Abstract
Background and objective:
Transjugular intrahepatic portosystemic shunt (TIPS) is an important intervention for relieving portal hypertension-related complications in patients with decompensated cirrhosis. However, over-hepatic encephalopathy (OHE) after TIPS is common and significantly impacts patients’ prognosis and quality of life. There is an urgent need for an effective predictive model to evaluate the risk of OHE. This study aims to develop and validate a practical, accessible, and high-performance predictive model for OHE based on preoperative clinical parameters.
Methods:
A total of 440 patients with decompensated cirrhosis who underwent their first TIPS procedure between January 2017 and December 2023 were retrospectively enrolled and randomly divided into training (n = 310) and validation (n = 130) cohorts in a 7:3 ratio. Least absolute shrinkage and selection operator (LASSO) regression was used for variable selection, followed by multivariate logistic regression to construct the predictive model, which was visualized as a nomogram. The model’s performance was evaluated using the area under the receiver operating characteristic curve (AUC), calibration curves, decision curve analysis (DCA), and clinical impact curves (CIC).
Results:
LASSO regression selected five predictors from 34 variables: prognostic nutritional index (PNI), age, previous history of hepatic encephalopathy, serum ammonia, and creatinine. The model achieved an AUC of 0.8835 (95% CI: 0.8408–0.9262) in the training cohort, outperforming MELD (AUC: 0.7204) and CTP scores (AUC: 0.6576). In the validation cohort, the AUC was 0.858, indicating good discrimination. Calibration curves, DCA, and CIC also demonstrated strong model accuracy and clinical utility.
Conclusion:
The prediction model based on preoperative clinical parameters accurately assesses the 1-year risk of OHE after TIPS in patients with cirrhosis and may serve as a practical tool for clinical decision-making.
Introduction
Cirrhosis is a global public health issue. According to the World Health Organization, cirrhosis-related deaths account for approximately 2.4% of global mortality. In the decompensated stage, portal hypertension worsens and may lead to severe complications such as refractory ascites and variceal bleeding, with an annual mortality rate of 20–57% (1, 2). Transjugular intrahepatic portosystemic shunt (TIPS), a minimally invasive procedure, offers advantages such as low trauma, rapid recovery, and effective portal pressure reduction. It has become a first-line therapy for refractory ascites and secondary prevention of variceal bleeding (3).
However, the incidence of hepatic encephalopathy (HE) after TIPS remains high, with reported rates ranging from 23 to 54.5% (4, 5). The West Haven criteria categorize HE severity into covert HE (grades 0–1) and overt hepatic encephalopathy (OHE) (grades 2–4) (6, 7), with post-TIPS OHE incidence reaching 30–45% (8). This complication can necessitate shunt reduction or embolization, increasing economic burden and hospitalization time while impairing quality of life and long-term prognosis. It also complicates clinical decision-making regarding TIPS eligibility.
Clinical prediction models, which integrate clinical, laboratory, and imaging data, are increasingly used to predict disease progression and treatment response, supporting personalized and precision medicine (9). Although Child-Turcotte-Pugh (CTP) and Model for end-stage liver disease (MELD) scores are commonly used to quantify liver function and estimate prognosis, they were not specifically developed to predict post-TIPS OHE and have limitations in this setting—such as inclusion of subjective items (e.g., ascites, HE grade) and limited incorporation of nutritional and inflammatory markers that are increasingly recognized as relevant to HE pathogenesis.
Thus, this study aimed to build and validate a multivariate clinical prediction model using quantifiable preoperative indicators to estimate the 1-year risk of OHE in patients with decompensated cirrhosis after TIPS. The goal is to provide clinicians with an intuitive and practical preoperative tool for identifying high-risk patients and optimizing interventions to advance TIPS-based care.
Methods
Study population and grouping
This single-center retrospective study included 550 patients with decompensated cirrhosis who underwent first-time TIPS between January 2017 and December 2023 at the Department of Gastroenterology, Air Force Medical Center. After excluding patients with malignancies (n = 38), severe hematologic disorders (n = 2), systemic infections (n = 1), post-transplantation (n = 2), and missing data or loss to follow-up (n = 67), 440 patients were enrolled. Based on OHE occurrence within 1-year post-TIPS, patients were categorized into OHE and non-OHE groups and randomly split into training (n = 310) and validation (n = 130) cohorts (Figure 1).
Figure 1

Flow chart of participants selection.
Inclusion criteria
(1) Cirrhosis confirmed by pathology, imaging, or clinical criteria (10, 11); (2) First-time TIPS procedure; (3) Complete perioperative clinical data available.
Exclusion criteria
(1) Malignancies; (2) Severe renal insufficiency, hematologic diseases, uncontrolled systemic infections; (3) History of liver transplantation; (4) Recent psychiatric medication use or neuropsychiatric disorders; (5) Lost to follow-up postoperatively.
Data collection
Demographics (age, sex), lifestyle (smoking, alcohol), laboratory tests (liver/renal function, coagulation, lipids, white blood cell count, hemoglobin, body mass index, albumin/globulin ratio), and clinical history (diabetes, hypertension, cirrhosis etiology, TIPS indication, previous history of HE) were recorded. Prognostic nutritional index (PNI) = albumin (g/L) + 5 × lymphocyte count (109/L) (12). CTP score: total bilirubin, albumin, prothrombin time, ascites, HE (13). MELD score = 11.2 × ln(International Normalized Ratio) + 9.57 × ln(creatinine, mg/dL) + 3.78 × ln(total bilirubin, mg/dL) + 6.43 (14).
Outcome definition
The primary outcome was the occurrence of OHE (West Haven grade ≥2) within 1 year post-TIPS (6). Diagnosis was adjudicated by two independent hepatologists (blinded to model predictors) based on in-hospital medical records, outpatient evaluations, and structured telephone interviews during follow-up. The structured telephone questionnaire asked about specific symptoms/signs (e.g., confusion/disorientation, somnolence, asterixis, slurred speech, behavioral change). If telephone screening suggested possible OHE, investigators reviewed the hospital/emergency records and, when necessary, consulted an expert hepatologist; disagreements were resolved by a third senior hepatologist. The first OHE episode after TIPS during the 12-month follow-up was considered the study endpoint.
Ethical approval
Approved by the Ethics Committee of the Air Force Medical Center. All participants signed informed consent (Air Force Special Forces (Research) No. 2025-38-PJ01). The study adhered to the Declaration of Helsinki.
Statistical analysis
Analysis of variance and Chi-square tests were used to compare continuous and categorical variables. After random allocation to training and validation cohorts (7:3), to avoid overfitting and multicollinearity, variable selection using LASSO regression (10-fold cross-validation) was performed only within the training cohort t. Variables retained by LASSO were entered into multivariable logistic regression to derive the final model and to construct a nomogram. Model performance was evaluated in both the training and independent validation cohorts. Variables selected by LASSO were subjected to Spearman correlation and multivariate logistic regression to build the model and nomogram.
Model discrimination was assessed using the area under the receiver operating characteristic curve (AUC), calibration with calibration curves, and clinical utility with decision curve analysis (DCA), and clinical impact curves (CIC). All analyses were performed using R software (v4.1.3). A two-sided p < 0.05 was considered statistically significant.
Results
Baseline characteristics
Among 440 patients, the mean age was 54.55 ± 12.77 years; 284 were male (64.55%), 156 female (35.45%). TIPS indications included variceal bleeding (n = 271, 61.59%), refractory ascites (n = 139, 31.59%), and others (n = 30, 6.82%). Cirrhosis etiologies included hepatitis B (n = 205, 46.59%), alcohol (n = 64, 14.55%), autoimmune liver disease (n = 25, 5.68%), cavernous transformation of portal vein (n = 17, 3.86%), and others (n = 129, 29.32%). Compared to the non-OHE group, OHE patients had significantly higher age, ammonia, creatinine, uric acid, total bile acid, Prothrombin time, MELD, and CTP scores, and lower high density lipoprotein cholesterol, cholinesterase, PNI, and hemoglobin (p < 0.05) (Table 1).
Table 1
| Characteristic | Total patients (N = 440) | Non-OHE (N = 338) | OHE (N = 102) | p-value |
|---|---|---|---|---|
| Serum ammonia (μmol/L) | 62.63 ± 32.36 | 57.01 ± 29.98 | 81.26 ± 33.08 | <0.001 |
| Age (years) | 54.55 ± 12.77 | 51.70 ± 12.28 | 64.01 ± 9.41 | <0.001 |
| Body mass index (kg/m2) | 23.41 ± 12.72 | 23.58 ± 14.38 | 22.83 ± 3.55 | 0.601 |
| Urea nitrogen (mmol/L) | 6.92 ± 6.70 | 6.69 ± 7.21 | 7.69 ± 4.56 | 0.187 |
| Creatinine (μmol/L) | 70.93 ± 39.41 | 67.66 ± 38.74 | 81.79 ± 39.88 | 0.001 |
| Total cholesterol (mmol/L) | 3.73 ± 11.24 | 3.25 ± 1.04 | 5.32 ± 23.29 | 0.102 |
| Triglyceride (mmol/L) | 0.88 ± 0.61 | 0.89 ± 0.66 | 0.84 ± 0.44 | 0.469 |
| HDL-C (mmol/L) | 0.90 ± 0.35 | 0.92 ± 0.35 | 0.83 ± 0.34 | 0.034 |
| LDL-C (mmol/L) | 1.77 ± 0.68 | 1.80 ± 0.67 | 1.67 ± 0.72 | 0.096 |
| Alanine aminotransferase (U/L) | 26.91 ± 38.35 | 28.11 ± 42.46 | 22.91 ± 18.82 | 0.231 |
| Aspartate aminotransferase (U/L) | 37.11 ± 39.40 | 37.97 ± 43.83 | 34.24 ± 18.02 | 0.402 |
| Total bilirubin (μmol/L) | 27.87 ± 30.96 | 27.90 ± 33.40 | 27.75 ± 21.10 | 0.966 |
| Alkaline phosphatase (U/L) | 92.64 ± 51.21 | 90.87 ± 49.70 | 98.48 ± 55.76 | 0.189 |
| Gamma-glutamyl transferase (U/L) | 53.83 ± 62.52 | 56.02 ± 63.55 | 46.58 ± 58.68 | 0.181 |
| Total bile acid (μmol/L) | 41.22 ± 56.64 | 36.95 ± 45.47 | 55.38 ± 82.35 | 0.004 |
| Cholinesterase (U/L) | 143.47 ± 69.61 | 150.62 ± 70.82 | 119.78 ± 59.91 | <0.001 |
| Lactate dehydrogenase (U/L) | 203.08 ± 80.92 | 199.37 ± 82.20 | 215.36 ± 75.61 | 0.080 |
| Prothrombin time (ser) | 14.77 ± 3.14 | 14.61 ± 2.80 | 15.30 ± 4.03 | 0.049 |
| International normalized ratio | 1.34 ± 0.28 | 1.32 ± 0.26 | 1.38 ± 0.33 | 0.102 |
| APTT (ser) | 34.71 ± 13.73 | 34.83 ± 15.42 | 34.34 ± 5.10 | 0.756 |
| Fibrinogen (g/L) | 2.17 ± 1.04 | 2.22 ± 1.11 | 2.00 ± 0.77 | 0.062 |
| PNI | 40.01 ± 6.00 | 41.11 ± 5.73 | 36.37 ± 5.41 | <0.001 |
| Hemoglobin (g/L) | 91.47 ± 26.79 | 93.42 ± 27.90 | 85.01 ± 21.62 | 0.005 |
| Platelet count (×109 /L) | 94.14 ± 83.14 | 98.12 ± 88.46 | 80.96 ± 60.85 | 0.068 |
| White blood cell count (×109 /L) | 3.78 ± 2.89 | 3.83 ± 2.87 | 3.59 ± 2.96 | 0.457 |
| Albumin/Globulins | 1.47 ± 1.66 | 1.52 ± 1.88 | 1.30 ± 0.39 | 0.234 |
| MELD score | 10.89 ± 4.47 | 10.66 ± 4.40 | 11.65 ± 4.62 | 0.050 |
| CTP score | 6.90 ± 1.64 | 6.70 ± 1.53 | 7.60 ± 1.80 | <0.001 |
| Gender (%) | 0.365 | |||
| Male | 284 (64.55%) | 222 (65.68%) | 62 (60.78%) | |
| Female | 156 (35.45%) | 116 (34.32%) | 40 (39.22%) | |
| Smoking (%) | 0.375 | |||
| Yes | 313 (71.14%) | 244 (72.19%) | 69 (67.65%) | |
| No | 127 (28.86%) | 94 (27.81%) | 33 (32.35%) | |
| Drink (%) | 0.709 | |||
| Yes | 304 (69.09%) | 232 (68.64%) | 72 (70.59%) | |
| No | 136 (30.91%) | 106 (31.36%) | 30 (29.41%) | |
| Diabetes (%) | 0.150 | |||
| Yes | 350 (79.55%) | 274 (81.07%) | 76 (74.51%) | |
| No | 90 (20.45%) | 64 (18.93%) | 26 (25.49%) | |
| Hypertension (%) | 0.053 | |||
| Yes | 353 (80.23%) | 278 (82.25%) | 75 (73.53%) | |
| No | 87 (19.77%) | 60 (17.75%) | 27 (26.47%) | |
| Etiology (%) | 0.003 | |||
| Viral hepatica | 205 (46.59%) | 165 (48.82%) | 40 (39.22%) | |
| Alcoholic hepatitis | 64 (14.55%) | 44 (13.02%) | 20 (19.61%) | |
| Autoimmune hepatitis | 25 (5.68%) | 13 (3.85%) | 12 (11.76%) | |
| Portal vein cavernous degeneration | 17 (3.86%) | 17 (5.03%) | 0 (0.00%) | |
| Others | 129 (29.32%) | 99 (29.29%) | 30 (29.41%) | |
| Surgical indications (%) | <0.001 | |||
| Gastrointestinal bleeding | 271 (61.59%) | 222 (65.68%) | 49 (48.04%) | |
| Refractory ascites | 139 (31.59%) | 88 (26.04%) | 51 (50.00%) | |
| Others | 30 (6.82%) | 28 (8.28%) | 2 (1.96%) | |
| Previous history of HE (%) | <0.001 | |||
| Yes | 19 (4.32%) | 4 (1.18%) | 15 (14.71%) | |
| No | 421 (95.68%) | 334 (98.82%) | 87 (85.29%) |
Baseline characteristics of the study population.
HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; APTT, activated partial thromboplastin time; PNI, prognostic nutritional index; MELD, model for end-stage liver disease; CTP, Child-Turcotte-Pugh; HE, hepatic encephalopathy.
Variable selection
To reduce complexity and overfitting, 34 candidate variables were analyzed by LASSO regression (Figure 2A). Using 10-fold cross-validation (Figure 2B), λ.1se = 0.025 was selected, retaining five predictors: PNI, age, prior HE, ammonia, and creatinine. Spearman analysis (Figure 3) showed age had the strongest positive correlation with OHE, while creatinine was weakest. PNI showed a protective effect—higher PNI correlated with lower OHE risk.
Figure 2
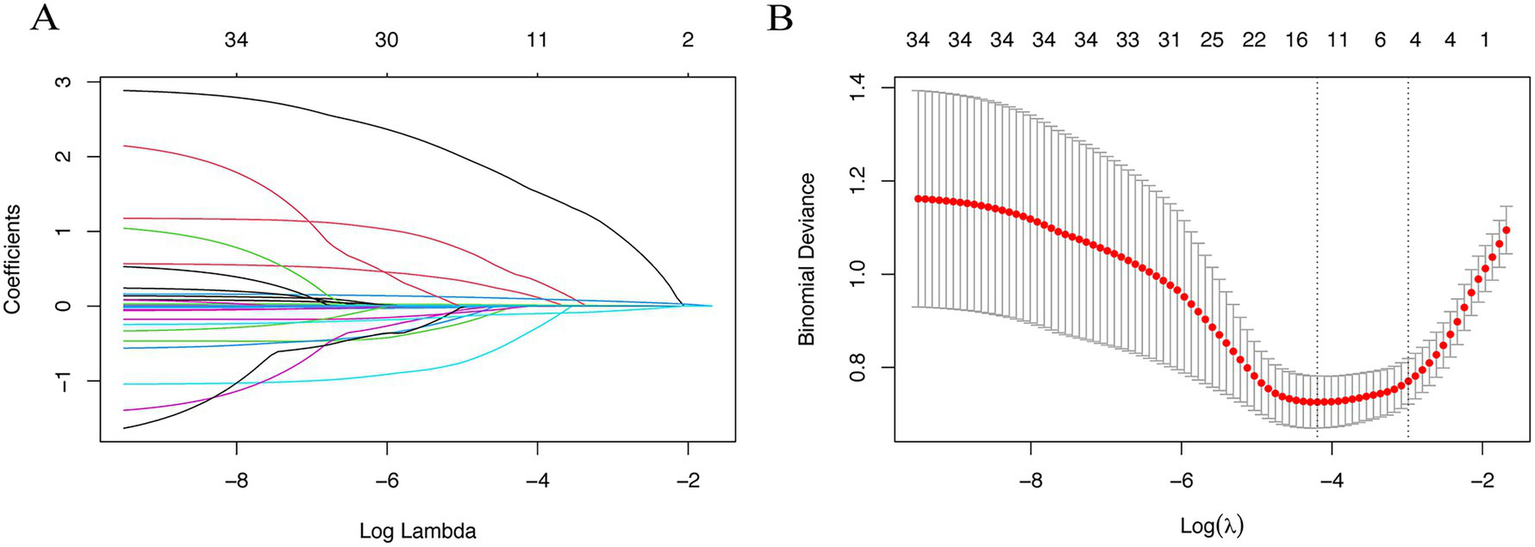
Lasso regression was used to screen predictors of overt hepatic encephalopathy within 1 year after TIPS in patients with cirrhosis. (A) Regression coefficients change curves versus log Lambda. (B) The optimal λ process was obtained by iterative analysis using 10-fold cross-validation.
Figure 3
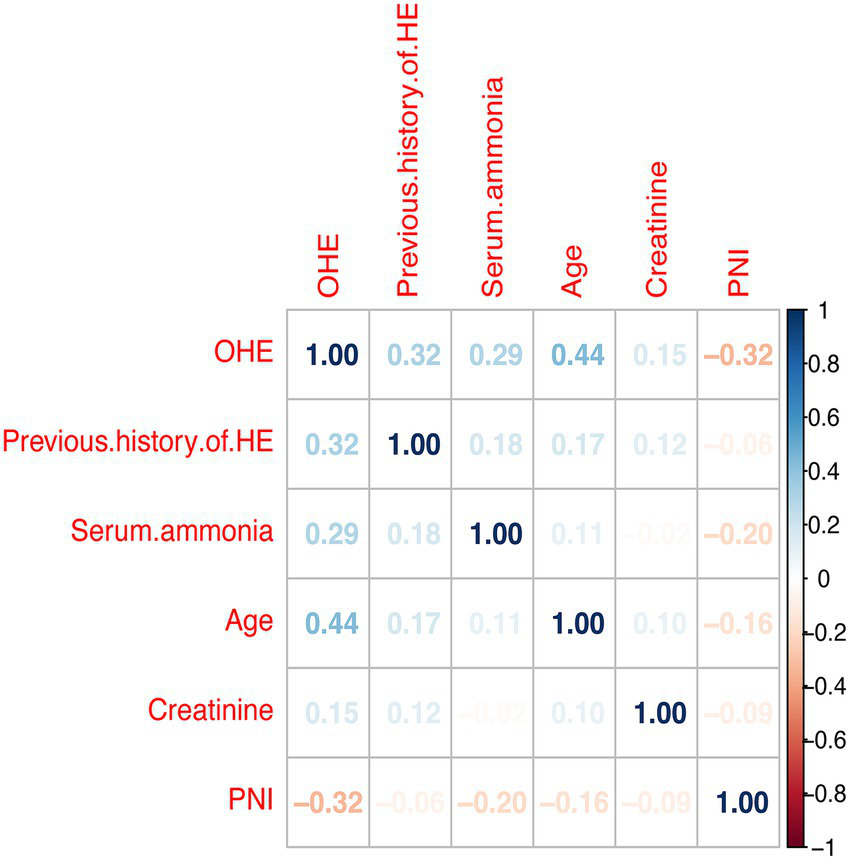
Pearson’s analysis of the occurrence of overt hepatic encephalopathy in cirrhotic patients 1 year after TIPS and its influencing factors. HE, Hepatic Encephalopathy; OHE, Overt Hepatic Encephalopathy; PNI, Prognostic nutritional index.
Model construction
A multivariate logistic regression model was constructed using the five variables, and a nomogram was drawn (Figure 4). Each predictor was assigned points, and total score mapped to 1-year OHE probability. For example, a score of 353 corresponded to a 91.2% risk (95% CI: 78.2–96.1%).
Figure 4
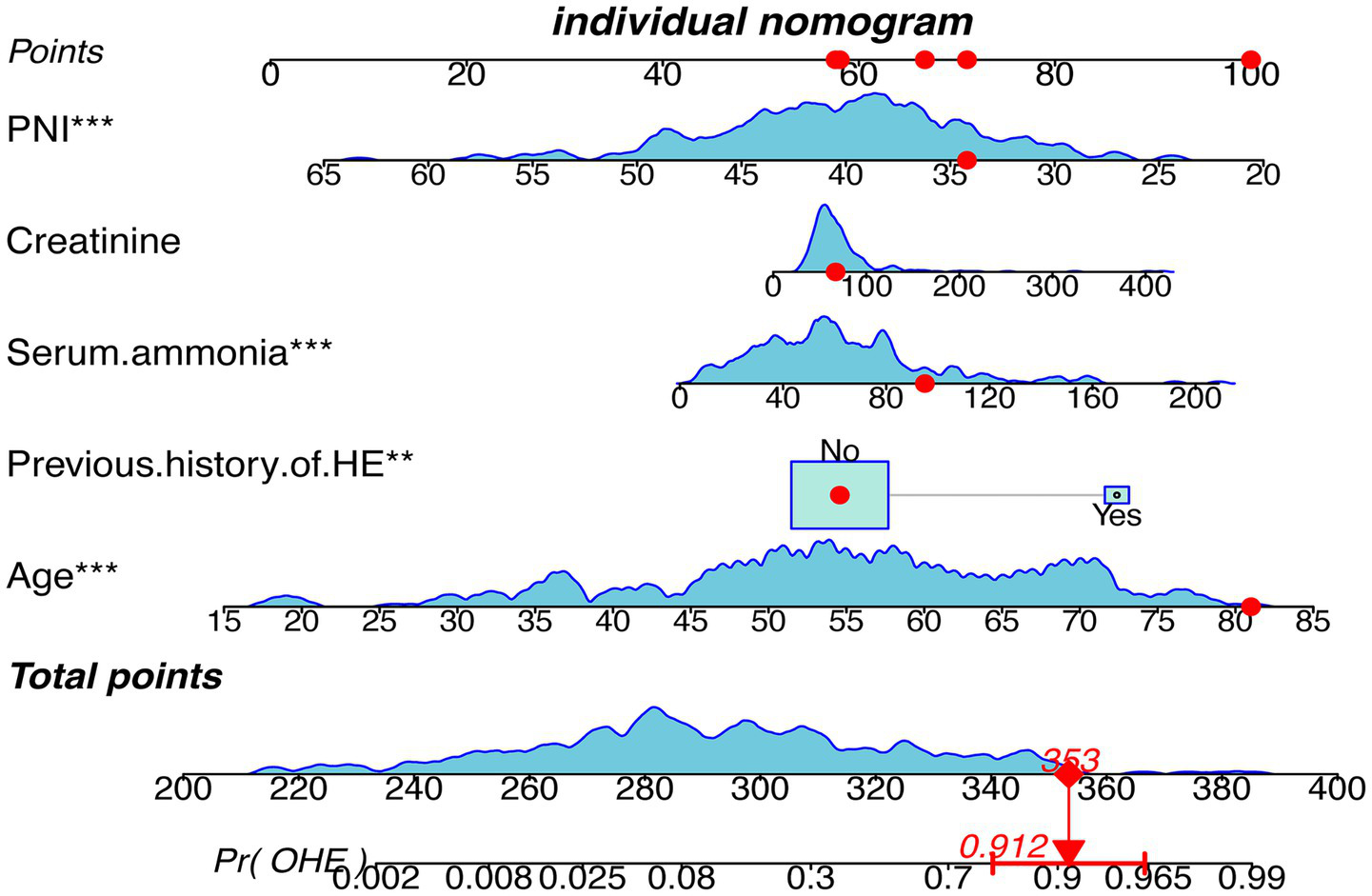
Nomogram depicting the clinical prediction model for the occurrence of OHE within 1 year after TIPS in patients with cirrhosis. HE, Hepatic Encephalopathy; OHE, Overt Hepatic Encephalopathy; PNI, Prognostic nutritional index.
Model validation and comparison
ROC curves (Figures 5A,B) demonstrated superior performance of the model vs. MELD and CTP. In the training cohort, AUC = 0.8835 (95% CI: 0.745–0.836) vs. MELD (0.7204) and CTP (0.6576). In the validation cohort, AUC = 0.858 vs. MELD (0.745) and CTP (0.6359). DeLong’s test was performed to assess the statistical significance of differences between paired ROC curves, and the results are presented in Supplementary Table S1. The nomogram showed significantly higher discrimination than MELD and CTP, whereas the difference between MELD and CTP was not statistically significant. Calibration curves (Figures 6A,B) showed high agreement between predicted and observed probabilities.
Figure 5
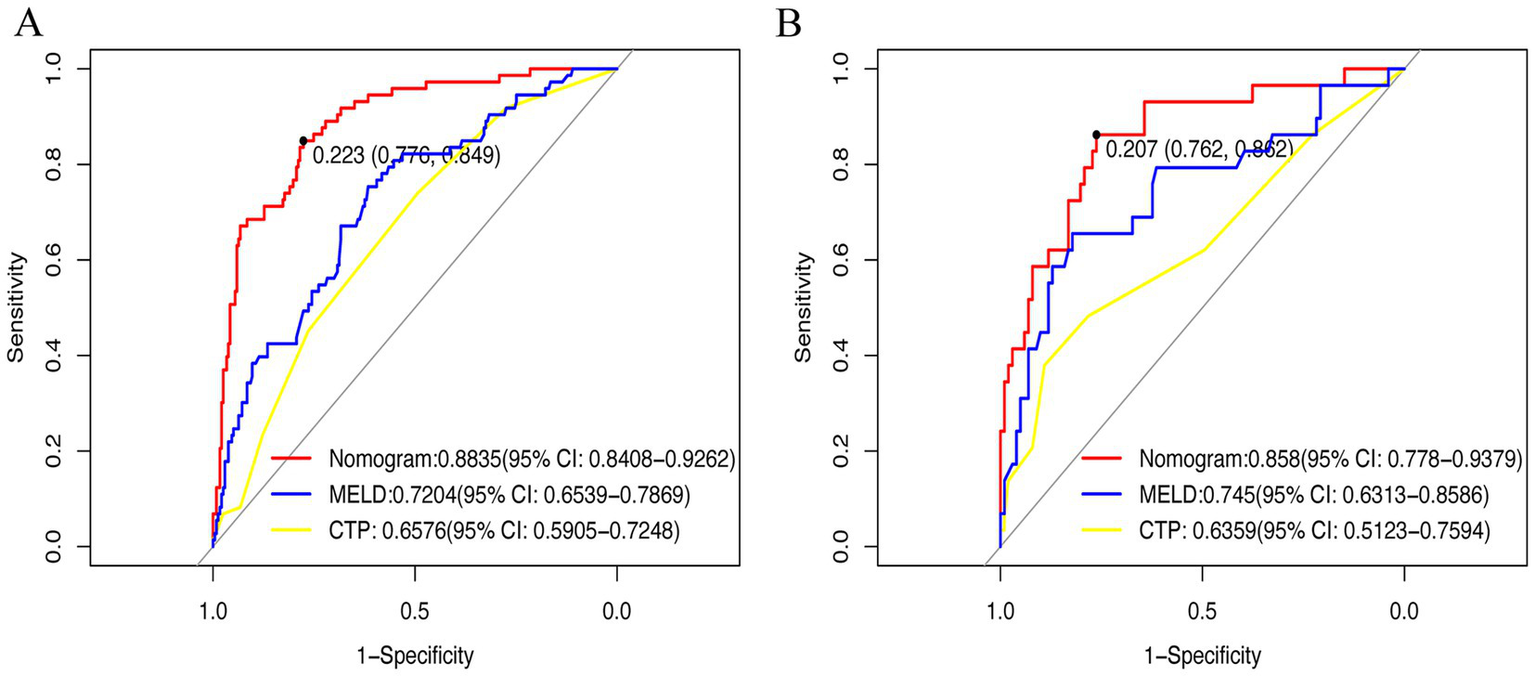
Receiver operating characteristic curve was used in clinical prediction models. (A) Training set; (B) Validation set. CTP, Child-Turcotte-Pugh; MELD: Model for End-Stage Liver Disease.
Figure 6
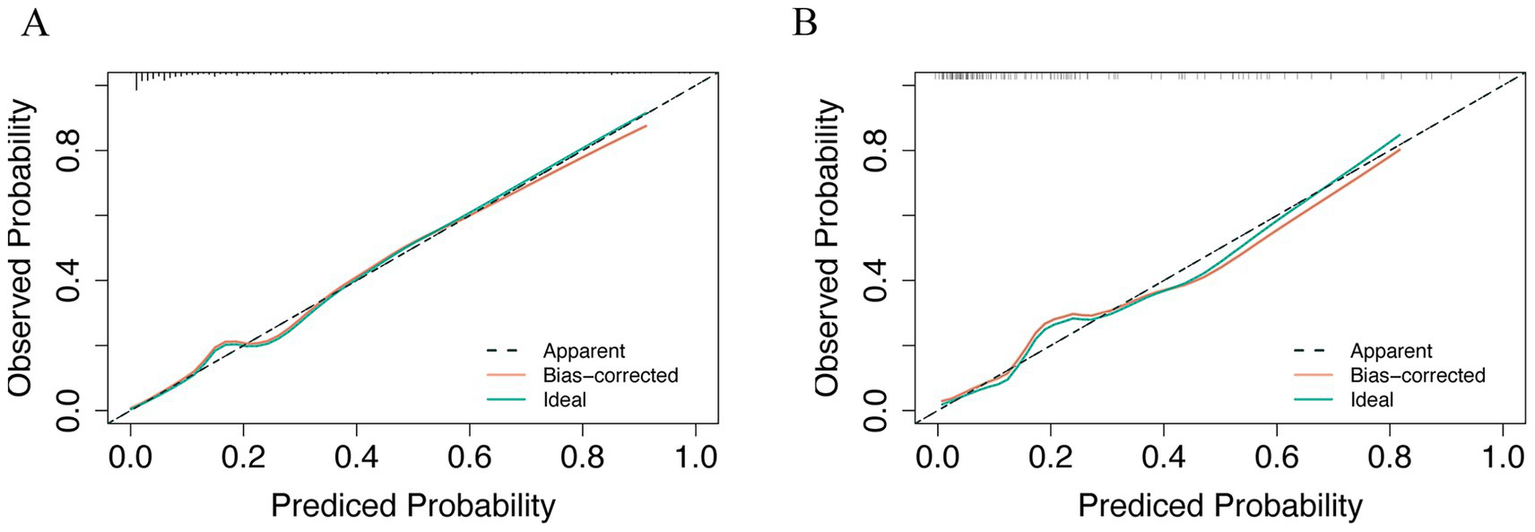
Calibration curve was used in clinical prediction models. (A) Training set; (B) Validation set.
Model evaluation
DCA (Figures 7A,B) demonstrated that the nomogram consistently outperformed the “treat all” and “treat none” strategies across the evaluated risk-threshold range (0.1–0.8). Within this interval, the net benefit values achieved by the model ranged approximately from 0 to 0.25. CIC (Figures 7C,D) indicated that above 60% threshold, the predicted high-risk population closely matched actual OHE cases, minimizing error and confirming clinical utility.
Figure 7
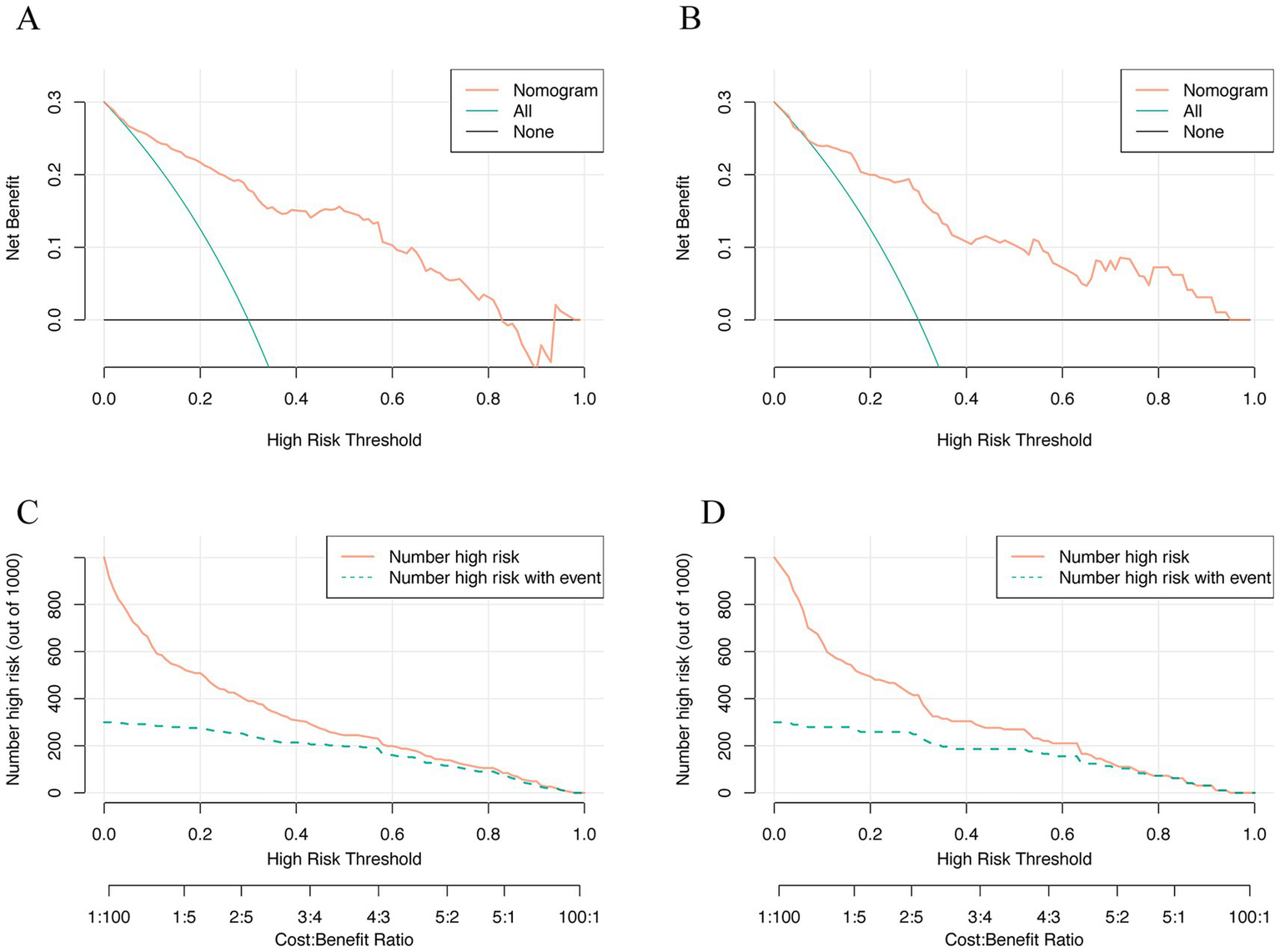
Decision curve analysis and clinical impact curve were used in clinical prediction models. (A) Training set; (B) Validation set; (C) Training set; (D) Validation set.
Discussion
In this single-center retrospective cohort of 440 patients undergoing first-time TIPS, we developed and internally validated a nomogram incorporating five preoperative variables (PNI, age, prior HE, serum ammonia, and creatinine) to predict the 1-year risk of OHE. The nomogram demonstrated good discrimination (AUC 0.88 training; 0.86 validation), favorable calibration, and superior clinical net benefit compared with MELD and CTP scoring systems.
These findings are clinically meaningful because, although TIPS effectively relieves portal hypertension and reduces complications such as variceal bleeding and refractory ascites, post-TIPS OHE remains a major challenge that compromises quality of life, increases healthcare costs, and may necessitate shunt reduction (15). Given the limited predictive tools for post-TIPS OHE, a simple, accurate model based on preoperative data is essential. Nomograms provide a user-friendly visual representation of complex models, integrating multiple predictors to estimate individualized risk, and are increasingly applied in clinical practice (16). This study identified five significant predictors—PNI, age, prior HE, serum ammonia, and creatinine—using LASSO regression. Elevated age, ammonia, creatinine, and prior HE were associated with increased OHE risk, consistent with previous studies (4, 17–20). Nevertheless, previous models for predicting post-TIPS OHE have primarily relied on conventional scores such as MELD, CTP, or single laboratory markers (e.g., ammonia). However, their discriminatory ability and clinical applicability have been limited. Our study extends this body of work by incorporating both traditional risk factors and the nutritional-inflammatory marker PNI, thereby improving predictive accuracy and providing a more practical tool for individualized risk stratification. Importantly, our findings additionally highlight nutritional and immune status, as reflected by PNI, as an independent protective factor, suggesting that good preoperative nutritional status may reduce OHE risk.
HE is a neuropsychiatric syndrome caused by liver dysfunction and portosystemic shunting, primarily driven by hyperammonemia (21). When liver detoxification is impaired (e.g., after TIPS), ammonia enters the systemic circulation, leading to cognitive impairment (22–24).
The present study also found that elevated serum creatinine levels were associated with the occurrence of OHE in TIPS in cirrhotic patients. Creatinine level is indicative of renal function, and research has identified renal insufficiency as a significant risk factor for HE (25, 26). In the presence of impaired hepatic function, impaired urea cycling limits hepatic clearance of ammonia, and the kidney becomes a key organ for compensatory ammonia excretion. As renal function deteriorates, renal tubular ammonia secretion is reduced, thereby exacerbating hyperammonemia. This suggests an important role for liver-kidney axis in ammonia metabolism.
A highlight of this study is incorporating the nutritional/inflammatory composite index PNI into post-TIPS OHE prediction. PNI (albumin + lymphocyte count) was originally used to assess nutritional status in gastric cancer (27) and has since been applied to cervical cancer (28), diabetic nephropathy (29), and cognitive decline in the elderly (30). Low PNI indicates malnutrition and immune compromise. Malnutrition elevates systemic inflammation, increasing HE risk (31). In recent years, the role of inflammation in the pathogenesis of HE has attracted increasing attention. Interleukin-6 (IL-6), a multifunctional cytokine involved in immune regulation, metabolic control, inflammatory response, and neuroregulation, has been shown to be closely associated with the development of HE (32–34). Elevated IL-6 levels in cirrhotic patients have been linked to a significantly increased risk of OHE following TIPS, underscoring the potential value of IL-6 as a biomarker for predicting post-TIPS HE risk (33). The interaction between nutritional and inflammatory status plays a critical role in the development of HE in patients after TIPS.
The relationship between nutritional status and HE has also become a research focus in recent years. Malnutrition-related sarcopenia may contribute to the pathogenesis of OHE by impairing muscle-based ammonia metabolism. In cirrhotic patients, skeletal muscle acts as an important extrahepatic route for ammonia detoxification through glutamine synthetase activity (35, 36). Loss of muscle mass compromises this compensatory pathway, leading to elevated blood ammonia levels. Furthermore, hyperammonemia itself can induce the expression of myostatin, a muscle growth inhibitor, thereby exacerbating sarcopenia and creating a vicious cycle. In this study, the inclusion of the PNI in the prediction model provides a simple, cost-effective, and practical surrogate for assessing preoperative nutritional and immunoinflammatory status, offering valuable guidance for clinical decision-making.
In this study, the model not only demonstrated strong discrimination and calibration in the training cohort but also maintained high performance in the independent validation cohort. Compared to traditional scores (MELD, CTP), the model significantly improved predictive accuracy and clinical utility. DCA and CIC confirmed that applying this model could provide greater net clinical benefit, aiding in early identification of high-risk patients and timely intervention, thus improving prognosis and reducing OHE-related healthcare burden. Nevertheless, several limitations should be acknowledged. First, as a single-center retrospective study, selection and information biases cannot be fully excluded. Second, although internal validation was performed, external validation using multicenter, prospective cohorts is needed to confirm the generalizability of the model. Additionally, our model relies solely on preoperative baseline variables, which may not capture dynamic changes in OHE risk over time. Future studies incorporating longitudinal data and emerging biomarkers could further enhance predictive accuracy and clinical applicability.
Conclusion
In conclusion, the present study developed and validated a predictive model to estimate the 1-year risk of OHE in patients with cirrhosis after TIPS. By providing an intuitive nomogram tool, our model can help clinicians make informed decisions regarding patient selection, preoperative optimization, and early postoperative management.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Air Force Medical Center. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
ZJ: Software, Writing – original draft. JC: Data curation, Methodology, Writing – review & editing. ZH: Supervision, Writing – review & editing. BW: Data curation, Writing – review & editing. ZX: Investigation, Writing – original draft. TZ: Writing – original draft. YZ: Writing – original draft. NX: Project administration, Supervision, Writing – review & editing. SN: Funding acquisition, Resources, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was funded by the Air Force Medical Center Outstanding Youth Program (2022YXQNNO36).
Acknowledgments
We would like to thank all participants in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1634368/full#supplementary-material
Abbreviations
AUC, Area under the (receiver operating characteristic) curve; CTP, Child-Turcotte-Pugh; MELD, Model for end-stage liver disease; HE, Hepatic encephalopathy; OHE, Overt hepatic encephalopathy; TIPS, Transjugular intrahepatic portosystemic shunt; LASSO, Least absolute shrinkage and selection operator; DCA, Decision curve analysis; CIC, Clinical impact curve; PNI, Prognostic nutritional index.
References
1.
Ginès P Krag A Abraldes JG Solà E Fabrellas N Kamath PS . Liver cirrhosis. Lancet. (2021) 398:1359–76. doi: 10.1016/S0140-6736(21)01374-X
2.
GBD 2021 Diseases and Injuries Collaborators . Global incidence, prevalence, years lived with disability (YLDs), disability-adjusted life-years (DALYs), and healthy life expectancy (HALE) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. (2024) 403:2133–61. doi: 10.1016/S0140-6736(24)00757-8
3.
Hung ML Lee EW . Role of transjugular intrahepatic portosystemic shunt in the management of portal hypertension: review and update of the literature. Clin Liver Dis. (2019) 23:737–54. doi: 10.1016/j.cld.2019.07.004
4.
Gairing SJ Müller L Kloeckner R Galle PR Labenz C . Review article: post-TIPSS hepatic encephalopathy-current knowledge and future perspectives. Aliment Pharmacol Ther. (2022) 55:1265–76. doi: 10.1111/apt.16825
5.
Fonio P Discalzi A Calandri M Doriguzzi Breatta A Bergamasco L Martini S et al . Incidence of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt (TIPS) according to its severity and temporal grading classification. Radiol Med. (2017) 122:713–21. doi: 10.1007/s11547-017-0770-6
6.
Weissenborn K . Hepatic encephalopathy: definition, clinical grading and diagnostic principles. Drugs. (2019) 79:5–9. doi: 10.1007/s40265-018-1018-z
7.
American Association for the Study of Liver Diseases; European Association for the Study of the Liver . Hepatic encephalopathy in chronic liver disease: 2014 Practice guideline by the European Association for the study of the liver and the American Association for the Study of Liver Diseases. J Hepatol. 61:642–59. doi: 10.1016/j.jhep.2014.05.042
8.
Rudler M Weiss N Bouzbib C Thabut D . Diagnosis and Management of Hepatic Encephalopathy. Clin Liver Dis. (2021) 25:393–417. doi: 10.1016/j.cld.2021.01.008
9.
Collins GS Reitsma JB Altman DG Moons KG . Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): the TRIPOD statement. BMJ. (2015) 350:g7594. doi: 10.1136/bmj.g7594
10.
D'Amico G Morabito A D'Amico M Pasta L Malizia G Rebora P et al . Clinical states of cirrhosis and competing risks. J Hepatol. (2018) 68:563–76. doi: 10.1016/j.jhep.2017.10.020
11.
European Association for the Study of the Liver . EASL clinical practice guidelines for the management of patients with decompensated cirrhosis. J Hepatol. (2018) 69:406–60. doi: 10.1016/j.jhep.2018.03.024
12.
Buzby GP Mullen JL Matthews DC Hobbs CL Rosato EF . Prognostic nutritional index in gastrointestinal surgery. Am J Surg. (1980) 139:160–7. doi: 10.1016/0002-9610(80)90246-9
13.
Pugh RN Murray-Lyon IM Dawson JL Pietroni MC Williams R . Transection of the oesophagus for bleeding oesophageal varices. Br J Surg. (1973) 60:646–9. doi: 10.1002/bjs.1800600817
14.
Freeman RB Jr . Model for end-stage liver disease (MELD) for liver allocation: a 5-year score card. Hepatology. (2008) 47:1052–7. doi: 10.1002/hep.22135
15.
Boike JR Thornburg BG Asrani SK Fallon MB Fortune BE Izzy MJ et al . North American practice-based recommendations for transjugular intrahepatic portosystemic shunts in portal hypertension. Clin Gastroenterol Hepatol. (2022) 20:1636–1662.e36. doi: 10.1016/j.cgh.2021.07.018
16.
Balachandran VP Gonen M Smith JJ DeMatteo RP . Nomograms in oncology: more than meets the eye. Lancet Oncol. (2015) 16:e173–80. doi: 10.1016/S1470-2045(14)71116-7
17.
Wang Z Wu YF Yue ZD Zhao HW Wang L Fan ZH et al . Comparative study of indocyanine green-R15, child-Pugh score, and model for end-stage liver disease score for prediction of hepatic encephalopathy after transjugular intrahepatic portosystemic shunt. World J Gastroenterol. (2021) 27:416–27. doi: 10.3748/wjg.v27.i5.416
18.
Peng Y Wei Q Liu Y Wu Z Zhang H Wu H et al . Prediction and risk factors for prognosis of cirrhotic patients with hepatic encephalopathy. Gastroenterol Res Pract. (2021) 2021:5623601. doi: 10.1155/2021/5623601
19.
Tranah TH Ballester MP Carbonell-Asins JA Ampuero J Alexandrino G Caracostea A et al . Plasma ammonia levels predict hospitalisation with liver-related complications and mortality in clinically stable outpatients with cirrhosis. J Hepatol. (2022) 77:1554–63. doi: 10.1016/j.jhep.2022.07.014
20.
Ballester MP Tranah TH Balcar L Fiorillo A Ampuero J Kerbert AJC et al . Development and validation of the AMMON-OHE model to predict risk of overt hepatic encephalopathy occurrence in outpatients with cirrhosis. J Hepatol. (2023) 79:967–76. doi: 10.1016/j.jhep.2023.05.022
21.
Lu K . Cellular pathogenesis of hepatic encephalopathy: an update. Biomolecules. (2023) 13:396. doi: 10.3390/biom13020396
22.
Li X Partovi S Coronado WM Gadani S Martin C Thompson D et al . Hepatic encephalopathy after TIPS placement: predictive factors, prevention strategies, and management. Cardiovasc Intervent Radiol. (2022) 45:570–7. doi: 10.1007/s00270-021-03045-3
23.
Zhu R Liu L Zhang G Dong J Ren Z Li Z . The pathogenesis of gut microbiota in hepatic encephalopathy by the gut-liver-brain axis. Biosci Rep. (2023) 43:2524. doi: 10.1042/BSR20222524
24.
Elsaid MI Rustgi VK . Epidemiology of hepatic encephalopathy. Clin Liver Dis. (2020) 24:157–74. doi: 10.1016/j.cld.2020.01.001
25.
Bigot A Tchan MC Thoreau B Blasco H Maillot F . Liver involvement in urea cycle disorders: a review of the literature. J Inherit Metab Dis. (2017) 40:757–69. doi: 10.1007/s10545-017-0088-5
26.
MacClean AJ Hayslett JP . Adaptive change in ammonia excretion in renal insufficiency. Kidney Int. (1980) 17:595–606.
27.
Onodera T Goseki N Kosaki G . Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. (1984) 85:1001–5.
28.
Chen JL Huang CY Shih IL Liou YM Tai YJ Chiang YC et al . Prognostic nutritional index and neutrophil-lymphocyte ratio predict toxicities and prognosis in patients with cervical cancer treated with curative radiochemotherapy. J Formos Med Assoc. (2024) 123:671–8. doi: 10.1016/j.jfma.2023.10.022
29.
Zhang J Chen Y Zou L Gong R . Prognostic nutritional index as a risk factor for diabetic kidney disease and mortality in patients with type 2 diabetes mellitus. Acta Diabetol. (2023) 60:235–45. doi: 10.1007/s00592-022-01985-x
30.
Zhou J Ma L Zhao L Sheng J Xu Y Chen J et al . Association between the prognostic nutritional index and cognitive function among older adults in the United States: a population-based study. J Alzheimer's Dis. (2021) 83:819–31. doi: 10.3233/JAD-210141
31.
Martínez de Toda I Ceprián N Díaz-Del Cerro E De la Fuente M . The role of immune cells in Oxi-Inflamm-aging. Cells. (2021) 10:2974. doi: 10.3390/cells10112974
32.
Labenz C Toenges G Huber Y Nagel M Marquardt JU Schattenberg JM et al . Raised serum Interleukin-6 identifies patients with liver cirrhosis at high risk for overt hepatic encephalopathy. Aliment Pharmacol Ther. (2019) 50:1112–9. doi: 10.1111/apt.15515
33.
Li J Liu Y Li M Rong X Yuan Z Ren C et al . Association of preoperative IL-6 levels with overt HE in patients with cirrhosis after TIPS. Hepatol Commun. (2023) 7:128. doi: 10.1097/HC9.0000000000000128
34.
Gairing SJ Anders J Kaps L Nagel M Michel M Kremer WM et al . Evaluation of IL-6 for stepwise diagnosis of minimal hepatic encephalopathy in patients with liver cirrhosis. Hepatol Commun. (2022) 6:1113–22. doi: 10.1002/hep4.1883
35.
Olde Damink SW Jalan R Redhead DN Hayes PC Deutz NE Soeters PB . Interorgan ammonia and amino acid metabolism in metabolically stable patients with cirrhosis and a TIPSS. Hepatology. (2002) 36:1163–71. doi: 10.1053/jhep.2002.36497
36.
Ganda OP Ruderman NB . Muscle nitrogen metabolism in chronic hepatic insufficiency. Metabolism. (1976) 25:427–35. doi: 10.1016/0026-0495(76)90075-5
Summary
Keywords
transjugular intrahepatic portosystemic shunt, overt hepatic encephalopathy, cirrhosis, prognostic nutritional index, nomogram, predictive model
Citation
Jiang Z, Chu J, Han Z, Wei B, Xia Z, Zhang T, Zhu Y, Xiao N and Ning S (2025) Development and validation of a preoperative clinical parameter-based nomogram to predict overt hepatic encephalopathy within 1 year after transjugular intrahepatic portosystemic shunt. Front. Med. 12:1634368. doi: 10.3389/fmed.2025.1634368
Received
24 May 2025
Accepted
08 October 2025
Published
20 October 2025
Volume
12 - 2025
Edited by
Dongbo Wu, Sichuan University, China
Reviewed by
Ying Li, Fudan University, China
Xingshun Qi, General Hospital of Northern Theater Command, China
Updates
Copyright
© 2025 Jiang, Chu, Han, Wei, Xia, Zhang, Zhu, Xiao and Ning.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Nianjun Xiao, xiao_nianjun@163.com; Shoubin Ning, ning-shoubin@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.