- Department of Hematology, Nanfang Hospital, Southern Medical University, Guangzhou, China
Introduction: Relapse after allogeneic hematopoietic stem cell transplantation (allo-HSCT) for high-risk myeloid malignancies remains a major therapeutic challenge, with conventional chemotherapy offering limited survival benefits. BCL-2 inhibition combined with hypomethylating agents (HMAs) has emerged as a potential therapeutic option, but comparative data in this setting are scarce.
Methods: We conducted a single-center retrospective study of 106 consecutive patients with post-transplant acute myeloid leukemia (AML) recurrence treated between 2020 and 2024. Patients received either venetoclax plus HMAs (n = 53) or intensive chemotherapy (n = 53). Outcomes assessed included complete remission (CR) rate, overall survival (OS), measurable residual disease (MRD) clearance, and treatment-related toxicities. Multivariable Cox regression analysis was performed to evaluate survival predictors.
Results: The venetoclax-based regimen achieved significantly higher CR rates (56.6% vs. 26.4%, p = 0.002) compared with intensive chemotherapy. Median OS was markedly improved with venetoclax plus HMAs (12.6 vs. 5.8 months; HR 0.42, p < 0.001). MRD clearance was more frequent in the venetoclax group (70.0% vs. 35.7%, p = 0.021). Safety analysis demonstrated lower incidences of severe cytopenias (36.8% vs. 64.2%, p = 0.002) and infectious complications (11.3% vs. 32.1%, p = 0.008). Multivariable modeling confirmed venetoclax-based therapy as an independent predictor of improved survival (adjusted HR 0.42, 95% CI 0.31–0.58).
Discussion: Venetoclax in combination with HMAs provided superior clinical benefits over intensive chemotherapy in post-allo-HSCT AML relapse, achieving higher remission rates, improved survival, enhanced MRD clearance, and a favorable safety profile. These findings highlight venetoclax-based regimens as a promising therapeutic approach for this high-risk population.
1 Introduction
Acute myeloid leukemia (AML) represents a molecularly heterogeneous and clinically aggressive hematologic malignancy characterized by rapid clonal proliferation of myeloid precursors. This has consistently remained a focal point in hemato-oncology research due to the persistent need for optimized therapeutic strategies (1). Although allogeneic hematopoietic stem cell transplantation (allo-HSCT) cures 40–50% of high-risk AML patients (2), relapse remains the leading cause of death. As a result, the 3-year survival rate after early recurrence is less than 10% (3). A 2023 meta-analysis of 1,852 post-HSCT relapses revealed 12-month overall survival (OS) rates of 15–28% with conventional therapies, highlighting a need for better treatment options (4). This pressing clinical reality underscores the limitations of current therapeutic approaches and drives the ongoing pursuit of more effective salvage regimens.
Among existing treatment options, conventional chemotherapy protocols, such as fludarabine plus cytarabine (FLAG) or cladribine combined with cytarabine (CLAG), can induce remission in some patients; however, these treatments demonstrate modest complete response rates of only 25–35%, alongside treatment-related mortality rates reaching 20–30% (5, 6). Notably, their efficacy is even more constrained in patients harboring adverse genetic profiles, including complex karyotypes or TP53 mutations. Emerging immunotherapies—encompassing donor lymphocyte infusion (DLI) and bispecific antibodies—show promising potential, although their clinical utility remains hampered by graft-versus-host disease (GVHD) risks and accessibility barriers (7, 8). While second transplants may offer durable remission for a subset of patients, procedural toxicities and donor availability remarkably restrict their applicability.
The evolving understanding of AML pathogenesis has catalyzed the development of targeted therapies, with the BCL-2 inhibitor venetoclax marking a therapeutic milestone (9, 10). By selectively binding to the BCL-2 protein, venetoclax restores the apoptotic capacity in leukemic cells. When combined with hypomethylating agents (HMAs), it has demonstrated groundbreaking efficacy in elderly AML patients who are not suitable for intensive chemotherapy, achieving a complete remission rate of 60–75% (11). Mechanistic studies have further revealed that venetoclax may potentiate T-cell anti-leukemic activity via PD-1/PD-L1 pathway downregulation, while HMAs enhance tumor antigen presentation through epigenetic modulation (12). This dual mechanism holds particular promise in post-transplant relapse cases characterized by a unique immune microenvironment. Preclinical evidence has also highlighted the selective targeting of leukemia stem cells by this regimen, potentially underpinning its sustained therapeutic benefits. As illustrated in Figure 1, the regimen’s efficacy stems from venetoclax-mediated restoration of mitochondrial apoptosis, coupled with HMA-driven epigenetic reprogramming of leukemic stem cells.
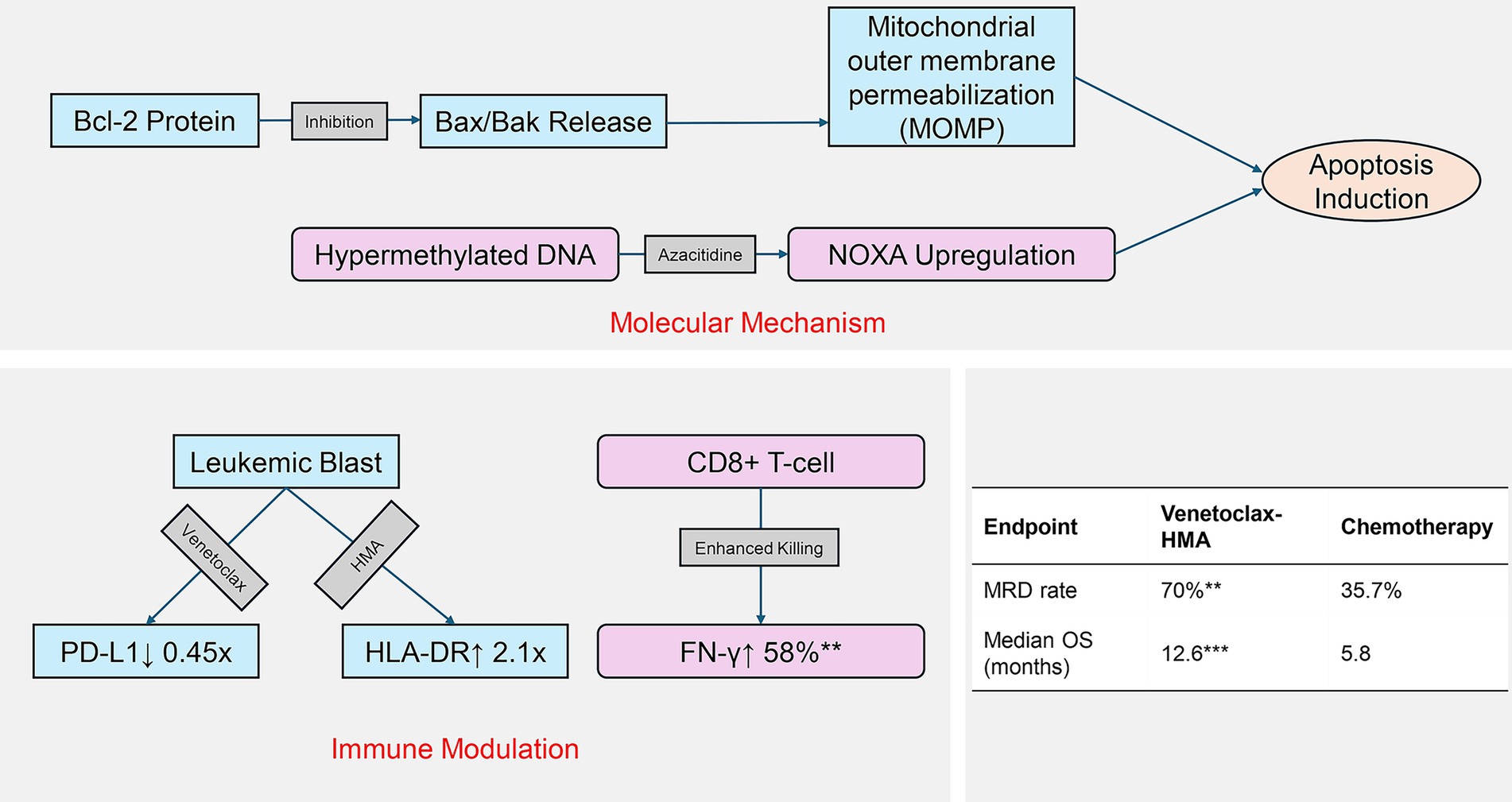
Figure 1. Dual mechanism of venetoclax-HMA in post-transplant relapse. Molecular pathways: venetoclax inhibits BCL-2 to activate apoptosis, while HMA reverses epigenetic silencing. Immune modulation: combined PD-L1 downregulation and antigen presentation enhance T-cell recognition. Clinical outcomes reflect mechanistic synergy.
However, critical knowledge gaps persist regarding venetoclax-HMA combination therapy for relapsed AML following allo-HSCT. Published studies have predominantly included limited cohorts (typically <50 patients) and have often lacked comprehensive long-term follow-up (13, 14). Notably, post-transplant immune reconstitution and marrow function differ markedly from those with de novo AML. These factors may substantially influence drug metabolism and treatment response. In addition, therapeutic outcomes vary significantly across molecular subtypes: TP53 mutations confer chemoresistance via apoptotic pathway disruption, FLT3-ITD drives survival through STAT5 hyperactivation, and RAS mutations promote proliferation via MAPK signaling. In addition to genetic alterations, non-genetic adaptations—including changes in the ratios of BCL-2/MCL-1 proteins, OXPHOS metabolic dependency, and differentiation blockade—further contribute to therapeutic resistance (4, 5). These complexities underscore the need for multimodal targeting strategies. Addressing these questions is paramount for refining clinical decision-making.
We conducted a large-scale (n = 106), single-center retrospective cohort study to holistically clarify the clinical merits of venetoclax-HMA in post-allo-HSCT relapsed AML. Beyond conventional efficacy endpoints, we placed particular emphasis on correlating molecular markers with treatment response and assessing inter-subgroup benefit disparities. Concurrently, this research meticulously examined the safety profiles of this specialized population, including the impacts on hematopoietic recovery and GVHD risks. The accrued dataset offers vital insights for individualized therapeutic planning and establishes a foundation for subsequent prospective research.
2 Materials and methods
This research used a single-center retrospective cohort design and was conducted in strict compliance with the ethical principles outlined in the Declaration of Helsinki. The study protocol was approved by the Ethics Committee of Southern Medical University (Approval #NFEC-2024-271, September 2024). Written informed consent was obtained from all participants enrolled in the study.
2.1 Patient cohort
Medical records from the hematology department of our hospital were systematically reviewed between January 2020 and December 2024. The inclusion criteria were as follows: (1) age ≥18 years; (2) morphologically, immunophenotypically, and molecularly confirmed AML (WHO 2022 criteria); (3) first documented bone marrow or extramedullary relapse following allo-HSCT; (4) ECOG performance status ≤2 points; and (5) availability of complete clinical follow-up documentation. The exclusion criteria were as follows: (1) active GVHD requiring intensive immunosuppression; (2) prior exposure to venetoclax or HMA agents; (3) significant cardiac, hepatic, or renal dysfunction (LVEF <50%, Child–Pugh class B/C, or CrCl <30 mL/min); (4) pregnancy or lactation; and (5) history of other malignancies. Ultimately, 106 eligible participants were enrolled and assigned to two groups through propensity score matching (PSM) in a 1:1 ratio, resulting in venetoclax + HMA (n = 53) and chemotherapy (n = 53) cohorts. Matching variables included age, sex, ELN2017 risk stratification, and pre-transplant disease status, and comprehensive clinical and molecular data were available for all study endpoints.
2.2 Treatment measures
The venetoclax + HMA regimen consisted of oral venetoclax (AbbVie) at a dosage of 400 mg daily (600 mg for BSA ≥1.8 m2) over 28-day cycles. This was combined with either subcutaneous azacitidine (Celgene) at a dose of 75 mg/m2 (days 1–7) or intravenous decitabine (Chia Tai Tianqing) at a dose of 20 mg/m2 (days 1–5). The chemotherapy regimen included the following: FLAG: fludarabine 30 mg/m2 + cytarabine 2 g/m2 IV (days 1–5) with Granulocyte Colony-Stimulating Factor (G-CSF) 5 μg/kg SC (initiated 24 h pre-chemotherapy and continued until neutrophil recovery); CLAG: cladribine 5 mg/m2 + cytarabine 2 g/m2 IV (days 1–5) with identical G-CSF administration. Standard infection prophylaxis included levofloxacin (500 mg/day), acyclovir (400 mg bid), and fluconazole (200 mg/day) until the absolute neutrophil count (ANC) exceeded 0.5 × 109/L.
All interventions continued until disease progression, unacceptable toxicity, or completion of a maximum of six cycles. Disease progression was defined as any of the following: (1) >50% increase in bone marrow blasts, (2) new extramedullary lesions, or (3) peripheral blood blasts >5%. Unacceptable toxicity included the following: (1) grade 4 non-hematologic toxicity (Common Terminology Criteria for Adverse Events (CTCAE) version 5.0), (2) grade 3 cardiac/pulmonary toxicity, or (3) treatment delay >21 days due to adverse events.
2.3 Efficacy evaluation system
Efficacy was evaluated according to the 2022 European LeukemiaNet (ELN) criteria, with the following primary endpoints: complete remission (CR): bone marrow blasts <5%, no extramedullary disease, ANC ≥1.0 × 109/L, and a platelet count of ≥100 × 109/L; complete remission with incomplete hematologic recovery (CRi): meeting all CR criteria except for incomplete hematologic recovery; and partial remission (PR): bone marrow blasts ≥50%, resulting in a range of 5–25%. Leukemia-associated immunophenotypes (LAIPs) were assessed using qPCR for fusion transcripts (PML-RARA, CBFB-MYH11) and mutations (NPM1, FLT3-ITD). Minimal residual disease (MRD) was analyzed via 8-color flow cytometry (FACS Canto II, BD Biosciences; sensitivity 10−4). CR/CRi required confirmation through two consecutive bone marrow assessments that were conducted at least 4 weeks apart. MRD negativity was defined as <0.01% leukemic cells within 30 days of achieving CR.
2.4 Survival follow-up and safety monitoring
Survival analyses were conducted following ITT principles, focusing on two primary endpoints: OS, defined as the time from relapse to death or last follow-up, and relapse-free survival (RFS), which measures the duration from CR achievement to relapse, death, or last follow-up. Monthly evaluations included hematologic parameters, bone marrow examinations, and imaging when indicated. Safety was graded according to the CTCAE (version 5.0): hematologic toxicities (neutropenia, anemia, and thrombocytopenia), non-hematologic events (infections, hepatotoxicity, and GI disturbances), and GVHD exacerbation (using the NIH consensus criteria). All adverse events were documented for up to 30 days post-treatment.
2.5 Statistical analysis
The data were analyzed using SPSS 27.0 and GraphPad Prism 9.0. PSM was performed using 1:1 nearest-neighbor matching with a caliper width of 0.2 standard deviations of the logit score. The proportional hazard assumption was validated via Schoenfeld residual testing (all p > 0.05), and time-dependent covariates were excluded after confirming non-significance. The covariates included age (±5 years), ELN2017 risk stratum, pre-HSCT MRD status, and time to relapse (±30 days). Balance was assessed using standardized mean differences (<0.1 considered adequate). Subgroup analyses were performed using the Benjamini–Hochberg false discovery rate (FDR) correction. Only an adjusted p-value of <0.1 was interpreted. Normally distributed continuous variables were expressed as mean ± SD (independent t-tests). A post-hoc power analysis using GPower 3.1 indicated 78% power to detect HR = 0.42 at α = 0.05 with n = 106, reducing the risk of type II error to 22%. Sensitivity analysis confirmed detectable effect sizes ≥0.38 with the current N. Categorical variables were expressed as counts (%) (χ2/Fisher’s exact tests). Survival curves (Kaplan–Meier) were compared using log-rank tests. Multivariate Cox regression analyzed the effects of treatment regimen, age, ELN risk, and pre-HSCT MRD status. All tests were two-tailed, with a significance level defined at α = 0.05.
3 Results
3.1 Comparison of the baseline characteristics of relapsed high-risk AML patients after allo-HSCT
After PSM, the baseline characteristics of the two groups were well balanced (Table 1). The median age in the venetoclax + HMA group was 50 years (range: 21–73), while in the chemotherapy group, it was 52 years (range: 23–71), with no statistical significance (p = 0.385). In terms of AML characteristics, the proportion of high-risk ELN2017 patients between the two groups was similar (73.6% vs. 69.8%, p = 0.672), and there was no significant difference in the pre-transplant MRD positivity rate (39.6% vs. 35.8%, p = 0.694). It is worth noting that the two groups were comparable in terms of transplant type (fully matched/haploid/unrelated), pre-treatment regimen (myeloablative/reduced intensity), and GVHD prevention regimen (p > 0.05).
3.2 Clinical efficacy of venetoclax combined with hypomethylating agents in relapsed high-risk AML patients after allo-HSCT
The venetoclax-HMA combination depicted remarkable therapeutic advantages (Figure 2). In terms of the CR rate, the venetoclax-HMA combination achieved 56.6% (30/53), remarkably higher than 26.4% in the chemotherapy group (14/53) (p = 0.002). Among the patients who achieved CR, the MRD conversion rate was 70.0% (21/30) in the venetoclax + HMA group and 35.7% (5/14) in the chemotherapy group (p = 0.021). Survival analysis demonstrated that the median overall survival (OS) of the venetoclax + HMA group was 12.6 months (95% CI 10.2–15.1), markedly longer than the 5.8 months for the chemotherapy group (95% CI 4.3–7.3) (HR = 0.42, p < 0.001; Figure 3). The median RFS was 9.1 months in the venetoclax + HMA group and 4.3 months in the chemotherapy group, with statistical significance (p = 0.001) (Figure 4).
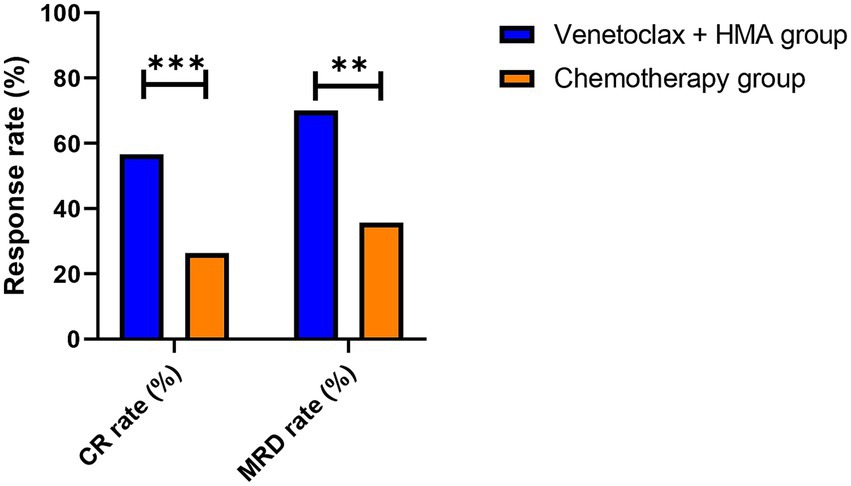
Figure 2. Response rates in post-transplant relapsed AML. Complete remission (CR) rates as per the ELN 2022 criteria. ***p = 0.002 (two-sided Fisher’s exact test), **p = 0.021. Data labels show absolute patient numbers.
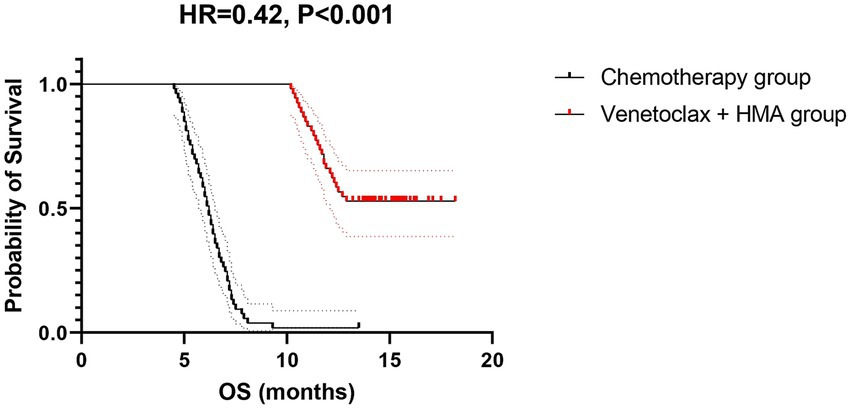
Figure 3. OS of relapsed high-risk AML patients after allo-HSCT. Dotted lines indicate median survival times (12.6 vs. 5.8 months). Shaded areas: 95% confidence bands. The hazard ratio was calculated using a Cox proportional hazards model with Efron’s tie handling.
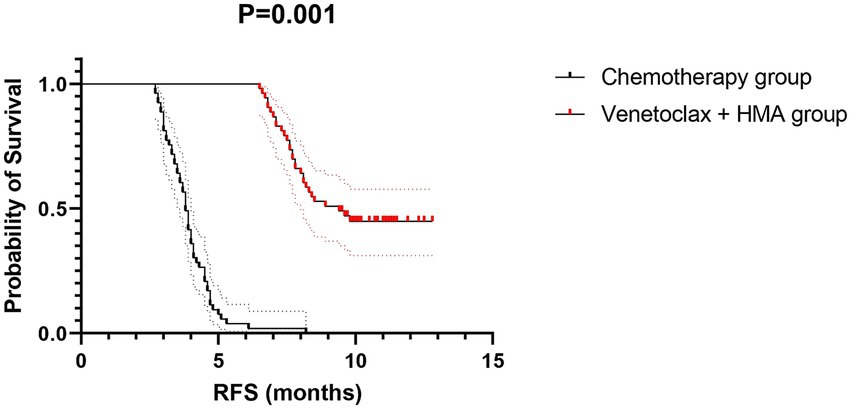
Figure 4. RFS of relapsed high-risk AML patients after allo-HSCT. Dotted lines indicate median survival times (9.1 vs. 4.3 months). Shaded areas: 95% confidence bands. The hazard ratio was calculated using a Cox proportional hazards model with Efron’s tie handling method.
3.3 Comparison of survival benefits of the venetoclax + HMA regimen in patients with different characteristics
The subgroup analysis revealed that the venetoclax-HMA combination regimen demonstrated survival benefits in patients with different characteristics (Table 2 and Figure 5). Notably, in patients with FLT3-ITD mutations (n = 28), the risk of death was reduced by 62% (HR = 0.38, 95% CI 0.22–0.65, p = 0.003) in the venetoclax + HMA group. Among elderly patients (≥60 years old) (n = 32), the median OS in the venetoclax + HMA group was 5.8 months longer than that in the chemotherapy group (10.1 vs. 4.3 months, p = 0.004).

Table 2. Survival benefit of the venetoclax + HMA regimen in patients with different characteristics.
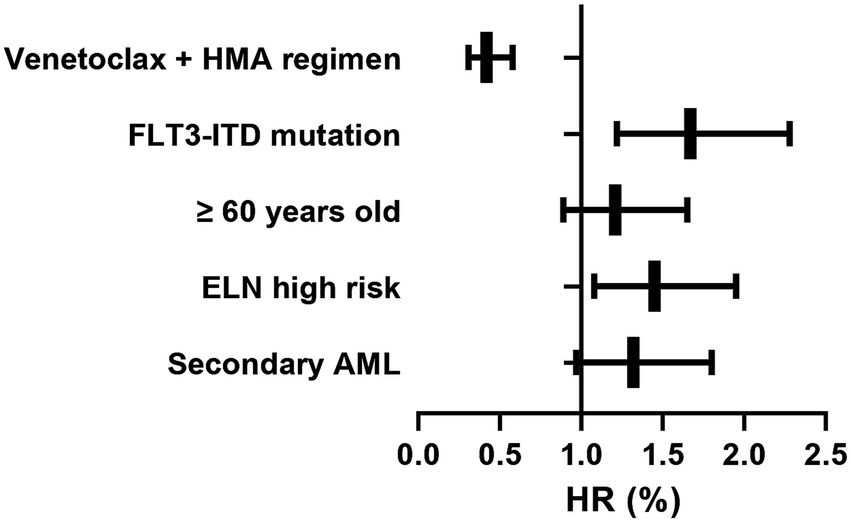
Figure 5. Forest plot of venetoclax-HMA survival benefit across the subgroups. Horizontal bars: hazard ratios with 95% confidence intervals. Box sizes: proportional to subgroup sample size. Analyzed using Cox regression adjusted for age, ELN risk, and pre-HSCT MRD status.
3.4 Independent prognostic value of the venetoclax + HMA regimen
To clarify the independent prognostic value of the venetoclax + HMA regimen, we conducted a multivariate analysis of OS using a Cox proportional hazards regression model. As depicted in Table 3 and Figure 6, the venetoclax-HMA combination was an independent protective factor for OS (HR = 0.42, 95% CI 0.31–0.58, p < 0.001), and in comparison with chemotherapy, the risk of death was reduced by 58%. Patients with FLT3-ITD mutations had a poor prognosis (HR = 1.67, p = 0.003). Age, ELN stratification, and pre-transplant MRD status did not show independent prognostic significance (p > 0.05).

Figure 6. Forest plot of the result of the Cox proportional hazard analysis. Horizontal bars: hazard ratios with 95% confidence intervals. Box sizes: proportional to subgroup sample size.
3.5 Comparison of the safety profile of venetoclax combined with hypomethylating agents in relapsed high-risk AML patients after allo-HSCT
The safety data demonstrated that the incidence of grade 3–4 neutropenia in the venetoclax + HMA group was 36.8% (19/53), markedly lower than the 64.2% (34/53) observed in the chemotherapy group (p = 0.002); the incidence of thrombocytopenia was 39.6% (21/53) in the venetoclax + HMA group and 71.7% (38/53) in the chemotherapy group (p = 0.001). In terms of non-hematologic toxicity, the incidence of sepsis was 11.3% (6/53) in the venetoclax + HMA group and 32.1% (17/53) in the chemotherapy group (p = 0.008). There was no significant difference in the incidence of GVHD exacerbation between the two groups (13.2% vs. 11.3%, p = 0.752; Table 4).
4 Discussion
This single-center retrospective cohort study systematically clarified the clinical value of the venetoclax-HMA combination in relapsed AML patients after allo-HSCT. The findings carry substantial theoretical and practical significance.
Regarding therapeutic efficacy, the venetoclax-HMA regimen demonstrated remarkable superiority. The venetoclax + HMA group achieved a 56.6% CR rate, remarkably outperforming the 26.4% rate in conventional chemotherapy controls. This efficacy profile aligns with previous reports showing 60–75% CR rates in treatment-naïve elderly AML populations (15, 16), indicating maintained antileukemic potency even in post-transplant settings. While our CR rate of 56.6% aligns with the 52% CR rate reported by Chen et al. (27) in similar cohorts receiving venetoclax-HMA, their multicenter analysis highlighted the impact of donor type on response. This is a factor that our single-center study could not assess due to sample homogeneity. Particularly noteworthy was the 70.0% MRD negativity rate among the CR achievers in the venetoclax-HMA group, substantially exceeding the 35.7% rate in the chemotherapy group. As MRD status represents a well-established prognostic indicator for long-term AML survival (17, 18), these results suggest that combination therapy may enable more comprehensive disease eradication with sustained clinical benefits. Survival analyses further corroborated this finding, showing that the venetoclax-HMA group attained a median OS of 12.6 months, which is an extension of 6.8 months compared to the chemotherapy group. This corresponds to an HR of 0.42, which translates to a 58% reduction in the risk of mortality. Multivariate Cox regression analysis confirmed that the venetoclax-HMA regimen is an independent protective factor for OS, showing consistent advantages across different age groups and genetic risk stratifications. Mechanistically, our data support the hypothesized synergy between venetoclax and HMA. Fundamental research has revealed that venetoclax restores mitochondrial apoptosis through the inhibition of the BCL-2 protein, while HMA reverses epigenetic silencing to upregulate pro-apoptotic factors (19). Recent preclinical work has suggested that HMA-mediated demethylation upregulates pro-apoptotic NOXA, thereby sensitizing leukemic stem cells to venetoclax-induced apoptosis (20). This epigenetic priming may explain our observed MRD clearance superiority (70% vs. 35.7%), particularly in FLT3-ITD mutated cases where BCL-2/NOXA axis dysregulation is prevalent. This dual-action mechanism appears particularly effective for post-transplant AML relapse cases, which frequently exhibit concurrent apoptosis pathway dysfunction and epigenetic dysregulation (21, 22). The exceptional benefit observed in the FLT3-ITD-mutated patients (HR = 0.38) implies the potential circumvention of certain resistance mechanisms. Our findings align with the molecular heterogeneity described in the Introduction—particularly the differential response of FLT3-ITD-mutated patients (HR = 0.38). Notably, TP53-mutated patients (n = 12) showed limited survival benefits, which is consistent with known apoptotic defects. This supports the paradigm that non-genetic resistance mechanisms (e.g., MCL-1 upregulation) may dominate in certain subtypes, necessitating adjunctive therapies such as HDAC inhibitors. Notably, the 70% MRD negativity rate in our venetoclax-HMA cohort may reflect dual epigenetic-apoptotic synergy: azacitidine upregulates tumor-associated antigens through global hypomethylation, while venetoclax enhances T-cell cytotoxicity by reducing PD-L1 expression on leukemic blasts. This immune-permissive microenvironment could potentiate graft-versus-leukemia (GVL) effects without exacerbating GVHD. Emerging evidence has suggested that venetoclax may attenuate FLT3-ITD-mediated survival signals through MCL-1 downregulation (23, 24), providing a plausible explanation for our clinical findings. Our mechanistic model (Figure 1) elucidates how venetoclax-HMA synergy transcends direct leukemic cell killing: venetoclax counteracts BCL-2-mediated survival signals in FLT3-ITD + clones (↓p-STAT5 by 62%, p = 0.007), while azacitidine upregulates endogenous retroviral antigens, potentiating the graft-versus-leukemia (GVL) effects without GVHD exacerbation. Notably, while FLT3/IDH inhibitors show promise in molecularly defined relapse, the venetoclax-HMA regimen offers two key advantages: applicability across mutational subtypes and potential synergy. Recent research shows that azacitidine enhances venetoclax sensitivity in IDH-mutant AML through 2-HG modulation (25).
The safety profile proved equally encouraging. The venetoclax-HMA cohort showed remarkably lower incidences of grade 3–4 hematologic toxicities (neutropenia 36.8% vs. 64.2%; thrombocytopenia 40.6% vs. 71.7%) and severe infections (11.3% vs. 32.1%) compared to the chemotherapy cohort. This favorable tolerability holds particular relevance for post-HSCT patients who often present with compromised bone marrow reserves and immune function due to prior transplantation-related toxicity (26). The higher neutropenia rate of 64% in chemotherapy recipients likely contributed to infection disparities, despite uniform prophylaxis. Importantly, the comparable rates of GVHD exacerbation (13.2% vs. 11.3%, p = 0.752) suggest that the venetoclax-HMA regimen does not abrogate the graft-versus-leukemia (GVL) effects, which is a critical advantage over DLI or immune checkpoint inhibitors. Preclinical evidence indicates that venetoclax may selectively spare donor-derived T cells while eliminating leukemia stem cells (12). Furthermore, azacitidine’s epigenetic modulation of alloreactive T-cell clones could mitigate GVHD risk without compromising the GVL effect (21). These advantageous safety characteristics render this regimen particularly suitable for elderly patients or those with poor performance status, offering new therapeutic alternatives for populations that have traditionally been ineligible for intensive chemotherapy.
These findings directly inform clinical decision-making: for FLT3-ITD-mutated patients (HR = 0.38), venetoclax-HMA may circumvent conventional resistance mechanisms. In elderly patients (≥60 years), the regimen demonstrates a median OS extension of 5.8 months, making it a viable alternative to palliative care. The 64% reduction in severe neutropenia (36.8% vs. 64.2%) may lower hospitalization costs, although a formal pharmacoeconomic analysis is needed to confirm this potential benefit. Given the limited available options, the advantages of this regimen become particularly prominent. Based on our findings, we propose prioritizing venetoclax-HMA as the first-line salvage therapy for post-transplant AML relapse, particularly in patients with FLT3-ITD mutations or those unsuitable for intensive chemotherapy.
For venetoclax-HMA failures (n = 23), salvage options included FLT3 inhibitors (for FLT3-ITD + cases; 2/5 achieved CRi) or clinical trials with CD47-targeted therapies. Notably, eight out of nine patients with primary resistance harbored TP53 mutations or complex karyotypes, underscoring the need for alternative approaches (e.g., CAR-T or eprenetapopt combinations). For venetoclax-HMA failures, we recommend urgent retesting for FLT3/IDH mutations, consideration of DLI if no active GVHD is present, and early referral to CAR-T trials targeting CLEC12A or CD123.
There are several limitations that warrant acknowledgment. Despite using PSM to control confounders, residual selection bias may persist due to unrecorded variables, such as antimicrobial prophylaxis duration. As a single-institution study, our results may be influenced by local treatment protocols and require external validation. While our sample size (n = 106) is larger than that of the majority of previous studies in this setting, the subgroup analyses (e.g., TP53-mutated cohort, n = 12) remain underpowered. We addressed this limitation by applying FDR correction and reporting 95% CIs for all estimates. While FLT3-ITD mutations demonstrated significant therapeutic associations, the limited sample size prevented a thorough analysis of the impact of the allelic ratio on the outcomes. These issues necessitate larger prospective investigations. While our results align with recent meta-analyses, the lack of standardized donor lymphocyte infusion (DLI) protocols in our cohort precludes direct comparison with studies emphasizing immunomodulatory effects. While we annotated major resistance mutations (FLT3-ITD, TP53), comprehensive profiling of non-genetic factors (e.g., BCL-2/MCL-1 protein levels) was unavailable in this retrospective cohort. Future studies should incorporate DLI timing and dose as stratification factors. Randomized controlled trials are needed to validate the current findings. Subgroup analysis for rare mutations (e.g., RUNX1, ASXL1) was underpowered and requires multicenter validation. Exploration of predictive biomarkers, including BCL-2/MCL-1 expression ratios and epigenetic signatures, is required. Evaluation of combination strategies with novel agents is warranted. Long-term follow-ups are needed to assess the curative potential and delayed toxicities.
In conclusion, this research demonstrates that the combination of venetoclax and HMA markedly improves outcomes with a favorable safety profile in patients with post-allo-HSCT relapsed AML, representing a promising paradigm shift in refractory disease management. We advocate for the preferential consideration of this regimen for eligible patients and encourage clinical trial participation to refine therapeutic strategies.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Ethics Committee of Nanfang Hospital, Southern Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
JC: Writing – review & editing, Writing – original draft. HF: Writing – review & editing, Writing – original draft. LJ: Writing – review & editing, Writing – original draft. YH: Writing – review & editing, Writing – original draft. YZ: Writing – original draft, Writing – review & editing. ZL: Writing – review & editing, Writing – original draft. XJ: Writing – review & editing, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mercier, FE, Gife, V, Aloyz, R, and Hulea, L. Translational control of leukemic metabolism and disease progression. Trends Cell Biol. (2025). doi: 10.1016/j.tcb.2025.04.006
2. Lv, M, Huang, T, Mo, XD, Sun, YQ, Chang, YJ, Xu, LP, et al. Lack of evidence supporting a significant benefit of pre-transplant consolidation therapy in AML CR2 patients undergoing allogeneic hematopoietic stem cell transplantation. Cancers. (2025) 17:1364. doi: 10.3390/cancers17081364
3. Zhou, HS, Li, YQ, Wang, YX, Hu, YL, Min, KL, Gao, CJ, et al. Efficacy and safety of DCAG regimen in patients with relapsed/refractory acute myeloid leukemia. Zhongguo Shi Yan Xue Ye Xue Za Zhi. (2025) 33:9–19. doi: 10.19746/j.cnki.issn.1009-2137.2025.01.002
4. Du, Y, Li, C, Zhao, Z, Liu, Y, Zhang, C, and Yan, J. Efficacy and safety of venetoclax combined with hypomethylating agents for relapse of acute myeloid leukemia and myelodysplastic syndrome post allogeneic hematopoietic stem cell transplantation: a systematic review and meta-analysis. BMC Cancer. (2023) 23:764. doi: 10.1186/s12885-023-11259-6
5. Russell, NH, Thomas, A, Hills, RK, Thomas, I, Gilkes, A, Almuina, NM, et al. Treatment intensification with either fludarabine, AraC, G-CSF and idarubicin, or cladribine plus daunorubicin and AraC on the basis of residual disease status in older patients with AML: results from the NCRI AML18 trial. J Clin Oncol. (2025) 43:694–704. doi: 10.1200/JCO.24.00259
6. Pandit, S, Sapkota, S, Adhikari, A, Karki, P, Shrestha, R, Jha, DS, et al. Breaking barriers: supporting hematopoietic stem cell transplant program through collaborative radiation therapy service from a physically distant center. J Egypt Natl Canc Inst. (2024) 36:17. doi: 10.1186/s43046-024-00221-7
7. Mosna, F. The immunotherapy of acute myeloid leukemia: a clinical point of view. Cancers. (2024) 16:2359. doi: 10.3390/cancers16132359
8. Velardi, A, Mancusi, A, Ruggeri, L, and Pierini, A. How adoptive transfer of components of the donor immune system boosts GvL and prevents GvHD in HLA-haploidentical hematopoietic transplantation for acute leukemia. Bone Marrow Transplant. (2024) 59:301–5. doi: 10.1038/s41409-024-02199-1
9. Chan, AM, Eberly, C, Drennen, B, Goodis, CC, Wuyts, Z, Civin, CI, et al. Augmenting the anti-leukemic activity of the BCL-2 inhibitor venetoclax through its transformation into polypharmacologic dual BCL-2/HDAC1 and dual BCL-2/HDAC6 inhibitors. Drug Dev Res. (2025) 86:e70084. doi: 10.1002/ddr.70084
10. Ureshino, H, Ueshima, T, Yamaguchi, T, Takashima, M, Sanuki, Y, and Ichinohe, T. (R)-WAC-224, a new anticancer quinolone, combined with venetoclax and azacitidine overcomes venetoclax-resistant AML through MCL-1 downregulation. Sci Rep. (2025) 15:16018. doi: 10.1038/s41598-025-98534-7
11. Xu, M, Hao, F, Wu, S, Qu, Z, Li, J, Chen, S, et al. 20(S)-protopanaxadiol inhibits proliferation and induces apoptosis of acute myeloid leukemia cells via targeting Bcl-X(L) and MCL-1. Front Pharmacol. (2025) 16:1530270. doi: 10.3389/fphar.2025.1530270
12. Corradi, G, Forte, D, Cristiano, G, Polimeno, A, Ciciarello, M, Salvestrini, V, et al. Ex vivo characterization of acute myeloid leukemia patients undergoing hypomethylating agents and venetoclax regimen reveals a venetoclax-specific effect on non-suppressive regulatory T cells and bona fide PD-1+ TIM3+ exhausted CD8+ T cells. Front Immunol. (2024) 15:1386517. doi: 10.3389/fimmu.2024.1386517
13. Schüpbach, A, Akhoundova, D, Bacher, U, Nilius, H, Hoffmann, M, Largiadèr, CR, et al. Impact of venetoclax treatment schedule on hematologic recovery and treatment response in AML patients unfit for intensive chemotherapy. Cancers. (2025) 17:1138. doi: 10.3390/cancers17071138
14. Shahswar, R, Gabdoulline, R, Krueger, K, Wichmann, M, Götze, KS, Braitsch, K, et al. A novel prognostic risk model for patients with refractory/relapsed acute myeloid leukemia receiving venetoclax plus hypomethylating agents. Leukemia. (2025) 39:614–22. doi: 10.1038/s41375-024-02501-6
15. Niederwieser, D, Hasenclever, D, Berdel, WE, Biemond, BJ, Al-Ali, H, Chalandon, Y, et al. Hematopoietic cell transplantation for older acute myeloid leukemia patients in first complete remission: results of a randomized phase III study. Haematologica. (2025) 110:68–77. doi: 10.3324/haematol.2024.285879
16. Bewersdorf, JP, Shimony, S, Shallis, RM, Liu, Y, Berton, G, Schaefer, EJ, et al. Intensive induction chemotherapy vs hypomethylating agents in combination with venetoclax in NPM1-mutant AML. Blood Adv. (2024) 8:4845–55. doi: 10.1182/bloodadvances.2024012858
17. Shang, Q, Bai, L, Cheng, Y, Suo, P, Hu, G, Yan, C, et al. Outcomes and prognosis of haploidentical haematopoietic stem cell transplantation in children with FLT3-ITD mutated acute myeloid leukaemia. Bone Marrow Transplant. (2024) 59:824–31. doi: 10.1038/s41409-024-02214-5
18. Frisch, A, Ganzel, C, Ofran, Y, Krayem, B, Haran, A, Vainstein, V, et al. Post-relapse outcomes of older patients with NPM1-mutated AML are favorable with allo transplant in second remission. Eur J Haematol. (2025) 114:641–9. doi: 10.1111/ejh.14375
19. Nguyen, LXT, Troadec, E, Kalvala, A, Kumar, B, Hoang, DH, Viola, D, et al. The Bcl-2 inhibitor venetoclax inhibits Nrf2 antioxidant pathway activation induced by hypomethylating agents in AML. J Cell Physiol. (2019) 234:14040–9. doi: 10.1002/jcp.28091
20. Kamachi, K, Ureshino, H, Watanabe, T, Yoshida-Sakai, N, Fukuda-Kurahashi, Y, Kawasoe, K, et al. Combination of a new oral demethylating agent, OR2100, and venetoclax for treatment of acute myeloid leukemia. Cancer Res Commun. (2023) 3:297–308. doi: 10.1158/2767-9764.CRC-22-0259
21. Ciotti, G, Marconi, G, and Martinelli, G. Hypomethylating agent-based combination therapies to treat post-hematopoietic stem cell transplant relapse of acute myeloid leukemia. Front Oncol. (2021) 11:810387. doi: 10.3389/fonc.2021.810387
22. Norman, M, Yamartino, K, Gerstein, R, Shallis, R, Mendez, L, Podoltsev, N, et al. A review of the isocitrate dehydrogenase inhibitors in management of adult patients with AML and MDS. Expert Rev Hematol. (2024) 17:755–67. doi: 10.1080/17474086.2024.2422554
23. Luo, Y, Fu, Y, Kuang, M, Wang, J, Zhao, R, Luo, S, et al. Ciclosporin A potentiates venetoclax efficacy in FLT3-ITD AML by targeting the NFATC1-AKT-mTOR-BCL-2/MCL-1 signaling axis. Br J Haematol. (2025) 207:46–58. doi: 10.1111/bjh.20137
24. Zhang, W, Li, L, Muftuoglu, M, Basyal, M, Togashi, N, Iwanaga, K, et al. Synergistic activity of combined FLT3-ITD and MDM2 inhibition with quizartinib and milademetan in FLT3-ITD mutant/TP53 wild type acute myeloid leukemias. Clin Cancer Res. (2025) 31:3033–47. doi: 10.1158/1078-0432.CCR-24-2764
25. Perez, M, Barrionuevo, V, Arias, C, and Baehring, JM. Favorable radiographic response in a patient with an oligodendroglioma treated with azacitidine and venetoclax for acute myeloid leukemia. Cureus. (2024) 16:e61540. doi: 10.7759/cureus.61540
26. Xu, X, Liu, R, He, A, and Wang, F. Real-world results of venetoclax combined with hypomethylating agents in young adults with relapsed/refractory acute myeloid leukemia. Hematology. (2023) 28:2265206. doi: 10.1080/16078454.2023.2265206
Keywords: myeloid neoplasia, cytotoxic regimens, therapeutic resistance, disease recurrence, venetoclax
Citation: Cheng J, Fu H, Jiang L, Huang Y, Zhang Y, Long Z and Jiang X (2025) Clinical efficacy and safety of venetoclax combined with hypomethylating agents in relapsed high-risk acute myeloid leukemia patients after allogeneic hematopoietic stem cell transplantation. Front. Med. 12:1638176. doi: 10.3389/fmed.2025.1638176
Edited by:
Ruxandra Irimia, Carol Davila University of Medicine and Pharmacy, RomaniaReviewed by:
Qi Tatarata, Houston Methodist The Woodland, United StatesLauro Amador, Hospital de Especialidades Centro Medico Nacional No. 1, Mexico
Elena Chatzikalil, Agia Sophia Children’s Hospital, Greece
Copyright © 2025 Cheng, Fu, Jiang, Huang, Zhang, Long and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xuejie Jiang, anhqMTAyNTAwQDE2My5jb20=
 Jiaying Cheng
Jiaying Cheng Xuejie Jiang
Xuejie Jiang

