- Department of Interventional Medicine, The Third Affiliated Hospital of Gansu University of Chinese Medicine, Baiyin, China
Malignant tumors pose a significant global health burden, necessitating innovative treatment approaches. Personalized peripheral vascular interventional embolization emerges as a promising strategy to enhance outcomes in tumor therapy. This review consolidates evidence on the principles, influencing factors, implementation processes, and clinical applications of this approach. By analyzing tumor vascular anatomy and biological behavior, along with patient-specific factors, clinicians can tailor embolization techniques and materials to maximize efficacy and minimize complications. In practical clinical settings, personalized embolization has shown remarkable potential to enhance patient outcomes. For instance, in patients with hepatocellular carcinoma, personalized chemoembolization not only significantly improved survival rates but also reduced post-procedure complications, thereby improving quality of life. Similarly, in colorectal cancer liver metastases, the combination of embolization with anti-angiogenic agents has proven effective in controlling disease progression, offering a new therapeutic option where conventional treatments fall short. Despite challenges such as technical complexity and treatment costs, advancements in imaging technology, genomics, and novel embolic agent development offer substantial opportunities to refine and expand the application of personalized interventional embolization, potentially transforming the landscape of cancer treatment.
1 Introduction
Malignant tumors exact a heavy toll on global health, with cancer being the second leading cause of death worldwide, claiming nearly 10 million lives in 2020 (1). In the United States, approximately 1.9 million new cancer cases were diagnosed in 2023. In China, cancer incidence and mortality rates have been rising over the years, resulting in about 4.06 million new cases and 2.41 million deaths in 2022 (2). Despite advancements in radiation therapy, chemotherapy, targeted therapy, and immunotherapy, these treatments often fall short for advanced malignant solid tumors (3). Patients frequently suffer severe side effects, and tumor heterogeneity makes them respond variably to therapies (4). Consequently, there is an urgent imperative to develop new technologies for personalized treatment (5).
Interventional embolization therapy has emerged as a promising approach in cancer treatment (6, 7). However, most existing reviews have broadly summarized embolization techniques without critically addressing how personalized strategies—based on tumor biology, vascular architecture, and patient-specific factors—can significantly alter clinical outcomes. For example, transarterial chemoembolization (TACE) utilizing drug-eluting beads (DEBs) has demonstrated favorable clinical outcomes (8). DEBs microspheres can be loaded with doxorubicin and have shown clinical efficacy in treating colorectal cancer metastases to the liver (6). HepaSphere microspheres, approved by Conformité Européenne Mark for TACE of hepatocellular carcinoma (HCC) in combination with doxorubicin, can swell to occlude vessels and deliver drugs (9). Additionally, Ding et al. developed alginate-chitosan microspheres loaded with Norcantharidin, which showed significant tumor growth suppression in vitro (10). Choi et al. (11) fabricated poly lactic acid-polyglycolic acid (PLGA) microspheres loaded with sorafenib for HCC embolization. In a Renca tumor mouse model, combined cisplatin and sorafenib-loaded microspheres achieved tumor shrinkage, underscoring the potential of interventional embolization therapy.
Emerging evidence suggests that programmed cell death pathways, including PANoptosis—a novel inflammatory form of regulated cell death—may play a pivotal role in modulating tumor immune microenvironment and therapeutic response in HCC. Xiang et al. (12) recently proposed that targeting PANoptosis-related molecular signatures could serve as a promising diagnostic and therapeutic strategy, potentially enhancing the efficacy of interventional embolization by sensitizing tumor cells to ischemia-induced death and modulating immune surveillance. However, due to the heterogeneity of tumors across individuals, a one-size-fits-all approach to treatment is suboptimal. Tumors vary in vascular anatomy, biological behavior, and genetic background, necessitating personalized treatment (13). Research found that patients with HCC receiving personalized chemoembolization exhibited significantly improved survival rates and quality of life compared to conventional treatment (14, 15). Similarly, Vogl et al. (16) revealed that personalized embolization for colorectal cancer liver metastases effectively controlled disease progression and prolonged patient survival.
While existing studies have explored the use of embolization in tumor treatment, our review uniquely consolidates evidence on the principles, influencing factors, implementation processes, and clinical applications of personalized peripheral vascular interventional embolization. By integrating tumor biology, vascular anatomy, and patient-specific factors, this approach offers a more precise and effective treatment strategy compared to conventional methods. This review uniquely synthesizes the latest advancements in personalized embolization, highlighting its potential to overcome the limitations of traditional therapies through tailored treatment strategies. Compared to prior reviews, our work provides a comprehensive, evidence-based framework that links tumor biology, vascular mapping, and patient-specific factors to clinical decision-making in interventional oncology.
2 Principles of personalized peripheral vascular interventional embolization for tumors
To achieve such personalized outcomes, a deep understanding of the principles underlying peripheral vascular interventional embolization is essential. Precision in treatment is paramount for effective tumor management, and personalized peripheral vascular interventional embolization exemplifies this approach (17). The principles guiding this method have evolved considerably, driven by a deeper understanding of tumor biology and vascular anatomy (18). Initially, interventional embolization was employed as a palliative measure to alleviate symptoms and control bleeding (19). However, as experience grew and technology advanced, its potential as a curative option became evident. Research demonstrated that the efficacy of chemoembolization could be enhanced by optimizing the size and composition of embolic agents to match tumor vascular characteristics (20, 21). Similarly, Choi et al. (22) highlighted the importance of considering tumor blood flow dynamics and vascular permeability when selecting embolic materials and determining drug doses. These findings highlight the necessity of a personalized approach, wherein treatment strategies are specifically tailored to the unique vascular and biological characteristics of each tumor. The development of these principles marked a shift from a one-size-fits-all model to one that recognizes the unique requirements of different tumors, ultimately aiming to maximize therapeutic outcomes while minimizing complications.
The vascular anatomy of tumors is not only crucial for shaping interventional embolization strategies but also determines the choice of embolic agents and techniques (23). For instance, HCC typically has a hypervascular nature, with abundant arterial blood supply. This characteristic makes HCC amenable to TACE, where embolic agents can be delivered through the hepatic artery to induce tumor ischemia and necrosis (24). A study by Long et al. showed that using smaller embolic particles could more effectively occlude the tumor’s feeding arteries, achieving better tumor shrinkage and improving patient survival rates (18). Conversely, renal cell carcinoma (RCC) often has a more complex vascular network with variability in the number and size of feeding arteries. In such cases, a more precise approach is required (25). Calvo et al. (26) indicated that using a combination of embolic agents with different particle sizes could achieve more complete vascular occlusion, thereby enhancing treatment efficacy. For colorectal cancer liver metastases, the vascular anatomy differs from primary liver tumors, with a more homogeneous distribution of blood flow (22). Here, Choi et al. (22) found that using DEBs loaded with chemotherapy drugs could achieve sustained drug release and more uniform drug distribution within the tumor. This approach not only improved treatment efficacy but also reduced systemic drug toxicity. These examples illustrate how understanding the vascular anatomy of different tumors allows for the selection of appropriate embolic agents and techniques to maximize treatment outcomes.
The biological behavior of tumors also significantly influences interventional embolization strategies (27). Tumors vary in growth rate, invasive capacity, and angiogenesis characteristics, all of which must be considered when planning treatment. Tumors with rapid growth rates, such as pancreatic neuroendocrine tumors (NETs), often have high metabolic demands and are more reliant on neoangiogenesis (27). Research has shown that combining anti-angiogenic drugs with embolization therapy can effectively inhibit tumor growth and improve patient survival (28). For example, Liu et al. (29) demonstrated that using embolic agents loaded with anti-angiogenic drugs could not only block the tumor’s blood supply but also inhibit the formation of new vessels, thereby suppressing tumor growth. Tumors with strong invasive capabilities, such as hepatocellular carcinoma, are more likely to invade surrounding tissues and blood vessels (30). In such cases, the embolization treatment must not only target the tumor itself but also consider the potential impact on surrounding tissues. Nam et al. (31) indicated that using embolic agents with a more controlled release profile could reduce the risk of drug leakage into surrounding tissues, thereby minimizing complications. Tumors with high angiogenesis activity, such as RCC, rely heavily on neoangiogenesis for growth and metastasis (32). Research has shown that combining embolization therapy with anti-angiogenic drugs can effectively inhibit tumor angiogenesis and improve treatment outcomes (33). For instance, a study demonstrated that using embolic agents loaded with anti-angiogenic drugs could not only block the tumor’s blood supply but also inhibit the formation of new vessels, leading to significant tumor shrinkage and improved patient survival (34).
3 Factors influencing personalized treatment decisions
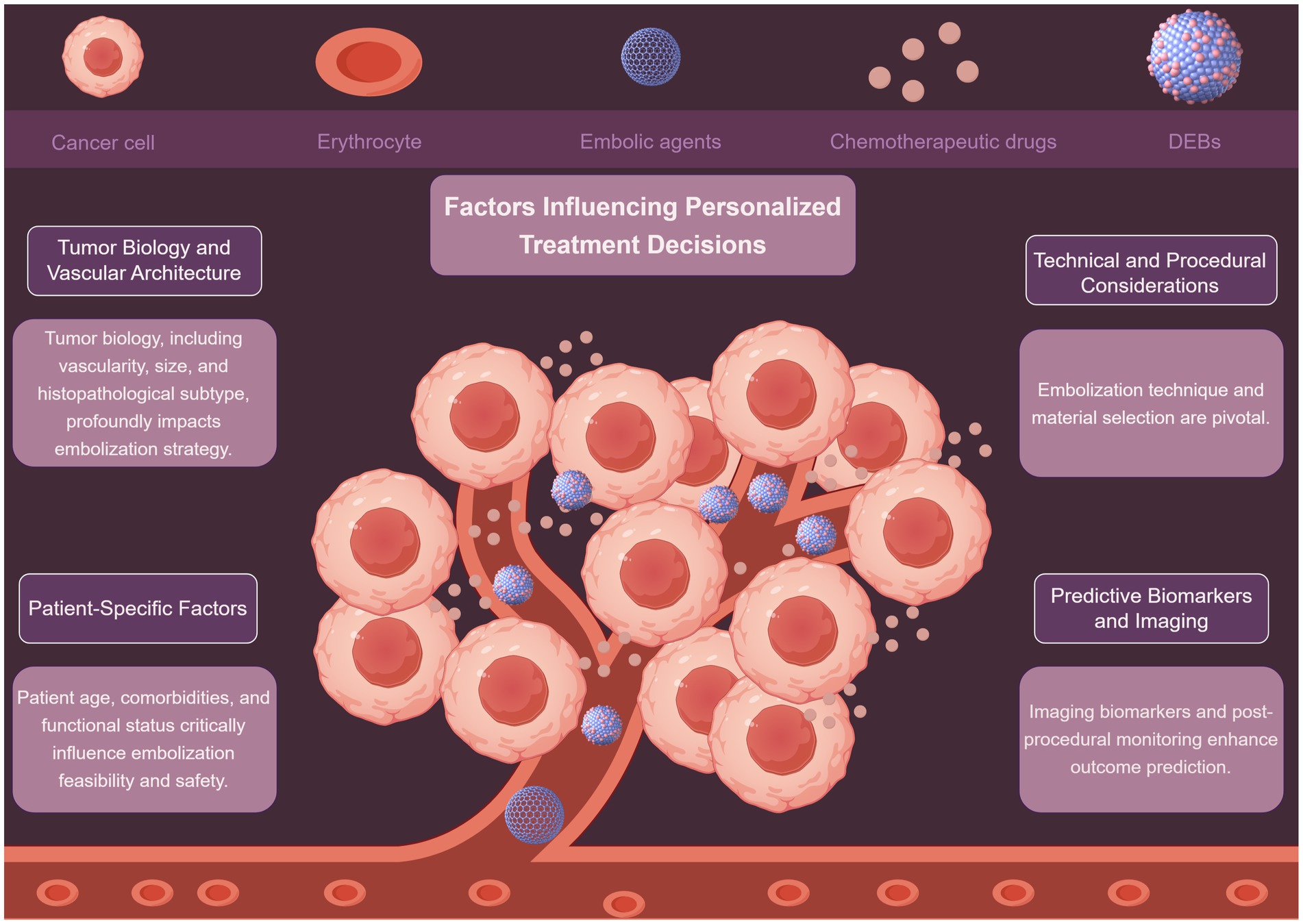
Personalized peripheral vascular interventional embolization for tumors requires a comprehensive decision-making process that integrates multiple factors (Figure 1). These factors include tumor biology, patient-specific characteristics, and technical considerations, among others. A detailed discussion of these factors is presented below.
3.1 Tumor biology and vascular architecture
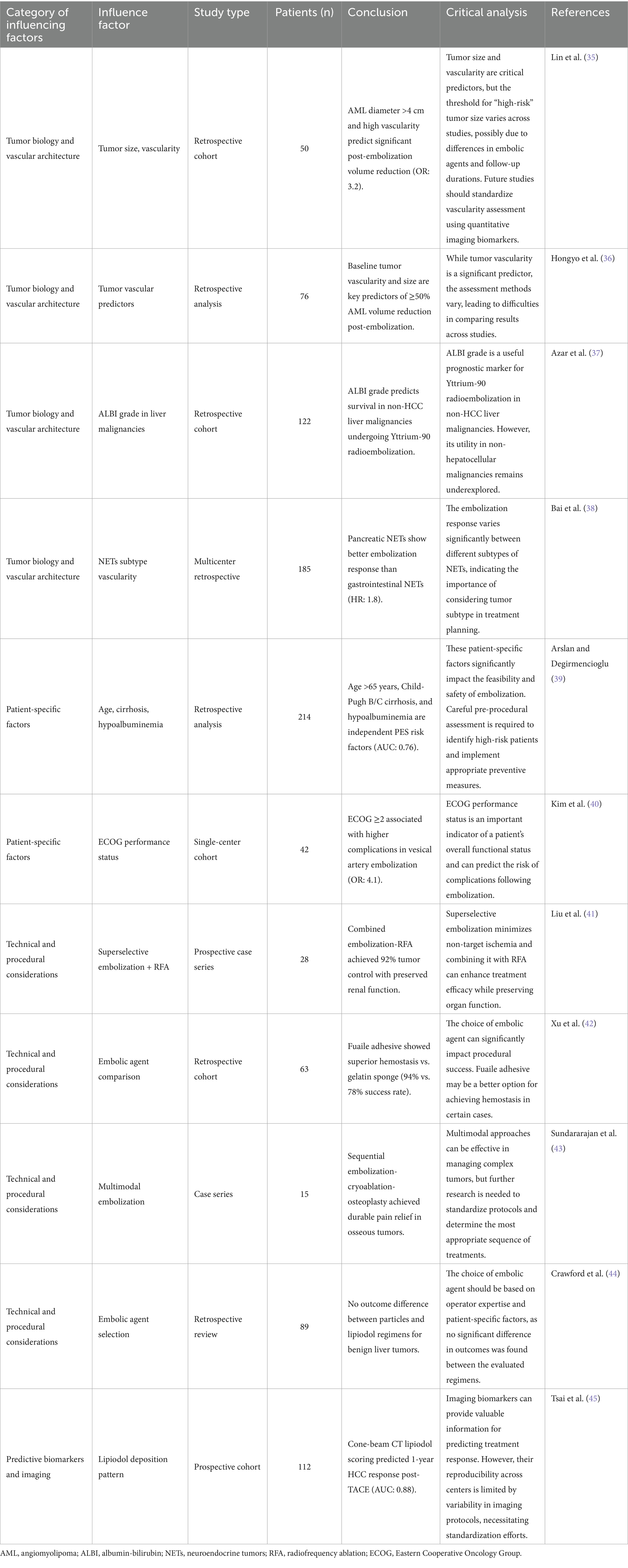
Tumor biology plays a vital role in shaping interventional embolization strategies. Tumors exhibit diverse vascular patterns, and understanding these differences is crucial for effective treatment. Hypervascular tumors, such as renal angiomyolipoma (AML), respond well to embolization, with tumor shrinkage rates correlating with baseline size and vascular density (35, 36). Mechanistically, high vascular density increases the delivery of embolic agents to the tumor site, enhancing ischemia-induced necrosis. Conversely, hypovascular tumors may require adjunctive strategies such as radioembolization or systemic therapy to overcome limited perfusion-mediated drug delivery. Lin et al. (35) demonstrated that AMLs diameter >4 cm and high intratumoral vascularity predicted significant post-embolization volume reduction (OR: 3.2, p < 0.01). Conversely, hypovascular metastases (e.g., colorectal liver metastases) may require combination therapies like radioembolization with Yttrium-90 to enhance efficacy (37). Notably, NETs exhibit heterogeneous vascular patterns; Bai et al. (38) found that embolization outcomes varied significantly between NETs subtypes, with pancreatic NETs showing better response than gastrointestinal counterparts (HR: 1.8, p = 0.03). This biological heterogeneity underscores the need for pre-procedural imaging biomarkers (e.g., perfusion CT, DCE-MRI) to quantify vascularity and predict embolic agent distribution, enabling a mechanistically informed selection of embolization strategy. While tumor size and vascularity are consistently identified as predictors (35, 36), discrepancies exist in thresholds for “high-risk” tumor size (e.g., 4 cm vs. 5 cm), likely due to differences in embolic agents and follow-up durations. Future studies should standardize vascularity assessment using quantitative imaging biomarkers.
3.2 Patient-specific factors
Patient-specific factors also significantly impact the feasibility and safety of embolization. Patient age, comorbidities, and functional status must be carefully evaluated. Arslan and Degirmencioglu (39) identified age >65 years, Child-Pugh B/C cirrhosis, and hypoalbuminemia as independent risk factors for post-embolization syndrome after TACE (AUC: 0.76). From a mechanistic perspective, hypoalbuminemia reflects reduced hepatic synthetic function and increased systemic inflammation, which may impair post-embolization recovery and increase susceptibility to infection or hepatic decompensation. Similarly, patients with ECOG ≥2 often have reduced systemic reserve, limiting their ability to tolerate procedural stress or post-embolization inflammation. Similarly, Kim et al. (40) reported higher complication rates in bladder cancer patients with ECOG ≥2 undergoing superselective vesical artery embolization (OR: 4.1, p < 0.001). Conversely, Hongyo et al. (36) emphasized that renal function preservation in AML patients favored selective embolization over surgery, particularly for those with chronic kidney disease (eGFR <60 mL/min/1.73 m2). The heterogeneity in comorbidity assessment complicates cross-study comparisons. Notably, while albumin-bilirubin (ALBI) grade predicts Yttrium-90 radioembolization survival in metastatic liver tumors, its utility in non-hepatocellular malignancies remains underexplored.
3.3 Technical and procedural considerations
Embolization technique and material selection are pivotal. Liu et al. (41) showed that superselective embolization minimizes non-target ischemia. They combined radiofrequency ablation with embolization for renal AMLs and achieved 92% tumor control with preserved renal function. Mechanistically, superselective catheterization enables targeted delivery of embolic agents to the tumor-feeding vessels while preserving adjacent normal tissue perfusion. This reduces the risk of post-embolization syndrome and organ dysfunction, particularly in patients with limited functional reserve. For gastrointestinal hemorrhage, Xu et al. (42) demonstrated superior hemostasis with Fuaile adhesive compared to gelatin sponge (success rate: 94% vs. 78%, p = 0.02). Emerging approaches, like sequential embolization-cryoablation-osteoplasty for osseous tumors, highlight the role of multimodal strategies in complex cases (43).
Despite advances, embolic agent selection remains contentious. Crawford et al. (44) noted no significant difference in benign liver tumor outcomes between particles and lipiodol-based regimens, suggesting agent choice should align with operator expertise. However, particle size impacts distal penetration; smaller particles (<100 μm) may increase PES risk4, necessitating personalized sizing. Importantly, the interaction between particle size and vascular resistance is a key determinant of embolic distribution. Smaller particles penetrate deeper into the tumor microvasculature but may also pass into collateral or shunt vessels, increasing the risk of non-target embolization. Thus, a mechanistic understanding of tumor hemodynamics (e.g., arteriovenous shunting, pressure gradients) is essential for safe and effective agent selection.
3.4 Predictive biomarkers and imaging
Imaging biomarkers and post-procedural monitoring enhance outcome prediction. Tsai et al. (45) developed a cone-beam CT-based lipiodol deposition scoring system to predict 1-year HCC response post-TACE (AUC: 0.88). Similarly, Azar et al. (37) validated ALBI grade as a prognostic marker for Yttrium-90 radioembolization in non-HCC liver malignancies (median OS: 15.2 vs. 8.1 months for ALBI grade 1 vs. 2, p = 0.004). This supports the hypothesis that hepatic reserve influences not only treatment tolerance but also the regenerative response to embolization-induced injury. Patients with poor liver function may have impaired compensatory regeneration, leading to worse outcomes even after technically successful procedures. While imaging biomarkers show promise, their reproducibility across centers is limited by variability in imaging protocols. Standardization efforts, such as the Quantitative Imaging Biomarkers Alliance guidelines, are essential for clinical adoption.
Personalized embolization strategies hinge on integrating tumor biology, patient physiology, technical precision, and predictive analytics. While consensus exists on factors like tumor vascularity and comorbidities, variability in study designs and outcome measures underscores the need for standardized protocols. Future research should prioritize prospective validation of biomarkers and comparative effectiveness studies to refine decision-making frameworks.
4 Process of personalized peripheral vascular interventional embolization for tumors
The process of personalized peripheral vascular interventional embolization for tumors involves a comprehensive pre-treatment evaluation, tailored treatment planning, careful intraoperative management, and rigorous post-treatment follow-up. This approach ensures that each patient receives the most appropriate treatment based on their individual characteristics and tumor features, ultimately leading to improved treatment outcomes and enhanced quality of life.
4.1 Pre-treatment evaluation
The pre-treatment evaluation phase is critical for establishing a comprehensive baseline of both the patient and the tumor. Imaging examinations such as computed tomography (CT) and magnetic resonance imaging (MRI) provide detailed anatomical information about the tumor, including its location, size, and vascular supply (46). CT angiography is particularly useful for visualizing the tumor’s blood supply and identifying potential feeding arteries (47). A study demonstrated that multi-phase CT imaging could accurately identify the vascular characteristics of hepatocellular carcinoma, guiding the selection of embolization techniques and embolic agents (21).
Laboratory tests are equally important. Liver function tests, such as albumin and bilirubin levels, help assess the patient’s ability to tolerate the procedure and the potential for post-embolization complications (48). For example, patients with Child-Pugh B cirrhosis may have a higher risk of liver failure following traditional TACE, making DEB-TACE a more suitable option (49). Similarly, renal function tests are essential for determining the patient’s ability to excrete contrast agents used during the procedure. Aoe et al. found that patients with impaired renal function had a higher incidence of contrast-induced nephropathy following TACE, highlighting the need for careful pre-treatment assessment (50).
Tumor biomarker assessments provide additional information about tumor activity and prognosis. Elevated levels of alpha-fetoprotein (AFP) in patients with HCC can indicate tumor burden and aggressiveness (51). He et al. showed that patients with high AFP levels often had poorer treatment outcomes following TACE, emphasizing the importance of incorporating biomarker data into treatment planning (52).
4.2 Treatment planning
Based on the pre-treatment evaluation, a tailored treatment plan is developed. This includes selecting the most appropriate embolization technique and determining the optimal embolic agents and drug dosages (53). Conventional TACE involves mixing chemotherapeutic drugs with lipiodol and embolic agents, while DEB-TACE uses drug-eluting microspheres loaded with chemotherapeutic drugs (54). A meta-analysis by Wang et al. found that DEB-TACE demonstrated better radiological tumor response and progression-free survival compared to conventional TACE. This is likely due to the more controlled drug release and reduced systemic toxicity of DEB-TACE (55).
The choice of embolic agents also depends on the tumor’s vascular characteristics and the desired treatment outcome (56). Gelatin sponge particles are biodegradable and commonly used in conventional TACE, but their non-uniform particle size may lead to non-target embolization. Polyvinyl alcohol particles are non-biodegradable and provide long-term vessel occlusion, but their use may increase the risk of post-embolization syndrome (56). Microspheres, such as DEBs, offer controlled drug release and reduced systemic toxicity. A retrospective study showed that DEB-TACE using microspheres achieved better tumor response and survival outcomes compared to conventional TACE with gelatin sponge particles (57).
4.3 Intraoperative management
The intraoperative phase involves the implementation of the treatment plan. During the procedure, the interventional radiologist carefully navigates the catheter to the tumor-feeding arteries and administers the embolic agents according to the pre-determined plan. Real-time imaging, such as fluoroscopy and angiography, is used to monitor the progress of the procedure and ensure accurate delivery of the embolic agents (58). Intraoperative complications, such as vessel perforation or non-target embolization, must be promptly identified and managed. Studies have shown that the combined use of embolizing agents with different particle sizes can achieve more complete vascular occlusion and reduce the risk of complications (18, 59).
4.4 Post-treatment follow-up
Short-term and long-term follow-up schedules are essential for monitoring treatment outcomes and complications. Short-term follow-up typically includes clinical assessments and imaging studies within the first few weeks to evaluate the immediate response to treatment and detect any complications (60). Scheau et al. (61) showed that early follow-up imaging could identify cases of incomplete tumor necrosis, allowing for timely re-treatment. Long-term follow-up involves regular monitoring of tumor recurrence and patient survival. Studies have demonstrated that patients receiving personalized embolization therapy often have improved survival rates and quality of life (62). For example, Garin et al. (63) found that personalized chemoembolization for HCC significantly improved patient survival rates compared to conventional treatment (Tables 1–4).
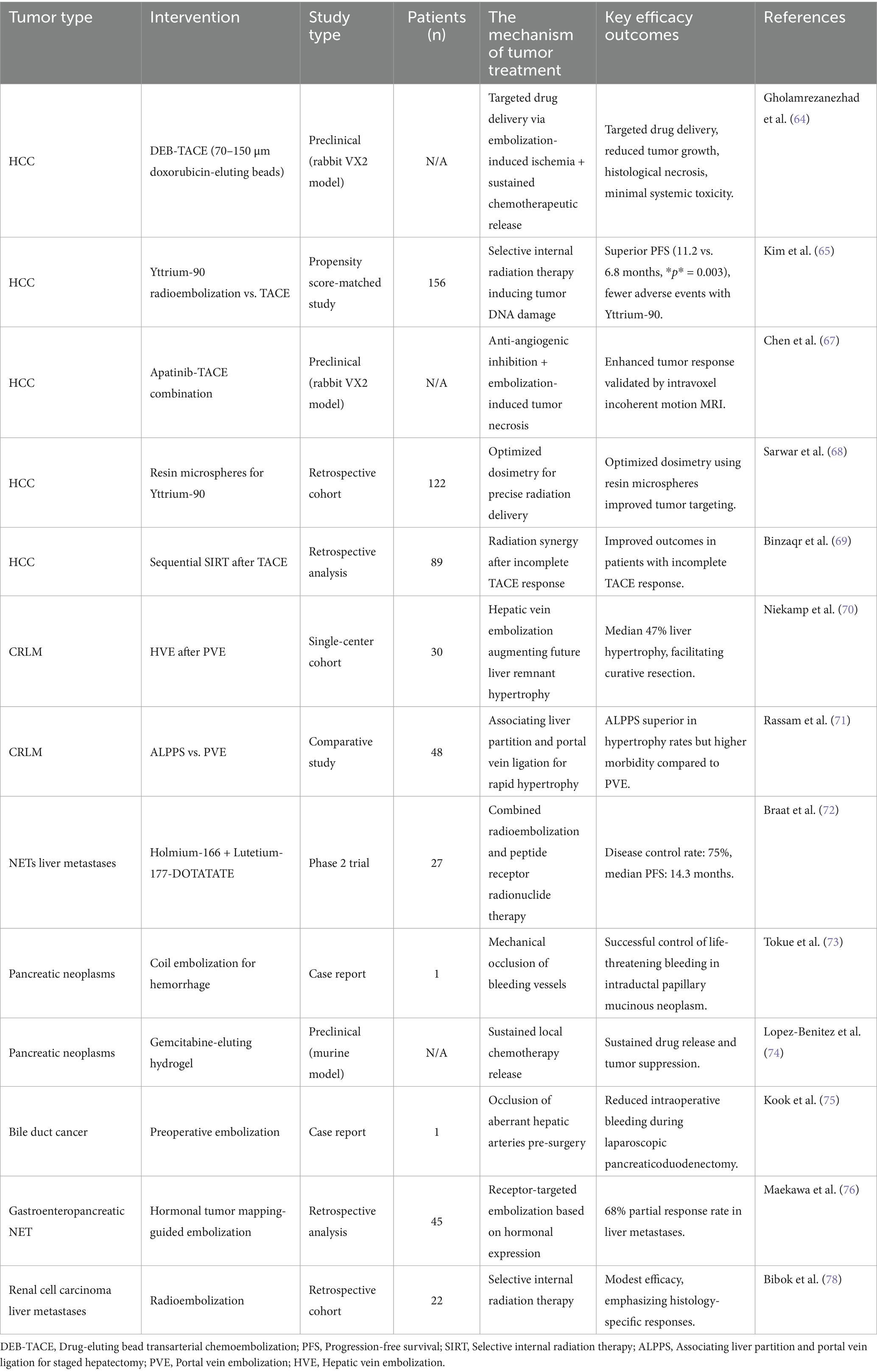
Table 2. The research of individualized peripheral vascular interventional embolization in the treatment of digestive system tumors.
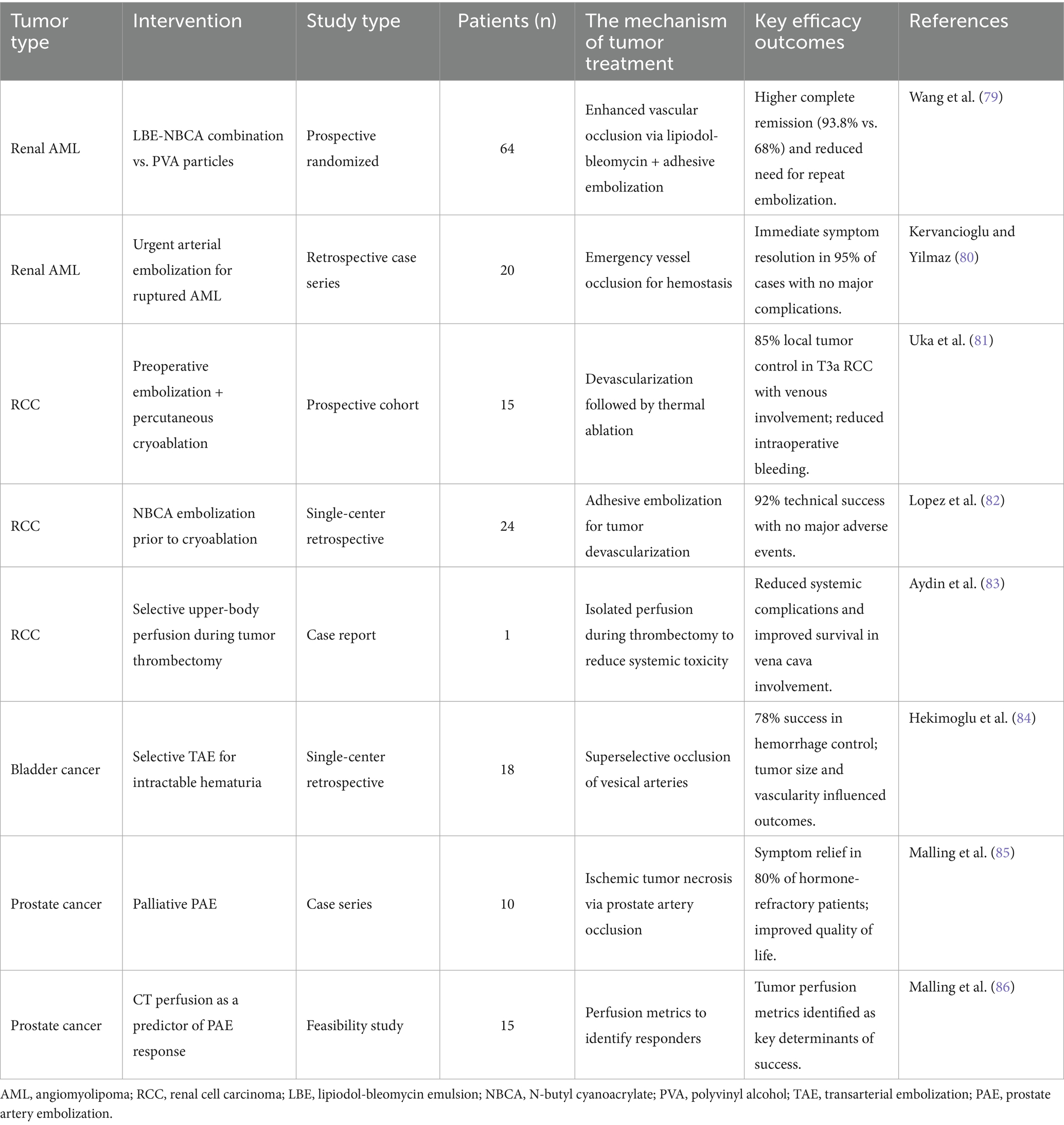
Table 3. The research of individualized peripheral vascular interventional embolization in the treatment of urinary system tumor.
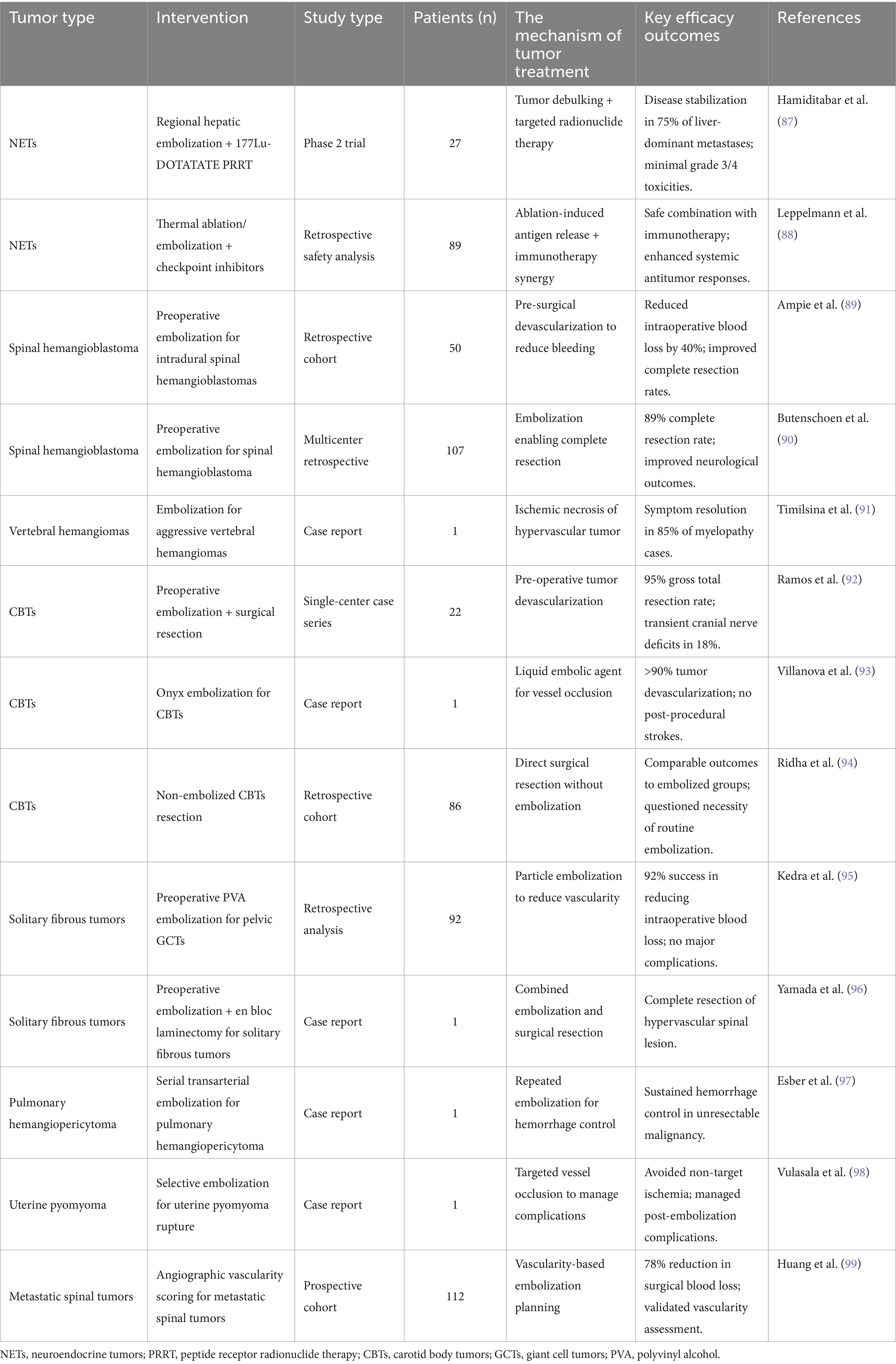
Table 4. The research of individualized peripheral vascular interventional embolization in the treatment of urinary system tumor.
5 Clinical applications and outcomes of personalized peripheral vascular interventional embolization for tumors
Personalized peripheral vascular interventional embolization offers a tailored approach to tumor treatment, enhancing outcomes through strategies aligned with individual tumor characteristics and patient needs. As illustrated in Figure 2, this method varies significantly across tumor types. For hepatocellular carcinoma (HCC), personalized chemoembolization improves survival while reducing complications. In colorectal cancer liver metastases, combining embolization with anti-angiogenic agents effectively controls disease progression. For pancreatic tumors and neuroendocrine tumor liver metastases, new techniques and agents are showing promise in early studies. These applications highlight the versatility and potential of personalized embolization in improving patient outcomes across a range of malignancies.
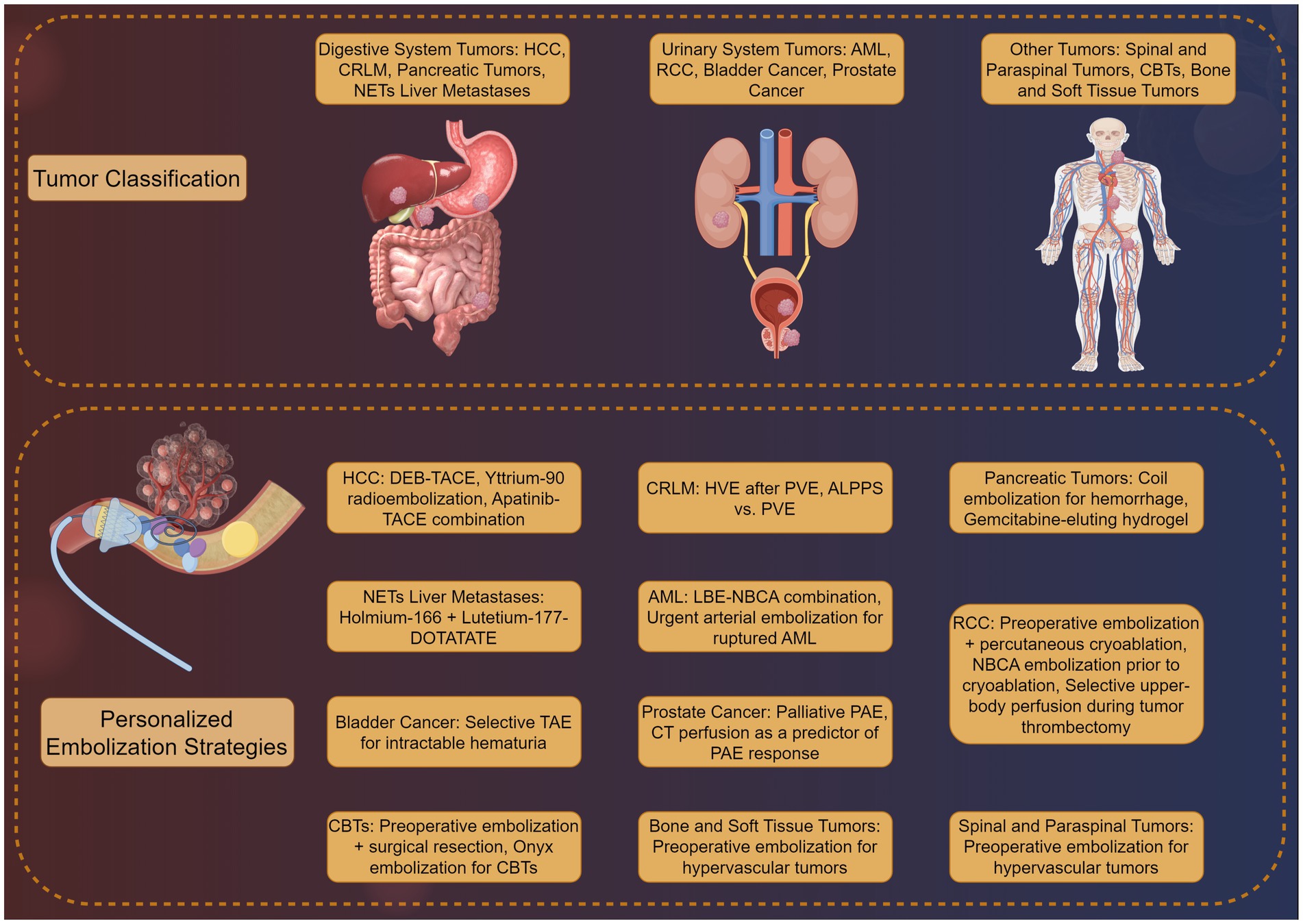
Figure 2. Clinical applications of personalized peripheral vascular interventional embolization in tumor therapy by Figdraw.
5.1 Digestive system tumors
Personalized TACE has revolutionized HCC management. Gholamrezanezhad et al. (64) demonstrated that 70–150 μm DEBs achieved targeted drug delivery and reduced tumor growth in a rabbit VX2 liver tumor model, with histological confirmation of necrosis and minimal systemic toxicity. In clinical practice, Kim et al. (65) compared Yttrium-90 radioembolization with conventional TACE in a propensity score-matched study (n = 156), showing superior progression-free survival (11.2 vs. 6.8 months, p = 0.003) and fewer adverse events with Yttrium-90. However, Lee et al. (66) highlighted regional variations in TACE refractoriness definitions, emphasizing the need for standardized criteria. Emerging strategies include combining TACE with anti-angiogenic agents; Chen et al. (67) reported enhanced tumor response in rabbit VX2 models using apatinib-TACE combination therapy, validated by intravoxel incoherent motion MRI. While DEB-TACE and Yttrium-90 show efficacy, discrepancies exist in patient selection and embolic agent protocols. For instance, Sarwar et al. (68) advocated for resin microspheres in Yttrium-90 to optimize dosimetry, whereas Binzaqr et al. (69) observed improved outcomes in HCC patients post-SIRT after incomplete TACE response, suggesting sequential therapy benefits. These variations underscore the need for personalized protocols based on tumor vascularity and liver reserve.
Portal vein embolization (PVE) and hepatic vein embolization (HVE) are critical for resectability in colorectal cancer liver metastases (CRLM). Niekamp et al. (70) demonstrated that HVE after PVE induced additional liver hypertrophy (median 47% volume increase) in metastatic colorectal cancer patients, facilitating curative resection. Comparatively, Rassam et al. (71) found associating liver partition and portal vein ligation (ALPPS) superior to PVE in hypertrophy rates but with higher morbidity. For unresectable cases, Braat et al. (72) combined lutetium-177-DOTATATE with holmium-166 radioembolization in neuroendocrine liver metastases, achieving disease control in 75% of patients. PVE and ALPPS differ in risk–benefit profiles, necessitating individualized planning. While ALPPS offers rapid hypertrophy, its morbidity limits use in frail patients. Radioembolization combinations (e.g., holmium-166) show promise but require validation in larger cohorts.
Embolization plays a role in managing bleeding and unresectable pancreatic tumors. Tokue et al. (73) successfully controlled life-threatening hemorrhage in intraductal papillary mucinous neoplasms using coil embolization. For advanced disease, Lopez-Benitez et al. (74) developed gemcitabine-eluting hydrogel devices, demonstrating sustained drug release and tumor suppression in murine models. Kook et al. (75) reported laparoscopic pancreaticoduodenectomy with preoperative embolization of aberrant hepatic arteries, reducing intraoperative bleeding in mid-bile duct cancer. While embolization effectively manages hemorrhage, therapeutic applications (e.g., drug-eluting hydrogels) remain experimental. Heterogeneity in pancreatic tumor biology complicates standardized protocols, necessitating biomarker-driven approaches.
Liver-dominant NETs metastases benefit from radioembolization and multimodal approaches. Maekawa et al. (76) introduced hormonal tumor mapping to guide embolization of gastroenteropancreaticNETs liver metastases, achieving 68% partial response rates. Drescher et al. (77) combined holmium-166 TARE with systemic therapy, reporting median progression-free survival of 14.3 months in a multicenter study. In contrast, Bibok et al. (78) observed modest efficacy of radioembolization in RCC liver metastases, emphasizing histology-specific responses. NETs heterogeneity demands subtype-specific strategies. While holmium-166 shows promise, its utility in non-NET metastases is limited, highlighting the importance of molecular profiling.
Personalized embolization strategies improve outcomes across digestive tumors by integrating tumor biology, vascular anatomy, and patient-specific factors. While HCC and CRLM have robust evidence, pancreatic and NETs applications require further refinement. Discrepancies in study designs (e.g., DEB-TACE vs. Yttrium-90) highlight the need for comparative trials. Future directions include biomarker-driven protocols and advanced embolic materials to optimize therapeutic precision.
5.2 Urinary system tumor
Personalized peripheral vascular interventional embolization has emerged as a critical strategy for managing urinary system tumors, particularly in cases where surgical resection is contraindicated or for palliative care. This section synthesizes evidence from recent studies, focusing on renal AML, RCC, bladder cancer, and prostate cancer, while evaluating the consistency and limitations of current research.
AML, a benign tumor composed of fat, smooth muscle, and blood vessels, often requires embolization to manage hemorrhage or reduce tumor burden. A landmark prospective randomized study by Wang et al. (79) compared polyvinyl alcohol (PVA) particles versus a combination of lipiodol-bleomycin emulsion (LBE) and NBCA-lipiodol emulsion for AML embolization. The LBE-NBCA group demonstrated superior outcomes, including higher rates of complete remission (CR, 93.8% vs. 68% in PVA) and reduced need for repeat embolization, attributed to enhanced vascular occlusion and tumor necrosis. These findings underscore the importance of tailored embolic agent selection based on tumor vascularity and clinical presentation. For acute scenarios, urgent embolization of ruptured AML achieves rapid hemostasis. Kervancioglu and Yilmaz (80) reported immediate symptom resolution in 95% of cases, with no major complications, highlighting its role in emergency management. However, long-term follow-up data remain limited, necessitating further studies to validate durability.
In locally advanced RCC, embolization is often combined with surgical or ablative therapies. Uka et al. (81) demonstrated that preoperative embolization followed by percutaneous cryoablation achieved 85% local tumor control in T3a RCC with venous involvement, minimizing intraoperative bleeding and enhancing procedural safety. Similarly, Lopez et al. (82) utilized NBCA embolization prior to cryoablation, achieving 92% technical success with no major adverse events, suggesting synergistic benefits in tumor devascularization. Selective embolization also plays a role in managing unresectable RCC. Aydin et al. (83) employed upper-body perfusion techniques during tumor thrombectomy, reducing systemic complications and improving survival in patients with vena cava involvement. These studies emphasize the need for individualized approaches based on tumor stage and vascular anatomy.
For advanced bladder cancer with intractable hematuria, selective transarterial embolization (TAE) offers effective palliation. Hekimoglu et al. (84) reported a 78% success rate in controlling hemorrhage, with minimal procedural morbidity, in patients unfit for surgery. Notably, tumor size and vascularity influenced outcomes, with larger tumors requiring higher embolic doses. While these results align with earlier studies, heterogeneity in embolic agents (e.g., gelatin sponge vs. coils) across trials complicates direct comparisons, underscoring the need for standardized protocols.
Prostate artery embolization (PAE) has gained traction for palliative management of localized prostate cancer. Malling et al. (85) observed significant symptom relief in 80% of patients with hormone-refractory disease, with reduced urinary obstruction and improved quality of life. A follow-up feasibility study by the same group proposed CT perfusion as a predictor of PAE response, identifying tumor perfusion metrics as key determinants of success (86). However, small sample sizes and single-center designs limit generalizability, warranting multicenter validation.
Current evidence supports the efficacy of personalized embolization across urinary tumors, yet inconsistencies persist. For AML, while LBE-NBCA combinations outperform PVA 10, cost and availability may limit adoption in resource-constrained settings. In RCC, combined embolization-ablation strategies show promise, but long-term oncologic outcomes remain understudied. For bladder and prostate cancers, standardization of embolic agents and dosages is crucial to enhance reproducibility. Future research should prioritize randomized trials comparing embolic materials, integrate biomarkers for response prediction, and explore combination therapies with immunotherapy or targeted agents. Such efforts will refine personalization paradigms and solidify embolization’s role in multidisciplinary tumor management.
5.3 Other tumors
Personalized peripheral vascular interventional embolization has demonstrated efficacy in managing a diverse range of tumors beyond the urinary and digestive systems. This section evaluates its role inNETs, spinal and soft tissue neoplasms, carotid body tumors, and other rare malignancies, focusing on clinical outcomes, technical nuances, and areas requiring further standardization.
5.3.1 NETs
For somatostatin receptor-positive NETs, combining embolization with peptide receptor radionuclide therapy (PRRT) enhances tumor control. Hamiditabar et al. (87) reported that regional hepatic embolization followed by 177Lu-DOTATATE PRRT achieved disease stabilization in 75% of patients with liver-dominant metastases, with minimal grade 3/4 toxicities. This approach leverages embolization to reduce tumor bulk and PRRT for targeted radiation delivery. Emerging strategies, such as combining embolization with immunotherapy, have shown potential synergies. Leppelmann et al. (88) demonstrated the safety of combining thermal ablation or embolization with checkpoint inhibitors, suggesting enhanced systemic antitumor responses. Future studies should standardize embolization protocols to optimize combination strategies.
5.3.2 Spinal and paraspinal tumors
Preoperative embolization significantly reduces intraoperative bleeding in hypervascular spinal tumors. For intradural spinal hemangioblastomas, Ampie et al. (89) demonstrated that preoperative embolization reduced blood loss by 40% compared to non-embolized cases. Similarly, Butenschoen et al. (90) reported improved neurological outcomes in a multicenter cohort of spinal hemangioblastoma patients, where embolization enabled complete resection in 89% of cases. For aggressive vertebral hemangiomas, Timilsina et al. (91) highlighted the role of embolization in alleviating myelopathy, achieving symptom resolution in 85% of patients. However, variability in embolic agents (e.g., Onyx vs. PVA particles) and timing (preoperative vs. palliative) underscores the need for tumor-specific protocols.
5.3.3 Carotid body tumors (CBTs)
Embolization prior to CBTs resection minimizes intraoperative hemorrhage. Ramos et al. (92) reported a 95% gross total resection rate in 22 CBTs patients undergoing preoperative embolization, with transient cranial nerve deficits in 18%. Villanova et al. (93) further validated the safety of Onyx embolization, achieving >90% tumor devascularization without post-procedural strokes. In contrast, Ridha et al. (94) observed comparable outcomes in non-embolized CBTs resections, questioning the necessity of routine preoperative embolization. These discrepancies may stem from differences in tumor size (embolization favored for tumors >3 cm) and surgeon expertise, highlighting the importance of individualized decision-making.
5.3.4 Bone and soft tissue tumors
Preoperative embolization is critical for managing hypervascular musculoskeletal tumors. Kedra et al. (95) reported a 92% success rate in reducing intraoperative blood loss for pelvic giant cell tumors (GCTs) using polyvinyl alcohol (PVA) particles, with no major complications. Scheurer et al. (93) further emphasized the role of embolization in iliosacral GCTs, achieving 80% tumor necrosis and facilitating safer resection. For solitary fibrous tumors, Yamada et al. (96) combined preoperative embolization with en bloc laminectomy, achieving complete resection of a hypervascular spinal lesion. Despite these successes, standardized embolic protocols (e.g., particle size, timing) remain lacking, necessitating multicenter trials.
5.3.5 Rare and uncommon tumors
Embolization serves as a palliative or adjunctive measure in rare malignancies. For unresectable pulmonary hemangiopericytoma, Esber et al. (97) achieved sustained hemorrhage control using serial transarterial embolization. In uterine pyomyoma rupture post-embolization, Vulasala et al. (98) underscored the importance of selective embolization to avoid non-target ischemia. For metastatic spinal tumors, Huang et al. (99) proposed angiographic vascularity scoring to predict embolization efficacy, achieving 78% reduction in surgical blood loss. These studies highlight embolization’s versatility but also reveal gaps in evidence for ultra-rare tumors.
Current evidence supports the role of personalized embolization in diverse tumors, yet challenges persist. For NETs, combining embolization with PRRT or immunotherapy (e.g., checkpoint inhibitors) shows promise but requires validation in larger cohorts (88). In spinal and CBTs, standardization of embolic materials (e.g., Onyx vs. PVA) and timing is critical to reduce variability. For bone tumors, predictive biomarkers (e.g., perfusion CT) may refine patient selection.
6 Challenges and opportunities in personalized peripheral vascular interventional embolization for tumors
While the clinical applications highlight the potential of personalized embolization, several challenges must be overcome to ensure its broader and more effective implementation. Personalized peripheral vascular interventional embolization for tumors faces several significant challenges (100). Technical limitations are a major hurdle. The procedure requires a deep understanding of vascular anatomy, knowledge of pathology, and experience with endovascular catheters and microvascular navigation skills (101). Unexpected non-target embolization may lead to tissue ischemia, organ failure, and, in rare cases, death. The complexity of the procedure necessitates a high level of expertise from the interventional radiologist (18). For instance, embolization for arteriovenous malformations (AVMs) requires the use of liquid embolic agents that can penetrate deeply into the AVMs and embolize the nidus. This demands precise control and extensive experience (102).
Treatment costs are another concern. The development and utilization of advanced embolic agents and technologies incur significant costs. For example, drug-eluting microspheres and nanoparticles are promising but can increase the overall cost of treatment (103). A study comparing stent-based and non-stent-based strategies in endovascular revascularization found that stent implantation was associated with significantly higher procedure costs (34). While this study focused on peripheral artery disease, the principle applies to tumor embolization as well.
The complexity of drug selection and dosing also poses challenges. Different tumors respond variably to embolic agents and chemotherapeutic drugs. Determining the optimal drug combination and dosage for each patient requires careful consideration of tumor biology, vascular characteristics, and patient comorbidities (104). For example, a study demonstrated that alginate-chitosan microspheres loaded with Norcantharidin showed favorable sustained drug release and significantly decreased tumor growth rates in vitro (34). However, translating these findings into clinical practice requires further research to determine the optimal dosing and administration protocols.
Despite these challenges, there are numerous opportunities for the advancement of personalized interventional embolization. Imaging technology continues to evolve, providing more detailed and accurate information about tumor vascular anatomy and blood flow dynamics (105). Advanced imaging techniques, such as computed tomography (CT) angiography and magnetic resonance imaging (MRI), enable precise identification of tumor-feeding arteries and assessment of tumor response to treatment. For example, multi-phase CT imaging can accurately identify the vascular characteristics of hepatocellular carcinoma, guiding the selection of embolization techniques and embolic agents (47).
Genomics and biomarker research offer exciting possibilities for tailoring embolization therapy to individual patients. By analyzing tumor genetics and biomarker profiles, clinicians can better predict tumor behavior and response to treatment (106). This information can be used to select the most appropriate embolic agents and drugs, potentially improving treatment efficacy and reducing complications. For instance, research into the proliferative activity of cancer cells observed on immunohistochemistry can help determine the likelihood of tumor progression after embolization (107). This can guide treatment planning and patient selection for embolization procedures.
The development of new embolic agents and technologies is another promising area. Drug-eluting microspheres and nanoparticles are being actively researched and developed. These agents offer advantages such as controlled drug release, reduced systemic toxicity, and the potential for targeted delivery (108). Park et al. (109) showed that PLGA microspheres loaded with sorafenib could achieve tumor shrinkage in a Renca tumor mouse model, highlighting the potential of these novel agents. The advent of smaller calibrated microspheres and nanoparticle technology may allow for more distal penetration into tumors, potentially improving treatment outcomes. Additionally, biodegradable particles used for tumor therapy may maintain vessel patency for future interventions.
Personalized peripheral vascular interventional embolization for tumors faces challenges related to technical complexity, treatment costs, and drug selection (110). However, advancements in imaging technology, genomics, and biomarker research, along with the development of new embolic agents and technologies, provide significant opportunities to overcome these challenges and enhance the effectiveness of this treatment approach (111). By addressing these challenges and leveraging these opportunities, the field of personalized interventional embolization can continue to evolve and improve outcomes for patients with cancer.
7 Future directions for personalized peripheral vascular interventional embolization in tumor therapy
The future of personalized peripheral vascular interventional embolization is promising, with significant advancements expected in techniques and materials. Robotic-assisted endovascular embolization represents a cutting-edge development in the field (18). This technology enhances procedural precision and reproducibility, enabling more accurate delivery of embolic agents to target sites. Studies have shown that robotic assistance can improve the stability and control of catheter navigation, potentially reducing procedure time and radiation exposure for both patients and healthcare providers (18).
Innovations in embolic materials are also expected to enhance treatment efficacy and safety. For instance, a novel liquid embolic agent composed of a coenzyme-based polymer (poly lipoic acid, PLA) and a biocompatible solvent (deep eutectic solvent, DES) has demonstrated favorable hemocompatibility and cytocompatibility in preclinical studies (112). This agent undergoes phase transformation to form a stable hydrogel upon contact with blood, providing effective and safe embolization. Additionally, the incorporation of liquid-metal nanoparticles enhances radiopacity, improving imaging during procedures (113).
Multidisciplinary collaboration among oncology, interventional radiology, and pathology is crucial for optimizing personalized treatment strategies. Multidisciplinary teams (MDTs) can integrate expertise from various fields to develop comprehensive treatment plans tailored to individual patients (114). Research has shown that MDT care can significantly improve overall survival in patients with HCC. A meta-analysis of 12 studies including 15,365 HCC patients suggested that MDT is associated with improved overall survival, with a hazard ratio of 0.63 (95% confidence interval, 0.45–0.88). MDT care ensures that patients receive the most appropriate combination of therapies, including surgery, interventional procedures, and systemic treatments, based on their specific tumor characteristics and overall health status (114).
The integration of genetic and molecular profiling with clinical data holds immense potential for achieving true personalized tumor treatment. Advances in genomics and biomarker research enable clinicians to better understand tumor biology and predict treatment responses (115). For example, analyzing tumor genetics can help identify patients who are more likely to benefit from specific embolization techniques or drug combinations. This information can be used to select the most appropriate treatment approach, potentially improving efficacy and reducing complications (116).
Moreover, the development of trackable polymeric embolic agents and stem cell delivery systems offers exciting possibilities for enhancing treatment outcomes (117). Trackable embolic agents allow for real-time monitoring of embolization procedures, ensuring accurate delivery and improving safety. Stem cell therapy, when combined with embolization, may help reduce inflammation and promote tissue repair, providing additional benefits for patients with tumors (117).
8 Conclusion
This review uniquely synthesizes the latest advancements in personalized embolization, highlighting its potential to overcome the limitations of traditional therapies through tailored treatment strategies. By leveraging innovations in imaging technology, genomics, and novel embolic agents, our work provides a forward-looking perspective that distinguishes itself from prior research. This approach improves outcomes and quality of life. Despite challenges like technical complexity and costs, advancements in imaging, genomics, and new embolic agents offer opportunities to enhance efficacy. Future directions include robotic assistance, innovative materials, and multidisciplinary collaboration, promising more precise and effective therapies for cancer patients.
Author contributions
XQ: Conceptualization, Data curation, Formal analysis, Investigation, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Conceptualization, Project administration, Supervision, Validation, Writing – review & editing. TL: Conceptualization, Formal analysis, Methodology, Software, Writing – review & editing. HH: Conceptualization, Funding acquisition, Investigation, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung, H, Ferlay, J, Siegel, RL, Laversanne, M, Soerjomataram, I, Jemal, A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. (2021) 71:209–49. doi: 10.3322/caac.21660
2. Islami, F, Ward, EM, Sung, H, Cronin, KA, Tangka, FKL, Sherman, RL, et al. Annual report to the nation on the status of cancer, part 1: national cancer statistics. J Natl Cancer Inst. (2021) 113:1648–69. doi: 10.1093/jnci/djab131
3. Wu, Y, He, S, Cao, M, Teng, Y, Li, Q, Tan, N, et al. Comparative analysis of cancer statistics in China and the United States in 2024. Chin Med J. (2024) 137:3093–100. doi: 10.1097/cm9.0000000000003442
4. Zhang, X, Xie, J, Yang, Z, Yu, CKW, Hu, Y, and Qin, J. Tumour heterogeneity and personalized treatment screening based on single-cell transcriptomics. Comput Struct Biotechnol J. (2025) 27:307–20. doi: 10.1016/j.csbj.2024.12.020
5. Wang, QB, Li, J, Zhang, ZJ, Li, YK, Liang, YB, Chen, XM, et al. The effectiveness and safety of therapies for hepatocellular carcinoma with tumor thrombus in the hepatic vein, inferior vena cave and/or right atrium: a systematic review and single-arm meta-analysis. Expert Rev Anticancer Ther. (2025) 25:561–70. doi: 10.1080/14737140.2025.2489651
6. Chen, Y, Yang, Y, Xu, WJ, Xin, YJ, Wang, YN, Zhou, X, et al. Clinical application of interventional embolization in tumor-associated hemorrhage. Ann Transl Med. (2020) 8:394. doi: 10.21037/atm.2020.03.69
7. Li, YK, Wu, S, Wu, YS, Zhang, WH, Wang, Y, Li, YH, et al. Portal venous and hepatic arterial coefficients predict post-hepatectomy overall and recurrence-free survival in patients with hepatocellular carcinoma: a retrospective study. J Hepatocell Carcinoma. (2024) 11:1389–402. doi: 10.2147/jhc.S462168
8. Zhang, Y, Zhang, MW, Fan, XX, Mao, DF, Ding, QH, Zhuang, LH, et al. Drug-eluting beads transarterial chemoembolization sequentially combined with radiofrequency ablation in the treatment of untreated and recurrent hepatocellular carcinoma. World J Gastrointest Surg. (2020) 12:355–68. doi: 10.4240/wjgs.v12.i8.355
9. Malagari, K, Pomoni, M, Moschouris, H, Kelekis, A, Charokopakis, A, Bouma, E, et al. Chemoembolization of hepatocellular carcinoma with HepaSphere 30-60 μm. Safety and efficacy study. Cardiovasc Intervent Radiol. (2014) 37:165–75. doi: 10.1007/s00270-013-0777-x
10. Ding, J, and Guo, Y. Recent advances in chitosan and its derivatives in cancer treatment. Front Pharmacol. (2022) 13:888740. doi: 10.3389/fphar.2022.888740
11. Choi, JW, Park, J-H, Cho, HR, Chung, JW, Kim, D-D, Kim, H-C, et al. Sorafenib and 2,3,5-triiodobenzoic acid-loaded imageable microspheres for transarterial embolization of a liver tumor. Sci Rep. (2017) 7:554. doi: 10.1038/s41598-017-00709-4
12. Xiang, J, Li, Y, Mei, S, Ou, Z, Wang, L, Ke, Y, et al. Novel diagnostic and therapeutic strategies based on PANoptosis for hepatocellular carcinoma. Cancer Biol Med. (2025) 22:1–12. doi: 10.20892/j.issn.2095-3941.2025.0150
13. Krzyszczyk, P, Acevedo, A, Davidoff, EJ, Timmins, LM, Marrero-Berrios, I, Patel, M, et al. The growing role of precision and personalized medicine for cancer treatment. Technology (Singap World Sci). (2018) 6:79–100. doi: 10.1142/s2339547818300020
14. Zhong, C, Niu, Y, Liu, W, Yuan, Y, Li, K, Shi, Y, et al. S100A9 derived from chemoembolization-induced hypoxia governs mitochondrial function in hepatocellular carcinoma progression. Adv Sci (Weinh). (2022) 9:e2202206. doi: 10.1002/advs.202202206
15. Peng, Z, Fan, W, Zhu, B, Wang, G, Sun, J, Xiao, C, et al. Lenvatinib combined with transarterial chemoembolization as first-line treatment for advanced hepatocellular carcinoma: a phase III, randomized clinical trial (LAUNCH). J Clin Oncol. (2023) 41:117–27. doi: 10.1200/jco.22.00392
16. Vogl, TJ, Marko, C, Langenbach, MC, Naguib, NNN, Filmann, N, Hammerstingl, R, et al. Transarterial chemoembolization of colorectal cancer liver metastasis: improved tumor response by DSM-TACE versus conventional TACE, a prospective, randomized, single-center trial. Eur Radiol. (2021) 31:2242–51. doi: 10.1007/s00330-020-07253-2
17. Guelfi, S, Hodivala-Dilke, K, and Bergers, G. Targeting the tumour vasculature: from vessel destruction to promotion. Nat Rev Cancer. (2024) 24:655–75. doi: 10.1038/s41568-024-00736-0
18. Wang, CY, Hu, J, Sheth, RA, and Oklu, R. Emerging embolic agents in endovascular embolization: an overview. Prog Biomed Eng (Bristol). (2020) 2. doi: 10.1088/2516-1091/ab6c7d
19. Papageorgiou, NM, Palaiodimou, L, Melanis, K, Theodorou, A, Stefanou, M-I, Tsalouchidou, P-E, et al. Embolization of middle meningeal artery in patients with chronic subdural hematoma: a systematic review and meta-analysis of randomized-controlled clinical trials. J Clin Med. (2025) 14:2862. doi: 10.3390/jcm14092862
20. Iezzi, R, Posa, A, Bargellini, I, and Spreafico, C. Transarterial chemoembolization with BioPearls for the treatment of hepatocellular carcinoma: a preliminary experience. Pharmaceuticals. (2025) 18:307. doi: 10.3390/ph18030307
21. Sutanto, H, Adytia, GJ, Elisa, E, and Maimunah, U. Advances in transarterial chemoembolization for hepatocellular carcinoma: integration with systemic therapies and emerging treatment strategies. Cancer Pathog Ther. (2025). doi: 10.1016/j.cpt.2025.04.004
22. Choi, Y, and Jung, K. Normalization of the tumor microenvironment by harnessing vascular and immune modulation to achieve enhanced cancer therapy. Exp Mol Med. (2023) 55:2308–19. doi: 10.1038/s12276-023-01114-w
23. Patel, RK, Tripathy, T, Maivizhi Shruthi, M, and Pal, T. Embolization in interventional radiology In: SH Chandrashekhara, editor. Textbook of interventional radiology. Singapore: Springer Nature Singapore (2024)
24. Miyayama, S, Arai, Y, and Matsui, O. Transarterial chemoembolization for hepatocellular carcinoma with vascular invasion. Br J Radiol. (2022) 95:20211316. doi: 10.1259/bjr.20211316
25. Munetomo, K, Matsui, Y, Tomita, K, Uka, M, Umakoshi, N, Kawabata, T, et al. Depiction rate of feeding arteries of renal cell carcinoma on four-dimensional computed tomography angiography. Jpn J Radiol. (2024) 42:648–55. doi: 10.1007/s11604-024-01538-y
26. Calvo, E, Porta, C, Grünwald, V, and Escudier, B. The current and evolving landscape of first-line treatments for advanced renal cell carcinoma. Oncologist. (2019) 24:338–48. doi: 10.1634/theoncologist.2018-0267
27. Hadjigeorgiou, AG, and Stylianopoulos, T. Hybrid model of tumor growth, angiogenesis and immune response yields strategies to improve antiangiogenic therapy. NPJ Biol Phys Mech. (2024) 1:4. doi: 10.1038/s44341-024-00002-2
28. Yu, J, Yu, J, Chen, Y, Yang, Y, and Yi, P. PD-1 inhibitors improve the efficacy of transcatheter arterial chemoembolization combined with apatinib in advanced hepatocellular carcinoma: a meta-analysis and trial sequential analysis. BMC Cancer. (2025) 25:564. doi: 10.1186/s12885-025-13932-4
29. Liu, X, Zhang, J, Yi, T, Li, H, Tang, X, Liu, D, et al. Decoding tumor angiogenesis: pathways, mechanisms, and future directions in anti-cancer strategies. Biomark Res. (2025) 13:62. doi: 10.1186/s40364-025-00779-x
30. Lencioni, R, Petruzzi, P, and Crocetti, L. Chemoembolization of hepatocellular carcinoma. Semin Intervent Radiol. (2013) 30:3–11. doi: 10.1055/s-0033-1333648
31. Nam, HC, Jang, B, and Song, MJ. Transarterial chemoembolization with drug-eluting beads in hepatocellular carcinoma. World J Gastroenterol. (2016) 22:8853–61. doi: 10.3748/wjg.v22.i40.8853
32. Lugano, R, Ramachandran, M, and Dimberg, A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cell Mol Life Sci. (2020) 77:1745–70. doi: 10.1007/s00018-019-03351-7
33. Zhou, C, Shi, Q, Liu, J, Huang, S, Yang, C, and Xiong, B. Effect of inhibiting tumor angiogenesis after embolization in the treatment of HCC with apatinib-loaded p(N-isopropyl-acrylamide-co-butyl methyl acrylate) temperature-sensitive nanogel. J Hepatocell Carcinoma. (2020) 7:447–56. doi: 10.2147/jhc.S282209
34. Jia, G, Van Valkenburgh, J, Chen, AZ, Chen, Q, Li, J, Zuo, C, et al. Recent advances and applications of microspheres and nanoparticles in transarterial chemoembolization for hepatocellular carcinoma. Wiley Interdiscip Rev Nanomed Nanobiotechnol. (2022) 14:e1749. doi: 10.1002/wnan.1749
35. Lin, L, Wang, C, Pei, R, Guan, H, Wang, J, Yang, M, et al. Prophylactic selective arterial embolization for renal angiomyolipomas: efficacy and evaluation of predictive factors of significant shrinkage. Int Urol Nephrol. (2018) 50:1765–70. doi: 10.1007/s11255-018-1953-3
36. Hongyo, H, Higashihara, H, Osuga, K, Kashiwagi, E, Kosai, S, Nagai, K, et al. Efficacy of prophylactic selective arterial embolization for renal angiomyolipomas: identifying predictors of 50% volume reduction. CVIR Endovasc. (2020) 3:84. doi: 10.1186/s42155-020-00179-2
37. Azar, A, Devcic, Z, Paz-Fumagalli, R, Vidal, LLC, McKinney, JM, Frey, G, et al. Albumin-bilirubin grade as a prognostic indicator for patients with non-hepatocellular primary and metastatic liver malignancy undergoing Yttrium-90 radioembolization using resin microspheres. J Gastrointest Oncol. (2020) 11:715–23. doi: 10.21037/jgo.2020.04.01
38. Bai, J, Song, J, Zhang, Y, Li, X, Yan, L, Hu, P, et al. Transcatheter arterial embolization in patients with neuroendocrine neoplasms related to liver metastasis with different blood supplies. Cancer Med. (2023) 12:18578–87. doi: 10.1002/cam4.6464
39. Arslan, M, and Degirmencioglu, S. Risk factors for postembolization syndrome after transcatheter arterial chemoembolization. Curr Med Imaging Rev. (2019) 15:380–5. doi: 10.2174/1573405615666181122145330
40. Kim, MS, Hong, HP, Kang, KA, Lee, YR, Joo, KJ, Cho, YS, et al. Superselective vesical artery embolization for intractable bladder hemorrhage related to pelvic malignancy. Acta Radiol. (2021) 62:1229–37. doi: 10.1177/0284185120952781
41. Liu, F, Yuan, H, Li, X, Tang, J, Tian, X, and Ji, K. A new management strategy for renal angiomyolipomas: superselective arterial embolization in combination with radiofrequency ablation. Ann Transl Med. (2019) 7:766. doi: 10.21037/atm.2019.11.63
42. Xu, M, Zhu, X, Liu, Y, Li, Z, An, T, Jiang, T, et al. Embolization of arterial gastrointestinal hemorrhage with Fuaile medical adhesive. J Chin Med Assoc. (2018) 81:636–42. doi: 10.1016/j.jcma.2017.10.006
43. Sundararajan, SH, Calamita, S, Girgis, P, Ngo, G, Ranganathan, S, Giglio, M, et al. Sequential interventional management of osseous neoplasms via embolization, cryoablation, and osteoplasty. J Oncol. (2019) 2019:1–6. doi: 10.1155/2019/5247837
44. Crawford, D, Naidu, S, Patel, I, Knuttinen, G, Alzubaidi, S, and Oklu, R. Bland embolization of benign liver tumors: review of the literature and a single center experience. J Clin Med. (2021) 10:658. doi: 10.3390/jcm10040658
45. Tsai, YC, Shih, JH, Hwang, HE, Chiu, NC, Lee, RC, Tseng, HS, et al. Early prediction of 1-year tumor response of hepatocellular carcinoma with lipiodol deposition pattern through post-embolization cone-beam computed tomography during conventional transarterial chemoembolization. Eur Radiol. (2021) 31:7464–75. doi: 10.1007/s00330-021-07843-8
46. Azadbakht, J, Condos, A, Haynor, D, Gibbs, WN, Jabehdar Maralani, P, Sahgal, A, et al. The role of CT and MR imaging in stereotactic body radiotherapy of the spine: from patient selection and treatment planning to post-treatment monitoring. Cancer. (2024) 16:3692. doi: 10.3390/cancers16213692
47. Abou Khadrah, RS, Abedelmalik, MH, Alameldeen, MAE, and Elbarbary, AA. Hepatocellular carcinoma vascularization: CT angiography variations identifying arteries feeding the tumour. Egypt J Radiol Nucl Med. (2023) 54:183. doi: 10.1186/s43055-023-01133-7
48. Johansson, P, Alig, S, Richter, J, Hanoun, C, Rekowski, J, Dürig, J, et al. Outcome prediction by interim positron emission tomography and IgM monoclonal gammopathy in diffuse large B-cell lymphoma. Ann Hematol. (2023) 102:3445–55. doi: 10.1007/s00277-023-05393-1
49. Deng, Z, and Wang, Y. Predictors of liver failure after transarterial chemoembolization in patients with spontaneously ruptured hepatocellular carcinoma: a retrospective study. J Interv Med. (2023) 6:35–40. doi: 10.1016/j.jimed.2022.10.003
50. Aoe, M, Kanemitsu, T, Ohki, T, Ambe, H, Furukawa, E, Kurata, Y, et al. MP205incidence and risk factors of contrast−induced nephropathy after transcatheter arterial chemoembolization in hepatocellular carcinoma. Nephrol Dial Transplant. (2016) 31:i408–9. doi: 10.1093/ndt/gfw187.11
51. Lu, Y, Lin, B, and Li, M. The role of alpha-fetoprotein in the tumor microenvironment of hepatocellular carcinoma. Front Oncol. (2024) 14:1363695. doi: 10.3389/fonc.2024.1363695
52. He, C, Zhang, X, Li, C, Peng, W, Wen, TF, Yan, LN, et al. Changes of alpha-fetoprotein levels could predict recurrent hepatocellular carcinoma survival after trans-arterial chemoembolization. Oncotarget. (2017) 8:85599–611. doi: 10.18632/oncotarget.20343
53. Peng, X, Tang, H, Zhao, Z, Zheng, Y, Gui, X, Jiang, A, et al. Intelligent generic high-throughput oscillatory shear technology fabricates programmable microrobots for real-time visual guidance during embolization. Small. (2024):e2408613. doi: 10.1002/smll.202408613
54. Melchiorre, F, Patella, F, Pescatori, L, Pesapane, F, Fumarola, E, Biondetti, P, et al. DEB-TACE: a standard review. Future Oncol. (2018) 14:2969–84. doi: 10.2217/fon-2018-0136
55. Wang, Z-Y, Xie, C-F, Feng, K-L, Xiong, C-M, Huang, J-H, Chen, Q-L, et al. Drug-eluting beads versus conventional transarterial chemoembolization for the treatment of unresectable hepatocellular carcinoma: a meta-analysis. Medicine. (2023) 102:e34527. doi: 10.1097/md.0000000000034527
56. Zeng, J, Wang, Y, Wang, H, Hou, Y, and He, D. Comparison of 8spheres polyvinyl alcohol microsphere and gelatin sponge particle efficacy for transcatheter arterial chemoembolization in stages A to B patients with hepatocellular carcinoma. J Cancer Res Ther. (2022) 18:1981–7. doi: 10.4103/jcrt.jcrt_736_22
57. Song, JE, and Kim, DY. Conventional vs drug-eluting beads transarterial chemoembolization for hepatocellular carcinoma. World J Hepatol. (2017) 9:808–14. doi: 10.4254/wjh.v9.i18.808
58. Shu, C, Lim, M, and Fang, A. Transarterial embolization and percutaneous ablation of primary and metastatic soft tissue Tumors. Life (Basel). (2023) 13:1485. doi: 10.3390/life13071485
59. Vaidya, S, Tozer, KR, and Chen, J. An overview of embolic agents. Semin Intervent Radiol. (2008) 25:204–15. doi: 10.1055/s-0028-1085930
60. Volteas, P, and Labropoulos, N. Follow-up and surveillance after peripheral interventions In: G Geroulakos, E Avgerinos, JP Becquemin, GC Makris, and A Froio, editors. Mastering endovascular techniques: Tips and tricks in endovascular surgery. Cham: Springer International Publishing (2024)
61. Scheau, A-E, Jurca, SO, Scheau, C, and Lupescu, IG. Non-surgical treatment for hepatocellular carcinoma: what to expect at follow-up magnetic resonance imaging—a pictorial review. Appl Sci. (2024) 14:9159. doi: 10.3390/app14209159
62. Sidali, S, Trépo, E, Sutter, O, and Nault, JC. New concepts in the treatment of hepatocellular carcinoma. United European Gastroenterol J. (2022) 10:765–74. doi: 10.1002/ueg2.12286
63. Garin, E, Tselikas, L, Guiu, B, Chalaye, J, Edeline, J, de Baere, T, et al. Personalised versus standard dosimetry approach of selective internal radiation therapy in patients with locally advanced hepatocellular carcinoma (DOSISPHERE-01): a randomised, multicentre, open-label phase 2 trial. Lancet Gastroenterol Hepatol. (2021) 6:17–29. doi: 10.1016/S2468-1253(20)30290-9
64. Gholamrezanezhad, A, Mirpour, S, Geschwind, JF, Rao, P, Loffroy, R, Pellerin, O, et al. Evaluation of 70-150-μm doxorubicin-eluting beads for transcatheter arterial chemoembolization in the rabbit liver VX2 tumour model. Eur Radiol. (2016) 26:3474–82. doi: 10.1007/s00330-015-4197-y
65. Kim, MA, Jang, H, Choi, NR, Nam, JY, Lee, YB, Cho, EJ, et al. Yttrium-90 radioembolization is associated with better clinical outcomes in patients with hepatocellular carcinoma compared with conventional chemoembolization: a propensity score-matched study. J Hepatocell Carcinoma. (2021) 8:1565–77. doi: 10.2147/jhc.S335879
66. Lee, JS, Kim, BK, Kim, SU, Park, JY, Ahn, SH, Seong, JS, et al. A survey on transarterial chemoembolization refractoriness and a real-world treatment pattern for hepatocellular carcinoma in Korea. Clin Mol Hepatol. (2020) 26:24–32. doi: 10.3350/cmh.2018.0065
67. Chen, C, Liu, X, Deng, L, Liao, Y, Liu, S, Hu, P, et al. Evaluation of the efficacy of transcatheter arterial embolization combined with apatinib on rabbit VX2 liver tumors by intravoxel incoherent motion diffusion-weighted MR imaging. Front Oncol. (2022) 12:951587. doi: 10.3389/fonc.2022.951587
68. Sarwar, A, Kudla, A, Weinstein, JL, Ali, A, Malik, R, Bullock, A, et al. Yttrium-90 radioembolization using MIRD dosimetry with resin microspheres. Eur Radiol. (2021) 31:1316–24. doi: 10.1007/s00330-020-07231-8
69. Binzaqr, S, Debordeaux, F, Blanc, JF, Papadopoulos, P, Hindie, E, Lapouyade, B, et al. Efficacy of selective internal radiation therapy for hepatocellular carcinoma post-incomplete response to chemoembolization. Pharmaceuticals (Basel). (2023) 16:1676. doi: 10.3390/ph16121676
70. Niekamp, AS, Huang, SY, Mahvash, A, Odisio, BC, Ahrar, K, Tzeng, CD, et al. Hepatic vein embolization after portal vein embolization to induce additional liver hypertrophy in patients with metastatic colorectal carcinoma. Eur Radiol. (2020) 30:3862–8. doi: 10.1007/s00330-020-06746-4
71. Rassam, F, Olthof, PB, van Lienden, KP, Bennink, RJ, Erdmann, JI, Swijnenburg, RJ, et al. Comparison of functional and volumetric increase of the future remnant liver and postoperative outcomes after portal vein embolization and complete or partial associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Ann Transl Med. (2020) 8:436. doi: 10.21037/atm.2020.03.191
72. Braat, A, Bruijnen, RCG, van Rooij, R, Braat, M, Wessels, FJ, van Leeuwaarde, RS, et al. Additional holmium-166 radioembolisation after lutetium-177-dotatate in patients with neuroendocrine tumour liver metastases (HEPAR PLuS): a single-centre, single-arm, open-label, phase 2 study. Lancet Oncol. (2020) 21:561–70. doi: 10.1016/s1470-2045(20)30027-9
73. Tokue, H, Morita, H, Tokue, A, and Tsushima, Y. Successful management of life-threatening bleeding of intraductal papillary mucinous neoplasms in the pancreatic head. SAGE Open Med Case Rep. (2017) 5:2050313x17741014. doi: 10.1177/2050313x17741014
74. Lopez-Benitez, R, Benz, D, Wu, Y, Wu, X, Chen, S, and Cruise, GM. New interventional treatment model for pancreatic neoplasms using gemcitabine-eluting hydrogel devices: in vitro and in vivo results. Cardiovasc Intervent Radiol. (2017) 40:1246–54. doi: 10.1007/s00270-017-1627-z
75. Kook, Y, Choi, M, Park, JY, Hwang, HK, Lee, WJ, Kim, MD, et al. Laparoscopic pancreaticoduodenectomy with excision of aberrant right hepatic artery after preoperative segmental embolization in mid-bile duct cancer. J Minim Invasive Surg. (2021) 24:104–8. doi: 10.7602/jmis.2021.24.2.104
76. Maekawa, A, Kudo, A, Kishino, M, Murase, Y, Watanabe, S, Ishikawa, Y, et al. Hormonal tumor mapping for liver metastases of gastroenteropancreatic neuroendocrine neoplasms: a novel therapeutic strategy. J Cancer Res Clin Oncol. (2022) 148:697–706. doi: 10.1007/s00432-021-03650-2
77. Drescher, R, Köhler, A, Seifert, P, Aschenbach, R, Ernst, T, Rauchfuß, F, et al. Clinical results of transarterial radioembolization (TARE) with Holmium-166 microspheres in the multidisciplinary oncologic treatment of patients with primary and secondary liver cancer. Biomedicine. (2023) 11:1831. doi: 10.3390/biomedicines11071831
78. Bibok, A, Mhaskar, R, Jain, R, Zhang, J, Frakes, J, Hoffe, S, et al. Role of radioembolization in the management of liver-dominant metastatic renal cell carcinoma: a single-center, retrospective study. Cardiovasc Intervent Radiol. (2021) 44:1755–62. doi: 10.1007/s00270-021-02925-y
79. Wang, MQ, Duan, F, Zhang, H, Zhang, JL, Fu, J, Ye, HY, et al. Comparison of polyvinyl alcohol versus combination of Lipiodol-bleomycin emulsion and NBCA-lipiodol emulsion for renal angiomyolipoma embolization: a prospective randomized study. AJR Am J Roentgenol. (2023) 220:873–83. doi: 10.2214/ajr.22.28587
80. Kervancioglu, S, and Yilmaz, F. Urgent arterial embolization of ruptured renal angiomyolipoma. Open Med (Wars). (2015) 10:233–7. doi: 10.1515/med-2015-0035
81. Uka, M, Iguchi, T, Okawa, N, Matsui, Y, Tomita, K, Umakoshi, N, et al. Percutaneous cryoablation for clinical T3a renal cell carcinoma (< 7 cm) with segmental vein involvement or perinephric fat invasion based on preoperative evaluation of high-resolution multidetector computed tomography scan. Jpn J Radiol. (2022) 40:1201–9. doi: 10.1007/s11604-022-01297-8
82. Lopez, O, Chevallier, O, Guillen, K, Comby, PO, Pellegrinelli, J, Tinel, C, et al. Selective arterial embolization with N-butyl cyanoacrylate prior to CT-guided percutaneous cryoablation of kidney malignancies: a single-center experience. J Clin Med. (2021) 10:4986. doi: 10.3390/jcm10214986
83. Aydin, S, Cengiz, B, Gokay, BV, Mammadov, A, Emiroglu, R, Eskicorapci, S, et al. Selective upper-body perfusion technique for removal of renal cell carcinoma extending into the inferior vena cava and right atrium. Tex Heart Inst J. (2017) 44:283–6. doi: 10.14503/thij-16-6011
84. Hekimoglu, A, Ergun, O, and Hekimoglu, B. Selective transarterial embolization for intractable hematuria due to bladder carcinomas: a single-center experience. Urol Res Pract. (2023) 49:334–7. doi: 10.5152/tud.2023.22224
85. Malling, B, Røder, MA, Lindh, M, Frevert, S, Brasso, K, and Lönn, L. Palliative prostate artery embolization for prostate cancer: a case series. Cardiovasc Intervent Radiol. (2019) 42:1405–12. doi: 10.1007/s00270-019-02227-4
86. Malling, B, Røder, MA, Lauridsen, C, and Lönn, L. Can computed tomography perfusion predict treatment response after prostate artery embolization: a feasibility study. Diagnostics (Basel). (2020) 10:304. doi: 10.3390/diagnostics10050304
87. Hamiditabar, M, Ali, M, Bolek, L, Vahdati, G, Tworowska, I, and Delpassand, ES. Safety and effectiveness of 177Lu-DOTATATE peptide receptor radionuclide therapy after regional hepatic embolization in patients with somatostatin-expressing neuroendocrine Tumors. Clin Nucl Med. (2017) 42:822–8. doi: 10.1097/rlu.0000000000001818
88. Leppelmann, KS, Mooradian, MJ, Ganguli, S, Uppot, RN, Yamada, K, Irani, Z, et al. Thermal ablation, embolization, and selective internal radiation therapy combined with checkpoint inhibitor cancer immunotherapy: safety analysis. J Vasc Interv Radiol. (2021) 32:187–95. doi: 10.1016/j.jvir.2020.09.014
89. Ampie, L, Choy, W, Khanna, R, Smith, ZA, Dahdaleh, NS, Parsa, AT, et al. Role of preoperative embolization for intradural spinal hemangioblastomas. J Clin Neurosci. (2016) 24:83–7. doi: 10.1016/j.jocn.2015.09.006
90. Butenschoen, VM, Schwendner, M, Hubertus, V, Onken, J, Koegl, N, Mohme, T, et al. Preoperative angiographic considerations and neurological outcome after surgical treatment of intradural spinal hemangioblastoma: a multicenter retrospective case series. J Neuro-Oncol. (2023) 161:107–15. doi: 10.1007/s11060-022-04213-2
91. Timilsina, K, Shrestha, S, Bhatta, OP, Paudel, S, Lakhey, RB, and Pokharel, RK. Atypical aggressive hemangioma of thoracic vertebrae associated with thoracic myelopathy-a case report and review of the literature. Case Rep Orthop. (2024) 2024:2307950. doi: 10.1155/2024/2307950
92. Ramos, A, Carnevale, JA, Majeed, K, Kocharian, G, Hussain, I, Goldberg, JL, et al. Multidisciplinary management of carotid body tumors: a single-institution case series of 22 patients. J Neurosurg. (2023) 138:95–103. doi: 10.3171/2022.3.Jns22136
93. Villanova, I, Zimatore, DS, Dell'Atti, C, Lucarelli, NM, Lorusso, G, De Toma, V, et al. Percutaneous preoperative embolization with onyx in the management of a carotid body tumor: a case report. Radiol Case Rep. (2024) 19:5922–5. doi: 10.1016/j.radcr.2024.09.032
94. Ridha, B, Aram, V, Baram, A, Hama Baqi, SY, and Yaldo, F. Surgical outcomes for carotid body tumour resection without preoperative embolization: a 10-year experience. Ann Med Surg (Lond). (2024) 86:2181–8. doi: 10.1097/ms9.0000000000001847
95. Kedra, A, Dohan, A, Biau, D, Belbachir, A, Dautry, R, Lucas, A, et al. Preoperative arterial embolization of musculoskeletal tumors: a tertiary center experience. Cancers (Basel). (2023) 15:2657. doi: 10.3390/cancers15092657
96. Yamada, T, Hasegawa, T, Shido, Y, Yamato, Y, Yoshida, G, Yasuda, T, et al. Combination therapy with preoperative embolization and en block laminectomy using thread saw for spinous process solitary fibrous tumor: a case report. Radiol Case Rep. (2020) 15:2607–12. doi: 10.1016/j.radcr.2020.10.008
97. Esber, Z, Schwarze, E, Critchfield, J, and Soubani, AO. Serial transarterial embolization for the management of unresectable malignant pulmonary hemangiopericytoma: a case report and review of the literature. Respir Med Case Rep. (2019) 28:100920. doi: 10.1016/j.rmcr.2019.100920
98. Vulasala, SSR, Gopireddy, DR, Mohamed, K, and Lall, C. Ruptured pyomyoma - rare complication of post-uterine artery embolization: a case report. J Clin Imaging Sci. (2021) 11:67. doi: 10.25259/jcis_188_2021
99. Huang, YC, Tsuang, FY, Lee, CW, and Lin, YH. Efficacy of preoperative embolization for metastatic spinal tumor surgery using angiographic vascularity assessment. Eur Radiol. (2023) 33:2638–46. doi: 10.1007/s00330-022-09276-3
100. Papalexis, N, Peta, G, Carta, M, Quarchioni, S, Di Carlo, M, Miceli, M, et al. How arterial embolization is transforming treatment of oncologic and degenerative musculoskeletal disease. Curr Oncol. (2024) 31:7523–54. doi: 10.3390/curroncol31120555
101. Liu, C, Wu, X, Hu, X, Wu, L, Guo, K, Zhou, S, et al. Navigating complexity: a comprehensive review of microcatheter shaping techniques in endovascular aneurysm embolization. Front Neurol. (2023) 14:1245817. doi: 10.3389/fneur.2023.1245817
102. Jordan, O, Doelker, E, and Rüfenacht, DA. Biomaterials used in injectable implants (liquid embolics) for percutaneous filling of vascular spaces. Cardiovasc Intervent Radiol. (2005) 28:561–9. doi: 10.1007/s00270-004-0238-7
103. Mikhail, AS, Negussie, AH, Mauda-Havakuk, M, Owen, JW, Pritchard, WF, Lewis, AL, et al. Drug-eluting embolic microspheres: state-of-the-art and emerging clinical applications. Expert Opin Drug Deliv. (2021) 18:383–98. doi: 10.1080/17425247.2021.1835858
104. Brown, KT, Do, RK, Gonen, M, Covey, AM, Getrajdman, GI, Sofocleous, CT, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. (2016) 34:2046–53. doi: 10.1200/jco.2015.64.0821
105. Frandon, J, and Beregi, JP. Special issue: present and future perspectives of vascular interventional radiology. J Pers Med. (2023) 13:1131. doi: 10.3390/jpm13071131
106. Kasprzak, A. Prognostic biomarkers of cell proliferation in colorectal cancer (CRC): from immunohistochemistry to molecular biology techniques. Cancers (Basel). (2023) 15:4570. doi: 10.3390/cancers15184570
107. Liu, SV, Nagasaka, M, Atz, J, Solca, F, and Müllauer, L. Oncogenic gene fusions in cancer: from biology to therapy. Signal Transduct Target Ther. (2025) 10:111. doi: 10.1038/s41392-025-02161-7
108. Rahmani, F, Naderpour, S, Nejad, BG, Rahimzadegan, M, Ebrahimi, ZN, Kamali, H, et al. The recent insight in the release of anticancer drug loaded into PLGA microspheres. Med Oncol. (2023) 40:229. doi: 10.1007/s12032-023-02103-9
109. Park, W, Cho, S, Ji, J, Lewandowski, RJ, Larson, AC, and Kim, DH. Development and validation of Sorafenib-eluting microspheres to enhance therapeutic efficacy of Transcatheter arterial chemoembolization in a rat model of hepatocellular carcinoma. Radiol Imaging Cancer. (2021) 3:e200006. doi: 10.1148/rycan.2021200006
110. Hu, J, Albadawi, H, Chong, BW, Deipolyi, AR, Sheth, RA, Khademhosseini, A, et al. Advances in biomaterials and technologies for vascular embolization. Adv Mater. (2019) 31:e1901071. doi: 10.1002/adma.201901071
111. Chang, L, Liu, J, Zhu, J, Guo, S, Wang, Y, Zhou, Z, et al. Advancing precision medicine: the transformative role of artificial intelligence in immunogenomics, radiomics, and pathomics for biomarker discovery and immunotherapy optimization. Cancer Biol Med. (2025) 22:33–47. doi: 10.20892/j.issn.2095-3941.2024.0376
112. Günkan, A, Ferreira, MY, Vilardo, M, Scarcia, L, Bocanegra-Becerra, JE, Alexandre, A, et al. Safety and efficacy of newer liquid embolic agents squid and PHIL in endovascular embolization of cerebral arteriovenous malformations and dural arteriovenous fistulas: a systematic review and meta-analysis. Interv Neuroradiol. (2024):15910199241288897. doi: 10.1177/15910199241288897
113. Marnat, G, Barreau, X, Januel, A-C, Spelle, L, Piotin, M, Mounayer, C, et al. Efficacy and safety of the PHIL embolic agent in the treatment of intracranial dural arteriovenous fistulas: results of the PHIL-dAVF study. J NeuroIntervent Surg. (2025):jnis-2024-022630. doi: 10.1136/jnis-2024-022630
114. Seif El Dahan, K, Reczek, A, Daher, D, Rich, NE, Yang, JD, Hsiehchen, D, et al. Singal: multidisciplinary care for patients with HCC: a systematic review and meta-analysis. Hepatol Commun. (2023) 7:e0143. doi: 10.1097/hc9.0000000000000143
115. Zhang, Q, Fu, Q, Bai, X, and Liang, T. Molecular profiling-based precision medicine in cancer: a review of current evidence and challenges. Front Oncol. (2020) 10:532403. doi: 10.3389/fonc.2020.532403
116. Berger, MF, and Mardis, ER. The emerging clinical relevance of genomics in cancer medicine. Nat Rev Clin Oncol. (2018) 15:353–65. doi: 10.1038/s41571-018-0002-6
Keywords: personalized medicine, tumor embolization, interventional oncology, peripheral vascular disease, drug-eluting beads, tumor biology, therapeutic outcomes, precision treatment
Citation: Qi X, Liu J, Liu T and Hao H (2025) Personalized peripheral vascular interventional embolization for tumor: tailoring treatment to improve outcomes. Front. Med. 12:1621148. doi: 10.3389/fmed.2025.1621148
Edited by:
Victoria McGilligan, School of Medicine, Ulster, United KingdomReviewed by:
Yang Ke, Kunming Medical University, ChinaXing-Ming Chen, The Second Affiliated Hospital of Kunming Medical University, China
Copyright © 2025 Qi, Liu, Liu and Hao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huaxin Hao, MTczNTgxODYwMDdAMTYzLmNvbQ==
 Xinyue Qi
Xinyue Qi Huaxin Hao
Huaxin Hao