Abstract
Introduction:
Sarcoidosis is often associated with psychiatric symptoms and syndromes (PSS). This systematic review and meta-analysis aimed to summarize available literature on the prevalence of PSS in sarcoidosis, as well as their potential associations.
Methods:
A systematic search was conducted across PubMed, including case reports and studies that investigated PSS in sarcoidosis. A meta-analysis was performed on studies that assessed the association between sarcoidosis and PSS, using odds ratios (OR).
Results:
We included 43 studies and 53 cases on PSS in patients with sarcoidosis. The weighted average prevalence was 24.9% for depression, 28.7% for anxiety, 29.2% for neurocognitive symptoms, 54.4% for fatigue, 50.5% for excessive daytime sleepiness and 26.9% for sleep disturbances and insomnia, with the best available evidence for depression, anxiety and fatigue. The meta-analysis (n = 962) confirms that patients with sarcoidosis have a significant increased risk of developing PSS when compared with healthy controls (OR = 5.498, CI = 0.430–70.238, p < 0.001). Depressive symptoms and fatigue were most reported on and demonstrated the strongest associations as well (resp. OR = 4.855, z = 2.401, p=0.016 and OR = 20.231, z = 2.868, p = 0.004). Significant associations with anxiety and neurocognitive symptoms were also observed, although with less available evidence. Case reports reveal a diagnostic diversity not reflected in study populations, including psychosis and catatonia.
Conclusion:
Sarcoidosis is associated with a higher prevalence of PSS. Nonetheless research in this area remains limited. Systematic use of standardized psychiatric assessment tools is recommended.
1 Introduction
Sarcoidosis is a granulomatous multisystem disease of unknown origin with predominant lung and lymph node involvement, a heterogeneous clinical presentation and a variable clinical course (1). It is a global disease, with a prevalence of about 4.7–64 in 100,000, and an incidence of 1.0–35.5 in 100,000 per year (1). The diagnosis of sarcoidosis is based on three major criteria: a compatible clinical presentation, the finding of nonnecrotizing granulomatous inflammation in one or more tissue samples, and the exclusion of alternative causes. The process is not standardized, and clinicians must use a combination of clinical judgment and available evidence to make a diagnosis (2). Sarcoidosis generally has a favorable prognosis, with approximately 60% of cases experiencing spontaneous remission within 2 to 5 years and a 5-year survival rate of 95.4%. However, 10% of patients may develop chronic disease or serious complications. The overall mortality rate is about 5%, varying by region (3). Extrapulmonary disorders of sarcoidosis have been described with central nervous system (CNS) involvement occurring in 3 to 10% of all sarcoidosis patients, in which case the diagnosis of neurosarcoidosis (NS) is made (4).
Psychiatric symptoms and syndromes (PSS) such as depression, anxiety and fatigue, are common in sarcoidosis and other symptoms like psychosis have been described as presenting features on rare occasions (4). Fatigue and neurocognitive symptoms are overarching symptoms in sarcoidosis that – together with many other comorbidities—significantly lower the quality of life (QoL). This necessitates a multidisciplinary approach that inherently includes a psychiatric perspective on fatigue, neurocognitive symptoms and QoL (5).
In NS, PSS might occur more frequently due to the neurocognitive disruption inherent to the disease process, although it has been hypothesized that PSS in sarcoidosis may arise independently of specific neurological lesions and that other psychopathological mechanisms may be involved (5).
To the best of our knowledge, a comprehensive and systematic review on PSS in sarcoidosis is lacking. We performed a systematic review of studies reporting on PSS in patients with sarcoidosis discussing diagnostics, prevalence, pathophysiology and treatment options. A subsequent meta-analysis was performed on studies including an association analysis between sarcoidosis and PSS.
2 Methods
2.1 Search strategy and selection criteria
A systematic search was conducted across PubMed using the following search string: (neurosarcoidosis OR sarcoidosis) AND (psychiatr* OR psychosis OR psychotic OR cataton* OR cognitive OR delir* OR depress* OR manic OR mania OR anxi* OR obsessive OR compulsive). The search included studies published from January 1950 until December 2024 and was restricted to English, French and Dutch-language articles, human studies and peer-reviewed publications. The reference lists of included articles were screened for additional studies. Efforts were made to include all available studies by contacting the corresponding authors for a full-text copy of their study, if necessary.
The study was conducted in accordance with the 2020 PRISMA guidelines (6). The protocol was submitted to the Department of Medicine of the University of Antwerp prior to execution. The review was not registered.
2.2 Inclusion criteria and exclusion criteria
Inclusion criteria were studies and case-reports: (a) focusing on individuals with a primary diagnosis of sarcoidosis or NS; (b) reporting PSS (e.g., depressed mood, hallucinations, etc…) or syndromes (e.g., mood disorders, psychotic episodes, etc…) in patients with sarcoidosis; (c) using original data. Studies and case-reports were excluded if they: (a) Focused exclusively on organic neurological symptoms without any PSS; (b) Were duplicate publications or secondary analyses; (c) No full text was available; (d) Were written in another language than English, French or Dutch.
2.3 Study selection and data extraction
Two independent reviewers performed the screening. In case of disagreement, papers were retained for full-text evaluation. Where needed, a third reviewer was available in case of disagreements or uncertainties concerning eligibility. All titles and abstracts were screened to select potentially relevant case reports and studies. It was decided whether, based on the full texts of all individual studies, they fulfilled the eligibility criteria. Reports of case-series were treated as multiple individual case-reports and evaluated accordingly.
One author extracted the following data from the studies: Author, year of publication, in- and exclusion criteria, study design, study focus, study outcome, psychiatric assessment tools used, data on the interactions of PSS, treatment interventions, sample sizes of sarcoidosis patients and healthy controls, mean scores of the psychiatric assessment tools and standard deviation (SD) or interquartile range (IQR) of the psychiatric assessment tools. For the subsequent meta-analysis, the outcome measure was the OR of psychiatric symptom in patients diagnosed with sarcoidosis. In case a treatment effect was measured, only the pre-treatment data was included.
One author extracted the following data from case reports: author, year of publication, gender, age, psychiatric symptom(s) described and information concerning treatment.
The methodological quality of the included studies was assessed using the Newcastle-Ottawa Quality Assessment Scale, designed for cohort studies (7). The certainty of the evidence was appraised using the Grading of Recommendations, Assessment, Development, and Evaluation approach (8).
2.4 Data synthesis and analysis
All prevalence data on PSS found in the systematic review was synthesized in range, average, mean and weighted averages for each scale and psychiatric symptom if there were 2 or more datapoints.
Data in the meta-analysis were synthesized using a random-effects model to account for heterogeneity across studies. OR, and 95% confidence intervals (CI) were calculated for all included studies and for the studies in categories that allowed further analysis. We recalculated the SD from the IQR when the SD was not reported, using the formula: SD=IQR/1.35, based on the assumption of a normal distribution. Heterogeneity was assessed using the I2 statistic. Statistical analyses for the meta-analysis were conducted using Comprehensive Meta-Analysis version 4.0.000 (2022, Biostat, Inc.). Funnel plots and Egger’s test were used to assess publication bias. The primary meta-analysis was performed on the prevalence of all PSS in patients with sarcoidosis. Secondary analysis was performed for each psychiatric symptom if at least two studies were available.
3 Results
3.1 Systematic review
3.1.1 Literature search and study selection
The initial search of the PubMed database yielded 571 records. The screening and exclusion process ultimately resulted in the inclusion of 43 studies reporting PSS in individuals with sarcoidosis (9–51) (Figure 1): 39 studies included patients with sarcoidosis without reporting on the possible differentiation with NS, 2 studies only included patients with NS (16, 17), 2 studies actively excluded NS (42, 46). The 45 included case-reports and case-series revealed 53 relevant cases (52–96).
Figure 1

PRISMA flow diagram of literature search and study selection.
Eventually, 6 studies were found eligible for subsequent meta-analysis (Table 1) (10, 23, 41, 42, 45, 48).
Table 1
| Author | Year | Sample size | Controls | NOS | Grade | Tools used | Study design | Population of sarcoidosis patients |
|---|---|---|---|---|---|---|---|---|
| Voortman et al. (48) | 2019 | 443 | 62 | 7 | 3 | CESD, CFQ, FAS, STAI, PSSS, WHOQOL Bref | Cross-sectional | Patients diagnosed per WASOG guidelines |
| Saligan et al. (42) | 2014 | 14 | 13 | 6 | 1 | HAMD, FAS | Prospective cohort | Biopsy-confirmed without cardiac or neurosarcoidosis |
| Patterson et al. (41) | 2013 | 62 | 1,005 | 7 | 3 | CESD, ESS | Retrospective cohort | Patients undergoing polysomnography for suspected OSA |
| Elfferich et al. (23) | 2010 | 343 | 343 | 8 | 2 | CFQ, CESD, FAS | Cross-sectional | Diagnosis based on clinical, radiologic, and histological criteria |
| Antoniou et al. (10) | 2006 | 75 | 30 | 5 | 2 | HADS | Cross-sectional | Histologically confirmed with active disease |
| Spruit et al. (45) | 2005 | 25 | 21 | 7 | 3 | HADS, SF36 | Cross-sectional | Patients with fatigue diagnosed per ATS/ERS/WASOG criteria |
Studies included in the meta-analysis.
ATS, American Thoracic Society; CESD, Center for Epidemiologic Studies Depression Scale; CFQ, Cognitive Failure Questionnaire; ERS, European Respiratory Society; ESS, Epworth Sleepiness Scale; FAS, Fatigue Assessment Scale; HADS, Hospital Anxiety and Depression Scale; HAMD, Hamilton Depression Rating Scale; PSSS, Perceived Social Support Scale; SF36, Short Form 36 Health Survey; STAI, State–Trait Anxiety Inventory; WASOG, World Association of Sarcoidosis and Other Granulomatous Disorders; WHOQOLBref, World Health Organization Quality of Life – BREF.
3.1.2 Study population, design and methodology
Study populations varied from the two biggest cohorts presented by Sikjaer et al. (n = 7,302) (44) and the German Sarcoidosis Society (n = 1,197) (14, 15, 28–30), to 17 large samples (n = 100–1000) and 20 smaller samples (n < 100). All studies either recruited from specialized centers for the treatment of sarcoidosis or gathered patient data from large epidemiological databases. Of the 43 studies 4 were interventional studies: 3 randomized controlled trials (RCT) (35, 40, 49) and 1 cohort study (38). The other 39 observational studies comprised of 30 cross-sectional studies, 7 cohort studies and 2 case–control studies. A wide variety of assessment tools was used for screening PSS. General screening for PSS with the “International Classification of Diseases, Ninth or Tenth Revision” (ICD-9/10), the “Mini International Neuropsychiatric Interview Plus” (MINI-Plus), “Symptom Checklist-90” (SCL-90) or clinical assessment was used in only 6 (14.0%) of the studies. Standardizing general screening for PSS was suggested in 10 (23.3%) studies (11, 15, 18, 24, 26, 27, 29, 33, 37, 47). PSS were the main study focus in 21 (48,8%) studies (11, 12, 14, 18, 20, 21, 24, 26, 27, 29, 31, 32, 34, 35, 38, 40, 43, 47, 48, 50, 51), of which 9 (20.9%) and 6 (14.0%) specifically investigated depression and anxiety, respectively. Fatigue and sleep-related disturbances were the main study focus in 17 (39.5%) studies (12–15, 21, 22, 24, 27, 28, 30, 35–37, 40–42, 51). Neurocognitive symptoms were a main focus in 7 (16.3%) studies (12, 16, 21, 27, 33, 46). QoL was investigated in 14 (32.6%) studies (9, 10, 13, 19, 20, 24, 26, 33, 35, 38, 40, 45, 48, 51).
3.1.3 Prevalence of PSS
We found 29 (67.4%) studies mentioning prevalence in of PSS in patients with sarcoidosis (11–15, 17–20, 23–26, 28–30, 33, 34, 37–39, 41, 43–45, 47, 50, 51) (Table 2). Sample sizes varied (Range = 20–7301 avg. = 580.4 median = 148). All but 4 used self-reporting scales (26, 44, 47, 50).
Table 2
| Author | Year | Sample | Study type | Prevalence per assessment tool |
|---|---|---|---|---|
| Sikjaer et al. (44) | 2024 | 7,301 | Retrospective cohort | ICD10 anxiety and depression: 6.9% in sarcoidosis |
| Kahlmann et al. (35) | 2023 | 99 | RCT | HADS: Anxiety: 52%, Depression: 51% |
| Byg et al. (17) | 2022 | 20 | Prospective cohort | FAS: Fatigue: 80%, BDI: Depression: 35% |
| Hoth et al. (33) | 2022 | 315 | Cross-sectional | CFQ: Cognitive Difficulties: 25% |
| Bloem et al. (13) | 2021 | 60 | Cross-sectional | HADS: Anxiety: 12%, Depression: 8% |
| Froidure et al. (25) | 2019 | 53 | Prospective cohort | HADS: Anxiety: 26%, Depression: 20%; FAS: Fatigue: 41% |
| Sharp et al. (43) | 2019 | 112 | Cross-sectional | PHQ8: Depression: 54% (20% moderate–severe); GAD7: Anxiety: 37% (13% moderate–severe) |
| Lingner et al. (38) | 2018 | 296 | Prospective cohort | FAS: Fatigue: 66%; HADS: Anxiety: 30.4%, Depression: 28.4% |
| Moor et al. (39) | 2018 | 210 | Cross-sectional | GADSI: Anxiety 48% |
| Bosse-Henck et al. (15) | 2017 | 1,197 | Cross-sectional | HADS: Anxiety: 27%, Depression: 19.7%; ESS: Excessive Daytime Sleepiness: 16.5%, FAS: Fatigue: 16.4% |
| Ungprasert et al. (47) | 2017 | 345 | Retrospective cohort | Clinical diagnosis: Depression: 13.8% |
| de Boer et al. (20) | 2014 | 56 | Cross-sectional | HADS: Depression: 10.9%, Anxiety: 25.4%, |
| Balaji et al. (11) | 2012 | 148 | Cross-sectional | HADS: Depression: 25.7% |
| Hinz et al. (29) | 2012 | 1,197 | Cross-sectional | HADS: Anxiety: 26%, Depression: 18% |
| Elfferich et al. (24) | 2011 | 588 | Cross-sectional | FAS: Fatigue: 79.4%; CESD: Depression: 32.7% |
| Hinz et al. (30) | 2011 | 1,197 | Cross-sectional | FAS: Fatigue: 70% MFI: Fatigue: 68% |
| Korenromp et al. (37) | 2011 | 75 | Cross-sectional | CDC-criteria: Chronic Fatigue: 47% |
| Elfferich et al. (23) | 2010 | 343 | Cross-sectional | CFQ: Cognitive failure: 33%, FAS: Fatigue: 80%, CESD: Depression: 27% |
| Ireland & Wilsher (34) | 2010 | 81 | Cross-sectional | HADS: Anxiety: 32%, Depression: 23% |
| Goracci et al. (26) | 2008 | 80 | Cross-sectional | MINI-Plus: Major Depressive Disorder: 25%, Panic Disorder: 6.3%, Bipolar Disorder: 6.3%, Generalized Anxiety Disorder: 5%, Obsessive Compulsive Disorder: 1.3% |
| Westney et al. (50) | 2007 | 165 | Cross-sectional | ICD9: Depression: 13% |
| Spruit et al. (45) | 2005 | 25 | Cross-sectional | HADS: Depression: 38%, Anxiety: 24% |
| Cox et al. (19) | 2004 | 111 | Cross-sectional | CESD: Depression: 66% |
| Chang et al. (18) | 2001 | 154 | Cross-sectional | CESD: Depression: 60% |
| Wirnsberger et al. (51) | 1998 | 64 | Cross-sectional | BDI: Depression: 18% |
Studies reporting prevalence of PSS in patients with sarcoidosis.
BDI, Beck Depression Inventory; CDC-criteria, Centers for Disease Control and Prevention criteria; CESD, Center for Epidemiologic Studies Depression Scale; CFQ, Cognitive Failure Questionnaire; ESS, Epworth Sleepiness Scale; FAS, Fatigue, assessment Scale; GAD7, Generalized Anxiety Disorder 7-item scale; GADSI, Generalized Anxiety Disorder Severity Index; HADS, Hospital Anxiety and Depression Scale; ICD9/10, International Classification of Diseases 9th/10th Revision; MINI-Plus, Mini International Neuropsychiatric Interview Plus version; PHQ8, Patient Health Questionnaire-8; PSS, Perceived Stress Scale; QoL: Quality of Life.
Prevalence of depression in total was measured in 20 studies (n = 5,194, range = 8.0–66.0%, avg. = 29.4%, mean = 25.4%, weighted avg. = 24.9%). Results per used method differed: HADS-depression (n = 3,212, range = 8.0–51.0%, avg. = 24.3.%, mean = 21.5%, weighted avg. = 21.0%), CESD (n = 1,196, range = 27.0–66.0%, avg. = 46.4%, mean = 46.4%, weighted avg. = 37.7%), BDI (n = 84, range = 18.0–35.0%, avg. = 26.5%, mean = 26.5%, weighted avg. = 22.0%) and non-self-reporting tools: MINI-Plus, ICD9 and clinical diagnosis (n = 590, range = 13.0–25.0%, avg. = 17.3%, mean = 13.8%, weighted avg. = 15.1%).
Prevalence of anxiety was assessed in 12 studies (n = 3,466, range = 12.0–52.0%, avg. = 29.4%, mean = 26.5%, weighted avg. = 28.7%), primarily using HADS-anxiety (n = 3,064, range = 12.0–52.0%, avg. = 28.3%, mean = 26.0%, weighted avg. = 27.5%); exceptions included three studies using Generalized Anxiety Disorder 7-item Scale (GAD-7) (37.0% n = 112), Generalized Anxiety Disorder Severity Index (GADSI) (48.0% n = 210) and MINI-Plus (12.6% n = 80) respectively (n = 402, range = 12.6–48.0%, avg. = 32.5%, mean = 37.0%, weighted avg. = 37.9%). The different MINI-Plus-diagnoses for anxiety (generalized anxiety disorder, obsessive compulsive disorder and panic disorder) were combined into a single percentage of 12.6%. Sikjaer et al. (44) reported a hazard ratio of anxiety and depression over a period of 15 years of 6.9% in 3701 patients from the Danish National Patient Register with newly diagnoses sarcoidosis versus 4.9% in 26,145 matched controls, mainly identifying patients on the basis of prescription-data; see also: limitations.
Neurocognitive symptoms occurrence was measured in only 2 studies, using the Cognitive Failures Questionnaire (CFQ) (n = 658, range = 25.0–33.0%, avg. = 29.0%, mean = 29.0%, weighted avg. = 29.2%).
The prevalence of fatigue was measured in 7 studies using the Fatigue Assessment Scale (FAS) (n = 3,694, range = 16.4–80.0%, avg. = 61.8%, mean = 70.0%, weighted avg. = 54.4%).
Excessive daytime sleepiness was reported on in 3 studies using the Epworth Sleepiness Scale (ESS): 16.5% (n = 1,197, ESS > 16) (15), 50.0% (n = 1,197, ESS > 10) (28), 60.0% (n = 62, ESS > 10). Since a cutoff of ESS > 10 is considered the standard threshold for excessive daytime sleepiness, the following statistics were calculated using only the latter two studies (n = 1,259, range = 50.0–60.0%, avg. = 55.0%, mean = 55.0%, weighted avg. = 50.5%).
As to sleep disturbances (n = 1,281 range = 25.0–54.0% avg. = 39.5%, mean = 39.5%, weighted avg. = 26.9%), 54% of sarcoidosis patients reported frequent and occasional sleep disturbance compared to only 17% of healthy controls (p < 0.0001) in the study by Benn et al. (12). Insomnia defined as a Pittsburgh Sleep Quality Index of more than 10 was reported in 25% (14).
One study reported on the relative risk of developing schizophrenia (n = 345, HR = 2.15, CI = 0.39–11.76) and substance abuse disorder (n = 345, HR = 1.55 CI = 0.44–5.49) over 5 years in sarcoidosis (47). However, neither association reached statistical significance.
We found no comprehensive reporting of other PSS.
3.1.4 Interactions among PSS in sarcoidosis
PSS in sarcoidosis have an assumed multifactorial etiology. In our systematic review we could only find anecdotal evidence to support this in 8 (18.6%) studies.
One study mentioned an higher relapse rate of neurocognitive symptoms in patients with parenchymal lesions (n = 9) (16). Another shows a difference with fMRI in brain activity in the absence of apparent neurological lesions when comparing patients with sarcoidosis with and without chronic fatigue (n = 30) (36).
Overall fatigue was a key symptom in 5 (11.9%) of the studies and has been linked to neurocognitive symptoms and depressive symptoms (n = 292) (27). When compared to controls, individuals with sarcoidosis consistently exhibit higher levels of fatigue and depression (n = 27) (42). Notably, fatigue often persists even in the context of clinical remission and is associated with psychological distress (n = 75) (37).
Obstructive sleep apnea (OSA) is very prevalent in sarcoidosis patients and several clinical manifestations like PSS are common to both OSA and sarcoidosis (97). Although abnormal lung function in sarcoidosis may contribute to OSA, sarcoidosis remains independently associated with excessive daytime sleepiness (n = 62) (41).
Physical fitness and particularly muscle strength showed a similar three-way association with either sarcoidosis and PSS in small cohorts (n < 112) (37, 42, 43, 45).
3.1.5 Treatment interventions
Research specifically addressing treatment interventions for PSS in patients with sarcoidosis was found to be scarce, with only 8 studies (18.6%) reporting on this topic. A RCT (n = 99) showed online mindfulness-based cognitive therapy significantly improved fatigue, anxiety and depression in sarcoidosis patients (35). A RCT (n = 18) showed 12 week exercise training significantly improved QoL, anxiety and fatigue (40). One study (n = 296) reported that fatigue, anxiety, depression, and QoL was significantly improved in sarcoidosis patients after a 3 week pulmonary rehabilitation program (38). Another study, however, failed to observe a significantly increase in daily life physical activity in patients with chronic sarcoidosis 10 months after a 2 months pulmonary rehabilitation suggesting the need for long-term behavioral interventions (49). A retrospective cohort study (n = 9) compared different treatment strategies of NS and among others discussed their effect on cognition with mixed results (16). A prospective cohort study (n = 20) showed that after one-year immunosuppression 75% of patients experienced important clinical improvement but no difference in fatigue and depression (17). A cross-sectional study (n = 343) showed anti-TNF-alpha treatment improved neurocognitive function and reduced fatigue in some patients, but effects varied (23). The role of physical rehabilitation has been suggested as a way to reduce PSS (42, 45). Sikjaer et al. (44) reported on 80.5% of patients with sarcoidosis taking antidepressants without having a diagnosis of anxiety or depression.
3.1.6 Characteristics of the included case reports
The 53 included cases revealed a gender distribution of 56.6% female cases and large variation of age (range = 16–79 years, avg. = 44.6 years mean = 41 years). The following PSS were reported: Neurocognitive symptoms (54.7%), psychosis (39.6%), mood disturbances (24.5%), delirium (17.0%), behavioral changes (15.1%), bipolar symptoms (11.3%), anxiety (9.4%), and catatonia (5.7%). We found no cases reports on addiction or eating disorders in patients with sarcoidosis. PSS were the presenting feature of sarcoidosis in 15.1% of all case reports.
Case reports detailing treatment strategies predominantly emphasized supportive therapies for sarcoidosis, supplemented by symptomatic management of comorbid PSS. Treatment for PSS was mentioned in 28 cases among which: antipsychotics (71.4%), antidepressants (32.1%), anti-epileptics (28.6%), benzodiazepines (25.0%), lithium (7.1%), electroconvulsive therapy (7.1%) and psychotherapy (7.1%).
In 80% of these cases, psychiatric and physical symptoms of sarcoidosis followed a synchronous course, with both improving or worsening in parallel according to the response to the primary treatment of sarcoidosis.
3.2 Meta-analysis
3.2.1 Study characteristics
The meta-analysis incorporated 6 studies from the systematic review investigating the association between sarcoidosis and PSS (10, 23, 41, 42, 45, 48). These studies collectively assessed 1,119 patients diagnosed with sarcoidosis and 2,605 healthy controls. The studies employed 11 different psychiatric assessment tools to evaluate depressive symptoms, anxiety, neurocognitive symptoms, and fatigue (Table 1). While one study explicitly excluded individuals with NS from its analysis (42), the remaining five studies did not specify differentiation between sarcoidosis and NS. Sample sizes varied across studies, ranging from small-scale cohorts to larger population-based analyses. The diagnostic methodologies and psychiatric screening tools differed significantly. The methodological quality of the included studies, as evaluated by the Newcastle-Ottawa Scale, was deemed acceptable (Table 1).
3.2.2 Association of PSS and sarcoidosis
All included studies reported a higher prevalence of PSS among sarcoidosis patients compared to control groups. The primary overarching analysis of the meta-analysis demonstrated that patients with sarcoidosis exhibit significantly more PSS compared to healthy controls (OR = 5.498, 95% CI = 0.430–70.238; p < 0.001) (Figure 2).
Figure 2
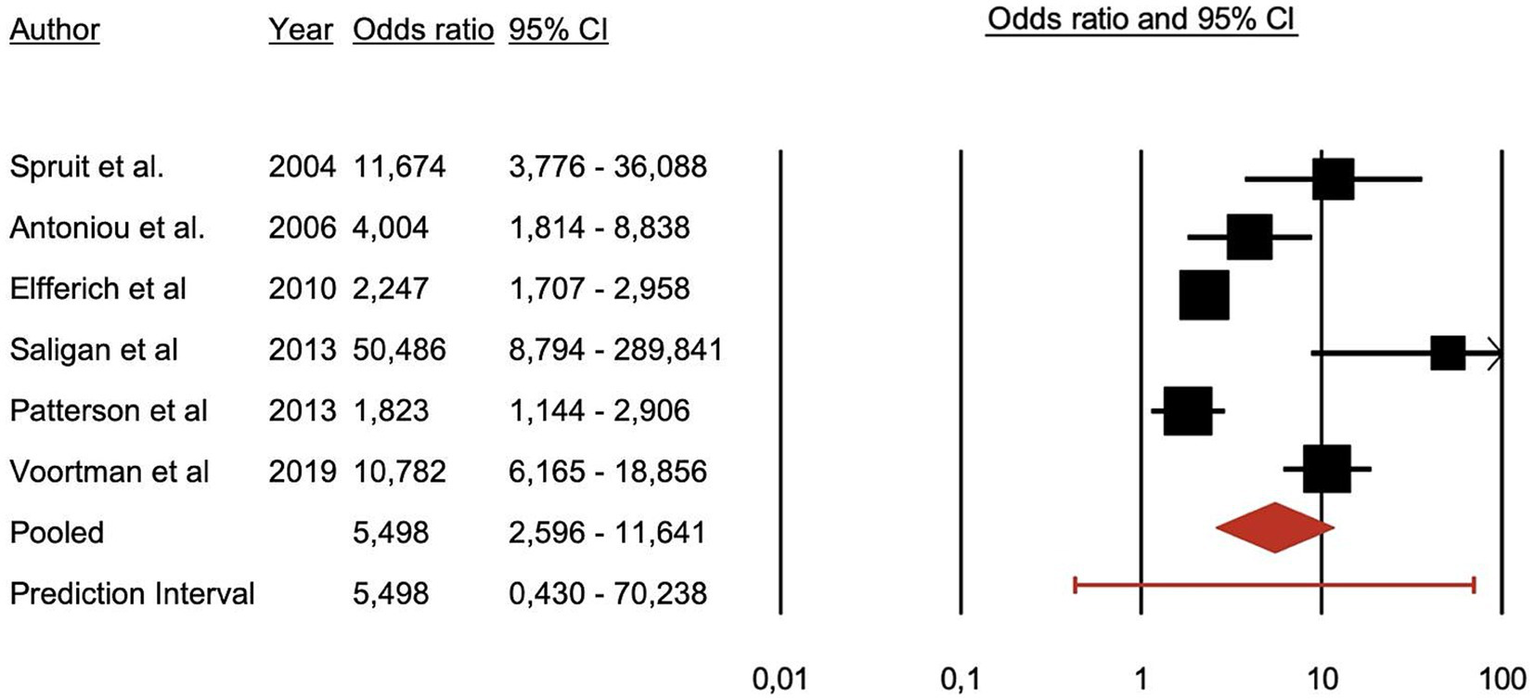
Forest plot illustrating the association between psychiatric symptoms and syndromes and sarcoidosis.
Secondary analyses by symptom category demonstrate a significant association of sarcoidosis with depressive symptoms and fatigue. Anxiety, and neurocognitive symptoms were also associated with sarcoidosis although with less available evidence as these two categories had only two studies each in their analysis. Depressive symptoms: patients = 180, controls = 1,069, OR = 4.855, z = 2.401, p = 0.016. Anxiety disorders: patients n = 104, controls n = 51, OR = 6.372, z = 5.600, p < 0.001. Neurocognitive symptoms: patients = 551, controls = 405, OR = 2.796, z = 3.911, p < 0.001. Fatigue: patients = 284, controls = 1,080, OR = 20.231, z = 2.868, p = 0.004.
3.2.3 Sensitivity analyses and certainty of evidence
Funnel plots were examined to assess potential publication bias in the meta-analysis. The plots indicate evidence of publication bias, as studies with positive and significant results appear to be overrepresented (Figure 3). This observation is supported by Egger’s test, which yielded a statistically significant result (t = 2.44, p = 0.0371), further suggesting the presence of publication bias.
Figure 3
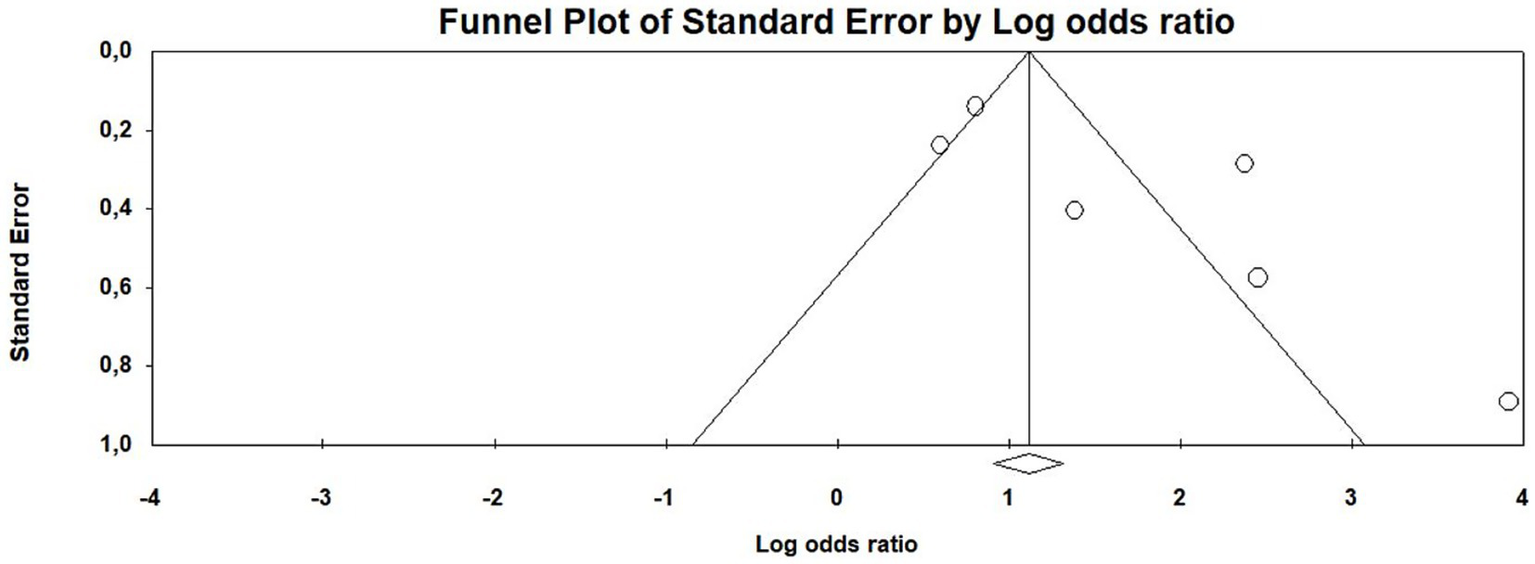
Funnel plot of publication bias of the meta-analysis.
The certainty of evidence of our meta-analysis was determined as low to moderate with a weighted average GRADE score of 2.54 on a scale of 1 to 4. This is mainly due to the observational study design and the publication bias detected.
4 Discussion
4.1 Main findings
In this systematic review on PSS in patients with sarcoidosis, prevalence of PSS varied widely depending on the study design with a weighted average of 24.9% for depression, 28.7% for anxiety, 29.2% for neurocognitive symptoms, 54.4% for fatigue, 50.5% for excessive daytime sleepiness (ESS > 10) and 26.9% for sleep disturbances and insomnia.
The subsequent meta-analysis of 6 eligible studies confirms that patients with sarcoidosis have an increased risk of developing PSS when compared with healthy controls (OR = 5.498, CI = 0.430–70.238, p < 0.001). Depressive symptoms and fatigue were most reported and demonstrated the strongest associations (resp. OR = 4.855, z = 2.401, p = 0.016 and OR = 20.231, z = 2.868, p = 0.004). Associations with anxiety and neurocognitive symptoms were also observed.
The case reports revealed a diagnostic diversity not reflected in study populations, pointing to heterogeneity and the need for a more systematic use of comprehensive psychiatric assessment tools.
4.2 Etiopathogenesis
Multiple principal yet interplaying mechanisms have been proposed to underlie sarcoidosis-related PSS including neuroinflammatory processes, immune-mediated pathways, disturbances in calcium-metabolism, disruption of neuronal functioning and psychosocial factors (88). However, as mentioned in the results, the evidence on the etiopathological factors is still scarce and mainly consists of data on interacting and mediating factors.
Direct inflammatory involvement of the CNS may lead to the formation of non-caseating granulomas in the parenchyma or meninges, triggering the release of pro-inflammatory cytokines such as interleukin-6 (IL-6), tumor necrosis factor alpha (TNF-α), and interferon-gamma (IFN-γ), which disrupt neurotransmitter systems and impair limbic and frontal lobe structures (88). Interestingly, increases in pro-inflammatory markers such as IL-6, TNF-a and IFN-g have been associated to mood and psychotic symptoms (98).
In addition, blood–brain barrier disruption may facilitate immune cell infiltration into the brain. Inflammatory processes and an altered immune response have been implicated in the pathogenesis in sarcoidosis, other CNS inflammatory and auto-immune disorders, as well as psychosis, delirium, and depression (99, 100). A shared feature across sarcoidosis and psychosis, particularly in later stages, appears to be a blunted cellular immune response combined with an over-activated type-2 immune response (88). IL-6, IL-8, and TNF-α concentrations have shown to be elevated with the kynurenine pathway in depression and schizophrenia (101). Pro-inflammatory cytokines can stimulate the activity of indoleamine 2,3-dioxygenase and lead to tryptophan deficiency and thus a reduction in the production of 5HT and melatonin, with a shift to the production of kynurenine and other neurotoxic tryptophan-derived metabolites (101). These higher values could then explain the white and grey matter degeneration in schizophrenia and in sarcoidosis patients in granuloma formation, with the possible development of fibrosis in the CNS This supports the hypothesis of a shared etiopathogenic link between sarcoidosis and psychosis (88).
Hypercalcemia is a recognized complication in sarcoidosis, occurring in about 6–18% of cases. This condition is often due to increased production of 1,25-dihydroxyvitamin D by activated macrophages within granulomas, leading to enhanced calcium absorption. Hypercalcemia can contribute to neurological symptoms, including encephalopathy, which may present with neurocognitive symptoms and altered mental status (102, 103).
As previously noted, up to 10% of patients with sarcoidosis develop some form of NS with neurological lesions present in half of these cases, potentially disturbing neurological functioning on a systemic level (4). The implied multilevel etiology suggests that PSS may occur in sarcoidosis both with and without overt neurocognitive disruption caused by disease-related lesions. According to Focke et al. (104), patients with cerebral vasculitis related to NS are significantly more likely to present with neurological symptoms, including cognitive and/or behavioral changes. Notably, the CNS involvement may be asymptomatic and the diagnosis of NS is often complicated by the heterogeneous disease presentation and the absence of specific diagnostic tests (5).
4.3 Psychosocial suffering and fatigue
Psychosocial suffering and fatigue is considered a possible cause and consequence of PSS in sarcoidosis. The chronic burden of sarcoidosis, accompanied by persistent fatigue and pain, may cause chronic somatic and psychosocial stress and reactive depression and anxiety, with a severe impairment and impact on social and occupational functioning may further enhance these complaints (105). In the study by Hinz et al. (29), the number of affected organs, the number of concomitant diseases and the degree of dyspnea significantly predicted anxiety and depression scores. Furthermore, sleep apnea, restless legs syndrome, and arterial hypertension were associated with anxiety as well as depression (29). Moreover, additional predictors for depressive symptoms were female sex, decreased access to medical care, and increased dyspnea (18). Furthermore, sarcoidosis patients with PSS showed a higher odds for emergency department visit in the previous 6 months and a worse health-related quality of life, compared with patients without PSS (43).
Fatigue in sarcoidosis is a multifactorial symptom influenced by a complex interplay of physiological and psychological factors and seems, in part, intrinsic to the disease. This impairs daily coping mechanisms and disrupts social interactions in patients that are often of a younger age, making them less resilient to this often chronic and burdensome disease (10). Fatigue has been linked to neurocognitive and depressive symptoms, small fiber neuropathy, and dyspnea (27, 41, 42), as well as sleep disturbances (12). Fatigue often persists even in the context of clinical remission and is associated with psychological distress, diminished health status, decreased physical activity and muscle weakness (37, 42, 43, 45). Which are all features also associated with chronic fatigue syndrome (106). Muscle involvement in sarcoidosis is common and physical fitness has been shown to be associated to PSS and fatigue (37, 42, 43, 45).
Sleep disturbance significantly correlated with fatigue, depression, cognitive dysfunction, and poor quality of life, independent of disease severity (12). Poor sleep quality is highly prevalent in patients with sarcoidosis and strongly associated with fatigue, anxiety, and depression (14). OSA is very prevalent in sarcoidosis patients and several clinical manifestations like PSS are common to both OSA and sarcoidosis (97). Although abnormal lung function in sarcoidosis may contribute to OSA, sarcoidosis remains independently associated with excessive daytime sleepiness, again emphasizing a multifactorial etiology (n = 62) (41).
All the above supports other research indicating that psychosocial suffering and fatigue – like PSS – in sarcoidosis requires multimodal approach and individual care strategies (107).
4.4 PSS as an effect of sarcoidosis treatments
Commonly used treatments for sarcoidosis have been associated with PSS. Corticosteroids, commonly have been associated with psychiatric disturbances such as depression, mania, and psychosis. The overall incidence of psychiatric symptoms from corticosteroids occurs in approximately 25% of patients (108). As it can even increase suicidal behavior patient education is warranted (109). Corticosteroids are also known to induce CYP3A4 activity although with extensive intersubject variability, lowering plasma concentrations of many antidepressants and antipsychotics (110). Anti-TNF-alpha therapy can, in rare cases, induce psychiatric side effects including mania, hypomania, and psychosis, even in patients without prior psychiatric history (111). These effects are generally reversible upon discontinuation of the drug (112).
4.5 Psychiatric screening within a multidisciplinary framework
As discussed PSS in sarcoidosis may occur in sarcoidosis with and without overt neurocognitive disruption caused by disease-related lesions (5). Comparing diagnostic diversity between studies and case reports, we found a notable discrepancy between the high diversity of PSS that have been identified in case reports on one hand and the limited focus of diagnostic and screening tools used in studies on sarcoidosis on the other. Indeed, whereas 39.6% of the cases reported psychotic symptoms, only 5 (11.6%) of the included studies actually employed psychiatric screening capable of capturing such symptoms. A higher association was investigated by Ungprasert et al. (47) but did not reach statistical significance. Psychotic symptoms rarely become clinically apparent in the early stages of the disease, because of the rather atypical clinical presentation with mainly affective symptoms or fatigue, ubiquitous in sarcoidosis (113). Notably, studies relying on retrospective medical file analysis reported lower depression prevalence—only up to 13.8% (44, 47, 50)—highlighting the importance of active screening.
Taken together, these findings add to existing literature highlighting the need for standardized psychiatric screening in sarcoidosis research and care using dedicated assessment tools. While routine psychiatric screening may not be necessary for many sarcoidosis patients without chronic or severe disease, it should nonetheless be applied with a low threshold when somatic or psychological distress is suspected; ideally imbedded in a multidisciplinary framework, for example through a proactive, team-based consultation-liaison approach that fosters collaboration between psychiatry and other specialties (114, 115). This allows coordinating internal medicine specialists to better mediate between disciplines, and ensuring a comprehensive, patient-centered management strategy integrating the complex somatic and psychological aspects of sarcoidosis (107).
4.6 Treatment of PSS in sarcoidosis
Evidence based treatment strategies for PSS in sarcoidosis remain limited, with only a few studies addressing potential interventions (35, 38, 40, 49). Overall, the current practice is limited to supportive therapies for sarcoidosis, added by symptomatic management of comorbid PSS as needed. Pharmacological treatment of depression and anxiety in sarcoidosis includes SSRIs or SNRIs as first-line options, with a recommended treatment duration of at least 6–12 months to reduce relapse risk (44).
Cognitive Behavioral Therapy is the preferred psychotherapeutic approach for depression and anxiety, particularly for patients experiencing fatigue or maladaptive coping patterns. Combining pharmacotherapy with psychotherapy yields superior outcomes compared to either modality alone (43). Psychotherapeutic interventions have been shown to enhance physical rehabilitation, and vice versa in small studies, encouraging further research in multidisciplinary treatment approaches (35, 38, 40, 49). However, a recent meta-analysis pulmonary rehabilitation failed to show any added value in treating fatigue (116). The European Respiratory Society’s clinical guidelines do, however, still formulate conditional recommendations for treating fatigue in sarcoidosis—stating low quality of evidence—regarding the use of pulmonary rehabilitation program and/or inspiratory muscle strength training for 6–12 weeks, and, if deemed useful, a trial with d-methylphenidate or armodafinil (117).
No additional therapies specific to certain PSS in sarcoidosis were identified necessitating already established consultation-liaison psychiatry guidelines for the management of these presentations. Case reports seemed to show that the most promising improvements in PSS tend to follow a successful therapeutic response to sarcoidosis. It remains clear however that untreated PSS are detrimental to not only QoL but also—to an unknown extent - to clinical progression of sarcoidosis, whether those PSS are reactive or comorbid.
Additionally, patient and healthcare provider education play a crucial role in accurately assessing symptoms and their impact (19, 31, 32, 34), ensuring a more comprehensive understanding and management of PSS in sarcoidosis again highlighting the importance of an integrated treatment strategy.
4.7 Limitations
In general, research on sarcoidosis presents significant challenges. The disease exhibits a heterogeneous presentation with variable severity and an unpredictable course. Diagnostically, there is no golden standard, and it remains a diagnosis of exclusion with a prolonged diagnostic process and requiring histological confirmation (1).
Diagnostic variance also presents a challenge for research on PSS. For instance, in the study by Sikjur et al. (44), more than 80% of the patients with sarcoidosis suffering from anxiety and/or depression in their cohort were defined as more than one redeemed prescription of antidepressants within 1 year, instead of a clear diagnosis based on criteria (44).
Many of the studies are of cross-sectional design, so a statement about causal relationships is not possible. The majority of data are based on the subjective self-reporting questionnaires.
The primary meta-analytic outcome showed a significant association, but the wide confidence interval (OR = 5.498, 95% CI = 0.430–70.238) reflects uncertainty. This likely stems from heterogeneity and small sample sizes and underscores the need for more robust studies to confirm these findings.
Differentiating between sarcoidosis and NS is complex. Research on PSS in sarcoidosis was largely constrained to observational studies. This may contribute to the underreporting of NS; however, as previously discussed, its distinction may be questioned in the context of PSS. The design of the individual studies is highly heterogeneous, with generally a small sample size and a lack of control for important confounding factors. As a consequence, the number of studies and the sample size of the meta-analysis was relatively small. Furthermore, the available data also did not permit adjustment of the results to account for a potential subpopulation of patients with NS, which may have influenced the outcomes.
Research on PSS in sarcoidosis focusses on depression, anxiety and fatigue. For example, less research has been done on neurocognitive symptoms, sleep and psychotic symptoms. Notably, we did not assess anorexia, as this symptom it is often associated with the disease and the treatment drugs can cause gastro-intestinal side effects and reduced appetite (5).
Finally, the methodological quality of included case-reports was not evaluated using a quality assessment tool.
5 Conclusion
Despite methodological variations across studies we describe in our systematic review a consistent trend indicating patients with sarcoidosis exhibit a significant higher prevalence of PSS. Our meta-analysis confirmed the significance of this higher prevalence when compared to healthy controls. Secondary analyses by symptom category also showed a significant association of sarcoidosis with depressive symptoms and fatigue. Anxiety, and neurocognitive symptoms were also associated with sarcoidosis although with less available evidence. The etiopathogenesis is multifactorial and complex with among others. PSS interacting with each other and treatment options for sarcoidosis possibly causing PSS themselves.
Nonetheless, research in this area remains limited by methodological heterogeneity, small sample sizes, and a lack of interventional studies. Standardized psychiatric screening tools should be integrated into routine sarcoidosis management protocols, and future research should focus on targeted therapeutic interventions and interdisciplinary care models.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
AF: Data curation, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. GVH: Conceptualization, Investigation, Writing – review & editing. XVM: Validation, Writing – review & editing. MM: Formal analysis, Methodology, Supervision, Visualization, Validation, Writing – review & editing. FVDE: Conceptualization, Methodology, Supervision, Validation, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We sincerely thank the reviewers for their valuable comments and suggestions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Valeyre D Prasse A Nunes H Uzunhan Y Brillet P-Y Müller-Quernheim J . Sarcoidosis. Lancet. (2014) 383:1155–67. doi: 10.1016/S0140-6736(13)60680-7
2.
Bernardinello N Petrarulo S Balestro E Cocconcelli E Veltkamp M Spagnolo P . Pulmonary sarcoidosis: diagnosis and differential diagnosis. Diagnostics. (2021) 11:1558. doi: 10.3390/diagnostics11091558
3.
Lopes MC Amadeu TP Ribeiro-Alves M Da CCH Silva BRA Rodrigues LS et al . Defining prognosis in sarcoidosis. Medicine (Baltimore). (2020) 99:e23100. doi: 10.1097/MD.0000000000023100
4.
Bradshaw MJ Pawate S Koth LL Cho TA Gelfand JM . Neurosarcoidosis: pathophysiology, diagnosis, and treatment. Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1084. doi: 10.1212/NXI.0000000000001084
5.
Tana C Bernardinello N Raffaelli B Garcia-Azorin D Waliszewska-Prosół M Tana M et al . Neuropsychiatric manifestations of sarcoidosis. Ann Med. (2025) 57:2445191. doi: 10.1080/07853890.2024.2445191
6.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
7.
Wells GA Shea B O’Connell D Peterson J Welch V Losos M et al . The Newcastle-Ottawa scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. (2013) Available online at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (Accessed December 23, 2024).
8.
Balshem H Helfand M Schünemann HJ Oxman AD Kunz R Brozek J et al . GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol. (2011) 64:401–6. doi: 10.1016/j.jclinepi.2010.07.015
9.
AlRyalat SA Malkawi L Abu-Hassan H Al-Ryalat N . The impact of skin involvement on the psychological well-being of patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. (2019) 36:53–9. doi: 10.36141/svdld.v36i1.7270
10.
Antoniou KM Tzanakis N Tzouvelekis A Samiou M Symvoulakis EK Siafakas NM et al . Quality of life in patients with active sarcoidosis in Greece. Eur J Intern Med. (2006) 17:421–6. doi: 10.1016/j.ejim.2006.02.024
11.
Balaji AL Abhishekh HA Kumar NC . Depression in sarcoidosis patients of a tertiary care general hospital. Aust N Z J Psychiatry. (2012) 46:1199–200. doi: 10.1177/0004867412450472
12.
Benn BS Lehman Z Kidd SA Miaskowski C Sunwoo BY Ho M et al . Sleep disturbance and symptom burden in sarcoidosis. Respir Med. (2018) 144S:S35–40. doi: 10.1016/j.rmed.2018.03.021
13.
Bloem AEM Mostard RLM Stoot N Vercoulen JH Peters JB Spruit MA . Perceptions of fatigue in patients with idiopathic pulmonary fibrosis or sarcoidosis. J Thorac Dis. (2021) 13:4872–84. doi: 10.21037/jtd-21-462
14.
Bosse-Henck A Wirtz H Hinz A . Subjective sleep quality in sarcoidosis. Sleep Med. (2015) 16:570–6. doi: 10.1016/j.sleep.2014.12.025
15.
Bosse-Henck A Koch R Wirtz H Hinz A . Fatigue and excessive daytime sleepiness in sarcoidosis: prevalence, predictors, and relationships between the two symptoms. Respiration. (2017) 94:186–97. doi: 10.1159/000477352
16.
Bou GA El Sammak S Chien L-C Cavanagh JJ Hutto SK . Tumefactive brain parenchymal neurosarcoidosis. J Neurol. (2023) 270:4368–76. doi: 10.1007/s00415-023-11782-3
17.
Byg K-E Illes Z Sejbaek T Nguyen N Möller S Lambertsen KL et al . A prospective, one-year follow-up study of patients newly diagnosed with neurosarcoidosis. J Neuroimmunol. (2022) 369:577913. doi: 10.1016/j.jneuroim.2022.577913
18.
Chang B Steimel J Moller DR Baughman RP Judson MA Yeager H Jr et al . Depression in sarcoidosis. Am J Respir Crit Care Med. (2001) 163:329–34. doi: 10.1164/ajrccm.163.2.2004177
19.
Cox CE Donohue JF Brown CD Kataria YP Judson MA . Health-related quality of life of persons with sarcoidosis. Chest. (2004) 125:997–1004. doi: 10.1378/chest.125.3.997
20.
de Boer S Kolbe J Wilsher ML . The relationships among dyspnoea, health-related quality of life and psychological factors in sarcoidosis. Respirology. (2014) 19:1019–24. doi: 10.1111/resp.12359
21.
De Kleijn WPE Drent M Vermunt JK Shigemitsu H De Vries J . Types of fatigue in sarcoidosis patients. J Psychosom Res. (2011) 71:416–22. doi: 10.1016/j.jpsychores.2011.09.006
22.
De Vries J Michielsen H Van Heck GL Drent M . Measuring fatigue in sarcoidosis: the fatigue assessment scale (FAS). Br J Health Psychol. (2004) 9:279–91. doi: 10.1348/1359107041557048
23.
Elfferich MD Nelemans PJ Ponds RW De Vries J Wijnen PA Drent M . Everyday cognitive failure in sarcoidosis: the prevalence and the effect of anti-TNF-alpha treatment. Respiration. (2010) 80:212–9. doi: 10.1159/000314225
24.
Elfferich MDP De Vries J Drent M . Type D or “distressed” personality in sarcoidosis and idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. (2011) 28:65–71. PMID:
25.
Froidure S Kyheng M Grosbois JM Lhuissier F Stelianides S Wemeau L et al . Daily life physical activity in patients with chronic stage IV sarcoidosis: a multicenter cohort study. Health Sci Rep. (2019) 2:e109. doi: 10.1002/hsr2.109
26.
Goracci A Fagiolini A Martinucci M Calossi S Rossi S Santomauro T et al . Quality of life, anxiety and depression in sarcoidosis. Gen Hosp Psychiatry. (2008) 30:441–5. doi: 10.1016/j.genhosppsych.2008.04.010
27.
Hendriks C Drent M De Kleijn W Elfferich M Wijnen P De Vries J . Everyday cognitive failure and depressive symptoms predict fatigue in sarcoidosis: a prospective follow-up study. Respir Med. (2018) 138S:S24–30. doi: 10.1016/j.rmed.2017.11.008
28.
Hinz A Geue K Zenger M Wirtz H Bosse-Henck A . Daytime sleepiness in patients diagnosed with sarcoidosis compared with the general population. Can Respir J. (2018) 2018:1–6. doi: 10.1155/2018/6853948
29.
Hinz A Brähler E Möde R Wirtz H Bosse-Henck A . Anxiety and depression in sarcoidosis: the influence of age, gender, affected organs, concomitant diseases and dyspnea. Sarcoidosis Vasc Diffuse Lung Dis. (2012) 29:139–46. PMID:
30.
Hinz A Fleischer M Brähler E Wirtz H Bosse-Henck A . Fatigue in patients with sarcoidosis, compared with the general population. Gen Hosp Psychiatry. (2011) 33:462–8. doi: 10.1016/j.genhosppsych.2011.05.009
31.
Holas P Szymańska J Dubaniewicz A Farnik M Jarzemska A Krejtz I et al . Association of anxiety sensitivity-physical concerns and FVC with dyspnea severity in sarcoidosis. Gen Hosp Psychiatry. (2017) 47:43–7. doi: 10.1016/j.genhosppsych.2017.04.013
32.
Holas P Kowalski J Dubaniewicz A Farnik M Jarzemska A Maskey-Warzechowska M et al . Relationship of emotional distress and physical concerns with fatigue severity in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. (2018) 35:160–4. doi: 10.36141/svdld.v35i2.6604
33.
Hoth KF Simmering J Croghan A Hamzeh NY Investigators GRADS . Cognitive difficulties and health-related quality of life in sarcoidosis: an analysis of the GRADS cohort. J Clin Med. (2022) 11:3594. doi: 10.3390/jcm11133594
34.
Ireland J Wilsher M . Perceptions and beliefs in sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. (2010) 27:36–42. PMID:
35.
Kahlmann V Moor CC van Helmondt SJ Mostard RLM van der Lee ML Grutters JC et al . Online mindfulness-based cognitive therapy for fatigue in patients with sarcoidosis (TIRED): a randomised controlled trial. Lancet Respir Med. (2023) 11:265–72. doi: 10.1016/S2213-2600(22)00387-3
36.
Kettenbach S Radke S Müller T Habel U Dreher M . Neuropsychobiological fingerprints of chronic fatigue in sarcoidosis. Front Behav Neurosci. (2021) 15:633005. doi: 10.3389/fnbeh.2021.633005
37.
Korenromp IHE Heijnen CJ Vogels OJM van den Bosch JMM Grutters JC . Characterization of chronic fatigue in patients with sarcoidosis in clinical remission. Chest. (2011) 140:441–7. doi: 10.1378/chest.10-2629
38.
Lingner H Buhr-Schinner H Hummel S van der Meyden J Grosshennig A Nowik D et al . Short-term effects of a multimodal 3-week inpatient pulmonary rehabilitation programme for patients with sarcoidosis: the ProKaSaRe study. Respiration. (2018) 95:343–53. doi: 10.1159/000486964
39.
Moor CC van Manen MJG van Hagen PM Miedema JR van den Toorn LM Gür-Demirel Y et al . Needs, perceptions and education in sarcoidosis: a live interactive survey of patients and partners. Lung. (2018) 196:569–75. doi: 10.1007/s00408-018-0144-4
40.
Naz I Ozalevli S Ozkan S Sahin H . Efficacy of a structured exercise program for improving functional capacity and quality of life in patients with stage 3 and 4 sarcoidosis: a RANDOMIZED CONTROLLED TRIAL. J Cardiopulm Rehabil Prev. (2018) 38:124–30. doi: 10.1097/HCR.0000000000000307
41.
Patterson KC Huang F Oldham JM Bhardwaj N Hogarth DK Mokhlesi B . Excessive daytime sleepiness and obstructive sleep apnea in patients with sarcoidosis. Chest. (2013) 143:1562–8. doi: 10.1378/chest.12-1524
42.
Saligan LN . The relationship between physical activity, functional performance and fatigue in sarcoidosis. J Clin Nurs. (2014) 23:2376–9. doi: 10.1111/jocn.12490
43.
Sharp M Brown T Chen E Rand CS Moller DR Eakin MN . Psychological burden associated with worse clinical outcomes in sarcoidosis. BMJ Open Respir Res. (2019) 6:e000467. doi: 10.1136/bmjresp-2019-000467
44.
Sikjær MG Hilberg O Farver-Vestergaard I Ibsen R Løkke A . Risk of depression and anxiety in 7.302 patients with sarcoidosis: a nationwide cohort study. Sarcoidosis Vasc Diffuse Lung Dis. (2024) 41:e2024009. doi: 10.36141/svdld.v41i1.15213
45.
Spruit MA Thomeer MJ Gosselink R Troosters T Kasran A Debrock AJT et al . Skeletal muscle weakness in patients with sarcoidosis and its relationship with exercise intolerance and reduced health status. Thorax. (2005) 60:32–8. doi: 10.1136/thx.2004.022244
46.
Topçuoğlu ÖB Kavas M Alibaş H Afşar GÇ Arınç S Midi İ et al . Executive functions in sarcoidosis: a neurocognitive assessment study. Sarcoidosis Vasc Diffuse Lung Dis. (2018) 35:26–34. doi: 10.36141/svdld.v35i1.5940
47.
Ungprasert P Matteson EL Crowson CS . Increased risk of multimorbidity in patients with sarcoidosis: a population-based cohort study 1976 to 2013. Mayo Clin Proc. (2017) 92:1791–9. doi: 10.1016/j.mayocp.2017.09.015
48.
Voortman M Hendriks CMR Lodder P Drent M De Vries J . Quality of life of couples living with sarcoidosis. Respiration. (2019) 98:373–82. doi: 10.1159/000501657
49.
Wallaert B Kyheng M Labreuche J Stelianides S Wemeau L Grosbois JM . Long-term effects of pulmonary rehabilitation on daily life physical activity of patients with stage IV sarcoidosis: a randomized controlled trial. Respir Med Res. (2020) 77:1–7. doi: 10.1016/j.resmer.2019.10.003
50.
Westney GE Habib S Quarshie A . Comorbid illnesses and chest radiographic severity in African-American sarcoidosis patients. Lung. (2007) 185:131–7. doi: 10.1007/s00408-007-9008-z
51.
Wirnsberger RM de Vries J Breteler MH van Heck GL Wouters EF Drent M . Evaluation of quality of life in sarcoidosis patients. Respir Med. (1998) 92:750–6. doi: 10.1016/S0954-6111(98)90007-5
52.
Alakhras H Goodman BD Zimmer M Aguinaga S . Confusion, hallucinations, and primary polydipsia: a rare presentation of neurosarcoidosis. Cureus. (2022) 14:e21687. doi: 10.7759/cureus.25103
53.
Berrios I Jun-O’Connell A Ghiran S Ionete C . A case of neurosarcoidosis secondary to treatment of etanercept and review of the literature. BMJ Case Rep. (2015) 2015:bcr2014208188. doi: 10.1136/bcr-2014-208188
54.
Besur S Bishnoi R Talluri SK . Neurosarcoidosis: rare initial presentation with seizures and delirium. QJM. (2011) 104:801–3. doi: 10.1093/qjmed/hcq194
55.
Bona JR Fackler SM Fendley MJ Nemeroff CB . Neurosarcoidosis as a cause of refractory psychosis: a complicated case report. Am J Psychiatry. (1998) 155:1106–8. doi: 10.1176/ajp.155.8.1106
56.
Bourgeois JA Maddock RJ Rogers L Greco CM Mangrulkar RS Saint S . Neurosarcoidosis and delirium. Psychosomatics. (2005) 46:148–50. doi: 10.1176/appi.psy.46.2.148
57.
Brouwer MC de Gans J Willemse RB van de Beek D . Sarcoidosis presenting with hydrocephalus. J Neurol Neurosurg Psychiatry. (2009) 80:550–1. doi: 10.1136/jnnp.2008.163725
58.
Bryant BS Marsh KA Beuerlein WJ Kalil D Shah KK . A case of sarcoidosis mimicking lymphoma confounded by cognitive decline. Cureus. (2021) 13:e13667. doi: 10.7759/cureus.13667
59.
Chandra SR Viswanathan LG Wahatule R Shetty S . The tale of the storyteller and the painter: the paradoxes in nature. Indian J Psychol Med. (2017) 39:817–20. doi: 10.4103/IJPSYM.IJPSYM_43_17
60.
Chao DC Hassenpflug M Sharma OP . Multiple lung masses, pneumothorax, and psychiatric symptoms in a 29-year-old African-American woman. Chest. (1995) 108:871–3. doi: 10.1378/chest.108.3.871
61.
Cordingley G Navarro C Brust JC Healton EB . Sarcoidosis presenting as senile dementia. Neurology. (1981) 31:1148–51. doi: 10.1212/WNL.31.9.1148
62.
de Almeida Marcelino AL Streit S Homeyer MA Bauknecht H-C Radbruch H Ruprecht K et al . Hypertrophic pachymeningitis with persistent intrathecal inflammation secondary to neurosarcoidosis treated with intraventricular chemotherapy: a case report. Case Rep Neurol. (2023) 15:87–94. doi: 10.1159/000531229
63.
de Deus-Silva L . Queiroz L de S, Zanardi Vd V de A, Ghizoni E, Pereira H da C, Malveira GLS, Pirani C Jr, Damasceno BP, Cendes F. Hypertrophic Pachymeningitis. (2003) 61:107–11. doi: 10.1590/s0004-282x2003000100021
64.
De Mulder D Vandenberghe J . Neurosarcoidosis as a cause of manic psychosis. Tijdschr Psychiatr. (2008) 50:741–5. PMID:
65.
Douglas AC Maloney AF . Sarcoidosis of the central nervous system. J Neurol Neurosurg Psychiatry. (1973) 36:1024–33. doi: 10.1136/jnnp.36.6.1024
66.
Endres D Frye BC Schlump A Kuzior H Feige B Nickel K et al . Sarcoidosis and obsessive-compulsive symptoms. J Neuroimmunol. (2022) 373:577989. doi: 10.1016/j.jneuroim.2022.577989
67.
Fortes GCC Oliveira MCB Lopes LCG Tomikawa CS Lucato LT Castro LHM et al . Rapidly progressive dementia due to neurosarcoidosis. Dement Neuropsychol. (2013) 7:428–34. doi: 10.1590/S1980-57642013DN74000012
68.
Friedman SH Gould DJ . Neurosarcoidosis presenting as psychosis and dementia: a case report. Int J Psychiatry Med. (2002) 32:401–3. doi: 10.2190/UYUB-BHRY-L06C-MPCP
69.
Greene JJ Naumann IC Poulik JM Nella KT Weberling L Harris JP et al . The protean neuropsychiatric and vestibuloauditory manifestations of neurosarcoidosis. Audiol Neurootol. (2017) 22:205–17. doi: 10.1159/000481681
70.
Joseph FG Scolding NJ . Neurosarcoidosis: a study of 30 new cases. J Neurol Neurosurg Psychiatry. (2009) 80:297–304. doi: 10.1136/jnnp.2008.151977
71.
Joy TM Ayirolimeethal HT . Bipolar affective disorder in a patient with neurosarcoidosis – a case report. Kerala J Psychiatry. (2024) 37:420. doi: 10.30834/kjp.37.1.2024.420
72.
Kaur G Cameron L Syritsyna O Coyle P Kowalska A . A case report of neurosarcoidosis presenting as a lymphoma mimic. Case Rep Neurol Med. (2016) 2016:7464587. doi: 10.1155/2016/7464587
73.
Mariani M Shammi P . Neurosarcoidosis and associated neuropsychological sequelae: a rare case of isolated intracranial involvement. Clin Neuropsychol. (2010) 24:286–304. doi: 10.1080/13854040903347942
74.
Matsumoto K Awata S Matsuoka H Nakamura S Sato M . Chronological changes in brain MRI, SPECT, and EEG in neurosarcoidosis with stroke-like episodes. Psychiatry Clin Neurosci. (1998) 52:629–33. doi: 10.1111/j.1440-1819.1998.tb02711.x
75.
McLoughlin D McKeon P . Bipolar disorder and cerebral sarcoidosis. Br J Psychiatry. (1991) 158:410–3. doi: 10.1192/bjp.158.3.410
76.
Oh J Stokes K Tyndel F Freedman M . Progressive cognitive decline in a patient with isolated chronic neurosarcoidosis. Neurologist. (2010) 16:50–3. doi: 10.1097/NRL.0b013e31819f959b
77.
Pandey A Stoker T Adamczyk LA Stacpoole S . Aseptic meningitis and hydrocephalus secondary to neurosarcoidosis. BMJ Case Rep. (2021) 14:e242312. doi: 10.1136/bcr-2021-242312
78.
Popli AP . Risperidone for the treatment of psychosis associated with neurosarcoidosis. J Clin Psychopharmacol. (1997) 17:132–3. doi: 10.1097/00004714-199704000-00021
79.
Posada J Mahan N Abdel Meguid AS . Catatonia as a presenting symptom of isolated Neurosarcoidosis in a woman with schizophrenia. Journal of the academy of consultation-liaison. Psychiatry. (2021) 62:546–50. doi: 10.1016/j.jaclp.2020.11.003
80.
Prüter C Kunert HJ Hoff P . ICD-10 mild cognitive disorder following meningitis due to neurosarcoidosis. Psychopathology. (2001) 34:326–7. doi: 10.1159/000049332
81.
Ptak R Birtoli B Imboden H Hauser C Weis J Schnider A . Hypothalamic amnesia with spontaneous confabulations: a clinicopathologic study. Neurology. (2001) 56:1597–600. doi: 10.1212/WNL.56.11.1597
82.
Quertermous BP Kavuri S Walsh DW . An old disease with a new twist: CNS and thyroid sarcoidosis presenting as subacute dementia. Clin Case Rep. (2021) 9:e04829. doi: 10.1002/ccr3.4829
83.
Rudkin AK Wilcox RA Slee M Kupa A Thyagarajan D . Relapsing encephalopathy with headache: an unusual presentation of isolated intracranial neurosarcoidosis. J Neurol Neurosurg Psychiatry. (2007) 78:770–1. doi: 10.1136/jnnp.2006.104703
84.
Sabaawi M Gutierrez-Nunez J Fragala MR . Neurosarcoidosis presenting as schizophreniform disorder. Int J Psychiatry Med. (1992) 22:269–74. doi: 10.2190/4AHB-AH4N-URK2-T22J
85.
Shoib S Das S Saleem SM Nagendrappa S Khan KS Ullah I . Neurosarcoidosis masquerading as mania with psychotic features-a case report. Ind Psychiatry J. (2023) 32:187–9. doi: 10.4103/ipj.ipj_2_22
86.
Sommer N Weller M Petersen D Wiethölter H Dichgans J . Neurosarcoidosis without systemic sarcoidosis. Eur Arch Psychiatry Clin Neurosci. (1991) 240:334–8. doi: 10.1007/BF02279763
87.
Spiegel DR Thomas CS Shah P Kent KD . A possible case of mixed mania due to neurosarcoidosis treated successfully with methylprednisolone and ziprasidone: another example of frontal-subcortical disinhibition?Gen Hosp Psychiatry. (2010) 32:e1–3. doi: 10.1016/j.genhosppsych.2009.07.010
88.
Spiegel DR Morris K Rayamajhi U . Neurosarcoidosis and the complexity in its differential diagnoses: a review. Innov Clin Neurosci. (2012) 9:10–6. PMID:
89.
Steriade C Shumak SL Feinstein A . A 54-year-old man with hallucinations and hearing loss. CMAJ. (2014) 186:1315–8. doi: 10.1503/cmaj.131535
90.
Tokumitsu K Demachi J Yamanoi Y Oyama S Takeuchi J Yachimori K et al . Cardiac sarcoidosis resembling panic disorder: a case report. BMC Psychiatry. (2017) 17:1184. doi: 10.1186/s12888-016-1184-z
91.
Tsitos S Niederauer LC Albert GP Mueller J Straube A Von Baumgarten L . Case report: drug-induced (neuro) sarcoidosis-like lesion under IL4 receptor blockade with dupilumab. Front Neurol. (2022) 13:881144. doi: 10.3389/fneur.2022.881144
92.
Tumialán LM Gupta M Hunter S Tumialán L . A 55-year-old man with liver failure, delirium and seizures. Brain Pathol. (2007) 17:475. doi: 10.1111/j.1750-3639.2007.00091_6.x
93.
Van Hoye G Willekens B Vanden Bossche S Morrens M Van Den Eede F . Case report: psychosis with catatonia in an adult man: a presentation of neurosarcoidosis. Front Psych. (2024) 15:1276744. doi: 10.3389/fpsyt.2024.1276744
94.
Walbridge DG . Rapid-cycling disorder in association with cerebral sarcoidosis. Br J Psychiatry. (1990) 157:611–3. doi: 10.1192/bjp.157.4.611
95.
Westhout FD Linskey ME . Obstructive hydrocephalus and progressive psychosis: rare presentations of neurosarcoidosis. Surg Neurol. (2008) 69:68. doi: 10.1016/j.surneu.2007.01.068
96.
Zhang J Waisbren E Hashemi N Lee AG . Visual hallucinations (Charles bonnet syndrome) associated with neurosarcoidosis. Middle East Afr J Ophthalmol. (2013) 20:369–71. doi: 10.4103/0974-9233.119997
97.
Lal C . Obstructive sleep apnea and sarcoidosis interactions. Sleep Med Clin. (2024) 19:295–305. doi: 10.1016/j.jsmc.2024.02.010
98.
Goldsmith DR Rapaport MH Miller BJ . A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry. (2016) 21:1696–709. doi: 10.1038/mp.2016.3
99.
Al-Diwani AAJ Pollak TA Irani SR Lennox BR . Psychosis: an autoimmune disease?Immunology. (2017) 152:388–401. doi: 10.1111/imm.12795
100.
Morrens M Overloop C Coppens V Loots E Van Den Noortgate M Vandenameele S et al . The relationship between immune and cognitive dysfunction in mood and psychotic disorder: a systematic review and a meta-analysis. Mol Psychiatry. (2022) 27:3237–46. doi: 10.1038/s41380-022-01582-y
101.
Pedraz-Petrozzi B Elyamany O Rummel C Mulert C . Effects of inflammation on the kynurenine pathway in schizophrenia - a systematic review. J Neuroinflammation. (2020) 17:56. doi: 10.1186/s12974-020-1721-z
102.
Gandhi T Fiorletta Quiroga E Atchley W . Hypercalcemia in sarcoidosis: a case with inappropriately normal levels of 1,25-dihydroxy vitamin d. Chest. (2022) 162:A1253. doi: 10.1016/j.chest.2022.08.1000
103.
Ray A Kar A Ray BK Dubey S . Hypercalcaemic encephalopathy as a presenting manifestation of sarcoidosis. BMJ Case Rep. (2021) 14:e241246. doi: 10.1136/bcr-2020-241246
104.
Focke JK Brokbals M Becker J Veltkamp R van de Beek D Brouwer MC et al . Cerebral vasculitis related to neurosarcoidosis: a case series and systematic literature review. J Neurol. (2025) 272:135. doi: 10.1007/s00415-024-12868-2
105.
Henderson C Noblett J Parke H Clement S Caffrey A Gale-Grant O et al . Mental health-related stigma in health care and mental health-care settings. Lancet Psychiatry. (2014) 1:467–82. doi: 10.1016/S2215-0366(14)00023-6
106.
Drent M Lower EE De Vries J . Sarcoidosis-associated fatigue. Eur Respir J. (2012) 40:255–63. doi: 10.1183/09031936.00002512
107.
Drent M Russell A-M Saketkoo LA Spagnolo P Veltkamp M Wells AU . Representatives of the sarcoidosis community. Breaking barriers: holistic assessment of ability to work in patients with sarcoidosis. Lancet Respir Med. (2024) 12:848–51. doi: 10.1016/S2213-2600(24)00297-2
108.
Goldman C Judson MA . Corticosteroid refractory sarcoidosis. Respir Med. (2020) 171:106081. doi: 10.1016/j.rmed.2020.106081
109.
Fardet L Petersen I Nazareth I . Suicidal behavior and severe neuropsychiatric disorders following glucocorticoid therapy in primary care. Am J Psychiatry. (2012) 169:491–7. doi: 10.1176/appi.ajp.2011.11071009
110.
McCune JS Hawke RL LeCluyse EL Gillenwater HH Hamilton G Ritchie J et al . In vivo and in vitro induction of human cytochrome P4503A4 by dexamethasone. Clin Pharmacol Ther. (2000) 68:356–66. doi: 10.1067/mcp.2000.110215
111.
Miola A Dal Porto V Meda N Perini G Solmi M Sambataro F . Secondary mania induced by TNF-α inhibitors: a systematic review. Psychiatry Clin Neurosci. (2022) 76:15–21. doi: 10.1111/pcn.13302
112.
Yelnik CM Gaboriau L Petitpain N Théophile H Delaporte E Carton L et al . TNF-α inhibitors and psychiatric adverse drug reactions in the spectrum of bipolar (manic) or psychotic disorders: analysis from the French pharmacovigilance database. Therapie. (2021) 76:449–54. doi: 10.1016/j.therap.2020.09.003
113.
Kapoor S . Psychosis: a rare but serious psychiatric anomaly in patients with sarcoidosis. Sarcoidosis Vasc Diffuse Lung Dis. (2014) 31:76–7. PMID:
114.
Oldham MA Desan PH Lee HB Bourgeois JA Shah SB Hurley PJ et al . Proactive consultation-liaison psychiatry: American Psychiatric Association resource document. J. Acad Consult Liaison Psychiatry. (2021) 62:169–85. doi: 10.1016/j.jaclp.2021.01.005
115.
Sharpe M Toynbee M Walker J . Proactive integrated consultation-liaison psychiatry: a new service model for the psychiatric care of general hospital inpatients. Gen Hosp Psychiatry. (2020) 66:9–15. doi: 10.1016/j.genhosppsych.2020.06.005
116.
Alsina-Restoy X Torres-Castro R Caballería E Gimeno-Santos E Solis-Navarro L Francesqui J et al . Pulmonary rehabilitation in sarcoidosis: a systematic review and meta-analysis. Respir Med. (2023) 219:107432. doi: 10.1016/j.rmed.2023.107432
117.
Baughman RP Valeyre D Korsten P Mathioudakis AG Wuyts WA Wells A et al . ERS clinical practice guidelines on treatment of sarcoidosis. Eur Respir J. (2021) 58:2004079. doi: 10.1183/13993003.04079-2020
Summary
Keywords
sarcoidosis, neurosarcoidosis, depression, anxiety, fatigue, psychosis, neuroinflammatory diseases
Citation
Frans A, Van Hoye G, Van Meerbeeck X, Morrens M and Van Den Eede F (2025) Psychiatric symptoms and syndromes in sarcoidosis: a systematic review and meta-analysis. Front. Med. 12:1634175. doi: 10.3389/fmed.2025.1634175
Received
23 May 2025
Accepted
01 September 2025
Published
29 September 2025
Volume
12 - 2025
Edited by
Ogugua Ndili Obi, East Carolina University, United States
Reviewed by
Luis Rafael Moscote-Salazar, Colombian Clinical Research Group in Neurocritical Care, Colombia
Claudio Tana, SS Annunziata Polyclinic Hospital, Italy
Updates
Copyright
© 2025 Frans, Van Hoye, Van Meerbeeck, Morrens and Van Den Eede.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andreas Frans, andreas.frans@uza.be
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.