Abstract
Rheumatoid arthritis is a chronic inflammatory joint disease with low treatment coverage in sub-Saharan Africa. Effective treatment strategies are available. We aimed to evaluate the cost effectiveness of six disease modifying anti-rheumatic drug (DMARD) treat-to-target treatment strategies for patients with rheumatoid arthritis in Zanzibar. A Markov model was used to calculate the cost-effectiveness of various DMARD strategies in the treatment of rheumatoid arthritis over a 3-year period. A health-provider perspective was used and only outpatient costs were considered. The Clinical Disease Activity Index (CDAI) was utilized for measurement of efficacy and values were obtained from literature. Quality Adjusted Life Years (QALYs) were obtained from 122 patients attending the rheumatology clinic at Mnazi Mmoja Hospital. Data on costs were obtained from the central medical stores and hospital administration. Treatment strategies were given in sequential approach based on treat to target goals of therapy. This included methotrexate monotherapy, methotrexate + sulfasalazine + hydroxychloroquine, methotrexate followed by one or two biologic/targeted-synthetic DMARDs (b/tsDMARDs). Probabilistic and one way sensitivity analysis were performed. Scenario analysis was undertaken comparing drug prices from India and Scandinavia. Costs of therapy/patient/3 years ranged from USD 634 for methotrexate monotherapy and USD 5011 for methotrexate and two consecutive b/tsDMARDs. The highest and lowest effects were 2.209 and 2.079 QALYs gained from methotrexate therapy + two consecutive b/tsDMARDs and methotrexate monotherapy, respectively. From a healthcare provider perspective methotrexate monotherapy was the cost-effective option at a willingness to pay of USD 282. Pairwise comparison also favored methotrexate monotherapy as the feasible option. We found that increasing the willingness to pay led to a change in the most acceptable option from methotrexate monotherapy to methotrexate followed by b/tsDMARD. Methotrexate monotherapy is the cost-effective option for the management of rheumatoid arthritis in Zanzibar. Other options may be feasible if the willingness to pay threshold is increased or the drug prices are lowered, particularly for the b/tsDMARDs.
Background
Rheumatoid arthritis (RA) is a chronic inflammatory joint disease characterized by pain, swelling, and stiffness that leads to joint destruction. In sub-Saharan African (SSA) populations, the prevalence of RA is estimated to be between 0.6 and 1.0% (1, 2). With low treatment coverage, RA is considered a neglected chronic disease (3). People with RA experience reduced physical functioning, quality of life, and life expectancy (4). The 2019 Global Burden of Disease estimated that RA caused 3.3 million disability adjusted life years (DALYs) and 44,000 deaths globally (5), with increasing incidence over the last decade (6).
Effective therapies for RA are available. When initiated early and aggressively, based on the treat-to-target (T2T) strategy, they improve overall outcomes and prevent disability (7–9). Recommended drugs include the affordable conventional synthetic disease modifying anti-rheumatic drugs (csDMARDS) such as methotrexate, sulfasalazine, and hydroxychloroquine, and the costlier and less accessible biological disease modifying anti-rheumatic drugs (bDMARDS) such as tumor necrosis factor alfa inhibitors (TNFi) and rituximab. Additionally, targeted synthetic DMARDs (tsDMARDS), for example, the JAK-inhibitors tofacitinib and baricitinib, have recently become widely available (10). These therapies are available in high income countries but are limited in low- and low-middle income countries (LLMICs) (11).
While economic evaluations of advanced RA treatments for high-income settings are available (12–14), only few exist for LLMICs (15, 16). Recent cost-effectiveness analyses from high-income countries focused on costly bDMARDs (17, 18), which may face implementation challenges in resource constrained settings compared to relatively basic treatments such as methotrexate and triple therapy with csDMARDs. In such settings, the opportunity costs are extremely high and fair priority setting of RA management alongside essential and very cost-effective services like basic obstetric care is important. Therefore, policy-relevant economic evaluations for SSA should compare feasible RA treatment options in regionally contextualized analyses.
Although one third of RA patients eventually require biologic therapy (19), the cost-effectiveness of biologic/targeted synthetic disease modifying anti-rheumatic drugs (b/tsDMARDs) in African settings is not yet researched and the drugs are often unavailable due to their relatively high treatment costs and fragile price negotiation systems (11). The Disease Control Priorities-3 (DCP3) review estimated the cost effectiveness of DMARD therapy in 2001 for developing countries based on available literature from Western populations. They reported possible effectiveness for corticosteroids at low doses, and for combination therapy of methotrexate, sulfasalazine, and prednisolone. For bDMARD therapy, the costs were considered prohibitive (20).
This study aims to evaluate the cost-effectiveness of six DMARD treat-to-target treatment strategies for patients with rheumatoid arthritis in Zanzibar. The strategies include combinations of methotrexate, sulfasalazine, hydroxychloroquine, prednisolone, and b/tsDMARDs. Some strategies may be more commonly practiced in resource-limited settings (21, 22) while others are practiced in developed countries (23, 24) where b/tsDMARDs are more readily available.
Methods
Model
Six Markov models were developed to compare the cost-effectiveness of the six RA treatment strategies using TreeAge® Pro Healthcare 2022 (Figure 1). Results were reported according to the 2022 Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement (25). We used a validated model (26) similar to that of Schipper et al (27) with an additional death state to include patients who died during the modelling period (Supplementary material 1).
Figure 1

Treatment algorithm of the six included strategies. Each treatment strategy is initiated with methotrexate as the first-line drug. The arrows depict the next drug option if patients did not achieve treatment target. In all arms patients are placed on rescue therapy (methotrexate+daily prednisolone <10 mg) if treatment goal was not reached. Triple therapy indicates combination of methotrexate+sulfasalazine+hydroxychloroquine, b/tsDMARD—biologic/targeted synthetic disease modifying anti-rheumatic drug.
The Markov design was used to evaluate the differences in transitions between five health states for each strategy (Figure 2). We used Markov cycle lengths of 6 months, reflecting international guidelines on treatment duration before drug change (23, 28). We further defined a time horizon of 3 years based on the assumption that patients’ disease is less likely to show much change to available therapy after this period.
Figure 2

Illustration of the transition possibilities between the health states used in the model.
Based on primary data (see Study Population, below), we determined that the modelled cohort started with moderate disease activity at baseline. Disease remission or low disease activity were the desired health states (treatment target), death was an absorbing state. At the end of each cycle, disease activity was determined. If the treatment target was achieved, patients remained on the same drug therapy in the subsequent cycle. If treatment target was not achieved, they progressed to the next drug option within the same strategy. Those who failed to achieve target on all available drugs within the strategy were placed on methotrexate with low dose prednisolone (<10 mg/day), termed rescue therapy.
Interventions and costs
We determined methotrexate as the comparator because it is the recommended first line DMARD, by both European and American guidelines unless contraindicated (29, 30). Costs included in the analysis are shown in Table 1. All cost data collected were converted into USD using the exchange rates of December 2021. We assumed a healthcare provider perspective to estimate the direct and indirect costs of RA interventions, limited to outpatient clinic visits. These included four clinic visits during the first 6 months of therapy: at diagnosis, at 1 month, at 3 months and at 6 months. After this, patients who reached treatment target had one clinic visit per cycle and those not at target (moderate or high disease activity) required two visits per cycle. Costs of adverse drug events or inpatient care were not considered, particularly because inpatient admissions for RA have decreased, especially among those in whom RA was the primary diagnosis such as our cohort (31). Additionally, compared to Caucasian populations, there are lower rates of DMARD intolerance and side effects among sub-Saharan Black patients with RA (32). Systematic review and meta-analysis from the high-income countries, found no increased risk for serious infection or hospitalization among patients using b/tsDMARDs although there was potential in patients on long-term therapy of more than 6 months (33, 34). Similar data from Africa was lacking. Experience from the Zanzibar Chronic Inflammatory Joint Disease (Zan-CIJD) cohort showed that over the study period only one patient developed infection requiring antibiotics (unpublished data) and three patients developed tuberculosis (35), none of whom required hospital admission. Laboratory costs were obtained from the hospital database. Costs of tests that were not offered at the hospital were obtained from private laboratories. We used three cost categories: costs at diagnosis, costs to start b/tsDMARDs, and costs to continue therapy (Table 1). Drug costs were obtained from the Tanzania Medical Stores Department (MSD) pricelist (36) which is the main drug supplier for the Ministry of Health. Where unavailable, we acquired wholesale prices from private pharmacies. For the b/tsDMARD we listed all locally available drugs and compared prices from various pharmaceutical suppliers and retailers in the market and selected the cheapest options available(Table 1). For the scenario analysis, drug prices for Scandinavia were obtained from the 2022 tender prices for Norway. Due to a governmental confidentiality clause, only average prices, and no specific-drug prices, of the two cheapest b/tsDMARDs are provided. Drug prices for India were obtained from the National Pharmaceutical Pricing Authority database website (37). This is the primary authority responsible for regulating and controlling drug prices in India. Where drug prices were not available from the database, retail vendor websites were explored and the cheapest price selected (38, 39).
Table 1
| Item | Zanzibar | Range | |
|---|---|---|---|
| Unit costs, 6-months (USD) | Low | High | |
| Methotrexate (+folic acid)1 | 45 | 22.5 | 67.5 |
| Bridging therapy2 | 6 | 3 | 9 |
| Sulfasalazine1 | 127 | 63.5 | 190.5 |
| Hydroxychloroquine1 | 111 | 55.5 | 166.5 |
| 1st b/tsDMARD3 | 94* | 47 | 141 |
| 2nd b/tsDMARD3 | 5456* | 2,728 | 8,184 |
| Prednisolone4 | 20 | 10 | 30 |
| Costs at diagnosis5 | 125 | 62.5 | 187.5 |
| Investigations to continue csDMARDs (1st cycle) | 57 | 27.5 | 82.5 |
| Investigations when starting b/tsDMARDs | 157 | 77.5 | 232.5 |
| Costs to continue csDMARD therapy6 | 25 | 20.5 | 61.5 |
| Costs to continue b/tsDMARDs7 | 56 | 3.5 | 10.5 |
| Clinic visit costs (per visit)8 | 4 | 2 | 6 |
Key costs for the management of RA in Zanzibar.
USD, United States dollar; csDMARDs, conventional synthetic disease modifying anti-rheumatic drugs; b/tsDMARD, biologic/targeted-synthetic disease modifying anti-rheumatic drugs. All costs are aggregated for 6 months except for clinic visit costs.
Costs for hydroxychloroquine 400 mg/day, sulfasalazine 2000 mg/day, and methotrexate 25 mg/week (+folic acid 5 mg once weekly).
Costs of prednisolone at 15 mg tapered over 6 weeks, calcium + vitamin D supplement and omeprazole.
Approximate prices for conventional dosage of the two cheapest b/tsDMARD in 2022 administered at recommended adult doses for rheumatoid arthritis therapy.
Prednisolone as rescue therapy at 5 mg/day. Used with Calcium + Vitamin D which contribute USD 18 to the total.
Costs of investigations required to diagnose, start therapy, and contraception.
Costs of investigations once/cycle for patients at target and twice/cycle for patients not at target.
Costs of pneumococcus vaccine, prophylaxis with cotrimoxazole, isoniazid preventive therapy and contraception as well as screening for pulmonary tuberculosis with chest radiograph.
Costs of staff, building, supplies and utilities per patient per clinic visit.
Capital costs were obtained from the Mnazi Mmoja Hospital Engineering and Nursing departments (Supplementary material 2). Costs of buildings, supplies and electricity were calculated per patient and combined as overhead costs (Table 1). We further assumed that the clinician, nurse, and pharmacist would each require 20, 10 and 5 min per outpatient visit, respectively. The personnel costs were obtained from the FairChoices DCP-4 analytic tool evidence brief for low-income country rates (40). All costs were point estimates. We used a range of ±50% for the univariate sensitivity analysis, and gamma distributions with 95% confidence intervals within these ranges for probabilistic sensitivity analyses (PSA).
Effectiveness
Data on intervention effectiveness was obtained from previously published studies, conducted largely in high-income settings (41–47). When multiple studies on patients with RA for at least 1 year (established RA) reported CDAI outcomes, the data were pooled. The effects were translated into transition probabilities for each strategy, capturing the relative proportions of patients in the different health states at 6 months from treatment initiation (Table 2). We included only studies with established RA cohorts in order tailor the available efficacy data from high-income settings to the Zanzibar Chronic Inflammatory Joint Disease (Zan-CIJD) cohort, where patients tend to have suffered RA relatively longer before seeking care. More than 80% of patients who achieved the treatment target within the first 6 months remained in the target state receiving the same therapy over the next 6 months (47). For those on rescue therapy about 43% could reach treatment target at the end of each cycle.
Table 2
| Intervention | Health state | Transition probability1 | Range High Low |
Source | |
|---|---|---|---|---|---|
| Methotrexate | Remission LDA MDA HDA |
0.09 0.34 0.38 0.19 |
0.0675 0.255 0.285 0.1425 |
0.1125 0.425 0.475 0.2375 |
(32, 33) |
| Triple therapy | Remission LDA MDA HDA |
0.10 0.41 0.33 0.16 |
0.75 0.3075 0.2475 0.12 |
1.25 0.5125 0.4125 0.20 |
(34) |
| b/tsDMARD 1 (early)2 | Remission LDA MDA HDA |
0.12 0.46 0.28 0.14 |
0.15 0.345 0.21 0.105 |
0.18 0.575 0.35 0.175 |
(35) |
| b/tsDMARD 1 (late)3 | Remission LDA MDA HDA |
0.11 0.41 0.32 0.16 |
0.0825 0.3075 0.24 0.12 |
0.1375 0.5125 0.4 0.20 |
(36) |
| b/tsDMARD2 (early)2 | Remission LDA MDA HDA |
0.12 0.30 0.39 0.19 |
0.09 0.225 0.2925 0.1425 |
0.15 0.375 0.4875 1.125 |
(37) |
| b/tsDMARD 2 (late)3 | Remission LDA MDA HDA |
0.12 0.25 0.42 0.21 |
0.09 0.1875 0.315 0.1575 |
0.15 0.3125 0.525 0.2625 |
(37) |
Transition probabilities of health states on different drug therapies for 6 months for patients with RA in Zanzibar.
Transition probability from one health state to another. All patients start at moderate disease activity.
Early—drug is started after methotrexate failure.
Late—treatment after both methotrexate and triple therapy failure.
Given the limited evidence, we made assumptions to determine some efficacy values. Once patients had exhausted all available treatment options within a strategy, they were categorized as either having reached the treatment target, or not, for the remaining cycles. Based on expert opinion, in the target group, patients were distributed in a ratio of 1:2 between remission and low disease activity, while in the non-target group, patients were distributed in a ratio of 2:1 between moderate and high disease activity.
We obtained mortality data specific to Tanzanian women from the World Health Organization’s health repository (48) and converted it into 6-monthly mortality rates. To account for the higher mortality rate among patients with RA, we applied adjustments of 1.29 and 1.42 for low and moderate disease activity, respectively (49).
Study population
The Zan-CIJD study contains data collected from 1st September 2019 to 28th February 2022 from a total of 102 patients with RA attending the rheumatology outpatient clinic at Mnazi Mmoja referral hospital. The majority were female (84%), with a mean age of 45 years (SD 13.5) and the mean disease duration was 6.4 years (CI 1.3). Their baseline CDAI was 19.8 (SD 12.8), indicating moderate disease activity.
Utilities
Quality-adjusted life-years (QALYs) are a combined measure of the mortality and morbidity caused by a disease. They are calculated as a function of the time spent in a health state and the health-related quality of life (HRQoL) weight (i.e., utility score) associated with that health state (50). HRQoL utility scores for each level of disease activity were derived from the Zan-CIJD cohort using the EuroQol® 5 dimensions and 5 levels (EQ-5D5L) questionnaire. Data was collected from the ZAN-CIJD cohort over the entire study period, totaling 538 questionnaires. These were tabulated and converted to utilities using the healthy Ugandan population utility scores (51) for reference. At baseline, patients had a mean utility score of 0.62 (95% CI: 0.54, 0.70).
In RA, HRQoL is mainly affected by disease activity regardless of therapy used (52, 53). Based on the CDAI scores for the Zan-CIJD population, patients were grouped into remission, low, moderate, and high disease activity. The average utility score for each of the groups was determined based on each health state (see Table 3).
Table 3
| Disease activity (CDAI) | Health-related quality of life (utility score) | Range | |
|---|---|---|---|
| Remission | 0.87 | 0.6525 | 1.0875 |
| LDA | 0.72 | 0.54 | 0.90 |
| MDA | 0.57 | 0.4275 | 0.7125 |
| HDA | 0.21 | 0.1575 | 0.2625 |
| Treatment target1 | 0.77 | 0.5775 | 0.9625 |
| Not at treatment target2 | 0.45 | 0.3375 | 0.5625 |
Utility values based on RA disease activity in the Zan-CIJD population.
CDAI, Clinical Disease Activity Index; LDA, low disease activity; MDA, moderate disease activity; HDA, high disease activity.
Patients who reached remission or low-disease activity.
Patients who did not reach remission or low disease activity.
Analysis
We report Incremental Cost-Effectiveness Ratios (ICERS), the ratio of the incremental costs divided by the incremental utilities gained (54). The baseline comparator used was methotrexate monotherapy. Discounting for costs and utilities was estimated at 3% per annum and half-cycle correction was done. We determined the willingness to pay (WTP) threshold at USD 228/QALY based on a recent study determining cost-effectiveness thresholds by Pichon-Riviere et al (55).
Univariate deterministic sensitivity analysis was performed for the key input parameters such as costs of drugs and probabilities of reaching treatment target. A range of ±25% of the point estimates was used to describe uncertainties around treatment effectiveness and associated utilities. We assumed wider ranges of ±50% to capture uncertainties around costs variables, which tend to show greater variation. We report the univariate analyses as a Tornado diagram.
We further performed probabilistic sensitivity analysis (PSA) using Monte Carlo simulation with 10,000 iterations. We adopted the ranges from the univariate deterministic sensitivity analysis to conduct the PSA and assumed specific distribution shapes for parameters on costs (gamma), probabilities (beta) and utilities (normal). Incremental cost-effectiveness scatter plots for pairwise comparison of strategy 1 as base case analysis with strategy 2 and strategy 3 were also plotted. For visualization purposes we reduced the corresponding scatterplots to 750 iterations.
We performed the analysis with varying WTP-thresholds equal to one GDP per capita and twice the GDP per capita at USD 1136 and 2,272, respectively. An analysis of the optimal treatment strategy using a WTP of USD 282 was also performed using Monte Carlo simulation. We also conducted price threshold analysis comparing methotrexate with the other treatment options to assess whether changes in drug prices would result in change in what is determined as optimal treatment strategy. Finally, scenario analysis using one-way sensitivity was done was performed drug costs from Scandinavian countries, and India (Table 4).
Table 4
| Drug | Scandinavia | India |
|---|---|---|
| Unit costs, 6-months (USD) | Unit costs, 6-months (USD) | |
| Methotrexate (+folic acid 5 mg once weekly) | 49 USD1 | 181 |
| Sulfasalazine | 1201 | 391 |
| Hydroxychloroquine | 581 | 291 |
| 1st b/tsDMARD2 | 486 | 129 |
| 2nd b/tsDMARD2 | 1,022 | 1,644 |
| Prednisolone | 383 | 193 |
Drug costs for the management of RA from Scandinavia and India (six-monthly).
All b/tsDMARDs are calculated based on their recommended doses for adults with rheumatoid arthritis.
Costs for hydroxychloroquine 400 mg/day, sulfasalazine 2000 mg/day, methotrexate 25 mg/week, and prednisolone 5 mg/day.
Approximate prices for conventional dosage of the two cheapest b/tsDMARD in 2022.
An additional cost of USD 18 was added as cost of Calcium + Vitamin D therapy in the rescue therapy arm.
Results
The difference in costs between the strategies were largely driven by the drug costs, with b/tsDMARDs costing significantly more than the conventional synthetic DMARDs. The lowest treatment cost was for strategy 1 (methotrexate therapy) at USD 634/patient/3 years and strategy six had the highest cost (methotrexate with two consecutive b/tsDMARDs) at USD 5,011/patient/3 years (Table 5).
Table 5
| Strategy | Cost (USD) | Incremental cost | QALYs gained | Incremental effect | ICER |
|---|---|---|---|---|---|
| Strategy 1 (Methotrexate) | 634 | 2.079 | 322 | ||
| Strategy 2 (Methotrexate then TT) | 980 | 243 | 2.150 | −0.012 | Abs dominated1 |
| Strategy 3 (Methotrexate then b/tsDMARD) | 737 | 103 | 2.161 | 0.083 | 1,251 |
| Strategy 4 (Methotrexate then TT then 1-b/tsDMARD) | 1,026 | 289 | 2.177 | 0.016 | 18,359 |
| Strategy 5 (Methotrexate then TT then two consecutive b/tsDMARDs) | 3,063 | 2037 | 2.181 | 0.004 | Ext dominated2 |
| Strategy 6 (Methotrexate then 2 consecutive b/tsDMARDs) | 5,011 | 3,984 | 2.209 | 0.032 | 124,877 |
Three-year costs (mean USD per patient), effects (mean QALY gained per patient) and cost effectiveness of six treatment strategies for rheumatoid arthritis using Monte Carlo simulation with 10,000 iterations and WTP threshold of USD 282.
QALYs, quality-adjusted life years; ICER, incremental cost-effectiveness ratio; WTP, willingness to pay; USD, United States Dollar; TT, triple therapy (methotrexate + sulfasalazine + hydroxychloroquine); b/tsDMARDs, biologic/targeted synthetic disease modifying anti-rheumatic drugs.
Abs dominated indicates absolute dominance (costlier and less effective than the next strategy).
Ext dominated indicates extended dominance (ICER greater than the next more effective strategy).
The highest effectiveness amounted to 2.209 QALYs gained from treatment according to strategy 6 (methotrexate therapy + two consecutive b/tsDMARDs) while the lowest treatment effectiveness was obtained using strategy 1 (methotrexate alone), 2.079 QALYs (Table 5).
From a Zanzibar healthcare provider perspective, for a WTP threshold of USD 282, strategy 1 was cost-effective while strategies 3, 4 and 6 were suboptimal options. ICERs ranged from 322 to 124,877 for all six strategies (Table 5). Strategy 2 was found to be both more costly and less effective than strategy 3 and therefore not a rational choice from the healthcare provider’s perspective (absolutely dominated). Strategy 5 had an ICER greater than strategy 6 despite strategy 6 being more effective and was therefore excluded (Table 5).
Deterministic sensitivity analysis
One-way sensitivity analysis comparing the three strategies considerable under the set WTP thresholds (methotrexate monotherapy (strategy 1) compared to methotrexate + TT (strategy 2) and methotrexate+ b/tsDMARD1 (strategy 3) showed that the most influential parameter on the ICERs was the cost of the TT and b/tsDMARD1 (Figures 3a,b). Compared to strategy one, the mean ICERs when considering single variables’ ranges remain largely above the WTP thresholds. However, comparing strategies 1 and 3, the assumed cost of the b/tsDMARD, at the lower end of its range, includes the option that strategy 3 is optimal in Zanzibar at the baseline WTP threshold, assuming all other variables remain constant (Figure 3b). Comparisons with other strategies found no single parameter had a significant effect on the ICER to cross the WTP threshold.
Figure 3

One-way sensitivity analysis comparing ICERS of methotrexate monotherapy vs. methotrexate + TT (a) and methotrexate + b/tsDMARD1 (b). Each variable is arranged by decreasing impact on the ICER. A willingness to pay threshold of USD 282 was used.
Probabilistic sensitivity analysis
Pairwise comparison of strategy 1 (methotrexate monotherapy) as the base case, with strategy 2 (methotrexate + TT) and strategy 3 (methotrexate+ b/tsDMARD1) showed that the iterations largely favored strategy 1 as the cost-effective option despite being less effective (Figures 4a,b).
Figure 4

(a) and (b). Incremental cost-effectiveness scatterplots, showing pairwise comparison of strategy 2 (methotrexate + TT (a)) and strategy 3 (methotrexate + b/tsDMARD1 (b)), both compared to strategy 1 (methotrexate monotherapy) as the base case. The expected values for cost and effectiveness for strategy 1 are located at the intercept of the y and x axis, 0 cost, and 0 effectiveness. All iterations of strategies 2 and 3, shown as dots, indicate the incremental difference in cost and effectiveness to the average values for strategy 1. In most iterations, both strategies are more effective and more costly than the base case, indicated by the majority of iterations location in the North-East quadrant of the graphs. The dotted line delineates the willingness to pay threshold for Zanzibar (WTP) set at USD 282. With the estimated WTP, the more effective treatments are, with high probability, not cost-effective treatment options for Zanzibar, shown as red dots. While more costly and more effective, the incremental gain in effectiveness can hardly be justified with the corresponding incremental cost, when assuming the ICER threshold for Zanzibar. Only 4.7% of iterations suggested strategy 2 as cost-effective treatment in this comparison. Comparing strategy 3 to strategy 2, 23.5%, a higher share of iterations favor strategy 3. This suggests less certainty for a potential decision in favor of strategy 1 in this comparison with the uncertainty we introduced in the model.
The evaluation of the cost effectiveness when applying other WTP thresholds for Zanzibar of USD 1136 and 2,272 indicated that with increasing WTP thresholds strategy 3 (methotrexate + b/tsDMARD) became a probable cost-effective treatment alongside strategy 1, and, less so, strategy 4 at the highest thresholds. (Figure 5).
Figure 5
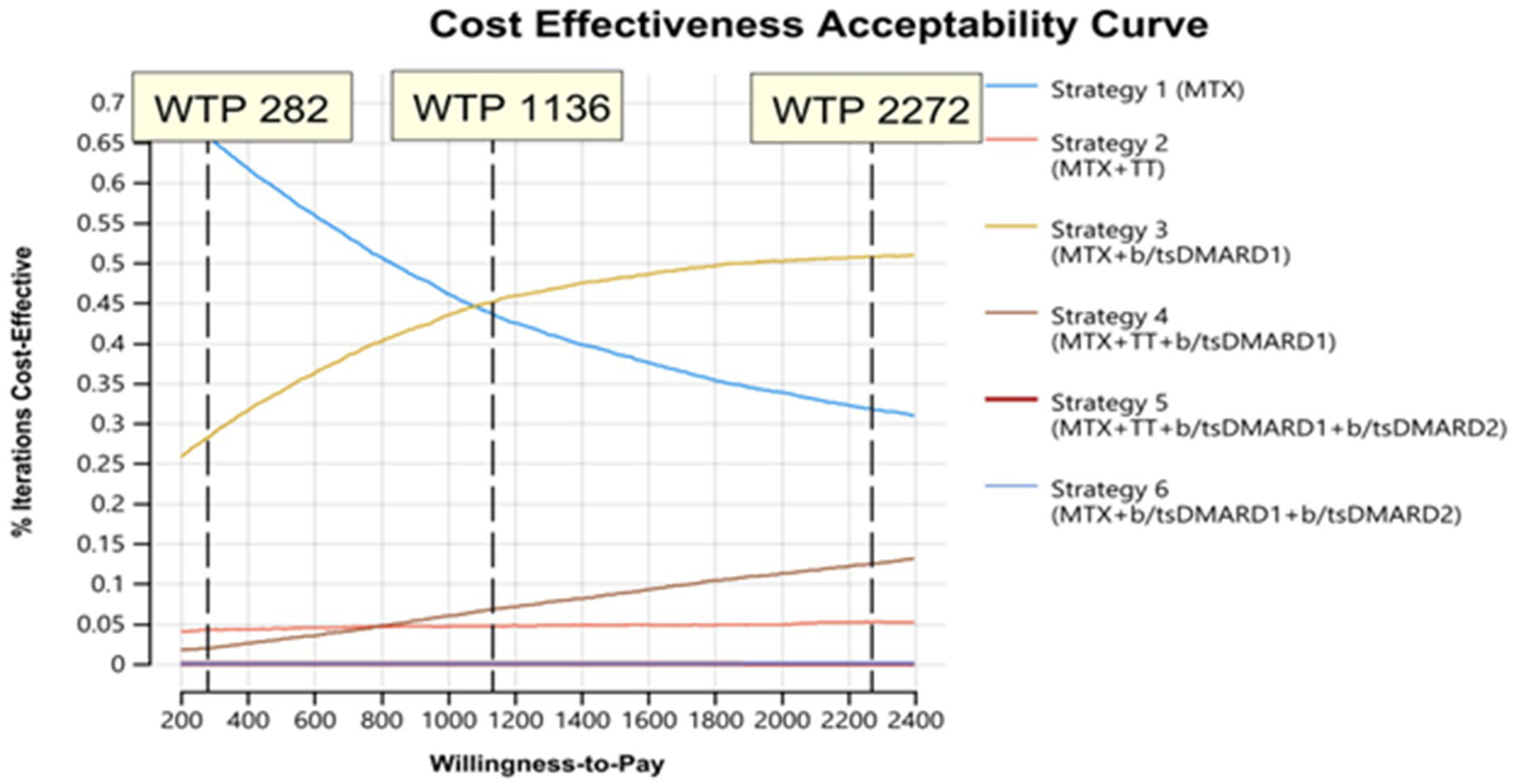
Cost effectiveness acceptability curve assessing feasible treatment strategies with variation in willingness to pay (WTP) thresholds.
Assuming the baseline WTP-threshold of USD 282, strategy 1 (72%) was likely the optimal strategy, with more than 20% of iterations favoring strategy 3 (Figure 5). Strategy 5 and 6 were not considered acceptable at any considered WTP.
At a WTP threshold of USD 282, price threshold analysis found that optimal prices for TT and b/tsDMARD1 were USD 102 and 61.5, respectively, for the other treatment strategies to be considered cost-effective. There was no optimal price threshold for b/tsDMARD2 at a WTP of USD 282 (Supplementary material 3).
Scenario analysis
We performed two scenario analyses by including drug prices from Scandinavia and India in the Zanzibar-based model. Strategy 1 was still the probably cost-effective option in both, but due to global differences in prices, particularly for TT and the b/tsDMARDs, strategies 2,4 and 5 became considerable, though suboptimal, options in Zanzibar once price changes were assumed. Strategies 3 and 6 were always dominated.
Discussion
To our knowledge, this is the first study in Sub-Saharan Africa looking at the cost-effectiveness of multiple DMARD therapies. The study evaluated the cost-effectiveness of providing treatment in a stepwise approach, which simulates a clinical setting and is in accordance with recommendations in international clinical guidelines. We do believe that using a T2T approach which predefines treatment goals and applies tight control via regular, appropriate treatment adjustment (56) enables limitation of costs as it is more effective in reaching treatment target than usual care (57). All patients are started on the most cost-effective drug and the least cost-effective therapies are reserved for few and select patients. The model is likely to be relevant in many African settings due to similarities across the region in delayed presentation and high disease activity among patients with RA at presentation (11, 58, 59).
In this study we found that methotrexate was the cost-effective RA treatment option in Zanzibar. A systematic review and meta-analysis looking at cost-effectiveness of b/DMARD with csDMARDs as comparator also found that for LMIC, bDMARDs were not cost-effective (60). In high-income settings, bDMARDS were found to be cost-effective (6, 61), because of the higher cost-effectiveness threshold, particularly for those with low costs (62). Our findings suggest that when WTP is increased to USD 2272 equivalent to twice the GDP per capita then regimens with b/tsDMARDs become the optimal treatment strategy.
Most reference studies used to determine efficacies were from high-income populations. In the Zanzibar setting, majority of the patients present with established disease. For this reason, we included studies with patients with established RA and long-disease activity in our analyses, although there were relatively few studies for consideration.
LMICs tend to have higher drug prices compared to high-income countries (63). Currently, the cost of b/tsDMARDs makes them unfeasible for clinical use in the Zanzibar setting. However, the availability of biosimilar b/tsDMARDs for several TNF-inhibitors, as well as rituximab, has led to price reductions where they are accessible. Over time, this may increase the utilization of these drugs in LMICs. In Scandinavian countries, where the efficacies of b/tsDMARDs are considered comparable, costs play a significant role in the choice of which b/tsDMARD to use in therapy (64). As a comparison, the cost of triple therapy and bDMARDs was much lower in Scandinavian countries as compared to Zanzibar while csDMARDs were cheaper in India. To reduce costs in SSA, adopting confidential drug tendering schemes, as is common in Scandinavia (64) and other European countries (65), could be beneficial. Institutionalization of drug price negotiations is important to enable governments to negotiate discounted prices from the pharmaceutical companies, ensuring both cost-effectiveness and fair distribution globally (66). Additionally, neighboring African countries could explore bulk purchases to qualify for large discounts. In India, b/tsDMARDs are not included in the government standard treatment guidelines and patients purchase them privately which allows the pharmaceutical companies to hike prices.
With RA, early diagnosis and early initiation of treatment increases likelihood of response to therapy and slows disease progression. The majority of our patients had severe RA at presentation, with moderate to high disease activity when seeking hospital care. Programs to increase RA community awareness as well as health care provider training on early detection and T2T therapies could potentially improve patient outcomes (67). Additionally, the younger age of our patient population would suggest more gains in terms of both morbidity and mortality.
Efficacy data used in the analysis were acquired from RCTs carried out in high-income populations (41–47). They vary from our group in age of onset, disease duration and disease activity at presentation. There may also be discrepancies in therapeutic DMARD doses and treatment response in Black African compared to Caucasian populations. Such factors may have influenced our findings and underline the need for more research on rheumatological conditions in SSA, particularly on the efficacy of DMARDs, to obtain reliable data from LLMIC settings. Such studies may also highlight the risk of b/tsDMARD side effects in resource-constrained regions where infectious illnesses, notably tuberculosis, are prevalent and pose a considerable risk to patients. Our findings must therefore be interpreted with caution, given this limitation.
Furthermore, in most of the RCTs on RA, the ACR-20 is used as a measure of efficacy of therapy. However, ACR-20 is not a commonly used target in clinical settings. For this reason, we chose to use CDAI as disease activity measure, even though for majority of the trials, CDAI was only reported as a secondary outcome. This reflects a knowledge deficit regarding efficacy of treatment in clinical settings.
Despite the high costs of bDMARDs, rituximab (RTX) is available in Zanzibar for the treatment of cancer. The availability of RTX was largely due to political goodwill and interest in establishing oncology care on the island. With the government policy of free health care for all, this means that it is available to patients with rheumatic diseases as well, without prior assessment of rationale or fairness. Unfortunately, due to its high cost it is not cost-effective. With several effective b/tsDMARDs becoming widely available globally, this emphasizes the need for decision-makers to evaluate the cost-effectiveness of therapeutic options before they are made available in clinical settings. This also underlines the need for a universal health care package, particularly in resource-constrained settings, that determine which options give the best value for money as has recently been rolled out in the Zanzibar essential health care package (ZEHCP) (68).
Cost-effectiveness is one of the most important criteria in health care priority setting. Our analysis indicates that methotrexate is the only feasible treatment option for patients with RA. However, our study showed that only around 15% would achieve the treatment target and remain on monotherapy. This is much lower than findings from real-world data, which show that with multiple options, around 50% of RA patients reach treatment target (69–71). Although these studies were undertaken in settings treating early RA, who respond much better to DMARDs compared to those with established RA, serious consideration must be given to the low number of patients who will achieve treatment target with methotrexate monotherapy. In 2022, the Zanzibar health budget was USD 39/capita (68) which is quite low. Within this budget, RA competes with several other interventions.
Given that RA is a severe disease, affecting HRqoL with pain over a long period of time, perhaps allowing for higher WTP thresholds are acceptable and strategy 3 is a more fair priority. This shows the limitations of a pure health maximizing perspective, and a Distributive Cost-Effectiveness (DCEA) study is perhaps more suitable. Although not explored by our analysis, other studies suggest that the high costs of care can be offset by the costs associated with lost productivity (72), which have a significant impact in RA patients because majority of patients are of working age. It is noteworthy that in SSA, for breast cancer therapy, costs of up to USD 20,000/QALY are considered acceptable in SSA (73), which is higher than the costs found for strategy 1, 3 and 4 in our study for RA treatment. Other options to consider for patients with RA requiring expensive drugs include tapering off expensive drugs and prioritizing only those with the most severe disease (74).
Our study also had implications for the Zanzibar Ministry of Health during the Zanzibar Essential Health Care Package revision in 2022 where RA care was included. The package consisted of steroid bridging and DMARD therapy, including methotrexate. Methotrexate monotherapy, although cost-effective in our setting, may not be the most suitable option given the low number of patients who reach treatment target. Apart from cost-effectiveness, other considerations such as equity, severity of the disease, relatively younger age of our patients and burden of disease must be considered before RA care decisions are made. With other competing healthcare interests within a limited budget, this is often the dilemma in setting priorities in LMIC. We believe that our study findings are of benefit not only for Zanzibar but for other countries across the continent where CEA of RA care has not been performed.
Conclusion
Although b/tsDMARD therapy is considered optimal treatment in developed countries it is still not a cost-effective option in resource limited settings, mainly due to the high cost of drugs. Allowing for a higher WTP threshold would ensure that these options become more acceptable. Until they are made affordable, methotrexate monotherapy is considered cost-effective for the management of patients with RA in Zanzibar.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Zanzibar Health Research Institute. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
SS: Writing – review & editing, Formal analysis, Project administration, Writing – original draft, Methodology, Visualization, Validation, Conceptualization, Investigation, Data curation, Software, Resources. M-JK: Validation, Methodology, Visualization, Software, Writing – review & editing, Formal analysis. B-TF: Supervision, Conceptualization, Writing – review & editing, Validation. TN: Validation, Supervision, Writing – review & editing. KJ: Software, Conceptualization, Funding acquisition, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. SS received funding for the software from The Department of International Collaboration, Haukeland University Hospital and the Bergen Center for Ethics and Priority Settings (BCEPS) through the Trond Mohn Foundation grant (BFS2019TMT02). No other funding was received. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgments
We acknowledge the contributions of many colleagues who helped in one way or another in finalizing this manuscript, in particular the registrars at the rheumatology clinic (Noorein A Omar, Faiza M Juma, and Mariam A Mzee). Perez Ochanda provided valuable assistance with the utility scores and Jutta Jorgensen who gave valuable insight throughout the process.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1618493/full#supplementary-material
References
1.
Almutairi K Nossent J Preen D Keen H Inderjeeth C . The global prevalence of rheumatoid arthritis: a meta-analysis based on a systematic review. Rheumatol Int. (2021) 41:863–77. doi: 10.1007/s00296-020-04731-0
2.
Rudan I Sidhu S Papana A Meng S Xin-Wei Y Wang W et al . Prevalence of rheumatoid arthritis in low– and middle–income countries: a systematic review and analysis. J Glob Health. (2015):5. doi: 10.7189/jogh.05.010409
3.
Nicholas A Alare K AbdulBasit Opeyemi M Oluwatosin A . The outlook of rheumatological care in Africa: current state, challenges, and recommendation. Ann Med Surg. (2022) 82:104689. doi: 10.1016/j.amsu.2022.104689
4.
Gullick NJ Scott DL . Co-morbidities in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. (2011) 25:469–83. doi: 10.1016/j.berh.2011.10.009
5.
Black RJ Cross M Haile LM Culbreth GT Steinmetz JD Hagins H et al . Global, regional, and national burden of rheumatoid arthritis, 1990–2020, and projections to 2050: a systematic analysis of the global burden of disease study 2021. Lancet Rheumatol. (2023) 5:e594–610. doi: 10.1016/S2665-9913(23)00211-4
6.
Cai Y Zhang J Liang J Xiao M Zhang G Jing Z et al . The burden of rheumatoid arthritis: findings from the 2019 global burden of diseases study and forecasts for 2030 by Bayesian age-period-cohort analysis. J Clin Med. (2023) 12:1291. doi: 10.3390/jcm12041291
7.
Smolen JS Landewé R Breedveld FC Buch M Burmester G Dougados M et al . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2013 update. Ann Rheum Dis. (2014) 73:492–509. doi: 10.1136/annrheumdis-2013-204573
8.
Smolen JS Breedveld FC Burmester GR Bykerk V Dougados M Emery P et al . Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. (2016) 75:3–15. doi: 10.1136/annrheumdis-2015-207524
9.
Haavardsholm EA Aga AB Olsen IC Lillegraven S Hammer HB Uhlig T et al . Ultrasound in management of rheumatoid arthritis: ARCTIC randomised controlled strategy trial. BMJ. (2016) 354:i4205. doi: 10.1136/bmj.i4205
10.
Harrington R Ahmad S Nokhatha A Conway R . JAK Inhibitors in rheumatoid arthritis: An evidence-based review on the emerging clinical data. J Inflamm Res. (2020) 13:519–31. doi: 10.2147/JIR.S219586
11.
Adelowo O Mody GM Tikly M Oyoo O Slimani S . Rheumatic diseases in Africa. Nat Rev Rheumatol. (2021) 17:363–74. doi: 10.1038/s41584-021-00603-4
12.
Shi ZC Fei HP Wang ZL . Cost-effectiveness analysis of etanercept plus methotrexate vs triple therapy in treating Chinese rheumatoid arthritis patients. Medicine (Baltimore). (2020) 99:e16635. doi: 10.1097/MD.0000000000016635
13.
Benucci M Saviola G Manfredi M Sarzi-Puttini P Atzeni F . Cost effectiveness analysis of disease-modifying antirheumatic drugs in rheumatoid arthritis. A systematic review literature. Int. J Rheumatol. (2011) 2011:845496. doi: 10.1155/2011/845496
14.
Zhao J Zhou W Wu Y Ji P Yang L Yan X et al . The efficacy, safety and cost-effectiveness of hydroxychloroquine, sulfasalazine, methotrexate triple therapy in preventing relapse among patients with rheumatoid arthritis achieving clinical remission or low disease activity: the study protocol of a randomized controlled clinical trial (ESCoRT study). BMC Med Inform Decis Mak. (2021) 21:1–10. doi: 10.1186/s12911-021-01449-2
15.
Fellous S Rkain H Ahid S Abouqal R Tahiri L Hmamouchi I et al . One-year direct costs of biological therapy in rheumatoid arthritis and its predictive factors: data from the Moroccan RBSMR registry. Rheumatol Int. (2021) 41:787–93. doi: 10.1007/s00296-020-04762-7
16.
Mittal G Bisht M Pai VS Handu S . The cost-effectiveness of and adherence to disease-modifying antirheumatic drug (DMARD) therapy in rheumatoid arthritis patients in a tertiary care teaching Hospital in Uttarakhand, India. Cureus. (2023) 15:e34664. doi: 10.7759/cureus.34664
17.
Harigane K Mochida Y Shimazaki T Kobayashi N Inaba Y . Pharmacoeconomic analysis of biologics and methotrexate for rheumatoid arthritis from the standpoint of the number needed to treat concept under the Japanese health insurance system. Cost Eff Resour Alloc. (2022) 20:13. doi: 10.1186/s12962-022-00347-2
18.
Schlueter M Finn E Díaz S Dilla T Inciarte-Mundo J Fakhouri W . Cost-effectiveness analysis of baricitinib versus adalimumab for the treatment of moderate-to-severe rheumatoid arthritis in Spain. Clin Outcomes Res. (2019) 11:395–403. doi: 10.2147/CEOR.S201621
19.
Aletaha D Smolen JS . The rheumatoid arthritis patient in the clinic: comparing more than 1300 consecutive DMARD courses. Rheumatology. (2002) 41:1367–74. doi: 10.1093/rheumatology/41.12.1367
20.
Connelly LB Woolf A Brooks P . Chapter 51 cost-effectiveness of interventions for musculoskeletal conditions. In: Disease control priorities in developing countries 2nd edition (2004). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK11713/
21.
Ministry of Health K . Clinical guidelines for management and referral of common conditions at level 4–6 hospitals. 2009th ed. World Health Organisation (2009).
22.
National department of Health . Hospital level (adults) standard treatment guidelines and essential medicines list for South Africa. 2019th ed. Republic of South Africa: National Department of Health (2019).
23.
Smolen JS Landewé RBM Bijlsma JWJ Burmester GR Dougados M Kerschbaumer A et al . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. (2020) 79:685–99. doi: 10.1136/annrheumdis-2019-216655
24.
Choi HK Seeger JD Kuntz KM . A COST-effectiveness analysis of treatment options for patients with methotrexate-resistant rheumatoid arthritis. Arthritis Rheum. (2000) 43:2316–27. doi: 10.1002/1529-0131(200010)43:10<2316::AID-ANR20>3.0.CO;2-6
25.
Husereau D Drummond M Augustovski F de Bekker-Grob E Briggs AH Carswell C et al . Consolidated health economic evaluation reporting standards 2022 (CHEERS 2022) statement: updated reporting guidance for health economic evaluations. BMC Med. (2022) 20:23. doi: 10.1186/s12916-021-02204-0
26.
Welsing PMJ Severens JL Hartman M van Gestel AM van Riel PLCM Laan RFJM . The initial validation of a Markov model for the economic evaluation of (new) treatments for rheumatoid arthritis. PharmacoEconomics. (2006) 24:1011–20. doi: 10.2165/00019053-200624100-00008
27.
Schipper LG Kievit W den Broeder AA van der Laar MA Adang EMM Fransen J et al . Treatment strategies aiming at remission in early rheumatoid arthritis patients: starting with methotrexate monotherapy is cost-effective. Rheumatology. (2011) 50:1320–30. doi: 10.1093/rheumatology/ker084
28.
Saunders W . Treatment of rheumatoid arthritis In: Kelley’s textbook of rheumatology. 9th ed. Elsevier (2013)
29.
Smolen JS Landewé RBM Bergstra SA Kerschbaumer A Sepriano A Aletaha D et al . EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2022 update. Ann Rheum Dis. (2023) 82:e76. doi: 10.1136/ard-2022-223356corr1
30.
Mikuls TR Johnson SR Fraenkel L Arasaratnam RJ Baden LR Bermas BL et al . American College of Rheumatology Guidance for the Management of Rheumatic Disease in adult patients during the COVID-19 pandemic: version 1. Arthritis Rheumatol. (2020) 72:1241–51. doi: 10.1002/art.41301
31.
Iyer P Gao Y Field EH Curtis JR Lynch CF Vaughan-Sarrazin M et al . Trends in hospitalization rates, major causes of hospitalization, and in-hospital mortality in rheumatoid arthritis in the United States from 2000 to 2014. ACR Open Rheumatol. (2020) 2:715–24. doi: 10.1002/acr2.11200
32.
Mkhize PE Viljoen AJ . Leflunomide as an alternative csDMARD for rheumatoid arthritis in a resource-constrained setting: a real-life experience. South African Med J. (2025) 115:e2536. doi: 10.7196/SAMJ.2025.v115i4.2536
33.
Kurek E. Rheumatology advisor (2024). Treatment with b/ts DMARDs moderately increases the risk for nonserious infections in Spondyloarthritis. Available online at: https://www.rheumatologyadvisor.com/news/b-tsdmards-linked-to-slight-increased-risk-for-global-infections-in-axspa/#:~:text=Axial Spondyloarthritis-,Treatment With b/ts DMARDs Moderately Increases the Risk for,fungal infection%2C excluding serious infecti
34.
Tarp S Eric Furst D Boers M Luta G Bliddal H Tarp U et al . Risk of serious adverse effects of biological and targeted drugs in patients with rheumatoid arthritis: a systematic review meta-analysis. Rheumatology. (2017) 56:417–25. doi: 10.1093/rheumatology/kew442
35.
Said SS Johansson KA Nystad TW Sadiq HS Fevang BTS . Establishing rheumatoid arthritis Care in Zanzibar: a year of clinical outcomes and challenges. Journal of Kenya Association of Physicians (JOKAP) (2025) 7:3–11. Available at: https://kapjournal.com/index.php/kap/en/article/view/73
36.
MSD . Tanzania Medical Stores Department. Available online at: https://www.msd.go.tz/ (Accessed February 2023).
37.
NPPM . National Pharmaceutical Pricing Authority. Available online at: https://www.nppaindia.nic.in/ (Accessed February 2023).
38.
Online pharmacy . Online Pharmacy India (2023).
39.
NetMeds . Indian Online Pharmacy. (2023). (Accessed February 18, 2023) Available online at: netmeds.com
40.
Hubbers J Ahmed S Watkins D Coates M Økland J Haaland Ø et al . Evidence brief: epilepsy, treatment FairChoices DCP analytics tool epilepsy, diagnostics and treatment (2021). Bergen Open Research Archives. Available at: https://www.uib.no/sites/w3.uib.no/files/attachments/neur01_epilepsy.pdf
41.
Chatzidionysiou K Sfikakis PP . Low rates of remission with methotrexate monotherapy in rheumatoid arthritis: review of randomised controlled trials could point towards a paradigm shift. RMD Open. (2019) 5:e000993. doi: 10.1136/rmdopen-2019-000993
42.
Rubbert-Roth A Aletaha D Devenport J Sidiropoulos PN Luder Y Edwardes MD et al . Effect of disease duration and other characteristics on efficacy outcomes in clinical trials of tocilizumab for rheumatoid arthritis. Rheumatology. (2021) 60:682–91. doi: 10.1093/rheumatology/keaa259
43.
Fleischmann R Strand V Wilkinson B Kwok K Bananis E . Original article: relationship between clinical and patient-reported outcomes in a phase 3 trial of tofacitinib or MTX in MTX-naïve patients with rheumatoid arthritis. RMD Open. (2016) 2:e000232. doi: 10.1136/rmdopen-2015-000232
44.
Curtis JR Palmer JL Reed GW Greenberg J Pappas DA Harrold LR et al . Real-world outcomes associated with methotrexate, sulfasalazine, and hydroxychloroquine triple therapy versus tumor necrosis factor inhibitor/methotrexate combination therapy in patients with rheumatoid arthritis. Arthritis Care Res. (2021) 73:1114–24. doi: 10.1002/acr.24253
45.
D’Angelo S Tirri E Giardino AM Mattucci-Cerinic M Dagna L Santo L et al . Effectiveness of golimumab as second anti-TNFα drug in patients with rheumatoid arthritis, psoriatic arthritis and axial Spondyloarthritis in Italy: GO-BEYOND, a prospective real-world observational study. J Clin Med. (2022) 11:4178. doi: 10.3390/jcm11144178
46.
Aletaha D Gomez-Reino J Burmester G van Vollenhoven R Rivas J Benkhalifa S et al . AB0417 efficacy and safety of tofacitinib in patients with rheumatoid arthritis according to duration of prior CSDMARD treatment and number of prior CSDMARDS: a post hoc analysis of phase 3 and phase 3B/4 trials. Ann Rheum Dis. (2019) 78:1671–2. doi: 10.1136/annrheumdis-2019-eular.417
47.
Reed GW Collier DH Koenig AS Saunders KC Pappas DA Litman HJ et al . Clinical and demographic factors associated with change and maintenance of disease severity in a large registry of patients with rheumatoid arthritis. Arthritis Res Ther. (2017) 19:81. doi: 10.1186/s13075-017-1289-x
48.
WHO Life expectancy (Accessed June 30, 2014). Available online at: http://www.who.int/gho/mortality_burden_disease/life_tables/situation_trends_text/en/
49.
Naz SM Symmons DPM . Mortality in established rheumatoid arthritis. Best Pract Res Clin Rheumatol. (2007) 21:871–83. doi: 10.1016/j.berh.2007.05.003
50.
Whitehead SJ Ali S . Health outcomes in economic evaluation: the QALY and utilities. Br Med Bull. (2010) 96:5–21. doi: 10.1093/bmb/ldq033
51.
Yang F Katumba KR Roudijk B Yang Z Revill P Griffin S et al . Developing the EQ-5D-5L value set for Uganda using the ‘lite’ protocol. PharmacoEconomics. (2022) 40:309–21. doi: 10.1007/s40273-021-01101-x
52.
Martinec R Pinjatela R Balen D . Quality of LIFE in patients with rheumatoid arthritis - a preliminary study. Acta Clin Croat. (2019) 58:157–66. doi: 10.20471/acc.2019.58.01.20
53.
Goma SH Razek MRA Abdelbary NM . Impact of rheumatoid arthritis on the quality of life and its relation to disease activity. Egypt Rheumatol Rehabil. (2019) 46:304–12. doi: 10.4103/err.err_39_19
54.
Rawlins M . Chapter 5 - health technology assessment In: BennettPNBrownMJSharmaP, editors. Clinical Pharmacology. 11th ed. Oxford: Churchill Livingstone (2012). 55–61. doi: 10.1016/B978-0-7020-4084-9.00044-6
55.
Pichon-Riviere A Drummond M Palacios A Garcia-Marti S Augustovski F . Determining the efficiency path to universal health coverage: cost-effectiveness thresholds for 174 countries based on growth in life expectancy and health expenditures. Lancet Glob Health. (2023) 11:e833–42. doi: 10.1016/S2214-109X(23)00162-6
56.
National Guideline Centre (UK) . Treat-to-target: Rheumatoid arthritis in adults: Diagnosis and management. London: National Institute for Health and Care Excellence (NICE) (2018).
57.
Hock ES Martyn-St James M Wailoo A Scott DL Stevenson M Rawdin A et al . Treat-to-target strategies in rheumatoid arthritis: a systematic review and cost-effectiveness analysis. SN Compr Clin Med. (2021) 3:838–54. doi: 10.1007/s42399-021-00727-4
58.
Halabi H Alarfaj A Alawneh K Alballa S Alsaeid K Badsha H et al . Challenges and opportunities in the early diagnosis and optimal management of rheumatoid arthritis in Africa and the Middle East. Int J Rheum Dis. (2015) 18:268–75. doi: 10.1111/1756-185X.12320
59.
Elshafie AI Elkhalifa AD Elbagir S Aledrissy MIE Elagib EM Nur MAM et al . Active rheumatoid arthritis in Central Africa: a comparative study between Sudan and Sweden. J Rheumatol. (2016) 43:1777–86. doi: 10.3899/jrheum.160303
60.
Kumar SS Bagepally BS Sasidharan A . Cost effectiveness of rituximab therapy for rheumatoid arthritis: a systematic review and Meta-analysis of cost-utility studies. Clin Drug Investig. (2023) 43:97–108. doi: 10.1007/s40261-022-01238-3
61.
Ghabri S Binard A Pers YM Maunoury F Caro JJ . Economic evaluation of sequences of biological treatments for patients with moderate-to-severe rheumatoid arthritis and inadequate response or intolerance to methotrexate in France. Value Heal J Int Soc Pharmacoeconomics Outcomes Res. (2020) 23:461–70. doi: 10.1016/j.jval.2019.12.003
62.
Kuwana M Tamura N Yasuda S Fujio K Shoji A Yamaguchi H et al . Cost-effectiveness analyses of biologic and targeted synthetic disease-modifying anti-rheumatic diseases in patients with rheumatoid arthritis: three approaches with a cohort simulation and real-world data. Mod Rheumatol. (2023) 33:302–11. doi: 10.1093/mr/roac038
63.
Jönsson B Kobelt G Smolen J . The burden of rheumatoid arthritis and access to treatment: uptake of new therapies. Eur J Health Econ. (2008) 8:61–86. doi: 10.1007/s10198-007-0089-7
64.
Brkic A Diamantopoulos AP Haavardsholm EA Fevang BTS Brekke LK Loli L et al . Exploring drug cost and disease outcome in rheumatoid arthritis patients treated with biologic and targeted synthetic DMARDs in Norway in 2010–2019 – a country with a national tender system for prescription of costly drugs. BMC Health Serv Res. (2022) 22:1–15. doi: 10.1186/s12913-021-07425-w
65.
Dranitsaris G Jacobs I Kirchhoff C Popovian R Shane LG . Drug tendering: drug supply and shortage implications for the uptake of biosimilars. Clinicoecon Outcomes Res. (2017) 9:573–84. doi: 10.2147/CEOR.S140063
66.
Tranvåg EJ Haaland ØA Robberstad B Norheim OF . Appraising drugs based on cost-effectiveness and severity of disease in Norwegian drug coverage decisions. JAMA Netw Open. (2022) 5:e2219503. doi: 10.1001/jamanetworkopen.2022.19503
67.
Hodkinson B Tikly M Adebajo A . Rheumatoid arthritis in the developing world: stepping up to the challenge. Clin Rheumatol. (2014) 33:1195–6. doi: 10.1007/s10067-014-2690-3
68.
Ministry of Health Zanzibar . Zanzibar essential health care package report (2022). Available online at: https://mohz.go.tz/eng/zanzibar-essential-health-care-package-report/
69.
Thomas K Lazarini A Kaltsonoudis E Drosos A Papalopoulos I Sidiropoulos P et al . Treatment patterns and achievement of the treat-to-target goals in a real-life rheumatoid arthritis patient cohort: data from 1317 patients. Ther Adv Musculoskelet Dis. (2020) 12:1759720X20937132. doi: 10.1177/1759720X20937132
70.
Östör A Feist E Sidiropoulos P Avouac J Rebella M Namas R et al . Achievement of treatment targets and maintenance of response with upadacitinib in patients with moderate-to-severe rheumatoid arthritis in real-world practice: 1-year outcomes from the UPHOLD observational study. Arthritis Res Ther. (2025) 27:84. doi: 10.1186/s13075-025-03528-5
71.
Alten R Burmester GR Matucci-Cerinic M Salmon JH Östör A Ng KJ et al . Comparative effectiveness, time to discontinuation, and patient-reported outcomes with baricitinib in rheumatoid arthritis: 2-year data from the multinational, prospective observational RA-BE-REAL study in European patients. Rheumatol Ther. (2023) 10:1575–95. doi: 10.1007/s40744-023-00597-3
72.
Sruamsiri R Mahlich J Tanaka E Yamanaka H . Productivity loss of Japanese patients with rheumatoid arthritis – a cross-sectional survey. Mod Rheumatol. (2018) 28:482–9. doi: 10.1080/14397595.2017.1361893
73.
Gershon N Berchenko Y Hall PS Goldstein DA . Cost effectiveness and affordability of trastuzumab in sub-Saharan Africa for early stage HER2-positive breast cancer. Cost Eff Resour Alloc. (2019) 17:5. doi: 10.1186/s12962-019-0174-7
74.
Westhovens R Annemans L . Costs of drugs for treatment of rheumatic diseases. RMD Open. (2016) 2:e000259. doi: 10.1136/rmdopen-2016-000259
Summary
Keywords
cost-effectiveness analysis, Zanzibar, rheumatoid arthritis, DMARDs, biologics
Citation
Said SS, Kühl M-J, Fevang B-TS, Nystad TW and Johansson KA (2025) Treating rheumatoid arthritis in Zanzibar: a cost effectiveness study comparing conventional, biologic, and targeted-synthetic disease modifying anti-rheumatic drugs. Front. Med. 12:1618493. doi: 10.3389/fmed.2025.1618493
Received
26 April 2025
Accepted
31 July 2025
Published
20 August 2025
Volume
12 - 2025
Edited by
Cristina Martinez-Molina, Hospital Clínic de Barcelona, Spain
Reviewed by
Stefka Stoilova, University General Hospital “Palmed” Plovdiv, Bulgaria
Maldonado-Restrepo Angela Isabel, Universidad del Rosario, Colombia
Updates
Copyright
© 2025 Said, Kühl, Fevang, Nystad and Johansson.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sanaa S. Said, wih013@uib.no
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.