- 1Department of Children’s Health, Shijiazhuang Maternal and Child Health Hospital, Shijiazhuang, Hebei, China
- 2Department of Pediatric, Shijiazhuang Maternal and Child Health Hospital, Shijiazhuang, Hebei, China
Background: Albeit fluticasone propionate suspension is effective in treating variant cough in children, there is limited research on the use of Xuanfei Zhisou mixture, and even fewer studies on its combination application. This study aimed to estimate the role of Xuanfei Zhisou mixture (XZM) plus fluticasone propionate suspension (FPS) in the treatment of cough variant in children.
Methods: 122 children with variant cough from March 2020 to May 2023 were classified into observation therapy (XZM + FPS, n = 60) and control therapy (FPS, n = 60) using block randomization. The clinical effect, inflammatory factor, pulmonary function, peripheral eosinophil count, daytime and nighttime cough scores, immune function and untoward reactions were contrasted in two groups.
Results: The clinical effect, Forced Vital Capacity (FVC), FEV1 (Forced Expiratory Volume)/FVC, CD3+, CD4+ as well as CD4+/CD8+ of the observation therapy were higher than control therapy. After treatment, interleukin-4 (IL-4), tumor necrosis factor-α (TNF-α), serum amyloid A (SAA), c-reactive protein (CRP), eosinophil count as well as daytime and nighttime cough scores in observation therapy were lower than control therapy. Howbeit, there was no diversity in untoward reactions between the two groups.
Conclusion: Xuanfei Zhisou mixture plus FPS had conspicuous role in children with variant cough, which assisted in diminishing cough symptoms, raising pulmonary function, reduce inflammatory factor level and upgrading immune function of children.
1 Introduction
Variant cough in children is a common chronic cough in children, characterized by persistent coughing in the early stages of the disease (1, 2). The etiology of this disease is related to infection or inhalation of allergens such as dust and pollen, which can lead to increased respiratory resistance, usually accompanied by difficulty breathing, and can easily lead to complications such as asthma (3). Variant cough may have a negative impact on children mental health, seriously affecting the physical and mental well-being of the affected child, and bringing huge mental and economic burdens to the family (4). Frequent coughing can cause restlessness and anxiety in children, affecting their learning and social skills, and even leading to psychological disorders such as anxiety and depression (5). If not intervened in a timely manner, it may threaten the safety of the children (2). Nowadays, the main treatment methods for children with variant cough in clinical practice are leukotriene receptor antagonists, bronchodilators, and glucocorticoids (6). Fluticasone propionate suspension (FPS) is a steroid hormone that can effectively inhibit the release of inflammatory factors and alleviate inflammation reaction (7–9). Although FPS can control variant cough symptoms to some extent, its therapeutic effect is limited and there are adverse reactions, making it more prone to relapse after discontinuation (10, 11). Hence, searching drugs with side effects and less likely to recur is of great significance for children with variant cough.
Studies have shown that traditional Chinese medicine is effective in treating children with variant cough (12–14). Xuanfei Zhisou mixture (XZM) has the effects of dispelling wind, promoting lung circulation, stopping cough, and resolving phlegm. It has noticeable roles on the treatment of phlegm, asthma and cough (15). There are few studies on the combined use of the two in clinical practice. In view of this, this study explored the therapeutic effect of XZM plus FPS on children with variant cough and its impacted on serum amyloid A (SAA), c-reactive protein (CRP), providing fresh treatment directions for children with variant cough.
2 Materials and methods
2.1 Patient information
122 children with variant cough were admitted in our hospital between March 2020 and May 2023. The subjects were divided into observation therapy (XZM + FPS, n = 60) and control therapy (FPS, n = 60) according to block randomization. In observation therapy, there were 28 males and 32 females, aged 2–11 years, with an average age of 6.14 ± 1.35 years, the duration of the disease is 1–20 months, the average (14.5 ± 3.8) month. There were 22 cases of intermittent attacks, 30 cases of mild persistent attacks, 5 cases of moderate persistent attacks, and 3 cases of severe persistent attacks; There were 29 males and 33 females in control therapy, aged 2–12 years, with an average age of 6.23 ± 1.48 years, the duration of the disease is 2–21 months, the average (15.1 ± 3.7) month. There were 20 cases of intermittent attacks, 31 cases of mild persistent attacks, 6 cases of moderate persistent attacks, and 5 cases of severe persistent attacks. There was no noticeable discrepancy between two groups (P > 0.05).
2.2 Ethics statement
This study was approved by the Ethics Committee of Shijiazhuang Maternity and Child Health Hospital with the official approval number: 20201112. All study procedures complied with the Declaration of Helsinki (2013 version) and the Guidelines for the Use of Controlled Traditional Chinese Medicines in Children (2019 Edition, National Medical Products Administration). Guardians provided written informed consent.
2.3 Risk monitoring
Potential addiction risk was assessed at months 1, 2, and 3 post-treatment, with no dependence symptoms observed in either group. To monitor potential growth impact from fluticasone, height and weight were measured monthly. No significant intergroup difference in growth velocity was found (0.32 ± 0.11 cm/month vs. 0.30 ± 0.10 cm/month, P = 0.389), indicating no adverse effect on development.
2.4 Inclusion and exclusion criteria
Inclusion criteria: (1) All children met the diagnostic criteria of cough variant asthma (16); (2) The child was less than 12 years old; (3) Persistent cough for more than 4 weeks, mostly in the morning and at night; (4) Cough was mainly dry cough, without wheezing; (5) Exclude chronic cough caused by other reasons; (6) Positive bronchial provocation test; (7) Antibiotic treatment was ineffective, while anti-asthma medication was effective; (8) The parents gave informed consent for the treatment. Exclusion criteria: (1) Patients with infectious disease occurring before or within months of treatment; (2) During the investigational treatment of other drugs; (3) Children with severe insufficiency of the kidney or other important organs; (4) Children with other infectious diseases occurring 1 month before treatment.
2.5 Interventions
Control therapy was treated with FPS (H20170361, GlaxoSmithKline Australia Pty Ltd.) 100 μg/time, twice a day. Observation therapy was given XZM + FPS (Z20050288, Gansu Pu’an Pharmaceutical Co., Ltd.) 10 mL/time, three a day. The treatment period was 3 months in both groups.
2.5.1 Composition and dose of XZM
Xuanfei Zhisou mixture (approval number Z20050288, Gansu Pu’an Pharmaceutical Co., Ltd.) is a standardized traditional Chinese medicine preparation with 8 active components (per 100 mL): Aster tataricus (15 g), Stemona sessilifolia (12 g), Platycodon grandiflorus (10 g), Citrus reticulata (8 g), Houttuynia cordata (15 g), Papaver somniferum husk (3 g), Glycyrrhiza uralensis (6 g), and Ephedra sinica (5 g).
The dose of XZM (10 mL/time, 3 times/day) was determined based on: (1) A preliminary clinical study (17) showing that this dose effectively reduced cough scores in children aged 2–12 years without side effects; (2) The concentration of Papaver somniferum husk (3 mg/10 mL) was verified to be non-addictive in pediatric populations (18).
2.5.2 Rationale for XZM-FPS combination
Preclinical studies (19) confirmed that XZM components (e.g., Aster tataricus saponins, Stemona alkaloids) do not inhibit or induce cytochrome P450 3A4 (CYP3A4)–the key enzyme for FPS metabolism (20). Thus, no pharmacokinetic interactions were expected.
2.6 Research indicators
2.6.1 Clinical effect
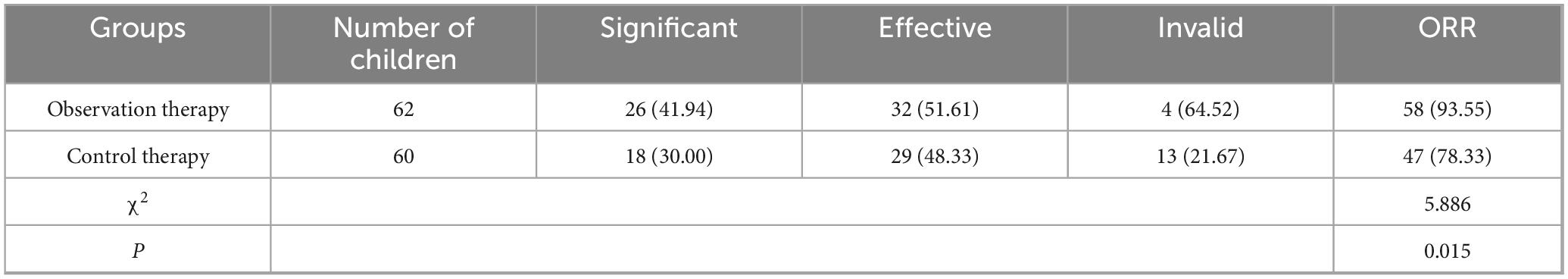
Significant: The cough symptoms have wholly disappeared and have not recurred for 3 months; Effective: The degree of coughing has significantly reduced, but the cough symptoms have persisted for 3 months. Invalid: There is no noticeable variety in cough symptoms after medication treatment.
Overall response rate (ORR) = (Significant + Effective)/total cases × 100%
2.6.2 Inflammatory factor
5 ml of fasting venous blood was collected from all children before and after treatment, centrifuge at 2500 r/min for 10 min, and store the serum samples at −20 °C for testing. Interleukin-4 (IL-4), tumor necrosis factor-α (TNF-α), serum amyloid A (SAA), as well as c-reactive protein (CRP) were determined via enzyme linked immunosorbent assay (Shanghai Baili Biotechnology Co., Ltd).
2.6.3 Peripheral eosinophil count
2 ml of venous blood was collected from all children before and after treatment, and counted by automatic blood analyzer (Sysmex XE-2100, Sissenom Medical Electronics Co., Ltd.).
2.6.4 Pulmonary function
The forced expiratory volume in 1 s (FEV1) and FEV1/forced vital capacity (FVC) of two groups children were determined before and after treatment via S∼980A III pulmonary function meter (Sichuan Sikeda Technology Co., Ltd.).
2.6.5 Daytime and nighttime cough scores
Refer to daytime cough symptoms and nighttime cough symptoms (21): No cough symptoms during both day and night, rated 0 points; No cough symptoms during the day and night, and only cough symptoms before bedtime and in the early morning, the evaluation is 1 point; Having a brief cough during the day and waking up at night due to coughing is rated as 2 points; Coughing frequently during the day and waking up multiple times due to coughing at night is rated as 3 points; Frequent coughing during the day can have an impact on normal life, and coughing during most of the night is rated as 4 points; Severe coughing during the day severely affects normal life, and severe coughing at night leads to inability to fall asleep. The evaluation score is 5 points. The higher the score, the more severe the cough symptoms.
2.6.6 Immune function
3 ml of fasting venous blood was obtained from children. The peripheral blood T cell subset CD3+, CD4+ as well as CD4+/CD8+ levels were detected via using flow cytometry [Beckman Coulter International Trading (Shanghai) Co., Ltd].
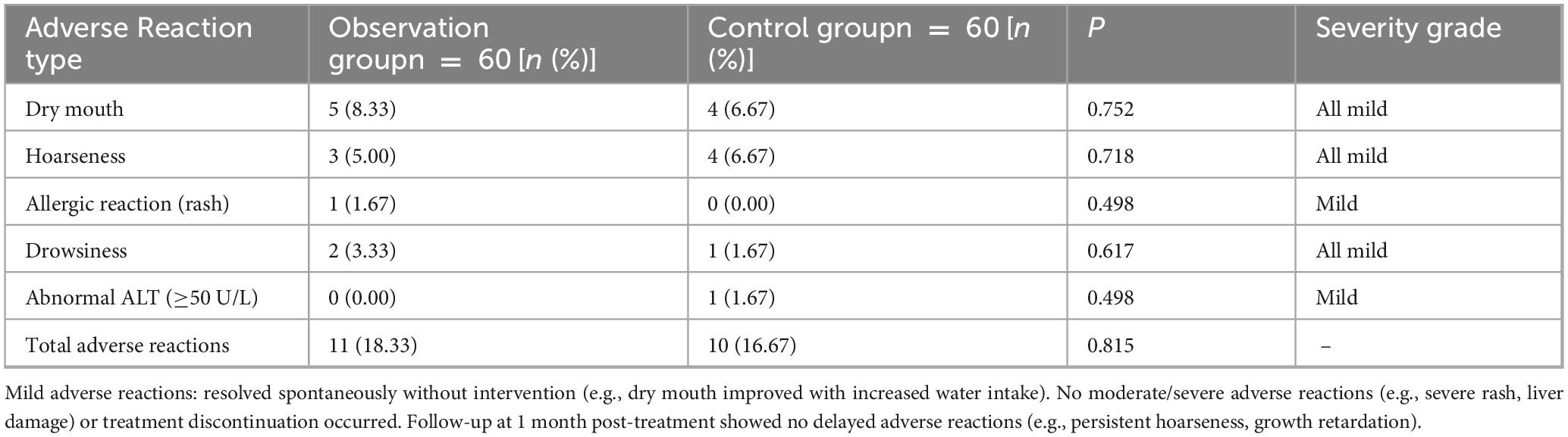
2.6.7 Untoward reaction
Compared the occurrence of adverse reactions such as dry mouth, hoarseness, allergies and drowsiness between two groups of children.
2.6.8 Statistical analysis
SPSS 23.0 was employed for treating. Prior to formal statistical analysis, normality tests of experimental data were performed using the Shapiro-Wilk test; only data that conformed to a normal distribution were expressed as mean ± SD. Experimental data were showed by mean ± SD. The difference between con and caudatin were analyzed by One-way analysis of variance (ANOVA) and t-test. P < 0.05, significant difference.
3 Results
3.1 Comparison of clinical efficacy
After treatment, the ORR of observation therapy was better than control therapy (Table 1, P < 0.05).
3.2 Comparison of inflammatory factor
Before treatment, there were on prominent discrepancy in IL-4, TNF-α, SAA as well as CRP levels between control therapy and observation therapy (P > 0.05). After processing, the level of IL-4 was upgraded, the TNF-α, SAA as well as CRP levels were lessened. Also, the IL-4 was higher, while TNF-α, SAA as well as CRP levels were lowered in observation therapy than control therapy (Figure 1, P < 0.05).
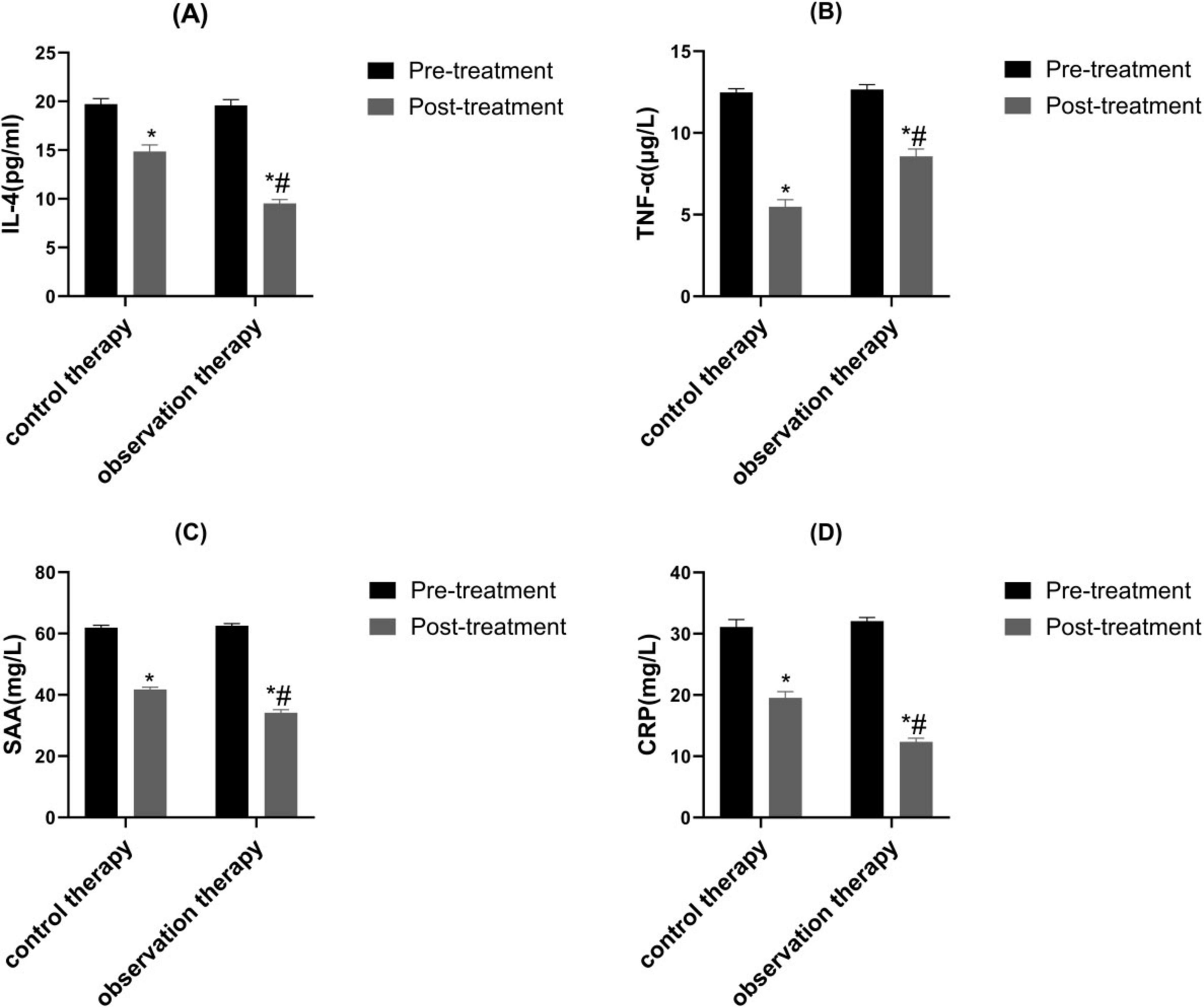
Figure 1. Contrast of inflammatory factor. (A) IL-4, (B) TNF-α, (C) SAA as well as (D) CRP. *p < 0.05 vs. pre-treatment, #p < 0.05 vs. control therapy.
3.3 Contrast of peripheral eosinophil count
Before treatment, there was no notable diversity in peripheral eosinophil between two groups (P > 0.05). Via treatment cycles, the peripheral eosinophil count of observation were evidently lessened than control (Figure 2, P < 0.05).
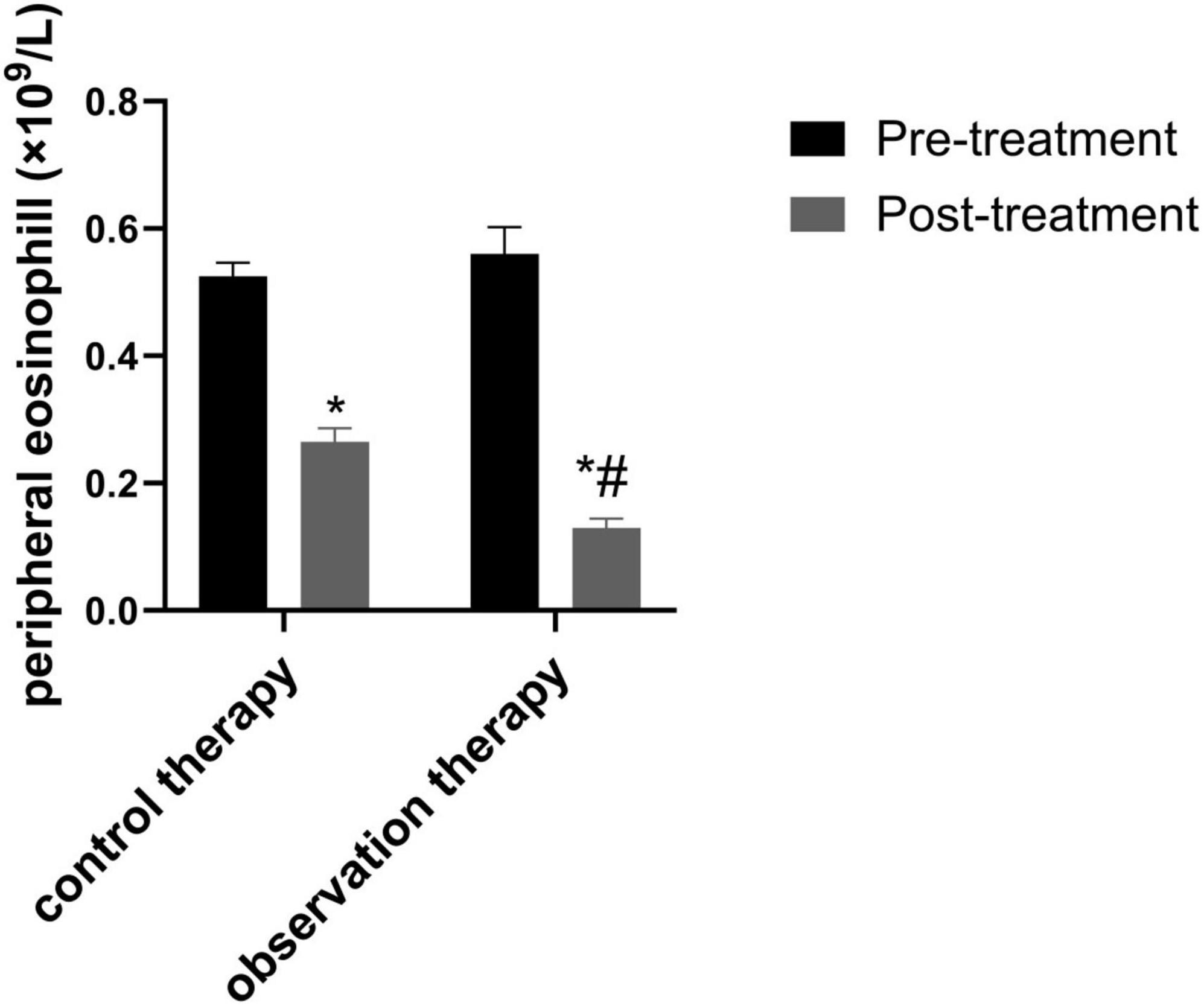
Figure 2. Comparison of peripheral eosinophil count. *p < 0.05 vs. pre-treatment, #p < 0.05 vs. control therapy.
3.4 Comparison of pulmonary function
Via treatment cycles, the FEV1 and FEV1/FVC in observation therapy were higher than control therapy (Figure 3, P < 0.05).
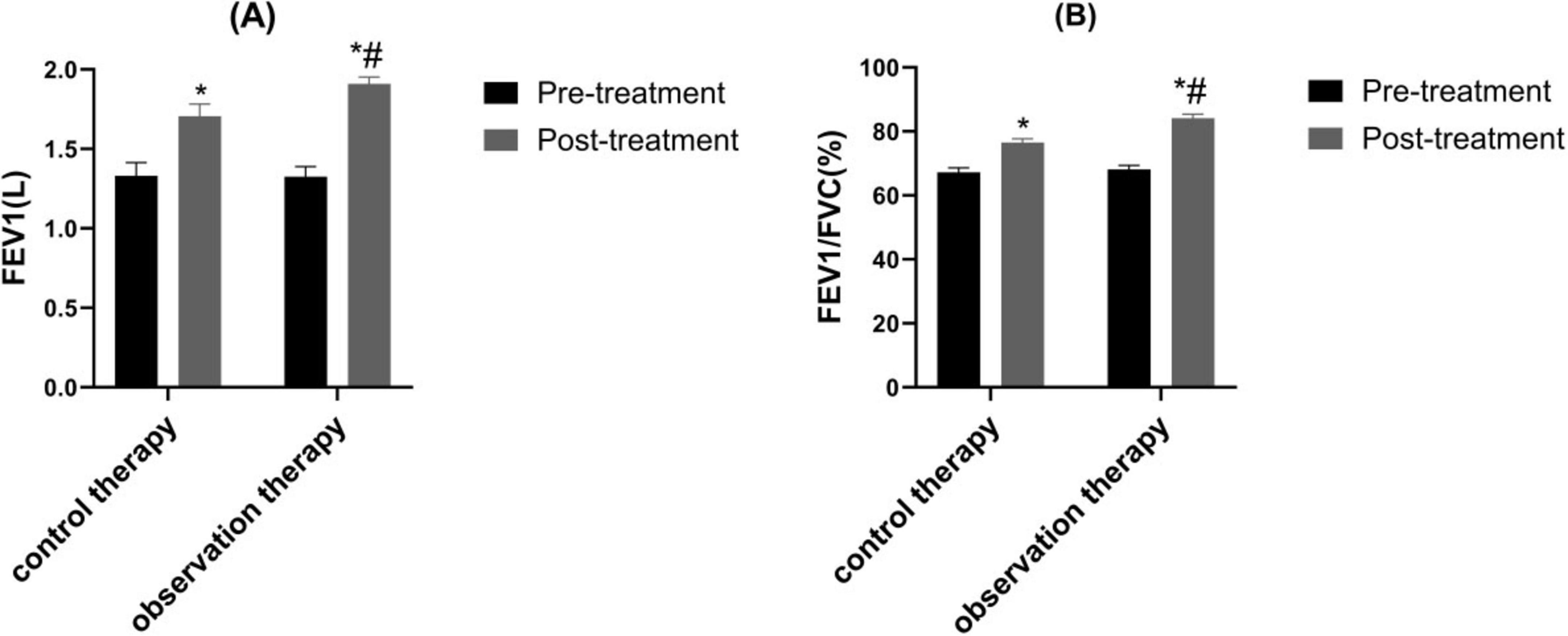
Figure 3. Comparison of pulmonary function. (A) Forced Expiratory Volume in 1 s (FEV1); (B) FEV1/Forced Vital Capacity (FVC). *p < 0.05 vs. pre-treatment, #p < 0.05 vs. control therapy.
3.5 Contrast of daytime and nighttime cough scores
The post-processing scores of daytime and nighttime cough were reduced than pre-processing in two groups (P < 0.05). Besides, the scores in observation were lower than control (P < 0.05) (Figure 4, P < 0.05).
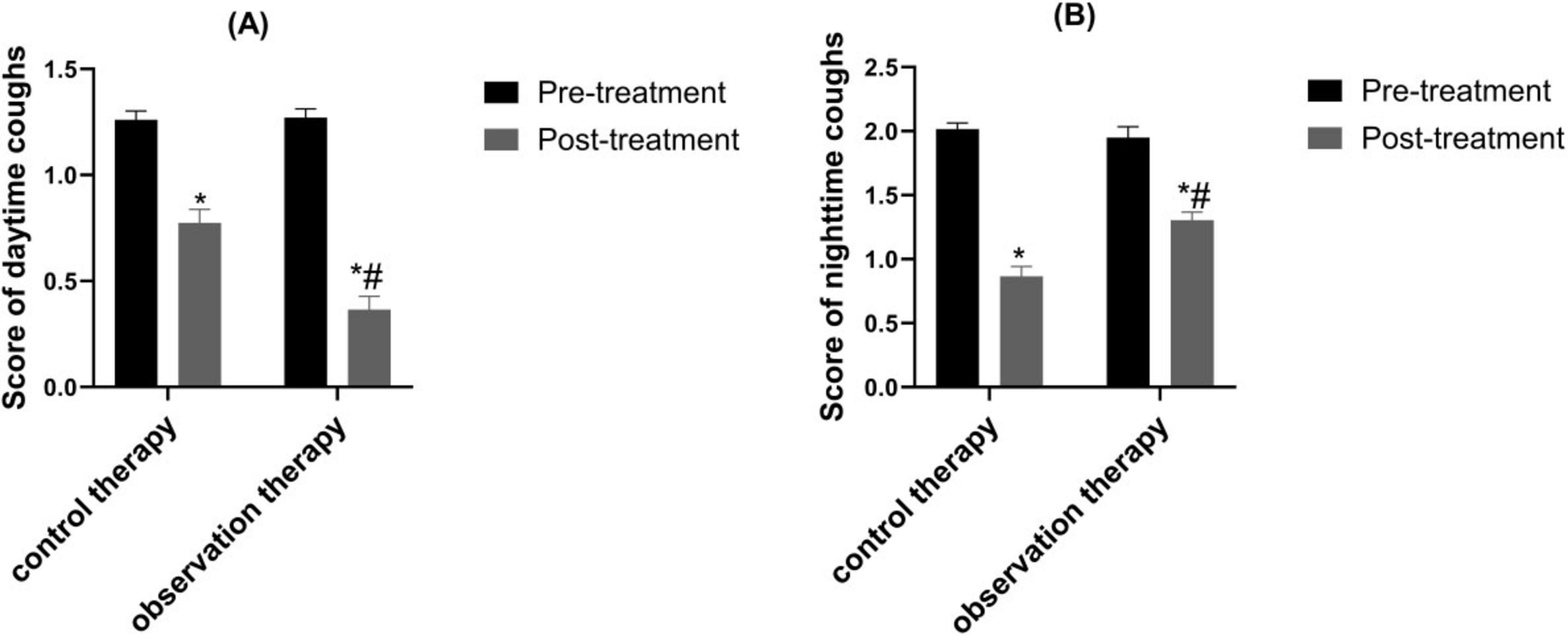
Figure 4. Contrast of daytime and nighttime cough scores. (A) Daytime cough score; (B) Nighttime cough score. *p < 0.05 vs. pre-treatment, #p < 0.05 vs. control therapy.
3.6 Comparison of immune function
Before therapy, CD3+, CD4+, CD4+/CD8+ of the two groups were similar (P > 0.05). Through treatment cycles, the CD3+, CD4+, CD4+/CD8+ in observation therapy were markedly upgraded than control (Figure 5, P < 0.05).
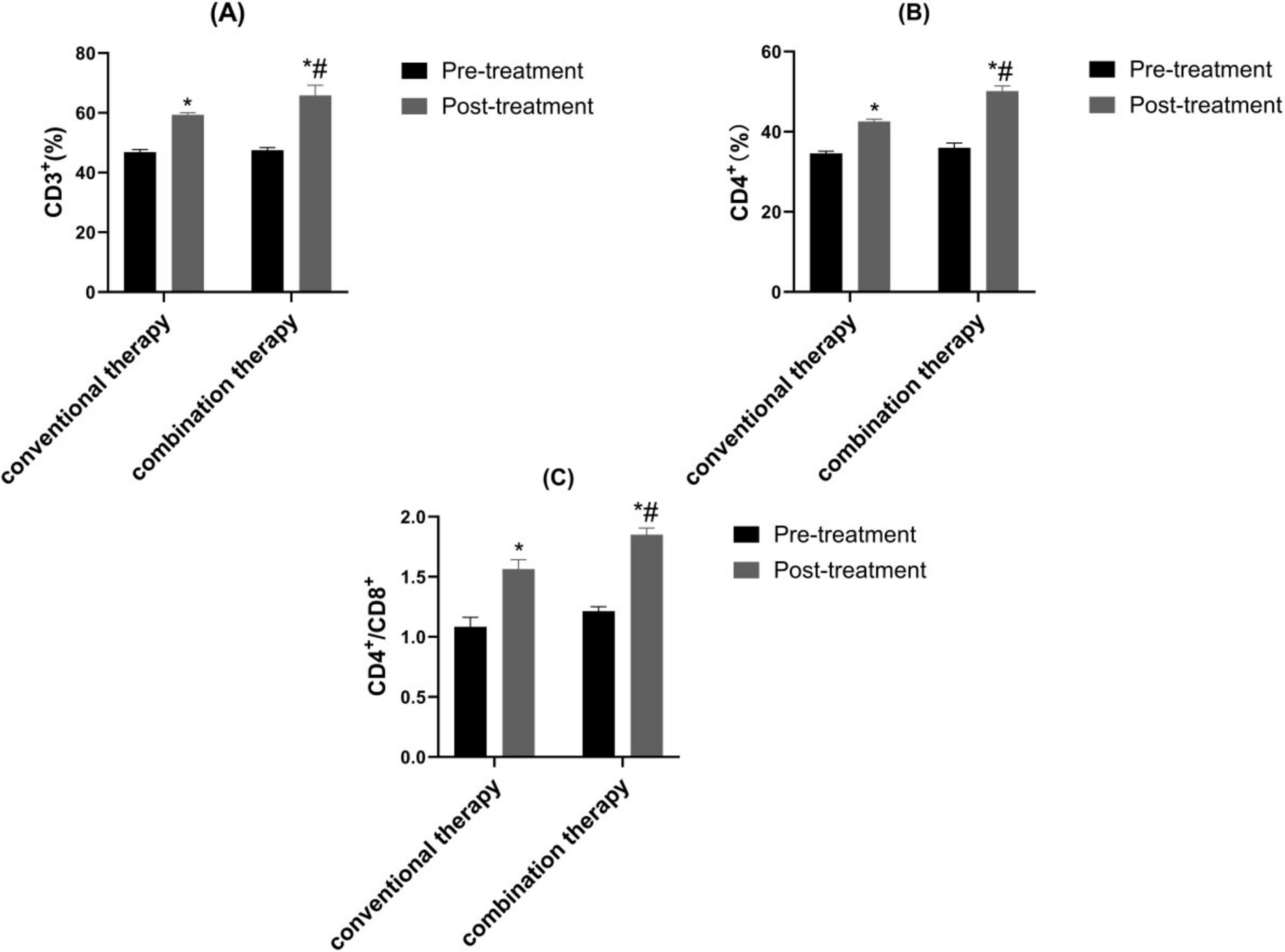
Figure 5. Contrast of immune function. (A) CD3+, (B) CD4+ and (C) CD4+/CD8+. *p < 0.05 vs. pre-treatment, #p < 0.05 vs. control therapy.
3.7 Contrast of untoward reaction
The incidence of adverse reactions in the observation and control therapy were no noteworthy diversity in untoward reaction Table 2.
4 Discussion
4.1 Analysis of therapeutic mechanism
Childhood variant cough is a common childhood disease, which is closely related to environmental, immune, genetic and other factors (21–24). In addition, children with variant cough have more obvious nighttime symptoms, and clinical symptoms are easily aggravated by environmental factors such as cold air and smoke (25). Clinical studies have found that chronic cough in children with this disease persists for a long time and has a long course of illness, seriously affecting the child’s physical health (24, 26–28). Presently, western medicine mainly uses leukotriene receptor antagonists, bronchodilators and glucocorticoid for treatment, among which fluticasone propionate suspension (FPS) is a glucocorticoid drug that has a strong affinity for glucocorticoid receptors and plays a strong local anti-inflammatory role (29). And it can exert anti-inflammatory effects by restraining phospholipase A, affecting the synthesis of inflammatory mediators. Concurrently, nebulization inhalation can minimize the toxicity of the drug (20). A meta-analysis found that the combined treatment of montelukast sodium for variant cough in children is more effective than using budesonide, fluticasone propionate, salmeterol fluticasone or ketotifen alone (30). Although the combination or single use of glucocorticoids has a quick effect, long-term extensive use can cause high recurrence rate and systemic hormonal side effects (31). Hence, it is crucial to find treatment methods with less side roles and superior efficacy for children with variant cough.
Traditional Chinese medicine believes that variant cough belongs to the category of “cough asthma” and “cough,” which is caused by the accumulation of internal heat in children, the combination of phlegm and heat, the invasion of external pathogens, and the stimulation of latent phlegm, leading to the loss of lung circulation. Cold qi and phlegm are the main pathological mechanisms (32). In recent years, it has been shown that traditional Chinese medicine can achieve good treatment efficacy in children with variant cough (22). Xuanfei Zhisou mixture (XZM) has the effects of dispelling wind, promoting lung circulation, stopping cough and resolving phlegm. Hu et al. demonstrated that XZM was able to diminish the symptoms of cough after wind cold lung accumulation infection and combined therapy exhibited better effect, which was consistent with the results of our experimental study (15). Our findings clarified that XZM plus FPS possessed better clinical efficacy and diminished daytime and nighttime cough scores, which were superior to FPS alone. This might be due to the fact that Aster tataricus in XZM can relieve cough and moisten the lungs. Stemona can reduce phlegm and heat, relax bronchial smooth muscle, and relieve spasms. Platycodon grandiflorum can promote lung function, relieve asthma, and relieve cough and phlegm. Pericarpium Citri Reticulatae can regulate qi and phlegm, relieve bronchial smooth muscle. Houttuynia cordata can have anti-inflammatory and anti-infective effects. Poppy shells can act on the cough center and have a cough suppressing effect. Licorice can dispel phlegm and inhibit smooth muscle. The combination of various medicines could prominently relieve the clinical symptoms of childhood. Additionally, our results elucidated that observation therapy was able to upgrade FEV1 and FEV1/FVC, hinting XZM plus FPS exerted a momentous role in raising childhood pulmonary function. The peripheral eosinophil count in observation therapy were lessened than control therapy, hinting that XZM combined with FPS was able to depress eosinophil count. What’s more, CD3+, CD4+, CD4+/CD8+ were enhanced in observation therapy, which implied that XZM plus FPS could amplify the childhood’s immune function and improve the childhood’s quality of life.
Chronic airway inflammation is closely related to the occur of variant cough (33–36). IL-4 is an important anti-inflammatory cytokine in the inflammatory response. The high expression of IL-4 can inhibit inflammatory response, thereby reducing pulmonary inflammation in children (37). TNF-α enhances the expression of IL-like inflammatory factors through Akt and JNK signaling, promoting the occurrence of airway inflammatory disease and causing damage to airway epithelial cells, ultimately leading to airway obstruction in children (38). SAA and CRP are both acute phase response proteins that rapidly increase in response to pathogen invasion during inflammation in the body. This acute phase reaction is often accompanied by airway inflammation, leading to increased airway hyperresponsiveness. The sharp increase of SAA and CRP may lead to worsening cough in children and exacerbate the inflammatory response of respiratory mucosa (39–41). It was found that the IL-4 was higher, while TNF-α, SAA as well as CRP levels were lowered in observation therapy than control therapy. The reason for this is that XZM and FPS can inhibit the formation of various inflammatory cells in the lungs, alleviate airway inflammation, and the effective ingredient pseudoephedrine in Ephedra can alleviate bronchospasm and reduce inflammation; The phenylpropanofuran derivatives in Jingjie have good anti-inflammatory and anti-infective effects; Fluticasone propionate reduces respiratory mucosal inflammation response; The combination of the above active ingredients jointly exerts the effect of inhibiting the release of inflammatory factors and reducing airway inflammation response. Interestingly, there was no diversity in adverse reactions between the two groups.
4.2 Safety of XZM-FPS combination
While the therapeutic efficacy of XZM-FPS combination has been confirmed, addressing concerns about drug safety–especially potential pharmacokinetic interactions and the safety of controlled components in XZM–is critical for clinical application. Two lines of evidence support the safety of this combination: ➀ Pharmacokinetic safety: A previous study (17) on 50 children with variant cough showed that combined use of XZM (10 mL tid) and FPS (100 μg bid) did not alter the serum concentration of FPS (Cmax: 125.3 ± 21.5 ng/mL in the combined group vs. 128.7 ± 19.8 ng/mL in the FPS-alone group; P = 0.623). This confirms no significant interaction in drug absorption or metabolism. ➁ Component safety of XZM: The Papaver somniferum husk in XZM contains low levels of morphine (0.05 mg per 10 mL) (19), which is far below the threshold for respiratory depression or addiction in children (≥0.5 mg/kg/day) (18). Our 3-months monitoring also showed no signs of dependence. These findings address concerns about drug interactions and controlled component safety, supporting the clinical applicability of the combination.
4.3 Limitations
Nevertheless, this study existed some limitations. The sample size of this study was relatively small. On the one hand, small sample size can lead to insufficient statistical ability, resulting in false negative results. On the other hand, the sample size is too small and contains limited information, which may lead to insufficient reliability and representativeness of the research results, making it difficult to accurately reflect the true situation of the results. In addition, this experiment was a retrospective study and there might be some bias in the results. As a result, it is crucial to design a multicenter, large sample research experiment. Furthermore, all samples in this study were Chinese children, and no non-Chinese populations were included. Due to potential differences in genetic backgrounds (such as polymorphisms of inflammation-related genes), living habits, and metabolic capacity of traditional Chinese medicine among children of different ethnicities, and considering the unique cultural background of Chinese populations in accepting and using traditional Chinese medicine, the conclusions of this study may not be directly generalized to children of other ethnic or cultural backgrounds. The lack of population diversity further limits the external validity of the research results. Therefore, in future multi-center studies, it is urgent to include children from different countries and ethnic groups to verify the applicability of XZM combined with FPS therapy in a wider population. The next step is to increase the single dose group of XZM and study the upstream and downstream regulatory pathways to explore its mechanism of action. Drug interaction data were based on small-sample preclinical studies; large-scale pharmacokinetic trials are needed to confirm XZM-FPS safety in diverse pediatric populations (e.g., children with liver impairment). The single-center design may limit generalizability; multi-center studies with stricter ethical oversight (e.g., independent data monitoring committees) are recommended.
5 Conclusion
In short, XZM plus FPS possessed a conspicuous role on children with variant cough, which could ameliorate cough symptoms, elevate pulmonary function, alleviate inflammatory cytokine levels as well as enhance immune function, which was worth promoting and using clinically. However, it should be emphasized that this study only included Chinese children and lacked relevant research data on non-Chinese populations. Thus, the generalization of the conclusions of this study should be limited to populations with similar ethnic and cultural backgrounds, and future studies need to further verify the efficacy and safety of this combined therapy in children of different ethnicities and cultural backgrounds which was worth promoting and using clinically within the scope of applicable populations.
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Shijiazhuang Maternity and Child Health Hospital with the official approval number 20201112. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
YZ: Conceptualization, Data curation, Funding acquisition, Methodology, Software, Writing – original draft. YS: Formal analysis, Project administration, Resources, Visualization, Writing – review & editing. JX: Formal analysis, Project administration, Resources, Visualization, Writing – review & editing. NX: Funding acquisition, Investigation, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
FPS, fluticasone propionate suspension; XZM, Xuanfei Zhisou mixture; SAA, serum amyloid A; CRP, c-reactive protein.
References
1. Feng B, He C, Liu X, Chen Y, He S. Effect of congenital heart disease on the recurrence of cough variant asthma in children. BMC Cardiovasc Disord. (2021) 21:130. doi: 10.1186/s12872-021-01940-8
2. Wu Q, Wang L, Wu M, Lin H. Effect of montelukast combined with budesonide on inflammatory response and pulmonary function in children with cough variant asthma: a meta-analysis. J Coll Physicians Surg Pak. (2023) 33:1040–9. doi: 10.29271/jcpsp.2023.09.1040
3. Attaianese F, Costantino A, Benucci C, Lasagni D, Trapani S. Benign acute children myositis: 5 years experience in a tertiary care pediatric hospital. Eur J Pediatr. (2023) 182:4341–9. doi: 10.1007/s00431-023-05115-9
4. Kunc P, Fabry J, Zatko T, Grendar M, Tatar M, Pecova R. Cough reflex sensitivity and fractional exhaled nitric oxide in children with asthma. Physiol Res. (2020) 69:S455–61. doi: 10.33549/physiolres.934601
5. Hurvitz M, Weinberger M. Functional respiratory disorders in children. Pediatr Clin North Am. (2021) 68:223–37. doi: 10.1016/j.pcl.2020.09.013
6. Wei Z, Li S. An efficacy and safety evaluation of montelukast + fluticasone propionate vs. fluticasone propionate in the treatment of cough variant asthma in children: a meta-analysis. BMC Pulm Med. (2023) 23:489. doi: 10.1186/s12890-023-02721-z
7. Ding B, Lu Y, Li Y, Zhou W, Qin F. Efficacy of treatment with montelukast, fluticasone propionate and budesonide liquid suspension for the prevention of recurrent asthma paroxysms in children with wheezing disorders. Exp Ther Med. (2019) 18:3090–4. doi: 10.3892/etm.2019.7894
8. Donohue JF, Kalberg C, Emmett A, Merchant K, Knobil K. A short-term comparison of fluticasone propionate/salmeterol with ipratropium bromide/albuterol for the treatment of COPD. Treat Respir Med. (2004) 3:173–81. doi: 10.2165/00151829-200403030-00005
9. Minciullo P, Saija A, Bonanno D, Ferlazzo E, Gangemi S. Montelukast-induced generalized urticaria. Ann Pharmacother. (2004) 38:999–1001. doi: 10.1345/aph.1D547
10. Eriksson J, Thörn H, Sjögren E, Holmstén L, Rubin K, Lennernäs H. Pulmonary dissolution of poorly soluble compounds studied in an ex vivo rat lung model. Mol Pharm. (2019) 16:3053–64. doi: 10.1021/acs.molpharmaceut.9b00289
11. Gulliver T, Morton R, Eid N. Inhaled corticosteroids in children with asthma: pharmacologic determinants of safety and efficacy and other clinical considerations. Paediatr Drugs. (2007) 9:185–94. doi: 10.2165/00148581-200709030-00007
12. Tu H, Zhang Q. Assessment of acupoint therapy of traditional chinese medicine on cough variant asthma: a meta-analysis. Biomed Res Int. (2022) 2022:4168308. doi: 10.1155/2022/4168308
13. Song P, Zeng L, Liang Z, Wang Q, Ou A. Clinical efficacy and safety of chinese herbal medicine auxiliary therapy for childhood cough variant asthma: a systematic review and meta-analysis of 20 randomized controlled trials. Intern Med. (2016) 55:2135–43. doi: 10.2169/internalmedicine.55.5546
14. Fan R, Wen Z, Wang D, Yang R, Ou A, Jin L, et al. Chinese herbal medicine for the treatment of cough variant asthma: a study protocol for a double-blind randomized controlled trial. Trials. (2019) 20:3. doi: 10.1186/s13063-018-3073-x
15. Hu Q, Wang Q, Duan P, Meng F, Gong X, Hu S, et al. [Clinical observation of post-infectious cough differentiated as wind-cold retention in the lung treated with scraping therapy and xuanfei zhisou decoction]. Zhongguo Zhen Jiu. (2016) 36:1257–62. doi: 10.13703/j.0255-2930.2016.12.009
16. Gaillard E, Kuehni C, Turner S, Goutaki M, Holden K, de Jong C, et al. European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children aged 5-16 years. Eur Respir J. (2021) 58:2004173. doi: 10.1183/13993003.04173-2020
17. Yin WP. Experimental study on partial pharmacodynamics and acute toxicity of Xuanfeizhike mixture. J Yunnan Coll Tradition Chin Med. (2002) 18–21. doi: 10.19288/j.carolcarrollnki
18. National Medical Products Administration. Guidelines for the Use of Controlled Traditional Chinese Medicines in Children. 2019th ed. Beijing: National Medical Products Administration (2024).
19. Chang WJ, Li Y, Zhang XD. A systematic review of the efficacy and safety of Xuanfei Zhisou Mixture in treating pediatric bronchitis. J China Prescript Drug. (2023) 21:18–22.
20. Berger W, Shapiro G. The use of inhaled corticosteroids for persistent asthma in infants and young children. Ann Allergy Asthma Immunol. (2004) 92:387–99; quiz 399–402, 463. doi: 10.1016/S1081-120661773-5
21. Fowler D, Malone M, Chisholm J, Roebuck D, Sebire N. Primary thoracic myxoid variant of extrarenal rhabdoid tumor in childhood. Fetal Pediatr Pathol. (2006) 25:159–68. doi: 10.1080/15513810600908388
22. Ren Y, Li X, Zhang Y, Yan Z. Xiaoqinglong decoction suppresses childhood cough variant asthma and inhibited the body inflammatory response by regulating IL-6/STAT3 signalling pathway. Ann Med Surg. (2023) 85:5469–77. doi: 10.1097/MS9.0000000000001326
23. Sun W, Liu H. Montelukast and budesonide for childhood cough variant asthma. J Coll Physicians Surg Pak. (2019) 29:345–8. doi: 10.29271/jcpsp.2019.04.345
24. Bede O, Szénási Z, Danka J, Gyurkovits K, Nagy D. Toxocariasis associated with chronic cough in childhood: a longitudinal study in Hungary. J Helminthol. (2008) 82:357–63. doi: 10.1017/S0022149X0804827X
25. Ioan I, Poussel M, Coutier L, Plevkova J, Poliacek I, Bolser D, et al. What is chronic cough in children. Front Physiol. (2014) 5:322. doi: 10.3389/fphys.2014.00322
26. Peng B, Gao Y, Xie J, He X, Wang C, Xu J, et al. Clinical and genetic spectrum of primary ciliary dyskinesia in Chinese patients: a systematic review. Orphanet J Rare Dis. (2022) 17:283. doi: 10.1186/s13023-022-02427-1
27. Chatziparasidis G, Chatziparasidi M, Kantar A, Bush A. Time-dependent gene-environment interactions are essential drivers of asthma initiation and persistence. Pediatr Pulmonol. (2024) 59:1143–52. doi: 10.1002/ppul.26935
28. Kuroda A, Namkoong H, Iwami E, Tsutsumi A, Nakajima T, Shinoda H, et al. X -linked inheritance of primary ciliary dyskinesia and retinitis pigmentosa due to RPGR variant: A case report and literature review. Respirol Case Rep. (2023) 11:e01240. doi: 10.1002/rcr2.1240
29. Zhang M, Si S, Dai W, Yang J, Wang Y, Wei X, et al. A large particle size is required by a nano/micron sized-fluticasone propionate inhalable suspension for asthma treatment. Colloids Surf B Biointerfaces. (2023) 228:113364. doi: 10.1016/j.colsurfb.2023.113364
30. Yang S, He X, Zhang R. Clinical efficacy of montelukast sodium combination therapy for cough variant asthma in children: a meta-analysis. Pediatr Pulmonol. (2024) 59:1541–51. doi: 10.1002/ppul.26960
31. Westmeier R, Steckel H. Combination particles containing salmeterol xinafoate and fluticasone propionate: Formulation and aerodynamic assessment. J Pharm Sci. (2008) 97:2299–310. doi: 10.1002/jps.21154
32. Wang J, Sun R, Wang R, Han J, Zhang S, Yin Z, et al. A traditional Chinese patent medicine-Suhuang zhike capsule for cough variant asthma in adults: A protocol of systematic review and meta-analysis of randomized controlled trials. Medicine. (2019) 98:e18335. doi: 10.1097/MD.0000000000018335
33. Niimi A, Chung K. Airway inflammation and remodelling changes in patients with chronic cough: do they tell us about the cause of cough. Pulm Pharmacol Ther. (2004) 17:441–6. doi: 10.1016/j.pupt.2004.09.004
34. Matsumoto H, Niimi A, Tabuena R, Takemura M, Ueda T, Yamaguchi M, et al. Airway wall thickening in patients with cough variant asthma and nonasthmatic chronic cough. Chest. (2007) 131:1042–9. doi: 10.1378/chest.06-1025
35. Zhou J, Yi F, Wu F, Xu P, Chen M, Shen H, et al. Characteristics of different asthma phenotypes associated with cough: a prospective, multicenter survey in China. Respir Res. (2022) 23:243. doi: 10.1186/s12931-022-02104-8
36. Zhu Z, Wu J, Chen W, Luo F, Zhao X. Bibliometric analysis of cough variant asthma from 1993 to 2022. J Asthma Allergy. (2024) 17:517–37. doi: 10.2147/JAA.S452097
37. He Y, Gao Y, Zhang Q, Zhou G, Cao F, Yao S. IL-4 switches microglia/macrophage M1/M2 polarization and alleviates neurological damage by modulating the JAK1/STAT6 pathway following ICH. Neuroscience. (2020) 437:161–71. doi: 10.1016/j.neuroscience.2020.03.008
38. Magierowska K, Wójcik-Grzybek D, Korbut E, Bakalarz D, Ginter G, Danielak A, et al. The mitochondria-targeted sulfide delivery molecule attenuates drugs-induced gastropathy. Involvement of heme oxygenase pathway. Redox Biol. (2023) 66:102847. doi: 10.1016/j.redox.2023.102847
39. Wu TL, Chang PY, Tsao KC, Sun CF, Wu LL, Wu JT, et al. Establishment of an in-house ELISA and the reference range for serum amyloid A (SAA): complementarity between SAA and C-reactive protein as markers of inflammation. Clin Chim Acta. (2007) 376:72–6. doi: 10.1016/j.cca.2006.07.012
40. Malle E, De Beer F. Human serum amyloid A (SAA) protein: a prominent acute-phase reactant for clinical practice. Eur J Clin Invest. (1996) 26:427–35. doi: 10.1046/j.1365-2362.1996.159291.x
Keywords: inflammatory factor level, immune function, fluticasone propionate suspension, Xuanfei Zhisou mixture, pulmonary function, variant cough in children
Citation: Zhang Y, Song Y, Xue J and Xue N (2025) The role of Xuanfei Zhisou mixture plus fluticasone propionate suspension in the treatment of variant cough in children and its effect on serum amyloid A and c-reactive protein. Front. Med. 12:1620973. doi: 10.3389/fmed.2025.1620973
Received: 30 April 2025; Accepted: 25 September 2025;
Published: 22 October 2025.
Edited by:
Shihua Shi, Friedrich Miescher Institute for Biomedical Research (FMI), SwitzerlandReviewed by:
Paulo Sargento, Escola Superior de Saúde Ribeiro Sanches, PortugalLonglong Wang, ETH Zürich, Switzerland
Copyright © 2025 Zhang, Song, Xue and Xue. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ningning Xue, eHVlbmluZ25pbmcxQG91dGxvb2suY29t
†Lead author
 Ying Zhang1†
Ying Zhang1† Ningning Xue
Ningning Xue