Abstract
Background:
COPD exacerbations are important events for disease management. The incidence of exacerbations impacts prognosis, guides treatment, and predicts future exacerbations. Despite their importance, exacerbations are often underdiagnosed and underreported. The aim of our study was to test and evaluate the effectiveness of a structured checklist for detecting past exacerbations that we developed and that would be suitable for routine clinical practice.
Methods:
350 patients with COPD and FEV1 < 80% of the predicted value were enrolled in 35 centers. Each patient completed a structured checklist and underwent an interview with the physician. The number of exacerbations, their symptoms and duration, and the treatments were evaluated. Clinical data on exacerbations in the previous 12 months were retrieved from the patient’s medical records and analyzed retrospectively. The data obtained using the structured checklist were compared with the data from the interviews and medical records.
Results:
Compared to the patient-physician interview, the structured checklist detected more exacerbations since the previous visit (p = 0.025). The difference was significant also for severe exacerbations (p = 0.003). In patients reporting only one event, the structured checklist was more sensitive in detecting mild events than the interview (p < 0.001). The structured checklist detected mild exacerbations in 10 patients in whom the interview detected none. Compared to the number of exacerbations in the medical records, the structured checklist detected more than twice as many events. The mean duration of an exacerbation was 9.7 days, and the most prominent symptoms were dyspnea and productive cough.
Conclusion:
Proposed structured checklist improved the detection of past exacerbations, including usually unreported events. Moreover, the structured checklist allows the severity and other clinical characteristics of past exacerbations to be specified and used to direct further COPD therapy.
Introduction
Exacerbations of COPD are acute events characterized by increased respiratory symptoms (1, 2). Their impact and consequences are substantial for the patient, the disease management, and the healthcare system. Exacerbations negatively impact the patient’s health status, often for a prolonged time, and contribute to disease progression as they accelerate the decline of lung functions. Patients with frequent exacerbations, defined as two or more exacerbations per year, have worse health status and morbidity than patients with less frequent exacerbations. The frequency and severity of exacerbations are associated with the risk of hospitalizations, all-cause mortality, and COPD-related mortality (1–5). Exacerbations account for the most significant proportion of healthcare costs of COPD (6).
The number of exacerbations in the prior year is the strongest predictor of a patient’s future exacerbation frequency (1, 7). The history of these acute events is essential for optimizing the treatment. For example, the administration of inhaled corticosteroids is indicated in patients with an increased number of eosinophils in the peripheral blood and a history of exacerbations. Similarly, exacerbations are required to indicate treatment with biologics.
Despite their importance, about 50 to 67% of COPD exacerbations are unreported to physicians (8, 9). Although often shorter in duration, unreported events also have a significant impact on health status (1).
The history of exacerbations is usually screened during a physician-patient interview and/or from the medical records. The use of other instruments is limited in routine clinical practice. Established questionnaires and scales, such as The COPD Assessment Test (CAT), Clinical COPD Questionnaire score (CCQ), Modified Medical Research Council Dyspnea Scale (mMRC), or St. George’s Respiratory Questionnaire (SGRQ), are focused more on symptoms, quality of life, or early detection of exacerbations (10, 11), but are not designed to screen exacerbation history.
Exacerbations can also be detected and monitored using diaries completed by the patient on a daily basis (12). However, patients using paper-based data recording in studies of respiratory diseases often falsify data (13–15). Patients are probably more compliant with electronic records. In one randomized trial of electronic symptom recording vs. monitored paper-based recording, actual compliance was 94% in the electronic group, whereas compliance with paper-based recording was 73% (13, 16). Smartphone-based diary of COPD symptoms enables near-complete identification of exacerbations at onset (13), and this tool was employed in the FLAME (17) and SPARK (18) studies. The EXAcerbations of Chronic pulmonary disease Tool (EXACT) is another standard, validated instrument for quantifying the frequency, severity, and duration of exacerbations of COPD in clinical trials. Nevertheless, smartphone-based e-diaries and EXACT are still used in research rather than clinical practice (19, 20).
Our study aimed to test and evaluate the effectiveness of a structured checklist that we developed to detect past exacerbations and that would be suitable for routine clinical practice, by comparing it with patient-physician interviews and medical records.
Materials and methods
Research
The study was conducted during May and June 2023 and involved 35 outpatient pneumology centers. There are 14 administrative regions in the Czech Republic and 2–3 centers were selected from each region to cover the entire Czech Republic. Each center enrolled 10 consecutive COPD patients. Inclusion criteria were COPD with forced expiratory volume in 1 s (FEV1) < 80% of predicted value, duration of COPD at least 2 years, and follow-up at the respective center with complete medical records for at least 2 years. No other eligibility criteria were defined.
During the study visit, a routine patient-physician interview took place in which the investigator asked about the number of exacerbations the patient had experienced since the last visit. After a standard examination, each patient completed the structured checklist individually or with the help of a nurse available on request. At the end of the study visit, the investigator retrospectively recorded data on exacerbations over the previous 12 months and additional clinical and demographical information about the patient from the medical records.
The study was approved by the Ethics Committee of University Hospital Olomouc, Reference number 80/25. All patients gave informed consent for completing and processing the structured checklist and using their medical records data for this retrospective study.
The structured checklist
The structured checklist was designed by expert panel, including the authors of this article, and based on our clinical experience, experience with the established scales and questionnaires, GOLD guidelines and practical considerations of the routine pulmonology practice (1, 2, 4). While the original two-page structured checklist is in Czech, Figure 1 gives the English translation of second page used by patients. The first page provides layperson-friendly information about the concept of exacerbation. This is not included in this paper. The second page, filled by the patient, collects data on past events. The structured checklist on the second page asks about the number of exacerbations since the last visit (Part A) and the symptoms selected by the patient (Part B). Part C covers event management, including pharmacotherapy and contact with a physician. Part D asks about the duration of past event(s) in days.
Figure 1

Structured checklist for the detection of past COPD exacerbations (English translation of the second page completed by patients).
Based on the information provided by the patient, whether from a structured checklist or an interview, the reported exacerbations can be categorized according to the treatment used (1).
Mild exacerbations were treated with short-acting bronchodilators only, moderate events were managed with antibiotics and/or systemic corticosteroids, and severe exacerbations required hospitalization.
Study outcomes
The main aim of our study was to test and evaluate the proposed structured checklist and its ability to detect past exacerbations, suitable for routine clinical practice. We compared the number of exacerbations (all and grouped by severity) that the patient had experienced since the last visit and that the patient reported in the structured checklist with the number of exacerbations obtained using usual conventional methods, i.e., a doctor-patient interview. The time since the last visit was also determined. Furthermore, we collected data from the medical records that were extracted for the last 12 months. Then we recalculated and converted the number of exacerbations in 12 months for each individual patient to the same period that had elapsed since their last visit. Based on the data provided by the patient in the structured checklist or interview, each exacerbation was classified by severity as mild, moderate, or severe.
To evaluate the structured checklist’s effectiveness, we compared the number of exacerbations since the last visit detected by the structured checklist with the number of exacerbations detected by interview. Furthermore, we compared the number of exacerbations since last visit detected by the structured checklist with the number of exacerbations recorded in medical records and converted to the period corresponding to the period since the last visit.
Statistical analysis
Descriptive data are reported as means ± standard deviations (SD) or percentages, as appropriate. The incidence of exacerbations was summarized as per-year and per-person rate. Data from the entire cohort and from subgroups defined by the number of exacerbations reported in the structured checklist were analyzed. Differences between paired data within one group were tested using paired t-test, e.g., differences between the structured checklist, the interview or between medical records for each patient. Differences in continuous parameters between the two groups were tested by a two-sample t-test or Mann–Whitney U test, depending on the normality of the data. All hypotheses were tested on a 5% level of significance. The analyses were performed using Microsoft Excel and IBM SPSS Statistics, version 28.
Results
A total of 350 COPD patients were enrolled. The characteristics of the patient cohort are shown in Table 1. 61.4% were male, and the mean age of the patients was 56.5 ± 14.8 years. Most patients had COPD GOLD stage 2 (64.3%) and stage 3 (28.6%). Mean FEV1 was 56.5 ± 14.8% of the predicted value. Among the comorbidities, hypertension (86.3%), hypercholesterolemia (48.0%), diabetes mellitus (29.4%), and atrial fibrillation (12.3%) were the most frequent. According to the medical records, patients had 494 exacerbations in the last year, with an average of 1.41 ± 1.6 per patient.
Table 1
| Characteristic | Analyzed population (N = 350) |
|---|---|
| Mean age (SD), years | 56.5 (14.8) |
| Male patients, n (%) | 215 (61.4) |
| Severity according to GOLD stages, n (%) | |
| Stage 1 | 8 (2.3) |
| Stage 2 | 225 (64.3) |
| Stage 3 | 100 (28.6) |
| Stage 4 | 17 (4.9) |
| Percentage of FEV1 predicted (mean, SD) | 56.5 (14.8) |
| Number of exacerbations in the previous 12 months | |
| All exacerbations | 494 |
| Mild | 120 |
| Moderate | 305 |
| Severe | 69 |
| Mean number of exacerbations per patient in the previous 12 months (SD) | |
| All exacerbations | 1.41 (1.6) |
| Mild | 0.34 (0.9) |
| Moderate | 0.87 (1.2) |
| Severe | 0.20 (0.6) |
| Phenotypes according to Czech guidelines,2n (%) | |
| Bronchitic phenotype | 194 (55.4) |
| COPD and asthma overlap | 105 (30.0) |
| Emphysematous phenotype | 76 (21.7) |
| Frequent exacerbator phenotype | 33 (9.4) |
| COPD and bronchiectasis overlap | 23 (6.6) |
| Pulmonary cachexia phenotype | 5 (1.4) |
| Comorbidities, n (%) | |
| Hypertension | 302 (86.3) |
| Hypercholesterolemia | 168 (48.0) |
| Diabetes mellitus | 103 (29.4) |
| Atrial fibrillation | 43 (12.3) |
| Ischemic heart disease | 33 (9.4) |
| Peripheral vascular disease | 33 (9.4) |
| History of myocardial infarction | 32 (9.1) |
| History of stroke | 12 (3.4) |
| History of transient cerebral ischemia | 6 (1.7) |
| COPD treatment during the last 12 months, n (%) | |
| €Treatment containing ICS | 227 (64.9) |
| Fixed combination of LABA+LAMA+ICS | 111 (31.7) |
| Fixed combination of LABA+LAMA | 99 (28.3) |
| Fixed combination of LABA+ICS | 94 (26.9) |
| Inhaler containing LAMA | 50 (14.3) |
| Inhaler containing LABA | 33 (9.4) |
| Inhaler containing ICS | 32 (9.1) |
| Free combination of LABA+LAMA+ICS | 20 (5.7) |
Baseline characteristics of the analyzed patients’ population.
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; SD, standard deviation.
All patients completed the structured checklist without difficulties in approximately 1–2 min. Patients went through the form independently or asked the nurse for an explanation.
Concordance between the structured checklist and the interview
The number of events detected by the structured checklist is shown in Table 2 and Figure 2. Supplementary Table S1 shows the exacerbation rates per patient. In the entire population of 350 patients, a total of 401 exacerbations since the last visit were detected by the structured checklist, while 370 exacerbations were detected by the interviews. The difference was statistically significant (p = 0.025). Using the structured checklist, approximately one third of patients (123; 35.1%) reported no exacerbation since their last visit, one third (116; 33.1%) experienced one exacerbation, and the last third reported two or more events (111; 31.7%) (Figure 3).
Table 2
| Structured checklist | Interview | p-value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Pts (N) |
All events (n) | Mild (n) |
Mod (n) |
Sev (n) |
All events (n) |
Mild (n) |
Mod (n) |
Sev (n) | Number of patients reporded no event in interview (N) | All events | Mild | Mod | Sev |
| All patients | 350 | 401 | 144 | 212 | 45 | 370 | 121 | 222 | 27 | 124 | 0.025 | 0.113 | 0.418 | 0.003 |
| Subgroups by number of reported exacerbations in the structured checklist | ||||||||||||||
| No events | 123 | 0 | 0 | 0 | 0 | 14 | 1 | 11 | 2 | 112 | 0.001 | 0.319 | 0.007 | 0.158 |
| 1 event | 116 | 116 | 53 | 56 | 7 | 110 | 34 | 71 | 5 | 10 | 0.109 | <0.001 | <0.001 | 0.158 |
| 2 events | 63 | 126 | 44 | 69 | 13 | 104 | 36 | 59 | 9 | 2 | <0.001 | 0.261 | 0.105 | 0.159 |
| 3 events | 33 | 99 | 21 | 63 | 15 | 87 | 18 | 63 | 6 | 0 | 0.008 | 0.62 | 1 | 0.027 |
| 4 events | 15 | 60 | 26 | 24 | 10 | 55 | 32 | 18 | 5 | 0 | 0.642 | 0.567 | 0.32 | 0.055 |
Comparison of effectiveness in the detection of past exacerbations between the structured checklist and the patient-physician interview.
Numbers of exacerbation since last visit assessed by structured checklist and by physician-patients interview.
Mod, moderate; N, number of patients; n, number of events; Pts, patients; Sev, severe. Bolding indicates significant data. The severity of exacerbations according to GOLD was determined based on the treatment of the exacerbation that the patient reported in the structured checklist or that the physician identified during the interview.
Figure 2
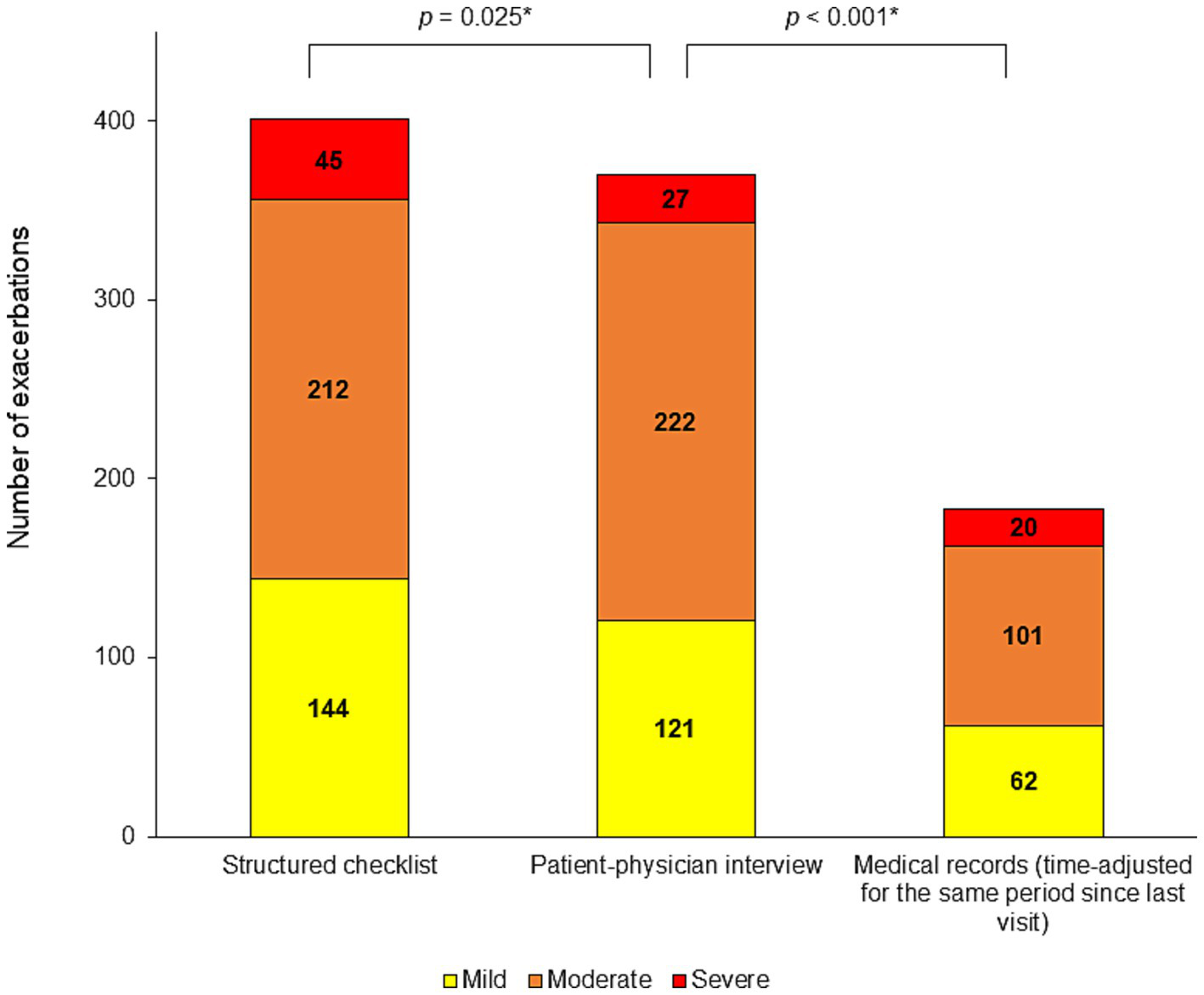
Number of detected exacerbations. Mild, moderate and, severe exacerbations since the last visit detected by the structured checklist, by the patient-physician interview and in the medical records (time-adjusted to cover the same period since the last visit as the other methods). *p-value for the comparison of all exacerbations number.
Figure 3
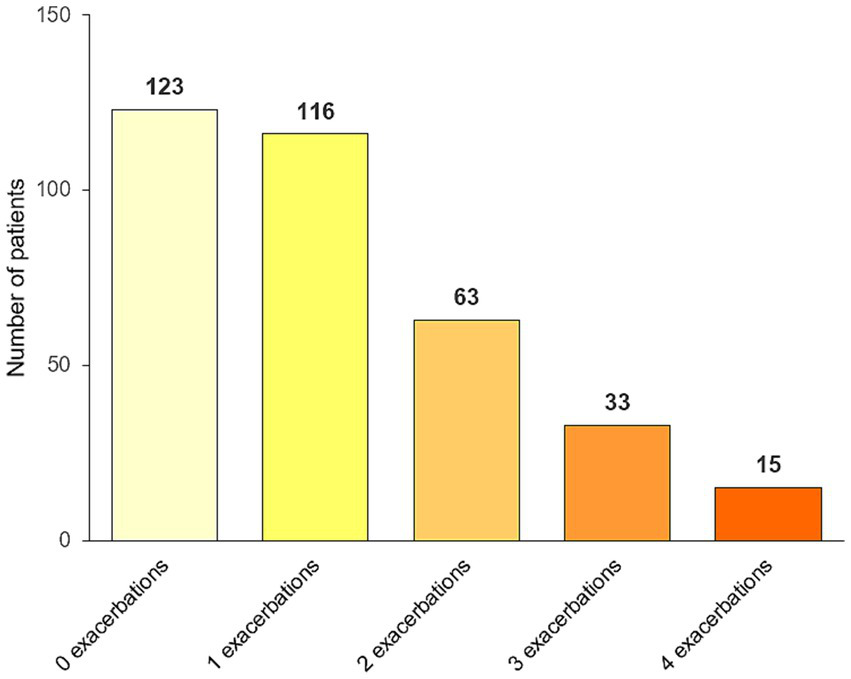
Proportion of patients with none, one, two, three, and four or more exacerbations since the last visit detected by the structured checklist.
In the overall study population, the structured checklist captured numerically more mild exacerbations (144 vs. 121; p = 0.113) and significantly more severe exacerbations (45 vs. 27; p = 0.003) compared to the patient-physician interview. In subgroups defined by the number of exacerbations reported in the structured checklist, significantly higher numbers of all exacerbations were detected by the structured checklist compared to the interview in patients who reported two or three exacerbations (126 vs. 104, p < 0.0001; 99 vs. 87, p = 0.008, respectively). In patients with one exacerbation since their last visit, the structured checklist identified only a numerically higher number of all exacerbations (116 vs. 110; p = 0.109) but a significantly higher number of mild exacerbations (53 vs. 34; p = 0.0001) and a significantly lower number of moderate exacerbations (56 vs. 71; p = 0.0009) compared with the patient-physician interview.
Of the 53 patients who reported only 1 mild exacerbation on the structured checklist, there were 10 patients in whom the interview did not detect any exacerbation, in another 31 patients the physician interview detected one mild exacerbation consistent with the structured checklist, and in 12 patients the physician interview rated the exacerbations as moderate. In addition, the interview then rated 3 other patients as having mild exacerbations, although the structured checklist rated these exacerbations differently as mild (Table 2).
In the group of 123 patients who reported no exacerbation in the structured checklist, the interview with the physician also did not detect any exacerbation in 112 patients, while in 11 patients (8.9%), the interview detected some exacerbations, including one mild, 11 moderate, and two severe events. The difference between structured checklist and the interview was significant for all and moderate exacerbations (p = 0.001 and 0.007, respectively) (Table 2).
Among the 116 patients who reported one exacerbation in the structured checklist, there were 10 patients (8.6%) in whom the interview did not detect any event and four patients in whom the interview detected two exacerbations. In addition, the patient-physician interview did not detect any exacerbation in two patients who reported two exacerbations in the structured checklist (Table 2).
Concordance between the structured checklist and the medical records
The mean time since the last visit to the physician was 149 days (5 months) in the overall study population. Table 3 shows the number of exacerbations identified in the medical chart review of the last 12 months, adjusted to cover the same period since the last visit, as assessed by the structured checklist and the interview. The exacerbation rates per patient are shown in Supplementary Table S2, and the number of exacerbations from which the time-adjusted numbers and rates were calculated are shown in Supplementary Table S3.
Table 3
| Structured checklist | Mean time since last visit, in days (SD) | Medical records (time-adjusted) | p-value | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Pts (N) | All events (n) | Mild (n) | Mod (n) | Sev (n) | All events (n) | Mild (n) | Mod (n) | Sev (n) | All events | Mild | Mod | Sev | |
| All patients | 350 | 401 | 144 | 212 | 45 | 149 (107) | 183 | 62 | 101 | 20 | <0.001 | <0.001 | <0.001 | <0.001 |
| Subgroups by number of reported exacerbations in the structured checklist | ||||||||||||||
| No event | 123 | 0 | 0 | 0 | 0 | 146 (122) | 21 | 5 | 13 | 3 | <0.001 | 0.004 | <0.001 | 0.0026 |
| 1 event | 116 | 116 | 53 | 56 | 7 | 154 (102) | 45 | 17 | 25 | 3 | <0.001 | <0.001 | <0.001 | 0.1 |
| 2 events | 63 | 126 | 44 | 69 | 13 | 156 (102) | 50 | 17 | 27 | 6 | <0.001 | <0.001 | <0.001 | 0.023 |
| 3 events | 33 | 99 | 21 | 63 | 15 | 142 (89) | 44 | 12 | 27 | 4 | <0.001 | 0.121 | <0.001 | 0.021 |
| 4 events | 15 | 60 | 26 | 24 | 10 | 115 (53) | 23 | 11 | 9 | 3 | <0.001 | 0.046 | 0.002 | 0.014 |
Comparison of effectiveness in the detection of past exacerbations between the structured checklist and medical records.
Mod, moderate; N, number of patients; n, number of events; Pts, patients; Sev, severe; SD, standard deviation. Bolding indicates significant data. The severity of exacerbations according to GOLD was determined based on the treatment of the exacerbation that the patient reported in the structured checklist or that was listed in the medical records.
Number of exacerbations since the last visit according to the structured checklist, time elapsed since the last visit, and number of exacerbations obtained from medical records and recalculated to the same period corresponding to the time since the last visit.
The structured checklist, asking about the period since the last visit, detected more exacerbations than the review of medical records, recalculated over the same period. The structured checklist identified more than twice as many events in the overall study population and in patients that reported one or more events. The differences reached statistical significance (Table 3; Figure 2).
In the subgroup of 123 patients who reported no exacerbation in the structured checklist, there were 78 patients who had no exacerbation in the past year also in their medical records. Other 45 of these patients had a record of at least one exacerbation in the last year; the time-adjusted number of events in these patients was 21 since last visit, and moderate intensity predominated. In 10 of these patients, exacerbations were also noted during the interview.
Symptoms and duration of exacerbations
The presence of symptoms (cough, shortness of breath, and feeling cold) and the duration of each exacerbation that the patient experienced since the last visit and reported on the structured checklist are shown in Table 4. The most prevalent symptoms were dyspnea (81.3%) and productive cough (61.1%). Feeling cold and dry cough were present in 44.1 and 32.2% of events, respectively. Two symptoms were present simultaneously in 53.9% of events. The mean duration of exacerbation was 9.7 days in the overall population.
Table 4
| Parameter | Exacerbations (n) | Dyspnea (n, %) | Productive cough (n, %) | Feeling cold (n, %) | Dry cough (n, %) | Mean duration in days (SD) |
|---|---|---|---|---|---|---|
| All patients | 401 | 326 (81.3) | 249 (62.1) | 177 (44.1) | 129 (32.2) | 9.7 (7.3) |
| Subgroups by number of reported exacerbations in the structured checklist | ||||||
| 1 event | 116 | 92 (79.3) | 63 (54.3) | 48 (41.4) | 47 (40.5) | 10.9 (9.8) |
| 2 events | 126 | 105 (83.3) | 80 (63.5) | 60 (47.6) | 42 (33.3) | 10.0 (6.8) |
| 3 events | 99 | 86 (86.9) | 76 (76.8) | 56 (56.6) | 29 (29.3) | 9.0 (4.4) |
| 4 events | 60 | 43 (71.7) | 30 (50.0) | 13 (21.7) | 11 (18.3) | 7.8 (6.0) |
Symptoms and duration of exacerbations since last visit, reported using the structured checklist.
n, number of events; SD, standard deviation.
A total of 139 of 225 patients with moderate COPD (61.8%), 70 of 100 patients with severe COPD (70.0%), and 10 of 17 patients with very severe COPD (58.8%) experienced at least one exacerbation since the previous visit, with a mean number of detected exacerbations of 1.07, 1.26 and 1.24 events per patient, respectively (Table 5).
Table 5
| COPD stage | Pts (N) | Structured checklist | First year (24 to 12 months) before the study visit | Second year (last 12 months) before the study visit | |||
|---|---|---|---|---|---|---|---|
| Pts with ≥ 1 event (%) | Event rate per patient | Pts with ≥ 1 event (%) | Event rate per patient and year | Pts with ≥ 1 event (%) | Event rate per patient and year | ||
| Mild | 8 | 8 (100) | 1.75 | 5 (62.5) | 1 | 5 (62.5) | 1.13 |
| Moderate | 225 | 139 (61.8) | 1.07 | 133 (59.1) | 1.08 | 147 (65.3) | 1.32 |
| Severe | 100 | 70 (70.0) | 1.26 | 54 (54.0) | 0.81 | 73 (73.0) | 1.30 |
| Very severe | 17 | 10 (58.8) | 1.24 | 15 (88.2) | 2.31 | 16 (94.1) | 3.35 |
| All stages | 350 | 227 (64.9) | 1.15 | 207 (59.1) | 1.05 | 241 (68.9) | 1.41 |
Exacerbation rates since the last visit according to COPD stages detected by the structured checklist and during first and second year before the study visit in the medical records.
COPD, chronic obstructive pulmonary disease; N, number of patients; Pts, patients.
Discussion
We have tested and evaluated a structured checklist for detection of COPD exacerbation history that is suitable for routine clinical practice, and tested its effectiveness in outpatient settings in the cohort of 350 patients from 35 centers across the Czech Republic. Study participants mostly had moderate and severe COPD. Completing the structured checklist did not significantly burden the patients, as it took approximately 1–2 min, comparable to other established tools, such as the CAT questionnaire.
Compared with the standard method of screening exacerbation history, i.e., the patient-physician interview, our structured checklist detected 8.4% more overall exacerbations (p = 0.025). We also focused on mild exacerbations, that may not require a visit to a physician and may remain unreported to healthcare professionals. Although not statistically significant, the structured checklist detected 19.0% more mild exacerbations than the interview (p = 0.113) in the overall study population.
Using the structured checklist, a higher number of exacerbations was found compared to the patient-physician interview, regardless of how many exacerbations the patient had experienced since the previous visit. However, the structured checklist seemed even more sensitive than the interview in those patients who reported two or three exacerbations since the last visit (p = 0.00002; p = 0.008, respectively) and specifically in those who reported only one mild exacerbation (p = 0.0001). The interview detected no event in 18.9% of patients who declared one mild exacerbation in the structured checklist. For these patients, the structured checklist was more sensitive than the interview. On the contrary, in 8.9% of all patients who declared no event in the structured checklist, the exacerbation was identified during the interview with the physician. In these patients, the structured checklist result was considered a false negative.
The patient-physician interview and structured checklist completion occurred during one study visit, so it can be assumed that the physicians focused more carefully on detecting exacerbations during the interviews than usual. Therefore, we also compared the number of exacerbations detected using the structured checklist with those recorded in the patient’s medical charts in the last 12 months, which our study procedure could not have influenced. The numbers of exacerbations obtained from medical records were adjusted to cover the same time period as the structured checklist and interview. Our structured checklist identified more than twice as many events in overall study population and in most subgroups by number of reported exacerbations, compared to data from medical records, which were recalculated for the same period corresponding to the time since the last visit for each patient.
The incidence of exacerbations in patients with COPD varies across studies. Data from an extensive UK database including 340,515 COPD patients published in 2022 show that in the previous year, 53.2% of patients experienced no exacerbations, 20.0% experienced one moderate exacerbation, 9.4% experienced two moderate exacerbations, 9.9% experienced three or more moderate exacerbations, 5.6% experienced one severe exacerbation, 1.2% experienced two severe exacerbations and 0.7% experienced three or more severe exacerbations (5). Another study published in 2021 that examined the effect of exacerbation history on subsequent exacerbations using the data from a large population of 250,723 COPD patients summarized that 78% of patients had no prior exacerbation in the previous year, 11% had one moderate and 11% had severe or multiple exacerbations at the baseline (21). Data from the ECLIPSE study (2010) involving 2,138 patients showed that 53% of patients had no exacerbation in the previous year, 47% had at least one exacerbation in the previous year, and 29% had at least two exacerbations. In this study, 39, 52, and 62% of patients with COPD stages 2, 3, and 4, respectively, experienced at least one exacerbation (7). In our study, 61.8, 70, and 58.8% of patients with moderate, severe, and very severe COPD, respectively, had experienced at least one exacerbation since the previous visit. The number of exacerbations detected by our structured checklist was higher than the number of exacerbations per year in the cited studies (5, 7, 21). It could be due to the larger representation of patients with more severe stages of COPD and the fact that the structured checklist was completed from May to June 2023, covering approximately five previous months (time since the last visit), during which higher incidence of respiratory infections is common and increases the frequency of exacerbations (22).
Mild exacerbations accounted for 73 to 74% of all exacerbations in the FLAME and SPARK studies (17, 18), and approximately 38% of all events in the RESTORE study (10). In our study, mild exacerbations detected by the questionnaire accounted for 35.9% of all exacerbations, similar to the RESTORE study and significantly less than in the SPARK and FLAME studies. Using our structured checklist, we found 19% more mild exacerbations than in the patient-physician interview and 2.3 times more than in the review of medical records. Compared to SPARK and FLAME studies, the incidence of mild exacerbations remained underestimated even using our structured checklist. It seems that it is still difficult to establish an accurate history of COPD exacerbations at present (23), and the result of our study shows that using a simple structured checklist could improve the detection of past exacerbations.
Our study has several limitations. First, the structured checklist we tested was not developed according to the precise and strict criteria required for creating a questionnaire. This is related to some of its characteristics. To keep the structured checklist as simple and practical as possible, we decided to leave out several details about the exacerbation, such as sputum purulence, emergency room visits, or duration of systemic corticosteroids or antibiotic treatment. Consequently, the results of the structured checklist cannot be used for the Anthonisen classification of exacerbations (24), and the physician gets rather superficial knowledge about the treatment of the reported event, although this will allow the severity of the exacerbation to be determined according to GOLD. These limitations can be improved by future modifications of the structured checklist, eg, by adding new questions. In addition, the structured checklist does not allow for collecting details about the fourth and subsequent events. Nevertheless, it still alerts the physician to the higher number of exacerbations and gives the opportunity to ask the patient for more information.
Another important limitation is that the effectiveness of detecting exacerbations using the structured checklist in our study was compared with the doctor-patient interview, as a usual method in clinical practice, while no standard method for detecting exacerbations used in clinical trials (e.g., diaries or electronic diaries) was used for comparison. Bias may be introduced by the timing of the structured checklist’s completion and the interview with the physician. Both procedures took place at the same visit, so they may have influenced each other. It is also likely that the physician was more intently and carefully focused on detecting exacerbations during the interview than he or she would have done in everyday clinical practice.
The final limitation we would like to mention is that the structured checklist was created and tested by respiratory doctors in the Czech Republic, among Czech patients, and in the Czech language. Its transferability to other languages and countries has not yet been tested.
Conclusion
We have tested a structured checklist that is easy to use in routine clinical practice to detect past COPD exacerbations. Using standard patient-physician interview as a reference tool to screen exacerbation history, we demonstrated that our structured checklist significantly improved the detection of past exacerbations, including mild events. Compared to the records in the medical charts, which reflect the detection of exacerbations in routine clinical practice, our structured checklist detected more than twice as many exacerbations. The structured checklist allows the determination of severity and other clinical characteristics of exacerbations and also records the treatment.
While it is still difficult to establish an accurate history of COPD exacerbations, our work suggests that using a simple structured checklist for detection of exacerbation history like ours could reduce the number of undetected events and improve the management of COPD. At the same time, our work shows some requirements for future research. In the future, it will be necessary to develop a questionnaire based on strict and precise criteria. It will also be necessary to test the questionnaire by comparing it with standard methods for detecting exacerbations used in clinical trials (e.g., diaries or e-diaries).
Statements
Data availability statement
The data supporting this manuscript may be shared upon reasonable request to the corresponding author.
Ethics statement
The study was approved by the Ethics Committee of University Hospital Olomouc, Czechia. Reference number 80/25. All patients gave informed consent for completing and processing the structured checklist and using their medical records data for this retrospective study. The studies were conducted in accordance with the local legislation and institutional requirements. Article does not contain identifiable human images.
Author contributions
JZ: Conceptualization, Methodology, Investigation, Data curation, Formal analysis, Writing – original draft, Writing – review & editing, Funding acquisition, Project administration. EV: Investigation, Writing – review & editing. JK: Investigation, Writing – review & editing. MK: Writing – review & editing. MM: Project administration, Writing – review & editing, Funding acquisition. BH: Funding acquisition, Project administration, Writing – review & editing. VK: Conceptualization, Methodology, Formal analysis, Writing – review & editing, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the Cooperatio INDI Program (research area Internal disciplines), which is the institutional support of the Charles University, Prague, Czech Republic; by the Ministry of Health, Czech Republic -conceptual development of research organization (FNOl, 00098892 and UHHK, 00179906) and by AstraZeneca Czech Republic, s.r.o., Prague, Czech Republic. AstraZeneca Czech Republic, s.r.o., Prague, Czech Republic, funded the medical writing support. This study was supported by institutional funding from the Faculty of Medicine and Dentistry at Palacký University in Olomouc, Czech Republic. The funders had no role in data collection, analysis, or interpretation.
Acknowledgments
We thank the physicians, nurses, and patients from all participating centers for careful data collection. Jiri Kryza from IQVIA Technology Solutions, s.r.o. (Prague, Czech Republic) has our gratitude for his help with study coordination and consultations regarding the data analysis. We also thank Jana Kubátová, MSc, PhD and ANOVA CRO, s.r.o. (Prague, Czech Republic) for medical writing support.
Conflict of interest
JZ received fees for presentation at symposia and or publications from Angelini CZ, AstraZeneca CZ, Sanofi CZ, GlaxoSmithKline CZ, received fees for advisory board participation from Berlin-Chemie CZ, AstraZeneca CZ, Sanofi CZ, GlaxoSmithKline CZ and reports travel grant from Angelini CZ. EV has received COPD research funding/travel grants from AstraZeneca CZ and consulting/lectures/advisory board payment from AstraZeneca CZ, GlaxoSmithKline CZ and Sanofi CZ. VK reports non-financial support from Meditech Media, UK, Boehringer Ingelheim RCV Vienna, AUT, Charles University, Czech Republic, and Ministry of the Health, Czech Republic, during the study. He gave presentations at symposia and sponsored and received fees for advisory board participation and travel grants from Angelini, AstraZeneca, Berlin-Chemie CZ, Boehringer Ingelheim CZ, Chiesi CZ and Menarini IL. He received research grants from Angelini CZ, AstraZeneca CZ, Boehringer Ingelheim CZ, RCV, Chiesi CZ, and an honorarium from the Roche Europe and Sanofi CZ advisory boards. All the above-mentioned interactions are outside the submitted work. MM and BH are employees of AstraZeneca Czech Republic, s.r.o.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1630338/full#supplementary-material
Abbreviations
CAT, COPD Assessment Test; CCQ, Clinical COPD Questionnaire score; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; LABA, long-acting beta2-agonist; LAMA, long-acting muscarinic antagonist; mMRC, Modified Medical Research Council Dyspnea Scale; SD, standard deviation; SGRQ, St. George’s Respiratory Questionnaire.
References
1.
2025 GOLD Report . (2024) Global initiative for chronic obstructive lung disease - GOLD. https://goldcopd.org/2025-gold-report/ [Accessed December 29, 2024]
2.
Zatloukal J Brat K Neumannová K et al . Chronic obstructive pulmonary disease - diagnosis and management of stable disease; a personalized approach to care, using the treatable traits concept based on clinical phenotypes. Position paper of the Czech Pneumological and Phthisiological society. Biomed Pap. (2020) 164:325–56. doi: 10.5507/bp.2020.056
3.
Voláková E Koblížek V Svoboda M Zatloukal J et al . Nutritional status and muscle mass loss in patients with COPD, association with lung function, symptoms, comorbidities and long-term survival: data from the National Database Study. Biomed Pap. (2025) 169:XX. doi: 10.5507/bp.2025.001
4.
Soler-Cataluña JJ Martínez-García MA Román Sánchez P Salcedo E Navarro M Ochando R . Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. (2005) 60:925–31. doi: 10.1136/thx.2005.040527
5.
Whittaker H Rubino A Müllerová H Morris T Varghese P Xu Y et al . Frequency and severity of exacerbations of COPD associated with future risk of exacerbations and mortality: a UK routine health care data study. Int J Chron Obstruct Pulmon Dis. (2022) 17:427–37. doi: 10.2147/COPD.S346591
6.
Villegas CG Paz-Zulueta M Herrero-Montes M Parás-Bravo P Pérez MM . Cost analysis of chronic obstructive pulmonary disease (COPD): a systematic review. Health Econ Rev. (2021) 11:31. doi: 10.1186/s13561-021-00329-9
7.
Hurst JR Vestbo J Anzueto A Locantore N Müllerova H Tal-Singer R et al . Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. (2010) 363:1128–38. doi: 10.1056/NEJMoa0909883
8.
Seemungal TA Hurst JR Wedzicha JA . Exacerbation rate, health status and mortality in COPD – a review of potential interventions. Int J Chron Obstruct Pulmon Dis. (2009) 4:203–23. doi: 10.2147/COPD.S3385
9.
Langsetmo L Platt RW Ernst P Bourbeau J . Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med. (2008) 177:396–401. doi: 10.1164/rccm.200708-1290OC
10.
Dal Negro RW Wedzicha JA Iversen M Fontana G Page C Cicero AF et al . Effect of erdosteine on the rate and duration of COPD exacerbations: the RESTORE study. Eur Respir J. (2017) 50:1700711. doi: 10.1183/13993003.00711-2017
11.
Agustí A Soler JJ Molina J Muñoz MJ García-Losa M Roset M et al . Is the CAT questionnaire sensitive to changes in health status in patients with severe COPD exacerbations?COPD. (2012) 9:492–8. doi: 10.3109/15412555.2012.692409
12.
Oliveira AS Munhá J Bugalho A Guimarães M Reis G Marques A . Identification and assessment of COPD exacerbations. Pulmonology. (2018) 24:42–7. doi: 10.1016/j.rppnen.2017.10.006
13.
Johnston NW Lambert K Hussack P de Verdier MG Higenbottam T Lewis J et al . Detection of COPD exacerbations and compliance with patient-reported daily symptom diaries using a smartphone-based information system. Chest. (2013) 144:507–14. doi: 10.1378/chest.12-2308
14.
Côté J Cartier A Malo JL Rouleau M Boulet LP . Compliance with peak expiratory flow monitoring in home Management of Asthma. Chest. (1998) 113:968–72. doi: 10.1378/chest.113.4.968
15.
Chowienczyk PJ Parkin DH Lawson CP Cochrane GM . Do asthmatic patients correctly record home spirometry measurements?BMJ. (1994) 309:1618. doi: 10.1136/bmj.309.6969.1618
16.
Stone AA Shiffman S Schwartz JE Broderick JE Hufford MR . Patient compliance with paper and electronic diaries. Control Clin Trials. (2003) 24:182–99. doi: 10.1016/s0197-2456(02)00320-3
17.
Wedzicha JA Banerji D Chapman KR Vestbo J Roche N Ayers RT et al . Indacaterol–Glycopyrronium versus Salmeterol–Fluticasone for COPD. N Engl J Med. (2016) 374:2222–34. doi: 10.1056/NEJMoa1516385
18.
Wedzicha JA Decramer M Ficker JH Niewoehner DE Sandström T Taylor AF et al . Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomized, double-blind, parallel-group study. Lancet Respir Med. (2013) 1:199–209. doi: 10.1016/S2213-2600(13)70052-3
19.
Leidy NK Wilcox TK Jones PW Roberts L Powers JH Sethi S . Standardizing measurement of chronic obstructive pulmonary disease exacerbations. Am J Respir Crit Care Med. (2011) 183:323–9. doi: 10.1164/rccm.201005-0762OC
20.
Mackay AJ Donaldson GC Patel ARC Singh R Kowlessar B Wedzicha JA . Detection and severity grading of COPD exacerbations using the exacerbations of chronic pulmonary disease tool (EXACT). Eur Respir J. (2014) 43:735–44. doi: 10.1183/09031936.00110913
21.
Vogelmeier CF Diesing J Kossack N Pignot M Friedrich FW . COPD exacerbation history and impact on future exacerbations – 8-year retrospective observational database cohort study from Germany. Int J Chron Obstruct Pulmon Dis. (2021) 16:2407–17. doi: 10.2147/COPD.S322036
22.
Donaldson GC Wedzicha JA . The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. (2014) 9:1101–10. doi: 10.2147/COPD.S54475
23.
Aaron SD . Measuring and quantifying acute exacerbations of COPD: pitfalls and practicalities. Eur Respir J. (2014) 43:662–4. doi: 10.1183/09031936.00158613
24.
Anthonisen NR Manfreda J Warren CP Hershfield ES Harding GK Nelson NA . Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Ann Intern Med. (1987) 106:196–204. doi: 10.7326/0003-4819-106-2-196
Summary
Keywords
obstructive lung disease, structured checklist, questionnaire, flare-up, disease history, dyspnea, cough
Citation
Zatloukal J, Volakova E, Kovacikova J, Kulirova M, Maruscak M, Hytychova B and Koblizek V (2025) New instrument for effective detection of a history of COPD exacerbations, including usually unreported events. Front. Med. 12:1630338. doi: 10.3389/fmed.2025.1630338
Received
12 June 2025
Accepted
02 September 2025
Published
19 September 2025
Volume
12 - 2025
Edited by
Francisco Epelde, Parc Taulí Foundation, Spain
Reviewed by
Zarko Vrbica, Dubrovnik General Hospital, Croatia
Celine Pribil, GlaxoSmithKline France, France
Updates
Copyright
© 2025 Zatloukal, Volakova, Kovacikova, Kulirova, Maruscak, Hytychova and Koblizek.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Vladimir Koblizek, vladimir.koblizek@fnhk.cz
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.