Abstract
Importance:
It is critical to identify novel biomarkers for the prediction and prognosis of rheumatoid arthritis-associated interstitial lung disease (RA-ILD).
Objective:
The objective of the study was to investigate the predictive and prognostic significance of serum uric acid (UA) in RA-ILD.
Design, setting, and participants:
In this multicenter retrospective cohort study, demographic data, medical history, and laboratory results of 829 RA patients were extracted from electronic medical records between December 2018 and January 2024. The cohort was divided into an RA-ILD group (n = 351) and an RA-no-ILD group (n = 478).
Main outcomes and measures:
The primary endpoint was the predictive capacity of baseline UA levels for the occurrence of ILD in RA patients. The secondary endpoints were all-cause mortality and the rehospitalization rate in RA-ILD patients.
Results:
The univariate analysis identified elevated levels of UA as a significant risk factor for the development of ILD in RA patients (OR [95% CI]: 1.68 [1.23–2.28], p = 0.001). The multivariate analysis confirmed that elevated levels of UA remained an independent risk factor. The subgroup analysis demonstrated a stronger predictive utility of elevated UA levels in younger RA patients (age < 65), particularly those with shorter disease duration and no comorbidities (OR [95% CI]: 3.66 [1.66–8.05], p = 0.001; AUCROC = 0.718). During follow-up, RA-ILD patients with elevated UA levels exhibited significantly higher all-cause mortality (22.1% vs. 13.1%; HR [95% CI]: 1.80 [1.03–3.17], p = 0.040) and rehospitalization rates (51.9% vs. 44.4%; HR [95% CI]: 1.41 [1.00–1.98], p = 0.047) compared to those with lower UA levels.
Conclusion and relevance:
Elevated levels of serum UA may serve as a predictive marker for ILD development in RA patients, particularly in younger individuals without comorbidities, and a prognostic indicator for increased mortality and rehospitalization rates in RA-ILD patients.
Clinical trial registration:
ClinicalTrials.gov, identifier NCT06036537.
Introduction
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by chronic synovial inflammation and progressive joint destruction, often accompanied by extra-articular manifestations. Among these comorbidities, rheumatoid arthritis-associated interstitial lung disease (RA-ILD) has emerged as a key determinant of mortality, although its clinical significance remains underrecognized (1, 2). Epidemiologic studies estimate that 5–10% of RA patients develop clinically significant ILD, with mortality rates 2- to 10-fold higher compared to RA patients without pulmonary involvement (3). Current diagnostic biomarkers, including anti-citrullinated protein antibodies (ACPA), MUC5B promoter polymorphisms, and Krebs von den Lungen-6 (KL-6), demonstrate limited prognostic utility for stratifying disease progression and survival outcomes (4). This diagnostic inadequacy underscores the urgent need to identify novel predictive biomarkers for ILD development in RA populations and elucidate prognostic determinants in established RA-ILD cases. Addressing these knowledge gaps may facilitate early intervention strategies that attenuate pulmonary deterioration and improve survival rates in this high-risk cohort.
Uric acid (UA), a crystallizable metabolite of purine catabolism, functions as an endogenous damage-associated molecular pattern (DAMP) that activates the NLR family pyrin domain containing 3 (NLRP3) inflammasome through crystal-dependent and independent mechanisms (5). Mechanistic studies by Gasse et al. demonstrated that UA exacerbates pulmonary inflammation and fibrotic remodeling through TLR2/TLR4-mediated NLRP3 inflammasome activation in alveolar macrophages, establishing a molecular link between hyperuricemia and lung pathology (6). Notably, the NLRP3 inflammasome has been pharmacologically validated as a therapeutic target in RA, where its aberrant activation drives synovitis and bone erosion, while targeted inhibition significantly ameliorates disease severity in preclinical models (7, 8). These pathophysiological connections suggest that UA may serve as a dual modulator influencing both ILD development in RA patients and clinical trajectories in established RA-ILD. Supporting this hypothesis, Andersen et al. conducted a retrospective cohort study involving 212 ILD patients and identified serum UA levels exceeding the 90th percentile as an independent predictor of ILD-related mortality (9). Nevertheless, the specific prognostic impact of hyperuricemia on RA-ILD mortality remains uncharacterized. This study aims to address two critical knowledge gaps: (1) Whether elevated levels of UA constitute a novel risk factor for ILD incidence in RA populations and (2) how UA dynamics modulate clinical outcomes in RA-ILD patients.
Materials and methods
Study design
We conducted a multicenter retrospective cohort study registered under clinicaltrials.gov (identifier NCT06036537). We selected cases for this study using strict inclusion and exclusion criteria based on a continuous sampling method at the First Affiliated Hospital of Chongqing Medical University and Chongqing General Hospital between December 2018 and January 2024. Patients with a confirmed RA diagnosis were enrolled. The exclusion criteria were as follows: (1) Age <18 years, (2) treatment with uric acid-lowering drugs, (3) systemic autoimmune diseases other than RA, such as systemic lupus erythematosus (SLE), ANCA-associated vasculitis, primary Sjogren’s syndrome (pSS), systemic sclerosis (SSc), and myositis, (4) cancer, (5) pulmonary tuberculosis, and (6) advanced chronic kidney disease or severe loss of kidney function, defined as an estimated glomerular filtration rate (eGFR) less than 30 mL/min/1.73 m2, which significantly impairs the excretion of metabolic substances (10, 11). This study was approved by the Institutional Ethics Board of Chongqing General Hospital (approval NO. KY S2023-024-01). All methods were performed following the relevant guidelines and regulations.
Data collection
We consecutively collected electronic medical records of patients hospitalized for the first time between December 2018 and January 2024 to obtain their demographic characteristics, medical history, RA disease duration, laboratory test results, comorbidities, and treatment information. These data were collected independently by two investigators and then checked by another two investigators. RA-ILD patients were followed up via telephone by two investigators until the end of August 2024.
Definition and study outcomes
Confirmed RA cases were classified in accordance with the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) classification criteria. The diagnosis of interstitial lung disease (ILD) in RA patients was consecutively confirmed through a multidisciplinary consensus review of high-resolution computed tomography (HRCT) findings, in accordance with the Fleischner Society guidelines (12). The study participants were stratified into hyperuricemic and normouricemic groups based on an optimized serum UA cutoff value derived from receiver operating characteristic (ROC) curve analysis for ILD prediction. The Youden index-derived cutoff maximized both sensitivity and specificity for discriminating ILD. The primary endpoint was the predictive capacity of baseline UA levels for incident ILD in RA patients. The secondary endpoints were all-cause mortality and rehospitalization rates in RA-ILD patients. RA-ILD patients were prospectively enrolled in a longitudinal observational cohort and were followed up through August 2024. The exposure period for mortality was defined as the number of days from study inclusion to death or the end of follow-up. The exposure period for rehospitalization was defined as the number of days from study inclusion to the first rehospitalization within 6 months.
Statistical analysis
Sample size determination was conducted using power analysis for logistic regression with a single binary predictor variable, employing the Wald test for calculating the odds ratio (OR) (13). The calculation assumed 80% power to detect a clinically meaningful effect size at the 0.05 significance level (two-tailed), yielding a minimum sample requirement of 745 participants. Categorical variables were described as frequency rates and percentages. Continuous variables were described as mean (standard deviation: [SD]) if normally distributed and as median (interquartile range: [IQR]) if not normally distributed. The χ2 test was performed to assess differences in the categorical variables between the two groups. A t-test or Mann–Whitney U test was performed to compare the continuous variables, depending on whether they were normally distributed. Binary and multiple logistic regression analyses were performed to estimate the odds ratio (OR) for risk factors. The Kaplan–Meier product-limit estimation method was used to estimate survival rates of RA-ILD patients. Univariate and multivariate Cox proportional hazards models were used to estimate hazard ratios (HRs) and 95% confidence intervals (CIs). To investigate whether UA provides additional value in predicting ILD in RA patients, we performed receiver operating characteristic (ROC) curve analysis and assessed the equality of the area under the curve (AUC). We considered that a p-value of < 0.050 is statistically significant, and all p-values were three-tailed. Statistical analyses were performed using R software (version 4.3) and SPSS software (version 22.0).
Results
Patients
The study initially enrolled 952 consecutive hospitalized patients with RA. Patients without UA data (n = 27) were excluded. Patients who had other systemic autoimmune diseases (n = 34), tumors (n = 41), and pulmonary tuberculosis (n = 5) were also excluded. In addition, patients who used uric acid-lowering drugs (n = 7) and those with an eGFR less than 30 mL/min/1.73 m2 (n = 9) were excluded. Finally, a total of 829 patients were included in the study. The patients were divided into two groups: RA-ILD group (351 patients) and RA-no-ILD group (478 patients). A total of 351 patients with RA-ILD were followed up by telephone, and 49 of them were lost to follow-up. Finally, 302 RA-ILD patients were analyzed for all-cause mortality and rehospitalization rates (Figure 1).
Figure 1

Flow diagram of patient recruitment.
Significant sex disparity was observed between the cohorts, with a female predominance in the RA-no-ILD patient group (78.87% [95% CI 75.2–82.3%]) compared to the RA-ILD patient group (54.70% [95% CI 49.5–59.8%]; χ2 = 48.7, p < 0.001). Age distribution differed significantly between the groups, demonstrating a mean difference of 4.17 years (RA-no-ILD: 59.76 ± 13.01 years vs. RA-ILD: 63.93 ± 11.19 years; t = 5.32, p < 0.001). Biochemical analysis revealed clinically significant intergroup variations in UA levels (RA-no-ILD: 277.20 ± 92.00 μmol/L vs. RA-ILD: 293.60 ± 97.80 μmol/L; mean difference 16.4 μmol/L, p = 0.010). Comprehensive baseline characteristics, including inflammatory markers and serological profiles, are presented in Table 1.
Table 1
| Characteristics | All (n = 829) | p-value | |
|---|---|---|---|
| RA-ILD (n = 351) | RA-no-ILD (n = 478) | ||
| General demographics | |||
| Sex (female) | 192 (54.70%) | 377 (78.87%) | <0.001 |
| Age (mean ± SD, years) | 63.91 ± 11.28 | 60.19 ± 12.59 | <0.001 |
| Smoking | 97 (27.64%) | 95 (19.87%) | 0.009 |
| RA duration (IQR, months) | 72 (24, 120) | 60 (12, 132) | 0.312 |
| Laboratory results | |||
| RF (IU/mL) | 197.00 (34.43, 609.50) | 81.35 (20.55, 287.50) | <0.001 |
| Anti-CCP (RU/mL) | 122.00 (17.85, 347.06) | 85.75 (11.57, 200.00) | 0.003 |
| ESR (mm/h) | 66.00 (43.00, 94.75) | 48.00 (25.00, 75.00) | <0.001 |
| DAS28 | 6.75 (5.64, 7.63) | 5.83 (4.55, 7.10) | <0.001 |
| CRP (mg/L) | 20.60 (8.50, 65.20) | 11.30 (3.74, 39.61) | <0.001 |
| UA (μmol/L) | 293.60 ± 97.80 | 277.20 ± 92.00 | 0.010 |
| Urea (mmol/L) | 5.50 (4.30, 6.80) | 5.10 (4.10, 6.30) | 0.008 |
| Crea (μmol/L) | 62.00 (53.00, 75.00) | 57.00 (49.00, 67.00) | <0.001 |
| ALP (U/L) | 78.00 (65.00, 98.55) | 79.50 (66.00, 97.00) | 0.897 |
| ALT(U/L) | 16.00 (10.50, 23.00) | 14.00 (10.00, 21.00) | 0.056 |
| AST (U/L) | 18.85 (15.00, 25.00) | 18.00 (14.00, 22.80) | 0.004 |
| WBC (109/L) | 7.32 (5.85, 9.54) | 6.24 (4.92, 7.82) | <0.001 |
| NEUT (%) | 70.53 ± 11.24 | 67.74 ± 10.74 | 0.001 |
| LYMPH (%) | 18.65 (14.25, 24.62) | 21.40 (16.10, 27.70) | <0.001 |
| MONO (%) | 6.70 (5.30, 8.50) | 6.90 (5.30, 8.70) | 0.962 |
| PLT (109/L) | 245.50 (186.75, 316.00) | 235.00 (188.00, 301.00) | 0.294 |
| HGB (g/L) | 120.00 (103.00, 132.00) | 118.00 (103.00, 130.00) | 0.394 |
| TC (mmol/L) | 4.22 (3.48, 4.72) | 4.24 (3.62, 5.02) | 0.104 |
| TG (mmol/L) | 1.14 (0.87, 1.53) | 1.11 (0.84, 1.52) | 0.320 |
| HDL-C (mmol/L) | 1.15 (0.95, 1.39) | 1.25 (1.00, 1.55) | 0.001 |
| LDL-C (mmol/L) | 2.51 (2.05, 3.13) | 2.47 (1.97, 3.01) | 0.383 |
| APOE(mg/dL) | 2.22 (1.94, 2.52) | 2.59 (2.08, 4.33) | <0.001 |
| Lp(a) (mg/L) | 175.00 (67.00, 358.00) | 162.00 (80.50, 419.00) | 0.624 |
| PCT (ng/mL) | 0.07 (0.05, 0.18) | 0.06 (0.03, 0.15) | 0.106 |
| IL-6 (pg/mL) | 20.91 (7.26, 67.76) | 20.94 (4.64, 63.83) | 0.569 |
| D-DIMER (mg/LFEU) | 1.43 (0.61, 3.12) | 0.91 (0.28, 2.34) | <0.001 |
| FIB (g/L) | 4.59 (3.72, 5.92) | 4.13 (3.14, 5.33) | <0.001 |
| TP (g/L) | 68.00 (62.25, 73.25) | 68.00 (64.00, 74.00) | 0.372 |
| ALB (g/L) | 37.00 ± 5.33 | 38.72 ± 5.66 | <0.001 |
| GLB (g/L) | 30.00 (26.00, 35.55) | 29.00 (26.00, 33.00) | 0.053 |
| Cys-C (mg/L) | 1.14 (0.97, 1.44) | 1.07 (0.90, 1.31) | 0.002 |
| eGFR (mL/min/1.73 m2) | 98.90 (85.03, 109.00) | 97.70 (86.54, 109.00) | 0.706 |
| Current treatments | |||
| GCs use | 191 (54.42%) | 73 (15.27%) | <0.001 |
| ISD use | 294 (83.76%) | 251 (52.51%) | <0.001 |
| Diuretics use | 15 (4.27%) | 9 (1.88%) | 0.043 |
| Concomitant diseases | |||
| HTN | 138 (39.32%) | 158 (33.05%) | 0.063 |
| DM | 68 (19.37%) | 69 (14.44%) | 0.059 |
| CLD | 53 (15.10%) | 39 (8.16%) | 0.002 |
| CAD | 33 (9.40%) | 53 (11.09%) | 0.432 |
Baseline characteristics of the included patients.
ALB, Albumin; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; Anti-CCP, Anti-cyclic citrulline polypeptide antibody; APOE, Apolipoprotein E; AST, Aspartate aminotransferase; CLD, Chronic lung disease; CRP, C-reactive protein; CAD, Coronary artery disease; Cys-C, Cystatin C; DAS28, Disease Activity Score 28; DM, Diabetes mellitus; ESR, Erythrocyte sedimentation rate; eGFR, Estimate glomerular filtration rate; FIB, Fibrinogen; GCs, Glucocorticoids; GLB, Globulin; HDL-C, High density lipoprotein cholesterol; HGB, Hemoglobin; HTN, Hypertension; ISD, Immunosuppressive drug; IL-6, Interleukin 6; Lp(a), Lipoprotein a; LDL-C, Low density lipoprotein cholesterol; LYMPH, Lymphocyte percentage; MONO, Monocyte percentage; NEUT, Neutrophil percentage; PCT, Procalcitonin; PLT, Platelet count; RF, Rheumatoid factor; TC, Total cholesterol; TG, Triglyceride; TP, Total protein; UA, Uric acid; WBC, White blood cell count.
Logistic regression analysis of risk factors for incident ILD in RA patients
Univariate logistic regression analysis was performed to determine risk factors for ILD incidence in RA patients. The analysis showed that female sex, age, smoking, rheumatoid factor (RF), erythrocyte sedimentation rate (ESR), disease activity score 28 (DAS28), C-reactive protein (CRP), high UA, aspartate aminotransferase (AST), white blood cell count (WBC), neutrophil ratio (NEUT %), fibrinogen (FIB), albumin (ALB), globulin (GLB), cystatin C (Cys-C), glucocorticoids (GCs) use, immunosuppressive drug (ISD) use, diuretics use, and chronic lung disease (CLD) were all statistically significant risk factors for the incidence of ILD in RA patients (Table 2).
Table 2
| General demographics | Odds ratio (95% CI) | p-value |
|---|---|---|
| General demographics | ||
| Sex (female) | 3.098 (2.303–4.169) | <0.001 |
| Age (IQR, years) | 1.028 (1.017–1.040) | <0.001 |
| Smoking | 1.544 (1.037–2.299) | 0.032 |
| RA duration (month) | 1.000 (0.998–1.001) | 0.461 |
| Laboratory results | ||
| RF (IU/mL) | 1.000 (1.000–1.001) | <0.001 |
| Anti-CCP (RU/mL) | 1.000 (1.000–1.000) | 0.828 |
| ESR (mm/h) | 1.014 (1.009–1.018) | <0.001 |
| DAS28 | 1.237 (1.133–1.351) | <0.001 |
| CRP (mg/L) | 1.004 (1.002–1.007) | 0.002 |
| High UA* (μmol/L) | 1.676 (1.230, 2.283) | 0.001 |
| Urea (mmol/L) | 1.042 (0.992–1.094) | 0.101 |
| Crea (μmol/L) | 1.004 (0.998, 1.010) | 0.173 |
| ALP (U/L) | 1.003 (0.995, 1.011) | 0.430 |
| AST (U/L) | 1.016 (1.002–1.030) | 0.023 |
| WBC (109/L) | 1.166 (1.108–1.226) | <0.001 |
| NEUT (%) | 1.027 (1.014–1.041) | <0.001 |
| LYMPH (%) | 0.959 (0.943–0.975) | <0.001 |
| MONO (%) | 1.014 (0.984–1.044) | 0.374 |
| PLT (109/L) | 1.001 (0.999–1.002) | 0.384 |
| HGB (g/L) | 1.002 (0.998–1.007) | 0.282 |
| CHO (mmol/L) | 0.890 (0.773–1.024) | 0.103 |
| TG (mmol/L) | 0.986 (0.858–1.134) | 0.847 |
| HDL-C (mmol/L) | 1.013 (0.978–1.049) | 0.482 |
| LDL-C (mmol/L) | 1.134 (0.969–1.326) | 0.117 |
| APOE (mg/dL) | 0.968 (0.936–1.002) | 0.062 |
| Lp(a) (mg/L) | 1.000 (0.999–1.000) | 0.721 |
| PCT (ng/mL) | 1.260 (0.810–1.960) | 0.305 |
| IL-6 (pg/mL) | 1.000 (0.997–1.003) | 0.900 |
| D-DIMER (mg/LFEU) | 1.034 (0.984–1.087) | 0.189 |
| FIB (g/L) | 1.146 (1.047–1.255) | 0.003 |
| TP (g/L) | 0.998 (0.990–1.005) | 0.540 |
| ALB (g/L) | 0.947 (0.923–0.972) | <0.001 |
| GLB (g/L) | 1.027 (1.005–1.050) | 0.015 |
| Cys-C (mg/L) | 1.422 (1.063–1.901) | 0.018 |
| eGFR (mL/min/1.73 m2) | 0.993 (0.986–1.000) | 0.064 |
| Current treatments | ||
| GCs use | 3.990 (2.806–5.672) | <0.001 |
| ISD use | 0.555 (0.246–1.247) | 0.154 |
| Diuretics use | 2.326 (1.006–5.379) | 0.048 |
| Concomitant diseases | ||
| HTN | 0.782 (0.584–1.048) | 0.100 |
| DM | 0.837 (0.575–1.218) | 0.353 |
| CLD | 1.249 (0.693–2.252) | 0.459 |
| CAD | 0.495 (0.311–0.790) | 0.003 |
Univariate logistics regression analysis of risk factors for incident ILD in RA patients.
ALB, Albumin; ALP, Alkaline phosphatase; ALT, Alanine aminotransferase; Anti-CCP, Anti-cyclic citrulline polypeptide antibody; APOE, Apolipoprotein E; AST, Aspartate aminotransferase; CRP, C-reactive protein; CLD, Chronic lung disease; CAD, Coronary artery disease; Cys-C, Cystatin C; DAS28, Disease Activity Score 28; DM, Diabetes mellitus; eGFR, Estimate glomerular filtration rate; ESR, Erythrocyte sedimentation rate; FIB, Fibrinogen; GCs, Glucocorticoids; GLB, Globulin; HGB, Hemoglobin; HTN, Hypertension; IL-6, Interleukin 6; ISD, Immunosuppressive drug; LDL-C, Low density lipoprotein cholesterol; Lp(a), Lipoprotein a; LYMPH, Lymphocyte percentage; MONO, Monocyte percentage; NEUT, Neutrophil percentage; PCT, Procalcitonin; PLT, Platelet count; RF, Rheumatoid factor; TP, Total protein; TC, Total cholesterol; TG, Triglyceride; UA, Uric acid; WBC, White blood cell count; *, UA ≥ 227.00 μmol/L.
Patients with high uric acid levels were defined as those with a serum UA concentration ≥ 227.00 μmol/L upon admission, according to the ROC curve–derived cutoff for ILD prediction. The univariate logistic regression analysis showed that high levels of UA was a risk factor for incident ILD in RA patients (OR [95% CI]: 1.68 [1.23–2.28], p = 0.001). To rule out potential confounding variables affecting the results, we performed multivariate logistic regression analyses with sequential adjustments for the following factors: (1) Baseline characteristics: age, sex, and smoking (adjusted OR [95% CI]: 1.71 [1.16–2.52], p = 0.007); (2) comorbidities: hypertension (HTN), diabetes mellitus (DM), coronary artery disease (CAD), and chronic lung disease (CLD) (adjusted OR [95% CI]: 1.78 [1.23–2.57], p = 0.002); (3) inflammatory markers: ESR, WBC, PLT, and DAS28 (adjusted OR [95% CI]: 2.02 [1.42–2.88], p < 0.001); and (4) medications: glucocorticoids (GCs), immunosuppressive drug (ISD), and diuretics use (adjusted OR [95% CI]: 1.82 [1.24–2.69], p = 0.002). After adjustment for these covariates, elevated UA levels remained an independent risk factor for incident ILD in RA patients (Figure 2).
Figure 2
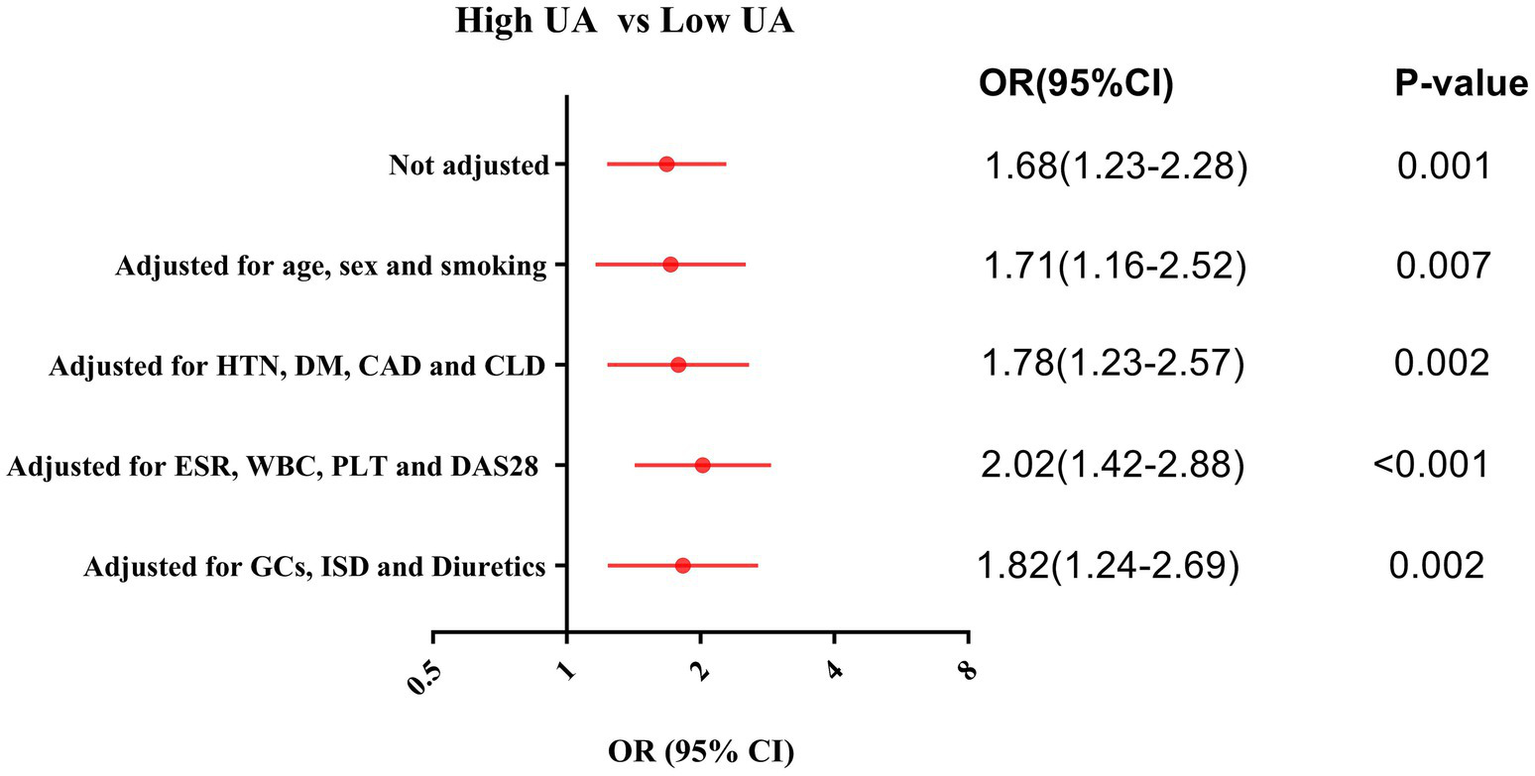
Logistic regression adjusted analysis for the risk of incident ILD in the RA patients with high UA. CAD, Coronary artery disease; CLD, Chronic lung disease; DAS28, Disease Activity Score 28; DM, Diabetes mellitus; ESR, Erythrocyte sedimentation rate; GCs, Glucocorticoids; HTN, Hypertension; ISD, Immunosuppressive drug; PLT, Platelet count; WBC, White blood cell count.
Subgroup analysis of the effect of UA levels on the risk of incident ILD in RA patients
To further investigate the relationship between UA and RA-ILD, subgroup analyses of several variables were performed. Although there were no statistical differences between the groups, it appeared that UA for the prediction of RA-ILD tended to be of greater value in patients who were younger, had shorter disease duration, and had fewer comorbidities (Figure 3).
Figure 3
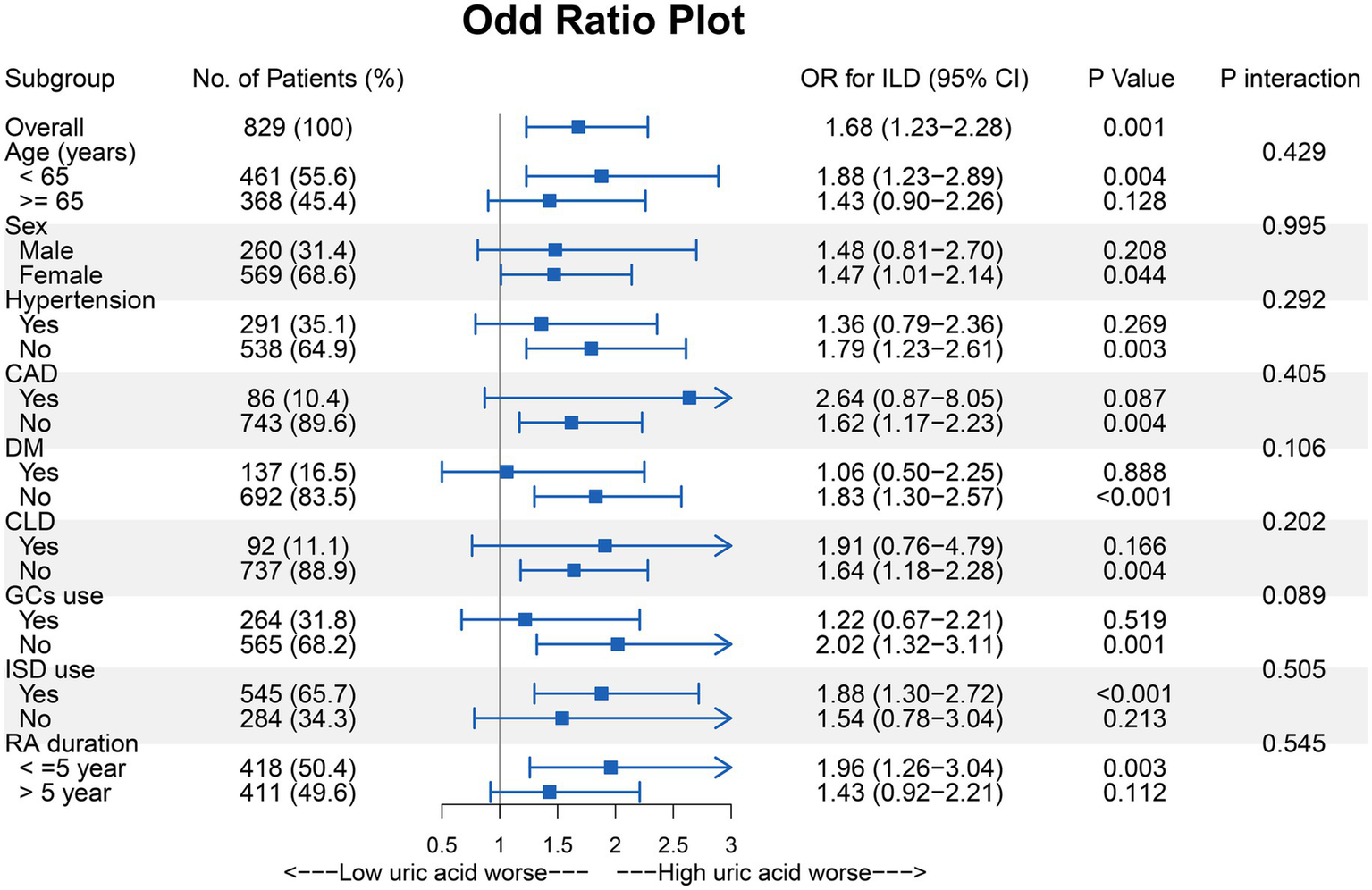
Subgroup analysis of the effect of UA on the risk of incident ILD in the RA patients. CAD, Coronary artery disease; CLD, Chronic lung disease; DM, Diabetes mellitus; GCs, Glucocorticoids; ISD, Immunosuppressive drug.
Therefore, we established a refined RA cohort (n = 152) based on stringent inclusion criteria: age <65 years, absence of comorbidities (HTN, DM, CAD, CLD), disease duration ≤5 years, and glucocorticoid-naïve status. In this homogeneous subpopulation, elevated UA levels demonstrated enhanced predictive capacity for incident ILD development (OR [95% CI]: 3.66 [1.66–8.05], p = 0.001). Further validation using ROC curve analysis revealed significant discriminative power of UA for ILD prediction (AUC = 0.718, p < 0.001), with an optimal cutoff value of 295.00 μmol/L (sensitivity 68.6%, specificity 69.2%) (Figure 4).
Figure 4
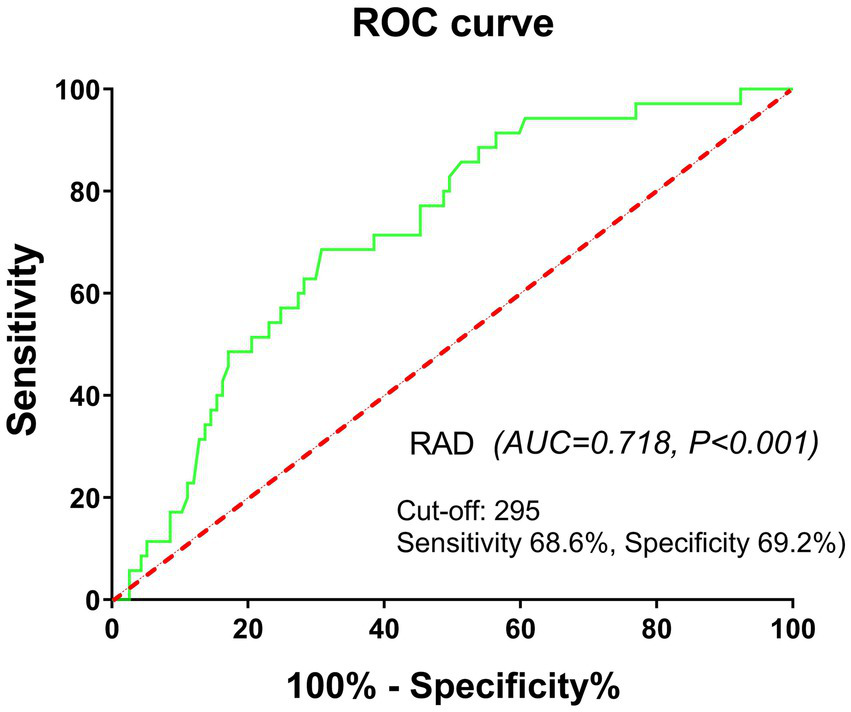
ROC curve analysis showing significant power of UA for ILD prediction in a refined RA cohort. Refined RA cohort: age <65 years, absence of concomitant diseases, disease duration ≤5 years, and glucocorticoid-naïve status.
Cumulative survival curves of all-cause mortality and rehospitalization rates
The cohort comprised 302 RA-ILD patients who completed follow-up, demonstrating distinct mortality and rehospitalization trajectories: the mean follow-up duration for mortality was 34.0 ± 21.1 months and 4.0 ± 2.3 months for rehospitalization. All-cause mortality occurred in 49 patients (16.2%), with a significant between-group disparity in crude incidence rates (high-UA: 22.1% [23/104] vs. low-UA: 13.1% [26/198]). The Cox proportional hazards models revealed an elevated mortality risk in hyperuricemic patients (HR [95%CI]: 1.80 [1.03, 3.17], p = 0.040). The rehospitalization analysis demonstrated a similar risk elevation (high-UA: 51.9% [54/104] vs. low-UA: 44.4% [88/198]), corresponding to an elevated rehospitalization risk in hyperuricemic patients (HR [95% CI]: 1.41 [1.00–1.98], p = 0.047). Temporal risk patterns were visualized using stratified Kaplan–Meier curves (Figures 5A,B).
Figure 5
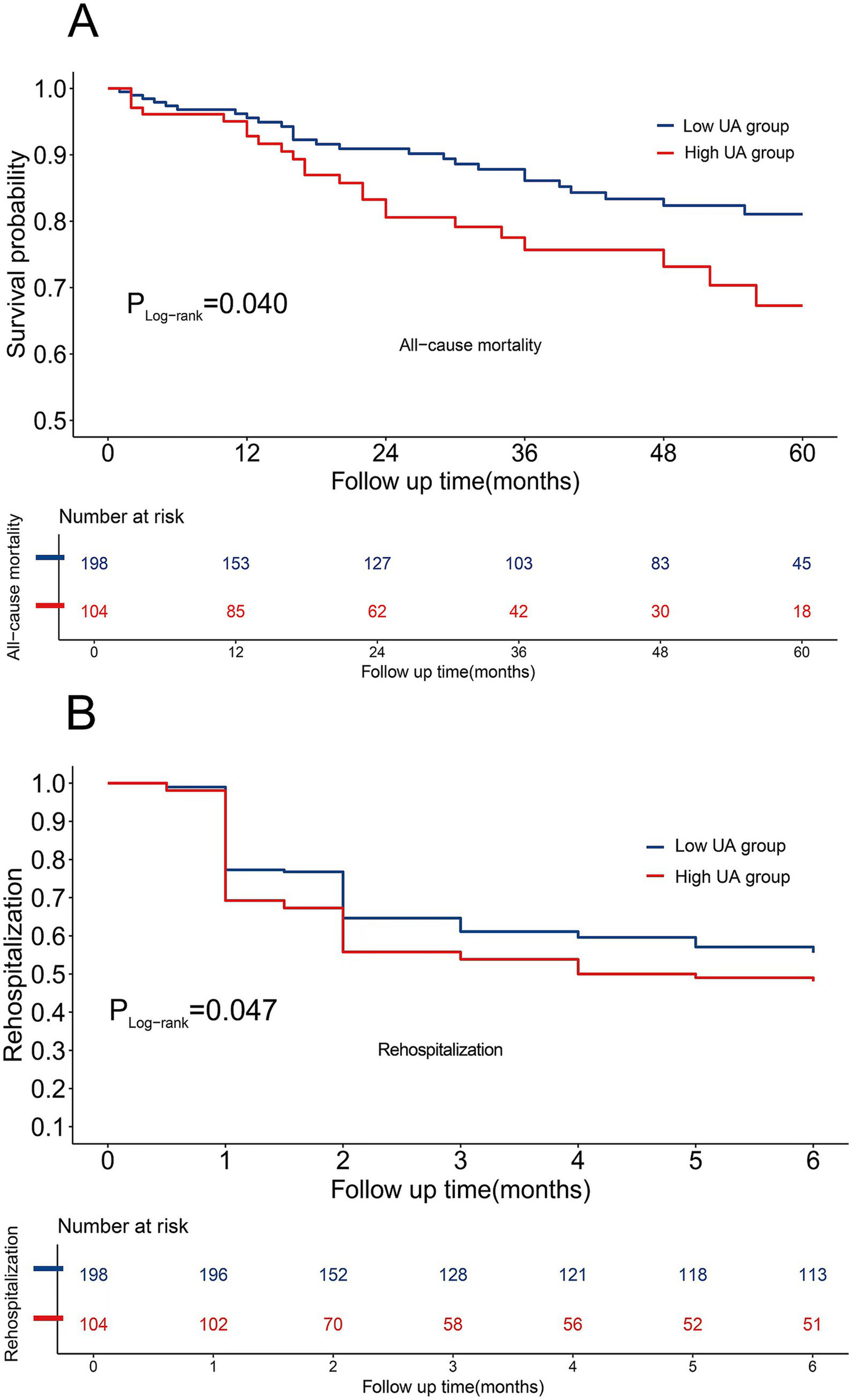
(A) Cumulative survival curves of all-cause mortality. (B) Cumulative survival curves of rehospitalization rates.
Discussion
Our investigation yielded four principal findings: (1) RA-ILD patients exhibited significantly elevated serum UA concentrations compared to RA-no-ILD counterparts; (2) hyperuricemia was an independent predictor of incident ILD in RA patients after multivariable-adjusted analyses; (3) this association was particularly strong in younger RA patients with shorter disease duration and no comorbidities; and (4) importantly, elevated UA levels was associated with an increased risk of mortality and rehospitalization in the RA-ILD cohort.
The enzymatic machinery for purine catabolism, particularly xanthine oxidase-mediated UA generation, demonstrates ubiquitous tissue distribution including pulmonary parenchyma, where it serves as the terminal pathway for purine nucleotide degradation. Preclinical evidence suggests that hyperuricemia may exacerbate RA autoimmune pathogenesis through dual mechanisms: crystal-dependent NLRP3 inflammasome activation and crystal-independent enhancement of Th17 differentiation (14). Mechanistically, genetic polymorphisms in inflammatory mediators significantly modulate UA homeostasis. For instance, Patel et al. reported that RA patients harboring the TGF-β1 + 869 T allele (rs1982073)exhibited 28% higher serum UA concentrations and heightened systemic inflammation compared to wild-type counterparts—findings potentially mediated through enhanced TGF-β1 signaling and impaired UA renal excretion (15). Clinico-pathological correlations further reveal that sustained UA elevation may perpetuate chronic inflammation through positive feedback loops involving CRP (r = 0.42, p < 0.001), TNF-α (r = 0.38, p = 0.002), and IL-6 (r = 0.35, p = 0.005), suggesting that hyperuricemia serves as a biomarker and a pathogenic indicator in RA-related systemic inflammation (16).
ILD, a prevalent yet underrecognized extra-articular manifestation of RA, contributes substantially to disease-related morbidity and mortality (17, 18). Emerging evidence indicates that diagnostic delays in RA-ILD correlate with accelerated mortality rates, underscoring the critical need for early detection strategies (19). While established risk factors, including tobacco exposure, advanced age at RA onset, and severe extra-articular manifestations, have been identified (20), novel predictive biomarkers remain underexplored. Our multicenter cohort study involving 829 RA patients found a statistical difference in serum UA levels between RA-ILD and RA-no-ILD patients. Multivariate logistic regression analyses demonstrated that hyperuricemia independently predicted ILD development after comprehensive adjustment for baseline characteristics (age, sex, and smoking), comorbidities (HTN, DM, CAD, and CLD), inflammatory markers (ESR, WBC, PLT, and DAS28), and medications (GCs, ISD, and diuretics). However, the absolute difference in UA levels between the two groups was less than 1 mg/dL. This minor discrepancy may be related to the higher comorbidity burden and the widespread use of antirheumatic medications in the included patient cohort.
Current evidence regarding UA’s role in RA-ILD pathogenesis remains sparse, with only Wang et al.’s small sample case–control study (n = 162 RA-ILD vs. 104 RA-no-ILD) preliminarily suggesting UA’s predictive potential for RA-ILD development (21). While concordant with our findings, our multicenter cohort study advances this evidence through three critical dimensions: (1) enhanced statistical power via expanded enrollment (N = 829); (2) demonstration of UA’s independence as a risk factor after multivariable adjustment for inflammatory markers, DAS28, concomitant diseases, and other confounders; and (3) identification of a high-risk subpopulation in which hyperuricemia confers a 3.66-fold increased risk of ILD (95%CI 1.66–8.05, p = 0.001)—specifically, RA patients aged <65 years, with disease duration <5 years, and without major comorbidities. This amplified effect in the high-risk subpopulation likely reflects minimized confounding from polypharmacy and preserved endogenous purine metabolism integrity, whereas geriatric patients’ metabolic dysregulation and multidrug regimens may attenuate UA’s biomarker fidelity through pharmacokinetic interactions and cumulative oxidative burden.
Current evidence identifies several prognostic determinants of RA-ILD progression, including advanced age, male sex, elevated DAS28, a usual interstitial pneumonia (UIP) pattern on HRCT, extensive pulmonary involvement, and anti-cyclic citrulline polypeptide (CCP) antibody titers (12). Risk stratification models incorporating age ≥60 years and HRCT radiomics features have demonstrated robust predictive accuracy for 5-year mortality (22). Despite these advances, the pathophysiological role of UA in RA-ILD remains insufficiently characterized. While Wang et al. (21) established significant inverse correlations between serum/bronchoalveolar lavage fluid (BALF) UA levels and forced vital capacity, our study extends this paradigm by elucidating UA’s dual clinical relevance (1) as an independent predictor of incident ILD in RA patients across multivariable-adjusted models and (2) as a prognostic biomarker for mortality and rehospitalization in established RA-ILD. Mechanistically, UA-mediated NLRP3 inflammasome activation induces IL-1β overproduction, perpetuating pulmonary inflammation through fibroblast-to-myofibroblast transition and extracellular matrix deposition—a pathway corroborated in murine models of silica-induced lung fibrosis (6).
This investigation has several limitations that warrant acknowledgment. Firstly, the retrospective design introduces potential selection bias and residual confounding despite multivariable adjustments. Secondly, while all-cause mortality and rehospitalization rates provide clinically relevant endpoints, the absence of serial pulmonary function tests (e.g., FVC% predicted) and quantitative imaging biomarkers (e.g., HRCT fibrosis scores) limits the precise stratification of ILD progression severity. Thirdly, the exclusive enrollment of East Asian participants restricts external validity, highlighting the need for validation in multiethnic cohorts due to known ethnic disparities in urate-handling polymorphisms. Fourthly, due to incomplete medical records of outpatients, we only included newly hospitalized RA patients, which introduces selection bias that limits the applicability of the results to outpatients with more stable conditions. Finally, the enhanced UA prognostic utility observed in patients aged <65 years, with disease duration of <5 years, and without comorbidities represents an exploratory finding requiring confirmation through prospective trials powered for subgroup interactions.
Conclusion
A major insight from our data is that elevated levels of serum UA may serve as a predictive marker for ILD development in RA patients, particularly in younger individuals without comorbidities, and a prognostic indicator for increased mortality and rehospitalization in RA-ILD. Further prospective studies are warranted to validate these findings and explore the underlying mechanisms.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the institutional ethics board of Chongqing General Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QH: Data curation, Investigation, Methodology, Writing – original draft, Project administration, Formal analysis. XC: Writing – original draft, Project administration, Formal analysis, Methodology, Data curation, Investigation. YiL: Data curation, Writing – original draft, Investigation. HL: Investigation, Data curation, Writing – original draft. CN: Conceptualization, Writing – review & editing, Supervision, Software. YaL: Supervision, Writing – review & editing, Conceptualization, Software.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Natural Science Foundation of Chongqing (CSTB2024NSCQ-MSX1099), the Chongqing Special Project for Technological Innovation and Application Development (CSTB2023TIAD-KPX0063-1), and the Joint project of Chongqing Health Commission and Science and Technology Bureau (No. 2024MSXM105).
Acknowledgments
We would like to thank the staff and the participants of this study for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Sullivan DI Ascherman DP . Rheumatoid arthritis-associated interstitial lung disease (RA-ILD): update on prevalence, risk factors, pathogenesis, and therapy. Curr Rheumatol Rep. (2024) 26:431–49. doi: 10.1007/s11926-024-01155-8
2.
Solomon JJ Danoff SK Woodhead FA Hurwitz S Maurer R Glaspole I et al . Safety, tolerability, and efficacy of pirfenidone in patients with rheumatoid arthritis-associated interstitial lung disease: a randomised, double-blind, placebo-controlled, phase 2 study. Lancet Respir Med. (2023) 11:87–96. doi: 10.1016/S2213-2600(22)00260-0
3.
Kronzer VL Hayashi K Yoshida K Davis JM 3rd McDermott GC Huang W et al . Autoantibodies against citrullinated and native proteins and prediction of rheumatoid arthritis-associated interstitial lung disease: a nested case-control study. Lancet Rheumatol. (2023) 5:e77–87. doi: 10.1016/s2665-9913(22)00380-0
4.
Florescu A Gherghina FL Mușetescu AE Pădureanu V Roșu A Florescu MM et al . Novel biomarkers, diagnostic and therapeutic approach in rheumatoid arthritis interstitial lung disease-a narrative review. Biomedicine. (2022) 10:1367. doi: 10.3390/biomedicines10061367
5.
Wang M Lin X Yang X Yang Y . Research progress on related mechanisms of uric acid activating NLRP3 inflammasome in chronic kidney disease. Ren Fail. (2022) 44:615–24. doi: 10.1080/0886022X.2022.2036620
6.
Gasse P Riteau N Charron S Girre S Fick L Pétrilli V et al . Uric acid is a danger signal activating NALP3 inflammasome in lung injury inflammation and fibrosis. Am J Respir Crit Care Med. (2009) 179:903–13. doi: 10.1164/rccm.200808-1274OC
7.
Lee J Sasaki F Koike E Cho M Lee Y Dho SH et al . Gelsolin alleviates rheumatoid arthritis by negatively regulating NLRP3 inflammasome activation. Cell Death Differ. (2024) 31:1679–94. doi: 10.1038/s41418-024-01367-6
8.
Wu D Xu J Jiao W Liu L Yu J Zhang M et al . Suppression of macrophage activation by sodium danshensu via HIF-1α/STAT3/NLRP3 pathway ameliorated collagen-induced arthritis in mice. Molecules. (2023) 28:1551. doi: 10.3390/molecules28041551
9.
Andersen CU Mellemkjær S Nielsen-Kudsk JE Bendstrup E Simonsen U Hilberg O . Diagnostic and prognostic role of biomarkers for pulmonary hypertension in interstitial lung disease. Respir Med. (2012) 106:1749–55. doi: 10.1016/j.rmed.2012.09.003
10.
Neuen BL Weldegiorgis M Herrington WG Ohkuma T Smith M Woodward M . Changes in GFR and albuminuria in routine clinical practice and the risk of kidney disease progression. Am J Kidney Dis. (2021) 78:350–360.e1. doi: 10.1053/j.ajkd.2021.02.335
11.
Zarbock A Larsson TE Noiseux N Mazer CD Böhm J Laflamme M et al . Efficacy and safety of therapeutic alpha-1-microglobulin RMC-035 in reducing kidney injury after cardiac surgery: a multicentre, randomised, double-blind, parallel group, phase 2a trial. EClinicalMedicine. (2024) 76:102830. doi: 10.1016/j.eclinm.2024.102830
12.
Koduri G Solomon JJ . Identification, monitoring, and management of rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. (2023) 75:2067–77. doi: 10.1002/art.42640
13.
Demidenko E . Sample size and optimal design for logistic regression with binary interaction. Stat Med. (2008) 27:36–46. doi: 10.1002/sim.2980
14.
El Ridi R Tallima H . Physiological functions and pathogenic potential of uric acid: a review. J Adv Res. (2017) 8:487–93. doi: 10.1016/j.jare.2017.03.003
15.
Patel SL Prakash J Gupta V . TGF-β1 +869C/T polymorphism increases susceptibility to rheumatoid arthritis in north Indian population. Clin Rheumatol. (2020) 39:2881–8. doi: 10.1007/s10067-020-05064-w
16.
Makhe PA Vagga AA . Comparison of adenosine deaminase, C-reactive protein, uric acid, and rheumatoid arthritis levels in patients with rheumatoid arthritis and those without arthritis: a review. Cureus. (2024) 16:e57433. doi: 10.7759/cureus.57433
17.
Raimundo K Solomon JJ Olson AL Kong AM Cole AL Fischer A et al . Rheumatoid arthritis-interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J Rheumatol. (2019) 46:360–9. doi: 10.3899/jrheum.171315.C1
18.
Zamora-Legoff JA Krause ML Crowson CS Ryu JH Matteson EL . Patterns of interstitial lung disease and mortality in rheumatoid arthritis. Rheumatology (Oxford). (2017) 56:344–50. doi: 10.1093/rheumatology/kew391
19.
Cano-Jiménez E Vázquez Rodríguez T Martín-Robles I Castillo Villegas D Juan García J Bollo de Miguel E et al . Diagnostic delay of associated interstitial lung disease increases mortality in rheumatoid arthritis. Sci Rep. (2021) 11:9184. doi: 10.1038/s41598-021-88734-2
20.
Samhouri BF Vassallo R Achenbach SJ Kronzer VL Davis JM 3rd Myasoedova E et al . Incidence, risk factors, and mortality of clinical and subclinical rheumatoid arthritis-associated interstitial lung disease: a population-based cohort. Arthritis Care Res (Hoboken). (2022) 74:2042–9. doi: 10.1002/acr.24856
21.
Wang Z Wang W Xiang T Gong B Xie J . Serum uric acid as a diagnostic biomarker for rheumatoid arthritis-associated interstitial lung disease. Inflammation. (2022) 45:1800–14. doi: 10.1007/s10753-022-01661-w
22.
Kim HC Lee JS Lee EY Ha YJ Chae EJ Han M et al . Risk prediction model in rheumatoid arthritis-associated interstitial lung disease. Respirology. (2020) 25:1257–64. doi: 10.1111/resp.13848
Summary
Keywords
rheumatoid arthritis, interstitial lung disease, uric acid, predictive value, prognostic impact
Citation
Hu Q, Cheng X, Lan Y, Lei H, Niu C and Luo Y (2025) Serum uric acid predictive value and prognostic impact in rheumatoid arthritis’ associated interstitial lung disease. Front. Med. 12:1623557. doi: 10.3389/fmed.2025.1623557
Received
06 May 2025
Accepted
21 July 2025
Published
05 August 2025
Volume
12 - 2025
Edited by
Francisca Sivera, Hospital General de Elda, Spain
Reviewed by
Ramcés Falfán-Valencia, National Institute of Respiratory Diseases-Mexico (INER), Mexico
Virginia Villaverde Garcia, Hospital Universitario de Móstoles, Spain
Updates
Copyright
© 2025 Hu, Cheng, Lan, Lei, Niu and Luo.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yang Luo, luoy@cqu.edu.cnChangchun Niu, bright_star20001@sina.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.