- 1Graduate School, Heilongjiang University of Chinese Medicine, Harbin, China
- 2Graduate School, Zhejiang University of Chinese Medicine, Wenzhou, China
- 3Graduate School, Anhui University of Chinese Medicine, Hefei, China
- 4Department of Nephrology, First Affiliated Hospital of Heilongjiang University of Chinese Medicine, Harbin, China
The escalating global burden of end-stage renal disease (ESRD), driven by aging populations and rising metabolic comorbidities, underscores the urgent need for innovative therapeutic strategies. Emerging evidence highlights the gut microbiome as a pivotal modulator of renal pathophysiology through the gut-kidney axis, with microbial dysbiosis exacerbating gut microbial metabolites (e.g., uremic toxins), systemic inflammation, and multi-organ damage. This narrative review explores the divergent impacts of kidney replacement therapies (KRT)—hemodialysis (HD) and peritoneal dialysis (PD)—on gut microbiota dynamics: HD is associated with Firmicutes and Proteobacteria enrichment, reduced butyrate-producing taxa (e.g., Faecalibacterium, Roseburia), and systemic microbial translocation; whereas PD-driven glucose absorption and iron supplementation foster pathogenic proliferation (e.g., Enterobacteriaceae) and impair short-chain fatty acid (SCFA) metabolism. Current interventions, including probiotics, prebiotics, plant-based diets (PBDs), and fecal microbiota transplantation (FMT), demonstrate potential in mitigating dysbiosis and uremic toxin accumulation. PBDs reduce inflammatory markers (IL-6, CRP) and lower all-cause mortality risk by 24% in PD patients; synbiotics (e.g., Lactobacillus casei + galactooligosaccharides) reduce serum p-cresyl sulfate by 20% in HD patients; and FMT increases levels of short-chain fatty acids (propionate, butyrate) and lowers trimethylamine N-oxide (TMAO) concentrations in streptozotocin-induced diabetic nephropathy mouse models. However, clinical translation remains challenged by small sample sizes, heterogeneous outcomes, and a lack of hard endpoints. Future research must prioritize standardized protocols, personalized microbial profiling, and synergistic integration of dietary and microbiome-targeted therapies. Bridging mechanistic insights with clinical validation will advance precision medicine in ESRD management, offering transformative potential for patients burdened by this therapeutic impasse.
1 Introduction
The 2019 Global Kidney Health Atlas (GKHA) cross-sectional epidemiological survey reveals an escalating global burden of end-stage renal disease (ESRD), with an annual incidence of 144 cases per million population (1). This trajectory is exacerbated by demographic aging and the mounting prevalence of diabetes, hypertension, and obesity. ESRD not only compromises patients’ quality of life but imposes staggering socioeconomic burdens—exemplified by annual Medicare expenditures exceeding $49.2 billion for ESRD-related care in the United States, a financial strain that has persistently intensified over the past decade (2).
While kidney replacement therapy (KRT)—encompassing hemodialysis (HD), peritoneal dialysis (PD), and renal transplantation—has prolonged survival, ESRD continues to markedly truncate life expectancy. Five-year survival rates post-ESRD onset remain suboptimal at 41.4% for HD and 46.9% for PD patients (2). HD necessitates vascular access via arteriovenous fistulas or central venous catheters, coupled with rigorous dietary constraints, and remains the predominant modality, particularly in resource-limited settings (3). PD, despite its advantages in home-based convenience and residual renal function preservation, is intrinsically limited by refractory complications including peritonitis, uremic toxin accumulation, and chronic low-grade inflammation (4). Renal transplantation, though the gold standard for ESRD, remains inaccessible to most patients due to critical donor shortages (5). This therapeutic impasse has catalyzed a paradigm shift toward investigating systemic regulation of the gut-kidney metabolic axis, transcending conventional neuropathological frameworks.
Emerging evidence suggests that the gut microbiota, as a key component of the human “meta-organ,” serves as a critical regulator of renal pathophysiology (6, 7). Patients with end-stage renal disease (ESRD) exhibit severe microbial dysbiosis: opportunistic pathogens (e.g., Staphylococcus, Enterococcus, Helicobacter) show significantly increased abundance in the gut, while short-chain fatty acid (SCFA)-producing bacteria with renoprotective effects (e.g., Faecalibacterium, Lachnospira) are significantly reduced (8, 9). Among these, pathogen-derived uremic toxins (e.g., indoxyl sulfate, p-cresol) disrupt the integrity of the intestinal barrier (“intestinal leakage”), trigger systemic inflammatory cascades, and accelerate renal deterioration (10). Notably, different KRT modalities exhibit marked heterogeneity in reshaping gut microbial ecology: PD patients, due to prolonged exposure to high-glucose dialysate, demonstrate fundamentally distinct microbiota profiles compared to HD patients, while renal transplant recipients under immunosuppressive states may experience exacerbated microbial dysbiosis (11, 12). These modality-dependent microbial signatures unveil novel targets for precision interventions.
This review pioneers a systematic dissection of KRT-driven gut microbiome remodeling patterns in ESRD. Through the lens of gut-kidney crosstalk mechanisms, we critically evaluate therapeutic boundaries of probiotics, prebiotics, dietary fiber, and fecal microbiota transplantation (FMT), proposing a translational roadmap for personalized ESRD management.
.2 Methods
To establish the evidence base for this review, we systematically searched three core databases: PubMed, Web of Science, and Embase, supplemented by manual searches in Google Scholar to capture gray literature. The search strategy employed a combination of Medical Subject Headings (MeSH terms) and free-text terms, with a focus on “end-stage kidney disease (ESKD),” “gut microbiome,” “kidney replacement therapy (KRT),” “hemodialysis (HD),” “peritoneal dialysis (PD),” “gut-targeted interventions (probiotics/prebiotics/synbiotics/plant-based diets/FMT),” “uremic toxins,” and “short-chain fatty acids (SCFA),” ensuring coverage of multi-dimensional associations among gut microbiome remodeling, KRT effects, and intervention strategies in ESKD.
The search logic was optimized using the PICO framework (Population: ESKD patients; Intervention: HD/PD treatment, probiotics/prebiotics/plant-based diets/FMT; Comparison: differences between KRT modalities or intervention types; Outcome: gut microbiota composition/function, SCFA metabolism, uremic toxin levels, inflammatory markers, and clinical outcomes) to precisely extract target studies.
Given the rapid advancements in gut microbiome research, the literature search was limited to the period 2014–2024 to focus on the latest mechanistic explorations and clinical translational evidence in this field. Inclusion criteria included: ➀ clinical interventional studies [e.g., randomized controlled trials (RCTs), cohort studies, cross-sectional studies]; ➁ animal models (e.g., adenine-induced CKD models, streptozotocin-induced diabetic nephropathy models); and ➂ mechanistic studies (e.g., microbiome sequencing).
3 The gut microbiome
3.1 Importance of the gut microbiota and its metabolites
Contemporary scientific discourse has elevated the gut microbiome to the status of a quasi-autonomous organ, with emerging evidence demonstrating its metabolic capacity surpasses hepatic functionality in orchestrating host metabolic regulation, immune homeostasis, and systemic physiology (13, 14). This intricate ecosystem derives its biological significance from staggering biodiversity—the human gut harbors over 100 trillion microbial cells encompassing ≥ 35,000 species, predominantly composed of Firmicutes and Bacteroidetes phyla, which together with Proteobacteria and Actinobacteria constitute a dynamically balanced microbial community (15, 16).
Within the approximately 5–7 m-long intestinal tract, microbial communities interact with the host environment to form highly differentiated ecological niches. The small intestine, characterized by its acidic conditions, rapid food transit, and antimicrobial molecule restrictions, is predominantly colonized by facultative anaerobes. In contrast, the colon, with its anaerobic environment and abundant undigested dietary residues, serves as the primary habitat for complex microbial populations such as Prevotellaceae, Rikenellaceae, and Lachnospiraceae (17). However, this spatial distribution is not static—factors including dietary patterns, medication use, and intestinal motility frequency can dynamically reshape microbial composition (18).
Microbial-host interactions extend beyond structural characteristics to exert broad physiological effects through metabolic activities. In nutrient metabolism, gut microbiota break down dietary fibers and complex polysaccharides to generate short-chain fatty acids (SCFAs) such as acetate, propionate, and butyrate (19). These metabolites not only serve as energy sources for the host but also regulate metabolic balance and anti-inflammatory responses (20). Notably, experiments by Lanza et al. demonstrated that orally administered SCFAs act as significant modulators of neuroinflammation in migraine pathology and potent regulators of perivascular neural fiber activation in the gut system (21). Additionally, gut microbiota participate in bile acid transformation and recycling, as well as the synthesis of nutrients like folate, vitamin K, and vitamin B12 (22). For instance, Prevotella and Ruminococcus dominate folate and vitamin K/B12 production, while Bifidobacterium and Lactobacillus function as primary producers of folate and B12, respectively. Microbial deconjugation of conjugated bile acids occurs through hydroxyl group oxidation at C-3, C-7, and C-12 positions, forming oxidized bile acids, which are subsequently reduced to α- or β-configurations (23–26).
Beyond their role in metabolic support, gut microbiota play a critical role in regulating the host immune system (27). They recognize microbial molecular patterns through pattern recognition receptors [e.g., Toll-like receptors (TLRs), NOD-like receptors (NLRs)], activate immune signaling pathways, and dynamically balance inflammatory responses with immune tolerance (28, 29). Meanwhile, a healthy microbial equilibrium enhances intestinal epithelial barrier function to defend against pathogen invasion by promoting the expression and stabilization of tight junction proteins and regulating mucus properties. Peterson et al. observed that germ-free mice exhibit extremely thin colonic mucus layers, but exposure to bacterial products (peptidoglycan or lipopolysaccharide) rapidly restores mucus thickness to levels seen in conventionally raised mice (30).
3.2 Gut dysbiosis: a catalyst for systemic pathogenesis
Gut microbiota dysbiosis, characterized by reduced diversity, an abnormal Firmicutes/Bacteroidetes ratio, and excessive proliferation of pathogenic bacteria, is closely associated with various diseases.
Dysbiosis leads to reductions in beneficial metabolites [e.g., short-chain fatty acids (SCFAs)] and promotes the production of pathogenic metabolites [e.g., indoxyl sulfate (IS), p-cresyl sulfate (pCS), trimethylamine N-oxide (TMAO)] (31, 32). In patients with chronic kidney disease (CKD), uremia and reduced renal clearance lead to elevated gastrointestinal urea levels, which are hydrolyzed into ammonia by bacterial ureases. This alkaline shift suppresses SCFA-producing commensal bacteria while promoting excessive proliferation of proteolytic pathogens (e.g., Enterobacteriaceae, Clostridium) capable of producing uremic toxins (indoxyl sulfate, p-cresyl sulfate) (33). These toxins impair intestinal barrier function, allowing endotoxins [e.g., lipopolysaccharide (LPS)] to translocate into systemic circulation. Once absorbed, they activate Toll-like receptor 4 (TLR4) and nuclear factor kappa-B (NF-κB) pathways, exacerbating chronic inflammation and oxidative stress—major contributors to renal fibrosis and cardiovascular complications (34, 35).
The impact of gut dysbiosis extends beyond the kidneys to systemic organs. Trimethylamine N-oxide (TMAO), a metabolite of dietary choline, promotes macrophage foam cell formation and platelet aggregation, thereby exacerbating atherosclerosis (36, 37). Similarly, p-cresyl sulfate (pCS) disrupts the integrity of the blood-brain barrier via epidermal growth factor receptor (EGFR)/STAT3 signaling, linking gut-derived toxins to cerebrovascular risk in CKD patients (38).
Furthermore, with the deepening of research, gut dysbiosis has been found to be associated with various gastrointestinal diseases (39), metabolic disorders (e.g., obesity, insulin resistance, type 2 diabetes) (40), and even neurological disorders (e.g., Alzheimer’s disease, Parkinson’s disease) (41).
In summary, gut microbiota are not merely passive participants in human physiological activities but act as critical drivers of disease pathogenesis. Therefore, systematically elucidating the core mechanisms of gut microbiota in renal function is crucial for providing new insights to break through traditional renal pathophysiological frameworks.
4 Core mechanisms of the gut-kidney axis
4.1 Microbial-derived uremic toxin metabolism
The gut microbiome serves as the principal bioreactor for uremic toxin generation, with its metabolic byproducts directly interfacing with renal pathophysiology through the gut-kidney axis. Characterized toxins of microbial origin include indoxyl sulfate (IS), p-cresyl sulfate (PCS), phenylacetylglutamine, and trimethylamine N-oxide (TMAO) (31, 42). While biosynthetically distinct, these compounds collectively orchestrate a pathogenic network through convergent mechanisms of renal and systemic toxicity.
4.1.1 Aromatic amino acid metabolism and toxin generation
Indoxyl sulfate (IS) originates from microbial degradation of tryptophan in the colon. Tryptophan is metabolized by Escherichia coli and other proteolytic bacteria into indole, which undergoes hepatic hydroxylation and sulfation to form IS (32). IS exhibits high albumin-binding affinity, and its clearance via hemodialysis is restricted to the unbound fraction due to protein-binding limitations (43). Consequently, IS accumulates and induces endothelial oxidative stress and renal fibrosis through activation of the aryl hydrocarbon receptor (AhR)/nuclear factor kappa-B (NF-κB) pathway (44).
Aryl hydrocarbon receptor, as a ligand-activated transcription factor, relies on dual regulation by both endogenous and exogenous ligands for its function. Exogenous ligands primarily originate from the metabolism of environmental pollutants. For example, 1-methoxypyrene (a metabolite of polycyclic aromatic hydrocarbons) can directly enter the circulatory system and bind with high affinity to the PAS-B domain of AhR (45, 46).
Among endogenous ligands, indoxyl sulfate (IS) serves as a core activator of AhR in chronic kidney disease (CKD): IS is actively transported into the cytoplasm via organic anion transporters (e.g., OAT1/3) on the membrane of proximal tubular epithelial cells (38). Upon entering the cell, IS binds to cytosolic AhR, triggering its conformational changes and release of chaperone proteins (e.g., HSP90), exposing the nuclear localization signal (NLS), and promoting AhR translocation into the nucleus (38). Within the nucleus, AhR forms a heterodimer with ARNT, directly binds to the XRE element (5′-GCGTG-3′) in the promoter regions of profibrotic genes (e.g., TGF-β1, Cyp1a1), and activates the transcription of target genes (47). This process significantly upregulates the expression of α-smooth muscle actin (α-SMA) and collagen type I (Col-I), induces epithelial-mesenchymal transition (EMT) of renal tubules, and ultimately exacerbates renal interstitial fibrosis (48, 49).
Activated AhR amplifies renal injury through dual pathological mechanisms: First, it forms a dynamic regulatory network with the NF-κB pathway—early IS-activated AhR binds to the p65 subunit of NF-κB, leading to mutual inhibition of both in the cytoplasm and limiting the initial release of pro-inflammatory factors; subsequently, AhR induces the expression of suppressor of cytokine signaling 2 (Socs2), relieving the inhibition of NF-κB, and ultimately, AhR directly binds to the TNF-α promoter region, driving the transcription of pro-inflammatory factors such as IL-6 and TNF-α, thereby exacerbating systemic micro-inflammation (44). Second, AhR mediates the ubiquitination and degradation of peroxisome proliferator-activated receptor γ coactivator 1-α (PGC1α) via the ubiquitin-proteasome system (47). As a core regulator of mitochondrial biogenesis, the loss of PGC1α leads to impaired mitochondrial DNA (mtDNA) replication, decreased activity of respiratory chain complexes (e.g., complexes I-IV), and reduced ATP production, ultimately inducing senescence and functional loss of renal tubular cells (44).
Aromatic amino acids tyrosine and phenylalanine undergo bacterial deamination, transamination, and decarboxylation in the distal colon to form phenolic compounds such as phenol and p-cresol (50). The majority of intestinal p-cresol is absorbed into systemic circulation, while a residual fraction undergoes glucuronidation by host epithelial UDP-glucuronosyltransferases to form p-cresyl glucuronide. Systemic p-cresol is sulfated in the liver to produce p-cresyl sulfate (pCS) (51). The free fraction of these compounds undergoes glomerular filtration, while conjugated forms are secreted via tubular epithelial cells, both ultimately excreted in urine (52, 53). The pathological impacts of pCS are increasingly elucidated:
A 2015 CKD rat model demonstrated pCS-induced overproduction of reactive oxygen species (ROS), activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and increased caspase-3 activity, promoting cardiomyocyte apoptosis (54).
A meta-analysis by Lin et al. (n = 1,572) confirmed significant associations between pCS and all-cause mortality/cardiovascular events in CKD patients (55).
Animal models by Sun et al. revealed that pCS-mediated activation of the renin-angiotensin system (RAS)/transforming growth factor-β (TGF-β) pathway induces renal tubular epithelial-mesenchymal transition (EMT), leading to renal fibrosis (49). Recent studies identified circulating pCS levels as a risk factor for cerebrovascular events in CKD patients. pCS activates epidermal growth factor receptor (EGFR) signaling, sequentially triggering intracellular annexin A1 and signal transducer and activator of transcription 3 (STAT3), which mobilize matrix metalloproteinases (MMP-2/9) and disrupt blood-brain barrier integrity (38).
4.1.2 Choline metabolism and the controversial role of TMAO
Distinct from other uremic toxins, trimethylamine N-oxide (TMAO) exhibits unique metabolic characteristics and pathological effects. Choline, phosphatidylcholine, and L-carnitine are metabolized by gut microbiota (e.g., Proteobacteria) into trimethylamine (TMA), which is absorbed and oxidized by hepatic flavin monooxygenase to form TMAO (56). Unlike protein-bound toxins like indoxyl sulfate and p-cresyl sulfate, TMAO can be effectively removed via dialysis. However, its residual toxicity remains clinically significant. Studies demonstrate that TMAO exacerbates atherosclerosis and thrombosis by promoting platelet Ca2 + release (36) and upregulating macrophage CD36/scavenger receptor-A1 (SR-A1) expression (37). Although its pathogenic mechanisms remain incompletely understood, TMAO is widely recognized as an early biomarker for cardiovascular risk in CKD patients (57).
4.2 Drivers of gut dysbiosis and intestinal barrier dysfunction
4.2.1 Urea-ammonia cycle: the initiation of microbial imbalance
Urea, a primary metabolic waste product of the kidneys, is hydrolyzed by bacterial ureases into ammonia and carbamate. Ammonia is further converted to ammonium hydroxide, elevating intestinal pH and compromising epithelial barrier integrity (58, 59). This eroded barrier triggers leukocyte infiltration, local inflammation, and proinflammatory cytokine release (TNF-α, IFN-γ, IL-1β, IL-12), which disrupt tight junction integrity and increase barrier permeability (59, 60).
In CKD patients, heightened gastrointestinal urea secretion creates a dysbiotic environment that suppresses beneficial bacteria (Lactobacillus, Bifidobacterium) while promoting pathogenic colonization (Enterococcus, Proteobacteria) (33, 61). Over 50% of gut microbiota in these patients encode urease genes, accelerating ammonia and uremic toxin production. Wong et al. systematically categorized bacterial families harboring urease, uricase, p-cresol/indole-producing enzymes, and SCFA-synthesizing genes in 12 healthy controls and 24 ESRD patients. Their analysis revealed significant enrichment of urease/uricase/indole/p-cresol-producing taxa and depletion of butyrate-producing Lachnospiraceae in ESRD cohorts (33).
4.2.2 SCFAs: bidirectional regulators of intestinal barrier homeostasis
Chronic kidney disease patients frequently experience intestinal wall congestion, edema, antibiotic overuse, metabolic acidosis, and reduced dietary fiber intake (62). Commensal bacteria (Bifidobacterium, Faecalibacterium) ferment dietary fiber to produce SCFAs (acetate, propionate, butyrate), which activate G protein-coupled receptors to enhance tight junction protein expression (ZO-1, occludin) and suppress inflammatory cytokines (63–65). However, low-fiber diets diminish SCFA production, while oral iron supplements and antibiotics exacerbate microbial imbalance. Reduced SCFA-producing taxa (Bifidobacterium, Lachnospiraceae) in CKD patients increase intestinal permeability, enabling endotoxin (e.g., monocyte-derived LPS) and bacterial DNA translocation into systemic circulation. These factors activate TLR4/TLR9 signaling, triggering NF-κB/NLRP3 inflammasome pathways and releasing proinflammatory cytokines (IL-1β, IL-6), which exacerbate systemic microinflammation and organ damage (66, 67). Wang et al. confirmed using a 5/6 nephrectomy rat model that bacterial translocation induces systemic microinflammation (68). Recent breakthroughs highlight SCFAs’ extraintestinal roles:Larraufie et al. demonstrated that propionate and butyrate regulate appetite and glucose metabolism via PYY upregulation, with SCFA deficiency exacerbating obesity and diabetes (69).
Pluznick et al. revealed SCFA-mediated blood pressure regulation through Olfr78 and Gpr41 receptors, with propionate exerting receptor-dependent antihypertensive effects (70).
These findings directly link SCFAs to CKD risk factors (hypertension, diabetes), underscoring their multidimensional regulatory value (71).
The above pathomechanisms of the gut-kidney axis indicate that gut dysbiosis is not merely a consequence of ESRD but may dynamically evolve with therapeutic interventions. As the most common renal replacement therapies, hemodialysis (HD) and peritoneal dialysis (PD) exert modality-specific impacts on gut microbiota structure and function through distinct solute clearance mechanisms. These differences may hold the key to optimizing ESRD management strategies.
5 Impact of hemodialysis on microbiome dynamics
The interaction between hemodialysis (HD) and the gut microbiome extends beyond solute clearance, manifesting as bidirectional dysregulation of the gut-kidney axis (72). This dysregulation is first evident in phylum-level microbial alterations: studies demonstrate significant enrichment of Proteobacteria, Actinobacteria, and Firmicutes alongside reduced Bacteroidetes abundance in HD patients compared to healthy individuals (61, 73). Direct evidence comes from Chao et al. who analyzed fecal samples from 30 healthy controls, 15 HD patients with well-controlled calcium/phosphorus levels, and 16 hyperphosphatemic HD patients using 16S rRNA amplicon sequencing. Their findings confirmed pronounced enrichment of Firmicutes, Actinobacteria, and Proteobacteria in HD cohorts (74). Notably, microbial changes exhibit population-specific heterogeneity: Crespo-Salgado et al. reported increased Bacteroidetes abundance in pediatric ESRD patients undergoing HD via 16S rRNA sequencing (75).
Beyond changes at the phylum level, diversity indices further revealed hemodialysis (HD)-induced ecological perturbation. Studies by Wu et al. demonstrated that α-diversity indices in HD patients were similar to those in healthy individuals, whereas β-diversity analysis showed significant differences in microbiota structure between HD patients and healthy populations (73). Crespo-Salgado’s research team recently compared the gut microbiomes of pediatric patients undergoing peritoneal dialysis (PD), hemodialysis (HD), post-renal transplantation, and the healthy control group. The authors noted that the gut microbiome of HD patients was similar to that of the control group and exhibited higher diversity than that of PD patients (75).
This dysbiosis is exacerbated by depletion of key functional taxa, particularly butyrate-producing genera (Faecalibacterium, Roseburia). As butyrate serves as a critical energy source for colonocytes, its deficiency leads to colonic metabolic dysfunction and directly correlates with indoxyl sulfate accumulation and intestinal barrier impairment (64, 76).
Hemodialysis-induced microbial disruption is not confined to the gut but triggers systemic microbial translocation. In the metagenomic study by Shi et al. bacterial DNA (predominantly Firmicutes, Bacteroidetes, and Proteobacteria) was detected in the bloodstream of 27% of HD patients, and this DNA concentration positively correlated with CRP and IL-6 levels (77). The mechanism underlying this “microbe-blood interface breach” involves HD-associated uremic toxins downregulating tight junction proteins (occludin, ZO-1), thereby compromising gut-vascular barrier integrity (35).
6 Impact of peritoneal dialysis on gut microbiota structure and function
Peritoneal dialysis (PD), through cyclical exchanges of glucose-based dialysate for solute clearance, exerts modality-specific effects on gut microbiota composition and functionality (78). However, existing studies on PD populations remain limited and exhibit marked heterogeneity in outcomes. While some reports indicate significantly reduced α- and β-diversity in PD patients compared to healthy controls (75, 79), Gao et al. recently observed comparable α-diversity between PD patients and healthy individuals (80). Teixeira et al. further demonstrated no statistical differences in α/β-diversity between PD patients and their cohabitating household contacts (81). These contradictory findings suggest confounding influences from variables such as ESRD disease duration and comorbid conditions.
To disentangle whether gut microbiota alterations in ESRD patients arise from dialysis or uremia itself, controlled comparative studies have been conducted. Wang et al. identified distinct microbial abundance and structural differences between PD patients and non-dialyzed ESRD counterparts, particularly in Lactobacillus species (82). Conversely, Luo et al. reported no significant phylum/genus-level variations between PD and pre-dialysis cohorts (83). Despite conflicting conclusions, all studies confirm characteristic functional metabolic deviations in PD-associated microbiota compared to healthy populations.
At the phylum level, PD patients consistently exhibit microbial remodeling marked by reduced Firmicutes and Actinobacteria abundance alongside Proteobacteria enrichment (84). This dysbiotic shift may correlate with long-term oral iron supplementation—known to suppress SCFA-producing taxa (e.g., Faecalibacterium) and promote proteolytic bacterial proliferation (85)—coupled with systemic glucose absorption from hypertonic dialysate. Approximately 15%–20% of dialysate glucose permeates peritoneal capillaries, creating a carbon-rich milieu that favors bacterial overgrowth (86). Of particular concern is Enterobacteriaceae expansion, as its secreted lipopolysaccharide (LPS) activates TLR4/NF-κB signaling, directly correlating with increased Gram-negative peritonitis incidence in PD patients (87). Milan Manani et al. provided clinical evidence linking elevated dialysate LPS levels to peritonitis risk, with dialysate leukocyte counts proportionally rising alongside LPS concentrations (87).
Metabolomic analyses further elucidate core functional deficits in PD-associated microbiota. PD patients show significant reductions in fecal butyrate (BA) and caprylate (CA) levels due to depleted SCFA-producing genera (Faecalibacterium, Roseburia) (88). As previously discussed, SCFA deficiency impairs intestinal barrier integrity via downregulation of tight junction proteins (e.g., occludin) (64) and induces systemic metabolic dysregulation through disrupted energy metabolism and immunomodulatory dysfunction (12, 64).
6.1 Comparison of HD and PD in SCFA-mediated downstream effects
Reductions in short-chain fatty acid (SCFA)-producing bacteria (e.g., Faecalibacterium, Roseburia) in hemodialysis (HD) patients lead to downstream effects similar to those observed in peritoneal dialysis (PD) patients. SCFAs—particularly butyrate—serve as the primary energy source for colonic epithelial cells, which can promote the expression of tight junction proteins (e.g., ZO-1, occludin) via activation of G protein-coupled receptors (e.g., GPR41/43), thereby maintaining the integrity of the intestinal mucosal barrier (34, 64). In HD patients, reduced butyrate-producing bacteria result in colonic metabolic dysfunction, exacerbated intestinal barrier “leakage,” and translocation of bacterial DNA and endotoxins (e.g., lipopolysaccharide, LPS) into the circulatory system (77). PD patients similarly exhibit downregulated tight junction proteins and increased intestinal permeability due to SCFA deficiency (34, 88). Both patient groups show elevated levels of inflammatory factors such as C-reactive protein (CRP) and interleukin-6 (IL-6) in the blood (77, 87).
Moreover, disrupted intestinal barriers allow translocated microbial components (e.g., LPS, bacterial DNA) to activate TLR4/TLR9 signaling pathways, triggering NF-κB/NLRP3 inflammasome activation and the release of pro-inflammatory factors like IL-1β and IL-6 (34, 77). In HD patients, this micro-inflammation directly correlates with an increased risk of cardiovascular events (55, 89); in PD patients, peritoneal microbial translocation (e.g., overgrowth of Enterobacteriaceae) elevates the risk of peritonitis (87).
Notably, despite similar underlying mechanisms, HD and PD patients differ in the specific manifestations of SCFA reduction and related complications due to distinct treatment modalities. Microbial translocation in HD patients is more directly associated with blood bacterial DNA levels (77), potentially linked to uremic toxin accumulation [e.g., indoxyl sulfate (IS), p-cresyl sulfate (PCS)] induced by HD itself, which damages the gut-vascular barrier (35). In contrast, PD patients—due to glucose absorption from high-glucose dialysate and iron supplementation—are more prone to overgrowth of pathogenic bacteria like Enterobacteriaceae (84), with microbial translocation potentially influencing inflammation via the peritoneal-blood interface (e.g., the peritoneum) (87).
Short-chain fatty acids-producing bacterial reduction in HD patients is primarily associated with uremic status (disruption of the urea-ammonia cycle, which impairs microbial balance) and HD treatment (partial removal of beneficial metabolites) (61, 72, 73). In PD patients, this reduction is more influenced by dual factors: a high-glucose environment (promoting pathogenic bacterial proliferation) and iron supplementation (inhibiting SCFA-producing bacteria) (85, 86).
In summary, reductions in SCFA-producing bacteria in both HD and PD patients trigger similar downstream effects (intestinal barrier disruption, bacterial translocation, inflammation), but the specific mechanisms and related complications differ due to variations in treatment modalities (Figure 1). These findings suggest that interventions targeting SCFA metabolism may benefit both patient groups, though strategies must be adjusted according to treatment type. Currently, clinically validated therapeutic approaches include probiotics, prebiotics, synbiotics, plant-based diets, and fecal microbiota transplantation (FMT). By restoring microbial ecology and SCFA homeostasis, these methods hold promise as core components of personalized kidney replacement therapy (KRT) management, synergizing with traditional dialysis regimens.
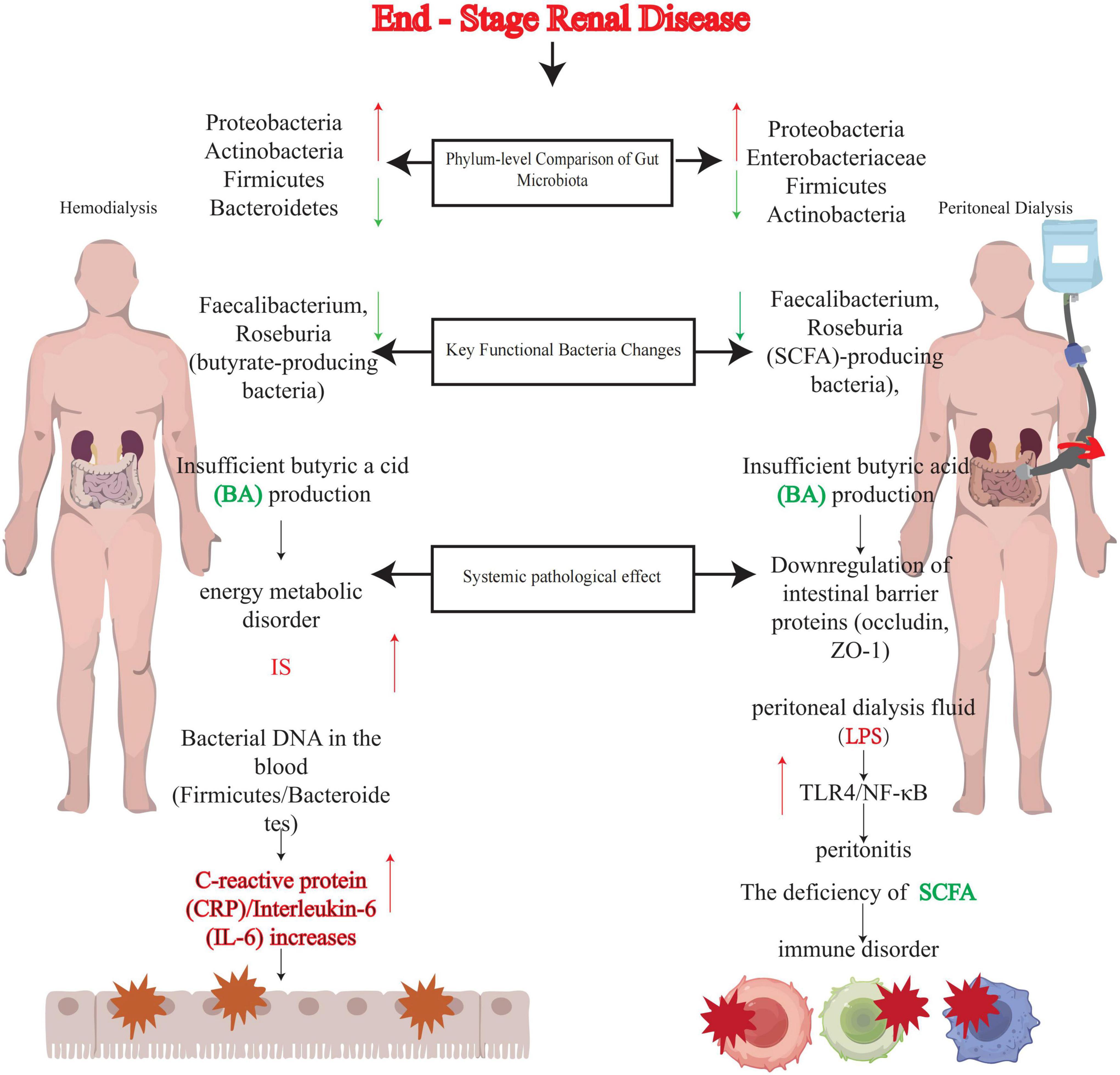
Figure 1. Comparison of the effects of hemodialysis (HD) vs. peritoneal dialysis (PD) on gut microbiota. This figure illustrates the comparison of gut microbiota dysbiosis in end-stage renal disease (ESRD) patients undergoing hemodialysis (HD) or peritoneal dialysis (PD), highlighting phylum-level compositional changes, alterations in functional bacteria, and systemic pathological consequences. Green arrows: Indicate positive regulation or increase. Red arrows: Indicate negative regulation or decrease. SCFA, short-chain fatty acids; BA, butyric acid; IS, indoxyl sulfate; CRP, C-reactive protein; IL-6, interleukin-6; LPS, lipopolysaccharide; TLR4, toll-like receptor 4; NF-κB, nuclear factor κB; ZO-1, zonula occludens-1.
7 Clinical significance and therapeutic approaches
7.1 Effects of probiotics, prebiotics, and synbiotics on patients with chronic kidney disease (CKD)
According to definitions established by the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus and academic literature, probiotics are “live microorganisms that, when administered in adequate amounts, confer a health benefit on the host.” Examples include Bifidobacterium longum, Lactobacillus acidophilus, and Enterococcus faecalis. Prebiotics are “a substrate that is selectively utilized by host microorganisms conferring a health benefit,” such as pectic oligosaccharides, oligosaccharides, inulin, and resistant starch (90). Synbiotics are defined as “a mixture comprising live microorganisms and substrate(s) selectively utilized by host microorganisms that confers a health benefit on the host.” Foods like yogurt, fermented milk, and kefir represent early applications introducing the concept of probiotics and their health benefits into the diets of both healthy individuals and CKD patients (91).
As research into gut microbiota functions deepens, the role of prebiotics in disease is receiving increasing attention. In animal models of CKD, prebiotics (such as resistant starch HAM-RS2 and arabic gum) enhance gut barrier function through fermentation and production of short-chain fatty acids (SCFAs). For instance, Kieffer et al. observed in an adenine-induced CKD rat model that feeding HAM-RS2 reduced cecal pH, altered microbial diversity (e.g., increased Bacteroidetes/Firmicutes ratio), and significantly lowered uremic retention solutes, including serum indoxyl sulfate (IS) (−36%), urinary IS (−66%), and urinary p-cresol (−47%) (92). Vaziri et al. confirmed that fermentable dietary fiber HAM-RS2 attenuated histological abnormalities in CKD rats, including tubular damage, interstitial fibrosis, and inflammatory cell infiltration (93). In human studies, arabic gum supplementation (10–40 g/day) significantly reduced inflammatory markers (e.g., TNF-α, IL-1β) in CKD patients, improved systemic inflammation, and potentially reduced morbidity and mortality (94). Notably, prebiotics may require > 5 g/day to modulate microbial diversity and 15–20 g/day to significantly reduce uremic toxins (95).
Advances in probiotic research reveal that Lactobacillus johnsonii may reduce serum creatinine levels and renal fibrosis area in CKD rats—potentially reversing CKD progression—by elevating serum indole-3-carboxaldehyde (IAld) and inhibiting aryl hydrocarbon receptor (AhR) pathway nuclear translocation (96). The same team found that Lactobacillus spp. ameliorate membranous nephropathy by suppressing the AhR pathway via tryptophan-derived indole metabolites (97).
Synbiotics demonstrate favorable microbiota-modulating effects in CKD patients. Rossi et al. showed that supplementing 37 Stage IV CKD patients for 6 weeks with a synbiotic (9 probiotic strains + 15 g prebiotic) reduced indoxyl sulfate (IS) and p-cresyl sulfate (PCS) levels by 22%–28%, increased fecal Bifidobacterium and Lachnospiraceae abundance, decreased Clostridia, and improved nutritional status (elevated serum albumin) (98). Another trial found that Stage III–V CKD patients treated for 6 months with a low-protein diet combined with a synbiotic (Streptococcus thermophilus, Lactobacillus acidophilus, Bifidobacterium longum) showed no biochemical improvements but experienced attenuated decline in estimated glomerular filtration rate (eGFR) (99).
McFarlane et al. conducted a 12 months feasibility double-blind placebo-controlled randomized trial in Stage 3–4 CKD adults, demonstrating that long-term synbiotic supplementation is feasible and acceptable. Synbiotics altered fecal microbiota, enriching Bifidobacterium and Blautia (100). Cosola et al. tested an innovative synbiotic (NATUREN G®) in a randomized single-blind placebo-controlled pilot trial involving Stage IIIb–IV CKD patients and healthy controls. Only CKD patients exhibited reduced free IS levels after a 2 months intervention. Though divergent trends in other gut-derived uremic toxins were observed (increasing in placebo, decreasing in synbiotic), changes lacked statistical significance, suggesting CKD-specific effects requiring further validation (101).
However, inconsistencies exist: a study of 30 non-dialysis CKD patients observed no changes in renal function or uremic status after 30 days of probiotics (102). Another 6 weeks synbiotic trial reduced serum p-cresyl sulfate but not serum IS in pre-dialysis CKD patients (103). These discrepancies may relate to strain specificity and individual factors.
In summary, current evidence is insufficient to confirm the efficacy of probiotics, prebiotics, or synbiotics on hard clinical endpoints (e.g., renal function decline rate, cardiovascular events) in CKD patients or establish relative advantages among supplement types. Future rigorously designed randomized controlled trials must define optimal protocols (strain selection, dose optimization, treatment duration) to develop cost-effective gut microbiota-targeted strategies for improving CKD prognosis.
7.2 Application of prebiotics, probiotics, and synbiotics in esrd patients
Current evidence indicates that both peritoneal dialysis (PD) and hemodialysis (HD) patients frequently develop gut dysbiosis due to declining residual renal function, dialysis procedures, and constipation (104).
Interventions with prebiotics, probiotics, and synbiotics may alleviate symptoms by modulating the intestinal microenvironment (105).
Research on prebiotics has primarily focused on their potential to clear uremic toxins, but results have shown significant heterogeneity. This heterogeneity may be closely associated with factors such as baseline microbiota characteristics in different populations (e.g., HD vs. PD patients), prebiotic dosage, strain types, and intervention duration.
In HD patients, the effects of inulin exhibit pronounced population specificity and individual variability: Meijers et al. reported that HD patients supplemented with inulin for 4 weeks showed a 20% reduction in serum PCS levels, but no change in IS (106); however, in the trial by Biruete et al. involving 12 HD patients who received 4-week inulin supplementation, no significant changes were observed in fecal or plasma toxin levels, nor were there major alterations in the fecal microbiota (107). In PD patients, the intervention effects of inulin-type prebiotics are directly related to microbiota modulation mechanisms, and intervention duration significantly impacts outcomes. A randomized double-blind crossover trial (n = 16) by a Chinese research team showed that continuous ambulatory peritoneal dialysis (CAPD) patients supplemented with inulin-type prebiotics for 24 weeks exhibited a significant decrease in Bacteroidetes abundance, an increase in Firmicutes abundance, and an elevated Firmicutes/Bacteroidetes (F/B) ratio (positively correlated with fecal uric acid degradation capacity). The authors suggested that long-term (24 weeks) intervention may promote uric acid metabolism by enhancing Firmicutes function, thereby reducing serum uric acid levels (108). Another PD patient study (n = 21) further found that 36 weeks intervention with inulin-type fructans could target and inhibit the abundance of indole-producing bacteria—Bacteroides thetaiotaomicron. This change correlated with reduced fecal indole levels, potentially suppressing the production of uremic toxins (e.g., indoxyl sulfate) (109). However, short-term (3 months) inulin-type fructan intervention (n = 22) increased the F/B ratio in PD patients but did not significantly reduce plasma TMAO levels, indicating that intervention duration may be a key factor influencing the toxin-clearing efficacy of inulin-type prebiotics in PD patients (110).
The toxin-clearing effect of resistant starch is more definitive: A 2,010 trial involving 56 HD patients showed that resistant starch supplementation significantly reduced serum indoxyl sulfate (IS) and p-cresyl sulfate (PCS) levels (111); Esgalhado et al. further validated the reduction of IS through resistant starch-enriched biscuit intervention (112). Animal experiments also support its role: CKD rats fed high-amylose resistant starch (HAM-RS2) for 3 weeks exhibited significant improvements in renal interstitial fibrosis, NF-κB activation, and oxidative stress markers, suggesting delayed CKD progression (93).
Probiotics have demonstrated multi-dimensional metabolic benefits. Oral administration of Bifidobacterium longum (Bifina strain) can reduce serum homocysteine, triglycerides, and IS levels in HD patients (113); Climent et al. further found that probiotic supplementation may improve depressive symptoms in malnourished HD patients (114). These benefits are likely closely related to probiotics’ modulation of gut microbiota:
In HD patients, probiotic intervention exerts effects by altering microbial composition and the abundance of specific taxa. A Korean randomized double-blind study (n = 22) showed that 3 months supplementation with Bifidobacterium bifidum BGN4 and Bifidobacterium longum BORI significantly increased the relative abundance of Prevotella, Enterococcus, Allistipes, Clostridium, Escherichia-Shigella, Klebsiella, and Bifidobacterium, while reducing the abundance of Bacteroides, Faecalibacterium, Eubacterium siraeum, Tyzzerella, Sutterella, and Akkermansia (115). A Chinese research team (n = 50) further found that long-term (6 months) supplementation with Bifidobacterium longum NQ1501, Lactobacillus acidophilus YIT2004, and Enterococcus faecalis YIT0072 increased the abundance of Bacteroidaceae and Enterococcaceae, while reducing the abundance of Ruminococ- caceae, Halomonadaceae, Peptostreptococ- caceae, Clostridiales XIII, and Erysipelotrichaceae (largely associated with uremic toxin production) (116). However, Borges’ research team noted that short-term (3 months) probiotic supplementation (e.g., Streptococcus thermophilus, Lactobacillus acidophilus, and Bifidobacterium longum) had limited effects on microbiota structure in HD patients, with no significant differences in microbiota band counts (reflecting composition) between the probiotic and placebo groups (117).
In PD patients, probiotic intervention primarily modulates phylum-level microbiota and reduces opportunistic pathogens. A single-center study by Liu et al. (n = 57) showed that 12 weeks supplementation with Lactobacillus paracasei N1115 (containing fructooligosaccharides) significantly increased Firmicutes abundance, decreased Bacteroidetes abundance, and reduced the abundance of opportunistic pathogens (e.g., Fusobacterium, Bilophila). Although microbiota diversity (α/β) showed no significant changes, these alterations correlated with improvements in gastrointestinal symptoms (e.g., dyspepsia, constipation) (118).
Despite the metabolic and symptomatic benefits of probiotics through microbiota modulation, their role in cardiovascular disease (CVD) prevention remains controversial: Some studies suggest they may reduce CVD risk via cholesterol regulation, blood pressure modulation, and anti-inflammatory effects (119, 120), but mechanisms such as strain-specific immune regulatory pathways remain unclear, and data on hard endpoints (e.g., mortality, myocardial infarction) are lacking.
Synbiotic formulations (probiotic-prebiotic combinations) show unique advantages. A 2 weeks regimen combining Lactobacillus casei Shirota, Bifidobacterium breve Yakult, and galactooligosaccharides significantly reduced serum PCS in HD patients (121). Iranian researchers observed superior reductions in IL-6, CRP, and endotoxemia with synbiotics versus probiotics alone (122, 123). Paradoxically, another Iranian study reported synbiotic-induced IS elevation in HD patients (122).
The peritoneal microenvironment in PD patients may reshape intervention efficacy. Continuous dialysate exposure alters intestinal osmolarity and microbial colonization, though evidence remains scarce. Preliminary data suggest probiotics (B. longum, Lactobacillus plantarum) in PD patients reduce serum endotoxin, proinflammatory cytokines (TNF-α, IL-6, IL-5), and elevate anti-inflammatory IL-10 while preserving residual renal function (124). Additional studies highlight oral probiotics’ ability to lower serum uric acid (108) and endotoxin (125) in PD patients, affirming the safety and gastrointestinal symptom-relieving benefits of pre/pro/synbiotic supplementation.
In summary, current research is constrained by small sample sizes, short intervention durations, and strain/dosage variability. Although systematic reviews suggest prebiotics modestly reduce serum urea and synbiotics modulate microbial composition, the lack of hard clinical endpoint data—particularly in PD cohorts—remains the foremost translational challenge (Table 1).
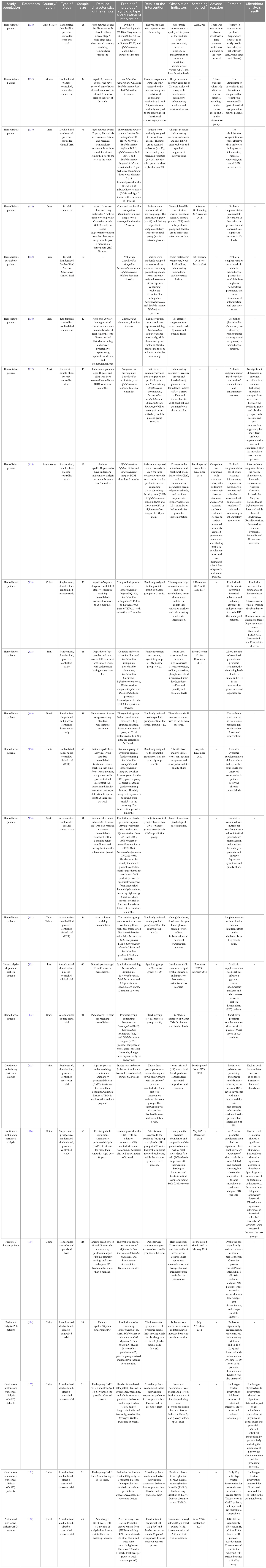
Table 1. Summary of clinical trials on the use of prebiotics, probiotics, and synbiotics supplements in d-stage renal disease (ESRD) patients.
7.3 Translational potential and clinical therapeutic approaches of plant-based diets in peritoneal dialysis (PD) and hemodialysis (HD) patients
Plant-based diets (PBDs), characterized by a focus on unprocessed or minimally processed plant foods such as whole grains, legumes, nuts, fruits, and vegetables, while strictly limiting animal products (meat, fish, eggs, dairy) and processed foods (e.g., refined vegetable oils, plant-based meat substitutes), offer multifaceted benefits in dialysis populations. These diets are distinguished by two core components: dietary fibers and phytochemicals (138).
Dietary fibers in plant-based foods include insoluble fibers (hemicellulose, cellulose, lignin) and soluble carbohydrates (pectins, gums, mucilages), as well as indigestible resistant starch and oligosaccharides (139). These non-digestible components possess strong water-absorbing properties, expanding fecal volume within the colon to mechanically stimulate intestinal peristalsis and accelerate bowel transit time (140). Additionally, anaerobic microbial fermentation of these fibers generates gaseous byproducts and short-chain fatty acids (SCFAs)—primarily acetate, propionate, and butyrate—which play critical roles in maintaining human health and regulating gut homeostasis (141).
Phytochemicals, bioactive non-nutrient compounds found in plants, exhibit anti-inflammatory and antioxidant properties. A Swedish cross-sectional study of dialysis patients revealed that higher proportions of linoleic acid (a plant-derived fatty acid) in plasma were inversely correlated with IL-6 levels and all-cause mortality, suggesting clinical benefits from increased plant-based oil consumption (142). Polyphenols, another class of phytochemicals widely distributed in plants, mitigate cardiovascular risk—a leading cause of death in dialysis patients—by enhancing nitric oxide (NO)-mediated endothelial function and reducing low-density lipoprotein (LDL) oxidation, thereby preventing atherosclerosis. In cardiomyocytes, polyphenols suppress the expression and production of inflammatory markers, exerting anti-inflammatory effects (143).
The alkaline nature of plant-based foods, particularly fruits and vegetables, reduces dietary acid load. Animal-derived foods (e.g., cheese, meat, fish) typically generate higher acid loads compared to plant-based alternatives (144). PBDs also reshape the spectrum of uremic toxins through microbial modulation. Stanford et al. further observed that HD patients adhering to PBDs exhibited reduced abundances of IS/PCS-associated pathogenic bacteria (Haemophilus, Haemophilus parainfluenzae), while increased animal fat and sweets consumption correlated with elevated toxin levels (145).
Clinical studies validate survival benefits associated with PBDs. A retrospective analysis of 884 PD patients revealed that higher plant protein intake (> 57.5% vs. < 47.7% of total protein) was linked to a 24% reduction in mortality. Each 10% increase in plant protein intake correlated with 71% and 89% lower risks of all-cause and cardiovascular mortality, respectively (146). Similarly, a multinational cohort of 8,078 HD patients demonstrated that the highest tertile of fruit/vegetable intake was associated with 20% lower all-cause mortality (95% CI: 9%–29%) and 23% reduced non-cardiovascular mortality (95% CI: 9%–34%) compared to the lowest tertile (147).
High fiber intake improves clinical outcomes through dual metabolic and anti-inflammatory pathways. A cross-sectional study of 52 PD patients found that daily fiber intake > 12.2 g reduced serum and dialysate IL-6 and CRP levels (148). In a 45 months follow-up of 881 PD patients, higher fiber consumption was associated with increased serum albumin over time. Among non-diabetic patients, each 1 g/day increase in fiber intake correlated with a 13% lower all-cause mortality, though no cardiovascular mortality benefits were observed in the overall cohort or subgroups (149).
A major concern in advocating PBDs for dialysis patients is the perceived risk of hyperkalemia. Despite clinical recommendations to restrict dietary potassium, current evidence does not support an increased hyperkalemia risk with plant-based diets. Data from 8,043 HD patients in Europe and South America showed no significant association between dietary potassium intake and serum potassium levels or all-cause mortality (150). Similarly, a PD cohort study (n = 881) found no independent correlation between dietary potassium intake and mortality over 45 months (151) (Table 2). Potassium-rich plant foods (e.g., bananas, spinach) may confer cardiorenal protection through urinary alkalinization and enhanced sodium excretion, though their long-term risk-benefit ratio in dialysis populations requires further validation (152).
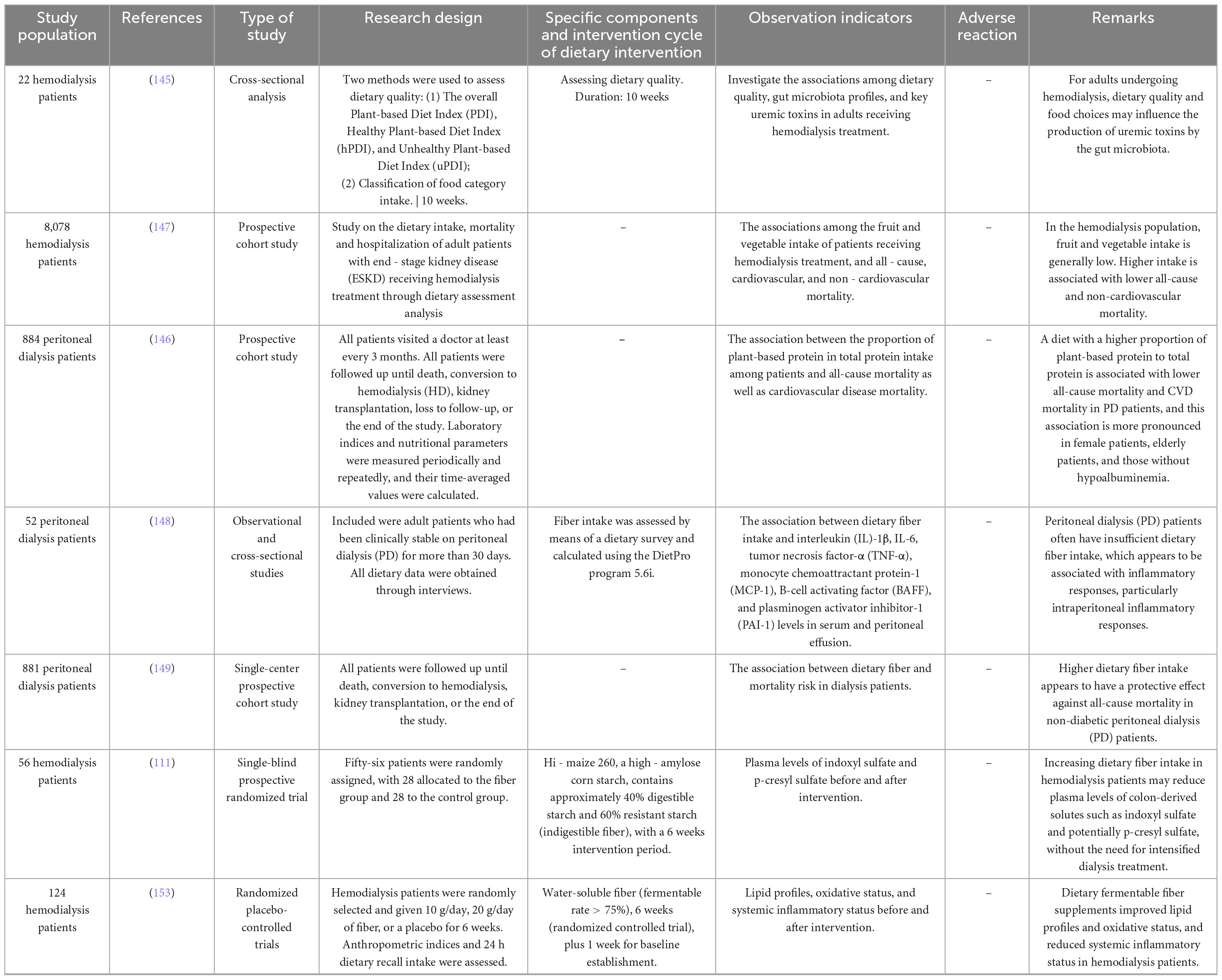
Table 2. Summary of clinical trials on plant-based diets in peritoneal dialysis (PD) and hemodialysis (HD) patients.
7.4 Mechanisms and Clinical translation of fecal microbiota transplantation (fmt) in dialysis patients
Fecal microbiota transplantation (FMT), by transplanting gut microbiota from healthy donors, offers targeted intervention for microbial dysbiosis in dialysis patients, aiming to reduce uremic toxin accumulation, systemic inflammation, and metabolic disturbances. Animal studies have revealed differential effects of FMT on toxin clearance. In adenine-induced CKD mouse models, FMT significantly reduced serum p-cresyl sulfate (PCS) levels and improved glucose tolerance, though no changes in renal function were observed (154). In streptozotocin-induced diabetic nephropathy mouse models, FMT lowered lipopolysaccharide (LPS) and trimethylamine N-oxide (TMAO) concentrations while increasing beneficial short-chain fatty acids (SCFAs) such as propionate and butyrate (155). In a traumatic brain injury (TBI) rat model, FMT rescued gut microbiota alterations induced by TBI at 8 days post-injury, alleviated neurological deficits, reduced fecal trimethylamine (TMA) levels, and decreased TMAO concentrations in both the ipsilateral brain and serum (156). Bastos et al. demonstrated that FMT prevented weight gain, reduced proteinuria, and lowered TNF-α levels in BTBR ob/ob mice, confirming its non-pharmacological therapeutic potential for diabetic nephropathy (157) (Table 3).
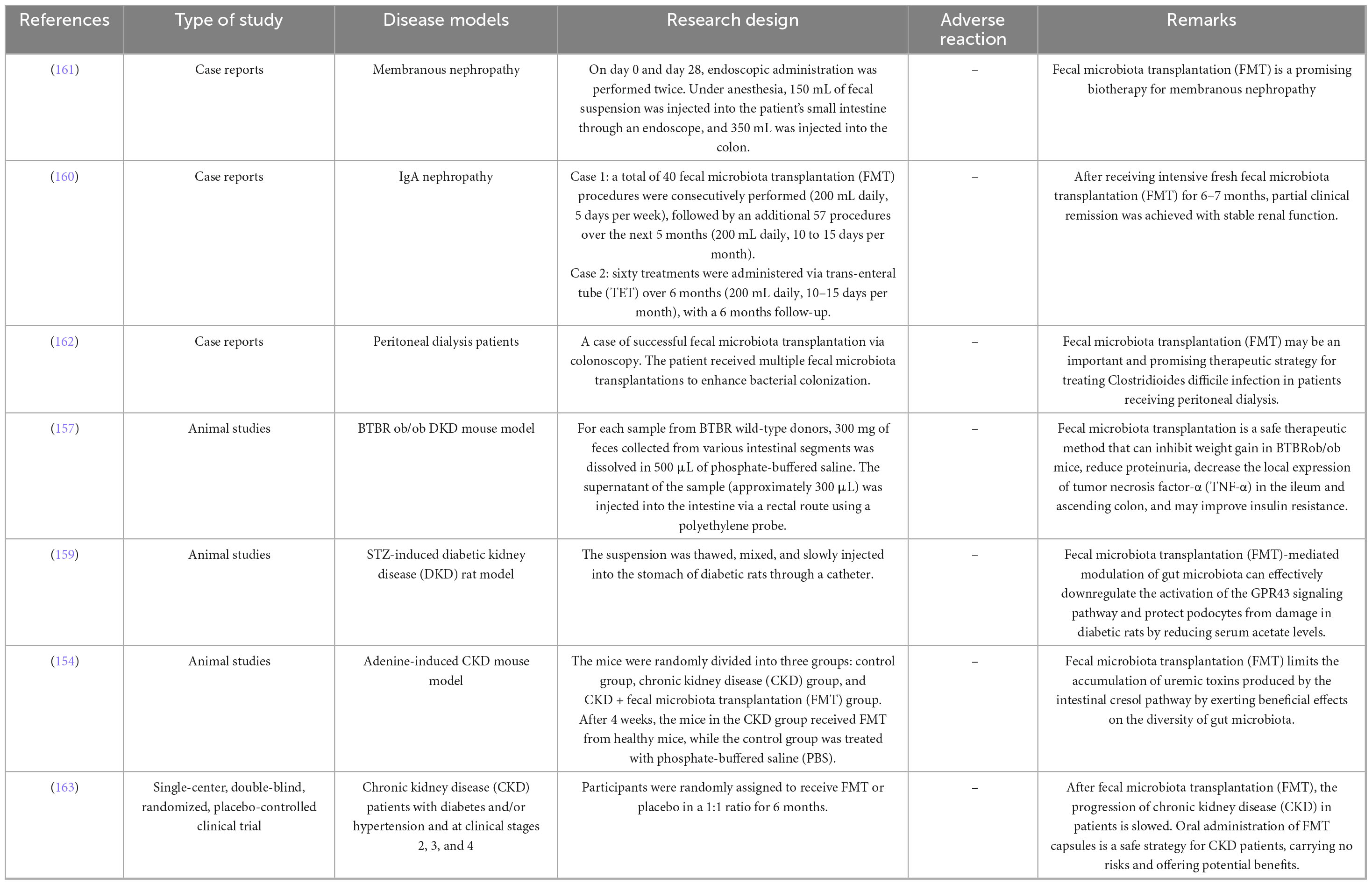
Table 3. Experimental and clinical studies of fecal microbiota transplantation (FMT) in chronic kidney disease (CKD) patients.
Crucially, the quality of donor microbiota determines therapeutic efficacy. Wang et al. found that transplanting microbiota from ESRD patients into germ-free CKD mice exacerbated uremic toxin accumulation, oxidative stress, and renal interstitial fibrosis (158), highlighting the importance of donor microbiota selection. Lu et al. showed that FMT from healthy donors restored podocyte insulin sensitivity and mitigated glomerular damage in diabetic rats with antibiotic-induced microbiota depletion (159), advancing mechanistic insights into microbiota-host metabolic interactions at the cellular level. Clinical evidence for FMT in renal diseases remains limited but includes notable case reports. Zhao et al. described two Chinese female IgA nephropathy patients who received intensive fresh FMT for 6–7 months, resulting in 24 h urinary protein (24 hUP) levels reduced to less than half of baseline, increased serum albumin (sAlb), stable renal function, and restored gut microbial balance (160). Zhou et al. reported a refractory membranous nephropathy patient who, after two FMT treatments, exhibited elevated serum albumin and total protein levels, reduced creatinine and 24 hUP, decreased anti-phospholipase A2 receptor (PLA2R) antibody titers, and resolution of edema and diarrhea (161). These cases suggest FMT may modulate autoimmune kidney diseases via the microbiota-immune axis. In dialysis-related infections, Marasco et al. successfully treated a peritoneal dialysis patient with recurrent Clostridioides difficile infection using colonoscopic FMT (162).
Despite its established role in infection control, FMT’s efficacy in ESRD/CKD populations lacks robust clinical validation. Current research focuses on animal models (e.g., diabetic nephropathy, TBI) and small pilot studies, with a complete absence of hard clinical endpoints such as improved eGFR or reduced cardiovascular events. The successful application of FMT in IgA nephropathy and membranous nephropathy may support further exploration of its therapeutic potential in ESRD. However, short-term adverse effects (diarrhea, abdominal pain, bloating, constipation, fever) and theoretical long-term risks (e.g., rheumatoid arthritis, irritable bowel syndrome) remain concerns (15). Thus, widespread adoption of FMT in dialysis populations requires bridging the gap between mechanistic plausibility and clinical evidence.
7.5 Emerging therapies in ESRD management
Novel therapeutic approaches are gaining attention for ESRD patients. For example:
AST-120: Developed in Japan, this porous carbon particle (0.2–0.4 mm diameter) adsorbs tryptophan metabolites (e.g., indole), reducing precursors for hepatic indoxyl sulfate synthesis and slowing renal deterioration (164). Wu et al. confirmed that AST-120 alleviates uremic pruritus severity in ESRD patients (165). A retrospective cohort study by Kweon et al. showed that pre-dialysis use of AST-120 for ≥ 4 months significantly reduced post-dialysis cardiovascular events and composite outcomes (166).
Sevelamer: A large cationic polymer that binds phosphate via protonated amine groups, forming non-absorbable complexes to lower serum phosphorus. Sevelamer also reduces endotoxin and CD14 levels in HD patients, potentially improving microinflammation and oxidative stress (167). Zeng Q et al. demonstrated in a randomized open-label trial that sevelamer delays vascular calcification, thereby reducing post-dialysis cardiovascular risks (168).
Although the diabetic population is not the primary focus of this review, it is noteworthy that a higher proportion of individuals with diabetes develop chronic kidney disease (CKD), with diabetic nephropathy being a leading cause of end-stage renal disease (169).
A recent breakthrough study revealed that diphenyl diselenide (DPDS) selectively increases the relative abundance of probiotics and enhances microbial diversity. It significantly improves the gut microbiota composition in streptozotocin (STZ)-induced type 1 diabetic rats, regulates intestinal microbial homeostasis, and ameliorates renal function in STZ-treated rats (170). This research demonstrates the potential application value of diphenyl diselenide in kidney diseases.
Beyond the above, gut microbiota modulation-based Chinese herbal medicines and plant-derived natural products have also demonstrated unique potential, offering multi-dimensional intervention strategies for end-stage renal disease (ESRD) management. In recent years, multiple clinical studies have confirmed that natural products represented by compound Chinese herbal formulas or active plant components can provide adjuvant therapy for ESRD patients by regulating gut microbiota dysbiosis, reducing uremic toxin production, and improving intestinal barrier function (171, 172).
For example:
Researchers have identified that mild-natured, sweet-flavored traditional Chinese medicines (TCMs) enrich Lactobacillaceae and Bifidobacterium, downregulate Enterobacteriaceae, and remodel short-chain fatty acid (SCFA) metabolism (173).
Concurrently, the herbal medicine Clerodendranthus spicatus (syn. Orthosiphon aristatus) enriches Lachnospiraceae and Alloprevotella, inhibits the URAT1 urate transporter, reduces serum uric acid levels, suppresses renal NLRP3 inflammasome activation, and restrains renal inflammation in hyperuricemic nephropathy (174).
Lin et al. conducted a randomized controlled trial investigating the effects of Fushen Granule (FSG) in patients with peritoneal dialysis-related peritonitis (PDRP). FSG enriched metabolism-related beneficial bacteria (e.g., Ruminococcus), significantly reduced blood urea nitrogen (BUN) and serum creatinine (Scr) levels in PDRP patients, increased albumin (ALB), and improved patients’ nutritional status (175). Plant-derived active components such as curcumin (a turmeric extract) have shown clear toxin clearance and anti-inflammatory effects in HD patients: 3 months curcumin supplementation significantly reduced serum p-cresyl sulfate (PCS) levels (176). Animal model studies have further corroborated the potential mechanisms of natural products: Ji’s research team confirmed in a 5/6 nephrectomy rat model that rhubarb enema treatment inhibited the overgrowth of conditionally pathogenic gut bacteria, including Akkermansia, Methanosphaera, and Clostridiaceae. Rhubarb enema improved the intestinal barrier, regulated gut microbiota dysbiosis, inhibited systemic inflammation, and alleviated renal fibrosis (177). Zhang et al. found that thonningianin A (TA) may exert protective effects against renal interstitial fibrosis in diabetic nephropathy rats by regulating gut microbiota dysbiosis, ameliorating intestinal mucosal barrier damage, reducing the production and release of LPS, inhibiting NLRP3/ASC/Caspase-1 signaling pathway activation, and suppressing renal inflammatory responses (178).
In summary, these natural products and synthetic compounds, through multi-acting mechanisms such as “modulating microbiota-reducing toxins-repairing barriers,” synergize with traditional kidney replacement therapies (e.g., HD/PD), providing more personalized treatment options for ESRD patients (Figure 2).
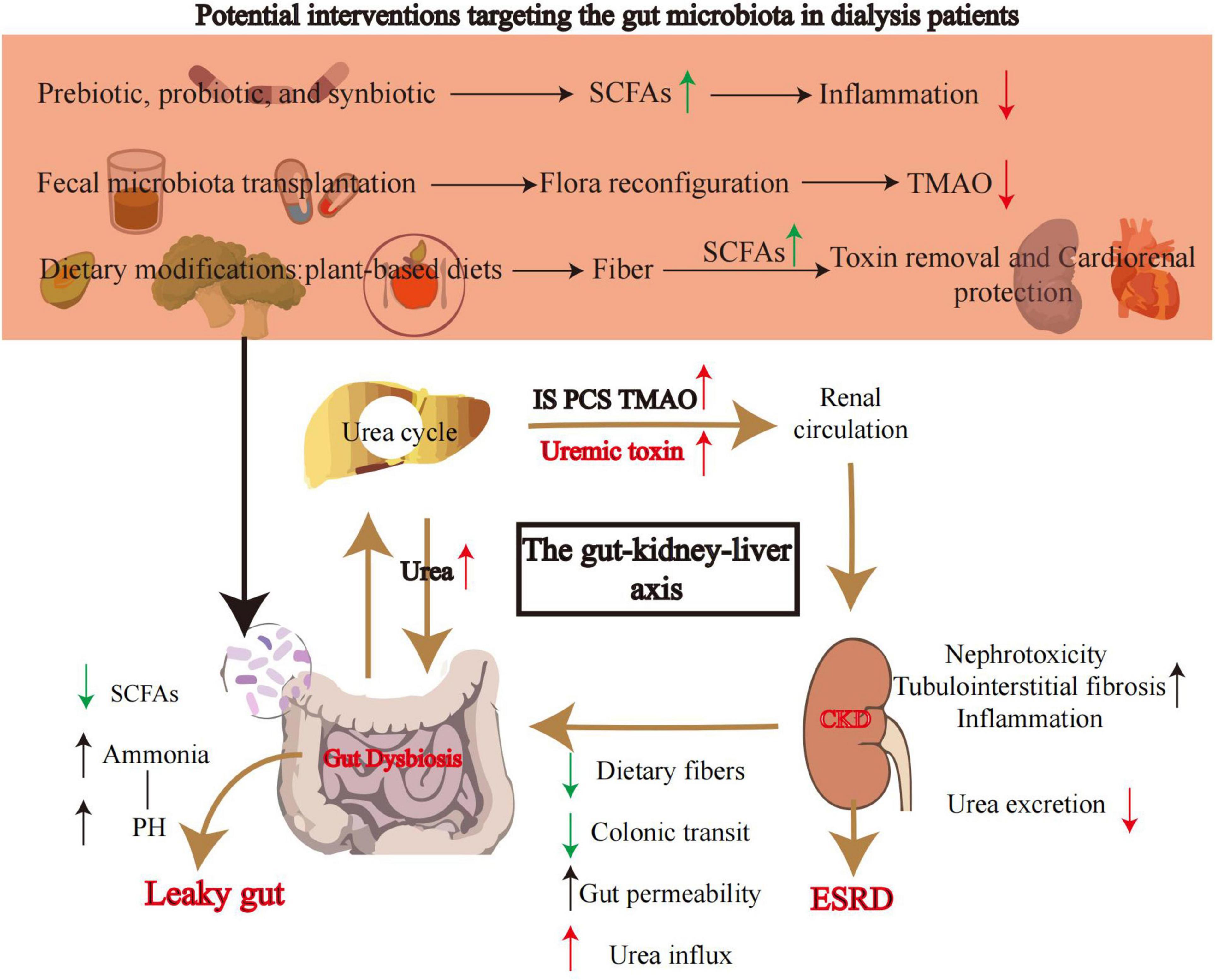
Figure 2. Core mechanisms of the gut-kidney axis and potential gut microbiota-targeted interventions. This diagram illustrates the core role of the gut microbiota in the progression of chronic kidney disease (CKD) to end-stage renal disease (ESRD) and the targeted gut microbiota intervention strategies. Prebiotics/probiotics/synbiotics: regulate microbial composition to promote the production of short-chain fatty acids (SCFAs). SCFAs enhance the intestinal mucosal barrier and suppress inflammatory factors (e.g., IL-6), reducing systemic microinflammation. Fecal microbiota transplantation (FMT): reconstructs the gut microbial ecosystem with healthy donor microbiota to inhibit TMAO-producing bacteria and reduce circulating TMAO levels. Plant-based diet (PBD): increases dietary fiber intake to promote SCFA production and reduce uremic toxin precursors, achieving dual benefits of “toxin clearance-cardiorenal protection.” Gut-kidney-liver axis mechanisms - urea and toxin accumulation: urea from hepatic metabolism enters the gut via renal circulation and is hydrolyzed into ammonia by bacterial urease (leading to increased gut pH). Uremic toxins [e.g., indoxyl sulfate (IS), p-cresyl sulfate (PCS), TMAO[accumulate due to reduced renal function. Intestinal leakage formation: in CKD patients, reduced dietary fiber, slowed colonic transit (constipation), and increased urea influx lead to decreased SCFA production and overgrowth of pathogenic bacteria (gut dysbiosis). Ammonia and toxins disrupt the intestinal mucosal barrier, increasing gut permeability (“intestinal leakage”) and exacerbating endotoxin translocation. Vicious cycle of renal injury: intestinal leakage causes endotoxin translocation into the circulatory system, triggering systemic inflammation (Inflammation). Circulating toxins (IS, PCS) induce tubulointerstitial fibrosis and nephrotoxic damage via the aryl hydrocarbon receptor (AhR) pathway. The interplay between inflammation and toxin accumulation ultimately accelerates the progression of CKD to ESRD. Arrow colors in the diagram - green arrows: positive regulation; red arrows: negative regulation. CKD, chronic kidney disease; ESRD, end-stage renal disease; SCFAs, short-chain fatty acids; IS, indoxyl sulfate; PCS, p-cresyl sulfate; TMAO, trimethylamine N-oxide.
8 Conclusion
Targeted interventions focusing on the gut microbiome offer a novel perspective for the precision management of end-stage renal disease (ESRD). Current research reveals that hemodialysis (HD) patients are characterized by enrichment of Firmicutes and Proteobacteria, while peritoneal dialysis (PD) patients exhibit reduced microbial diversity and increased pathogenic bacterial proliferation due to long-term exposure to high-glucose dialysate and iron supplementation. Probiotics, prebiotics, and plant-based diets demonstrate clinical potential by modulating short-chain fatty acid (SCFA) metabolism and suppressing pathogenic bacteria, though their efficacy is significantly influenced by host microbial profiles, intervention dosages, and kidney replacement therapy (KRT) modalities. Although fecal microbiota transplantation (FMT) has validated toxin clearance and metabolic improvements in animal models, its long-term safety and hard clinical endpoint benefits in immunocompromised ESRD populations still require large-scale clinical validation.
The core challenge in current research lies in the complexity of interactions among therapeutic interventions, host factors, and the microbiome. The dynamic impact of KRT on microbial communities has not been fully elucidated. Both HD and PD not only directly alter gut microbiota composition but may also trigger multi-organ pathological changes through mechanisms such as blood microbial translocation (HD) or peritoneal microbiome imbalance (PD). Future studies must adopt standardized microbial strain selection, larger sample sizes, and extended follow-up periods to clarify the clinical impact of prebiotics, probiotics, and synbiotics in ESRD patients. Simultaneously, it is imperative to reassess the rationale behind traditional dietary restrictions and explore synergistic effects between plant-based diets and microbiome-targeted therapies. In the era of personalized medicine, microbiota-specific research will undoubtedly occupy a central position. Overcoming the gap between mechanistic understanding and clinical translation will pave the way for individualized KRT management strategies, ultimately bringing profound benefits to ESRD patients.
Author contributions
QW: Writing – review and editing, Writing – original draft. YH: Writing – original draft, Data curation. LP: Writing – original draft, Methodology. ZZ: Writing – original draft, Methodology. LD: Writing – review and editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Thurlow JS, Joshi M, Yan G, Norris K, Agodoa L, Yuan C, et al. Global epidemiology of end-stage kidney disease and disparities in kidney replacement therapy. Am J Nephrol. (2021) 52:98–107. doi: 10.1159/000514550
2. Gupta R, Woo K, Yi J. Epidemiology of end-stage kidney disease. Semin Vasc Surg. (2021) 34:71–8. doi: 10.1053/j.semvascsurg.2021.02.010
3. Tandukar S, Palevsky P. Continuous renal replacement therapy: Who. When, Why, and How. Chest. (2019) 155:626–38. doi: 10.1016/j.chest.2018.09.004
4. Bello AK, Okpechi IG, Osman MA, Cho Y, Cullis B, Htay H, et al. Epidemiology of peritoneal dialysis outcomes. Nat Rev Nephrol. (2022) 18:779–93. doi: 10.1038/s41581-022-00623-7
5. Voora S, Adey DB. Management of kidney transplant recipients by general nephrologists: Core curriculum 2019. Am J Kidney Dis. (2019) 73:866–79. doi: 10.1053/j.ajkd.2019.01.031
6. Pantazi AC, Kassim MAK, Nori W, Tuta LA, Mihai CM, Chisnoiu T, et al. Clinical perspectives of gut microbiota in patients with chronic kidney disease and end-stage kidney disease: Where do we stand? Biomedicines. (2023) 11:2480. doi: 10.3390/biomedicines11092480
7. Tao P, Huo J, Chen L. Bibliometric analysis of the relationship between gut microbiota and chronic kidney disease from 2001–2022. Integr Med Nephrol Androl. (2024) 11:e00017. doi: 10.1097/imna-d-23-00017
8. Li XJ, Shan QY, Wu X, Miao H, Zhao YY. Gut microbiota regulates oxidative stress and inflammation: A double-edged sword in renal fibrosis. Cell Mol Life Sci. (2024) 81:480. doi: 10.1007/s00018-024-05532-5
9. Amini Khiabani S, Haghighat S, Tayebi Khosroshahi H, Asgharzadeh M, Samadi Kafil H. Diversity of bacteroidaceae family in gut microbiota of patients with chronic kidney disease and end stage renal disease. Health Promot Perspect. (2023) 13:237–42. doi: 10.34172/hpp.2023.29
10. Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. (2013) 83:1010–6. doi: 10.1038/ki.2012.440
12. Zaza G, Dalla Gassa A, Felis G, Granata S, Torriani S, Lupo A. Impact of maintenance immunosuppressive therapy on the fecal microbiome of renal transplant recipients: Comparison between an everolimus- and a standard tacrolimus-based regimen. PLoS One. (2017) 12:e0178228. doi: 10.1371/journal.pone.0178228
13. Khanna S, Tosh PK. A clinician’s primer on the role of the microbiome in human health and disease. Mayo Clin Proc. (2014) 89:107–14. doi: 10.1016/j.mayocp.2013.10.011
14. Nallu A, Sharma S, Ramezani A, Muralidharan J, Raj D. Gut microbiome in chronic kidney disease: Challenges and opportunities. Transl Res. (2017) 179:24–37. doi: 10.1016/j.trsl.2016.04.007
15. Ramezani A, Raj DS. The gut microbiome, kidney disease, and targeted interventions. J Am Soc Nephrol. (2014) 25:657–70. doi: 10.1681/asn.2013080905
16. Jandhyala S, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. (2015) 21:8787–803. doi: 10.3748/wjg.v21.i29.8787
17. Donaldson GP, Lee SM, Mazmanian SK. Gut biogeography of the bacterial microbiota. Nat Rev Microbiol. (2016) 14:20–32. doi: 10.1038/nrmicro3552
18. Cani PD. Human gut microbiome: Hopes, threats and promises. Gut. (2018) 67:1716–25. doi: 10.1136/gutjnl-2018-316723
19. Cummings JH, Macfarlane GT. Role of intestinal bacteria in nutrient metabolism. JPEN J Parenter Enteral Nutr. (1997) 21:357–65. doi: 10.1177/0148607197021006357
20. Layden BT, Angueira AR, Brodsky M, Durai V, Lowe WL Jr. Short chain fatty acids and their receptors: New metabolic targets. Transl Res. (2013) 161:131–40. doi: 10.1016/j.trsl.2012.10.007
21. Lanza M, Filippone A, Ardizzone A, Casili G, Paterniti I, Esposito E, et al. SCFA treatment alleviates pathological signs of migraine and related intestinal alterations in a mouse model of NTG-Induced migraine. Cells. (2021) 10:2756. doi: 10.3390/cells10102756
22. Valencia S, Zuluaga M, Florian Pérez MC, Montoya-Quintero KF, Candamil-Cortés MS, Robledo S. Human gut microbiome: A connecting organ between nutrition, metabolism, and health. Int J Mol Sci. (2025) 26:4112. doi: 10.3390/ijms26094112
23. D’Aimmo MR, Satti M, Scarafile D, Modesto M, Pascarelli S, Biagini SA, et al. Folate-producing bifidobacteria: Metabolism, genetics, and relevance. Microbiome Res Rep. (2024) 3:11. doi: 10.20517/mrr.2023.59
24. De Angelis M, Bottacini F, Fosso B, Kelleher P, Calasso M, Di Cagno R, et al. Lactobacillus rossiae, a vitamin B12 producer, represents a metabolically versatile species within the Genus Lactobacillus. PLoS One. (2014) 9:e107232. doi: 10.1371/journal.pone.0107232
25. Zhao M, Zhao J, Yang H, Ouyang Z, Lv C, Geng Z, et al. The bile acid-gut microbiota axis: A central hub for physiological regulation and a novel therapeutic target for metabolic diseases. Biomed Pharmacother. (2025) 188:118182. doi: 10.1016/j.biopha.2025.118182
26. Hossain KS, Amarasena S, Mayengbam SB. Vitamins and their roles in gut health. Microorganisms. (2022) 10:1168. doi: 10.3390/microorganisms10061168
27. Hevia A, Delgado S, Sánchez B, Margolles A. Molecular players involved in the interaction between beneficial bacteria and the immune system. Front Microbiol. (2015) 6:1285. doi: 10.3389/fmicb.2015.01285
28. Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. (2004) 118:229–41. doi: 10.1016/j.cell.2004.07.002
29. Cario E, Gerken G, Podolsky DK. Toll-like receptor 2 controls mucosal inflammation by regulating epithelial barrier function. Gastroenterology. (2007) 132:1359–74. doi: 10.1053/j.gastro.2007.02.056
30. Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO, et al. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. (2011) 300:G327–33. doi: 10.1152/ajpgi.00422.2010
31. Rosner MH, Reis T, Husain-Syed F, Vanholder R, Hutchison C, Stenvinkel P, et al. Classification of uremic toxins and their role in kidney failure. Clin J Am Soc Nephrol. (2021) 16:1918–28. doi: 10.2215/cjn.02660221
32. Leong SC, Sirich TL. Indoxyl sulfate-review of toxicity and therapeutic strategies. Toxins. (2016) 8:358. doi: 10.3390/toxins8120358
33. Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. (2014) 39:230–7. doi: 10.1159/000360010
34. Hand TW, Vujkovic-Cvijin I, Ridaura VK, Belkaid Y. Linking the microbiota, chronic disease, and the immune system. Trends Endocrinol Metab. (2016) 27:831–43. doi: 10.1016/j.tem.2016.08.003
35. Rysz J, Franczyk B, Ławiński J, Olszewski R, Ciałkowska-Rysz A, Gluba-Brzózka A. The Impact of CKD on uremic toxins and gut microbiota. Toxins. (2021) 13:252. doi: 10.3390/toxins13040252
36. Zhu W, Gregory JC, Org E, Buffa JA, Wang Z, et al. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. (2016) 165:111–24. doi: 10.1016/j.cell.2016.02.011
37. Stubbs JR, House JA, Ocque AJ, Zhang S, Johnson C, Kimber C, et al. Serum Trimethylamine-N-Oxide is elevated in CKD and correlates with coronary atherosclerosis burden. J Am Soc Nephrol. (2016) 27:305–13. doi: 10.1681/asn.2014111063
38. Shah SN, Knausenberger TB, Pontifex MG, Connell E, Le Gall G, Hardy TAJ. Cerebrovascular damage caused by the gut microbe/host co-metabolite p-cresol sulfate is prevented by blockade of the EGF receptor. Gut Microbes. (2024) 16:2431651. doi: 10.1080/19490976.2024.2431651
39. Quaglio AEV, Grillo TG, De Oliveira ECS, Di Stasi LC, Sassaki LY. Gut microbiota, inflammatory bowel disease and colorectal cancer. World J Gastroenterol. (2022) 28:4053–60. doi: 10.3748/wjg.v28.i30.4053
40. Wang L, Li L, Liu J, Sheng C, Yang M, Hu Z, et al. Associated factors and principal pathophysiological mechanisms of type 2 diabetes mellitus. Front Endocrinol (Lausanne). (2025) 16:1499565. doi: 10.3389/fendo.2025.1499565
41. Ray D, Bose P, Mukherjee S, Roy S, Kaity S. Recent drug delivery systems targeting the gut-brain-microbiome axis for the management of chronic diseases. Int J Pharm. (2025) 680:125776. doi: 10.1016/j.ijpharm.2025.125776
42. Glorieux G, Gryp T, Perna A. Gut-derived metabolites and their role in immune dysfunction in chronic kidney disease. Toxins. (2020) 12:245. doi: 10.3390/toxins12040245
43. Sánchez-Ospina D, Mas-Fontao S, Gracia-Iguacel C, Avello A, González de Rivera M, Mujika-Marticorena M, et al. Displacing the burden: A review of protein-bound uremic toxin clearance strategies in chronic kidney disease. J Clin Med. (2024) 13:1428. doi: 10.3390/jcm13051428
44. Xie H, Yang N, Lu L, Sun X, Li J, Wang X, et al. Uremic toxin receptor AhR facilitates renal senescence and fibrosis via suppressing mitochondrial biogenesis. Adv Sci. (2024) 11:e2402066. doi: 10.1002/advs.202402066
45. Cao G, Miao H, Wang YN, Chen DQ, Wu XQ, Chen L, et al. Intrarenal 1-methoxypyrene, an aryl hydrocarbon receptor agonist, mediates progressive tubulointerstitial fibrosis in mice. Acta Pharmacol Sin. (2022) 43:2929–45. doi: 10.1038/s41401-022-00914-6
46. Curran CS, Kopp JB. Aryl hydrocarbon receptor mechanisms affecting chronic kidney disease. Front Pharmacol. (2022) 13:782199. doi: 10.3389/fphar.2022.782199
47. Liu JR, Miao H, Deng DQ, Vaziri ND, Li P, Zhao YY. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol Life Sci. (2021) 78:909–22. doi: 10.1007/s00018-020-03645-1
48. Mo Y, Lu Z, Wang L, Ji C, Zou C, Liu X. The aryl hydrocarbon receptor in chronic kidney disease: Friend or foe? Front Cell Dev Biol. (2020) 8:589752. doi: 10.3389/fcell.2020.589752
49. Sun CY, Chang SC, Wu MS. Uremic toxins induce kidney fibrosis by activating intrarenal renin-angiotensin-aldosterone system associated epithelial-to-mesenchymal transition. PLoS One. (2012) 7:e34026. doi: 10.1371/journal.pone.0034026
50. Ramakrishna BS, Gee D, Weiss A, Pannall P, Roberts-Thomson IC, Roediger WE. Estimation of phenolic conjugation by colonic mucosa. J Clin Pathol. (1989) 42:620–3. doi: 10.1136/jcp.42.6.620
51. Poesen R, Evenepoel P, de Loor H, Kuypers D, Augustijns P, Meijers B. Metabolism, protein binding, and renal clearance of microbiota-derived p-Cresol in patients with CKD. Clin J Am Soc Nephrol. (2016) 11:1136–44. doi: 10.2215/cjn.00160116
52. Gryp T, Vanholder R, Vaneechoutte M, Glorieux G. p-Cresyl sulfate. Toxins. (2017) 9:3390. doi: 10.3390/toxins9020052
53. Liu WC, Tomino Y, Lu KC. Impacts of indoxyl sulfate and p-Cresol sulfate on chronic kidney disease and mitigating effects of AST-120. Toxins. (2018) 10:367. doi: 10.3390/toxins10090367
54. Han H, Zhu J, Zhu Z, Ni J, Du R, Dai Y, et al. p-Cresyl sulfate aggravates cardiac dysfunction associated with chronic kidney disease by enhancing apoptosis of cardiomyocytes. J Am Heart Assoc. (2015) 4:e001852. doi: 10.1161/jaha.115.001852
55. Lin CJ, Wu V, Wu PC, Wu CJ. Meta-analysis of the associations of p-Cresyl sulfate (PCS) and indoxyl sulfate (IS) with cardiovascular events and all-cause mortality in patients with chronic renal failure. PLoS One. (2015) 10:e0132589. doi: 10.1371/journal.pone.0132589
56. Gatarek P, Kaluzna-Czaplinska J. Trimethylamine N-oxide (TMAO) in human health. EXCLI J. (2021) 20:301–19. doi: 10.17179/excli2020-3239
57. Koeth RA, Wang Z, Levison BS, Buffa JA, Org E, Sheehy BT, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med. (2013) 19:576–85. doi: 10.1038/nm.3145
59. Vaziri ND, Goshtasbi N, Yuan J, Jellbauer S, Moradi H, Raffatellu M, et al. Uremic plasma impairs barrier function and depletes the tight junction protein constituents of intestinal epithelium. Am J Nephrol. (2012) 36:438–43. doi: 10.1159/000343886
60. Al-Sadi R, Boivin M, Ma T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front Biosci (Landmark Ed). (2009) 14:2765. doi: 10.2741/3413
61. Vaziri ND, Wong J, Pahl M, Piceno YM, Yuan J, DeSantis TZ, et al. Chronic kidney disease alters intestinal microbial flora. Kidney Int. (2013) 83:308–15. doi: 10.1038/ki.2012.345
62. Chen YY, Chen DQ, Chen L, Liu JR, Vaziri ND, Guo Y, et al. Microbiome-metabolome reveals the contribution of gut-kidney axis on kidney disease. J Transl Med. (2019) 17:5. doi: 10.1186/s12967-018-1756-4
63. Wang H, Huang X, Tan H, Chen X, Chen C, Nie S. Interaction between dietary fiber and bifidobacteria in promoting intestinal health. Food Chem. (2022) 393:133407. doi: 10.1016/j.foodchem.2022.133407
64. Mann ER, Lam YK, Uhlig HH. Short-chain fatty acids: Linking diet, the microbiome and immunity. Nat Rev Immunol. (2024) 24:577–95. doi: 10.1038/s41577-024-01014-8
65. Benus RF, van der Werf TS, Welling GW, Judd PA, Taylor MA, Harmsen HJ, et al. Association between Faecalibacterium prausnitzii and dietary fibre in colonic fermentation in healthy human subjects. Br J Nutr. (2010) 104:693–700. doi: 10.1017/s0007114510001030
66. Martin-Gallausiaux C, Marinelli L, Blottière H, Larraufie P, Lapaque NSCFA. mechanisms and functional importance in the gut. Proc Nutr Soc. (2021) 80:37–49. doi: 10.1017/s0029665120006916
67. Yu W, Sun S, Fu Q. The role of short-chain fatty acid in metabolic syndrome and its complications: Focusing on immunity and inflammation. Front Immunol. (2025) 16:1519925. doi: 10.3389/fimmu.2025.1519925
68. Wang F, Zhang P, Jiang H, Cheng S. Gut bacterial translocation contributes to microinflammation in experimental uremia. Dig Dis Sci. (2012) 57:2856–62. doi: 10.1007/s10620-012-2242-0
69. Larraufie P, Martin-Gallausiaux C, Lapaque N, Dore J, Gribble F, Reimann F, et al. SCFAs strongly stimulate PYY production in human enteroendocrine cells. Sci Rep. (2018) 8:74. doi: 10.1038/s41598-017-18259-0
70. Pluznick J. A novel SCFA receptor, the microbiota, and blood pressure regulation. Gut Microbes. (2014) 5:202–7. doi: 10.4161/gmic.27492
71. Zhang D, Jian YP, Zhang YN, Li Y, Gu LT, Sun HH, et al. Short-chain fatty acids in diseases. Cell Commun Signal. (2023) 21:212. doi: 10.1186/s12964-023-01219-9
72. Zhang ZH, Mao JR, Chen H, Su W, Zhang Y, Zhang L, et al. Removal of uremic retention products by hemodialysis is coupled with indiscriminate loss of vital metabolites. Clin Biochem. (2017) 50:1078–86. doi: 10.1016/j.clinbiochem.2017.09.012
73. Wu HY, Lin YT, Tsai WC, Chiu YL, Ko MJ, Yang JY, et al. Microbiota analysis in the hemodialysis population - Focusing on Enterobacteriaceae. J Microbiol Immunol Infect. (2023) 56:311–23. doi: 10.1016/j.jmii.2022.12.001
74. Chao YT, Lin YK, Chen LK, Huang P, Hsu YC. Role of the gut microbiota and their metabolites in hemodialysis patients. Int J Med Sci. (2023) 20:725–36. doi: 10.7150/ijms.82667
75. Crespo-Salgado J, Vehaskari VM, Stewart T, Ferris M, Zhang Q, Wang G, et al. Intestinal microbiota in pediatric patients with end stage renal disease: A Midwest Pediatric Nephrology Consortium study. Microbiome. (2016) 4:50. doi: 10.1186/s40168-016-0195-9
76. Koshida T, Gohda T, Sugimoto T, Asahara T, Asao R, Ohsawa I, et al. Gut microbiome and microbiome-derived metabolites in patients with end-stage kidney disease. Int J Mol Sci. (2023) 24:11456. doi: 10.3390/ijms241411456
77. Shi K, Wang F, Jiang H, Liu H, Wei M, Wang Z, et al. Gut bacterial translocation may aggravate microinflammation in hemodialysis patients. Dig Dis Sci. (2014) 59:2109–17. doi: 10.1007/s10620-014-3202-7
78. Mehrotra R, Devuyst O, Davies SJ, Johnson DW. The current state of peritoneal dialysis. J Am Soc Nephrol. (2016) 27:3238–52. doi: 10.1681/asn.2016010112
79. Bao M, Zhang P, Guo S, Zou J, Ji J, Ding X, et al. Altered gut microbiota and gut-derived p-cresyl sulfate serum levels in peritoneal dialysis patients. Front Cell Infect Microbiol. (2022) 12:639624. doi: 10.3389/fcimb.2022.639624
80. Gao B, Alonzo-Palma N, Brooks B, Jose A, Barupal D, Jagadeesan M, et al. A pilot study on the effect of prebiotic on host-microbial co-metabolism in peritoneal dialysis patients. Kidney Int Rep. (2020) 5:1309–15. doi: 10.1016/j.ekir.2020.05.023
81. Teixeira RR, de Andrade LS, Pereira NBF, Montenegro H, Hoffmann C, Cuppari L. Gut microbiota profile of patients on peritoneal dialysis: Comparison with household contacts. Eur J Clin Nutr. (2023) 77:90–7. doi: 10.1038/s41430-022-01190-7
82. Wang J, Wu S, Zhang J, Li Y, Wu Y, Qi X. Correlation between gut microbiome and cognitive impairment in patients undergoing peritoneal dialysis. BMC Nephrol. (2023) 24:360. doi: 10.1186/s12882-023-03410-z
83. Luo D, Zhao W, Lin Z, Wu J, Lin H, Li Y, et al. The effects of hemodialysis and peritoneal dialysis on the gut microbiota of end-stage renal disease patients, and the relationship between gut microbiota and patient prognoses. Front Cell Infect Microbiol. (2021) 11:579386. doi: 10.3389/fcimb.2021.579386
84. Wang IK, Lai HC, Yu CJ, Liang CC, Chang CT, Kuo HL, et al. Real-time PCR analysis of the intestinal microbiotas in peritoneal dialysis patients. Appl Environ Microbiol. (2012) 78:1107–12. doi: 10.1128/aem.05605-11
85. Ribeiro M, Fonseca L, Anjos JS, Capo-Chichi JCC, Borges NA, Burrowes J, et al. Oral iron supplementation in patients with chronic kidney disease: Can it be harmful to the gut microbiota? Nutr Clin Pract. (2022) 37:81–93. doi: 10.1002/ncp.10662
86. Szeto CC, Johnson DW. Low GDP solution and glucose-sparing strategies for peritoneal dialysis. Semin Nephrol. (2017) 37:30–42. doi: 10.1016/j.semnephrol.2016.10.005
87. Milan Manani S, Virzì GM, Giuliani A, Baretta M, Corradi V, De Cal M, et al. Lipopolysaccharide evaluation in peritoneal dialysis patients with peritonitis. Blood Purif. (2020) 49:434–9. doi: 10.1159/000505388
88. Li J, Xing H, Lin W, Yu H, Yang B, Jiang C, et al. Specific gut microbiome and metabolome changes in patients with continuous ambulatory peritoneal dialysis and comparison between patients with different dialysis vintages. Front Med (Lausanne). (2023) 10:1302352. doi: 10.3389/fmed.2023.1302352
89. Matsushita K, Ballew SH, Wang AY, Kalyesubula R, Schaeffner E, Agarwal R. Epidemiology and risk of cardiovascular disease in populations with chronic kidney disease. Nat Rev Nephrol. (2022) 18:696–707. doi: 10.1038/s41581-022-00616-6
90. Hill C, Guarner F, Reid G, Gibson GR, Merenstein DJ, Pot B, et al. Expert consensus document. The international scientific association for probiotics and prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat Rev Gastroenterol Hepatol. (2014) 11:506–14. doi: 10.1038/nrgastro.2014.66
91. Chilton SN, Burton JP, Reid G. Inclusion of fermented foods in food guides around the world. Nutrients. (2015) 7:390–404. doi: 10.3390/nu7010390
92. Kieffer DA, Piccolo BD, Vaziri ND, Liu S, Lau WL, Khazaeli M, et al. Resistant starch alters gut microbiome and metabolomic profiles concurrent with amelioration of chronic kidney disease in rats. Am J Physiol Renal Physiol. (2016) 310:F857–71. doi: 10.1152/ajprenal.00513.2015
93. Vaziri ND, Liu SM, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS One. (2014) 9:e114881. doi: 10.1371/journal.pone.0114881
94. Elamin S, Alkhawaja MJ, Bukhamsin AY, Idris MAS, Abdelrahman MM, Abutaleb NK, et al. Gum arabic reduces C-reactive protein in chronic kidney disease patients without affecting urea or indoxyl sulfate levels. Int J Nephrol. (2017) 2017:9501470. doi: 10.1155/2017/9501470
95. Rossi M, Johnson DW, Campbell KL. Pre-, pro-, and synbiotics: Do they have a role in reducing uremic toxins? A systematic review and meta-analysis. Int J Nephrol. (2012) 2012:673631. doi: 10.1155/2012/673631
96. Miao H, Liu F, Wang YN, Yu XY, Zhuang S, Guo Y, et al. Targeting Lactobacillus johnsonii to reverse chronic kidney disease. Signal Transduct Target Ther. (2024) 9:195. doi: 10.1038/s41392-024-01913-1
97. Miao H, Wang YN, Yu XY, Zou L, Guo Y, Su W, et al. Lactobacillus species ameliorate membranous nephropathy through inhibiting the aryl hydrocarbon receptor pathway via tryptophan-produced indole metabolites. Br J Pharmacol. (2024) 181:16219. doi: 10.1111/bph.16219
98. Rossi M, Johnson DW, Morrison M, Pascoe E, Coombes JS, Forbes JM, et al. SYNbiotics easing renal failure by improving Gut microbiologY (SYNERGY): A protocol of placebo-controlled randomised cross-over trial. BMC Nephrol. (2014) 15:106. doi: 10.1186/1471-2369-15-106
99. Pavan M. Influence of prebiotic and probiotic supplementation on the progression of chronic kidney disease. Minerva Urol Nefrol. (2016) 68:222–6.
100. McFarlane C, Krishnasamy R, Stanton T, Savill E, Snelson M, Mihala G, et al. Synbiotics easing renal failure by improving gut microbiology II (SYNERGY II): A feasibility randomized controlled trial. Nutrients. (2021) 13:4481. doi: 10.3390/nu13124481
101. Cosola C, Rocchetti MT, di Bari I, Acquaviva PM, Maranzano V, Corciulo S, et al. An innovative synbiotic formulation decreases free serum indoxyl sulfate, small intestine permeability and ameliorates gastrointestinal symptoms in a randomized pilot trial in stage IIIb-IV CKD patients. Toxins. (2021) 13:334. doi: 10.3390/toxins13050334
102. de Faria Barros A, Borges NA, Nakao LS, Dolenga CJ, do Carmo FL, de Carvalho Ferreira D, et al. Effects of probiotic supplementation on inflammatory biomarkers and uremic toxins in non-dialysis chronic kidney patients: A double-blind, randomized, placebo-controlled trial. J Funct Foods. (2018) 46:378–83. doi: 10.1016/j.jff.2018.05.018
103. Rossi M, Johnson DW, Morrison M, Pascoe E, Coombes JS, Forbes JM, et al. Synbiotics easing renal failure by improving gut microbiology (SYNERGY): A randomized trial. Clin J Am Soc Nephrol. (2016) 11:223–31. doi: 10.2215/cjn.05240515
104. Ikee R, Sasaki N, Yasuda T, Fukazawa S. Chronic kidney disease, gut dysbiosis, and constipation: A burdensome triplet. Microorganisms. (2020) 8:1862. doi: 10.3390/microorganisms8121862
105. Stepanova N. Probiotic interventions in peritoneal dialysis: A review of underlying mechanisms and therapeutic potentials. World J Nephrol. (2024) 13:98719. doi: 10.5527/wjn.v13.i4.98719
106. Meijers BK, De Preter V, Verbeke K, Vanrenterghem Y, Evenepoel P. p-Cresyl sulfate serum concentrations in haemodialysis patients are reduced by the prebiotic oligofructose-enriched inulin. Nephrol Dial Transplant. (2010) 25:219–24. doi: 10.1093/ndt/gfp414
107. Biruete A, Cross TL, Allen JM, Kistler BM, de Loor H, Evenepoel P, et al. Effect of dietary inulin supplementation on the gut microbiota composition and derived metabolites of individuals undergoing hemodialysis: A pilot study. J Ren Nutr. (2021) 31:512–22. doi: 10.1053/j.jrn.2020.10.003
108. He S, Xiong Q, Tian C, Li L, Zhao J, Lin X, et al. Inulin-type prebiotics reduce serum uric acid levels via gut microbiota modulation: A randomized, controlled crossover trial in peritoneal dialysis patients. Eur J Nutr. (2022) 61:665–77. doi: 10.1007/s00394-021-02669-y
109. Lopes R, Theodoro JMV, da Silva BP, Queiroz VAV, de Castro Moreira ME, Mantovani HC, et al. Synbiotic meal decreases uremic toxins in hemodialysis individuals: A placebo-controlled trial. Food Res Int. (2019) 116:241–8. doi: 10.1016/j.foodres.2018.08.024
110. Lydia A, Indra T, Rizka A, Abdullah M. The effects of synbiotics on indoxyl sulphate level, constipation, and quality of life associated with constipation in chronic haemodialysis patients: A randomized controlled trial. BMC Nephrol. (2022) 23:259. doi: 10.1186/s12882-022-02890-9
111. Sirich TL, Plummer NS, Gardner CD, Hostetter TH, Meyer TW. Effect of increasing dietary fiber on plasma levels of colon-derived solutes in hemodialysis patients. Clin J Am Soc Nephrol. (2014) 9:1603–10. doi: 10.2215/cjn.00490114
112. Esgalhado M, Kemp JA, Paiva BR, Brito JS, Cardozo L, Azevedo R, et al. Resistant starch type-2 enriched cookies modulate uremic toxins and inflammation in hemodialysis patients: A randomized, double-blind, crossover and placebo-controlled trial. Food Funct. (2020) 11:2617–25. doi: 10.1039/c9fo02939g
113. Taki K, Takayama F, Niwa T. Beneficial effects of Bifidobacteria in a gastroresistant seamless capsule on hyperhomocysteinemia in hemodialysis patients. J Ren Nutr. (2005) 15:77–80. doi: 10.1053/j.jrn.2004.09.028
114. Climent E, Hevilla F, Padial M, Barril-Cuadrado G, Blanca M, Jiménez-Salcedo T, et al. Psychobiotic protection of nutritional supplements and probiotics in patients undergoing hemodialysis: A randomized trial. Nutrients. (2025) 17:652. doi: 10.3390/nu17040652
115. Choi E, Yang J, Ji G, Park MS, Seong Y, Oh SW, et al. The effect of probiotic supplementation on systemic inflammation in dialysis patients. Kidney Res Clin Pract. (2022) 41:89–101. doi: 10.23876/j.krcp.21.014
116. Zhou S, Yan Y, Chu R, Chen N, Wang L, Zhang H, et al. Probiotic treatment induces changes in intestinal microbiota but does not alter SCFA levels in peritoneal dialysis patients-a randomized, placebo-controlled trial. Sci Rep. (2024) 14:31413. doi: 10.1038/s41598-024-83056-5
117. Borges NA, Carmo FL, Stockler-Pinto MB, de Brito JS, Dolenga CJ, Ferreira DC, et al. Probiotic supplementation in chronic kidney disease: A double-blind, randomized, placebo-controlled trial. J Ren Nutr. (2018) 28:28–36. doi: 10.1053/j.jrn.2017.06.010
118. Liu S, Liu H, Chen L, Liang S, Shi K, Meng W, et al. Effect of probiotics on the intestinal microbiota of hemodialysis patients: A randomized trial. Eur J Nutr. (2020) 59:3755–66. doi: 10.1007/s00394-020-02207-2
119. López-Yerena A, de Santisteban Villaplana V, Badimon L, Vilahur G, Padro T. Probiotics: A potential strategy for preventing and managing cardiovascular disease. Nutrients. (2024) 17:52. doi: 10.3390/nu17010052
120. Oniszczuk A, Oniszczuk T, Gancarz M, Szymańska J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules. (2021) 26:1172. doi: 10.3390/molecules26041172
121. Nakabayashi I, Nakamura M, Kawakami K, Ohta T, Kato I, Uchida K, et al. Effects of synbiotic treatment on serum level of p-cresol in haemodialysis patients: A preliminary study. Nephrol Dial Transplant. (2011) 26:1094–8. doi: 10.1093/ndt/gfq624
122. Mirzaeian S, Saraf-Bank S, Entezari MH, Hekmatdoost A, Feizi A, Atapour A. Effects of synbiotic supplementation on microbiota-derived protein-bound uremic toxins, systemic inflammation, and biochemical parameters in patients on hemodialysis: A double-blind, placebo-controlled, randomized clinical trial. Nutrition. (2020) 73:110713. doi: 10.1016/j.nut.2019.110713
123. Haghighat N, Mohammadshahi M, Shayanpour S, Haghighizadeh MH. Effects of synbiotics and probiotics supplementation on serum levels of endotoxin, heat shock protein 70 antibodies and inflammatory markers in hemodialysis patients: A randomized double-blinded controlled trial. Probiotics Antimicrob Proteins. (2020) 12:144–51. doi: 10.1007/s12602-018-9509-5
124. Wang IK, Wu YY, Yang YF, Ting IW, Lin CC, Yen TH, et al. The effect of probiotics on serum levels of cytokine and endotoxin in peritoneal dialysis patients: A randomised, double-blind, placebo-controlled trial. Benef Microbes. (2015) 6:423–30. doi: 10.3920/bm2014.0088
125. Yu Z, Zhao J, Qin Y, Wang Y, Zhang Y, Sun S. Probiotics, prebiotics, and synbiotics improve uremic, inflammatory, and gastrointestinal symptoms in end-stage renal disease with dialysis: A network meta-analysis of randomized controlled trials. Front Nutr. (2022) 9:850425. doi: 10.3389/fnut.2022.850425
126. Natarajan R, Pechenyak B, Vyas U, Ranganathan P, Weinberg A, Liang P, et al. Randomized controlled trial of strain-specific probiotic formulation (Renadyl) in dialysis patients. Biomed Res Int. (2014) 2014:568571. doi: 10.1155/2014/568571
127. Viramontes-Hörner D, Márquez-Sandoval F, Martín-del-Campo F, Vizmanos-Lamotte B, Sandoval-Rodríguez A, Armendáriz-Borunda J, et al. Effect of a symbiotic gel (Lactobacillus acidophilus + Bifidobacterium lactis + inulin) on presence and severity of gastrointestinal symptoms in hemodialysis patients. J Ren Nutr. (2015) 25:284–91. doi: 10.1053/j.jrn.2014.09.008
128. Shariaty Z, Mahmoodi Shan G, Farajollahi M, Amerian M, Behnam Pour N. The effects of probiotic supplement on hemoglobin in chronic renal failure patients under hemodialysis: A randomized clinical trial. J Res Med Sci. (2017) 22:74. doi: 10.4103/jrms.JRMS_614_16
129. Soleimani A, Zarrati Mojarrad M, Bahmani F, Taghizadeh M, Ramezani M, Tajabadi-Ebrahimi M, et al. Probiotic supplementation in diabetic hemodialysis patients has beneficial metabolic effects. Kidney Int. (2017) 91:435–42. doi: 10.1016/j.kint.2016.09.040
130. Eidi F, Poor-Reza Gholi F, Ostadrahimi A, Dalili N, Samadian F, Barzegari A. Effect of Lactobacillus Rhamnosus on serum uremic toxins (phenol and P-Cresol) in hemodialysis patients: A double blind randomized clinical trial. Clin Nutr ESPEN. (2018) 28:158–64. doi: 10.1016/j.clnesp.2018.08.010
131. Lim PS, Wang HF, Lee MC, Chiu LS, Wu MY, Chang WC, et al. The efficacy of lactobacillus-containing probiotic supplementation in hemodialysis patients: A randomized, double-blind, placebo-controlled trial. J Ren Nutr. (2021) 31:189–98. doi: 10.1053/j.jrn.2020.07.002
132. Soleimani A, Motamedzadeh A, Zarrati Mojarrad M, Bahmani F, Amirani E, Ostadmohammadi V, et al. The effects of synbiotic supplementation on metabolic status in diabetic patients undergoing hemodialysis: A randomized, double-blinded, placebo-controlled trial. Probiotics Antimicrob Proteins. (2019) 11:1248–56. doi: 10.1007/s12602-018-9499-3
133. Borges NA, Stenvinkel P, Bergman P, Qureshi AR, Lindholm B, Moraes C, et al. Effects of probiotic supplementation on trimethylamine-N-Oxide plasma levels in hemodialysis patients: A pilot study. Probiotics Antimicrob Proteins. (2019) 11:648–54. doi: 10.1007/s12602-018-9411-1
134. Pan Y, Yang L, Dai B, Lin B, Lin S, Lin E. Effects of probiotics on malnutrition and health-related quality of life in patients undergoing peritoneal dialysis: A randomized controlled trial. J Ren Nutr. (2021) 31:199–205. doi: 10.1053/j.jrn.2020.04.008
135. Li L, Xiong Q, Zhao J, Lin X, He S, Wu N, et al. Inulin-type fructan intervention restricts the increase in gut microbiome-generated indole in patients with peritoneal dialysis: A randomized crossover study. Am J Clin Nutr. (2020) 111:1087–99. doi: 10.1093/ajcn/nqz337
136. Xiong Q, Li L, Xiao Y, He S, Zhao J, Lin X, et al. The effect of inulin-type fructans on plasma trimethylamine N-oxide levels in peritoneal dialysis patients: A randomized crossover trial. Mol Nutr Food Res. (2023) 67:e2200531. doi: 10.1002/mnfr.202200531
137. de Andrade LS, Sardá FAH, Pereira NBF, Teixeira RR, Rodrigues SD, de Lima JD, et al. Effect of unripe banana flour on gut-derived uremic toxins in individuals undergoing peritoneal dialysis: A randomized, double-blind, placebo-controlled, crossover trial. Nutrients. (2021) 13:646. doi: 10.3390/nu13020646
138. Adair KE, Bowden RG. Ameliorating chronic kidney disease using a whole food plant-based diet. Nutrients. (2020) 12:1007. doi: 10.3390/nu12041007
139. Chassard C, Lacroix C. Carbohydrates and the human gut microbiota. Curr Opin Clin Nutr Metab Care. (2013) 16:453–60. doi: 10.1097/MCO.0b013e3283619e63
140. Birt DF, Boylston T, Hendrich S, Jane J, Hollis J, Li L, et al. Resistant starch: Promise for improving human health. Adv Nutr. (2013) 4:587–601. doi: 10.3945/an.113.004325
141. Huang W, Zhou L, Guo H, Xu Y, Xu Y. The role of short-chain fatty acids in kidney injury induced by gut-derived inflammatory response. Metabolism. (2017) 68:20–30. doi: 10.1016/j.metabol.2016.11.006
142. Huang X, Stenvinkel P, Qureshi AR, Risérus U, Cederholm T, Bárány P, et al. Essential polyunsaturated fatty acids, inflammation and mortality in dialysis patients. Nephrol Dial Transplant. (2012) 27:3615–20. doi: 10.1093/ndt/gfs132
143. Iqbal I, Wilairatana P, Saqib F, Nasir B, Wahid M, Latif M, et al. Plant polyphenols and their potential benefits on cardiovascular health: A review. Molecules. (2023) 28:6403. doi: 10.3390/molecules28176403
144. Passey C. Reducing the dietary acid load: How a more alkaline diet benefits patients with chronic kidney disease. J Ren Nutr. (2017) 27:151–60. doi: 10.1053/j.jrn.2016.11.006
145. Stanford J, Charlton K, Stefoska-Needham A, Zheng H, Bird L, Borst A, et al. Associations among plant-based diet quality, uremic toxins, and gut microbiota profile in adults undergoing hemodialysis therapy. J Ren Nutr. (2021) 31:177–88. doi: 10.1053/j.jrn.2020.07.008
146. Liu X, Hu Z, Xu X, Li Z, Chen Y, Dong J. The associations of plant-based protein intake with all-cause and cardiovascular mortality in patients on peritoneal dialysis. Nutr Metab Cardiovasc Dis. (2020) 30:967–76. doi: 10.1016/j.numecd.2020.03.003
147. Saglimbene VM, Wong G, Ruospo M, Palmer SC, Garcia-Larsen V, Natale P, et al. Fruit and vegetable intake and mortality in adults undergoing maintenance hemodialysis. Clin J Am Soc Nephrol. (2019) 14:250–60. doi: 10.2215/cjn.08580718
148. Erthal Leinig C, Pecoits-Filho R, Kunii L, Claro L, Merlin J, Almeida N, et al. Low-fiber intake is associated with high production of intraperitoneal inflammation biomarkers. J Ren Nutr. (2019) 29:322–7. doi: 10.1053/j.jrn.2018.10.013
149. Xu X, Li Z, Chen Y, Liu X, Dong J. Dietary fibre and mortality risk in patients on peritoneal dialysis. Br J Nutr. (2019) 122:996–1005. doi: 10.1017/s0007114519001764
150. Bernier-Jean A, Wong G, Saglimbene V, Ruospo M, Palmer SC, Natale P, et al. Dietary potassium intake and all-cause mortality in adults treated with hemodialysis. Clin J Am Soc Nephrol. (2021) 16:1851–61. doi: 10.2215/cjn.08360621
151. Pan J, Xu X, Wang Z, Ma T, Dong J. Dietary potassium and clinical outcomes among patients on peritoneal dialysis. Nutrients. (2023) 15:4271. doi: 10.3390/nu15194271
152. Bardhi O, Clegg DJ, Palmer B. The role of dietary potassium in the cardiovascular protective effects of plant-based diets. Semin Nephrol. (2023) 43:151406. doi: 10.1016/j.semnephrol.2023.151406
153. Xie LM, Ge YY, Huang X, Zhang YQ, Li JX. Effects of fermentable dietary fiber supplementation on oxidative and inflammatory status in hemodialysis patients. Int J Clin Exp Med. (2015) 8:1363–9.
154. Barba C, Soulage C, Caggiano G, Glorieux G, Fouque D, Koppe L. Effects of fecal microbiota transplantation on composition in mice with CKD. Toxins. (2020) 12:741. doi: 10.3390/toxins12120741
155. Li Y, Su X, Gao Y, Lv C, Gao Z, Liu Y, et al. The potential role of the gut microbiota in modulating renal function in experimental diabetic nephropathy murine models established in same environment. Biochim Biophys Acta Mol Basis Dis. (2020) 1866:165764. doi: 10.1016/j.bbadis.2020.165764
156. Du D, Tang W, Zhou C, Sun X, Wei Z, Zhong J, et al. Fecal microbiota transplantation is a promising method to restore gut microbiota dysbiosis and relieve neurological deficits after traumatic brain injury. Oxid Med Cell Longev. (2021) 2021:5816837. doi: 10.1155/2021/5816837
157. Bastos RMC, Simplício-Filho A, Sávio-Silva C, Oliveira LFV, Cruz GNF, Sousa EH, et al. Fecal microbiota transplant in a pre-clinical model of type 2 diabetes mellitus, obesity and diabetic kidney disease. Int J Mol Sci. (2022) 23:3842. doi: 10.3390/ijms23073842
158. Wang X, Yang S, Li S, Zhao L, Hao Y, Qin J, et al. Aberrant gut microbiota alters host metabolome and impacts renal failure in humans and rodents. Gut. (2020) 69:2131–42. doi: 10.1136/gutjnl-2019-319766
159. Lu J, Chen PP, Zhang JX, Li XQ, Wang GH, Yuan BY, et al. GPR43 deficiency protects against podocyte insulin resistance in diabetic nephropathy through the restoration of AMPKα activity. Theranostics. (2021) 11:4728–42. doi: 10.7150/thno.56598
160. Zhao J, Bai M, Yang X, Wang Y, Li R, Sun S. Alleviation of refractory IgA nephropathy by intensive fecal microbiota transplantation: The first case reports. Ren Fail. (2021) 43:928–33. doi: 10.1080/0886022x.2021.1936038
161. Zhou G, Zeng J, Peng L, Wang L, Zheng W, Di W, et al. Fecal microbiota transplantation for membranous nephropathy. CEN Case Rep. (2021) 10:261–4. doi: 10.1007/s13730-020-00560-z
162. Marasco G, Cannarile DC, Cremon C, Papalia G, Marangoni A, Zucchelli A, et al. Fecal microbiota transplantation for Clostridioides difficile infection in a peritoneal dialysis patient: A case report. Perit Dial Int. (2025). doi: 10.1177/08968608251316165 [Online ahead of print].
163. Arteaga-Muller GY, Flores-Treviño S, Bocanegra-Ibarias P, Robles-Espino D, Garza-González E, Fabela-Valdez GC, et al. Changes in the progression of chronic kidney disease in patients undergoing fecal microbiota transplantation. Nutrients. (2024) 16:1109. doi: 10.3390/nu16081109
164. Takkavatakarn K, Wuttiputinun T, Phannajit J, Praditpornsilpa K, Eiam-Ong S, Susantitaphong P. Protein-bound uremic toxin lowering strategies in chronic kidney disease: A systematic review and meta-analysis. J Nephrol. (2021) 34:1805–17. doi: 10.1007/s40620-020-00955-2
165. Wu CC, Tian YC, Lu CL, Wu MJ, Lim PS, Chiu YW, et al. AST-120 improved uremic pruritus by lowering indoxyl sulfate and inflammatory cytokines in hemodialysis patients. Aging. (2024) 16:4236–49. doi: 10.18632/aging.205580
166. Kweon T, Kee YK, Shin DH, Oh J, Jeon HJ. Impact of using oral spherical carbon adsorbent in predialysis chronic kidney disease period on cardiovascular outcome after dialysis therapy. Sci Rep. (2025) 15:6315. doi: 10.1038/s41598-025-90779-6
167. De la Cruz EN, Cerrillos-Gutiérrez JI, García-Sánchez A, Prado-Nevárez CG, Andrade-Sierra J, Jalomo-Martínez B, et al. The influence of sevelamer hydrochloride and calcium carbonate on markers of inflammation and oxidative stress in hemodialysis at six months of follow-up. Front Med (Lausanne). (2021) 8:714205. doi: 10.3389/fmed.2021.714205
168. Zeng Q, Zhong Y, Yu X. Meta-analysis of the efficacy and safety of sevelamer as hyperphosphatemia therapy for hemodialysis patients. Ren Fail. (2023) 45:2210230. doi: 10.1080/0886022x.2023.2210230
169. Chu C, Behera TR, Huang Y, Qiu W, Chen J, Shen Q. Research progress of gut microbiome and diabetic nephropathy. Front Med (Lausanne). (2024) 11:1490314. doi: 10.3389/fmed.2024.1490314
170. Wang X, Long D, Peng X, Li J, Zhou M, Wang Y, et al. Diphenyl diselenide protects against diabetic kidney disease through modulating gut microbiota dysbiosis in streptozotocin-induced diabetic rats. Front Pharmacol. (2024) 15:1506398. doi: 10.3389/fphar.2024.1506398
171. Zheng L, Luo M, Zhou H, Chen J. Natural products from plants and microorganisms: Novel therapeutics for chronic kidney disease via gut microbiota regulation. Front Pharmacol. (2022) 13:1068613. doi: 10.3389/fphar.2022.1068613
172. Zhao H, Zhao T, Li P. Gut microbiota-derived metabolites: A new perspective of traditional chinese medicine against diabetic kidney disease. Integr Med Nephrol Androl. (2024) 11:e23–4. doi: 10.1097/imna-d-23-00024
173. Li L, Yang YN, Cao Y, Zhan J, Wu Y, Wu C. Perspective on the modern interpretation of the property theory of mild-natured and sweet-flavored traditional chinese medicine via gut microbiota modulation. Integr Med Nephrol Androl. (2023) 10:e00012. doi: 10.1097/imna-d-23-00012
174. Wang Y, Li K, Yan S, Li G, Cheng M, Chen Q, et al. Clerodendranthus spicatus [Orthosiphon aristatus (Blume) Miq.] maintains uric acid homeostasis via regulating gut microbiota and restrains renal inflammation in hyperuricemic nephropathy. Front Pharmacol. (2024) 15:1485861. doi: 10.3389/fphar.2024.1485861
175. Lin W, Jiang C, Yu H, Wang L, Li J, Liu X, et al. The effects of fushen granule on the composition and function of the gut microbiota during peritoneal dialysis-related peritonitis. Phytomedicine. (2021) 86:153561. doi: 10.1016/j.phymed.2021.153561
176. Salarolli RT, Alvarenga L, Cardozo L, Teixeira KTR, de SGML, Lima JD, et al. Can curcumin supplementation reduce plasma levels of gut-derived uremic toxins in hemodialysis patients? A pilot randomized, double-blind, controlled study. Int Urol Nephrol. (2021) 53:1231–8. doi: 10.1007/s11255-020-02760-z
177. Ji C, Deng Y, Yang A, Lu Z, Chen Y, Liu X, et al. Rhubarb enema improved colon mucosal barrier injury in 5/6 nephrectomy rats may associate with gut microbiota modification. Front Pharmacol. (2020) 11:1092. doi: 10.3389/fphar.2020.01092
Keywords: end-stage renal disease, gut microbial metabolites, plant-based diet, probiotics, fecal microbiota transplantation
Citation: Wang Q, Han Y, Pang L, Zhou Z and Dai L (2025) Gut microbiome remodeling in chronic kidney disease: implications of kidney replacement therapies and therapeutic interventions. Front. Med. 12:1620247. doi: 10.3389/fmed.2025.1620247
Received: 29 April 2025; Accepted: 09 June 2025;
Published: 15 July 2025.
Edited by:
Ying-Yong Zhao, Northwest University, ChinaReviewed by:
Hailing Zhao, China-Japan Friendship Hospital, ChinaHsiao-Wen Huang, Synbio Tech Inc, Taiwan
Copyright © 2025 Wang, Han, Pang, Zhou and Dai. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijuan Dai, NzY0ODg2MUBxcS5jb20=
 Qianwei Wang
Qianwei Wang Yucheng Han1
Yucheng Han1 Liang Pang
Liang Pang