Abstract
Introduction:
Current evidence from randomized controlled trials (RCTs) supports the anti-osteoporotic properties of Chinese Herbal Medicine (CHM); however, its therapeutic advantages over conventional treatments remain inconclusive. This study aimed to compare the therapeutic effects of CHM with those of conventional therapy in patients with osteoporosis, using a meta-analysis approach.
Methods:
A systematic search of PubMed, Embase, Cochrane Library, CNKI, and Wanfang databases was conducted through March 2025 to identify eligible RCTs. The weighted mean difference (WMD) and 95% confidence intervals (CI) were used as effect estimates, with pooled analyses calculated using a random-effects model. Additional exploratory analyses included sensitivity and subgroup analyses.
Results:
Eighteen RCTs involving a total of 1,816 patients with osteoporosis were included in the meta-analysis. CHM was associated with increased bone mineral density (BMD) at the lumbar spine (WMD: 0.09; 95% CI: 0.04 to 0.13; p < 0.001), femoral neck (WMD: 0.09; 95% CI: 0.02 to 0.17; p = 0.015), and Ward’s triangle area (WMD: 0.08; 95% CI: 0.01 to 0.15; p = 0.025). However, CHM showed no significant effect on BMD at the greater trochanter of the femur (WMD: 0.01; 95% CI: −0.03 to 0.05; p = 0.698). Additionally, CHM was not associated with changes in alkaline phosphatase (WMD: 0.98; 95% CI: −6.88 to 8.83; p = 0.808), serum calcium (WMD: 0.08; 95% CI: −0.09 to 0.25; p = 0.372), or serum phosphorus (WMD: -0.05; 95% CI: −0.22 to 0.12; p = 0.574).
Conclusion:
Chinese Herbal Medicine was associated with significant improvements in BMD at the lumbar spine, femoral neck, and Ward’s triangle area compared to conventional therapies, though the evidence is limited by moderate study quality and high heterogeneity. The findings suggest potential benefits of CHM in specific skeletal sites, but further rigorous trials are needed to confirm efficacy.
Systematic review registration:
INPLASY platform (number: INPLASY202530115).
Introduction
Osteoporosis is a systemic skeletal disorder characterized by bone loss and deterioration of bone microarchitecture, resulting in increased fragility and a heightened risk of fractures (1). Data indicate that approximately 21.7% of the domestic population meets the World Health Organization (WHO) diagnostic criteria for osteoporosis. The prevalence reaches 35.3% among postmenopausal women and 12.5% among men over 65 years of age (2). WHO has ranked osteoporosis as the second-leading cause of disability-adjusted life year (DALY) loss worldwide, following only cardiovascular diseases (3).
As a typical age-related condition, osteoporosis incidence increases exponentially with age: cross-sectional studies show a prevalence of 24.1% among individuals aged 50–64, rising to 51.8% in those over 80 (4). Patients with osteoporosis face a 3.2–6.1-fold increased risk of hip fractures (5). With the global population aging, osteoporotic fragility fractures have become a significant public health issue, with 8.9 million cases occurring worldwide each year. Among these, the 1-year mortality rate following a hip fracture is as high as 20–24%, and the disability rate exceeds 40%, imposing a considerable economic burden (6).
A critical concern is the significant gap between diagnosis and treatment: fewer than 30% of high-risk individuals are screened, and only 18.5% of patients receive standardized anti-osteoporosis therapy within 12 months following a fracture. This paradox of high disease incidence and low intervention rates severely hampers the effectiveness of osteoporosis prevention and control systems (7, 8).
Clinical management of osteoporosis adheres to a hierarchical treatment strategy, with pharmacological intervention at its core. According to the “World Health Organization Guidelines for the Prevention and Treatment of Osteoporosis,” standardized drug therapy can increase lumbar spine bone mineral density (BMD) by 6–8% and reduce the relative risk of fragility fractures by 40–70% (9). Current medications are categorized by their mechanisms of action into bone resorption inhibitors (e.g., bisphosphonates, RANKL monoclonal antibodies, estrogen modulators), bone formation promoters (e.g., parathyroid hormone analogs), dual-action agents (e.g., romosozumab), and others with alternative mechanisms (e.g., vitamin K2) (10).
Despite their efficacy in reducing vertebral fracture risk, safety concerns surrounding these medications have grown. Data from the U.S. Food and Drug Administration’s Adverse Event Reporting System (FAERS) show that 23.7% of patients discontinue treatment due to adverse drug reactions (11). This underscores the need for safe, effective, and reliable alternatives in the management of osteoporosis.
Chinese Herbal Medicine (CHM), with its multi-component and synergistic therapeutic properties, has been used clinically since the era of the Huangdi Neijing (Inner Canon of Huangdi). CHM is grounded in the traditional principle of “tonifying the kidney and strengthening the bones” to treat bone metabolism disorders. Modern clinical applications of CHM include single-herb extracts, compound formulations, and sequential combinations with bisphosphonates. However, despite numerous clinical trials investigating the anti-osteoporotic effects of CHM, many studies fall short of the Consolidated Standards of Reporting Trials (CONSORT) criteria.
While previous research has primarily focused on changes in BMD to assess CHM efficacy, data regarding its effects on bone turnover markers remain limited (12). Therefore, we conducted this systematic review and meta-analysis to comprehensively evaluate the therapeutic effectiveness of CHM in the treatment of patients with osteoporosis.
Methods
Data sources, search strategy, and selection criteria
This meta-analysis was conducted in full compliance with the 2020 Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (13). Our study was registered in INPLASY platform (number: INPLASY202530115). As all data were derived from previously published studies, ethical approval and review were not required for this research.
A comprehensive literature search was performed using PubMed, Embase, Cochrane Library, China National Knowledge Infrastructure (CNKI), and Wanfang databases through March 2025. The search strategy incorporated both subject headings and free-text terms. Keywords included combinations of “Chinese herbal medicine,” “Herbal therapy,” “Traditional Chinese medicine,” “Osteoporosis,” and “Fracture” (see Supplementary File 1). Randomized controlled trials (RCTs) evaluating the effects of CHM on osteoporosis or related fractures were systematically identified. No restrictions were placed on the language of publication. Despite searching international databases, all RCTs evaluating CHM for osteoporosis were conducted in China, likely reflecting the regional focus of such research. To ensure thoroughness, reference lists of included articles were also manually reviewed to capture additional relevant studies not found during the initial database search.
A double-blind, independent screening process was employed. Two researchers independently reviewed study titles, abstracts, and full texts in a stepwise manner. Any discrepancies between reviewers were resolved by a third researcher, who served as an arbitrator to reach a consensus.
Inclusion criteria were established according to the PICOS framework:
-
Participants: Patients diagnosed with osteoporosis based on dual-energy X-ray absorptiometry (DEXA) and meeting the World Health Organization (WHO) criteria (T-score ≤ − 2.5) (14).
-
Interventions: The experimental group received a systematic traditional Chinese medicine regimen.
-
Comparators: The control group received standard anti-osteoporosis pharmacologic therapies or injectable treatments.
-
Outcomes: Primary outcomes included changes in BMD and bone metabolism markers.
-
Study Design: Only RCTs utilizing standardized randomization techniques (e.g., computer-generated random sequences or random number tables) were included.
Data collection and quality assessment
Two authors independently extracted data from each study. Extracted variables included: the first author’s surname, year of publication, study location, sample size, proportion of male participants, mean patient age, clinical condition, intervention protocol, control treatment, reported outcomes, and follow-up duration. Following data extraction, both authors independently evaluated the methodological quality of each study using the Cochrane Risk of Bias Tool. Assessment domains included: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential sources of bias (15). Any inconsistencies in data extraction or quality ratings were resolved through discussion with a third reviewer, who made final determinations based on the original study texts.
Statistical analysis
Therapeutic outcomes of CHM were treated as continuous variables, with effect sizes expressed as weighted mean differences (WMDs) and corresponding 95% confidence intervals (CIs). Meta-analyses were conducted using a random-effects model to account for heterogeneity across studies (16, 17). Heterogeneity was assessed using the I2 statistic and Cochran’s Q test, with significant heterogeneity defined as I2 > 50% or a Q-test p-value < 0.10 (18, 19). Robustness of the results was evaluated via leave-one-out sensitivity analysis, in which each study was sequentially excluded to assess its impact on the overall effect size (20). Meta-regression were performed to identify potential source of heterogeneity on the basis of publication year, proportion of male participants, average patient age, clinical disease status, follow-up, and baseline BMD. Then subgroup analyses were performed and the differences between subgroups were tested using interaction t-tests, assuming normal distribution of data (21). Potential publication bias was assessed visually using funnel plot asymmetry and statistically using Egger’s linear regression test and Begg’s rank correlation test (22, 23). All analyses were conducted using STATA version 12.0 (StataCorp LLC, College Station, TX, USA), following Cochrane Collaboration guidelines for conducting and reporting meta-analyses. Two-tailed p-values were reported, with statistical significance set at α = 0.05.
Results
Literature search
A total of 2,240 relevant studies were initially identified through a comprehensive database search. After removing 868 duplicates using reference management software, 1,372 records remained for preliminary screening. Following title and abstract screening, 1,309 studies that did not meet the predefined eligibility criteria were excluded. The remaining 63 articles were subjected to full-text evaluation.
Through a double-blind, independent full-text review by two researchers, 18 studies were confirmed to meet the PICOS inclusion criteria and were included in the quantitative synthesis (24–41). To enhance the comprehensiveness of the literature coverage, reference lists of the included studies were manually searched. Two additional potentially eligible studies were identified. However, after duplicate verification using the CrossCheck database, both were confirmed to have already been included in the initial retrieval and were excluded due to redundancy. The final analysis included 18 studies (Figure 1).
Study characteristics
Table 1 summarizes the basic characteristics of the included RCTs and the demographic details of the study populations. Across the 18 studies, a total of 1,816 patients diagnosed with osteoporosis were included. The median sample size of single-center studies was 98 participants (range: 62–140). Gender distribution was as follows: one study (5.6%) included only male patients, five studies (27.8%) enrolled only postmenopausal women, and 12 studies (66.6%) included mixed-gender cohorts. Regarding follow-up duration, six studies (33.3%) conducted short-term follow-up of 3 months, 11 studies (61.1%) had a 6-month follow-up, and one study (5.6%) reported outcomes at multiple time points (12 and 24 months).
Table 1
| Study | Country | Sample size | Male (%) | Age (years) | Baseline BMD (g/cm2) | Disease status | Intervention | Control | Follow-up (months) |
|---|---|---|---|---|---|---|---|---|---|
| Xie 1997 (24) | China | 80 (50/30) | 51.3 | 68.1 | Lumbar spine: 0.968; Femoral neck: 0.795; Greater trochanter of the femur: 0.647; Ward’s triangle area: 0.601 | Primary osteoporosis with kidney-yang deficiency syndrome | Bugushengsui capsules | Vitamin D and calcium tablet | 6.0 |
| Shi 2001 (25) | China | 140 (80/60) | 37.1 | 62.3 | Lumbar spine: 0.72 | Primary osteoporosis with fracture | Bushenjiangu decoction | Calcium tablet | 3.0 |
| Lv 2002 (26) | China | 90 (48/42) | 42.2 | 65.1 | Lumbar spine: 0.66 | Primary osteoporosis | Fortified Bushenzhuangjin decoction | Active calcium cranules | 3.0 |
| Wang 2005 (27) | China | 100 (50/50) | 39.0 | 63.1 | Lumbar spine: 0.67 | Primary osteoporosis | Bushenzhuanggu capsules | Calcium tablet | 3.0 |
| Ke 2005 (28) | China | 90 (46/44) | 100.0 | 68.1 | Lumbar spine: 0.47 | Primary osteoporosis with kidney- deficiency | Bone strengthening formula | Alendronate sodium | 6.0 |
| Pan 2006 (29) | China | 120 (60/60) | 0.0 | 48.6 | Lumbar spine: 0.751 | Post-menopause osteoporosis | Tonifying the kidney and replenishing essence | Alendronate sodium | 6.0 |
| Luo 2008 (30) | China | 215 (108/107) | 37.7 | 65.9 | Lumbar spine: 0.925; Femoral neck: 0.678; Greater trochanter of the femur: 0.668; Ward’s triangle area: 0.693 | Primary osteoporosis | Strong bone granules | Calcium Gluconate | 6.0 |
| Li 2009 (31) | China | 93 (47/46) | 0.0 | 67.4 | Lumbar spine: 0.290 | Post-menopause osteoporosis | Yiguyin | Vitamin D and calcium tablet | 6.0 |
| Ouyang 2012 (32) | China | 82 (42/40) | 32.9 | 46.4 | Lumbar spine: 0.805; Femoral neck: 0.725 | Osteoporosis induced from rheumatoid arthritis | Qianggu capsules and DMARD | DMARD | 6.0 |
| Ma 2012 (33) | China | 62 (31/31) | NA | NA | Lumbar spine: 0.900; Femoral neck: 0.713; Greater trochanter of the femur: 0.576; Ward’s triangle area: 0.767 | Osteoporosis | Bugu capsule and Xianlinggubao | Elcatonin and alfacalcidol | 12.0 |
| Li 2013 (34) | China | 80 (40/40) | 0.0 | 59.5 | NA | Post-menopause osteoporosis | Bushenhuoxue granules | Vitamin D and calcium tablet | 6.0 |
| Zhang 2013 (35) | China | 80 (41/39) | 0.0 | 66.4 | Lumbar spine: 0.541 | Post-menopause osteoporosis | Bushenzhuanggu decoction | Salmon Calcitonin | 6.0 |
| Liang 2014 (36) | China | 100 (50/50) | 47.0 | 62.0 | Lumbar spine: 0.805 | Osteoporosis | Jiangu Bushen decoction | Caltrate D tablets | 3.0 |
| Ma 2014 (37) | China | 90 (45/45) | 54.4 | 63.1 | Lumbar spine: 0.52 | Osteoporosis | Buzhongyiqi decoction | Salmon Calcitonin | 3.0 |
| Hu 2020 (38) | China | 66 (33/33) | 31.1 | 45.8 | Lumbar spine: 0.716; Femoral neck: 0.714 | Glucocorticoid-induced osteoporosis | Nourishing liver and kidney decoction | Alfacalcidol soft capsules and calcium supplement with vitamin D | 6.0 |
| Gong 2020 (39) | China | 106 (53/53) | 32.1 | 65.3 | Lumbar spine: 0.39 | Primary osteoporosis | Consolidating origin and improving bone formula | Calcium carbonate D3 chewable tablet and calcitriol capsules | 6.0 |
| Chen 2023 (40) | China | 120 (60/60) | 0.0 | 67.9 | Lumbar spine: 0.705; Femoral neck: 0.62; Ward’s triangle area: 0.725 | Post-menopause osteoporosis | Bushenzhuanggu tablet | Calcium and vitamin D | 6.0 |
| Zhang 2024 (41) | China | 102 (51/51) | 31.4 | 78.0 | Lumbar spine: 0.655; Femoral neck: 0.526 | Osteoporosis | Yishengushu formula | Alendronate sodium and Calcium Carbonate and Vitamin D3 Granules | 3.0 |
The baseline characteristics of identified trials and involved patients.
Methodological quality, assessed using the Cochrane Risk of Bias Tool, indicated that the overall quality of the included studies was moderate (Table 2). The primary limitations involved allocation concealment and implementation of blinding procedures.
Table 2
| Study | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Xie 1997 (24) | Low | Unclear | Unclear | Low | Low | High | Unclear |
| Shi 2001 (25) | Low | Unclear | Unclear | Low | Low | High | Unclear |
| Lv 2002 (26) | Low | Unclear | Unclear | Low | Low | High | Unclear |
| Wang 2005 (27) | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Ke 2005 (28) | Low | Unclear | Unclear | Low | Low | High | Unclear |
| Pan 2006 (29) | Low | Unclear | Unclear | Low | Low | High | Unclear |
| Luo 2008 (30) | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Li 2009 (31) | Low | Unclear | Unclear | Low | Low | High | Unclear |
| Ouyang 2012 (32) | Low | Unclear | Unclear | Low | Low | High | Unclear |
| Ma 2012 (33) | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Li 2013 (34) | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Zhang 2013 (35) | Low | Unclear | Unclear | Low | Low | High | Unclear |
| Liang 2014 (36) | Low | Unclear | Unclear | Low | Low | High | Unclear |
| Ma 2014 (37) | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Hu 2020 (38) | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Gong 2020 (39) | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Chen 2023 (40) | Low | Unclear | Low | Low | Low | Unclear | Unclear |
| Zhang 2024 (41) | Low | Unclear | Low | Low | Low | Unclear | Unclear |
The methodological quality assessment of included trials.
Figure 1
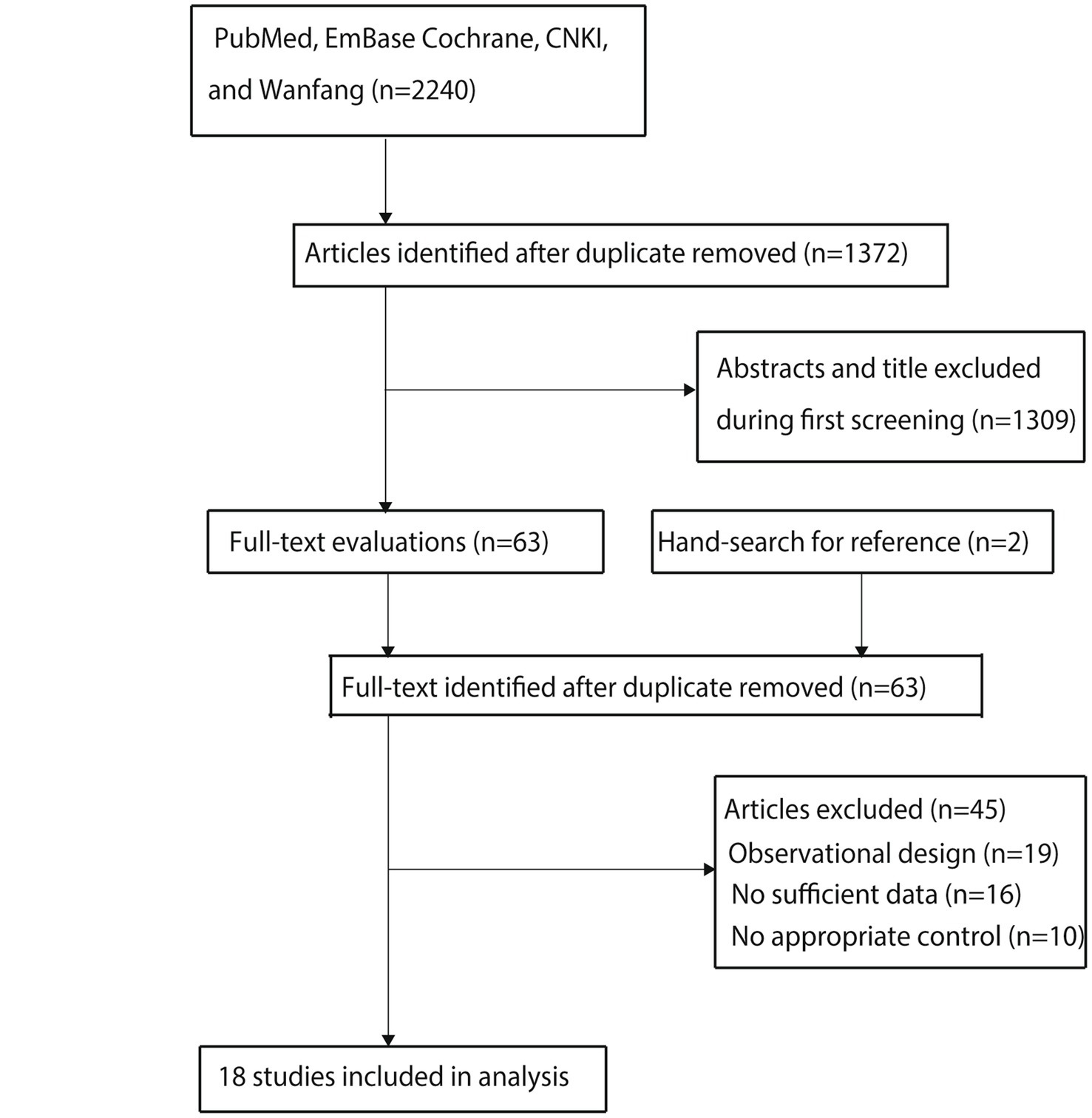
Flow diagram of the literature search and study selection process.
BMD at various sites
Pooled analysis of all included trials showed that CHM was associated with increased BMD at the lumbar spine compared to conventional therapy (WMD: 0.09; 95% CI: 0.04 to 0.13; p < 0.001; Figure 2). However, there was significant heterogeneity among studies (I2 = 97.0%; p < 0.001). Sensitivity analysis indicated the overall result remained stable when each study was sequentially removed (Supplementary File 2). Meta-regression found publication year, proportion of male participants, average patient age, follow-up, and baseline BMD were not significant factors contributing to the association between CHM and BMD at the lumbar spine, while clinical disease status that contributed to the association between CHM and BMD at the lumbar spine (p = 0.021) (Supplementary File 3). Subgroup analyses revealed that CHM significantly improved lumbar spine BMD in most subgroups. However, no statistically significant differences were observed when the proportion of male participants was ≥ 50%, the mean patient age was < 65 years, or the patients had secondary osteoporosis (Table 3). No significant publication bias was detected for lumbar spine BMD (Egger’s test p = 0.457; Begg’s test p = 0.256; Supplemental File 4).
Figure 2
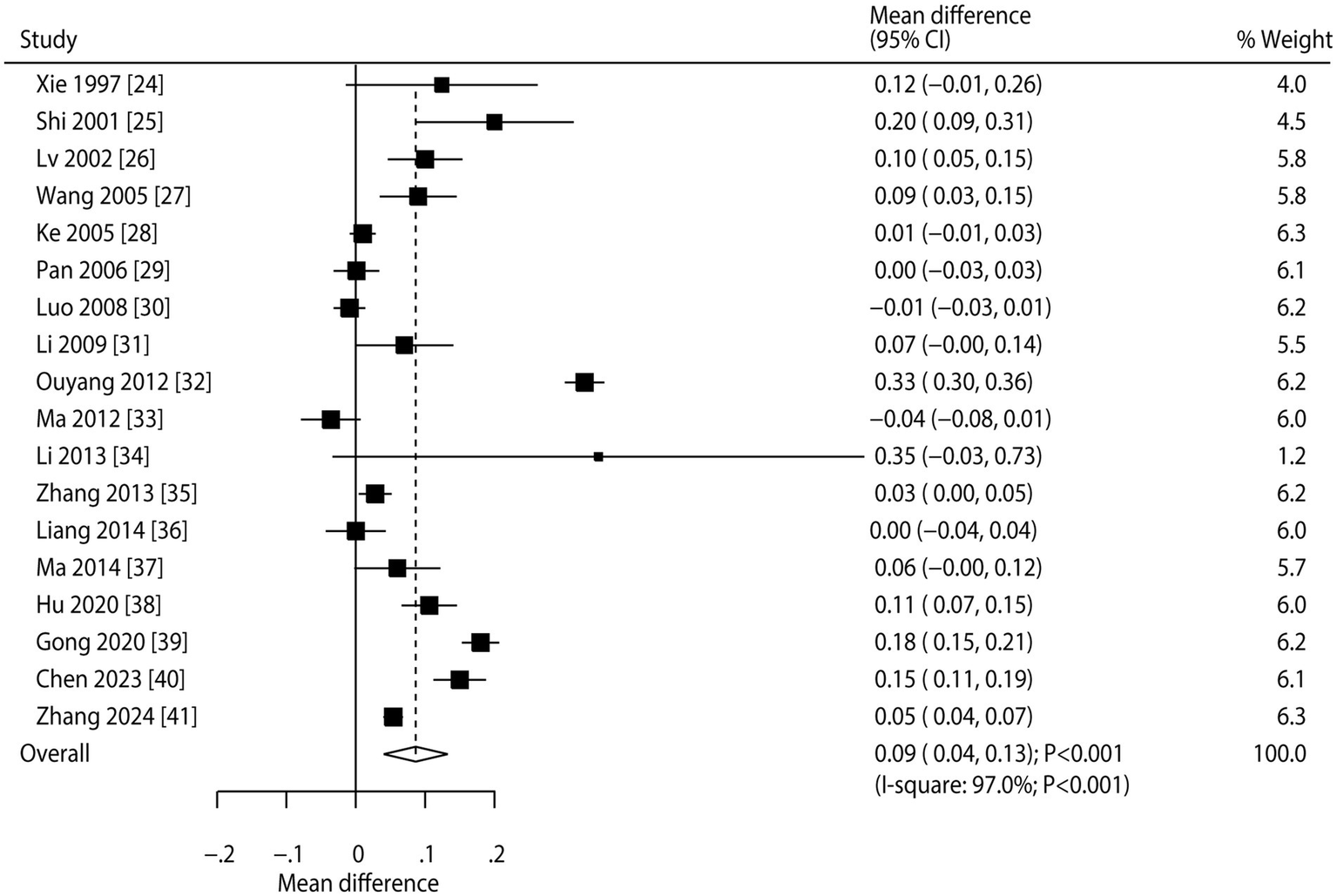
Effect of CHM on changes in BMD at the lumbar spine.
Table 3
| Outcomes | Factors | Subgroups | WMD and 95%CI | p-value | I2 (%) | p-value for heterogeneity | p-value between subgroups |
|---|---|---|---|---|---|---|---|
| Lumbar spine | Publication year | Before 2010 | 0.05 (0.02 to 0.09) | 0.004 | 80.8 | <0.001 | <0.001 |
| 2010 or after | 0.10 (0.03 to 0.17) | 0.004 | 97.9 | <0.001 | |||
| Male (%) | ≥ 50.0% | 0.04 (−0.01 to 0.09) | 0.140 | 57.2 | 0.097 | <0.001 | |
| < 50.0% | 0.09 (0.04 to 0.15) | 0.001 | 97.3 | <0.001 | |||
| Mean age (years) | ≥ 65.0 | 0.07 (0.03 to 0.12) | 0.001 | 95.2 | <0.001 | <0.001 | |
| < 65.0 | 0.10 (−0.00 to 0.21) | 0.052 | 97.7 | <0.001 | |||
| Disease status | Primary | 0.06 (0.03 to 0.10) | <0.001 | 92.8 | <0.001 | <0.001 | |
| Secondary | 0.22 (−0.00 to 0.44) | 0.051 | 98.8 | <0.001 | |||
| Follow-up (months) | 3.0 | 0.07 (0.03 to 0.10) | <0.001 | 69.9 | 0.005 | 0.029 | |
| 6.0 or 12.0 | 0.09 (0.02 to 0.16) | 0.008 | 98.0 | <0.001 | |||
| Femoral neck | Publication year | Before 2010 | 0.02 (−0.05 to 0.09) | 0.594 | 39.7 | 0.198 | < 0.001 |
| 2010 or after | 0.11 (0.04 to 0.19) | 0.003 | 98.7 | <0.001 | |||
| Male (%) | ≥50.0% | 0.09 (−0.05 to 0.23) | 0.191 | – | – | 0.740 | |
| <50.0% | 0.09 (0.01 to 0.17) | 0.020 | 99.3 | <0.001 | |||
| Mean age (years) | ≥65.0 | 0.09 (0.01 to 0.17) | 0.036 | 98.6 | <0.001 | <0.001 | |
| <65.0 | 0.10 (−0.03 to 0.23) | 0.119 | 98.6 | <0.001 | |||
| Disease status | Primary | 0.08 (0.01 to 0.14) | 0.022 | 97.7 | <0.001 | < 0.001 | |
| Secondary | 0.13 (−0.03 to 0.29) | 0.116 | 98.9 | <0.001 | |||
| Follow-up (months) | 3.0 | 0.08 (0.07 to 0.09) | <0.001 | – | – | <0.001 | |
| 6.0 or 12.0 | 0.10 (0.00 to 0.19) | 0.044 | 99.2 | <0.001 | |||
| Greater trochanter of the femur | Publication year | Before 2010 | 0.03 (−0.02 to 0.09) | 0.239 | 21.2 | 0.260 | 0.005 |
| 2010 or after | −0.03 (−0.06 to 0.01) | 0.118 | 0.0 | 0.818 | |||
| Male (%) | ≥50.0% | 0.11 (−0.04 to 0.27) | 0.155 | – | – | 0.231 | |
| <50.0% | 0.00 (−0.04 to 0.04) | 0.961 | 74.8 | 0.019 | |||
| Mean age (years) | ≥65.0 | 0.03 (−0.02 to 0.09) | 0.239 | 21.2 | 0.260 | 0.005 | |
| <65.0 | −0.03 (−0.06 to 0.01) | 0.118 | 0.0 | 0.818 | |||
| Disease status | Primary | 0.01 (−0.03 to 0.05) | 0.698 | 68.0 | 0.025 | – | |
| Secondary | – | – | – | – | |||
| Follow-up (months) | 3.0 | – | – | – | – | – | |
| 6.0 or 12.0 | 0.01 (−0.03 to 0.05) | 0.698 | 68.0 | 0.025 | |||
| Ward’s triangle area | Publication year | Before 2010 | 0.07 (0.07 to 0.08) | <0.001 | 0.0 | 0.895 | 0.512 |
| 2010 or after | 0.09 (−0.10 to 0.27) | 0.363 | 97.9 | <0.001 | |||
| Male (%) | ≥50.0% | 0.07 (−0.05 to 0.19) | 0.267 | – | – | 0.909 | |
| <50.0% | 0.08 (0.00 to 0.16) | 0.041 | 95.9 | <0.001 | |||
| Mean age (years) | ≥65.0 | 0.11 (0.03 to 0.20) | 0.009 | 91.6 | <0.001 | <0.001 | |
| <65.0 | −0.01 (−0.04 to 0.03) | 0.636 | – | – | |||
| Disease status | Primary | 0.08 (0.01 to 0.15) | 0.025 | 93.9 | <0.001 | – | |
| Secondary | – | – | – | – | |||
| Follow-up (months) | 3.0 | – | – | – | – | – | |
| 6.0 or 12.0 | 0.08 (0.01 to 0.15) | 0.025 | 93.9 | <0.001 | |||
| ALP (u/L) | Publication year | Before 2010 | 0.97 (−1.33 to 3.28) | 0.409 | 0.0 | 0.637 | 0.806 |
| 2010 or after | 0.31 (−16.79 to 17.40) | 0.972 | 97.0 | <0.001 | |||
| Male (%) | ≥50.0% | −1.72 (−10.00 to 6.56) | 0.684 | – | – | 0.545 | |
| <50.0% | 1.30 (−7.30 to 9.90) | 0.767 | 94.9 | <0.001 | |||
| Mean age (years) | ≥65.0 | −4.90 (−12.14 to 2.34) | 0.184 | 87.9 | <0.001 | <0.001 | |
| < 65.0 | 9.55 (−2.70 to 21.80) | 0.127 | 93.0 | <0.001 | |||
| Disease status | Primary | −3.81 (−9.93 to 2.32) | 0.223 | 86.8 | <0.001 | <0.001 | |
| Secondary | 16.17 (1.02 to 31.32) | 0.036 | 93.3 | <0.001 | |||
| Follow-up (months) | 3.0 | – | – | – | – | – | |
| 6.0 or 12.0 | 0.98 (−6.88 to 8.83) | 0.808 | 94.2 | <0.001 | |||
| Serum Ca | Publication year | Before 2010 | −0.00 (−0.03 to 0.03) | 0.945 | 33.6 | 0.211 | <0.001 |
| 2010 or after | 0.13 (−0.23 to 0.50) | 0.481 | 99.4 | <0.001 | |||
| Male (%) | ≥50.0% | 0.10 (−0.05 to 0.25) | 0.186 | – | – | 0.817 | |
| <50.0% | 0.08 (−0.11 to 0.26) | 0.426 | 99.0 | <0.001 | |||
| Mean age (years) | ≥65.0 | −0.05 (−0.17 to 0.06) | 0.359 | 84.7 | <0.001 | <0.001 | |
| < 65.0 | 0.21 (−0.07 to 0.50) | 0.143 | 99.4 | <0.001 | |||
| Disease status | Primary | −0.02 (−0.08 to 0.03) | 0.444 | 78.6 | <0.001 | <0.001 | |
| Secondary | 0.41 (−0.22 to 1.05) | 0.202 | 99.7 | <0.001 | |||
| Follow-up (months) | 3.0 | 0.01 (−0.04 to 0.06) | 0.711 | – | – | 0.004 | |
| 6.0 or 12.0 | 0.09 (−0.11 to 0.29) | 0.386 | 99.0 | <0.001 | |||
| Serum P | Publication year | Before 2010 | 0.02 (−0.06 to 0.11) | 0.572 | 0.0 | 0.446 | 0.005 |
| 2010 or after | −0.09 (−0.31 to 0.13) | 0.405 | 98.2 | <0.001 | |||
| Male (%) | ≥50.0% | – | – | – | – | – | |
| < 50.0% | −0.05 (−0.22 to 0.12) | 0.574 | 97.2 | < 0.001 | |||
| Mean age (years) | ≥65.0 | −0.11 (−0.47 to 0.24) | 0.531 | 97.4 | <0.001 | <0.001 | |
| <65.0 | 0.01 (−0.04 to 0.06) | 0.665 | 55.9 | 0.104 | |||
| Disease status | Primary | −0.09 (−0.35 to 0.16) | 0.475 | 97.8 | <0.001 | <0.001 | |
| Secondary | 0.04 (−0.04 to 0.11) | 0.376 | 59.5 | 0.116 | |||
| Follow-up (months) | 3.0 | −0.02 (−0.07 to 0.03) | 0.385 | – | – | <0.001 | |
| 6.0 or 12.0 | −0.05 (−0.28 to 0.17) | 0.648 | 97.5 | <0.001 |
Subgroup analyses.
Eight, four, and four trials, respectively, reported on CHM’s effects on BMD at the femoral neck, greater trochanter of the femur, and Ward’s triangle area (Figure 3). Pooled results indicated CHM was associated with higher BMD at the femoral neck (WMD: 0.09; 95% CI: 0.02 to 0.17; p = 0.015) and Ward’s triangle area (WMD: 0.08; 95% CI: 0.01 to 0.15; p = 0.025). However, CHM was not associated with changes in BMD at the greater trochanter of the femur (WMD: 0.01; 95% CI: −0.03 to 0.05; p = 0.698). Significant heterogeneity was noted for BMD at the femoral neck (I2 = 99.1%; p < 0.001), greater trochanter (I2 = 68.0%; p = 0.025), and Ward’s triangle area (I2 = 93.9%; p < 0.001). Sensitivity analyses suggested variable stability of results, likely due to the small number of included trials and wide confidence intervals (Supplementary File 2). Meta-regression analyses found publication year, proportion of male participants, average patient age, clinical disease status, follow-up, and baseline BMD were not significant factors contributing to the association between CHM and BMD at the femoral neck, greater trochanter of the femur, and Ward’s triangle area (Supplementary File 3). Subgroup analyses found that CHM significantly increased femoral neck BMD in studies published in 2010 or later, with <50% male participants, mean age ≥ 65 years, patients with primary osteoporosis, and across all follow-up durations. CHM did not affect BMD at the greater trochanter in any subgroup. For Ward’s triangle area, significant improvements were seen in studies published before 2010, with <50% male participants, mean age ≥ 65 years, patients with primary osteoporosis, and follow-up durations of 6 or 12 months (Table 3). No significant publication bias was observed for BMD at the femoral neck (Egger’s test p = 0.658; Begg’s test p = 0.711), greater trochanter (Egger’s p = 0.787; Begg’s p = 0.734), or Ward’s triangle area (Egger’s p = 0.965; Begg’s p = 1.000; Supplemental File 4).
Figure 3
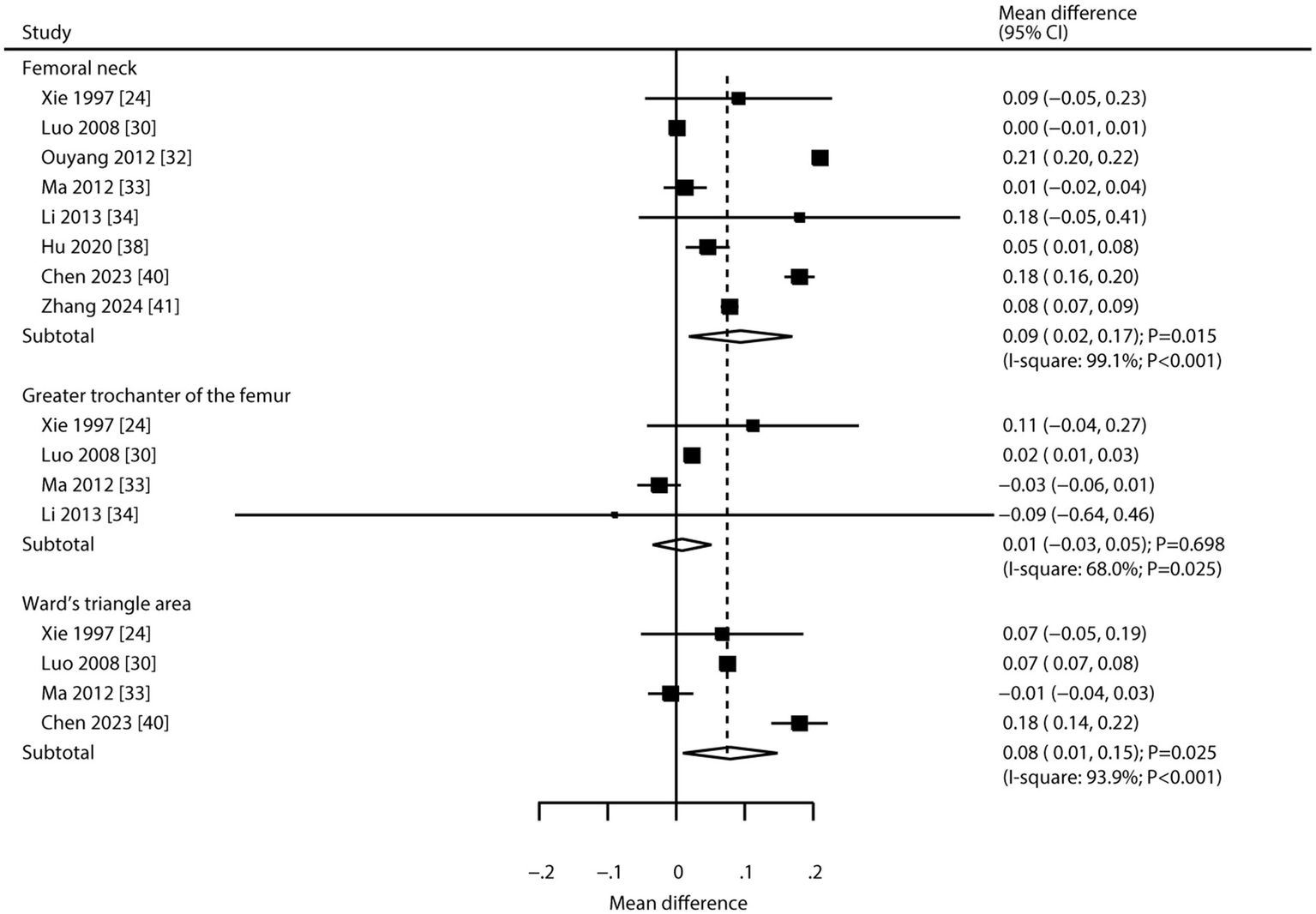
Effect of CHM on changes in BMD at the femoral neck, greater trochanter of the femur, and Ward’s triangle area.
Bone turnover markers
Nine studies reported the effect of CHM on alkaline phosphatase. The pooled result showed no significant association between CHM and alkaline phosphatase levels (WMD: 0.98; 95% CI: −6.88 to 8.83; p = 0.808; Figure 4). Notably, there was significant heterogeneity across studies (I2 = 94.2%; p < 0.001). Sensitivity analysis confirmed the overall conclusion was stable, with no individual study altering the pooled effect (Supplementary File 2). Meta-regression analyses found average patient age (p = 0.029) and disease status (p = 0.018) contributed to the association between CHM and alkaline phosphatase levels (Supplementary File 3). Subgroup analysis indicated CHM was associated with increased alkaline phosphatase levels in patients with secondary osteoporosis (Table 3). No significant publication bias was detected (Egger’s test p = 0.933; Begg’s test p = 0.917; Supplementary File 4).
Figure 4
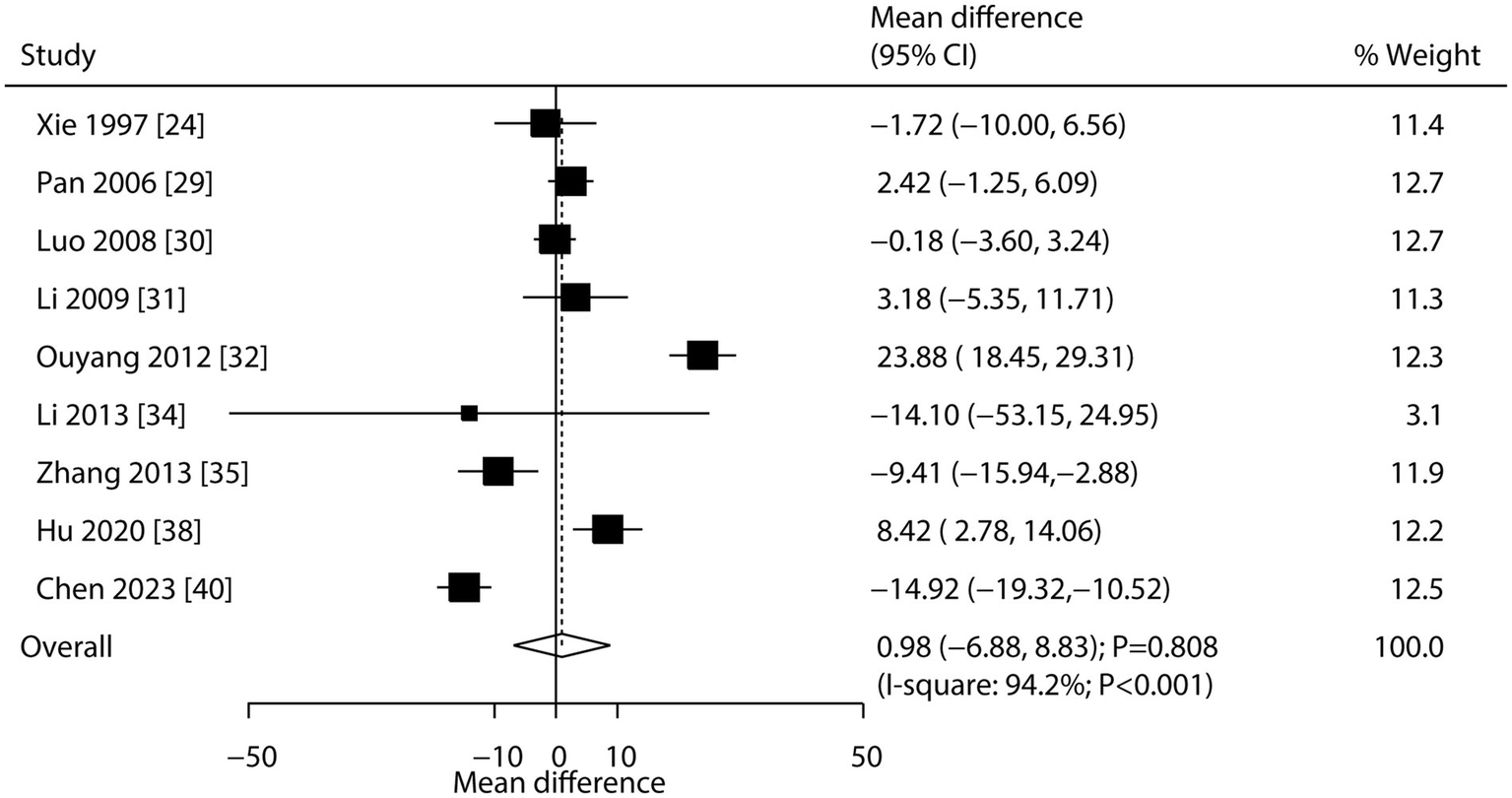
Effect of CHM on changes in alkaline phosphatase levels.
Eight and six studies reported the effects of CHM on serum calcium and serum phosphorus, respectively (Figure 5). CHM had no significant effect on serum calcium (WMD: 0.08; 95% CI: −0.09 to 0.25; p = 0.372) or serum phosphorus (WMD: -0.05; 95% CI: −0.22 to 0.12; p = 0.574). Substantial heterogeneity was observed for both serum calcium (I2 = 98.8%; p < 0.001) and phosphorus (I2 = 97.2%; p < 0.001). Sensitivity analysis indicated stable pooled conclusions for serum calcium, while the effect on serum phosphorus was more variable (Supplementary File 2). Meta-regression analyses found publication year, proportion of male participants, average patient age, clinical disease status, follow-up, and baseline BMD were not significant factors contributing to the association of CHM with serum calcium and serum phosphorus (Supplementary File 3). Subgroup analyses showed CHM was not associated with serum calcium or phosphorus levels in any subgroup (Table 3). No significant publication bias was found for either outcome (serum calcium: Egger’s p = 0.832, Begg’s p = 0.536; serum phosphorus: Egger’s p = 0.694, Begg’s p = 0.707; Supplementary File 4).
Figure 5
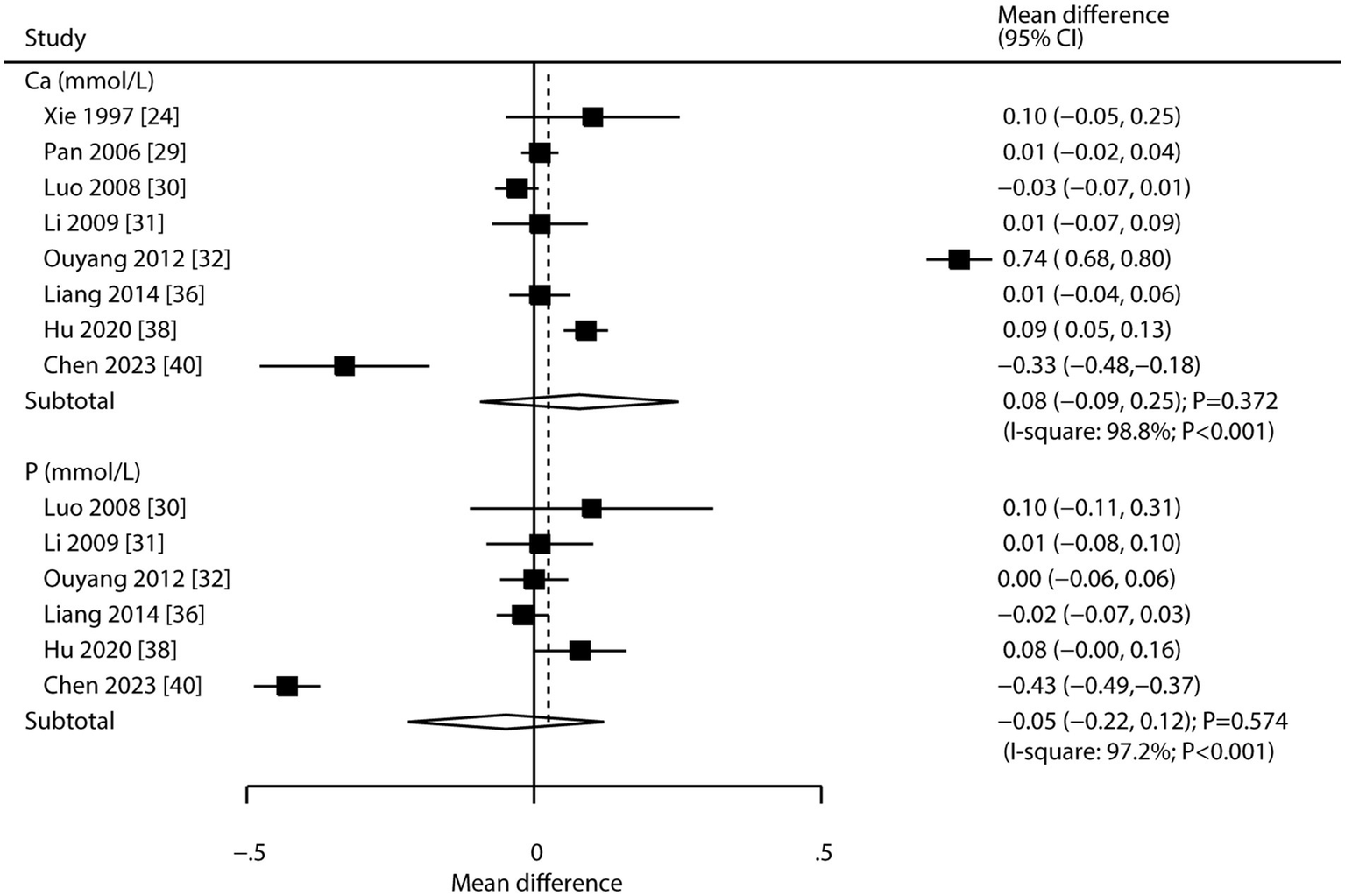
Effect of CHM on changes in serum calcium and serum phosphorus levels.
Discussion
This systematic review included 18 RCTs, encompassing 1,816 patients with osteoporosis, identified through rigorous screening and quantitative synthesis. The meta-analysis demonstrated that CHM significantly improved BMD at the lumbar spine, femoral neck, and Ward’s triangle area when compared with conventional therapies. However, no statistically significant differences were observed in BMD at the greater trochanter. Secondary outcomes indicated no clinically meaningful differences in bone turnover markers, including alkaline phosphatase, serum calcium, and serum phosphorus levels. The methodological limitations of included studies, specifically unclear allocation concealment and inadequate blinding, introduce potential performance and detection biases. In trials comparing CHM to conventional therapy, lack of blinding may lead to observer bias in assessing BMD changes, particularly if evaluators were aware of treatment assignments. Additionally, unblinded participants might report outcomes subjectively, potentially overestimating CHM efficacy.
Regarding BMD outcomes, CHM was found to significantly increase lumbar spine BMD in most cases. Additionally, CHM also improved BMD at the femoral neck and Ward’s triangle area, but had no significant effect at the greater trochanter. CHM’s osteoprotective effects are attributed to bioactive components such as flavonoids, saponins, and polysaccharides, supported by preclinical studies: (1) Flavonoids: In vitro studies show icariin activates the Wnt/β-catenin pathway in osteoblasts, increasing Runx2 expression and promoting bone formation (42). Animal models of osteoporosis demonstrate icariin reduces bone loss via suppression of NF-κB/RANKL signaling in osteoclasts (43); (2) Saponins: These compounds enhance osteoblast differentiation by upregulating BMP-2/Smad signaling, as shown in murine pre-osteoblastic cells (44). Moreover, osteoporosis exhibited improved BMD after notoginsenoside R1 treatment, associated with decreased TNF-α levels (45); and (3) Polysaccharides: In vitro, these promote mesenchymal stem cell osteogenesis through Wnt/β-catenin pathway activation, while inhibiting adipogenesis (46). A rat model of postmenopausal osteoporosis showed Astragalus polysaccharides increased trabecular bone density via estrogen receptor α-mediated signaling (47). For secondary osteoporosis, CHM’s immunomodulatory effects on alkaline phosphatase may involve: (1) Triptolide: This component suppresses NF-κB signaling in activated T cells, reducing TNF-α-induced osteoclastogenesis (48); (2) Curcuminoids: These enhance BMP-2 expression in osteoblasts while inhibiting RANKL secretion from immune cells (49); and (3) Glycyrrhizic acid: This compound modulates the Th17/Treg balance, reducing IL-17-mediated bone resorption (50).
This review also found that patient heterogeneity may influence the therapeutic efficacy of CHM, particularly in the following contexts: (1) Male patients with osteoporosis—Bone loss in men is largely attributed to age-related testosterone decline. Phytoestrogen components in CHM may have limited effects on androgen-regulated bone metabolism (51). Furthermore, men tend to have a higher proportion of cortical bone and lower bone turnover rates, which may attenuate the effects of CHM, especially when its benefits are more pronounced in trabecular bone (52). (2) Younger patients—In this population, active bone remodeling may make them more responsive to conventional anti-resorptive therapies, whereas the gradual regulatory effects of CHM may require longer durations to manifest (53). (3) Patients with secondary osteoporosis—Secondary osteoporosis, often linked to glucocorticoid use or endocrine disorders, involves complex mechanisms such as inflammatory cytokine overactivation. Standard CHM formulations may be insufficient to specifically target these inflammatory pathways (54).
The pooled analysis showed no significant effects of CHM on key bone turnover markers, including alkaline phosphatase, serum calcium, and serum phosphorus. These findings suggest that CHM, in its conventional forms, may not markedly influence the systemic balance between osteoblast-driven bone formation and osteoclast-mediated resorption. While alkaline phosphatase serves as a surrogate for osteoblast activity, and calcium/phosphorus levels reflect mineralization processes, the pleiotropic effects of CHM—primarily involving Wnt/β-catenin activation and RANKL/OPG axis modulation—may not translate into measurable changes in these biomarkers at a population level (55, 56).
However, subgroup analysis revealed that patients with secondary osteoporosis receiving CHM showed elevated alkaline phosphatase levels. This may reflect CHM’s immunomodulatory potential in inflammatory environments, as seen in secondary osteoporosis. Bioactive components such as triptolide and curcuminoids may suppress NF-κB signaling, reduce TNF-α-driven osteoclastogenesis, and promote BMP/Smad-mediated osteoblast differentiation (57, 58).
This study has several methodological limitations that should be considered when interpreting the results: (1) notably, all included studies were conducted in China, introducing significant geographical and potential ethnic biases. This limitation may affect the generalizability of our findings to populations with different genetic backgrounds, lifestyle factors, and healthcare practices. Future research should prioritize multinational, multilingual RCTs to evaluate CHM efficacy in diverse populations. Standardizing CHM formulations and reporting herb compositions in detail will facilitate cross-cultural comparisons and enhance evidence generalizability; (2) Follow-up durations in included studies ranged from 3 to 12 months, with only 1 study lasting 12 months. This short-term observation may underestimate CHM’s long-term effects on bone remodeling and fracture risk. Osteoporosis treatment typically requires ≥2 years to demonstrate significant fracture risk reduction. Additionally, rare adverse events may not emerge within short follow-ups, necessitating long-term safety surveillance; (3) despite conducting sensitivity and subgroup analyses, residual heterogeneity remained; (4) the marginal statistical significance observed in BMD improvements at the greater trochanter and in bone marker changes reflects limited statistical power; post-hoc analysis suggested inadequate power to detect small but potentially relevant differences; and (5) as with all meta-analyses based on published literature, there is a risk of publication bias and limitations in the depth of individual patient data, which restricted the ability to perform more granular analyses.
Conclusion
Our findings suggest that CHM may offer adjunctive benefit in improving BMD at specific skeletal sites among osteoporosis patients. However, the moderate-quality evidence, high heterogeneity, and regional limitations of included studies necessitate cautious interpretation. CHM’s efficacy relative to conventional therapies remains uncertain and requires validation by large, multicenter RCTs with rigorous methodology.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
JW: Data curation, Project administration, Methodology, Formal analysis, Conceptualization, Investigation, Writing – original draft, Supervision. FT: Writing – review & editing, Investigation, Data curation, Methodology, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1620264/full#supplementary-material
References
1.
Hadji P Esterberg E Obermüller D Bartsch R . Bone evaluation study-2: update on the epidemiology of osteoporosis in Germany. Arch Osteoporos. (2024) 19:26. doi: 10.1007/s11657-024-01380-9
2.
Cui Z Meng X Feng H Zhuang S Liu Z Zhu T et al . Estimation and projection about the standardized prevalence of osteoporosis in mainland China. Arch Osteoporos. (2019) 15:2. doi: 10.1007/s11657-019-0670-6
3.
Sözen T Özışık L Başaran NÇ . An overview and management of osteoporosis. Eur J Rheumatol. (2017) 4:46–56. doi: 10.5152/eurjrheum.2016.048
4.
Zhu JY Gao M Song QY Ji P Li HY Zhong ZM et al . Prevalence of osteoporosis in Chinese elderly people: a meta-analysis. Chin General Pract. (2022) 25:346–53.
5.
Kanis JA Johnell O Oden A de Laet C Dawson A Jonsson B . Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. (2001) 12:989–95. doi: 10.1007/s001980170006
6.
Migliorini F Giorgino R Hildebrand F Spiezia F Peretti GM Alessandri-Bonetti M et al . Fragility fractures: risk factors and management in the elderly. Medicina (Kaunas). (2021) 57:1119. doi: 10.3390/medicina57101119
7.
Panneman MJ Lips P Sen SS Herings RM . Undertreatment with anti-osteoporotic drugs after hospitalization for fracture. Osteoporos Int. (2004) 15:120–4. doi: 10.1007/s00198-003-1544-7
8.
Wilk A Sajjan S Modi A Fan CPS Mavros P . Post-fracture pharmacotherapy for women with osteoporotic fracture: analysis of a managed care population in the USA. Osteoporos Int. (2014) 25:2777–86. doi: 10.1007/s00198-014-2827-x
9.
Nakamura T World Health Organization . Absolute risk for fracture and WHO guideline. Fracture risk assessments recommended by World Health Organization and Japanese guidelines for prevention and treatment of osteoporosis 2006. Clin Calcium. (2007) 17:1022–8.
10.
Fuggle N Laslop A Rizzoli R al-Daghri N Alokail M Balkowiec-Iskra E et al . Treatment of osteoporosis and osteoarthritis in the oldest old. Drugs. (2025) 85:343–60. doi: 10.1007/s40265-024-02138-w
11.
Edwards BJ Bunta AD Lane J Odvina C Rao DS Raisch DW et al . Bisphosphonates and nonhealing femoral fractures: analysis of the FDA adverse event reporting system (FAERS) and international safety efforts: a systematic review from the research on adverse drug events and reports (RADAR) project. J Bone Joint Surg Am. (2013) 95:297–307. doi: 10.2106/JBJS.K.01181
12.
Jin YX Wu P Mao YF Wang B Zhang JF Chen WL et al . Chinese herbal medicine for osteoporosis: a meta-analysis of randomized controlled trials. J Clin Densitom. (2017) 20:516–25. doi: 10.1016/j.jocd.2017.07.003
13.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
14.
World Health Organization . Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Technical report series no. 843. Geneva, Switzerland: WHO (1994).
15.
Higgins J Green S . Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated march 2011). Cochrane Collaboration. Chichester, UK: John Wiley and Sons. (2011).
16.
DerSimonian R Laird N . Meta-analysis in clinical trials. Control Clin Trials. (1986) 7:177–88. doi: 10.1016/0197-2456(86)90046-2
17.
Ades AE Lu G Higgins JP . The interpretation of random-effects metaanalysis in decision models. Med Decis Mak. (2005) 25:646–54. doi: 10.1177/0272989X05282643
18.
Deeks JJ Higgins JPT Altman DG . Analyzing data and undertaking meta-analyses In: HigginsJGreenS, editors. Cochrane handbook for systematic reviews of interventions 5.0.1. Oxford, UK: The Cochrane Collaboration (2008)
19.
Higgins JPT Thompson SG Deeks JJ Higgins JP Altman DG . Measuring inconsistency in meta-analyses. BMJ. (2003) 327:557–60. doi: 10.1136/bmj.327.7414.557
20.
Tobias A . Assessing the influence of a single study in the meta-analysis. Stata Tech Bull. (1999) 47:15–7.
21.
Altman DG Bland JM . Interaction revisited: the difference between two estimates. BMJ. (2003) 326:219. doi: 10.1136/bmj.326.7382.219
22.
Egger M Davey Smith G Schneider M Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
23.
Begg CB Mazumdar M . Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 50:1088–101. doi: 10.2307/2533446
24.
Xie YM Zhang FZ Zhou WQ Gao P Fu RJ Zhao TY et al . Clinical study of Bugu Shengsui capsule in treating primary osteoporosis with kidney-Yang deficiency syndrome. Chin J Integr Tradit Western Med. (1997) 17:526–30.
25.
Shi J . Efficacy observation of bushen jiangu tang in the treatment of primary osteoporosis complicated with fracture. J Guangxi Med Univ. (2001) 18:679–80.
26.
Lv ZH Wen ZJ Wu SP Qiu JM Qiu JX . Clinical observation of Jiawei bushen Zhuangjintang on the treatment of primary osteoporosis. China J Orthop Trauma. (2002) 15:263–4.
27.
Wang Q Chen JF Lin LF Kong TH . Effects of bushenzhuanggu capsule on primary osteoporosis. Harbin Med J. (2005) 25:69–71.
28.
Ke Q Liu HQ Chen XC Wu DF . Clinical observation of Bushen (Tonifying the kidney) prescription in the treatment of 95 cases of primary osteoporosis of kidney deficiency type. ITCM. (2005) 22:26–7.
29.
Pan WJ Zeng ZM Huang HX . Clinical observation on the treatment of osteoporosis in menopausal women by the method of tonifying the kidney and nourishing essence. J Shanxi Univ Tradit Chin Med. (2006) 17:30–1.
30.
Luo HX Zhao XW Ji HY Fan WZ Tang XD . Multicenter randomized controlled trial of Zhuanggu granules in the treatment of primary osteoporosis. Liaoning J Tradit Chin Med. (2008) 35:1037–8.
31.
Li Y Cai WP Hu YW Xu ZX An MH . Clincial observation on post-menopause osteoporosis treated with yiguyin. J Zhejiang College Tradit Chin Med. (2009) 33:53–4.
32.
Ouyang GL Feng XH Xiao LB Huang Z Xia Q Zhu F . Effects of Chinese herbal medicine Qianggu capsule on patients with rheumatoid arthritis-induced osteoporosis: a report of 82 cases. J Chin Integr Med. (2012) 10:1394–9. doi: 10.3736/jcim20121210
33.
Ma JL Hou QW Shi L Yang DX Guo HY Huang SX et al . Comparison of three therapy results in a 2-year research on osteoporosis. Chin Prim Health Care. (2012) 26:108–10.
34.
Li JP . A randomized, double-blind, placebo controlled clinical study on Bushenhuoxue granul treatment for postmenopausal osteoporosis. Beijing, China: China Academy of Chinese Medical Sciences (2013).
35.
Zhang RS Ma ML Sun QH An F Liu YB Jiang B . Effect of Bushen Zhuanggu decoction in the treatment of postmenopausal osteoporosis randomized parallel controlled study. J Pract Tradit Chin Int Med. (2013) 27:20–2.
36.
Liang T . Clinical observation on treating 100 cases of OP by the Jianpi Bushen method. CJCM. (2014) 6:103–4.
37.
Ma HX Wang XH Tang XL Chen J . Clinical observation of Buzhongyiqi decoction on senile osteoporosis. Hebei J Tradit Chin Med. (2014) 36:696–697.711.
38.
Hu JJ . Clinical observation of nourishing liver and kidney decoction to treat glococorticoid-induced osteoporosis. Guangzhou University of Chinese Medicine (2020).
39.
Gong YL Song M Dong WT Dong P Song ZJ Huang K et al . Randomized controlled clinical study on the treatment of osteoporosis with consolidating origin and improving bone formula in the elderly. Chin J Osteoporos. (2020) 26:676–679.701.
40.
Chen T Li G Xu Y . Study on the effect of Bushen Zhuanggu tablet combined with conventional regimen on bone mineral density improvement, functional recovery and fracture risk prevention in patients with postmenopausal osteoporosis. Comput Math Methods Med. (2023) 2023:4846392. doi: 10.1155/2023/4846392
41.
Zhang XY Liu H Chai Y Chen F Zeng H Gao ZG et al . Effect of Yishen Gushu formula on bone metabolic markers and clinical efficacy in patients with osteoporosis of kidney deficiency and blood stasis type. Chin J Tissue Eng Res. (2024) 28:1155–60.
42.
Xu Q Chen G Liu X Dai M Zhang B . Icariin inhibits RANKL-induced osteoclastogenesis via modulation of the NF-κB and MAPK signaling pathways. Biochem Biophys Res Commun. (2019) 508:902–6. doi: 10.1016/j.bbrc.2018.11.201
43.
Zhang XY Li HN Chen F Chen YP Chai Y Liao JZ et al . Icariin regulates miR-23a-3p-mediated osteogenic differentiation of BMSCs via BMP-2/Smad5/Runx2 and WNT/β-catenin pathways in osteonecrosis of the femoral head. Saudi Pharm J. (2021) 29:1405–15. doi: 10.1016/j.jsps.2021.10.009
44.
Choi CW Choi SW Kim HJ Lee KS Kim SH Kim SL et al . Germinated soy germ with increased soyasaponin ab improves BMP-2-induced bone formation and protects against in vivo bone loss in osteoporosis. Sci Rep. (2018) 8:12970. doi: 10.1038/s41598-018-31118-w
45.
Wang T Wan D Shao L Dai J Jiang C . Notoginsenoside R1 stimulates osteogenic function in primary osteoblasts via estrogen receptor signaling. Biochem Biophys Res Commun. (2015) 466:232–9. doi: 10.1016/j.bbrc.2015.09.014
46.
Liu J An J Jiang N Yang K Guan C Zhao N et al . Codonopsis pilosula polysaccharides promote osteogenic differentiation and inhibit lipogenic differentiation of rat bone marrow stem cells by activating β-catenin. Chem Biol Interact. (2023) 385:110721. doi: 10.1016/j.cbi.2023.110721
47.
Huo J Sun X . Effect of Astragalus polysaccharides on ovariectomy-induced osteoporosis in mice. Genet Mol Res. (2016) 15:1–9. doi: 10.4238/gmr15049169
48.
Wang X Ni T Miao J Huang X Feng Z . The role and mechanism of triptolide, a potential new DMARD, in the treatment of rheumatoid arthritis. Ageing Res Rev. (2025) 104:102643. doi: 10.1016/j.arr.2024.102643
49.
Pengjam Y Syazwani N Inchai J Numit A Yodthong T Pitakpornpreecha T et al . High water-soluble curcuminoids-rich extract regulates osteogenic differentiation of MC3T3-E1 cells: involvement of Wnt/β-catenin and BMP signaling pathway. Chin Herb Med. (2021) 13:534–40. doi: 10.1016/j.chmed.2021.01.003
50.
Huo M Liu H Chen S Xiu L Yu X Zhong G . Kansui-liquorice enhances the "water-expelling" effect of Gansui Banxia decoction in rats with malignant ascites by targeting the NPs/NPRs/cGMP/PKGII pathway and T cell immunity. Front Pharmacol. (2025) 16:1557717. doi: 10.3389/fphar.2025.1557717
51.
Cheung EY Ho AY Lam KF Tam S Kung AW . Determinants of bone mineral density in Chinese men. Osteoporos Int. (2005) 16:1481–6. doi: 10.1007/s00198-005-2000-7
52.
Osipov B Paralkar MP Emami AJ Cunningham HC Tjandra PM Pathak S et al . Sex differences in systemic bone and muscle loss following femur fracture in mice. J Orthop Res. (2022) 40:878–90. doi: 10.1002/jor.25116
53.
Abu-Nada L Liu Y Saleh Al-Hamed F Ouliass B Millecamps M Tran SD et al . Young bone marrow transplantation delays bone aging in old mice. Exp Gerontol. (2025) 202:112704. doi: 10.1016/j.exger.2025.112704
54.
Wang Y Jiang Z Deng L Zhang G Xu X Alonge E et al . Dendrobium offificinale polysaccharides prevents glucocorticoids-induced osteoporosis by destabilizing KEAP1-NRF2 interaction. Int J Biol Macromol. (2023) 253:126600. doi: 10.1016/j.ijbiomac.2023.126600
55.
Kim SK Lee MH Rhee MH . Studies on the effects of biomedicinal agents on serum concentration of Ca2+, P and ALP activity in osteoporosis-induced rats. J Vet Sci. (2003) 4:151–4. doi: 10.4142/jvs.2003.4.2.151
56.
Mettwally WSA Hussein RA Abdel Jaleel GA Hassan A Saleh DO el-Beih AA . Cardenolides; calotropin and gomphogenin from Calotropis procera (Aiton) mitigate bone turnover in ovariectomized osteoporotic rats: targeting RANKL/OPG axis and estrogen receptor-alpha. Fitoterapia. (2024) 179:106226. doi: 10.1016/j.fitote.2024.106226
57.
Liu X Fan JB Hu J Li F Yi R Tan F et al . Lactobacillus fermentum ZS40 prevents secondary osteoporosis in Wistar rat. Food Sci Nutr. (2020) 8:5182–91. doi: 10.1002/fsn3.1824
58.
Xie B Wu J Li Y Wu X Zeng Z Zhou C et al . Geniposide alleviates glucocorticoid-induced inhibition of osteogenic differentiation in MC3T3-E1 cells by ERK pathway. Front Pharmacol. (2019) 10:411. doi: 10.3389/fphar.2019.00411
Summary
Keywords
Chinese herbal medicine, osteoporosis, systematic review, meta-analysis, bone mineral density
Citation
Wang J and Teng F (2025) Efficacy of Chinese herbal medicine in patients with osteoporosis: a systematic review and meta-analysis. Front. Med. 12:1620264. doi: 10.3389/fmed.2025.1620264
Received
29 April 2025
Accepted
24 June 2025
Published
25 July 2025
Volume
12 - 2025
Edited by
Hongzhe Li, United States Department of Veterans Affairs, United States
Reviewed by
Tomislav Tosti, University of Belgrade, Serbia
Xu Liu, Fudan University, China
Updates
Copyright
© 2025 Wang and Teng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jin Wang, Wangjin20230506@outlook.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.