Abstract
Background:
The genetic etiology of hypertrophic cardiomyopathy (HCM) and the critical role of sarcomeric variants in its pathogenesis are well recognized (1). Among these, loss-of-function variants in the myosin binding protein C gene (MYBPC3) are the most prevalent, resulting in protein insufficiency when compared to healthy controls (1). Preclinical studies have shown that recombinant adeno-associated virus serotype 9 (rAAV9) carrying the full-length MYBPC3 transgene can increase protein expression and improve cardiac function. However, pre-existing anti-AAV9 antibodies—neutralizing (NAb) and total (TAb)—may limit gene transfer efficacy and eligibility for gene therapy. We sought to evaluate the prevalence of anti-AAV9 antibodies in patients with MYBPC3-associated HCM to optimize patient selection for MyPEAK-1, an ongoing trial evaluating the safety, tolerability, and pharmacodynamics of TN-201, an AAV9: MYBPC3 gene therapy.
Methods:
This was a prospective, cross-sectional study of 100 adults (aged 18–65 years) with symptomatic MYBPC3-associated HCM (NYHA II–IV). Blood samples were analyzed for anti-AAV9 NAb (transduction inhibition assay) and TAb (electrochemiluminescence assay). Titers ≥1:10 were considered positive. Associations between antibody levels and demographic and clinical characteristics were explored using statistical tests.
Results:
Pre-existing anti-AAV9 NAb were undetectable in 50% of patients. Among those with detectable titers (range: 1:10–1:720), only 16% exceeded 1:40. TAb were undetectable in 53%; titers ranged from 1:10 to 1:65,600. A strong correlation was observed between NAb and TAb titers (r = 0.671, p < 0.001). Serostatus was not significantly associated with age, sex, NYHA class, or ethnicity (all p > 0.05).
Conclusion:
Pre-existing immunity to AAV9 was absent or low in most MYBPC3-associated HCM patients, with only a small proportion exceeding standard NAb thresholds for AAV gene therapy trials. These findings support the feasibility of a clinical trial of TN-201, an AAV9-based MYBPC3 gene replacement therapy, in this population. Given the concordance between NAb and TAb assays, either may be suitable for screening.
Introduction
The genetic etiology of hypertrophic cardiomyopathy (HCM) and the critical role of sarcomeric variants in its pathogenesis are well-recognized (1). Among these, loss-of-function variants in the myosin binding protein C gene (MYBPC3) are the most prevalent, resulting in ~60% of the normal protein levels necessary for optimal cardiac function (1). TN-201 is an adeno-associated virus serotype 9 (AAV9)-based gene therapy designed to deliver a working MYBPC3 gene to heart muscle cells via a single intravenous infusion, increasing MyBP-C protein levels to address the underlying cause of MYBPC3-associated HCM with the aim of halting or even reversing disease after a single dose. Preclinical in vivo and in vitro studies have demonstrated that administration of a recombinant adeno-associated virus serotype 9 (rAAV9) carrying the full-length MYBPC3 transgene restored missing protein and improved cardiac function, offering a strong rationale for a gene therapy to reverse disease progression, potentially in MYBPC3-associated HCM (2).
The first interim clinical data from the TN-201 gene therapy trial—designed to evaluate the safety, tolerability, pharmacodynamics, and efficacy of TN-201—show promising results. In the initial three patients who received a one-time infusion of TN-201 at the 3E13 vg/kg dose, the therapy demonstrated a compelling safety profile, robust cardiac transduction, encouraging levels of MYBPC3 expression, and early signs of clinical improvement or disease stability, all of which represent positive early signals of clinical impact of TN-201 (3, 4).
Adeno-associated viruses (AAVs) are naturally occurring and non-pathogenic. AAV9 has shown relatively high affinity for cardiomyocytes among all serotypes. However, pre-existing immunity to AAVs, manifesting as neutralizing (NAb) or total (TAb) antibodies, may impact vector transduction efficiency or possibly increase the risk of an immune response following administration of an AAV-based gene therapy. Therefore, neutralizing antibodies may represent a major barrier in gene transfer, which could potentially result in clearance of the vector before it reaches the target cell (5, 6). The impact of preexisting immunity suggests that screening patients for serostatus may help identify those most likely to benefit from gene transfer. Rare, pre-existing antibodies may increase the risk of immune responses post-treatment, such as complement activation, hepatotoxicity, and inflammatory responses; however, serious AAV-related immune events are uncommon in clinical trials (7–11).
Prior studies in patients, including those with heart failure, showed increasing AAV seroprevalence with older age (12, 13). Since the MYBPC3-associated HCM population is, on average, older than all other populations enrolled in intravenously administered AAV9 gene therapy programs, it was expected that more patients may not be eligible for AAV gene therapy due to pre-existing immunity to the serotype than expected (14). Although AAV9 has not been evaluated as extensively as AAV2 for pre-existing NAb, several studies suggest a 30–60% seroprevalence reported by others across various populations (Table 1).
Table 1
| Characteristic | Total (N = 100) |
|---|---|
| Age (years) | |
| Median (Min–Max) | 48.0 (19–65) |
| Age Groups (years) [n (%)] | |
| ≥18 to <30 | 19 (19.0) |
| ≥30 to <40 | 14 (14.0) |
| ≥40 to <50 | 23 (23.0) |
| ≥50 to ≤65 | 44 (44.0) |
| Sex [n (%)] | |
| Female | 34 (34.0) |
| Male | 66 (66.0) |
| Race [n (%)] | |
| American Indian or Alaska Native | 0 |
| Asian | 3 (3.0) |
| Black or African American | 10 (10.0) |
| Native Hawaiian or Other Pacific Islander | 1 (1.0) |
| White | 80 (80.0) |
| Not reported/Unknown | 6 (6.0) |
| Ethnicity [n (%)] | |
| Hispanic or Latino | 5 (5.0) |
| Not Hispanic or Latino | 92 (92.0) |
| Not reported/Unknown | 3 (3.0) |
| MYBPC3 variant [n (%)] | |
| Pathogenic/likely pathogenic (P/LP), truncating | 100 (100) |
| Additional sarcomeric P/LP variant | 11 (11.0) |
| HCM diagnosis [n (%)] | |
| Obstructive | 44 (44.0) |
| Nonobstructive | 48 (48.0) |
| Non-obstructive status post-SRT | 8 (8.0) |
| Implantable cardiac defibrillator | 56 (56.0) |
| New York Heart Association Class [n (%)] | |
| Class I | 0 |
| Class II | 87 (87.0) |
| Class III | 13 (13.0) |
| Septal myectomy | 9 (9.0) |
Baseline demographics and medical history of study population.
HCM, hypertrophic cardiomyopathy; MYBPC3, myosin-binding protein C gene; AAV9, adeno-associated virus serotype 9.
We sought to evaluate the prevalence of pre-existing immunity to AAV9 in MYBPC3-associated HCM to estimate the seroeligibility of MYBPC3 + HCM patients and support patient selection for MyPEAK-1 (NCT05836259), a trial evaluating the safety, tolerability, and pharmacodynamics of TN-201, an AAV9 MYBPC3 gene therapy (4).
Methods
This was a prospective, cross-sectional, noninterventional, multicenter (n = 12) cohort study. The inclusion criteria were the following: symptomatic adult patients (New York Heart Association [NYHA] class II-IV), aged 18–65, with HCM diagnosis and confirmed pathogenic or likely pathogenic (P/LP) MYBPC3 truncating variant. Patients were excluded if they were receiving immunomodulating therapy or chemotherapy, had a history of clinically significant liver disease, or had prior exposure to gene therapy. All patients attended a single study visit where a blood sample was collected to assess the presence of anti-AAV9 antibodies. No investigational product was administered. Institutional review board approval was obtained, and all patients signed informed consent.
NAb assay
AAV9 NAb were assessed by a transduction inhibition assay (BioAgilytix, NC, USA). Chinese hamster ovary (CHO) Lec2 cells were incubated with a mixture of participant serum (minimum dilution 2) and a custom AAV9 virus with luminescent reporter gene (multiplicity of infection 6,000). Luminescence was measured after 24 h of incubation at 37 °C (One-Glo, Promega). Luminescence from each well was normalized to the plate negative control and compared to a statistically established cut-off point for positivity (1% false positive rate, FPR). Titer was determined through serial dilution of participant serum. Results were reported as not detected (titer <1:10) and detected (≥1:10), with titer when present.
TAb assay
AAV9 TAb was detected by electrochemiluminescent (ECL) bridging assay (BioAgilytix, NC, USA). Assay plates were coated with AAV9 capsid (Tenaya Therapeutics, Inc.). Participant serum was diluted 10-fold and incubated on the plate for 1 h at room temperature to bind TAb. Labeled AAV9 capsid (Tenaya Therapeutics) was added to detect the bound TAb through ECL reaction (Meso Scale Diagnostics). Sample luminescence was normalized to the plate negative control and compared to a statistically determined cut-off point for positivity (1% FPR). Titer was determined through serial dilution of participant serum. Results were reported as not detected (<1:10) and detected (≥1:10), with titer when present.
Statistical analysis
Patients’ demographic and baseline characteristics were summarized using descriptive statistics. Medical history included NYHA class, ICD placement, and type of HCM (obstructive, non-obstructive, or non-obstructive post-myectomy). NAb and TAb titer values were provided for samples with detectable antibody levels. To assess the relationship between NAb and TAb titers, Pearson’s correlation coefficient was calculated. Subgroup analyses were conducted to explore potential associations between pre-existing immunity to AAV9 (as measured by TAb and NAb titers) and key demographic factors. Comparison between subgroups was performed using the Chi-square test or Fisher’s exact test, as appropriate. A p-value of <0.05 was considered significant.
Results
A total of 100 adult HCM patients (aged 18–65 years) were enrolled in the study (Table 1). All participants were symptomatic (NYHA class II or III) and had MYBPC3 P/LP truncating variants; 44% had obstructive HCM, and ICD placement in 55% of patients.
NAb against AAV9 were detected in 50% of patients (N = 50). Among those with detectable NAb, titers ranged from 1:10 to 1:720 with a median of 1:20 (Figure 1). Notably, only 16% of patients exhibited titers exceeding 1:40, indicating a relatively low prevalence of high-titer responses. TAb against AAV9 were detected in 47% of patients (N = 47), with titers ranging from 1:10 to 1:65,600 and a median titer of 1:640 (Figure 2). A significant correlation was observed between TAb and NAb levels (r = 0.671, p < 0.001), indicating a high degree of concordance. This finding suggests that measuring AAV9 TAb titer could be a surrogate method to determine eligibility to receive an AAV9 gene therapy, as ECL-based assays for TAb are easier and more standardized to perform than transduction inhibition assays.
Figure 1

Distribution of neutralizing antibodies to AAV9.
Figure 2
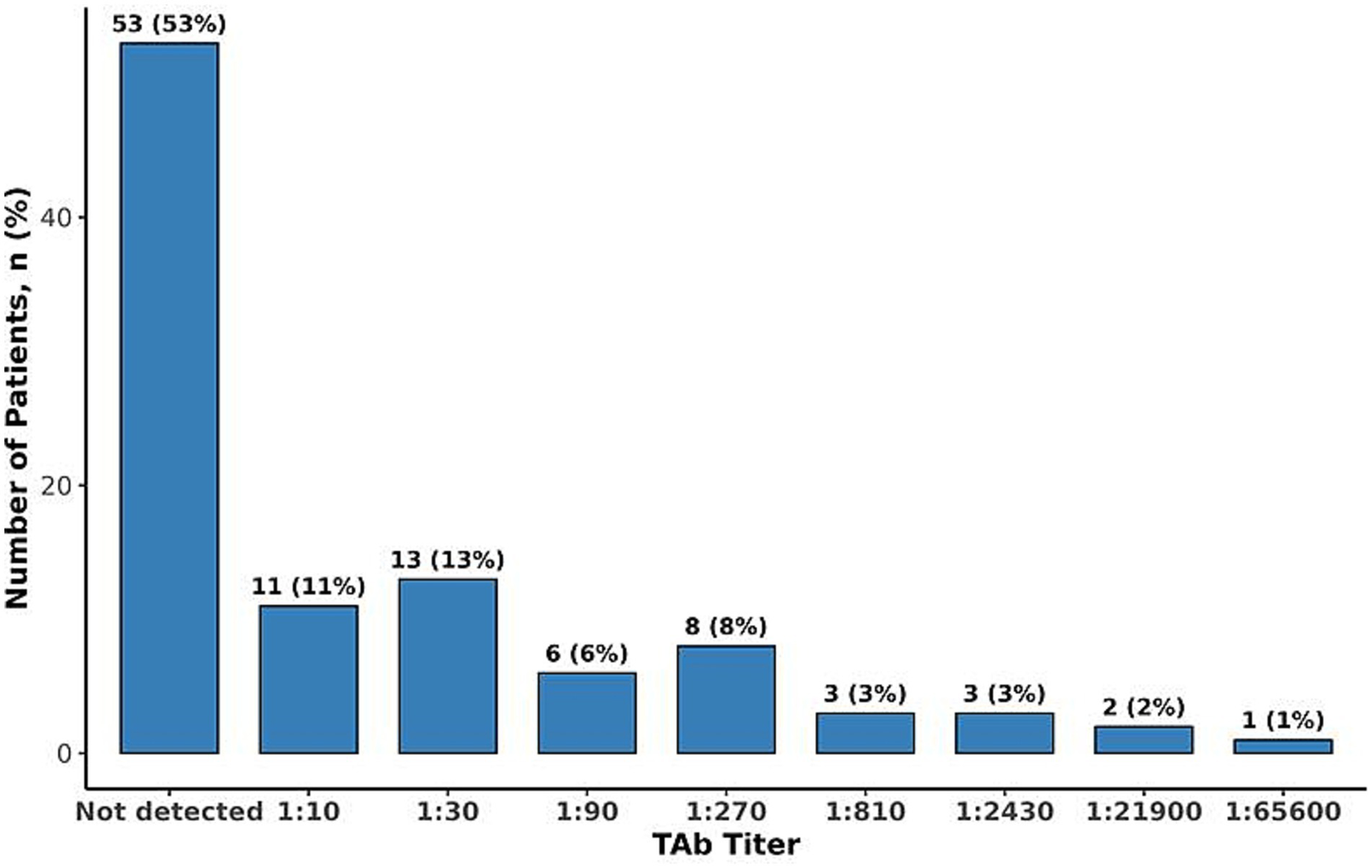
Distribution of total antibodies to AAV9.
Exploratory subgroup analyses were conducted to evaluate potential associations between clinical characteristics and antibody levels. No meaningful differences in the prevalence of detectable NAb titers were observed by age (p-value = 0.791), sex (p = 0.398), or NYHA Class >II (p = 0.766). Similarly, prevalence of detectable TAb titers showed no differences by age (p = 0.917), sex (p = 0.666), or NYHA Class >II (p = 0.596) (all p = ns). Furthermore, no discernible trends existed across ethnic groups (p = 0.837 for NAb, p = 0.586 for TAb).
Discussion
Findings from this seroprevalence study suggest that approximately half of the participants with MYBPC3-associated HCM exhibited detectable NAb or TAb titers to AAV9. Moreover, only 16% had NAb titers exceeding 1:40, a threshold relevant for TN-201 gene therapy eligibility, and only 6% had NAb titers exceeding 1:80, the maximum allowable AAV9 titer threshold in the TN-201 gene therapy trial. Gene therapy trials have prioritized enrolling patients with low anti-AAV NAb or TAb titers to maximize the likelihood of effective transgene delivery and efficacy while mitigating potential immune responses, despite minimal data linking adverse events to pre-existing antibodies, despite thousands treated with AAVs (13). Currently, there is a lack of standardization of assays that measure neutralizing activity and total antibodies; therefore, there is a variation in cut-off titer values where participants are considered positive. Previous studies set the cut-off for seropositivity at 1:50 (15, 16). It is also important to note that for previous studies, the cut-off was determined in healthy US donors (15, 17). In addition, the cut-off threshold for determining seroprevalence may change with geographical location and/or the utilization of patient-specific samples during validation of an assay. The MyPEAK-1 gene therapy clinical trial of TN-201 currently requires patients to have an AAV9 NAb titer ≤1:40 for inclusion into the study and may allow titers ≤1:80 in the future. Importantly, we found no association with patient age and serostatus, indicating that older subgroups are as likely to be seroeligible as younger cohorts. Sex, ethnicity, and NYHA class were not associated with NAb or TAb status, although prior studies have observed differences in serostatus by ethnicity (14, 18). This study population was predominantly White and exclusively adult and symptomatic, which may limit conclusions regarding differences in serostatus across all MYBPC3-associated HCM subgroups. Other relevant seroprevalence studies have shown comparable overall lower seropositivity rates for AAV9 compared to other serotypes, though several of these studies show that rates are higher among African American and Hispanic donors than other races/ethnicities (Table 2) (14–20). Importantly, several of these studies did not show increasing seropositivity over time.
Table 2
| Study | Population | Sample size | NAb titer threshold | Ethnicity | AAV9 seropositive (%) |
|---|---|---|---|---|---|
| Verma et al. (18) | DMD | 101 | 1:5 | African American | 59 |
| Southeast Asian Indian | 50 | ||||
| Hispanic | 36 | ||||
| Caucasian | 32 | ||||
| Boutin et al. (15) | Healthy adults | 226 | 1:20 | Caucasian (France) | 34 |
| Mumiro et al. (17) | Healthy adults | 85 | 1:14 | Asian (Japan) | 37 |
| Hemophilia | 59 | Asian (Japan) | 27 | ||
| Khatri et al. (14) | Healthy adults | 100 | 1:5 | African American | 79 |
| Hispanic | 50 | ||||
| Caucasian | 38 | ||||
| Navarro-Oliveros et al. (19) | Healthy adults | 100 | 1:20 | Caucasian (Spain) | 17 |
| Sierra-Delgado et al. (20) | Healthy adults | 60 | 1:50 | Hispanic (Colombia) | 23 |
| Heart failure | 60 | Hispanic (Colombia) | 20 |
Baseline demographics and medical history of study population.
In our study, the strong correlation between TAb and NAb titers suggests that TAb could become a reliable measure of pre-existing immunity. As more AAV gene therapy clinical trials are conducted, sponsors should advocate standardized screening assays and antibody titer cut-offs for future trials.
In conclusion, while approximately 50% of patients with MYBPC3-associated HCM had detectable anti-AAV9 antibody titers, 84% had low or no NAb titers to AAV9. The low prevalence of pre-existing immunity in this population indicates that most MYBPC3-associated HCM patients could be eligible for an AAV9 gene therapy, such as TN-201.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by Cleveland Clinic IRB, All IRBs from various institutions. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
MD: Writing – original draft, Formal analysis, Data curation, Supervision, Methodology, Conceptualization, Investigation, Writing – review & editing. DM: Writing – review & editing, Investigation. HW: Writing – review & editing, Investigation. TW: Investigation, Writing – review & editing. OW-P: Investigation, Writing – review & editing. NL: Investigation, Writing – review & editing. JG: Investigation, Writing – review & editing. TA: Investigation, Writing – review & editing. SN: Writing – review & editing, Investigation. FR: Investigation, Writing – review & editing. AM: Writing – review & editing, Investigation. ET: Formal analysis, Writing – review & editing, Methodology, Investigation. PM: Formal analysis, Investigation, Writing – review & editing. EB: Methodology, Investigation, Writing – review & editing, Formal analysis. WH: Writing – review & editing, Investigation, Methodology. NS-P: Conceptualization, Project administration, Investigation, Writing – review & editing. GA: Formal analysis, Methodology, Writing – review & editing, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was funded by Tenaya Therapeutics, Inc, San Francisco.
Conflict of interest
MD consulting fees from Bristol Myers Squibb, Tenaya, Edgewise, VizAI and Cytokinetics. DM consulting fees from Chiesi Pharmaceuticals, Cytokinetics, Tenaya and Sanofi. TW site principal investigator for clinical trials related to myosin inhibitors sponsored by Bristol Myers Squibb and Cytokinetics and serves in an unpaid capacity on advisory committees sponsored by Bristol Myers Squibb and Cytokinetics. NL consulting fees from Tenaya, Cytokinetics, BMS, Nuevocore, Bayer, Alexion, Pfizer and grant support from BMS and Pfizer. TA research grants to institutions from BMS, Cytokinetics, Tenaya, Edgewise and serves as a consultant for Edgewise. FR consulting fees from Bristol Myer Squibb, Cytokinetics, Medtronic, ReCor Medical, Idorsia, Mineralys and Astra Zeneca. AM reports research grants from Pfizer, Ionis, Attralus, Cytokinetics and Janssen and consulting fees from Cytokinetics, BMS, BridgeBio, Pfizer, Ionis, Lexicon, Attralus, Alnylam, Haya, Alexion, Akros, Edgewise, Rocket, Lexeo, Prothena, BioMarin, AstraZeneca, and Tenaya. PM and EB employees of BioAgilytix. WH, NS-P, and GA employees of Tenaya Therapeutics.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Ommen SR Ho CY Asif IM Balaji S Burke MA Day SM et al . 2024 AHA/ACC/AMSSM/HRS/PACES/SCMR guideline for the management of hypertrophic cardiomyopathy: a report of the American Heart Association/American College of Cardiology Joint Committee on clinical practice guidelines. Circulation. (2024) 149:1239–1311. doi: 10.1161/CIR.0000000000001250
2.
Greer-Short A Greenwood A Leon EC Qureshi TN von Kraut K Wong J et al . AAV9-mediated MYBPC3 gene therapy with optimized expression cassette enhances cardiac function and survival in MYBPC3 cardiomyopathy models. Nat Commun. (2025) 16:2196. doi: 10.1038/s41467-025-57481-7
3.
Desai M. (2025) American College of Cardiology Scientific Sessions (ACC.25)
4.
ClinicalTrials.gov . Study of safety and tolerability of TN-201 in adults with symptomatic MYBC3 mutation-associated HCM (MyPEAK-1). (2025). Available online at: https://clinicaltrials.gov/study/NCT05836259 (accessed 4/18/2023).
5.
Manno CS Pierce GF Arruda VR Glader B Ragni M Rasko JJ et al . Successful transduction of liver in hemophilia by AAV-factor IX and limitations imposed by the host immune response. Nat Med. (2006) 12:342–7. doi: 10.1038/nm1358
6.
Jiang H Couto LB Patarroyo-White S Liu T Nagy D Vargas JA et al . Effects of transient immunosuppression on adeno-associated, virus-mediated, liver-directed gene transfer in rhesus macaques and implications for human gene therapy. Blood. (2006) 108:3321–8. doi: 10.1182/blood-2006-04-017913
7.
Mingozzi F High KA . Immune responses to AAV vectors: overcoming barriers to successful gene therapy. Blood. (2013) 122:23–36. doi: 10.1182/blood-2013-01-306647
8.
Hinderer C Katz N Buza EL Dyer C Goode T Bell P et al . Severe toxicities in nonhuman primates and piglets following high-dose intravenous administration of an adeno-associated virus vector expressing human SMN. Hum Gene Ther. (2018) 29:285–98. doi: 10.1089/hum.2018.015
9.
Wang D Tai PW Gao G . Adeno-associated virus vector as a platform for gene therapy delivery. Nat Rev Drug Discov. (2019) 18:358–78. doi: 10.1038/s41573-019-0012-9
10.
Calcedo R Wilson JM . AAV natural infection induces broad cross-neutralizing antibody responses to multiple AAV serotypes in humans. Gene Ther. (2013) 20:527–36. doi: 10.1038/gt.2012.73
11.
Chand DH et al . Immune responses in AAV gene therapy: current understanding and future perspectives. Front Immunol. (2022) 13:940928. doi: 10.3389/fimmu.2022.940928
12.
Schulz M Levy DI Petropoulos CJ Bashirians G Winburn I Mahn M et al . Binding and neutralizing anti-AAV antibodies: detection and implications for rAAV-mediated gene therapy. Mol Ther. (2023) 31:616–30. doi: 10.1016/j.ymthe.2023.01.010
13.
Shen W Liu S Ou L . rAAV immunogenicity, toxicity, and durability in 255 clinical trials: a meta-analysis. Front Immunol. (2022) 13:1001263. doi: 10.3389/fimmu.2022.1001263
14.
Khatri A Shelke R Guan S Somanathan S . Higher Seroprevalence of anti-adeno associated viral vector neutralizing antibodies among racial minorities in the United States. Hum Gene Ther. (2022) 33:442–50. doi: 10.1089/hum.2021.243
15.
Boutin S Monteilhet V Veron P Leborgne C Benveniste O Montus MF et al . Prevalence of serum IgG and neutralizing factors against adeno-associated virus (AAV) types 1, 2, 5, 6, 8, and 9 in the healthy population: implications for gene therapy using AAV vectors. Hum Gene Ther. (2010) 21:704–12. doi: 10.1089/hum.2009.182
16.
Li C Narkbunnam N Samulski RJ Asokan A Hu G Jacobson LJ et al . Neutralizing antibodies against adeno-associated virus examined prospectively in pediatric patients with hemophilia. Gene Ther. (2012) 19:288–94. doi: 10.1038/gt.2011.90
17.
Mimuro J Mizukami H Shima M Matsushita T Taki M Muto S et al . The prevalence of neutralizing antibodies against adeno-associated virus capsids is reduced in young Japanese individuals. J Med Virol. (2014) 86:1990–7. doi: 10.1002/jmv.23818
18.
Verma S Nwosu SN Razdan R Upadhyayula SR Phan HC Koroma AA et al . Seroprevalence of adeno-associated virus neutralizing antibodies in males with Duchenne muscular dystrophy. Hum Gene Ther. (2022). doi: 10.1089/hum.2022.081
19.
Navarro-Oliveros M Vidaurrazaga A Soares Guerra G Castellana D Embade N Millet O et al . Seroprevalence of adeno-associated virus types 1, 2, 3, 4, 5, 6, 8, and 9 in a Basque cohort of healthy donors. Sci Rep. (2024) 14:15941. doi: 10.1038/s41598-024-66546-4
20.
Sierra-Delgado JA Likhite S Bautista PK Gómez-Ochoa SA Echeverría LE Guío E et al . Prevalence of neutralizing antibodies against adeno-associated virus serotypes 1, 2, and 9 in non-injected Latin American patients with heart failure—ANVIAS study. Int J Mol Sci. (2023) 24:5579. doi: 10.3390/ijms24065579
Summary
Keywords
MYBPC3, seroprevalence, HCM, adenoassociated virus 9, gene therapy
Citation
Desai MY, Massera D, Wang H, Wong TC, Wever-Pinzon O, Lakdawala NK, Giudicessi JR, Abraham T, Nagueh S, Rader F, Masri A, Turer E, Mushonga P, Flores EB, Harrison W, Sonicheva-Paterson N and Argast G (2025) High rate of seroeligibility among MYBPC3-associated hypertrophic cardiomyopathy patients for TN-201, an adeno-associated virus serotype 9 MYBPC3 gene therapy. Front. Med. 12:1635586. doi: 10.3389/fmed.2025.1635586
Received
30 June 2025
Accepted
20 August 2025
Published
12 September 2025
Volume
12 - 2025
Edited by
Xingjie Gao, Tianjin Medical University, China
Reviewed by
Soontaree Petchdee, Kasetsart University, Thailand
Jiali Nie, Huazhong University of Science and Technology, China
Updates
Copyright
© 2025 Desai, Massera, Wang, Wong, Wever-Pinzon, Lakdawala, Giudicessi, Abraham, Nagueh, Rader, Masri, Turer, Mushonga, Flores, Harrison, Sonicheva-Paterson and Argast.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Milind Y. Desai, desaim2@ccf.org
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.