- 1Department of Thoracic Surgery, The First Affiliated Hospital of Ningbo University, Ningbo, China
- 2Department of General Surgery, Shanghai Changhai Hospital, Shanghai, China
- 3Department of Radiology, Shanghai Changhai Hospital, Shanghai, China
- 4Department of Radiotherapy and Oncology, Affiliated Kunshan Hospital of Jiangsu University, Kunshan, China
Objective: To evaluate and compare the clinical value of computed tomography (CT) and magnetic resonance imaging (MRI) in the preoperative TNM staging of esophageal carcinoma.
Methods: A total of 1,209 patients with histologically confirmed esophageal cancer who underwent preoperative CT (n = 868) or MRI (n = 341) scanning from January 2014 to December 2024 were retrospectively analyzed. Tumor location, histologic type, and postoperative pathological TNM stage were recorded. CT and MRI findings were compared with pathological results as the standard.
Results: For T-stage, the overall accuracy of CT was 86%, with a sensitivity and specificity of 53 and 89%, respectively. MRI achieved an accuracy of 82%, with sensitivity and specificity of 57 and 93%, respectively. For N-stage, CT showed a sensitivity, specificity, and accuracy of 85, 83, and 84% for mediastinal lymph nodes, and 87, 82, and 83% for abdominal lymph nodes. There was no significant difference in the total accuracy rate of TN staging diagnosis of esophageal cancer between the two groups.
Conclusion: Both CT and MRI demonstrate high diagnostic value in the preoperative TNM stage of esophageal carcinoma. While CT is more economic and easier to operate, MRI offers superior contrast of connective tissues and multiplanar imaging, making it particularly valuable in evaluating T4 stage and assessing tumor invasion of adjacent organs. Limitations exist in early T-stage and N-stage assessments due to difficulty distinguishing mucosal infiltration and differentiating metastatic from non-metastatic lymphadenopathy. Multimodal imaging strategies and tumor-targeted contrast agents may enhance staging precision.
Introduction
Early-stage esophageal cancer patients usually show no obvious symptoms, by the time they are diagnosed, most are already in the late stage, leading to a large variation in prognosis (1). Early-stage esophageal cancer, if treated with radical resection, is expected to achieve long-term survival. In contrast, for some advanced cases, surgery may be futile or even harmful, increasing patient trauma and hastening death (2). Therefore, how to diagnose esophageal cancer early and accurately stage it preoperatively has become a major and difficult task in the field of esophageal surgery. Accurate preoperative staging helps surgeons select appropriate treatment strategies (3).
To allow early-stage esophageal cancer patients to undergo radical surgical treatment, and to avoid unnecessary or palliative surgery and chemoradiotherapy in late-stage patients, many imaging methods have been applied in clinical diagnosis of esophageal cancer, such as CT (computed tomography), MRI (magnetic resonance imaging), EUS (endoscopic ultrasonography), PET (positron emission tomography), thoracoscopy, and laparoscopy (4). Researchers have been conducting studies on the accuracy of these methods in TNM staging of esophageal cancer, but there is still no consensus. Among these, CT and MRI are the most widely used (5). This study compares preoperative CT and MRI findings with intraoperative and postoperative pathological results, identifying their respective advantages and limitations, in order to guide clinical practice.
Methods
Clinical data
This is a retrospective study included patients from January 2014 to December 2024, a total of 1,209 patients with esophageal cancer underwent preoperative TNM staging via CT (868 cases) or MRI (341 cases). All patients underwent postoperative gross pathology examination for TNM staging, which was compared with the preoperative CT and MRI results. Two radiologists jointly examined the CT and MRI images. When there was a dispute, a third senior radiologist would re-examine the images.
CT scanning
CT examinations were performed using a spiral CT scanner (Hispeed CT/i, GE, USA), with a slice thickness and pitch of 5 mm. The scanning range extended from the supraclavicular apices to the upper pole of the adrenal glands, including the entire liver. Contrast-enhanced scans were performed in dual phases, with intravenous injection of 50 mL of iohexol. For lower esophageal lesions, 300 mL of deionized water was administered orally before the scan to distend the stomach.
MRI scanning
MRI was conducted by a 1.5 T superconducting scanner (Gyroscan 5NT, Philips, Netherlands). Multisection spin echo sequences were obtained with TRs of 500, 1800, or 2,500 ms and TEs of 35, 90, or 120 ms. T1-weighted images (T1W) used TR 500 ms and TE 30 ms, while T2-weighted images (T2W) used TR 1500–1800 ms and TE 90–120 ms. Slice thickness was 4–6 mm, with a 1,024 × 1,024 matrix. Coronal, sagittal, and axial views were routinely obtained, with the scan range identical to CT. To ensure gastric distention, patients ingested 0.59 g/kg of Gd-DTPA before scanning.
CT and MRI staging criteria
The T staging criteria were based on the method of AJCC TNM staging. Tumor thickness of 3–5 mm was classified as T1, 5–15 mm as T2, and >15 mm with irregular outer esophageal margins as T3 (6). Involvement of adjacent organs such as the trachea, aorta, or vertebrae was defined as T4. Lymph nodes with short-axis diameters >10 mm were considered abnormal. Figure 1 shows images without lymph node metastasis, cardiac lymph node metastasis, and left gastric lymph node metastasis (Figure 1).
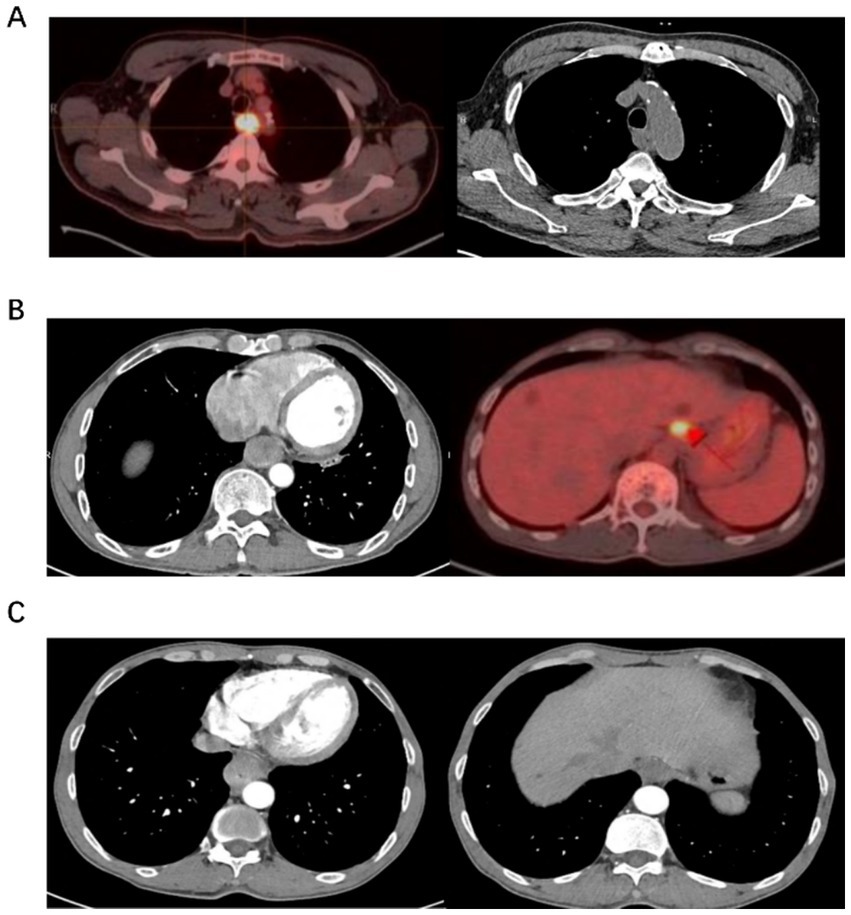
Figure 1. Examples of diagnostic diagram. (A) It shows a PET/CT scan with a bright area indicating activity in the chest, alongside a CT scan of the chest. (B) It displays a CT scan highlighting internal organs and a PET scan with a bright spot in the liver area. (C) It presents two CT scans, one showing the heart and another showing diferent sections of the torso.
Statistical analysis
Postoperative gross pathology results were used as the final standard to calculate the sensitivity, specificity, and accuracy of CT and MRI. Statistical significance was assessed with the t test and chi-square test, with p < 0.05 considered significant.
Results
From January 2014 to December 2024, a total of 1,209 patients with esophageal cancer underwent preoperative TNM staging via CT (868 cases) or MRI (341 cases). There were no differences in gender, age, BMI, tumor height and pathological types between the two groups (Table 1).
Comparison of CT and MRI accuracy in T staging
Among 868 patients who underwent CT, the distribution of preoperative T stages was T1 (57), T2 (114), T3 (340), and T4 (357). Of 57 patients with pathological T1 disease, 14 were overstaged, 13 were not identified, 30 was accurately diagnosed as T1 (53%), and 72 as T2 (63%). Among 340 pathological T3 cases, 12 were understaged as T2, 2 as T1, 13 as T4, and 303 correctly staged (89%). Of the 357 T4 cases, 26 were misdiagnosed, yielding a correctly staging rate of 93%. The overall accuracy of 86% (Table 2).
For MRI (341 patients), T1 (32), T2 (53), T3 (161), T4 (95). Among 32 pathological T1 cases, 7 was missed, and 8 overstaged, with a correct diagnosis rate of 53%. For 53 T2 cases, 8 was understaged, 9 overstaged, 36 correctly staged (66%). Among 161 T3 cases, 11 was understaged, 12 overstaged, and 138 correctly staged (83%). 93 T4 cases were accurately staged. MRI overall accuracy of 82% (Table 2).
Comparison of CT and MRI accuracy in N staging
Among the patients who underwent CT examination before the operation, 597 cases were pathologically confirmed mediastinal metastasis. 383 cases (84%) were diagnosed by CT. Among the 612 cases without lymph node metastasis, 363 cases were correctly diagnosed by CT (88%). There were 346 cases of abdominal lymph node metastasis, and 255 cases (90%) were diagnosed correctly by CT. The accuracy of CT in diagnosing abdominal lymph node metastasis was 90%.
Among the 341 patients who underwent MRI examination before the operation, 141 cases of mediastinal lymph node metastasis and 96 cases of abdominal lymph node metastasis were confirmed by pathology. The accuracy of MRI in diagnosing lymph node metastasis is in the mediastinum and abdomen are 84 and 86%, respectively (Table 3).
Discussion
After the diagnosis of esophageal cancer, it is very important for clinical surgeons to accurately determine the TNM stage of the tumor to choose the individualized treatment plan (7). In recent years, with the popularity of CT and MRI, it has brought many benefits for esophageal surgeons and patients to choose the appropriate treatment mode, but its accuracy in preoperative TN staging of esophageal cancer has been controversial (8).
It can be seen from the results of this study that there is no difference between CT and MRI in preoperative TN staging of esophageal cancer (with postoperative gross pathological results as the gold standard) in terms of sensitivity, specificity and accuracy (x test, p > 0.05). The advantage of CT is that the imaging technology is simple, the parameters are less, and it is easy to be grasped by clinicians. The price is relatively low. Enhanced CT scan can also show the relationship between the mass and the large blood vessels of the heart, making up for the lack of traditional X-ray examination, and the vast majority of cases can be diagnosed with X-ray and CT results. However, for some special cases, conventional CT scan has limitations, so MRI examination is necessary. MRI has the advantages of being multi-directional (axial, sagittal, coronal or even oblique), showing tumor components, the relationship between tumor and blood vessels, and the blood vessels within the tumor (9). The disadvantages are that MRI has many imaging parameters, is susceptible to interference by external factors (breathing, heartbeat), and is expensive (10).
A large number of literatures have reported that the accuracy of CT and MRI for preoperative T staging of esophageal cancer ranges from 45 to 73% (11). The results of this study showed that for T1 and T2 tumors, the diagnostic coincidence rate of CT and MRI was not high, especially when T1 tumors were confined to the esophageal mucosa or submucosa, (12) the tumors may only cause changes in the local motor function of the esophagus without changes in the thickness of the esophageal wall, while CT and MRI could only perform static imaging of the esophagus, but could not perform dynamic observation. Therefore, there is a high error rate (13). The low accuracy for T1/T2 staging may due to the atypical imaging features of early-stage lesions. Quint et al. (14) proposed that the normal wall thickness of the esophagus was 3 mm, the anteroposterior diameter was 14 mm, and the left and right diameter was 18 mm when the esophagus collapsed. At present, most domestic and foreign scholars still determine the depth of tumor invasion by observing the thickness of the esophageal wall according to the standard, which is obviously unable to keep up with the development of modern science and technology. How to improve the diagnostic technology of CT and MRI, determine more accurate diagnostic criteria of CT and MRI for esophageal cancer, and achieve early detection of esophageal cancer and screen the depth of tumor invasion still needs to be further discussed by radiologists. Of course, this is one of the main reasons for the current limitations on CT and MRI as alternatives to esophageal barium meal and endoscopy. EUS examination is recommended for patients with suspected early esophageal cancer to improve the accuracy of preoperative staging (15). In the future, we will further combine the EUS with MRI to improve the accuracy.
Although neither CT nor MRI can accurately distinguish each layer of the esophagus and judge the depth of tumor invasion, when esophageal cancer develops to the T3 and T4 stages, CT and MRI can not only show the subtle difference in the density of the tumor and its surrounding tissues, but also display the tumor in multiple directions and carefully distinguish its degree of external invasion in spatial orientation (16). In particular, the accuracy of T4 esophageal cancer was especially high (the T coincidence rate of CT and MRI diagnosis in this group was as high as 92 and 100%, respectively). In addition, CT and MRI can accurately determine whether esophageal cancer has invaded adjacent organs (trachea, bronchus, aorta, pericardium, etc.) and the extent of invasion. Since results demonstrated similar accuracy of CT and MRI, we recommend CT scan for initial screening for its economical and quick characteristic, and MRI for suspected T4 or complex anatomical sites, such as aortic invasion. If cases of suspected T4 stage located at regions with limited medical resources, CT can be the alternative for diagnosis.
CT and MRI have incomparable advantages over traditional X-ray in N staging of esophageal cancer. It is generally believed that pathological enlargement of thoracic lymph nodes >1 cm, subclavian lymph nodes > 0.6 cm, and abdominal lymph nodes > 0.8 cm are pathological enlargement. Due to the high resolution of CT and MRI, the diagnosis rate of mediastinal and abdominal enlarged lymph nodes is relatively high (specificity, sensitivity and diagnostic coincidence rate are >80%), which can provide clinical reference. However, CT and MRI still have high false negative and false positive results. In clinical practice, enlarged lymph nodes are not metastatic lymph nodes, while metastatic lymph nodes are not enlarged. Previous literature reports also confirmed the existence of high false negative and false positive rates in CT and MRI. Therefore, there is still a serious shortage in judging the N stage of esophageal cancer from the size of lymph node findings on CT and MRI alone. The development of tumorophilic contrast imaging is expected to further improve the accuracy of CT and MRI in preoperative TNM staging of esophageal cancer. However, we did not compare the equipment heterogeneity across hospitals. In the future, we will promote the results of this research to other medical centers and conduct a multi-centered prospective study.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the First Affiliated Hospital of NingBo University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements.
Author contributions
WZ: Writing – original draft, Software. JH: Methodology, Writing – review & editing. YC: Writing – original draft, Resources, Writing – review & editing. JL: Writing – original draft, Funding acquisition, Validation. HW: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Domper Arnal, MJ, Ferrández Arenas, Á, and Lanas Arbeloa, Á. Esophageal cancer: risk factors, screening and endoscopic treatment in Western and eastern countries. World J Gastroenterol. (2015) 21:7933–43. doi: 10.3748/wjg.v21.i26.7933
2. Kato, H, and Nakajima, M. Treatments for esophageal cancer: a review. Gen Thorac Cardiovasc Surg. (2013) 61:330–5. doi: 10.1007/s11748-013-0246-0
3. Yang, H, Wang, F, Hallemeier, CL, Lerut, T, and Fu, J. Oesophageal cancer. Lancet. (2024) 404:1991–2005. doi: 10.1016/S0140-6736(24)02226-8
4. Mwachiro, M, and White, R. Management of Esophageal Cancer Treatment in resource-limited settings. Thorac Surg Clin. (2022) 32:397–404. doi: 10.1016/j.thorsurg.2022.04.007
5. Harada, K, Rogers, JE, Iwatsuki, M, Yamashita, K, Baba, H, and Ajani, JA. Recent advances in treating oesophageal cancer. F1000Res. (2020) 9:1189. doi: 10.12688/f1000research.22926.1
6. Yeung, JC, and Elimova, E. Overview of the Management of Esophageal Cancer. Thorac Surg Clin. (2022) 32:xiii-xiv. doi: 10.1016/j.thorsurg.2022.08.001
7. Zhou, N, Rajaram, R, and Hofstetter, WL. Management of locally advanced esophageal cancer. Surg Oncol Clin N Am. (2020) 29:631–46. doi: 10.1016/j.soc.2020.06.003
8. Demarest, CT, and Chang, AC. The landmark series: multimodal therapy for esophageal Cancer. Ann Surg Oncol. (2021) 28:3375–82. doi: 10.1245/s10434-020-09565-5
9. Lin, Z, Cai, W, Hou, W, Chen, Y, Gao, B, Mao, R, et al. CT-guided survival prediction of esophageal Cancer. IEEE J Biomed Health Inform. (2022) 26:2660–9. doi: 10.1109/JBHI.2021.3132173
10. Bruzzi, JF, Munden, RF, Truong, MT, Marom, EM, Sabloff, BS, Gladish, GW, et al. PET/CT of esophageal cancer: its role in clinical management. Radiographics. (2007) 27:1635–52. doi: 10.1148/rg.276065742
11. Lu, J, Sun, XD, Yang, X, Tang, XY, Qin, Q, Zhu, HC, et al. Impact of PET/CT on radiation treatment in patients with esophageal cancer: a systematic review. Crit Rev Oncol Hematol. (2016) 107:128–37. doi: 10.1016/j.critrevonc.2016.08.015
12. Kröger, K, Pepper, NB, Ventura, D, Troschel, FM, Backhaus, P, Rahbar, K, et al. FAPI-PET/CT guided radiotherapy for patients with esophageal cancer. Radiat Oncol. (2025) 20:29. doi: 10.1186/s13014-025-02606-x
13. Karaosmanoğlu, AD, and Blake, MA. Applications of PET-CT in patients with esophageal cancer. Diagn Interv Radiol. (2012) 18:171–82. doi: 10.4261/1305-3825.DIR.4309-11.1
14. Quint, LE, Glazer, GM, and Orringer, MB. Esophageal imaging by MR and CT: study of normal anatomy and neoplasms. Radiology. (1985) 156:727–31. doi: 10.1148/radiology.156.3.4023234
15. Huang, FL, and Yu, SJ. Esophageal cancer: risk factors, genetic association, and treatment. Asian J Surg. (2018) 41:210–5. doi: 10.1016/j.asjsur.2016.10.005
Keywords: esophageal cancer, CT, MR, diagnosis, radiology
Citation: Zhao W, Huang J, Chen Y, Lu J and Hu W (2025) The clinical value of CT and MRI in preoperative TNM staging of esophageal cancer. Front. Med. 12:1637764. doi: 10.3389/fmed.2025.1637764
Edited by:
Yiqun Sun, Fudan University Shanghai Cancer Center, ChinaReviewed by:
Anfu Pan, Affiliated to Zhejiang Chinese Medical University, ChinaJiawei Wu, Jiangsu University, China
Copyright © 2025 Zhao, Huang, Chen, Lu and Hu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jingjing Lu, bWFydmVsX2pAMTYzLmNvbQ==; Wentao Hu, bmluZ2JvaHd0QDE2My5jb20=
†These authors have contributed equally to this work
 Weijun Zhao
Weijun Zhao Jing Huang2†
Jing Huang2† Jingjing Lu
Jingjing Lu Wentao Hu
Wentao Hu