- Department of Gastrointestinal Surgery, The Second Qilu Hospital of Shandong University, Jinan, China
Ménétrier’s disease is a rare, progressive disorder of unclear etiology, typically affecting middle-aged men and characterized by giant gastric mucosal folds, mainly in the fundus and body, with occasional antral involvement. Diagnosis is challenging due to its rarity and the need to differentiate from hypertrophic lymphocytic gastritis, Zollinger-Ellison syndrome, gastric cancer, and lymphoma. We report a young male with Menetrier’s disease presenting as generalized edema due to hypoproteinemia. After a challenging diagnostic process and ineffective medical management, the patient underwent robot-assisted total gastrectomy with Roux-en-Y esophagojejunostomy, which corrected the hypoproteinemia. He recovered uneventfully and was discharged on postoperative day 12. At 23 months post-surgery, he showed no hypoalbuminemia, edema, or related symptoms; body weight and serum albumin remained normal. Robot-assisted total gastrectomy is a surgical method for treating Meniere’s disease; however, its cost-effectiveness should be carefully considered, and in the long term, more high-quality studies may be required to validate its feasibility.
1 Introduction
Ménétrier’s disease, also known as giant hypertrophic gastritis or protein-losing hypertrophic gastropathy, is a rare and severe gastric disorder associated with neoplastic potential (1, 2). Fewer than 1,000 cases have been reported since its initial description in 1888, with a predilection for males aged 30–60 years (3). The precise etiology remains incompletely understood, though it is generally considered an acquired condition. In pediatric cases, Ménétrier’s disease has been linked to cytomegalovirus infection and typically follows a self-limited course (4), whereas in adults, Helicobacter pylori infection is implicated (5, 6). Overexpression of transforming growth factor alpha (TGF-α) in the gastric epithelium also contributes to pathogenesis (7–9), and SMAD4 mutations have been reported as a potential etiological factor (10). The disease predominantly involves the gastric body and fundus, though antral involvement has also been documented (11–13). Endoscopically, the gastric mucosa exhibits characteristic gyriform thickening (3, 13), while histopathology reveals foveolar hyperplasia, glandular atrophy, and a reduction in acid-secreting parietal cells (14). Common clinical manifestations include epigastric pain, fatigue, anorexia, weight loss, edema, and vomiting (2, 3, 15, 16). Given its progressive nature and classification as a premalignant lesion (17), coupled with diagnostic challenges, a comprehensive understanding of its management is essential. Here, we present a case of Ménétrier’s disease in a young male, characterized by generalized edema secondary to hypoalbuminemia, detailing the complex diagnostic and therapeutic course, and providing insights into the diagnosis and management of this rare entity.
2 Case presentation
2.1 Complex medical history
A 38-year-old male with a history of external hemorrhoidectomy (4 years prior) and a family history of paternal lung cancer presented with generalized edema, predominantly in the lower extremities, and abdominal distension for over 10 days. Initial workup revealed hypoalbuminemia (albumin 23.4 g/L), hypocalcemia (calcium 1.79 mmol/L), with unremarkable urinalysis, tumor markers, BNP, viral panel, and chest-abdominal CT. Gastrointestinal endoscopy identified lesions in the gastric fundus and body; biopsy showed acute and chronic gastritis, reduced gland density, epithelial hyperplasia, and focal diffuse large cells. Pathology review at a tertiary center revealed chronic mucosal inflammation with focal high-grade intraepithelial neoplasia; immunohistochemistry: Ki-67 (hotspot ~40%), CK(AE1/AE3)(+), CEA(−), P53 (wild-type+). Liver function showed further decreased albumin (17.8 g/L); immunological and rheumatological tests were unremarkable. Symptomatic treatment with albumin infusion led to symptom improvement and discharge.
After discharge, the patient’s symptoms worsened and he was readmitted locally. Tests showed 24-h urinary protein at 0.19 g, albumin at 17.7 g/L, and no abnormalities in rheumatologic markers. Symptomatic treatment with diuretics and albumin improved abdominal distension, but bilateral lower limb edema with erythema, warmth, pain, and fever up to 38.6°C persisted. Ceftriaxone and other therapies yielded no significant improvement.
The patient presented to the nephrology department with eyelid edema and mild pitting edema of both lower limbs. Laboratory evaluation revealed hypoalbuminemia (18.4 g/L), decreased globulin (15.6 g/L), and reduced A/G ratio (1.18); fecal occult blood was positive. Infectious soft tissue disease of the lower limbs was considered the cause of fever. Despite albumin infusion, diuretics, and anti-infective therapy, hypoalbuminemia persisted, though hypocalcemia was corrected after albumin supplementation. Multidisciplinary consultation excluded cardiac, hepatic, and renal dysfunction as causes of systemic edema. Further investigations, including peripheral blood morphology, Brucella, C. difficile, tuberculosis, CMV, H. pylori testing, and enhanced CT, were performed. Pathology review of gastric biopsies from an outside hospital revealed severe chronic active gastritis with marked erosion, glandular destruction, and prominent regenerative changes; low-grade gastric adenocarcinoma could not be excluded. Immunohistochemistry: CEA (−), CK (+), Ki67 (high in proliferative zone), P53 (wild-type). Repeat endoscopy showed abundant gelatinous exudate, diffuse congestion, edema, and thickened folds in the gastric fundus and body. Large EMR biopsy was performed (Supplementary Figures S1A–E). The chief gastroenterologist considered gastric disease the likely cause of hypoalbuminemia. During hospitalization, lower limb infection improved with antibiotics, and albumin, IVIG, and PPI were administered for hypoalbuminemia-related edema, with partial symptomatic improvement. The patient was discharged while awaiting pathology results.
One week post-discharge, the patient developed generalized edema and abdominal distension, and was readmitted to the Department of Gastroenterology. Gastroscopic biopsy revealed severe chronic active gastritis with erosion, congestion, edema, marked proliferation and elongation of foveolar and glandular neck regions, partial glandular dilatation, significant reduction of oxyntic glands, and focal pyloric gland metaplasia, consistent with Menetrier’s disease based on clinical and endoscopic findings. Immunohistochemistry: CEA (foveolar surface membrane +), CK (broad-spectrum +), Ki67 (normal proliferative zone pattern), MUC-2 (−), MUC-5 AC (foveolar epithelium +), MUC-6 (metaplastic pyloric glands +), P53 (wild-type expression) (Supplementary Figure S1F). During hospitalization, albumin supplementation, acid suppression, and octreotide were administered with poor response. Multidisciplinary discussion concluded the disease is difficult to treat, optimal therapy remains unclear, and prognosis is poor; further evaluation for gastrectomy was recommended. After being informed, the patient chose to discharge voluntarily.
After discharge, the patient visited a higher-level gastroenterology department outside the province. Further rheumatologic, CMV, H. pylori, gastrointestinal tumor markers, lymphocyte subsets, and comprehensive humoral immunity tests remained unremarkable. Enhanced retroperitoneal CT showed mild gastric wall thickening, prompting gastroscopy. Gastroscopy revealed diffuse lesions in the gastric fundus and body, circumferentially thickened folds with gyriform changes, surface congestion and erosion, abundant white mucus in the lumen, poor response to chymotrypsin-protease irrigation, marked mucosal friability with contact bleeding, and diffuse submucosal thickening (10–15 mm), highly suggestive of hypertrophic gastritis. Large mucosal and deep gastric biopsies were obtained. Pathology reviewed by multiple gastrointestinal pathologists indicated Menetrier’s disease, with surgical intervention recommended. The patient subsequently self-discharged and presented to our department.
After admission, upper gastrointestinal contrast study and contrast-enhanced CT of the chest, abdomen, and pelvis confirmed the diagnosis of Menetrier’s disease involving the gastric fundus and body (Figure 1). Multidisciplinary discussion determined the lesion was too extensive for ESD, recommending surgical intervention with total gastrectomy. Following preoperative optimization and exclusion of surgical contraindications, and in accordance with the patient’s wishes, robot-assisted total gastrectomy with Roux-en-Y esophagojejunostomy was planned.
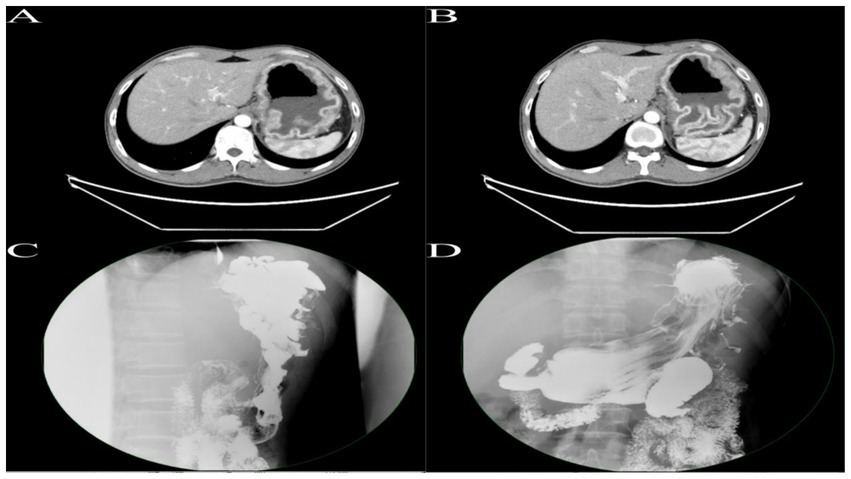
Figure 1. (A,B) Enhanced CT of the upper abdomen reveals gastric distension with significant mucosal thickening exhibiting a corrugated appearance, with pronounced enhancement on contrast imaging. (C,D) Upper gastrointestinal series demonstrates marked hypertrophy of the mucosal folds in the fundus and body of the stomach, presenting with polypoid and convoluted characteristics, with some interfold spaces appearing irregular.
2.2 Surgical procedure
1. After successful general anesthesia, a gastric tube, urinary catheter, and central venous catheter were placed. The patient was positioned supine with legs apart, and standard sterile draping was performed. A 1 cm infraumbilical incision was made for pneumoperitoneum with CO2 insufflation maintained at 12 mmHg. A trocar was inserted as the camera port (C, 8 mm). The patient was placed in a 15° Trendelenburg position. Exploration assessed the diaphragm, liver, paracolic gutters, pelvis, small intestine, peritoneum, omentum, and mesentery. Findings included diffuse gastric wall thickening and edema, firm texture, significant gastric enlargement, multiple soft perigastric lymph nodes (~5 mm), extensive adhesions, marked perigastric vascular proliferation, and mild pelvic effusion. Robotic-assisted total gastrectomy was deemed feasible. Additional ports were placed: R1 (8 mm, left anterior axillary line, subcostal), for harmonic scalpel; R2 (8 mm, right midclavicular line, umbilical level), for Maryland bipolar forceps; R3 (8 mm, right anterior axillary line, subcostal), for Cadiere forceps; and A (12 mm, left midclavicular line, umbilical level), as an assistant port. The “arc-shaped five-port” configuration was used (Figure 2A). The liver was suspended to fully expose the subphrenic cardia and esophagus.
2. After entering the lesser sac, the omentum is dissected leftward along the gastric wall, ligating and dividing the left gastroepiploic vessels near the lower pole of the spleen. Several short gastric vessels are divided superiorly. The gastric fundus is retracted inferolaterally to expose the left cardia and left crus of the diaphragm. Dissection continues along the greater curvature to the right, exposing the pancreatic head at the lower pancreatic border. The right gastroepiploic vessels are exposed, ligated, and divided at their roots, followed by ligation and division of the inferior pyloric vessels. The duodenal bulb is mobilized up to 2 cm below the pylorus and transected with a 60 mm laparoscopic linear stapler (white cartridge, 2.6 mm staple height). The assistant retracts the gastric stump superolaterally to expose the pancreas, and the pancreatic capsule is dissected to the upper border. The right gastric vessels are exposed, ligated, and divided, then the stomach is further retracted to expose and divide the left gastric vessels. The posterior gastric wall is elevated, and posterior gastric vessels are ligated and divided. The lesser omentum is dissected along the liver margin to the right cardia and right crus. The esophagus is fully mobilized and transected with a 60 mm laparoscopic linear stapler (blue cartridge, 3.6 mm staple height). Both sides of the esophageal stump are suspended with 3–0 absorbable knotless sutures (15 cm) to prevent retraction. A 0.5 cm incision is made in the posterior wall of the esophageal stump with electrocautery, followed by exploration and dilation with dissecting forceps, and a gastric tube is advanced through the incision.
3. A 6–8 cm upper midline incision was made below the xiphoid, with layered entry into the abdomen and placement of a wound protector for specimen retrieval. The jejunum was transected approximately 25 cm distal to the ligament of Treitz using a 60 mm laparoscopic linear stapler (white cartridge, 2.6 mm staple height). The distal jejunum was brought up retrocolically to the esophageal stump without tension. A 0.5 cm mesenteric-side enterotomy was made and dilated at the jejunal limb, 40 cm from the distal end, and at the proximal and distal stumps. Side-to-side jejunojejunostomy was performed with a 60 mm linear stapler (white cartridge, 2.6 mm), and the common enterotomy and stump were closed with 3–0 absorbable knotless sutures, reinforcing the duodenal stump. Mesenteric defects were closed. After re-establishing pneumoperitoneum, an esophagojejunostomy (overlap technique) was performed at the distal jejunal stump, with closure and reinforcement using 3–0 absorbable knotless sutures. Resection and anastomosis diagrams are shown in Figure 2B.
4. The abdominal cavity was irrigated with warm saline and hemostasis achieved. Two drains were placed—one behind the esophagojejunostomy and one in the left upper abdomen—exteriorized and secured through lateral abdominal wall trocar sites (Supplementary Video S1). Instrument and gauze counts were accurate. The abdomen and trocar sites were closed in layers. Gross specimen showed diffusely thickened gastric wall, coarse and disordered mucosal folds, and abundant yellow-white viscous secretions in the gastric lumen (Figures 3A–C). The procedure was uneventful, lasting 245 min with approximately 20 mL intraoperative blood loss.
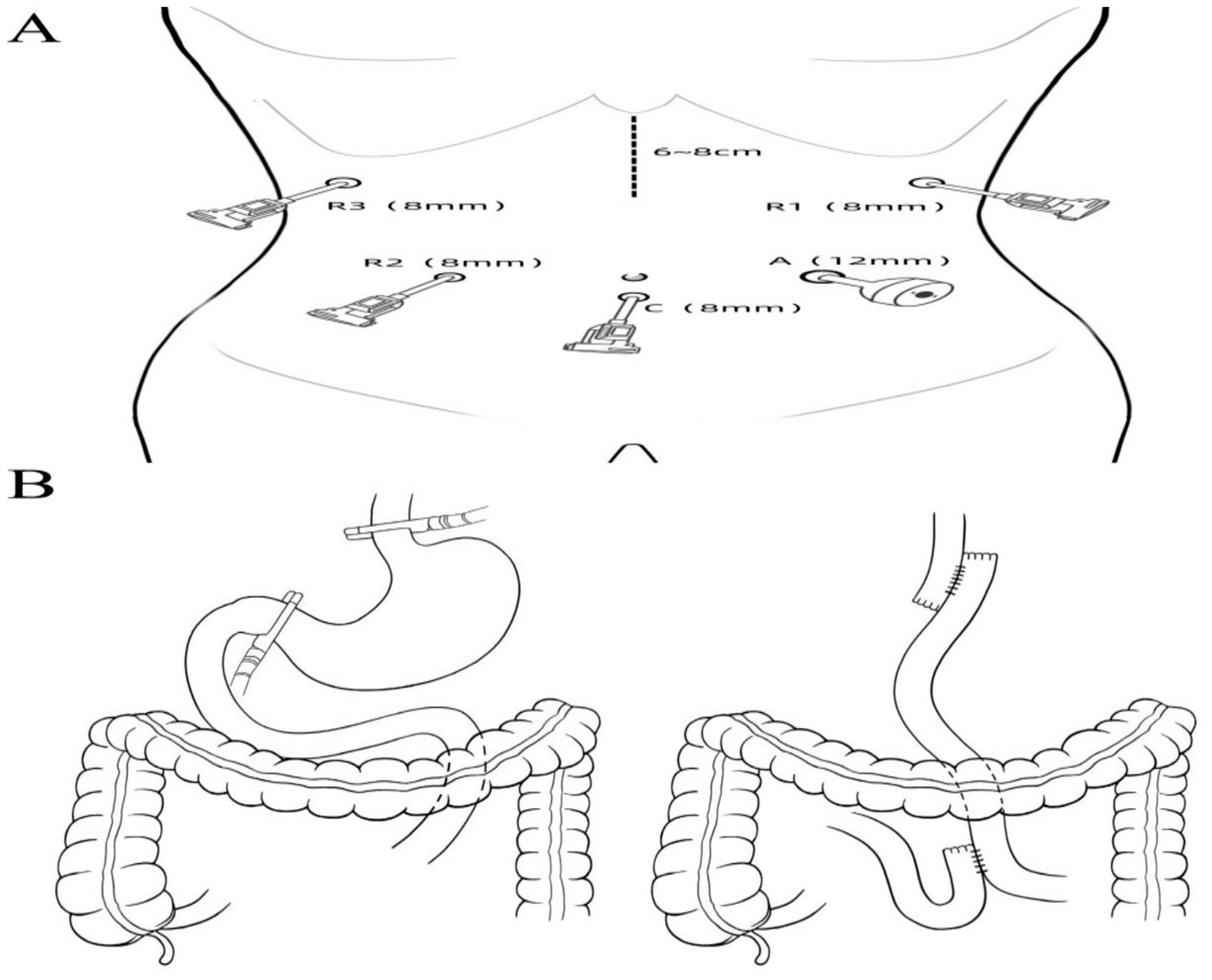
Figure 2. (A) Trocar placement for surgical access. (B) Schematic illustration of the extent of surgical resection and digestive tract reconstruction.
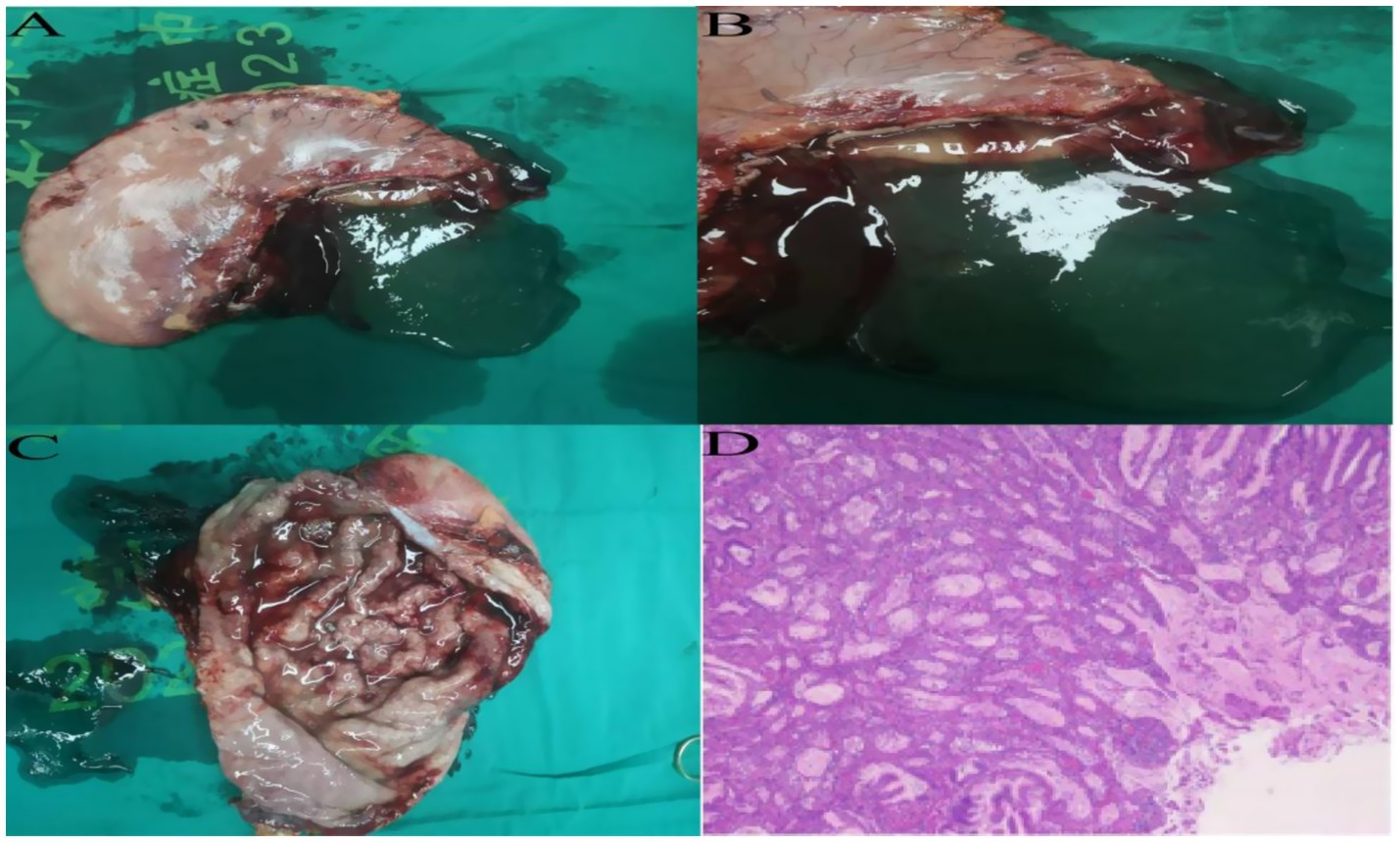
Figure 3. (A–C) Postoperative total gastrectomy specimen reveals diffuse thickening of the gastric wall, with prominent and disorganized mucosal folds, and a significant accumulation of yellow-white viscous secretions within the gastric lumen. (D) Postoperative specimen routine pathology results, hematoxylin and eosin staining, high-power microscopy.
2.3 Postoperative outcome
On postoperative day 3, the patient passed flatus and gradually resumed oral intake. Upper GI contrast on day 6 showed a patent anastomosis with smooth passage (Figure 4A). Sutures and the left upper abdominal drain were removed at 1 week. On day 12, abdominal CT confirmed anastomotic patency with no significant abnormalities (Figure 4B). The posterior esophagojejunostomy drain was removed, and the patient was discharged. Supportive care included albumin supplementation, nutritional support, regular dressing changes, and monitoring of liver and renal function. The patient recovered well, tolerated diet, albumin normalized, and no complications occurred. Pathology revealed marked mucosal fold hypertrophy, severe chronic active inflammation with erosion, congestion, edema, prominent foveolar hyperplasia with mucinous change, distortion, and dilation, foveolar extension to the muscularis mucosae, and reduced acid glands, consistent with Menetrier’s disease (Figure 3D). Immunohistochemistry: CEA (partial+), CK (broad-spectrum+), Ki67 (normal proliferative pattern), MUC-2(−), MUC-5 AC (foveolar+), MUC-6 (focal+), P53 (wild-type).
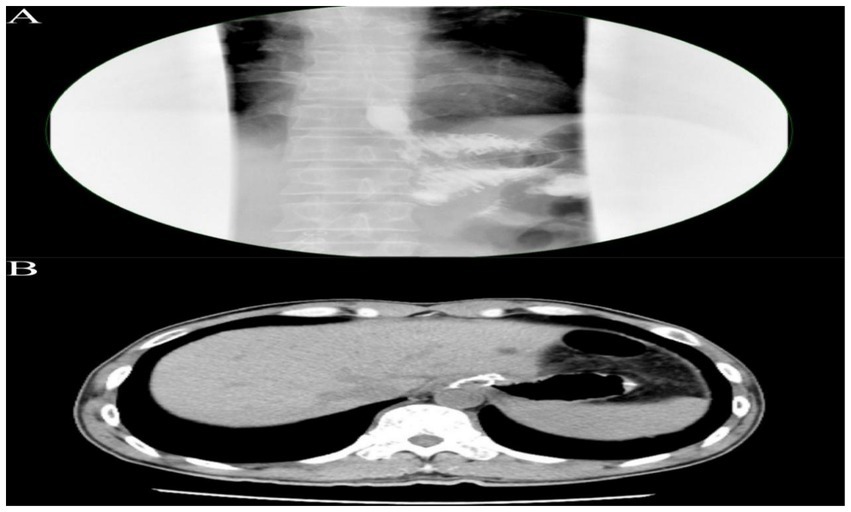
Figure 4. (A) Upper gastrointestinal contrast study demonstrating an esophagojejunostomy with an anastomotic diameter of approximately 0.6 cm. The contrast agent traverses slowly but smoothly, with no significant leakage observed around the anastomosis. (B) Abdominal CT scan reveals the esophagojejunostomy, showing a circumferential metallic density shadow, with the anastomosis appearing patent.
At 23 months post-surgery, the patient showed no hypoalbuminemia, generalized edema, or related symptoms. Body weight recovered gradually, and serum albumin remained normal.
3 Discussion
Hypertrophic gastropathy is a rare, acquired precancerous gastric disorder of unclear etiology (15), predominantly affecting the fundus and body (12), with occasional cases in the antrum (18). Since its first report in 1888, only several hundred cases have been documented worldwide (6). Etiology differs between children and adults; in adults, it typically occurs in males aged 30–60, is progressive, and is associated with Helicobacter pylori infection (13, 19). Overexpression of TGF-α in gastric epithelium, leading to increased EGFR signaling (20), and comorbidity with ulcerative colitis have been implicated (20, 21). SMAD4 mutations are also reported as a possible cause (10). The clinical manifestations of giant hypertrophic gastritis are often nonspecific, including epigastric pain, fatigue, anorexia, and edema (2, 3, 15, 16), necessitating differentiation from conditions such as hypertrophic lymphocytic gastritis, hypersecretory gastritis, Zollinger-Ellison syndrome, and gastric cancer (12, 19). Diagnosis typically requires comprehensive endoscopic full-thickness mucosal biopsy and laboratory tests (serum albumin, gastrin, Helicobacter pylori, cytomegalovirus) (12). Pathology usually reveals mucosal thickening, pit cell hyperplasia, and oxyntic gland atrophy (14). There is no standard treatment; options include non-surgical and surgical approaches. Eradication of H. pylori and antiviral therapy for cytomegalovirus can benefit some patients (2, 22); somatostatin analogs (23, 24) and EGFR monoclonal antibodies are effective in select cases (15, 20, 25). Other medications, such as H2R antagonists and PPIs, may alleviate symptoms (12, 26). For non-surgical patients, endoscopic monitoring every 6 months is recommended (27–29). Surgical treatment, primarily partial or total gastrectomy (via laparoscopy or robotic assistance), is considered the first-line therapy (12, 30–35). Robotic surgery is considered one of the optional approaches (35). Some cases may be cured by endoscopic submucosal dissection (ESD) (36).
We report a case of a young male with Menetrier’s disease presenting as generalized edema due to hypoproteinemia. After a complex diagnostic process, definitive diagnosis was achieved. Given poor response to medical therapy and extensive lesions, we performed robot-assisted total gastrectomy with Roux-en-Y esophagojejunostomy. Based on the patient’s clinical course, our experience with robotic treatment, and relevant literature, we summarize the following insights:
1. Giant hypertrophic gastritis presents with non-specific clinical features and a broad differential diagnosis, making definitive diagnosis challenging (16). This patient underwent seven consultations, extensive disease-related investigations, three endoscopies with biopsies, before diagnosis and optimal treatment were established, highlighting diagnostic difficulty and therapeutic uncertainty.
2. Single endoscopic biopsy may be insufficient due to inadequate depth and sampling; multiple, large, and deep biopsies from various sites may be necessary to improve diagnostic yield (15, 37). Endoscopic ultrasonography may offer diagnostic advantages (38).
3. Due to the rarity of this disease, clinicians, endoscopists, and pathologists may lack experience. Medical professionals should enhance their knowledge and consider this diagnosis in patients with extensive negative workups, pursuing targeted investigations.
4. Based on our center’s experience with the da Vinci Xi robotic system for gastrointestinal diseases, we find that patients with hypertrophic gastritis often exhibit diffuse gastric wall thickening and prominent perigastric vascular proliferation. The da Vinci robot’s precision and flexibility offer advantages in such cases (39). Careful dissection is required to avoid bleeding or vascular compromise; liver suspension aids exposure and frees the assistant. Stapler height should be selected according to gastric wall thickness to prevent misfiring or staple line disruption.
5. We used the “curved five-port” trocar layout, but due to the extensive resection range in total gastrectomy, trocar positions should be adjusted according to patient body habitus.
6. The procedure involves extensive organ mobilization and transection with complex local anatomy, requiring high technical proficiency from assistants, especially for exposure and dissection without direct vision. Team coordination is essential to enhance surgical safety.
7. Postoperative specimens showed diffuse gastric wall thickening, coarse and disorganized mucosal folds, and abundant yellow-white viscous secretions. Literature suggests that imbalance between mucus and acid secretion leads to protein malabsorption and subsequent hypoproteinemia (16).
8. As no effective treatment exists, a multidisciplinary team involving gastroenterologists, pathologists, nutritionists, oncologists, and surgeons is recommended for management (15).
9. Intraoperative complications such as bleeding or splenic injury require clear visualization and gentle manipulation; splenectomy may be necessary for uncontrolled splenic bleeding, and conversion to open surgery should be considered when needed. Early postoperative complications mainly include duodenal stump and anastomotic leaks; intraoperative assessment of tissue quality, secure suturing, adequate blood supply, and tension-free anastomosis, along with postoperative nutritional support, are essential.
10. Although surgical management of Menétrier’s disease is well-established, robotic surgery remains rare, with only one case reported in the literature (35). Although the report indicates that robotic systems could improve surgeon ergonomics and suggests potential safety and efficacy, as well as the possibility of robotics being the optimal therapeutic option, these conclusions require cautious interpretation. Due to the disease’s rarity and reliance on single-case reports, systematic comparative data on perioperative safety and long-term outcomes between robotic, laparoscopic, and open surgery are lacking. Furthermore, the relatively higher costs and potentially prolonged operative times associated with robotic surgery are critical factors that must be carefully weighed in clinical decision-making (40, 41). Although some studies indicate that workflow optimization may improve the efficiency of robotic procedures, the actual impact of these factors requires thorough evaluation (42). Demonstrating technical feasibility in isolated cases does not suffice to prove the safety of robotic platforms or their superiority to conventional methods. Future research should focus on long-term follow-up of robotic surgery patients to evaluate late outcomes and complications, and on conducting large, rigorously designed multicenter studies to systematically assess the safety, long-term efficacy, and cost-effectiveness of robotic surgery.
4 Conclusion
Menetrier’s disease, a rare precancerous condition, often presents with hypoalbuminemia and is easily missed. Diagnosis requires multidisciplinary collaboration, repeated deep biopsies, and imaging. While medical therapy benefits some patients, extensive disease necessitates total gastrectomy. This case highlights the need to raise awareness, standardize endoscopic biopsy, and advance minimally invasive techniques; Robot-assisted total gastrectomy is a surgical method for treating Meniere’s disease; however, its cost-effectiveness should be carefully considered, and in the long term, more high-quality studies may be required to validate its feasibility.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the Second Qilu Hospital of Shandong University (approval number KYLL2025458). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW: Conceptualization, Investigation, Writing – original draft, Data curation, Formal analysis, Visualization, Methodology. ZL: Writing – review & editing, Visualization, Methodology, Writing – original draft, Investigation, Conceptualization. JinZ: Methodology, Writing – original draft, Investigation, Conceptualization, Writing – review & editing, Visualization. XZ: Writing – original draft, Investigation, Conceptualization, Methodology. SZ: Investigation, Conceptualization, Writing – original draft. CZ: Conceptualization, Investigation, Writing – original draft. HL: Writing – original draft, Investigation, Conceptualization. JieZ: Conceptualization, Investigation, Writing – original draft. TG: Conceptualization, Writing – original draft, Investigation. YD: Conceptualization, Validation, Writing – review & editing, Project administration, Writing – original draft, Methodology, Supervision, Data curation, Formal analysis, Investigation, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work is supported by Development Center for Medical Science & Technology National Health Commission of the People’s Republic of China (W2018RY9).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1634451/full#supplementary-material
References
1. Scharschmidt, BF. The natural history of hypertrophic gastrophy (Menetrier's disease). Report of a case with 16 year follow-up and review of 120 cases from the literature. Am J Med. (1977) 63:644–52. doi: 10.1016/0002-9343(77)90210-8
2. Almazar, AE, Penfield, JD, Saito, YA, and Talley, NJ. Survival times of patients with Menetrier's disease and risk of gastric Cancer. Clin Gastroenterol Hepatol. (2021) 19:707–12. doi: 10.1016/j.cgh.2020.03.017
3. Gore, M, Bansal, K, and Jadhav, N. Menetrier Disease In: StatPearls. Treasure Island, FL: StatPearls Publishing (2023)
4. Tard, C, Madhi, F, Verlhac, S, Hagège, H, Epaud, R, and Jung, C. Protein-losing gastropathy associated with cytomegalovirus in two sisters - case reports and review of the literature. Arch Pediatr. (2019) 26:232–5. doi: 10.1016/j.arcped.2019.03.005
5. Iguchi, E, Tsumura, T, Sekikawa, A, Wakasa, T, Maruo, T, Okabe, Y, et al. Cap-polyposis-like gastropathy with hypoproteinemia treated with H. pylori eradication. Intern Med. (2013) 52:2215–8. doi: 10.2169/internalmedicine.52.0700
7. Coffey, RJ, Romano, M, and Goldenring, J. Roles for transforming growth factor-alpha in the stomach. J Clin Gastroenterol. (1995) 21:S36–9.
8. Dempsey, PJ, Goldenring, JR, Soroka, CJ, Modlin, IM, McClure, RW, Lind, CD, et al. Possible role of transforming growth factor alpha in the pathogenesis of Ménétrier's disease: supportive evidence form humans and transgenic mice. Gastroenterology. (1992) 103:1950–63. doi: 10.1016/0016-5085(92)91455-d
9. Nomura, S, Settle, SH, Leys, CM, Means, AL, Peek, RM Jr, Leach, SD, et al. Evidence for repatterning of the gastric fundic epithelium associated with Ménétrier's disease and TGFalpha overexpression. Gastroenterology. (2005) 128:1292–305. doi: 10.1053/j.gastro.2005.03.019
10. Burmester, JK, Bell, LN, Cross, D, Meyer, P, and Yale, SH. A SMAD4 mutation indicative of juvenile polyposis syndrome in a family previously diagnosed with Menetrier's disease. Dig Liver Dis. (2016) 48:1255–9. doi: 10.1016/j.dld.2016.06.010
11. Stamm, B. Localized hyperplastic gastropathy of the mucous cell- and mixed cell-type (localized Ménétrier's disease): a report of 11 patients. Am J Surg Pathol. (1997) 21:1334–42. doi: 10.1097/00000478-199711000-00008
12. Chen, YA, Tsai, HL, Liang, PI, and Wang, JY. A rare case of Menetrier's disease-induced gastric outlet obstruction. Kaohsiung J Med Sci. (2023) 39:1054–5. doi: 10.1002/kjm2.12735
13. Wang, HH, Zhao, CC, Wang, XL, Cheng, ZN, and Xie, ZY. Menetrier's disease and differential diagnosis: a case report. World J Clin Cases. (2021) 9:6943–9. doi: 10.12998/wjcc.v9.i23.6943
14. Huh, WJ, Coffey, RJ, and Washington, MK. Ménétrier's disease: its mimickers and pathogenesis. J Pathol Transl Med. (2016) 50:10–6. doi: 10.4132/jptm.2015.09.15
15. Ramrakhiani, H, and Triadafilopoulos, G. Erasing Giant folds: Ménétrier's disease treated with Cetuximab. Dig Dis Sci. (2022) 67:5006–9. doi: 10.1007/s10620-022-07692-5
16. Keener, M, Waack, A, Ranabothu, M, Ranabothu, A, and Vattipally, VR. Clinical and radiological features of Menetrier's disease: a case report and review of the literature. Cureus. (2023) 15:e45537. doi: 10.7759/cureus.45537
17. Grantham, T, Ramachandran, R, Parvataneni, S, Budh, D, Gollapalli, S, and Gaduputi, V. Epidemiology of gastric Cancer: global trends, risk factors and premalignant conditions. J Community Hosp Intern Med Perspect. (2023) 13:100–6. doi: 10.55729/2000-9666.1252
18. Sweeney, AR, and Lynch, MK. A case of Ménétrier's disease localized to the gastric antrum without helicobacter infection or hypoalbuminemia. Int J Surg Case Rep. (2013) 4:839–41. doi: 10.1016/j.ijscr.2013.07.004
19. Kmiecik, M, Walczak, A, Samborski, P, Paszkowski, J, Dobrowolska, A, Karczewski, J, et al. Upper gastrointestinal bleeding as an unusual manifestation of localized Ménétrier's disease with an underlying lipoma: a case report. World J Gastrointest Endosc. (2023) 15:10–8. doi: 10.4253/wjge.v15.i1.10
20. Hussameddin, A, Sedano, R, Ramsewak, D, Driman, D, and Jairath, V. Vedolizumab-induced endoscopic and histologic improvement in gastric Menetrier's disease in a patient with ulcerative colitis. Inflamm Bowel Dis. (2021) 27:e132–3. doi: 10.1093/ibd/izab129
21. Kayes, T, Bonnichsen, M, Willmann, L, Lorenzo, A, Das, A, El-Haddad, C, et al. Menetrier's disease exacerbating ulcerative colitis and relieved by gastrectomy. BMJ Open Gastroenterol. (2021) 8:e000806. doi: 10.1136/bmjgast-2021-000806
22. Setakhr, V, Muller, G, Hoang, P, Lambert, AS, and Geubel, A. Cytomegalovirus-associated protein losing gastropathy in an immunocompetent adult: a case report. Acta Gastroenterol Belg. (2007) 70:296–9.
23. Heurgué-Berlot, A, Féron, T, Jazeron, JF, Hoeffel, C, Diebold, MD, and Cadiot, G. Ménétrier's disease: long-term remission with lanreotide. Clin Res Hepatol Gastroenterol. (2016) 40:e5–9. doi: 10.1016/j.clinre.2015.07.007
24. Salmón Olavarría, P, Irabien Ortiz, M, de la Fuente Molinero, I, Ugarte Bilbao, A, Cubillas Urkiola, S, and Erdozain Castiella, JG. Ménétrier disease: a rare cause of severe chronic diarrhea difficult to diagnose with response to treatment with octreotide. Rev Esp Enferm Dig. (2024) 119. doi: 10.17235/reed.2024.10668/2024
25. Nalle, SC, and Turner, JR. Menetrier's disease therapy: rebooting mucosal signaling. Sci Transl Med. (2009) 1:8ps10. doi: 10.1126/scitranslmed.3000438
26. Bradburn, DM, Redwood, NF, Venables, CW, and Gunn, A. Medical therapy of Ménétrier's disease with omeprazole. Digestion. (1992) 52:204–8. doi: 10.1159/000200954
27. Țanțu, DE, Dimitriu, AM, Becheanu, G, Diculescu, M, and Gheorghe, C. An atypical case of Menetrier's disease with antral-duodenal extension. J Gastrointestin Liver Dis. (2024) 33:450. doi: 10.15403/jgld-5823
28. Xiong, LS, and Gong, YY. Natural history of adult-onset Ménétrier's disease: report of a case with 9-year follow-up. Exp Ther Med. (2016) 11:2462–6. doi: 10.3892/etm.2016.3181
29. Ravilla, J, He, P, Ali, M, and Patel, A. Menetrier disease - a rare presentation of gastrointestinal bleeding. J Community Hosp Intern Med Perspect. (2024) 14:57–9. doi: 10.55729/2000-9666.1377
30. Parianos, C, Aggeli, C, Sourla, A, and Zografos, GN. Total gastrectomy for the treatment of Menetrier's disease persistent to medical therapy: a case report. Int J Surg Case Rep. (2020) 73:95–9. doi: 10.1016/j.ijscr.2020.06.033
31. Roy, S, Neogi, S Sr, Chaturvedi, A, and Tomar, R Sr. The great mimicker of gastric cancer: a case report of Ménétrier's disease. Cureus. (2024) 16:e55076. doi: 10.7759/cureus.55076
32. Pepa, P, Uehara, T, Wonaga, A, Redondo, A, Avagnina, A, Mazzocchi, O, et al. Menetrier's disease. A diagnostic and therapeutic challenge. Medicina. (2021) 81:470–3.
33. Byun, J, Kwon, S, Oh, SY, Lee, KG, Suh, YS, Kong, SH, et al. Laparoscopic management of hypertrophic hypersecretory gastropathy with protein loss: a case report. Asian J Endosc Surg. (2014) 7:48–51. doi: 10.1111/ases.12062
34. Rodríguez Gonzalez, O, José, R, Génesis, J, Luis, M, Liumariel, V, Raquel, F, et al. Robot-assisted laparoscopic gastrectomy for Menetrier's disease. J Robot Surg. (2015) 9:257–61. doi: 10.1007/s11701-015-0512-5
35. Fukushi, K, Goda, K, Kino, H, Kondo, M, Kanazawa, M, Kashima, K, et al. Curative resection with endoscopic submucosal dissection of early gastric cancer in Helicobacter pylori-negative Ménétrier's disease: a case report. World J Gastroenterol. (2022) 28:594–601. doi: 10.3748/wjg.v28.i5.594
36. Sundt, TM 3rd, Compton, CC, and Malt, RA. Ménétrier's disease. A trivalent gastropathy. Ann Surg. (1988) 208:694–701. doi: 10.1097/00000658-198812000-00004
37. Mei, M, Jingmei, N, Zongming, C, Mei, J, and Leimin, S. Diffuse type gastric carcinoma presenting as giant gastric folds: lessons learned from six miss diagnosed cases. Clin Res Hepatol Gastroenterol. (2012) 36:505–9. doi: 10.1016/j.clinre.2012.04.009
38. Xia, C, and Xu, G. Endoscopic ultrasound in the diagnosis of Ménétrier’s disease: unique contribution. Rev Esp Enferm Dig. (2025). doi: 10.17235/reed.2025.11012/2024
39. Guerrini, GP, Esposito, G, Magistri, P, Serra, V, Guidetti, C, Olivieri, T, et al. Robotic versus laparoscopic gastrectomy for gastric cancer: the largest meta-analysis. Int J Surg. (2020) 82:210–28. doi: 10.1016/j.ijsu.2020.07.053
40. Bobo, Z, Xin, W, Jiang, L, Quan, W, Liang, B, Xiangbing, D, et al. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: meta-analysis and trial sequential analysis of prospective observational studies. Surg Endosc. (2019) 33:1033–48. doi: 10.1007/s00464-018-06648-z
41. Lu, J, Wu, D, Huang, JB, Lin, J, Xu, BB, Xue, Z, et al. Comparison of robotic versus laparoscopic versus open distal gastrectomy for locally advanced gastric cancer: a prospective trial-based economic evaluation. Surg Endosc. (2023) 37:7472–85. doi: 10.1007/s00464-023-10147-1
Keywords: Ménétrier’s disease, robotic-assisted total gastrectomy, refractory hypoproteinemia, Roux-en-Y esophagojejunostomy, case report
Citation: Wang L, Liu Z, Zhang J, Zhu X, Zhao S, Zhao C, Liang H, Zhang J, Gao T and Ding Y (2025) Robotic-assisted total gastrectomy for refractory hypoproteinemia in Menetrier’s disease: a case report with operative video and literature review. Front. Med. 12:1634451. doi: 10.3389/fmed.2025.1634451
Edited by:
Angel Lanas, University of Zaragoza, SpainReviewed by:
Chrysanthi Aggeli, General Hospital of Athens G. Genimatas, GreeceNida Ali Safdar, Osmania University, India
Copyright © 2025 Wang, Liu, Zhang, Zhu, Zhao, Zhao, Liang, Zhang, Gao and Ding. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yinlu Ding, ZGluZ3lpbmx1QDEyNi5jb20=
†These authors have contributed equally to this work
 Lang Wang
Lang Wang Ziping Liu†
Ziping Liu† Xianglin Zhu
Xianglin Zhu Shijun Zhao
Shijun Zhao Cheng Zhao
Cheng Zhao Jie Zhang
Jie Zhang Yinlu Ding
Yinlu Ding