Abstract
Mooren’s Ulcer (MU) is a rare, chronic, and painful form of autoimmune peripheral ulcerative keratitis (PUK), with an elusive etiology and a risk of corneal perforation and vision loss. Despite numerous proposed triggers, including parasitic infections, hepatitis C virus, ocular trauma, and surgery, the pathogenesis of MU remains unclear, and diagnosis continues to rely heavily on exclusion. A key controversy in current clinical practice lies in the absence of standardized diagnostic criteria and consensus treatment protocols. This review addresses this gap by presenting a comprehensive and structured diagnostic framework for MU, particularly emphasizing laboratory and immunological testing strategies to facilitate accurate differential diagnosis. To our knowledge, this is the first review to systematize these diagnostic components in detail. In addition to summarizing the latest findings on epidemiology, etiology, pathology, and classification, the work also review the evolving role of advanced imaging, histopathology, and tear-based markers in MU diagnosis and monitoring. Treatment options, ranging from immunosuppressive therapy to surgical intervention, are discussed based on disease severity. This work recommend a tiered, individualized approach to treatment and advocate for future multicenter studies to validate diagnostic protocols and establish evidence-based clinical guidelines.
1 Introduction
Mooren’s ulcer (MU) is a significant subset of autoimmune peripheral ulcerative keratitis (PUK), accounting for approximately 35% of cases (1). As with other forms of PUK, MU is characterized by a progressive inflammatory process affecting the juxtalimbal cornea, resulting in crescent-shaped stromal thinning, epithelial defects, and infiltration of inflammatory cells within the corneal stroma (2, 3). First proposed by Bowman in 1849 and later described in greater detail by Mooren in 1867, MU has since been recognized as a chronic, idiopathic, progressive, and painful variant of PUK (3, 4). Typically, MU presents as a grey, swollen patch located about 2–3 mm from the limbus. This lesion rapidly progresses, forming a furrow that moves toward the central cornea, potentially involving the entire corneal surface while usually sparing the sclera (5, 6). The ongoing progression of these ulcers can lead to corneal perforation. Notably, approximately one-third of MU patients exhibit bilateral involvement.
MU is believed to involve genetic and autoimmune components, but its exact pathogenesis remains unknown. Several factors have been implicated in its development, including intestinal parasite infections (7), chronic hepatitis C infection (8), ocular trauma (9), surgical interventions (10), and pterygium, particularly following pterygium surgery at the donor site after conjunctival limbal graft (11). Despite the use of various therapeutic strategies, including local and systemic immunosuppressants, cytotoxic drugs, anti-inflammatory agents, and surgical treatment such as conjunctival resection and keratoplasty, there is no established standardized treatment protocol for MU. Therefore, this review delves into the intricate details of epidemiology, etiology, risk factors, pathology, clinical manifestations and classification, diagnosis, differential diagnosis, investigational techniques, treatment, efficacy evaluation, and prognosis, aiming to bridge the existing gaps and controversy, especially in full diagnostic techniques and different options of stepladder approach, in the management of MU patients and provide a clearer guidelines for clinicians and researchers in their efforts to understand and treat this complex condition.
2 Epidemiology
MU occurs infrequently in the northern hemisphere, whereas it is more prevalent in regions such as the southern hemisphere, the Indian subcontinent, China, and central Africa (12–14). The incidence, clinical characteristics, and severity of MU vary significantly across geographical regions and among different racial groups (15). Chen et al. (16) analyzed 550 cases (715 eyes) of MU treated in China from 1960 to 1996. The study reported a mean onset age of 48.4 years and a male-to-female ratio of 1:0.74, suggesting that men were 1.35 times more likely to be affected than women. In Ibadan, southwest Nigeria, a study reported a male-to-female ratio of 1:0.28, indicating a significantly higher number of male patients (17). Wood and Kaufman (18) suggest that MU has a higher prevalence in men. Consistent with this observation, Raghav et al. recently reported that males account for 90% of MU patients in rural India (19). While the exact reasons for this discrepancy are still uncertain, contributing factors may include a higher incidence of ocular injuries among men and potential biological differences.
3 Etiology and risk factors
MU is associated with a wide range of possible causes and risk factors (Figure 1), most of which fall into the categories outlined below:
Figure 1

The etiology and pathology of Mooren’s ulcer. Autoimmunity, intestinal parasite infection, hepatitis C virus infection, ocular trauma or surgery, coexistence with pterygium, and genetic predisposition are recognized risk factors. Corneal injury or infection may expose the cornea-associated antigen (CO-Ag), inducing anti-CO-Ag antibodies and immune cross-reactivity. Activated inflammatory cells and circulating immune complexes (CICs) promote collagenase release and corneal lysis, while adhesion molecules (LFA-1, ICAM-1) and costimulatory pathways (B7/CD28/CTLA-4) sustain local hyperimmunity. Upregulation of innate immune cascades, including NLRP3-CASP1-IL-1β and cGAS/STING signaling, further contributes to progressive stromal destruction. Created in BioRender. Wu, Y. (2025) https://BioRender.com/xn992hu.
3.1 Autoimmunity
Accumulating evidence suggests that MU is associated with immune dysfunction (13, 20, 21). The main evidence for this inference includes:
-
(a) Serum analyses of MU patients have revealed the presence of cornea-associated antigen (CO-Ag) and circulating autoantibodies targeting corneal tissue (22–25). In addition, indirect immunofluorescence testing revealed that 75% of MU patients had serum antibodies against rabbit corneal epithelial cells, and 37.5% had antibodies against human corneal epithelial cells, further supporting the presence of autoantibodies in MU patients (26).
-
(b) MU patients’ serum exhibits elevated levels of IgG and IgA (27).
-
(c) Immunological analyses of peripheral blood have shown an elevated helper-to-suppressor T cell (Th/Ts) ratio, accompanied by a reduction in suppressor T cells (Ts) (28, 29). Consistently, another study reported significantly reduced OKT8 + T cells and elevated OKT4+/OKT8 + ratios in MU patients compared to controls, suggesting regulatory imbalance in cellular immunity (26).
-
(d) Abnormally high levels of circulating immune complexes (CICs) have been observed in the serum (29).
-
(e) High expression of lymphocyte function-associated antigen-1 (LFA-1) and intercellular adhesion molecule-1 (ICAM-1) has been observed in the bulbar conjunctival tissue adjacent to the ulcer, along with significant infiltration of polymorphonuclear granulocytes, plasma cells, and lymphocytes (30).
-
(f) Additionally, immunoreactive cells and cytokines are present at the corneal limbus, with complement 1 (C1) more concentrated in the periphery than at the center of the cornea. Antigen–antibody complexes within the corneal limbal vessels can activate C1, initiating a classical complement cascade reaction (31, 32).
-
(g) The fact that the erosive corneal ulcer originates at the corneal limbus further supports the theory that it is an immune-related disease (21).
3.2 Intestinal parasite infection
Intestinal parasite infection is a risk factor for inducing MU. Zelefsky et al. identified a link between hookworm infection and the development of MU, especially among elderly men (7). The amino acid sequence encoded by the cDNA of CO-Ag, a stromal protein possibly involved in MU pathogenesis, was shown by Gottsch et al. to be identical to calgranulin C, a neutrophil protein present on filarial nematodes (23, 33). So, they considered that calgranulin C on the surface of parasites after parasite infection, such as filarial nematodes and hookworm, stimulates the body to produce cross immune response to cornea, resulting in the occurrence of MU (7, 23, 33). However, more than 50% of patients did not show hookworm infection (7), and the correlation with hookworm infection should be further confirmed.
Although the hypothesis of molecular mimicry between corneal CO-Ag and parasite-derived calgranulin C is intriguing, current evidence remains limited to case reports and small, uncontrolled series from endemic areas. Over half of reported MU patients had no confirmed parasitic infection, and diagnostic methods for helminth detection varied widely. Therefore, the existing data suggests a possible association rather than a proven causal link. Larger, controlled studies using standardized parasitological testing are needed to clarify this relationship.
3.3 Hepatitis C infection
The relationship between hepatitis C virus (HCV) infection and MU is controversial. Successful treatment of HCV infection with ribavirin and interferon therapy has been associated with remission of corneal symptoms in MU patients (34). However, some research considered that effect may be related to the immunomodulatory effects of interferon (35). What’s more, Pluznik et al. found that corneal symptoms worsened with anti-HCV treatment for chronic HCV infection in MU patients (36). Furthermore, Wang et al. performed serological screening for HCV infection on eight MU patients and found that all patients tested negative for HCV serology (37). Although the precise link between HCV infection and MU pathogenesis remains unclear, MU patients with HCV infection should be carefully managed.
Reports describing improvement of MU after antiviral therapy and others showing disease exacerbation during interferon treatment indicate that the observed effects may reflect immunomodulatory mechanisms rather than a direct viral association. In addition, several cohort studies failed to detect HCV infection in MU patients. Taken together, the evidence remains inconsistent and of low certainty. Current evidence suggests that HCV infection could be an associated comorbidity, although a direct causal role has not yet been established.
3.4 Ocular trauma or surgery
Ocular trauma or surgery is increasingly recognized as a contributing factor to MU. According to Chen et al., 10.7% of MU cases (550 patients, 715 eyes) in China were preceded by surgical procedures or ocular trauma (16). Additionally, Kim et al. reported that 41.7% (10/24) of patients had prior infection, trauma, or ocular surgery (15). These findings collectively imply a potential role of corneal injury in the development of MU. Given the current lack of uniform diagnostic standards for MU, clinicians should exercise caution when assessing corneal ulcers post-trauma or surgery. Furthermore, the mechanism through which ocular trauma contributes to MU remains unclear. In a case reported by Toyokawa et al., bilateral MU developed after EX-PRESS glaucoma implantation, implicating a possible autoimmune mechanism affecting the peripheral cornea (38). Surgical procedures or ocular traumas to the cornea disrupt its local structures, exposing normally hidden collagen type I, CO-Ag, thus triggering an autoimmune response and heightening the likelihood of MU development (7, 14). Further investigation into these aspects is warranted.
3.5 Combined with pterygium
Pterygium is a common, benign growth of conjunctival tissue that extends in a wedge-shaped form onto the cornea (39, 40). Ulcerations can occur at the donor site following pterygium excision (11). Several cases of MU occurring in conjunction with pterygium have been reported (41, 42). Pterygium could impair the integrity of the cornea and lead to the exposure of CO-Ag, potentially triggering MU (42). Although the exact mechanism is unclear, the presence of corneal ulceration in conjunction with pterygium should raise suspicion for MU.
3.6 Genetic predisposition
Through molecular biological methods, the cause of corneal ulcers, such as MU, can be located to certain genetic changes (43). In recent years, several researchers have reported a strong correlation between MU and the expression of ocular surface inflammation marker, human leukocyte antigen (HLA) (43). Taylor et al. reported elevated frequencies of HLA-DR17 and HLA-DQ2 alleles, with statistically significant differences [3]. Zelefsky et al. later found that HLA-DR17 was more strongly associated with MU than HLA-DQ2 (44). Additionally, Liang et al. found that HLA-DQ5 was also associated with MU (45). However, the mechanism by which HLA genotypes confer susceptibility to MU remains unclear and warrants further investigation through genetic studies involving diverse populations and larger sample sizes.
3.7 Other potential immunogens
Some reports suggest that external immunogens, such as vaccines, can potentially induce corneal diseases through immune-mediated mechanisms (46). Consequently, vaccines may also influence the occurrence or progression of MU. In a Moroccan case reported by Alliti et al., MU developed in a patient with prolonged thalidomide exposure shortly after an inactivated COVID-19 vaccination. Vision loss occurred a week after the second dose, pointing to a possible immune-mediated link (47). However, isolated case reports do not necessarily establish a definitive clinical link between vaccines and MU. The patient may have had other comorbidities or underlying health and immunological conditions that were not thoroughly documented prior to vaccination (48).
4 Pathology
While the underlying mechanisms of MU remain elusive, it is generally recognized as an autoimmune disease involving both cellular and humoral pathways (Figure 1). The cornea and conjunctiva near ulcer sites have been found to contain CO-Ag (33), HLA class II molecules, and infiltrating inflammatory cells, indicating localized immune activation (14, 44, 45). The conjunctiva and its associated vasculature also appear to contribute to corneal destruction (49).
CO-Ag, localized within the corneal stroma and sharing sequence identity with human neutrophil calgranulin C (23, 33), is regarded as a potential key autoantigen in MU. High serum levels of anti-CO-Ag autoantibodies have been reported in MU patients (24). Immune responses to CO-Ag may be triggered by corneal trauma or surgery, or through molecular mimicry with pathogens such as HCV or intestinal parasites, thereby initiating or amplifying autoimmune injury. Although the precise biological role of CO-Ag remains uncertain, it likely serves as an immunogenic stimulus that sustains chronic inflammation. Elucidating its properties may help identify disease activity early and inform targeted therapies.
Histopathologic studies have demonstrated marked infiltration of T lymphocytes, together with smaller numbers of B cells, macrophages, neutrophils, NK cells, and mast cells (20, 28). Hyperactive immune responses in the peripheral cornea and adjacent bulbar conjunctiva are mediated through adhesion molecules such as ICAM-1 and LFA-1. The colocalization of CD28/CTLA-4 with B7-1 and B7-2 beneath the epithelium suggests further immune-regulatory interactions (13). Elevated circulating immune complexes may enhance inflammatory cell recruitment and collagenase release, leading to stromal lysis and progressive ulceration (50).
Recent molecular studies have expanded understanding of MU pathogenesis. Li et al. reported upregulation of the NLRP3-CASP1-IL-1β pathway in conjunctival tissue (51). Hao et al. generated a proteome atlas showing enrichment of immune-related pathways, including lysosomal activity, antigen presentation, platelet activation, oxidative phosphorylation, and phagosomes (52). Zhang et al. found increased expression of cGAS/STING signaling components in corneal epithelial cells of MU patients (53). These findings highlight multiple innate immune cascades that may converge to drive corneal inflammation, although their precise contributions require further clarification.
5 Clinical manifestations and classification
MU typically presents with severe ocular pain, marked redness, photophobia, and excessive tearing. Typical features include intense limbal inflammation and ulceration of the corneal rim with a disrupted advancing edge containing many blood vessels [Figures 2A (a,b)] (54). In most cases, stromal involvement ranges from one-third to two-thirds of the corneal thickness, and the sclera remains unaffected. Studies have shown that 45% of corneal lesions involve half of the limbus, 21% extend to the entire limbus, and 70.1% involve the eyelid fissure (16). It may relate to eyelid fissure exposure and a relative deficiency of limbal stem cells in the affected area.
Figure 2
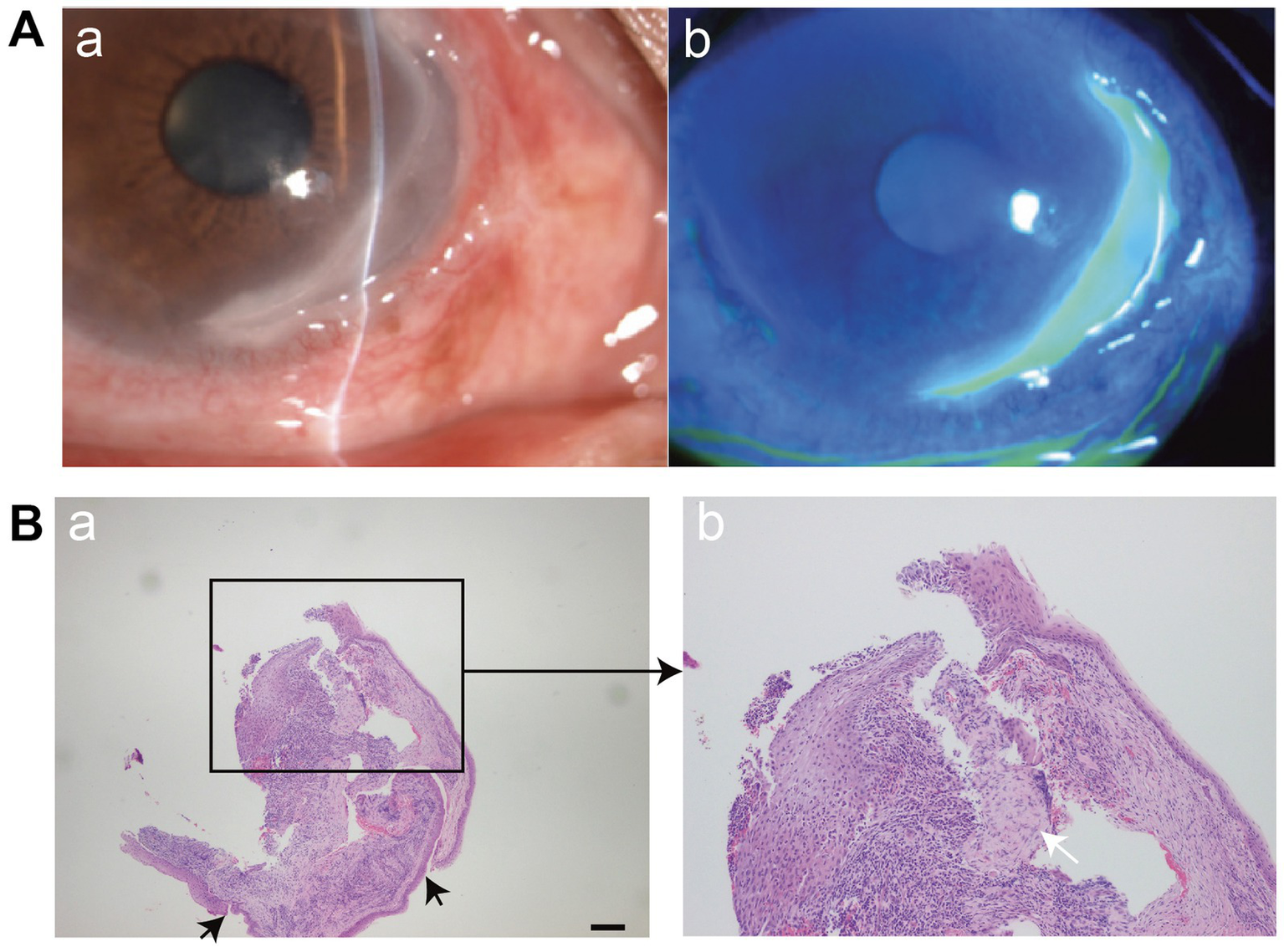
(A) (a,b) Slit-lamp microscopy demonstrates the typical morphology of MU, including steeply overhanging central and leading edges, and a thin, vascularized ulcer base. The silt lamp image was reproduced and permitted from Ou S, et al (copyright © 2024 The Authors. Published by Elsevier Ltd. CC BY-NC 4.0) (54). (B) Histopathological features of MU. (a) Typical findings include epithelial detachment and loss of the anterior elastic membrane (black arrows). Scale bar = 200 μm. (b) Dense infiltration of inflammatory cells (white arrow) and stromal degeneration are evident in the corneal tissue. The image was reproduced and permitted from Zhang et al. (56) [copyright © 2022 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group. CC BY-NC-ND 4.0].
Wood and Kaufman (18) proposed a two-subtype classification of MU based on factors such as prognosis, clinical presentation, and age at onset. Type 1, considered the benign variant, typically affects only one eye, presents with relatively mild symptoms, and occurs in individuals over 35 years old. Patients in this group generally respond well to both pharmacological and surgical interventions. Type 2, in contrast, represents the more aggressive form. It often involves both eyes, is associated with severe symptoms, and is seen predominantly in patients under the age of 35. This type is typically resistant to conventional treatment approaches.
Currently, the commonly used classification, according to clinical images Watson (12) publication, based on clinical manifestations and low-dose anterior segment fluorescein angiography, divides MU into three types (Table 1) (3):
-
(a) Unilateral Mooren’s ulceration (UM): UM is rare and typically occurs in patients over 60 years old, characterized by the rapid onset of redness and severe pain in one eye. UM is characterized by poor response to analgesics, intense inflammation, conjunctival congestion, but no scleral inflammation or necrosis. Anterior segment fluorescein angiography found occluded conjunctival vessels and local episcleral venular, along with structural disruption and vascular leakage at the limbus and ulcer base.
-
(b) Bilateral aggressive Mooren’s ulceration (BAM): Commonly observed in the Indian subcontinent and East Africa, BAM usually affects patients from 14 to 40 years of age and presents with bilateral disease accompanied by mild eye pain. When one eye is diagnosed, the other eye may not have obvious symptoms, even though the conjunctiva is congested. If left untreated, the unaffected eye will develop a gray patch within the corneal stroma, followed by a gray patch forming along the limbus edge approximately 2 mm from the edge. Over a few days, these gray spots coalesce and tissue loss occurs, resulting in the typical appearance. Unlike UM, ulcers in BAM can perforate spontaneously. Angiography reveals closure of episcleral vessels in some areas.
-
(c) Bilateral indolent Mooren’s ulceration (BIM): It is more common in middle-aged patients with discomfort in both eyes. Ulcers in both eyes typically occur almost simultaneously or within a few days of each other, although usually, one eye exhibits a more severe reaction. Many ulcers will heal spontaneously. Angiography shows no detectable changes in episcleral or conjunctival vessels, but there is an abnormality in the limbal circulation, with new blood vessels extending to the base of the ulcer.
Table 1
| Features | BIM | BAM | UM |
|---|---|---|---|
| Affected Sides | Involves both sides | Typically affects both sides | Generally one-sided |
| Gender | No strong gender association | More frequent in males than females | More frequent in females than males |
| Age | Primarily middle-aged to elderly | Common in younger individuals | Mostly found in older adults |
| Progression | Slow | Slow, aggressive at presentation | Rapid |
| Pain Severity | Mild discomfort | Moderate to severe pain | Severe pain |
| Recurrence Risk | Rare | Common | Common |
| Complications | Rare | Perforation | Rare |
Watson’s classification of MU types.
6 Diagnosis
MU is primarily diagnosed through a process of exclusion (Table 2 introduces normal ocular examination, imaging examination, and laboratory test for MU in previous publications; Table 3 introduces potential autoimmune markers for excluding other diseases) (9, 10, 35, 38, 41–43, 49, 55–65). Any disease capable of causing PUK must first be excluded (α1-antitrypsin deficiency, Beçhet’s disease, malignancy, infections (AIDS, bacillary dysentery, borreliosis, gonorrhea, hepatitis C, herpes zoster, syphilis, tuberculosis), inflammatory bowel disease, polyarteritis nodosa, progressive systemic sclerosis, systemic lupus erythematosus, sjögren syndrome, scleroderma, sarcoidosis, rheumatoid arthritis, relapsing polychondritis, and Wegener granulomatosis) (4, 35, 50), and the diagnosis of MU relies on a detailed medical history and typical ulcer morphology.
Table 2
| Type of examination | Specific test/Procedure | Purpose and potential findings |
|---|---|---|
| Ocular Examination | Slit-lamp biomicroscopy | To identify peripheral corneal ulceration, epithelial defects, and characteristic overhanging edges of Mooren’s ulcer. Helps rule out infectious keratitis or trauma-related ulcers. |
| AS-OCT (Anterior segment optical coherence tomography) | Provides detailed corneal structure visualization, including stromal thinning and depth of ulceration. Supports diagnosis and tracks healing. Helps rule out deeper infectious or structural pathologies. | |
| Fundus examination | To assess posterior segment and exclude other intraocular pathologies. | |
| Imaging | Chest X-ray | To rule out systemic diseases like TB or sarcoidosis. |
| Ultrasound B-scan | Used when media opacity precludes fundus exam, especially in severe MU. | |
| Sinus and KUB radiographs | Performed to rule out other systemic inflammatory causes. | |
| Laboratory test | Complete blood count (CBC) | Assesses systemic inflammation, anemia, infection. Helps rule out systemic causes of ulceration. |
| ESR and CRP | Markers of systemic inflammation; elevated in some MU patients. Help differentiate from systemic autoimmune diseases. | |
| Hepatitis B and C serology | Investigates possible viral associations with MU. Some cases had positive Hep C. | |
| HIV serology | To assess for immunosuppression as a possible contributing factor. | |
| Urinalysis and stool exam | To assess for systemic infection or parasitic infestation. | |
| Liver and renal function tests | To evaluate for systemic health and exclude metabolic contributors. | |
| Serum ACE and QuantiFERON test | To rule out sarcoidosis and tuberculosis. | |
| Corneal scraping and culture | To exclude infectious keratitis. All cases with this test returned negative microbiology. | |
| Corneal or conjunctival biopsy | Histological confirmation of MU features. Helps rule out neoplastic or infectious causes. | |
| Autoimmune markers | To exclude autoimmune diseases which can mimic or contribute to PUK. Negative in all MU cases confirming idiopathic etiology. |
MU diagnostic workup: examination types, procedures, and clinical rationale.
Table 3
| Autoimmune marker | Disease(s) ruled out |
|---|---|
| ANA (Antinuclear antibodies) | Systemic sclerosis, Sjögren’s syndrome, systemic lupus erythematosus |
| ANCA (Anti-neutrophil cytoplasmic antibodies) | Granulomatosis with polyangiitis, microscopic polyangiitis, IBD-related vasculitis |
| RF (Rheumatoid factor) | Rheumatoid arthritis |
| Anti-CCP (Anti-cyclic citrullinated peptide) | Rheumatoid arthritis |
| Anti-dsDNA (Double-stranded DNA Antibody) | Systemic lupus erythematosus |
| SSA/Ro (Anti-Ro antibodies) | Systemic lupus erythematosus, Sjögren’s syndrome |
| SSB/La (Anti-La antibodies) | Sjögren’s syndrome |
| Cardiolipin antibody | Antiphospholipid syndrome, systemic lupus erythematosus |
| Complement (C3, C4) | SLE, Immune complex-mediated diseases, Infection-related immune activation |
| MPO (Myeloperoxidase antibody) | Microscopic polyangiitis |
| PR3 (Proteinase 3 antibody) | Granulomatosis with polyangiitis |
| Lupus anticoagulant | Antiphospholipid syndrome |
| RNP (Anti-ribonucleoprotein antibody) | Mixed connective tissue disease |
Autoimmune serology panel: disease exclusion in differential diagnosis of MU.
Infectious corneal disease can be ruled out by scraping and culture of corneal and conjunctival secretions. Comprehensive laboratory tests, including antistreptococcal antibodies, rheumatoid factor, circulating immune complexes, antinuclear antibodies erythrocyte sedimentation rate, and complete blood count, can be conducted to exclude other causes of PUK (66, 67). In recent years, histopathological examination has been increasingly utilized to aid in the diagnosis of MU through typical histopathological manifestations.
7 Differential diagnosis
MU is an exclusionary disease, requiring the exclusion of other diseases causing peripheral corneal ulcers through detailed clinical presentation and laboratory tests (Tables 2, 4).
Table 4
| Diseases | Pain | Visual loss | Epithelial defect | Location | Disease progression | Association |
|---|---|---|---|---|---|---|
| MU | + | + | + | Starts in the peripheral region, with progression both circumferentially and centrally; typically bilateral in the malignant form and unilateral in the limited form. | Progressive | Inflamed conjunctiva |
| CVD-associated PUK | + | + | + | Anywhere of the peripheral cornea, bilateral | Progressive | Constitutional symptoms and other systemic manifestations |
| TMD | − | ± | − | Mostly affect superior cornea, and rarely involving the inferior, unilateral or bilateral | Slow | none |
| Fuchs’ superficial marginal keratitis | + | + | − | Irregular | Mild | Remissions and relapse |
| Marginal keratitis | + | + | + | Anywhere of the cornea, unilateral | Benign, microbial keratitis is rapid progress | Hypersensitivity caused by blepharoconjunctivitis associated organisms |
| PMD | − | ± | − | Mostly affect Inferior cornea, bilateral, asymmetric | Mild | Corneal ectatic diseases |
| Senile marginal furrow degeneration | − | − | − | Circumferential | Mild | None |
| Arcus senilis | − | − | − | Circumferential, bilateral and symmetric | Mild | Hypercholesterolemia |
Differential diagnosis of MU.
*“+” means yes, “-” means no, “±” means not sure. CVD-associated PUK: collagen vascular diseases-associated peripheral ulcerative keratitis, MU, Mooren’s ulcer; PMD, pellucid marginal degeneration; TMD, terrien’s marginal degeneration.
7.1 Collagen vascular disease-associated PUK
Collagen vascular diseases (CVDs), including systemic lupus erythematosus, relapsing polychondritis, polyarteritis nodosa, Wegener’s granulomatosis, and rheumatoid arthritis, need to be considered. In CVD-associated PUK, inflammation typically extends to adjacent conjunctiva, episclera, and sclera, whereas MU generally lacks scleral involvement. Unlike MU, CVD-associated PUK is often a manifestation of systemic disease. CVD-associated PUK represents an ocular manifestation of an underlying systemic condition (68). In contrast, MU occurs in the absence of any diagnosable systemic disorder (4).
7.2 Terrien’s marginal degeneration
Terrien’s marginal degeneration (TMD) typically presents as bilateral thinning of the superior peripheral cornea (69, 70), progressing more slowly compared to MU. TMD presents with superficial vascularization and a distinct opaque line of fine cholesterol crystals separating it from the central cornea (71, 72). Acute painful inflammation is rare in TMD cases (73).
7.3 Fuchs’ superficial marginal keratitis
Fuchs’ superficial marginal keratitis manifests with recurrent episodes of ocular irritation and progressive thinning of the marginal superficial stroma, occasionally accompanied by pseudopterygium (74, 75). The progressing ulcer is bordered by a gray demarcation line separating it from the central cornea (72).
7.4 Marginal keratitis
Marginal keratitis is an inflammatory condition caused by various organisms, including viruses, bacteria, fungi, parasites, and chlamydiae. Bacterial corneas with peripheral involvement often display purulent infiltrates. Although corneal cultures may be negative, pathogenic organisms can sometimes be isolated from the ipsilateral lid margin or conjunctiva (76). Unlike MU, which involves a sterile inflammatory response, marginal keratitis is infectious in nature. For instance, staphylococcal marginal keratitis typically manifests as a peripheral corneal infiltrate accompanied by epithelial disruption. A distinct separation between the infiltrate and the limbus is usually observed, and the condition is commonly associated with underlying blepharitis. While patients may report symptoms such as photophobia and ocular discomfort, the intense and incapacitating pain characteristic of Mooren’s ulcer is generally absent.
7.5 Degenerative corneal diseases
This category includes arcus senilis, senile marginal furrow degeneration, and pellucid marginal degeneration (PMD). PMD predominantly involves the inferior peripheral cornea, presenting as crescent-shaped thinning while maintaining corneal transparency (77). Arcus senilis and senile marginal furrow degeneration are physiological changes that, unlike MU, are usually not visually significant (76).
8 Application of new and investigational techniques in MU
8.1 Three-dimensional anterior segment optical coherence tomography
Conventional corneal topography methods face challenges in analyzing corneal shape due to ulcer infiltration and corneal clouding, particularly in severe cases (78, 79). Three-dimensional anterior segment optical coherence tomography (3D AS-OCT) offers enhanced imaging depth and higher-definition cross-sectional images of the cornea (80, 81). Masahito Yoshihara et al. used 3D AS-OCT to utilize 3D AS-OCT to analyze corneal topography and visual function in MU cases, dividing the patients’ axial power maps into arcuate, crab-claw and intermediate maps. They observed decreased best-corrected visual acuity (BCVA) and increased regular and irregular astigmatism when the lesion was near the corneal center (82). Future applications of 3D AS-OCT hold promise in quantitatively assessing the impact of corneal thinning on optical quality and visual function in MU. However, it is important to note that 3D AS-OCT cannot always provide a definitive differential diagnosis of MU. For instance, both TMD and MU may present with similar imaging features, such as a central flattened zone surrounded by steeper regions and a crab-claw pattern on the corneal axial power map (82, 83).
8.2 In vivo confocal microscopy
In vivo confocal microscopy (IVCM) is a non-invasive diagnostic tool valuable for evaluating various ocular surface diseases (84, 85). Recent research efforts have explored the utility of IVCM in MU patients (86, 87). Shin Hatou et al. (87) have shown that patients with active ulcers exhibit higher inflammatory cell density (ICD) compared to those in remission. Furthermore, ICD tends to decrease over time with immunosuppressive therapy. Additionally, numerous dark cysts containing polymorphs were observed in patients with active ulcers, but not in those in remission (87). The observation of a large number of fluid-filled cysts in the IVCM is likely to suggest imminent perforation. Thus, IVCM can aid in assessing inflammation severity, treatment response, and predicting corneal perforation risk in MU patients.
8.3 Histopathological examination
Several studies have conducted histopathological examinations of corneal tissues from MU patients. Hematoxylin–eosin (HE) staining revealed epithelial hyperplasia in adjacent conjunctiva and inflammatory infiltration in stroma (20, 56) (Figure 2B). Immunohistochemical (IHC) staining demonstrated strong expression of inflammatory markers such as CD34, c-kit, STRO-1 cells (88), ICAM-1, LFA-1, NLRP3 (51) in MU specimens. Li et al. founded high levels of GPR91 protein were observed in conjunctival and/or corneal tissues of MU patients (89). The histopathological findings from the conjunctiva adjacent to ulcer, limbus and ulcerated area of the cornea in Table 5 (90). So the histopathological examinations revealing an increase in local inflammatory vesicles or immunohistochemical staining demonstrating high expression of inflammatory factors can be instrumental in confirming the diagnosis of MU when clinical diagnosis is inconclusive.
Table 5
| Section | Performance | |
|---|---|---|
| Adjacent conjunctiva | Epithelium and basement membrane | Normal |
| Stroma | Hyperemia and edema | |
| Inflammatory infiltrations | Less numerous eosinophils, neutrophils and mast cells; Mainly by plasma cells and lymphocytes |
|
| Corneal stroma at limbus | Superficial zone | Vascularized with perivascular infiltration (mainly by lymphocytes and plasma cells of various densities) |
| Central zone | Fibroblastic activity | |
| Deepest zone | Macrophages infiltration | |
| Corneal ulcer | Ulcer base | Necrobiotic material and inflammatory cell infiltration |
| Central edge of the ulcer | Thickened stroma, absence of inflammatory infiltration | |
| Peripheral edge of the ulcer | Dense inflammatory infiltration including plasma cells, lymphocytes, neutrophils, histiocytes and mast cells | |
Histopathological findings in MU.
8.4 Ocular surface microflora testing
The ocular surface microbiota establishes a unique microecology on the ocular surface, closely intertwined with human immune defense mechanisms and the development of ocular diseases (91, 92). The innate immune activity of the ocular surface epithelium, as highlighted by Ueta et al. (93), contributes to establishing symbiotic interactions with commensal bacteria. In recent years, advancements in 16S rDNA sequencing technology have facilitated the detection of ocular surface microbiota (94). Presently, 16S rDNA sequencing can distinguish bacterial flora variations in patients with conditions like infectious keratitis, dry eye, and conjunctivitis. However, research on the ocular surface bacterial flora in immune-related keratitis, including MU, remains scarce. Thus, investigating the ocular surface microecology in MU ulcer patients holds promise for deeper insights into the disease.
9 Treatment
Some researchers advocate for a “stepladder approach (55, 95)” in treating MU (Figure 3), tailoring interventions based on the severity of the disorder. This approach typically involves a combination of medication [both topical and systemic, Table 6 (95–101)] and surgical therapy. Among them, due to the irreversible destruction of the corneal anatomy by surgical therapy, surgical therapy is often carried out when the medication can not control the MU symptoms.
Figure 3
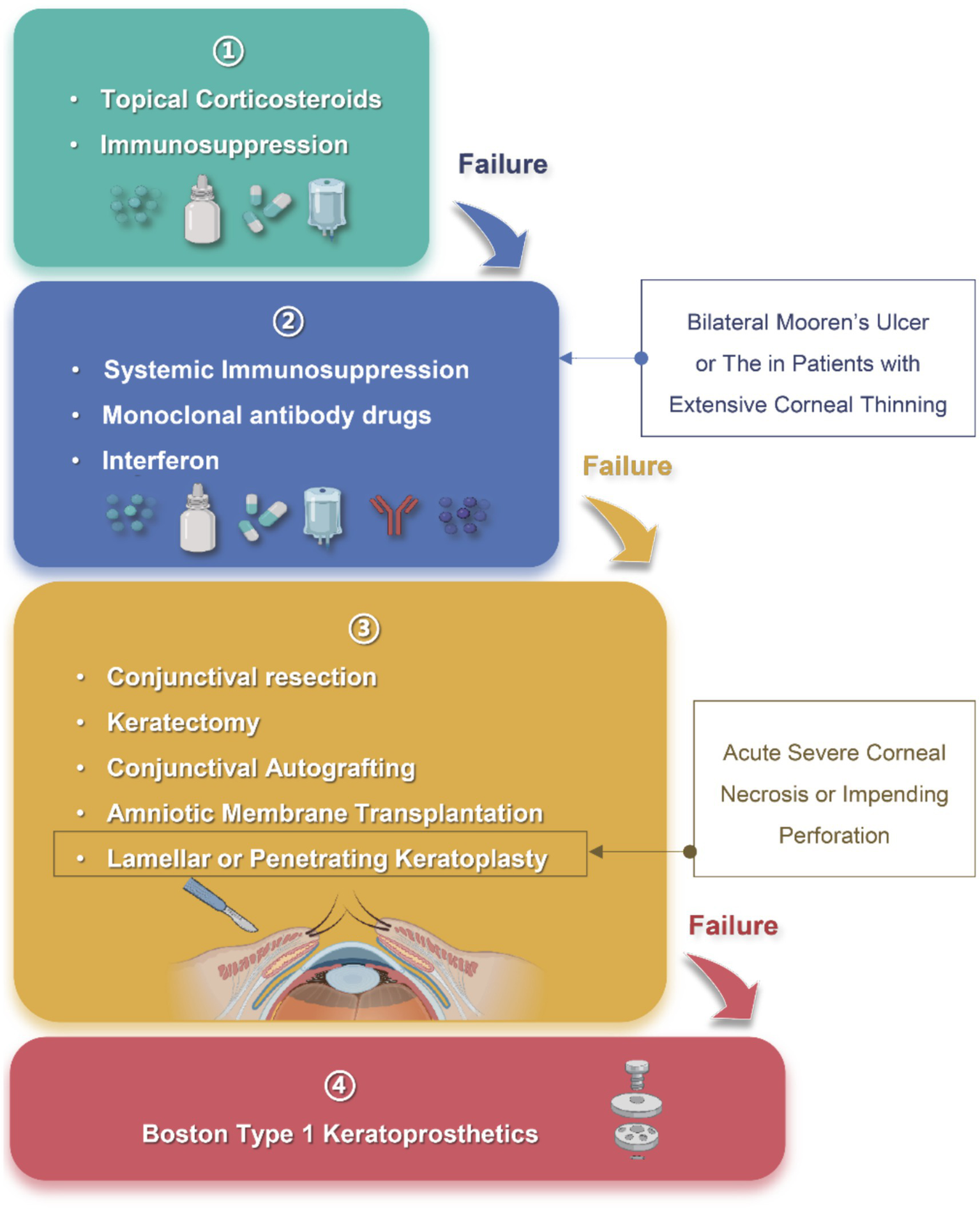
“The stepladder approach” in the treatment of Mooren’s ulcer. Step 1: Topical corticosteroids and immunosuppressants are used as first-line therapy for mild, unilateral disease; Step 2: Systemic immunosuppression, monoclonal antibodies and interferon are indicated for bilateral or refractory cases with progressive corneal thinning; Step 3: Surgical procedures, including conjunctival resection, keratectomy, conjunctival autografting, amniotic membrane transplantation, or lamellar/penetrating keratoplasty, are considered when medical therapy fails or when acute corneal necrosis or impending perforation develops; Step 4: Boston type 1 keratoprosthesis serves as a last-resort option after repeated graft failure or end-stage corneal destruction. Arrows indicate escalation to the next level of intervention when disease control is inadequate. Created in BioRender. Wu, Y. (2025) https://BioRender.com/x9geu1w.
Table 6
| Clinical features | Treatment modality | Dosage and administration | Follow-up protocol |
|---|---|---|---|
| Unilateral involvement, <2 quadrants of peripheral corneal damage, <50% stromal thinning | Topical corticosteroids | Dexamethasone eye drops (1.5 mg/mL), 6 times daily | Every 3 days during acute phase until healing; every 3 months for 6 months; thereafter as needed |
| Bilateral involvement, >2 quadrants of peripheral corneal damage, >50% stromal thinning | Systemic corticosteroids | Oral prednisolone 1–1.5 mg/kg/day | Alternate-day monitoring in acute phase; monthly for 6 months; then every 3 months |
| Steroid-intolerant patients; age <40 years; monocular patients; or bilateral cases with side effects from systemic corticosteroids | Immunosuppressants | Oral methotrexate 7.5–12.5 mg weekly | Daily in acute phase until healing; monthly for 6 months; then every 3 months |
| Bilateral cases with >3 quadrants affected, >50% stromal loss, impending perforation | High-dose systemic corticosteroids | Intravenous methylprednisolone; dose titrated based on clinical response | Inpatient care with internist collaboration; daily monitoring during acute phase; follow-up every 1–2 weeks for 3 months; then monthly for 3 months; every 3 months thereafter |
| Perforation or post-keratoplasty phase in severe bilateral cases | Combination immunosuppressive therapy | Intravenous methylprednisolone + Intravenous cyclophosphamide; dosage individualized per disease severity | Inpatient monitoring with multidisciplinary care; daily during acute phase; every 1–2 weeks for 3 months; monthly for 3 months; every 3 months thereafter |
| Refractory cases unresponsive to conventional immunosuppressants | Infliximab | Intravenous infusion at weeks 0, 2, and 6; monthly thereafter | Adhere to infusion schedule; hospital visits required for each dose and follow-up |
| Refractory cases unresponsive to conventional immunosuppressants | Adalimumab | Subcutaneous: 80 mg on days 0, 1, and 7; then 40 mg biweekly | Regular follow-up aligned with injection schedule |
| Refractory cases unresponsive to conventional immunosuppressants | Rituximab | Two Intravenous infusions of 1,000 mg each, 2 weeks apart | Follow infusion timeline; regular follow-up |
| Cases associated with systemic disease (e.g., Hepatitis C) | Interferon alpha-2b and ribavirin | Interferon alpha-2b 100 μg weekly; ribavirin 1,000 mg daily in divided doses (400 mg + 600 mg) | Monitor disease progression and liver function; regular follow-up; consult gastroenterologist if necessary |
Classic treatment strategies and follow-up protocols for different clinical presentations of MU.
9.1 Medical treatment
Medical therapy follows a stepwise escalation: corticosteroids as first-line, conventional immunosuppressants as second-line, and biologics or monoclonal antibodies reserved for refractory disease.
9.1.1 Corticosteroids
Topical corticosteroids (95), such as dexamethasone (55), betamethasone (38) and prednisolone (43), are commonly considered the initial treatment for MU, as same as other PUK (102). However, despite high-dose steroid therapy, there have been reported cases of progressive corneal melting (corneal lysis) in some patients [60].
9.1.2 Immunosuppression
Immunosuppressive agents, such as cyclosporin A [CsA, topical 1% cyclosporin A eye drops, 2–4 times daily for 6–12 months (16) or systemic cyclosporin 10 mg/kg/day if necessary (103)], methotrexate (Table 6), cyclophosphamide (Table 6), tacrolimus [FK506, topically 0.02% tacrolimus ointment 1 to 3 times daily (104), azathioprine orally 2 mg/kg/day (103)] and mycophenolate mofetil [500 mg orally twice daily (105)], have demonstrated efficacy in treating MU in most studies and also considered another routine treatment for MU (9, 95, 103, 105–109). The choice between topical, oral, or intravenous immunosuppressive therapy should be tailored to the severity of the disease. However, systemic immunosuppressive therapy is related to the risk of secondary infections (109), underscoring its importance of rheumatologic or internal medicine guidance when administering such treatment.
All systemic immunosuppressive regimens require close internal-medicine supervision and regular hematologic and hepatic monitoring as summarized in Table 6 (101).
-
(a) Methotrexate is associated with gastrointestinal symptoms, cytopenia, and elevated liver enzymes. Warning signs such as fever, bruising, pallor, mouth ulcers, or respiratory complaints should prompt clinical attention, and patients should undergo biweekly liver function tests and blood counts during the first month, then monthly for six months, and subsequently every 2–3 months if stable.
-
(b) Cyclophosphamide may cause opportunistic infections, pneumonia, cystitis, mucositis, and cytopenia; thus, urinalysis, liver function tests, and complete blood counts should be conducted 10 days after the last dose and 2 days prior to the next dose.
-
(c) Cyclosporine and tacrolimus, both T-cell inhibitors, share common complications such as hypertension, cytopenia, and nephrotoxicity. Clinical signs like peripheral edema, mouth ulcers, rash, or elevated blood pressure warrant further evaluation. Monitoring includes blood counts every 2 days for the initial 6 weeks, then monthly, along with regular liver function tests and lipid profiles every 6 months.
-
(d) Azathioprine can lead to cytopenia, hepatotoxicity, and gastrointestinal disturbances; monthly liver function tests and blood counts are recommended for the initial 6–12 months, with reduced frequency to every 6–8 weeks upon stabilization.
-
(e) Mycophenolate mofetil may cause gastrointestinal symptoms, cytopenia, liver dysfunction, respiratory issues, or hematuria. Monitoring involves weekly blood counts during the first month and monthly assessments thereafter, including C-reactive protein, erythrocyte sedimentation rate, and liver function tests.
9.1.3 Monoclonal antibody drugs
-
(a) Infliximab: Infliximab, a chimeric monoclonal antibody used for certain autoimmune diseases, is designed to bind TNF-α and prevent it from interacting with its target (110, 111). Two earlier reports from different European countries have simultaneously documented successful use of Infliximab in treating MU. Both studies suggest that Infliximab could be a significant option for preserving corneal when conventional immunosuppressive therapies fail (97, 98). Additionally, this therapy was recently applied in the treatment of two female MU patients, further substantiating its efficacy (96). Notably, all three reports emphasize that Infliximab was considered only after conventional immunosuppressants had proven ineffective. Infliximab may lead to congestive heart failure, multiple sclerosis, cutaneous vasculitis, injection site reactions, lymphoma, and opportunistic infections (101). Warning signs of these complications include pallor, bruising, chills, fever, and mouth ulcers. To ensure early detection, liver function tests and blood counts should be performed prior to every infusion, with annual dsDNA testing recommended.
-
(b) Adalimumab: The treatment mechanism of Adalimumab closely mirrors that of Infliximab, targeting TNF-α to inhibit its activity (110). Miguel et al. adopted this therapeutic approach for MU patients after conventional immunosuppressive treatments had failed. Their findings indicated that Adalimumab significantly alleviated the eye symptoms of MU patients and did not exhibit notable side effects during a 10-month period of long-term treatment (99). This suggests that Adalimumab, like Infliximab, could be a viable alternative for MU patients who do not respond to traditional immunosuppressive therapies. Adalimumab is associated with risks such as cytopenias, worsening or new onset of congestive heart failure, drug-induced lupus, lymphoma, opportunistic infections, and neurological disorders including multiple sclerosis (101). Alarming symptoms include pallor, bruising, chills, fever, and mouth ulcers. Monitoring protocols include monthly liver function tests and blood counts for the first three months, followed by testing every three months, with yearly dsDNA testing.
-
(c) Rituximab: Rituximab, as a chimeric monoclonal antibody against CD20 for treating certain autoimmune diseases and types of cancer, has emerged as a potential therapeutic option for MU (110). Guindolet et al. reported its effectiveness in managing severe cases of MU (100). Supriya et al. (112) emphasized its importance in controlling inflammation before surgery in MU patients. Rituximab can cause infusion reactions, opportunistic infections, cytopenia, and cardiovascular and dermatological complications (101). Pruritus, abdominal pain, chills, fever, dyspnea, pallor, and fatigue may signal the onset of adverse events. Monitoring includes vital sign assessment at each ophthalmic visit and regular blood counts.
9.1.4 Interferon
Studies have shown that the combination of interferon alpha-2b and ribavirin is effective in treating MU in patients with hepatitis C virus infection (35, 36). The effectiveness of interferon in this context may stem from its immunomodulatory properties or from potential associations between MU and hepatitis C.
9.2 Surgical treatment
Surgical intervention is usually considered in the presence of severe corneal necrosis or the threat of perforation. It is advisable to proceed with surgery once inflammation is under control (113).
9.2.1 Conjunctival resection
In cases where ulceration is attributed to autoimmunity, with the cornea serving as an antigen and the limbal conjunctiva containing antibodies and enzymes that contribute to corneal destruction, conjunctival resection may be considered. By limiting stromal antigen access to systemic circulation, conjunctival resection helps suppress immune infiltration and promotes ulcer repair. Consequently, perilimbal conjunctival excision has been proposed as a strategy to control inflammation. Brown et al. (114) performed conjunctival resection on 10 eyes diagnosed with MU, with only one eye experiencing ulcer recurrence post-surgery. However, Ikeda Lal et al. (115) argue that conjunctival resection fails to effectively halt disease progression or prevent recurrence. Controversy surrounds the efficacy of conjunctival resection due to limited case numbers and short follow-up periods. Larger studies are warranted to validate these clinical observations.
9.2.2 Keratectomy
According to Brown et al. (114), keratectomy may be considered when medical treatments fail to resolve local corneal ulcers. This surgical intervention aims to remove the affected corneal tissue, thereby controlling corneal inflammation and preventing further development of corneal perforation.
9.2.3 Amniotic membrane transplantation
Amniotic membrane (AM) transplantation facilitates epithelial cell adhesion and migration, induces epithelial differentiation, and suppresses interleukin secretion, thereby reducing corneal inflammation (116–118). Several studies have confirmed AM transplantation is effective in managing MU and other corneal ulcers unresponsive to medical therapy (117–119). Additionally, AM transplantation can be combined with other MU treatments. Lavaju P et al. (63) suggested that combining AM transplantation with autologous serum eye drops is an effective approach for MU. For MU patients at risk of or already experiencing corneal perforation, combining corneal transplantation with AM transplantation is feasible (120, 121). However, the effectiveness of AM transplantation for treating MU remains a topic of debate. Schallenberg et al. (122) concluded that while AM transplantation may not cure severe cases of MU, it can be beneficial in acute situations, such as critical corneal thinning, alongside immunosuppressive therapy.
9.2.4 Lamellar keratoplasty
In addition to systemic immunosuppression, MU with severe corneal thinning or perforations often necessitates keratoplasty to remove the inflamed cornea and reconstruct its structure. However, penetrating keratoplasty (PK) frequently encounters challenges. Marta Jerez-Peña reported a case with persistent epithelial defects in the graft post-PK (113). Consequently, various forms of lamellar keratoplasty (LK) techniques have been proposed based on the extent and severity of corneal lesions:
-
(a) Semilunar/crescentic/biconvex LK: When the ulcer affects less than 6 clock hours of the limbus and does not involve the central cornea (Figures 4a,b), semilunar LK is the preferred technique. If the ulcer involves less than 3 clock hours but extends into the central cornea (Figure 4c), biconvex LK is selected to ensure adequate excision. When the ulcer spans 3 to 6 clock hours and the chord between both ends encompasses a substantial area of healthy cornea (Figure 4d), crescentic LK is chosen to preserve the uninvolved tissue.
-
(b) Annular LK: If the ulcer extends beyond 6 clock hours of the limbus without central corneal involvement (Figure 4e), annular LK is performed to remove the peripheral lesion while preserving the central cornea.
-
(c) Total LK: When the ulcer involves more than 6 clock hours and includes the central cornea (Figure 4f), total LK is required to excise both peripheral and central lesions. In such cases, large-diameter grafts (9–9.5 mm) are used, which carry a higher risk of immune rejection than standard-sized grafts. Therefore, enhanced local and systemic immunosuppressive therapy is necessary.
-
(d) Double-layer peripheral keratoplasty: Continued progression of MU can lead to corneal perforation and peripheral staphyloma formation. AM are too thin to repair corneal perforation and PK carries a higher risk of immune rejection. Therefore, double-layer keratoplasty has been proposed. The perforation is first repaired with a thin layer of lamellar graft incorporating the Descemet membrane, followed by an additional lamellar graft layer, the shape of which is determined by the ulcer’s configuration (Figure 4g). Shi et al. (123) performed double-layer peripheral keratoplasty in four patients with peripheral staphyloma (one of them with MU), all achieved favorable outcomes. It seems to be an ideal surgical intervention for MU with perforation or peripheral staphyloma.
Figure 4
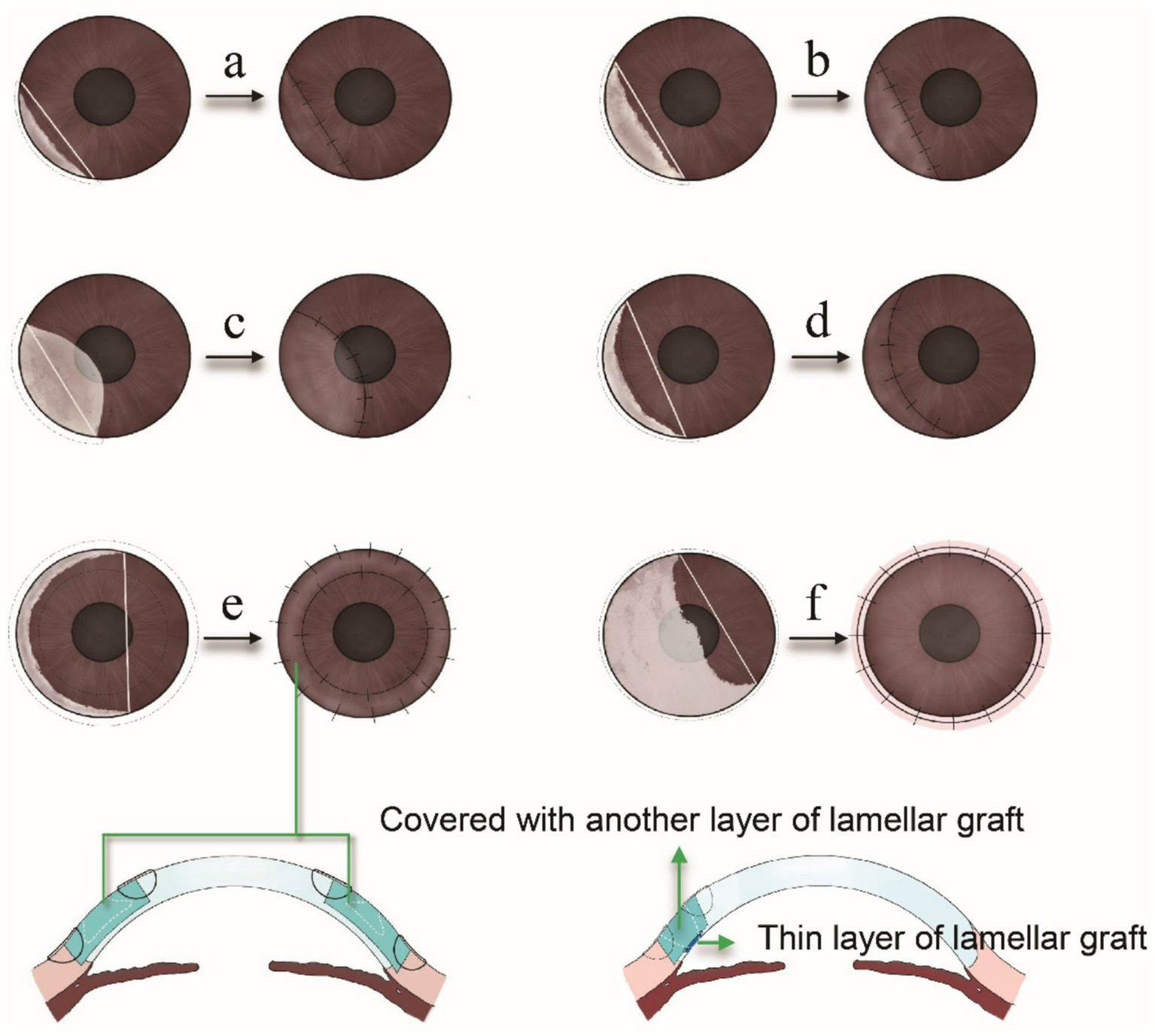
(a) When the ulcer involves less than 3 clock hours of the limbus and the chord connecting the lesion margins fully contains the corneal defect, a semilunar lamellar keratoplasty (LK) is performed. (b) For ulcers spanning 3 to 6 clock hours of the limbus, with the chord still encompassing the entire lesion, semilunar LK remains the preferred approach. (c) If the ulcer is less than 3 clock hours but involves part of the central cornea, biconvex LK is selected to ensure adequate excision. (d) When the ulcer spans 3 to 6 clock hours and the chord includes a significant portion of healthy cornea, crescentic LK is used to preserve uninvolved tissue within the chord. (e) For ulcers involving more than 6 clock hours without central corneal involvement, annular LK is employed to excise the peripheral lesion while preserving the central cornea. (f) If the ulcer exceeds 6 clock hours and extends into the central cornea, total LK is chosen to remove both peripheral and central lesions. (g) The corneal perforation or peripheral staphyloma formation, double-layer keratoplasty is chosen to repair the perforation and reconstruct the structure.
Due to the complexity of MU, some recent reports have proposed non-traditional procedures that build upon PK or LK. Supriya et al. (112) combined PK with glaucoma valve surgery to achieve long-term visual recovery in MU patients. It should be noted, however, that such “customized surgical procedures (112)” are not standard and may even be a dangerous approach for other patients. Ashok et al. customized a new deep anterior lamellar keratoplasty for MU patients. They used trephines of two different diameters to achieve optimal donor sizing and avoid manual dissection (124). Notably, based on clinical experience, manual cutting of donor tissue is often challenging. Therefore, it is advisable to convert the shape of the ulcer into a geometric form, allowing for the use of two corneal trephines to harvest donor tissue of the desired shape and size. Additionally, a lamellar patch graft may be considered in cases involving both corneal melt and large corneal perforation.
In addition, Chen et al. (16) conducted a study involving 550 cases (715 eyes) of MU, where they combined LK with 1% cyclosporine A eye drops. They assert that LK represents an effective therapeutic approach for MU. However, some studies have reported higher recurrence rates associated with LK (125). It’s important to note that cases treated with LK are typically severe, and the observed increase in recurrence risk may not solely be attributed to the LK procedure.
9.2.5 Boston type 1 keratoprosthesis
Boston type 1 keratoprosthesis (BKPro) represents a viable option for MU patients who have experienced failure with multiple corneal transplants (113). However, postoperative complications such as recurrence of MU, corneal necrosis, and uncontrolled high intraocular pressure might impact long-term treatment outcomes, necessitating prompt and intensive local and systemic interventions (126). While Sayan Basu et al. (127) reported promising short-term outcomes with BKPro in treating end-stage MU, the long-term efficacy of this approach requires further evaluation.
9.3 Other treatments
Autologous serum, rich in nutrients and fibronectin, accelerates epithelial regeneration and repair. It contains α1 and α2 macroglobulins and the metalloproteinase inhibitor TIMP-1, which help prevent corneal ulcers and perforations. Consequently, many investigators have utilized autologous serum in MU patients to expedite corneal epithelial healing and prevent perforation (63, 128, 129). Bandage contact lenses are also recommended to alleviate patient discomfort, promote epithelial healing, and maintain corneal structural stability (115, 130). Immediate post-surgical use of bandage contact lenses can prevent damage to the Descemet’s membrane after conjunctival resection (131). Therefore, autologous serum eye drops and bandage contact lenses can be employed as a combined treatment approach for MU patients (Figure 5).
Figure 5
The treatment for Mooren’s ulcer. Created in BioRender. Wu, Y. (2025) https://BioRender.com/naj09b0.
In addition, cyanoacrylate glue can be effectively used to repair corneal damage or perforations caused by MU. When MU leads to small, localized corneal ulcers, cyanoacrylate adhesive can be used for sealing, with a bandage contact lens subsequently applied to maintain corneal integrity (132). Notably, cyanoacrylate adhesive alone has been proven effective in managing corneal perforations ≤2.0 mm, providing prompt tectonic stabilization and preventing aqueous leakage (95). However, in MU patients with larger perforations (>2.0 mm), there is a significant risk that the adhesive may enter the anterior chamber and trigger severe intraocular inflammation. To mitigate this risk, a scleral patch graft can first be placed over the site of perforation (95), acting as a mechanical barrier to prevent intraocular extension of the glue. The scleral patch also serves as a biological scaffold for keratocytes, facilitating stromal regeneration and defect closure. In such cases, cyanoacrylate adhesive is subsequently applied over the patch to secure the repair (133). While these approaches have been shown to be effective in both the early and late stages of MU, they do not prevent the recurrence or ongoing progression of the disease (115).
10 Efficacy evaluation
The treatment of MU poses a considerable challenge, with systemic immunomodulatory therapy being pivotal for achieving successful functional and visual outcomes. Typically, changes in pain levels, photophobia, and inflammatory signs such as conjunctival hyperemia and edema are employed to assess alterations in the inflammatory response and treatment efficacy in MU patients (131). Shin Hatou et al. utilized IVCM to quantify ICD as a means of evaluating treatment response in MU patients (87). They observed that the mean ICD in patients with active MU was significantly higher than in remission, with effective treatment resulting in a gradual decrease in ICD over time. Limbal cysts detected during IVCM examination may serve as an indicator of impending perforation, necessitating close patient monitoring.
Recently, tear-based analysis has emerged as a promising approach in clinical and experimental practice (134). Given the localized immune hyperactivity implicated in MU pathogenesis, researchers have assessed corneal inflammatory status by analyzing levels of pro-inflammatory factors in MU patients’ tears (135, 136). This avenue holds potential as a novel method for evaluating treatment efficacy in MU patients. Future large-scale prospective studies are necessary to validate treatment outcomes, with careful consideration of disease severity, determined by age at onset, laterality, the extent (in clock hours) of corneal involvement, and stromal depth (4).
11 Prognosis
The progression of MU is closely intertwined with the healing process, involving neovascularization and epithelial regeneration. The process is thought to result from the coordinated proliferation and migration of endothelial progenitor cells and multipotent mesenchymal cells originating from the bone marrow (76, 88). Effective control of inflammation typically leads to ulcer healing, corneal vascularization, and subsequent corneal scar formation, resulting in reduced vision quality (137). However, if inflammation persists, it can culminate in corneal perforation.
The association between age and MU perforation remains a subject of debate. While Watson proposed a higher perforation rate in young men with BAM (12). But this is controversial in some studies. Muthaiah Srinivasan reported a perforation rate of 19% in 242 eyes of 166 patients, noting that it was not significantly more prevalent in bilateral cases or among young patients (138). Similarly, a study involving 715 eyes of 550 patients in China found no significant difference in perforation rates between young and old patients but observed a markedly higher rate in bilateral cases compared to unilateral ones (16). Kim (15) research revealed a significant association between younger age and corneal perforation, though whether the disease was bilateral or unilateral had no impact on perforation incidence. Given the rarity of MU, conducting large prospective studies on this disease is challenging. Consequently, further investigation into risk factors influencing the perforation rate is warranted. However, patients with MU who are younger or present with bilateral onset should be carefully monitored due to their potential for a higher perforation rate.
A recurrence of an epithelial defect associated with stromal infiltration is defined as a relapse in MU. Reports indicate a very high postoperative recurrence rate of 25.6%, with the first recurrence occurring mostly within 6 months after surgery. Recurrences may occur at the original lesion site or along the interface between the lamellar bed and the donor graft (16). Reducing the relapse rate is crucial for improving the cure rate of MU. Several factors have been associated with the early recurrence of MU. Yang et al. linked corneal infection and perforation to early recurrence (139), and Dong et al. identified male gender and severe cases requiring surgical treatment as risk factors (125). It is suggested that the mechanisms underlying recurrence may differ from those of the initial onset, necessitating further research to elucidate the risk factors, mechanisms, and optimal treatment strategies for MU recurrence.
Schallenberg et al. found disparities in the severity and prognosis of MU among different racial groups, proposing the expression of HLA-DR17 and/or HLA-DQ2 might influence the prognosis of MU (122). Although additional studies are needed to validate these findings, clinicians are advised to consider systemic immunosuppression for MU patients with high HLA-DR17 and/or HLA-DQ2 expression as early as possible.
12 Summary and future perspectives
The cornea, integral to the ocular surface ecosystem, collaborates with surrounding tissues to maintain ocular function and homeostasis (140–142). While minor corneal microenvironment abnormalities may go unnoticed due to compensatory mechanisms from neighboring tissues, the accumulation of severe dysfunction or abnormalities, as seen in conditions like MU, can trigger a cascade of complications. This may initiate a detrimental cycle of decompensation, exacerbating ocular pathology. MU has been recognized for over 170 years, yet its exact etiology, pathogenesis, and causal mechanisms remain unclear. Despite being identified as an autoimmune-related, genetically susceptible corneal disease leading to blindness, MU diagnosis remains one of exclusion, necessitating the exclusion of other causes of peripheral corneal ulcers (3, 20, 66). It is also one of the important reasons that may promote the process of MU in clinic. To address this, the work newly introduced Tables 2, 3 in this review after summarizing recent 19 case reports of MU, offering a structured summary of diagnostic examinations and an autoimmune serological panel, respectively. These tables aim to provide clinicians with practical guidance for differentiating MU from other causes of peripheral ulcerative keratitis, based on traditional diagnostic methods, and represent one of the novel contributions of this work.
In some new diagnostic technologies, although tools like 3D AS-OCT and IVCM have improved early detection, they have not overcome the core diagnostic challenge, reliably distinguishing MU from other causes of peripheral corneal ulceration. Similar inflammatory keratopathies may present comparable findings using these techniques, such as increased ICD and reduced visual quality in patients (143, 144). Furthermore, studies have indicated that these methods may not be as effective in examining the corneas of patients in MU remission compared to those with active corneal ulcers (87). Our previous work (56) introduced a histopathology-based diagnosis protocol that integrates AS-OCT and IVCM. While this protocol continues to rule out other similar diseases, it has proven effective in accurately diagnosing MU even when MU is obscured by other corneal conditions, such as pterygium. This protocol is a crucial tool for diagnosing MU in patients with complex ocular surface conditions. Future diagnostic criteria might include proteomic approach (52) and histopathological markers (51, 88, 89), like cathepsins, anti-citrullinated protein antibody (ACPA), catalase (CAT), CD34, CD74, KIT proto-oncogene, receptor tyrosine kinase (c-kit), G protein-coupled receptor 91 (GPR91), heat shock protein family A (Hsp70) member 5 (HSPA5), ICAM-1, leucine aminopeptidase 3 (LAP3), LFA-1, matrix metalloproteinase-10 (MMP-10), myocilin (MYOC), marginal zone B and B1 cell-specific protein (MZB1), NLR Family Pyrin Domain Containing 3 (NLRP3), peptidyl arginine deiminase 4 (PADI4), polymeric immunoglobulin receptor (PIGR), superoxide dismutase 2 (SOD2), superoxide dismutase 3 (SOD3), stromal cell surface marker 1 (STRO-1), and tissue inhibitor of metalloproteinase 3 (TIMP3), which could enhance the diagnostic accuracy and provide new insights into the underlying pathophysiology of MU.
In terms of treatment strategy, management of MU primarily aims to improve vision and corneal integrity, with varied approaches based on different subtypes’ clinical manifestations, prognosis, and treatment responses. Presently, similar to the treatment of most PUK, the majority of treatments focus on immune regulation, while surgical interventions prioritize lesion removal and corneal restoration (3).
Although MU can be managed with the intervention of advanced diagnostic protocols and treatment strategies aimed at restoring visual quality, the acute phase presentation and variable prognosis of MU patients pose significant challenges to the development of standardized treatment protocols. Notably, MU symptoms are significantly influenced by factors, including the patient’s race and age. Additionally, there are greater prognostic challenges associated with corneal perforation during exacerbations of MU, and there is a persistently high postoperative recurrence rate. Fortunately, recent advancements in diagnostic tools, such as 3D AS-OCT, IVCM, and proteomic markers, along with the use of immunosuppressants and monoclonal antibody therapies, have significantly improved the early detection and management of MU. These developments have enabled more effective disease control in many cases, potentially reducing the reliance on surgical interventions. However, despite these technological improvements, current diagnostic approaches still rely heavily on exclusion, and there remains no universally accepted clinical guideline or standardized treatment algorithm. Further research is needed to refine diagnostic criteria and establish evidence-based, consensus-driven treatment protocols.
13 Conclusion
Mooren’s ulcer (MU) is a rare but sight-threatening autoimmune corneal disease. Despite advances in immunopathology and imaging, its diagnosis still relies largely on exclusion, and management remains challenging. Early recognition and prompt initiation of immunosuppressive therapy are crucial to prevent corneal perforation and vision loss. A tiered, individualized “stepladder” approach, beginning with corticosteroids and escalating to systemic immunomodulators, biologics, or surgery as required, offers the most effective management strategy. For clinicians, establishing a structured diagnostic workup and close interdisciplinary collaboration between ophthalmologists and immunologists is essential. For researchers, future efforts should focus on defining standardized diagnostic criteria, identifying reliable immunologic biomarkers, and validating evidence-based treatment algorithms through multicenter studies.
14 Methods of literature search
The search of this comprehensive review was performed in the PubMed and Web of Science databases (up to Oct 2025), without limitations on publication date or type. Articles not published in English or lacking peer review were excluded. This narrative review incorporated a range of relevant keywords and phrases, including but not limited to: “Mooren,” “peripheral ulcerative keratitis,” or “Mooren’s ulcer.” All articles deemed pertinent to the study, as determined by the two authors’ discretion, were included in the review.
Statements
Author contributions
SO: Funding acquisition, Supervision, Methodology, Conceptualization, Writing – original draft. YZ: Formal analysis, Writing – original draft. YF: Writing – review & editing, Visualization. XZ: Data curation, Writing – review & editing. YL: Data curation, Writing – review & editing. LZ: Investigation, Writing – review & editing. SZ: Writing – review & editing, Investigation. YS: Visualization, Investigation, Writing – review & editing. HC: Writing – review & editing, Visualization. LL: Writing – review & editing, Visualization. HG: Writing – review & editing, Supervision, Project administration. HW: Supervision, Writing – review & editing, Project administration. YW: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Guizhou Provincial Basic Research Program (QKHJC-ZK[2024]ZD043 and QKHJC-ZK[2025]MS473), Fujian Provincial Science Fund for Distinguished Young Scholars (2023J06053), the Natural Science Foundation of China (82101084), Medical Research Union Fund for High-quality health development of Guizhou Province (2024GZYKYJJKM0043), National Natural Science Foundation of China Cultivation Project of Guizhou Medical University (gyfynsfc[2024]-01), Xiamen Science and Technology Program for Public Wellbeing (3502Z20214ZD1208), and China Scholarship Council (202306310049).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- 3D AS-OCT
Three-dimensional anterior segment optical coherence tomography
- ACPA
Anti-citrullinated protein antibody
- AM
Amniotic membrane
- BAM
Bilateral aggressive mooren’s ulceration
- BCVA
Best-corrected visual acuity
- BIM
Bilateral indolent mooren’s ulceration
- BKPro
Boston type 1 keratoprosthesis
- C1
Complement 1
- CAGC
Calcium granule protein C
- CAT
Catalase
- CICs
Circulating immune complexes
- c-kit
KIT proto-oncogene, receptor tyrosine kinase
- CO-Ag
Cornea-associated antigen
- CsA
Cyclosporin A
- CVDs
Collagen vascular diseases
- GPA
Granulomatosis with polyangiitis
- GPR91
G protein-coupled receptor 91
- HCV
Hepatitis c virus
- HE
Hematoxylin–eosin
- HLA
Human leukocyte antigen
- HSPA5
Heat shock protein family A (Hsp70) member 5
- ICAM-1
Intercellular adhesion molecule-1
- ICD
Inflammatory cell density
- IHC
Immunohistochemical
- IVCM
In vivo confocal microscopy
- LAP3
Leucine aminopeptidase 3
- LFA-1
Lymphocyte function-associated antigen-1
- LK
Lamellar keratoplasty
- MMP-10
Matrix metalloproteinase-10
- MU
Mooren’s ulcer
- MYOC
Myocilin
- MZB1
Marginal zone B and B1 cell-specific protein
- NLRP3
NLR family pyrin domain containing 3
- PADI4
Peptidyl arginine deiminase 4
- PIGR
Polymeric immunoglobulin receptor
- PK
Penetrating keratoplast
- PMD
Pellucid marginal degeneration
- PUK
Peripheral ulcerative keratitis
- SOD2
Superoxide dismutase 2
- SOD3
Superoxide dismutase 3
- STRO-1
Stromal cell surface marker 1
- Th
Helper T cells
- TIMP3
Tissue inhibitor of metalloproteinase 3
- TMD
Terrien’s marginal degeneration
- Ts
T cells
- UM
Unilateral Mooren’s ulceration
Glossary
References
1.
Sharma N Sinha G Shekhar H Titiyal JS Agarwal T Chawla B et al . Demographic profile, clinical features and outcome of peripheral ulcerative keratitis: A prospective study. Br J Ophthalmol. (2015) 99:1503–8. doi: 10.1136/bjophthalmol-2014-306008
2.
Messmer EM Foster CS . Vasculitic peripheral ulcerative keratitis. Surv Ophthalmol. (1999) 43:379–96. doi: 10.1016/s0039-6257(98)00051-4
3.
Gupta Y Kishore A Kumari P Balakrishnan N Lomi N Gupta N et al . Peripheral ulcerative keratitis. Surv Ophthalmol. (2021) 66:977–98. doi: 10.1016/j.survophthal.2021.02.013
4.
Sangwan VS Zafirakis P Foster CS . Mooren's ulcer: current concepts in management. Indian J Ophthalmol. (1997) 45:7–17. PMID:
5.
Lian XF Wang CX Yang SJ Zhou SY . Evaluation of Mooren's corneal ulcer by anterior segment optical coherence tomography. Photodiagn Photodyn Ther. (2023) 44:103806. doi: 10.1016/j.pdpdt.2023.103806
6.
Cornea Group of Ophthalmology Branch of Chinese Medical A . Chinese expert consensus on clinical diagnosis and treatment of immune-related peripheral keratopathy (2022). Zhonghua Yan Ke Za Zhi. (2022) 58:90–5. doi: 10.3760/cma.j.cn112142-20211123-00559
7.
Zelefsky JR Srinivasan M Kundu A Lietman T Whitcher JP Wang K et al . Hookworm infestation as a risk factor for Mooren's ulcer in South India. Ophthalmology. (2007) 114:450–3. doi: 10.1016/j.ophtha.2006.08.014
8.
Patel R Nair S Choudhry H Jaffry M Dastjerdi M . Ocular manifestations of liver disease: an important diagnostic aid. Int Ophthalmol. (2024) 44:177. doi: 10.1007/s10792-024-03103-y
9.
Khakshoor H Moshirfar M Simpson RG Gharaee H Vejdani AH Christiansen SM et al . Anesthetic keratopathy presenting as bilateral Mooren-like ulcers. Clin Ophthalmol. (2012) 6:1719–22. doi: 10.2147/OPTH.S36611
10.
Li S Deng Y Du C Huang H Zhong J Chen L et al . Rapid deterioration of Mooren's ulcers after conjunctival flap: A review of 2 cases. BMC Ophthalmol. (2017) 17:93. doi: 10.1186/s12886-017-0488-1
11.
Kim EC Jun AS Kim MS Jee D . Mooren ulcer occurring at donor site after contralateral Conjunctivolimbal autograft for recurrent pterygium. Cornea. (2012) 31:1357–8. doi: 10.1097/ICO.0b013e31824d7041
12.
Watson PG . Management of Mooren's ulceration. Eye. (1997) 11:349–56. doi: 10.1038/eye.1997.74
13.
Kafkala C Choi J Zafirakis P Baltatzis S Livir-Rallatos C Rojas B et al . Mooren ulcer: an immunopathologic study. Cornea. (2006) 25:667–73. doi: 10.1097/01.ico.0000214216.75496.7e
14.
Taylor CJ Smith SI Morgan CH Stephenson SF Key T Srinivasan M et al . Hla and Mooren's ulceration. Br J Ophthalmol. (2000) 84:72–5. doi: 10.1136/bjo.84.1.72
15.
Kim DH Kim MK Wee WR Oh JY . Mooren's ulcer in a cornea referral practice in Korea. Ocul Immunol Inflamm. (2016) 24:55–9. doi: 10.3109/09273948.2014.926938
16.
Chen J Xie H Wang Z Yang B Liu Z Chen L et al . Mooren's ulcer in China: A study of clinical characteristics and treatment. Br J Ophthalmol. (2000) 84:1244–9. doi: 10.1136/bjo.84.11.1244
17.
Fasina O Ogundipe A Ezichi E . Mooren's ulcer in Ibadan, Southwest Nigeria. J West Afr Coll Surg. (2013) 3:102–19.
18.
Wood TO Kaufman HE . Mooren's ulcer. Am J Ophthalmol. (1971) 71:417–22. doi: 10.1016/0002-9394(71)90420-x
19.
Peraka RP Murthy SI Rathi VM . Strategies for successful Management of Moderate and Severe Mooren's ulcer at a rural Center during the Covid-19 pandemic: lessons learnt. Indian J Ophthalmol. (2024) 72:195–200. doi: 10.4103/IJO.IJO_1057_23
20.
Lee HJ Kim MK Wee WR Oh JY . Interplay of immune cells in Mooren ulcer. Cornea. (2015) 34:1164–7. doi: 10.1097/ICO.0000000000000471
21.
Gomes BF Santhiago MR . Biology of peripheral ulcerative keratitis. Exp Eye Res. (2021) 204:108458. doi: 10.1016/j.exer.2021.108458
22.
Gottsch J. D. Stark W. J. Liu S. H. Cloning and sequence analysis of human and bovine corneal antigen (co-ag) Cdna: identification of host-parasite protein calgranulin C. Trans Am Ophthalmol Soc (1997) 95:111–125; discussion 26–9.
23.
Gottsch JD Liu SH . Cloning and expression of human corneal calgranulin C (co-ag). Curr Eye Res. (1998) 17:870–4. doi: 10.1076/ceyr.17.9.870.5136
24.
Gottsch JD Liu SH Minkovitz JB Goodman DF Srinivasan M Stark WJ . Autoimmunity to a cornea-associated stromal antigen in patients with Mooren's ulcer. Invest Ophthalmol Vis Sci. (1995) 36:1541–7.
25.
Schaap OL Feltkamp TE Breebaart AC . Circulating antibodies to corneal tissue in a patient suffering from Mooren's ulcer (Ulcus Rodens Corneae). Clin Exp Immunol. (1969) 5:365–70.
26.
Zhao JC Jin XY . Etiopathological investigation of Mooren's ulcer. Chin Med J. (1993) 106:57–60. PMID:
27.
Mondino BJ Brown SI Rabin BS . Autoimmune phenomena of the external eye. Ophthalmology. (1978) 85:801–17. doi: 10.1016/s0161-6420(78)35618-9
28.
Shinomiya K Ueta M Sotozono C Inatomi T Yokoi N Koizumi N et al . Immunohistochemical analysis of inflammatory limbal conjunctiva adjacent to Mooren's ulcer. Br J Ophthalmol. (2013) 97:362–6. doi: 10.1136/bjophthalmol-2012-302631
29.
Murray PI Rahi AH . Pathogenesis of Mooren's ulcer: some new concepts. Br J Ophthalmol. (1984) 68:182–7. doi: 10.1136/bjo.68.3.182
30.
Berkowitz PJ Arentsen JJ Felberg NT Laibson PR . Presence of circulating immune complexes in patients with peripheral corneal disease. Arch Ophthalmol. (1983) 101:242–5. doi: 10.1001/archopht.1983.01040010244012
31.
Ung L Chodosh J . Urgent unmet needs in the Care of Bacterial Keratitis: an evidence-based synthesis. Ocul Surf. (2023) 28:378–400. doi: 10.1016/j.jtos.2021.08.013
32.
Mondino BJ . Inflammatory diseases of the peripheral cornea. Ophthalmology. (1988) 95:463–72. doi: 10.1016/s0161-6420(88)33164-7
33.
Gottsch JD Li Q Ashraf F O'Brien TP Stark WJ Liu SH . Cytokine-induced calgranulin C expression in keratocytes. Clin Immunol. (1999) 91:34–40. doi: 10.1006/clim.1998.4681
34.
Wilson SE Lee WM Murakami C Weng J Moninger GA . Mooren's corneal ulcers and hepatitis C virus infection. N Engl J Med. (1993) 329:62. doi: 10.1056/NEJM199307013290118
35.
Aaltonen V Alavesa M Pirila L Vesti E Al-Juhaish M . Case report: bilateral Mooren ulcer in association with hepatitis C. BMC Ophthalmol. (2017) 17:239. doi: 10.1186/s12886-017-0633-x
36.
Pluznik D Butrus SI . Hepatitis C-associated peripheral corneal ulceration: rapid response to intravenous steroids. Cornea. (2001) 20:888–9. doi: 10.1097/00003226-200111000-00023
37.
Wang QS Yuan J Zhou SY Chen JQ . Chronic hepatitis C virus infection is not associated with Mooren's ulcer. Eye (Lond). (2008) 22:697–700. doi: 10.1038/sj.eye.6702788
38.
Toyokawa N Araki-Sasaki K Kimura H Kuroda SI . Case of bilateral Mooren's ulcers following filtering surgery using ex-Press glaucoma filtering devices. Am J Ophthalmol Case Rep. (2020) 17:100588. doi: 10.1016/j.ajoc.2020.100588
39.
Zhang X Han P Qiu J Huang F Luo Q Cheng J et al . Single-cell Rna sequencing reveals the complex cellular niche of pterygium. Ocul Surf. (2024) 32:91–103. doi: 10.1016/j.jtos.2024.01.013
40.
Oke I Elze T Miller JW Lorch AC Hunter DG Traish AS et al . The prevalence and recurrence risk of bare sclera pterygium surgery in the United States. Ocul Surf. (2023) 29:547–9. doi: 10.1016/j.jtos.2023.05.006
41.
He N Song W Gao Y . Treatment of Mooren's ulcer coexisting with a pterygium using an intrastromal lenticule obtained from small-incision lenticule extraction: case report and literature review. J Int Med Res. (2021) 49:3000605211020246. doi: 10.1177/03000605211020246
42.
Touge C Mihara E Funakoshi T Saeki Y Hasegawa J Miyazaki D et al . A case of Mooren's ulcer associated with a pterygium. Jpn J Ophthalmol. (2005) 49:543–5. doi: 10.1007/s10384-004-0233-1
43.
Selvan H Samantaray PP Rana M . Bilateral aggressive Mooren ulcer in the setting of bilateral pterygia and pregnancy: A unique case. Cornea. (2024) 43:1573–7. doi: 10.1097/ICO.0000000000003591
44.
Zelefsky JR Taylor CJ Srinivasan M Peacock S Goodman RS Key T et al . Hla-Dr17 and Mooren's ulcer in South India. Br J Ophthalmol. (2008) 92:179–81. doi: 10.1136/bjo.2007.127050
45.
Liang CK Chen KH Hsu WM Chen KH . Association of Hla Type and Mooren's ulcer in Chinese in Taiwan. Br J Ophthalmol. (2003) 87:797–8. doi: 10.1136/bjo.87.6.797
46.
Singh RB Li J Parmar UPS Jeng BH Jhanji V . Vaccine-associated corneal graft rejection following Sars-Cov-2 vaccination: A Cdc-Vaers database analysis. Br J Ophthalmol. (2023) 108:17–22. doi: 10.1136/bjo-2022-322512
47.
Alliti F McHachi A Benhmidoune L Chakib A Rachid R El Belhadji M . Corneal perforation on Mooren ulcer after anti-Covid-19 vaccination: A case report. J Fr Ophtalmol. (2023) 46:e1–3. doi: 10.1016/j.jfo.2022.07.007
48.
Sookaromdee P Wiwanitkit V . Corneal perforation on Mooren ulcer after anti-Covid-19 vaccination: correspondence. J Fr Ophtalmol. (2023) 46:e138–9. doi: 10.1016/j.jfo.2023.02.001
49.
Chen KH Hsu WM Liang CK . Relapsing Mooren's ulcer after amniotic membrane transplantation combined with conjunctival autografting. Ophthalmology. (2004) 111:792–5. doi: 10.1016/j.ophtha.2003.06.024
50.
Dana MR Qian Y Hamrah P . Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection. Cornea. (2000) 19:625–43. doi: 10.1097/00003226-200009000-00008
51.
Li Z Wei C Wang S Liu T Zhai H Shi W . Upregulation of Nlrp3 inflammasome components in Mooren's ulcer. Graefes Arch Clin Exp Ophthalmol. (2017) 255:607–12. doi: 10.1007/s00417-016-3516-6
52.
Chi H Hao W Qi X Zhang T Dong Y Gao H et al . A proteomic approach towards understanding the pathogenesis of Mooren's ulcer. Exp Eye Res. (2021) 205:108509. doi: 10.1016/j.exer.2021.108509
53.
Zhang YN Dong YL Hao WP Bai XF Qi X Liu T et al . Increased Cgas/Sting signaling components in patients with Mooren's ulcer. Int J Ophthalmol. (2021) 14:1660–5. doi: 10.18240/ijo.2021.11.03
54.
Ou S Zhang Y Lin Y Fang X Xie Z Shi K et al . Risk factors for recurrence of Mooren's ulcer after keratoplasty. Heliyon. (2024) 10:e39623. doi: 10.1016/j.heliyon.2024.e39623
55.
Lucchino L Mastrogiuseppe E Giovannetti F Bruscolini A Marenco M Lambiase A . Anterior segment optical coherence tomography for the tailored treatment of Mooren's ulcer: A case report. J Clin Med. (2024) 13:5384. doi: 10.3390/jcm13185384
56.
Zhang Y Fang X Lin Z Xie Z Wu H Ou S . Histopathology-based diagnosis of Mooren's ulcer concealed beneath the pterygium on eye. J Histotechnol. (2022) 45:195–201. doi: 10.1080/01478885.2022.2137666
57.
Husain A Sr Saleem A Zaidi ZA Kazmi Z Khawaja UA . Treating Mooren's ulcer - squeezing water from a stone. Cureus. (2020) 12:e12248. doi: 10.7759/cureus.12248
58.
Acharya M Dave A Mathur U . Unusual case of acute corneal Hydrops in Mooren's ulcer treated with intracameral injection of Perfluoropropane. Middle East Afr J Ophthalmol. (2017) 24:106–8. doi: 10.4103/meajo.MEAJO_23_16
59.
Urkude J Singh R Chaniyara M Pujari A . Customised crescentic corneal transplant with conjunctival resection in an atypical case of malignant Mooren's ulcer. BMJ Case Rep. (2017) 2017:bcr2017220973. doi: 10.1136/bcr-2017-220973
60.
Kim J Kim MK Wee WR Oh JY . Mooren ulcer in a child wearing orthokeratology contact lenses. Eye Contact Lens. (2018) 44:e13–5. doi: 10.1097/ICL.0000000000000341
61.
Al Motowa S Al Zobidi M . Amniotic membrane transplant with a special technique (Motowa's sandwich technique) in Mooren's ulcer. Middle East Afr J Ophthalmol. (2015) 22:386–8. doi: 10.4103/0974-9233.159776
62.
Du Toit SH Smit DP . Mooren's ulcer of the cornea after immune reconstitution. AIDS. (2014) 28:139–40. doi: 10.1097/QAD.0000000000000044
63.
Lavaju P Sharma M Sharma A Chettri S . Use of amniotic membrane and autologous serum eye drops in Mooren's ulcer. Nepal J Ophthalmol. (2013) 5:120–3. doi: 10.3126/nepjoph.v5i1.7839
64.
Agarwal P Singh D Sinha G Sharma N Titiyal JS . Bilateral Mooren's ulcer in a child secondary to helminthic infestation of the gastrointestinal tract. Int Ophthalmol. (2012) 32:463–6. doi: 10.1007/s10792-012-9520-x
65.
Wagoner MD Islam SI Riley F . Intracorneal hematoma in Mooren ulceration. Am J Ophthalmol. (2000) 129:251–3. doi: 10.1016/s0002-9394(99)00423-7
66.
Xie H Chen J Wang Z Yang B Gong X Feng C et al . Microsurgical treatment of Mooren's corneal ulcer. Microsurgery. (2003) 23:27–31. doi: 10.1002/micr.10091
67.
Alhassan MB Rabiu M Agbabiaka IO . Interventions for Mooren's ulcer. Cochrane Database Syst Rev. (2014) 2014:CD006131. doi: 10.1002/14651858.CD006131.pub3
68.
Yagci A . Update on peripheral ulcerative keratitis. Clin Ophthalmol. (2012) 6:747–54. doi: 10.2147/OPTH.S24947
69.
Ruutila M Immonen AT Turunen JA Fagerholm P Lagali N Hjortdal J et al . Prospective in vivo confocal microscopy of the central cornea in Terrien marginal degeneration. Cornea. (2024) 44:1283–90. doi: 10.1097/ICO.0000000000003724
70.
Ruutila M Fagerholm P Lagali N Hjortdal J Bram T Yamaguchi T et al . Does corneal topography using 3-dimensional optical coherence tomography suggest different subtypes of Terrien marginal degeneration?Cornea. (2024) 43:571–7. doi: 10.1097/ICO.0000000000003409
71.
Harthan JS Reeder RE . Peripheral ulcerative keratitis in association with sarcoidosis. Cont Lens Anterior Eye. (2013) 36:313–7. doi: 10.1016/j.clae.2013.07.013
72.
Keenan JD Mandel MR Margolis TP . Peripheral ulcerative keratitis associated with Vasculitis manifesting asymmetrically as Fuchs superficial marginal keratitis and Terrien marginal degeneration. Cornea. (2011) 30:825–7. doi: 10.1097/ICO.0b013e3182000c94
73.
Chan AT Ulate R Goldich Y Rootman DS Chan CC . Terrien marginal degeneration: clinical characteristics and outcomes. Am J Ophthalmol. (2015) 160:867–872.e1. doi: 10.1016/j.ajo.2015.07.031
74.
Harada S Mohamed YH Kusano M Inoue D Uematsu M . Bilateral Fuchs' superficial marginal keratitis diagnosis and treatment. Life. (2024) 14:1644. doi: 10.3390/life14121644
75.
Arnalich-Montiel F . Systemic treatment and surgical intervention in inflammatory Terrien disease. Taiwan J Ophthalmol. (2024) 14:108–11. doi: 10.4103/tjo.TJO-D-23-00176
76.
Robin JB Schanzlin DJ Verity SM Barron BA Arffa RC Suarez E et al . Peripheral corneal disorders. Surv Ophthalmol. (1986) 31:1–36. doi: 10.1016/0039-6257(86)90049-4
77.
Jinabhai A Radhakrishnan H O'Donnell C . Pellucid corneal marginal degeneration: a review. Cont Lens Anterior Eye. (2011) 34:56–63. doi: 10.1016/j.clae.2010.11.007
78.
Altan-Yaycioglu R Pelit A Akova YA . Comparison of ultrasonic pachymetry with Orbscan in corneal haze. Graefes Arch Clin Exp Ophthalmol. (2007) 245:1759–63. doi: 10.1007/s00417-007-0578-5
79.
Boscia F La Tegola MG Alessio G Sborgia C . Accuracy of Orbscan optical pachymetry in corneas with haze. J Cataract Refract Surg. (2002) 28:253–8. doi: 10.1016/s0886-3350(01)01162-2
80.
Luisi J Kraft ER Giannos SA Patel K Schmitz-Brown ME Reffatto V et al . Longitudinal assessment of alkali injury on mouse cornea using anterior segment optical coherence tomography. Transl Vis Sci Technol. (2021) 10:6. doi: 10.1167/tvst.10.3.6
81.
Aschauer J Klimek M Donner R Lammer J Roberts P Schranz M et al . Non-invasive quantification of corneal vascularization using anterior segment optical coherence tomography angiography. Sci Rep. (2024) 14:2124. doi: 10.1038/s41598-024-52598-z
82.
Yoshihara M Maeda N Soma T Fuchihata M Hayashi A Koh S et al . Corneal topographic analysis of patients with Mooren ulcer using 3-dimensional anterior segment optical coherence tomography. Cornea. (2015) 34:54–9. doi: 10.1097/ICO.0000000000000237
83.
Wilson SE Lin DT Klyce SD Insler MS . Terrien's marginal degeneration: corneal topography. Refract Corneal Surg. (1990) 6:15–20. doi: 10.3928/1081-597X-19900101-05
84.
Gu Y Liu X Yu X Qin Q Yu N Ke W et al . Corneal in vivo confocal microscopy for assessment of non-neurological autoimmune diseases: a meta-analysis. Front Med. (2022) 9:809164. doi: 10.3389/fmed.2022.809164
85.
Przybek-Skrzypecka J Armstrong M Kim J Walkden A Au L Brahma A et al . Diagnostic features of Acanthamoeba keratitis via in vivo confocal microscopy. Sci Rep. (2025) 15:10940. doi: 10.1038/s41598-025-94567-0
86.
Liu J Shi W Li S Gao H Wang T . Modified lamellar keratoplasty and immunosuppressive therapy guided by in vivo confocal microscopy for perforated Mooren's ulcer. Br J Ophthalmol. (2015) 99:778–83. doi: 10.1136/bjophthalmol-2014-306012
87.
Hatou S Dogru M Ibrahim OM Wakamatsu T Sato EA Shimmura S et al . The application of in vivo confocal scanning laser microscopy in the diagnosis and evaluation of treatment responses in Mooren's ulcer. Invest Ophthalmol Vis Sci. (2011) 52:6680–9. doi: 10.1167/iovs.10-5906
88.
Ye J Chen J Kim JC Yao K . Bone marrow-derived cells are present in Mooren's ulcer. Ophthalmic Res. (2004) 36:151–5. doi: 10.1159/000077328
89.
Li L Dong YL Liu T Luo D Wei C Shi WY . Increased succinate receptor Gpr91 involved in the pathogenesis of Mooren's ulcer. Int J Ophthalmol. (2018) 11:1733–40. doi: 10.18240/ijo.2018.11.01
90.
Kalogeropoulos CD Malamou-Mitsi VD Aspiotis MB Psilas KG . Bilateral Mooren's ulcer in six patients: diagnosis, surgery and histopathology. Int Ophthalmol. (2004) 25:1–8. doi: 10.1023/b:inte.0000018510.06715.c9
91.
Kong W Cheng G Cao J Yu J Wang X Xu Z . Ocular mucosal homeostasis of teleost fish provides insight into the coevolution between microbiome and mucosal immunity. Microbiome. (2024) 12:10. doi: 10.1186/s40168-023-01716-6
92.
Zysset-Burri DC Schlegel I Lincke JB Jaggi D Keller I Heller M et al . Understanding the interactions between the ocular surface microbiome and the tear proteome. Invest Ophthalmol Vis Sci. (2021) 62:8. doi: 10.1167/iovs.62.10.8
93.
Ueta M Kinoshita S . Innate immunity of the ocular surface. Brain Res Bull. (2010) 81:219–28. doi: 10.1016/j.brainresbull.2009.10.001
94.
Yousefi A Ma Y Roberts CJ Moroi SE Reilly MA . Hydrodynamic interaction between tear film and air puff from noncontact tonometry. Transl Vis Sci Technol. (2022) 11:2. doi: 10.1167/tvst.11.2.2
95.
Ashar JN Mathur A Sangwan VS . Immunosuppression for Mooren's ulcer: evaluation of the stepladder approach--topical, oral and intravenous immunosuppressive agents. Br J Ophthalmol. (2013) 97:1391–4. doi: 10.1136/bjophthalmol-2012-302627
96.
Cakmak AI Akova Y Yildirim N . The complete success in refractory Mooren's ulcer treated with infliximab. Ocul Immunol Inflamm. (2023) 31:682–8. doi: 10.1080/09273948.2022.2091615
97.
Saw VP Cornelius N Salama AD Pusey C Lightman SL . Infliximab therapy for aggressive Mooren ulceration. Arch Ophthalmol. (2008) 126:734. doi: 10.1001/archopht.126.5.734
98.
Fontana L Parente G Neri P Reta M Tassinari G . Favourable response to infliximab in a case of bilateral refractory Mooren's ulcer. Clin Experiment Ophthalmol. (2007) 35:871–3. doi: 10.1111/j.1442-9071.2007.01609.x
99.
Cordero-Coma M Benito MF Fuertes CL Antolín SC Ruíz JMG . Adalimumab for Mooren's ulcer. Ophthalmology. (2009) 116:1589–90. doi: 10.1016/j.ophtha.2009.03.019
100.
Guindolet D Reynaud C Clavel G Belange G Benmahmed M Doan S et al . Management of severe and refractory Mooren's ulcers with rituximab. Br J Ophthalmol. (2017) 101:418–22. doi: 10.1136/bjophthalmol-2016-308838
101.
Hassanpour K ElSheikh RH Arabi A Frank CR Elhusseiny AM Eleiwa TK et al . Peripheral ulcerative keratitis: a review. J Ophthalmic Vis Res. (2022) 17:252–75. doi: 10.18502/jovr.v17i2.10797
102.
Katz EA Sunshine S Mun C Sarwar M Surenkhuu B Pradeep A et al . Combinatorial therapy with immunosuppressive, immunomodulatory and tear substitute eyedrops ("triple play") in recalcitrant immunological ocular surface diseases. Ocul Surf. (2022) 23:1–11. doi: 10.1016/j.jtos.2021.11.002
103.
Wakefield D Robinson LP . Cyclosporin therapy in Mooren's ulcer. Br J Ophthalmol. (1987) 71:415–7. doi: 10.1136/bjo.71.6.415
104.
Lee YJ Kim SW Seo KY . Application for tacrolimus ointment in treating refractory inflammatory ocular surface diseases. Am J Ophthalmol. (2013) 155:804–813.e1. doi: 10.1016/j.ajo.2012.12.009
105.
Furundaoturan O Akcay P Selver OB . Use of systemic mycophenolate mofetil therapy in ocular surface inflammatory pathologies at the initiative and responsibility of the ophthalmologist. Middle East Afr J Ophthalmol. (2022) 29:209–15. doi: 10.4103/meajo.meajo_109_23
106.
Tiev KP Borderie VM Briant M Ziani M Morvant C Baret M et al . Severe Moorens ulcer: efficacy of monthly cyclophosphamide intravenous pulse treatment. Rev Med Interne. (2003) 24:118–22. doi: 10.1016/s0248-8663(02)00021-8
107.
Tandon R Chawla B Verma K Sharma N Titiyal JS . Outcome of treatment of Mooren ulcer with topical cyclosporine a 2%. Cornea. (2008) 27:859–61. doi: 10.1097/ICO.0b013e3181702d0c
108.
Zhao JC Jin XY . Immunological analysis and treatment of Mooren's ulcer with cyclosporin A applied topically. Cornea. (1993) 12:481–8. doi: 10.1097/00003226-199311000-00004
109.
Saw VP . Immunotherapy for corneal inflammatory disorders: stepping up and down the ladder. Br J Ophthalmol. (2013) 97:1364–7. doi: 10.1136/bjophthalmol-2013-303359
110.
Rodrigues EB Farah ME Maia M Penha FM Regatieri C Melo GB et al . Therapeutic monoclonal antibodies in ophthalmology. Prog Retin Eye Res. (2009) 28:117–44. doi: 10.1016/j.preteyeres.2008.11.005
111.
Wang J Zhang Q Shi W Pang Y Li S . Peripheral ulcerative keratitis, nodular episcleritis, and pulmonary nodules as the initial signs of rheumatic arthritis: A case report. Front Immunol. (2022) 13:1048889. doi: 10.3389/fimmu.2022.1048889
112.
Sharma S Hoshing A Senthil S Murthy SI . Penetrating keratoplasty and Glaucoma valve surgery in recurrent Mooren's disease: A multidisciplinary approach. Int J Surg Case Rep. (2023) 106:108135. doi: 10.1016/j.ijscr.2023.108135
113.
Jerez-Pena M Salvador-Culla B de la Paz MF Barraquer RI . Bilateral Boston keratoprosthesis type 1 in a case of severe Mooren's ulcer. Eur J Ophthalmol. (2021) 31:NP33–8. doi: 10.1177/1120672120909768
114.
Brown SI . Mooren's ulcer. Treatment by conjunctival excision. Br J Ophthalmol. (1975) 59:675–82. doi: 10.1136/bjo.59.11.675
115.
Lal I Shivanagari SB Ali MH Vazirani J . Efficacy of conjunctival resection with cyanoacrylate glue application in preventing recurrences of Mooren's ulcer. Br J Ophthalmol. (2016) 100:971–5. doi: 10.1136/bjophthalmol-2015-307350
116.
Hopkinson A Figueiredo FC . A narrative review of amniotic membrane transplantation in ocular surface repair: unveiling the immunoregulatory pathways for timely intervention. Ophthalmol Ther. (2025) 14:1385–409. doi: 10.1007/s40123-025-01143-w
117.
Said DG Rallis KI Al-Aqaba MA Ting DSJ Dua HS . Surgical Management of Infectious Keratitis. Ocul Surf. (2023) 28:401–12. doi: 10.1016/j.jtos.2021.09.005
118.
Yaici R Solecki L Ballonzoli L Gaucher D Sauer A Bourcier T . Innovative use of lyophilized umbilical cord amniotic membrane in the treatment of Mooren's pseudo-ulcer. J Fr Ophtalmol. (2025) 48:104497. doi: 10.1016/j.jfo.2025.104497
119.
Ngan ND Chau HT . Amniotic membrane transplantation for Mooren's ulcer. Clin Experiment Ophthalmol. (2011) 39:386–92. doi: 10.1111/j.1442-9071.2010.02479.x
120.
Lambiase A Sacchetti M Sgrulletta R Coassin M Bonini S . Amniotic membrane transplantation associated with conjunctival peritomy in the management of Mooren's ulcer: a case report. Eur J Ophthalmol. (2005) 15:274–6. doi: 10.1177/112067210501500217
121.
Bhandari V Siddharthan KS . Bilateral Mooren's ulcer - customised corneal graft with additional amniotic membrane graft. Saudi J Ophthalmol. (2015) 29:235–7. doi: 10.1016/j.sjopt.2014.12.005
122.
Schallenberg M Westekemper H Steuhl KP Meller D . Amniotic membrane transplantation ineffective as additional therapy in patients with aggressive Mooren's ulcer. BMC Ophthalmol. (2013) 13:81. doi: 10.1186/1471-2415-13-81
123.
Shi W Li S Gao H Xie L . Clinical features and surgical treatment of peripheral staphyloma. Cornea. (2011) 30:395–9. doi: 10.1097/ICO.0b013e3181f22b8e
124.
Sharma A Sharma R . Customized therapeutic deep anterior lamellar keratoplasty in perforated Mooren's ulcer: A novel technique. Indian J Ophthalmol. (2024) 72:130–3. doi: 10.4103/IJO.IJO_964_23
125.
Dong Y Zhang Y Xie L Ren J . Risk factors, clinical features, and treatment outcomes of recurrent Mooren ulcers in China. Cornea. (2017) 36:202–9. doi: 10.1097/ICO.0000000000001084
126.
Nonpassopon M Niparugs M Cortina MS . Boston type 1 keratoprosthesis: updated perspectives. Clin Ophthalmol. (2020) 14:1189–200. doi: 10.2147/OPTH.S219270
127.
Basu S Taneja M Sangwan VS . Boston type 1 keratoprosthesis for severe blinding vernal keratoconjunctivitis and Mooren's ulcer. Int Ophthalmol. (2011) 31:219–22. doi: 10.1007/s10792-011-9438-8
128.
Mavrakanas NA Kiel R Dosso AA . Autologous serum application in the treatment of Mooren's ulcer. Klin Monatsbl Augenheilkd. (2007) 224:300–2. doi: 10.1055/s-2007-962935
129.
Soifer M Tovar A Wang M Mousa HM Yennam S Sabater AL et al . A Multicenter report of the use of plasma rich in growth factors (Prgf) for the treatment of patients with ocular surface diseases in North America. Ocul Surf. (2022) 25:40–8. doi: 10.1016/j.jtos.2022.04.007
130.
Tuli SS Schultz GS Downer DM . Science and strategy for preventing and managing corneal ulceration. Ocul Surf. (2007) 5:23–39. doi: 10.1016/s1542-0124(12)70050-2
131.
Brown SI Mondino BJ . Therapy of Mooren's ulcer. Am J Ophthalmol. (1984) 98:1–6. doi: 10.1016/0002-9394(84)90179-x
132.
Agrawal V Kumar A Sangwan V Rao GN . Cyanoacrylate adhesive with conjunctival resection and superficial keratectomy in Mooren's ulcer. Indian J Ophthalmol. (1996) 44:23–7.
133.
Sharma A Mohan K Sharma R Nirankari VS . Scleral patch graft augmented cyanoacrylate tissue adhesive for treatment of moderate-sized noninfectious corneal perforations (3.5-4.5 mm). Cornea. (2013) 32:1326–30. doi: 10.1097/ICO.0b013e31829cb625
134.
Ramos RCF Barbosa ADS Aleixo A Arruda IF Amendoeira MRR . Comparison of immunological and molecular methods for laboratory diagnosis of ocular toxoplasmosis in blood, serum and tears in Brazil. PLoS One. (2024) 19:e0298393. doi: 10.1371/journal.pone.0298393
135.
Yamaguchi T Calvacanti BM Cruzat A Qazi Y Ishikawa S Osuka A et al . Correlation between human tear cytokine levels and cellular corneal changes in patients with bacterial keratitis by in vivo confocal microscopy. Invest Ophthalmol Vis Sci. (2014) 55:7457–66. doi: 10.1167/iovs.14-15411
136.
Yamaguchi T Hamrah P Shimazaki J . Bilateral alterations in corneal nerves, dendritic cells, and tear cytokine levels in ocular surface disease. Cornea. (2016) 35:S65–70. doi: 10.1097/ico.0000000000000989
137.
Fortingo N Melnyk S Sutton SH Watsky MA Bollag WB . Innate immune system activation, inflammation and corneal wound healing. Int J Mol Sci. (2022) 23:14933. doi: 10.3390/ijms232314933
138.
Srinivasan M Zegans ME Zelefsky JR Kundu A Lietman T Whitcher JP et al . Clinical characteristics of Mooren's ulcer in South India. Br J Ophthalmol. (2007) 91:570–5. doi: 10.1136/bjo.2006.105452
139.
Yang L Xiao J Wang J Zhang H . Clinical characteristics and risk factors of recurrent Mooren's ulcer. J Ophthalmol. (2017) 2017:8978527. doi: 10.1155/2017/8978527
140.
Lin JB Shen X Pfeifer CW Shiau F Santeford A Ruzycki PA et al . Dry eye disease in mice activates adaptive corneal epithelial regeneration distinct from constitutive renewal in homeostasis. Proc Natl Acad Sci USA. (2023) 120:e2204134120. doi: 10.1073/pnas.2204134120
141.
Ou SK Jeyalatha MV Mao Y Wang JQ Chen C Zhang MJ et al . The role of ectodysplasin a on the ocular surface homeostasis. Int J Mol Sci. (2022) 23:12. doi: 10.3390/ijms232415700
142.
Lin Y Zhang X Sun D Wang Q Dou S Zhou Q . Decoding the corneal immune microenvironment in healthy and diabetic mice during corneal wound healing. Ocul Surf. (2025) 37:68–79. doi: 10.1016/j.jtos.2025.02.010
143.
Geboes K Abu el-Asrar A Missotten L . Immune cells in a case of postherpetic marginal trophic ulcer. Br J Ophthalmol. (1989) 73:191–6. doi: 10.1136/bjo.73.3.191
144.
Chang JY Huang TY Kuo YS Lin PY . Pseudopterygia in Fuchs superficial marginal keratitis: clinical course and surgical outcome. Cornea. (2024) 43:1468–72. doi: 10.1097/ICO.0000000000003498
Summary
Keywords
autoimmune corneal diseases, corneal melt, filarial nematodes, hepatitis C, HLA, hookworm, Mooren’s ulcer, peripheral ulcerative keratitis
Citation
Ou S, Zhang Y, Feng Y, Zheng X, Lin Y, Zhang L, Zhao S, Su Y, Cai H, Lin L, Gu H, Wu H and Wu Y (2025) Mooren’s ulcer: a multifactorial autoimmune peripheral ulcerative keratitis and current treatment protocols. Front. Med. 12:1630585. doi: 10.3389/fmed.2025.1630585
Received
04 August 2025
Accepted
27 October 2025
Published
11 November 2025
Volume
12 - 2025
Edited by
Karim Mohamed-Noriega, Autonomous University of Nuevo León, Mexico
Reviewed by
Fernando Morales-Wong, Autonomous University of Nuevo León, Mexico
Guillermo Raul Vera-Duarte, Instituto de Oftalmología Fundación de Asistencia Privada Conde de Valenciana, Mexico
Updates
Copyright
© 2025 Ou, Zhang, Feng, Zheng, Lin, Zhang, Zhao, Su, Cai, Lin, Gu, Wu and Wu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huping Wu, wuhuping123@163.com; Yiming Wu, y.wu.13@pgr.bham.ac.uk
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.