- 1Department of Emergency, Clinical Medical College & Affiliated Hospital of Chengdu University, Chengdu, China
- 2Department of Hospital Infection Management, Clinical Medical College & Affiliated Hospital of Chengdu University, Chengdu, China
- 3Department of Nursing, Pengzhou Peoples’s Hospital, Pengzhou, China
Background: This systematic review and meta-analysis assessed the analgesic efficacy of ultrasound-guided ESPB in metabolic surgery.
Methods: A systematic literature search of PubMed, the Cochrane Library, Web of Science, and Embase was conducted from database inception to February 2025 to identify randomized controlled trials (RCTs) comparing ultrasound-guided erector spinae plane block (ESPB) with either no block, sham block, or alternative regional analgesic techniques in patients undergoing metabolic surgery. Primary outcomes included 24 h postoperative opioid consumption, while secondary outcomes encompassed pain scores, time to first analgesic requirement, postoperative nausea and vomiting, and patient satisfaction. Risk of bias was assessed using the Cochrane Risk of Bias Tool, and evidence quality was evaluated using GRADE.
Results: Ten RCTs involving 729 patients were included. ESPB significantly reduced 24 h opioid consumption [mean difference (MD): −6.68; 95% CI: −10.75, −2.61; p = 0.001] and resting pain scores at 24 h [MD: −0.78; 95% CI: −1.10, −0.46; p < 0.00001]. Movement pain scores were also reduced to 6, 12, 24, and 48 h (p < 0.00001 for all). ESPB prolonged the time to first rescue analgesic [MD: 14.17; 95% CI: 5.50, 22.85; p = 0.001]. However, no significant differences were observed in PONV incidence or patient satisfaction scores.
Conclusion: Ultrasound-guided ESPB is an effective and safe analgesic technique for metabolic surgery, significantly reducing opioid consumption and pain scores while delaying the need for rescue analgesics.
Systematic review registration: PROSPERO 2025 CRD420251000358. Available from https://www.crd.york.ac.uk/PROSPERO/view/CRD420251000358.
Highlights
• Metabolic surgery is associated with considerable postoperative pain, which can impede recovery, delay ambulation, and increase the risk of pulmonary complications.
• Effective pain management is therefore critical to enhancing postoperative outcomes and patient satisfaction.
• Ultrasound-guided ESPB significantly reduces postoperative opioid consumption at 24 h compared to non-block care and sham block in patients undergoing metabolic surgery.
• ESPB prolonged the time to first rescue analgesic requirement, indicating its potential to reduce the need for additional opioid administration.
1 Introduction
Metabolic surgery, including bariatric procedures such as laparoscopic sleeve gastrectomy and Roux-en-Y gastric bypass, has become a cornerstone in the management of obesity and related metabolic disorders (1). While these surgeries offer significant health benefits, they are associated with considerable postoperative pain, which can impede recovery, delay ambulation, and increase the risk of pulmonary complications (2, 3). Effective pain management is therefore critical to enhancing postoperative outcomes and patient satisfaction (4).
Opioids have traditionally been the mainstay of postoperative analgesia, but their use is fraught with significant side effects, including respiratory depression, sedation, nausea, vomiting, and the potential for addiction (5). In the context of metabolic surgery, where patients often have comorbidities such as obstructive sleep apnea and obesity hypoventilation syndrome, the risks associated with opioid use are particularly pronounced (6, 7). Consequently, there is a growing interest in opioid-sparing analgesic strategies, with regional anesthesia techniques playing a pivotal role. The erector spinae plane block (ESPB) has gained attention as a novel regional anesthesia technique that provides effective analgesia for a variety of surgical procedures (8–10). By injecting local anesthetic into the fascial plane deep to the erector spinae muscle, ESPB can block the dorsal rami of spinal nerves, providing both somatic and visceral analgesia (11, 12). Its ease of performance under ultrasound guidance, combined with a favorable safety profile, makes ESPB an attractive option for postoperative pain management in metabolic surgery (13, 14).
Despite its potential, the efficacy of ESPB in metabolic surgery has not been comprehensively evaluated. Although a meta-analysis encompassing six studies, including both randomized controlled trials (RCTs) and observational cohort studies, demonstrated that bilateral ESPB offers opioid-sparing analgesia and superior pain scores compared to the control group, the limited sample size and heterogeneity of the included studies may compromise the overall quality of the research (2). Additionally, existing studies have reported conflicting results regarding its impact on opioid consumption, pain scores, and patient-reported outcomes such as quality of recovery and satisfaction. Furthermore, the comparative effectiveness of ESPB relative to other regional anesthesia techniques, such as the transversus abdominis plane block (TAPB) and quadratus lumborum block (QLB), remains unclear.
This systematic review and meta-analysis aimed to synthesize the available evidence from RCTs to evaluate the analgesic efficacy and safety of ultrasound-guided ESPB in patients undergoing metabolic surgery. By addressing these questions, we hope to provide clinicians with evidence-based recommendations for incorporating ESPB into multimodal analgesic regimens, ultimately improving postoperative outcomes in this high-risk patient population.
2 Methods
This study was conducted in accordance with the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) Guidelines (15). The meta-analysis was prospectively registered in the PROSPERO database (CRD420251000358).
2.1 Search strategy
A comprehensive search was performed across multiple electronic databases, including PubMed, The Cochrane Library, Web of Science Citation Index, and Embase, from their inception to February 2025. The search aimed to identify randomized controlled trials (RCTs) meeting predefined inclusion criteria. Search terms were selected based on the PICOS framework and included: “laparoscopic bariatric surgery,” “bariatric surgery,” “metabolic surgery,” and “erector spinae plane block.” Additionally, reference lists of identified articles were screened to ensure thorough search. No language restrictions were applied during the search process. The detailed search strategy is provided in the Supplementary Digital Content. Grey literature was also searched through manual screening, focusing on ESPB-related studies, which were first introduced in 2016.
2.2 Study selection criteria
Two independent investigators conducted the literature search and screening. Disagreements were resolved through discussion with a third reviewer. Eligible studies included full-text RCTs comparing the analgesic efficacy of ESPB with non-block care or other blocks in adult patients undergoing metabolic surgery. Case reports, non-RCT studies, incomplete clinical trials, and conference abstracts lacking sufficient study design or data (even after contacting authors) were excluded. No language restrictions were applied during study selection.
2.3 Data extraction
Data extraction was performed systematically and included the following variables: authors, publication year, country, surgical procedure, use of ultrasound guidance, puncture location, type and dosage of local anesthetics, postoperative pain scores, postoperative pain management, opioid-related side effects (e.g., postoperative nausea and vomiting, PONV), and complications associated with ESPB (e.g., nerve damage, local anesthetic toxicity, pneumothorax, hematoma, or infection at the puncture site). Pain scores were assessed using either a visual analogue scale (VAS) or a numeric rating scale (NRS), which were standardized to a 0–10 scale for statistical analysis (0 = no pain, 10 = extreme pain).
The primary outcome was postoperative opioid consumption (measured in morphine equivalents) at 24 h. Secondary outcomes included age, BMI, postoperative pain scores at rest and during movement at various time points, duration of surgery and anesthesia, time to first analgesic requirement, time to first ambulation, length of hospital stay, PONV, patient satisfaction scores, quality of recovery15/40 (QoR15/40), and regional blocks related complications. For studies presenting data graphically, numerical data were extracted using WebPlot Digitizer (16). Perioperative opioid consumption was converted to intravenous morphine equivalents using a standardized conversion calculator (17).
2.4 Risk of bias assessment
Methodological quality assessment was independently assessed by two authors, with any disagreements resolved by a third author, according to the Cochrane Risk of Bias Tool 2 (each article was recorded either as low risk of bias, some concerns, or high risk of bias). Finally, the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology was performed to assess the quality of evidence for each outcome (18). Evidence quality was rated as low, moderate, or high based on outcome-specific and comparison-specific criteria. A flow chart was used to illustrate the study selection process and reasons for exclusion.
2.5 Statistical analysis
All statistical analyses were performed using Review Manager (version 5.4; The Nordic Cochrane Center, Copenhagen). Studies with similar outcome measures were included in the meta-analysis. Continuous data, such as postoperative pain scores and opioid consumption, were expressed as means and standard deviations (SD). Mean differences (MD) with 95% confidence intervals (CI) were calculated using a random-effects model. For studies reporting medians and interquartile ranges (IQR), these values were converted to means and SDs using the method described by Hozo et al. (19). Dichotomous data, such as PONV, were analyzed as relative risks (RRs) with 95% CIs using the Mantel–Haenszel method (20). To avoid redundant sample size assessments in multi-arm studies, the number of participants is evenly distributed. In cases where there are one intervention group and two control groups, the number of patients in the intervention group is proportionally allocated to enable comparisons with each of the control groups.
Heterogeneity was assessed using the I2 statistic. A random-effects model was applied if I2 > 50%, indicating substantial heterogeneity; otherwise, a fixed-effects model was used (20, 21). Sensitivity analyses were conducted using the leave-one-out approach to identify potential sources of heterogeneity for primary outcomes (postoperative 24 h resting pain score and opioid consumption). The methodological quality of individual studies was evaluated using the Cochrane Risk of Bias Tool 2 for RCTs (22), focusing on sequence generation, allocation concealment, blinding of participants and outcome assessors, incomplete data, and selective reporting.
3 Results
3.1 Results of literature search
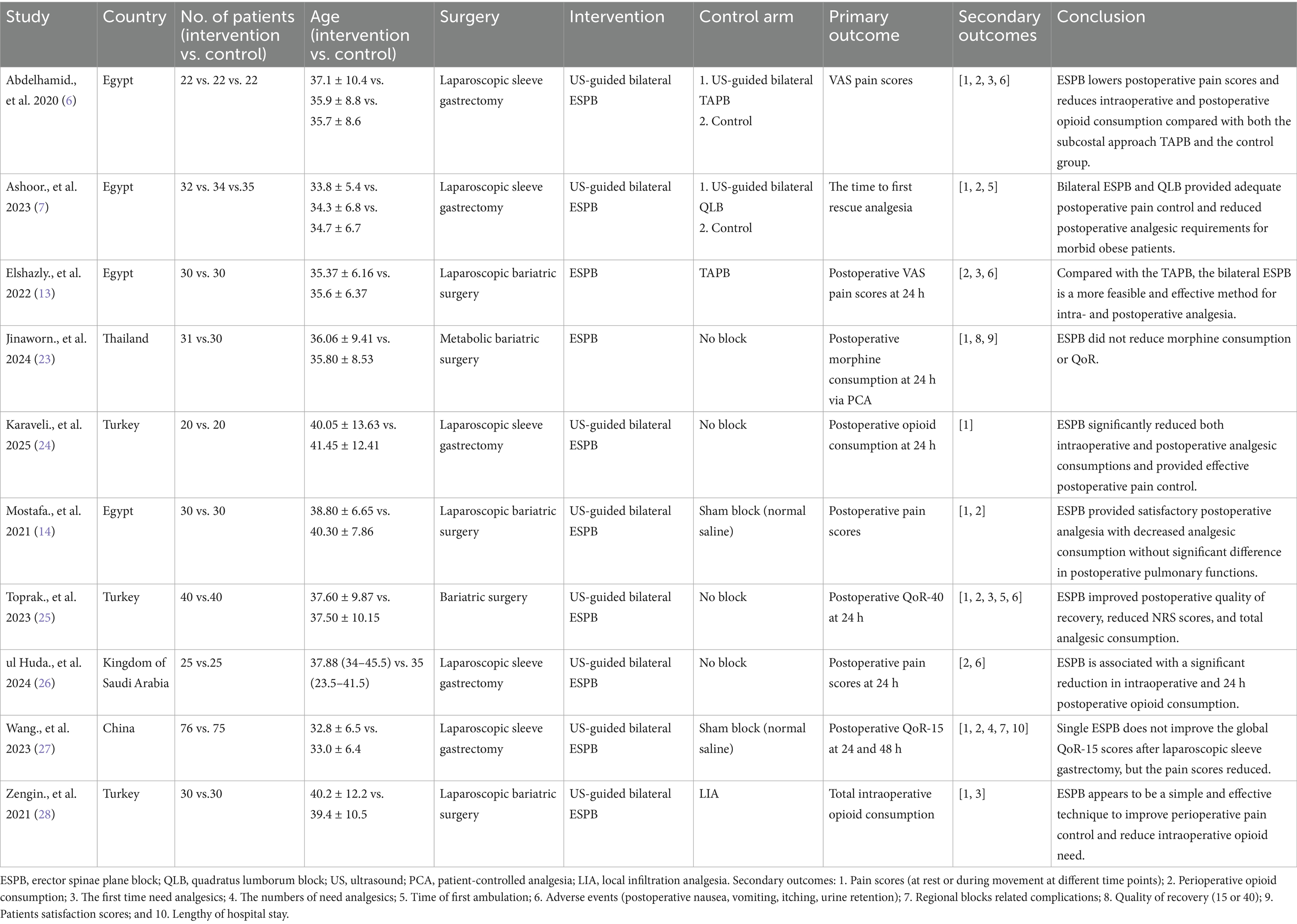
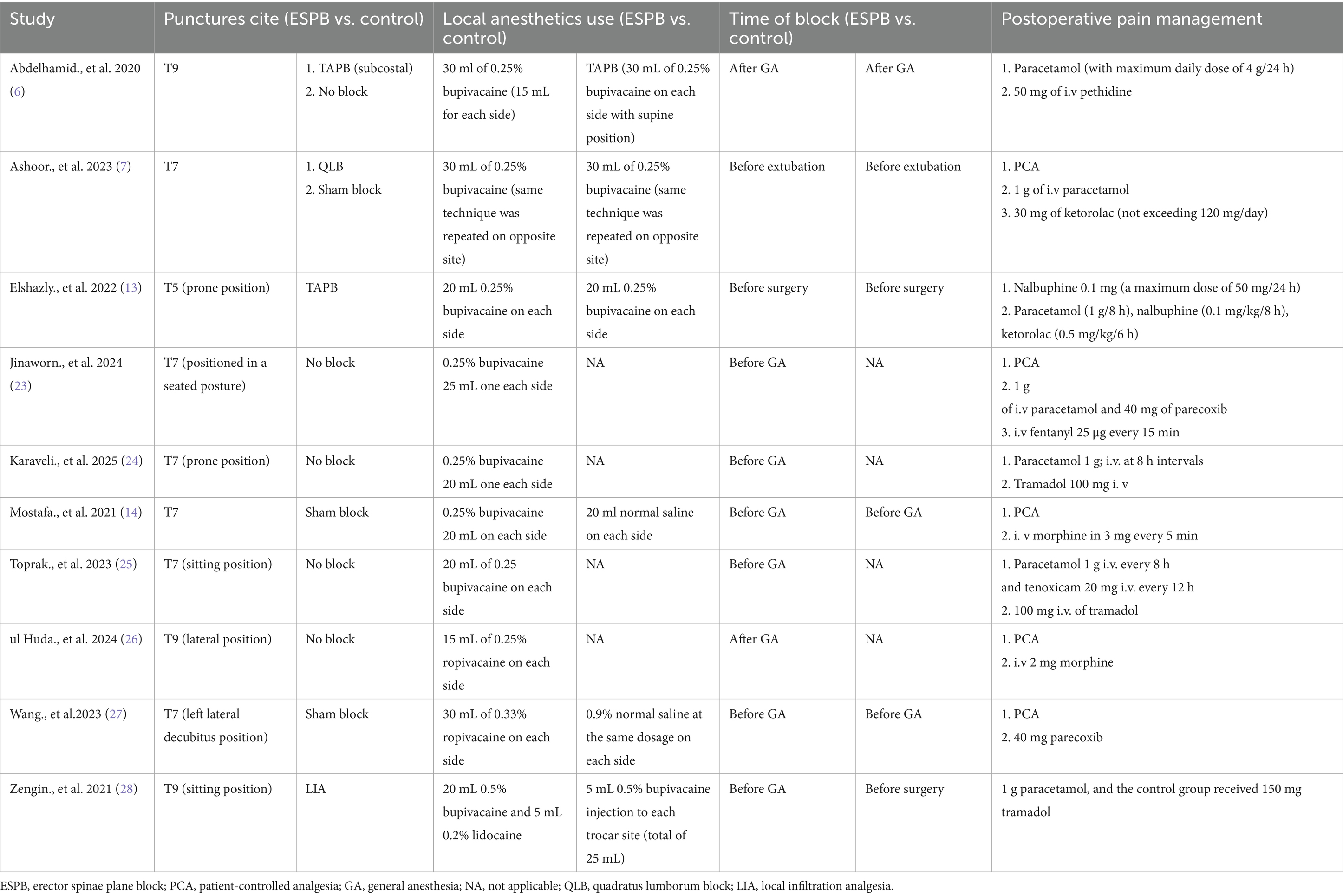
The study selection process is illustrated in the PRISMA flow diagram (Figure 1). The initial literature search identified 111 citations across four electronic databases. After full-text review and application of exclusion criteria, 10 RCTs involving 729 patients were included in the analysis (6, 7, 13, 14, 23–28). Table 1 summarizes the characteristics and details of these studies. All 10 articles were published between 2020 and 2025 and were written in English. The studies originated from Egypt (n = 4) (6, 7, 13, 14), Turkey (n = 3) (24, 25, 28), Thailand (n = 1) (23), Kingdom of Saudi Arabia (n = 1) (26), and China (n = 1) (27).
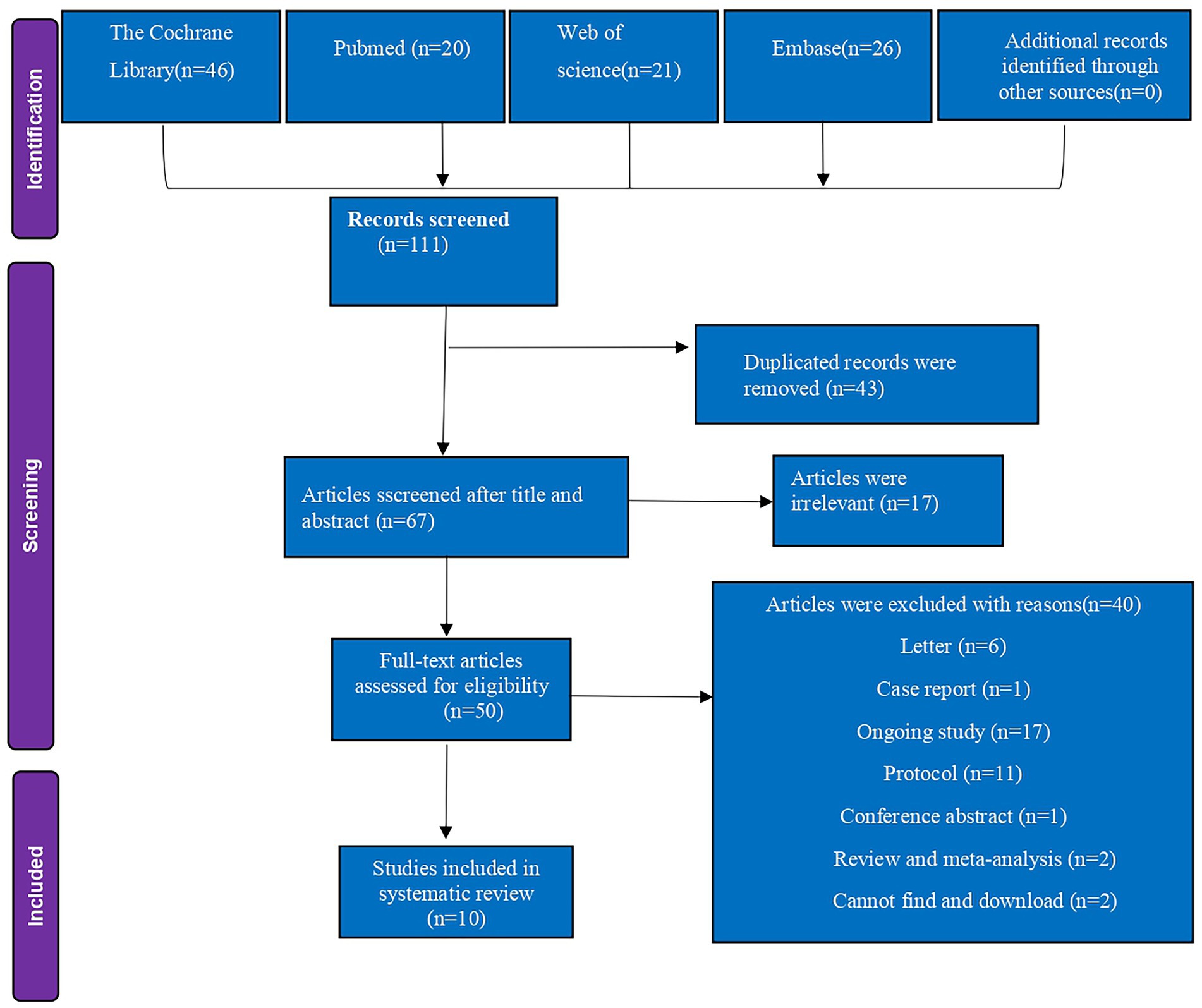
Figure 1. PRISMA flow diagram of included and excluded studies. PRISMA, preferred reporting items for systematic reviews and meta-analyses.
Among the included studies, two were designed as three-arm comparators (6, 7), while the remaining eight were two-arm comparators (13, 14, 23–28). Four RCTs compared erector spinae plane block (ESPB) with non-block care (23–26), two compared ESPB with sham block (14, 27), and the remaining four compared ESPB with other regional anesthesia techniques, including TAPB (6, 13), QLB (7), and local infiltration analgesia (LIA) (28), respectively (Table 2).
3.2 Primary outcome
3.2.1 Postoperative opioid consumption (morphine equivalent) at 24 h
Three studies compared ESPB with control groups (non-block care or sham block) in terms of cumulative opioid consumption (intravenous morphine equivalents, mg) at 24 h postoperatively (Figure 2) (14, 23, 26). Pooled data from these RCTs demonstrated that ESPB significantly reduced postoperative opioid consumption compared to the control group [MD −6.68; 95% CI −10.75, −2.61; p = 0.001; I2 = 96%]. Due to the limited number of studies, subgroup analysis based on different regional anesthesia techniques was not performed. Sensitivity analysis, conducted by sequentially removing two studies (14, 26), identified the source of heterogeneity, which remained high (Supplementary Table 1).
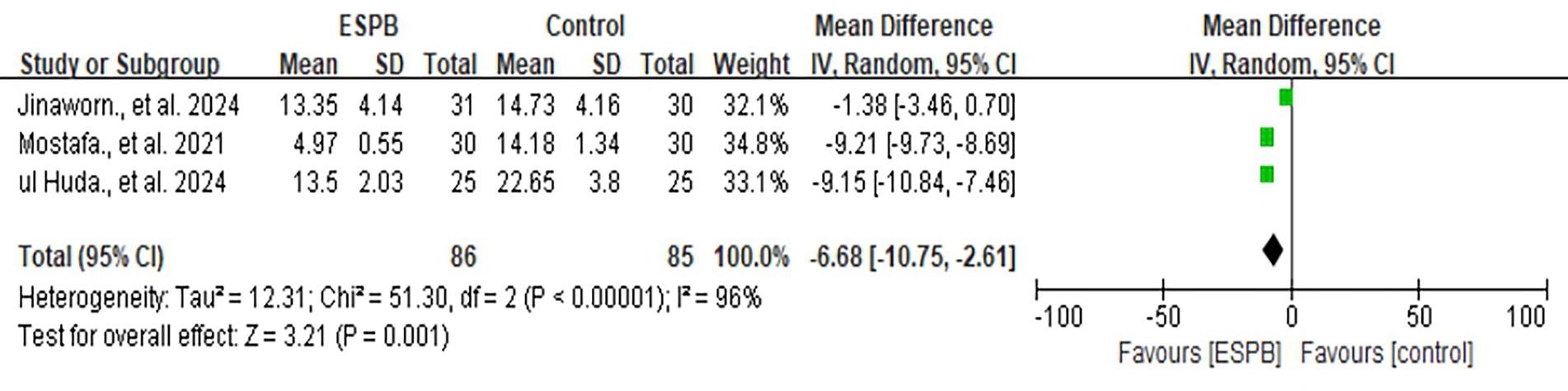
Figure 2. The forest plot of postoperative opioid consumption (morphine equivalent) at 24 h between ESPB and control group.
3.3 Secondary outcomes
3.3.1 Age and BMI
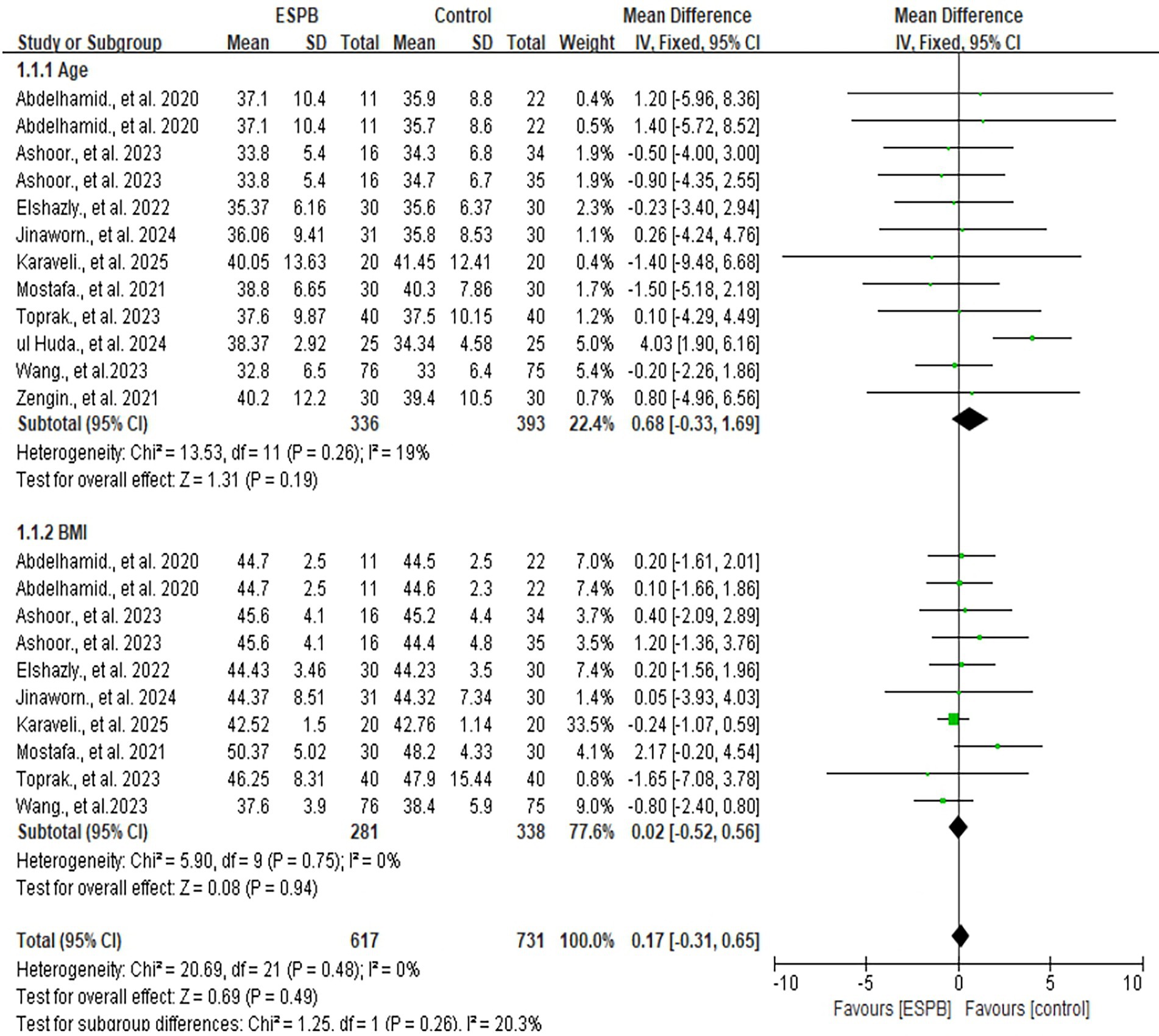
All included studies reported data on age [MD 0.68; 95% CI −0.33, 1.69; p = 0.19; I2 = 19%] and BMI [MD 0.02; 95% CI −0.52, 0.56; p = 0.94; I2 = 0%] (6, 7, 13, 14, 23–28). No significant differences were observed between the ESPB and control groups for either variable (Figure 3). Considering the I2<50%, a fixed model was applied.
3.3.2 Postoperative rest and movement pain scores at different time points
No significant differences were observed in postoperative rest pain scores at 0 h [MD −0.79; 95% CI −4.52, 2.94; p = 0.68; I2 = 70%], 30 min [MD −0.83; 95% CI −1.92, 0.25; p = 0.13; I2 = 100%], 1 h [MD −0.16; 95% CI −1.23, 0.90; p = 0.76; I2 = 62%], 2 h [MD −0.50; 95% CI −1.55, 0.55; p = 0.35; I2 = 100%], 6 h [MD −0.43; 95% CI −1.02, 0.16; p = 0.15; I2 = 77%], 12 h [MD −0.57; 95% CI −1.27, 0.14; p = 0.11; I2 = 80%], 18 h [MD −1.64; 95% CI −4.65, 1.36; p = 0.28; I2 = 79%], and 48 h [MD −0.31; 95% CI −1.01, 0.39; p = 0.38; I2 = 86%]. However, at 24 h, ESPB was associated with significantly lower rest pain scores compared to the control group [MD −0.78; 95% CI −1.10, −0.46; p<0.00001; I2 = 41%] in favor of ESPB compared with control group (Supplementary Figure 1).
Similarly, no significant differences were observed in movement pain scores at 0 h [MD −0.20; 95% CI −3.36, 2.96; p = 0.90; I2 = 50%], 30 min [MD −0.29; 95% CI −0.99, 0.41; p = 0.42; I2 = 78%], 1 h [MD −0.33; 95% CI −0.96, 0.30; p = 0.30; I2 = 11%], 2 h [MD −0.56; 95% CI −1.27, 0.16; p = 0.13; I2 = 83%], and 18 h [MD −1.89; 95% CI −5.44, 1.67; p = 0.30; I2 = 78%] also demonstrated that no significant difference between ESPB and control group. However, ESPB was associated with significantly lower movement pain scores at 6 h [MD −1.02; 95% CI −1.12, −0.92; p<0.00001; I2 = 0%], 12 h [MD −1.00; 95% CI −1.13, −0.87; p<0.0001; I2 = 42%], 24 h [MD −0.82; 95% CI −1.23, −0.42; p<0.00001; I2 = 0%], and 48 h [MD −0.80; 95% CI −1.07, −0.54; P<0.0001; I2 = 37%] respectively (Supplementary Figure 2).
3.3.3 Duration of anesthesia and surgery time
Three studies compared the duration of anesthesia time between ESPB and control groups (ESPB vs. QLB, no-block care) (7, 23, 24), revealing no significant differences [MD −0.57; 95% CI −13.06, 11.92; p = 0.97; I2 = 97%]. Similarly, no significant differences were observed in surgery time [MD −1.04; 95% CI −4.11, 2.03; p = 0.51; I2 = 66%] (Supplementary Figure 3).
3.3.4 Stay in PACU, the first time need analgesics, first ambulation time, and length of hospital stay
No significant differences were observed in PACU stay [MD −0.75; 95% CI −3.00, 1.49; p = 0.51; I2 = 95%] (7, 27), first ambulation [MD −0.41; 95% CI −1.30, 0.48; p = 0.36; I2 = 90%] (7, 25, 27), and lengthy of hospital stay [MD −0.16; 95% CI −0.55, 0.24; p = 0.44; I2 = 95%] (7, 24, 27) between ESPB and control group. However, patients receiving ESPB had a significantly prolonged time to first analgesic requirement [MD 14.17; 95% CI 5.50, 22.85; p = 0.001; I2 = 100%] (6, 7, 13, 14) (Supplementary Figure 4).
3.3.5 PONV and patients’ satisfaction scores
Two studies compared the incidence of PONV between ESPB and control groups (24, 26) (ESPB vs. no block-care), revealing no significant differences [RR 0.77; 95% CI 0.39, 1.51; p = 0.45; I2 = 0%] (Supplementary Figure 5). Similarly, three studies found no significant differences in patient satisfaction scores between ESPB and control groups (7, 23, 24) (ESPB vs. QLB, no-block care) [MD 0.79; 95% CI −0.09, 1.67; p = 0.08; I2 = 97%] (Supplementary Figure 6).
3.3.6 Postoperative QoR-15/40
One study reported higher QoR-15 scores at 24 h in the ESPB group compared to non-block care (175.02 ± 11.25 vs. 167.78 ± 18.59, p < 0.05) (25). However, another study comparing ESPB with sham block found no significant differences in QoR-40 scores at 24 h [115 (103–132) vs. 114 (101–126), p = 0.26] or 48 h [132 (110–144) vs. 129 (118–136), p = 0.22] found no significance different between two groups (27).
3.3.7 Regional blocks related to complications
No procedure-related complications, such as nerve injury, pneumothorax, hematoma formation, or local anesthetic systemic toxicity, were reported in any of the included studies.
3.4 Publication bias
Due to the high heterogeneity (I2 > 50%) and limited number of included RCTs, publication bias was not assessed using funnel plots. Sensitivity analysis using the leave-one-out approach revealed no significant changes in the pooled effect size. The risk of bias was assessed using the Cochrane Risk of Bias Tool 2 (Supplementary Table 2). Using GRADE, the certainty of evidence for both primary and secondary outcomes ranged from moderate to high (Supplementary Table 3). Specifically, the key end-points—24 h opioid consumption, pain scores at rest and on movement, and time to first rescue analgesic—were all supported by moderate- to high-certainty evidence.
4 Discussion
This meta-analysis demonstrates that ultrasound-guided ESPB significantly reduces postoperative opioid consumption at 24 h compared to non-block care and sham block in patients undergoing metabolic surgery (p = 0.001). The observed 6.68 mg reduction in 24 h morphine consumption is clinically relevant. Although it falls short of the 10 mg threshold identified as the minimal clinically important difference after arthroplasty, this reduction remains meaningful in the context of metabolic surgery, where patients with obesity are particularly susceptible to opioid-related respiratory depression and other adverse events. Additionally, ESPB was associated with lower resting pain scores at 24 h (p < 0.00001) and reduced movement pain scores at 6, 12, 24, and 48 h postoperatively (p < 0.00001 for all time points). These findings suggest that ESPB provides effective postoperative analgesia, which is particularly relevant in metabolic surgery, where pain management is crucial for early mobilization and recovery. Furthermore, ESPB prolonged the time to first rescue analgesic requirement (p = 0.001), indicating its potential to reduce the need for additional opioid administration, which is beneficial in minimizing opioid-related side effects such as PONV. Interestingly, despite the analgesic benefits, ESPB did not significantly reduce the incidence of PONV or improve patient satisfaction scores (p = 0.45 and p = 0.08, respectively). This may be attributed to several factors. First, the limited number of studies reporting PONV outcomes could introduce bias (24, 26). Second, the postoperative pain management protocols in most studies included opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), which are known to contribute to PONV. This highlights the need for future studies to explore multimodal analgesic regimens that minimize opioid use while maximizing the benefits of regional anesthesia techniques like ESPB (29). The safety profile of ESPB was notable, as none of the included studies reported complications such as nerve injury, pneumothorax, hematoma formation, or local anesthetic systemic toxicity (LAST). No complications were reported, but studies lacked systematic assessment (e.g., neurological exams, local anesthetic toxicity screens). Small samples and short follow-up preclude definitive safety conclusions. The safety of ESPB in obesity requires larger trials with protocolized monitoring. Notably, all included RCTs employed a bilateral single-injection technique, obviating the need for continuous catheterization and thereby reducing the risk of catheter-related complications and enhancing the practical applicability of ESPB in routine metabolic surgical care.
Four studies compared ESPB with no block (23–26), yielding conflicting results. Reported that ESPB did not significantly reduce morphine consumption or improve quality of recovery (23). In contrast, the other three studies demonstrated that ESPB significantly reduced both intraoperative and postoperative analgesic consumption, provided effective postoperative pain control, and enhanced postoperative quality of recovery (24–26). Similarly, two studies comparing ESPB with sham block found that ESPB offered satisfactory postoperative analgesia and reduced analgesic consumption (14). However, while ESPB effectively lowered pain scores, it did not improve global QoR-15 scores (27). When compared to other regional anesthesia techniques, such as TAPB and QLB, ESPB provided comparable or superior pain control, as evidenced by reduced pain scores and opioid consumption in patients undergoing metabolic surgery (6, 7, 13, 28). The variability in clinical outcomes across studies may also be influenced by factors such as patient positioning during block administration, injection speed, local anesthetic volume, and comparator type. Recent imaging studies have shown that prone positioning and higher injection speeds can lead to wider spread of local anesthetics, potentially enhancing the analgesic efficacy (30). These factors should be considered when designing future trials to optimize ESPB protocols (8). Thus, these reasons also may contribute to the different clinical results. In the current study, seven studies recorded the ESPB injection positions (13, 23–28) (prone position, seated posture, and lateral position). Moreover, most of included studies used 30 mL of 0.25% bupivacaine 15 to 30 mL on each side before or after general anesthesia induction. In addition, performing ESPB in obesity may also pose several challenges. First, deep fascial planes (>4–6 cm) limit ultrasound penetration, requiring low-frequency curvilinear probes (10). Second, prone positioning may be impractical; lateral decubitus optimizes ergonomics. Third, targeting T7–T9 achieves optimal dermatomal coverage for upper abdominal surgery (11). Last but not least, heterogeneity may also arise from center-specific expertise or equipment. Future studies should standardize those imaging protocols.
Several study limitations should be considered when interpreting our results: First, this study only included 10 RCTs and included moderate samples. The lack of quantity analysis did not assess publication bias by Egger or Begg regression. Second, the results of this study showed ESPB may not have a promising analgesia effect compared with non-block care, TAPB, QLB, and LIA in patients undergoing metabolic surgery. Third, all the included studies only described different metabolic surgeries but did not classify the types of incisions and surgical techniques involved, so selection bias may underestimate the analgesic efficacy of the ESPB. Last but not the least, heterogeneity stems from methodological diversity, including control types (sham vs. no-block), anesthetic volumes (15–30 mL), and administration timing. Most of the literature is composed of small and moderate sample sizes, with the largest included experimental group including 151 patients. Future studies should perform more analyses of ESPB, which would provide great incentive for setting guidelines for perioperative pain management.
5 Conclusion
In summary, this meta-analysis offers moderate evidence that ultrasound-guided ESPB constitutes an effective and safe analgesic strategy for patients undergoing metabolic surgery. ESPB significantly reduces postoperative opioid consumption and pain scores, particularly at 24 h postoperatively, while prolonging the time to first rescue analgesic requirement. Although ESPB did not significantly reduce PONV or improve patient satisfaction scores in this analysis, its favorable safety and ease of performance make it a valuable addition to multimodal analgesic regimens in metabolic surgery.
Future research should focus on standardizing ESPB techniques and exploring its role in multimodal analgesia to further minimize opioid use and enhance recovery outcomes. Additionally, larger, well-designed RCTs are needed to evaluate the impact of ESPB on patient-reported outcomes, such as quality of recovery and satisfaction, as well as its comparative effectiveness against other regional anesthesia techniques. Until then, ESPB should be considered a promising option for postoperative pain management in metabolic surgery, particularly in settings where opioid-sparing strategies are prioritized.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YW: Project administration, Funding acquisition, Writing – review & editing, Resources, Methodology, Formal analysis, Writing – original draft, Software, Visualization, Conceptualization, Supervision, Validation, Data curation, Investigation. YL: Supervision, Formal analysis, Software, Writing – original draft, Data curation, Writing – review & editing, Project administration, Conceptualization, Resources, Visualization, Investigation, Validation. WJ: Resources, Investigation, Validation, Software, Writing – review & editing, Supervision, Conceptualization, Writing – original draft, Project administration, Visualization, Data curation. QY: Writing – original draft, Supervision, Formal analysis, Resources, Writing – review & editing, Investigation, Data curation, Visualization, Methodology, Conceptualization, Software. FJ: Data curation, Validation, Methodology, Formal analysis, Visualization, Writing – review & editing, Investigation, Resources, Conceptualization, Supervision, Software, Writing – original draft, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1630657/full#supplementary-material
References
1. Hayes, K, and Eid, G. Laparoscopic sleeve gastrectomy: surgical technique and perioperative care. Surg Clin North Am. (2016) 96:763–71. doi: 10.1016/j.suc.2016.03.015
2. Nair, A, Rangaiah, M, Dudhedia, U, and Borkar, N. Analgesic efficacy and outcomes of ultrasound-guided erector spinae plane block in patients undergoing bariatric and metabolic surgeries: a systematic review. J Med Ultrason. (2023) 31:178–87. doi: 10.4103/jmu.jmu_112_22
3. Achi, N, Wang, H, Hao, J, and Chen, W. Innovative approaches to managing postoperative complications in laparoscopic sleeve gastrectomy: a scoping review. J Laparoendosc Adv Surg Tech A. (2025) 35:6–14. doi: 10.1089/lap.2024.0227
4. Macfater, H, Xia, W, Srinivasa, S, Hill, AG, Van De Velde, M, and Joshi, GP. Evidence-based management of postoperative pain in adults undergoing laparoscopic sleeve gastrectomy. World J Surg. (2019) 43:1571–80. doi: 10.1007/s00268-019-04934-y
5. Benyamin, R, Trescot, AM, Datta, S, Buenaventura, R, Adlaka, R, Sehgal, N, et al. Opioid complications and side effects. Pain Physician. (2008) 11:S105–20.
6. Abdelhamid, BM, Khaled, D, Mansour, MA, and Hassan, MM. Comparison between the ultrasound-guided erector spinae block and the subcostal approach to the transversus abdominis plane block in obese patients undergoing sleeve gastrectomy: a randomized controlled trial. Minerva Anestesiol. (2020) 86:816–26. doi: 10.23736/S0375-9393.20.14064-1
7. Ashoor, TM, Jalal, AS, Said, AM, Ali, MM, and Esmat, IM. Ultrasound-guided techniques for postoperative analgesia in patients undergoing laparoscopic sleeve gastrectomy: erector spinae plane block vs. quadratus lumborum block. Pain Physician. (2023) 26:245–56.
8. Huang, X, Wang, J, Zhang, J, Kang, Y, Sandeep, B, and Yang, J. Ultrasound-guided erector spinae plane block improves analgesia after laparoscopic hepatectomy: a randomised controlled trial. Br J Anaesth. (2022) 129:445–53. doi: 10.1016/j.bja.2022.05.013
9. Huang, X, Sandeep, B, and Yang, J. Mapping structural and research trends in surgical use of ultrasound-guided erector spinae plane block: a bibliometric analysis – correspondence. Int J Surg. (2022) 106:106904. doi: 10.1016/j.ijsu.2022.106904
10. Forero, M, Adhikary, SD, Lopez, H, Tsui, C, and Chin, KJ. The erector spinae plane block: a novel analgesic technique in thoracic neuropathic pain. Reg Anesth Pain Med. (2016) 41:621–7. doi: 10.1097/AAP.0000000000000451
11. Chin, KJ, and El-Boghdadly, K. Mechanisms of action of the erector spinae plane (ESP) block: a narrative review. Can J Anesth/J Can Anesth. (2021) 68:387–408. doi: 10.1007/s12630-020-01875-2
12. Chin, KJ, Malhas, L, and Perlas, A. The erector Spinae plane block provides visceral abdominal analgesia in bariatric surgery: a report of 3 cases. Reg Anesth Pain Med. (2017) 42:372–6. doi: 10.1097/AAP.0000000000000581
13. Elshazly, M, El-Halafawy, YM, Mohamed, DZ, Wahab, KAE, and Mohamed, TMK. Feasibility and efficacy of erector spinae plane block versus transversus abdominis plane block in laparoscopic bariatric surgery: a randomized comparative trial. Korean J Anesthesiol. (2022) 75:502–9. doi: 10.4097/kja.22169
14. Mostafa, SF, Abdelghany, MS, and Abu Elyazed, MM. Ultrasound-guided erector Spinae plane block in patients undergoing laparoscopic bariatric surgery: a prospective randomized controlled trial. Pain Pract. (2021) 21:445–53. doi: 10.1111/papr.12975
15. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg. (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
16. Drevon, D, Fursa, SR, and Malcolm, AL. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav Modif. (2017) 41:323–39. doi: 10.1177/0145445516673998
17. Svendsen, K, Borchgrevink, P, Fredheim, O, Hamunen, K, Mellbye, A, and Dale, O. Choosing the unit of measurement counts: the use of oral morphine equivalents in studies of opioid consumption is a useful addition to defined daily doses. Palliat Med. (2011) 25:725–32. doi: 10.1177/0269216311398300
18. Kerwin, AJ, Haut, ER, Burns, JB, Como, JJ, Haider, A, Stassen, N, et al. The eastern Association of the Surgery of trauma approach to practice management guideline development using grading of recommendations, assessment, development, and evaluation (GRADE) methodology. J Trauma Acute Care Surg. (2012) 73:S283–7. doi: 10.1097/TA.0b013e31827013e
19. Hozo, SP, Djulbegovic, B, and Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. (2005) 5:13. doi: 10.1186/1471-2288-5-13
20. Chen, B, and Benedetti, A. Quantifying heterogeneity in individual participant data meta-analysis with binary outcomes. Syst Rev. (2017) 6:243. doi: 10.1186/s13643-017-0630-4
21. Bowden, J, Tierney, JF, Copas, AJ, and Burdett, S. Quantifying, displaying and accounting for heterogeneity in the meta-analysis of RCTs using standard and generalised Q statistics. BMC Med Res Methodol. (2011) 11:41. doi: 10.1186/1471-2288-11-41
22. Sterne, JAC, Savović, J, Page, MJ, Elbers, RG, Blencowe, NS, Boutron, I, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
23. Jinaworn, P, Pannangpetch, P, Bunanantanasan, K, Manomaisantiphap, S, Udomsawaengsup, S, Thepsoparn, M, et al. Efficacy of erector Spinae plane block on postoperative analgesia for patients undergoing metabolic bariatric surgery: a randomized controlled trial. Obes Surg. (2024) 34:4211–9. doi: 10.1007/s11695-024-07515-8
24. Karaveli, A, Kaplan, S, Kavakli, AS, Kosar, MN, and Mayir, B. The effect of ultrasound-guided erector Spinae plane block on postoperative opioid consumption and respiratory recovery in laparoscopic sleeve gastrectomy: a randomized controlled study. Obes Surg. (2025) 35:112–21. doi: 10.1007/s11695-024-07576-9
25. Toprak, H, Başaran, B, Toprak, ŞS, Et, T, Kumru, N, Korkusuz, M, et al. Efficacy of the erector Spinae plane block for quality of recovery in bariatric surgery: a randomized controlled trial. Obes Surg. (2023) 33:2640–51. doi: 10.1007/s11695-023-06748-3
26. ul Huda, A, Alshahrani, AS, Yasir, M, Sawilah, A, and Alharthi, AAN. Erector spinae block reduces intraoperative and postoperative opioid consumption in patients undergoing laparoscopic sleeve gastrectomy: a randomized controlled trial. Qatar Med J. (2024) 2024:58. doi: 10.5339/qmj.2024.58
27. Wang, Y, Zuo, S, Ma, Y, Shen, J, Chu, Q, and Yang, Z. Effect of ultrasound-guided erector Spinae plane block on recovery after laparoscopic sleeve gastrectomy in patients with obesity: a randomized controlled trial. Clin Ther. (2023) 45:894–900. doi: 10.1016/j.clinthera.2023.07.010
28. Zengin, SU, Ergun, MO, and Gunal, O. Effect of ultrasound-guided erector Spinae plane block on postoperative pain and intraoperative opioid consumption in bariatric surgery. Obes Surg. (2021) 31:5176–82. doi: 10.1007/s11695-021-05681-7
29. Kushner, BS, Freeman, D, Sparkman, J, Salles, A, Eagon, JC, and Eckhouse, SR. Assessment of postoperative nausea and vomiting after bariatric surgery using a validated questionnaire. Surg Obes Relat Dis. (2020) 16:1505–13. doi: 10.1016/j.soard.2020.05.017
Keywords: metabolic surgery, erector spinae plane block, regional anesthesia, meta-analysis, bariatric procedures
Citation: Wang Y, Liu Y, Jie W, Yang Q and Jiang F (2025) Analgesic efficacy of ultrasound-guided ESPB on metabolic surgery. Front. Med. 12:1630657. doi: 10.3389/fmed.2025.1630657
Edited by:
Francisco Lopez-Munoz, Camilo José Cela University, SpainReviewed by:
Malgorzata Reysner, Poznan University of Medical Sciences, PolandRobert Stenberg, Cleveland Clinic, United States
Sevim Cesur, Kocaeli University, Türkiye
Copyright © 2025 Wang, Liu, Jie, Yang and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fenglin Jiang, amlhbmdmZW5nbGluMDEyNUAxNjMuY29t
†These authors have contributed equally to this work and share first authorship
 Ying Wang1†
Ying Wang1† Fenglin Jiang
Fenglin Jiang