Abstract
Background:
The fields of dysphagia is progressively acknowledging the transformative capacity of artificial intelligence (AI). The implementation of this technology is profoundly impacting research directions, clinical practices, and healthcare systems. However, existing studies remain scattered and predominantly focus on specific techniques or case applications, lacking a systematic synthesis of global research output, influential contributors, collaboration networks, and evolving thematic trends. A comprehensive bibliometric review is therefore essential to map the current landscape and guide future interdisciplinary research.
Methods:
This study applies bibliometric and visual analysis methods to comprehensively review the global research activities in AI in dysphagia. Data from 633 articles published by 3,533 authors in 292 journals from January 2000 to February 2025 in Web of Science Core Collection (WoSCC) database were collected and analyzed to identify top publications, sources, authors, institutions, countries/regions, and keywords.
Results:
The research activity of AI in dysphagia, which shows an overall upward trend that can be divided into three distinct periods: the first phase (2000–2012), the second phase (2013–2017) and the third phase (2018-Present). The most cited article was Radiotherapy vs. transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomized trial (344 citations). The most prolific journal was Head and Neck—Journal for the Sciences and Specialties of the Head and Neck with 30 publications. Sejdic Ervin (28 articles), the University of Pittsburgh (39 articles), and the USA (255 articles) were the leading author, institution, and country, respectively. Dysphagia was the most frequently occurring keyword (286 occurrences), while emerging terms included machine learning (ML) and deep learning (DL).
Conclusion:
This bibliometric analysis reveals the evolving landscape of AI research in dysphagia, highlighting current hotspots and future directions. AI is driving significant shifts in both research and clinical practice in dysphagia; however, challenges such as interdisciplinary integration and ethical considerations remain to be addressed.
1 Introduction
Dysphagia is a clinical symptom of swallowing dysfunction, impairing the safe and transport of solids and/or liquids from the oral cavity to the stomach (1). It affects 13.4% of the global population (2), with prevalence exceeding 40% in specific patient populations including stroke, sarcopenia, parkinson's disease, dementia, and geriatric patients (3). Anatomically, dysphagia is typically categorized into oropharyngeal dysphagia (OD) and esophageal dysphagia (ED) (1). OD is often attributed to neurologic diseases or head and neck malignancies, and is clinically manifested by coughing, choking, nasal regurgitation and aspiration pneumonia (4). ED commonly resulting from motility disorders, strictures or esophageal tumors, presents a sensation of food sticking in the throat or chest (5). In addition to dysphagia, including dryness, silent aspiration, protein-energy malnutrition and recurrent pulmonary infections (3), recent epidemiologic data reveal a significant increase in adverse outcomes: the intubation rate in the cohort was more than twice as high in patients with dysphagia (34%) compared to patients without dysphagia (6). There were also longer length of hospital stay and mechanical ventilation in the dysphagic patients, which may be attributed to factors such as inadequate oral intake, aspiration pneumonia, and secondary infections (7).
Current diagnostic paradigms rely heavily on a range of instrumental assessments, including videofluoroscopic swallow studies (VFSS), fiberoptic endoscopic evaluation of swallowing (FEES), high-resolution manometry (HRM), electromyography (EMG), electrokinesiographic study of swallowing (EKSS), and computed tomography (CT) (8). Among these, VFSS and FEES are widely regarded as the gold standards, providing dynamic visualization of pharyngeal phase physiology and objective quantification of penetration-aspiration events (9). However, their widespread application is often limited by the availability of equipment and trained personnel, particularly in resource-constrained settings, leading to geographical and socioeconomic disparities in care (10).
The treatment of dysphagia is highly individualized, depending on the etiology, anatomical site and severity. Current therapeutic approaches generally fall into four categories: compensatory, facilitative, rehabilitative and restorative techniques (11). Compensatory strategies such as postural adjustments such chin-tuck or head rotation, and dietary or bolus modification, aim to reduce aspiration risk without altering swallowing physiology (12, 13). Facilitation approaches, including thermal tactile stimulation (TTS) and sour bolus, are used to enhance reflexive swallowing response (14, 15). Rehabilitative methods emphasize neuromuscular retraining through targeted exercises such as the Mendelsohn maneuver and Shaker exercise (16). Restorative techniques, such as neuromuscular electrical stimulation, biofeedback-assisted therapy, or task-specific swallowing training, seek to improve biomechanical coordination and promote long-term recovery (17, 18). In severe cases, when dysphagia is so severe that the nutritional demands cannot be covered orally, artificial nutrition has to be considered (16).
In recent years, artificial intelligence (AI) has begun to reshape the dysphagia research landscape through three transformative pathways: (1) automated interpretation of multidimensional clinical metrics (19), (2) predictive modeling of therapeutic outcomes (20), and (3) improving clinical decision support (21). Lee et al. (22) used a deep learning (DL) model to detect airway invasion from VFSS images, without clinician input, with 97.2% accuracy in classifying image frames and 93.2% in classifying video files. Beyond traditional computer vision applications, Li et al. (23) used multi-layer perceptron (MLP), convolutional neural network (CNN), and convolutional recurrent neural network (CRNN) models to classify and identify swallowing-related activities based on their acoustic signatures, achieving classification accuracies of 74% (MLP), 68% (CNN), and 54% (CRNN), respectively. These technologies not only promise improved diagnostic accuracy and early detection, but also open new avenues for real-time monitoring and individualized therapy. However, the integration of AI into dysphagia care is still at an early stage, and progress is uneven across disciplines and geographic regions.
To date, there has been no systematic, data-driven evaluation of how AI is being deployed in the study and management of dysphagia. While interest in this domain is growing, the lack of a comprehensive overview makes it difficult to identify leading contributors, technological trajectories, and emergent research priorities. Bibliometric and science mapping approaches offer a powerful lens through which to assess the structure and dynamics of this interdisciplinary field, revealing both the strengths and gaps in current knowledge (24).
In this study, we conduct the first global bibliometric and visualization analysis of research on AI in dysphagia, covering a 25-year span from 2000 to early 2025. Using tools such as VOSviewer and the Bibliometrix package in R, we quantitatively map the evolution of publications, authorship networks, institutional and national contributions, and thematic clusters of research. By doing so, we aim to (1) characterize the structure and dynamics of the existing research landscape, (2) identify dominant and emerging themes in the integration of AI with dysphagia, and (3) inform future directions at the intersection of digital health, clinical neuroscience, and rehabilitation medicine.
2 Methods
2.1 Data source
The Web of Science Core Collection (WoSCC) was selected as the primary data source for this bibliometric analysis. WoSCC, maintained by Clarivate Analytics, is widely recognized as one of the most authoritative and commonly used databases for academic literature retrieval and citation analysis (25). The dataset used in this study is publicly available on the Open Science Framework (OSF) platform (doi: 10.17605/OSF.IO/S3HNW).
2.2 Search strategies
This study defined appropriate keywords for the search after reviewing related literature on AI and dysphagia research (26, 27). All data were retrieved on February 10, 2025 from WoSCC with the following strategies: (1) TS = (“artificial intelligence” OR “robotic*” OR “expert* system*” OR “intelligent learning” OR “feature* extraction” OR “feature* mining” OR “feature* learning” OR “machine learning” OR “feature* selection” OR “unsupervised clustering” OR “image* segmentation” OR “supervised learning” OR “semantic segmentation” OR “deep network*” OR “Bayes* network” OR “deep learning” OR “neural network*” OR “neural learning” OR “neural nets model” OR “artificial neural network” OR “data mining” OR “graph mining” OR “data clustering” OR “big data” OR “knowledge graph”) AND (“dysphagia” OR “swallowing disorder” OR “swallowing difficulty” OR “swallowing impairment” OR “swallowing dysfunction” OR “swallowing problem” OR “aphagia” OR “deglutition difficulty” OR “ingurgitation difficulty”); (2) Language = English; (3) Timespan = 2000–2025; (4) Document Type = article and review article. Full records and cited references are exported and downloaded in plain text format for analysis. The retrieval, screening, and enrollment process is shown in Figure 1.
Figure 1

Publication selection flow diagram. This diagram illustrates the screening process and final inclusion of publications related to AI in dysphagia.
2.3 Analysis tools
Microsoft Excel is a widely accessible data analysis and visualization tool, commonly provided by institutions for research and administrative purposes (28). Excel (version 16.90) was employed to analyze the annual publication volume and trends, as well as to generate corresponding line charts.
VOSviewer (version 1.6.20), developed by Nees Jan van Eck and Ludo Waltman at Leiden University in 2010, is a freely available software tool widely used for constructing and visualizing bibliometric networks (29). In this study, VOSviewer was utilized to analyze and visualize co-authorship, co-occurrence, and bibliographic coupling relationships across publications, journals, authors, countries, institutions, and keywords.
Bibliometrix, an open-source R package developed by Aria and Cuccurullo (30), was used for advanced bibliometric analysis and science mapping. R (version 2024.12.11) was specifically applied to visualize the Three-Field Plot, international collaboration networks, and the scientific output of countries and affiliations.
3 Results
3.1 Analysis of publications
From 2000 to 2025, the number of publications on AI in dysphagia research exhibited an overall upward trajectory, which can be divided into three distinct phases (Figure 2). The first phase (2000–2012) was marked by a limited number of publications, reflecting the nascent stage of research in this domain. The second phase (2013–2017) showed irregular fluctuations in publication volume, indicating a period of exploration and gradual development. The third phase (2018–present) has been characterized by a sustained and significant increase in the number of articles, suggesting growing interest and advancements in this field.
Figure 2

The Annual Number and Trend of Publications of AI in Dysphagia (2000–2025). The number of publications shows an overall increasing trend, with a marked acceleration after 2018, reflecting growing research interest in this field.
A total of 633 articles have been published, with an average annual growth rate of 13.6%, reflecting the accelerating pace of research in this area. Among these, 16 publications have received more than 100 citations. Table 1 lists the top 10 most-cited articles, led by the study “Radiotherapy vs. transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomized trial” (31), published in 2019, which has accrued 369 citations.
Table 1
| Ranking | Title | Author (Year) | DOI | IF | Citation |
|---|---|---|---|---|---|
| 1 | Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomized trial | Nichols et al. (2019) | 10.1016/S1470-2045(19)30410-3 | 41.6 | 369 |
| 2 | Phase II Randomized Trial of Transoral Surgery and Low-Dose Intensity Modulated Radiation Therapy in Resectable p16+Locally Advanced Oropharynx Cancer: An ECOG-ACRIN Cancer Research Group Trial (E3311) | Ferris et al. (2022) | 10.1200/JCO.21.01752 | 42.1 | 267 |
| 3 | Functional outcomes after transoral robotic surgery for head and neck cancer | Iseli et al. (2009) | 10.1016/j.otohns.2009.05.014 | 2.6 | 190 |
| 4 | Functional outcomes after TORS for oropharyngeal cancer: a systematic review | Hutcheson et al. (2014) | 10.1007/s00405-014-2985-7 | 1.9 | 163 |
| 5 | A new paradigm for the diagnosis and management of unknown primary tumors of the head and neck: A role for transoral robotic surgery | Mehta et al. (2013) | 10.1002/lary.23562 | 2.2 | 136 |
| 6 | Quality of life in survivors of oropharyngeal cancer: A systematic review and meta-analysis of 1,366 patients | Roets et al. (2018) | 10.1016/j.ejca.2017.03.006 | 2.8 | 132 |
| 7 | Repetitive transcranial magnetic stimulation in stroke rehabilitation: review of the current evidence and pitfalls | Fisicaro et al. (2019) | 10.1177/1756286419878317 | 4.7 | 127 |
| 8 | Cardiovascular causes of airway compression | Kussman et al. (2004) | 10.1046/j.1460-9592.2003.01192.x | 1.7 | 127 |
| 9 | Transoral Endoscopic Head and Neck Surgery and Its Role Within the Multidisciplinary Treatment Paradigm of Oropharynx Cancer: Robotics, Lasers, and Clinical Trials | Holsinger et al. (2015) | 10.1200/JCO.2015.62.3157 | 42.1 | 132 |
| 10 | Functional Swallowing Outcomes Following Transoral Robotic Surgery vs Primary Chemoradiotherapy in Patients With Advanced-Stage Oropharynx and Supraglottis Cancers | More et al. (2013) | 10.1001/jamaoto.2013.1074 | 6.0 | 117 |
The top 10 cited publications of AI in dysphagia.
3.2 Analysis of sources
A total of 292 journals have published articles related to the application of artificial intelligence in dysphagia. Table 2 presents the top 10 journals ranked by the number of publications, among which 8 journals have published at least 10 articles. Notably, Head and Neck—Journal for the Sciences and Specialties of the Head and Neck ranks first, with a total of 30 (4.74%) documents in this field.
Table 2
| Ranking | Journal | Country | JCR | IF | Documents | Total link strength |
|---|---|---|---|---|---|---|
| 1 | Head and Neck-Journal for the Sciences and Specialties of the Head and Neck | USA | Q1 | 2.4 | 30 (4.74%) | 494.06 |
| 2 | Surgical Endoscopy and Other Interventional Techniques | USA | Q1 | 2.4 | 21 (3.32%) | 121.28 |
| 3 | Dysphagia | USA | Q2 | 2.2 | 19 (3.00%) | 181.9 |
| 4 | Laryngoscope | USA | Q2 | 2.2 | 17 (2.69%) | 192.27 |
| 5 | European Archives of Oto-Rhino-Laryngology | Germany | Q2 | 1.9 | 14 (2.21%) | 290.69 |
| 6 | Oral Oncology | UK | Q1 | 4.0 | 14 (2.21%) | 204.37 |
| 7 | Scientific Reports | UK | Q2 | 3.8 | 13 (2.05%) | 127.18 |
| 8 | JAMA Otolaryngology-Head & Neck Surgery | USA | Q1 | 6.1 | 11 (1.74%) | 202.76 |
| 9 | Auris Nasus Larynx | Netherlands | Q3 | 1.6 | 8 (1.26%) | 123.29 |
| 10 | Frontiers in Neurology | Switzerland | Q2 | 2.7 | 7 (1.11%) | 154.04 |
The top 10 journals of AI in dysphagia.
3.3 Analysis of authors
A total of 3,533 authors have contributed to research articles on artificial intelligence in dysphagia, and the institutions and countries of high contributing authors are visualized in Figure 3. Table 3 lists the top 10 most productive authors in this domain, among whom 5 have published at least 10 articles. Notably, Ervin Sejdić emerges as the most prolific contributor, with a total of 28 (4.42%) documents, highlighting his prominent role in advancing research in this area.
Figure 3

Three-field plot visualized using bibliometrix. This bibliometric map displays the interrelationships among the most productive authors (left), institutions (center), and countries (right). Thicker lines and larger nodes indicate stronger collaboration and higher publication volume.
Table 3
| Ranking | Author | Organization | Country | Subject categories | Documents (%) | Total link strength |
|---|---|---|---|---|---|---|
| 1 | Sejdic Ervin (28) | University of Toronto | Canada | Engineering, Computer Science, Neurosciences & Neurology, Science & Technology, Medical Informatics | 28 (4.42%) | 28.00 |
| 2 | Coyle James L. (21) | University of Pittsburgh | USA | Engineering, Computer Science, Otorhinolaryngology, Rehabilitation, Neurosciences & Neurology | 21 (3.32%) | 21.00 |
| 3 | Khalifa Yassin (13) | University of Pittsburgh | USA | Engineering, Computer Science, Cardiovascular System & Cardiology, Otorhinolaryngology, Science & Technology | 12 (2.05%) | 13.00 |
| 4 | Hutcheson Katherine A. (11) | UTMD Anderson Cancer Center | USA | Oncology, Radiology, Nuclear Medicine & Medical Imaging, Otorhinolaryngology, Surgery, Research & Experimental Medicine | 11 (1.74%) | 9.00 |
| 5 | Mao Shitong (10) | UTMD Anderson Cancer Center | USA | Engineering, Computer Science, Otorhinolaryngology, Science & Technology, Materials Science | 10 (1.58%) | 10.00 |
| 6 | Donohue Cara (9) | Vanderbilt University Medical Center | USA | Neurosciences & Neurology, Rehabilitation, Linguistics, Audiology & Speech-Language Pathology, Otorhinolaryngology | 9 (1.42%) | 9.00 |
| 7 | Clifton D. Fuller (7) | UTMD Anderson Cancer Center | USA | Oncology, Radiology, Nuclear Medicine & Medical Imaging, Otorhinolaryngology, Surgery, Science & Technology | 7 (1.11%) | 7.00 |
| 8 | Chau Tom (7) | Holland Bloorview Kids Rehabilitation Hospital | Canada | Engineering, Neurosciences & Neurology, Rehabilitation, Computer Science, Sport Sciences | 7 (1.11%) | 6.00 |
| 9 | Quon Harry (7) | Johns Hopkins University | USA | Oncology, Otorhinolaryngology, Radiology, Nuclear Medicine & Medical Imaging, Surgery, Research & Experimental Medicine | 7 (1.11%) | 6.00 |
| 10 | Meccariello Giuseppe (6) | Azienda USL della Romagna | Italy | Otorhinolaryngology, Surgery, General & Internal Medicine, Oncology, Neurosciences & Neurology | 6 (0.95%) | 6.00 |
The top 10 productive authors of AI in Dysphagia.
3.4 Analysis of institutions and countries
A total of 1,055 institutions have contributed to research on artificial intelligence in dysphagia. As shown in Table 4, the top 10 most productive institutions are identified, among which 8 have published at least 10 articles. The University of Pittsburgh ranks first with 39 publications (6.16%), highlighting its leading influence in the field. Notably, the Pennsylvania Commonwealth System of Higher Education (PCSHE) has recently emerged as the most productive affiliation, with its increasing output over time further confirmed by the trends shown in Figure 4C.
Table 4
| Ranking | Institution | Documents (%) | Total link strength | Country | Documents (%) | Total link strength |
|---|---|---|---|---|---|---|
| 1 | University of Pittsburgh | 39 (6.16%) | 15.00 | USA | 255 (40.28%) | 60.00 |
| 2 | University of Toronto | 31 (4.90%) | 23.00 | China | 81 (12.80%) | 13.00 |
| 3 | UTMD Anderson Cancer Center | 24 (3.79%) | 15.00 | Canada | 51 (8.07%) | 28.00 |
| 4 | Soul National University | 12 (1.90%) | 3.00 | Japan | 50 (7.90%) | 4.00 |
| 5 | North York General Hospital | 10 (1.58%) | 10.00 | South Korea | 47 (7.42%) | 6.00 |
| 6 | Newcastle University | 10 (1.58%) | 8.00 | England | 46 (7.27%) | 22.00 |
| 7 | University of California San Diego | 10 (1.58%) | 3.00 | Italy | 39 (6.16%) | 16.00 |
| 8 | Northwestern University | 10 (1.58%) | 1.00 | Australia | 25 (3.95%) | 15.00 |
| 9 | Korea University | 9 (1.42%) | 4.00 | Germany | 21 (3.32%) | 10.00 |
| 10 | University of Pennsylvania | 9 (1.42%) | 4.00 | Netherlands | 17 (2.69%) | 5.00 |
The top institutions and countries of AI in dysphagia.
Figure 4
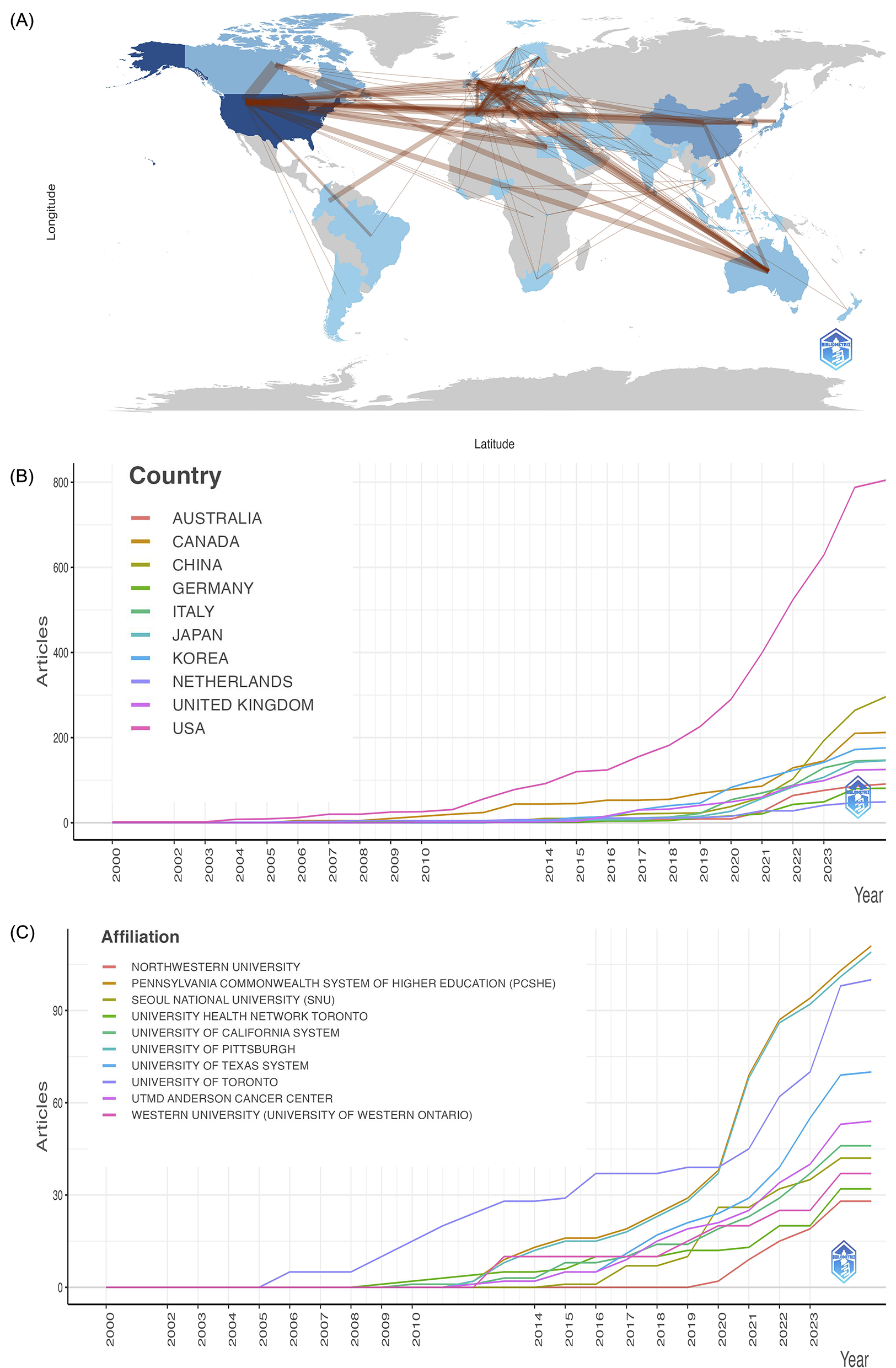
(A) Countries' collaboration world map visualized using bibliometrix. Deeper blues signal more extensive collaboration, with thicker lines indicating stronger collaborative ties. (B) Countries' production over time. (C) Affiliations' production over time.
Research in this area spans across 65 countries, with 11 of them having published 10 or more articles. Table 4 and Figure 4B list the top 10 countries/regions by publication volume. The United States stands out as the most prolific contributor, accounting for 255 publications (40.28%), underscoring its dominant role in advancing AI-related dysphagia research globally. In addition, Figure 4A visualizes the global collaboration network, indicating that the lack of knowledge sharing and cooperation across countries and the USA demonstrated the strongest international collaboration.
3.5 Analysis of keywords
A keyword co-occurrence knowledge map related to AI in dysphagia is presented in Figure 5A. Among the 2,586 keywords identified, 14 appeared 50 times or more. These high-frequency keywords, listed in descending order of occurrence, include: dysphagia, head, transoral robotic surgery, radiotherapy, squamous-cell carcinoma, quality of life, neck cancer, outcomes, robotic surgery, swallowing, machine learning, aspiration, oropharyngeal cancer, and cancer. Table 5 displays the top 10 most frequently occurring keywords, with dysphagia ranking first, appearing 286 times.
Figure 5

Keywords of AI in Dysphagia visualized using VOSviewer. (A) Knowledge map of keywords. Larger circles indicate higher frequency; thicker lines represent stronger connections; different colors correspond to distinct clusters. (B) Timeline of keywords. Keywords closer to yellow represent more recently appearing terms. (C) Hotpots of Keywords. Keywords closer to red indicate higher research intensity or popularity.
Table 5
| Ranking | Keywords | Occurrences (%) | Total link strength |
|---|---|---|---|
| 1 | Dysphagia | 286 (11.06%) | 267.00 |
| 2 | Head | 99 (3.83%) | 97.00 |
| 3 | Transoral robotic surgery | 96 ()3.71% | 92.00 |
| 4 | Radiotherapy | 78 (3.02%) | 75.00 |
| 5 | Squamous-cell carcinoma | 75 (2.90%) | 75.00 |
| 6 | Quality of life | 75 (2.90%) | 75.00 |
| 7 | Neck cancer | 72 (2.78%) | 72.00 |
| 8 | Outcomes | 62 (2.40%) | 61.00 |
| 9 | Robotic surgery | 58 (2.24%) | 53.00 |
| 10 | Swallowing | 56 (2.17%) | 54.00 |
The top 10 keywords of AI in dysphagia.
As shown in Figure 5B, the primary research trends in AI and dysphagia have undergone substantial evolution over time. Early research predominantly emphasized surgical interventions, including robotic surgery, minimally invasive surgery, and Heller myotomy. In contrast, recent years have seen the emergence of three prominent thematic trends: (1) A growing focus on diagnostic and rehabilitative approaches, with increasing attention to voice, speech, clinical scales, and transcranial magnetic stimulation; (2) Deeper investigation into the etiology and complications of dysphagia, addressing aspects such as classification, risk factors, stroke, oropharyngeal dysphagia, and pneumonia; (3) Advancement of AI-based analytical techniques, particularly emphasizing machine learning (ML) and DL methodologies.
The current research hotspots in the field, as visualized in Figure 5C, cluster around three major thematic areas: (1) Etiology and complications of dysphagia, involving terms such as head, neck cancer, stroke, oropharyngeal cancer, carcinoma, swallowing, and speech. (2) Therapeutic strategies for dysphagia, including radiotherapy, transoral robotic surgery, and laser microsurgery. (3) AI models and algorithms, with a particular focus on machine learning, deep learning, and intelligent modeling approaches.
4 Discussion
This study analysis highlights the dynamic evolution of global research on AI in dysphagia since the beginning of the 21st century. The developmental trajectory can be broadly categorized into three phases: a period of slow growth (2000–2012), a stage of fluctuating progress (2013–2017), and a phase of exponential expansion (2018–present). These stages reflect both the opportunities and limitations inherent in applying AI technologies to the complex clinical landscape of dysphagia. Notably, the steep rise in publications after 2018 coincides with significant advancements in ML and deep DL, as well as their expanding applications in biomedical research domains (32–34). The United States has emerged as the most productive country, contributing 255 publications, underscoring its leadership in this interdisciplinary field. However, the relatively fragmented global collaboration network suggests a critical gap in cross-national knowledge sharing and cooperative development.
5 Shifting study paradigm
The keyword co-occurrence and temporal analysis reveal a marked paradigm shift in the research landscape—from early investigations centered on surgical techniques such as transoral robotic surgery to AI-driven approaches in diagnosis, therapy, and rehabilitation. For example, a landmark article with 190 citations reported on functional outcomes following transoral robotic surgery for head and neck cancer, reflecting the emphasis on surgical innovation in earlier years (35). More recent studies, however, have increasingly focused on non-invasive, technology-assisted methods such as acoustic signal analysis (36, 37) and AI-based predictive modeling (38).
This thematic shift is not solely driven by artificial intelligence itself, but rather by the growing integration of AI into existing medical frameworks, enhancing data interpretation, risk stratification, and clinical decision-making. It reflects a methodological evolution: from unidisciplinary problem-solving toward multidisciplinary and eventually interdisciplinary frameworks. In the backdrop of a new round of scientific and industrial revolutions, scientific research is going through a paradigm shift, making interdisciplinarity a necessity for disciplinary development in the era of big science, and this evolution enables more robust exploration of complex, multifactorial conditions like dysphagia, which involves neuromuscular, structural, and behavioral components (39). The growing prominence of ML and DL as keywords in recent years further supports the notion that computational modeling is becoming central to the study of swallowing pathophysiology.
Notably, the role of AI varies depending on the specific clinical situation. For instance, Radiotherapy vs. transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomized trial exemplifies both the broad and narrow scopes of AI application in dysphagia research. From a broad perspective, robotic surgery can be considered part of AI's extended definition, reflecting the increasing role of intelligent systems in surgical interventions (40–42). From a narrower clinical standpoint, AI does not directly replace management of surgically treated diseases but serves an auxiliary role, supporting postoperative rehabilitation, risk assessment, and follow-up monitoring (43, 44).
6 Interdisciplinary gaps and collaborative opportunities
Interdisciplinarity, which addresses the complexity innate to nature and society, is the signature of complexity science (39). Despite notable advances in AI for dysphagia, the field faces significant challenges in achieving true interdisciplinary integration. Engineering and computer science currently dominate the authorship landscape, as exemplified by Sejdic Ervin, the most prolific author with 28 (4.42%) publications—while key clinical specialties such as speech-language pathology, rehabilitation, geriatric medicine and nursing remain underrepresented. Additionally, the concentration of high-impact studies in journals such as Head and Neck, and institutions like the University of Pittsburgh underscores the role of specialized academic hubs in shaping the discourse. However, countries' collaboration world map reveals a lack of meaningful collaboration: the USA ranks first among countries worldwide with 255 (40.28%) publications, accounting for almost half of the total. The top three institutions in terms of publication rankings are all from the USA, with very few from Asian countries and none from Africa and South America.
This lack of integration may slow the translational pipeline from algorithm development to clinical application, and exacerbate the problem of unequal distribution of global medical resources, especially in the field of swallowing disorders. Previous evidence has shown that interdisciplinary videoconferencing, as opposed to siloed consultation, can significantly reduce patient length of stay, streamline decision-making, and improve care efficiency (45). Interdisciplinarity is essential for disciplines to achieve development and solve problems, and is also valuable in breaking down disciplinary silos, enriching respective disciplines, achieving sustainable development, and producing multitalented dysphagia professionals (46), including those from gastroenterology, neurology, otolaryngology (ENT), speech-language pathology (SLP), nursing sci and clinical nutrition. Future efforts should thus prioritize cross-disciplinary, cross-institutional and cross-national collaboration, particularly between fields such as AI, medicine, nursing, and rehabilitation science.
7 Clinical and ethical significance
The prominence of the keyword dysphagia (286 occurrences), and its frequent co-occurrence with terms related to etiology and complications, suggests that AI holds promise not only in classification but also in mechanistic understanding and early risk detection. However, the paucity of highly cited studies addressing the clinical utility, ethical oversight, and real-world deployment of AI systems raises significant concerns (47, 48). While retrospective studies have demonstrated high diagnostic accuracy, the external validity and generalizability of these models, especially in older adults and neurodiverse populations—remain largely untested (49).
Moreover, ethical and legal implications, including data privacy, model transparency, and the potential for algorithmic bias, have yet to be adequately addressed (50). As AI systems begin to influence clinical decision-making, it is imperative that ethical frameworks evolve in parallel with technical advancements. Ensuring explainability, equity, and patient autonomy will be essential for the responsible and sustainable integration of AI into dysphagia care.
8 Limitations
This study has several limitations. First, only the Web of Science Core Collection was used, potentially omitting relevant studies indexed in other databases such as Scopus or PubMed. Second, non-English publications were excluded, possibly biasing the global landscape of AI in dysphagia. Third, citation-based metrics may not fully reflect the quality or impact of recent studies due to time-lag effects. Lastly, bibliometric tools may oversimplify complex interdisciplinary relationships, and this study did not assess algorithm performance or clinical applicability directly.
9 Conclusion
This study reveals the rapid growth and shifting research paradigm of AI in dysphagia, evolving from surgical interventions to intelligent, non-invasive diagnostics and rehabilitation. The United States and institutions like the University of Pittsburgh lead in output, yet international and interdisciplinary collaborations remain limited. Emerging focus on machine learning and deep learning signals a data-driven future, though clinical validation and ethical considerations remain underexplored. Moving forward, stronger cross-disciplinary collaboration is essential to translate AI innovations into effective and equitable dysphagia care.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Author contributions
TL: Visualization, Software, Writing – original draft, Conceptualization, Investigation, Writing – review & editing, Methodology, Data curation. YR: Conceptualization, Writing – review & editing, Methodology, Data curation, Writing – original draft, Investigation, Software, Visualization. DL: Methodology, Conceptualization, Software, Writing – original draft, Investigation, Writing – review & editing. HZ: Writing – original draft, Methodology, Conceptualization, Investigation, Software, Writing – review & editing. BL: Writing – original draft, Resources, Funding acquisition, Writing – review & editing, Formal analysis, Validation, Supervision, Project administration. GC: Writing – review & editing, Project administration, Formal analysis, Validation, Writing – original draft, Resources, Supervision, Funding acquisition. SS: Formal analysis, Resources, Writing – original draft, Supervision, Writing – review & editing, Project administration, Funding acquisition, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Peking University Third Hospital-Yantai International Joint Research Center for Rehabilitation and Elderly Care (No. H79463-06).
Acknowledgments
This manuscript was language-polished with the assistance of OpenAI's ChatGPT (version GPT-4-turbo, May 2024), accessed via ChatGPT Plus (https://chat.openai.com). The AI was used solely to improve grammar, clarity, and fluency without altering the intellectual content. The authors reviewed and approved all modifications.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Gen AI was used in the creation of this manuscript. This manuscript was language-polished with the assistance of OpenAI's ChatGPT (version GPT-4-turbo, May 2024), accessed via ChatGPT Plus (https://chat.openai.com). The AI was used solely to improve grammar, clarity, and fluency without altering the intellectual content. The author(s) reviewed and approved all modifications.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Clavé P Shaker R . Dysphagia: current reality and scope of the problem. Nat Rev Gastroenterol Hepatol. (2015) 12:259–70. 10.1038/nrgastro.2015.49
2.
Rajati F Ahmadi N Naghibzadeh ZA Kazeminia M . The global prevalence of oropharyngeal dysphagia in different populations: a systematic review and meta-analysis. J Transl Med. (2022) 20:175. 10.1186/s12967-022-03380-0
3.
Baijens LW Clavé P Cras P Ekberg O Forster A Kolb G et al . European society for swallowing disorders – European union geriatric medicine society white paper: oropharyngeal dysphagia as a geriatric syndrome. Clin Interv Aging. (2016) 11:1403–28. 10.2147/CIA.S107750
4.
Ribeiro M Miquilussi PA Gonçalves FM Taveira KVM Stechman-Neto J Nascimento WV et al . The prevalence of oropharyngeal dysphagia in adults: a systematic review and meta-analysis. Dysphagia. (2024) 39:163–76. 10.1007/s00455-023-10608-8
5.
Mascarenhas A Mendo R O'Neill C Franco AR Mendes R Simao I et al . Current approach to dysphagia: a review focusing on esophageal motility disorders and their treatment. GE Port J Gastroenterol. (2023) 30:403–13. 10.1159/000529428
6.
Labeit B Kremer A Muhle P Claus I Warnecke T Dziewas R et al . Costs of post-stroke dysphagia during acute hospitalization from a health-insurance perspective. Eur Stroke J. (2023) 8:361–9. 10.1177/23969873221147740
7.
Bordeje Laguna L Marcos-Neira P de Lagran Zurbano IM Mor Marco E Pollan Guisasola C Vinas Soria CD et al . Dysphagia and mechanical ventilation in SARS-CoV-2 pneumonia: it's real. Clin Nutr. (2022) 41:2927–33. 10.1016/j.clnu.2021.11.018
8.
Cosentino G Todisco M Giudice C Tassorelli C Alfonsi E . Assessment and treatment of neurogenic dysphagia in stroke and Parkinson's disease. Curr Opin Neurol. (2022) 35:741–52. 10.1097/WCO.0000000000001117
9.
Audag N Goubau C Toussaint M Reychler G . Screening and evaluation tools of dysphagia in adults with neuromuscular diseases: a systematic review. Ther Adv Chronic Dis. (2019) 10:2040622318821622. 10.1177/2040622318821622
10.
Ferrari de Castro MA Dedivitis RA Luongo de Matos L Baraúna JC Kowalski LP de Carvalho Moura K et al . Endoscopic and videofluoroscopic evaluations of swallowing for dysphagia: a systematic review. Braz J Otorhinolaryngol. (2025) 91:101598. 10.1016/j.bjorl.2025.101598
11.
Speyer R Baijens L Heijnen M Zwijnenberg I . Effects of therapy in oropharyngeal dysphagia by speech and language therapists: a systematic review. Dysphagia. (2010) 25:40–65. 10.1007/s00455-009-9239-7
12.
Wheeler-Hegland K Ashford J Frymark T McCabe D Mullen R Musson N et al . Evidence-based systematic review: oropharyngeal dysphagia behavioral treatments. Part II–impact of dysphagia treatment on normal swallow function. J Rehabil Res Dev. (2009) 46:185–94. 10.1682/JRRD.2008.08.0094
13.
Cichero JAY Steele C Duivestein J Clavé P Chen J Kayashita J et al . The need for international terminology and definitions for texture-modified foods and thickened liquids used in dysphagia management: foundations of a global initiative. Curr Phys Med Rehabil Rep. (2013) 1:280–91. 10.1007/s40141-013-0024-z
14.
de Lama Lazzara G Lazarus C Logemann JA . Impact of thermal stimulation on the triggering of the swallowing reflex. Dysphagia. (1986) 1:73–7. 10.1007/BF02407117
15.
Logemann JA Pauloski BR Colangelo L Lazarus C Fujiu M Kahrilas PJ . Effects of a sour bolus on oropharyngeal swallowing measures in patients with neurogenic dysphagia. J Speech Hear Res. (1995) 38:556–63. 10.1044/jshr.3803.556
16.
Wirth R Dziewas R Beck AM Clave P Hamdy S Heppner HJ et al . Oropharyngeal dysphagia in older persons - from pathophysiology to adequate intervention: a review and summary of an international expert meeting. Clin Interv Aging. (2016) 11:189–208. 10.2147/CIA.S97481
17.
Shigematsu T Fujishima I Ohno K . Transcranial direct current stimulation improves swallowing function in stroke patients. Neurorehabil Neural Repair. (2013) 27:363–9. 10.1177/1545968312474116
18.
Jayasekeran V Singh S Tyrrell P Michou E Jefferson S Mistry S et al . Adjunctive functional pharyngeal electrical stimulation reverses swallowing disability after brain lesions. Gastroenterology. (2010) 138:1737–46. 10.1053/j.gastro.2010.01.052
19.
Ibrahim M Khalil YA Amirrajab S Sun C Breeuwer M Pluim J et al . Generative AI for synthetic data across multiple medical modalities: a systematic review of recent developments and challenges. Comput Biol Med. (2025) 189:109834. 10.1016/j.compbiomed.2025.109834
20.
Liu X Rivera SC Moher D Calvert MJ Denniston AK . Reporting guidelines for clinical trial reports for interventions involving artificial intelligence: the CONSORT-AI extension. Lancet Digit Health. (2020) 2:E537–48. 10.1136/bmj.m3164
21.
Liu S Wright AP Patterson BL Wanderer JP Turer RW Nelson SD et al . Using AI-generated suggestions from ChatGPT to optimize clinical decision support. J Am Med Inform Assoc. (2023) 30:1237–45. 10.1093/jamia/ocad072
22.
Lee SJ Ko JY Kim HI Choi SI . Automatic detection of airway invasion from videofluoroscopy via deep learning technology. Appl Sci. (2020) 10:6179. 10.3390/app10186179
23.
Li D Zhang Y Wu J Luo W Liu T Lyu B et al . Development of the swallowing activity classification model for old adults based on acoustic analysis. Innov Aging. (2024) 8(Supplement_1):927. 10.1093/geroni/igae098.2991
24.
Donthu N Kumar S Mukherjee D Pandey N Lim WM . How to conduct a bibliometric analysis: an overview and guidelines. J Bus Res. (2021) 133:285–96. 10.1016/j.jbusres.2021.04.070
25.
Zhu J Liu W . A tale of two databases: the use of web of science and scopus in academic papers. Scientometrics. (2020) 123:321–35. 10.1007/s11192-020-03387-8
26.
Shen Z Wu H Chen Z Hu J Pan J Kong J et al . The global research of artificial intelligence on prostate cancer: a 22-year bibliometric analysis. Front Oncol. (2022) 12:843735. 10.3389/fonc.2022.843735
27.
Sun W Kang X Zhao N Dong X Li S Zhang G et al . Study on dysphagia from 2012 to 2021: a bibliometric analysis via CiteSpace. Front Neurol. (2022) 13:1015546. 10.3389/fneur.2022.1015546
28.
LaPolla FWZ . Excel for data visualization in academic health sciences libraries: a qualitative case study. J Med Libr Assoc JMLA. (2020) 108:67–75. 10.5195/jmla.2020.749
29.
Pan X Yan E Cui M Hua W . Examining the usage, citation, and diffusion patterns of bibliometric mapping software: a comparative study of three tools. J Informetr. (2018) 12:481–93. 10.1016/j.joi.2018.03.005
30.
Aria M Cuccurullo C . Bibliometrix: an R-tool for comprehensive science mapping analysis. J Informetr. (2017) 11:959–75. 10.1016/j.joi.2017.08.007
31.
Nichols AC Theurer J Prisman E Read N Berthelet E Tran E et al . Radiotherapy versus transoral robotic surgery and neck dissection for oropharyngeal squamous cell carcinoma (ORATOR): an open-label, phase 2, randomised trial. Lancet Oncol. (2019) 20:1349–59. 10.1016/S1470-2045(19)30410-3
32.
Janiesch C Zschech P Heinrich K . Machine learning and deep learning. Electron Mark. (2021) 31:685–95. 10.1007/s12525-021-00475-2
33.
Choi RY Coyner AS Kalpathy-Cramer J Chiang MF Campbell JP . Introduction to machine learning, neural networks, and deep learning. Transl Vis Sci Technol. (2020) 9:14. 10.1167/tvst.9.2.14
34.
Castiglioni I Rundo L Codari M Di Leo G Salvatore C Interlenghi M et al . AI applications to medical images: from machine learning to deep learning. Phys Med. (2021) 83:9–24. 10.1016/j.ejmp.2021.02.006
35.
Iseli TA Kulbersh BD Iseli CE Carroll WR Rosenthal EL Magnuson JS . Functional outcomes after transoral robotic surgery for head and neck cancer. Otolaryngol Head Neck Surg. (2009) 141:166–71. 10.1016/j.otohns.2009.05.014
36.
Li D Wu J Jin X Li Y Tong B Zeng W et al . A review on intelligent aid diagnosis for dysphagia using swallowing sounds. Interdiscip Nurs Res. (2023) 2:250–6. 10.1097/NR9.0000000000000040
37.
Kim H Park HY Park D Im S Lee S . Non-invasive way to diagnose dysphagia by training deep learning model with voice spectrograms. Biomed Signal Process Control. (2023) 86:105259. 10.1016/j.bspc.2023.105259
38.
Lienhart AM Kramer D Jauk S Gugatschka M Leodolter W Schlegl T . Multivariable risk prediction of dysphagia in hospitalized patients using machine learning. Stud Health Technol Inform. (2020) 271:31–8. 10.3233/SHTI200071
39.
Qiao J . Interdisciplinarity for life sciences. Interdiscip Nurs Res. (2022) 1:1. 10.1097/NR9.0000000000000005
40.
Chatterjee S Das S Ganguly K Mandal D . Advancements in robotic surgery: innovations, challenges and future prospects. J Robot Surg. (2024) 18:28. 10.1007/s11701-023-01801-w
41.
Knudsen JE Ghaffar U Ma R Hung AJ . Clinical applications of artificial intelligence in robotic surgery. J Robot Surg. (2024) 18:102. 10.1007/s11701-024-01867-0
42.
Haidegger T Speidel S Stoyanov D Satava RM . Robot-assisted minimally invasive surgery-surgical robotics in the data age. Proc IEEE. (2022) 110:835–46. 10.1109/JPROC.2022.3180350
43.
Jae LS . Application of artificial intelligence in the area of dysphagia. J Korean Dysphagia Soc. (2020) 10:4–9. 10.34160/jkds.2020.10.1.002
44.
Alter IL Dias C Briano J Rameau A . Digital health technologies in swallowing care from screening to rehabilitation: a narrative review. Auris Nasus Larynx. (2025) 52:319–26. 10.1016/j.anl.2025.05.002
45.
Reeves S Pelone F Harrison R Goldman J Zwarenstein M . Interprofessional collaboration to improve professional practice and healthcare outcomes. Cochrane Database Syst Rev. (2017) 6:CD000072. 10.1002/14651858.CD000072.pub3
46.
Shang S Li D Hou L Yu M Wang Z . Interdisciplinarity catalyzes sustained development of nursing discipline. Interdiscip Nurs Res. (2022) 1:3–5. 10.1097/NR9.0000000000000009
47.
Roldan-Vasco S Restrepo-Uribe JP Orozco-Duque A Suarez-Escudero JC Orozco-Arroyave JR . Analysis of electrophysiological and mechanical dimensions of swallowing by non-invasive biosignals. Biomed Signal Process Control. (2023) 82:104533. 10.1016/j.bspc.2022.104533
48.
Girardi AM Cardell EA Bird SP . Artificial intelligence in the interpretation of videofluoroscopic swallow studies: implications and advances for speech-language pathologists. Big Data Cogn Comput. (2023) 7:178. 10.3390/bdcc7040178
49.
Ashok M Madan R Joha A Sivarajah U . Ethical framework for artificial intelligence and digital technologies. Int J Inf Manag. (2022) 62:102433. 10.1016/j.ijinfomgt.2021.102433
50.
Cartolovni A Tomičić A Lazić Mosler E . Ethical, legal, and social considerations of AI-based medical decision-support tools: a scoping review. Int J Med Inf. (2022) 161:104738. 10.1016/j.ijmedinf.2022.104738
Summary
Keywords
dysphagia, swallowing, artificial intelligence, bibliometric, VOSviewer
Citation
Liu T, Rong Y, Li D, Zhang H, Li B, Cui G and Shang S (2025) Artificial intelligence in dysphagia since the 21st century: a bibliometric and visualization study. Front. Med. 12:1624381. doi: 10.3389/fmed.2025.1624381
Received
07 May 2025
Accepted
24 July 2025
Published
18 August 2025
Volume
12 - 2025
Edited by
Rahul Kashyap, WellSpan Health, United States
Reviewed by
Harikrishna Choudary Ponnam, Summa Health System, Akron, United States
Dheera Tamvada, Golden Valley Health Centers, United States
Updates
Copyright
© 2025 Liu, Rong, Li, Zhang, Li, Cui and Shang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Baohua Li lianglbh@126.comGuoqing Cui drcuiguoqing1964@126.comShaomei Shang shangshaomei@126.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.