2024) 65:100485. doi: 10.1016/j.jlr.2023.100485
91. Alboraie, M, Butt, AS, Piscoya, A, Dao Viet, H, Hotayt, B, Al Awadhi, S, et al. Why MASLD lags behind MAFLD: a critical analysis of diagnostic criteria evolution in metabolic dysfunction-associated liver diseases. Med Sci Monit. (2024) 30:e945198. doi: 10.12659/MSM.945198
92. Cai, W, Qiu, T, Hu, W, and Fang, T. Changes in the intestinal microbiota of individuals with non-alcoholic fatty liver disease based on sequencing: An updated systematic review and meta-analysis. PLoS One. (2024) 19:e0299946. doi: 10.1371/journal.pone.0299946
93. Jasirwan, COM, Muradi, A, Hasan, I, Simadibrata, M, and Rinaldi, I. Correlation of gut Firmicutes/Bacteroidetes ratio with fibrosis and steatosis stratified by body mass index in patients with non-alcoholic fatty liver disease. Biosci Microbiota Food Health. (2021) 40:50–8. doi: 10.12938/bmfh.2020-046
94. Tsai, MC, Liu, YY, Lin, CC, Wang, CC, Wu, YJ, Yong, CC, et al. Gut microbiota Dysbiosis in patients with biopsy-proven nonalcoholic fatty liver disease: a cross-sectional study in Taiwan. Nutrients. (2020) 12:820. doi: 10.3390/nu12030820
95. Yu, JS, Youn, GS, Choi, J, Kim, CH, Kim, BY, Yang, SJ, et al. Lactobacillus lactis and Pediococcus pentosaceus-driven reprogramming of gut microbiome and metabolome ameliorates the progression of non-alcoholic fatty liver disease. Clin Transl Med. (2021) 11:e634. doi: 10.1002/ctm2.634
96. Gao, LL, Ma, JM, Fan, YN, Zhang, YN, Ge, R, Tao, XJ, et al. Lycium barbarum polysaccharide combined with aerobic exercise ameliorated nonalcoholic fatty liver disease through restoring gut microbiota, intestinal barrier and inhibiting hepatic inflammation. Int J Biol Macromol. (2021) 183:1379–92. doi: 10.1016/j.ijbiomac.2021.05.066
97. Aron-Wisnewsky, J, Vigliotti, C, Witjes, J, Le, P, Holleboom, AG, Verheij, J, et al. Gut microbiota and human NAFLD: disentangling microbial signatures from metabolic disorders. Nat Rev Gastroenterol Hepatol. (2020) 17:279–97. doi: 10.1038/s41575-020-0269-9
98. Amrousy, DE, Ashry, HE, Maher, S, Elsayed, Y, and Hasan, S. Non-alcoholic fatty liver disease and the gut microbiota in adolescents: is there a relationship? BMC Pediatr. (2024) 24:779. doi: 10.1186/s12887-024-05268-y
99. Rodriguez-Diaz, C, Taminiau, B, García-García, A, Cueto, A, Robles-Díaz, M, Ortega-Alonso, A, et al. Microbiota diversity in nonalcoholic fatty liver disease and in drug-induced liver injury. Pharmacol Res. (2022) 182:106348. doi: 10.1016/j.phrs.2022.106348
100. Abenavoli, L, Giubilei, L, Procopio, AC, Spagnuolo, R, Luzza, F, Boccuto, L, et al. Gut microbiota in non-alcoholic fatty liver disease patients with inflammatory bowel diseases: a complex interplay. Nutrients. (2022) 14:5323. doi: 10.3390/nu14245323
101. Lee, G, You, HJ, Bajaj, JS, Joo, SK, Yu, J, Park, S, et al. Distinct signatures of gut microbiome and metabolites associated with significant fibrosis in non-obese NAFLD. Nat Commun. (2020) 11:4982. doi: 10.1038/s41467-020-18754-5
102. Juárez-Fernández, M, Goikoetxea-Usandizaga, N, Porras, D, García-Mediavilla, MV, Bravo, M, Serrano-Maciá, M, et al. Enhanced mitochondrial activity reshapes a gut microbiota profile that delays NASH progression. Hepatology. (2023) 77:1654–69. doi: 10.1002/hep.32705
103. Yuan, J, Chen, C, Cui, J, Lu, J, Yan, C, Wei, X, et al. Fatty liver disease caused by high-alcohol-producing Klebsiella pneumoniae. Cell Metab. (2019) 30:675–688.e7. doi: 10.1016/j.cmet.2019.08.018
104. Caussy, C, Tripathi, A, Humphrey, G, Bassirian, S, Singh, S, Faulkner, C, et al. A gut microbiome signature for cirrhosis due to nonalcoholic fatty liver disease. Nat Commun. (2019) 10:1406. doi: 10.1038/s41467-019-09455-9
105. Cornejo-Pareja, I, Amiar, MR, Ocaña-Wilhelmi, L, Soler-Humanes, R, Arranz-Salas, I, Garrido-Sánchez, L, et al. Non-alcoholic fatty liver disease in patients with morbid obesity: the gut microbiota axis as a potential pathophysiology mechanism. J Gastroenterol. (2024) 59:329–41. doi: 10.1007/s00535-023-02075-7
106. Leung, H, Long, X, Ni, Y, Qian, L, Nychas, E, Siliceo, SL, et al. Risk assessment with gut microbiome and metabolite markers in NAFLD development. Sci Transl Med. (2022) 14:eabk0855. doi: 10.1126/scitranslmed.abk0855
107. Fei, N, Bruneau, A, Zhang, X, Wang, R, Wang, J, Rabot, S, et al. Endotoxin producers overgrowing in human gut microbiota as the causative agents for nonalcoholic fatty liver disease. mBio. (2020) 11:e03263-19. doi: 10.1128/mBio.03263-19
108. Nian, F, Chen, Y, Xia, Q, Zhu, C, Wu, L, and Lu, X. Gut microbiota metabolite trimethylamine N-oxide promoted NAFLD progression by exacerbating intestinal barrier disruption and intrahepatic cellular imbalance. Int Immunopharmacol. (2024) 142:113173. doi: 10.1016/j.intimp.2024.113173
109. Ma, R, Shi, G, Li, Y, and Shi, H. Trimethylamine N-oxide, choline and its metabolites are associated with the risk of non-alcoholic fatty liver disease. Br J Nutr. (2024) 131:1915–23. doi: 10.1017/S0007114524000631
110. Zhen, J, Zhou, Z, He, M, Han, HX, Lv, EH, Wen, PB, et al. The gut microbial metabolite trimethylamine N-oxide and cardiovascular diseases. Front Endocrinol (Lausanne). (2023) 14:1085041. doi: 10.3389/fendo.2023.1085041
111. Mao, Q, Lin, B, Zhang, W, Zhang, Y, Zhang, Y, Cao, Q, et al. Understanding the role of ursodeoxycholic acid and gut microbiome in non-alcoholic fatty liver disease: current evidence and perspectives. Front Pharmacol. (2024) 15:1371574. doi: 10.3389/fphar.2024.1371574
112. Matsumoto, Y, Fujii, H, Harima, M, Okamura, H, Yukawa-Muto, Y, Odagiri, N, et al. Severity of liver fibrosis is associated with the Japanese diet pattern and skeletal muscle mass in patients with nonalcoholic fatty liver disease. Nutrients. (2023) 15:1175. doi: 10.3390/nu15051175
113. Gallage, S, Ali, A, Barragan Avila, JE, Seymen, N, Ramadori, P, Joerke, V, et al. A 5:2 intermittent fasting regimen ameliorates NASH and fibrosis and blunts HCC development via hepatic PPARα and PCK1. Cell Metab. (2024) 36:1371–1393.e7. doi: 10.1016/j.cmet.2024.04.015
114. Lee, NK, Lee, Y, Shin, DS, Ra, J, Choi, YM, Ryu, BH, et al. Hepatoprotective effect of Lactiplantibacillus plantarum DSR330 in mice with high fat diet-induced nonalcoholic fatty liver disease. J Microbiol Biotechnol. (2024) 34:399–406. doi: 10.4014/jmb.2310.10026
115. Li, H, Wang, XK, Tang, M, Lei, L, Li, JR, Sun, H, et al. Bacteroides thetaiotaomicron ameliorates mouse hepatic steatosis through regulating gut microbial composition, gut-liver folate and unsaturated fatty acids metabolism. Gut Microbes. (2024) 16:2304159. doi: 10.1080/19490976.2024.2304159
116. Ahn, SB, Jun, DW, Kang, BK, Lim, JH, Lim, S, and Chung, MJ. Randomized, double-blind, placebo-controlled study of a multispecies probiotic mixture in nonalcoholic fatty liver disease. Sci Rep. (2019) 9:5688. doi: 10.1038/s41598-019-42059-3
117. Silva-Sperb, AS, Moraes, HA, Barcelos, STA, de Moura, BC, Longo, L, Michalczuk, MT, et al. Probiotic supplementation for 24 weeks in patients with non-alcoholic steatohepatitis: the PROBILIVER randomized clinical trial. Front Nutr. (2024) 11:1362694. doi: 10.3389/fnut.2024.1362694
118. Mofidi, F, Poustchi, H, Yari, Z, Nourinayyer, B, Merat, S, Sharafkhah, M, et al. Synbiotic supplementation in lean patients with non-alcoholic fatty liver disease: a pilot, randomised, double-blind, placebo-controlled, clinical trial. Br J Nutr. (2017) 117:662–8. doi: 10.1017/S0007114517000204
119. Wang, G, Jiao, T, Xu, Y, Li, D, Si, Q, Hao, J, et al. Bifidobacterium adolescentis and Lactobacillus rhamnosus alleviate non-alcoholic fatty liver disease induced by a high-fat, high-cholesterol diet through modulation of different gut microbiota-dependent pathways. Food Funct. (2020) 11:6115–27. doi: 10.1039/c9fo02905b
120. Yilmaz, Y. Postbiotics as Antiinflammatory and immune-modulating bioactive compounds in metabolic dysfunction-associated Steatotic liver disease. Mol Nutr Food Res. (2024) 68:e2400754. doi: 10.1002/mnfr.202400754
121. Ginès, P, Krag, A, Abraldes, JG, Solà, E, Fabrellas, N, and Kamath, PS. Liver cirrhosis. Lancet. (2021) 398:1359–76. doi: 10.1016/S0140-6736(21)01374-X
122. Trebicka, J, Hernaez, R, Shawcross, DL, and Gerbes, AL. Recent advances in the prevention and treatment of decompensated cirrhosis and acute-on-chronic liver failure (ACLF) and the role of biomarkers. Gut. (2024) 73:1015–24. doi: 10.1136/gutjnl-2023-330584
123. Luan, YT, Liu, CH, Jiang, SL, Gu, HT, Lyu, J, Xing, F, et al. Comparative analysis of intestinal microbiota distribution characteristics based on metagenomics in patients with hepatitis B cirrhosis with or without ascites. Zhonghua Gan Zang Bing Za Zhi. (2023) 31:974–85. doi: 10.3760/cma.j.cn501113-20220830-00440
124. Tang, Y, Zhou, H, Xiang, Y, and Cui, F. The diagnostic potential of gut microbiome for early hepatitis B virus-related hepatocellular carcinoma. Eur J Gastroenterol Hepatol. (2021) 33:e167–75. doi: 10.1097/MEG.0000000000001978
125. Efremova, I, Maslennikov, R, Medvedev, O, Kudryavtseva, A, Avdeeva, A, Krasnov, G, et al. Gut microbiota and biomarkers of intestinal barrier damage in cirrhosis. Microorganisms. (2024) 12:463. doi: 10.3390/microorganisms12030463
126. Bajaj, JS, Vargas, HE, Reddy, KR, Lai, JC, O'Leary, JG, Tandon, P, et al. Association between intestinal microbiota collected at hospital admission and outcomes of patients with cirrhosis. Clin Gastroenterol Hepatol. (2019) 17:e3:756–65. doi: 10.1016/j.cgh.2018.07.022
127. Efremova, I, Alieva, A, Maslennikov, R, Poluektova, E, Zharkova, M, Kudryavtseva, A, et al. Akkermansia muciniphila is associated with normal muscle mass and Eggerthella is related with sarcopenia in cirrhosis. Front Nutr. (2024) 11:1438897. doi: 10.3389/fnut.2024.1438897
128. Efremova, I, Maslennikov, R, Kudryavtseva, A, Avdeeva, A, Krasnov, G, Diatroptov, M, et al. Gut microbiota and cytokine profile in cirrhosis. J Clin Transl Hepatol. (2024) 12:689–700. doi: 10.14218/JCTH.2024.00090
129. Woodhouse, CA, Patel, VC, Singanayagam, A, and Shawcross, DL. Review article: the gut microbiome as a therapeutic target in the pathogenesis and treatment of chronic liver disease. Aliment Pharmacol Ther. (2018) 47:192–202. doi: 10.1111/apt.14397
130. Huang, L, Yu, Q, Peng, H, and Zhen, Z. Alterations of gut microbiome and effects of probiotic therapy in patients with liver cirrhosis: a systematic review and meta-analysis. Medicine (Baltimore). (2022) 101:e32335. doi: 10.1097/MD.0000000000032335
131. Nishimura, N, Kaji, K, Kitagawa, K, Sawada, Y, Furukawa, M, Ozutsumi, T, et al. Intestinal permeability is a mechanical rheostat in the pathogenesis of liver cirrhosis. Int J Mol Sci. (2021) 22:6921. doi: 10.3390/ijms22136921
132. Zafar, H, Jimenez, B, and Schneider, A. Small intestinal bacterial overgrowth: current update. Curr Opin Gastroenterol. (2023) 39:522–8. doi: 10.1097/MOG.0000000000000971
133. Maslennikov, R, Pavlov, C, and Ivashkin, V. Is small intestinal bacterial overgrowth a cause of hyperdynamic circulation in cirrhosis? Turk J Gastroenterol. (2019) 30:964–75. doi: 10.5152/tjg.2019.18551
134. Midori, Y, Nosaka, T, Hiramatsu, K, Akazawa, Y, Tanaka, T, Takahashi, K, et al. Isolation of mucosa-associated microbiota dysbiosis in the ascending colon in hepatitis C virus post-sustained virologic response cirrhotic patients. Front Cell Infect Microbiol. (2024) 14:1371429. doi: 10.3389/fcimb.2024.1371429
135. Hu, YC, Ding, XC, Liu, HJ, Ma, WL, Feng, XY, and Ma, LN. Effects of Lactobacillus paracasei N1115 on gut microbial imbalance and liver function in patients with hepatitis B-related cirrhosis. World J Gastroenterol. (2024) 30:1556–71. doi: 10.3748/wjg.v30.i11.1556
136. Leitner, U, Brits, A, Xu, D, Patil, S, and Sun, J. Efficacy of probiotics on improvement of health outcomes in cirrhotic liver disease patients: a systematic review and meta-analysis of randomised controlled trials. Eur J Pharmacol. (2024) 981:176874. doi: 10.1016/j.ejphar.2024.176874
137. Kang, EJ, Cha, MG, Kwon, GH, Han, SH, Yoon, SJ, Lee, SK, et al. Akkermansia muciniphila improve cognitive dysfunction by regulating BDNF and serotonin pathway in gut-liver-brain axis. Microbiome. (2024) 12:181. doi: 10.1186/s40168-024-01924-8
138. Chang, G, Sun, J, Li, J, and Li, T. Effect of probiotics on portal hypertension (PH) with cirrhosis: a systematic review and meta-analysis. Clin Res Hepatol Gastroenterol. (2024) 48:102361. doi: 10.1016/j.clinre.2024.102361
139. Román, E, Kaür, N, Sánchez, E, Poca, M, Padrós, J, Nadal, MJ, et al. Home exercise, branched-chain amino acids, and probiotics improve frailty in cirrhosis: a randomized clinical trial. Hepatol Commun. (2024) 8:e0443. doi: 10.1097/HC9.0000000000000443
140. Bloom, PP, Donlan, J, Torres Soto, M, Daidone, M, Hohmann, E, and Chung, RT. Fecal microbiota transplant improves cognition in hepatic encephalopathy and its effect varies by donor and recipient. Hepatol Commun. (2022) 6:2079–89. doi: 10.1002/hep4.1950
141. Hong, AS, Tun, KM, Hong, JM, Batra, K, and Ohning, G. Fecal microbiota transplantation in decompensated cirrhosis: a systematic review on safety and efficacy. Antibiotics (Basel). (2022) 11:838. doi: 10.3390/antibiotics11070838
142. Bajaj, JS, Salzman, NH, Acharya, C, Sterling, RK, White, MB, Gavis, EA, et al. Fecal microbial transplant capsules are safe in hepatic encephalopathy: a phase 1, randomized, placebo-controlled trial. Hepatology. (2019) 70:1690–703. doi: 10.1002/hep.30690
143. Chen, R, Wu, F, Zeng, G, Chen, Y, Lu, S, and Huang, H. Therapeutic effect of fecal microbiota transplantation on rats with liver cirrhosis and its influence on gut microbiota. Iran J Basic Med Sci. (2024) 27:1148–54. doi: 10.22038/IJBMS.2024.74280.16142
144. Liu, J, MacNaughtan, J, Kerbert, AJC, Portlock, T, Martínez Gonzalez, J, Jin, Y, et al. Clinical, experimental and pathophysiological effects of Yaq-001: a non-absorbable, gut-restricted adsorbent in models and patients with cirrhosis. Gut. (2024) 73:1183–98. doi: 10.1136/gutjnl-2023-330699
145. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global Cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
146. Schwabe, RF, and Greten, TF. Gut microbiome in HCC - mechanisms, diagnosis and therapy. J Hepatol. (2020) 72:230–8. doi: 10.1016/j.jhep.2019.08.016
147. Yang, J, Gao, H, Zhang, T, Fan, Y, Wu, Y, Zhao, X, et al. In vitro lactic acid Bacteria anti-hepatitis B virus (HBV) effect and modulation of the intestinal microbiota in fecal cultures from HBV-associated hepatocellular carcinoma patients. Nutrients. (2024) 16:600. doi: 10.3390/nu16050600
148. Jinato, T, Anuntakarun, S, Satthawiwat, N, Chuaypen, N, and Tangkijvanich, P. Distinct alterations of gut microbiota between viral- and non-viral-related hepatocellular carcinoma. Appl Microbiol Biotechnol. (2024) 108:34. doi: 10.1007/s00253-023-12845-1
149. Zheng, C, Lu, F, Chen, B, Yang, J, Yu, H, Wang, D, et al. Gut microbiome as a biomarker for predicting early recurrence of HBV-related hepatocellular carcinoma. Cancer Sci. (2023) 114:4717–31. doi: 10.1111/cas.15983
150. Peng, YC, Xu, JX, You, XM, Huang, YY, Ma, L, Li, LQ, et al. Specific gut microbiome signature predicts hepatitis B virus-related hepatocellular carcinoma patients with microvascular invasion. Ann Med. (2023) 55:2283160. doi: 10.1080/07853890.2023.2283160
151. Ma, H, Yang, L, Liang, Y, Liu, F, Hu, J, Zhang, R, et al. B. thetaiotaomicron-derived acetic acid modulate immune microenvironment and tumor growth in hepatocellular carcinoma. Gut Microbes. (2024) 16:2297846. doi: 10.1080/19490976.2023.2297846
152. Ma, J, Li, J, Jin, C, Yang, J, Zheng, C, Chen, K, et al. Association of gut microbiome and primary liver cancer: a two-sample Mendelian randomization and case-control study. Liver Int. (2023) 43:221–33. doi: 10.1111/liv.15466
153. Zheng, R, Wang, G, Pang, Z, Ran, N, Gu, Y, Guan, X, et al. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. (2020) 9:4232–50. doi: 10.1002/cam4.3045
154. Alharthi, J, Latchoumanin, O, George, J, and Eslam, M. Macrophages in metabolic associated fatty liver disease. World J Gastroenterol. (2020) 26:1861–78. doi: 10.3748/wjg.v26.i16.1861
155. Miura, K, Ishioka, M, Minami, S, Horie, Y, Ohshima, S, Goto, T, et al. Toll-like receptor 4 on macrophage promotes the development of steatohepatitis-related hepatocellular carcinoma in mice. J Biol Chem. (2016) 291:11504–17. doi: 10.1074/jbc.M115.709048
156. Wang, X, Fang, Y, Liang, W, Cai, Y, Wong, CC, Wang, J, et al. Gut-liver translocation of pathogen Klebsiella pneumoniae promotes hepatocellular carcinoma in mice. Nat Microbiol. (2025) 10:169–84. doi: 10.1038/s41564-024-01890-9
157. Tilg, H, Adolph, TE, and Trauner, M. Gut-liver axis: pathophysiological concepts and clinical implications. Cell Metab. (2022) 34:1700–18. doi: 10.1016/j.cmet.2022.09.017
158. Wu, XQ, Ying, F, Chung, KPS, Leung, CON, Leung, RWH, So, KKH, et al. Intestinal Akkermansia muciniphila complements the efficacy of PD1 therapy in MAFLD-related hepatocellular carcinoma. Cell Rep Med. (2025) 6:101900. doi: 10.1016/j.xcrm.2024.101900
159. Hu, C, Xu, B, Wang, X, Wan, WH, Lu, J, Kong, D, et al. Gut microbiota-derived short-chain fatty acids regulate group 3 innate lymphoid cells in HCC. Hepatology. (2023) 77:48–64. doi: 10.1002/hep.32449
160. Schneider, KM, Mohs, A, Gui, W, Galvez, EJC, Candels, LS, Hoenicke, L, et al. Imbalanced gut microbiota fuels hepatocellular carcinoma development by shaping the hepatic inflammatory microenvironment. Nat Commun. (2022) 13:3964. doi: 10.1038/s41467-022-31312-5
161. Lee, CG, Cha, KH, Kim, GC, Im, SH, and Kwon, HK. Exploring probiotic effector molecules and their mode of action in gut-immune interactions. FEMS Microbiol Rev. (2023) 47:fuad046. doi: 10.1093/femsre/fuad046, Erratum in: FEMS Microbiol Rev. 2023;47(5):fuad055. 10.1093/femsre/fuad055
162. Guo, M, Liu, H, Yu, Y, Zhu, X, Xie, H, Wei, C, et al. Lactobacillus rhamnosus GG ameliorates osteoporosis in ovariectomized rats by regulating the Th17/Treg balance and gut microbiota structure. Gut Microbes. (2023) 15:2190304. doi: 10.1080/19490976.2023.2190304
163. Zhou, P, Chang, WY, Gong, DA, Xia, J, Chen, W, Huang, LY, et al. High dietary fructose promotes hepatocellular carcinoma progression by enhancing O-GlcNAcylation via microbiota-derived acetate. Cell Metab. (2023) 35:1961–1975.e6. doi: 10.1016/j.cmet.2023.09.009
164. Li, G, Hou, Y, Zhang, C, Zhou, X, Bao, F, Yang, Y, et al. Interplay between drug-induced liver injury and gut microbiota: a comprehensive overview. Cell Mol Gastroenterol Hepatol. (2024) 18:101355. doi: 10.1016/j.jcmgh.2024.05.003
165. Garcia-Cortes, M, Robles-Diaz, M, Stephens, C, Ortega-Alonso, A, Lucena, MI, and Andrade, RJ. Drug induced liver injury: an update. Arch Toxicol. (2020) 94:3381–407. doi: 10.1007/s00204-020-02885-1
166. Xia, J, Lv, L, Liu, B, Wang, S, Zhang, S, Wu, Z, et al. Akkermansia muciniphila ameliorates acetaminophen-induced liver injury by regulating gut microbial composition and metabolism. Microbiol Spectr. (2022) 10:e0159621. doi: 10.1128/spectrum.01596-21
167. Xu, B, Hao, K, Chen, X, Wu, E, Nie, D, Zhang, G, et al. Broussonetia papyrifera polysaccharide alleviated acetaminophen-induced liver injury by regulating the intestinal Flora. Nutrients. (2022) 14:2636. doi: 10.3390/nu14132636
168. Román-Sagüillo, S, Quiñones Castro, R, Juárez-Fernández, M, Soluyanova, P, Stephens, C, Robles-Díaz, M, et al. Idiosyncratic drug-induced liver injury and amoxicillin-Clavulanate: spotlight on gut microbiota, fecal metabolome and bile acid profile in patients. Int J Mol Sci. (2024) 25:6863. doi: 10.3390/ijms25136863
169. Ali, AQ, Sabir, DK, Dawood, AF, Abu-Rashed, M, Hasari, A, Gharqan, F, et al. The potential liver injury induced by metronidazole-provoked disturbance of gut microbiota: modulatory effect of turmeric supplementation. Naunyn Schmiedeberg's Arch Pharmacol. (2024) 397:9845–58. doi: 10.1007/s00210-024-03242-0
170. Tulstrup, MV, Christensen, EG, Carvalho, V, Linninge, C, Ahrné, S, Højberg, O, et al. Antibiotic treatment affects intestinal permeability and gut microbial composition in Wistar rats dependent on antibiotic class. PLoS One. (2015) 10:e0144854. doi: 10.1371/journal.pone.0144854
171. Fu, H, Zhao, S, Song, S, and Xie, Q. Gut microbiota causally affects drug-induced liver injury via plasma metabolites: a Mendelian randomization study. Front Microbiol. (2024) 15:1432049. doi: 10.3389/fmicb.2024.1432049
172. Wang, C, Zhao, S, Xu, Y, Sun, W, Feng, Y, Liang, D, et al. Integrated microbiome and metabolome analysis reveals correlations between gut microbiota components and metabolic profiles in mice with methotrexate-induced Hepatoxicity. Drug Des Devel Ther. (2022) 16:3877–91. doi: 10.2147/DDDT.S381667
173. Li, J, Lian, X, Li, B, Ma, Q, Yang, L, Gao, G, et al. Pharmacodynamic material basis of licorice and mechanisms of modulating bile acid metabolism and gut microbiota in cisplatin-induced liver injury based on LC-MS and network pharmacology analysis. J Ethnopharmacol. (2025) 340:119293. doi: 10.1016/j.jep.2024.119293
174. Niu, M, Luo, Z, Gong, S, Win, S, Kaplowitz, N, Jiang, Y, et al. Intestinal epithelial chemokine (C-C motif) ligand 7 overexpression enhances acetaminophen-induced hepatotoxicity in mice. Am J Pathol. (2020) 190:57–67. doi: 10.1016/j.ajpath.2019.09.009
175. Tao, W, Fan, Q, and Wei, J. Gut-liver Axis as a therapeutic target for drug-induced liver injury. Curr Issues Mol Biol. (2024) 46:1219–36. doi: 10.3390/cimb46020078
176. Sun, J, Zhao, F, Lin, B, Feng, J, Wu, X, Liu, Y, et al. Gut microbiota participates in Antithyroid drug induced liver injury through the lipopolysaccharide related signaling pathway. Front Pharmacol. (2020) 11:598170. doi: 10.3389/fphar.2020.598170
177. Li, ZM, Kong, CY, Mao, YQ, Huang, JT, Chen, HL, Han, B, et al. Ampicillin exacerbates acetaminophen-induced acute liver injury by inducing intestinal microbiota imbalance and butyrate reduction. Liver Int. (2023) 43:865–77. doi: 10.1111/liv.15512
178. Zeng, Y, Wu, R, Wang, F, Li, S, Li, L, Li, Y, et al. Liberation of daidzein by gut microbial β-galactosidase suppresses acetaminophen-induced hepatotoxicity in mice. Cell Host Microbe. (2023) 31:766–780.e7. doi: 10.1016/j.chom.2023.04.002
179. Yang, CJ, Chang, HC, Sung, PC, Ge, MC, Tang, HY, Cheng, ML, et al. Oral fecal transplantation enriches Lachnospiraceae and butyrate to mitigate acute liver injury. Cell Rep. (2024) 43:113591. doi: 10.1016/j.celrep.2023.113591
180. Li, D, Chen, Y, Wan, M, Mei, F, Wang, F, Gu, P, et al. Oral magnesium prevents acetaminophen-induced acute liver injury by modulating microbial metabolism. Cell Host Microbe. (2024) 32:48–62.e9. doi: 10.1016/j.chom.2023.11.006
181. Hong, MK, Liu, HH, Chen, GH, Zhu, JQ, Zheng, SY, Zhao, D, et al. Oridonin alters hepatic urea cycle via gut microbiota and protects against acetaminophen-induced liver injury. Oxidative Med Cell Longev. (2021) 2021:3259238. doi: 10.1155/2021/3259238
182. Hu, S, Tang, B, Lu, C, Wang, S, Wu, L, Lei, Y, et al. Lactobacillus rhamnosus GG ameliorates triptolide-induced liver injury through modulation of the bile acid-FXR axis. Pharmacol Res. (2024) 206:107275. doi: 10.1016/j.phrs.2024.107275
183. Gawlik-Kotelnicka, O, Burzyński, J, Rogalski, J, Skowrońska, A, and Strzelecki, D. Probiotics may be useful for drug-induced liver dysfunction in patients with depression - a secondary analysis of a randomized clinical trial. Clin Nutr ESPEN. (2024) 63:604–14. doi: 10.1016/j.clnesp.2024.07.1024
184. Shi, H, Wang, Q, Xu, B, Liu, Y, Zhao, J, Yang, X, et al. Research on gut microbiota characteristics of PBC patients at different ALBI grades based on machine learning. Front Microbiol. (2024) 15:1495170. doi: 10.3389/fmicb.2024.1495170
185. Liu, Q, Huang, B, Zhou, Y, Wei, Y, Li, Y, Li, B, et al. Gut microbiome pattern impacts treatment response in primary biliary cholangitis. Med. (2025) 6:100504. doi: 10.1016/j.medj.2024.08.003
186. Santiago, P, Quinn, KP, Chen, J, Friton, JJ, Rypstra, CR, Kashyap, PC, et al. Altered bile acid and pouch microbiota composition in patients with chronic Pouchitis. Inflamm Bowel Dis. (2024) 30:1062–70. doi: 10.1093/ibd/izad288
187. Fukui, H. Role of gut Dysbiosis in liver diseases: what have we learned So far? Diseases. (2019) 7:58. doi: 10.3390/diseases7040058
188. Cui, Y, Guo, Y, Kong, Y, and Zhang, G. Association between gut microbiota and autoimmune cholestatic liver disease, a Mendelian randomization study. Front Microbiol. (2024) 15:1348027. doi: 10.3389/fmicb.2024.1348027
189. Costello, SP, Hughes, PA, Waters, O, Bryant, RV, Vincent, AD, Blatchford, P, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. (2019) 321:156–64. doi: 10.1001/jama.2018.20046
190. Sun, C, Zhu, D, Zhu, Q, He, Z, Lou, Y, and Chen, D. The significance of gut microbiota in the etiology of autoimmune hepatitis: a narrative review. Front Cell Infect Microbiol. (2024) 14:1337223. doi: 10.3389/fcimb.2024.1337223
191. Cai, X, Dai, J, Xie, Y, Xu, S, and Liu, M. Multi-omics study unravels gut microbiota and metabolites alteration in patients with Wilson's disease. Sci Rep. (2024) 14:21025. doi: 10.1038/s41598-024-71740-5
192. Cao, CF, Zhang, XM, Li, XC, and Lin, W. Relationship between changes of intestinal flora and HBV-DNA level in patients with hepatitis B cirrhosis. Chin J Microecol. (2022) 34:1436–47. doi: 10.13381/j.cnki.cjm.202212013
193. Smirnova, E, Puri, P, Muthiah, MD, Daitya, K, Brown, R, Chalasani, N, et al. Fecal microbiome distinguishes alcohol consumption from alcoholic hepatitis but does not discriminate disease severity. Hepatology. (2020) 72:271–86. doi: 10.1002/hep.31178
194. Lang, S, Fairfied, B, Gao, B, Duan, Y, Zhang, X, Fouts, DE, et al. Changes in the fecal bacterial microbiota associated with disease severity in alcoholic hepatitis patients. Gut Microbes. (2020) 12:1785251. doi: 10.1080/19490976.2020.1785251
195. Grander, C, Meyer, M, Steinacher, D, Claudel, T, Hausmann, B, Pjevac, P, et al. 24-Norursodeoxycholic acid ameliorates experimental alcohol-related liver disease and activates hepatic PPARγ. JHEP Rep. (2023) 5:100872. doi: 10.1016/j.jhepr.2023.100872
196. Zhang, H, Li, C, Han, L, Xiao, Y, Bian, J, Liu, C, et al. MUP1 mediates urolithin a alleviation of chronic alcohol-related liver disease via gut-microbiota-liver axis. Gut Microbes. (2024) 16:2367342. doi: 10.1080/19490976.2024.2367342
197. Wang, Y, Zou, Z, Wang, S, Ren, A, Ding, Z, Li, Y, et al. Golden bile powder prevents drunkenness and alcohol-induced liver injury in mice via the gut microbiota and metabolic modulation. Chin Med. (2024) 19:39. doi: 10.1186/s13020-024-00912-2
198. Yin, R, Wang, T, Sun, J, Dai, H, Zhang, Y, Liu, N, et al. Postbiotics from Lactobacillus johnsonii activates gut innate immunity to mitigate alcohol-associated liver disease. Adv Sci (Weinh). (2025) 12:e2405781. doi: 10.1002/advs.202405781
199. Li, SQ, Wang, YR, Xie, ZL, Wang, Y, Feng, ZH, Xu, JH, et al. NLRP3 activation maintains intestinal epithelial barrier and reduces liver injury in alcoholic liver disease mice. Clin Transl Med. (2024) 14:e70099. doi: 10.1002/ctm2.70099
Keywords: gut microbiota, viral hepatitis, metabolic dysfunction-associated steatotic liver disease, alcoholic fatty liver disease, liver cirrhosis
Citation: Yu J-X, Wu J, Chen X, Zang S-g, Li X-b, Wu L-P and Xuan S-h (2025) Gut microbiota in liver diseases: initiation, development and therapy. Front. Med. 12:1615839. doi: 10.3389/fmed.2025.1615839
Edited by:
Xuyong Wei, Hangzhou First People’s Hospital, ChinaReviewed by:
Yuzhu Dai, The 903rd Hosipital of the PLA, ChinaLan Huang, Suzhou Municipal Hospital, China
Copyright © 2025 Yu, Wu, Chen, Zang, Li, Wu and Xuan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li-Pei Wu, d2xwODI4NDE0QDE2My5jb20=; Shi-hai Xuan, eHNoLmp5a0AxNjMuY29t
 Jian-Xiu Yu
Jian-Xiu Yu Jun Wu
Jun Wu Xin Chen
Xin Chen