Abstract
Background::
Hepatic sinusoidal obstruction syndrome (HSOS) is a rare but life-threatening liver condition. The clinical utility of transjugular intrahepatic portosystemic shunt (TIPS) for HSOS remains unclear.
Methods:
A systematic review and meta-analysis was performed across five databases (up to November 2024) to evaluate the efficacy and safety of TIPS in HSOS patients. Key outcomes included technical success, clinical response, portal pressure changes, survival, and complications.
Results:
Nineteen studies involving 465 patients were included. Pooled technical success and clinical response rates were 100 and 94.2%, respectively. TIPS significantly reduced portal pressure (mean PPG: −13.5 mmHg; PVP: −12.3 mmHg). The 3-month and 1-year survival rates were both 91.6%. Hepatic encephalopathy occurred in 13.2% of patients.
Conclusion:
TIPS is a feasible and relatively safe treatment for HSOS, particularly in patients with pyrrolizidine alkaloid–induced HSOS (PA-HSOS). Further research is warranted to confirm its long-term benefits.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/view/CRD42024620323, identifier CRD42024620323.
Introduction
Hepatic sinusoidal obstruction syndrome (HSOS), formerly known as hepatic veno-occlusive disease (HVOD), is a hepatic vascular disorder characterized by sinusoidal endothelial cell injury, leading to edema, necrosis, endothelial cell detachment, and microthrombosis within the hepatic sinusoids, central venules, and interlobular veins (1). These hemodynamic alterations can lead to portal hypertension and impaired liver function, potentially progressing to multi-organ failure and death. In developed countries, HSOS is most commonly observed in patients undergoing hematopoietic stem cell transplantation (HSCT) following myeloablative conditioning, those receiving chemotherapy for solid malignancies, or liver transplant recipients on immunosuppressive therapy. However, in contrast to developed countries, HSOS in China is primarily attributed to the consumption of pyrrolizidine alkaloid (PA)-containing herbs, such as Gynura segetum, due to their widespread use in traditional medicine (2–4).
Although there are a large number of HSCT cases in China, HVOD is rarely reported in HSCT patients, likely due to insufficient awareness of the condition. Due to potential differences in ethnicity, etiology, and underlying diseases, there is no global consensus on the diagnosis and treatment of HSOS. At present, the treatment of HSOS mainly focuses on anticoagulation therapy and symptomatic supportive measures, including liver protection, diuretics, and improving microcirculation (5). In critically ill patients, the mortality rate remains high with supportive treatment alone (6). Therefore, the prognosis for patients with severe HSOS remains poor, and new strategies are needed to improve their outcomes. TIPS is an effective interventional procedure. This procedure alleviates hepatic congestion by shunting portal venous blood flow, thereby reducing portal pressure and improving liver function, with an extremely low procedure-related mortality rate (7, 8). Studies have shown that TIPS can alleviate portal hypertension and improve liver function in HSOS patients, providing additional time for liver transplantation (9).
Nevertheless, due to the absence of sufficiently robust randomized controlled trials, the efficacy and safety of TIPS in HSOS patients remain inconclusive. To address this knowledge gap, the present study conducts a meta-analysis of existing published studies to systematically evaluate the efficacy and safety of TIPS in the management of HSOS.
Methods
This systematic review and meta-analysis of single-arm studies was conducted in strict accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (10).
Literature search
Five databases, including PubMed, Embase, Scopus, Web of Science and the Cochrane Library, were searched to identify eligible studies. The search period covered all records from database inception to November 30, 2024. The search keywords were: “Portasystemic Shunt, Transjugular Intrahepatic; Shunt, Transjugular Intrahepatic Portasystemic; TIPSS; Shunt, Transjugular Intrahepatic Portosystemic; Portosystemic Shunt, Transjugular Intrahepatic; TIPS; Transjugular Intrahepatic Portasystemic Shunt; Hepatic Veno-Occlusive Disease; Disease, Hepatic Veno-Occlusive; Hepatic Veno-Occlusive Diseases; Sinusoidal Obstruction Syndrome; Syndrome, Sinusoidal Obstruction; Hepatic Veno Occlusive Disease; Veno-Occlusive Disease, Hepatic; Veno Occlusive Disease, Hepatic” For example, the search strategy in PubMed was as follows: ((“Portasystemic Shunt, Transjugular Intrahepatic”[Mesh]) OR (((((((Portasystemic Shunt, Transjugular Intrahepatic[Title/Abstract]) OR (Shunt, Transjugular Intrahepatic Portasystemic[Title/Abstract])) OR (TIPSS[Title/Abstract])) OR (Shunt, Transjugular Intrahepatic Portosystemic[Title/Abstract])) OR (Portosystemic Shunt, Transjugular Intrahepatic[Title/Abstract])) OR (TIPS[Title/Abstract])) OR (Transjugular Intrahepatic Portasystemic Shunt[Title/Abstract]))) AND ((“Hepatic Veno-Occlusive Disease”[Mesh]) OR ((((((((Hepatic Veno-Occlusive Disease[Title/Abstract]) OR (Disease, Hepatic Veno-Occlusive[Title/Abstract])) OR (Hepatic Veno-Occlusive Diseases[Title/Abstract])) OR (Sinusoidal Obstruction Syndrome[Title/Abstract])) OR (Syndrome, Sinusoidal Obstruction[Title/Abstract])) OR (Hepatic Veno Occlusive Disease[Title/Abstract])) OR (Veno-Occlusive Disease, Hepatic[Title/Abstract])) OR (Veno Occlusive Disease, Hepatic[Title/Abstract]))). No language restrictions were applied during the search. Additionally, gray literature and the references of the included articles were also searched to ensure that no eligible studies were overlooked.
Study selection
All studies were independently evaluated by two authors, with any disagreements resolved through discussion with a third author. The selection process involved searching the literature using predefined keywords, screening study titles and abstracts, and reviewing the full text of potentially eligible studies. Reasons for exclusion and the number of excluded studies were documented. The inclusion criteria were as follows: (1) Population: Patients diagnosed with HSOS, (2) Intervention: Treatment with TIPS, (3) Outcomes: Efficacy and safety of TIPS in HSOS. The exclusion criteria were: (1) Pediatric patients (age < 10 years), (2) Studies lacking relevant outcome measures or with incomplete data, (3) Conference abstracts, case reports, review articles, letters, and editorials, (4) Duplicate publications.
Data extraction
Data extraction was independently performed by two authors, with a third author assessing the clinical outcomes. Discrepancies were resolved through discussion and consensus. Key data extracted from the original studies included both general information and clinical outcomes. General information encompassed the first author’s name, publication year, country, etiology, study design, interventions, sample size, patient age, and follow-up duration. Clinical outcomes were divided into two categories: safety and efficacy. Efficacy endpoints included the technical success rate, clinical response rate, and mean changes in portal pressure gradient (PPG) and portal vein pressure (PVP). Safety endpoints included the 3-month survival rate, 1-year survival rate, and the incidence of HE.
Risk assessment
Given the single-arm design of this study without a control group, the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Case Series was used to assess the risk of bias (11). To ensure consistency, all included studies were independently evaluated, and any disagreements were resolved through consensus.
Statistical analysis
Data were collected and standardized from various reports, and a single-arm meta-analysis was performed using Stata version 16.0 to evaluate the efficacy and safety of TIPS in the treatment of HSOS. Heterogeneity among studies was assessed using Cochrane’s Q chi-squared test and the I2 statistic. For low to moderate heterogeneity (p ≥ 0.10 and I2 < 50%), a fixed-effects model was applied, whereas a random-effects model was used for high heterogeneity (p < 0.10 or I2 > 50%). To address high heterogeneity in the pooled results, sensitivity analyses were conducted by excluding studies one at a time. Publication bias was evaluated using funnel plots, with Egger’s and Begg’s tests. Additionally, subgroup analyses were performed to explore the influence of different etiologies on the efficacy and safety of TIPS in the treatment of HSOS.
Results
Study selection and characteristics
Our initial search across five databases identified a total of 373 articles, comprising 107 from Embase, 64 from PubMed, 135 from Web of Science, 1 from the Cochrane Library, and 66 from Scopus. After removing duplicate studies, screening the titles and abstracts of the remaining records, and reviewing the full text of potentially eligible studies, 19 studies involving a total of 465 patients met the inclusion criteria and were included in this meta-analysis (12–30). All included studies were retrospective in design. The study selection process is illustrated in Figure 1, and the details of each included study are provided in Table 1. The review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) under registration number CRD42024620323.
FIGURE 1

Flow diagram of the selection process.
TABLE 1
| Study | Country | Study design | Etiology | Patients, n | Gender | Age (years) | Follow-up (months) |
AT before TIPS |
| Gómez-Centurión et al. (12) | Spain | Retrospective | HSCT-HSOS | 7 | All Male | 22–36 | 4–84 | 6Y, 1N |
| Zhou et al. (13) | China | Retrospective | PA-HSOS | 37 | 28M, 9F | 62.3 (Mean) | 3–48 | YES |
| Zhang et al. (14) | China | Retrospective | PA-HSOS | 9 | 6F, 3F | 49–78 | 5–39 | 7Y, 2N |
| Huang et al. (15) | China | Retrospective | PA-HSOS | 12 | 7M, 5F | 61.5 (Mean) | 10.5 (Median) | YES |
| Wang et al. (16) | China | Retrospective | PA-HSOS | 69 | 39M, 30F | 52.2 (Mean) | 38.5 (Median) | NO |
| Li et al. (17) | China | Retrospective | PA-HSOS | 13 | 10M, 3F | 49.5–66 | NA | NO |
| Wu et al. (18) | China | Retrospective | PA-HSOS | 4 | All Female | 48–61 | NA | YES |
| Zhuge et al. (19) | China | Retrospective | PA-HSOS | 29 | NA | NA | NA | 27Y, 2N |
| Huang (20) | China | Retrospective | PA-HSOS | 20 | 11M, 9F | 64.1 (Mean) | 2–69 | NO |
| Azoulay et al. (21) | France | Retrospective | HSCT-HSOS | 10 | 8M, 2F | 11–52 | NA | NA |
| Fried et al. (22) | America | Retrospective | HSCT-HSOS | 6 | 4M, 2F | 42 (Mean) | NA | YES |
| Wang et al. (23) | China | Retrospective | PA-HSOS | 27 | 16M, 11F | 21–60 | NA | NO |
| Wang et al. (24) | China | Retrospective | PA-HSOS | 7 | 4M, 3F | 29–74 | NA | YES |
| Changlong et al. (25) | China | Retrospective | PA-HSOS | 21 | 16M, 5F | 25–86 | NA | YES |
| Wei et al. (26) | China | Retrospective | PA-HSOS | 9 | 5M, 4F | 53–75 | 1–17 | NO |
| Yi et al. (27) | China | Retrospective | PA-HSOS | 21 | NA | NA | NA | YES |
| Xiao et al. (28) | China | Retrospective | PA-HSOS | 116 | 72M, 44F | 63.6 (Mean) | NA | 86Y, 30N |
| Xu et al. (30) | China | Retrospective | PA-HSOS | 15 | 7M, 8F | 30–85 | NA | NO |
| Zhang et al. (29) | China | Retrospective | PA-HSOS | 54 | NA | NA | NA | YES |
Characteristics of the included studies.
PA-HSOS, pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome; HSCT-HSOS, hematopoietic stem cell transplantation-induced hepatic sinusoidal obstruction syndrome; AT, anticoagulant treatment; TIPS, transjugular intrahepatic portosystemic shunt; NA, not available.
Quality assessment
All studies were evaluated using the JBI Critical Appraisal Checklist for Case Series. Detailed results of the assessment are presented in Table 2.
TABLE 2
| Study | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Overall appraisal |
| Gómez-Centurión et al. (12) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | Included |
| Zhou et al. (13) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | Included |
| Zhang et al. (14) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | Included |
| Huang et al. (15) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | Included |
| Wang et al. (16) | YES | YES | YES | YES | YES | YES | Unclear | YES | YES | YES | Included |
| Li et al. (17) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | Included |
| Wu et al. (18) | YES | YES | YES | YES | YES | YES | YES | YES | YES | Unclear | Included |
| Zhuge et al. (19) | YES | YES | YES | YES | YES | YES | YES | Unclear | YES | YES | Included |
| Huang et al. (20) | YES | YES | YES | YES | YES | YES | YES | Unclear | YES | YES | Included |
| Azoulay et al. (21) | YES | YES | YES | Unclear | YES | YES | Unclear | YES | YES | Unclear | Included |
| Fried et al. (22) | YES | YES | YES | YES | YES | YES | YES | YES | YES | Unclear | Included |
| Wang et al. (23) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | Included |
| Wang et al. (24) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | Included |
| Changlong et al. (25) | YES | YES | YES | YES | YES | YES | YES | Unclear | YES | YES | Included |
| Wei et al. (26) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | Included |
| Yi et al. (27) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | Included |
| Xiao et al. (28) | YES | YES | YES | Unclear | YES | YES | Unclear | YES | YES | YES | Included |
| Xu et al. (30) | YES | YES | YES | YES | YES | YES | YES | YES | YES | YES | Included |
| Zhang et al. (29) | YES | YES | YES | YES | YES | YES | YES | YES | YES | Unclear | Included |
Quality assessment of individual studies according to the JBI critical appraisal checklist.
JBI: Joanna Briggs Institute; Possible answers: Yes, no, unclear, not applicable. No means high risk of bias; Q1: Were there clear criteria for inclusion in the case series? Q2: Was the condition measured in a standard, reliable way for all participants included in the case series? Q3: Were valid methods used for identification of the condition for all participants included in the case series? Q4: Did the case series have consecutive inclusion of participants? Q5: Did the case series have complete inclusion of participants? Q6: Was there clear reporting of the demographics of the participants in the study? Q7: Was there clear reporting of clinical information of the participants? Q8: Were the outcomes or follow-up results of cases clearly reported? Q9: Was there clear reporting of the presenting site(s)/clinic(s) demographic information? Q10: Was statistical analysis appropriate?
Meta-analysis results
Rates of technical success and clinical response
The establishment of a shunt between the hepatic vein and an intrahepatic branch of the portal vein is considered a technical success for TIPS (17). A total of fifteen studies involving 340 patients reported the technical success rate, with a pooled success rate of 100% (95% CI: 99.6–100%, I2 = 0%). Clinical response was defined as a marked alleviation or resolution of clinical symptoms within 2 weeks of medical treatment, accompanied by improvements in laboratory and imaging findings (19). A total of eleven studies involving 231 patients reported the clinical remission rate, with a pooled remission rate of 94.2% (95% CI: 87.1–99.0%, I2 = 59.5%). Additionally, subgroup analyses were performed based on etiology to explore potential factors contributing to the significant heterogeneity in clinical remission rates. The results revealed significant overall heterogeneity in effect size. However, after subgroup analysis, within-group heterogeneity was no longer significant, suggesting that the subgrouping factor was likely a major contributor to the observed heterogeneity (Figure 2).
FIGURE 2
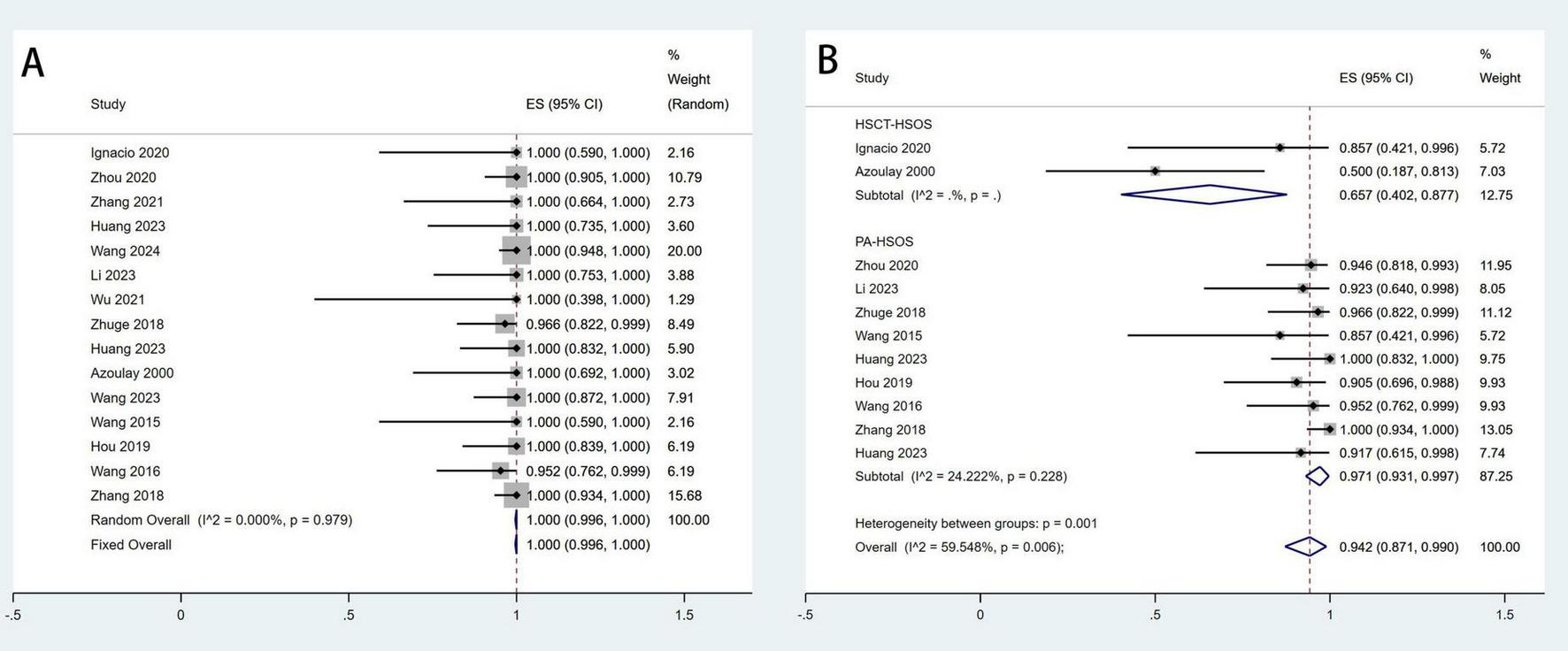
Meta-analysis of the rates of technical success (A) and clinical response (B) after TIPS in patients with HSOS.
Change in portal pressure gradient and portal venous pressure
Eleven studies involving 355 patients reported data on changes in PPG, while five studies with a total of 82 patients provided data on PVP. The pooled mean change in PPG was −13.5 mmHg (95% CI: −14.6 to −12.4 mmHg, I2 = 72.3%), and the pooled mean change in PVP was −12.3 mmHg (95% CI: −15.1 to −9.15 mmHg, I2 = 87.1%) (Figure 3). Sensitivity analyses were performed by systematically excluding individual studies from the pooled results, and these analyses demonstrated that such exclusions did not materially affect the overall findings (Figure 4).
FIGURE 3
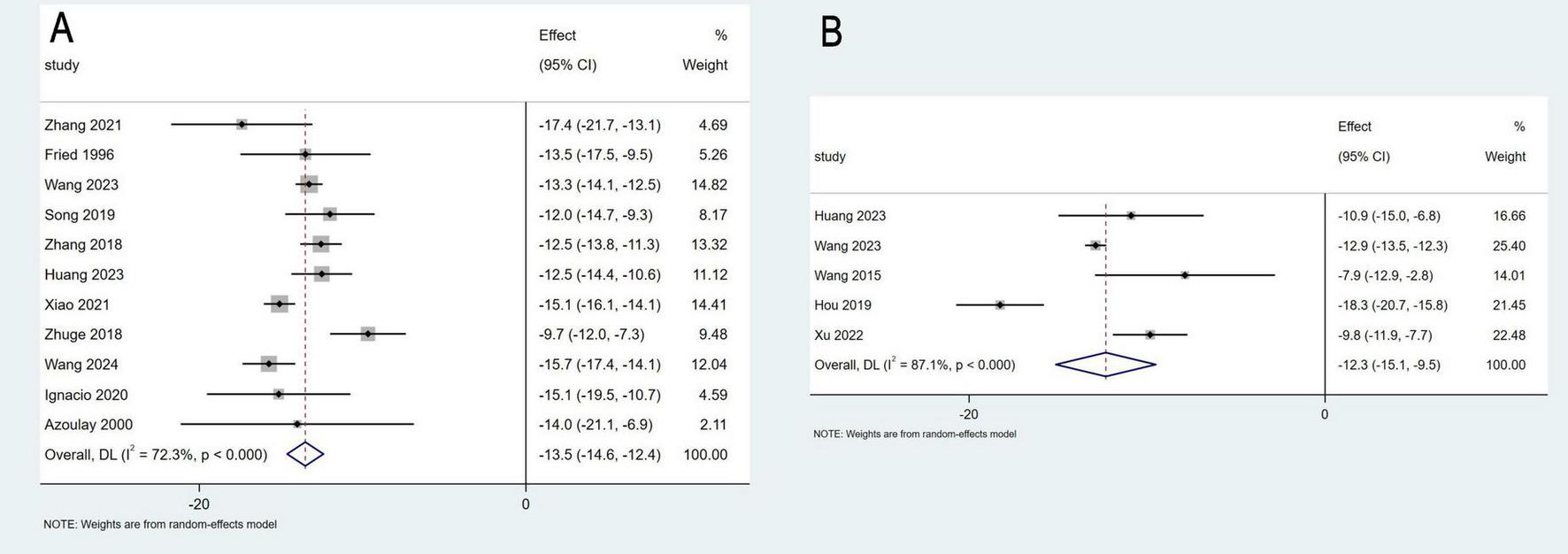
Meta-analysis of the change in portal pressure gradient (A), and portal venous pressure (B).
FIGURE 4
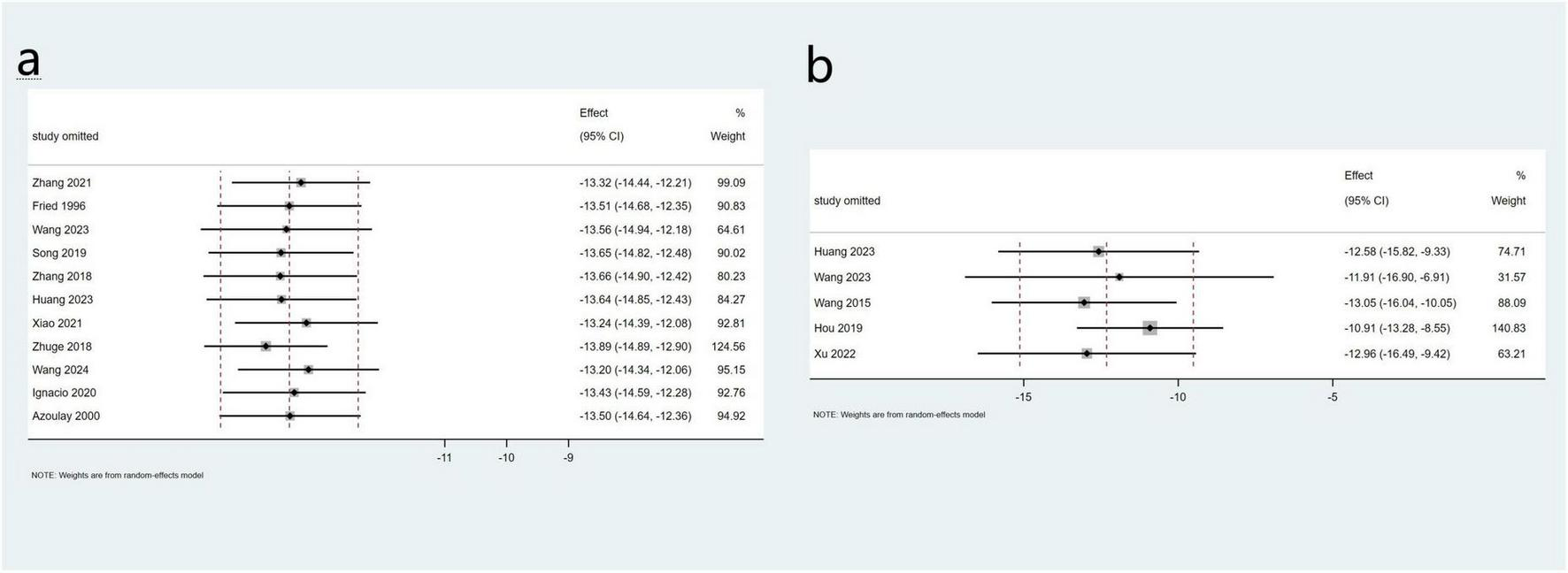
Sensitivity analysis of PPG (a) and PVP (b) in the total study.
Potential publication bias among the included studies was assessed using Begg’s and Egger’s tests. For the pooled PPG results, Egger’s test (P = 0.828) and Begg’s test (P = 0.755) indicated no significant publication bias. Similarly, for the pooled portal PVP results, Egger’s test (P = 0.809) and Begg’s test (P = 0.806) also showed no evidence of publication bias. A meta-regression analysis was performed for the pooled PPG results, incorporating etiology as a covariate. The p-value for etiology was 0.674 (P > 0.05), suggesting that etiology was not a significant contributor to heterogeneity. Due to the limited number of studies reporting PVP data, a meta-regression analysis for PVP was not conducted.
Rates of survival and hepatic encephalopathy
A total of ten studies involving 359 patients reported 3-month survival rates, with a pooled survival rate of 91.6% (95% CI: 82.7–97.9%, I2 = 77.9%). Seven studies with 329 patients provided data on 1-year survival rates, resulting in a pooled survival rate of 91.6% (95% CI: 88.1–94.6%, I2 = 6.9%) (Figure 5). Additionally, eight studies involving 225 patients reported the incidence of HE, with a pooled incidence rate of 13.2% (95% CI: 7.7–19.6%, I2 = 0%) (Figure 6).
FIGURE 5
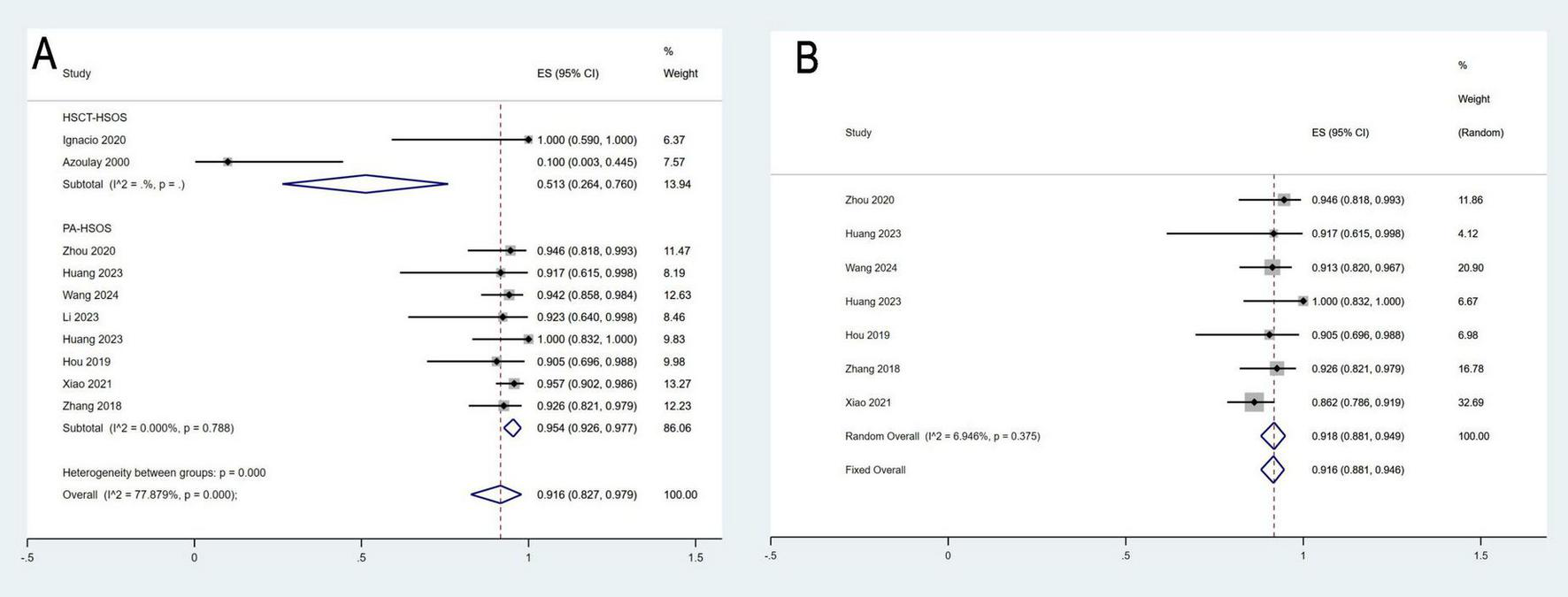
Meta-analysis of the survival rates of 3-month (A) and 1-year (B).
FIGURE 6
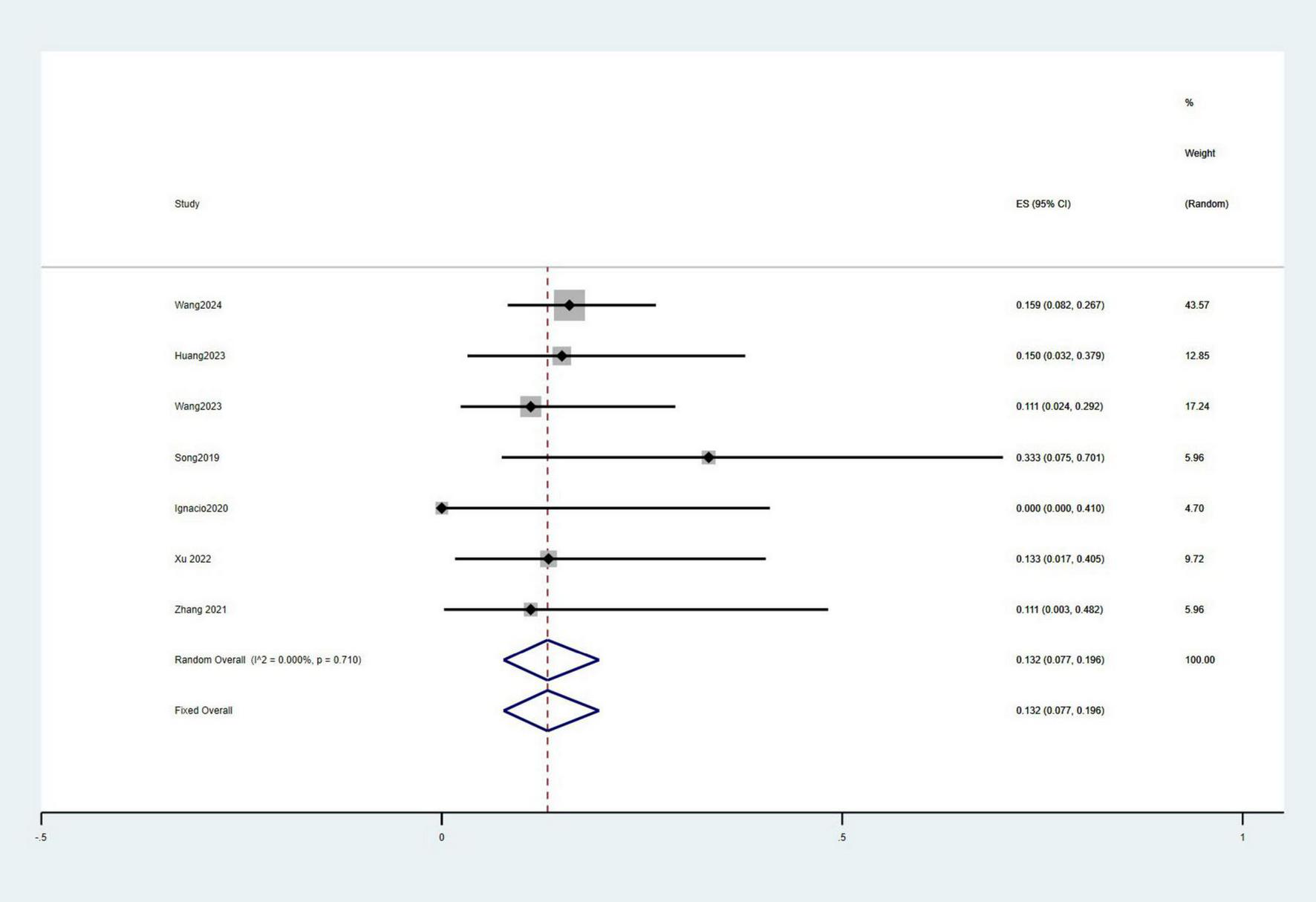
Meta-analysis of the incidence of HE.
To explore potential factors contributing to heterogeneity in the 3-month survival rate, a subgroup analysis based on etiology was conducted. As shown in the results, although overall heterogeneity in the effect size was significant, subgroup analyses revealed no significant heterogeneity within the subgroups. These findings suggest that etiology may be a significant source of heterogeneity.
Subgroup analysis by etiology
To address the observed heterogeneity in treatment outcomes, we conducted a subgroup analysis based on etiological characteristics (PA-HSOS vs. HSCT-HSOS). The results showed that the pooled clinical response rate for PA-HSOS patients was 97.1%, whereas it was only 65.7% for HSCT-HSOS patients. Similarly, the pooled 3-month survival rate was 95.4% in the PA-HSOS subgroup, compared to just 51.3% in the HSCT-HSOS subgroup. These findings are illustrated in Figures 2B, 5A and are systematically summarized in Table 3. These results suggest a significant association between etiology and treatment efficacy, indicating that etiology may be a key contributor to the overall heterogeneity observed in the analysis.
TABLE 3
| Outcome | PA-HSOS (n = 10 studies) | HSCT-HSOS (n = 2 studies) |
| Clinical response rate | 205/214 = 95.8% | 11/17 = 64.7% |
| 3-month survival rate | 323/342 = 94.4% | 8/17 = 47.1% |
Summary of pooled outcomes by etiology.
PA-HSOS, pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome; HSCT-HSOS, hematopoietic stem cell transplantation-induced hepatic sinusoidal obstruction syndrome.
Discussion
Portal hypertension and liver failure are the key pathological mechanisms driving disease progression in patients with HSOS. Common clinical manifestations include abdominal distension, ascites, and hepatomegaly (1). Current treatment strategies primarily focus on symptomatic management. However, in severe cases, symptomatic and supportive treatment alone is often insufficient to significantly reduce mortality. (6, 31, 32). For critically ill patients, liver transplantation can also be considered. However, it has inherent limitations, including organ shortages, surgical risks, and high costs (33). For patients who do not respond to symptomatic treatment and are awaiting liver transplantation, TIPS may serve as a viable alternative. The results of this study show that TIPS treatment led to a mean reduction in PPG of 13.5 mmHg and a mean reduction in PVP of 12.3 mmHg. This significant decrease in pressure effectively alleviated clinical symptoms associated with portal hypertension, such as abdominal distension, ascites, and hepatomegaly. Moreover, the pooled 3-month and 1-year survival rates reached 91.6%, suggesting that TIPS may improve the survival prognosis in these patients.
In patients with hematopoietic stem cell transplantation-induced hepatic sinusoidal obstruction syndrome (HSCT-HSOS), the only drug currently approved is defibrotide, which has a 100-day survival rate of approximately 50% (34, 35). However, the efficacy of TIPS in patients who are unresponsive to defibrotide remains controversial. Early studies in the 2000s suggested that TIPS did not significantly improve survival in these patients, with a 3-month survival rate of only 10% (36). Nevertheless, more recent studies have indicated that TIPS may improve survival and provide additional time for liver transplantation, reflecting advancements in interventional techniques and supportive care. Currently, research on the use of TIPS for HSCT-HSOS remains limited and requires further validation through large-scale randomized controlled trials.
In PA-HSOS patients, since defibrotide has not been approved for use in China, no studies have evaluated its efficacy in this population (20). In clinical practice, a stepwise approach of initial anticoagulation followed by TIPS is widely adopted. Existing evidence suggests that in mild cases, symptomatic or anticoagulation therapy may achieve comparable efficacy to TIPS; however, in severe cases, TIPS tends to show better clinical benefit (20). In this study, TIPS treatment for PA-HSOS patients demonstrated good safety and efficacy, with a 3-month survival rate and technical success rate reaching 95% and 100%, respectively. These results indicate that TIPS is both feasible and potentially valuable for broader application in PA-HSOS patients.
The efficacy of TIPS differs between PA-HSOS and HSCT-HSOS patients, primarily because mortality in HSCT-HSOS is often linked to multi-organ failure, sepsis, and hemorrhage, typically resulting from underlying hematological disorders. In contrast, PA-HSOS patients usually do not have severe hematological diseases, presenting with milder conditions and a slower progression of the disease (13).
In addition, regarding concerns about TIPS-related complications, the incidence of HE in this study was 13.2%, which is comparable to the incidence observed in other end-stage liver disease patients undergoing TIPS treatment, with no significant increase observed (37).
This study is the first systematic review to evaluate the efficacy of TIPS in the treatment of HSOS patients using quantitative meta-analysis. Like any meta-analysis, this study has several limitations. First, all included studies were retrospective observational in nature, which limits causal inference and introduces selection bias. TIPS is typically reserved for patients with more severe disease, leading to potential confounding by indication. Second, only single-arm studies were included, without direct comparisons to other treatments such as anticoagulation or defibrotide. Third, although our etiology-based subgroup analysis partially explained the clinical heterogeneity in treatment response (I2 = 59.5%), other factors—such as age, baseline liver function, coagulation status, and portal vein velocity—may also contribute. However, most included studies did not consistently report these variables, limiting further subgroup or meta-regression analyses. Lastly, most studies focused on PA-HSOS, while data on HSCT-HSOS were limited; thus, our findings are more generalizable to the PA-HSOS population. Future prospective, multicenter studies with standardized reporting are needed to validate these results and clarify the role of TIPS in current treatment algorithms.
Conclusion
Overall, TIPS treatment for HSOS is both feasible and effective, significantly reducing portal pressure and lowering the risk of major complications. Therefore, TIPS should be considered a viable treatment option for HSOS patients, particularly for those with PA-HSOS. However, due to the current lack of randomized controlled trials, further studies are needed to assess its long-term safety and efficacy.
Statements
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
XJ: Writing – original draft, Conceptualization. XM: Writing – review and editing, Investigation. ST: Investigation, Writing – review and editing. LP: Validation, Writing – review and editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the project ZR2023MH295 supported by Shandong Provincial Natural Science Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Mohty M Malard F Abecassis M Aerts E Alaskar A Aljurf M et al Sinusoidal obstruction syndrome/veno-occlusive disease: current situation and perspectives-a position statement from the European society for blood and marrow transplantation (EBMT). Bone Marrow Transplant. (2015) 50:781–9. 10.1038/bmt.2015.52
2.
Helmy A . Review article: updates in the pathogenesis and therapy of hepatic sinusoidal obstruction syndrome.Aliment Pharmacol Ther. (2006) 23:11–25. 10.1111/j.1365-2036.2006.02742.x
3.
Mohty M Malard F Abecassis M Aerts E Alaskar A Aljurf M et al Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: a new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. (2016) 51:906–12. 10.1038/bmt.2016.130
4.
Triolo M Poggi F Pasulo L Leonard F Vigano L Luca M et al Clinical impact and treatment of veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after liver transplant (LT). The role of transjugular intrahepatic porto-systemic shunt (TIPS). Digestive Liver Dis. (2023) 55:S60–60. 10.1016/j.dld.2023.01.118
5.
Wang Y Qiao D Li Y Xu F . Risk factors for hepatic veno-occlusive disease caused by Gynura segetum: a retrospective study.BMC Gastroenterol. (2018) 18:156. 10.1186/s12876-018-0879-7
6.
Zhuge Y Liu Y Xie W Zou X Xu J Wang J et al Expert consensus on the clinical management of pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome. J Gastroenterol Hepatol. (2019) 34:634–42. 10.1111/jgh.14612
7.
Boyer T . Transjugular intrahepatic portosystemic shunt: current status.Gastroenterology. (2003) 124:1700–10. 10.1016/s0016-5085(03)00377-9
8.
Khuroo M Al-Suhabani H Al-Sebayel M Al Ashgar H Dahab S Khan M et al Budd-Chiari syndrome: long-term effect on outcome with transjugular intrahepatic portosystemic shunt. J Gastroenterol Hepatol. (2005) 20:1494–502. 10.1111/j.1440-1746.2005.03878.x
9.
Annaloro C Robbiolo L Pozzoli E Ibatici A Nicolini A Salerno F et al Four-year survival after trans-jugular intrahepatic porto-systemic shunt for veno-occlusive disease following autologous bone marrow transplantation. Leuk Lymphoma. (2004) 45:1485–7. 10.1080/10428190410001663626
10.
Page M McKenzie J Bossuyt P Boutron I Hoffmann T Mulrow C et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. 10.1136/bmj.n71
11.
Munn Z Moola S Lisy K Riitano D Tufanaru C . Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data.Int J Evid Based Healthc. (2015) 13:147–53. 10.1097/XEB.0000000000000054
12.
Gómez-Centurión I Bailén R Oarbeascoa G Muñoz C Luque AÁ Boyra ME et al Transjugular intrahepatic portosystemic shunt for very severe veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS) after unmanipulated haploidentical hematopoietic stem cell transplantation with post-transplantation cyclophosphamide. Biol Blood Marrow Transplant. (2020) 26:2089–97. 10.1016/j.bbmt.2020.08.006
13.
Zhou C Wang R Lv W Fu Y Cheng D Zhu Y et al Transjugular intrahepatic portosystemic shunt for pyrrolizidine alkaloid-related hepatic sinusoidal obstruction syndrome. World J Gastroenterol. (2020) 26:3472–83. 10.3748/wjg.v26.i24.3472
14.
Zhang L Li Q Makamure J Zhao D Liu Z Zheng C et al Transjugular intrahepatic portosystemic shunt for hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum. BMC Gastroenterol. (2021) 21:26. 10.1186/s12876-021-01599-7
15.
Huang T Zhang X Yan K Lou D He Y Dai S et al Transjugular intrahepatic portosystemic shunt for pyrrolidine alkaloids-induced hepatic sinusoidal obstruction syndrome: a retrospective cohort study. Eur J Gastroenterol Hepatol. (2023) 35:1004–11. 10.1097/MEG.0000000000002591
16.
Wang C Wang Y Zhao J Yang C Zhu X Niu H et al Transjugular intrahepatic portosystemic shunt for the treatment of hepatic sinusoidal obstruction syndrome caused by pyrrolizidine alkaloids: a multicenter retrospective study. Heliyon. (2024) 10:e23455. 10.1016/j.heliyon.2023.e23455
17.
Li S Li Y Zhou C Li H Chen C Peng C et al Transjugular intrahepatic portosystemic shunt benefits for hepatic sinusoidal obstruction syndrome associated with consumption of Gynura segetum: a propensity score-matched analysis. Cardiovasc Intervent Radiol. (2023) 46:931–42. 10.1007/s00270-023-03451-9
18.
Wu F Yu J Gan H Zhang H Tian D Zheng D . Timing and efficacy of transjugular intrahepatic portosystemic shunt in patients with pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome.Sci Rep. (2021) 11:21743. 10.1038/s41598-021-01201-w
19.
Zhuge Y Wang Y Zhang F Zhu C Zhang W Zhang M et al Clinical characteristics and treatment of pyrrolizidine alkaloid-related hepatic vein occlusive disease. Liver Int. (2018) 38:1867–74. 10.1111/liv.13684
20.
Huang Q Zhang Q Xu H Zu M Xiao J Shen B . Mid- to long-term outcomes of initial transjugular intrahepatic portosystemic shunt versus anticoagulation for pyrrolizidine alkaloid-induced hepatic sinusoidal obstruction syndrome.Eur J Gastroenterol Hepatol. (2023) 35:445–52. 10.1097/MEG.0000000000002509
21.
Azoulay D Castaing D Lemoine A Hargreaves G Bismuth H . Transjugular intrahepatic portosystemic shunt (TIPS) for severe veno-occlusive disease of the liver following bone marrow transplantation.Bone Marrow Transplant. (2000) 25:987–92. 10.1038/sj.bmt.1702386
22.
Fried M Connaghan D Sharma S Martin L Devine S Holland K et al Transjugular intrahepatic portosystemic shunt for the management of severe venoocclusive disease following bone marrow transplantation. Hepatology. (1996) 24:588–91. 10.1002/hep.510240321
23.
Wang L Zhang Y Wang XQ Yue ZD Fan ZH Wu YF et al Evaluation of the efficacy of TIPS in 27 patients with hepatic sinus obstruction syndrome in the near and medium term. Zhonghua Gan Zang Bing Za Zhi. (2023) 31:842–6. 10.3760/cma.j.cn501113-20221012-00493
24.
Wang Y Zhang F Zhang M Zou X Zhu-Ge Y . Efficacy of transjugular intrahepatic portosystemic stent-shunt in treatment of gynura segetum induced hepatic veno-occlusive disease with refractory ascites.World Chinese J Digestol. (2015) 23:4261–5. 10.11569/wcjd.v23.i26.4261
25.
Changlong H Jun X Hanlin Q Xianhai Z Yipeng F Lei Z . Efficacy of transjugular intrahepatic portosystemic shunt in the treatment of 21 patients with gynura segetum-related hepatic sinusoidal obstruction syndrome.Chinese J Digestion. (2019) 39:251–6. 10.3760/cma.j.issn.0254-1432.2019.04.006
26.
Wei S Qingqiao Z Hao X Ning W Hongtao L Jinchang X et al Efficacy of transjugular intrahepatic portosystemic shunt in treatment of sinusoidal obstruction syndrome caused by gynura segetum. Chinese J Hepatobiliary Surg. (2019) 25:418–21. 10.3760/cma.j.issn.1007-8118.2019.06.005
27.
Yi W Yuzheng Z Feng Z Ming Z Wei Z Qibin H et al Treatment for Gynura segetum caused hepatic vein occlusive disease: a single-center retrospective study. Chinese J Digestion. (2016) 36:811–5. 10.3760/cma.j.issn.0254-1432.2016.12.003
28.
Xiao J Tu J Zhang H Zhang F Zhang W Xu H et al Risk factors of poor prognosis in patients with pyrrolidine alkaloid-induced hepatic sinusoidal obstruction syndrome after transjugular intrahepatic portosystemic shunt. Hepatol Int. (2021) 15:720–9. 10.1007/s12072-020-10126-x
29.
Zhang W Zhang M Zhang F Zhu C Wang Y Xiao J et al Tips might be effective for patients with acute hepatic veno-occlusive disease caused by pyrrolizidine alkaloids with no response to anticoagulation therapy: A single center retrospective study. Hepatology. (2018) 68:204A–5A.
30.
Xu R Zhang K Cui M Li W Zhang D Liang K et al Clinical impact of transjugular intrahepatic portosystemic shunt on refractory hepatic sinus obstruction syndrome caused by Gynura segetum. Chinese J Hepatobiliary Surg. (2022) 28:491–4. 10.3760/cma.j.cn113884-20220305-00088
31.
Richardson P Riches M Kernan N Brochstein J Mineishi S Termuhlen A et al Phase 3 trial of defibrotide for the treatment of severe veno-occlusive disease and multi-organ failure. Blood. (2016) 127:1656–65. 10.1182/blood-2015-10-676924
32.
Tsirigotis P Resnick I Avni B Grisariu S Stepensky P Or R et al Incidence and risk factors for moderate-to-severe veno-occlusive disease of the liver after allogeneic stem cell transplantation using a reduced intensity conditioning regimen. Bone Marrow Transplant. (2014) 49:1389–92. 10.1038/bmt.2014.168
33.
European Association for the Study of the Liver. EASL Clinical Practice Guidelines on liver transplantation. J Hepatol. (2024) 81:1040–86. 10.1016/j.jhep.2024.07.032
34.
Yang L Qi J Pan T You T Ruan C Han Y . Efficacy and safety of defibrotide for the treatment of hepatic veno-occlusive disease after hematopoietic stem cell transplantation: a systematic review and meta-analysis.Semin Thromb Hemost. (2019) 45:767–77. 10.1055/s-0039-1698763
35.
Richardson P Grupp S Pagliuca A Krishnan A Ho V Corbacioglu S . Defibrotide for the treatment of hepatic veno-occlusive disease/sinusoidal obstruction syndrome with multiorgan failure.Int J Hematol Oncol. (2017) 6:75–93. 10.2217/ijh-2017-0015
36.
Zenz T Rössle M Bertz H Siegerstetter V Ochs A Finke J . Severe veno-occlusive disease after allogeneic bone marrow or peripheral stem cell transplantation–role of transjugular intrahepatic portosystemic shunt (TIPS).Liver. (2001) 21:31–6. 10.1034/j.1600-0676.2001.210105.x
37.
de Wit K Schaapman J Nevens F Verbeek J Coenen S Cuperus F et al Prevention of hepatic encephalopathy by administration of rifaximin and lactulose in patients with liver cirrhosis undergoing placement of a transjugular intrahepatic portosystemic shunt (TIPS): a multicentre randomised, double blind, placebo controlled trial (PEARL trial). BMJ Open Gastroenterol. (2020) 7:e000531. 10.1136/bmjgast-2020-000531
Summary
Keywords
hepatic sinusoidal obstruction syndrome, hepatic veno-occlusive disease, transjugular intrahepatic portosystemic shunt, portal hypertension, meta-analysis
Citation
Jiang X, Ma X, Tian S and Peng L (2025) Efficacy and safety of transjugular intrahepatic portosystemic shunt in hepatic sinusoidal obstruction syndrome: systematic review and meta-analysis. Front. Med. 12:1625825. doi: 10.3389/fmed.2025.1625825
Received
09 May 2025
Accepted
23 July 2025
Published
13 August 2025
Volume
12 - 2025
Edited by
Babak Pakbin, Texas A&M University, United States
Reviewed by
Yuzheng Zhuge, Nanjing Drum Tower Hospital, China
Feng Zhang, Nanjing Drum Tower Hospital, China
Updates
Copyright
© 2025 Jiang, Ma, Tian and Peng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lijun Peng, plj8353@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.