- 1College of Pharmacy, Gansu University of Traditional Chinese Medicine, Lanzhou, China
- 2Pharmaceutical Research Institute of Gansu Academy of Medical Sciences, Lanzhou, China
- 3Gansu Medical College, Pingliang, China
- 4Affiliated Hospital of Gansu University of Traditional Chinese Medicine, Lanzhou, China
In recent years, with the continuous development of social productivity and improvements in living standards, the issue of population aging has intensified. As the elderly are susceptible to cardiovascular diseases, osteoporosis, Alzheimer’s disease, and other conditions, the economic and resource burdens associated with treating, caring for, and supporting this demographic rise with their growing numbers, imposing significant pressure on societal development. In light of these challenges, research on natural products possessing anti-aging properties for treating aging-related chronic diseases has gained prominence. Flavonoids, a class of natural bioactive compounds abundantly found in plants, exhibit diverse pharmacological effects, including antioxidant, anti-inflammatory, and anti-osteoporosis activities. Therefore, studying the anti-aging effects of flavonoids is of significant value. This paper reviews recent research on the anti-aging effects of flavonoids by searching databases including CNKI, PubMed, Web of Science, and Google Scholar. The findings are summarized based on research subjects (C. elegans, animal models, and cells), effects, and mechanisms, to provide references for future in-depth research on the anti-aging effects of flavonoids.
1 Introduction
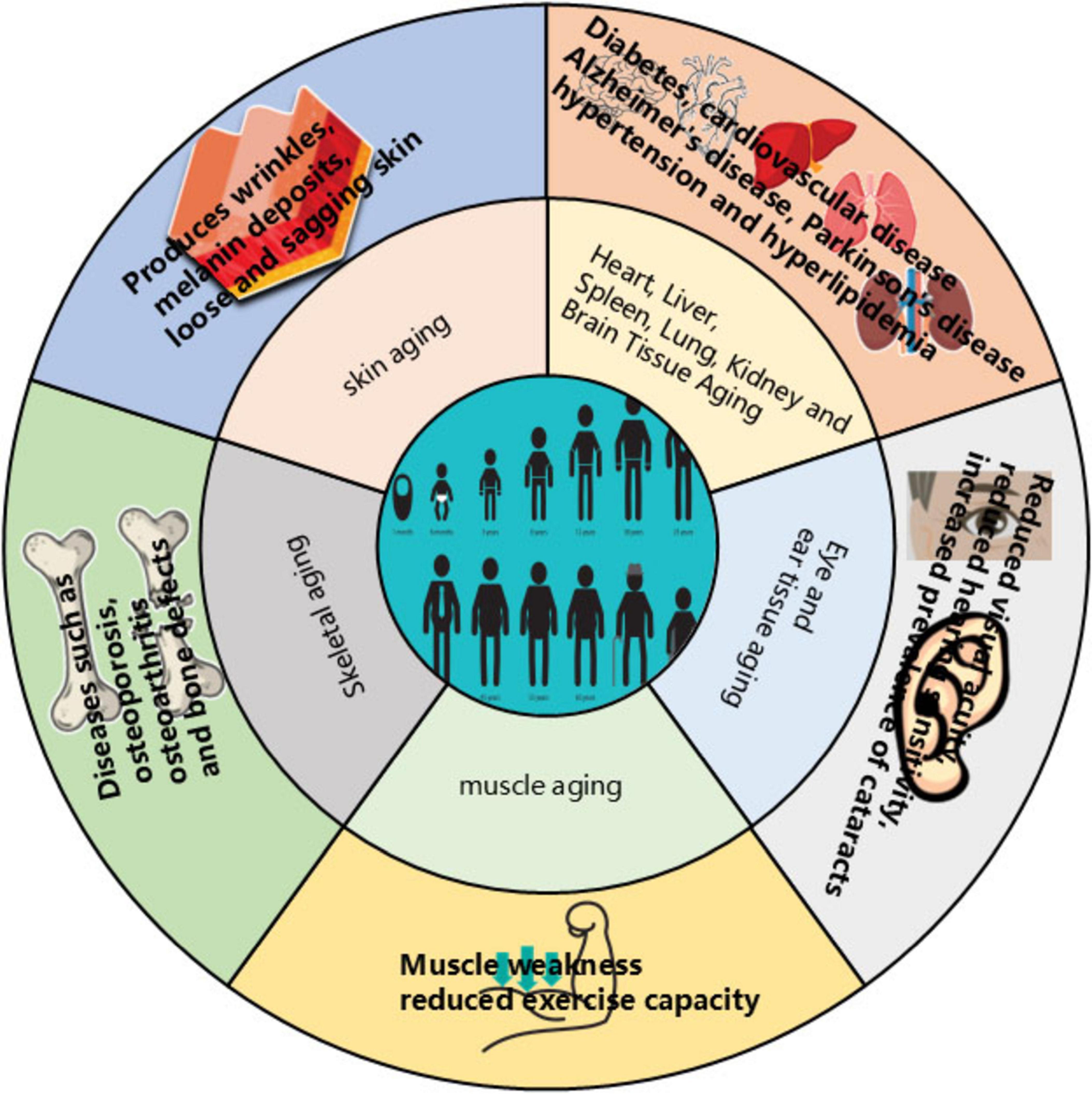
Aging represents an inevitable biological process characterized by a progressive decline in metabolic function and deterioration of organs and tissues. Due to this systemic degradation, the elderly exhibit heightened susceptibility to cardiovascular and neurological disorders, including hypertension, hyperlipidemia, diabetes, and Alzheimer’s disease. Visible manifestations of aging encompass sensory decline (hearing loss, impaired vision), integumentary changes (skin sagging, increased wrinkles), diminished physical fitness and endurance, and gradual muscle weakening. Additionally, elderly women face an increased risk of osteoporosis (1, 2) (see Figure 1). Currently, population aging is intensifying globally, notably in China and numerous other nations (3), Driven
by persistently declining birth rates and steadily increasing life expectancy, the global population aged 65 and above is projected to reach 1.1 billion by 2035, constituting 13% of the world’s population. This demographic shift will significantly reduce the proportion of younger individuals. Developed countries are already confronting substantial labor shortages, while developing nations face unprecedented pressures and challenges from accelerated aging. The impact permeates multiple societal domains, including employment, healthcare, economic systems, and service provision. Consequently, ensuring comfortable aging while alleviating the economic burden on younger generations and mitigating associated socioeconomic and healthcare pressures has emerged as an urgent societal imperative. Given the strong association between aging and chronic diseases such as cardiovascular disorders and Alzheimer’s disease, elucidating the aging process is fundamental to understanding these conditions. Therefore, the development of safe, effective, environmentally sustainable, and economically viable anti-aging therapeutics and medical products is critically important.
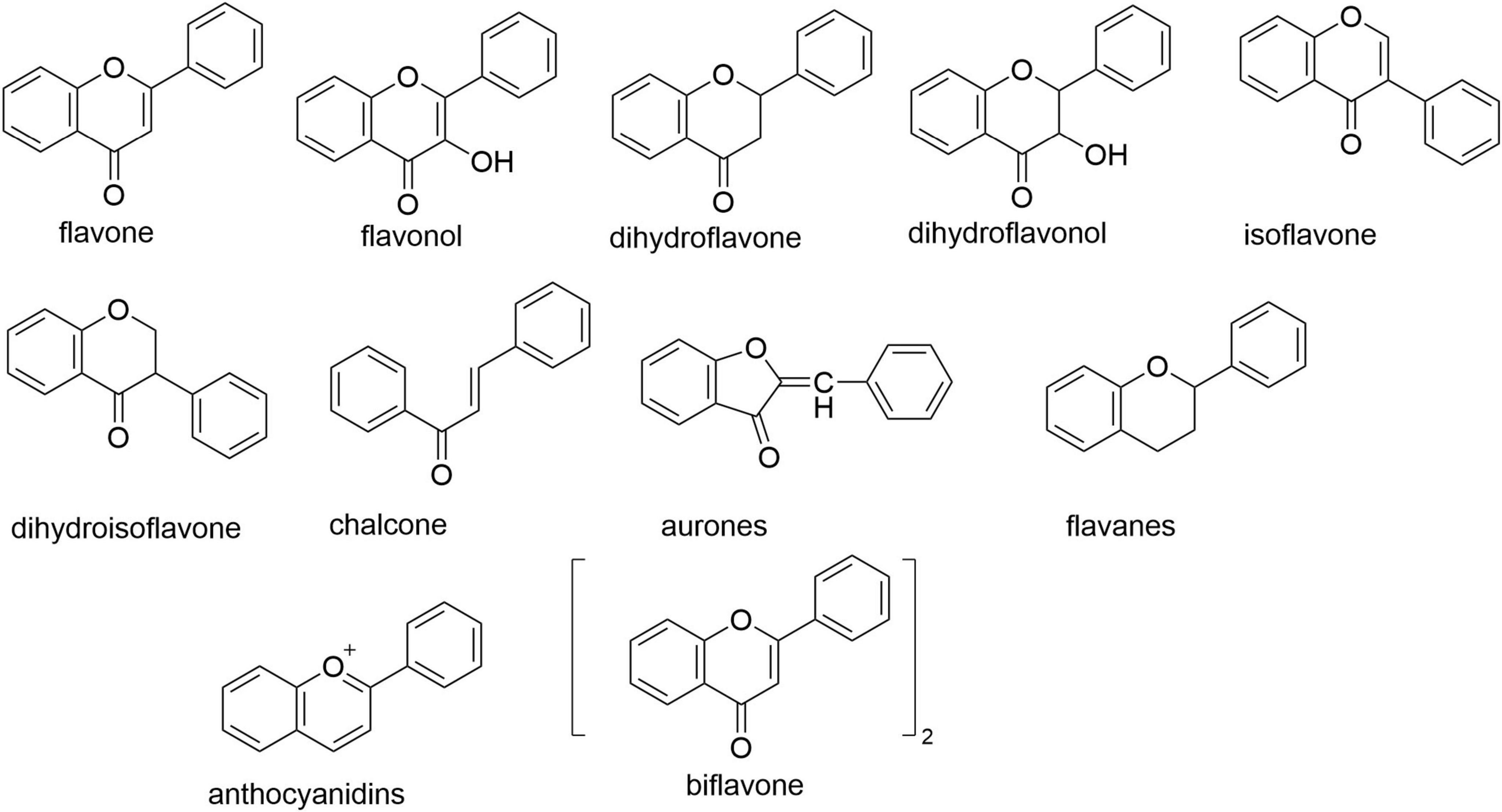
Flavonoids comprise a class of compounds characterized by a 2-phenylchromone (flavone) core structure. They are primarily classified into core subclasses: flavanones, flavanols, dihydroflavonoids, dihydroflavonols, isoflavonoids, dihydroisoflavonoids, chalcones, aurones, flavones, and anthocyanins (see Figure 2 for parent nucleus structures). Functionally, flavonoids exist predominantly as glycosides (bound to sugar moieties), with a minority occurring as free aglycones. As ubiquitous plant secondary metabolites, flavonoids are distributed throughout stems, leaves, flowers, fruits, and seeds. Their presence contributes to the vibrant pigmentation of many common foods and botanicals, including citrus fruits, legumes, and medicinal herbs such as liquorice, black goji berries, and ginger (4). This widespread occurrence underscores their status as green, safe, inexpensive, and readily accessible compounds. Modern pharmacological research confirms flavonoids possess significant antioxidant activity—a property critically linked to aging and age-related metabolic diseases (5–7), Accumulating evidence further demonstrates their therapeutic potential against diabetes, neurodegenerative disorders, cardiovascular diseases, and skeletal system pathologies (8–11). Consequently, global research efforts increasingly employ extraction, separation, purification, and synthesis techniques to obtain flavonoid fractions or specific compounds for anti-aging investigations (12), highlighting their substantial promise in this field.
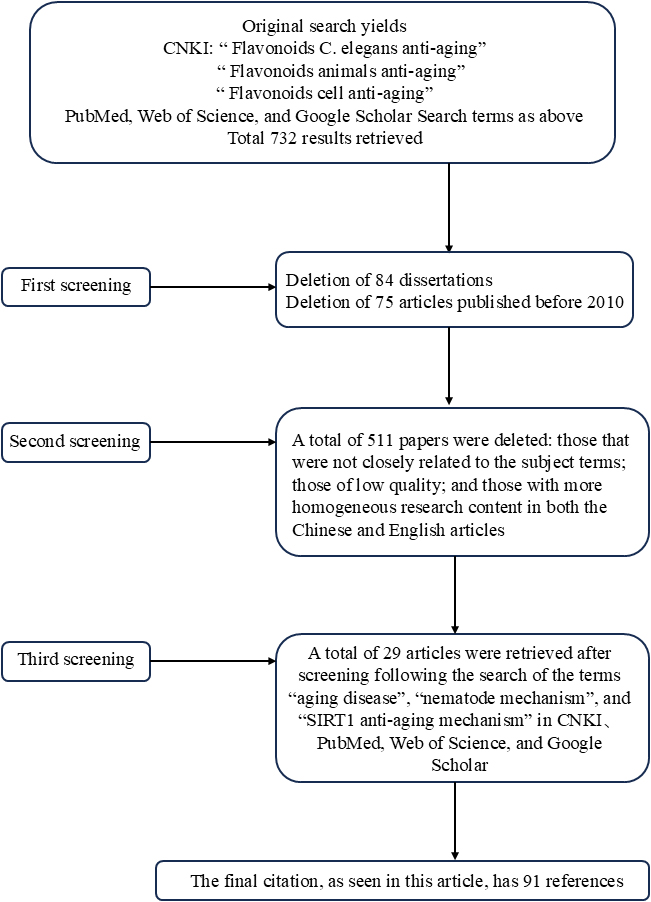
While previous reviews have outlined flavonoids’ anti-aging bioactivities, these often emphasize specific roles and mechanisms (13, 14), with relatively limited systematic organization. Recent advances in model organism applications have accelerated research in this domain. This review synthesizes findings from the most extensively studied anti-aging models—C. elegans, rodent species (rats/mice), and cellular systems—to systematically summarize flavonoid effects and mechanisms. We searched the CNKI, PubMed, Web of Science and Google Scholar databases using relevant keywords, as shown in Figure 3. This structured approach aims to provide future investigators with conceptual frameworks, stimulate novel research directions, and enhance contextual understanding of existing data.
2 Studies on the anti-aging effects of flavonoids based on different models
2.1 Anti-aging effects of flavonoids in Caenorhabditis elegans models
As eukaryotic organisms, C. elegans share skin, muscular, digestive, nervous, and reproductive system homology with higher organisms, along with significant genetic conservation—including with humans. Their short lifespan and high reproductive capacity have established C. elegans as a fundamental model for longevity research since the 1980s (15). making it a cornerstone organism in aging studies. The anti-aging effects and mechanistic pathways of flavonoids in this C. elegans model are summarized in Table 1.
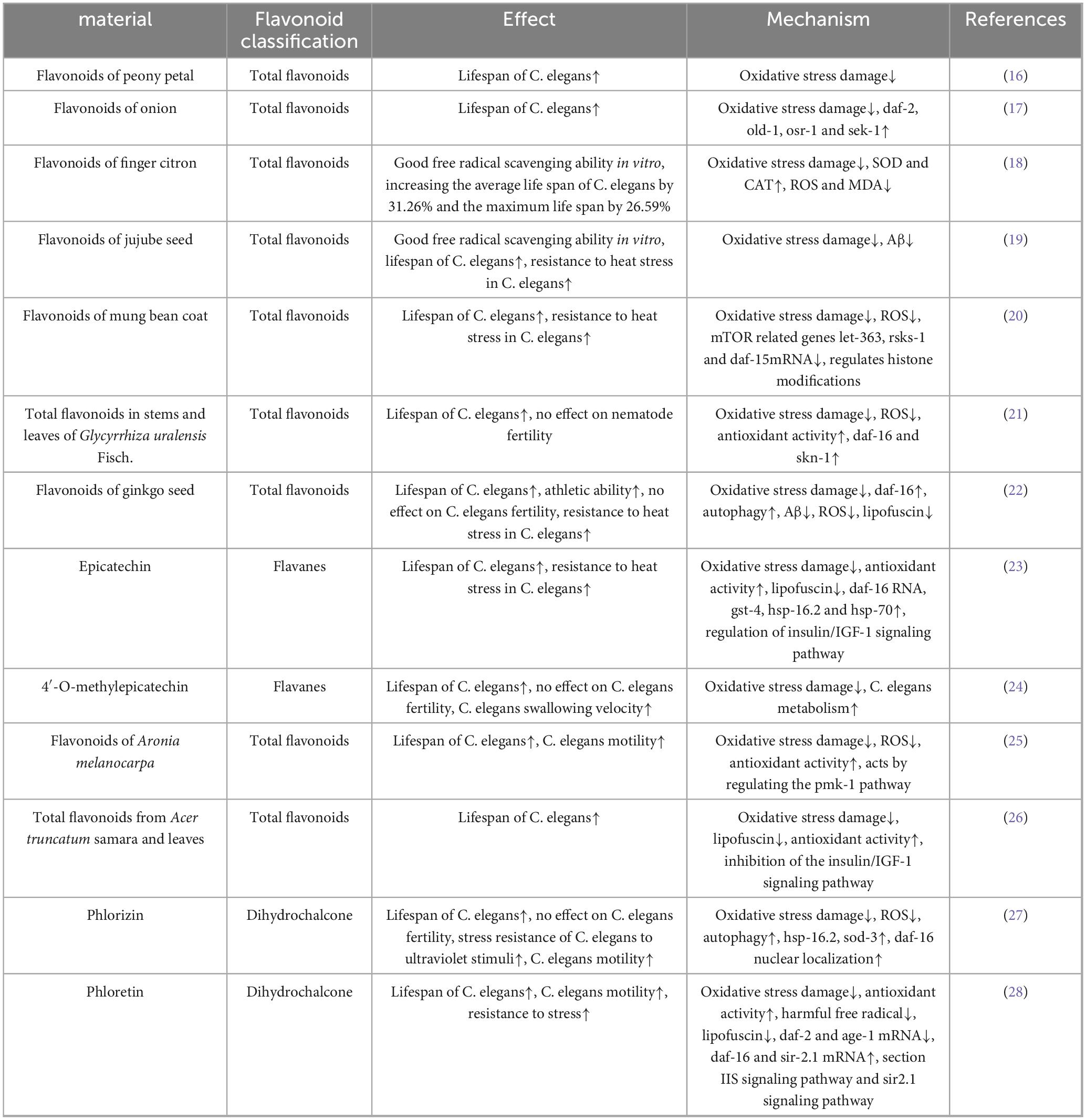
Table 1. Anti aging effects and mechanisms of natural flavonoids in models of Caenorhabditis elegans.
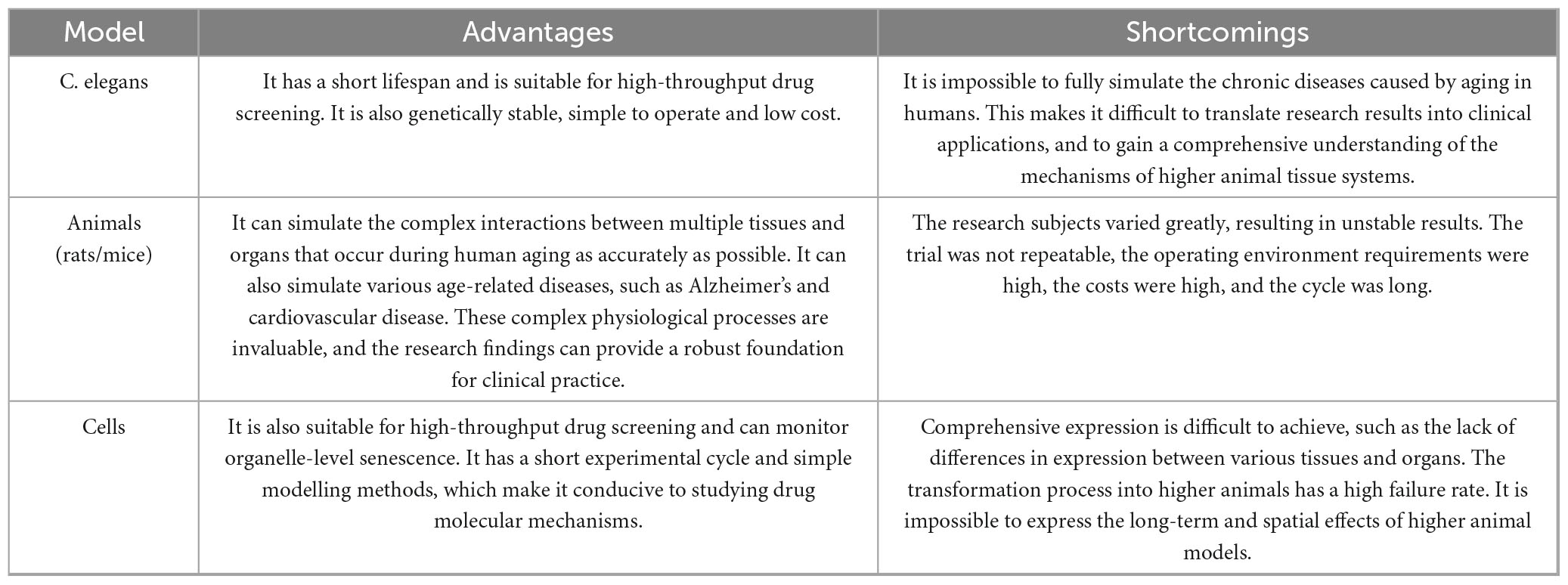
Compared to other model organisms, such as higher animals and cells, Caenorhabditis elegans offers advantages including genetic stability, a simple individual structure, a short life cycle, a large population size and low operational costs. It has unique applications in the study of aging and genetic diseases. However, it has limitations in terms of aging phenotypes when compared to higher animals, making it difficult to simulate human skin aging or neurodegenerative disease phenotypes. There are also fundamental differences between C. elegans and higher organisms in terms of physiology and living environments. Therefore, while it is highly promising for the initial screening of flavonoid compounds for anti-aging effects, further validation through animal and cell models is necessary for in-depth research.
2.2 Anti-aging effects of flavonoids in animal models
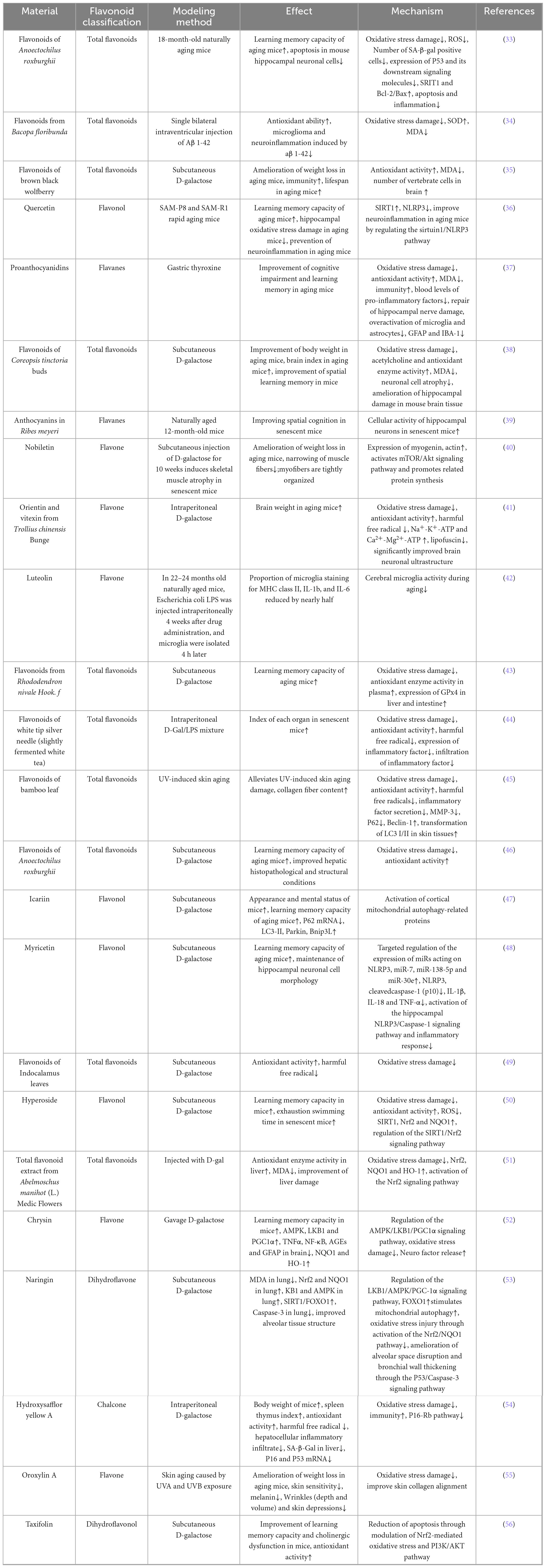
The D-galactose-induced subacute aging model is widely employed in animal studies. High-dose D-galactose administration generates excessive reactive oxygen species (ROS), inducing oxidative stress that exacerbates mitochondrial dysfunction, apoptosis, and tissue damage (29–31). Owing to its procedural simplicity and minimal adverse effects, this model has gained prominence in aging research (32). Flavonoid compounds tested in animal models, along with their specific actions and mechanisms, are detailed in Table 2.
2.3 Anti-aging effects of flavonoids in cellular models
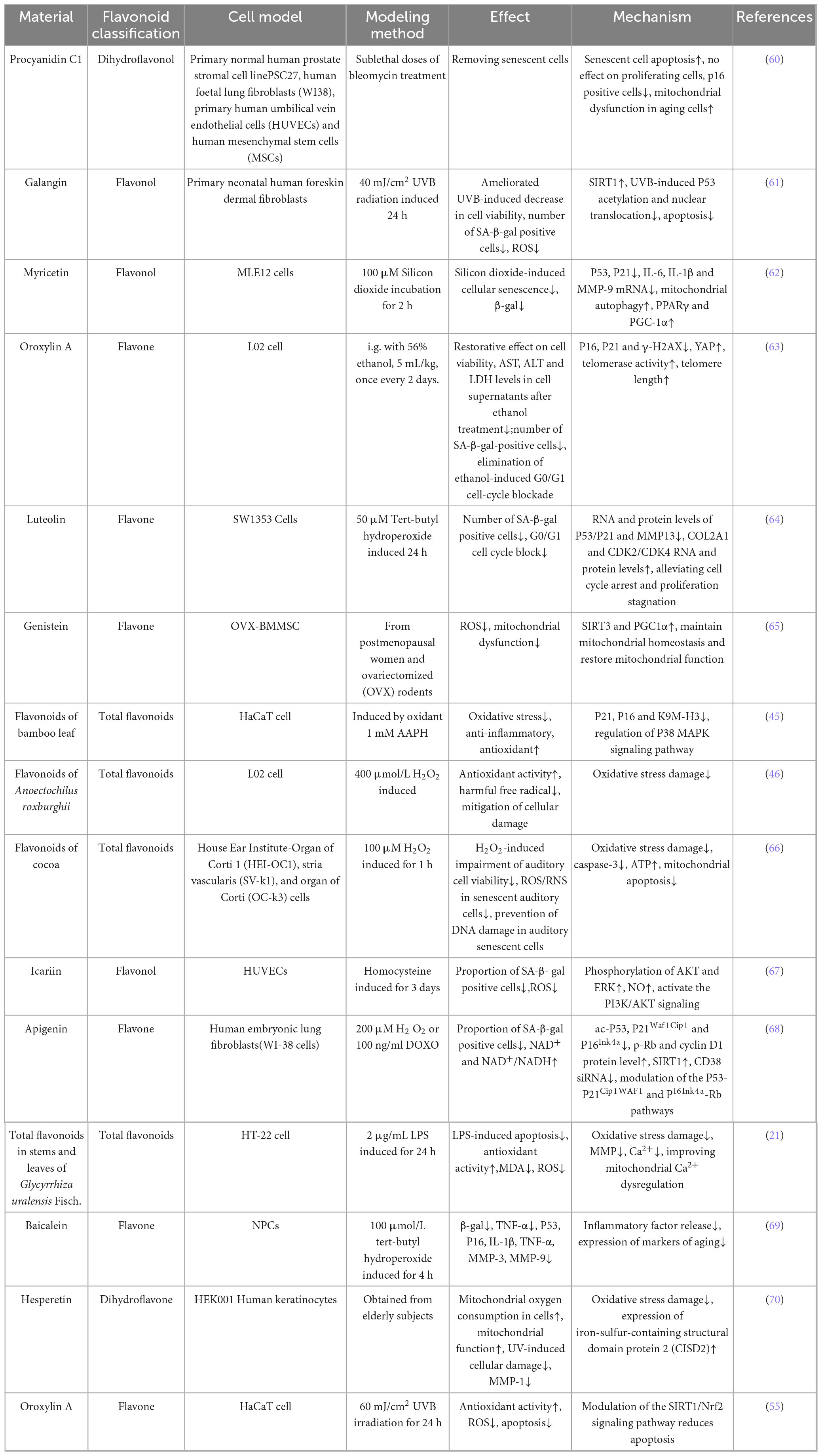
As the fundamental functional unit of human biology, cells represent condensed microcosms of organismal physiology. Cellular senescence triggers senescence-associated secretory phenotype (SASP) expression in critical tissues including heart, liver, and kidneys (57–59). Growing interest in natural anti-aging compounds has expanded research at the cellular level (1). The availability of diverse cellular models and the feasibility of molecular-level mechanistic investigations make this system ideal for anti-aging studies. Flavonoid mechanisms and functional outcomes in cellular models are cataloged in Table 3.
Our summary shows that flavonoids exhibit significant anti-aging activity in C. elegans, animal and cellular aging models. The advantages and disadvantages of each model are summarized in Table 4.
2.4 Other models and roles
Alternative model systems further demonstrate flavonoids’ anti-aging potential. Pu et al. (71) utilized network pharmacology to predict anti-aging mechanisms in Lantana camara, identifying quercetin, apigenin, baicalein, kaempferol, naringenin, and lignans as key components that modulate aging- and tumor-related pathways. Li et al. (72) reported potent in vitro antioxidant activity in Ginkgo biloba seed kernel extracts, suggesting anti-aging effects requiring further validation. In yeast models, sacred lotus stamen flavonoids extended the chronological lifespan of Saccharomyces cerevisiae DBY746 by enhancing mitochondrial function, antioxidant enzymes, and NADH/ATP production (73). Concurrently, Guo et al. (74) found neohesperidin prolonged stationary-phase survival in S. cerevisiae BY4742 following nutrient depletion, though its ROS-scavenging capacity was limited and mechanisms remain uncharacterized. Complementing these findings, Lycium ruthenicum (black wolfberry) flavonoid extract significantly extended Drosophila melanogaster lifespan by alleviating H2O2-induced oxidative damage (35).
3 Study on the mechanism of anti-aging effects of natural flavonoids
3.1 Free radical scavenging mechanisms
Human physiological processes inherently generate oxidative reactions. An imbalance between oxidation and antioxidant defenses induces oxidative stress, producing harmful free radicals that damage cellular components. Chronic oxidative stress causes cumulative DNA, protein, and tissue damage, accelerating aging and disease pathogenesis (75). Most flavonoids counteract aging through antioxidant mechanisms - reducing oxidative stress and enhancing antioxidant capacity across model systems including C. elegans, cellular models, and D-galactose-induced animal models. This aligns with Harman’s Free Radical Theory of Aging (1956), which posits that age-related accumulation of free radicals drives DNA damage, metabolic dysregulation, and degenerative conditions including neurodegeneration and cancer (76–78). As illustrated in Figure 4, flavonoids reduce oxidative stress-induced damage by enhancing the activity of antioxidant enzymes, such as SOD and GSH. They do this by eliminating excess ROS and H2O2, which are harmful free radicals, and by regulating redox balance to exert anti-aging effects.
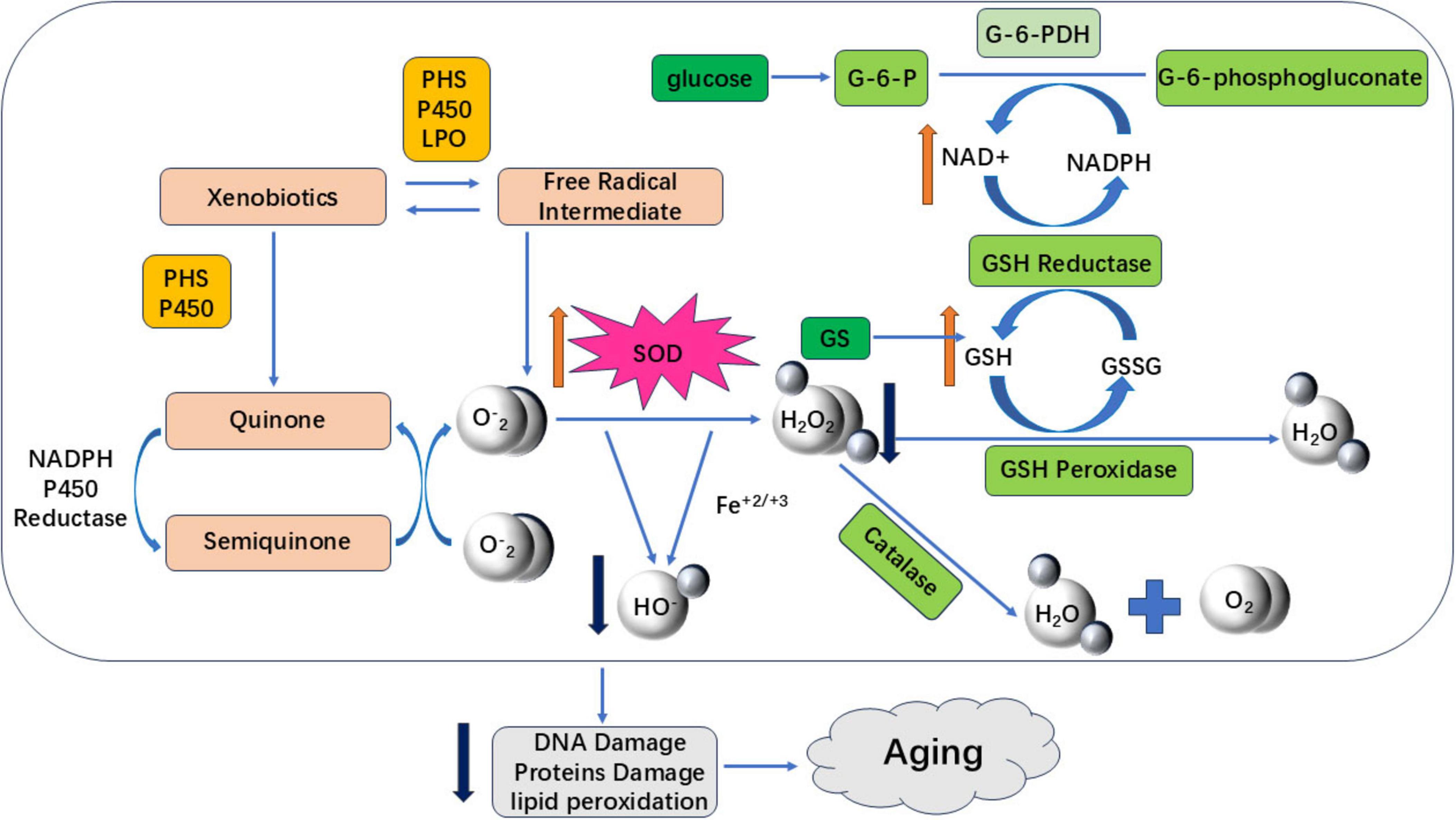
Figure 4. Aging induced by oxidative stress. The red upward arrow in the figure indicates an increase in antioxidant enzyme activity, a decrease in ROS and harmful free radical accumulation, and a delay in aging; The dark blue downward arrow indicates a reduction in harmful free radical content or oxidative stress damage, delaying aging.
3.2 Nutrient-sensing pathway regulation
Beyond oxidative stress mitigation, flavonoids extend lifespan in C. elegans primarily through insulin/insulin-like signaling (IIS) pathway modulation (Figure 5) (79). This regulatory pathway is similar to those found in higher organisms. In Caenorhabditis elegans, under nutrient-rich conditions, insulin-like growth factor ligands bind to receptors. This triggers a series of protein kinase cascades, resulting in the phosphorylation and modification of DAF-16 by AKT-1 and AKT-2. DAF-16 that has been phosphorylated by AKT cannot enter the nucleus and is inactivated as a transcription factor. However, in DAF-2 or AGE-1 mutant C. elegans, this cascade is not activated and unphosphorylated DAF-16 is transported into the nucleus. There, it regulates the expression of downstream genes, thereby extending lifespan. The key regulatory factors DAF-2 and DAF-16 control the expression of downstream genes during the dauer larval stage. Flavonoids can enhance their anti-aging functions. Key regulators DAF-2 and DAF-16 control downstream effectors during the dauer larval stage (80, 81), flavonoids enhance their anti-aging functions by increasing the expression of daf-2 and daf-16 (82). While these genes respond to multiple factors (Figure 5), further research is needed to elucidate: (1) their downstream regulatory mechanisms, and (2) their role in nutrient-sensing mediated lipid/glucose metabolism.
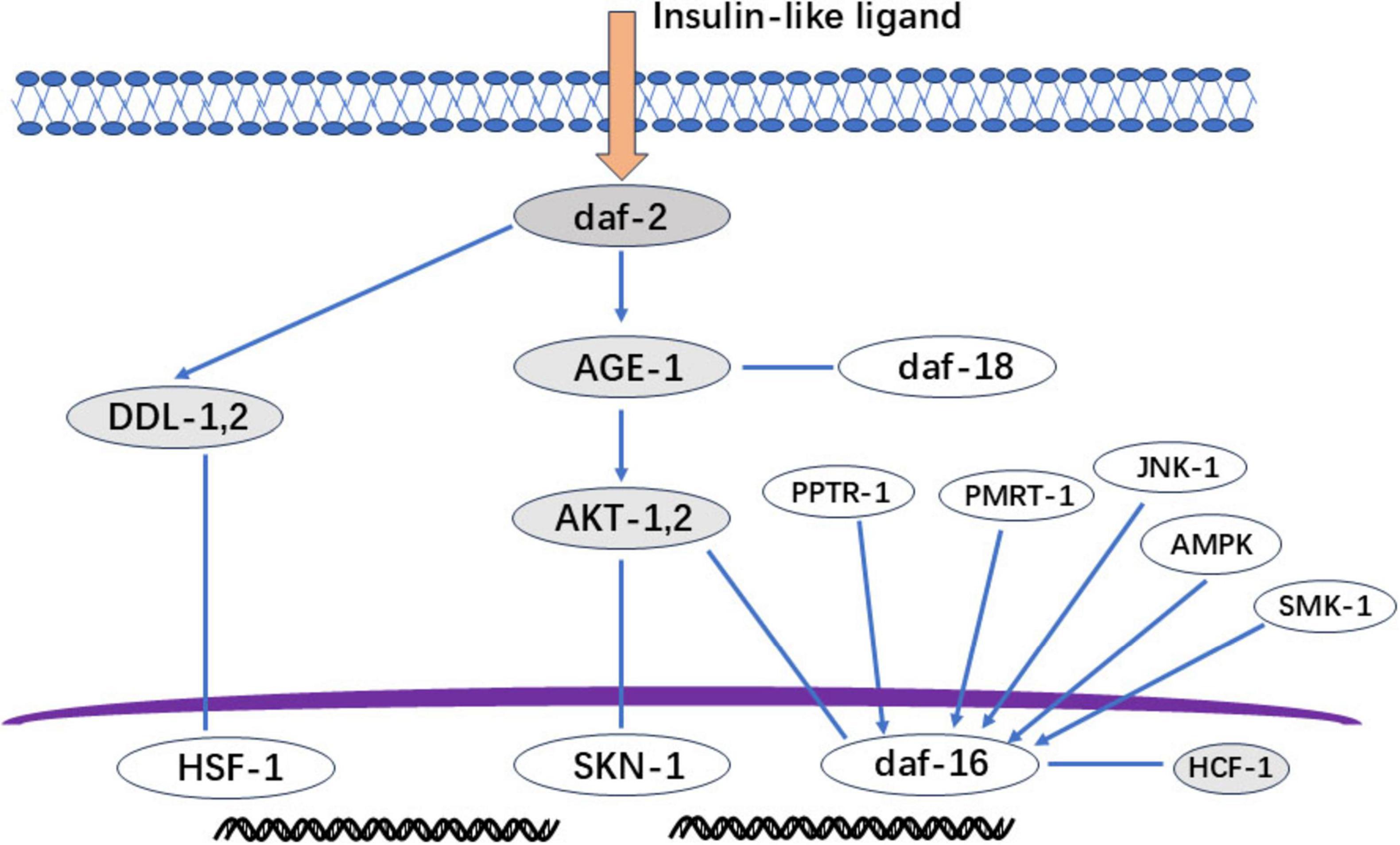
Figure 5. IIS signal regulation diagram. Gray section: The deletion mutation of daf-2 (insulin-like growth factor receptor) can cause long-term nematode aging and death; Age-1 (3-Phosphatidylinositol Kinase): Mutation prolongs lifespan; AKT-1 and AKT-2 (protein kinase): daf-16 not phosphorylated by AKT will be transported to the nucleus to regulate the expression of downstream genes and play a role in prolonging life; DDL-1, 2: The heat shock factor HSF-1 forms a trimeric DHIC with two other proteins, DDL-1 and DDL-2, but HSF-1 needs to dissociate from DDL1,2 to form a monomer for activation activity; HCF-1: A protein highly homologous to host cell factor 1 in C. elegans, but HCF-1 interacts with daf-16 to prevent daf-16 from binding to the promoter of downstream genes regulating aging. Therefore, knocking down HCF-1 can stimulate daf-16 to exert anti-aging effects.
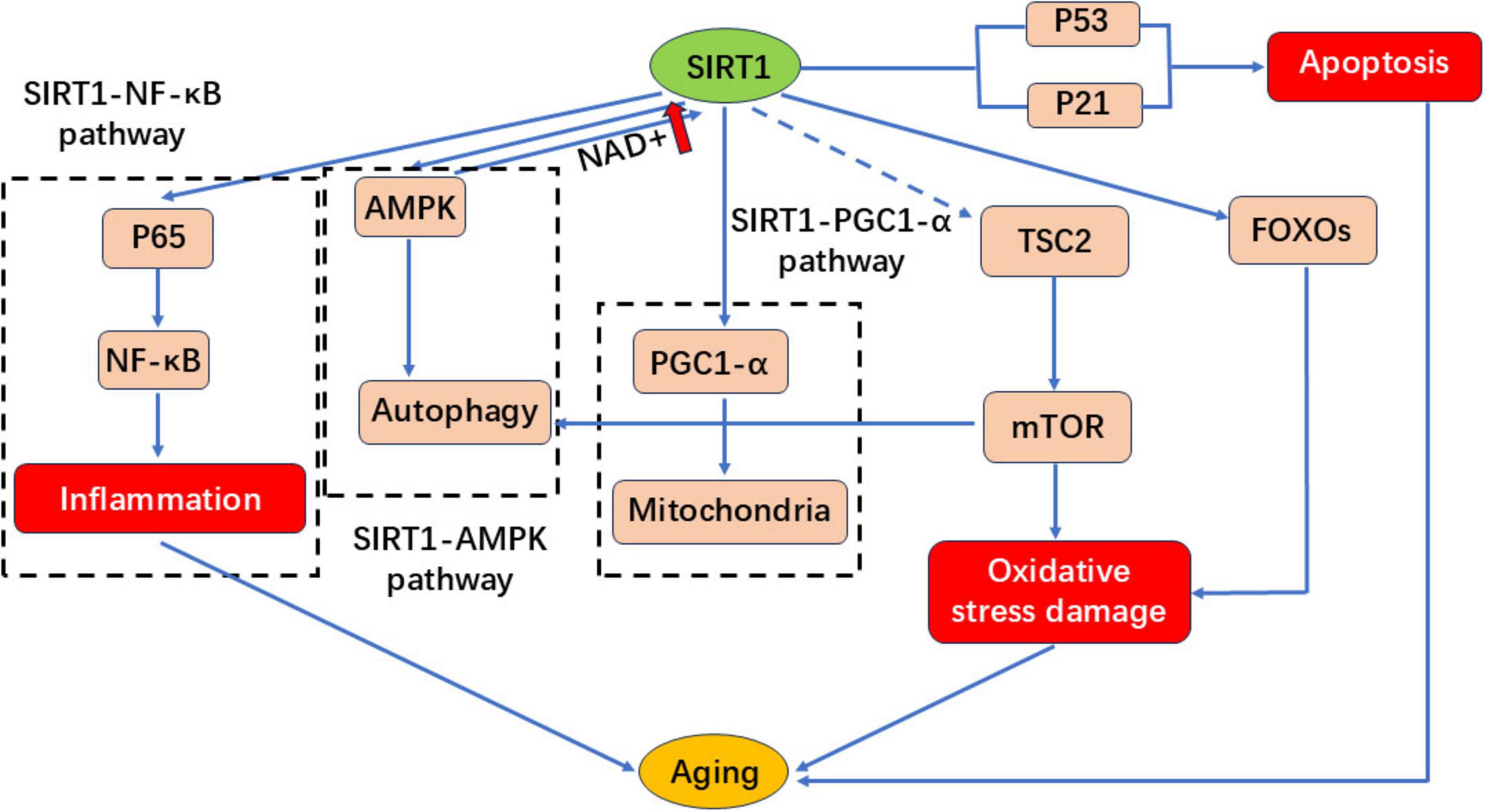
3.3 Sirtuin pathway activation
Sirtuins (NAD+-dependent deacylases) sense metabolic cues, modulate mitochondrial function, exert neuroprotection, and mitigate age-related pathologies (83). SIRT1 activation has emerged as a promising therapeutic strategy for delaying aging and improving metabolic health (84–86). As depicted in Figure 6, flavonoids combat cellular senescence by enhancing SIRT1 expression and activity (87). In the SIRT1-NF-κB signaling pathway, P65 acetylation enhances NF-κB activity, triggering inflammatory responses and leading to aging. However, SIRT1 can inhibit NF-κB activity by mediating P65 deacetylation, thereby reducing inflammation and delaying the aging process. In the SIRT1-AMPK signaling pathway, AMP-activated protein kinase (AMPK) plays a key role in cellular energy metabolism and survival. AMPK activation can enhance autophagy and prolong lifespan. SIRT1 interacts with AMPK and can enhance its own activity by increasing NAD+ levels. In the SIRT1-PGC1α signaling pathway, peroxisome proliferator-activated receptor coactivator-1α (PGC-1α) is the primary transcriptional coactivator responsible for regulating mitochondrial function and maintaining mitochondrial homeostasis. SIRT1 regulates mitochondrial function and metabolic balance by increasing mitochondrial biosynthesis and oxygen consumption through the deacetylation of PGC1α, thereby delaying aging. It has been shown that inhibiting mTOR activity can prolong the lifespan of various organisms. SIRT1 delays aging by blocking the mTOR pathway and activating autophagy. SIRT1 can also interact with tuberous sclerosis protein 2 (TSC2), an upstream inhibitor of TORC1 that negatively regulates mTOR signaling in a TSC2-dependent manner. P53 activation is involved in cell apoptosis, cell cycle arrest and aging. SIRT1 leads to the deacetylation of P53, thereby inhibiting DNA damage and stress-induced cell aging. P21 is a P53 target, and reducing the expression of both P53 and P21 can delay aging. FOXOs are a family of proteins that act as sensors in the insulin signaling pathway and are a type of transporter protein. They are involved in oxidative stress, DNA damage repair, autophagy and cell cycle arrest. FOXOs bind to SIRT1 to regulate cellular aging. In mammalian cells, SIRT1 reduces oxidative stress damage by regulating FOXOs.
3.4 Other mechanism of action
Flavonoids combat aging through multifaceted mechanisms beyond oxidative stress mitigation and nutrient-sensing pathway modulation. Aging hallmarks—including genomic instability, telomere attrition, epigenetic alterations, proteostasis collapse, impaired autophagy, dysregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, and chronic inflammation (88)-are counteracted by flavonoids via apoptosis regulation, suppression of inflammatory factor release, enhanced autophagic flux, and telomerase activation. Critically, flavonoids exhibit pleiotropic regulation, with single compounds concurrently engaging mTOR, IIS, and AMPK pathways. For instance, mTOR signaling modulates MAPK expression and senescence-associated secretory phenotypes (SASP), integrating these pathways into a coordinated anti-aging network (89–91). While flavonoids demonstrably ameliorate aging phenotypes across cellular, tissue, and organismal levels, their comprehensive mechanistic landscape remains an active research frontier.
4 Discussion
The continuous growth of the global elderly population imposes substantial pressure and poses significant challenges for society, the economy, and service industries. Developing natural and effective anti-aging drugs and health products represents a promising approach to mitigate this issue. This paper reviews the anti-aging effects of flavonoid compounds—a subject of considerable research interest. By synthesizing available data, we summarize the roles of flavonoids in aging across model organisms (e.g., Caenorhabditis elegans), animal models, and cellular systems. From both in vivo and in vitro perspectives, this work elucidates the value and potential of flavonoid compounds in anti-aging research.
In summary, future research on flavonoid anti-aging effects requires optimization in several areas. First, isolating and identifying individual compounds remains time-consuming; most current studies focus on total flavonoids, with limited reports on single constituents. Subsequent studies should employ advanced chromatographic techniques for efficient compound separation and analysis to better understand their chemical profiles and anti-aging properties. Second, current models primarily utilize C. elegans, mice, and cells, while organisms like Drosophila and yeast are underutilized—likely due to higher costs and specialized expertise requirements for the latter. Future efforts should increase investment, implementing a three-tiered “model organisms-animals-cells” strategy combined with in vivo/in vitro approaches for comprehensive validation. Third, most animal aging models rely on D-galactose injection, whereas naturally aged or genetic defect models are scarce due to long experimental cycles, multifactorial instability, and high costs. More cost-effective, operationally simple modeling methods are needed to enhance scientific rigor. Regarding mechanisms, current research emphasizes the free radical theory targeting oxidative stress reduction. However, as oxidative stress is not the sole driver of aging, future studies should adopt integrated strategies—combining genomics, proteomics, and multi-level (cellular/tissue) analyses—to identify novel targets and signaling pathways, thereby establishing a holistic understanding of anti-aging mechanisms.
Author contributions
W-yL: Writing – review & editing, Writing – original draft. LZ: Writing – original draft. X-wX: Funding acquisition, Writing – review & editing. X-fS: Project administration, Verification, Writing – review & editing. Q-hM: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Gansu Provincial Science and Technology Major Project (22ZD6FA021-4), Gansu Province Chinese Medicine Major Scientific Research Project (GZKZ-2020-5), and the Natural Science Foundation of Gansu Province (23JRRA1802).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
A β, amyloid β-protein; AMPK, Adenosine 5’-monophosphate (AMP)-activated protein kinase; AGEs, advanced glycation end products; Bcl-2, B-cell lymphoma-2; BMMSCs, Bone marrow mesenchymal stem cells; Caspase-3, cysteinyl aspartate specific proteinase-3; Gal, galactose; GPx4, Glutathione Peroxidase 4; GFAP, glial fibrillary acidic protein; HO-1, heme oxygenase-1; HUVECs, human umbilical vein endothelial cells; LPS, Lipopolysaccharide; LC3 I/II, Microtubule-associated protein 1 light chain 3; LKB1, Liver kinase B1; MMP-3, matrix metalloproteinase-3; MDA, Malondialdehyde; MAO-B, monoamine oxidase; Nrf2, nuclearfactorerythroid-2-relatedfactor2; NQO1, NAD(P)H: quinone oxidoreductase 1; NF- κ B, nuclear factor kappa-light-chain-enhancer of activated B; NPMSCs, nucleus pulposus mesenchymal stem cells; NPCs, Neural progenitor cells; PGC1 α, Peroxisome proliferator-activated receptor y coactivator 1 α; ROS, reactive oxygen species; SIRT1, Sirtuin 1; SA- β-Gal, Senescence-associated β-galactosidase; TNF- α, tumor necrosis factor- α; TORC1, target of rapamycin complex 1.
References
1. Kritsilis M, Rizou S, Koutsoudaki PN, Evangelou K, Gorgoulis VG, Papadopoulos D. Ageing, cellular senescence and neurodegenerative disease. Int J Mol Sci. (2018) 19:1–37. doi: 10.3390/ijms19102937
2. Partridge L, Deelen J, Slagboom P. Facing up to the global challenges of ageing. Nature. (2018) 561:45–56. doi: 10.1038/s41586-018-0457-8
3. Bao J, Zhou L, Liu G, Tang J, Lu X, Cheng C, et al. Current state of care for the elderly in China in the context of an aging population. Biosci Trends. (2022) 16:107–18. doi: 10.5582/bst.2022.01068
4. Shen N, Wang T, Gan Q, Liu S, Wang L, Jin B. Plant flavonoids: classification, distribution, biosynthesis, and antioxidant activity. Food Chem. (2022) 383:132531. doi: 10.1016/j.foodchem.2022.132531
5. Catarino M, Alves-Silva J, Pereira O, Cardoso S. Antioxidant capacities of flavones and benefits in oxidative-stress related diseases. Curr Top Med Chem. (2015) 15: 105–19. doi: 10.2174/1568026615666141209144506
6. Jomova K, Raptova R, Alomar S, Alwasel S, Nepovimova E, Kuca K, et al. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: chronic diseases and aging. Arch Toxicol. (2023) 97:2499–574. doi: 10.1007/s00204-023-03562-9
7. Dias M, Pinto D, Silva A. Plant flavonoids: chemical characteristics and biological activity. Molecules. (2021) 26:1–16. doi: 10.3390/molecules26175377
8. Yi X, Dong M, Guo N, Tian J, Lei P, Wang S, et al. Flavonoids improve type 2 diabetes mellitus and its complications: a review. Front Nutr. (2023) 10:1192131. doi: 10.3389/fnut.2023.1192131
9. Bo S, Chang S, Chen Y, Sheng Z, Jiang Y, Yang B. The structure characteristics, biosynthesis and health benefits of naturally occurring rare flavonoids. Crit Rev Food Sci Nutr. (2022) 64:2490–512. doi: 10.1080/10408398.2022.2124396
10. Hasan S, Khatri N, Rahman Z, Menezes A, Martini J, Shehjar F, et al. Neuroprotective potential of flavonoids in brain disorders. Brain Sci. (2023) 13:1–16. doi: 10.3390/brainsci13091258
11. Bellavia D, Dimarco E, Costa V, Carina V, De Luca A, Raimondi L, et al. Flavonoids in bone erosive diseases: perspectives in osteoporosis treatment. Trends Endocrinol Metab. (2021) 32:76–94. doi: 10.1016/j.tem.2020.11.007
12. Liga S, Paul C, Péter F. Flavonoids: overview of biosynthesis, biological activity, and current extraction techniques. Plants. (2023) 12:1–25. doi: 10.3390/plants12142732
13. Fan X, Fan Z, Yang Z, Huang T, Tong Y, Yang D, et al. Flavonoids-natural gifts to promote health and longevity. Int J Mol Sci. (2022) 23:1–17. doi: 10.3390/ijms23042176
14. Păcularu-Burada B, Cîrîc A, Begea M. Anti-aging effects of flavonoids from plant extracts. Foods. (2024) 13:1–30. doi: 10.3390/foods13152441
15. Johnson T, Wood W. Genetic analysis of life-span in Caenorhabditis elegans. Proc Natl Acad Sci U S A. (1982) 79:6603–7. doi: 10.1073/pnas.79.21.6603
16. Wang S, Xue J, Zhang S, Zheng S, Xue Y, Xu D, et al. Composition of peony petal fatty acids and flavonoids and their effect on Caenorhabditis elegans lifespan. Plant Physiol Biochem. (2020) 155:1–12. doi: 10.1016/j.plaphy.2020.06.029
17. Xue Y, Ahiko T, Miyakawa T, Amino H, Hu F, Furihata K, et al. Isolation and Caenorhabditis elegans lifespan assay of flavonoids from onion. J Agric Food Chem. (2011) 59:5927–34. doi: 10.1021/jf104798n
18. Luo X, Wang J, Chen H, Zhou A, Song M, Zhong Q, et al. Identification of flavoanoids from finger citron and evaluation on their antioxidative and antiaging activities. Front Nutr. (2020) 7:584900. doi: 10.3389/fnut.2020.584900
19. Yang T, Fang L, Lin T, Li J, Zhang Y, Zhou A, et al. Ultrasonicated sour Jujube seed flavonoids extract exerts ameliorative antioxidant capacity and reduces Aβ-induced toxicity in Caenorhabditis elegans. J Ethnopharmacol. (2019) 239:111886. doi: 10.1016/j.jep.2019.111886
20. Tao M, Li R, Xu T, Zhang Z, Wu T, Pan S, et al. Flavonoids from the mung bean coat promote longevity and fitness in Caenorhabditis elegans. Food Funct. (2021) 12:8196–207. doi: 10.1039/d1fo01322j
21. Chen W, He L, Pei H, Li J, Zhao Y, Zong Y, et al. Neuroprotective effect of total flavonoids in stems and leaves of Glycyrrhiza uralensis Fisch. on oxidative stress in HT-22 cells and Caenorhabditis elegans. Aging. (2023) 15:5290–303. doi: 10.18632/aging.204627
22. Shen N, Zeng W, Leng F, Lu J, Lu Z, Cui J, et al. Ginkgo seed extract promotes longevity and stress resistance of Caenorhabditis elegans. Food Funct. (2021) 12:12395–406. doi: 10.1039/d1fo02823e
23. Ayuda-Durán B, González-Manzano S, Miranda-Vizuete A, Dueñas M, Santos-Buelga C, González-Paramás A. Epicatechin modulates stress-resistance in C. elegans via insulin/IGF-1 signaling pathway. PLoS One. (2019) 14:e0199483. doi: 10.1371/journal.pone.0199483
24. Surco-Laos F, Duenas M, González-Manzano S, Cabello J, Santos-Buelga C, Gonzalez-Paramás A. Influence of catechins and their methylated metabolites on lifespan and resistance to oxidative and thermal stress of Caenorhabditis elegans and epicatechin uptake. Food Res Int. (2012) 46:514–21. doi: 10.1016/j.foodres.2011.10.014
25. Zhang H, Zhu Z, Wei W, Liu Z, Zhou H, Gong Y, et al. Aronia melanocarpa extract extends the lifespan and health-span of Caenorhabditis elegans via mitogen-activated protein kinase 1. Food Funct. (2024) 15:11020–35. doi: 10.1039/d4fo02479f
26. Zhang X, Wang D, Li X, Miao Y, Gao J, Zhang Q. A new utilization of total flavonoids from Acer truncatum samara and leaves: anti-aging and metabolic regulation. Ind Crops Prod. (2023) 2023:1–11. doi: 10.1016/j.indcrop.2023.117207
27. Park S, Park S. Anti-Oxidant and anti-aging effects of phlorizin are mediated by DAF-16-induced stress response and autophagy in Caenorhabditis elegans. Antioxidants. (2022) 11:1–16. doi: 10.3390/antiox11101996
28. Zhang X, Zhao J, Han Y, Diao CR, Wang H. Effect of Phloretin on lifespan of Caenorhabditis elegans. Food Sci. (2021) 42:187–96. doi: 10.7506/spkx1002-6630-20191206-075
29. Rajendran P, Al-Saeedi F, Ammar R, Abdallah B, Ali E, Al Abdulsalam N, et al. Geraniol attenuates oxidative stress and neuroinflammation-mediated cognitive impairment in D galactose-induced mouse aging model. Aging. (2024) 16:5000–26. doi: 10.18632/aging.205677
30. Zhang Y, Ma R, Deng Q, Wang W, Cao C, Yu C, et al. S-adenosylmethionine improves cognitive impairment in D-galactose-induced brain aging by inhibiting oxidative stress and neuroinflammation. J Chem Neuroanat. (2023) 128:102232. doi: 10.1016/j.jchemneu.2023.102232
31. Yang Y, Lu S, Liang Y, Tu X, Zeng X, Wang L, et al. Recombinant protein LPxT-GYLEQ attenuates cognitive impairment by ameliorating oxidative stress in D-galactose-induced aging mice model. Food Biosci. (2024) 62:105079. doi: 10.1016/j.fbio.2024.105079
32. Hong J, Zhang Y, Yan S, Li X, Tian X, Cao H, et al. Research progress of the D-galactose-induced aging mouse model. Chin J Comp Med. (2023) 33:136–42. doi: 10.3969/J.issn.1671-7856.2023.03.018
33. Zeng Z, Chen C, SiTu Y, Shen Z, Chen Y, Zhang Z, et al. Anoectochilus roxburghii flavonoids extract ameliorated the memory decline and reduced neuron apoptosis via modulating SIRT1 signaling pathway in senescent mice. J Ethnopharmacol. (2022) 296:115361. doi: 10.1016/j.jep.2022.115361
34. Oyeleke M, Owoyele B. Saponins and flavonoids from Bacopa floribunda plant extract exhibit antioxidant and anti-inflammatory effects on amyloid beta 1-42-induced Alzheimer’s disease in BALB/c mice. J Ethnopharmacol. (2022) 288:114997. doi: 10.1016/j.jep.2022.114997
35. Zhang J, Liu X, Pan J, Zhao Q, Li Y, Gao W, et al. Anti-aging effect of brown black wolfberry on Drosophila melanogaster and D-galactose-induced aging mice. J Funct Foods. (2020) 65:103724. doi: 10.1016/j.jff.2019.103724
36. Li H, Chen F, Yang W, Qiao H, Zhang S. Quercetin improves cognitive disorder in aging mice by inhibiting NLRP3 inflammasome activation. Food Funct. (2021) 12:717–25. doi: 10.1039/d0fo01900c
37. Yuan C, Ren H, Hu K, Chen L, Yue K, He K, et al. Effect of proanthocyanidins on cognitive improvement in thyroxin-induced aging mice. Food Funct. (2025) 16:207–18. doi: 10.1039/d4fo03987d
38. He X, Tian Y, Lei L, Zhi Q, Zhao J, Ming J. Protective effects of Coreopsis tinctoria buds extract against cognitive impairment and brain aging induced by D-galactose. J Funct Foods. (2020) 73:104089. doi: 10.1016/j.jff.2020.104089
39. Gao J, Wu Y, He D, Zhu X, Li H, Liu H, et al. Anti-aging effects of Ribes meyeri anthocyanins on neural stem cells and aging mice. Aging. (2020) 12:17738–53. doi: 10.18632/aging.103955
40. Wang H, Zhang Y, Qu T, Sang X, Li Y, Ren F, et al. Nobiletin improves D-galactose-induced aging mice skeletal muscle atrophy by regulating protein homeostasis. Nutrients. (2023) 15:1–12. doi: 10.3390/nu15081801
41. An F, Yang G, Tian J, Wang S. Antioxidant effects of the orientin and vitexin in Trollius chinensis Bunge in D-galactose-aged mice. Neural Regen Res. (2012) 7:2565–75. doi: 10.3969/j.issn.1673-5374.2012.33.001
42. Burton M, Rytych J, Amin R, Johnson R. Dietary luteolin reduces proinflammatory microglia in the brain of senescent mice. Rejuvenation Res. (2016) 19:286–92. doi: 10.1089/rej.2015.1708
43. Guo X, Dong Z, Li Q, Wan D, Zhong J, Dongzhi D, et al. Flavonoids from Rhododendron nivale Hook. f delay aging via modulation of gut microbiota and glutathione metabolism. Phytomedicine. (2022) 104:154270. doi: 10.1016/j.phymed.2022.154270
44. Li C, He J, Yang Y, Gou Y, Wang Z, Chen H, et al. White tip silver needle (slightly fermented white tea) flavonoids help prevent aging via antioxidative and anti-inflammatory effects. Drug Des Devel Ther. (2021) 15:1441–57. doi: 10.2147/DDDT.S304885
45. Gu Y, Xue F, Xiao H, Chen L, Zhang Y. Bamboo leaf flavonoids suppress oxidative stress-induced senescence of HaCaT cells and UVB-induced photoaging of mice through p38 MAPK and autophagy signaling. Nutrients. (2022) 14:1–19. doi: 10.3390/nu14040793
46. Wang L, Chen Q, Zhuang S, Wen Y, Cheng W, Zeng Z, et al. Effect of Anoectochilus roxburghii flavonoids extract on H2O2 - Induced oxidative stress in LO2 cells and D-gal induced aging mice model. J Ethnopharmacol. (2020) 254:112670. doi: 10.1016/j.jep.2020.112670
47. Zhou Y, Liu J, Chen G. Effects of icariin on cortical mitochondrial autophagy in mice with D-galactose-induced brain aging model. Chung-hua Chung I Yao Tsa Chih. (2019) 34:5674–8.
48. Zhang X, Xia S, Chen X. Improvement of cognitive impairment by myricetin in aging mice. Acta Nutr Sin. (2024) 46:365–71. doi: 10.13325/j.cnki.acta.nutr.sin.2024.04.008
49. Jin S, Yin Y. In vivo antioxidant activity of total flavonoids from indocalamus leaves in aging mice caused by D-galactose. Food Chem Toxicol. (2012) 50:3814–8. doi: 10.1016/j.fct.2012.07.046
50. Gao J, Niu S, Li K, Li D, Chen N, Li H. Antioxidant effects of hyperoside in aging mice induced by D-galactose. Pharmacol Clin Chin Mater Med. (2017) 33:57–9. doi: 10.13412/j.cnki.zyyl.2017.01.016
51. Qiu Y, Ai P, Song J, Liu C, Li Z. Total flavonoid extract from abelmoschus manihot (L.) medic flowers attenuates d-galactose-induced oxidative stress in mouse liver through the Nrf2 pathway. J Med Food. (2017) 20:557–67. doi: 10.1089/jmf.2016.3870
52. Salama A, Elgohary R. Influence of chrysin on D-galactose induced-aging in mice: up regulation of AMP kinase/liver kinase B1/peroxisome proliferator-activated receptor-γ coactivator 1-α signaling pathway. Fundam Clin Pharmacol. (2023) 37:947–59. doi: 10.1111/fcp.12895
53. Salama A, Yassen N, Mansour H. Naringin protects mice from D-galactose-induced lung aging and mitochondrial dysfunction: implication of SIRT1 pathways. Life Sci. (2023) 324:121471. doi: 10.1016/j.lfs.2023.121471
54. Min F, Sun H, Wang B, Ahmad N, Guo H, Gao H, et al. Hepatoprotective effects of hydroxysafflor yellow A in D-galactose-treated aging mice. Eur J Pharmacol. (2020) 881:173214. doi: 10.1016/j.ejphar.2020.173214
55. Liu T, Zhu S, Yang Y, Qin W, Wang Z, Zhao Z, et al. Oroxylin A ameliorates ultraviolet radiation-induced premature skin aging by regulating oxidative stress via the Sirt1 pathway. Biomed Pharmacother. (2024) 171:116110. doi: 10.1016/j.biopha.2023.116110
56. Liu X, Zhao Y, Zhu H, Wu M, Zheng Y, Yang M, et al. Taxifolin retards the D-galactose-induced aging process through inhibiting Nrf2-mediated oxidative stress and regulating the gut microbiota in mice. Food Funct. (2021) 12:12142–58. doi: 10.1039/d1fo01349a
57. Lucas V, Cavadas C, Aveleira C. Cellular senescence: from mechanisms to current biomarkers and senotherapies. Pharmacol Rev. (2023) 75:675–713. doi: 10.1124/pharmrev.122.000622
58. Marino F, Scalise M, Salerno N, Salerno L, Molinaro C, Cappetta D, et al. Diabetes-induced cellular senescence and senescence-associated secretory phenotype impair cardiac regeneration and function independently of age. Diabetes. (2022) 71:1081–98. doi: 10.2337/db21-0536
59. Kumari R, Jat P. Mechanisms of cellular senescence: cell cycle arrest and senescence associated secretory phenotype. Front Cell Dev Biol. (2021) 9:645593. doi: 10.3389/fcell.2021.645593
60. Xu Q, Fu Q, Li Z, Liu H, Wang Y, Lin X, et al. The flavonoid procyanidin C1 has senotherapeutic activity and increases lifespan in mice. Nat Metab. (2021) 3:1706–26. doi: 10.1038/s42255-021-00491-8
61. Wen S, Ng S, Chiu Y, Tai P, Chen T, Chen C, et al. Enhanced SIRT1 activity by galangin mitigates UVB-induced senescence in dermal fibroblasts via p53 acetylation regulation and activation. J Agric Food Chem. (2024) 72:23286–94. doi: 10.1021/acs.jafc.4c05945
62. Xie W, Deng L, Zhang X, Huang X, Ding J, Liu W, et al. Myricetin alleviates silica-mediated lung fibrosis via PPARγ-PGC-1α loop and suppressing mitochondrial senescence in epithelial cells. J Agric Food Chem. (2024) 72:27737–49. doi: 10.1021/acs.jafc.4c04887
63. Jin H, Lian N, Bian M, Zhang C, Chen X, Shao J, et al. Oroxylin A inhibits ethanol-induced hepatocyte senescence via YAP pathway. Cell Prolif. (2018) 51:e12431. doi: 10.1111/cpr.12431
64. Long L, Tang X, Wang Y, Gu J, Xiong J, Luo H, et al. Network pharmacology and experimental validation to elucidate the pharmacological mechanisms of luteolin against chondrocyte senescence. Comb Chem High Throughput Screen. (2025) 28:291–305. doi: 10.2174/0113862073273675231114112804
65. Li M, Yu Y, Xue K, Li J, Son G, Wang J, et al. Genistein mitigates senescence of bone marrow mesenchymal stem cells via ERRα-mediated mitochondrial biogenesis and mitophagy in ovariectomized rats. Redox Biol. (2023) 61:102649. doi: 10.1016/j.redox.2023.102649
66. Del Mar Rivas-Chacón L, Yanes-Díaz J, de Lucas B, Riestra-Ayora JI, Madrid-García R, Sanz-Fernández R, et al. Preventive effect of cocoa flavonoids via suppression of oxidative stress-induced apoptosis in auditory senescent cells. Antioxidants. (2022) 11:1–21. doi: 10.3390/antiox11081450
67. Xiao-Hong D, Chang-Qin X, Jian-Hua H, Wen-Jiang Z, Bing S. Icariin delays homocysteine-induced endothelial cellular senescence involving activation of the PI3K/AKT-eNOS signaling pathway. Pharm Biol. (2013) 51:433–40. doi: 10.3109/13880209.2012.738332
68. Li B, Zhu R, Lim S, Seo J, Choi B. Apigenin alleviates oxidative stress-induced cellular senescence via modulation of the SIRT1-NAD[Formula: see text]-CD38 axis. Am J Chin Med. (2021) 49:1235–50. doi: 10.1142/S0192415X21500592
69. Can Y, He J, Shang Q, Chen G, Chen H, Zhang X, et al. Baicalein protects mouse nucleus pulposus cells from TBHP-induced senescence. Chin J Comp Med. (2023) 33:61–8. doi: 10.3969/j.issn.1671-7856.2023.05.008
70. Shen Z, Chang C, Yeh C, Lu C, Hung H, Wang T, et al. Hesperetin activates CISD2 to attenuate senescence in human keratinocytes from an older person and rejuvenates naturally aged skin in mice. J Biomed Sci. (2024) 31:1–20. doi: 10.1186/s12929-024-01005-w
71. Pu Y, Su C, Zhu Y, Xiao Q, Zhang T, Li H, et al. Study on the anti-aging mechanism of Erigeron breviscapus based on network pharmacology and molecular docking. Nat Prod Res Dev. (2021) 33:1758–68. doi: 10.16333/j.1001-6880.2021.10.017
72. Li J, Hao Q, Zhao S, Zhang R. Ultrasonic extraction and anti-aging effect of total flavonoids from white fruit (Hibiscus esculentus). Chin J Gerontol. (2015) 35:3252–4. doi: 10.3969/j.issn.1005-9202.2015.12.029
73. Tungmunnithum D, Drouet S, Hano C. Flavonoids from sacred lotus stamen extract slows chronological aging in yeast model by reducing oxidative stress and maintaining cellular metabolism. Cells. (2022) 11:1–15. doi: 10.3390/cells11040599
74. Guo C, Zhang H, Guan X, Zhou Z. The anti-aging potential of neohesperidin and its synergistic effects with other citrus flavonoids in extending chronological lifespan of saccharomyces cerevisiae BY4742. Molecules. (2019) 24:4093. doi: 10.3390/molecules24224093
75. Akki R, Siracusa R, Cordaro M, Remigante A, Morabito R, Errami M, et al. Adaptation to oxidative stress at cellular and tissue level. Arch Physiol Biochem. (2019) 128:521–31. doi: 10.1080/13813455.2019.1702059
76. Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. (2002) 11:298–300. doi: 10.1093/geronj/11.3.298
77. Harman D, Heidrick M, Eddy D. Free radical theory of aging: effect of free-radical-reaction inhibitors on the immune response. J Am Geriatr Soc. (1977) 25:400–7. doi: 10.1111/j.1532-5415.1977.tb00674.x
78. Harman D. Free-radical theory of aging. Increasing the functional life span. Ann N Y Acad Sci. (1994) 717:176–7. doi: 10.1111/j.1749-6632.1994.tb12069.x
79. Chen D. The nutrients-regulated insulin/IGF-1 and TOR pathways play an important role in C. elegans aging. Prog Biochem Biophys. (2014) 41: 305–12. doi: 10.3724/SP.J.1206.2014.00019
80. Henderson S, Bonafè M, Johnson T. daf-16 protects the nematode Caenorhabditis elegans during food deprivation. J Gerontol A Biol Sci Med Sci. (2006) 61:444–60. doi: 10.1093/gerona/61.5.444
81. Lin K, Hsin H, Libina N, Kenyon C. Regulation of the Caenorhabditis elegans longevity protein DAF-16 by insulin/IGF-1 and germline signaling. Nat Genet. (2001) 28:139–45. doi: 10.1038/88850
82. Albert P, Riddle D. Mutants of Caenorhabditis elegans that form dauer-like larvae. Dev Biol. (1988) 126:270–93. doi: 10.1016/0012-1606(88)90138-8
83. Ji Z, Liu G, Qu J. Mitochondrial sirtuins, metabolism, and aging. J Genet Genomics. (2022) 49:287–98. doi: 10.1016/j.jgg.2021.11.005
84. Tang B. Sirt1 and the mitochondria. Mol Cells. (2016) 39:87–95. doi: 10.14348/molcells.2016.2318
85. Guo X, Economides A, John Mann J, Kousteni S. A neuronal action of sirtuin 1 suppresses bone mass in young and aging mice. J Clin Invest. (2022) 132:1–13. doi: 10.1172/JCI152868
86. Sousa C, Mendes A. Monoterpenes as Sirtuin-1 activators: therapeutic potential in aging and related diseases. Biomolecules. (2022) 12:1–20. doi: 10.3390/biom12070921
87. Chen C, Zhou M, Ge Y, Wang X. SIRT1 and aging related signaling pathways. Mech Ageing Dev. (2020) 187:111215. doi: 10.1016/j.mad.2020.111215
88. López-Otín C, Blasco M, Partridge L, Serrano M, Kroemer G. Hallmarks of aging: an expanding universe. Cell. (2023) 186:243–78. doi: 10.1016/j.cell.2022.11.001
89. Jung G, Lee J, Han S, Woo J, Choi E, Jeon S, et al. Chrysin induces apoptosis via the MAPK pathway and regulates ERK/mTOR-mediated autophagy in MC-3 cells. Int J Mol Sci. (2022) 23:15747. doi: 10.3390/ijms232415747
90. Cui W, Yang X, Chen X, Xiao D, Zhu J, Zhang M, et al. Treating LRRK2-related Parkinson’s disease by inhibiting the mTOR signaling pathway to restore autophagy. Adv Funct Mater. (2021) 31:2105152. doi: 10.1002/adfm.202105152
Keywords: flavonoids, anti-aging effects, different models, antioxidants, mechanism of action, research progress
Citation: Li W-y, Zhang L, Xie X-w, Shi X-f and Ma Q-h (2025) Advancements in research on the anti-aging effects and mechanisms of flavonoids in natural products. Front. Med. 12:1637992. doi: 10.3389/fmed.2025.1637992
Received: 30 May 2025; Accepted: 04 August 2025;
Published: 19 August 2025.
Edited by:
Jie Li, Huazhong University of Science and Technology, ChinaReviewed by:
Lay Jing Seow, University of Kuala Lumpur, MalaysiaYue Wang, Zhejiang University, China
Rajan Logesh, JSS Academy of Higher Education and Research, India
Copyright © 2025 Li, Zhang, Xie, Shi and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xing-wen Xie, ODI3OTc1MjcyQHFxLmNvbQ==; Xiao-feng Shi, c2hpeGlhb2ZlbmcyMDA1QCBzaW5hLmNvbQ==
†These authors have contributed equally to this work
 Wan-yu Li
Wan-yu Li Liang Zhang3
Liang Zhang3