- 1School of Public Health, The Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, Guizhou Medical University, Guiyang, Guizhou, China
- 2Department of Infection, Guiyang Public Health Clinical Center, Guiyang, Guizhou, China
Background: Evidence regarding the use of dolutegravir plus lamivudine (DTG + 3TC) among patients with HIV infection who present late remains limited. This study aimed to evaluate the effectiveness and safety of DTG + 3TC therapy in patients with late presentation in Southwest China.
Methods: This single-center, retrospective cohort study included patients with late presentation who initiated DTG + 3TC anti-retroviral therapy (ART) between January 2020 and July 2023 (N = 176). Changes in immunologic and metabolic parameters as well as liver and kidney function, were assessed. The primary endpoint was the proportion of participants with HIV-1 RNA < 50 copies/mL at week 48. Late presentation was defined as CD4 < 350 cells/μL or the presence of AIDS-defining conditions.
Results: At weeks 24 and 48, 83.0% (146/176) and 90.9% (160/176) of the patients achieved HIV-1 RNA levels <50 copies/mL, respectively. At week 48, the median CD4 count increased by 139.5 cells/μL (120.5–158.5), and the CD4/CD8 ratio increased by 0.2 (0.1–0.3) (p < 0.001). No patient discontinued treatment owing to adverse events during the observation period.
Conclusion: DTG + 3TC demonstrated high virologic efficacy and good tolerability in patients with late presentation. However, the regimen may be associated with an increase in lipid levels and weight, highlighting the need for regular monitoring.
Introduction
People diagnosed late with HIV-1 infection are a vulnerable group and experience delayed diagnosis and treatment. Late presentation hinders epidemic control and is associated with high mortality rates (1), high transmission (2), and high treatment costs, all of which pose major challenges in global human immunodeficiency virus (HIV) prevention and control (3). Unfortunately, even with high-cost treatment, the health status of patients with late presentation remains worse than that of those who seek timely care, and their medical expenses continue to rise, placing significant burdens on both the individual patients and healthcare system (3). Untreated late presentation is a common issue in global HIV prevention and control. For instance, a study encompassing 17 European countries demonstrated that the late-presenting rate was 48.4% (4), with China’s total late-presenting rate at 43.26% between 2010 and 2020 (5). These data suggest that late presentation is one of the major challenges facing the global context of acquired immunodeficiency syndrome (AIDS).
Anti-retroviral therapy (ART) transforms AIDS from a fatal disease into a manageable chronic condition (6). Since there is no cure for AIDS, patients need to take medication for life. Long-term drug use may lead to drug toxicities, such as cardiovascular disease (7), liver and kidney damage (8, 9), and neurocognitive disorders (10). These drugs can seriously affect the quality of life of people living with HIV (PLWH). The elderly (≥50 years) are the most important high-risk group for late presentation in China (5). With universal access to antiretroviral treatment, more than 20% patients with late presentation worldwide are over 50 years old (11). Considering that the numbers of associated chronic comorbidities and medications increase with age (12), the combination of ART and non-ART medications can further exacerbate drug toxicities in this patient population. Therefore, suitable treatment options are needed for patients aged over 50 years with late presentation.
A standard ART initiation regimen typically comprises a three-drug combination (13–16). To reduce drug toxicity and drug–drug interactions, two-drug regimens (2DRs) have become a focus of recent research, with the aim of lowering the overall drug burden while maintaining an efficacy comparable to that of three-drug regimens (3DRs). Second-generation integrase strand transfer inhibitors (INSTIs), such as dolutegravir (DTG), are considered ideal core agents because of their high barrier to resistance, strong efficacy, favorable safety profile, and minimal drug–drug interactions (17). Currently, multiple international and Chinese guidelines recommend DTG + lamivudine (3TC) as a first-line regimen for the treatment of PLWH (13–16). Numerous studies have demonstrated the efficacy and safety of DTG + 3TC as an initial regimen (18–21), and others have shown that DTG + 3TC is non-inferior to 3DRs in treatment-naïve PLWH (20–23).
Theoretically, ART should be initiated in patients with late presentation as soon as possible, even before resistance test results and other test results are available. Therefore, the optimal regimen should have high efficacy and a high barrier to resistance, and 2DRs of DTG + 3TC may be a cost-effective and efficient option for those with late presentation. This study aimed to evaluate the efficacy and safety of the DTG + 3TC regimen in patients with late HIV presentation in Southwest China.
Materials and methods
Study design and participants
This was an observational, single-center, retrospective study. The study site was the Guiyang Public Health Clinical Center, which manages approximately 20% of the PLWH in Guizhou Province, China, and is one of the largest infectious disease hospitals in Southwest China. Newly reported cases of late presentation between 1 January 2020 and 31 July 2023 were recruited as the study population and followed until 31 July 2024. The inclusion criteria were as follows: (1) HIV-1 antibody confirmed positive by Western blot, (2) age ≥18 years, (3) treatment-naïve, late presentation of HIV-1 infection, (4) and ART regimen of DTG + 3TC [including DTG/3TC (compound single tablet preparation) or DTG + 3TC (two pills) simplified double regimen, including patients with 3TC reduction due to abnormal renal function]. The exclusion criteria were as follows: (1) presence of hepatitis B virus (HBV) infection, (2) pregnancy, (3) loss to follow-up or death, and (4) incomplete baseline information.
Study endpoints
The primary endpoint was the rate of virologic suppression after 48 weeks on the DTG + 3TC regimen, calculated as the proportion of patients with HIV-1 RNA < 50 copies/mL. The secondary endpoints were as follows: (1) virologic efficacy after 24 weeks with the DTG + 3TC regimen and (2) changes in immunologic efficacy (CD4, CD4/CD8), metabolic function, liver and kidney function and occurrence of adverse events (AEs) at week 48.
Definitions
According to international and Chinese guidelines, late presentation was defined as CD4 < 350 cells/μL or meeting the criteria for AIDS-defining conditions (4, 16, 24, 25). AIDS-defining conditions were as follows (16): persistent irregular fever of unknown cause, diarrhea for >1 month (>3 bowel movements/day), decrease in body mass >10% within 1 month, recurrent oral fungal infections, recurrent herpes simplex virus infection or herpes zoster virus infection, Pneumocystis jirovecii pneumonia (PJP), recurrent bacterial pneumonia, active tuberculosis (TB) or nontuberculosis mycobacteria disease, deep fungal infection, central nervous system space-occupying lesions, development of dementia as a middle-aged or young person, active cytomegalovirus infection, Toxoplasma gondii encephalopathy, Penicillium marneffei infection, recurrent sepsis, Kaposi sarcoma, and lymphoma.
Virologic suppression was defined as HIV-1 RNA < 50 copies/mL at week 48. Virologic failure was defined as HIV-1 RNA ≥ 200 copies/mL at week 48. When ART was initiated within 7 days of HIV-1 antibody confirmation, it was considered as rapid ART.
Dyslipidemia was defined as follows: total cholesterol (TC) ≥ 5.2 mmol/L, triglyceride (TG) level ≥1.7 mmol/L, high-density lipoprotein cholesterol (HDL-C) < 1 mmol/L, and low-density lipoprotein cholesterol (LDL-C) ≥ 3.4 mmol/L (26).
Abnormal liver function was defined as aspartate aminotransferase (AST) > 40 U/L and alanine aminotransferase (ALT) level >50 U/L (27).
Renal dysfunction was defined as estimated glomerular filtration rate (eGFR) < 60 mL/min (28, 29). The eGFR is calculated using the CKD-EPI formula, which includes sex, age, and serum creatinine (Scr), and can represent changes in renal function in a more comprehensive way (30).
Data collection and laboratory tests
Participants’ information was obtained from the China AIDS Prevention and Control Information System and the case system of the Guiyang Public Health Clinical Center. The following information was collected at baseline and weeks 24 and 48: (1) basic personal information: age, sex, infection characteristics, height, weight, date of HIV-1 diagnosis, date of ART initiation; (2) laboratory test results: HIV-1 RNA, CD4 count, complete blood count, lipid levels (four items), glucose (GLU), and amylase (AMY); and (3) other information: comorbidities (clinical diagnosis), AIDS-defining conditions (clinical diagnosis), primary reasons for use of DTG + 3TC, and AEs. To ensure data comparability, all tests were performed at the Guiyang Public Health Clinical Center, using standardized instruments, reagents, and methodologies. Participants with missing baseline data were excluded. For participants lacking data at the 24- and 48-week time points, the following procedures were applied: (1) results within the 30-day windows of the target time points were accepted; (2) if no window data were available, direct imputation was performed when missingness was <5%. The imputation method was based on the Kolmogorov–Smirnov test: mean imputation was applied to normally distributed data (p > 0.05), and median imputation, to non-normally distributed data.
Statistical analysis
Excel was used to input data, and R 4.3.1 was used for statistical analysis. Based on the data distribution type, qualitative variables were reported as frequency distributions, whereas quantitative variables were described as median (IQR) or mean (SD). The Kolmogorov–Smirnov test was used to determine whether the numerical variables fit the assumptions for normality of distribution. Paired t-test was used to compare normally distributed independent variables between the baseline and week 48, while Wilcoxon signed-rank test was used for continuous numeric variables that followed a non-normal distribution. Classification data were compared using the chi-square or Fisher’s exact tests. All statistical tests were two-tailed, and p < 0.05 was considered statistically significant.
Results
Patient characteristics
The patient selection process is shown in Figure 1. A total of 193 patients received DTG + 3TC between January 2020 and July 2023. Among these patients, one patient was lost to follow-up, four patients died (unrelated to the medication), five patients had no baseline data, and seven patients changed medications due to financial reasons. Finally, 176 participants were included.
Figure 1. Participant selection process DTG + 3TC, dolutegravir plus lamivudine, including DTG/3TC (compound single tablet preparation) or DTG + 3TC (two pills) simplified double regimen.
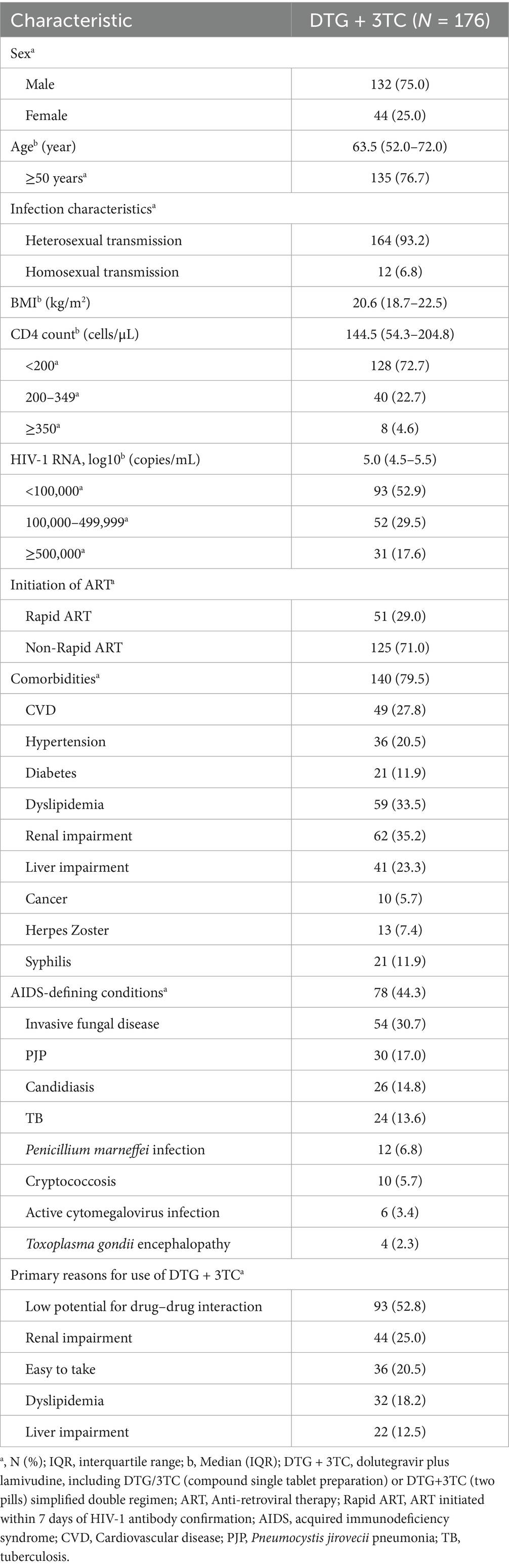
The baseline characteristics of the patients are described in Table 1. The patients were predominantly male (n = 132, 75.0%). The median age of the patients was 63.5 (52.0–72.0) years, and the proportion of older patients (≥50 years) was as high as 76.7% (135/176). At baseline, the median CD4 count was 144.5 (54.3–204.8) cells/μL, with 128 (72.7%) patients having a CD4 count of <200 cells/μL. The median log10 HIV-1 RNA was 5.0 (4.5–5.5) copies/mL; 31 (17.6%) patients had HIV-1 RNA ≥ 5 × 105 copies/mL; 29.0% (51/176) of the patients received rapid ART. In our patient group, 140 (79.5%) patients had comorbidities, the most common being renal impairment, at 35.2% (62/176), followed by dyslipidemia (n = 59, 33.5%), cardiovascular disease (CVD) (n = 49, 27.8%), liver impairment (n = 41, 23.3%), hypertension (n = 36, 20.5%), diabetes (n = 21, 11.9%), and cancer (n = 10, 5.7%). Among all AIDS-defining conditions (n = 78, 44.3%), the most common was invasive fungal disease (n = 54, 30.7%), followed by PJP (n = 30, 17.0%), candidiasis (n = 26, 14.8%), and TB (n = 24, 13.6%). The main reasons for choosing DTG + 3TC included low potential for drug–drug interaction (n = 93, 52.8%), renal impairment (n = 44, 25.0%), and ease of use (n = 36, 20.5%).
Virologic efficacy
At week 24, 83.0% (146/176) of the participants had HIV-1 RNA levels <50 copies/mL, while 4.5% (8/176) had levels ≥200 copies/mL. The virologic suppression rates were 64.5% (20/31) for those with baseline HIV-1 RNA ≥ 500,000 copies/mL and 86.9% (126/145) for those with HIV-1 RNA < 500,000 copies/mL. Participants with baseline CD4 counts <200 cells/μL had a virologic suppression rate of 80.5% (103/128), compared to 89.6% (43/48) for those with CD4 counts ≥200 cells/μL. Suppression rates for rapid and non-rapid ART initiation were 86.3% (44/51) and 81.6% (102/125), respectively. Among participants aged ≥50 years and <50 years, the suppression rates were 83.7% (113/135) and 80.5% (33/41), respectively (Figure 2).
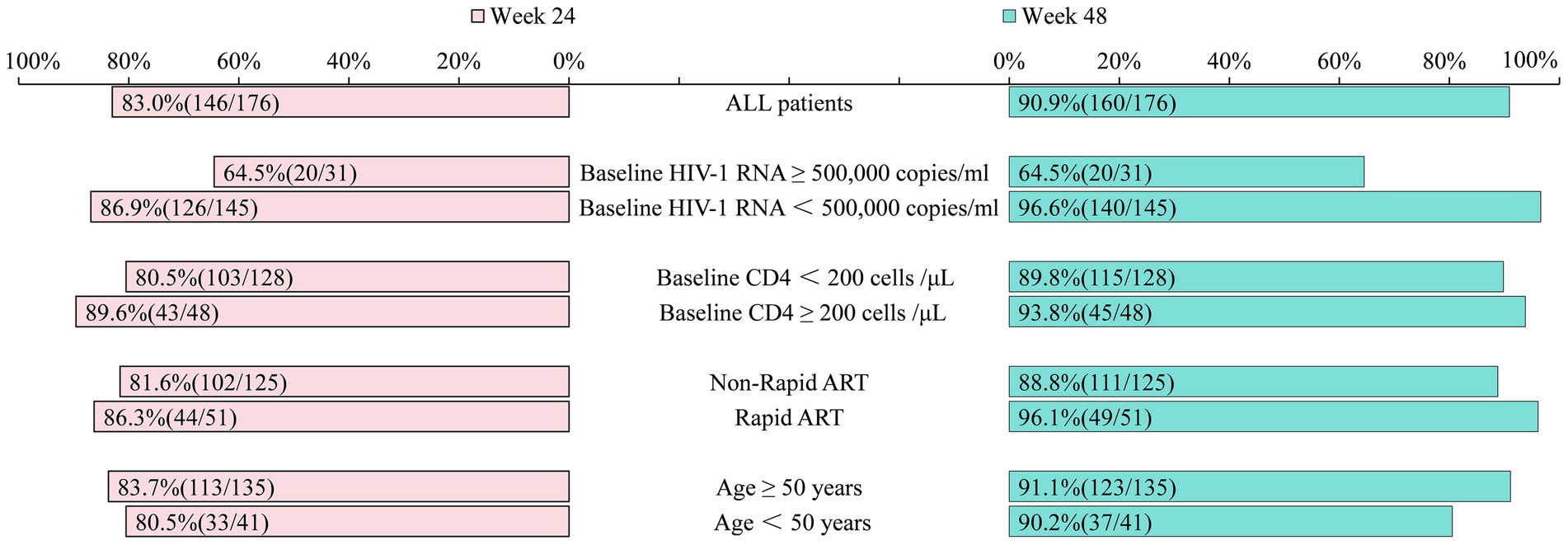
Figure 2. Comparison of the proportion of patients with HIV-1 RNA < 50 copies/mL at week 24 versus week 48 (%). The same standardized instruments, reagents, and methodologies were used throughout the process to detect viral load. ART, Anti-retroviral therapy; Rapid ART, ART initiated within 7 days of HIV-1 antibody confirmation.
At week 48, 90.9% (160/176) of the participants had HIV-1 RNA levels <50 copies/mL, and an intention to treat (snapshot) analysis showed that if all 193 patients who ever received DTG + 3TC were retained and the 17 now excluded (losses, deaths, baseline data gaps, economic switches) were counted as non-suppressed, the viral suppression rate at week 48 would be 160 /193 ≈ 83.0%, not 90.9% (160/176). 3.4% (6/176) of the participants would have RNA levels of ≥200 copies/mL. The virologic suppression rates were 64.5% (20/31) and 96.6% (140/145) for those with baseline HIV-1 RNA ≥ 500,000 and <500,000 copies/mL, respectively. The suppression rates for baseline CD4 < 200 cells/μL and CD4 ≥ 200 cells/μL were 89.8% (115/128) and 93.8% (45/48), respectively. Rapid ART initiation resulted in a suppression rate of 96.1% (49/51), compared to 88.8% (111/125) for non-rapid initiation. Among participants aged ≥50 years and <50 years, the suppression rates at baseline were 91.1% (123/135) and 90.2% (37/41), respectively (Figure 2).
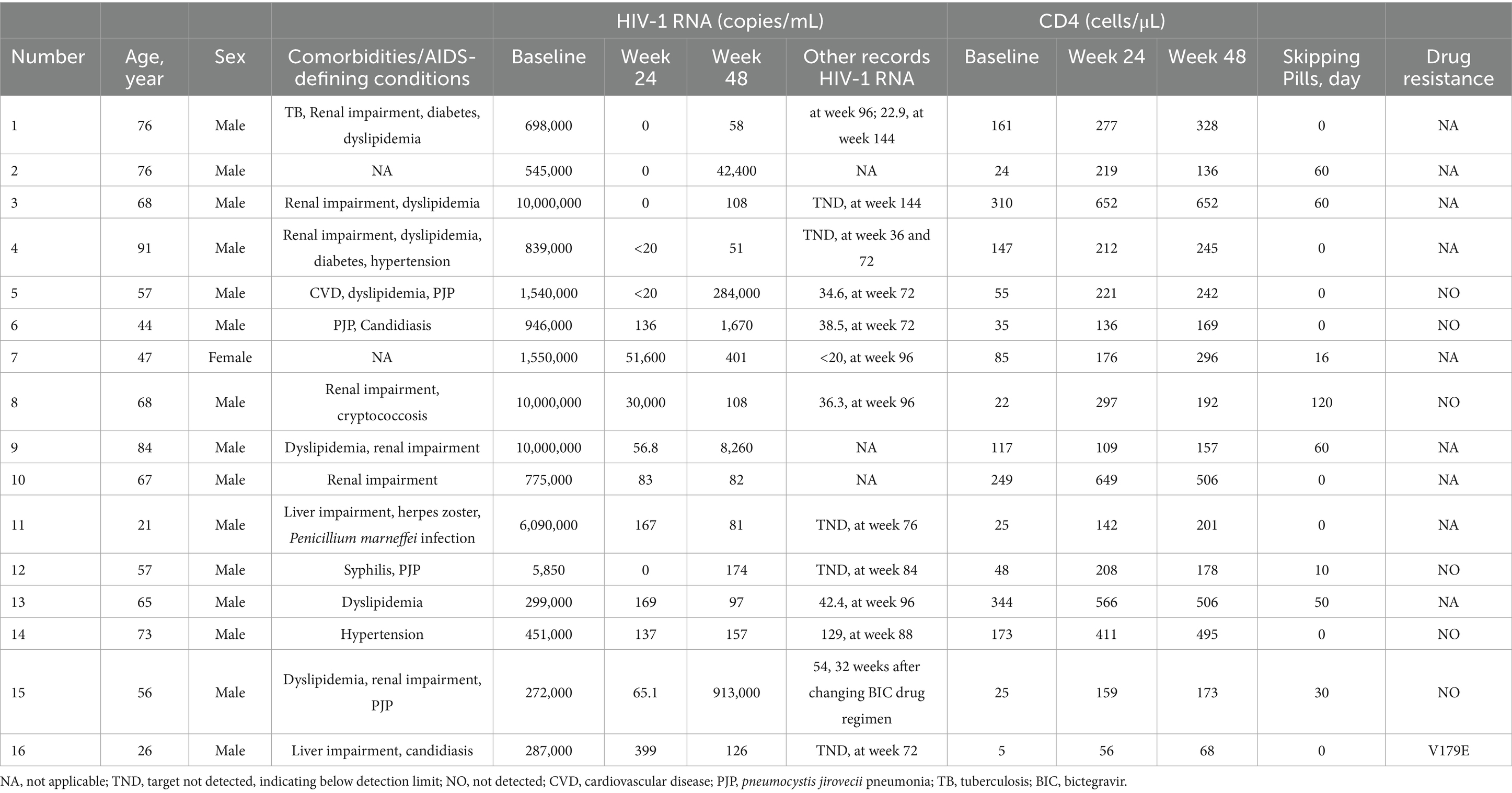
At week 48, 16 participants in the study had HIV-1 RNA ≥ 50 copies/mL. Among them, eight had documented treatment discontinuation, and 11 achieved virologic suppression during subsequent follow-up. Notably, in one patient with a 48-week HIV-1 RNA level of 913,000 copies/mL, who switched to a 3-drug bictegravir (BIC) regimen, the level declined to 54 copies/mL after 32 weeks. Three patients had no available viral load (VL) test results after week 48. At week 48, 11 patients with baseline HIV-1 RNA ≥ 500,000 copies/mL had HIV-1 RNA levels ≥50 copies/mL, of whom five exhibited treatment discontinuation. Among patients with baseline CD4 counts <200 cells/μL, 13 had HIV-1 RNA ≥ 50 copies/mL, and six of these had self-discontinuation behavior. Of the 16 virologically unsuppressed patients, seven underwent baseline resistance testing, and one was found to have resistance at locus V179E (Table 2).
Immunologic, metabolic, and organ function outcomes
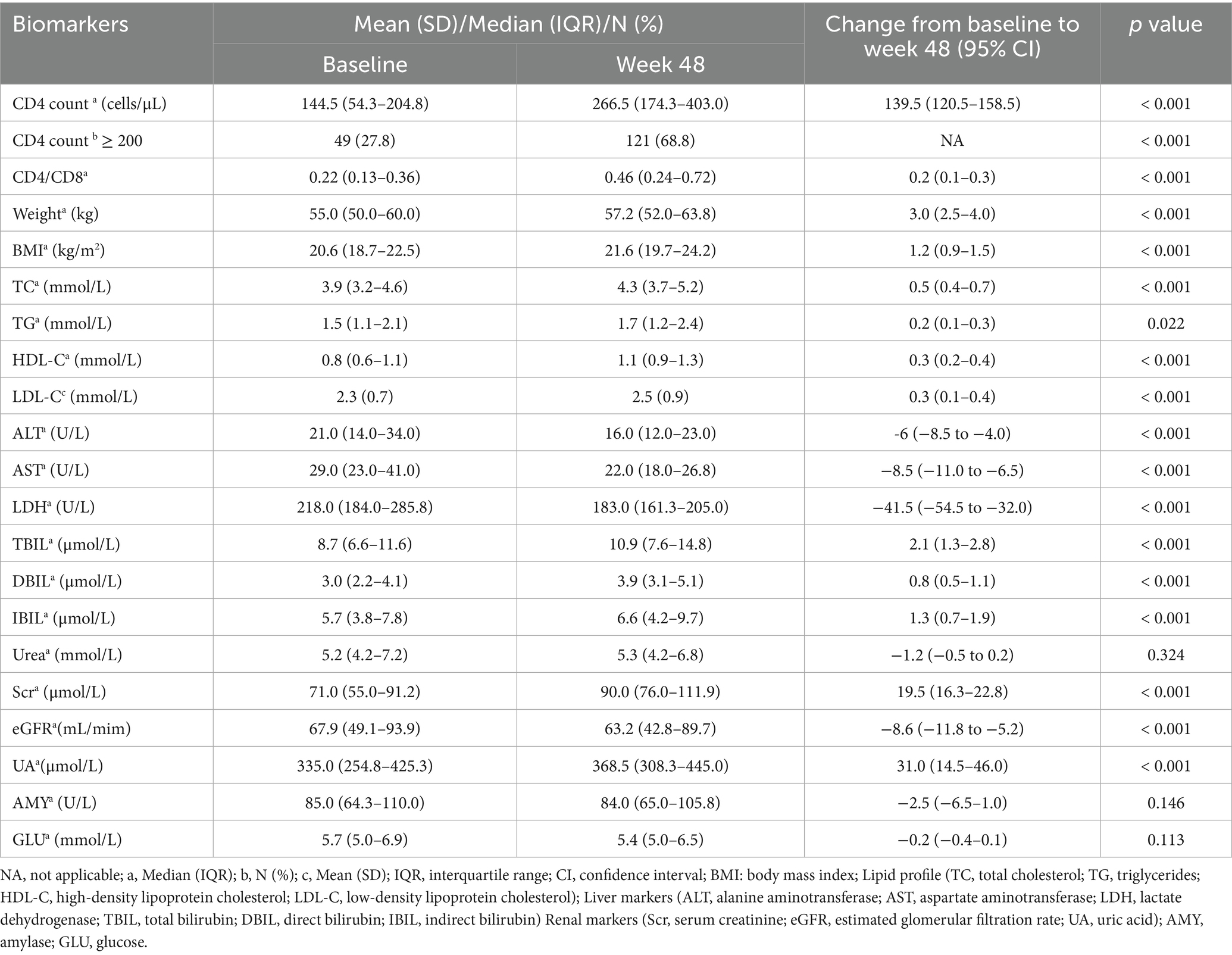
After 48 weeks, the mean CD4 count increased by 139.5 (120.5–158.5) cells/μL, and the CD4/CD8 ratio increased by 0.2 (0.1–0.3) (p < 0.001; Table 3). The number of patients with CD4 counts ≥200 cells/μL increased from 49 (27.8%) at baseline to 121 (68.8%) at week 48 (p < 0.001, Table 3). Metabolically, significant increments in weight [3.0 (2.5–4.0)]; body mass index [BMI; 1.2 (0.9–1.5)]; and TC [0.5 (0.4–0.7)], TG [0.2 (0.1–0.3)], HDL-C [0.3 (0.2–0.4)], and LDL-C [0.3 (0.1–0.4)] levels were observed. These changes were statistically significant (TG: p = 0.022, all others: p < 0.001). Regarding liver function, the levels of ALT [−6 (−8.5 to −4.0)], AST [−8.5 (−11.0 to −6.5)], and lactate dehydrogenase [LDH; −41.5 (−54.5 to −32.0)] decreased (p < 0.001). In contrast, total bilirubin [TBIL; 2.1 (1.3–2.8)], direct bilirubin [DBIL; 0.8 (0.5–1.1)], and indirect bilirubin [IBIL; 1.3 (0.7–1.9)] levels increased (p < 0.001), although these changes were not clinically significant. In terms of renal function, Scr [19.5 (16.3–22.8)] and uric acid levels [UA; 31.0 (14.5–46.0)] increased (p < 0.001), while eGFR level [−8.6 (−11.8 to −5.2)] decreased (p < 0.001). Urea levels did not change significantly (Table 3).
Adverse events
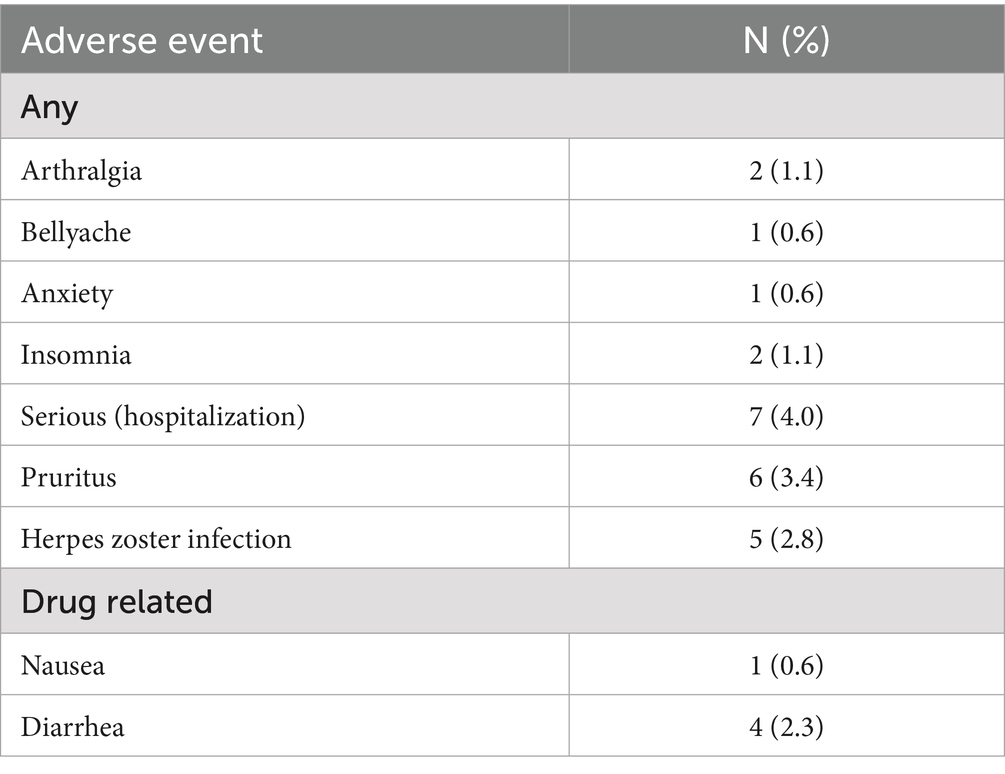
AEs occurred in 27 (15.3%) patients. Among them, seven (4.0%) patients were hospitalized for the following reasons: pulmonary issues (two cases), myocardial infarction, lung cancer, gastrointestinal bleeding, cytomegalovirus retinopathy, and osteophyte formation. Drug-related AEs occurred in five (2.8%) patients: nausea in one (0.6%) patient and diarrhea in four (2.3%). During the treatment period, no patient discontinued the medication owing to lack of drug efficacy or adverse effects (Table 4).
Discussion
This is the first retrospective cohort study in China to evaluate the efficacy and safety of DTG + 3TC in late-presenting individuals with HIV in a real-world setting. Virologic suppression rates were high at both 24 and 48 weeks, and no patient discontinued DTG + 3TC owing to adverse events.
At 24 weeks, the virologic suppression rate in the late-presentation cohort was 83.0% (146/176), which is lower than that reported in the STAT study (18). At week 48, the suppression rate increased to 90.9%, slightly below the rates observed in the GEMINI 1 and 2 trials (21–23). These discrepancies may be explained by the demographic and clinical characteristics of our cohort, including older age and low CD4 counts. Furthermore, patients with late presentation often experience greater challenges with medication adherence (31). In our study, among the 16 patients without virologic suppression, eight exhibited self-discontinuation of treatment for durations ranging from 10 to 120 days. Thus, non-adherence significantly impacted virologic outcomes, underscoring the importance of adherence education for individuals with late presentation of HIV.
Although DTG + 3TC is not currently recommended for treatment-naïve individuals with HIV-1 RNA ≥ 500,000 copies/mL (13–16), emerging evidence supports its use in this subgroup. The STAT study demonstrated suppression rates of 68% at week 24 and 89% at week 48 (18). In a study by Dou et al., suppression rates increased from 50% at 24 weeks to 78.3% at 48 weeks (20). In this study, virologic suppression reached 64.5% (20/31) at week 24, which was higher than that reported by Dou et al. (20) and lower than the rate observed in the STAT study (18), likely because high baseline viral loads are associated with delayed suppression (32). However, the suppression rate at week 48 remained at 64.5% (20/31), still lower than that reported in both the STAT study (18) and the study by Dou et al. (20). Notably, two of the five individuals with high baseline viral loads who achieved suppression at week 24 subsequently discontinued therapy and experienced viral rebound at week 48, suggesting that nonadherence may have contributed to the suboptimal outcomes. Whether DTG + 3TC is effective in reducing viral load in PLWH who present late with high viral burdens remains unclear and warrants further research.
The INSIGHT START study recently confirmed that early detection of HIV and timely initiation of ART are optimal strategies for preventing immune depletion and maintaining immune balance, thereby significantly lowering HIV-related morbidity and mortality (33). In our study, the patients who initiated ART within 7 days achieved a virologic suppression rate of 86.3% at week 24, which increased to 96.1% at week 48—findings consistent with those reported by Gan (34). These results suggest that rapid ART initiation in patients with late presentation not only enhances virologic control but also reduces the risk of HIV transmission.
As the prevalence of chronic comorbidities and polypharmacy increases with age, simplified ART regimens offer several advantages for older adults, including reduced drug–drug interactions, low cumulative toxicity, and improved tolerability and safety profiles. In our study, virologic suppression in patients aged ≥50 years increased from 83.7% (113/135) at week 24 to 91.1% (123/135) at week 48. These findings support the use of DTG + 3TC as an optimized option for late-presenting older adults with HIV.
The CD4 count and CD4/CD8 ratio are key indicators of immune system function. A reduced CD4/CD8 ratio reflects immune dysregulation and increased systemic inflammation (35) and is associated with a greater risk of AIDS-defining conditions and all-cause mortality (36). In our study, patients with baseline CD4 < 200 cells/μL achieved a virologic suppression rate of 89.8% (115/128) at week 48, consistent with the findings of Hou et al. (19). Additionally, after 48 weeks of treatment, both CD4 count and CD4/CD8 ratio increased significantly, with the proportion of patients having CD4 ≥ 200 cells/μL rising from 27.8 to 68.8%. These results indicate substantial immune restoration, aligning with the results of previous studies (18–21).
Long-term ART is known to negatively affect lipid metabolism, and dyslipidemia remains an independent risk factor for cardiovascular disease that requires careful monitoring (37). Current evidence suggests that INSTIs have relatively minor effects on lipid profiles (38). In our cohort, the levels of three lipid (TC, HDL-C, LDL-C) parameters increased by week 48 but remained within the normal reference ranges, consistent with findings from the GEMINI 1 and 2 studies (21) and of Deng et al. (39). Notably, GEMINI 1 and 2 (23) reported no dyslipidemia-related adverse events over a 3-year follow-up period, supporting the lipid-friendly profile of the DTG + 3TC regimen. In the present study, TG levels just reached the threshold for abnormal values (TG ≥ 1.7 mmol/L). TG levels are closely related to diet and lifestyle, and in cases where other lipid levels are normal and TG levels are not significantly elevated, only regular monitoring is required (26). In addition, TG levels tend to increase with age (26), and the median age of the patients in this study was 63.5 years (52.0–72.0), and 76.7% (135/176) of the patients were >50 years old; therefore, the high TG levels could be attributed to the age of our cohort. Based on the above analysis, we concluded that the changes in TG were not clinically significant and did not yet necessitate clinical intervention, but enhanced long-term monitoring of the four lipids is needed to facilitate timely intervention in the event of an abnormality. Similarly, an increase in weight and BMI observed at week 48 was consistent with the results of the GEMINI studies (21) and Wei et al. (40). This finding suggests improved overall health, considering that weight gain in individuals with HIV is often associated with reduced HIV-related inflammation and reversal of catabolic processes (41). Additionally, INSTIs have been shown to directly affect adipose tissue, particularly by inhibiting adipose shrinkage, which can lead to adipofibrosis and hypertrophy, ultimately contributing to weight gain (42). However, the observation period in this study was relatively short, and long-term monitoring of body weight and BMI is needed to further explore the relationships among weight, BMI, and metabolic changes.
DTG has been shown to inhibit organic cation transporter 2 (OCT2), leading to increased Scr levels. This mechanism reflects the non-pathological inhibition of proximal renal tubular secretion, indicating that the renal effects of DTG are reversible and clinically benign (43). After 48 weeks of treatment, a slight increase in Scr and slight decrease in eGFR were observed, consistent with the findings reported by Deng et al. (39). In our study, the patients’ eGFR was within the normal range (eGFR ≥ 60 mL/min) after 48 weeks, and no patient developed severe renal impairment or required regimen changes due to renal toxicity, suggesting that the observed renal function changes were not clinically significant.
This study has some limitations. It was a single-center, region-specific study (in Southwest China), retrospective analysis with a small sample size. Patients who were lost to follow-up, died, lacked baseline data, or switched medications for economic reasons were excluded, and this may have affected the representativeness and generalizability of the findings. Additionally, because resistance testing was cost-prohibitive for many patients, we could not precisely analyze the virologic failure mechanisms. Future studies should include multicenter cohorts, longer follow-up periods, and dedicated patient support funds to improve resistance testing access and ensure broader representativeness.
Conclusion
The DTG + 3TC regimen is an effective and well-tolerated treatment option for patients with late presentation of HIV infection. No patient in this study discontinued therapy owing to adverse events, and the two-drug regimen demonstrated a favorable safety profile. However, lipid levels and body weight should be monitored regularly to enable timely intervention in the event of metabolic abnormalities.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Guiyang Public Health Clinical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin because this was a retrospective study, so the ethics center waived informed consent.
Author contributions
QW: Writing – original draft, Conceptualization, Writing – review & editing, Methodology, Software. XX: Writing – review & editing, Conceptualization. YF: Writing – review & editing, Conceptualization. XY: Conceptualization, Writing – review & editing. LG: Writing – review & editing, Methodology, Data curation. SM: Methodology, Writing – review & editing, Data curation. PJ: Visualization, Writing – review & editing, Methodology. MW: Visualization, Writing – review & editing, Methodology. JL: Writing – review & editing, Supervision, Methodology. HL: Writing – review & editing, Methodology, Supervision, Data curation, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank the site staff who supported the study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Elgalib, A, Shah, S, Al-Wahaibi, A, Al-Habsi, Z, Al-Fouri, M, Lau, R, et al. Predictors of late and advanced HIV disease among people living with HIV in Oman (2000-2019). BMC Public Health. (2021) 21:2029–36. doi: 10.1186/s12889-021-12048-1
2. Komninakis, SV, Mota, ML, Hunter, JR, and Diaz, RS. Late presentation HIV/AIDS is still a challenge in Brazil and worldwide. AIDS Res Hum Retrovir. (2018) 34:129–31. doi: 10.1089/AID.2015.0379
3. Krentz, HB, and Gill, MJ. The direct medical costs of late presentation (<350/mm) of HIV infection over a 15-year period. AIDS Res Treat. (2012) 2012:757135–42. doi: 10.1155/2012/757135
4. Late presentation working groups in EuroSIDA and COHERE. Estimating the burden of HIV late presentation and its attributable morbidity and mortality across Europe 2010-2016. BMC Infect Dis. (2020) 20:728–38. doi: 10.1186/s12879-020-05261-7
5. Sun, C, Li, J, Liu, X, Zhang, Z, Qiu, T, Hu, H, et al. HIV/AIDS late presentation and its associated factors in China from 2010 to 2020: a systematic review and meta-analysis. AIDS Res Ther. (2021) 18:96–109. doi: 10.1186/s12981-021-00415-2
6. Antiretroviral Therapy Cohort Collaboration. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV. (2017) 4:e349–56. doi: 10.1016/S2352-3018(17)30066-8
7. Barbaro, G. Highly active antiretroviral therapy-associated metabolic syndrome: pathogenesis and cardiovascular risk. Am J Ther. (2006) 13:248–60. doi: 10.1097/01.mjt.0000162013.66614.16
8. Darge, T, Babusha, A, Chilo, D, Dukessa, A, and Teferi, S. Predictors of severe hepatotoxicity among retroviral infected adults on HAART regimen in Ilubabor zone, Southwest Ethiopia. Sci Rep. (2024) 14:8473–80. doi: 10.1038/s41598-024-57900-7
9. Cao, Y, Han, Y, Xie, J, Cui, Q, Zhang, L, Li, Y, et al. Impact of a tenofovir disoproxil fumarate plus ritonavir-boosted protease inhibitor-based regimen on renal function in HIV-infected individuals: a prospective, multicenter study. BMC Infect Dis. (2013) 13:301–8. doi: 10.1186/1471-2334-13-301
10. Heaton, RK, Clifford, DB, Franklin, DR Jr, Woods, SP, Ake, C, Vaida, F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER study. Neurology. (2010) 75:2087–96. doi: 10.1212/WNL.0b013e318200d727
11. Autenrieth, CS, Beck, EJ, Stelzle, D, Mallouris, C, Mahy, M, and Ghys, P. Global and regional trends of people living with HIV aged 50 and over: estimates and projections for 2000-2020. PLoS One. (2018) 13:e0207005–15. doi: 10.1371/journal.pone.0207005
12. Ruzicka, DJ, Imai, K, Takahashi, K, and Naito, T. Comorbidities and the use of comedications in people living with HIV on antiretroviral therapy in Japan: a cross-sectional study using a hospital claims database. BMJ Open. (2018) 8:e019985. doi: 10.1136/bmjopen-2017-019985
13. Ambrosioni, J, Levi, L, Alagaratnam, J, Van Bremen, K, Mastrangelo, A, Waalewijn, H, et al. EACS governing board. Major revision version 12.0 of the European AIDS clinical society guidelines 2023. HIV Med. (2023) 24:1126–36. doi: 10.1111/hiv.13542
14. DHHS. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV[M/OL]. (2021). Available online at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/AdultandAdolescentGL.pdf
15. Gandhi, RT, Bedimo, R, Hoy, JF, Landovitz, RJ, Smith, DM, Eaton, EF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2022 recommendations of the international antiviral society-USA panel. JAMA. (2023) 329:63–84. doi: 10.1001/jama.2022.22246
16. Acquired Immunodeficiency Syndrome Professional Group, Society of Infectious Diseases, Chinese Medical Association, Chinese Center for Disease Control and Prevention. Chinese guidelines for the diagnosis and treatment of human immunodeficiency virus infection/acquired immunodeficiency syndrome (2024 edition). Chin Med J Engl. (2024) 137:2654–80. doi: 10.1097/CM9.0000000000003383
17. Patel, R, Evitt, L, Mariolis, I, Di Giambenedetto, S, d'Arminio Monforte, A, Casado, J, et al. HIV treatment with the two-drug regimen dolutegravir plus lamivudine in real-world clinical practice: a systematic literature review. Infect Dis Ther. (2021) 10:2051–70. doi: 10.1007/s40121-021-00522-7
18. Rolle, CP, Berhe, M, Singh, T, Ortiz, R, Wurapa, A, Ramgopal, M, et al. Dolutegravir/lamivudine as a first-line regimen in a test-and-treat setting for newly diagnosed people living with HIV. AIDS. (2021) 35:1957–65. doi: 10.1097/QAD.0000000000002979
19. Hou, H, Ba, H, Jin, X, Luo, P, Zhang, Y, Li, J, et al. Effectiveness and safety of dolutegravir plus lamivudine in treating HIV in China, including outcomes of patients coinfected with tuberculosis. Medicine (Baltimore). (2024) 103:e38558–63. doi: 10.1097/MD.0000000000038558
20. Dou, Y, Liao, G, Lu, R, Su, L, Lan, K, Meng, Z, et al. DTG + 3TC dual therapy for the treatment naïve patients with viral load exceeding 500,000 copies/mL: a retrospective study. BMC Infect Dis. (2024) 24:720–6. doi: 10.1186/s12879-024-09624-2
21. Cahn, P, Madero, JS, Arribas, JR, Antinori, A, Ortiz, R, Clarke, AE, et al. GEMINI study team. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet. (2019) 393:132–55. doi: 10.1016/S0140-6736(18)33041-1
22. Cahn, P, Madero, JS, Arribas, JR, Antinori, A, Ortiz, R, Clarke, AE, et al. Durable efficacy of dolutegravir plus lamivudine in antiretroviral treatment-naive adults with HIV-1 infection: 96-week results from the GEMINI-1 and GEMINI-2 randomized clinical trials. J Acquir Immune Defic Syndr. (2020) 83:310–18. doi: 10.1097/QAI.0000000000002275
23. Cahn, P, Sierra Madero, J, Arribas, JR, Antinori, A, Ortiz, R, Clarke, AE, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy - naive adults with HIV-1 infection. AIDS. (2022) 36:39–48. doi: 10.1097/QAD.0000000000003070
24. Croxford, S, Stengaard, AR, Brännström, J, Combs, L, Dedes, N, Girardi, E, et al. Late diagnosis of HIV: an updated consensus definition. HIV Med. (2022) 23:1202–8. doi: 10.1111/hiv.13425
25. Antinori, A, Coenen, T, Costagiola, D, Dedes, N, Ellefson, M, Gatell, J, et al. Late presentation of HIV infection: a consensus definition. HIV Med. (2011) 12:61–4. doi: 10.1111/j.1468-1293.2010.00857.x
26. Zengwu, W, Jing, L, Jianjun, L, Naqiong, W, Guoping, L, Zhengyue, C, et al. Joint Committee on the Chinese Guidelines for Lipid Management, Chinese guidelines for lipid management. Chin Circ J. (2023) 38:237–71. doi: 10.3969/j.issn.1000-3614.2023.03.001
27. Yiming, M. Committee on DILI Prevention and Management, Chinese Medical Biotechnology Association Study Group of Drug-Induced Liver Disease, Chinese Medical Association for the Study of Liver Diseases. Chinese guideline for diagnosis and management of drug- induced liver injury (2023 version). Chin J Gastroenterol. (2023) 28:397–431. doi: 10.3760/cma.j.cn501113-20230419-00176
28. Li, H, Zhang, FJ, Lu, HZ, Cai, WP, Wu, H, Sun, YT, et al. Expert consensus on the management of HIV-infected patients with chronic kidney disease. Chin J AIDS STD. (2017) 23:578–81. doi: 10.13419/j.cnki.aids.2017.06.30
29. United States Department of Health and Human Services. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents, (2017): 1–288. Available online at: https://clinicalinfo.hiv.gov/sites/default/files/guidelines/documents/adult-adolescent-arv/guidelines-adult-adolescent-arv.pdf.
30. Chronic Kidney Disease Epidemiology Collaboration. New creatinine- and cystatin c-based equations to estimate GFR without race. N Engl J Med. (2021) 385:1737–49. doi: 10.1056/NEJMoa2102953
31. Wong, CS, Wei, L, and Kim, YS. HIV late presenters in Asia: management and public health challenges. AIDS Res Treat. (2023) 2023:9488051-9488058. doi: 10.1155/2023/9488051
32. Eron, J, Hung, CC, Baril, JG, Slim, J, Falcó, V, Bogner, J, et al. Brief report: virologic response by baseline viral load with dolutegravir plus lamivudine vs dolutegravir plus tenofovir disoproxil fumarate/emtricitabine: pooled analysis. J Acquir Immune Defic Syndr. (2020) 84:60–5. doi: 10.1097/QAI.0000000000002302
33. INSIGHT START Study GroupLundgren, JD, Babiker, AG, Gordin, F, Emery, S, Grund, B, et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N Engl J Med. (2015) 373:795–807. doi: 10.1056/NEJMoa1506816
34. Gan, L, Xie, X, Fu, Y, Yang, X, Ma, S, Kong, L, et al. Comparison of dolutegravir+lamivudine and bictegravir/emtricitabine/tenofovir alafenamide in antiretroviral therapy-naïve patients infected with HIV: preliminary results from clinical practice. Expert Rev Anti-Infect Ther. (2024) 22:877–84. doi: 10.1080/14787210.2023.2279719
35. Serrano-Villar, S, Sainz, T, Lee, SA, Hunt, PW, Sinclair, E, Shacklett, BL, et al. HIV-infected individuals with low CD4/CD8 ratio despite effective antiretroviral therapy exhibit altered T cell subsets, heightened CD8+ T cell activation, and increased risk of non-AIDS morbidity and mortality. PLoS Pathog. (2014) 10:e1004078–92. doi: 10.1371/journal.ppat.1004078
36. Mussini, C, Lorenzini, P, Cozzi-Lepri, A, Lapadula, G, Marchetti, G, Nicastri, E, et al. Icona foundation study group. CD4/CD8 ratio normalisation and non-AIDS-related events in individuals with HIV who achieve viral load suppression with antiretroviral therapy: an observational cohort study. Lancet HIV. (2015) 2:e98–e106. doi: 10.1016/S2352-3018(15)00006-5
37. Halcox, JP, Banegas, JR, Roy, C, Dallongeville, J, De Backer, G, Guallar, E, et al. Prevalence and treatment of atherogenic dyslipidemia in the primary prevention of cardiovascular disease in Europe: EURIKA, a cross-sectional observational study. BMC Cardiovasc Disord. (2017) 17:160. doi: 10.1186/s12872-017-0591-5
38. Diggins, CE, Russo, SC, and Lo, J. Metabolic consequences of antiretroviral therapy. Curr HIV/AIDS Rep. (2022) 19:141–53. doi: 10.1007/s11904-022-00600-6
39. Deng, L, Li, C, Chen, P, Luo, X, Zheng, X, Zhou, L, et al. Dolutegravir plus lamivudine versus efavirenz plus tenofovir disoproxil fumarate and lamivudine in antiretroviral-naive adults with HIV-1 infection. BMC Infect Dis. (2022) 22:17–25. doi: 10.1186/s12879-021-06991-y
40. Wei, Y, Li, J, Xu, R, Wen, L, Deng, Y, He, L, et al. Efficacy and safety profiles of dolutegravir plus lamivudine vs. bictegravir/emtricitabine/tenofovir alafenamide in therapy-naïve adults with HIV-1. Chin Med J Engl. (2023) 136:2677–85. doi: 10.1097/CM9.0000000000002907
41. Kumar, S, and Samaras, K. The impact of weight gain during HIV treatment on risk of pre-diabetes, diabetes mellitus, cardiovascular disease, and mortality. Front Endocrinol. (2018) 9:705. doi: 10.3389/fendo.2018.00705 doi: 10.3389/ fendo.2018.00705.
42. Capeau, J, Lagathu, C, and Béréziat, V. Recent data on the role of antiretroviral therapy in weight gain and obesity in persons living with HIV. Curr Opin HIV AIDS. (2024) 19:14–20. doi: 10.1097/COH.0000000000000833
43. Koteff, J, Borland, J, Chen, S, Song, I, Peppercorn, A, Koshiba, T, et al. A phase 1 study to evaluate the effect of dolutegravir on renal function via measurement of iohexol and Para-aminohippurate clearance in healthy subjects. Br J Clin Pharmacol. (2013) 75:990–6. doi: 10.1111/j.1365-2125.2012.04440.x
Glossary
DTG + 3TC - dolutegravir plus lamivudine, including DTG/3TC (compound single tablet preparation) or DTG + 3TC (two pills) simplified double regimen
IQR - Interquartile range
CI - Confidence interval
NA - Not applicable
TND - Target not detected indicating below detection limit
NO - Not detected
AIDS - Acquired immunodeficiency syndrome
ART - Anti-retroviral therapy
rapid ART - ART initiated within 7 days of HIV-1 antibody confirmation
CVD - Cardiovascular disease
PJP - Pneumocystis jirovecii pneumonia
TB - Tuberculosis
BIC - Bictegravir
BMI - Body mass index
TC - Total cholesterol
TG - Triglycerides
HDL-C - High-density lipoprotein cholesterol
LDL-C - Low-density lipoprotein cholesterol
ALT - Alanine aminotransferase
LDH - Lactate dehydrogenase
TBIL - Total bilirubin
DBIL - Direct bilirubin
IBIL - Indirect bilirubin
Scr - Serum creatinine
eGFR - Estimated glomerular filtration rate
UA - Uric acid
AMY - Amylase
GLU - Glucose
Keywords: human immunodeficiency virus 1, efficacy, dolutegravir plus lamivudine, late presentation, retrospective real-world cohort study
Citation: Wang Q, Xie X, Fu Y, Yang X, Gan L, Ma S, Jiao P, Wu M, Li J and Long H (2025) Efficacy and safety of dolutegravir plus lamivudine for patients with late presentation of HIV-1 infection: a retrospective real-world cohort study in Southwest China. Front. Med. 12:1630960. doi: 10.3389/fmed.2025.1630960
Edited by:
Carlos Brites, Federal University of Bahia (UFBA), BrazilReviewed by:
Adam Abdullahi, University of Cambridge, United KingdomEsteban Martinez, Hospital Clinic of Barcelona, Spain
Copyright © 2025 Wang, Xie, Fu, Yang, Gan, Ma, Jiao, Wu, Li and Long. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hai Long, bG9uZ2xvbmcxMjI1QDEyNi5jb20=
 Qing Wang
Qing Wang Xiaoxin Xie2
Xiaoxin Xie2 Xiaoyan Yang
Xiaoyan Yang Hai Long
Hai Long