Abstract
Background:
Androgenetic alopecia is the most prevalent form of progressive hair loss. Minoxidil is widely regarded as a standard treatment for this condition. Consequently, we assessed the effectiveness of minoxidil in combination with other pharmacological agents for the treatment of androgenetic alopecia.
Methods:
A comprehensive search was conducted across four databases–PubMed, Embase, Web of Science, and Cochrane Library–on December 10, 2024. Eligible studies were selected based on the PICOS framework. Data extraction and synthesis were carried out using a Bayesian network meta-analysis, focusing on mean difference and sample size data. League tables and Surface Under the Cumulative Ranking (SUCRA) values were employed to evaluate the relative efficacy of the interventions.
Results:
Among the 20 study groups analyzed, the combination of platelet-rich plasma and basic fibroblast growth factor with minoxidil demonstrated the highest overall efficacy (SUCRA = 93.06%). This combination resulted in a mean increase in hair density of 35.12 hairs/cm2 compared to the group treated with minoxidil alone. In male subgroups, finasteride combined with minoxidil was the most effective treatment (SUCRA = 80.18%). Among seven combination therapies for females, microneedle with minoxidil proved most effective (SUCRA = 87.18%).
Conclusion:
This study establishes a clinically actionable hierarchy of minoxidil-based combination therapies, providing evidence-based guidance for dermatologists to optimize androgenetic alopecia management.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/view/CRD42024623164, identifier CRD42024623164.
Introduction
Androgenetic alopecia (AGA), known as male or female pattern hair loss, is the most common form of alopecia worldwide, characterized by progressive hair loss post-puberty and worsening with age. While AGA affects both genders, it is more prevalent in men and impacts quality of life, often leading to low self-esteem (1). The condition is linked to the distribution of androgen receptors in the scalp, differing between males and females, male pattern baldness (MPHL) mainly affects the crown and frontal areas of the scalp, accompanied by a receding hairline, while female pattern baldness (FPHL) is diffuse hair loss, with the hairline often remaining normal (2–4). Despite the development of numerous therapies for androgenetic alopecia (AGA), many fail to meet expectations, and AGA remains a significant concern for affected individuals (5). Currently, the Food and Drug Administration (FDA) has approved two medications for AGA treatment: oral finasteride and topical minoxidil (6). However, the limited efficacy of these treatments when used independently has prompted research into the effectiveness of minoxidil when combined with other therapies. Studies indicate that combination therapies are more effective than monotherapy (7–10). Nonetheless, there is a paucity of comprehensive literature comparing the advantages and disadvantages of various minoxidil combination therapies. Therefore, this article aims to systematically compare and rank different minoxidil combination therapies, providing a valuable reference for the clinical management of androgenetic alopecia.
Method
The protocol for our work was published in the International Platform of Registered Systematic Review and Meta-Analysis Protocols (PROSPERO) database under the ID: CRD42024623164. Our work also followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA-NMA) guidelines (11).
Identification of eligible studies
The study was designed in accordance with the PICOS framework, focusing on patients with androgenic alopecia (P). The interventions compared included minoxidil in combination with other therapies versus minoxidil alone (I/C), with the primary outcome being the change in hair density at 24 weeks (O). All included studies were randomized controlled trials (S). Given the proximity of 24 weeks to 6 months, results from studies with a 6-months follow-up were incorporated into the final analysis. This outcome was selected due to its common usage as an endpoint and its relative objectivity.
A comprehensive search was conducted across four databases–PubMed, Embase, Web of Science, and the Cochrane Library–on December 10, 2024, with a publication cutoff of December 1, 2024. The search strategy employed both subject-specific and free-text terms. Literature selection was performed independently by two authors, who screened titles, abstracts, and full texts. Discrepancies were assessed and resolved by a third author. Data extraction included details such as author, year of publication, patient demographics (sex and age), severity of androgenetic alopecia, and treatment regimen.
Statistical analyses
In this study, direct comparisons between interventions across trials are illustrated using network plots, which are graphs consisting of nodes and edges. Each node represents a specific intervention, while an edge, depicted as a line connecting two nodes, signifies a direct comparison between the two interventions in a head-to-head trial. The thickness of an edge indicates the number of direct comparisons between the corresponding nodes.
A Bayesian network analysis was conducted, employing 50,000 iterations and a random effects model. All analyses were executed using RStudio software. The Surface Under the Cumulative Ranking (SUCRA) value was calculated for each intervention, allowing for the ranking of interventions and the generation of an optimized SUCRA line chart. A 95% confidence interval was estimated for each comparison measure, with a p-value of less than 0.05 denoting statistical significance. The quality of evidence in the study was assessed using the latest version of the Cochrane Collaboration’s Risk of Bias 2 tool.
Results
A total of 5,025 studies were identified, of which 18 were selected for inclusion in the analysis following a rigorous screening process (Figure 1). The results of the risk of bias assessment for each study are presented in Figure 2. The quality of evidence for each intervention is detailed in Supplementary Tables 1–3. The study encompassed 729 patients, 20 intervention comparisons, and 10 combinations involving minoxidil. The interventions included: minoxidil combined with low-level light therapy (LMX) (n = 4), monofilament thread therapy with minoxidil (PLLAMX) (n = 1), platelet-rich plasma with minoxidil (PMX) (n = 2), platelet-rich plasma plus basic fibroblast growth factor combined with minoxidil (PBMX) (n = 1), microneedling with minoxidil (MMX) (n = 4), finasteride with minoxidil (FMX) (n = 3), flutamide with minoxidil (FTMX) (n = 1), cetirizine with minoxidil (CMX) (n = 1), concentrated growth factors with minoxidil (CGFMX) (n = 1), and spironolactone with minoxidil (SPTMX) (n = 1). The characteristics of each research group are detailed in Table 1. A network plot illustrating the various interventions is shown in Figure 3. Minoxidil monotherapy was used as the reference group, as each study’s control group consisted of minoxidil alone.
FIGURE 1

Screening flow chart.
FIGURE 2
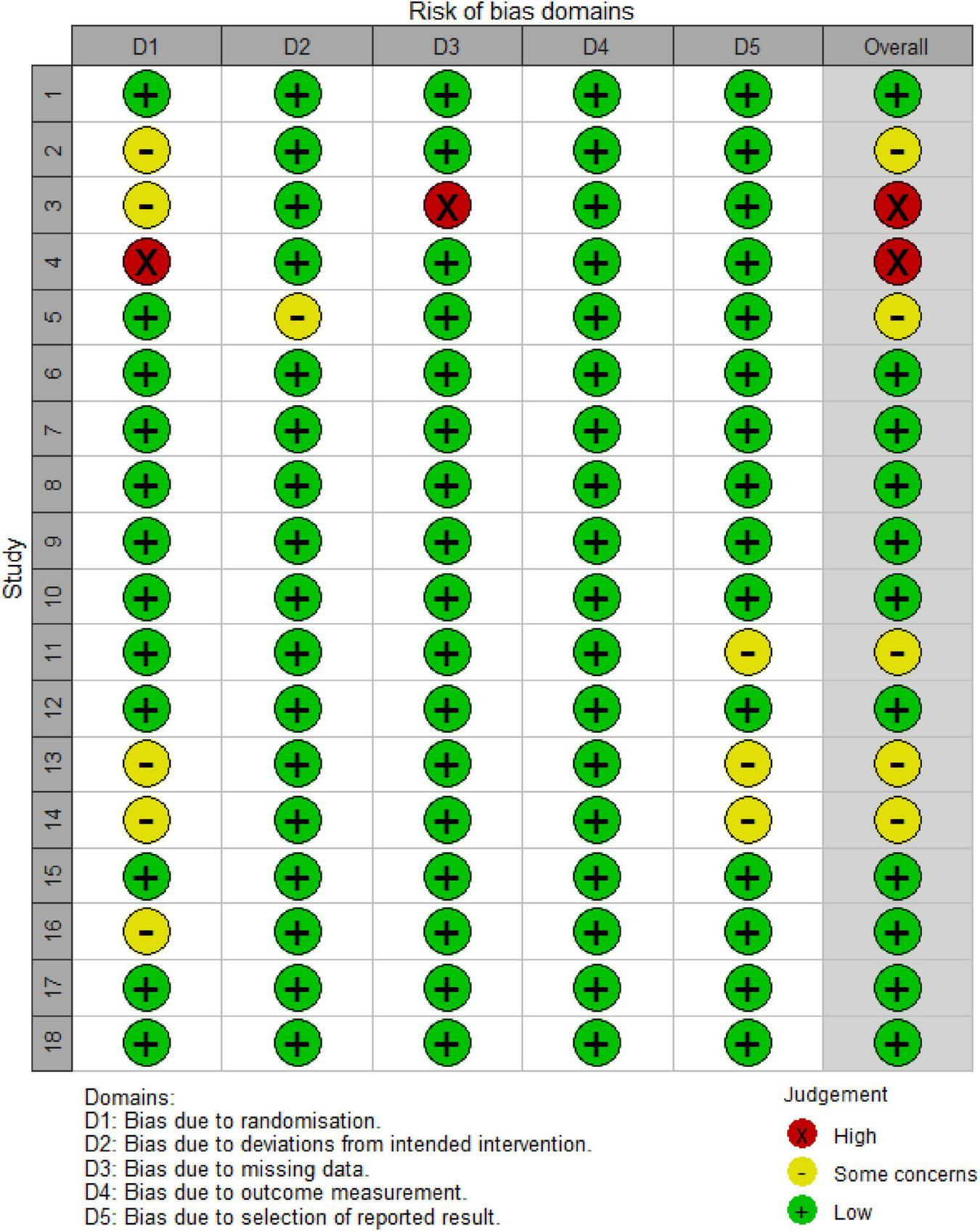
Risk of bias assessment chart. Literature Information: 1. Faghihi et al. (12); 2. Ferrara et al. (13); 3. Yang et al. (14); 4. Khattab and Bessar (16); 5. Singh et al. (17); 6. Wu et al. (19); 7. Suchonwanit et al. (24); 8, 9. Bao et al. (20); 10, 11. Suchonwanit et al. (25); 12. Rossi et al. (26); 13. Faghihi et al. (27); 14. Zhang et al. (22); 15. Bassiouny et al. (28); 16. Alves and Grimalt (18); 17. Tan et al. (29); 18. Liang et al. (23).
TABLE 1
| Study | Age | Sample size (percentage of male patients) | Severity mild/moderate/severe | Design (whole-scalp or split-scalp) | Treatment |
| Faghihi et al. (12) | NA | 23 | NA | Whole | Topical minoxidil 5%, 20 drops, twice per day for 6 months, low level light therapy (10–50 MW power and a 785 nm wavelength) per week for 24 weeks; |
| 22 | NA | Whole | Topical minoxidil 5%, 20 drops, twice per day for 6 months | ||
| Ferrara et al. (13) | 41.7 ± 6.76 | 19 (1) | 0/9/10 | Half | 5 mw of 660 nm light to the irradiated side when used for 24 min per day and 1 mL of 5% topical minoxidil for 6 months |
| 19 (1) | 0/9/10 | Half | 1 mL of 5% topical minoxidil for 6 months | ||
| Yang et al. (14) | 29.8 ± 5.74 | 30 (0) | NA | Whole | 1 ml 2% topical minoxidil solution twice daily for 24 weeks, laser hamlet treatment 20 min every other day for 24 weeks |
| 30 (0) | NA | Whole | 1 ml 2% topical minoxidil solution twice daily for 24 weeks | ||
| Suchonwanit et al. (15) | 35.4 ± 10.3 | 29 (1) | 0/16/13 | Half | 1 mL of 5% topical minoxidil solution twice daily for 24 weeks and 1550-nm fractional erbium-glass laser treatment at 2-weeks intervals for a total of 12 sessions |
| 29 (1) | 0/16/13 | Half | 1 mL of 5% topical minoxidil solution twice daily for 24 weeks | ||
| Khattab and Bessar (16) | 32 (21–49) | 27 (0) | NA | Half | 1 mL of 2% topical minoxidil solution twice daily for 6 months, ploy-L-lactic acid threads were implemented in the dermal layer. |
| 27 (0) | NA | Half | 1 mL of 2% topical minoxidil solution twice daily for 6 months, | ||
| Singh et al. (17) | 26.1 ± 4.2 | 20 (1) | NA | Whole | 1 mL of 5% topical minoxidil solution twice daily for 3 months, intradermal injection of PRP monthly for 3 months |
| 17 (1) | NA | Whole | 1 mL of 5% topical minoxidil solution twice daily for 3 months | ||
| Alves and Grimalt (18) | 39.9 (18–65) | 13 (0) | 3/9/1 | Half | 1 mL 5% topical minoxidil solution twice daily and intradermal injection of PRP monthly for 6 months |
| 13 (0) | 3/9/1 | Half | 1 mL 5% topical minoxidil solution twice daily for 6 months | ||
| Wu et al. (19) | 26.1 ± 4.2 | 20 (0.48) | NA | Whole | 1 mL of 5% topical minoxidil solution twice daily for 6 months, three PRP, BFGF treatment sessions at 1-month intervals |
| 17 (0.64) | NA | Whole | 1 mL of 5% topical minoxidil solution twice daily for 6 months, | ||
| Bao et al. (20) | 35.2 ± 3.3 | 20 (1) | 0/11/9 | Whole | 1 mL of 5% topical minoxidil solution twice daily for 24 weeks and microneedle 2-weeks intervals for a total of 12 times |
| 34.7 ± 6.9 | 18 (1) | 0/11/7 | Whole | 1 mL of 5% topical minoxidil solution twice daily for 24 weeks | |
| Bao et al. (21) | 36.33 ± 8.04 | 25 (1) | 0/13/12 | Whole | 1 mL of 5% topical minoxidil solution twice daily for 24 weeks and microneedle 2-weeks intervals for a total of 12 times |
| 37.01 ± 8.43 | 23 (1) | 0/13/10 | Whole | 1 mL of 5% topical minoxidil solution twice daily for 24 weeks | |
| Zhang et al. (22) | 31.68 ± 4.93 | 20 (0) | NA | Whole | 1 ml of topical 2% minoxidil solution twice a day and 24 sessions of weekly microneedle over a total period of 24 weeks |
| 30.05 ± 5.46 | 20 (0) | NA | Whole | 1 mL of 2% topical minoxidil solution twice daily for 24 weeks | |
| Liang et al. (23) | 30.83 ± 6.28 | 40 (0) | NA | Whole | Microneedle treatments with the delivery of 5% topical minoxidil every 2 weeks and 1 ml of topical 5% topical minoxidil once daily for 24 weeks |
| 31.62 ± 6.29 | 37 (0) | NA | Whole | Oral SPT of 80–100 mg/day and 1 ml of topical 5% minoxidil once daily for 24 weeks | |
| 31.08 ± 6.87 | 40 (0) | NA | Whole | 1 ml of topical 5% minoxidil once daily for 24 weeks | |
| Suchonwanit et al. (24) | 56.8 ± 6.6 | 15 (0) | 5/8/2 | Whole | 1 mL of 3% topical minoxidil and 0.25% finasteride solution twice daily for 24 weeks |
| 59.8 ± 7.7 | 15 (0) | 4/8/3 | Whole | 1 ml of topical 3% minoxidil once daily for 24 weeks | |
| Suchonwanit et al. (25) | 39.3 ± 11.9 | 19 (1) | 0/12/7 | Whole | 1 mL of 3% topical minoxidil and 0.25% finasteride solution twice daily for 24 weeks |
| 44.4 ± 12.5 | 18 (1) | 014/4 | Whole | 1 ml of topical 3% minoxidil once daily for 24 weeks | |
| Rossi and Caro (26) | 25.3 ± 2.6 | 19 (1) | 11/5/3 | Whole | 5% topical minoxidil in the morning and 0.25% topical finasteride spray in the evening |
| 23.5 ± 2.2 | 11 (1) | 10/1/0 | Whole | 5% topical minoxidil twice daily | |
| Faghihi et al. (27) | 27.15 ± 5.29 | 20 (0.3) | NA | Whole | 1 mL of 5% topical minoxidil and 2% flutamide solution twice daily for 6 months |
| 27.05 ± 4.75 | 20 (0.55) | NA | Whole | 1 ml of topical 5% minoxidil twice daily for 24 weeks | |
| Bassiouny et al. (28) | 38.61 ± 8.74 | 26 (0) | NA | Whole | 1 ml of topical minoxidil (5%) once daily in the morning and 1 ml of topical cetirizine (1%) once daily in the evening for 24 weeks |
| 36.74 ± 9.84 | 27 (0) | NA | Whole | 1 ml of topical minoxidil (5%) once daily in the morning and 1 ml of placebo once daily in the evening | |
| Tan et al. (29) | NA | 16 (1) | NA | Half | 1 mL 5% topical minoxidil solution twice daily for 24 weeks and intradermal injection of CGF (2–3 ml) at 0, 4, 8 weeks |
| 16 (1) | NA | Half | 1 mL 5% topical minoxidil solution twice daily for 24 weeks and intradermal injection of placebo normal saline (2–3 ml) at 0, 4, 8 weeks |
Basic information sheets.
FIGURE 3
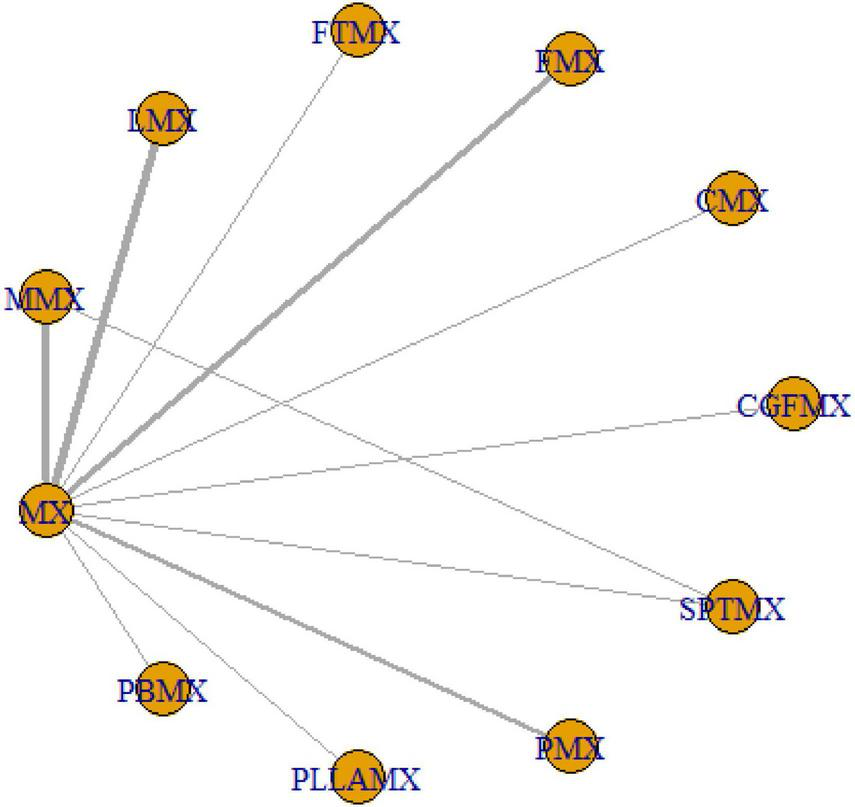
Network plot for mixed groups. One node corresponds to a given intervention; an edge is represented by a line between the two nodes, which corresponds to a direct comparison of the two interventions in a head-on trial. The thickness of an edge corresponds to the number of direct comparisons between the respective nodes.
Among the 20 study groups, the PBMX group demonstrated the highest overall efficacy, with a SUCRA value of 93.06%. In comparison to the group receiving minoxidil alone, the PBMX group exhibited a mean increase in hair density of 35.12 hairs/cm2. The group treated with microneedling combined with minoxidil showed an increase of 22.64 hairs/cm2 (SUCRA = 74.06%), while the PMX group had an increase of 22.14 hairs/cm2 (SUCRA = 71.53%). However, the study did not reveal any statistically significant differences in efficacy between the PBMX, MMX, and PMX groups. Most combination therapies demonstrated greater efficacy than the minoxidil alone group, with the exception of the cetirizine and minoxidil combination, which had a SUCRA value of 6.90%, as detailed in Supplementary Tables 4, 7. We refined the original SUCRA chart and represented it as a line graph, where a larger area under the curve corresponds to a higher SUCRA value and greater efficacy, as illustrated in Figure 4.
FIGURE 4
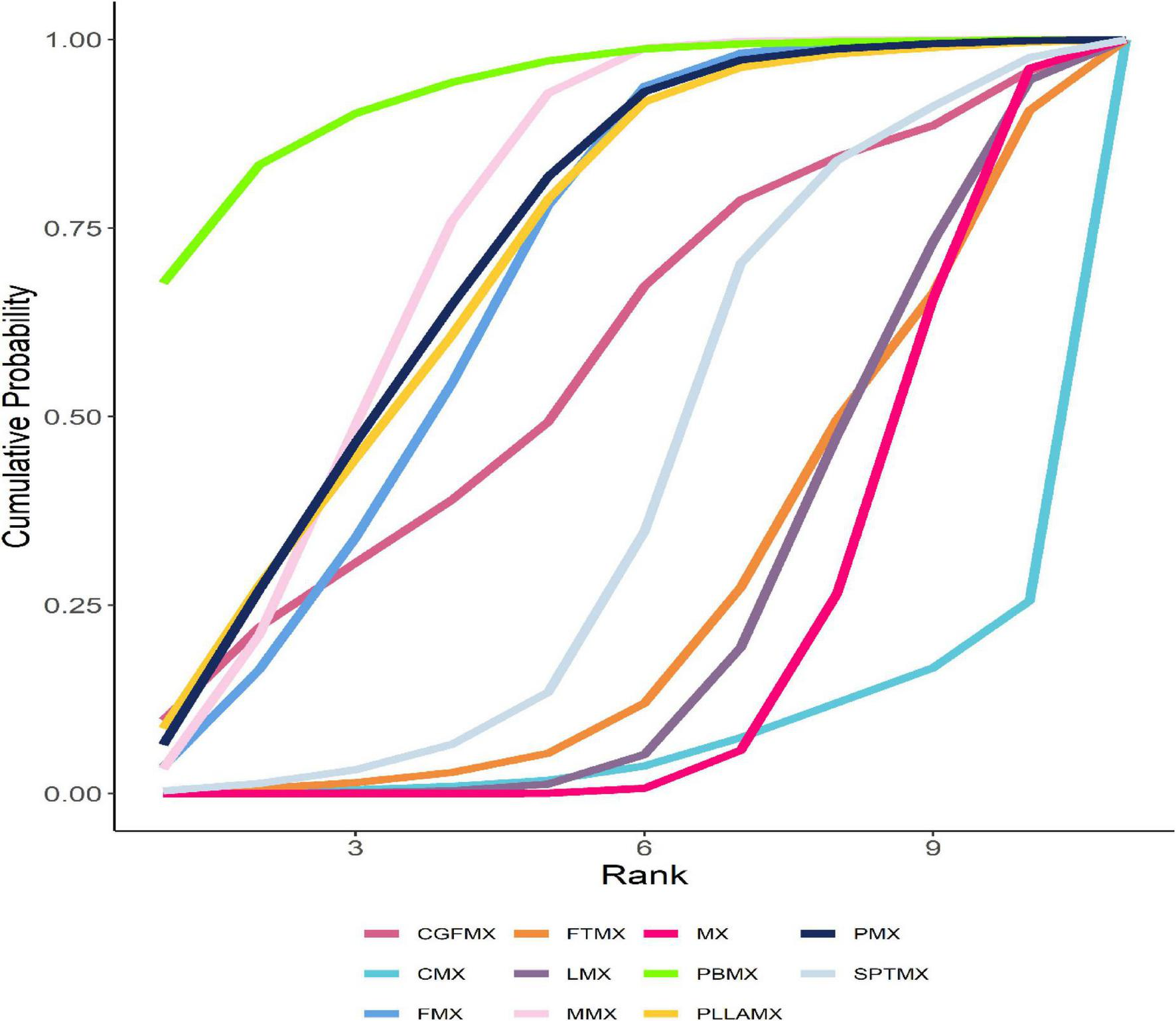
Optimized SUCRA folding plot for mixed groups. The area under the fold line represents the size of the sucra value, the better the efficacy, the larger the area.
Subgroup analysis
Given that the majority of research on androgenetic alopecia has been conducted separately for male and female subjects, subgroup analyses were performed based on the gender of the study participants. The network plots for these subgroups are presented in Supplementary Figures 1, 2.
In the male cohort, a total of five combined interventions were evaluated. The most efficacious treatment was the combination of finasteride and minoxidil, with a SUCRA value of 80.21%. This treatment resulted in an increase in hair density of 29.68 hairs/cm2 after 24 weeks, compared to the reference group. The second most effective treatment was the PMX group, with a SUCRA value of 73.00%, which achieved an increase in hair density of 27.18 hairs/cm2. Among male patients with androgenetic alopecia, all combination therapies demonstrated enhanced efficacy; however, only the FMX group exhibited a statistically significant difference in efficacy when compared to minoxidil alone, with the evidence being of moderate quality. The SUCRA ranking and corresponding league tables are provided in Supplementary Tables 5, 8, respectively, and the optimized fold plot is illustrated in Supplementary Figure 3.
Among the seven combination therapies evaluated in the female subgroup, the most effective treatments were microneedle combined with minoxidil (SUCRA = 87.20%) and silk thread combined with minoxidil (SUCRA = 84.51%). These combinations resulted in an increase in hair density of 22.02 hairs/cm2 and 21.63 hairs/cm2, respectively, after 24 weeks compared to minoxidil alone, with a statistically significant difference observed in the microneedle-minoxidil group, supported by moderate quality of evidence. The efficacy of spironolactone combined with minoxidil (SUCRA = 56.63%) and platelet-rich plasma combined with minoxidil (SUCRA = 53.88%) was comparable and also demonstrated superiority over minoxidil alone (SUCRA = 36.00%). Conversely, the combination of low-level light therapy and cetirizine with minoxidil exhibited reduced efficacy compared to their use as monotherapies. The quality of evidence within the female subgroups varied from very low to low, with only two comparisons achieving a moderate quality of evidence, as illustrated in Supplementary Figure 4 and Supplementary Tables 6, 9.
Discussion
Hair loss can result from various causes, with androgenetic alopecia (AGA) being the most prevalent. AGA significantly affects patients’ quality of life and may lead to severe psychological disorders (30). While Minoxidil is an FDA-approved standard treatment for AGA, its efficacy as a monotherapy often falls short of patient expectations. Research indicates that combination therapies are generally more effective than monotherapy for treating AGA (31). In our study, the majority of combination therapies demonstrated superior efficacy compared to Minoxidil alone, with PBMX emerging as the most effective treatment (SUCRA = 93.00%). Previous network meta-analyses have also shown similar results when comparing Minoxidil combined with microneedling or platelet-rich plasma (32). Our study, however, included a more extensive and comprehensive range of combination therapies, all of which were evaluated through RCTs, thereby enhancing the credibility of our findings. Although PBMX exhibited the highest efficacy, no significant difference in relative effectiveness was observed when compared to the combination of microneedling and Minoxidil (SUCRA = 74.10%) and platelet-rich plasma combined with minoxidil (SUCRA = 71.68%).
The pathogenesis of androgenetic alopecia is primarily attributed to an exaggerated response to androgens, particularly dihydrotestosterone (DHT) and 5-alpha-reductase type II (3, 33). Given that treatment protocols differ between men and women, and most clinical trials are conducted separately for each gender, our study accounted for these differences by analyzing subgroups based on gender.
In men with male pattern baldness, finasteride, a 5-alpha reductase inhibitor, has been shown to effectively reduce dihydrotestosterone levels, with prior research indicating that oral finasteride is the most efficacious treatment (34). Our analysis revealed that for men, the combination of finasteride and minoxidil was the most effective treatment modality, demonstrating a significant difference compared to minoxidil alone. However, the combination of finasteride and minoxidil did not show a significant difference when compared to other combination therapies. It is important to note that our findings were derived from indirect comparisons, and the quality of the evidence was predominantly very low to low. Therefore, further direct comparison studies are necessary to validate our results.
In the female subgroup, the efficacy ranking indicated that the most effective treatment modality was the combination of microneedling and minoxidil. Consistent with findings in the male subgroup, the relative effectiveness of microneedling combined with minoxidil did not significantly differ from other combination therapies. Our NMA revealed that two regimens were less effective than minoxidil alone; however, these differences were not statistically significant. Given that our study exclusively included RCTs, resulting in small sample sizes for each therapy, further RCTs are necessary to substantiate these conclusions.
Strengths and limitations
The relative efficacy of minoxidil combined with microneedling or platelet-rich plasma has been examined in only one prior study. For our analysis, we intentionally included only randomized controlled trials to enhance the reliability of our findings and consistently selected a 24-weeks follow-up period to reduce selection bias associated with varying outcome assessment time points. Our study not only assessed overall efficacy but also conducted subgroup analyses to investigate gender-specific effects in androgenic alopecia, thereby aiming to derive more comprehensive conclusions.
Nonetheless, our research has certain limitations. Firstly, the small sample size and the lack of direct comparisons among various combination therapies resulted in a low quality of evidence for relative comparisons. Therefore, larger sample sizes and more direct comparative studies are necessary to validate these findings in future research. Secondly, a significant portion of the existing literature does not explicitly detail the distribution of different severities of androgenic alopecia, which may have influenced our results. Thirdly, while dutasteride and oral minoxidil have demonstrated promising efficacy in treating AGA, our NMA lacks studies on these two medications. Future research should include these drugs to facilitate a more comprehensive comparative assessment of combination therapy efficacy. Lastly, the insufficient characterization of AGA severity in existing studies precludes further exploration of the impact of disease severity on treatment efficacy, which may affect the reliability of our results.
Conclusion
This study conducted an analysis to ascertain the relative efficacy rankings of minoxidil-based combination therapies, thereby offering evidence-based guidance for dermatologists aiming to improve the management of androgenetic alopecia. Nonetheless, additional research, particularly direct comparative studies of various combination therapies, is necessary to validate these findings.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YX: Writing – original draft, Methodology, Data curation, Conceptualization. HC: Writing – original draft, Data curation. YC: Writing – original draft, Data curation. ZC: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Fundamental Research Funds for the Central Universities (2022CDJYGRH-016).
Acknowledgments
We would like to thank all those who contributed to this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1638496/full#supplementary-material
References
1.
Huang C Fu Y Chi C . Health-related quality of life, depression, and self-esteem in patients with androgenetic alopecia: a systematic review and meta-analysis.JAMA Dermatol. (2021) 157:963–70. 10.1001/jamadermatol.2021.2196
2.
Piraccini B Alessandrini A . Androgenetic alopecia.G Ital Dermatol Venereol. (2014) 149:15–24.
3.
Price V . Androgenetic alopecia in women.J Investig Dermatol Symp Proc. (2003) 8:24–7. 10.1046/j.1523-1747.2003.12168.x
4.
Devjani S Ezemma O Kelley K Stratton E Senna M . Androgenetic alopecia: therapy update.Drugs. (2023) 83:701–15. 10.1007/s40265-023-01880-x
5.
Nestor M Ablon G Gade A Han H Fischer D . Treatment options for androgenetic alopecia: efficacy, side effects, compliance, financial considerations, and ethics.J Cosmet Dermatol. (2021) 20:3759–81. 10.1111/jocd.14537
6.
Ibrahim IM Hasan MS Elsabaa KI Elsaie M . Pumpkin seed oil vs. minoxidil 5% topical foam for the treatment of female pattern hair loss: a randomized comparative trial.J Cosmet Dermatol. (2021) 20:2867–73. 10.1111/jocd.13976
7.
Pakhomova EE Smirnova IO . Comparative evaluation of the clinical efficacy of PRP-therapy, minoxidil, and their combination with immunohistochemical study of the dynamics of cell proliferation in the treatment of men with androgenetic alopecia.Int J Mol Sci. (2020) 21:6516. 10.3390/ijms21186516
8.
Hu R Xu F Sheng Y Qi S Han Y Miao Y et al Combined treatment with oral finasteride and topical minoxidil in male androgenetic alopecia: a randomized and comparative study in Chinese patients. Dermatol Ther. (2015) 28:303–8. 10.1111/dth.12246
9.
Yu A Luo Y Xu X Bao L Tian T Li Z et al A pilot split-scalp study of combined fractional radiofrequency microneedling and 5% topical minoxidil in treating male pattern hair loss. Clin Exp Dermatol. (2018) 43:775–81. 10.1111/ced.13551
10.
Abdel-Raouf H Aly U Medhat W Ahmed S Abdel-Aziz RTA . A novel topical combination of minoxidil and spironolactone for androgenetic alopecia: clinical, histopathological, and physicochemical study.Dermatol Ther. (2021) 34:e14678. 10.1111/dth.14678
11.
Hutton B Salanti G Caldwell D Chaimani A Schmid C Cameron C et al The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. 10.7326/M14-2385
12.
Faghihi G Mozafarpoor S Asilian A Mokhtari F Esfahani A Bafandeh B et al The effectiveness of adding low-level light therapy to minoxidil 5% solution in the treatment of patients with androgenetic alopecia. Indian J Dermatol Venereol Leprol. (2018) 84:547–53. 10.4103/ijdvl.IJDVL_1156_16
13.
Ferrara F Kakizaki P de Brito FF . Efficacy of minoxidil combined with photobiomodulation for the treatment of male androgenetic alopecia. a double-blind half-head controlled trial.Lasers Surg Med. (2021) 53:1201–7. 10.1002/lsm.23411
14.
Yang X Qiao R Cheng W Lan X Li Y Jiang Y . Comparative efficacy of 2% minoxidil alone against combination of 2% minoxidil and low-level laser therapy in female pattern hair loss-A randomized controlled trial in Chinese females.Photodiagnosis Photodyn Ther. (2024) 45:103966. 10.1016/j.pdpdt.2024.103966
15.
Suchonwanit P Rojhirunsakool S Khunkhet S . A randomized, investigator-blinded, controlled, split-scalp study of the efficacy and safety of a 1550-nm fractional erbium-glass laser, used in combination with topical 5% minoxidil versus 5% minoxidil alone, for the treatment of androgenetic alopecia.Lasers Med Sci. (2019) 34:1857–64. 10.1007/s10103-019-02783-8
16.
Khattab F Bessar H . Accelerated hair growth by combining thread monofilament and minoxidil in female androgenetic alopecia.J Cosmet Dermatol. (2020) 19:1738–44. 10.1111/jocd.13228
17.
Singh S Kumar V Rai T . Comparison of efficacy of platelet-rich plasma therapy with or without topical 5% minoxidil in male-type baldness: a randomized, double-blind placebo control trial.Indian J Dermatol Venereol Leprol. (2020) 86:150–7. 10.4103/ijdvl.IJDVL_589_18
18.
Alves R Grimalt R . Platelet-rich plasma in combination with 5% minoxidil topical solution and 1 mg oral finasteride for the treatment of androgenetic alopecia: a randomized placebo-controlled, double-blind, half-head study.Dermatol Surg. (2018) 44:126–30. 10.1097/DSS.0000000000001198
19.
Wu S Liu S Chen J Dai D Liu W Le D et al Evaluation of platelet-rich plasma plus basic fibroblast growth factor combined with minoxidil in the treatment of androgenetic alopecia: a randomized controlled trial. J Cosmet Dermatol. (2023) 22:1995–2002. 10.1111/jocd.15825
20.
Bao L Gong L Guo M Liu T Shi A Zong H et al Randomized trial of electrodynamic microneedle combined with 5% minoxidil topical solution for the treatment of Chinese male Androgenetic alopecia. J Cosmet Laser Ther. (2020) 22:1–7. 10.1080/14764172.2017.1376094
21.
Bao L Zong H Fang S Zheng L Li Y . Randomized trial of electrodynamic microneedling combined with 5% minoxidil topical solution for treating androgenetic alopecia in Chinese males and molecular mechanistic study of the involvement of the Wnt/β-catenin signaling pathway.J Dermatolog Treat. (2022) 33:483–93. 10.1080/09546634.2020.1770162
22.
Zhang Y Sheng Y Zeng Y Hu R Zhao J Wang W et al Randomized trial of microneedling combined with 2% minoxidil topical solution for the treatment of female pattern hair loss in a Chinese population. J Cosmet Dermatol. (2022) 21:6985–91. 10.1111/jocd.15424
23.
Liang X Chang Y Wu H Liu Y Zhao J Wang L et al Efficacy and safety of 5% minoxidil alone, minoxidil plus oral spironolactone, and minoxidil plus microneedling on female pattern hair loss: a prospective, single-center, parallel-group, evaluator blinded, randomized trial. Front Med. (2022) 9:905140. 10.3389/fmed.2022.905140
24.
Suchonwanit P Iamsumang W Rojhirunsakool S . Efficacy of topical combination of 0.25% finasteride and 3% minoxidil versus 3% minoxidil solution in female pattern hair loss: a randomized, double-blind, controlled study.Am J Clin Dermatol. (2019) 20:147–53. 10.1007/s40257-018-0387-0
25.
Suchonwanit P Srisuwanwattana P Chalermroj N Khunkhet SA . randomized, double-blind controlled study of the efficacy and safety of topical solution of 0.25% finasteride admixed with 3% minoxidil vs. 3% minoxidil solution in the treatment of male androgenetic alopecia.J Eur Acad Dermatol Venereol. (2018) 32:2257–63. 10.1111/jdv.15171
26.
Rossi A Caro G . Efficacy of the association of topical minoxidil and topical finasteride compared to their use in monotherapy in men with androgenetic alopecia: a prospective, randomized, controlled, assessor blinded, 3-arm, pilot trial.J Cosmet Dermatol. (2024) 23:502–9. 10.1111/jocd.15953
27.
Faghihi G Iraji F Siadat AH Saber M Jelvan M Hoseyni M . Comparison between “5% minoxidil plus 2% flutamide” solution vs. “5% minoxidil” solution in the treatment of androgenetic alopecia.J Cosmet Dermatol. (2022) 21:4447–53. 10.1111/jocd.14788
28.
Bassiouny E El-Samanoudy S Abbassi M Nada H Farid S . Comparison between topical cetirizine with minoxidil versus topical placebo with minoxidil in female androgenetic alopecia: a randomized, double-blind, placebo-controlled study.Arch Dermatol Res. (2023) 315:1293–304. 10.1007/s00403-022-02512-2
29.
Tan P Zhang P Xie Y Gao Y Li Q Zhou S et al Autologous concentrated growth factors combined with topical minoxidil for the treatment of male androgenetic alopecia: a randomized controlled clinical trial. Facial Plast Surg Aesthet Med. (2021) 23:255–62. 10.1089/fpsam.2020.0288
30.
Cash T . The psychosocial consequences of androgenetic alopecia: a review of the research literature.Br J Dermatol. (1999) 141:398–405. 10.1046/j.1365-2133.1999.03030.x
31.
Chen D Yang X Liu X He Y Fan W Wang H et al Efficacy comparison of monotherapies and combination therapies for androgenetic alopecia: a Bayesian network meta-analysis. Dermatol Ther. (2022) 35:e15262. 10.1111/dth.15262
32.
Gupta A Wang T Bamimore M Polla Ravi S Talukder M . Relative effects of minoxidil 5%, platelet-rich plasma, and microneedling in pattern hair loss: a systematic review and network meta-analysis.Skin Appendage Disord. (2023) 9:397–406. 10.1159/000534196
33.
Saceda-Corralo D Domínguez-Santas M Vañó-Galván S Grimalt R . What’s new in therapy for male androgenetic alopecia?Am J Clin Dermatol. (2023) 24:15–24. 10.1007/s40257-022-00730-y
34.
Varothai S Bergfeld W . Androgenetic alopecia: an evidence-based treatment update.Am J Clin Dermatol. (2014) 15:217–30. 10.1007/s40257-014-0077-5
Summary
Keywords
androgenic alopecia, minoxidil, minoxidil combination therapy, male pattern hair loss, female pattern hair loss
Citation
Xia Y, Chen H, Chen Y and Chen Z (2025) Relative efficacy of minoxidil in combination with other treatments for androgenic alopecia: a network meta-analysis based on randomized controlled trials. Front. Med. 12:1638496. doi: 10.3389/fmed.2025.1638496
Received
30 May 2025
Accepted
01 September 2025
Published
17 September 2025
Volume
12 - 2025
Edited by
Jose Maria Ricart Vaya, Instituto Médico Ricart, Spain
Reviewed by
David Vega Diez, Servicio Madrileño de Salud (SERMAS), Spain
Blanca Ferrer Guillen, Instituto Médico Ricart, Spain
Cristian Valenzuela Oñate, Instituto Médico Ricart, Spain
Updates
Copyright
© 2025 Xia, Chen, Chen and Chen.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhiyong Chen, czy1002008@cqu.edu.cn
†ORCID: Yinfeng Xia, orcid.org/0009-0008-7610-2990
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.