- Department of Gastroenterology, Changzhou Tumor Hospital, Changzhou, Jiangsu, China
Objective: To explore the effects of bifidobacteria quadruple viable bacteria tablets (Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus) plus quadruple therapy on inflammatory response and Helicobacter pylori (Hp) eradication rate in patients with Hp-positive gastric ulcers.
Methods: One hundred patients with Hp-positive gastric ulcers admitted in our hospital from January 2022 to December 2024 were included and divided into a control group and a study group. The former accepted quadruple therapy (esomeprazole magnesium enteric-coated capsules + colloidal bismuth pectin capsule + clarithromycin tablets + amoxicillin capsule). Based on the quadruple therapy, the latter was added with bifidobacteria quadruple viable bacteria tablets. The clinical symptoms, clinical efficacy, Hp eradication rate, levels of gastrointestinal hormones and inflammatory factors, immune function, number of beneficial bacteria, and incidence of adverse reactions were compared in both groups.
Results: Following 2 weeks of treatment, the study group had lower scores across these symptoms compared to the control group (P < 0.05). Furthermore, the study group had a higher total treatment efficacy rate and a higher Hp eradication rate compared to the control group (P < 0.05). Compared to the control group, the study group had higher levels of serum somatostatin and lower levels of motilin, gastrin, and pepsinogen I following 2 weeks of treatment (P < 0.05). Additionally, the study group had lower levels of interleukin-6 (IL-6), C-reactive protein (CRP), interleukin-8 (IL-8), and matrix metalloproteinase-9 (MMP-9) compared to the control group (P < 0.05). In comparison to the control group, the study group had higher levels of CD4 + T-cells, higher CD4 + /CD8 + ratio and lower CD8 + T-cell levels after 2 weeks of treatment (P < 0.05). Moreover, the study group had a significant increase in the abundance of beneficial gut microbiota, specifically Enterococcus faecalis, Lactobacillus acidophilus, and Bifidobacterium compared to the control group (P < 0.05). Lastly, the study group had a lower incidence of adverse reactions than the control group (P < 0.05).
Conclusion: Bifidobacteria quadruple viable bacteria tablets plus quadruple therapy can improve the clinical symptoms, promote the clinical therapeutic effect and Hp eradication rate, improve the levels of gastrointestinal hormones and inflammatory factors, enhance the immune function, increase levels of beneficial bacteria and diminish the incidence of adverse reactions caused by quadruple therapy in patients suffered from Hp-positive gastric ulcer.
Introduction
Gastric ulcers are a common clinical digestive disease, mainly caused by excessive gastric acid secretion. This excessive secretion can damage the gastric mucosa, resulting in ulcers of varying degrees in the antrum and cardia regions of the stomach (1). Gastric ulcers are closely related to Helicobacter pylori (Hp) infection (2). Hp is the pathogenic bacterium that causes gastric ulcers that can grow and reproduce in acidic environments (3, 4). When the human body is infected with Hp, it will enter the stomach, penetrate the mucosal layer, and subsequently damage the function of the gastric mucosa, thereby causing gastric ulcers (5). It has been reported 67.0%–80.0% of gastric ulcers are caused by Hp infection (6). If an Hp-positive gastric ulcers cannot be treated promptly and effectively, the long-term parasitism of Hp in the body may lead to gastrointestinal tumors, thereby endangering the patient’s life (7, 8). Therefore, the eradication of Hp plays a key role in the treatment of gastric ulcers.
The main purpose of clinical treatment for Hp-positive gastric ulcers is to eradicate Hp and alleviate clinical symptoms (9). Quadruple therapy, the first choice for treating Hp-positive gastric ulcers, aims to achieve treatment goals through measures such as killing the bacteria, reducing acid production, and protecting the gastric mucosa (10). However, long-term use of this treatment method may lead to the development of drug resistance, thereby reducing its effectiveness in eradicating Hp. Moreover, it also disrupts the balance of the intestinal microbiota, thereby hindering the recovery of normal stomach functions (11).
Toll-like receptors (TLRs) play a significant role in the pathogenesis of Hp-positive gastric ulcers (12, 13). After Hp infection, TLRs on the gastric mucosal epithelial cells and immune cells recognize the pathogen-associated molecular patterns of the bacteria, activating downstream signaling pathways, leading to the activation of transcription factors such as nuclear factor kappaB (NF-κB), thereby mediating the transcription and expression of nuclear inflammatory-related genes (14, 15). This process triggers the release of a large amount of inflammatory cytokines, such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), leading to the formation of an inflammatory cascade and disruption of the immune function, thereby causing damage to the gastric mucosa and the formation of ulcers (16).
Studies have shown that the development of various human diseases is closely linked to an imbalance in the microbial flora (17, 18). Probiotics have gained increasing attention because of their non-toxicity, lack of side effects, and absence of residues. They are now being utilized to regulate the balance of normal microbial flora (19). There is a close regulatory relationship between probiotics and inflammation. Probiotics exert anti-inflammatory effects through various mechanisms, such as regulating intestinal microbiota, strengthening the immune barrier, inhibiting harmful bacteria, and regulating cytokines (18). Studies have shown that probiotics adjuvant therapy can efficiently promote the eradication of Hp, inhibit the growth of Hp, increase intestinal probiotics, promote intestinal peristalsis, and improve local immunity of the gastrointestinal tract (20, 21). Bifidobacteria quadruple viable bacteria tablets have been widely used in clinical practice in recent years, and have a good effect on chronic diarrhea, antibiotic-resistant constipation, and diarrhea resulting from intestinal flora imbalance (22). It contains Bifidobacterium infantis, Lactobacillus acidophilus, Enterococcus faecalis, and Bacillus cereus, which are normal intestinal flora in the body of healthy people and can multiply and grow rapidly in the intestines (23). Relevant studies have found that bifidobacteria quadruple viable bacteria tablets can adjust the balance of intestinal flora, directly supplement the normal physiological flora of the body, and inhibit and kill potentially harmful bacteria in the intestine (24). However, the clinical efficacy of bifidobacteria quadruple viable bacteria tablets plus quadruple therapy in patients with Hp-positive gastric ulcers remains largely unclear.
Therefore, our study aimed to assess the effects of bifidobacteria quadruple viable bacteria tablets plus quadruple therapy on inflammatory response and Hp eradication rate in patients with Hp-positive gastric ulcer.
Data and methods
General data
One hundred patients with Hp-positive gastric ulcers in our hospital from January 2022 to December 2024 were included and divided into a control group and a study group using the random number table method, with 50 patients in each group. The flowchart of the method process used in the current study was shown in Figure 1. Inclusion criteria: (1) Gastric ulcer was diagnosed by clinical symptoms and gastroscopy; (2) 14C urea breath test was Hp positive (25); (3) Age ≥ 18 years old. Exclusion criteria: (1) Use of antibiotics, probiotics, bismuth and other drugs in the past 2 weeks; (2) Recent history of gastrointestinal surgery; (3) Allergic to the study drugs; (4) Gastric malignant tumor, gastrointestinal perforation or gastrointestinal bleeding.
Randomization and blinding
A group randomization design was adopted for random grouping. The random allocation sequence was generated by a computer. The allocation confidentiality measures were achieved through sequential numbering, sealing, and opaque envelopes. After being deemed to meet the inclusion criteria, patients were randomly assigned to the control group or the study group in a 1:1 ratio. This study was single-blind, and the participants were unaware of the allocation.
Materials and methods
The control group was treated with quadruple therapy based on local Hp resistance patterns, including:
(1) Esomeprazole magnesium enteric-coated capsules (Chia Tai Tianqing Pharmaceutical Group Co., Ltd.; Specification: 20 mg/pill), oral before meals, 20 mg/time, twice a day.
(2) Colloidal bismuth pectin capsule (Hunan Dibo Pharmaceutical Co., Ltd.; Specification: 50 mg), 0.2 g/time, twice a day.
(3) Clarithromycin tablets (Changchun Lei Yun Shang Pharmaceutical Co., Ltd.; Specification: 0.25 g/tablet), oral after meals, 0.5 g/time, twice a day.
(4) Amoxicillin capsule (Sichuan Emeishan Pharmaceutical Co., Ltd.; Specification: 0.5 g), 1.0 g/time, twice a day.
Based on the quadruple therapy, the study group was added with bifidobacteria quadruple viable bacteria tablets (Hangzhou Yuanda Bio-Pharmaceutical Co., Ltd.; Specification: 0.5 g/tablet), 1.5 g/time, 3 times a day.
Both groups received treatment for 2 weeks as described previously (26).
At 4 weeks after treatment, we performed a 14C-urea breath test to evaluate Hp eradication.
Observation indicators
(1) The clinical symptoms including abdominal distension, acid reflux, belching and abdominal pain were scored as follows: (1) 0 point: no symptoms; (2) 1 point: the symptoms were mild and did not affect daily life, and the attack occurred once more than 5 days; (3) 2 points: the symptoms were slightly severe, the effect on daily life was slight, and the attack occurred once more than 3–5 days; (4) 3 points: severe symptoms, seriously affecting daily life, and the attack occurred every other day or every day. Scores for each symptom ranged from 0 to 3, with higher scores representing more severe symptoms.
(2) After 2 weeks of treatment, the total effective rate of was evaluated as described previously (27). The total effective rate of treatment refers to the percentage of patients whose condition has improved to a certain extent (including obviously effective, effective, and ineffective) after a certain treatment among all patients who received that treatment. It reflects the overall effectiveness of the treatment plan for most patients and is a comprehensive indicator for measuring the treatment outcome. (1) Obviously effective: abdominal distension, acid reflux and other symptoms disappeared, no mucosal edema was found, gastroscopy showed that ulcers disappeared or scar formed, and the clinical symptom score decreased by ≥80.0%. (2) Effective: Gastroscopy showed that the ulcer basically disappeared, with mild edema or inflammation. The clinical symptom score was reduced by 50.0%–79.0%. (3) Ineffective: patients did not meet the criteria of obviously effective or effective. Total effective rate = (Number of obviously effective + number of effective)/total number of cases × 100%.
(3) After 2 weeks of treatment, Hp was determined to be eradicated if the 14C urea breath test was negative, and eradication failure if the 14C urea breath test was positive.
(4) Before and following 2 weeks of treatment, 3 mL of venous blood was obtained from the patients, and the serum was collected by centrifugation. The serum levels of somatostatin (SS), motilin (MTL), pepsinogen I (PG I) and gastrin (GAS) were determined by immunoturbidimetry.
(5) Before and following 2 weeks of treatment, 3 mL of venous blood was obtained from the patients, and the serum was collected by centrifugation. The serum levels of C-reactive protein (CRP), interleukin-6 (IL-6), matrix metalloproteinase-9 (MMP-9) and interleukin-8 (IL-8) levels were measured by enzyme-linked immunosorbent assay (ELISA).
(6) Before and after 2 weeks of treatment, 3 mL of venous blood was obtained from the patients, and the serum was collected by centrifugation. Flow cytometry (Beckman Coulter Co., Ltd., USA) was used to detect cluster of differentiation 4 positive (CD4+) and cluster of differentiation 8 positive (CD8+), followed by calculation of CD4+/CD8+.
(7) Fresh stool samples were collected from the patients, 1 g and 9 mL glycerol were diluted 10-fold to 1 × 108, and inoculated and cultured. Enterococcus faecalis was cultured in an ordinary incubator at 37 °C for 24 h, Lactobacillus acidophilus and Bifidobacterium were cultured in an anaerobic incubator for 45 h, and the number of bacteria in each gram stool was calculated by l g colony-forming unit (CFU)/g.
(8) The total incidence of adverse reactions containing dizziness, vomiting, nausea along with diarrhea were calculated.
Statistical analysis
GraphPad Prism 10.0 statistical software was employed for analyzing the data. The measurement data were exhibited by mean ± standard deviation (x ± s), and t-test was conducted for comparison. The counting data were exhibited as number and rate (%), and χ2 test was applied for comparison. P < 0.05 was considered as statistically significant.
Results
The flowchart of the results was shown in Figure 2.
Patients’ general information in both groups
As Table 1 displayed, no difference was seen in general information containing gender, age, course of disease, diameter of ulcer, location of ulcer and BMI of patients between both groups (P > 0.05).
Clinical symptom scores in both groups
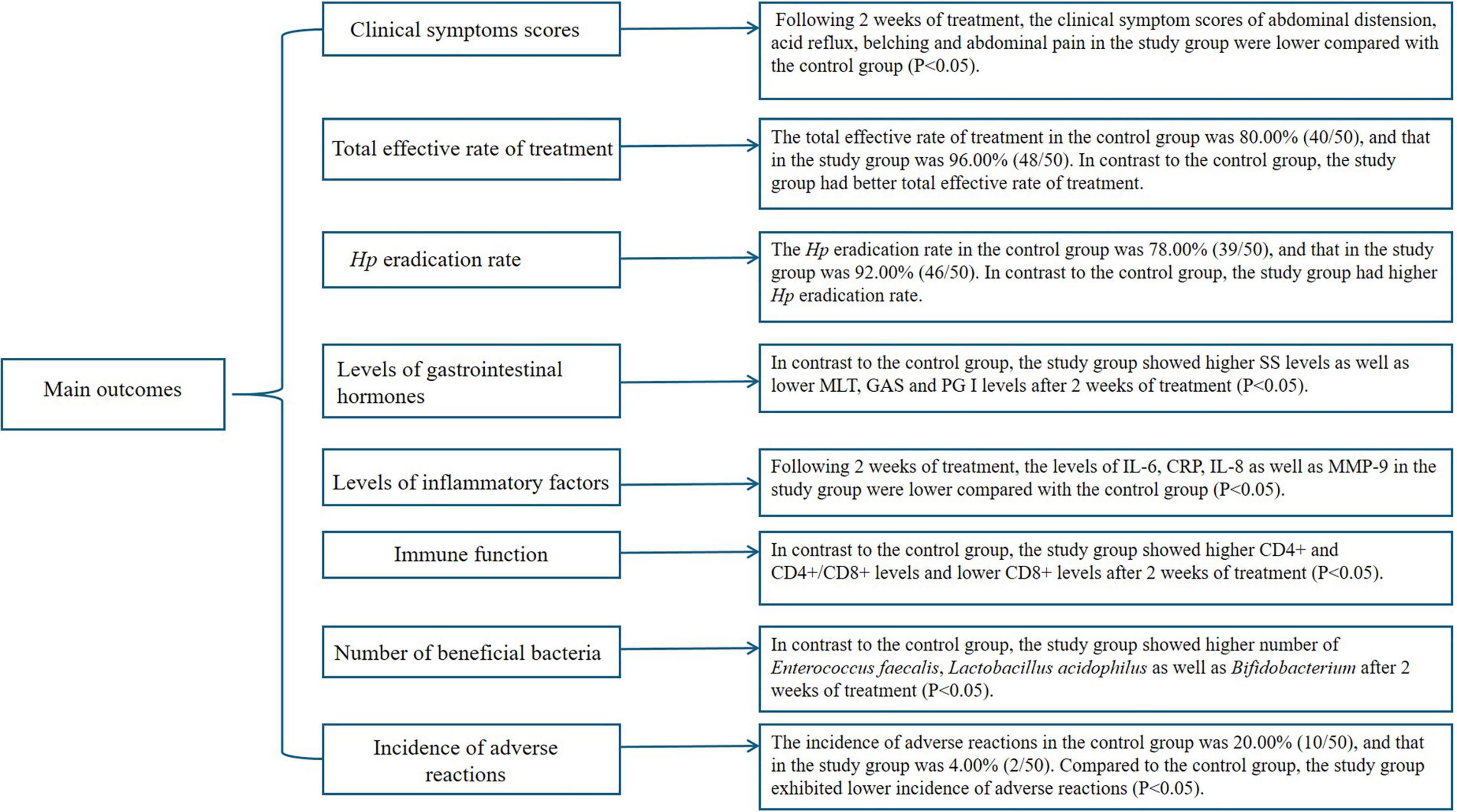
Prior to treatment, no differences were seen in the clinical symptom scores of abdominal distension, acid reflux, belching and abdominal pain between both groups (P > 0.05). Following 2 weeks of treatment, the clinical symptom scores of abdominal distension, acid reflux, belching and abdominal pain were diminished in both groups (P < 0.05), and those in the study group were lower compared with the control group (P < 0.05, Figure 3, and Supplementary Table 1).
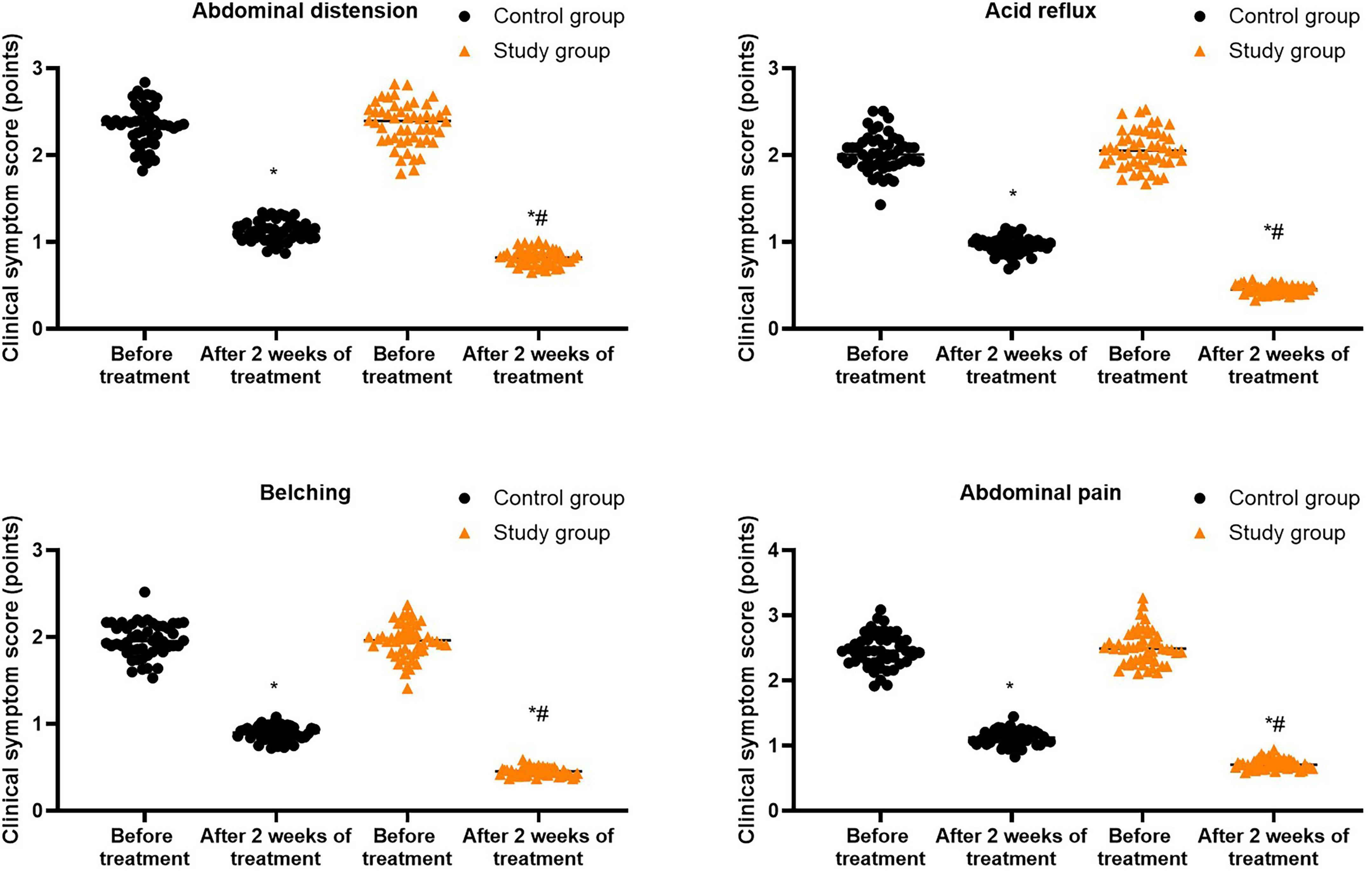
Figure 3. Clinical symptom scores in both groups. *P < 0.05 vs. before treatment; #P < 0.05 vs. control group.
Total effective rate of treatment in both groups
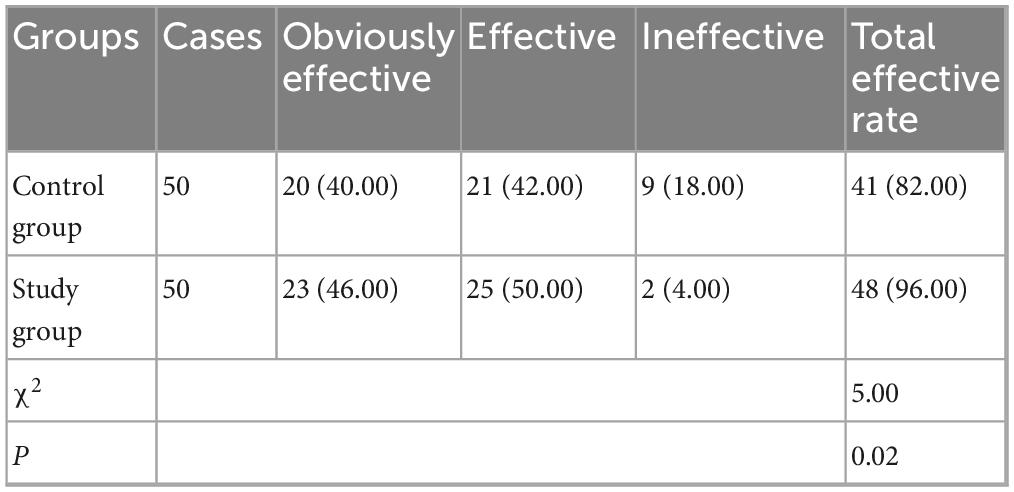
As Table 2 revealed, the total effective rate of treatment in the control group was 80.00% (40/50), and that in the study group was 96.00% (48/50). In contrast to the control group, the study group had better total effective rate of treatment (P < 0.05).
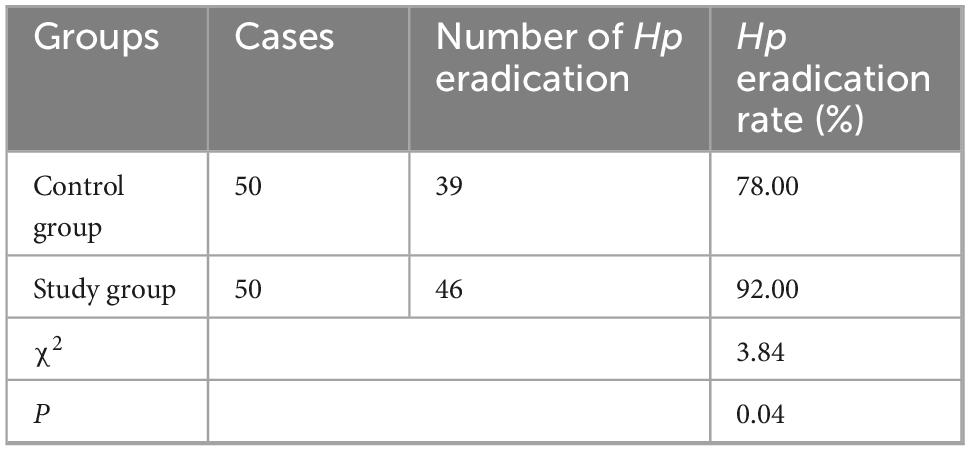
Hp eradication rate in both groups
As Table 3 revealed, the Hp eradication rate in the control group was 78.00% (39/50), and that in the study group was 92.00% (46/50). In contrast to the control group, the study group had higher Hp eradication rate (P < 0.05).
Gastrointestinal hormones in both groups
Prior to treatment, no differences were seen in SS, MLT, GAS and PG I levels between both groups (P > 0.05). Following 2 weeks of treatment, the SS levels were elevated while MLT, GAS and PG I levels were declined in both groups (P < 0.05). In contrast to the control group, the study group showed higher SS levels as well as lower MLT, GAS and PG I levels 2 weeks of treatment (P < 0.05, Figure 4, and Supplementary Table 2).
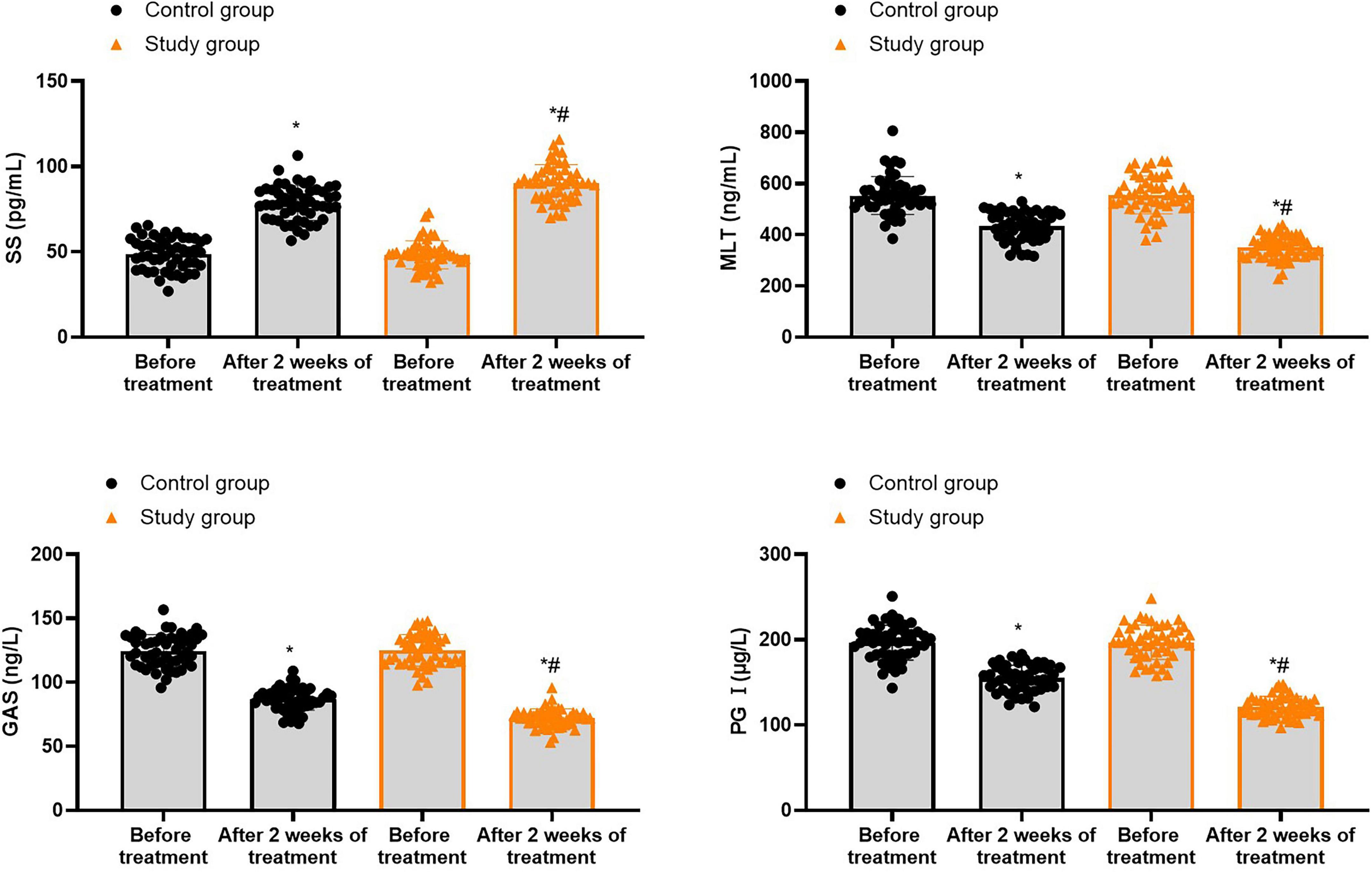
Figure 4. Gastrointestinal hormones in both groups. *P < 0.05 vs. before treatment; #P < 0.05 vs. control group.
Inflammatory response in both groups
Prior to treatment, no differences were seen in the levels of IL-6, CRP, IL-8 as well as MMP-9 between both groups (P > 0.05). Following 2 weeks of treatment, the levels of IL-6, CRP, IL-8 as well as MMP-9 were declined in both groups (P < 0.05), and those in the study group were lower compared with the control group (P < 0.05, Figure 5, and Supplementary Table 3).
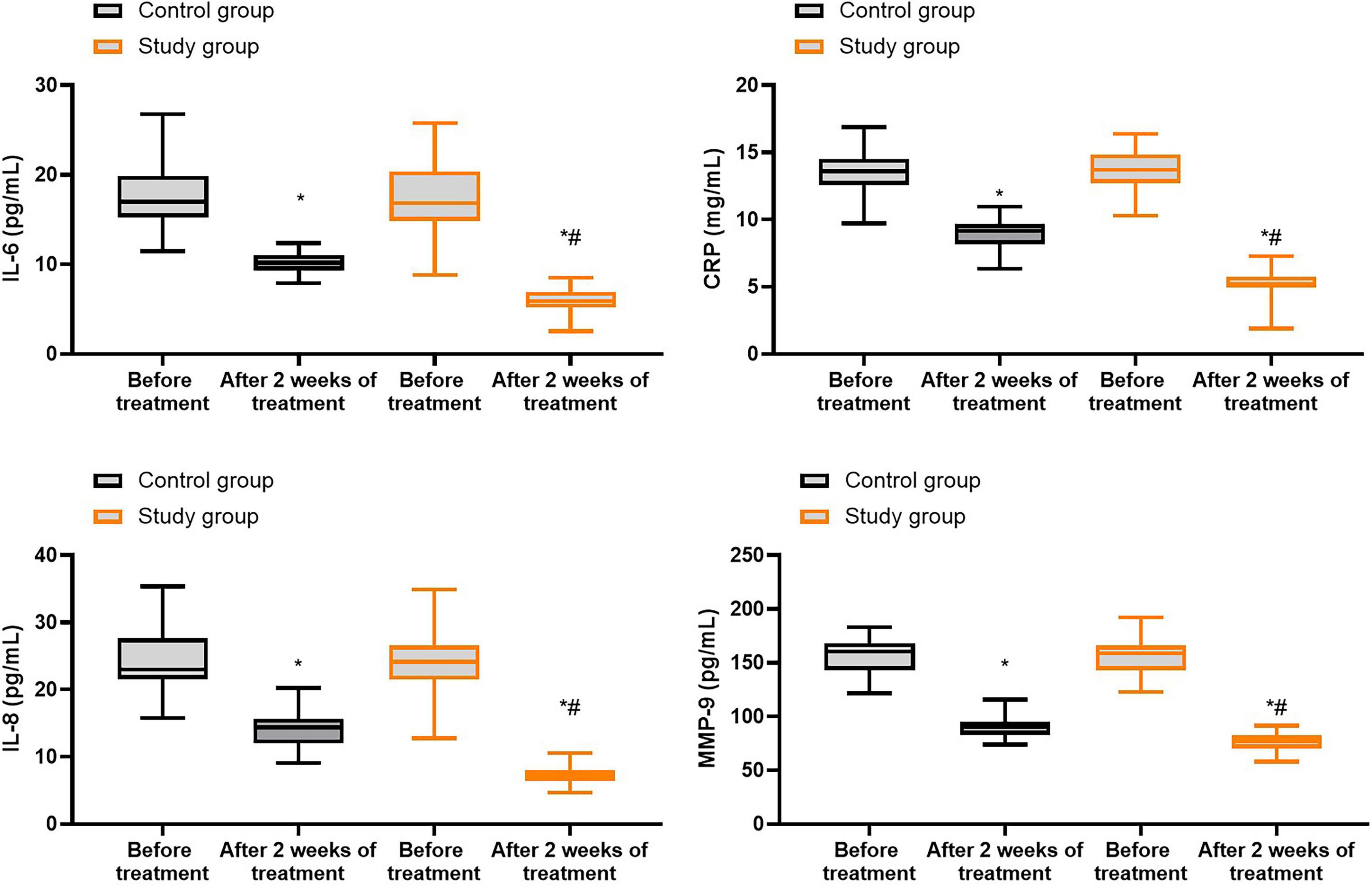
Figure 5. Inflammatory response in both groups. *P < 0.05 vs. before treatment; #P < 0.05 vs. control group.
Immune function in both groups
Prior to treatment, no differences were seen in CD4+, CD8+ and CD4+/CD8+ levels between both groups (P > 0.05). Following 2 weeks of treatment, the CD4+ and CD4+/CD8+ levels were elevated while CD8+ levels were declined in both groups (P < 0.05). In contrast to the control group, the study group showed higher CD4+ and CD4+/CD8+ levels and lower CD8+ levels following 2 weeks of treatment (P < 0.05, Figure 6, and Supplementary Table 4).
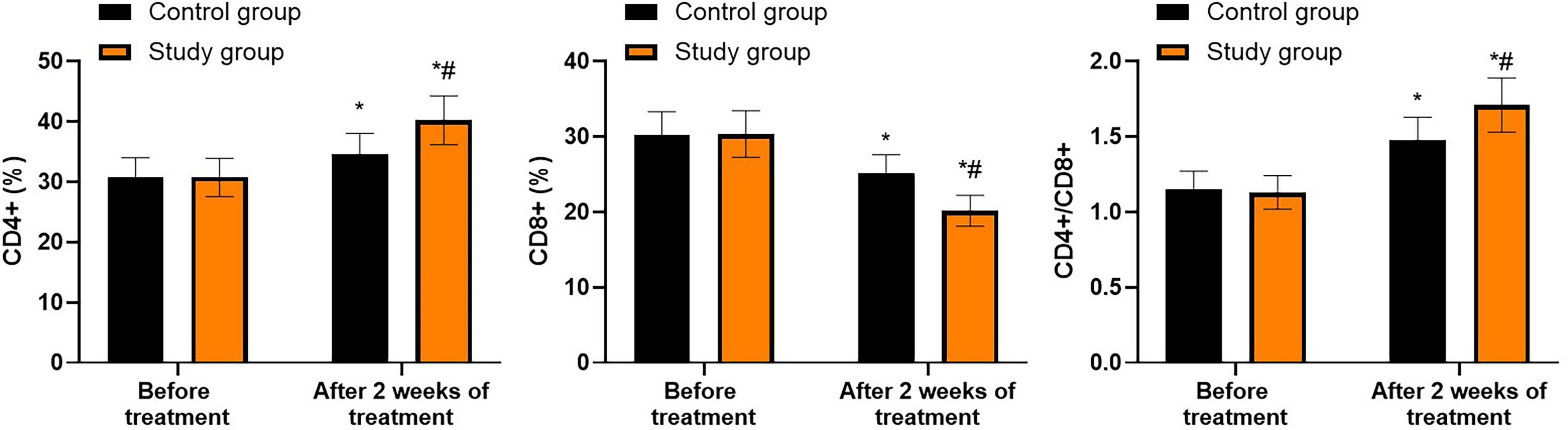
Figure 6. Immune function in both groups. *P < 0.05 vs. before treatment; #P < 0.05 vs. control group.
Number of beneficial bacteria in both groups
Prior to treatment, no differences were seen in the number of Enterococcus faecalis, Lactobacillus acidophilus as well as Bifidobacterium between both groups (P > 0.05). Following 2 weeks of treatment, the number of Enterococcus faecalis, Lactobacillus acidophilus and Bifidobacterium were elevated in the study group, and those were diminished in the control group (P < 0.05). In contrast to the control group, the study group had higher number of Enterococcus faecalis, Lactobacillus acidophilus and Bifidobacterium after 2 weeks of treatment (P < 0.05, Figure 7, and Supplementary Table 5).
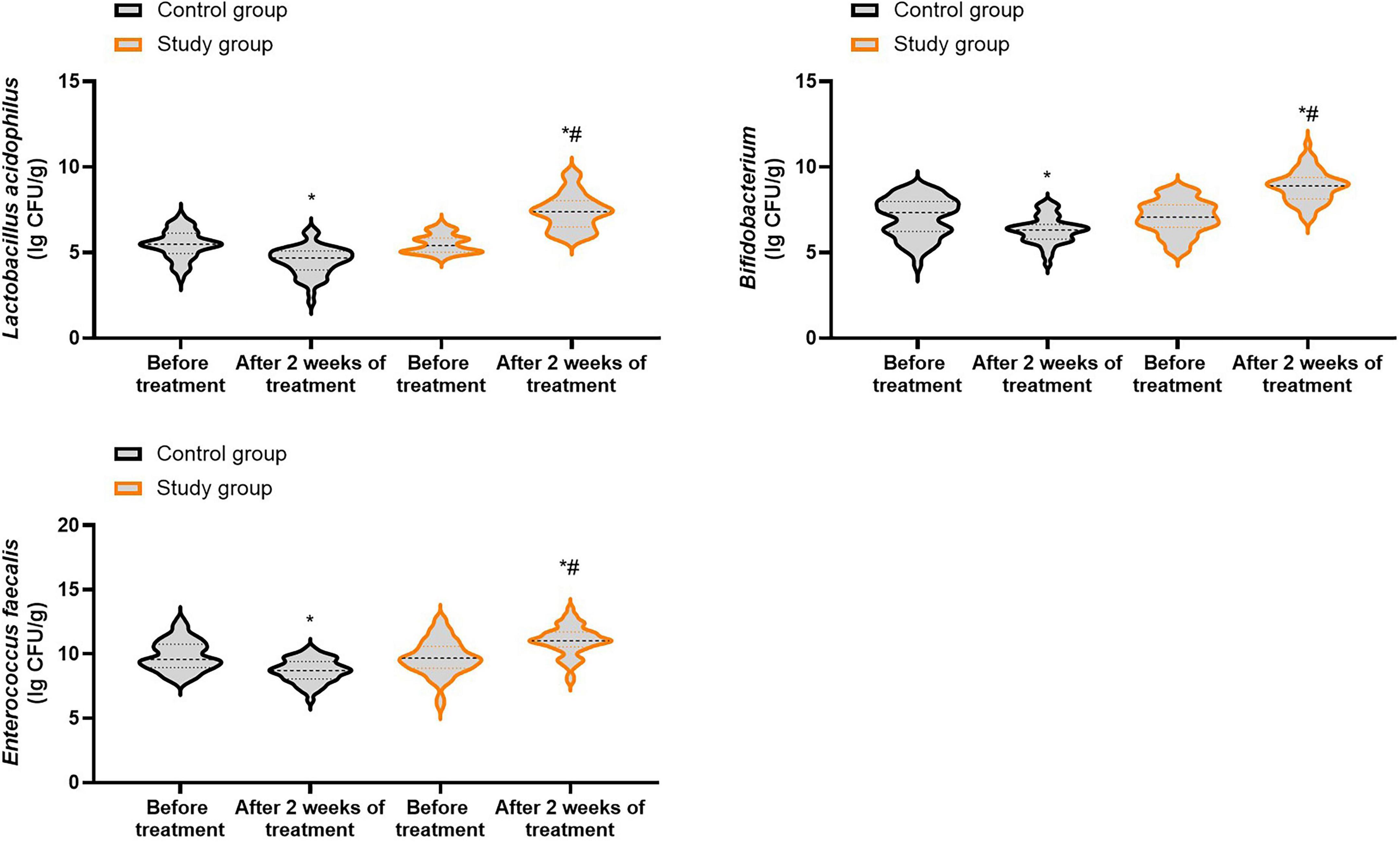
Figure 7. Number of beneficial bacteria in both groups. *P < 0.05 vs. before treatment; #P < 0.05 vs. control group.
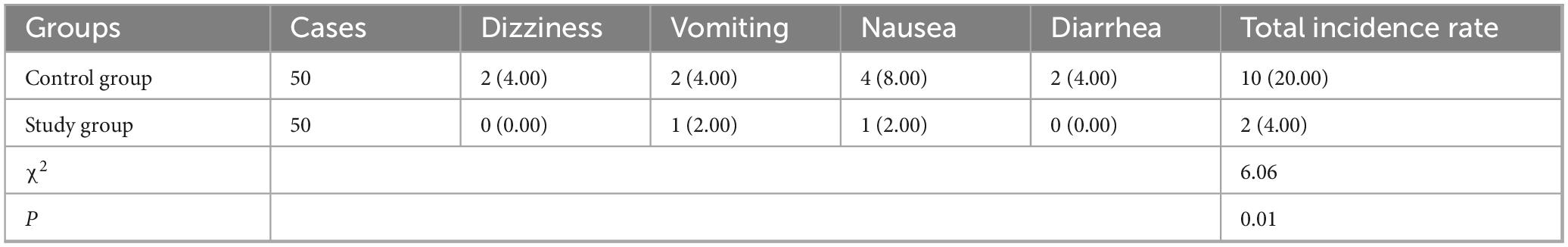
Incidence of adverse reactions in both groups
As Table 4 revealed, the incidence of adverse reactions in the control group was 20.00% (10/50), and that in the study group was 4.00% (2/50). Compared to the control group, the study group had lower incidence of adverse reactions (P < 0.05).
Discussion
Hp-positive gastric ulcers are characterized by gastric mucosa damage. The main cause is that the urease produced by Hp acts on the urea surrounding the gastric mucosa, leading to the decomposition of urea and thereby significantly increasing the ammonia concentration and Hp content in the gastric juice (28). In addition, Hp promotes pepsin and gastric acid secretion. Excessive secretion of these substances increases various factors that damage the gastric mucosa (29). If Hp-positive gastric ulcers are not treated promptly, they may damage the gastric mucosa and lead to atrophic gastritis (30). In severe cases, it may develop into gastric cancer, which poses a serious threat to life (31). Therefore, for patients with Hp-positive gastric ulcers, active and effective treatment measures are crucial for alleviating symptoms and reducing adverse reactions.
Quadruple therapy is the preferred treatment for Hp-positive gastric ulcers (32). However, the quadruple therapy involves the combined use of multiple antibiotics. This multi-drug combination approach may accelerate the development of bacterial resistance, thereby reducing the eradication effect of Hp and potentially causing an imbalance in the intestinal flora, which may adversely affect the recovery of gastric function (33). In recent years, the concept of “treating bacteria by bacteria” had provided a new direction for treating Hp-positive gastric ulcers (34). The combination of probiotics and antibiotics can not only enhance the therapeutic effect of antibiotics but also help maintain the balance of gastrointestinal flora (35).
In our study, we first compared the general data of patients between the two groups and found no difference in general information, including gender, age, course of disease, ulcer diameter, ulcer location and BMI of patients between both groups, suggesting that the general data of the two groups of patients were comparable.
Patients with Hp-positive gastric ulcers may experience clinical symptoms such as abdominal distension and pain, acid reflux, belching, and abdominal pain, which can impact their health (36). The results of this study showed that, compared to the control group, the study group had lower clinical symptom scores for abdominal distension, acid reflux, belching and abdominal pain, a higher total effective rate of treatment, and a higher Hp eradication rate following 2 weeks of treatment. These results suggested that bifidobacteria quadruple viable bacteria tablets plus quadruple therapy could improve clinical symptoms, facilitate the clinical therapeutic effect, and enhance the Hp eradication rate in patients with Hp-positive gastric ulcers. This is because bifidobacteria quadruple viable bacteria tablets can directly supplement the microbial flora on the mucosal surface of the host, improve the imbalance of the microecological environment, change the growth environment of Hp, fundamentally block the growth of Hp, increase the Hp eradication rate, and improve the clinical symptoms and treatment effect when combined with quadruple therapy. Consistently, Zhou et al. (37) suggested that Bifidobacterium quadruple viable tablets combined with quadruple therapy had good efficacy in Hp-associated peptic ulcer disease and could promote Hp clearance.
Gastrointestinal hormone levels can reflect the severity of gastric ulcers to a certain extent. Among them, SS can inhibit the release of gastrin (38). MTL can not only regulate gastrointestinal motility but also promote gastric acid secretion, thereby stimulating the smooth muscles of the gastrointestinal tract (39). At the same time, Hp infection can damage the gastric mucosa, causing monocytes and neutrophils in the gastric antrum to infiltrate the gastric mucosa, stimulating the secretion of pepsin (40). During this process, the content of PG I in the serum increases significantly, which aggravates the erosion of gastric mucosa and may also have a regulatory effect on the secretion of gastrointestinal hormones (41). Furthermore, some studies have found that TLRs are closely related to the occurrence and development of gastric ulcers. They can recognize the pathogen-associated molecular patterns of Hp, thereby activating the immune response and exacerbating inflammation (42). After TLRs are activated, they trigger the synthesis and secretion of inflammatory factors through pathways such as the NF-κB pathway. These inflammatory factors further amplify the immune response, forming a positive feedback loop (43). MMP-9 plays a crucial role in tissue healing. When ulcers occur, MMP-9 levels significantly increases, and pro-inflammatory factors such as IL-6 and IL-8 also abnormally increase (44, 45). The results of this study revealed that compared to the control group, the study group had higher SS levels, lower MLT, GAS and PG I levels, and lower IL-6, CRP, IL-8 and MMP-9 levels 2 weeks after treatment. These findings indicated that bifidobacteria quadruple viable bacteria tablets plus quadruple therapy could improve the levels of gastrointestinal hormones and inflammatory factors in patients with Hp-positive gastric ulcers. This is because the normal human gut flora contained in bifidobacteria quadruple viable bacteria tablets, such as Enterococcus and Bifidobacterium, can regulate the harmful bacteria in the gut, thereby maintaining the microecological balance and reducing inflammation of the gastric mucosa (46). In addition, bifidobacteria quadruple viable bacteria tablets have anti-inflammatory and anti-oxidant properties, which can effectively promote the secretion of mucin, thereby stabilizing the mucosal barrier function (47). Moreover, bifidobacteria quadruple viable bacteria tablets can regulate the secretion of gastrointestinal hormones, further promoting the repair process of the gastric mucosa (48). More importantly, bifidobacteria quadruple viable bacteria tablets plus quadruple therapy may improve the levels of gastrointestinal hormones and inflammatory factors in Hp-positive gastric ulcers through regulating that TLR/NF-κB signaling pathway. The probiotics in bifidobacteria quadruple viable bacteria tablets bind to the surface components (such as peptidoglycan, lipoteichoic acid) or metabolites (such as short-chain fatty acids, bacteriocins) of the host intestinal epithelial cells or immune cells’ surface TLR2/TLR4, thereby activating the NF-κB signaling pathway (49). Consistently, Niu et al. indicated that probiotic supplementation reduced the levels of inflammatory markers in the body, alleviated the adverse symptoms and gut microbiota disturbances caused by eradication therapy, providing a possible way to improve the overall health of patients (50).
The findings of this study also demonstrated that, compared to the control group, the study group showed higher CD4+ and CD4+/CD8+ levels, lower CD8+ levels, and a higher number of Enterococcus faecalis, Lactobacillus acidophilus, and Bifidobacterium 2 weeks after treatment. These results indicated that bifidobacteria quadruple viable bacteria tablets plus quadruple therapy could enhance the immune function and increase levels of beneficial bacteria of patients with Hp-positive gastric ulcers. We suggest that this is because a variety of beneficial bifidobacteria quadruple viable bacteria tablets directly supplement the gastrointestinal flora, thereby regulating the microecological balance and heightening patients’ immune function (51). Similarly, Chen et al. suggested that probiotic supplementation could relieve more gastrointestinal symptoms by inducing alterations in gut microbiota and host immune responses (52).
In addition, our study indicated that, compared to the control group, the study group had a lower incidence of adverse reactions, suggesting that bifidobacteria quadruple viable bacteria tablets plus quadruple therapy could diminish the incidence of adverse reactions caused by quadruple therapy in patients with Hp-positive gastric ulcers. This is because bifidobacteria quadruple viable bacteria tablets can regulate and balance the gastrointestinal flora of patients, improve the internal environment, and reduce the risk of antibiotic-induced adverse reactions. Jiang et al. conducted a comprehensive and methodical systematic review coupled with a meta-analysis, consistently demonstrating that the occurrence rate of adverse reactions was notably lower when compared to bismuth-based quadruple therapy (53).
Compared with other similar studies, this study further explored the effects of the combined treatment on the levels of gastrointestinal hormones and inflammatory factors. Gastrointestinal hormones play a crucial role in regulating gastrointestinal functions, and inflammatory factors are closely related to the onset and progression of gastric ulcers. By observing the changes in these indicators, we can gain a more comprehensive understanding of the mechanism of the combined treatment and provide a more solid theoretical basis for clinical application. In addition, this study not only focused on the eradication rate of Hp and the improvement of clinical symptoms, but also conducted a comprehensive assessment of the patients’ immune function and beneficial bacteria. The enhancement of immune function indicates that the patient’s body’s resistance to pathogens has increased, which is conducive to preventing the recurrence of the disease; while the increased levels of beneficial bacteria reflects the positive impact of this therapy on the balance of intestinal microecology. Currently, although some studies have mentioned that combined treatment has a certain effect on intestinal flora, our research adopted more advanced detection techniques to conduct a more precise analysis of the types and quantities of beneficial bacteria, clarifying which beneficial bacteria have increased, providing more detailed information for optimizing the treatment plan. In summary, the results of this study are expected to have a positive impact on the clinical treatment guidelines for Hp-positive gastric ulcers. As our understanding of the mechanism of the combined treatment and the optimization of the treatment plan deepen, this therapy is likely to be more frequently recommended as the first-line treatment option. At the same time, our research also provides new evidence and support for the update and improvement of clinical guidelines, which is conducive to promoting the standardization and development of clinical practice in this field.
Strengths and limitations
The strength of the study is that it includes multiple observation indicators related to clinical therapeutic effect of Hp-positive gastric ulcer. Our research has some limitations. Firstly, our sample size is relatively small, which may lead to deviations between the data results and the actual values. Secondly, our research was a single-center study, and the sample was not representative, which may not accurately reflect the characteristics of a broader population. Fourthly, the observation period for our study was only 2 weeks, and no longer-term follow-up observations were conducted. Therefore, more multi-center, large-scale, and long-term studies should be conducted in the future to further verify our findings.
Conclusion
Our study demonstrates that bifidobacteria quadruple viable bacteria tablets plus quadruple therapy can improve the clinical symptoms, promote the clinical therapeutic effect and Hp eradication rate, improve the levels of gastrointestinal hormones and inflammatory factors, enhance the immune function, increase levels of beneficial bacteria and diminish the incidence of adverse reactions caused by quadruple therapy in patients suffered from Hp-positive gastric ulcer.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in this article/Supplementary material.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Changzhou Tumor Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CJ: Writing – original draft, Writing – review & editing. JS: Writing – original draft, Writing – review & editing. XZ: Writing – review & editing, Writing – original draft. MZ: Writing – review & editing, Writing – original draft. HZ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1636039/full#supplementary-material
References
1. Gong H, Zhao N, Zhu C, Luo L, Liu S. Treatment of gastric ulcer, traditional Chinese medicine may be a better choice. J Ethnopharmacol. (2024) 324:117793. doi: 10.1016/j.jep.2024.117793
2. Malfertheiner P, Camargo M, El-Omar E, Liou J, Peek R, Schulz C, et al. Helicobacter pylori infection. Nat Rev Dis Primers. (2023) 9:19. doi: 10.1038/s41572-023-00431-8
3. Tshibangu-Kabamba E, Yamaoka Y. Helicobacter pylori infection and antibiotic resistance - from biology to clinical implications. Nat Rev Gastroenterol Hepatol. (2021) 18:613–29. doi: 10.1038/s41575-021-00449-x
4. Ranjbar R, Behzadi P, Farshad S. Advances in diagnosis and treatment of Helicobacter pylori infection. Acta Microbiol Immunol Hung. (2017) 64:273–92. doi: 10.1556/030.64.2017.008
5. Suzuki S, Kusano C, Horii T, Ichijima R, Ikehara H. The ideal Helicobacter pylori treatment for the present and the future. Digestion. (2022) 103:62–8. doi: 10.1159/000519413
6. Kamada T, Satoh K, Itoh T, Ito M, Iwamoto J, Okimoto T, et al. Evidence-based clinical practice guidelines for peptic ulcer disease 2020. J Gastroenterol. (2021) 56:303–22. doi: 10.1007/s00535-021-01769-0
7. Tran S, Bryant K, Cover T. The Helicobacter pylori cag pathogenicity island as a determinant of gastric cancer risk. Gut Microbes. (2024) 16:2314201. doi: 10.1080/19490976.2024.2314201
8. Niu C, Zhang J, Okolo P, Daglilar E. Plant polyphenols in gastric cancer: nature’s healing touch. Semin Oncol. (2025) 52:152333. doi: 10.1053/j.seminoncol.2025.01.002
9. Chey W, Howden C, Moss S, Morgan D, Greer K, Grover S, et al. ACG clinical guideline: treatment of Helicobacter pylori Infection. Am J Gastroenterol. (2024) 119:1730–53. doi: 10.14309/ajg.0000000000002968
10. Lee M, Kim G. Bismuth-based quadruple therapy for clarithromycin-resistant Helicobacter pylori Infection: effectiveness and cost-efficiency. Gut Liver. (2022) 16:807–8. doi: 10.5009/gnl220414
11. Aumpan N, Mahachai V, Vilaichone R. Management of Helicobacter pylori infection. JGH Open. (2023) 7:3–15. doi: 10.1002/jgh3.12843
12. Yang C, Scheibenbogen C, Bauer S, Kleinle C, Wex T, Bornschein J, et al. A frequent Toll-like receptor 1 gene polymorphism affects NK- and T-cell IFN-γ production and is associated with Helicobacter pylori-induced gastric disease. Helicobacter. (2013) 18:13–21. doi: 10.1111/hel.12001
13. Behzadi P, Chandran D, Chakraborty C, Bhattacharya M, Saikumar G, Dhama K, et al. The dual role of toll-like receptors in COVID-19: balancing protective immunity and immunopathogenesis. Int J Biol Macromol. (2025) 284:137836. doi: 10.1016/j.ijbiomac.2024.137836
14. Zhang X, Zhang K, Yan L, Wang P, Zhao F, Hu S. The role of toll-like receptors in immune tolerance induced by Helicobacter pylori infection. Helicobacter. (2023) 28:e13020. doi: 10.1111/hel.13020
15. Mukherjee S, Patra R, Behzadi P, Masotti A, Paolini A, Sarshar M. Toll-like receptor-guided therapeutic intervention of human cancers: molecular and immunological perspectives. Front Immunol. (2023) 14:1244345. doi: 10.3389/fimmu.2023.1244345
16. Hu Y, Liu J, Zhu Y, Lu N. The importance of toll-like receptors in NF-κB signaling pathway activation by Helicobacter pylori infection and the regulators of this response. Helicobacter. (2016) 21:428–40. doi: 10.1111/hel.12292
17. Zhao L, Luo J, Ali M, Spiekerkoetter E, Nicolls M. The human respiratory microbiome: current understandings and future directions. Am J Respir Cell Mol Biol. (2023) 68:245–55. doi: 10.1165/rcmb.2022-0208TR
18. Dodero V, Morré S, Behzadi P. Editorial: gut microbiota and immunity in health and disease: dysbiosis and eubiosis’s effects on the human body. Front Immunol. (2024) 15:1536258. doi: 10.3389/fimmu.2024.1536258
19. Zhou J, Li M, Chen Q, Li X, Chen L, Dong Z, et al. Programmable probiotics modulate inflammation and gut microbiota for inflammatory bowel disease treatment after effective oral delivery. Nat Commun. (2022) 13:3432. doi: 10.1038/s41467-022-31171-0
20. He C, Xie Y, Zhu Y, Zhuang K, Huo L, Yu Y, et al. Probiotics modulate gastrointestinal microbiota after Helicobacter pylori eradication: a multicenter randomized double-blind placebo-controlled trial. Front Immunol. (2022) 13:1033063. doi: 10.3389/fimmu.2022.1033063
21. Bai X, Zhu M, He Y, Wang T, Tian D, Shu J. The impacts of probiotics in eradication therapy of Helicobacter pylori. Arch Microbiol. (2022) 204:692. doi: 10.1007/s00203-022-03314-w
22. Bai T, Xu Z, Xia P, Feng Y, Liu B, Liu H, et al. The short-term efficacy of bifidobacterium quadruple viable tablet in patients with diarrhea-predominant irritable bowel syndrome: potentially mediated by metabolism rather than diversity regulation. Am J Gastroenterol. (2023) 118:1256–67. doi: 10.14309/ajg.0000000000002147
23. Luo M, Xiong L, Zhang L, Xu Q. Efficacy and safety of Bifidobacterium quadruple viable tablets combined with mosapride citrate in the treatment of constipation in China: a systematic review and meta-analysis. BMC Gastroenterol. (2023) 23:245. doi: 10.1186/s12876-023-02884-3
24. Bai T, Zeng H, Long Y, Li X, Sun X, Lan Y, et al. The efficacy of Bifidobacterium quadruple viable tablet in the treatment of diarrhea predominant irritable bowel syndrome: protocol for a randomized, double-blind, placebo-controlled, multicenter trial. Trials. (2020) 21:597. doi: 10.1186/s13063-020-04490-0
25. Chey W. Accurate diagnosis of Helicobacter pylori. 14C-urea breath test. Gastroenterol Clin North Am. (2000) 29:895–902. doi: 10.1016/s0889-8553(05)70157-6
26. Ding Y, Duan M, Han Z, Song X, Zhang F, Wang Z, et al. Bismuth-containing quadruple therapy for Helicobacter pylori eradication: a randomized clinical trial of 10 and 14 days. Dig Dis Sci. (2024) 69:2540–7. doi: 10.1007/s10620-024-08460-3
27. Song K, Lee Y, Fan D, Ge Z, Ji F, Chen M, et al. Healing effects of rebamipide and omeprazole in Helicobacter pylori-positive gastric ulcer patients after eradication therapy: a randomized double-blind, multinational, multi-institutional comparative study. Digestion. (2011) 84:221–9. doi: 10.1159/000329353
28. Katelaris P, Hunt R, Bazzoli F, Cohen H, Fock K, Gemilyan M, et al. Helicobacter pylori world gastroenterology organization global guideline. J Clin Gastroenterol. (2023) 57:111–26. doi: 10.1097/MCG.0000000000001719
29. Muzaheed. Helicobacter pylori oncogenicity: mechanism, prevention, and risk factors. ScientificWorldJournal. (2020) 2020:3018326. doi: 10.1155/2020/3018326
30. FitzGerald R, Smith S. An overview of Helicobacter pylori infection. Methods Mol Biol. (2021) 2283:1–14. doi: 10.1007/978-1-0716-1302-3_1
31. Lee Y, Dore M, Graham D. Diagnosis and treatment of Helicobacter pylori infection. Annu Rev Med. (2022) 73:183–95. doi: 10.1146/annurev-med-042220-020814
32. Zhou L, Lu H, Song Z, Lyu B, Chen Y, Wang J, et al. 2022 Chinese national clinical practice guideline on Helicobacter pylori eradication treatment. Chin Med J. (2022) 135:2899–910. doi: 10.1097/CM9.0000000000002546
33. Gupta A, Shetty S, Mutalik S, Chandrashekar HR, Mathew EM. Treatment of H. pylori infection and gastric ulcer: need for novel Pharmaceutical formulation. Heliyon. (2023) 9:e20406. doi: 10.1016/j.heliyon.2023.e20406
34. Viazis N, Argyriou K, Kotzampassi K, Christodoulou D, Apostolopoulos P, Georgopoulos S, et al. A four-probiotics regimen combined with a standard Helicobacter pylori-eradication treatment reduces side effects and increases eradication rates. Nutrients. (2022) 14:632. doi: 10.3390/nu14030632
35. Liang B, Yuan Y, Peng X, Liu X, Hu X, Xing D. Current and future perspectives for Helicobacter pylori treatment and management: from antibiotics to probiotics. Front Cell Infect Microbiol. (2022) 12:1042070. doi: 10.3389/fcimb.2022.1042070
36. Cho Y, Choi M, Choi S, Lee K, Kim T, Park S, et al. Randomised clinical trial: tegoprazan, a novel potassium-competitive acid blocker, or lansoprazole in the treatment of gastric ulcer. Aliment Pharmacol Ther. (2020) 52:789–97. doi: 10.1111/apt.15865
37. Zhou H, Li Y, Lu W. Treatment effects of bifidobacterium quadruple viable tablets combined with quadruple therapy on Helicobacter Pylori-infected peptic ulcer in children. Clin Pediatr. (2025) 64:64–71. doi: 10.1177/00099228241248717
38. Duan S, Rico K, Merchant J. Gastrin: from physiology to gastrointestinal malignancies. Function. (2022) 3:zqab062. doi: 10.1093/function/zqab062
39. Kitazawa T, Kaiya H. Motilin comparative study: structure, distribution, receptors, and gastrointestinal motility. Front Endocrinol. (2021) 12:700884. doi: 10.3389/fendo.2021.700884
40. Adeniyi A, Bello I, Mukaila T, Monono E, Hammed A. Developing rumen mimicry process for biological ammonia synthesis. Bioprocess Biosyst Eng. (2023) 46:1011–20. doi: 10.1007/s00449-023-02880-7
41. Sánchez-López J, Díaz-Herrera L, Rizo-de la Torre LDC. Pepsinogen I, pepsinogen II, gastrin-17, and Helicobacter pylori serological biomarkers in the diagnosis of precursor lesions of gastric cancer. Arch Med Sci. (2024) 20:1016–21. doi: 10.5114/aoms/189971
42. Moyat M, Velin D. Immune responses to Helicobacter pylori infection. World J Gastroenterol. (2014) 20:5583–93. doi: 10.3748/wjg.v20.i19.5583
43. Sharma V, Sharma P, Singh T. Therapeutic correlation of TLR-4 mediated NF-κB inflammatory pathways in ischemic injuries. Curr Drug Targets. (2024) 25:1027–40. doi: 10.2174/0113894501322228240830063605
44. Rudra D, Chatterjee S, Pal U, Mandal M, Chaudhuri S, Bhunia M, et al. Newly synthesized 3-Indolyl furanoid inhibits matrix Metalloproteinase-9 activity and prevents nonsteroidal anti-inflammatory drug-induced gastric ulceration. J Med Chem. (2023) 66:8917–28. doi: 10.1021/acs.jmedchem.3c00511
45. Li S, Feng W, Chen D, Yu L, Cui H, Li M. Revealing the gastric protective mechanism of Physochlaina physaloides (L) G. Don. with clinical orientation: ppar/nf-κb signalling pathway activation. Phytomedicine. (2025) 141:156642. doi: 10.1016/j.phymed.2025.156642
46. Yang J, Liu S, Zhao Q, Li X, Jiang K. Gut microbiota-related metabolite alpha-linolenic acid mitigates intestinal inflammation induced by oral infection with Toxoplasma gondii. Microbiome. (2023) 11:273. doi: 10.1186/s40168-023-01681-0
47. Cao F, Jin L, Gao Y, Ding Y, Wen H, Qian Z, et al. Artificial-enzymes-armed Bifidobacterium longum probiotics for alleviating intestinal inflammation and microbiota dysbiosis. Nat Nanotechnol. (2023) 18:617–27. doi: 10.1038/s41565-023-01346-x
48. Nabavi-Rad A, Sadeghi A, Asadzadeh Aghdaei H, Yadegar A, Smith S, Zali M. The double-edged sword of probiotic supplementation on gut microbiota structure in Helicobacter pylori management. Gut Microbes. (2022) 14:2108655. doi: 10.1080/19490976.2022.2108655
49. Yan Y, Liu C, Zhao S, Wang X, Wang J, Zhang H, et al. Probiotic Bifidobacterium lactis V9 attenuates hepatic steatosis and inflammation in rats with non-alcoholic fatty liver disease. AMB Express. (2020) 10:101. doi: 10.1186/s13568-020-01038-y
50. Niu Y, Li J, Qian H, Liang C, Shi X, Bu S. Evaluation of efficacy and safety of Lacticaseibacillus rhamnosus LRa05 in the eradication of Helicobacter pylori: a randomized, double-blind, placebo-controlled trial. Front Immunol. (2024) 15:1450414. doi: 10.3389/fimmu.2024.1450414
51. Mazziotta C, Tognon M, Martini F, Torreggiani E, Rotondo J. Probiotics mechanism of action on immune Cells and beneficial effects on human health. Cells. (2023) 12:184. doi: 10.3390/cells12010184
52. Chen L, Xu W, Lee A, He J, Huang B, Zheng W, et al. The impact of Helicobacter pylori infection, eradication therapy and probiotic supplementation on gut microenvironment homeostasis: an open-label, randomized clinical trial. EBioMedicine. (2018) 35:87–96. doi: 10.1016/j.ebiom.2018.08.028
Keywords: gastric ulcer, Helicobacter pylori, quadruple therapy, bifidobacteria quadruple viable bacteria tablets, gastrointestinal hormones, inflammatory factors
Citation: Jiang C, Shi J, Zhuang X, Zhou M and Zhong H (2025) Effects of a combination of bifidobacteria quadruple viable bacteria tablets and quadruple therapy on inflammatory response and Helicobacter pylori eradication rate in patients with Helicobacter pylori positive gastric ulcers. Front. Med. 12:1636039. doi: 10.3389/fmed.2025.1636039
Received: 27 May 2025; Accepted: 15 September 2025;
Published: 29 September 2025.
Edited by:
George Grant, Independent Researcher, Aberdeen, United KingdomReviewed by:
Bowen Yan, Nanjing Forestry University, ChinaDawn Israel, Vanderbilt University, United States
Chengu Niu, Rochester Regional Health, United States
Copyright © 2025 Jiang, Shi, Zhuang, Zhou and Zhong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Haiyan Zhong, WmhvbmdoYWl5YW5fZWR1QG91dGxvb2suY29t
 Chunying Jiang
Chunying Jiang Haiyan Zhong
Haiyan Zhong