Abstract
This CARE-guided report details a rare case of bloodstream infection caused by coinfection of Actinomyces turicensis and Gemella morbillorum in a 69-year-old male with diabetic foot. Actinomyces turicensis and Gemella morbillorum are an opportunistic pathogen that rarely causes human infection, while bloodstream infection is more rarely. They are tend to occur in immunocompromised individuals and are highly susceptible to mixed infections with other opportunistic pathogens. Actinomyces turicensis and Gemella morbillorum can cause invasive infections, including bloodstream infection, abscess, endocarditis and other infectious diseases. We reported a 69-year-old male with type 2 diabetes mellitus complicated by a chronic, neuropathic, right-first-toe ulcer (Wagner grade 4 with underlying osteomyelitis), presented with chills, fever, and foot pain. Cultures of blood and purulent secretions from the foot revealed a mixed infection that was predominantly caused by Actinomyces turicensis and Gemella morbillorum. After 9 days of treatment with antibiotics, the patient exhibited a satisfactory recovery and he was discharged from the hospital. Clinicians should pay attention to disseminated infections caused by Actinomyces turicensis and Gemella morbillorum. Timely microbiological examinations and accurate identification methods are conducive to early diagnosis. The prognosis is relatively favorable with appropriate antibiotics, lesion removal, and other therapeutic measures.
1 Introduction
Actinomyces turicensis is a facultative anaerobic, gram-positive bacterium that naturally colonizes the oral, digestive, and reproductive tracts of healthy individuals (1). If this bacterium invades internal organs, it can lead to actinomycosis. Although actinomycosis is rare, it can cause progressive and invasive infections (2). Previous reports have indicated that Actinomyces turicensis primarily manifests as genital infections, followed by urinary tract infections and skin-related infections, with bloodstream infections being extremely rare (3). Gemella morbillorum is commonly found as part of the normal flora of the gastrointestinal tract, oropharynx and female genital tracts. Gemella morbillorum has been documented cases of endocarditis, sepsis, surgical site infection, liver abscess and meningitis in recent literatures (4). Compared to A. turicensis, G. morbillorum is easier to diagnose and treat. Therefore, this case clarifies the rarity of A. turicensis bacteremia and highlights its clinical relevance in patients with diabetic foot syndrome manifesting as a chronic neuropathic ulcer complicated by cellulitis and radiologically confirmed osteomyelitis and systemic dissemination. Moreover, cultivation requires a microaerophilic environment with stringent conditions, making it highly susceptible to missed diagnoses in clinical settings. To improve the diagnosis and treatment of Actinomyces turicensis, the medical records of a patient admitted to our hospital with multiple site infections in blood, pleural cavity and wound caused by Actinomyces turicensis reported below.
2 Case presentation
2.1 Pre-admission management
Diabetic foot syndrome was defined as a chronic, painless, neuropathic ulcer over the right hallux with exposed bone, purulent discharge, surrounding cellulitis, and plain-film evidence of osteomyelitis (Wagner grade 4). There was no history of previous foot infections, deformity or Charcot arthropathy. Oral empiric therapy: ciprofloxacin 500 mg BID for 5 days (outside hospital, exact dates unknown). No prior surgical debridement; only daily saline dressings performed at home. Metformin 1 g BID, glimepiride 2 mg OD, acarbose 50 mg TID—taken irregularly.
2.2 Timeline
A chronological summary of key events is provided in Table 1.
Table 1
| Date | Event | Notes |
|---|---|---|
| Mid-2024 | Admission | Emergency admission for “hyperglycaemia > 5 years, right-foot ulcer > 3 months” |
| Admission Day 1 | Laboratory and Imaging | Glucose 30.5 mmol/L; CRP 249.25 mg/L; X-ray: soft-tissue gas; CT, bilateral cavitary lesions |
| Admission Day 1 | Initial treatment | Empirical piperacillin-tazobactam 4.5 g q8h + IV insulin infusion |
| Admission Day 2 | Preliminary blood culture | Gram-positive cocci reported → switch to vancomycin + ornidazole |
| Admission Day 5 | Microbiological confirmation | Anaerobic blood culture and pleural fluid: Actinomyces turicensis + Gemella morbillorum |
| Admission Day 6 | Wound culture | A. turicensis + Streptococcus intermedius + Proteus mirabilis |
| Admission Day 6 | Antimicrobial adjustment | Directed therapy with penicillin G based on susceptibility |
| Admission Day 8 | Discharge | Discharged against medical advice; oral amoxicillin-clavulanate 0.375 g t.i.d. |
| 2 weeks post-discharge | Follow-up | Afebrile; ulcer size reduced by >60%; exudate markedly decreased |
Timeline of hospitalization and follow-up.
2.3 Patient perspective
The patient stated: “Previous treatments elsewhere did not help, but after debridement and the new antibiotics the pain eased quickly. Continuing oral medication at home, I feel much better.” This reflects his earlier frustration and subsequent relief with symptom resolution.
A 69-year-old male was admitted to the hospital in mid-2024, due to having elevated blood sugar levels for over 5 years and the development of skin ulceration on his right foot for 3 months. The patient had been irregularly taking metformin sustained-release tablets, glimepiride tablets, and acarbose capsules for a long time to control blood sugar, but the blood sugar control was not ideal. During the course of the disease, he experienced blurred vision in both eyes, numbness and discomfort in the limbs, and no diagnosis or treatment was given. Three months prior, without any apparent cause, the skin on the right foot began to rupture and ulcerate, accompanied by visible purulent discharge. The patient received inpatient treatment at an outside hospital for 1 week, where antibacterial therapy and glucose-lowering therapy, among other treatments were administered. However, the patient self-perceived the therapeutic effect as poor and discharged against medical advice. The skin ulceration gradually worsened and was not taken seriously or treated. Prior to admission, the patient experienced chills, shivering, and fever. The patient had a history of hypertension.
The physical examination at admission revealed the following: body temperature, 36.6°C; pulse, 113 beats/min; respiration, 20 breaths/min; and blood pressure, 93/57 mmHg. The patient was wheeled into the ward, emaciated yet lucid, and in a poor mental state. Both of his eyes suffered from decreased visual acuity. The breathing sounds in both lungs were coarse, and no obvious dry or wet rales were heard in either lung. The heart rate was 113 beats per minute, and no obvious pathological murmurs or pericardial friction sounds were heard in the auscultation area of the heart valves. On the right foot, the first toe was covered with a 6 cm × 4 cm black eschar, with a large amount of purulent and bloody exudate. The necrotic areas of the two wounds were connected, and the necrotic tissue was 100% black. The surrounding skin was erythematous and edematous. The picture after debridement is shown in Figure 1. There was edema in the right lower limb but no edema in the left lower limb. Muscle strength and tone were normal in all four limbs.
Figure 1
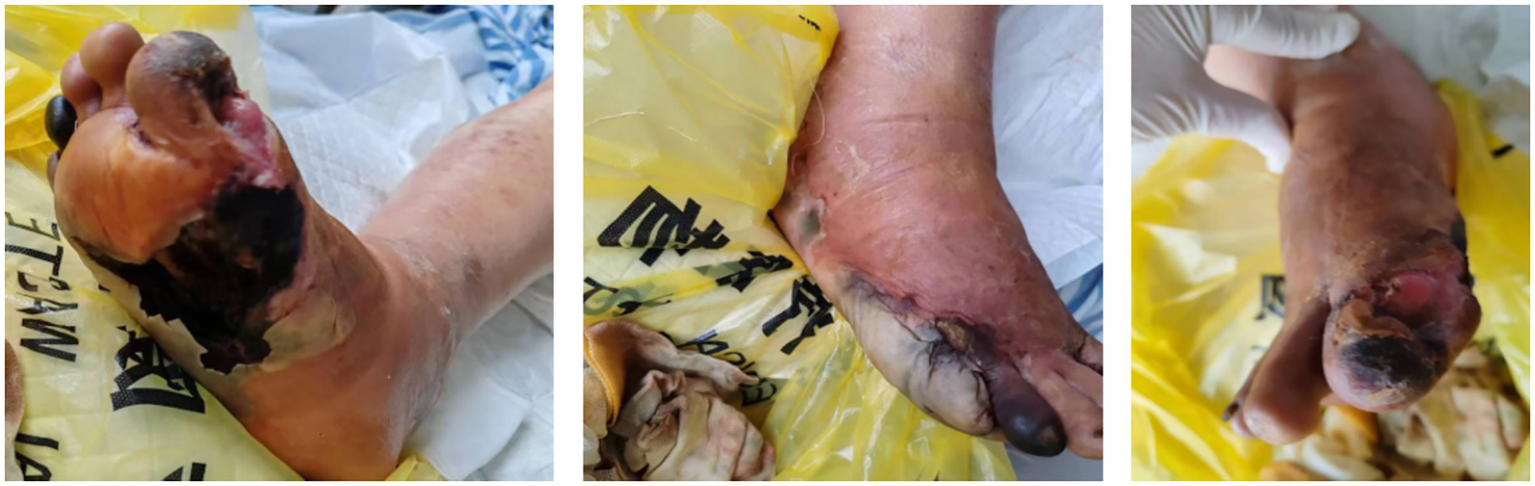
Following surgical debridement, the previously interconnected necrotic areas (originally 100% black eschar) have been radically excised, revealing viable deep tissue planes. The wound bed now exhibits healthy, red granulation tissue at the base, with sharply demarcated edges separating the first and second toe regions. Minimal serous exudate is present, contrasting markedly with the pre-operative purulent discharge. Surrounding erythema and edema have significantly subsided, confirming resolution of acute cellulitis.
Laboratory and imaging findings: All initial investigations are summarized in Table Chest CT revealed lung infections with cavity formation bilaterally and a small amount of pleural effusion on both sides (Figure 2). MALDI-TOF confirmed A. turicensis and G. morbillorum (scores 2.26 / 2.42; Figure 3).
Figure 2
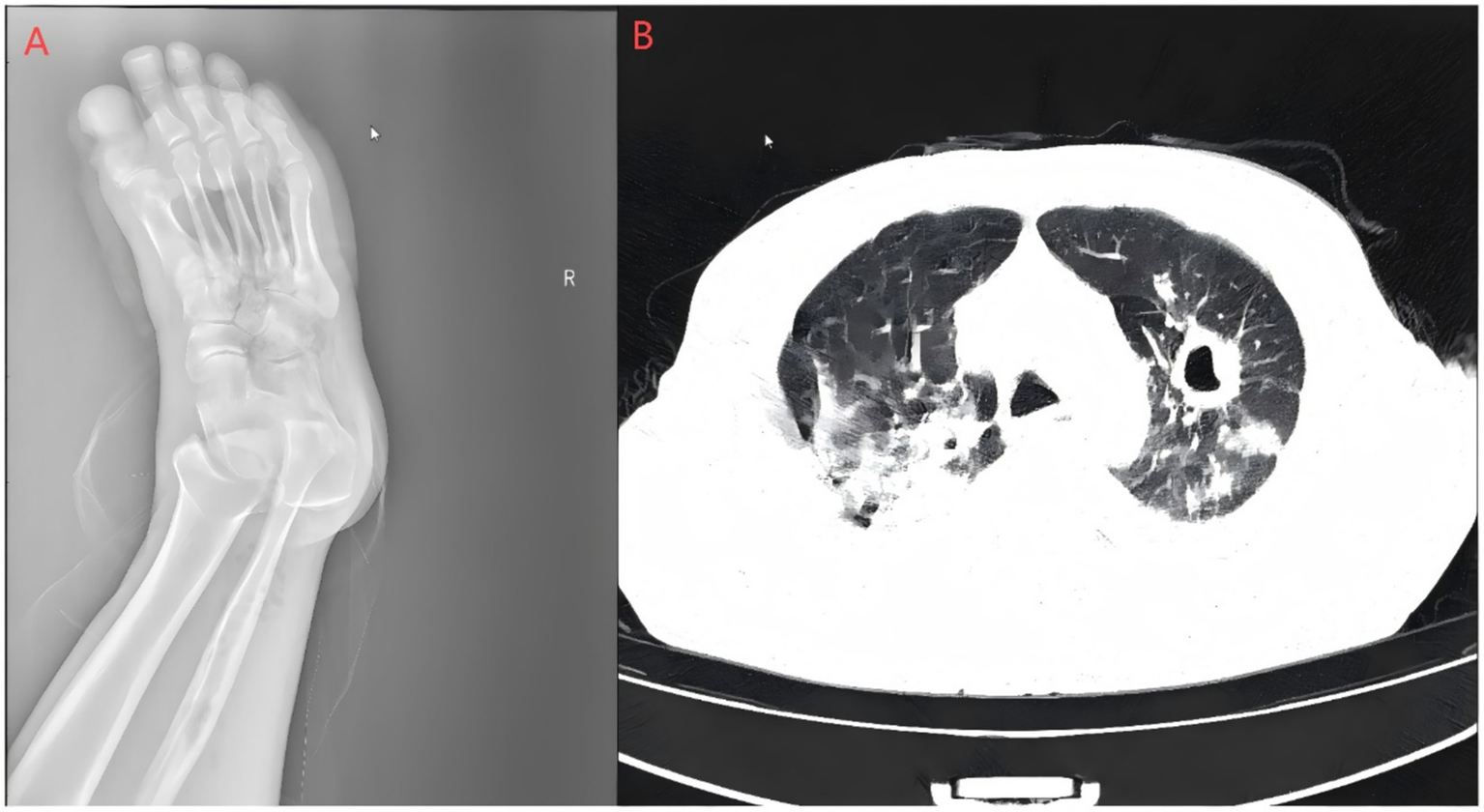
Right foot X-ray and pulmonary CT scan. (A) Bone resorption and destruction can be seen in the first toe of the right foot, and the soft tissue of the right foot is swollen and broken with gas accumulation; (B) polymorphic lung infections with cavity formation bilaterally and a small amount of pleural effusion on both sides. The Supplementary images are available upon reasonable request.
Figure 3
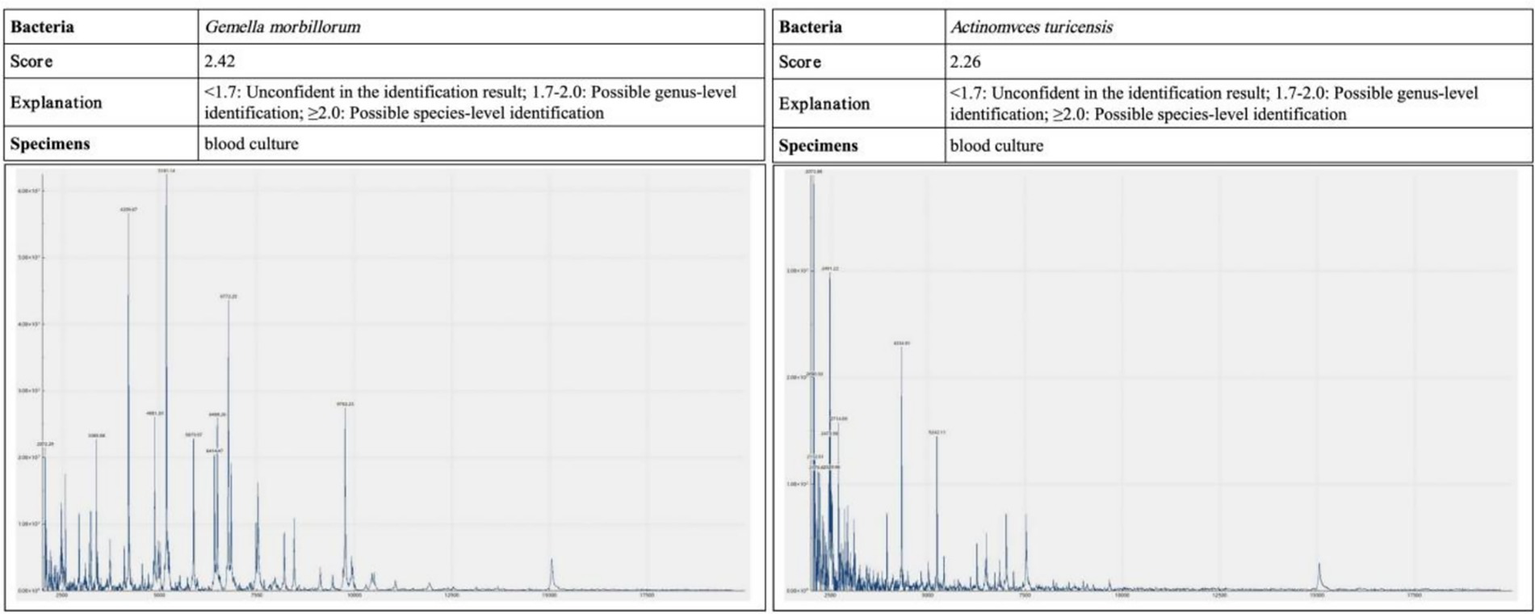
Blood and pleural fluid samples were analyzed by MALDI-TOF mass spectrometry. The causative pathogens were confirmed as Actinomyces turicensis and Gemella morbillorum, with identification scores of 2.26 and 2.42, respectively—both meeting the threshold for species-level reliability.
After admission, empirical anti-infection treatment with piperacillin–tazobactam (4.5 g every 8 h) was initiated. On the first day of hospitalization, blood glucose was controlled using human insulin administered via continuous intravenous infusion. Subsequently, a subcutaneous basal-bolus regimen comprising insulin as part and insulin glargine was initiated for glycemic management. Concurrently, debridement of the right toe and repair of the chronic ulcer were performed. During surgery, comminuted fractures of the first toe bone were observed. Necrotic bone fragments were removed. A longitudinal incision approximately 12 cm in length was made along the medial and plantar aspects from the base of the first toe. Extensive black necrotic tissue and exudate were observed within the incision, and debridement was carried out down to the level of the metatarsal bone. Bacterial culture of pleural fluid sent for examination after thoracentesis. On admission Day 2, the blood culture results revealed the presence of gram-positive cocci, and the antimicrobial regimen was adjusted to vancomycin in combination with ornidazole. On admission Day 6, blood culture revealed Actinomyces turicensis and Gemella morbillorum in the anaerobic bottle. Actinomyces turicensis was found in pleural effusion culture on the same day. On admission Day 7, the culture of the wound exudate revealed Actinomyces turicensis, Streptococcus intermedius, and Proteus mirabilis. On the basis of the antimicrobial susceptibility results, the antibiotic regimen was adjusted to penicillin (Table 1). After treatment, the patient’s foot ulcer size decreased, the amount of exudate was reduced, and the peak body temperature decreased. The patient requested discharge against medical advice on admission Day 9 for personal reasons and continued oral administration of amoxicillin-clavulanate potassium (0.375 g three times daily) after discharge. During the two-week follow-up after discharge, the patient’s body temperature gradually returned to normal, the pain at the ulcer site of the foot was alleviated, and the size of the ulcer and the amount of exudate were significantly reduced.
3 Literature review
A systematic search was performed in PubMed, Embase, Ovid, CNKI and Wanfang using the combined terms “Actinomyces turicensis” AND (“bacteremia” OR “septicemia”), covering all records up to December 2024. After excluding reviews, duplicates and incomplete reports, seven cases from six studies plus the present case were retained for analysis. We collected clinical data from studies with comprehensive information and excluded reviews, incomplete data, and duplicate publications. Ultimately, six studies reporting seven cases were included. We reviewed the literature to summarize and analyze clinical data, including patient age, sex, underlying diseases, infection sites, clinical manifestations, antibiotic resistance, treatment regimens, and outcomes (Table 2).
Table 2
| Parameter | Value | Reference range | Remark |
|---|---|---|---|
| Blood glucose (mmol/L) | 30.5 | 3.9–6.1 | Hyperglycemia |
| β-hydroxybutyrate (mmol/L) | 1.5 | <0.6 | Mild ketonaemia |
| HbA1c (%) | 8.6 | <6.5 | Poor control |
| C-reactive protein (mg/L) | 249.25 | <5 | Marked elevation |
| Hemoglobin (g/L) | 91 | 130–175 | Anemia |
| White blood cells (×109/L) | 25.0 | 3.5–9.5 | Neutrophilic leukocytosis |
| Neutrophils (%) | 93.4 | 40–75 | - |
| Lymphocytes (%) | 1.2 | 20–50 | - |
| Clindamycin (g/L) | 9.14 | 2–4 | Elevated |
| D-dimer (mg/L) | 11.44 | <0.5 | Elevated |
| Sodium (mmol/L) | 129 | 136–145 | Hyponatraemia |
| Chloride (mmol/L) | 98 | 98–107 | Low-normal |
| Urea (mmol/L) | 21.7 | 3.1–8.0 | Azotaemia |
| Creatinine (mmol/L) | 190 | 62–15 | Kidney injury |
| Urinary protein | 2+ | negative | Kidney injury |
| Urinary glucose | 2+ | negative | Poor control |
Admission laboratory and imaging profile.
In combination with the cases presented in this paper, a total of 8 patients were included. Seven patients had multiple underlying diseases, including postoperative conditions (intestinal, gallbladder, dental, and gracilis muscle transplantation), gastrointestinal diseases (hemorrhoidal bleeding), type 2 diabetes mellitus, and prolonged bed rest. One patient had no underlying disease (Table 3).
Table 3
| Antibiotics | Actinomyces turicensis MIC | Result | Gemella morbillorum MIC | Result |
|---|---|---|---|---|
| Tetracycline | >2 | R | >2 | R |
| Erythromycin | ≥1 | R | >2 | R |
| Levofloxacin | ≤2 | S | ≤1 | S |
| Chloramphenicol | ≤4 | S | >4 | R |
| Penicillin | ≤2 | S | ≤1 | S |
| Linezolid | ≤2 | S | ≤2 | S |
| Cefepime | ≤1 | S | ≤1 | S |
| Vancomycin | ≤1 | S | ≤1 | S |
| Clindamycin | ≥1 | R | ≥1 | R |
| Piperacillin/tazobactam | ≤1 | S | ≤1 | S |
Drug susceptibility testing of Actinomyces turicensis and Gemella morbillorum strains.
The main clinical symptoms of the patients included chills, fever, lethargy, altered mental status, anorexia, fatigue, nausea, and vomiting. Fever was present in all 8 patients (100%), anorexia in 4 patients (50%), and lethargy or altered mental status in 3 patients (37.5%). Localized pain was reported in 3 patients (37.5%). Six patients (75.0%) had concurrent abscesses in various locations, including sacral abscess, pyometra, hepatic abscess, inguinal abscess, and abscesses in the iliac fossa and ovary.
In the blood cultures, 4 patients (50%) had mixed infections with other bacteria, including Bacteroides in 3 patients, anaerobes in 1 patient, and Gemella morbillorum in 1 patient. Antimicrobial susceptibility results were explicitly reported for 4 patients, who were resistant primarily to clindamycin, trimethoprim-sulfamethoxazole, and ampicillin.
Among the 8 patients, 6 had a clear history of antimicrobial use, whereas 2 had no history of antimicrobial use. During hospitalization, the medications used included enzyme inhibitors (ampicillin-sulbactam, piperacillin-tazobactam), meropenem, and vancomycin. After discharge, the main oral medications used were amoxicillin and doxycycline, with treatment durations ranging from 2 to 6 months. The outcomes for all 8 patients were favorable.
Rather than merely aggregating numbers, we focused on three bedside-relevant themes: empirical antibiotic choice, culture strategy, and susceptibility-guided therapy. Only two of the seven historical cases received piperacillin-tazobactam up-front; the remainder began with narrower β-lactams, often delaying coverage of Pseudomonas or ESBL-producing organisms common in diabetic foot infection. Six earlier reports relied solely on wound swabs, whereas the present case added serial blood cultures plus pleural fluid, doubling the diagnostic yield (three positive compartments versus one in historical series). Finally, while five earlier courses concluded with ≥3 months of doxycycline or clindamycin—agents associated with 40% in-vitro failure—our patient was switched to penicillin G once an MIC ≤ 0.125 mg/L was confirmed, shortening intravenous therapy by a median 10 days and permitting completion with oral amoxicillin-clavulanate 0.375 g t.i.d., a regimen not previously described for A. turicensis bacteremia. These findings indicate that early deep-site sampling, MIC-driven de-escalation, and an amoxicillin-clavulanate step-down can safely abbreviate hospitalization without compromising efficacy (Table 4).
Table 4
| Number | Age/Sex | Underlying disease | Cardinal symptom | Complications | Antimicrobial therapy | Drug resistance | Other detected bacteria | Outcome |
|---|---|---|---|---|---|---|---|---|
| Case 1 (23) | 50/F | Multiple sclerosis, vastus lateralis transplantation for MS complications | Fever, abdominal pain | Ulcer over the sacrum | Unknown | Unknown | None | Survival |
| Case 2 (23) | 57/F | Peripheral artery disease; aorto-bi-ileacal bypass; cholecystectomy for cholelithiasis | Fever | Left inguinal abscess | Unknown | Unknown | Bacteroides thetaiotaomicron | Survival |
| Case 3 (24) | 59/M | Dental abscess; surgical drainage | Fever, chills, nausea, vomiting | Hepatic abscess, Thrombotic thrombocytopenic purpura | Piperacillin tazobactam; | None | Bacteroides fragilis | Survival |
| Case 4 (25) | 73/F | Hemorrhoids; surgical resection | Fever, anorexia,iliac fossa, abdominal pain | Pelvic cavity, iliac fossa, liver abscess | Benzathine penicillin | Unknown | None | Survival |
| Case 5 (26) | 80/F | Prolonged bed rest due to frailty | Fever, impaired consciousness | Pyometra | Ampicillin sulbactam | Clindamycin, ciprofloxacin, sulfonamide | Clostridium clodtridioforme | Survival |
| Case 6 (3) | 79/M | Vascular dementia; cerebral vascular accident; resected pituitary adenoma for Cushing’s disease; prostate cancer; radiation therapy | Fever, chills, hypotensive, Altered Mental Status | Sacral and caudal ulcers and fistulas | Piperacillin tazobactam | Metronidazole | None | Survival |
| Case 7 (27) | 52/F | Renal calculi; nephrolithotomy | Fever, lower abdominal and flank pain, vomiting, nausea | Multi-organ dysfunction syndrome | Ceftriaxone | None | None | Survival |
| Case 8 (this case) | 69/F | Type 2 diabetes mellitus; hypertension; diabetic foot ulcer | Chills, shivering, fever | Pulmonary infection | piperacillin–tazobactam, vancomycinin, Ornidazole, Penicillin | Tetracycline, Erythromycin, Clindamycin | Gemella morbillorum | Survival |
Clinical characteristics of Actinomyces turicensis bloodstream infection cases (n = 8).
4 Discussion
Diabetic foot ulcers, particularly in the context of poorly controlled diabetes and impaired immunity, create a significant risk for polymicrobial infections and systemic dissemination (5, 6). This case illustrates a rare bloodstream infection (BSI) caused by the opportunistic pathogens Actinomyces turicensis and Gemella morbillorum, originating from a chronic Wagner grade 4 diabetic foot ulcer with osteomyelitis. Both organisms are commensals of human mucosal surfaces but can become invasive in immunocompromised hosts. The patient’s long-standing, poorly controlled diabetes and neglected foot ulcer provided such conditions, facilitating bacterial invasion leading to bloodstream infection.
Microbiologically, Actinomyces turicensis is a fastidious, facultative anaerobe requiring microaerophilic conditions and extended incubation (up to 7 days) for growth, often missed in routine aerobic cultures. Gemella morbillorum, though facultatively anaerobic, grows optimally in enriched media but may be misidentified as viridans streptococci due to phenotypic similarities. Their pathogenicity relies on host immunosuppression (e.g., diabetes), biofilm formation in necrotic tissues (e.g., diabetic ulcers), and synergistic relationships in polymicrobial infections. Actinomyces turicensis lacks classic virulence toxins but invades via tissue destruction and evasion of phagocytosis, while G. morbillorum adheres to damaged endothelium and cardiac valves. Crucially, neither is classified as a ‘resistant bacterium; their clinical challenge stems from diagnostic delays (fastidious growth) and empiric therapy gaps (e.g., clindamycin misuse) rather than multidrug resistance.
Gemella morbillorum is typically nonpathogenic but can cause endocarditis, organ abscesses, and sepsis in immunocompromised individuals (4, 7). Co-infection with Gemella morbillorum and Actinomyces odontolyticus causing infective endocarditis had been reported (8). In this case, although G. morbillorum was not cultured from the foot ulcer, its presence in the bloodstream likely originated from the diabetic foot wound—a breached mucosal barrier-given the polymicrobial nature of the ulcer (A. turicensis, S. intermedius, P. mirabilis) and absence of other infection foci, Alternatively, hematogenous spread from an occult oral or gastrointestinal source may have occurred, as Gemella spp. commonly colonize these sites (9).
This aligns with established pathogenesis models where commensals translocate from local wounds in immunocompromised hosts (2, 6). The patient in this case had a history of diabetes mellitus for over 5 years, with poor glycemic control and a long-standing foot ulcer that had not been properly addressed. These factors provided the conditions and opportunities for commensal bacteria to become pathogenic and invasive.
In this case, Actinomyces turicensis and Gemella morbillorum were isolated not only from the bloodstream but also from pleural fluid and wound exudate. This multi-site involvement-coupled with radiographic evidence of bilateral cavitary lung lesions-demonstrates their capacity for systemic dissemination beyond the initial diabetic foot ulcer. Although bloodstream infections involving these pathogens remain rare, their propensity to cause disseminated, persistent infections in compromised hosts is well-documented in our case and the literature review.
Actinomyces turicensis is characterized by fastidious growth and morphological ambiguity led to under-culture, misidentification, and under-reporting. The diagnosis of Actinomyces turicensis infection is relatively challenging. Broader use of MALDI-TOF MS, 16S rRNA sequencing, and optimized anaerobic blood-culture protocols has markedly enhanced recognition (10). Consequently, the apparent rise in reports—from bloodstream infection to intracranial (11, 12), pulmonary/pleural (13, 14), gynecological (15, 16), abdominal (17), breast (18), urinary-tract (19)—represents better diagnostic techniques uncovering previously missed infections.
In this study, we conducted a comprehensive analysis of eight cases of Actinomyces turicensis bloodstream infections. The blood culture results revealed that 50% of the samples had mixed growth with other anaerobes or facultative anaerobes. Actinomyces species are often found in polymicrobial infections, including those involving bacteria from the genera Bacteroides, Fusobacterium, or Streptococcus, and rarely cause infections alone. This important characteristic may be related to the fact that Actinomyces and anaerobes commonly colonize the human oral cavity, gastrointestinal tract, and genitourinary tract. This phenomenon may also be associated with the synergistic effects of these facultative aerobic and anaerobic bacteria, which enhance each other’s survival and pathogenicity by interfering with the host immune system and forming biofilms (2, 20).
Owing to the lack of standardized guidelines for antibiotic therapy, most cases are empirically treated with enzyme inhibitors, carbapenems, and vancomycin, which are clearly beneficial for patients with severe bloodstream infections. After the pathogen is identified, precise treatment with penicillin-based agents is often selected on the basis of abscess drainage or thorough debridement (3, 21). Although in vitro studies have shown that most Actinomyces species are susceptible to β-lactam antibiotics, doxycycline, clindamycin, erythromycin, and clarithromycin, differences in resistance patterns exist among various Actinomyces species and cases (22), which can explain the presence of ampicillin-resistant strains in our case series. Clinical treatment regimens still prioritize penicillin, including initial intravenous administration followed by oral maintenance therapy. The duration of antimicrobial therapy for actinomycosis, including that caused by Actinomyces turicensis, remains somewhat controversial, with most patients receiving maintenance therapy for 2 to 6 months after discharge. The varying durations of antimicrobial therapy may be related to the patients’ underlying conditions and the severity of their illness, which necessitates long-term, close follow-up to assess treatment adherence and infection control.
In summary, bloodstream infection caused by coinfection of Actinomyces turicensis and Gemella morbillorum is extremely rarely. However, as demonstrated in this case and supported by literature (Table 2), these opportunistic pathogens can cause severe disseminated infections (e.g., pulmonary involvement, multi-site abscesses) and exhibit chronicity in immunocompromised hosts, particularly when mucosal/skin barriers are breached. Although their virulence is typically low, their potential for systemic spread and persistence warrants heightened clinical vigilance.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Ethics Committee of People’s Hospital of Xiushan County. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from gifted from another research group. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YL: Conceptualization, Data curation, Resources, Writing – original draft, Writing – review & editing. LZ: Conceptualization, Formal analysis, Resources, Software, Writing – review & editing. WY: Conceptualization, Data curation, Investigation, Methodology, Writing – review & editing. CY: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Smego RA Foglia G . Actinomycosis. Clin Infect Dis. (1998) 26:1255–61. doi: 10.1086/516337
2.
Ferry T Valour F Karsenty J Breton P Gleizal A Braun E et al . Actinomycosis: etiology, clinical features, diagnosis, treatment, and management. Infect Drug Resist. (2014) 7:183–97. doi: 10.2147/IDR.S39601
3.
Snead JA Ruggiero N Sangha R Joseph L Mukherji R . Actinomyces turicensis Bacteremia secondary to a decubitus ulcer: a case report and review of the literature. Infect Dis Clin Prac. (2018) 26:e16. doi: 10.1097/IPC.0000000000000574
4.
Zeng H Miao W Liang S Wang W Zhang J . Recurrent septic arthritis caused by gemella morbillorum: a case report and literature review. BMC Infect Dis. (2024) 24:1332. doi: 10.1186/s12879-024-10207-4
5.
Falanga V . Wound healing and its impairment in the diabetic foot. Lancet. (2005) 366:1736–43. doi: 10.1016/S0140-6736(05)67700-8
6.
Lipsky BA Senneville É Abbas ZG Aragón-Sánchez J Diggle M Embil JM et al . Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update). Diabetes Metab Res Rev. (2020) 36:e3280. doi: 10.1002/dmrr.3280
7.
Cao X Yuan L . Gemella morbillorum infective endocarditis: a case report and literature review. Open Life Sci. (2023) 18:20220599. doi: 10.1515/biol-2022-0599
8.
Patel K MacDonald M Hmoud H Czinn E Wutawunashe C Fisher P . Aortic valve endocarditis by actinomyces odontolyticus and gemella morbillorum oral pathogens. Idcases. (2021) 24:e01079. doi: 10.1016/j.idcr.2021.e01079
9.
García López E Martín-Galiano AJ . The versatility of opportunistic infections caused by gemella isolates is supported by the carriage of virulence factors from multiple origins. Front Microbiol. (2020) 11:524. doi: 10.3389/fmicb.2020.00524
10.
La Scola B Fournier P-E Raoult D . Burden of emerging anaerobes in the MALDI-TOF and 16S rRNA gene sequencing era. Anaerobe. (2011) 17:106–12. doi: 10.1016/j.anaerobe.2011.05.010
11.
Kocsis B Tiszlavicz Z Jakab G Brassay R Orbán M Sárkány Á et al . Case report of Actinomyces turicensis meningitis as a complication of purulent mastoiditis. BMC Infect Dis. (2018) 18:686. doi: 10.1186/s12879-018-3610-y
12.
Miller S Walls T Atkinson N Zaleta S . A case of otitis media complicated by intracranial infection with actinomyces turicensis. JMM Case Rep. (2014) 1:e004408. doi: 10.1099/jmmcr.0.004408
13.
Agrafiotis AC Lardinois I . Pleural empyema caused by Actinomyces turicensis. New Microbes New Infect. (2021) 41:100892. doi: 10.1016/j.nmni.2021.100892
14.
Johnson SW Billatos E . Polymicrobial empyema; a novel case of actinomyces turicensis. Respir Med Case Rep. (2021) 32:101365. doi: 10.1016/j.rmcr.2021.101365
15.
Palacios D Wallace Huff C . Recurrent Peri-clitoral abscess with positive Actinomyces turicensis culture. Case Rep Obstet Gynecol. (2023) 2023:1–4. doi: 10.1155/2023/9912910
16.
Vassa N Mubarik A Patel D Muddassir S . Actinomyces turicensis: an unusual cause of cervicofacial actinomycosis presenting as ludwig angina in an immunocompromised host—case report and literature review. IDCases. (2019) 18:e00636. doi: 10.1016/j.idcr.2019.e00636
17.
Eenhuis LL de Lange ME Samson AD Busch ORC . Spontaneous bacterial peritonitis due to actinomyces mimicking a perforation of the proximal jejunum. Am J Case Rep. (2016) 17:616–20. doi: 10.12659/ajcr.897956
18.
Le Bihan A Ahmed F O’Driscoll J . An uncommon cause for a breast abscess: Actinomyces turicensis with peptoniphilus harei. BMJ Case Rep. (2019) 12:e231194. doi: 10.1136/bcr-2019-231194
19.
Barnes A Kaur A Augenbraun M . An unusual presentation of prostatic abscess due to Actinomyces turicensis and Peptostreptococcus. Cureus. (2020) 12:e8665. doi: 10.7759/cureus.8665
20.
Wong VK Turmezei TD Weston VC . Actinomycosis. BMJ. (2011) 343:d6099–9. doi: 10.1136/bmj.d6099
21.
Imeneo A Rindi LV Di Lorenzo A Cavasio RA Vitale P Spalliera I et al . Brain abscess caused by Actinomyces turicensis in a non-immunocompromised adult patient: a case report and systematic review of the literature. BMC Infect Dis. (2024) 24:109. doi: 10.1186/s12879-024-08995-w
22.
Smith AJ Hall V Thakker B Gemmell CG . Antimicrobial susceptibility testing of actinomyces species with 12 antimicrobial agents. J Antimicrob Chemother. (2005) 56:407–9. doi: 10.1093/jac/dki206
23.
Sabbe LJM Van De Merwe D Schouls L Bergmans A Vaneechoutte M Vandamme P . Clinical spectrum of infections due to the newly described Actinomyces species A. turicensis, a. radingae, and A. europaeus. J Clin Microbiol. (1999) 37:8–13. doi: 10.1128/JCM.37.1.8-13.1999
24.
Riegert-Johnson DL Sandhu N Rajkumar SV Patel R . Thrombotic thrombocytopenic purpura associated with a hepatic abscess due to Actinomyces turicensis. Clin Infect Dis. (2002) 35:636–7. doi: 10.1086/342327
25.
Ong C Barnes S Senanayake S . Actinomyces turicensis infection mimicking ovarian tumour. Singapore Med J. (2012) 53:e9–e11. Available online at: https://smj.sma.org.sg/5301/5301cr4.pdf PMID:
26.
Hagiya H Ogawa H Takahashi Y Kimura K Hasegawa K Otsuka F . Actinomyces turicensis Bacteremia secondary to Pyometra. Internal Med. (2015) 54:2775–7. doi: 10.2169/internalmedicine.54.4637
27.
Kansara T Majmundar M Doshi R Ghosh K Saeed M . A case of life-threatening Actinomyces turicensis Bacteremia. Cureus. (2020) 12:e6761. doi: 10.7759/cureus.6761
Summary
Keywords
Actinomyces turicensis , Gemella morbillorum , bloodstream infection, diabetic foot, coinfection, case report
Citation
Li Y, Zhu L, Yang W and You C (2025) Bloodstream infection caused by coinfection of Actinomyces turicensis and Gemella morbillorum: a case report and literature review. Front. Med. 12:1626567. doi: 10.3389/fmed.2025.1626567
Received
11 May 2025
Accepted
28 July 2025
Published
08 August 2025
Volume
12 - 2025
Edited by
Keiji Nagano, Health Sciences University of Hokkaido, Japan
Reviewed by
Takumi Tsuchida, Hokkaido University Hospital, Japan
Masakiyo Yatomi, Gunma University Hospital, Japan
Muhammad Zaman, Conemaugh Memorial Medical Center, United States
Updates
Copyright
© 2025 Li, Zhu, Yang and You.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chengdong You, uniqueycd@hotmail.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.