Abstract
The Nocardia genus is an aerobic, Gram positive, opportunistic pathogen that primarily affects cell-mediated immunosuppressed patients. The clinical manifestation of nocardiosis varies widely, making it challenging to diagnose. In this report, we describe a 48 year old woman with a muscular abscess caused by Nocardia asteroides. Venous blood, skin biopsy specimen, muscle tissue, and inguinal lymph node puncture tissue cultures yielded negative results. Using metagenomic next-generation sequencing (mNGS), the pathogen was identified as Nocardia asteroides. Whole-exome sequencing of the peripheral blood showed that the patient had a monoallelic mutation in the lipopolysaccharide-responsive beige-like anchor protein (LRBA) gene. The mNGS detected Nocardia asteroides in the patient, and the administration of accurate treatment led to her complete recovery.
Introduction
The genus Nocardia is a ubiquitous group of environmental bacteria that usually cause opportunistic infections in immunocompromised hosts. Nocardia is widely distributed in plants, gardens, and soil and is classified into more than 80 species, over 50 of which can cause human diseases (1). Nocardiosis is an infectious disease caused by Nocardia species, and patients with compromised cell-mediated immunity are at a higher risk of developing this condition (1, 2). Nocardiosis exhibits variable clinical manifestations, complicating its diagnosis. Traditional diagnostic methods rely on culture techniques, which have limitations, such as low sensitivity (3). Recently, metagenomic next-generation sequencing (mNGS) has emerged as a superior alternative, offering high sensitivity and specificity for diagnosis. This advanced technique enables the detection of rare and hard-to-identify pathogens directly from clinical samples, demonstrating significant advantages in diagnosing infectious diseases such as nocardiosis (4). Here, we report a case of intramuscular abscesses caused by Nocardia asteroides, which was accurately identified using mNGS. Owing to the rapidity and high sensitivity of this diagnostic approach, the patient received timely antimicrobial therapy and full recovered. We present this case to highlight how a Nocardia infection can progress from superficial skin and soft tissue involvement to deeper muscle invasion, emphasizing the imperative for timely diagnosis and therapeutic intervention.
Case presentation
A 48-year-old woman presented to our clinic with a history of recurrent swelling, painful erythema on the right inner thigh, and fever lasting more than 1 year. Twelve months ago, the patient developed a low-grade fever that did not subside under despite unsupervised self-medication with amoxicillin and cefuroxime. Subsequently, an erythematous lesion with tenderness appeared on the medial aspect of the right thigh. Despite treatment with antibiotics (non-specified) and corticosteroids, the patient developed an overlying ecchymosis accompanied by significant local pain, followed shortly by episodes of fever (38.5 °C). Sporadic intake of antibiotics and prednisone provided some symptomatic relief, despite the persistence of the cutaneous lesion. Over the last 2 months, the patient noticed a progressive worsening of the local pain. She had no known underlying immunosuppressive disease.
Upon examination, the patient’s temperature was 39.3 °C, her pulse was 110 beats per min, blood pressure measured 129/76 mm Hg, and her respiratory rate was 21 breaths per min. Physical examination revealed enlarged cervical and inguinal lymph nodes. The dermatological examination revealed a 15 × 15 cm square-shaped edematous, infiltrative, well-demarcated erythema on the medial aspect of the right thigh, with a tough texture (Figure 1). Blood tests indicated the following results: the white blood cell count was 7.9 × 109/L, the percentage of neutrophils was 81.2%, the T cell count was 414.51/μL, the B cell count was 34.21/μL, the NK cell count was 35.55/μL, the procalcitonin level was 0.17 ng/mL, the erythrocyte sedimentation rate was 21 mm, and the C-reactive protein count was 20.83 mg/L. Tests for HIV, syphilis, and hepatitis B virus (HBV) were all negative. A muscle abscess was diagnosed and confirmed through magnetic resonance imaging (MRI) (Figure 1). The affected musculature included the right distal posterior femoral muscle group and adjacent intermuscular compartments. An enlarged lymph node in the right inguinal region was observed on local ultrasound (Figure 1). Histopathology of the skin from the right lower extremity showed lymphocytic infiltration (Figure 2). Additionally, histopathology of the muscles and lymph node (Figure 2) showed inflammatory changes and reactive lymphoid hyperplasia.
Figure 1

(A) 15 × 15 cm square-shaped edematous, infiltrative, well-demarcated erythema on the right medial femur with a tough texture. (B) Coronal MRI of the lower limbs showing abnormal signals in the right lower posterior femoral muscle group and muscle spaces. (C) Transverse axial MRI of the lower limbs showing abnormal signals in the right lower posterior femoral muscle group and muscle spaces. (D) Ultrasonography of the right groin showing an enlarged lymph node with a clear border (2.7 × 1.3 cm).
Figure 2
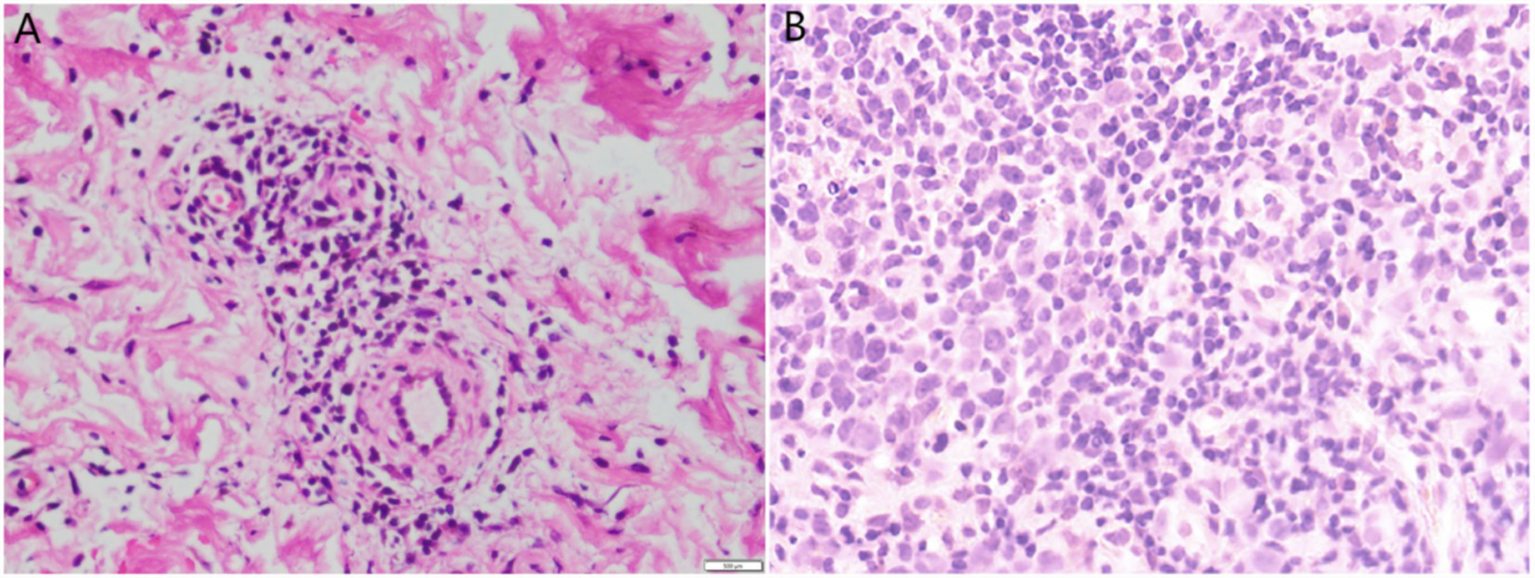
(A) Skin biopsy revealed lymphocytic infiltration around small blood vessels in the dermis (H & E, 400×). (B) Lymph node aspiration tissue biopsy revealed proliferative lesions of lymphoid tissue (H & E, 400×).
Venous blood, skin biopsy specimen, muscle tissue, and right inguinal lymph node puncture tissue cultures revealed no bacterial or fungal growth. The incubation period for microbiological culture of tissue samples is 2 weeks for fungi and 3 days for bacteria. Venous blood cultures incubated for 5 days reported no bacterial growth. Neither specialized culture media nor extended incubation periods were employed. Due to limited experience with uncommon bacterial infections, potential diagnostic omissions may have occurred. mNGS of the venous blood and muscle samples revealed no microbes. Finally, on day 13 after admission, mNGS of lymph node puncture tissue revealed N. asteroides. Microbiological analysis identified Nocardia at the genus level with 19 detected sequences, representing a relative abundance of 0.5% within the sampled microbial community. At the species level, Nocardia asteroides was specifically confirmed through eight distinct sequences. Notably, this isolate exhibited an RPM-ratio of 12.56, indicating significantly elevated detection relative to background microbial abundance (where RPM-ratio >1 suggests biological relevance). No other potential pathogens were detected. The reason why our patient developed a Nocardia abscess in the thigh remains unclear. The patient recalled an episode of temporary swelling and tenderness in the right thigh following friction with the ground 6 years ago. It is possible that our patient was infected with Nocardia at that time and developed the abscess after 6 years because of a latent infection. Why this infection developed 6 years post-trauma is difficult to explain. Whole-exome sequencing of peripheral blood showed that the patient had a monoallelic mutation in the lipopolysaccharide-responsive beige-like anchor protein (LRBA) gene. Genetic analysis revealed a compound variant in LRBA consisting of two heterozygous missense mutations: p.Val2066Met (c.6196G > A) and p.Ser952Leu (c.2855C > T). The initial empiric antimicrobial regimen for our patient was oral trimethoprim-sulfamethoxazole (TMP-SMX) (960 mg twice daily). During the first 3 days following admission, intravenous immunoglobulin (IVIG) was administered daily at a dose of 25 grams.
During the first 4 days of admission, the patient was successively administered TMP-SMX, azithromycin, minocycline, and itraconazole capsules. No significant clinical improvement was observed. Consequently, all medications except TMP-SMX were discontinued. On day 5, treatment was initiated with intravenous voriconazole and moxifloxacin. This regimen was subsequently discontinued following the onset of transient xanthopsia and the development of generalized erythema in conjunction with high fever. Current management is restricted to maintaining TMP-SMX therapy. The patient’s body temperature returned to normal by the 9th day of hospitalization. In addition, the laboratory tests, MRI, and ultrasound were in remission. During the third week of hospitalization, IVIG was readministered at 25 grams daily for three consecutive days. By the 28th day of hospitalization, the area of erythema on the medial aspect of the right thigh had decreased in size (Figure 3). Her mental state was markedly improved. The patient continued to be treated with oral TMP-SMX (960 mg twice daily for 1 year). The lesion on the limb disappeared completely without recurrence.
Figure 3

Erythema after 28 days of treatment.
Discussion
Common species of Nocardia include N. asteroides, N. brasiliensis, N. farcinica, N. otitidiscaviarum, and others, all of which may cause various diseases in humans. Primary cutaneous nocardiosis is most commonly caused by N. brasiliensis (5). Skin involvement in nocardiosis can be classified into four categories: primary cutaneous, lymphocutaneous, cutaneous manifestations of disseminated nocardia, and mycetoma. Primary cutaneous infection most often results from direct inoculation of the bacteria due to trauma or surgery (6, 7). N. asteroides is one of the most common pathogenic species within the Nocardia genus, known for causing localized and systemic infections. Cutaneous nocardiosis typically presents as abscesses, cellulitis, or mycetoma, with N. asteroides being a frequent etiological agent. Cutaneous manifestations of Nocardia have been reported in immunocompetent hosts. However, involvement of the muscle layer is rare, with only three previously reported cases, due to Nocardia brasiliensis and Nocardia farcinica (8–10). Despite its clinical significance, diagnosis is often delayed due to its resemblance to other pyogenic or granulomatous infections. Nocardia infection should be suspected in cutaneous infections that respond poorly to routine treatment.
The traditional diagnostic method for nocardiosis involves culturing the bacteria. The microbiological laboratory should be informed, as Nocardia species grow poorly on many culture media and need long-term incubation periods (11). The conventional culture-based identification of Nocardia species typically requires 2–7 days of incubation, potentially delaying definitive diagnosis. This diagnostic challenge is further compounded by the fact that the majority of nocardiosis patients receive empirical antibiotic therapy prior to specimen collection, which significantly diminishes microbial culturability (12). mNGS is a novel approach characterized by high sensitivity, rapid detection, and reduced susceptibility to interference from prior antibiotic usage (13, 14). mNGS can theoretically detect all types of pathogens, which makes it suitable for difficult and atypical infectious diseases (14). mNGS demonstrates superior sensitivity for Nocardia detection compared to conventional methods, especially in culture-negative cases. While not yet replacing culture, it serves as a powerful complementary tool for rapid diagnosis. Ongoing improvements in sequencing depth and bioinformatics will likely increase detection rates further. However, mNGS also has some limitations. Its high cost can be a burden for some patients. Moreover, the time of sample collection may influence its sensitivity. A recent study revealed that 14 samples were identified to be Nocardia spp. positive by mNGS, whereas only five of them yielded positive culture results (15). This patient’s negative blood culture may be due to the influence of prior antibiotic usage.
Given the Nocardia infection, the patient should be evaluated for underlying primary immunodeficiency (PID) or inborn immunity errors (16). LRBA deficiency was described as a novel primary immunodeficiency (PID) in 2012 (17). Patients with LRBA deficiency present with a broad spectrum of clinical phenotypes, including autoimmunity, enteropathy, hypogammaglobulinemia, and recurrent infections (18). Bi-allelic mutations in the LRBA gene represent an important but underrecognized risk factor for nocardiosis. Previous studies have documented the identification of patients harboring heterozygous LRBA mutations in patient cohorts. These individuals were observed to exhibit significantly milder clinical manifestations compared to those with biallelic mutations. Their phenotype aligns with milder forms of common variable immunodeficiency (CVID) or CVID-like presentations, characterized by features such as selective antibody deficiency, later disease onset, predominant yet milder autoimmune manifestations, and the absence of severe complications (19–21). Increased awareness, improved diagnostic tools, and personalized management approaches are needed for this patient population.
Treatment of nocardiosis is tailored to the individual patient (22). Currently, TMP-SMX is commonly used as the primary medication for treating Nocardia (23). In some cases, such as mycetoma and deep abscesses, a longer duration of therapy, potentially up to a year, may be necessary (2, 24). The duration of treatment should be based on the patient’s immune status and the extent of the disease. Early recognition and effective therapy are imperative for achieving successful outcomes.
Conclusion
In conclusion, Nocardia should be included in the differential diagnosis of cutaneous infections, particularly when there is no improvement with traditional antimicrobial regimens and when the infection is spreading into deeper muscle tissues. Both immunocompromised and immunocompetent patients are at risk. mNGS could help identify clinical infectious disease pathogens, especially in cases of culture-negative infections.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Shandong Provincial Hospital Affiliated to Shandong First Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
YY: Conceptualization, Methodology, Writing – original draft. JG: Conceptualization, Methodology, Writing – original draft. ND: Funding acquisition, Methodology, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82273527).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Lerner PI . Nocardiosis. Clin Infect Dis. (1996) 22:891–905. doi: 10.1093/clinids/22.6.891
2.
Wilson JW . Nocardiosis: updates and clinical overview. Mayo Clin Proc. (2012) 87:403–7. doi: 10.1016/j.mayocp.2011.11.016
3.
Welsh O Vera-Cabrera L Salinas-Carmona MC . Current treatment for nocardia infections. Expert Opin Pharmacother. (2013) 14:2387–98. doi: 10.1517/14656566.2013.842553
4.
Han D Li Z Li R Tan P Zhang R Li J . MNGS in clinical microbiology laboratories: on the road to maturity. Crit Rev Microbiol. (2019) 45:668–85. doi: 10.1080/1040841X.2019.1681933
5.
Dodiuk-Gad R Cohen E Ziv M Goldstein LH Chazan B Shafer J et al . Cutaneous nocardiosis: report of two cases and review of the literature. Int J Dermatol. (2010) 49:1380–5. doi: 10.1111/j.1365-4632.2010.04554.x
6.
Lederman ER Crum NF . A case series and focused review of nocardiosis: clinical and microbiologic aspects. Medicine (Baltimore). (2004) 83:300–13. doi: 10.1097/01.md.0000141100.30871.39
7.
Onuma K Crespo MM Dauber JH Rubin JT Sudilovsky D . Disseminated nocardiosis diagnosed by fine needle aspiration biopsy: quick and accurate diagnostic approach. Diagn Cytopathol. (2006) 34:768–71. doi: 10.1002/dc.20564
8.
Rehman A Olayiwola A Vu CA Bhatt P Joseph JA Ayoade F . Nocardia brasiliensis pyomyositis in an immunocompetent patient following gardening activity. J Investig Med High Impact Case Rep. (2024) 12:1636109252. doi: 10.1177/23247096241261508
9.
Nguyen NM Sink JR Carter AJ Asrani F . Nocardiosis incognito: primary cutaneous nocardiosis with extension to myositis and pleural infection. JAAD Case Rep. (2018) 4:33–5. doi: 10.1016/j.jdcr.2017.08.010
10.
Tramer L Mertz KD Huegli R Hinic V Jost L Burkhalter F et al . Intra-abdominal nocardiosis-case report and review of the literature. J Clin Med. (2020) 9:9. doi: 10.3390/jcm9072141
11.
Schaal KP Lee HJ . Actinomycete infections in humans--a review. Gene. (1992) 115:201–11. doi: 10.1016/0378-1119(92)90560-c
12.
Rouzaud C Rodriguez-Nava V Catherinot E Mechai F Bergeron E Farfour E et al . Clinical assessment of a nocardia PCR-based assay for diagnosis of nocardiosis. J Clin Microbiol. (2018) 56:56. doi: 10.1128/JCM.00002-18
13.
Miao Q Ma Y Wang Q Pan J Zhang Y Jin W et al . Microbiological diagnostic performance of metagenomic next-generation sequencing when applied to clinical practice. Clin Infect Dis. (2018) 67:S231–40. doi: 10.1093/cid/ciy693
14.
Goldberg B Sichtig H Geyer C Ledeboer N Weinstock GM . Making the leap from research laboratory to clinic: challenges and opportunities for next-generation sequencing in infectious disease diagnostics. MBio. (2015) 6:e01888–15. doi: 10.1128/mBio.01888-15
15.
Weng SS Zhang HY Ai JW Gao Y Liu YY Xu B et al . Rapid detection of nocardia by next-generation sequencing. Front Cell Infect Microbiol. (2020) 10:13. doi: 10.3389/fcimb.2020.00013
16.
Dumic I Brown A Magee K Elwasila S Kaljevic M Antic M et al . Primary lymphocutaneous Nocardia brasiliensis in an immunocompetent host: case report and literature review. Medicina (Kaunas). (2022) 58:488. doi: 10.3390/medicina58040488
17.
Lopez-Herrera G Tampella G Pan-Hammarstrom Q Herholz P Trujillo-Vargas CM Phadwal K et al . Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. (2012) 90:986–1001. doi: 10.1016/j.ajhg.2012.04.015
18.
Habibi S Zaki-Dizaji M Rafiemanesh H Lo B Jamee M Gamez-Diaz L et al . Clinical, immunologic, and molecular spectrum of patients with LPS-responsive beige-like anchor protein deficiency: a systematic review. J Allergy Clin Immunol Pract. (2019) 7:2379–2386.e5. doi: 10.1016/j.jaip.2019.04.011
19.
Gamez-Diaz L August D Stepensky P Revel-Vilk S Seidel MG Noriko M et al . The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. (2016) 137:223–30. doi: 10.1016/j.jaci.2015.09.025
20.
Li R Zheng Y Li Y Zhang R Wang F Yang D et al . Common variable immunodeficiency with genetic defects identified by whole exome sequencing. Biomed Res Int. (2018) 2018:1–7. doi: 10.1155/2018/3724630
21.
van Schouwenburg PA Davenport EE Kienzler AK Marwah I Wright B Lucas M et al . Application of whole genome and RNA sequencing to investigate the genomic landscape of common variable immunodeficiency disorders. Clin Immunol. (2015) 160:301–14. doi: 10.1016/j.clim.2015.05.020
22.
Munoz J Mirelis B Aragon LM Gutierrez N Sanchez F Espanol M et al . Clinical and microbiological features of nocardiosis 1997-2003. J Med Microbiol. (2007) 56:545–50. doi: 10.1099/jmm.0.46774-0
23.
Jiao M Deng X Yang H Dong J Lv J Li F . Case report: a severe and multi-site nocardia farcinica infection rapidly and precisely identified by metagenomic next-generation sequencing. Front Med (Lausanne). (2021) 8:669552. doi: 10.3389/fmed.2021.669552
24.
Margalit I Lebeaux D Tishler O Goldberg E Bishara J Yahav D et al . How do I manage nocardiosis?Clin Microbiol Infect. (2021) 27:550–8. doi: 10.1016/j.cmi.2020.12.019
Summary
Keywords
Nocardia asteroides , nocardiosis, muscle abscess, metagenomic next-generation sequencing, LRBA gene mutation
Citation
Yang YM, Guo J and Dang NN (2025) Case Report: Diagnosis of Nocardia asteroides infection using metagenomic next-generation sequencing of lymph node puncture tissue. Front. Med. 12:1626685. doi: 10.3389/fmed.2025.1626685
Received
11 May 2025
Accepted
18 August 2025
Published
12 September 2025
Volume
12 - 2025
Edited by
Paulo Filipe, Centro Hospitalar Lisboa Norte (CHLN), Portugal
Reviewed by
João Ferreira, Universidade de Lisboa, Portugal
Wei Shang, Nanjing University, China
Updates
Copyright
© 2025 Yang, Guo and Dang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ning Ning Dang, dangningning@sdfmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.