Abstract
Background and Objective:
Community-acquired pneumonia (CAP) is an acute lung infection disease with high morbidity and mortality. The treatment of CAP has become more and more challenging due to the gradual increase of antibiotic resistance and adverse events. Relevant evidence indicates that Maxing Shigan Decoction (MXSG) may play a unique therapeutic advantage. Our aim is to evaluate the overall effectiveness and safety of MXSG for CAP.
Methods:
Eight databases (PubMed, Embase, the Cochrane Library, CNKI, Wanfang, VIP, Yiigle, and Sinomed) were searched from their inception to January 20, 2025. Randomized controlled trials evaluating the effectiveness and safety of MXSG alone or in combination with conventional western medicine (WM) for CAP were included. We conducted meta-analysis by RevMan 5.4 software or just performed qualitative analysis.
Results:
We included 81 RCTs with 6682 participants in total. Compared with western medicine (WM) alone, MXSG plus WM showed a more beneficial effect on reducing the duration of fever (MD = −1.58 days, 95% CI: −1.88 to −1.29, p < 0.00001), cough (MD = −2.30 days, 95% CI: −2.61 to −1.99, p < 0.00001), phlegm (MD = −2.40 days, 95% CI: −2.56 to −2.23, p < 0.00001), dyspnea (MD = −2.11 days, 95% CI: −2.73 to −1.49, p < 0.00001), pulmonary crepitation (MD = −2.13 days, 95% CI: −2.47 to −1.79, p < 0.00001) and length of hospitalization (MD = −1.38 days, 95% CI: −2.54 to −0.23, p = 0.02). Furthermore, MXSG plus WM was significantly superior to WM in promoting the absorption of lung inflammation (MD = −3.31 days, 95% CI: −4.17 to −2.46, p < 0.00001) and improving forced expiratory volume in the first second (MD = 0.54 L, 95% CI: 0.21 to 0.87, p = 0.001). The incidence of adverse events was 3.60% in MXSG plus WM group and 5.38% in WM group, but the difference was not significant (p = 0.06).
Conclusion:
Moderate or low certainty of evidence suggested that compared with WM alone, MXSG combined with WM may have potential effectiveness on relieving the clinical symptoms, promoting the absorption of lung inflammation, improving lung function, and reducing hospitalization length with a good safety for patients with CAP. In the future, high-quality double-blind RCTs should be required to confirm the effectiveness and safety on CAP.
Systematic review registration:
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42023404693, identifier CRD42023404693.
1 Introduction
Community-acquired pneumonia (CAP) is an acute inflammation of lung parenchyma which occurs outside the hospital, including pneumonia that develops during the incubation period after hospital admission. Common clinical manifestations of CAP include fever, cough, expectoration, dyspnea, chest distress, chest pain, and localized auscultatory abnormalities (1). CAP is one of the leading causes of death from infectious diseases with high morbidity and mortality in all ages (2). In the United States, more than 1.5 million adults are hospitalized for CAP each year, 100,000 deaths of inpatients occur during hospitalization and approximately a third of hospitalization patients with CAP die within 1 year (1, 3). A study in 2020 showed that the annual incidence of CAP in China was about 713 patients per 100,000 people (4). Besides, CAP also becomes one of the major burdens on healthcare resources with high medical cost. For CAP inpatients, the mean healthcare cost is 11148 dollars in simple cases and 51219 dollars in complicated cases (5). CAP can be caused by a variety of pathogenic microorganisms such as bacteria, viruses, fungi, and other atypical pathogens. Streptococcus pneumoniae, human rhinovirus, and influenza virus are the most frequently identified pathogens (2). Other common pathogens include Staphylococcus aureus, Mycoplasma pneumoniae, Legionella, and Enterobacteriaceae (2).
The conventional treatments for CAP involve anti-infective treatment and symptomatic supportive treatment. Prior to pathogen identification, antibiotics should be selected according to the patient age, comorbidities, risk factors, severity of disease, antibiotic allergies, the most possible pathogen, and local epidemiological patterns (2). Referring to the guideline of American Thoracic Society and Infectious Diseases Society of America, amoxicillin, doxycycline, or a macrolide are recommended for CAP patients without comorbidities or risk factors (6). For patients with comorbidities, the choices of antibiotics involve a monotherapy with a respiratory fluoroquinolone as well as a combination therapy of amoxicillin/clavulanate or a cephalosporin and macrolide or doxycycline (6). However, the irrational use of antibiotics may result in potential adverse events (7). And the increasing antibiotic resistance has brought great difficulty to empiric treatment of CAP and has become a significant global public health problem (8). In this context, traditional Chinese medicine (TCM) has distinctive features and obvious advantages. Clinical evidence indicated that TCM alone could reduce antibiotic utilization in the treatment of none-severe CAP (9). Furthermore, the combination of TCM and western medicine may decrease the treatment failure rate and mortality in treating severe CAP (9).
Maxing Shigan Decoction (MXSG) is one of the classical TCM formulations which has been applied to treating respiratory infectious diseases for thousands of years. Originally documented in Treatise on Febrile and Miscellaneous Diseases, MXSG consists of Ephedrae Herba (Mahuang, Ephedra sinica Stapf), Armeniacae Semen Amarum (Xingren, Semen Armeniacae Amarum), Gypsum Fibrosum (Shigao, Gypsum Fibrosum), Radix Glycyrrhizae (Gancao, Glycyrrhiza uralensis Fisch.). The combination of these four kinds of Chinese herbal medicine plays the role of clearing heat, freeing lung and calming panting, which is suitable for the TCM syndrome of lung heat congestion characterized by fever, cough, asthma, thirst, thin white or yellow tongue coating, and slippery rapid pulse. Modern pharmacological studies have corroborated that MXSG may have antipyretic, anti-inflammatory, antibacterial, antiviral, and immunomodulatory effects (10–12). For instance, a preclinical study demonstrated the antiviral and anti-inflammatory pharmacodynamic functions and the mechanism of MXSG by network pharmacology and in vitro experimental verification, which also indicated the positive role of MXSG in combating COVID-19 (10). In another in vitro model of Mycoplasma pneumoniae infection in A549 cell culture, the experiment results revealed that MXSG played anti-inflammatory action by reducing NLRP3, pro-IL-1β, Caspase-1, pro-Caspase-1, and GSDMD-N (11).
Numerous clinical trials have provided evidence demonstrating positive therapeutic effect of MXSG on CAP (13, 14). Furthermore, Several Chinese clinical guidelines also recommended MXSG as a complementary medicine in CAP treatment (9, 15). In recent years, growing medical researchers have developed a large number of Chinese herbal formulations based on MXSG by adjusting composition and dosage of the original formulation, which brings more possibility and variety into clinical research. Therefore, this review aims to evaluate the total effectiveness and safety of MXSG for CAP by systematic review and try to explore the potential factors which may affect the therapeutic effect.
2 Materials and methods
2.1 Study registration
This systematic review was conducted referring to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 (16) and Cochrane Handbook for Systematic Reviews of Interventions (17). We have registered a protocol of this review in PROSPERO (CRD42023404693).
2.2 Eligibility criteria
2.2.1 Type of studies
All design modes of randomized controlled trials (RCTs) evaluating the effectiveness and safety of MXSG in the treatment of CAP were involved in this review, regardless of source or country. Duplicate publications were also included. However, we would only extract the data in publication with more complete information.
2.2.2 Type of participants
Participants meeting the diagnostic criteria for CAP (6) were eligible for enrollment: (1) community-acquired onset; (2) new chest imaging findings demonstrating patchy infiltrates, lobar or segmental consolidation, ground-glass opacities, or interstitial changes; and (3) at least one of the following clinical manifestations: (i) new-onset cough, sputum production, exacerbation of pre-existing respiratory symptoms, chest pain, dyspnea, or hemoptysis; (ii) fever; (iii) signs of pulmonary consolidation or the presence of wet rales on auscultation; or (iv) peripheral white blood cell count > 10 × 109/L or <4 × 109/L. This diagnosis requires the exclusion of alternative conditions including tuberculosis, lung tumors, non-infectious interstitial lung diseases, pulmonary edema, atelectasis and pulmonary embolism. Besides, patients with comorbidities such as tumors, tuberculosis, bronchiectasis, and other serious diseases were excluded. There were no restrictions on age, gender, or race of participants.
2.2.3 Type of interventions
Interventions involved oral MXSG or modified MXSG (including Ephedrae Herba, Armeniacae Semen Amarum, Gypsum Fibrosum and Radix Glycyrrhizae), without limitations on dosage, frequency or dosage form. The treatment group received MXSG alone or in combination with conventional western medicine (WM). WM included antibiotics, antipyretic, antitussive, expectorant, antiasthmatic and other symptomatic treatment. Control group received either no intervention, placebo, waiting-list management or WM alone. It should be noted that if the WM in both groups were different, the study was excluded. The control group could not contain TCM therapy.
2.2.4 Type of comparisons
The following comparisons were included in this review: MXSG vs. placebo, MXSG vs. WM, MXSG plus WM vs. WM, and MXSG+WM1 vs. WM1+WM2.
2.2.5 Type of outcome measures
The included studies were required to report at least one of the following outcomes:
(1) Main outcomes
Resolution time of symptoms (defined as the duration from treatment initiation until complete alleviation of target symptoms): Resolution time of fever, cough, phlegm, pulmonary crepitations, dyspnea, and chest pain.
Adverse events: The specific adverse events that occurred during treatment and the incidence of adverse events were recorded.
(2) Additional outcomes
Laboratory indicators: White blood cell, C-reactive protein and procalcitonin.
Improvement of chest radiographs: Improvement rate of chest radiograph (defined as the proportion of patients demonstrating improvement on chest imaging after treatment), the absorption time of lung inflammation (the duration from treatment initiation until complete clearance of pulmonary inflammatory lesions).
Lung function: Forced vital capacity (FVC), forced expiratory volume in the first second (FEV1) and peak expiratory flow (PEF).
Length of hospital stay.
All-cause mortality: The proportion of deceased patients to the total enrolled cohort during the study.
2.3 Search strategy
Eight databases were searched from their inception to January 20, 2025, including Chinese National Knowledge Infrastructure (CNKI), Wanfang Database, Chongqing VIP Database (VIP), Yiigle Database, Chinese Biomedical Literature Database (Sinomed), Cochrane Library, PubMed and Embase. We also retrieved the reference lists of included studies, relevant systematic reviews and clinical trial registers to find studies meeting the inclusion criteria. The detailed search strategies were in Supplementary File 1.
2.4 Data selection and extraction
Two reviewers independently screened titles and abstracts to identify potentially relevant studies using NoteExpress3.6.0 software and further judged possible relevant studies by reading the full text. Disagreements were resolved through discussion with a third author.
After the selection process, six reviewers independently performed data extraction by using pretested Excel data extraction forms. The data to extract includes (1) basic information of the eligible studies (such as author, year of publication, and sample size) (2) characteristics of participants (such as age and gender) (3) details of the interventions (such as dose, dosage form, and course) (4) outcome data.
2.5 Risk of bias
Five reviewers independently used the Cochrane Risk of Bias 2 tool (17) to evaluate the quality of included RCTs based on the following five items: randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome and selection of the reported result. We reported all the risks of bias described above, and judged each item from three levels: “high risk,” “low risk,” and “some concerns.” Each RCT was evaluated an overall bias by two reviewers independently. Any disagreement was resolved by consensus or with the discussion of a third review author.
2.6 Data syntheses
Meta-analysis was performed when studies had homogeneity. And qualitative analysis would be used when both subgroup analysis and sensitivity analysis could not explain the source of heterogeneity. RevMan 5.4 software was applied for meta-analysis. We evaluated effect size by using relative risk (RR) with 95% CI for dichotomous data, mean difference (MD) or standardized mean difference (SMD) with 95% CI for continuous data.
Because of the variation in TCM treatments (including composition, dosage, dosage form, and administration frequency), there was considerable clinical heterogeneity among the included studies. Therefore, we chose the random effects model to pool the overall effects. Heterogeneity between studies was evaluated using the statistic I2. When there was substantial heterogeneity (I2 > 50%), subgroup analysis or sensitivity analysis would be conducted to understand the source of heterogeneity or we just conducted qualitative integrated description.
Sensitivity analysis was performed to test the robustness of the results by excluding the included studies one by one to see if there were clinical differences. Funnel plot and Egger’s test were conducted to evaluate publication bias if there were at least 10 RCTs for certain outcome.
2.7 Subgroup analysis
Subgroup analysis would be performed when there was substantial heterogeneity (I2 > 50%). In consideration of participants’ characteristics, TCM therapy, and other clinical diversity, we would try to conduct subgroup analysis according to the following clinical factors: (1) age of participants (patients aged under 14 years old were regarded as children, in the range of 14–65 years old as adults, and over 65 years old as elders; (2) severity of CAP; (3) flavored quantity of Chinese herbal medicine (the flavored quantity referred to the modification amount of Chinese herbal medicine except for Ephedrae Herba, Semen Armeniacae Amarum, Gypsum Fibrosum and Radix Glycyrrhizae in Modified MXSG); (4) whether to take syndrome differentiation and treatment (syndrome differentiation and treatment meant researchers would add or cut several Chinese herbal medicine in the formulation according to the specific symptom or syndrome of each participant).
2.8 Trial sequential analysis
To reduce type I (false positive) and type II (false negative) errors, we conducted trial sequential analysis (TSA) using TSA Viewer version 0.9.5.10 (Copenhagen: Copenhagen trial Unit) to control the risk of random errors and estimated the required sample size for robust meta-analysis conclusion. We defined the risk of type I errors as 5% and risk of type II errors as 20%. For dichotomous outcomes, we applied 33.09% relative risk reduction (RRR) based on the results of previous RCTs to calculate required information size (RIS). For continuous outcomes, we used the built-in empirical algorithm in the software to calculate RIS. When the cumulative Z curve entered the futility area or crossed the trial sequential monitoring boundary, a sufficient level of evidence may have been reached to confirm the results. If the Z curve didn’t cross any boundaries and RIS has not been reached, more trials would be required.
2.9 Certainty of evidence
Two authors independently assessed the certainty of evidence by using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach in GRADEpro GDT software1. Any disagreement would be considered by a third author to make final decision.
3 Results
3.1 Screening
3804 records were identified initially from the databases and 1987 duplicates were removed before screening. After reading the titles and abstracts, we excluded 784 studies according to the eligibility criteria. Nine studies were excluded because of the lack of full text, so only 1024 studies were downloaded. And 78 studies were included after reading the whole text. Meanwhile, we also retrieved the references of included studies and found three studies meeting the eligibility. Eventually, 81 studies (18–98) were included in this review. More details were presented in the PRISMA flow chart (Figure 1).
FIGURE 1

PRISMA flow chart of studies searching and screening.
3.2 Characteristics of included studies
All RCTs included were conducted in China and published between 2006 and 2024, with 80 RCTs reported in Chinese and one in English. A total of 6682 participants aged from 2 months to 94 years old were enrolled. 15 RCTs focused on children, 10 RCTs only enrolled elders, 20 RCTs paid attention to adults, and the participants of the remaining 36 RCTs incorporated patients at different ages. In total, 20 studies reported severity of CAP, of which four studies recruited patients with severe CAP and the other 16 recruited patients with none-severe CAP. Only 10 studies reported the types of pathogen and participants in seven studies were infected with Mycoplasma pneumonia. The included studies covered a total of four comparison types, which were respectively, MXSG vs. placebo, MXSG vs. WM, MXSG plus WM vs. WM, and MXSG+WM1 vs. WM1+WM2.
In terms of intervention, only five studies used Standard MXSG (S-MXSG, the original prescription of MXSG), and the remaining used Modified MXSG (M-MXSG, the modified prescription of MXSG added with more Chinese herbal medicine based on S-MXSG). As for the composition, M-MXSG in 76 included studies were additionally supplemented with other kinds of Chinese herbal medicine. The additional Chinese herbal medicine mainly played the effects of clearing heat and detoxifying, relieving cough and reducing sputum, freeing lung and relieving asthma, thus assisting MXSG to play a more significant effect (Supplementary Table 1). Furthermore, more than 60% studies applied M-MXSG containing more than double the amount of Chinese herbal medicine than that in the original formulation. Almost half of the included studies took TCM syndrome differentiation and treatment based on the fixed prescription, according to the specific symptoms or TCM syndrome of each patient. The other studies gave the same fixed decoction to all participants. A total of 78 RCTs used antibiotics in WM group. And the kinds of antibiotics mainly incorporated macrolides (such as azithromycin), quinolones (such as levofloxacin and moxifloxacin), and cephalosporins (such as cefoperazone and ceftriaxone). For the report of primary outcomes, 31 studies evaluated the duration of symptoms and 33 studies recorded adverse events. As for secondary outcomes, 51 RCTs reported relevant laboratory indicators, 46 RCTs mentioned the improvement of chest radiographs, six RCTs evaluated lung function, seven recorded the hospitalization length, and only one reported mortality. The composition of Chinese herbal formulations could be seen in Supplementary Table 2. The details of the included studies were shown in Table 1.
TABLE 1
| Study ID | Sample size | Gender (male/female) | Age (years old) | Duration of symptoms before treatments | Intervention | Course of treatment (d) | Outcome | ||||
| T | C | T | C | T | C | T | C | ||||
| MXSG+WM vs. WM, 78 studies | |||||||||||
| Chen (18) | 53 | 14/13 | 15/11 | 65.3 ± 2.7 | 66.1 ± 2.5 | (7.1 ± 0.5) d | (7.3 ± 0.6) d | M- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 30 | ➀➂ |
| Cheng et al. (20) | 58 | 15/14 | 16/13 | 72.37 ± 3.26 | 73.38 ± 3.15 | (5.24 ± 1.27) d | (5.31 ± 1.42) d | M-MXSG+moxifloxacin | Moxifloxacin | 14 | ➁➂➃ |
| Cheng et al. (21) | 70 | 18/17 | 16/19 | 59.68 ± 15.98 | 58.77 ± 15.28 | NR | NR | M-MXSG+antibiotics | Antibiotics | 10 | ➂➃ |
| Cheng et al. (22) | 80 | 23/17 | 17/23 | 51.63 ± 20.00 | 54.43 ± 20.68 | (36.82 ± 18.06) h | (38.47 ± 19.73) h | N- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 7 | ➂➄ |
| Chu (23) | 94 | 25/22 | 26/21 | 41.87 ± 6.76 | 42.29 ± 6.82 | (7.15 ± 1.52) d | (7.38 ± 1.64) d | M-MXSG+cefuroxime sodium+symptomatic treatment | Cefuroxime sodium+symptomatic treatment | 7 | ➂➃ |
| Cui et al. (24) | 160 | 52/28 | 48/32 | 52.82 ± 6.61 | 52.45 ± 6.58 | (2.63 ± 1.73) m | (12.47 ± 1.71) m | M- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 7 | ➁➂➃➄ |
| Dai (25) | 110 | 32/23 | 30/25 | 53.32 ± 10.21 | 52.89 ± 9.92 | (7.45 ± 1.31) d | (7.49 ± 1.31) d | M-MXSG+symptomatic treatment | Symptomatic treatment | NR | ➁➂➃ |
| Deng (26) | 88 | 24/20 | 25/19 | 61.6 ± 7.8 | 61.2 ± 7.5 | (6.8 ± 2.1) d | (6.5 ± 2.2) d | N- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 10 | ➂ |
| Dong et al. (27) | 106 | 25/28 | 29/24 | 52.14 ± 10.93 | 51.36 ± 11.85 | (4.30 ± 0.82) d | (4.32 ± 0.84) d | M-MXSG+imipenem and cilastatin sodium+symptomatic treatment | Imipenem and cilastatin sodium+symptomatic treatment | 7 | ➃ |
| Du (28) | 300 | 82/68 | 79/71 | 2.29 ± 0.53 | 2.32 ± 0.49 | NR | NR | S-MXSG+azithromycin+symptomatic treatment | Azithromycin+symptomatic treatment | 21 | ➂ |
| Fang and Long (29) | 98 | 21/28 | 23/26 | 5.0 ± 1.2 | 5.2 ± 1.4 | (2.3 ± 0.4) d | (2.4 ± 0.4) d | M-MXSG+cefodizime sodium+symptomatic treatment | Cefodizime sodium+symptomatic treatment | 14 | ➀ |
| Fei (30) | 60 | 15/15 | 14/16 | 75.2 ± 11.85 | 73.3 ± 12.44 | NR | NR | M-MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 7 | ➂ |
| Gao (31) | 94 | 23/24 | 25/22 | 7.14 ± 1.49 | 6.93 ± 1.65 | (6.98 ± 1.02) d | (7.34 ± 0.96) d | M-MXSG+azithromycin | Azithromycin | 21 | ➃ |
| Guo (32) | 87 | 25/19 | 23/20 | 61.01 ± 8.34 | 61.64 ± 7.20 | (3.46 ± 0.59) d | (3.41 ± 0.64) d | M- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 12 | ➀➁➂ |
| He (33) | 60 | 20/19 | 11/9 | 67 ∼ 89 | 67 ∼ 90 | NR | NR | M- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 5 ∼ 7 | ➂ |
| Hu (34) | 60 | 30 | 30 | 52.63 ± 5.41 | 53.76 ± 5.44 | NR | NR | M- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 5 | ➂ |
| Hu (35) | 130 | 66 | 64 | 3 ∼ 7 | 3 ∼ 7 | NR | NR | S-MXSG+azithromycin+symptomatic treatment | Azithromycin+symptomatic treatment | 12 | ➂➃ |
| Huo et al. (36) | 78 | NR | NR | 4.77 ± 0.52 | 4.77 ± 0.52 | NR | NR | M-MXSG+cefodizime sodium+symptomatic treatment | Cefodizime sodium+symptomatic treatment | 14 | ➀➁➂➃ |
| Jin (37) | 84 | 9/31 | 14/26 | 49.73 ± 2.19 | 50.40 ± 2.18 | NR | NR | M-MXSG+levofloxacin+symptomatic treatment | Levofloxacin+symptomatic treatment | 10 (±3) | ➀➁➂➃ |
| Kong et al. (38) | 120 | 34/26 | 31/29 | 54.38 ± 9.97 | 55.91 ± 9.26 | (12.81 ± 3.73) d | (12.06 ± 3.84) d | S- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 21 | ➁➄ |
| Li (39) | 79 | 20/19 | 23/17 | 18 ∼ 65 | 20 ∼ 64 | (4.00 ± 2.12) d | (4.00 ± 2.02) d | M-MXSG+antibiotics | Antibiotics | 10 ∼ 14 | ➀➁➃ |
| Li (40) | 45 | 14/8 | 13/10 | 53.7 ± 2.8 | 51.2 ± 3.7 | (5.7 ± 1.1) d | (6.1 ± 0.9) d | M-MXSG+moxifloxacin+ symptomatic treatment |
Moxifloxacin+symptomatic treatment | 10 | ➀➁➂➃ |
| Li et al. (41) | 88 | 25/19 | 23/21 | 4.8 ± 0.7 | 4.9 ± 0.7 | (4.5 ± 0.8) d | (4.6 ± 0.7) d | M-MXSG+azithromycin | Azithromycin | 7 | ➁➂➃ |
| Li and Zhang (42) | 100 | 27/24 | 27/22 | 67.9 ± 12.7 | 65.8 ± 11.9 | (7.8 ± 4.2) d | (8.1 ± 3.9) d | M- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 10 | ➀➁➂ |
| Li et al. (43) | 84 | 24/18 | 23/19 | 37.4 ± 10.2 | 37.2 ± 10.1 | (6.5 ± 1.3) d | (6.4 ± 1.5) d | M-MXSG+cefoxitin sodium+symptomatic treatment | Cefoxitin sodium+symptomatic treatment | 7 | ➀➂ |
| Li and Huang (44) | 74 | 18/19 | 17/20 | 6.25 ± 1.28 | 6.12 ± 1.23 | (17.62 ± 3.74) d | (17.28 ± 3.44) d | M-MXSG+azithromycin+ symptomatic treatment |
Azithromycin+symptomatic treatment | 12 | ➀➁➃ |
| Liu (45) | 68 | 18/16 | 19/15 | 54.38 ± 4.83 | 54.44 ± 4.91 | (16.35 ± 4.92) h | (16.28 ± 4.87) h | M-MXSG+cefoperazone sodium and sulbactam sodium+azithromycin | Cefoperazone sodium and sulbactam sodium+azithromycin | 14 | ➀➃➅ |
| Liu (46) | 60 | 11/19 | 13/17 | 38.43 ± 5.28 | 35.24 ± 6.23 | (3.54 ± 1.02) d | (3.54 ± 1.03) d | M-MXSG+levofloxacin | Levofloxacin | 10 | ➃ |
| Liu et al. (47) | 80 | 24/16 | 23/17 | 53.29 ± 1.23 | 52.78 ± 1.04 | (65.23 ± 9.27) h | (64.83 ± 9.16) h | M-MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 7 ∼ 14 | ➀➄ |
| Liu et al. (51) | 62 | 17/14 | 15/16 | 55.59 ± 4.27 | 56.08 ± 4.19 | NR | NR | M-MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 14 | ➁➂ |
| Liu et al., (48) | 50 | 25 | 25 | 70.5 ± 4.7 | 70.5 ± 4.7 | NR | NR | M-MXSG+moxifloxacin | Moxifloxacin | 7 | ➀➂➃ |
| Liu (49) | 60 | 19/11 | 16/14 | 37.43 ± 13.70 | 39.47 ± 16.49 | (4.06 ± 1.26) d | (3.86 ± 1.09) d | M-MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 7 | ➁➃➅ |
| Huo et al. (36) | 78 | NR | NR | 4.77 ± 0.52 | 4.77 ± 0.52 | NR | NR | M-MXSG+cefodizime sodium+symptomatic treatment | Cefodizime sodium+symptomatic treatment | 14 | ➀➁➂➃ |
| Liu et al. (50) | 60 | 18/12 | 17/13 | 38.13 ± 13.70 | 39.27 ± 16.19 | (4.06 ± 1.26) d | (3.86 ± 1.09) d | M-MXSG+antibiotics+ symptomatic treatment | antibiotics+ symptomatic treatment | 7 | ➂ |
| Lu (52) | 74 | NR | NR | 13∼78 | 13∼78 | NR | NR | M-MXSG+ulinastain+symptomatic treatment | Ulinastain+symptomatic treatment | 7 | ➁ |
| Ma (53) | 106 | 31/22 | 30/23 | 55.01 ± 7.70 | 54.60 ± 7.52 | (3.11 ± 0.83) d | (3.02 ± 0.71) d | M-MXSG+cefuroxime sodium+symptomatic treatment | Cefuroxime sodium+symptomatic treatment | 14 | ➀➂➃➅ |
| Ma (54) | 60 | 15/14 | 16/13 | 51.97 ± 15.69 | 48.56 ± 17.09 | NR | NR | M-MXSG+levofloxacin+symptomatic treatment | Levofloxacin+symptomatic treatment | 7 | ➁➃ |
| Ma and Chen (55) | 62 | 18/13 | 16/15 | 35 ∼ 84 | 28 ∼ 86 | 2∼12 d | 2∼11 d | M-MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 15 | ➀➃ |
| Ma (56) | 100 | 26/24 | 31/19 | 2.711 ± 2.259 | 1.700 ± 1.801 | (7.600 ± 4.899) d | (8.020 ± 4.565) d | M-MXSG+cefuroxime sodium+symptomatic treatment | Cefuroxime sodium+symptomatic treatment | 7 | ➁➂ |
| Meng et al. (57) | 80 | 22/18 | 24/16 | 62.50 ± 8.30 | 64.20 ± 7.50 | (65.70 ± 9.50) h | (63.80 ± 8.60) h | M-MXSG+cefoperazone sodium and sulbactam sodium+symptomatic treatment | Cefoperazone sodium and sulbactam sodium+symptomatic treatment | 7 ∼ 14 | ➀➂ |
| Mo et al. (58) | 53 | 15/12 | 14/12 | 55.62 ± 4.50 | 55.24 ± 4.36 | NR | NR | M-MXSG+imipenem and cilastatin sodium+ulinastain+symptomatic treatment | Imipenem and cilastatin sodium+ulinastain+symptomatic treatment | 14 | ➂➃ |
| Mo (59) | 50 | 14/11 | 12/13 | 47.28 ± 13.21 | 46.44 ± 9.59 | NR | NR | M-MXSG+moxifloxacin+ambroxol hydrochloride | Moxifloxacin+ambroxol hydrochloride | 7 | ➁➂➃ |
| Ni (60) | 80 | 22/18 | 21/19 | 51.34 ± 3.23 | 51.36 ± 3.21 | (4.23 ± 0.34) d | (4.21 ± 0.32) d | M-MXSG+cefotaxime sodium | Cefotaxime sodium | 7 | ➀➁➄ |
| Ning et al. (61) | 86 | 27/16 | 26/17 | 58.15 ± 6.04 | 58.09 ± 6.01 | (4.22 ± 1.03) d | (4.29 ± 1.08) d | M-MXSG+cefuroxime sodium | Cefuroxime sodium | 7 | ➀➂➃ |
| Shen et al. (62) | 80 | 40 | 40 | 64.37 ± 11.35 | 64.71 ± 11.45 | (7.23 ± 4.28) d | (7.14 ± 4.33) d | M-MXSG+antibiotics+N-symptomatic treatment | Antibiotics+symptomatic treatment | 10 | ➁➂ |
| Shen and Zhou (63) | 50 | 13/12 | 14/11 | 35 ∼ 70 | 28 ∼ 72 | 2∼7 d | 2∼8 d | M-MXSG+antibiotics | Antibiotics | 10 | ➂➃ |
| Shi (64) | 100 | 27/23 | 26/24 | 44.56 ± 6.35 | 47.36 ± 7.65 | (4.51 ± 0.63) d | (4.62 ± 0.53) d | M-MXSG+levofloxacin | Levofloxacin | 10 | ➀ |
| Song (65) | 100 | 28/22 | 26/24 | 4.85 ± 1.12 | 4.76 ± 1.33 | (3.13 ± 1.03) d | (3.08 ± 1.01) d | M-MXSG+cefuroxime sodium or azithromycin+symptomatic treatment | Cefuroxime sodium or azithromycin+symptomatic treatment | 7 | ➀➁➂➃ |
| Su and Yang (66) | 60 | 20/10 | 16/14 | 62.7 ± 7.9 | 63.5 ± 8.3 | (65.3 ± 9.8) h | (64.6 ± 9.0) h | M-MXSG+cefoperazone sodium and sulbactam sodium | Cefoperazone sodium and sulbactam sodium | 7∼14 | ➃ |
| Su et al. (67) | 80 | 23/17 | 25/15 | 40 ± 5.6 | 43 ± 6.8 | (3 ± 1.2) d | (3 ± 1.1) d | S-MXSG+cefuroxime sodium | Cefuroxime sodium+symptomatic treatment | 14 | ➃ |
| Sun (68) | 64 | 17/15 | 18/14 | 67.15 ± 7.95 | 64.65 ± 8.25 | (7.15 ± 4.34) d | (7.32 ± 4.28) d | M- -MXSG+cefazolin sodium and levofloxacin+symptomatic treatment | Cefazolin sodium and levofloxacin+symptomatic treatment | 10 | ➂ |
| Sun et al. (69) | 108 | 28/26 | 29/25 | 9.6 ± 8.4 | 9.2 ± 8.8 | (1.6 ± 0.4) d | (1.4 ± 0.5) d | M-MXSG+cefoperazone sodium and sulbactam sodium+symptomatic treatment | Cefoperazone sodium and sulbactam sodium+symptomatic treatment | 7 | ➀➂➃➅ |
| Sun (70) | 130 | 38/27 | 35/30 | 67. 28 ± 5. 15 | 67. 28 ± 5. 15 | NR | NR | M-MXSG+cefuroxime sodium+symptomatic treatment | Cefuroxime sodium+symptomatic treatment | 20 | ➀➁➂➃ |
| Tang and Chen (71) | 90 | 25/20 | 24/21 | 69.60 ± 6.10 | 71.16 ± 6.51 | NR | NR | M-MXSG+cefoperazone sodium and sulbactam sodium+symptomatic treatment | Cefoperazone sodium and sulbactam sodium+symptomatic treatment | 10 | ➁➂➃ |
| Tian and Hu (73) | 76 | 23/15 | 25/13 | 42.6 ± 7.5 | 41.8 ± 7.9 | (2.3 ± 1.1) d | (2.0 ± 1.2) d | M-MXSG+levofloxacin+symptomatic treatment | Levofloxacin+symptomatic treatment | 10 | ➃ |
| Wang et al. (74) | 60 | 30 | 30 | NR | NR | NR | NR | M-MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 10 | ➂ |
| Wang and Zhou (75) | 50 | 14/11 | 15/10 | 50 | 41 | 3.5d | 4d | M-MXSG+azithromycin+ cefoperazone sodium and sulbactam sodium |
Azithromycin+cefoperazone sodium and sulbactam sodium | 10 | ➃ |
| Wang et al. (76) | 60 | 13/17 | 14/16 | 5.3 ± 1.4 | 5.2 ± 1.3 | (23.8 ± 3.2) d | (23.5 ± 2.8) d | M-MXSG+azithromycin+ symptomatic treatment |
Azithromycin+symptomatic treatment | 14 | ➀ |
| Wang (77) | 56 | 20/8 | 18/10 | 55.18 ± 4.7 | 54.23 ± 5.6 | NR | NR | M-MXSG+moxifloxacin | Moxifloxacin | 14 | ➃ |
| Wang (78) | 106 | 33/25 | 30/18 | 3.63 ± 1.54 | 3.87 ± 1.83 | 4.23 d | 4.52 d | M-MXSG+cefoperazone sodium and sulbactam sodium+symptomatic treatment | Cefoperazone sodium and sulbactam sodium+symptomatic treatment | 7 | ➀➅ |
| Wang (79) | 60 | 17/13 | 16/14 | 49.69 ± 4.38 | 49.75 ± 4.42 | NR | NR | M- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 7∼10 | ➁➂ |
| Wu et al. (80) | 123 | 33/30 | 32/28 | 7.6 ± 1.7 | 7.3 ± 1.8 | (4.3 ± 1.4) d | (4.2 ± 1.6) d | M-MXSG+azithromycin | Azithromycin | 21 | ➀➃ |
| Wu et al. (81) | 82 | 17/23 | 20/22 | 59.3 ± 14.6 | 61.2 ± 13.7 | (9.6 ± 2.4) d | (10.9 ± 2.4) d | N- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 10 | ➀➂➅ |
| Xiao (82) | 80 | 25/12 | 23/15 | 50.135 ± 13.039 | 49.711 ± 13.160 | NR | NR | M-MXSG+moxifloxacin+ symptomatic treatment |
Moxifloxacin+symptomatic treatment | 10 | ➁➂➃ |
| Xie (83) | 60 | 23/7 | 24/6 | 2.814 ± 1.503 | 2.883 ± 1.0228 | NR | NR | M-MXSG+ceftriaxone sodium+symptomatic treatment | Cefuroxime sodium+symptomatic treatment | 7 | ➀➅ |
| Xie et al. (84) | 96 | 29/19 | 26/22 | 73.47 ± 4.38 | 71.23 ± 6.16 | (3.78 ± 1.60) d | (3.60 ± 2.07) d | M- -MXSG+antibiotics+symptomatic treatment | Moxifloxacin+symptomatic treatment | 14 | ➂ |
| Xin (85) | 120 | 51 | 69 | 46.76 ± 13.72 | 46.76 ± 13.72 | (4.79 ± 2.36) d | (4.79 ± 2.36) d | M-MXSG+latamoxef sodium+symptomatic treatment | Latamoxef sodium+symptomatic treatment | 7 | ➁➂ |
| Xu (86) | 60 | 19/11 | 20/10 | 32 ∼ 78 | 33 ∼ 79 | NR | NR | M-MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 14 | ➂➃➆ |
| Xu (87) | 60 | 18/12 | 17/13 | 43.25 ± 4.35 | 44.10 ± 3.87 | NR | NR | M-MXSG+symptomatic treatment | Symptomatic treatment | 5 | ➁➂ |
| Yang (88) | 88 | NR | NR | NR | NR | 3.2 d | 3.2 d | M- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 10 | ➃ |
| Yang (89) | 60 | 18/12 | 17/13 | 45.5 ± 3.8 | 45.8 ± 3.9 | (4.32 ± 1.57) d | (4.29 ± 1.45) d | M-MXSG+cefoperazone sodium and sulbactam sodium+symptomatic treatment | Cefoperazone sodium and sulbactam sodium+symptomatic treatment | 14 | ➀➃ |
| Yang (90) | 62 | 21/10 | 22/9 | 65 ∼ 79 | 65 ∼ 80 | 5–10 d | 4–11 d | M-MXSG+cefoperazone sodium and sulbactam sodium+levofloxacin | Cefoperazone sodium and sulbactam sodium+levofloxacin | 7 | ➂ |
| Yuan (91) | 60 | 16/14 | 17/13 | 66.43 ± 8.23 | 64.73 ± 6.97 | (3.87 ± 1.66) d | (3.37 ± 1.59) d | N- -MXSG+levofloxacin+symptomatic treatment | Levofloxacin+symptomatic treatment | 10 | ➁➂ |
| Zhang (92) | 80 | 26/14 | 25/15 | 52.78 ± 3.52 | 52.78 ± 3.52 | (6.31 ± 2.76) d | (6.23 ± 1.89) d | M- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 10 | ➁➃ |
| Zhang (93) | 120 | NR | NR | 5.84 ± 1.47 | 5.67 ± 1.62 | (7.65 ± 3.14) d | (5.81 ± 1.76) d | M-MXSG+azithromycin+ symptomatic treatment |
Azithromycin+symptomatic treatment | 14 | ➀➁ |
| Zhou et al. (95) | 80 | 23/17 | 25/15 | 49.60 ± 6.10 | 50.16 ± 6.51 | (10.95 ± 1.60) d | (10.80 ± 1.79) d | M-MXSG+cefuroxime sodium+levofloxacin+symptomatic treatment | Cefuroxime sodium+ levofloxacin+symptomatic treatment |
10 | ➁➂➃ |
| Zhou (96) | 90 | 26/19 | 27/18 | 66.08 ± 6.13 | 66.32 ± 6.74 | NR | NR | M-MXSG+cefuroxime sodium | Cefuroxime sodium | 14 | ➀➃ |
| Zhu et al. (97) | 102 | 34/17 | 32/19 | 60.12 ± 19.97 | 60.12 ± 18.32 | (9.93 ± 2.02) d | (9.32 ± 1.77) d | M-MXSG+symptomatic treatment | Symptomatic treatment | 5 | ➁➂ |
| Zou et al. (98) | 60 | 18/12 | 16/14 | 42.1 ± 14.2 | 41.3 ± 15.1 | NR | NR | M- -MXSG+antibiotics+symptomatic treatment | Antibiotics+symptomatic treatment | 10 | ➂ |
| MXSG vs. WM, 1 study | |||||||||||
| Chen et al. (19) | 40 | 8/12 | 10/10 | 61.95 ± 12.37 | 60.15 ± 9.21 | (3.20 ± 1.01) d | (3.30 ± 1.17) d | M-MXSG+symptomatic treatment | Levofloxacin+symptomatic treatment | 10 | ➂➃ |
| MXSG+WM 1 vs. WM 1+WM 2, 1 study | |||||||||||
| Tian et al. (72) | 98 | 22/27 | 23/26 | 49.6 ± 2.35 | 49.8 ± 2.36 | (6.12 ± 3.1) d | (6.14 ± 3.13) d | M-MXSG+moxifloxacin | Moxifloxacin+symptomatic treatment | 14 | ➁➂➃ |
| MXSG vs. placebo, 1 study | |||||||||||
| Zheng et al. (94) | 80 | 16/20 | 17/18 | 4.28 ± 0.96 | 4.47 ± 1.09 | (37.00 ± 14.63) h | (39.26 ± 14.16) h | S-MXSG | Placebo | 10 | ➀➁➃ |
Characteristics of the included studies.
T, treatment group; C, control group; m, month; d, day; h, hour; NR, not report; S-MXSG, Standard-Maxing Shigan Decoction; M-MXSG, Modified-Maxing Shigan Decoction. ➀ Resolution time of clinical symptoms (fever, cough, phlegm, pulmonary crepitation, dyspnea, chest pain). ➁ Adverse events. ➂ Relevant laboratory indicators (WBC, CRP, PCT). ➃ Improvement of chest radiograph (improvement rate of chest radiograph, absorption time of lung inflammation). ➄ Lung function (FVC, FEV1, PEF). ➅ Length of hospitalization. ➆ All-cause mortality.
3.3 Risk of bias assessment
In the aspect of randomization, 42 RCTs used random number tables and one RCT used software to generate random sequences, but the other 38 RCTs failed to report random sequence generation. Besides, none of studies reported the information of allocation concealment. However, in all the included studies, there was no significant baseline difference among intervention groups. Therefore, all studies were determined to be of uncertain risk.
In terms of deviations from intended interventions, only one study conducted blind method by applying MXSG placebo in control group, but the other 80 studies failed to blinded participants. Furthermore, a total of three studies didn’t use appropriate analysis to estimate the effect of assignment to intervention. After comprehensive assessment, two studies were rated as high risk, while the others were considered to have an uncertain risk.
As for the bias of outcome measurement, only two studies were judged as low risk, among which one study blinded the outcome assessors, and the other used objective laboratory outcomes. The remaining 79 studies didn’t mention the blinding of outcome assessment so that were rated as high risk.
For incomplete outcome data, 77 RCTs had no or only a few missing data and were assessed as low risk. But the drop-out rates in three RCTs were over 5% and the other one RCT didn’t report the number of patients after treatment, which resulted in high risk for this item.
As to the selective reporting, none of studies mentioned registration protocol. Of which, two studies didn’t sufficiently report the expected outcome indicators, so were considered as unclear risk. In the remaining 79 studies, the outcomes in Section “3 Results” were the same as that in Section “2 Materials and methods,” resulting in low risk of bias.
In conclusion, only two RCTs were judged to have moderate risk of bias and the other RCTs were considered as high risk of bias (Figure 2).
FIGURE 2

Risk of bias assessment for eligible studies.
3.4 Primary outcomes
3.4.1 Resolution time of fever
3.4.1.1 MXSG+WM versus WM
A total of 28 RCTs (2399 participants) took this type of comparison. Compared with WM alone, MXSG plus WM significantly reduced fever duration (MD = −1.58 days, 95% CI: −1.88 to −1.29, p < 0.00001; I2 = 97%) (Figure 3).
FIGURE 3

Forest plot of resolution time of fever by age. Comparison: MXSG plus WM vs. WM. MXSG, Maxing Shigan Decoction; WM, western medicine.
For subgroup analysis, heterogeneity in all subgroups decreased when classified by age, but high heterogeneity still existed in children group and adults plus elders group. Furthermore, MXSG plus WM may be more effective for elders on fever resolution (MD = −3.12 days, 95% CI: −3.28 to −2.96, p < 0.00001; I2 = 0%). However, neither the application of syndrome differentiation nor the flavored quantity of Chinese herbal medicine accounted for the overall high heterogeneity. And subgroup analysis based on the severity of pneumonia was failed to performed as most studies didn’t report this information (Supplementary Table 3). Although sensitivity analysis demonstrated the robustness of the findings, it could not fully explain the sources of heterogeneity. Through a comprehensive analysis of the included studies, we found that the high heterogeneity might stem from the clinical diversity including inconsistent baseline disease severity, significant differences in baseline disease duration and variations in western medical treatment plans. Additionally, most studies did not clearly define the measurement standards and methods for the resolution time of fever, which might lead to methodological heterogeneity.
3.4.1.2 MXSG versus placebo
Only one study (80 participants) compared MXSG with placebo using median time to record the duration of fever. The median time to fever resolution in MXSG group was 0.5 (. to.) days, which was shorter than 1.0 (0.5–1.5) days in control group (p < 0.05).
3.4.2 Resolution time of cough
By comparing MXSG plus WM with WM, 25 RCTs (2157 participants) reported the resolution time of cough. The pooled data showed that the duration of cough in MXSG plus WM group was shorter than that in WM group (MD = −2.30 days, 95% CI: −2.61 to −1.99, p < 0.00001; I2 = 87%) (Figure 4).
FIGURE 4

Forest plot of resolution time of cough. Comparison: MXSG plus WM vs. WM. MXSG, Maxing Shigan Decoction; WM, western medicine.
When conducting subgroup analyses based on age, flavored quantity of Chinese medicine, and the application of syndrome differentiation, the heterogeneity within some subgroups did not decrease (Supplementary Table 3). To further explore heterogeneity sources, sensitivity analysis revealed that the exclusion of two trials by Huo HM (36) and Wang JH (76) reduced overall heterogeneity to I2 = 42%, indicating these studies were key contributors of heterogeneity. Distinctive features of these trials included: First, the included patients were all children with pneumonia aged 4–5 years old; second, the types of pneumonia were special, including bronchopneumonia and Mycoplasma pneumonia; third, the baseline symptom duration of cough was relatively long, with an average of 23.8 days. Additionally, unreported assessment methods for cough outcomes in most studies may introduce methodological measurement bias.
3.4.3 Resolution time of phlegm
Nine RCTs including 805 participants evaluated this outcome. When compared with WM group, the resolution time of phlegm in MXSG plus WM group was significantly shorter. (MD = −2.40 days, 95% CI: −2.56 to −2.23, p < 0.00001; I2 = 4%) (Figure 5).
FIGURE 5
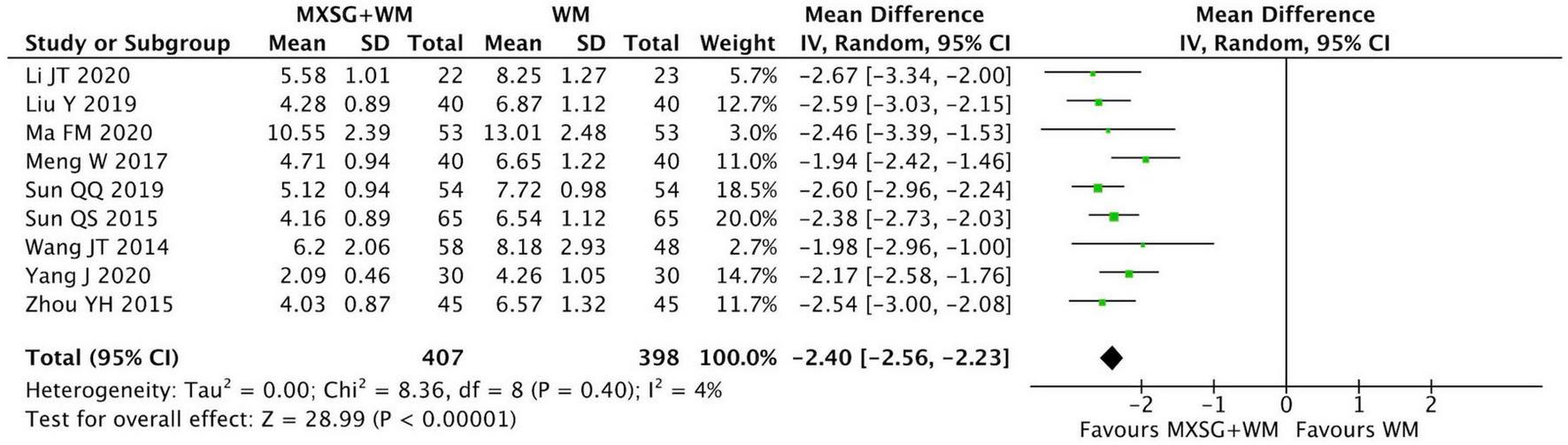
Forest plot of resolution time of phlegm. Comparison: MXSG plus WM vs. WM. MXSG, Maxing Shigan Decoction; WM, western medicine.
3.4.4 Resolution time of dyspnea
Five studies involving 490 participants compared MXSG plus WM with WM alone on this outcome. The pooled data indicated that the resolution time of dyspnea in MXSG plus WM group reduced more than that in WM group (MD = −2.11 days, 95% CI: −2.73 to −1.49, p < 0.00001; I2 = 91%) (Figure 6).
FIGURE 6
![Forest plot showing the mean difference in effectiveness of flavored Chinese medicine compared to control. Two subgroups are analyzed: flavored quantity less than or equal to four and less than eight. Both subgroups show significant negative mean differences, indicating greater effectiveness of the treatment. The overall mean difference is \(-2.11\) with a 95% confidence interval of \([-2.73, -1.49]\), favoring the experimental treatment.](https://www.frontiersin.org/files/Articles/1639027/xml-images/fmed-12-1639027-g006.webp)
Forest plot of resolution time of dyspnea by flavored quantity of Chinese medicine. Comparison: MXSG plus WM vs. WM. MXSG, Maxing Shigan Decoction; WM, western medicine.
The result of subgroup analysis revealed that MXSG plus WM may be more effective in relieving dyspnea when the number of additional Chinese medicine was in the range of four to eight (MD = −2.61 days, 95% CI: −2.92 to −2.30, p < 0.00001; I2 = 0%). And the heterogeneity in each group had a significant reduction (Supplementary Table 3).
3.4.5 Resolution time of chest pain
None of studies reported this outcome.
3.4.6 Resolution time of pulmonary crepitation
23 studies (2025 participants) reporting the resolution time of pulmonary crepitation were pooled in a meta-analysis. The result demonstrated that the pulmonary crepitation in MXSG plus WM group disappeared faster than that in WM group (MD = −2.13 days, 95% CI: −2.47 to −1.79, p < 0.00001; I2 = 89%) (Figure 7).
FIGURE 7

Forest plot of resolution time of pulmonary crepitation by age. Comparison: MXSG plus WM vs. WM. MXSG, Maxing Shigan Decoction; WM, western medicine.
Meanwhile, we observed that age may be the source of heterogeneity for this outcome. The data revealed that MXSG plus WM may reduce the duration of pulmonary crepitation more effectively on elders with CAP (MD = −3.41 days, 95% CI: −3.89 to −2.94, p < 0.00001; I2 = 33%) (Supplementary Table 3).
3.4.7 Adverse events
In total, 33 studies reported adverse events including nausea, inappetence, emesis, diarrhea, rash, dizziness, and other mild symptoms. Among these studies, 17 RCTs declared that no adverse events were found in both groups and the remaining 16 RCTs recorded the specific adverse reactions in detail (Table 2). The incidence of adverse reactions was 3.60% in MXSG plus WM group and 5.38% in WM group. (RR = 0.70, 95% CI: 0.48 to 1.01, p = 0.06; I2 = 0%) (Figure 8 and Supplementary Table 3).
TABLE 2
| Treatment group | Control group | |||
| Study ID | Number of adverse events | Specific adverse event | Number of adverse events | Specific adverse event |
| MXSG+WM vs. WM | ||||
| Cui et al. (24) | 8 | 4 for nausea; 1 for headache; 2 for sweating; 1 for dizzy | 6 | 3 for nausea; 1 for headache; 1 for sweating; 1 for dizzy |
| Cheng et al. (20) | 0 | NR | 0 | NR |
| Dai (25) | 4 | 3 for inappetence; 1 for diarrhea | 7 | 2 for nausea and emesis; 3 for inappetence; 1 for mild increase of AST; 1 for mild increase of ALT |
| Guo (32) | 3 | 3 for nausea, emesis and diarrhea | 4 | 2 for dizziness and headache; 1 for nausea, emesis and diarrhea; 1 for lethargy |
| Huo et al. (36) | 3 | 1 for mild laryngeal irritation; 1 for whiny; 1 for rash | 6 | 2 for hoarseness; 2 for mild increase of ALT; 1 for mild laryngeal irritation; 1 for pain of mouth and tongue |
| Jin (37) | 1 | 1 for loose stool | 2 | 1 for dizzy and hyperactive; 1 for nausea, stomach discomfort and abdominal distension |
| Kong et al. (38) | 8 | 2 for nausea and emesis; 2 for abdominal pain and diarrhea; 2 for inappetence; 2 for rash | 5 | 2 for nausea and emesis; 1 abdominal pain; 1 for inappetence; 1 for rash |
| Li (39) | 0 | NR | 0 | NR |
| Li (40) | 1 | 1 for dizzy | 2 | 1 for dizzy; 1 for nausea |
| Li et al. (41) | 0 | NR | 0 | NR |
| Li and Zhang (42) | 0 | NR | 1 | 1 for increased frequency of bowel movement |
| Li et al. (43) | 0 | NR | 0 | NR |
| Liu (51) | 3 | NR | 0 | NR |
| Liu (49) | 0 | NR | 0 | NR |
| Lu (52) | 5 | 2 for diarrhea; 1 for decrease of WBC; 2 for rash | 6 | 2 for decrease of WBC; 1 for diarrhea; 3 for rash |
| Ma (54) | 0 | NR | 0 | NR |
| Ma (56) | 0 | NR | 1 | 1 for considered allergic to cefuroxime |
| Mo (59) | 0 | NR | 0 | NR |
| Ni (60) | 0 | NR | 0 | NR |
| Shen and Zhou (63) | 0 | NR | 0 | NR |
| Song (65) | 0 | NR | 0 | NR |
| Sun (70) | 0 | NR | 0 | NR |
| Tang and Chen (71) | 3 | 1 for diarrhea; 2 for inappetence | 6 | 4 for inappetence and nausea; 2 for mild increase of ALT and AST |
| Wang (79) | 1 | 1 for nausea and emesis | 8 | 3 for abdominal discomfort; 3 for nausea and emesis; 2 for minor rash |
| Xiao (82) | 0 | NR | 0 | NR |
| Xin (85) | 0 | NR | 0 | NR |
| Xu (87) | 0 | NR | 0 | NR |
| Yuan (91) | 0 | NR | 0 | NR |
| Zhang (92) | 2 | 2 for increase of BUN | 1 | 1 for increase of BUN |
| Zhang (93) | 4 | 2 for mild nausea and emesis; 1 for abdominal pain; 1 for rash | 12 | 4 for mild nausea and emesis; 3 for mild diarrhea; 3 for mild abdominal pain; 2 for rash |
| Zhou et al. (95) | 1 | 1 for nausea and inappetence | 3 | 3 for nausea and inappetence |
| MXSG+WM 1 vs. WM 1+WM 2 | ||||
| Tian et al. (72) | 0 | NR | 0 | NR |
| MXSG vs. placebo | ||||
| Zheng et al. (94) | 0 | NR | 0 | NR |
Adverse events of MXSG for patients with CAP in the included studies.
T, treatment group; C, control group; NR, not report; AST, glutamic oxaloacetic transaminase; ALT, glutamic pyruvic transaminase; WBC, white blood cell; BUN, blood urea nitrogen.
FIGURE 8
![Forest plot displaying a meta-analysis of studies comparing MXSG+WM against WM alone. The plot includes risk ratios with 95 percent confidence intervals for individual studies, with weights and overall effects shown. Some entries are listed as “Not estimable.” The diamond represents the overall effect size of 0.70 with a 95 percent confidence interval of [0.48, 1.01], suggesting no significant difference. The heterogeneity is low, with an I2 of 0 percent.](https://www.frontiersin.org/files/Articles/1639027/xml-images/fmed-12-1639027-g008.webp)
Forest plot of the incidence of adverse events. Comparison: MXSG plus WM vs. WM. MXSG, Maxing Shigan Decoction; WM, western medicine.
3.5 Secondary outcomes
3.5.1 C-reactive protein (CRP)
3.5.1.1 MXSG+WM versus WM
38 RCTs involving 3293 participants compared the level of CRP between MXSG plus WM and WM alone. There was high heterogeneity (I2 = 96%) among the studies. And all of the planned subgroup analyses could not explain the source of heterogeneity (Supplementary Table 3). Consequently, we just performed a narrative synthesis instead of combining the data for a meta-analysis (Supplementary Table 4).
Among these 38 RCTs, the results of 34 studies all indicated that CRP in MXSG plus WM group decreased more than that in WM group. However, there was no significant change on CRP in the remaining four studies.
3.5.1.2 MXSG versus WM
Only one study (40 patients) evaluated the level of CRP by comparing MXSG with levofloxacin injection. The result showed that no significant difference was found between the two groups (p = 0.27).
3.5.1.3 MXSG+WM 1 versus WM 1+WM 2
The other study compared MXSG plus moxifloxacin injection with moxifloxacin injection plus antipyretic and expectorants. Result showed that MXSG plus antibiotic had more reduction in CRP compared with control group (MD = −4.03 mg/L, 95% CI: −4.43 to 3.63; p < 0.00001). The concrete results of CRP were presented in Supplementary Table 4.
3.5.2 White blood cell (WBC)
3.5.2.1 MXSG+WM versus WM
33 studies (2435 participants) reported WBC level in this comparison type, among which the results of 23 studies showed that the level of WBC in MXSG plus WM group reduced more than in WM group. In the remaining 10 studies, the difference between two groups was not notable (Supplementary Table 4). We did not conduct a meta-analysis because of the high heterogeneity (I2 = 92%) even after subgroup analyses (Supplementary Table 3).
3.5.2.2 MXSG versus WM
One RCT with 40 patients compared MXSG with levofloxacin injection on the level of WBC. The result indicated that there was no significant change between two groups (MD = −0.29 × 109/L, 95% CI: −1.46 to 0.88; p = 0.63).
3.5.2.3 MXSG+WM 1 versus WM 1+WM 2
The remaining one RCT (90 participants) took this comparison type. Compared with moxifloxacin injection plus antipyretic and expectorants, WBC level in MXSG plus moxifloxacin injection group reduced more (MD = −2.15 × 109/L, 95% CI: −3.43 to −0.87; p = 0.001). More details of the results on WBC were in Supplementary Table 4.
3.5.3 Procalcitonin (PCT)
3.5.3.1 MXSG+WM versus WM
By comparing MXSG plus WM with WM alone, 28 studies including 2379 participants evaluated this outcome. All the results of 26 RCTs demonstrated that MXSG plus WM was superior to WM alone on decreasing the level of PCT. However, in the other two studies, no significant difference was observed between two groups (Supplementary Table 4). The heterogeneity (I2 = 95%) among studies was too high to perform meta-analysis (Supplementary Table 3).
3.5.3.2 MXSG+WM 1 versus WM 1+WM 2
The result of the other study showed that MXSG plus moxifloxacin injection group had a lower level of PCT after treatment when compared with moxifloxacin injection plus antipyretic and expectorants (MD = −0.19 ng/ml, 95% CI: −0.29 to −0.09; p = 0.0003). The specific data of PCT could be seen in Supplementary Table 4.
3.5.4 Absorption time of lung inflammation
Nine studies reported the absorption time of lung inflammation and applied X-rays to observe the change of lung radiograph.
3.5.4.1 MXSG+WM versus WM
Eight RCTs incorporating 687 participants evaluated this outcome. The pooled data indicated that the absorption of lung inflammation in MXSG plus WM group was faster than that in WM group (MD = −3.31 days, 95% CI: −4.17 to −2.46, p < 0.00001; I2 = 72%) (Figure 9).
FIGURE 9

Forest plot of absorption time of lung inflammation by flavored quantity of Chinese medicine. Comparison: MXSG plus WM vs. WM. MXSG, Maxing Shigan Decoction; WM, western medicine.
When the flavored quantity of Chinese medicine was over eight, the combination of MXSG and WM seemed to be more effective in promoting the absorption of lung inflammation (MD = −4.75 days, 95% CI: −5.70 to −3.79, p < 0.00001; I2 = 0%). Besides, the heterogeneity decreased in each subgroup (Supplementary Table 3).
3.5.4.2 MXSG+WM 1 versus WM 1+WM 2
Only one study compared MXSG plus moxifloxacin injection with moxifloxacin injection plus symptomatic treatment on this outcome. The data indicated that there was no statistical difference between two groups in the absorption time of lung inflammation (MD = −4.17 days, 95% CI: −8.43 to 0.09, p = 0.06).
3.5.5 Improvement rate of chest radiograph
For this outcome, 35 studies applied X-rays to observe the absorption of lung inflammation, eight studies used chest CT, one study applied both measurement methods at the same time, and the remaining one study did not report the measurement method.
3.5.5.1 MXSG+WM versus WM
A total of 42 RCTs involving 2244 participants measured this outcome. The heterogeneity among studies was high (I2 = 73%) and we could not find the source of it by the predefined subgroup analyses (Supplementary Table 3). Among these RCTs, 25 studies declared that the difference in the improvement rate of chest radiographs was not significant between two groups. However, results of the remaining 17 studies demonstrated that MXSG plus WM was better in improving the chest radiograph of CAP patients than WM alone (Supplementary Table 4).
3.5.5.2 MXSG versus WM
One study compared the treatment of MXSG with levofloxacin injection and the improvement rates of chest radiographs in both groups were 100%.
3.5.5.3 MXSG+WM 1 versus WM 1+WM 2
One study took this comparison type and there was no statistical difference between the treatment of MXSG plus moxifloxacin injection and moxifloxacin injection plus antipyretic and expectorants on the improvement rate of chest radiograph (RR = 1.05, 95% CI: 0.96 to 1.15, p = 0.29).
3.5.5.4 MXSG versus placebo
Only one study took this comparison type and no significant change was found between MXSG group and placebo group on this outcome (RR = 1.04, 95% CI: 0.87 to 1.24, p = 0.69). The details of improvement rate of chest radiographs were presented in Supplementary Table 4.
3.5.6 Lung function: forced vital capacity (FVC)
Five RCTs compared MXSG plus WM with WM alone on this outcome. Among them, two studies demonstrated that MXSG plus WM group had a better effect on FVC than WM group. However, the other two studies showed that FVC in WM group improved more than MXSG plus WM group. And in the remaining one study, the difference between groups was not significant (Supplementary Table 4). Heterogeneity among studies was still high even after subgroup analyses, so meta-analysis was failed to perform (I2 = 98%) (Supplementary Table 3).
3.5.7 Lung function: forced expiratory volume in the first second (FEV1)
Four RCTs reported this outcome. The data indicated that MXSG plus WM had a better effect on FEV1 than WM alone (MD = 0.54L, 95% CI: 0.21 to 0.87, p = 0.001; I2 = 97%) (Figure 10). Heterogeneity in each group had an obvious reduction when subgroup analysis was classified by age. Furthermore, we found that MXSG plus WM seemed to be more effective for adults plus elders group on FEV1 compared with WM alone (MD = 0.64L, 95% CI: 0.56 to 0.71, p < 0.00001; I2 = 0%) (Supplementary Table 3).
FIGURE 10

Forest plot of FEV1 by age. Comparison: MXSG plus WM vs. WM. MXSG, Maxing Shigan Decoction; WM, western medicine.
3.5.8 Lung function: peak expiratory flow (PEF)
Two studies compared MXSG plus WM with WM on this outcome. The results of both studies indicated that the level of PEF in MXSG plus WM group was higher than that in WM group after treatment. However, the heterogeneity was too high to pool the data (I2 = 97%) (Supplementary Tables 3, 4).
3.5.9 Length of hospitalization
Seven RCTs involving 590 participants evaluated the length of hospitalization. The results demonstrated that MXSG plus WM may shorten the length of hospitalization when compared with WM alone (MD = −1.38 days, 95% CI: −2.54 to −0.23, p = 0.02; I2 = 94%) (Figure 11).
FIGURE 11

Forest plot of length of hospitalization by whether to take syndrome differentiation and treatment. Comparison: MXSG plus WM vs. WM. MXSG, Maxing Shigan Decoction; WM, western medicine.
We found that heterogeneity within each group reduced in subgroup analysis based on whether to take syndrome differentiation and treatment, but substantial heterogeneity (I2 = 91%) remained in the “without syndrome differentiation and treatment” subgroup. Furthermore, MXSG plus WM may show better effect on reducing the hospitalization time when taking syndrome differentiation and treatment (MD = −2.61 days, 95% CI: −3.72 to −1.49, p < 0.00001; I2 = 73%). Besides, age and the flavored quantity of Chinese medicine failed to substantially reduce heterogeneity within each subgroup (Supplementary Table 3). Sensitivity analysis revealed that when the studies by Xie JJ (83), Liu YR (49) and Sun QQ (69) were excluded, the overall heterogeneity decreased to I2 = 0%, indicating that these studies likely contributed to the observed heterogeneity. Further analysis of study characteristics demonstrated that the high heterogeneity may originate from the variation in antibiotic selection within western medicine regimens, the differences in herb dosage and compatibility during TCM interventions and the methodological limitation arising from ill-defined discharge criteria.
3.5.10 Mortality
Only one RCT recorded the outcome of mortality. There was no statistically significant difference in mortality between MXSG plus WM and WM alone (RR = 0.25, 95% CI: 0.06 to 1.08; p = 0.06).
3.6 Sensitivity analysis
The result of sensitivity analysis revealed that most of our findings were robust. However, for incidence of adverse events, the significance of difference (p = 0.06) changed to p ≤ 0.04 after excluding the studies of Cui CR (24) (n = 160, RR = 1.33, 95% CI: 0.48 to 3.67, p = 0.58), Kong FH (38) (n = 120, RR = 1.60, 95% CI: 0.56 to 4.61, p = 0.38), Liu YF (51) (n = 62, RR = 7.00, 95% CI: 0.38 to 130.1, p = 0.19) and Zhang (92) (n = 80, RR = 2.00, 95% CI: 0.19 to 21.18, p = 0.56), respectively. We found that the results of these studies all indicated the incidence of adverse events in MXSG plus WM group might be higher than WM group, but the difference was not statistically significant. By analyzing the study characteristics, we found the potential reasons for the instability of results: First, these studies had relatively small or moderate sample sizes compared to the other studies. The instability of their results might have an impact on the point estimate and confidence interval, especially when the event occurrence rate was already low; Second, the excluded studies had inconsistent definitions, collection and reporting of adverse events. Cui CR 2022 (24) and Kong FH 2023 (38) captured symptomatic adverse events, Zhang JJ 2020 (92) only reported the specific laboratory indicator abnormality of “serum urea nitrogen” as an adverse event, while Liu YF 2023 (51) reported unspecified events. Therefore, the certainty of findings may be influenced to some extent and our conclusion should be treated with caution (Supplementary Figure 1).
3.7 Publication bias
Egger’s test results indicated that there was no evidence of publication bias in the resolution time of fever (p = 0.156), resolution time of cough (p = 0.095) and the incidence of adverse events (p = 0.550). However, the resolution time of pulmonary crepitation had a significant publication bias (p = 0.019) (Supplementary Figure 2). And the above results were basically consistent with funnel plots (Supplementary Figure 3). Publication bias was failed to detected for other outcomes because of the insufficient number of the included studies.
3.8 Trial sequential analysis
According to TSA, Z-curve of length of hospitalization crossed conventional significant threshold, but didn’t reach the TSA line and RIS line, suggesting the existence of a false positive result. Z-curves of the incidence of adverse events neither crossed the conventional significant threshold and the futility boundaries, nor reached the RIS line, indicating the possibility of a false negative error and the need for more trials to prove the conclusion. TSA of other outcomes didn’t show false positive or negative error (Supplementary Figure 4).
3.9 Certainty of evidence
Grade method was applied to evaluate the certainty of evidence for all important outcomes. Certainty of evidence was rated as moderate or low mainly due to the risk of bias (lack of allocation concealment and blinding as well as the selective reporting), inconsistency (I square value was high), imprecision and the potential publication bias. More details about the certainty assessment were shown in Figure 12.
FIGURE 12

Certainty of evidence.
4 Discussion
4.1 Summary of main findings
This review demonstrated that compared with WM alone, the combination of MXSG and WM may have potential effective on decreasing the length of hospitalization as well as the duration of symptoms including fever, cough, phlegm, dyspnea, and pulmonary crepitation by 1∼3 days. It may also promote the absorption of lung inflammation and improve lung function better than WM alone. However, these findings should be interpreted with caution given the moderate-to-low certainty of evidence. For mortality, there was no statistically significant difference between MXSG plus WM and WM alone. The heterogeneities among studies on laboratory indicators and the improvement rate of chest radiographs were too high to pool the data. In addition, MXSG alone seemed to have a better antipyretic effect than placebo, but it didn’t show statistical difference in other outcomes compared with either WM group or placebo group. Regarding safety, no serious adverse events occurred during treatment and the incidence of adverse events in MXSG plus WM group was not higher than WM group.
4.2 Interpretation of the results
The pathogenesis of CAP primarily involved local and systemic inflammatory responses caused by pathogen invasion. These inflammatory responses accounted for most clinical symptoms, physical signs, and abnormalities in laboratory and imaging findings (99). In this regard, MXSG demonstrated anti-inflammatory and antipyretic effects by inhibiting leukocyte adhesion, suppressing the release of inflammatory factors and inflammatory cell infiltration, as well as improving endotoxin-induced pulmonary interstitial edema (100). Additionally, MXSG played an anti-infection effect by inhibiting the proliferation of pathogens, blocking the storm of inflammatory factors, and improving the imbalance of intestinal flora to show (101). Furthermore, MXSG presented a positive effect on relieving cough and asthma by inhibiting the release of allergic substances, reducing bronchial epithelial damage, and relieving bronchospasm (102). Consequently, adding MXSG with antibiotics had a better effect on relieving fever, cough and dyspnea, promoting the absorption of pulmonary inflammation as well as improving lung function, thereby potentially reducing hospitalization length. However, we couldn’t draw a conclusion of the effectiveness of MXSG on the laboratory indicators due to the high heterogeneity, which may result from the different detection methods, treatment course, detection time, and disease baseline in each study. Meanwhile, the influence of MXSG on mortality was also uncertain, because most of the included studies didn’t report this outcome.
For subgroup analyses, we found that when compared with WM alone, the effect of MXSG plus WM was more notable for elders on reducing the duration of fever (−3.12 vs. −1.45 days, interaction p < 0.00001). In addition, for adults plus elders group, MXSG plus WM significantly decreased the duration of pulmonary crepitation (−3.41 vs. −1.88 days, interaction p < 0.00001) and improved FEV1 (0.64L vs. 0.19L, interaction p < 0.00001). These findings suggested that age may influence the effectiveness of MXSG, possibly due to the different clinical features including etiology, risk factors, comorbidities, severity, and clinical presentation for CAP patients at different ages (103, 104). Besides, MXSG plus WM shortened the length of hospitalization more obviously in subgroup receiving syndrome differentiation and treatment (−2.61 vs. −0.91 days, interaction p = 0.04). TCM syndrome differentiation was known as a comprehensive analysis of clinical information obtained from observation, listening, questioning, and pulse (105). It provided more individual therapeutic schedules which could resolve different symptoms of each patient with pertinence, so that improving the effectiveness of intervention. In addition, the effectiveness of MXSG plus WM was more significant in relieving dyspnea when the flavored quantity of Chinese medicine was four to eight (−2.61 vs. −1.46 days, interaction p < 0.00001) and in reducing the absorption time of lung inflammation when the flavored quantity was over eight (−4.75 vs. −2.71 days, interaction p = 0.0008). The results indicated that compared with S-MXSG, M-MXSG seemed to be more suitable for complex clinical conditions in reality, because it contained more Chinese herbal medicine with different therapeutic actions. However, since subgroup analyses were exploratory and observational with no causal inferences should be drawn, the results only suggested potential associations between study characteristics and the intervention effect, and further studies were needed to verify the conclusion.
By analyzing the composition of M-MXSG, we found that in addition to the four fixed Chinese herbal medicine, the following Chinese herbal medicine were often added in clinical practice to achieve a synergistic effect: First, Heat-clearing and detoxifying Chinese herbal medicine, such as Baikal Skullcap Root (Huangqin, Scutellaria baicalensis Georgi) and Honeysuckle Flower (Jinyinhua, Lonicera japonica Thunb.), which have the antipyretic, anti-inflammatory, antibacterial and antiviral effects in pharmacology (106–108); Second, Wind-heat-dispersing Chinese herbal medicine, such as Peppermint Leaf (Bohe, Mentha haplocalyx Briq.) and Great Burdock Fruit (Niubangzi, Arctium lappa L.), demonstrating pharmacological effects such as diaphoretic, antipyretic, anti-pathogenic activity and anti-allergic action (109–111); Third, Lung-fire-clearing and asthma-relieving Chinese herbal medicine, such as Pepperweed Seed (Tinglizi, Lepidium apetalum Willd.) and White Mulberry Root Bark (Sangbaipi, Morus alba L.), which have pharmacological effects including relieving asthma, cough suppression and diuresis (112, 113); Forth, Cough-relieving and expectorant Chinese herbal medicine, such as Platycodon Root [Jiegeng, Platycodon grandiflorus (Jacq.) A.DC.] and Stemona Root [Baibu, Stemona sessilifolia (Miq.) Miq.], which have expectorant, cough-relieving and anti-inflammatory effects (114–116) (Supplementary Table 1).
4.3 Strengths and limitations
This systematic review presented three key methodological strengths: First, we have collected and extracted abundant clinical data. For example, we incorporated all Chinese herbal decoctions based on MXSG and a total of four comparison types. Second, we chose proper statistical methods to analyze data and deal with the heterogeneity among studies. Considering the diversity of clinical heterogeneity, we predefined several subgroup analyses to solve it and tried to explore more specific information to provide a reference for clinical practice. The subgroup analyses not only covered the common characteristics of participants but also considered the particularity of TCM treatment especially the influence of additional Chinese herbal medicine in MXSG as well as TCM syndrome differentiation and treatment. Furthermore, we observed that the grouping factors may be the source of heterogeneity, which confirmed our suspicions.
However, there were also some limitations. First, all studies were carried out in China which may result from that MXSG was mainly used in China and has not been popularized abroad. Second, the methodology quality of the included RCTs was generally medium to low, which resulted from the inadequate reporting of randomization process, the lack of double-blind method and the loss of registration protocol. As a consequence, the risk of bias was relatively high, resulting in a reduction on the certainty of conclusion. Third, the clinical heterogeneity of the included studies was high, which brought challenges to quantitative analysis and even resulted in the failure to conduct meta-analysis. Moreover, due to the differences in the composition and dosage of MXSG as well as TCM syndrome differentiation and treatment, it was hard to repeat the research and made the standardization of TCM difficult. The above differences mainly arose from the different ages and conditions of participants in each study, as well as the different clinical experiences and medication habits of physicians, which may lead to great clinical heterogeneity. Furthermore, this was also the problem and challenge of clinical trials related to TCM compounds. However, we could not thoroughly deny the effectiveness of TCM because of its diversity. The same and standardized therapy was not necessarily the most effective for each individual. And the unique feature of TCM precisely lies in its individualized treatment plan such as the modification in formulation as well as syndrome differentiation and treatment. Consequently, while our findings provided preliminary evidence regarding the effectiveness of MXSG for CAP, the study conclusion should be treated with appropriate caution because of these limitations.
4.4 Comparison with previous studies
Current systematic reviews of MXSG on CAP demonstrated that MXSG could shorten the duration of fever, cough, phlegm, pulmonary crepitation, and the absorption time of lung inflammation (13, 14, 117–119). The relevant evidence also showed that MXSG may reduce infection indices (such as CAP, WBC and PCT) and improve effective rate. Moreover, none of the reviews reported serious adverse reactions which could reflect the safety of MXSG. In conclusion, these findings were consistent with our review, which confirmed that MXSG may bring benefits in relieving the symptoms and signs of CAP patients with a great safety.
In contrast to previous studies with similar subject, our review included a larger amount of RCTs with wider research range. Meanwhile, this was an update and supplement to systematic reviews on MXSG for patients with CAP. Additionally, we included various comparison types including MXSG alone or MXSG plus WM in treatment groups and WM or placebo in control groups. Last but not least, we also designed several subgroup analyses based on the clinical diversity and characteristics of TCM therapy to explore the source of heterogeneity, which could enrich the thoughts for research and clinical treatment.
4.5 Implications
In future clinical practice, MXSG was suggested to be used in treating CAP as an adjunctive therapy to antibiotics. Importantly, the application of MXSG should adhere to TCM syndrome differentiation principle. The kind and dosage of Chinese herbal medicine were supposed to be adjusted according to the specific symptoms of patients, rather than employing a standardized formulation for all patients. In order to achieve a better curative effect, we recommended that based on S-MXSG, more Chinese herbal medicine demonstrating the effect of clearing lung heat, reducing phlegm or relieving cough should be added to the decoction.
To enhance the methodological quality, we advised strict adherence to CONSORT statement for the reporting of RCTs. It was also suggested that researchers register a protocol in advance and record the URL (Uniform Resource Locator) in articles to decrease the reporting bias. In order to improve the quality of future clinical trials, random sequence generation and allocation concealment were advised to be conducted strictly referring to Cochrane Handbook17. Besides, TCM placebo had better be given to control group to realize the blinding of participants and researchers. And the outcome of follow-up was supposed to be added in future trials to observe long-term curative effect of MXSG on CAP, such as mortality. This series of measures would improve the methodology quality and enhance the certainty of conclusion.
In consideration of the variety of TCM treatment, researchers could conduct dose-effect analysis by designing clinical trials to explore whether different dosages and ratios of Chinese herbal medicine would influence the effectiveness of MXSG on CAP patients. It was also recommended to perform data mining to analyze the composition principles of MXSG applied in modern times, which could provide guiding value for clinical practice. Furthermore, future clinical studies based on real world should also research the influence of TCM syndrome differentiation and treatment on the effectiveness of MXSG.
Traditional Chinese medicine clinical research is facing a great methodological challenge due to the complex composition principle and dose-effect relationship of TCM compounds. To address these challenges, we propose the following research strategies: First, future researchers should establish classification criteria for TCM formulations and clinical evidence reporting standards, so that TCM clinical research is consistent with TCM theories and reflects the general principles of evidence-based medicine at the same time. Second, future clinical research on TCM formulations need to be implemented gradually in phases on the premise of basic experiments, and the research methods should be continuously innovated to facilitate effective translation and promotion of clinical evidence for TCM compounds.
5 Conclusion
Evidence of limited quality indicated that in contrast to WM, MXSG combined with WM may have potential positive effect on the treatment of CAP with a good safety. The subgroup analyses indicated that age, TCM syndrome differentiation and treatment as well as the flavored quantity of Chinese medicine may be the sources of clinical heterogeneity among studies. Because of the poor methodological quality and substantial heterogeneity, the evidence supporting our findings remained uncertain. Additionally, due to the certainty of evidence was moderate or low, the results of this review were suggested to be interpreted and applied with caution. Future TCM clinical trials should pay attention to the methodological quality to improve the reliability of evidence. Furthermore, researchers were advised to explore whether the composition, dose, flavored quantity, TCM syndrome differentiation and other relevant factors would influence the effectiveness of MXSG on CAP, which could optimize its clinical application for CAP management.
Statements
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YL: Conceptualization, Data curation, Formal analysis, Investigation, Project administration, Visualization, Writing – original draft. XL: Conceptualization, Methodology, Project administration, Supervision, Writing – review & editing. BZ: Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. QX: Data curation, Formal analysis, Visualization, Writing – original draft. ZS: Data curation, Formal analysis, Visualization, Writing – original draft. HB: Data curation, Formal analysis, Writing – original draft. RC: Data curation, Formal analysis, Writing – original draft. SL: Data curation, Formal analysis, Writing – original draft. MY: Data curation, Formal analysis, Writing – original draft. YZ: Conceptualization, Funding acquisition, Project administration, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National High Level Chinese Medicine Hospital Clinical Research Funding and the National Natural Science Foundation of China (grant number 82360906).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1639027/full#supplementary-material
Footnotes
References
1.
Aliberti S Dela Cruz CS Amati F Sotgiu G Restrepo MI . Community-acquired pneumonia.Lancet. (2021) 398:906–19. 10.1016/s0140-6736(21)00630-9
2.
Rothberg MB . Community-acquired pneumonia.Ann Intern Med. (2022) 175:Itc49–64. 10.7326/aitc202204190
3.
Ramirez JA Wiemken TL Peyrani P Arnold FW Kelley R Mattingly WA et al Adults Hospitalized with Pneumonia in the United States: Incidence, Epidemiology, and Mortality. Clin Infect Dis. (2017) 65:1806–12. 10.1093/cid/cix647
4.
Sun Y Li H Pei Z Wang S Feng J Xu L et al Incidence of community-acquired pneumonia in urban China: A national population-based study. Vaccine (2020) 38:8362–70. 10.1016/j.vaccine.2020.11.004
5.
Sato R Gomez Rey G Nelson S Pinsky B . Community-acquired pneumonia episode costs by age and risk in commercially insured US adults aged =50 years.Appl Health Econ Health Policy. (2013) 11:251–8. 10.1007/s40258-013-0026-0
6.
Metlay JP Waterer GW Long AC Anzueto A Brozek J Crothers K et al Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med. (2019) 200:e45–67. 10.1164/rccm.201908-1581ST
7.
Huttner B Cappello B Cooke G Gandra S Harbarth S Imi M et al 2019 Community-acquired Pneumonia Treatment Guidelines: There Is a Need for a Change toward More Parsimonious Antibiotic Use. Am J Respir Crit Care Med. (2020) 201:1315–6. 10.1164/rccm.201911-2226LE
8.
Liapikou A Cilloniz C Palomeque A Torres T . Emerging antibiotics for community-acquired pneumonia.Expert Opin Emerg Drugs. (2019) 24:221–31. 10.1080/14728214.2019.1685494
9.
Li D Zhi Y . Clinical Practice Guidelines for Chinese Medicine Alone or Combined with Antibiotics in the Treatment of Community-acquired Pneumonia.J China-Japan Friendship Hosp. (2021) 35:11–5.
10.
Li Y Chu F Li P Johnson N Li T Wang Y et al Potential effect of Maxing Shigan decoction against coronavirus disease 2019 (COVID-19) revealed by network pharmacology and experimental verification. J Ethnopharmacol. (2021) 271:113854. 10.1016/j.jep.2021.113854
11.
Liu F Liu T Sun M Zhou J Xue F Chen S et al Maxing Shigan Decoction Mitigates Mycoplasma pneumonia-Induced Pyroptosis in A549 Cells via the NLRP3 Inflammasome. Infect Drug Resist. (2021) 14:859–67. 10.2147/idr.S292413
12.
Zhong Y Zhou J Liang N Liu B Lu R He Y et al Effect of Maxing Shigan Tang on H1N1 Influenza A Virus-Associated Acute Lung Injury in Mice. Intervirology. (2016) 59:267–74. 10.1159/000458726
13.
Li K Yang F Pang W Cai H Zhang M Zhang J . Systematic Review of Randomized Controlled Trial of Maxing Shigan Decoction in Treatment of Community-Acquired Pneumonia.China J Chinese Materia Med. (2021) 46:1268–75. 10.19540/j.cnki.cjcmm.20200714.501
14.
Yuan Y . Meta-analysis on the Curative Effect and Safety of Maxing Shigan Decoction in the Treatment of Senile Pneumonia.Guangming J Chinese Med. (2019) 34:495–9.
15.
Yu X Xie Y Li J . Guidelines for Traditional Chinese Medicine Diagnosis and Treatment of Community-Acquired Pneumonia (2018 Revision).J Traditional Chinese Med. (2019) 60:350–60.
16.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj (2021) 372:n71. 10.1136/bmj.n71
17.
Higgins JPT Thomas J Chandler J Cumpston M Li T Page MJ et al Cochrane Handbook for Systematic Reviews of Interventions version 6.3. (2022).
18.
Chen A . Effectiveness of Maxing Shigan Decoction on Community-acquired Pneumonia with Lung-Heat Syndrome and its Influence on Serum Procalcitonin.Special Health. (2018) 16:246. 10.3969/j.issn.2095-6851.2018.16.373
19.
Chen Z Chen B Yuan L Chen J Yan G Lian L . Observation of the Modified Decoction on Phlegm-heat Obstruction Lungs Cases with Community-Acquired Pneumonia.J Emerg Traditional Chinese Med. (2010) 19:725–33.
20.
Cheng F Liu Z Yang Z Hu X . Effects of Maxing Shigan Decoction and Moxifloxacin in the Treatment of 29 Cases of Elderly Patients with Community-acquired Pneumonia.Shanghai Med Pharm J. (2018) 39:29–31.
21.
Cheng YF He R Wei S Yang C . Ephedra, Apricot Kernel, Gypsum and Licorice Decoction Combined with Modified Phragmites Stem Decoction for the Treatment of Thirty-Five Cases with Wind-Warm and Lung-Heat Disease of Phlegm-Heat Accumulating the Lung Syndrome.Henan Traditional Chinese Med. (2017) 37:102–4.
22.
Cheng Y He R Yang C . Effect of modified Maxing Shigan Decoction and Qianjin Weijing Decoction on Lung Function and Serum Inflammatord Serum Inflammatory Factors in Patients with Community-Acquired Pneumonia of Phlegm Heat obstructing Lung Type.Clin J Traditional Chinese Med. (2021) 33:755–7. 10.16448/j.cjtcm.2021.0440
23.
Chu Z . Clinical Observation on the Combination of Traditional Chinese Medicine and Western Medicine in the Treatment of Acute Pneumonia.J Practical Traditional Chinese Med. (2019) 35:50–1.
24.
Cui C Liu H Cong X . Effect of Maxingshigan Decoction Combined with Western Medicine on Inflammatory Factors and Lung Function in Patients with Chronic Pneumonia.Traditional Chinese Med J. (2022) 21:47–50.
25.
Dai L . Clinical Observation of Modified Maxing Shigan Tang for Community-Acquired Pneumonia in Adults with Phlegm-Heat in Lung Syndrome.J New Chinese Med. (2018) 50:41–4.
26.
Deng S . Clinical Effectiveness of Maxing Shigan Decoction in the Treatment of Community-Acquired Pneumonia.Heilongjiang J Traditional Chinese Med. (2018) 47:32–3. 10.19540/j.cnki.cjcmm.20200714.501
27.
Dong LH Wang YJ Zhu XL Zhao HY He C. Study on the curative effect of qinghua recipe on severe pneumonia patients and the changes of IL-1 β and IL-13, lac in serum of patients with severe pneumonia. Chin Arch Tradit Chin Med. (2023) 41:34–7. 10.13193/j.issn.1673-7717.2023.08.007
28.
Du B . Clinical Effect of Azithromycin Combined with Maxing Shigan Decoction in the Treatment of Pneumonia in Children.Smart Healthc. (2020) 6:112–4. 10.19335/j.cnki.2096-1219.2020.31.044
29.
Fang F Long Y . Clinical Study on Maxing Shigan Tang Combined with Oxygen Atomization Therapy with Budesonide Suspension for Inhalation in the Treatment of Pediatric Pneumonia.New Chinese Med. (2022) 54:1–5. 10.13457/j.cnki.jncm.2022.18.001
30.
Fei X. Clinical Observation on Immune Regulation of Qingfeirefang in Treating CAP of patients. Shanghai: Shanghai University of Traditional Chinese Medicine (2014).
31.
Gao GL . Effectiveness of Huanglian Jiedu Decoction Combined with Maxingshigan Decoction in the Treatment of Refractory Mycoplasma Pneumonia.J Pract Traditional Chinese Med. (2022) 38:594–5.
32.
Guo JW . Maxing Shigan Decoction in the Treatment of Community-Acquired Pneumonia of Pulmonary Heat Type.Chinese Med Modern Distance Educ China. (2023) 21:109–11. 10.3969/j.issn.1672-2779.2023.11.037
33.
He X. Observation on the Effectiveness of Modified Maxing Shigan Decoction Combined with Antibiotics in the Treatment of Elderly Community-acquired Pneumonia. Master. Beijing: Beijing University of Chinese Medicine (2011).
34.
Hu P . Modified MaXing Shigan Decoction Combined with Western Medicine in the Treatment of Community -acquired Pneumonia of Pulmonary Heat Type on the Improvement of the Symptoms.Chinese Sci Technol Periodical Database (2024).
35.
Hu W . Effect of Maxing Shigan Decoction on Peripheral Venous Blood Immunoglobulin IgE Levels in Children with Mycoplasma Pneumonia.Inner Mongolia J Traditional Chinese Med. (2017) 3615:32–3. 10.16040/j.cnki.cn15-1101.2017.15.027
36.
Huo H Chen L Chen J . Effect of Serum CRP and Clinical Observation of Budesonide Inhalation Combined with Maxing Shigan Decoction in Children with Pneumonia.J Liaoning Univer Traditional Chinese Med. (2016) 18:186–8. 10.13194/j.issn.1673-842x.2016.07.056
37.
Jin Z. Clinical Efficacy and Safety Evaluation of Qingfei Mixture Combined with Western-medicine Routine Treatment in Treating Community-Acquired Pneumonia. Master. Shanghai: Shanghai University of Traditional Chinese Medicine (2020).
38.
Kong F Zhang X Liu J Wang M Zhao F Lin T et al Clinical Evaluation of Modified Maxing Shigan Decoction Combined with High-Flow Nasal Cannula Oxygen Therapy in the Treatment of Pneumonia Complicated with Type I Respiratory Failure. China Pharmaceuticals. (2023) 32:105–8. 10.3969/j.issn.1006-4931.2023.12.026
39.
Li HN . Observation on the Effect of Integrated TCM and WM in Treating Community-Acquired Pneumonia.Shanxi J Traditional Chinese Med. (2006) 22:25–6.
40.
Li JT . Safety and Efficacy of the Maxing Shigan Decoction plus Moxifloxacin Injection on Community-Acquired Pneumonia.Clin J Chinese Med. (2020) 12:47–9. 10.3969/j.issn.1674-7860.2020.14.017
41.
Li L Tan J Hu W Li Y . Effects of Modified Maxing Shigan Decoction Combined with Azithromycin on Serum Inflammatory Factors in Children with Bronchial Pneumonia.Acta Med Sin. (2021) 34:74–7. 10.19296/j.cnki.1008-2409.2021-05-019
42.
Li Y Zhang S . Efficacy Evaluation of Maxing Shigan Decoction on Community-Acquired Pneumonia and its Influence on Serum Procalcitonin.China J Traditional Chinese Med Pharm. (2014) 29:1272–5.
43.
Li Y Zhao J Wang Y . Observation on the Effectiveness of Maxing Ganshi Decoction in the Treatment of Community-acquired Pneumonia.Chinese J Clin Rational Drug Use. (2020) 13:40–2. 10.15887/j.cnki.13-1389/r.2020.02.021
44.
Li YE Huang H . Analysis on Clinical Efficacy of Modified Maxing Shigan Decoction in Adjuvant Treatment of Children with Mycoplasma Pneumonia.Clin Med Eng. (2020) 27:1521–2. 10.3969/j.issn.1674-4659.2020.11.1521
45.
Liu B . Effectiveness and Prognosis of Maxing Shigan Decoction Combined with Tingli Dazao Xiefei Decoction in the Treatment of Community-Acquired Pneumonia in Middle-Aged and Elderly Patients.J Front Med. (2018) 8:288–9. 10.3969/j.issn.2095-1752.2018.23.245
46.
Liu H . Effectiveness of Modified Maxing Shigan Decoction for Patients with Community-Acquired Pneumonia.Chinese J Urban Rural Enterprise Hygiene. (2016) 31:59–60. 10.16286/j.1003-5052.2016.02.024
47.
Liu Y Hu J Pan J Liu H . Effects of Modified Maxing Shigan Decoction on Pulmonary Function and Immune System in Community-Acquired Pneumonia with Phlegm Heat Obstructing Lung.World Chinese Med. (2019) 14:2295–9. 10.3969/j.issn.1673-7202.2019.09.015
48.
Liu YE Zhang F Wang C Zhang H Pei L Liu J et al Clinical Observation on Maxingshigan Decoction Combined with Moxifloxacin in the Treatment of Senile Pulmonary Infection. World Latest Med Information (2016) 16:166–8. 10.3969/j.issn.1671-3141.2016.62.132
49.
Liu YH. The Clinical Research on Treating Community-Acquired Pneumonia Wind-warm Evil Heat Obstructing Lung with Jia Wei Ma Xing Shi Gan Tang. Master. Guangzhou: Guangzhou University of Chinese Medicine (2011).
50.
Liu Y Yu J Zhan S . Clinical Study on Modified Maxing Shigan Tang for Community-acquired Pneumonia with Syndrome of Wind-Warm and Evil-Heat Accumulated in Lung.New Chinese Med. (2019) 51:78–81. 10.13457/j.cnki.jncm.2019.08.023
51.
Liu Y Wang Y Li N Zhang J Xu J . Analysis of Clinical Effectiveness of Maxing Shigan Decoction plus Qianjin Weijing Decoction in the Treatment of Pneumonia with the Syndrome of Heat Obstructing in the Lung.Med Health. (2023).
52.
Lu T . The Combination of Traditional Chinese Medicine and Western Medicine in the Treatment of Bacterial Pneumonia.J China Traditional Chinese Med Information. (2010) 2:279.
53.
Ma FM . Value of Maxing Shigan Decoction and Tingli Dazao Xiefei Decoction in the Adjuvant Treatment of Community-Acquired Pneumonia in Middle-Aged and Elderly Patients.Chinese Health Care. (2020) 38:33–4.
54.
Ma WJ. Clinical Observation of Curative Effect of Xianma Xiaotan Decoction in the Treatment of Community-Acquired Pneumonia (Heat Obstruct Pulmonary Syndrome). Master. Chengdu: Chengdu University of Traditional Chinese Medicine (2015).
55.
Ma X Chen L . 31 Cases of Modified Maxing Shigan Decoction Combined with Western Medicine in Treating Community-Acquired Pneumonia.Shaanxi J Traditional Chinese Med. (2011) 32:395–7.
56.
Ma Y. Clinical Study of Jiaweimaxingshigan Combined with Western Medicine Treatment of Pneumonia Cough Syndrome of Wind-Heat Blocking the Lung Syndrome. Master. Taiyuan: Shanxi University of Chinese Medicine (2017).
57.
Meng W Wang X Wang Y . Additional Maxingganshi Decoction Combined with Antibiotic Treating on the Effect and C-Reactive Protein in Community-Acquired Pneumonia with Phlegm-Heat Obstructing Lung Type.J Emerg Traditional Chinese. (2017) 26:1668–70. 10.3969/j.issn.1004-745X.2017.09.053
58.
Mo WS Su Y Chen X . Efficacy of the Maxing Shigan Decoction Plus the Qianjin Weijing Decoction on Severe Pneumonia and White Blood Cell Count, Procalcitonin and Oxygenation Index.Clin J Chinese Med. (2021) 13:87–9. 10.3969/j.issn.1674-7860.2021.19.029
59.
Mo ZH. Clinical Observation of Feirening Mixture in the Treatment of Community Acquired Pneumonia of Lung Phlegm-Heat Syndrome. Master. Wuhan: Hubei University of Chinese Medicine (2022).
60.
Ni CY . Observation of the Effectiveness of MaXing Shigan Decoction Combined with Cefotaxime Sodium in the Treatment of Community-acquired Pneumonia (Phlegm-heat Obstructing Lung Syndrome).Heilongjiang J Traditional Chinese Med. (2023) 52:95–8.
61.
Ning WJ Zeng Y Zhang Y . Effectiveness of Xiaochaihu Decoction Combined with Modified Maxing Shigan Decoction in the Treatment of Community-Acquired Pneumonia.Inner Mongolia J Traditional Chinese Med. (2022) 41:16–8. 10.16040/j.cnki.cn15-1101.2022.02.091
62.
Shen AY Jiang Z Zhu H . Observation on Effectiveness of Modified Maxing Shigan Decoction in the Treatment of Community-Acquired Pneumonia.Shandong Med J. (2014) 54:55–7.
63.
Shen RX Zhou J . 25 Cases of Modified Maxing Shigan Decoction Treating Community-Acquired Pneumonia Zhejiang.J Traditional Chinese Med. (2013) 48:173.
64.
Shi H . 50 Cases of Pneumonia Mixture Combined with Western Medicine in the Treatment of Community-Acquired Pneumonia (Syndrome of Phlegm-Heat Obstructing the Lung).Fujian J Traditional Chinese Med. (2010) 41:27–8. 10.13260/j.cnki.jfjtcm.010029
65.
Song D . Clinical Effect of Modified Maxing Shigan Decoction Combined with Western Medicine in the Treatment of Pneumonia in Children.Med Health. (2022) 8:173–6.
66.
Su Q Yang Y . Clinical Observation of the Effectiveness of Modified Maxing Shigan Decoction on Community-acquired Pneumonia (Phlegm-Heat Obstructing Lung Syndrome) which is Basically Treated with Antibiotics.China’s Naturopathy. (2016) 24:63–4. 10.19621/j.cnki.11-3555/r.2016.07.055
67.
Su S Huang L Pan X . Observation of Curative Effect of Maxing Shigan Decoction Combined with Zhuangyi Bamboo Pot Therapy on Community-Acquired Pneumonia.Guangxi J Traditional Chinese Med. (2016) 39:27–8.
68.
Sun K . Application of Maxingshigan Decoction in Patients with Community-Acquired Pneumonia and its Influence on the Curative Effect of TCM Syndrome.Med Health. (2022) 1:195–7.
69.
Sun Q Yang J Wang Y . Clinical Observation of Modified Maxing Ganshi Decoction Combined with Antibiotics in the Treatment of Bronchopulmonary Pneumonia.Hebei J Traditional Chinese Med. (2019) 41:1380–3. 10.3969/j.issn.1002-2619.2019.09.022
70.
Sun Q . Observation on Effectiveness of the Combination of Traditional Chinese Medicine and Western Medicine on Elderly Community-Acquired Pneumonia.J Sichuan Traditional Chinese Med. (2015) 33:104–6. 10.1186/s13020-025-01149-3
71.
Tang H Chen X . Clinical Observation of Modified Maxing Shigan Decoction in 45 Cases with Senile Pneumonia.Tianjin J Traditional Chinese Med. (2013) 30:84–6. 10.11656/j.issn.1672-1519.2013.02.08
72.
Tian XM Wang L Liu L Xu C Jiang Z . The Curative Effect of Modified Maxing Shigan Decoction Combined Moxifloxacin Injection on Community-Acquired Pneumonia (Syndrome of Phlegm-Heat Obstructing in Lung).Modern Chinese Clin Med. (2017) 24:18–22.
73.
Tian Y Hu M . Observation on Effectiveness of Modified Mangxing Ganshi Decoction on 38 Cases of Community-Acquired Pneumonia.Jilin Med J. (2013) 34:689–90.
74.
Wang C Zhang F Liu Y Zhou H . Clinical observation of Maxing Ganshi Decoction as an Adjuvant Treatment for Elderly Community-Acquired Pneumonia and its Influence on Inflammatory Indexes.World Latest Med Information. (2015) 15:160–1. 10.3969/j.issn.1671-3141.2015.40.133
75.
Wang CM Zhou Q . Clinical Study on the Treatment of Community Acquired Pneumonia with Combination of TCM and Western Medicine.Acta Chinese Med. (2014) 29:959–60. 10.16368/j.issn.1674-8999.2014.07.051
76.
Wang J He J Ding K Tao Q . Clinical Efficacy of Modified Maxing Shigan Tang in Adjuvant Treatment of Drug-Resistant Mycoplasma pneumoniae Pneumonia in Children with Wind-Heat Invading Lung Syndrome and its Effects on Inflammatory Factors.J Pediatr Traditional Chinese. (2020) 16:60–3. 10.16840/j.issn1673-4297.2020.04.16
77.
Wang J . Observation on the Effectiveness of Modified Maxing Shigan Decoction Combined with Moxifloxacin Injection in the Treatment of Community-Acquired Pneumonia (Syndrome of Phlegm-heat Obstructing Lung).J Clin Med. (2017) 4:20145–6. 10.16281/j.cnki.jocml.2017.a2.113
78.
Wang J . Clinical Observation on Self-Made Phlegm-Heat Cough Formula in the Treatment of 58 Cases of Pneumonia and Cough.Clin J Traditional Chinese Med. (2014) 26:1237–8. 10.16448/j.cjtcm.2014.12.012
79.
Wang XM. Clinical Study of Maxing Shigan Decoction Plus Qianjin Weijing Decoction in the Treatment of Community-acquired Pneumonia of Phlegm-Heat Blocking Lung Type Chinese Science and Technology Periodical Database (Citation Index). (2024).
80.
Wu T Wu J Geng X Zhou Y Wang A . Modified Maxing Shigan Decoction Combined with Azithromycin for Treating Mycoplasma Pneumonia in Children.Zhejiang J Integr Traditional Chinese Western Med. (2013) 23:238–9.
81.
Wu X Wang C Cheng M Wang J Wang B Liu G et al Clinical Evaluation of Detoxification-clearing Lung Mixture on Treatment of Community-Acquired Pneumonia in Phlegm-Heat Obstructing Lung Type and its Effects on Serum C-Reactive Protein and Interleukin 6 Expression. Hebei J Traditional Chinese Med. (2016) 38:1789–93.
82.
Xiao YL. Clinical Study of Yinhuangqingfei Decoction in the Treatment of Community-Acquired Pneumonia with Phlegm-Heat Obstructing Lung Syndrome. Master. Chengdu: Chengdu University of Traditional Chinese Medicine (2021).
83.
Xie JJ. Clinical Study on Treating the Phlegm Closed Lungs Pneumonia Asthma with the Self Maxing Mixture. Master. Guangzhou: Guangzhou University of Chinese Medicine (2010).
84.
Xie Y Gao J Ren M Wang T . Clinical Observation of Modified Qianjinweijing Decotion and Maxing Shigan Decoction Combined with Thymalfasin Treating Elderly Patients with Severe Pneumonia Hebei.J Traditional Chinese Med. (2023) 45:1292–301. 10.3969/j.issn.1002-2619.2023.08.014
85.
Xin D. Clinical Application of Maxing Shigan Decoction Plus Gegen Qinlian Decoctio Combined with Conventional Western Medicine in the Treatment of Community-acquired Pneumonia. Master. Beijing: Beijing University of Chinese Medicine (2018). 10.26973/d.cnki.gbjzu.2018.000009
86.
Xu G . Observation on Curative Effect of Maxing Shigan Decoction plus Qianjin Weijing Decoction in the Treatment of Severe Pneumonia.J Emerg Traditional Chinese Med. (2016) 25:1805–6. 10.3969/j.issn.1004-745X.2016.09.054
87.
Xu G . Clinical Observation on the Maxing Shigan Decoction on Non-Severe Community-Acquired Pneumonia.Clin J Chinese Med. (2020) 12:26–8. 10.3969/j.issn.1674-7860.2020.35.008
88.
Yang HG . Clinical Value of Modified Maxing Shigan Decoction in the Treatment of Community-Acquired Pneumonia.China Health Care Nutr. (2014) 24:1713–4. 10.3969/j.issn.1004-7484(S).2014.03.660
89.
Yang J . Clinical Observation of Modified Maxing Ganshi Decoction Combined with Antibiotics in the Treatment of Community-Acquired Pneumonia (Syndrome of Phlegm-heat Obstructing Lung).Yunnan J Traditional Chinese Med Materia Medica. (2020) 41:59–60. 10.16254/j.cnki.53-1120/r.2020.07.019
90.
Yang J . Observation on Effectiveness of Modified Maxing Shigan Decoction in the Treatment of Elderly CAP of Phlegm-Heat Obstructing Lung Syndrome.Diet Health. (2021) 18:104.
91.
Yuan L. Study on Curative Effect and Contribution to Indicatrix of Phlegmonosis by Treating Wind-Warm Lung-Heat Disease with Add Taste Ma Xing Shi Gan Tang Recipe. Master. Fuzhou: Fujian University of Traditional Chinese Medicine (2008).
92.
Zhang J . Curative Effect of Modified Maxing Shigan Decoction on Community-Acquired Pneumonia (Phlegm-heat Obstructing Lung).Electronic J Clin Med Literature. (2020) 7:141–2.
93.
Zhang L . Effects of Bawei Qingfei Decoction Combined with Azithromycin on Clinical Efficacy, TCM Syndromes Scores and Serum Inflammatory Factors in Children with Mycoplasma Pneumonia (Accumulation of Phlegm-heat Syndrome).J Sichuan Traditional Chinese Med. (2019) 37:104–8.
94.
Zheng Y Shi C Han Y Li X Dong L Li Y et al Efficacy and Safety of a Chinese Herbal Formula Maxing Ganshi Decoction in Children with Community-Acquired Pneumonia: A Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial. Front Pharmacol. (2022) 13:948831. 10.3389/fphar.2022.948831
95.
Zhou D Qin Y Zhang J . Clinical Observation on Modified Maxing Shigan Decoction in the Treatment of Community-Acquired Pneumonia with Phlegm-Heat Obstructing Lung Syndrome.Hebei J Traditional Chinese Med. (2016) 38:243–5.
96.
Zhou Y . Discuss Effect of Jiawei Maxingshigan Decoction Combined With Antibiotic in the Treatment of Aged Community Acquired Pneumonia.China Continuing Med Educ. (2015) 7:222–3. 10.3969/j.issn.1674-9308.2015.33.148
97.
Zhu J Luo L Chen J . Clinical Observation on Modified Maxing Shigan Decoction in the Treatment of Community Acquired Pneumonia of Syndrome of Phlegm-Heat Obstructing Lung.Chinese Med Modern Distance Educ China. (2022) 20:90–2. 10.3969/j.issn.1672-2779.2022.10.033
98.
Zou P Yuan K Cai H Jian X Lai X Chen W et al Efficacy of the Maxing Shigan Decoction on CAP. Clin J Chinese Med. (2018) 10:73–5. 10.3969/j.issn.1674-7860.2018.10.033
99.
File TM Ramirez JA . Community-Acquired Pneumonia.N Engl J Med. (2023) 389:632–41. 10.1056/NEJMcp2303286
100.
Ma LQ Pan CS Yang N Liu YY Yan L Sun K et al Posttreatment with Ma-Xing-Shi-Gan-Tang, a Chinese medicine formula, ameliorates lipopolysaccharide-induced lung microvessel hyperpermeability and inflammatory reaction in rat. Microcirculation. (2014) 21:649–63. 10.1111/micc.12144
101.
Jing Y Xing L Li X Yu H Zhang L Song X . Overview of Modern Research on Maxing Shigan Decoction.J Tianjin Univer Traditional Chinese Med. (2023) 42:264–72. 10.11656/j.issn.1673-9043.2023.02.22
102.
Wen D Wang M . Progress of Therapy about Maxing Shigan Decoction in Cough Variant Asthma.Chinese J Exp Traditional Med Formulae. (2012) 18:285–7. 10.13422/j.cnki.syfjx.2012.08.018
103.
Qin T Zhou H Ren H Meng J Du Y Mahemut M et al Incidence, Etiology, and Environmental Risk Factors of Community-Acquired Pneumonia Requiring Hospitalization in China: A 3-Year, Prospective, Age-Stratified, Multicenter Case-Control Study. Open Forum Infect Dis. (2021) 8:ofab499. 10.1093/ofid/ofab499
104.
Sahuquillo-Arce JM Menéndez R Méndez R Amara-Elori I Zalacain R Capelastegui A et al Age-related risk factors for bacterial aetiology in community-acquired pneumonia. Respirology. (2016) 21:1472–9. 10.1111/resp.12851
105.
Jiang M Lu C Zhang C Yang J Tan Y Lu A et al Syndrome differentiation in modern research of traditional Chinese medicine. J Ethnopharmacol. (2012) 140:634–42. 10.1016/j.jep.2012.01.033
106.
Shang X Pan H Li M Miao X Ding H . Lonicera japonica Thunb.: ethnopharmacology, phytochemistry and pharmacology of an important traditional Chinese medicine.J Ethnopharmacol. (2011) 138:1–21. 10.1016/j.jep.2011.08.016
107.
Ma W Liu T Ogaji OD Li J Du K Chang Y . Recent advances in Scutellariae radix: A comprehensive review on ethnobotanical uses, processing, phytochemistry, pharmacological effects, quality control and influence factors of biosynthesis.Heliyon. (2024) 10:e36146. 10.1016/j.heliyon.2024.e36146
108.
Wu J Sun J Liu M Zhang X Kong L Ma L et al Botany, Traditional Use, Phytochemistry, Pharmacology and Quality Control of Taraxaci herba: Comprehensive Review. Pharmaceuticals. (2024) 17:1113. 10.3390/ph17091113
109.
Amtaghri S Slaoui M Eddouks M . Mentha Pulegium: A Plant with Several Medicinal Properties.Endocr Metab Immune Disord Drug Targets. (2024) 24:302–20. 10.2174/1871530323666230914103731
110.
Dong Z Lu X Tong X Dong Y Tang L Liu M . Forsythiae Fructus: A Review on its Phytochemistry, Quality Control, Pharmacology and Pharmacokinetics.Molecules (2017) 22:1466. 10.3390/molecules22091466
111.
Yosri N Alsharif SM Xiao J Musharraf SG Zhao C Saeed A et al Arctium lappa (Burdock): Insights from ethnopharmacology potential, chemical constituents, clinical studies, pharmacological utility and nanomedicine. Biomed Pharmacother. (2023) 158:114104. 10.1016/j.biopha.2022.114104
112.
Kim HJ Lee HJ Jeong SJ Lee HJ Kim SH Park EJ . Cortex Mori Radicis extract exerts antiasthmatic effects via enhancement of CD4(+)CD25(+)Foxp3(+) regulatory T cells and inhibition of Th2 cytokines in a mouse asthma model.J Ethnopharmacol. (2011) 138:40–6. 10.1016/j.jep.2011.08.021
113.
Zhang BB Zeng MN Zhang QQ Wang R Jia JF Guo PL et al Mechanism of “Ephedrae Herba-Descurainiae Semen Lepidii Semen” combination in treatment of bronchial asthma based on network pharmacology and experimental verification. Zhongguo Zhong Yao Za Zhi. (2022) 47:4996–5007. 10.19540/j.cnki.cjcmm.20220211.403
114.
Zhang LL Huang MY Yang Y Huang MQ Shi JJ Zou L et al Bioactive platycodins from Platycodonis Radix: Phytochemistry, pharmacological activities, toxicology and pharmacokinetics. Food Chem. (2020) 327:127029. 10.1016/j.foodchem.2020.127029
115.
Li KJ Liu YY Wang D Yan PZ Lu DC Zhao DS . Radix Asteris: Traditional Usage, Phytochemistry and Pharmacology of An Important Traditional Chinese Medicine.Molecules (2022) 27:5388. 10.3390/molecules27175388
116.
Liu Y Shen Y Teng L Yang L Cao K Fu Q et al The traditional uses, phytochemistry, and pharmacology of Stemona species: A review. J Ethnopharmacol. (2021) 265:113112. 10.1016/j.jep.2020.113112
117.
Cheng M. A Systematic Review on Maxing Shigan Decoction in the Treatment of Community-acquired Pneumonia. Master. Shenyang: Liaoning University of Traditional Chinese Medicine (2016).
118.
Lai Q Li J Yuan Z . A Meta Analysis for Randomized Controlled Trial of the Maxing Shigan Decoctiom versus Western Medicine on Treating Pneumonia.Clinical J Chinese Med. (2016) 8:7–10.
119.
Zhao S Ma Y Fu Y Zhang L . Systematic Review and its Dose Effect Analysis on Randomized Control Trials of Maxing Shigan Decoction in Treatment of Pneumonia in Children.China J Traditional Chinese Med Pharm. (2013) 28:361–7.
Summary
Keywords
traditional Chinese medicine, Maxing Shigan Decoction, community-acquired pneumonia, systematic review, meta-analysis
Citation
Ling Y, Liu X, Zhang B, Xiao Q, Shuai Z, Bai H, Cai R, Li S, Yuan M and Zhang Y (2025) Effectiveness and safety of Maxing Shigan Decoction for community-acquired pneumonia: a systematic review and meta-analysis of randomized controlled trials. Front. Med. 12:1639027. doi: 10.3389/fmed.2025.1639027
Received
01 June 2025
Accepted
19 August 2025
Published
29 August 2025
Volume
12 - 2025
Edited by
Arinjita Bhattacharyya, University of Louisville, United States
Reviewed by
Yongliang Jia, Zhengzhou University, China
Xingyu Zong, China Academy of Chinese Medical Sciences, China
Updates
Copyright
© 2025 Ling, Liu, Zhang, Xiao, Shuai, Bai, Cai, Li, Yuan and Zhang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yanxia Zhang, zhangyx929@bucm.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.