Abstract
Background:
Extra-pulmonary emphysema is characterized by the presence of gas within loose soft tissues outside the lungs. This condition is frequently mistaken for acute cardiovascular diseases, leading to potential misdiagnosis. Enhancing clinical recognition of extra-pulmonary emphysema is critical for improving patient outcomes at high altitude. Notably, hyperbaric oxygen therapy has shown promise as a treatment modality for this condition.
Object:
To evaluate the feasibility of early detection and hyperbaric oxygen therapy for extra-pulmonary emphysema in high-altitude regions.
Method:
A retrospective analysis was conducted on non-trauma patients presenting with chest and back pain in areas exceeding 5,000 meters in altitude from May to December 2024. Patients with a confirmed diagnosis of extra-pulmonary emphysema based on computed tomography (CT) imaging were included. Clinical data and treatment details were collected, and prognostic outcomes were monitored.
Results:
Nine young male patients, averaging 23.56 ± 5.27 years, presented with symptoms of neck and chest pain. None had a history of chronic disease, surgery, or trauma. Initial misdiagnosis occurred in seven cases, with only two correctly identified as simple extra-pulmonary emphysema. Eight patients showed significantly improvement following hyperbaric oxygen therapy at 2.0 atmospheres absolute (ATA).
Conclusion:
Recognizing extra-pulmonary emphysema at high altitude is crucial, especially severe mediastinal emphysema. Hyperbaric oxygen therapy may serve as an effective treatment option for this condition.
Introduction
Extra-pulmonary emphysema is defined as gas accumulation within soft tissues, particularly in areas such as the neck and chest. This condition can be classified into subcutaneous emphysema (SE) and mediastinal emphysema (ME), depending on the location of the gas infiltration. The etiology of extra-pulmonary emphysema is multifactorial, encompassing trauma from blunt or penetrating injuries, surgical interventions, and infections. Furthermore, any condition that creates a pressure differential between the alveoli and the surrounding vascular interstitial tissue can precipitate this phenomenon (1–3).
The pathophysiology of extra-pulmonary emphysema is explained by the Macklin effect, in which elevated intrathoracic pressure leads to alveolar rupture, permitting gas to escape into the perivascular interstitium and subsequently dissect into the mediastinum (4). Common triggers include violent coughing, breath-holding, strenuous exercise, acute asthma attacks, and traumatic injury. This mechanism is of particular relevance in high-altitude settings. Hypoxia can induce pulmonary vasoconstriction and endothelial injury, thereby facilitating the entry of gas into perivascular and peribronchial sheaths. Furthermore, the compensatory deep and labored breathing characteristic of high-altitude exposure exacerbates the pressure gradient between alveoli and vascular structures, increasing the risk of alveolar rupture (5). Physical exertion under such conditions can further raise intrathoracic pressure, promoting the development of extra-pulmonary emphysema.
Diagnosis of extra-pulmonary emphysema typically involves clinical assessment of swelling and crepitus over affected areas, while imaging studies are essential for evaluating deep tissue involvement (6). Treatment strategies for mild cases often include oxygen therapy, rest, and close monitoring. Most cases resolving spontaneously within a week. However, significant cases necessitate intervention, such as needle decompression or closed drainage (7). Notably, while hyperbaric oxygen therapy is generally contraindicated in severe cases of pneumothorax and mediastinal emphysema (8), it may lead to gas compression of the heart and major blood vessels, and even endanger life. Some studies suggest that its potential efficacy in treating extra-pulmonary emphysema when other serious conditions are ruled out (9).
At high altitude, the challenges posed by limited medical facilities and the potential for misdiagnosis of extra-pulmonary emphysema as cardiovascular emergencies underscore the importance of accurate and timely diagnosis. This case report details the clinical presentation and management of nine patients diagnosed with extra-pulmonary emphysema at an altitude of 5,000 meters, highlighting the need for heightened awareness and appropriate treatment strategies in such environments. However, due to the small sample size and the different etiologies in each patient, the specific mechanisms still need to be further investigated.
Case description
Basic clinical information
This study included nine male patients diagnosed with subcutaneous emphysema or mediastinal emphysema who visited a high altitude medical station (at 3,700 meters) from May to December 2024. They all developed the condition in areas above 5,000 meters and were transported down to the medical station for further treatment. The basic information of the nine patients are shown in Tables 1, 2. None of them had a history of chronic diseases, nor did they have a history of surgery or immunosuppression. Before the onset of illness, three patients had never received oxygen therapy, two patients received oxygen therapy for 1 h daily, one patient for 2 h, one patient for 3 h, and two patients for 4 h. The duration of residence at altitude varied, ranging from 17 to 229 days. Upon arrival at the medical facility, patients reported discomfort including neck pain, chest pain, and chest tightness. Initial physical examinations revealed crepitus in some patients, with CT confirming the diagnosis of extra-pulmonary emphysema. Only two patients were diagnosed with isolated extra-pulmonary emphysema among all the patients, while the others had definite etiologies.
Table 1
| No | Age (years) |
Sex | HA (m) | Time at HA (days) | Oxygen inhalation time (h) | Primary diagnosis | Final diagnosis | Comorbidities | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 22 | Male | 5,100 | 30 | 0 | SE | SE、ME | Aortic Dissection、DVT | Oxygen Inhalation (Mask) + Blood Pressure Control (Sodium Nitroprusside) + Heart Rate Control (Esmolol) + Symptomatic Supportive Treatment + Transfer to Plain Area + Surgical Treatment (Artificial Aortic Arch Replacement) | Improvement |
| 2 | 23 | Male | 5,090 | 90 | 1 | SE | SE | Cerebral Edema、Tracheal Rupture | Oxygen Inhalation (Non-invasive Ventilator) + Sedation (Propofol) + Dehydration (Furosemide + Human Albumin) + Steroids (Dexamethasone) + Symptomatic Supportive Treatment + Hyperbaric Oxygen Therapy (2.0 ATA) | Improvement |
| 3 | 32 | Male | 5,100 | 94 | 4 | SE | SE | Tracheal Rupture | Oxygen Inhalation (Mask) + Symptomatic Supportive Treatment + Hyperbaric Oxygen Therapy (2.0 ATA) | Improvement |
| 4 | 21 | Male | 5,390 | 17 | 0 | ME | ME | Pericarditis | Oxygen Inhalation (Mask) + Anti-infection (Imipenem/cilastatin) + Symptomatic Supportive Treatment + Hyperbaric Oxygen Therapy (2.0 ATA) | Improvement |
| 5 | 20 | Male | 5,248 | 42 | 3 | ME | ME | ME | Oxygen Inhalation (Mask) + Symptomatic Supportive Treatment + Hyperbaric Oxygen Therapy (2.0 ATA) | Improvement |
| 6 | 22 | Male | 5,428 | 224 | 1 | SE | SE | Acute Appendicitis | Oxygen Inhalation (Mask) + Anti-infection (Levofloxacin) + Surgical Treatment + Symptomatic Supportive Treatment + Hyperbaric Oxygen Therapy (2.0 ATA) | Improvement |
| 7 | 33 | Male | 5,100 | 82 | 0 | SE | SE | Pneumonia、Pulmonary Edema、Pulmonary Emphysema | Oxygen Inhalation (Mask) + Dehydration (Furosemide) + Steroids (Dexamethasone) + Anti-infection (Levofloxacin) + Symptomatic Supportive Treatment + Hyperbaric Oxygen Therapy (2.0 ATA) | Improvement |
| 8 | 18 | Male | 5,100 | 229 | 4 | ME | ME | ME | Oxygen Inhalation (Nasal Cannula) + Symptomatic Supportive Treatment + Hyperbaric Oxygen Therapy (2.0 ATA) | Improvement |
| 9 | 21 | Male | 5,200 | 19 | 2 | ME | ME | ICN | Oxygen Inhalation (Nasal Cannula) + Pain Relief (Ibuprofen) + Symptomatic Supportive Treatment + Hyperbaric Oxygen Therapy (2.0 ATA) | Improvement |
Clinical summary of cases at high altitude.
SE: Subcutaneous Emphysema; ME: Mediastinal Emphysema; DVT: Deep Vein Thrombosis; BP: Blood Pressure; HR: Heart Rate; NIV: Non-Invasive Ventilation; HBOT: Hyperbaric Oxygen Therapy (2.0 ATA); ICN: Intercostal Neuralgia.
Table 2
| Variable | Mean or proportion |
|---|---|
| Age (years) | 23.56 ± 5.27 |
| Time at HA (days) | 82 ± 24.5 |
| Altitude (m) | 5195.11 ± 133.36 |
| Heart rate | 100.44 ± 17.89 |
| Systolic blood pressure | 133.56 ± 29.09 |
| Diastolic blood pressure | 83.67 ± 11.42 |
| Oxygen saturation | 81.33 ± 6.56 |
| Combined with pericarditis | 11.1% |
| Combined with aortic dissection | 11.1% |
| Combined with tracheal rupture | 22.2% |
| Combined with upper respiratory infection | 22.2% |
| Combined with pneumonia | 11.1% |
| Combined with acute appendicitis | 11.1% |
| Combined with cerebral edema | 11.1% |
| Pulmonary edema | 11.1% |
| Smoking | 33.3% |
| Alcohol consumption | 0 |
| Immunodeficiency | 0 |
| Surgical history | 0 |
| Symptoms | |
| Cough | 33.3% |
| Fever | 44.4% |
| Neck and back pain | 44.4% |
| Chest Pain | 33.3% |
Basic clinical characteristics of patients.
Case 1 is 22 years old, he suddenly experienced severe neck pain while straining during a bowel movement. The pain persisted and radiated to the back. Six hours later, the patient was transferred to our department. His blood pressure was 202/103 mmHg in the right upper limb and 190/95 mmHg in another limb. The electrocardiogram showed “sinus rhythm, left axis deviation, left anterior fascicular block, and T-wave changes in the anterior leads.” Ultrasound revealed the formation of deep vein thrombosis. Non-contrast CT revealed emphysema in the muscles of the neck and back and subcutaneously, enhanced CT scan confirmed a thoracoabdominal aortic dissection. After stabilizing the blood pressure and heart rate, the patient was urgently transferred to a higher-level hospital for “aortic arch artificial vessel replacement.” Follow-up examinations indicated that the patient recovered well.
Case 2 and Case 3 are both young males. While engaging in heavy physical labor, both patients suddenly experienced chest pain. The patients were urgently transported to our medical facility and underwent chest CT, they were diagnosed with subcutaneous emphysema and tracheal rupture. Among them, case 2 also developed acute high altitude cerebral edema. After received symptomatic support and hyperbaric oxygen therapy (10, 11), the symptoms of these two patients significantly improved, and the extra-emphysema was also markedly absorbed. Follow-up showed that the tracheal rupture had also healed.
Case 4 is 21 years old. One week prior, he had a history of upper respiratory tract infection, and then He visited our hospital due to chest discomfort. The electrocardiogram suggested: “sinus rhythm, T-wave changes in the anterior leads,” and there was a slight increase in lymphocyte count. After underwent echocardiography and chest CT, he was diagnosed with mediastinal emphysema and pericarditis. In addition to antibiotic treatment, we also provided hyperbaric oxygen therapy (12, 13), and the patient eventually recovered well.
Case 6 is 22 years old, he experienced chest discomfort and abdominal pain after excessive alcohol consumption. After undergoing chest and abdominal CT, he was diagnosed with acute appendicitis and subcutaneous emphysema. Following an emergency “appendectomy,” he received hyperbaric oxygen therapy and had a good prognosis.
Case 7 is 33 years old, who developed symptoms of cough and chest pain after engaging in heavy physical labor outdoors. He was diagnosed with pneumonia and pulmonary edema through chest CT. Following this diagnosis, he received oxygen therapy, diuretics, antibiotic treatment, and hyperbaric oxygen therapy, his symptoms improved and he recovered well.
Case 5, Case 8, and Case 9 are all young males. All three patients presented with unexplained chest pain. Initially, primary healthcare institutions diagnosed them with cardiovascular emergencies and transferred them to our medical facility. After a series of examinations, including electrocardiogram, echocardiography, and chest and abdominal CT, Case 9 received a diagnosis of intercostal neuralgia, while Cases 5 and 8 were diagnosed with mediastinal emphysema. Follow-up after hyperbaric oxygen therapy showed significant resolution of mediastinal emphysema in all patients.
Laboratory results and auxiliary
Examinations affected by the hypoxic environment of high altitudes, all patients exhibited varying degrees of increased red blood cell count, hemoglobin concentration, and uric acid (UA). Case 6 and 7, who were diagnosed with acute appendicitis and pulmonary infection, respectively, showed increased percentages of neutrophils. Case 1, in the acute phase of aortic dissection, also had an elevated white blood cell count. Additionally, we observed that D-dimer in the blood is a good indicator of myocardial or vascular damage. It showed varying degrees of elevation in patients with aortic dissection, while this indicator remained normal in other patients, maintaining a high negative predictive value. It is noteworthy that the serum potassium (K+) levels of all patients were high, with five patients having levels above the normal range. The average serum K+ level for all patients was 5.39 ± 0.73 mmol/L, this may be due to the increased metabolism in high altitude areas (Table 3). The diagnosis of extra-pulmonary emphysema for all patients was confirmed through CT examination. Case 1, 2, 3, 6, and 7 showed subcutaneous emphysema, while the others showed mediastinal emphysema. Specific imaging manifestations are shown in Figure 1.
Table 3
| Variable | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 |
|---|---|---|---|---|---|---|---|---|---|
| RBC (10^12/L) | 7.95 | 5.94 | 6.69 | 5.59 | 5.81 | 4.93 | 4.95 | 5.17 | 6.08 |
| HB (G/L) | 232 | 188 | 186 | 180 | 198 | 169 | 159 | 172 | 199 |
| WBC (10^9/L) | 12.9 | 6.26 | 8.5 | 8.13 | 8.04 | 14.06 | 6.98 | 9.8 | 7.6 |
| Neut count (10^9/L) | 11.74 | 3.82 | 4.44 | 4.87 | 5.43 | 12.05 | 5.72 | 6.78 | 5.22 |
| Neut (%) | 91 | 60.9 | 52.3 | 59.9 | 67.5 | 85.7 | 81.9 | 69.2 | 68.7 |
| PLT (10^9/L) | 119 | 198 | 350 | 243 | 160 | 287 | 159 | 335 | 177 |
| CK (U/L) | 58 | 125 | 199 | 80 | 261 | 95 | 94 | 90 | 75 |
| CK-MB (U/L) | 23.47 | 4.2 | 10.35 | 5.43 | 11.13 | 8.65 | 5.19 | 4.6 | 31.1 |
| LDH (U/L) | 347 | 244 | 339 | 221 | 233 | 272 | 126 | 312 | 159 |
| BUN (mmol/L) | 5.09 | 4.41 | 6.73 | 10.7 | 5.28 | 5.38 | 6.44 | 7.26 | 5.85 |
| UA (umol/L) | 443 | 345 | 478 | 473 | 454 | 357 | 457 | 170 | 483 |
| Cr (umol/L) | 63 | 100 | 63 | 106 | 85 | 83 | 95 | 81 | 94 |
| D-Dimer (mg/L) | 1.7 | 0.6 | 0.02 | 0.1 | 0.5 | 0.2 | 0.04 | 0.3 | 0.05 |
| GPT (U/L) | 30 | 17 | 41 | 20 | 25 | 42 | 21 | 24 | 13 |
| GOT (U/L) | 58 | 19 | 36 | 22 | 25 | 41 | 21 | 32 | 12 |
| TBil (umol/L) | 29.58 | 25.45 | 16.51 | 30.37 | 13.04 | 95.9 | 9.13 | 13.43 | 18.6 |
| DBIL (umol/L) | 4.08 | 15.77 | 2 | 5.89 | 3.51 | 13.19 | 2.21 | 3.12 | 4.49 |
| IBIL (umol/L) | 25.5 | 9.68 | 16.32 | 24.48 | 9.53 | 82.71 | 6.92 | 10.31 | 14.11 |
| TP (g/L) | 90.22 | 79.58 | 80.3 | 79.6 | 78.69 | 88.82 | 76.34 | 88.81 | 72.25 |
| ALB (g/L) | 54.62 | 54.38 | 49.69 | 51.9 | 44.3 | 52.31 | 48.71 | 48.42 | 45.94 |
| GLB (g/L) | 35.6 | 25.2 | 30.6 | 27.7 | 34.4 | 36.5 | 27.6 | 40.4 | 26.4 |
| TG (mmol/L) | 0.65 | 1.05 | 1.57 | 1.5 | 1.04 | 0.8 | 0.9 | 0.97 | 0.51 |
| TC (mmol/L) | 4.4 | 2.88 | 4.02 | 5.47 | 3 | 4.19 | 4.78 | 3.22 | 2.92 |
| K+ (mmol/L) | 5.84 | 4.55 | 5.3 | 5.52 | 5.68 | 5.76 | 4.68 | 6.7 | 4.46 |
| Na+ (mmol/L) | 136 | 139 | 138 | 136 | 137 | 131 | 152 | 124 | 144 |
Laboratory test results of patients.
RBC: Red Blood Cell Count; HB: Hemoglobin WBC: White Blood Cell Count; PLT: Platelet Count; BUN: Blood Urea Nitrogen; UA: Uric-Acid; Cr: Creatinine; GPT: Glutamic Pyruvic Transaminase; GOT: Glutamic Oxaloacetic Transaminase; TBil: Total Bilirubin; DBIL: Direct Bilirubin; IBIL: Indirect Bilirubin; TP: Total Protein; ALB: Albumin; GLB: Globulin; TG: Triglyceride; TC: Total Cholesterol.
Figure 1

CT comparison images of extra-pulmonary emphysema treatment before and after for Case 1 to Case 3.
Figure 2
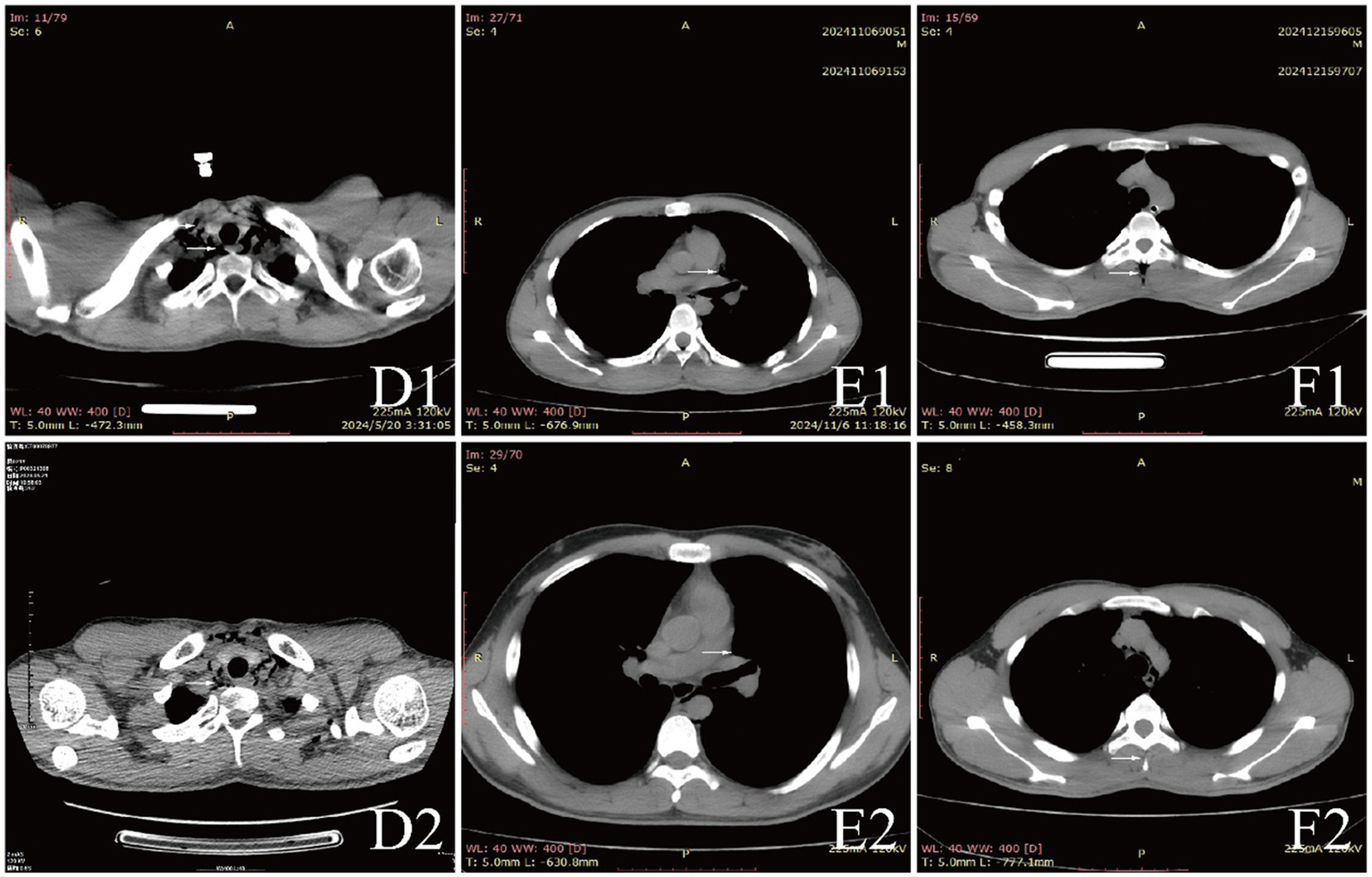
CT comparison images of extra-pulmonary emphysema treatment before and after for Case 4 to Case 6.
Diagnosis, treatment and prognosis
All patients were diagnosed with extra-pulmonary emphysema through CT scans, which showed gas infiltration in the mediastinum or subcutaneous tissue. The patient with aortic dissection was urgently transferred to a lower altitude area for “aortic arch artificial vessel replacement surgery.” Case 6 underwent “appendectomy” for treatment, and three patients received antibiotic therapy. All patients were given symptomatic and supportive treatment, including oxygen therapy. Six were given oxygen via a face mask, two received a nasal cannula, and one was assisted with non-invasive ventilation. In addition to case 1, they also received hyperbaric oxygen therapy at 2.0 ATA. Follow-up showed that all patients responded well to the treatment, their conditions improved, and the emphysema was largely absorbed (Figures 1–3).
Figure 3

CT comparison images of extra-pulmonary emphysema treatment before and after for Case 7 to Case 9.
Discussion
The occurrence of extra-pulmonary emphysema, particularly at high altitudes, represents a significant clinical challenge due to its rarity and potential for misdiagnosis (14–17). In our retrospective analysis of patients treated for this condition at altitudes exceeding 5,000 meters, we observed a concerning trend: many patients initially presented with symptoms that could easily be mistaken for cardiovascular emergencies, such as aortic dissection and pulmonary embolism. This underscores the critical need for heightened awareness and understanding of extra-pulmonary emphysema among healthcare providers, particularly in high-altitude settings where diagnostic resources may be limited. In the special environment of the plateau, medical resources are scarce, and most areas may only be equipped with X-ray examinations without conducting CT scans. This may lead to two types of misdiagnoses: either mistaking simple emphysema for cardiovascular emergencies such as pulmonary embolism or aortic dissection, or diagnosing emphysema solely based on X-ray examination while overlooking atypical critical illnesses. Both of these can result in excessive or insufficient treatment strategies. CT scans, through multiplanar imaging and 3D reconstruction analysis, can effectively avoid these situations. Through CT sections, we can observe vascular filling defects or intimal tears, thereby effectively obtaining diagnostic information for conditions such as pulmonary embolism and aortic dissection, which can guide subsequent medical procedures.
In our series, the majority of patients were young males with no significant medical histories, indicating that even previously healthy individuals can be susceptible to extra-pulmonary emphysema under extreme environmental stressors (18, 19). The clinical presentation varied, with symptoms such as neck pain, chest tightness, and crepitus upon examination. However, the diagnosis was often missed or delayed in primary care settings, reflecting a gap in knowledge regarding the manifestations of this condition at high altitudes. The initial misdiagnoses highlight a critical area for improvement in clinical practice, emphasizing the importance of considering extra-pulmonary emphysema in differential diagnoses for patients presenting with chest pain or dyspnea in such environments.
Furthermore, our findings support the efficacy of hyperbaric oxygen therapy as a treatment modality for extra-pulmonary emphysema. Eight out of nine patients showed improvement following this intervention, illustrating its potential in expediting recovery in high-altitude scenarios (20–22). Most cases of extra-pulmonary emphysema are asymptomatic or mildly symptomatic, and the standard treatment is typically the administration of pure oxygen under normal pressure. However, if hyperbaric oxygen therapy is administered, the volume of gas in the alveoli will change with the increase and decrease of ambient pressure during treatment. If the gas in bullae or air cysts cannot be normally expelled, it may lead to alveolar overexpansion and even rupture, thereby causing rare but serious complications such as pneumothorax and gas embolism. At the same time, the accumulation of a large amount of gas can affect the return flow of venous blood, leading to thrombotic diseases such as deep vein thrombosis or pulmonary embolism. In addition, if a patient with emphysema also has an untreated pneumothorax, this is an absolute contraindication for hyperbaric oxygen therapy, as the pneumothorax may rapidly worsen during treatment, leading to severe respiratory and circulatory dysfunction. Therefore, before undergoing hyperbaric oxygen therapy, patients with extra-pulmonary emphysema must undergo a comprehensive evaluation by a professional physician. During the treatment process, the general condition of the patient needs to be closely monitored to reduce the risk of pulmonary complications.
This analysis also draws attention to the limitations of medical facilities in high-altitude regions, where access to advanced imaging and definitive diagnostic capabilities may be restricted. The challenges of accurate diagnosis and timely intervention are compounded by the geographical isolation of many high-altitude communities, making early recognition of conditions such as extra-pulmonary emphysema crucial. Future studies should focus on developing standardized protocols for the diagnosis and management of extra-pulmonary emphysema in these settings, as well as exploring the long-term outcomes of patients treated at high altitudes.
In conclusion, our findings highlight the need for increased clinical awareness of extra-pulmonary emphysema, especially in high-altitude environments, where its presentation can mimic more serious cardiovascular conditions. Enhanced understanding and recognition of this condition may improve patient outcomes, as timely diagnosis and appropriate management strategies, such as hyperbaric oxygen therapy, can be life-saving (23–25). The lessons learned from our cases should inform future clinical practice and guide the development of emergency protocols for healthcare providers operating in high-altitude regions.
The limitations of this study are that the number of cases was small, and we do not have a control group. Meanwhile, after reviewing the relevant literature, we found that plasma sRAGE, CT images analyzed by deep learning, and blood-based transcriptomic and proteomic biomarkers can all serve as early warning indicators for the development of emphysema (26–28). Unfortunately, due to the limitations of our medical environment, we were unable to collect biological samples from the patients and thus could not conduct the relevant tests. In future work, we can pay more attention to research in this area.
Conclusion
In this study, a retrospective analysis of extra-pulmonary emphysema case data in extreme high altitude areas suggested that the diagnosis of extra-pulmonary emphysema should be emphasized in high altitude regions. After ruling out fatal diseases, administering hyperbaric oxygen therapy and symptomatic treatment is a reliable approach.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving humans were approved by the protocol of this study was approved by the Committee for Ethical Affairs of the General Hospital of Xinjiang Military Command, Urumqi, China. Informed consent was obtained from each patient. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
ZG: Methodology, Writing – original draft. JW: Data curation, Writing – original draft. JT: Writing – original draft. XLH: Data curation, Visualization, Writing – review & editing. QY: Writing – original draft. LZ: Writing – review & editing. XY: Writing – review & editing. XBH: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by grants from the Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. TSYC202301B046).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Fosi S Giuricin V Girardi V Di Caprera E Costanzo E Di Trapano R et al . Subcutaneous emphysema, pneumomediastinum, pneumoretroperitoneum, and pneumoscrotum: unusual complications of acute perforated diverticulitis. Case Rep Radiol. (2014) 2014:1–5. doi: 10.1155/2014/431563
2.
Kukuruza K. Aboeed A. (2025). Subcutaneous emphysema (Archived). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
3.
Talwar A Rajeev A Rachapudi S Khan S Singh V Talwar A . Spontaneous pneumomediastinum: a comprehensive review of diagnosis and management. Intractable Rare Dis Res. (2024) 13:138–47. doi: 10.5582/irdr.2024.01020
4.
Angelini M Belletti A Landoni G Zangrillo A De Cobelli F Palumbo D . Macklin effect: from pathophysiology to clinical implication. J Cardiothorac Vasc Anesth. (2024) 38:881–3. doi: 10.1053/j.jvca.2023.12.025
5.
Sydykov A Mamazhakypov A Maripov A Kosanovic D Weissmann N Ghofrani H et al . Pulmonary hypertension in acute and chronic high altitude maladaptation disorders. Int J Environ Res Public Health. (2021) 18:1692. doi: 10.3390/ijerph18041692
6.
Medeiros B. J. D. C. (2018). Subcutaneous emphysema, a different way to diagnose. Rev Assoc Med Bras (1992). 64:159–163. doi: 10.1590/1806-9282.64.02.159
7.
Iteen A. J. Bianchi W. Sharman T. (2025). Pneumomediastinum In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing.
8.
Howell RS Criscitelli T Woods JS Gillette BM Gorenstein S . Hyperbaric oxygen therapy: indications, contraindications, and use at a tertiary care center: 1.3 www.Aornjournal.Org/content/cme [Journal Article]. AORN J. (2018) 107:442–53. doi: 10.1002/aorn.12097
9.
Al-Njadat I. Obeidat M. El-Sukkar W. Swalgh M. (2022). Benign subcutaneous emphysema treated with hyperbaric oxygen therapy after surgical exploration. Med J Armed Forces India. 78:475–477. doi: 10.1016/j.mjafi.2020.01.001
10.
Ratzenhofer-Komenda B Offner A Kaltenböck F Maier A Pinter H Prause G et al . Differential lung ventilation and emergency hyperbaric oxygenation for repair of a tracheal tear. Canad J Anesthesia. (2000) 47:169–75. doi: 10.1007/BF03018855
11.
Schimmel S El Sayed B Lockard G Gordon J Young I D'Egidio F et al . Identifying the target traumatic brain injury population for hyperbaric oxygen therapy. Int J Mol Sci. (2023) 24:14612. doi: 10.3390/ijms241914612
12.
Memar MY Yekani M Alizadeh N Baghi HB . Hyperbaric oxygen therapy: antimicrobial mechanisms and clinical application for infections. Biomed Pharmacother. (2019) 109:440–7. doi: 10.1016/j.biopha.2018.10.142
13.
Price SM Price WD Johnston MJ USS EMORY S LAND, S. R. G Naval Hospital Guam, A. H. G USS FRANK CABLE, S. R. G et al . Pneumomediastinum and the use of hyperbaric oxygen treatment. Diving Hyperb Med. (2021) 51:119–23. doi: 10.28920/dhm51.1.119-123
14.
Aghajanzadeh M Dehnadi A Ebrahimi H Fallah Karkan M Khajeh Jahromi S Maafi A et al . Classification and Management of Subcutaneous Emphysema: a 10-year experience. Indian J Surg. (2015) 77:673–7. doi: 10.1007/s12262-013-0975-4
15.
Garcia P Pizanis A Massmann A Reischmann B Burkhardt M Tosounidis G et al . Bilateral pneumothoraces, pneumomediastinum, pneumoperitoneum, pneumoretroperitoneum, and subcutaneous emphysema after thoracoscopic anterior fracture stabilization. [case reports; journal article]. Spine. (2009) 34:E371–5. doi: 10.1097/BRS.0b013e3181995c87
16.
Kim WH Kim BH . Bilateral pneumothoraces, pneumomediastinum, pneumoperitoneum, pneumoretroperitoneum, and subcutaneous emphysema after percutaneous tracheostomy - a case report [Journal Article]. Korean J Anesthesiol. (2012) 62:488–92. doi: 10.4097/kjae.2012.62.5.488
17.
Muramori K Takahashi Y Handa N Aikawa H . Subcutaneous emphysema, pneumomediastinum, pneumothorax, pneumoperitoneum, and pneumoretroperitoneum by insufflation of compressed air at the external genitalia in a child. J Pediatr Surg. (2009) 44:e5–8. doi: 10.1016/j.jpedsurg.2008.12.025
18.
Li W Wang Y Xu L Yuan F . High altitude pulmonary edema combined with spontaneous pneumomediastinum: a case report. Curr Med Imaging Rev. (2024) 20:e1591029446. doi: 10.2174/0115734056234694230922101747
19.
Wang P Zuo Z Wu J Wang J Jiang R Du F . Short-term changes in chest CT images among individuals at low altitude after entering high altitude environments [Journal Article]. Front Public Health. (2024) 12:1392696. doi: 10.3389/fpubh.2024.1392696
20.
Crabbé A Jensen PØ Bjarnsholt T Coenye T . Antimicrobial tolerance and metabolic adaptations in microbial biofilms [Journal Article; Review]. Trends Microbiol. (2019) 27:850–63. doi: 10.1016/j.tim.2019.05.003
21.
Jensen PØ Møller SA Lerche CJ Moser C Bjarnsholt T Ciofu O et al . Improving antibiotic treatment of bacterial biofilm by hyperbaric oxygen therapy: not just hot air. Biofilm. (2019) 1:100008. doi: 10.1016/j.bioflm.2019.100008
22.
Sønderholm M Bjarnsholt T Alhede M Kolpen M Jensen PØ Kühl M et al . The consequences of being in an infectious biofilm: microenvironmental conditions governing antibiotic tolerance [Journal Article; Review]. Int J Mol Sci. (2017) 18:2688. doi: 10.3390/ijms18122688
23.
Halitim P Weisenburger G Bunel-Gourdy V Godet C Salpin M Mouren D et al . Spontaneous pneumomediastinum. Rev Mal Respir. (2022) 39:228–40. doi: 10.1016/j.rmr.2021.12.004
24.
Molla YD Abegaz SH Desita ZT . Spontaneous pneumomediastinum with subcutaneous emphysema in a young healthy female, a case report [Case Reports; Journal Article]. Heliyon. (2023) 9:e18326. doi: 10.1016/j.heliyon.2023.e18326
25.
Shahsavarinia K Rahvar G Soleimanpour H Saadati M Vahedi L Mahmoodpoor A . Spontaneous pneumomediastinum, pneumothorax and subcutaneous emphysema in critically ill COVID-19 patients: a systematic review. Pak J Med Sci. (2022) 38:730–5. doi: 10.12669/pjms.38.3.5529
26.
Gonzalez G Washko GR Estepar R . Deep learning for biomarker regression: application to osteoporosis and emphysema on chest CT scans. Proc SPIE Int Soc Opt Eng. (2018):10574. doi: 10.1117/12.2293455
27.
Sin S Lim MN Kim J Bak SH Kim WJ . Association between plasma sRAGE and emphysema according to the genotypes of AGER gene [Journal Article]. BMC Pulm Med. (2022) 22:58. doi: 10.1186/s12890-022-01848-9
28.
Suryadevara R Gregory A Lu R Xu Z Masoomi A Lutz SM et al . Blood-based transcriptomic and proteomic biomarkers of emphysema. Am J Respir Crit Care Med. (2024) 209:273–87. doi: 10.1164/rccm.202301-0067OC
Summary
Keywords
high altitude, extra-pulmonary emphysema, subcutaneous emphysema, mediastinal emphysema, management
Citation
Gan Z, Wu J, Tian J, Han X, Yang Q, Zhang L, Yang X and Han X (2025) Severe chest and back pain due to extra-pulmonary emphysema above 5,000 m: a series of case reports. Front. Med. 12:1640035. doi: 10.3389/fmed.2025.1640035
Received
05 June 2025
Accepted
25 September 2025
Published
06 October 2025
Volume
12 - 2025
Edited by
Agron Dogjani, University of Medicine, Tirana, Albania
Reviewed by
Brieuc Bonnemaison, Hospital Instruction of Armées Sainte-Anne, France
Udvass Kumar Kotokey, National Institute of Tuberculosis and Respiratory Diseases, India
Updates
Copyright
© 2025 Gan, Wu, Tian, Han, Yang, Zhang, Yang and Han.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaobo Han, hanxiaobo@tmmu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.