- Department of Gastroenterology, The First Medical Center of Chinese PLA General Hospital, Beijing, China
Acute pancreatitis (AP) is pancreatic inflammation caused by abnormal activation of trypsinogen, and moderately severe or severe acute pancreatitis (SAP) can lead to systemic inflammatory response syndrome (SIRS) and organ failure, associated with high mortality. Therefore, early prediction of the severity of acute pancreatitis is particularly important to improve patient survival rate and reduce complications. Currently, many scoring systems (e.g., Ranson scoring, etc) and classical biomarkers are available in the clinical practice, but there are still many limitations, such as low predictive value and time delay. Potential biomarkers for the prediction of SAP are still a hot topic in current research. In this review, we aim to summarize newly discovered biomarkers for the prediction of the severity of acute pancreatitis in the recent years, and provide an overview of serum markers, intestinal flora, and genetic markers. At the same time, the development of emerging detection technologies [e.g., Robust AP Identification and Diagnosis (RAPIDx) and droplet digital PCR (ddPCR)] also provides new possibilities for early prediction of SAP, allowing these biomarkers to be applied clinically.
Introduction
Acute pancreatitis (AP) is a rapidly developing inflammatory process of the pancreas and is one of the most common diseases of the digestive system (1). Most patients have only mild acute pancreatitis (MAP), with symptoms resolving within 1 week. About 20% of patients develop moderately severe or severe acute pancreatitis (SAP) with pancreatic necrosis and even organ failure (2, 3). Acute pancreatitis is featured by damage to the alveolar cells, which prompts inappropriate release and activation of trypsinogen. This triggers the activation of other digestive enzymes, the kinin system and the complement cascade, leading to the self-digestion of the pancreatic parenchyma and even systemic inflammatory response syndrome (SIRS) (4). Revision of the Atlanta classification in 2012 (5) categorizes acute pancreatitis into three degrees of severity: (1) mild acute pancreatitis (MAP): absence of organ failure and local or systemic complications, with early recovery and a very low mortality rate; (2) moderately severe acute pancreatitis: the presence of transient organ failure (<48 h) or local or systemic complications in the absence of persistent organ failure; and (3) severe acute pancreatitis (SAP): the presence of persistent organ failure (>48 h), and the development of infected necrosis among patients with persistent organ failure is associated with an extremely high mortality (20%−40%). Given the variable clinical course in acute pancreatitis and the significant mortality rate in severe cases, early prediction of SAP has become a very important clinical issue. Scoring systems such as the Ranson score, the Acute Physiology and Chronic Health Evaluation (APACHE) II score, Bedside Index of Severe Acute Pancreatitis, the Computed Tomography Severity Index, and classical serologic molecules have emerged to predict the severity of AP (4, 6, 7). Potential biomarkers for the prediction of SAP are still a hot topic in current research. In recent years, researches targeting the pathophysiological mechanisms of acute pancreatitis have identified a large number of emerging biomarkers. This article summarizes some emerging biomarkers, including serum markers, intestinal flora and genetic markers. At the same time, by analyzing the pathogenesis of AP, we find a potential molecule that may play a role in predicting SAP in the hope that it can be helpful in the subsequent clinical applications.
Serum markers
Serum amyloid A (SAA) protein
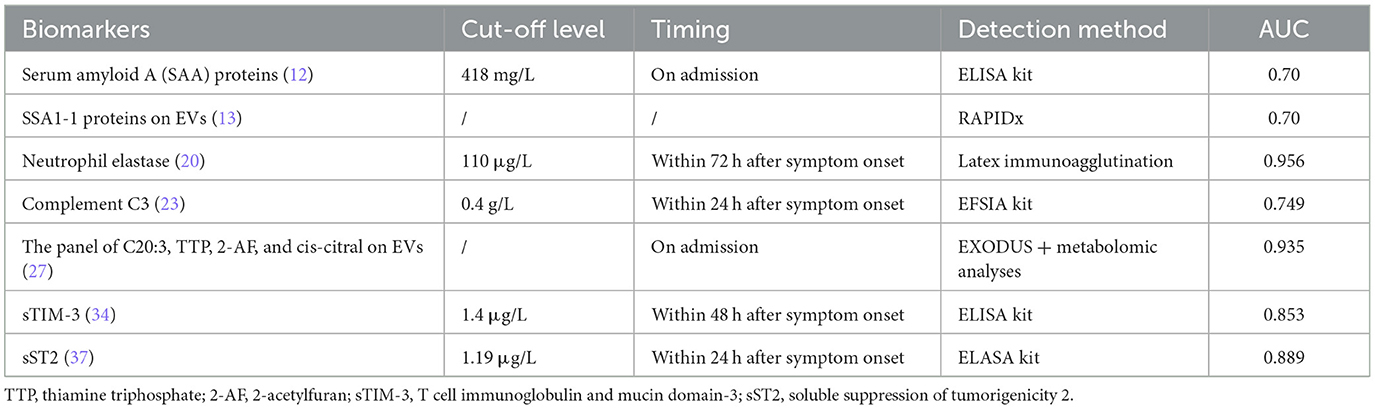
Serum amyloid A (SAA) protein is an early and sensitive biomarker of inflammatory diseases, with expression up-regulated 1,000-fold during inflammation, infection and tissue injury (8, 9). Increased transcription of the A-SAA gene in the acute phase response was demonstrated both in vivo and in vitro (10). In a caerulein-induced AP animal model, the expression of the specific SAA isoform, SAA3, was found to be significantly elevated. Compared with wild-type mice after administration of caerulein, the lower levels of serum amylase and lipase, milder tissue damage, and less production of pro-inflammatory cytokines in the pancreas were observed in SAA3 knockout (Saa3−/−) mice, suggesting a correlation between SAA and the severity of AP (11). As a biomarker of predicting AP severity detected at an early stage, SAA had a better performance than CRP in determining the severity of AP (10, 12). A multicenter prospective study that included 246 patients showed that SAA was superior to CRP in predicting the severity of pancreatitis on admission (area under curve 0.7 vs. 0.59, respectively; P = 0.02) and 24 h or less after the onset of symptoms (12). The commercially available kits for A-SAA detection in clinical laboratories are mainly based on immunoturbidimetric or immunonephelometric technology. But most of them are polyclonal antibody-based assays that cannot distinguish among the different A-SAA isotypes (10). It is worth mentioning that SSA proteins on extracellular vesicles (EVs) are also potential biomarkers for differential expression in patients with AP of varying severity. Recently a rapid and high-sensitive detection method, Robust AP Identification and Diagnosis (RAPIDx) method was constructed by proteomic fingerprinting of intact nanoscale extracellular vesicles. The RAPIDx allows a fast EV isolation step from plasma samples via EXODUS within 15 min followed by high-throughput MALDI-TOF-MS detection of AP in 1 min. By using this method to analyze the EVs of 115 clinical samples, the SAA proteins was demonstrated to be a promising biomarker to discriminate AP severity with an AUC of 83% (13).
Neutrophil extracellular trap
Neutrophils play a role in the progression of acute pancreatitis (AP). In addition to secreting antimicrobial compounds, activated neutrophils can also form extracellular meshwork structures known as neutrophil extracellular traps (NETs) through the expulsion of nuclear DNA and histone proteins. It has been demonstrated that neutrophil-derived NETs constitute a central component in the pathophysiology of severe AP and the plasma levels of NET components increased in patients with severe AP (14).
The potential role of NETs in the tissue damage in AP has been reported in numerous studies. NETs promote neutrophils infiltration which is a key component in AP, which can activate neutrophils directly or indirectly by upregulating Mac-1 expression on neutrophils. Mac-1 is an adhesion molecule that promotes extravascular accumulation of neutrophils at sites of inflammation. Moreover, NETs regulate signal transducer and activator of transcription 3 (STAT3) activity in acinar cells and promote trypsin activation in acinar cells through MMP-9 (14), exacerbating the degree of pancreatic tissue damage. A recent study showed that the formation of NETs in AP requires the participation of the myeloperoxidase–neutrophil elastase pathway, and the citrullination of histone H3 in SAP is affected by the activity of NE (15). In a mice model, Linders et al. (16) demonstrated that C3 induces neutrophil recruitment and the formation of neutrophil extracellular traps. A recent study explored the mechanism of the highly expressed P-selectin stimulating NETs formation in AP. They found that the levels of NETs and P-selectin in patients with AP was higher than that in healthy volunteers. And P-selectin induces NETs formation in neutrophils through PSGL-1 and its downstream Syk/Ca2+/PAD4 signaling pathway. Inhibiting p-selectin blunted NETs formation and ameliorated the severity of AP in mice (17, 18).
The quantitative determination of NETs was achieved by measuring cell free DNA (cf-DNA) levels or the concentration of MPO-DNA complexes, which limits the clinical application of NETs. Nowadays, a novel and comprehensive flow cytometry approach for the measurement of circulating cell appendant NETs has been developed (19). It is hoped that more research will focus on the application of this method in the early diagnosis of severe acute pancreatitis. At the same time, the discovery of these molecular pathways provides us with ideas for finding potential biomarkers in the future. The high sensitivity and specificity of neutrophil elastase (20) and complement C3 (21–23) in predicting SAP has been demonstrated, and we may be able to look for new biomarkers in these potential molecular pathways that may be serve as determining AP severity.
Extracellular vesicle
Extracellular vesicles (EVs) are nanoscale bioparticles that transport biomolecules (RNA, proteins, and metabolites) from disease-related organs or tissues to target cells for intercellular communication, mainly including exosomes (30–150 nm) and microvesicles (150–1,000 nm) (24, 25). Extracellular vesicles have been shown to be involved in the progression and exacerbation of many diseases, and are closely related to disease severity (26). Based on this characterization, EVs is a potential source for the discovery of new biomarkers. In acute pancreatitis, EVs can be directly released into peripheral blood circulation from the inflammatory pancreas in the early stages of the disease, carrying information relevant to disease progression before cell necrosis (27, 28). An efficient exosome detection method via the ultrafast-isolation system, EXODUS, has been established to achieve the isolation of EVs with high purity and yield from the plasma (29). This also laid the foundation for subsequent high-throughput detection. In addition to the SAA proteins described above, there are many other biomarkers in EVs that play a role in predicting the severity of acute pancreatitis. Four biomarkers for SAP prediction were identified from plasma EVs by EXODUS system and quantitative metabolomic analyses, including eicosatrienoic acid (C20:3), thiamine triphosphate, 2-acetylfuran, and cis-citral. The area under the curve (AUC) of the panel was >0.95 for both discovery (n = 30) and validation (n = 70) sets (27).
A comprehensive transcriptomic and metabolomic analysis of sEVs revealed that macrophage migration inhibitory factor and tubulin alpha 1b may be key variables that play an important role in AP progression, serving as potential markers for the diagnosis of SAP (30, 31). Increased amounts of S100A8 and S100A9 carried by extracellular vesicles in severe acute pancreatitis have been reported to activate NADPH oxidase to produce free radicals that promote inflammatory responses and exacerbate pancreatic damage (32).
Others
The progression of AP is closely related to immune function. Overactivation of inflammatory cells and their cytokines is one of the pathological mechanism of AP (33). Some immune-related molecules may be potential markers for predicting the severity of acute pancreatitis. T cell immunoglobulin and mucin domain-3 (TIM-3) is initially founded to be expressed in activated Th1 cells, and it has been thought to play a crucial role in the negative regulation of immune responses through interactions with its ligand galectin-9 (34, 35). In a retrospective study, sTIM-3 levels were detected by ELASA kits and proved to have good predictive efficacy with an AUC of 0.853 (34). Circulating extracellular nicotinamide phosphoribosyl transferase (eNAMPT) exerts the role of a cytokine regulator of innate immunity by binding to Toll-Like receptor 4 and nuclear factor-κB (NF-κB) activation. Pancreatitis circulating eNAMPT levels are significantly elevated in acute pancreatitis, and have positive correlation with disease severity (36). In addition, soluble suppression of tumorigenicity 2 (sST2) protein (37) regulates the function and differentiation of IL-33/ST2-mediated Th1 and Th2 Lymphocytes in AP homeostasis. And the optimal cut-off value of serum sST2 levels detected by ELASA kits as an indicator for prediction of SAP was projected to be 1,190 pg/ml, with the area under the curve 0.889. These biomarkers have been shown to be useful in clinical trials in predicting the severity of acute pancreatitis (Table 1).
A number of emerging serum markers have been identified based on the pathogenesis of SAP, including von Willebrand factor (38, 39), soluble mannose receptor (sCD206) (38), Plasma Osteopontin (40). These biomarkers provide new ideas for our future experiment, and large, prospective clinical trials are needed to further validate their predictive efficacy. Basic medicine experiments are also needed to determine potential molecular pathways of these molecular markers in predicting the severity of AP. And efficient and rapid detection methods are anticipated to achieve early identification of SAP, to be able to apply these biomarkers to the clinic as early as possible. Recently, developments in nanotechnology have improved the sensitivity of serologic diagnosis of AP and have provided a new modality for the treatment of acute pancreatitis (41).
Intestinal flora
The gut microbiota plays an important role not only in intestinal structure and function, but also in intestinal-associated immune system and epithelial cell function. More and more studies have shown that alterations in gut micro-ecology are associated with the progression of AP, including microbiota dysbiosis, intestinal barrier damage and immune dysfunction (42). Translocation of intestinal bacteria and endotoxins after intestinal barrier damage is the main cause for superinfection of pancreatic necrosis, which aggravated the severity of acute pancreatitis (43).
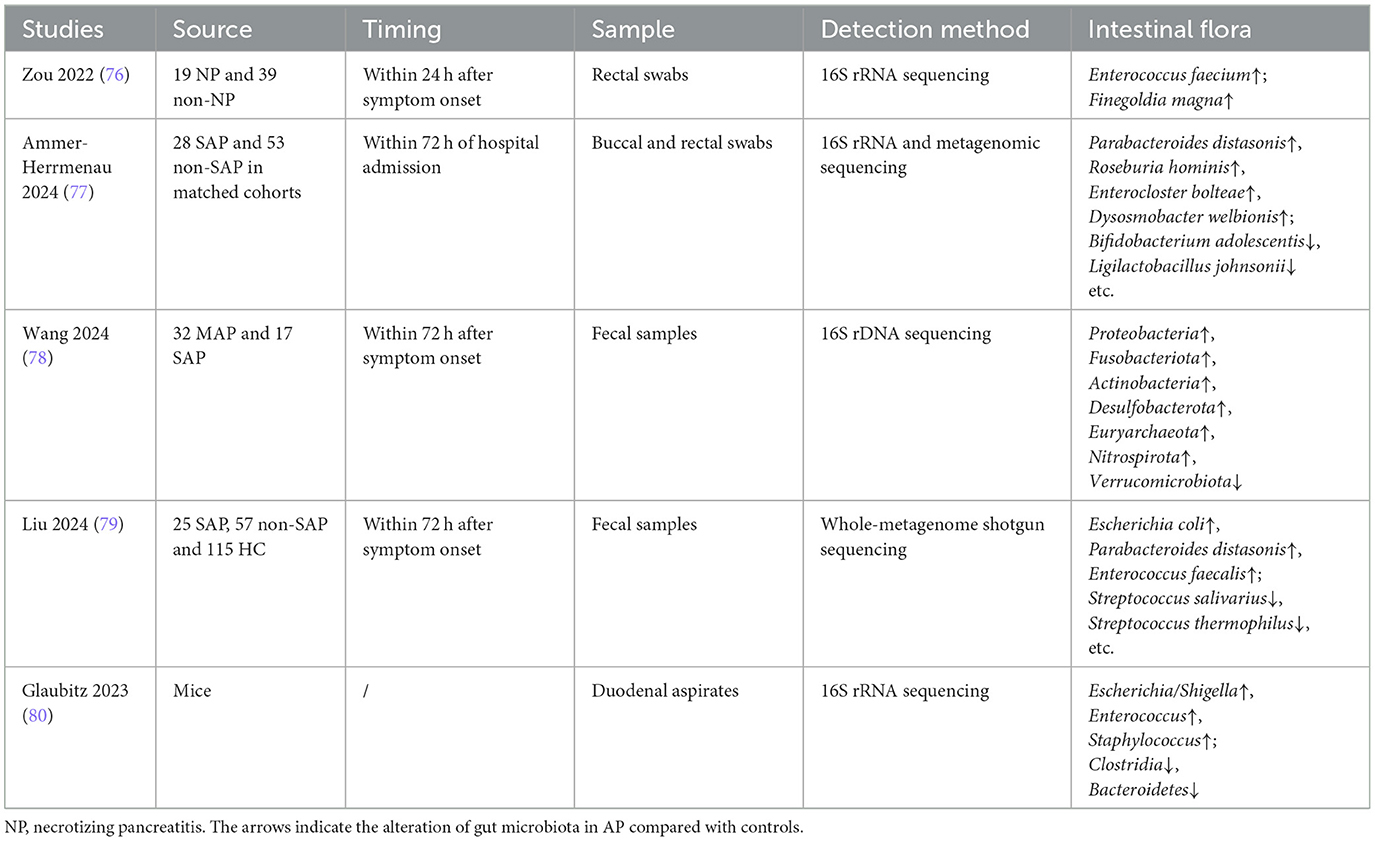
Multiple studies found that the intestinal flora have changed in SAP patients or mice models (Table 2). It has been demonstrated that the abundance of Enterococcus was significantly increased, and that of Bifidobacterium and Blautia was significantly decreased in patients with SAP (44, 45). Differential analysis of gut microbiota composition and functional enrichment was performed, and it found that representative pathways associated with the amino acid metabolism (valine, leucine, and isoleucine degradation) were enriched in SAP (45). Li et al. (46) has also demonstrated that hyper-triglyceridemic pancreatitis (HTGP) patients showed increased abundances of Escherichia Shigella and Enterococcus, and decreased abundances of Bacteroides and Faecalibacterium. The downregulated abundances of Faecalibacterium prausnitzii and Bacteroides uniformis indicated severe complications and poor outcome. The incidence of (HTGP) has been increasing in recent years, accounting for ~20%−30% of patients with acute pancreatitis. HTGP tends to have high incidence of complications and poor clinical prognosis, such as infected pancreatic necrosis and organ failure. Mice with long-term deficiency of Paneth cells exhibit more severe pancreatic pathological injuries and inflammation. The underlying mechanism may be intestinal flora dysbiosis and weakened intestinal mucosal barrier function. Mice lacking Paneth cells are accompanied by increases in Enterococcus and a decrease in Bifidobacterium (47, 48). And at the same time, the lack of antimicrobial peptides (AMPs) secreted by Paneth cells makes the intestinal barrier function reduced, leading to bacterial translocation (49). Restoring of partial Paneth cell function by supplementing lysozyme reduces the severity of acute pancreatitis and intestinal flora dysbiosis. These studies further confirmed the role that intestinal flora imbalance played in exacerbating pancreatic injury.
The severity of AP is also related to the metabolites of intestinal flora. Taurine plays an important role in maintaining neutrophil redox homeostasis and inhibiting NETs formation. As previously mentioned, NETs exacerbate pancreatic injury and systemic inflammation. The decreased abundance of Bacteroides in gut microbiota impairs taurine production. At the same time, it has been found that the taurine level in the serum decreases in severe HTGP patients. The mechanism is that taurine limits the activations of IL-17 and NF-κB signaling pathways in the neutrophils that repress NETs and pancreatic injury in HTGP (46). Short-chain fatty acids, one of the metabolites of intestinal microflora, have been confirmed to be reduced in AP patients (50). Reviews have described in detail the role of SCFA in AP (51). SCFA protects the intestinal barrier by regulating the expression and distribution of tight junction proteins and promoting the secretion of mucins on the intestinal surface (52). During the process of AP, dysbiosis of intestinal flora, SCFA reduction and intestinal barrier damage further aggravate pancreatic damage and promote the progression of AP. And the SCFA-producing bacterial flora and SCFA levels of SAP patients were significantly reduced compared with those of MAP patients (50). In a recent study, a total of three immunogenic cell death-related HUB genes (LY96, BCL2, and IFNGR1) were identified in SAP, and single-sample gene set enrichment analysis showed that the HUB genes were closely associated with the infiltration of specific immune cells, the activation of immune pathways, and the metabolism of single-chain fatty acids (especially butyric acid) (53), further confirming the importance of SCFA in identifying SAP.
Genetic markers
Mitochondrial DNA (mtDNA)
The loss of cell membrane integrity and release of intracellular contents gives necrotic cells the ability to induce inflammatory response. These immunogenic endogenous molecules are collectively called “damage-associated molecular patterns” (DAMPs) (54). In acute pancreatitis, dead pancreatic acini release intracellular damage-associated molecular patterns (DAMPs) including mtDNA, leading to the activation of various inflammatory signaling pathways [such as nuclear factor-κB (NF-κB), mitogen-activated protein kinase, signal transducer and activator of transcription 3 (STAT3), and inflammasome) and subsequent systemic inflammatory response syndrome, ultimately leading to organ damage in moderate to severe AP (55, 56). Unmethylated CpG regions in mtDNA make mtDNA resemble microbial DNA, which results in mitochondrial DNA being more immunogenic (57). At the same time, mtDNA can bind to pattern recognition receptors, thereby inducing the production of inflammatory cytokines (58). Yakah et al. (59) used a newly developed highly sensitive double droplet digital PCR (ddPCR), which achieves the absolute concentration of the mtDNA template (copy numbers/μl of plasma), to measure circulating plasma mtDNA fragments [D-Loop (DL), NADH ubiquinone oxidoreductase chain 1, and averaged mtDNA). The three types of plasma mtDNA fragments measured within 24 h of admission showed that the copy number of fragments in moderate severe or severe AP was higher than that in mild AP, and the difference was statistically significant. Additionally, receiver-operating characteristic curve analysis to distinguish AP severity yielded an area under the curve for mtDNA fragments of 0.91. An optimal cutoff value of 3.90, 5.62, and 4.76 copies/ml for DL, ND1, and averaged mtDNA fragments has been determined at a sensitivity of 0.90 and specificity of 0.90. Ezzat et al. (60) also found the familiar result.
Others
Cell free DNA (cfDNA) is derived from genomic DNA released during cell death (apoptosis or necrosis) and, therefore carries cell type-specific epigenetic features from its source tissue (61). A SAP prediction model based on cfDNA methylation levels has been established (62). However, the measurement of methylation levels takes 2–3 days to complete, which limits the application of this biomarker in early diagnosis, but it still provides a new idea for our future experiments. And we can conduct organ/tissue specific research in cfDNA methylation levels to achieve molecular diagnosis of specific organ damage.
MicroRNAs play an essential role in the occurrence and progression of acute pancreatitis. Several recently published articles have elaborated on the potential mechanisms and biomarkers of MicroRNAs in the progression of SAP (63–65). Studies have also found three types of differentially expressed exosomal miRNAs in patients with SAP. The complement component 3 (C3) gene is the target gene of one of the differentially expressed miRNAs, which also indicates that C3 may serve as an early biomarker of SAP (21).
Future perspectives
The development of bioinformatics and multi-omics analysis allows us to discover differentially expressed genes and proteins in SAP patients, but basic medical experiments are needed for validation. Moreover, researches on the molecular pathways and mechanisms of SAP can also help us predict emerging molecular markers. Early detection of biomarkers for severe acute pancreatitis will remain a problem we need to strive in the future, in order to achieve early clinical detection and timely intervention of SAP patients.
In searching the literature, we found that receptor-interacting protein kinase 1 (RIPK1) plays an important role in multiple inflammatory diseases (66–68). RIPK1 is a major mediator of multiple cell death pathways, including apoptosis and necroptosis, and inflammatory responses (69). The assembly and activation of multiple necrotic mechanisms, including RIPK1, are associated with NETosis, which in turn leads to the formation of neutrophil extracellular traps (NETs) (70). Activation of RIPK1 has been shown to promote the production of pro-inflammatory cytokines, including IL-6 and TNF, and inhibiting RIPK1 can block the occurrence of TNF-induced sepsis in animal models (71, 72). These mechanisms have been confirmed to play an important role in the pathophysiology of acute severe pancreatitis.
The in vivo results showed that inhibition of RIPK1 expression significantly reduced the extent of pancreatic tissue necrosis, inhibited the massive release of inflammatory mediators and oxidative stress damage, and ultimately effectively decreased the severity of AP (73). This process might be potentially regulated by the RIPK1/NF-κB/AQP8 pathway. Yao et al. (74) has founded that dexmedetomidine (Dex) attenuated SAP-induced pancreatic injury, infiltration of neutrophils and macrophages, and oxidative stress. Further mechanisms suggest that Dex inhibited the expression of necroptosis-associated proteins RIPK1, RIPK3, and MLKL and alleviated apoptosis in acinar cells (74). And disulfiram could also moderate the severity of mouse acute pancreatitis by inhibiting RIPK-dependent acinar cell necrosis and the following formation of NETs (75).
Serum levels of RIPK1 can be determined by ELISA kit. It has been demonstrated that serum RIPK1 levels were correlated positively with the severity of bulbar symptoms in the patients with amyotrophic lateral sclerosis, which can be used as clinical biomarker for the activation of RIPK1 in central nervous system (69). However, the level of serum RIPK1 is not currently used to predict the severity of acute pancreatitis. Further studies on the potential of RIPK1 as an emerging biomarker are still required.
Conclusion
Early identification of SAP will help us take more aggressive treatment measures to improve patient survival rates and reduce the occurrence complications. Currently, early prediction methods of the severity of acute pancreatitis include clinical scoring systems, inflammatory markers, and imaging assessments. The Ranson scoring, the APACHE II scoring and the bedside severity index scoring system are more widely used in clinical practice, but still have some limitations. Biomarkers of SAP has become a hot research topic since the last century, including some classic molecules, such as IL-6, CRP, etc. With the development of assay technologies, early, rapid, and sensitive detection of classical serum biomarkers has been achieved. In addition, the intestinal flora may play an important role in the development of SAP since most bacteria causing necrotic infection of pancreatic tissue are from the intestinal flora. Moreover, dead pancreatic acini release mtDNA leading to the activation of various inflammatory signaling pathways and subsequent systemic inflammatory response syndrome, ultimately leading to organ damage in moderate to severe AP. This review summarizes its predictive role in severe acute pancreatitis from the above three aspects, looking forward to early clinical application.
Author contributions
XH: Writing – original draft. ZW: Resources, Writing – review & editing. XN: Writing – review & editing, Resources. LL: Writing – review & editing. YB: Investigation, Writing – review & editing. NC: Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by Noncommunicable Chronic Diseases-National Science and Technology Major Project, grant number No. 2023ZD0500904.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tenner S Baillie J DeWitt J Vege SS and and American College of Gastroenterology. American College of Gastroenterology guideline: management of acute pancreatitis. Am J Gastroenterol. (2013) 108: 1400–15; 16. doi: 10.1038/ajg.2013.218
2. Boxhoorn L, Voermans RP, Bouwense SA, Bruno MJ, Verdonk RC, Boermeester MA, et al. Acute pancreatitis. Lancet. (2020) 396:726–34. doi: 10.1016/S0140-6736(20)31310-6
3. Schepers NJ, Bakker OJ, Besselink MG, Ahmed Ali U, Bollen TL, Gooszen HG, et al. Impact of characteristics of organ failure and infected necrosis on mortality in necrotising pancreatitis. Gut. (2019) 68:1044–51. doi: 10.1136/gutjnl-2017-314657
4. Mederos MA, Reber HA, Girgis MD. Acute pancreatitis: a review. Jama. (2021) 325:382–90. doi: 10.1001/jama.2020.20317
5. Banks PA, Bollen TL, Dervenis C, Gooszen HG, Johnson CD, Sarr MG, et al. Classification of acute pancreatitis−2012: revision of the Atlanta classification and definitions by international consensus. Gut. (2013) 62:102–11. doi: 10.1136/gutjnl-2012-302779
6. van den Berg FF, de Bruijn AC, van Santvoort HC, Issa Y, Boermeester MA. Early laboratory biomarkers for severity in acute pancreatitis; a systematic review and meta-analysis. Pancreatology. (2020) 20:1302–11. doi: 10.1016/j.pan.2020.09.007
7. Lee DW, Cho CM. Predicting severity of acute pancreatitis. Medicina. (2022) 58:787. doi: 10.3390/medicina58060787
8. Yu N, Zhang S, Lu J, Li Y, Yi X, Tang L, et al. Serum amyloid A, an acute phase protein, stimulates proliferative and proinflammatory responses of keratinocytes. Cell Prolif . (2017) 50:e12320. doi: 10.1111/cpr.12320
9. Connolly M, Marrelli A, Blades M, McCormick J, Maderna P, Godson C, et al. Acute serum amyloid A induces migration, angiogenesis, and inflammation in synovial cells in vitro and in a human rheumatoid arthritis/SCID mouse chimera model. J Immunol. (2010) 184:6427–37. doi: 10.4049/jimmunol.0902941
10. Zhang Y, Zhang J, Sheng H, Li H, Wang R. Acute phase reactant serum amyloid A in inflammation and other diseases. Adv Clin Chem. (2019) 90:25–80. doi: 10.1016/bs.acc.2019.01.002
11. Yang X, Li R, Xu L, Qian F, Sun L. Serum amyloid A3 is required for caerulein-induced acute pancreatitis through induction of RIP3-dependent necroptosis. Immunol Cell Biol. (2021) 99:34–48. doi: 10.1111/imcb.12382
12. Mayer JM, Raraty M, Slavin J, Kemppainen E, Fitzpatrick J, Hietaranta A, et al. Serum amyloid A is a better early predictor of severity than C-reactive protein in acute pancreatitis. Br J Surg. (2002) 89:163–71. doi: 10.1046/j.0007-1323.2001.01972.x
13. Zhu Q, Luo J, Li HP, Ye W, Pan R, Shi KQ, et al. Robust acute pancreatitis identification and diagnosis: RAPIDx. ACS Nano. (2023) 17:8564–74. doi: 10.1021/acsnano.3c00922
14. Merza M, Hartman H, Rahman M, Hwaiz R, Zhang E, Renström E, et al. Neutrophil extracellular traps induce trypsin activation, inflammation, and tissue damage in mice with severe acute pancreatitis. Gastroenterology. (2015) 149:1920–31.e8. doi: 10.1053/j.gastro.2015.08.026
15. Li H, Qiao C, Zhao L, Jing Q, Xue D, Zhang Y. Epigallocatechin-3-gallate reduces neutrophil extracellular trap formation and tissue injury in severe acute pancreatitis. J Leukoc Biol. (2022) 112:1427–43. doi: 10.1002/JLB.3A0322-151R
16. Linders J, Madhi R, Mörgelin M, King BC, Blom AM, Rahman M. Complement component 3 is required for tissue damage, neutrophil infiltration, and ensuring NET formation in acute pancreatitis. Eur Surg Res. (2020) 61:163–76. doi: 10.1159/000513845
17. Long L, Deng L, Wang L, Wen S, Luo L, Liang L, et al. P-selectin-based dual-model nanoprobe used for the specific and rapid visualization of early detection toward severe acute pancreatitis in vivo. ACS Biomater Sci Eng. (2020) 6:5857–65. doi: 10.1021/acsbiomaterials.0c00596
18. Xu Q, Shi M, Ding L, Xia Y, Luo L, Lu X, et al. High expression of P-selectin induces neutrophil extracellular traps via the PSGL-1/Syk/Ca(2+)/PAD4 pathway to exacerbate acute pancreatitis. Front Immunol. (2023) 14:1265344. doi: 10.3389/fimmu.2023.1265344
19. Jones C, La Flamme A, Larsen P, Hally K. CPHEN-017: comprehensive phenotyping of neutrophil extracellular traps (NETs) on peripheral human neutrophils. Cytometry A. (2024) 105:639–52. doi: 10.1002/cyto.a.24851
20. Domínguez-Muñoz JE, Villanueva A, Lariño J, Mora T, Barreiro M, Iglesias-Canle J, et al. Accuracy of plasma levels of polymorphonuclear elastase as early prognostic marker of acute pancreatitis in routine clinical conditions. Eur J Gastroenterol Hepatol. (2006) 18:79–83. doi: 10.1097/00042737-200601000-00014
21. Xu Y, Sun Y, Yin R, Dong T, Song K, Fang Y, et al. Differential expression of plasma exosomal microRNA in severe acute pancreatitis. Front Pharmacol. (2022) 13:980930. doi: 10.3389/fphar.2022.980930
22. Zhang X, Li Z, Liu W, Du J, Liu Y, Yu N, et al. The complement and coagulation cascades pathway is associated with acute necrotizing pancreatitis by genomics and proteomics analysis. J Inflamm Res. (2022) 15:2349–63. doi: 10.2147/JIR.S351416
23. Zhang L, Qiao Z, Feng H, Shen J. The early predictive role of complement C3 and C4 in patients with acute pancreatitis. J Clin Lab Anal. (2020) 34:e23205. doi: 10.1002/jcla.23205
24. Kalluri R, Lebleu VS. The biology, function, and biomedical applications of exosomes. Science. (2020) 367:eaau6977. doi: 10.1126/science.aau6977
25. Shao H, Im H, Castro CM, Breakefield X, Weissleder R, Lee H. New technologies for analysis of extracellular vesicles. Chem Rev. (2018) 118:1917–50. doi: 10.1021/acs.chemrev.7b00534
26. Deng Y, Zou Y, Song X, Jiang A, Wang M, Qin Q, et al. Potential of extracellular vesicles for early prediction of severity and potential risk stratification in critical inflammatory diseases. J Cell Commun Signal. (2023) 17:1283–92. doi: 10.1007/s12079-023-00763-w
27. Lou D, Shi K, Li HP, Zhu Q, Hu L, Luo J, et al. Quantitative metabolic analysis of plasma extracellular vesicles for the diagnosis of severe acute pancreatitis. J Nanobiotechnology. (2022) 20:52. doi: 10.1186/s12951-022-01239-6
28. Jia YC, Ding YX, Mei WT, Wang YT, Zheng Z, Qu YX, et al. Extracellular vesicles and pancreatitis: mechanisms, status and perspectives. Int J Biol Sci. (2021) 17:549–61. doi: 10.7150/ijbs.54858
29. Chen Y, Zhu Q, Cheng L, Wang Y, Li M, Yang Q, et al. Exosome detection via the ultrafast-isolation system: EXODUS. Nat Methods. (2021) 18:212–8. doi: 10.1038/s41592-020-01034-x
30. Zhu Q, Yang R, Luo J, Xu H, Li H, Liu X, et al. Identification of circulating extracellular vesicle long RNAs as diagnostic biomarkers for patients with severe acute pancreatitis. Clin Transl Med. (2022) 12:e1034. doi: 10.1002/ctm2.1034
31. Rahman SH, Menon KV, Holmfield JH, McMahon MJ, Guillou JP. Serum macrophage migration inhibitory factor is an early marker of pancreatic necrosis in acute pancreatitis. Ann Surg. (2007) 245:282–9. doi: 10.1097/01.sla.0000245471.33987.4b
32. Carrascal M, Areny-Balagueró A, de-Madaria E, Cárdenas-Jaén K, García-Rayado G, Rivera R, et al. Inflammatory capacity of exosomes released in the early stages of acute pancreatitis predicts the severity of the disease. J Pathol. (2022) 256:83–92. doi: 10.1002/path.5811
33. Minkov GA, Halacheva KS, Yovtchev YP, Gulubova MV. Pathophysiological mechanisms of acute pancreatitis define inflammatory markers of clinical prognosis. Pancreas. (2015) 44:713–7. doi: 10.1097/MPA.0000000000000329
34. Wang F, Zhu M, Meng Y, Lin M. Serum soluble T cell immunoglobulin mucin domain-3 as an early predictive marker for severity of acute pancreatitis; a retrospective analysis. BMC Gastroenterol. (2022) 22:522. doi: 10.1186/s12876-022-02537-x
35. Zhao L, Cheng S, Fan L, Zhang B, Xu S. TIM-3: an update on immunotherapy. Int Immunopharmacol. (2021) 99:107933. doi: 10.1016/j.intimp.2021.107933
36. Bime C, Casanova NG, Camp SM, Oita RC, Ndukum J, Hernon VR, et al. Circulating eNAMPT as a biomarker in the critically ill: acute pancreatitis, sepsis, trauma, and acute respiratory distress syndrome. BMC Anesthesiol. (2022) 22:182. doi: 10.1186/s12871-022-01718-1
37. Zhang Y, Cheng B, Wu ZW, Cui ZC, Song YD, Chen SY, et al. Serum soluble suppression of tumorigenicity 2 as a novel inflammatory marker predicts the severity of acute pancreatitis. World J Gastroenterol. (2021) 27:6489–500. doi: 10.3748/wjg.v27.i38.6489
38. Reuken PA, Brozat JF, Quickert S, Ibidapo-Obe O, Reißing J, Franz A, et al. Soluble mannose receptor CD206 and von Willebrand factor are early biomarkers to identify patients at risk for severe or necrotizing acute pancreatitis. J Intensive Care. (2022) 10:28. doi: 10.1186/s40560-022-00619-2
39. Li H, Xu Y, Zhou X, Jin T, Wang Z, Sun Y, et al. DIA-based proteomic analysis of plasma protein profiles in patients with severe acute pancreatitis. Molecules. (2022) 27:3880. doi: 10.3390/molecules27123880
40. Wirestam L, Nyberg PB, Dzhendov T, Gasslander T, Sandström P, Sjöwall C, et al. Plasma osteopontin reflects tissue damage in acute pancreatitis. Biomedicines. (2023) 11:1627. doi: 10.3390/biomedicines11061627
41. Jia W, Xu L, Xu W, Yang M, Zhang Y. Application of nanotechnology in the diagnosis and treatment of acute pancreatitis. Nanoscale Adv. (2022) 4:1949–61. doi: 10.1039/D2NA00020B
42. Fu Y, Mei Q, Yin N, Huang Z, Li B, Luo S, et al. Paneth cells protect against acute pancreatitis via modulating gut microbiota dysbiosis. mSystems. (2022) 7:e0150721. doi: 10.1128/msystems.01507-21
43. Fritz S, Hackert T, Hartwig W, Rossmanith F, Strobel O, Schneider L, et al. Bacterial translocation and infected pancreatic necrosis in acute necrotizing pancreatitis derives from small bowel rather than from colon. Am J Surg. (2010) 200:111–7. doi: 10.1016/j.amjsurg.2009.08.019
44. Tan C, Ling Z, Huang Y, Cao Y, Liu Q, Cai T, et al. Dysbiosis of intestinal microbiota associated with inflammation involved in the progression of acute pancreatitis. Pancreas. (2015) 44:868–75. doi: 10.1097/MPA.0000000000000355
45. Yu S, Xiong Y, Fu Y, Chen G, Zhu H, Mo X, et al. Shotgun metagenomics reveals significant gut microbiome features in different grades of acute pancreatitis. Microb Pathog. (2021) 154:104849. doi: 10.1016/j.micpath.2021.104849
46. Li G, Liu L, Lu T, Sui Y, Zhang C, Wang Y, et al. Gut microbiota aggravates neutrophil extracellular traps-induced pancreatic injury in hypertriglyceridemic pancreatitis. Nat Commun. (2023) 14:6179. doi: 10.1038/s41467-023-41950-y
47. Riba A, Olier M, Lacroix-Lamandé S, Lencina C, Bacquié V, Harkat C, et al. Paneth cell defects induce microbiota dysbiosis in mice and promote visceral hypersensitivity. Gastroenterology. (2017) 153:1594–606.e2. doi: 10.1053/j.gastro.2017.08.044
48. Qi-Xiang M, Yang F, Ze-Hua H, Nuo-Ming Y, Rui-Long W, Bin-Qiang X, et al. Intestinal TLR4 deletion exacerbates acute pancreatitis through gut microbiota dysbiosis and Paneth cells deficiency. Gut Microbes. (2022) 14:2112882. doi: 10.1080/19490976.2022.2112882
49. Teltschik Z, Wiest R, Beisner J, Nuding S, Hofmann C, Schoelmerich J, et al. Intestinal bacterial translocation in rats with cirrhosis is related to compromised Paneth cell antimicrobial host defense. Hepatology. (2012) 55:1154–63. doi: 10.1002/hep.24789
50. Li XY, He C, Zhu Y, Lu NH. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J Gastroenterol. (2020) 26:2187–93. doi: 10.3748/wjg.v26.i18.2187
51. Yan X, Li J, Wu D. The role of short-chain fatty acids in acute pancreatitis. Molecules. (2023) 28:4985. doi: 10.3390/molecules28134985
52. Wang HB, Wang PY, Wang X, Wan YL, Liu YC. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein Claudin-1 transcription. Dig Dis Sci. (2012) 57:3126–35. doi: 10.1007/s10620-012-2259-4
53. Wang Z, Liu J, Wang Y, Guo H, Li F, Cao Y, et al. Identification of key biomarkers associated with immunogenic cell death and their regulatory mechanisms in severe acute pancreatitis based on WGCNA and machine learning. Int J Mol Sci. (2023) 24:3033. doi: 10.3390/ijms24033033
54. Krysko DV, Agostinis P, Krysko O, Garg AD, Bachert C, Lambrecht BN, et al. Emerging role of damage-associated molecular patterns derived from mitochondria in inflammation. Trends Immunol. (2011) 32:157–64. doi: 10.1016/j.it.2011.01.005
55. Kang R, Lotze MT, Zeh HJ, Billiar TR, Tang D. Cell death and DAMPs in acute pancreatitis. Mol Med. (2014) 20:466–77. doi: 10.2119/molmed.2014.00117
56. Timmermans K, Kox M, Scheffer GJ, Pickkers P. Plasma nuclear and mitochondrial DNA levels, and markers of inflammation, shock, and organ damage in patients with septic shock. Shock. (2016) 45:607–12. doi: 10.1097/SHK.0000000000000549
57. Riley JS, Tait SW. Mitochondrial DNA in inflammation and immunity. EMBO Rep. (2020) 21:e49799. doi: 10.15252/embr.201949799
58. Kaczmarek A, Vandenabeele P, Krysko DV. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity. (2013) 38:209–23. doi: 10.1016/j.immuni.2013.02.003
59. Yakah W, Shah I, Skelton-Badlani D, Freedman SD, Popov YV, Sheth SG, et al. Circulating mitochondrial DNA as a diagnostic biomarker for predicting disease severity in patients with acute pancreatitis. Gastroenterology. (2023) 164:1009–11.e3. doi: 10.1053/j.gastro.2023.01.013
60. Ezzat GM, Nageb SM, Haredi MA, El-Masry MA. Mitochondrial DNA copy number, a damage-associated molecular pattern molecule, can predict pancreatic necrosis and is correlated with the severity of acute pancreatitis. Dig Dis Sci. (2023) 68:4175–85. doi: 10.1007/s10620-023-08049-2
61. Lehmann-Werman R, Neiman D, Zemmour H, Moss J, Magenheim J, Vaknin-Dembinsky A, et al. Identification of tissue-specific cell death using methylation patterns of circulating DNA. Proc Natl Acad Sci USA. (2016) 113:E1826–34. doi: 10.1073/pnas.1519286113
62. Sun HW, Dai SJ, Kong HR, Fan JX, Yang FY, Dai JQ, et al. Accurate prediction of acute pancreatitis severity based on genome-wide cell free DNA methylation profiles. Clin Epigenetics. (2021) 13:223. doi: 10.1186/s13148-021-01217-z
63. Patel HR, Diaz Almanzar VM, LaComb JF, Ju J, Bialkowska AB. The role of MicroRNAs in pancreatitis development and progression. Int J Mol Sci. (2023) 24:1057. doi: 10.3390/ijms24021057
64. Qu Y, Ding Y, Lu J, Jia Y, Bian C, Guo Y, et al. Identification of key microRNAs in exosomes derived from patients with the severe acute pancreatitis. Asian J Surg. (2023) 46:337–47. doi: 10.1016/j.asjsur.2022.04.032
65. Yang Y, Huang Q, Luo C, Wen Y, Liu R, Sun H, et al. MicroRNAs in acute pancreatitis: from pathogenesis to novel diagnosis and therapy. J Cell Physiol. (2020) 235:1948–61. doi: 10.1002/jcp.29212
66. Yang T, Xiang CG, Wang XH, Li QQ, Lei SY, Zhang KR, et al. RIPK1 inhibitor ameliorates pulmonary injury by modulating the function of neutrophils and vascular endothelial cells. Cell Death Discov. (2024) 10:152. doi: 10.1038/s41420-024-01921-8
67. Wang X, Chai Y, Guo Z, Wang Z, Liao H, Wang Z, et al. A new perspective on the potential application of RIPK1 in the treatment of sepsis. Immunotherapy. (2023) 15:43–56. doi: 10.2217/imt-2022-0219
68. Xu G, Li Y, Zhang S, Peng H, Wang Y, Li D, et al. SARS-CoV-2 promotes RIPK1 activation to facilitate viral propagation. Cell Res. (2021) 31:1230–43. doi: 10.1038/s41422-021-00578-7
69. Wei J, Li M, Ye Z, Hu X, He X, Wang J, et al. Elevated peripheral levels of receptor-interacting protein kinase 1 (RIPK1) and IL-8 as biomarkers of human amyotrophic lateral sclerosis. Signal Transduct Target Ther. (2023) 8:451. doi: 10.1038/s41392-023-01713-z
70. Mifflin L, Ofengeim D, Yuan J. Receptor-interacting protein kinase 1 (RIPK1) as a therapeutic target. Nat Rev Drug Discov. (2020) 19:553–71. doi: 10.1038/s41573-020-0071-y
71. Patel S, Webster JD, Varfolomeev E, Kwon YC, Cheng JH, Zhang J, et al. RIP1 inhibition blocks inflammatory diseases but not tumor growth or metastases. Cell Death Differ. (2020) 27:161–75. doi: 10.1038/s41418-019-0347-0
72. Tao P, Sun J, Wu Z, Wang S, Wang J, Li W, et al. A dominant autoinflammatory disease caused by non-cleavable variants of RIPK1. Nature. (2020) 577:109–14. doi: 10.1038/s41586-019-1830-y
73. Duan PY, Ma Y, Li XN, Qu FZ, Ji L, Guo XY, et al. Inhibition of RIPK1-dependent regulated acinar cell necrosis provides protection against acute pancreatitis via the RIPK1/NF-κB/AQP8 pathway. Exp Mol Med. (2019) 51:1–17. doi: 10.1038/s12276-019-0278-3
74. Yao J, Lan B, Ma C, Liu Y, Wu X, Feng K, et al. RNA-sequencing approach for exploring the protective mechanisms of dexmedetomidine on pancreatic injury in severe acute pancreatitis. Front Pharmacol. (2023) 14:1189486. doi: 10.3389/fphar.2023.1189486
75. Huang QY, Zhang R, Zhang QY, Dai C, Yu XY, Yuan L, et al. Disulfiram reduces the severity of mouse acute pancreatitis by inhibiting RIPK1-dependent acinar cell necrosis. Bioorg Chem. (2023) 133:106382. doi: 10.1016/j.bioorg.2023.106382
76. Zou M, Yang Z, Fan Y, Gong L, Han Z, Ji L, et al. Gut microbiota on admission as predictive biomarker for acute necrotizing pancreatitis. Front Immunol. (2022) 13:988326. doi: 10.3389/fimmu.2022.988326
77. Ammer-Herrmenau C, Antweiler KL, Asendorf T, Beyer G, Buchholz SM, Cameron S, et al. Gut microbiota predicts severity and reveals novel metabolic signatures in acute pancreatitis. Gut. (2024) 73:485–95.
78. Wang Z, Guo M, Yang S, Chen Y, Cheng J, Huang Z, et al. Intestinal microflora and metabolites affect the progression of acute pancreatitis (AP). Gut Pathog. (2024) 16:64. doi: 10.1186/s13099-024-00652-6
79. Liu J, Yan Q, Li S, Jiao J, Hao Y, Zhang G, et al. Integrative metagenomic and metabolomic analyses reveal the potential of gut microbiota to exacerbate acute pancreatitis. NPJ Biofilms Microbiomes. (2024) 10:29. doi: 10.1038/s41522-024-00499-4
Keywords: acute pancreatitis, biomarkers, extracellular traps, intestinal flora, mitochondrial DNA
Citation: Hao X, Wang Z, Niu X, Li L, Bi Y and Chai N (2025) Biomarkers to predict the severity of acute pancreatitis. Front. Med. 12:1619087. doi: 10.3389/fmed.2025.1619087
Received: 27 April 2025; Accepted: 23 July 2025;
Published: 06 August 2025.
Edited by:
Peter C. Ambe, Witten/Herdecke University, GermanyReviewed by:
Muhammad Daniyal Waheed, Maroof International Hospital, PakistanFaisal Ismail, Aga Khan University, Pakistan
Copyright © 2025 Hao, Wang, Niu, Li, Bi and Chai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ningli Chai, Y2hhaW5pbmdsaUB2aXAuMTYzLmNvbQ==; Yawei Bi, MTMwMjE5Nzc5NTNAMTYzLmNvbQ==
 Xinwei Hao
Xinwei Hao Zimeng Wang
Zimeng Wang Ningli Chai
Ningli Chai