Abstract
Background:
Complementary and integrative medicine (CIM) modalities, particularly probiotics, prebiotics, and fermentable dietary fibers (PPF) use in IBD patients is common and increasing, particularly for symptom management. This study aimed to assess the prevalence of CIM and PPF use among IBD patients and to identify potential demographic and clinical factors associated with utilization.
Methods:
This was a cross-sectional study of adult IBD patients at a tertiary IBD centre in Western Canada. A self-administered questionnaire and chart review were performed, focusing on demographic and clinical characteristics, CIM and PPF use in the past year (current) and/or lifetime, and sources of awareness about PPF products.
Results:
A total of 267 patients were included, 182 with CD and 85 with UC. Overall, 89.9% of participants reported CIM use in the current year, while the current and lifetime prevalence of PPF use was 51 and 63%, respectively. UC diagnosis was associated with increased likelihood of current PPF use (OR: 1.91, 95% CI: 1.10–3.12). Holding a university degree was associated with increased likelihood of lifetime PPF use (OR: 2.21, 95% CI: 1.07–4.55). PPF awareness through gastroenterologists (OR: 3.19, 95% CI: 1.55–6.58) was significantly associated with lifetime PPF use.
Conclusion:
Use of CIM modalities such as PPF is common among IBD patients. This study found that lifetime PPF use was associated with higher level of education and awareness through gastroenterologists. Healthcare providers, and specifically gastroenterology specialists, should routinely inquire about PPF use and educate IBD patients. Further studies are required to determine the benefit derived from these products.
Introduction
Inflammatory bowel disease (IBD) is a chronic, progressive, relapsing-remitting inflammation of the gastrointestinal tract, consisting primarily of Crohn’s disease (CD) and ulcerative colitis (UC). It is thought to be resulted from a complex interaction characterized by an abnormal immune response to altered gut microbiota in genetically susceptible individuals (1, 2). The goal of therapy is to induce and maintain a steroid-free deep remission, typically achieved with conventional medical therapies directed against acute and chronic intestinal inflammation and sometimes requiring surgical management (1, 2). Though mostly efficacious, pharmaceutical therapies are costly and can be limited by potential adverse effects prompting many patients to seek other treatments alongside conventional care (3).
According to the National Center for Complementary and Integrative Health (NCCIH), CAM is defined as a diverse group of medical and healthcare systems, practices, and products that are not generally considered part of conventional medicine. CAM approaches can be categorized based on their primary therapeutic input, including nutritional (e.g., special diets, dietary supplements, herbs, probiotics), psychological (e.g., mindfulness), physical (e.g., massage, spinal manipulation), and combined approaches (e.g., yoga, acupuncture, dance, mindful eating) (4). In conditions such as IBD, these non-mainstream approaches are used alongside conventional medical treatments rather than as replacements and are therefore not truly “alternative.” Following NCCIH recommendations, these approaches fall under integrative health, which combines conventional and complementary interventions in a coordinated, multimodal manner, with an emphasis on whole-person care rather than targeting individual organ systems. Accordingly, the preferred replacement for the term CAM in an IBD context is complementary and integrative medicine (CIM).
CIM use is common among adults with IBD, however supporting evidence of their effectiveness in IBD remains limited and conflicting (5–8). Probiotics, prebiotics, and fermentable dietary fibres (PPF) are amongst the most commonly forms of CIM used by IBD patients (8, 9). The rationale behind their use lies in the potential role of gut microbiota in the pathophysiology of IBD. Altered gut microbial composition and function, along with other contributing factors such as genetics, immune dysregulation, and environmental influences, play a major role in the development and perpetuation of the disease (10–12). Dysbiosis, an imbalance of the gut microbiota, associated with both endogenous (e.g., immune system interactions, epithelial cell responses) and exogenous factors (e.g., medications, surgery, diet), ultimately contributes to chronic intestinal inflammation and damage (10, 13). Therefore, it has been suggested that modulating the gut microbiota through approaches such as fecal microbial transplantation, dietary interventions, and PPF consumption may benefit IBD patients by restoring the dynamic balance between the gut microbiota and host mucosal immune system mechanisms (14). While clinical trials on the effectiveness of PPFs in IBD remain inconclusive (15, 16), a growing number of patients use them for different reasons such as the prevention of disease relapse, symptom control, and improvement of general health.
With growing use of CIM modalities such as PPFs, it is necessary to examine the potential factors that influence their utilization through well-designed epidemiological studies. Therefore, we conducted the present study to estimate the proportion of our IBD patients utilizing PPF, and to determine the demographic and clinical factors associated with use among our IBD patient population.
Methods
This cross-sectional study was conducted at the University of Alberta Gastroenterology outpatient IBD clinic and an infusion clinic in Edmonton, Alberta, Canada. Eligible participants were 18 years or older with an established IBD diagnosis, confirmed by a physician. Those unable to speak or write fluently in English were excluded. The estimated number of patients attending the IBD clinic was ≈2,800. A sample size of 287 was calculated to estimate the prevalence of PPF use [assumed 58% based on the proportion of probiotic and prebiotic use reported by Hedin et al. (17)] with a 5% margin of error and 93% confidence level, applying a population size adjustment.
All participants provided informed consent before completing the questionnaire. Care providers were not present during the data collection process. Responses were anonymized and kept confidential, with no identifying information recorded. The study protocol was approved by the University of Alberta Human Research Ethics Board (No. Pro00064575).
Participants were asked to complete a self-administered questionnaire in the clinic waiting room, either before or after their clinic visit. The questionnaire included twenty items covering demographic information (gender, age, ethnicity, and education level), summarized clinical details (disease diagnosis, duration, medications, and relapse history), and CIM usage in the past year (e.g., vitamins, minerals, herbal supplements, massage, chiropractic care, yoga, meditation, and acupuncture). To assess PPF use, participants were provided with a comprehensive list of PPF products available in Canada and asked to indicate if they had history of using any of the products (lifetime PPF use) or had used them within the past year (current PPF use). They were also asked to identify their sources of knowledge about PPF use (e.g., social media, family and friends, internet, advertisements, family physician, gastroenterologist, dietitian or nurse, and pharmacist). A trained interviewer was present during data collection to address participant questions and ensure the questionnaires were completed accurately and thoroughly.
Following the clinic visit, a detailed chart review was conducted to validate the accuracy of the data collected via the self-administered questionnaire and to gather additional clinical information. This included surgical history, current medications, past corticosteroid use, extraintestinal manifestations of IBD, current partial Mayo score for UC, Harvey-Bradshaw Index for CD, Montreal classification, disease flare frequency over the past 2 years, and average serum C-reactive protein (CRP) levels over the past year. Active disease in UC was defined as a current partial Mayo score between 2 and 9 (18), while in CD, it was defined as a Harvey-Bradshaw Index ≥ 5 (19). Elevated CRP was defined as serum CRP > 8 mg/L (20). For the purposes of this study, flares were defined as a combination of clinical symptoms and endoscopic and/or histological scores. Clinical symptoms pertaining to a flare included diarrhea ≥ 3 days, obvious blood in the stool, bloating and/or abdominal pain. Symptoms such as presence of fever > 38° C, loss of appetite, nausea/vomiting, fatigue and change in health status were also considered.
Quantitative variables are presented as mean ± standard deviation (SD). Normally distributed data were compared between groups using independent sample t-tests, while non-normally distributed data (assessed by the Kolmogorov-Smirnov test) were analyzed using the Mann-Whitney U test. Qualitative variables are expressed as frequencies and percentages and were compared using Chi-square or Fisher’s exact tests, as appropriate. Binary logistic regression was performed to identify predictors of CIM or PPF use. All statistical analyses were conducted using IBM SPSS Statistics software (version 26.0, Armonk, NY, USA: IBM Corp). A two-tailed p-value of < 0.05 was considered statistically significant.
Results
Demographics and clinical phenotypes
Of the 280 patients invited to participate, 13 were excluded from analysis: 4 lacked a confirmed IBD diagnosis, and 9 had IBD-unclassified. Among the remaining 267 individuals with confirmed diagnosis of IBD, 182 (68.2%) were diagnosed with CD and 85 (31.8%) with UC. The mean age was 42.6 ± 15.7 years and 53.9% were female. Demographic and clinical characteristics of participants and comparisons based on IBD subtype (UC vs. CD), are presented in Table 1. Patients with UC were significantly younger, had shorter disease duration, were less likely to be Caucasian, and more likely to be on 5-ASA therapy versus patients with CD. In contrast, those with CD were more likely to be on biologic therapy, have a history of IBD-related surgery, and to exhibit extraintestinal manifestations of IBD.
Table 1
| Total (n = 267) | Ulcerative colitis (n = 85) | Crohn’s disease (n = 182) | p-value | |
|---|---|---|---|---|
| Age, yr | 42.6 ± 15.7 | 39.6 ± 15.2 | 44.0 ± 15.8 | 0.04 |
| Age at diagnosis, yr | 29.3 ± 14.0 | 30.2 ± 13.6 | 28.8 ± 14.2 | 0.46 |
| Disease duration, yr | 13.3 ± 11.2 | 9.4 ± 9.2 | 15.1 ± 11.6 | <0.001 |
| Females, n (%) | 144 (53.9) | 45 (52.9) | 99 (54.4) | 0.82 |
| Education, n (%) | ||||
| Less than high school | 76 (28.5) | 19 (22.4) | 57 (31.3) | |
| High school or college | 119 (44.6) | 37 (43.5) | 82 (45.1) | 0.13 |
| University degree | 72 (27.0) | 29 (34.1) | 43 (23.6) | |
| Caucasian, n (%) | 244 (91.4) | 72 (84.7) | 172 (94.5) | 0.01 |
| UC Montreal classification, n (%) | ||||
| Proctitis | 5 (5.9) | |||
| Left-sided colitis | 18 (21.2) | |||
| Pancolitis | 62 (72.9) | |||
| CD Montreal classification, n (%) | ||||
| Ileal | 47 (25.8) | |||
| Colonic | 34 (18.7) | |||
| Ileocolonic | 101 (55.5) | |||
| Upper gastrointestinal | 38 (20.9) | |||
| Inflammatory | 72 (39.6) | |||
| Stricturing | 92 (50.5) | |||
| Penetrating | 42 (23.1) | |||
| Stricturing and penetrating | 15 (8.2) | |||
| Perianal | 61 (33.5) | |||
| IBD medication, n (%) | ||||
| No medication | 35 (13.1) | 10 (11.8) | 25 (13.7) | 0.66 |
| 5-aminosalicylic acid | 85 (31.8) | 55 (64.7) | 30 (16.5) | <0.001 |
| Immunomodulators | 94 (35.2) | 23 (27.1) | 71 (39.0) | 0.06 |
| Biologics | 157 (58.8) | 34 (40.0) | 123 (67.6) | <0.001 |
| Current corticosteroids | 10 (3.7) | 4 (4.7) | 6 (3.3) | 0.57 |
| Past corticosteroids | 215 (80.9) | 69 (81.2) | 146 (80.2) | 0.85 |
| Surgical history, n (%) | 79 (29.6) | 2 (2.4) | 77 (42.3) | <0.001 |
| Extraintestinal manifestation, n (%) | 133 (49.8) | 15 (17.6) | 118 (64.8) | <0.001 |
| Number of flares in the last 2 yr | 1.4 ± 1.2 | 1.7 ± 1.3 | 1.2 ± 1.2 | 0.01 |
| Harvey Bradshaw Index | 1.4 ± 0.7 | |||
| Partial Mayo score | 1.5 ± 0.8 | |||
| Active disease, n (%) | 80 (30.0) | 32 (37.6) | 48 (26.4) | 0.06 |
| CRP > 8 mg/L in the past year, n (%) | 81 (33.6) | 34 (43.0) | 70 (40.7) | 0.73 |
Comparison of demographic and clinical characteristics between ulcerative colitis (UC) and Crohn’s disease (CD) patients.
IBD, inflammatory bowel disease; CRP, C-reactive protein.
Bold values indicate statistically significant p-values (p < 0.05).
CIM use
Overall, 89.9% of participants reported using any CIM modalities within the past year. The most common CIM modalities included supplements (76.4%), PPFs (51.3%), and massage therapy (27.7%). Comparison of CIM and its modalities use between UC and CD patients is presented in Figure 1. PPF use was significantly higher in UC (61.2%) compared to CD patients (46.7%) (OR: 1.80, 95% CI: 1.06–3.04). The use of other CIM modalities was comparable between UC and CD patients.
Figure 1

Comparison of complementary and integrative medicine (CIM) use in the past year between ulcerative colitis and Crohn’s disease patients. Only p-values < 0.05 are shown.
The relationship between demographic and clinical characteristics and CIM use is presented in Table 2. None of the demographic or clinical factors showed a statistically significant association with CIM use neither in univariate nor in multivariable analysis.
Table 2
| Complementary and integrative medicine use | p-value | ||
|---|---|---|---|
| Yes (n = 240) | No (n = 27) | ||
| Age, yr | 43.0 ± 15.8 | 39.0 ± 14.8 | 0.21 |
| Females, n (%) | 134 (55.8) | 10 (37.0) | 0.06 |
| Caucasian, n (%) | 220 (91.7) | 24 (88.9) | 0.63 |
| Education, n (%) | |||
| Less than high school | 67 (27.9) | 9 (33.3) | |
| High school or college | 106 (44.2) | 13 (48.1) | 0.57 |
| University degree | 67 (27.9) | 5 (18.5) | |
| Ulcerative colitis (UC), n (%) | 79 (32.9) | 6 (22.2) | 0.26 |
| Crohn’s disease (CD), n (%) | 161 (67.1) | 21 (77.8) | |
| Disease duration, yr | 13.6 ± 11.3 | 10.9 ± 10.4 | 0.24 |
| UC Montreal classification, n (%) | |||
| Proctitis | 5 (6.3) | 0 (0.0) | 0.76 |
| Lef-sided colitis | 17 (21.5) | 1 (16.7) | |
| Pancolitis | 57 (72.2) | 5 (83.3) | |
| CD Montreal classification, n (%) | |||
| Ileal | 42 (26.1) | 5 (23.8) | 0.82 |
| Colonic | 31 (19.3) | 3 (14.3) | 0.58 |
| Ileocolonic | 88 (54.7) | 13 (61.9) | 0.63 |
| Upper gastrointestinal | 34 (21.1) | 4 (19.0) | 0.83 |
| Inflammatory | 67 (41.6) | 5 (23.8) | 0.12 |
| Stricturing | 81 (50.3) | 11 (52.4) | 0.86 |
| Penetrating | 35 (21.7) | 7 (33.3) | 0.24 |
| Stricturing and penetrating | 14 (8.7) | 1 (4.8) | 0.54 |
| Perianal | 53 (32.9) | 8 (38.1) | 0.64 |
| IBD medication, n (%) | |||
| No medication | 32 (13.3) | 3 (11.1) | >0.99 |
| 5-aminosalicylic acid | 80 (33.3) | 5 (18.5) | 0.12 |
| Immunomodulators | 82 (34.2) | 12 (44.4) | 0.29 |
| Biologics | 140 (58.3) | 17 (63.0) | 0.64 |
| Current corticosteroids | 6 (3.2) | 4 (5.0) | 0.49 |
| Past corticosteroids | 151 (80.7) | 64 (80.0) | 0. 89 |
| Surgical history, n (%) | 68 (28.3) | 11 (40.7) | 0.18 |
| Extraintestinal manifestation, n (%) | 120 (50.0) | 13 (48.1) | 0.86 |
| Number of flares in the last 2 yr | 1.4 ± 1.3 | 1.3 ± 1.2 | 0.92 |
| Active disease, n (%) | 53 (28.3) | 27 (33.8) | 0.38 |
| CRP > 8 mg/L in the past year, n (%) | 68 (38.4) | 36 (48.6) | 0.13 |
Association of complementary and integrative medicine use with demographic and clinical characteristics of inflammatory bowel disease patients.
IBD, inflammatory bowel disease; CRP, C-reactive protein.
PPF use
Overall, 51% of participants reported current PPF use, and 63% reported lifetime PPF use. Among current PPF users, probiotics were the most commonly used (90.2%), followed by dietary fiber supplements (23.5%) and prebiotics (11.8%). Since PPF use was significantly higher in UC patients and given the differences in demographic and clinical characteristics between UC and CD patients (Table 1), further analyses to identify determinants of PPF use were conducted separately for each IBD subtype.
In univariate analysis, none of the demographic or clinical characteristics were associated with current or lifetime PPF use in UC (Table 3) or CD (Table 4) patients. However, in multivariable analysis, a diagnosis of UC was associated with increased odds of current PPF use (OR: 1.91, 95% CI: 1.10–3.12, p = 0.02). Furthermore, a university degree was associated with higher odds of lifetime PPF use among IBD patients (OR: 2.21, 95% CI: 1.07–4.55, p = 0.03).
Table 3
| Current consumption | p-value | Lifetime consumption | p-value | |||
|---|---|---|---|---|---|---|
| Yes (n = 52) | No (n = 33) | Yes (n = 57) | No (n = 28) | |||
| Age, yr | 39.8 ± 15.3 | 39.5 ± 15.1 | 0.93 | 38.8 ± 15.1 | 41.4 ± 15.4 | 0.46 |
| Disease duration, yr | 9.5 ± 9.1 | 9.3 ± 9.3 | 0.92 | 9.3 ± 8.8 | 9.6 ± 10.0 | 0.89 |
| Females, n (%) | 30 (57.7) | 15 (45.5) | 0.27 | 33 (57.9) | 12 (42.9) | 0.19 |
| Education, n (%) | ||||||
| Less than high school | 11 (21.2) | 8 (24.2) | Ref | 11 (19.3) | 8 (28.6) | Ref |
| High school or college | 22 (42.3) | 15 (45.5) | 0.91 | 23 (40.4) | 14 (50.0) | 0.76 |
| University degree | 19 (36.5) | 10 (30.3) | 0.59 | 23 (40.4) | 6 (21.4) | 0.12 |
| University degree, n (%) | 19 (36.5) | 10 (30.3) | 0.56 | 23 (40.4) | 6 (21.4) | 0.08 |
| Caucasian, n (%) | 45 (86.5) | 27 (81.8) | 0.56 | 49 (86.0) | 23 (82.1) | 0.65 |
| Montreal classification, n (%) | ||||||
| Proctitis | 5 (9.6) | 0 (0.0) | 0.18 | 5 (8.8) | 0 (0.0) | 0.27 |
| Lef-sided colitis | 11 (21.2) | 7 (21.2) | 12 (21.1) | 6 (21.4) | ||
| Pancolitis | 36 (69.2) | 26 (78.8) | 40 (70.2) | 22 (78.6) | ||
| IBD medication, n (%) | ||||||
| No medication | 6 (11.5) | 4 (12.1) | 0.28 | 8 (14.0) | 2 (7.1) | 0.35 |
| 5-aminosalicylic acid | 32 (61.5) | 23 (69.7) | 0.44 | 35 (61.4) | 20 (71.4) | 0.36 |
| Immunomodulators | 16 (30.8) | 7 (21.2) | 0.84 | 17 (29.8) | 6 (21.4) | 0.41 |
| Biologics | 23 (44.2) | 11 (33.3) | 0.91 | 25 (43.9) | 9 (32.1) | 0.3 |
| Current corticosteroids | 2 (3.8) | 2 (6.1) | 0.64 | 2 (3.5) | 2 (7.1) | 0.46 |
| Past corticosteroids | 41 (78.8) | 28 (84.8) | 0.49 | 45 (78.9) | 24 (85.7) | 0.45 |
| Surgical history, n (%) | 1 (1.9) | 1 (3.0) | 0.74 | 2 (3.5) | 0 (0.0) | >0.99 |
| Extraintestinal manifestation, n (%) | 10 (19.2) | 5 (15.2) | 0.3 | 11 (19.3) | 4 (14.3) | 0.57 |
| Number of flares in the last 2 yr | 1.6 ± 1.2 | 1.8 ± 1.4 | 0.58 | 1.5 ± 1.2 | 2.0 ± 1.5 | 0.13 |
| Partial Mayo score | 1.5 ± 0.8 | 1.6 ± 0.8 | 0.75 | 1.5 ± 0.8 | 1.6 ± 0.8 | 0.81 |
| Active disease, n (%) | 19 (36.5) | 13 (39.4) | 0.79 | 21 (36.8) | 11 (39.3) | 0.83 |
| CRP > 8 mg/L in the year, n (%) | 19 (38.8) | 15 (50.0) | 0.33 | 21 (38.9) | 13 (52.0) | 0.27 |
Demographic and clinical characteristics of ulcerative colitis patients according to their current and lifetime probiotics, prebiotics, and fermentable dietary fibres consumption status.
IBD, inflammatory bowel disease; CRP, C-reactive protein.
Table 4
| Current consumption | p-value | Lifetime consumption | p-value | |||
|---|---|---|---|---|---|---|
| Yes (n = 85) | No (n = 97) | Yes (n = 111) | No (n = 71) | |||
| Age, yr | 44.3 ± 15.3 | 43.7 ± 16.3 | 0.78 | 44.7 ± 16.0 | 42.8 ± 15.7 | 0.41 |
| Disease duration, yr | 15.8 ± 12.4 | 14.5 ± 11.0 | 0.45 | 15.6 ± 12.1 | 14.3 ± 10.9 | 0.46 |
| Females, n (%) | 50 (58.8) | 49 (50.5) | 0.26 | 64 (57.7) | 35 (49.3) | 0.27 |
| Education, n (%) | ||||||
| Less than high school | 26 (30.6) | 31 (32.0) | Ref | 30 (27.0) | 27 (38.0) | Ref |
| High school or college | 41 (48.2) | 41 (42.3) | 0.61 | 52 (46.8) | 30 (42.3) | 0.21 |
| University degree | 18 (21.2) | 25 (25.8) | 0.71 | 29 (26.1) | 14 (19.7) | 0.14 |
| University degree | 18 (21.2) | 25 (25.8) | 0.47 | 29 (26.1) | 14 (19.7) | 0.32 |
| Caucasian, n (%) | 80 (94.1) | 92 (94.8) | 0.83 | 104 (93.7) | 68 (95.8) | 0.55 |
| Montreal classification, n (%) | ||||||
| Colonic | 18 (21.2) | 16 (16.5) | 0.42 | 22 (19.8) | 12 (16.9) | 0.62 |
| Ileocolonic | 46 (54.1) | 55 (56.7) | 0.73 | 59 (53.2) | 42 (59.2) | 0.43 |
| Upper gastrointestinal | 13 (15.3) | 25 (25.8) | 0.08 | 20 (18.0) | 18 (25.4) | 0.24 |
| Inflammatory | 33 (38.8) | 39 (40.2) | 0.85 | 43 (38.7) | 29 (40.8) | 0.78 |
| Stricturing | 44 (51.8) | 48 (49.5) | 0.76 | 59 (53.2) | 33 (46.5) | 0.38 |
| Penetrating | 20 (23.5) | 22 (22.7) | 0.89 | 23 (20.7) | 19 (26.8) | 0.35 |
| Stricturing and penetrating | 8 (9.4) | 7 (7.2) | 0.59 | 9 (8.1) | 6 (8.5) | 0.94 |
| Perianal | 28 (32.9) | 33 (34.0) | 0.88 | 36 (32.4) | 25 (35.2) | 0.7 |
| IBD medication, n (%) | ||||||
| No medication | 9 (10.6) | 16 (16.5) | 0.25 | 16 (14.4) | 9 (12.7) | 0.74 |
| 5-aminosalicylic acid | 18 (21.2) | 12 (12.4) | 0.11 | 19 (17.1) | 11 (15.5) | 0.77 |
| Immunomodulators | 33 (38.8) | 38 (39.2) | 0.96 | 39 (35.1) | 32 (45.1) | 0.18 |
| Biologics | 58 (68.2) | 65 (67.0) | 0.86 | 75 (67.6) | 48 (67.6) | >0.99 |
| Current corticosteroids | 4 (4.7) | 2 (2.1) | 0.32 | 5 (4.5) | 1 (1.4) | 0.41 |
| Past corticosteroids | 72 (84.7) | 74 (76.3) | 0.16 | 92 (82.9) | 54 (76.1) | 0.26 |
| Surgical history, n (%) | 38 (44.7) | 39 (40.2) | 0.54 | 52 (46.8) | 25 (35.2) | 0.12 |
| Extraintestinal manifestation, n (%) | 54 (63.5) | 64 (66.0) | 0.73 | 72 (64.9) | 46 (64.8) | 0.99 |
| Number of flares in the last 2 yr | 1.3 ± 1.3 | 1.2 ± 1.2 | 0.73 | 1.2 ± 1.2 | 1.2 ± 1.2 | 0.78 |
| Harvey Bradshaw Index | 1.4 ± 0.7 | 1.4 ± 0.7 | 0.73 | 1.4 ± 0.7 | 1.4 ± 0.8 | 0.44 |
| Active disease, n (%) | 21 (24.7) | 27 (27.8) | 0.63 | 28 (25.2) | 20 (28.2) | 0.66 |
| CRP > 8 mg/L in the past year, n (%) | 33 (41.3) | 37 (40.2) | 0.89 | 40 (38.1) | 30 (44.8) | 0.38 |
Demographic and clinical characteristics of Crohn’s disease patients according to their current and lifetime probiotics, prebiotics, and fermentable dietary fibres consumption status.
IBD, inflammatory bowel disease; CRP, C-reactive protein.
Sources of PPF awareness
Overall, 243 (91.0%) patients reported general awareness of PPFs. General awareness was significantly associated with female gender (OR: 2.54, 95% CI: 1.05–6.16). No other demographic or clinical characteristics were associated with general awareness of PPFs. General awareness of PPFs was significantly associated with both current (OR: 6.05, 95% CI: 2.00–18.21) and lifetime (OR: 7.74, 95% CI: 2.79–21.49) PPF use.
The primary sources of PPF awareness among participants were advertisements (32.2%), family or friends (30.3%), the internet (28.1%), and gastroenterologists (23.6%). Among participants, 39.0% of those without a university degree and 54.2% of those with a university degree reported being aware of PPFs through healthcare professionals (p = 0.03), suggesting that higher educational attainment may be associated with greater awareness of these modalities. In UC patients, no significant association was found between sources of PPF awareness and current or lifetime PPF use (Figure 2). However, in CD patients, awareness through the internet was significantly associated with increased current PPF use (OR: 2.48, 95% CI: 1.27–4.83). Additionally, among CD patients, awareness through gastroenterologists (OR: 2.79, 95% CI: 1.24–6.28) was significantly associated with higher odds of lifetime PPF use (Figure 3). Among participants, 39.0% of those without a university degree and 54.2% of those with a university degree reported being aware of PPFs through healthcare professionals (p = 0.03), suggesting that higher educational attainment may be associated with greater awareness of these modalities.
Figure 2
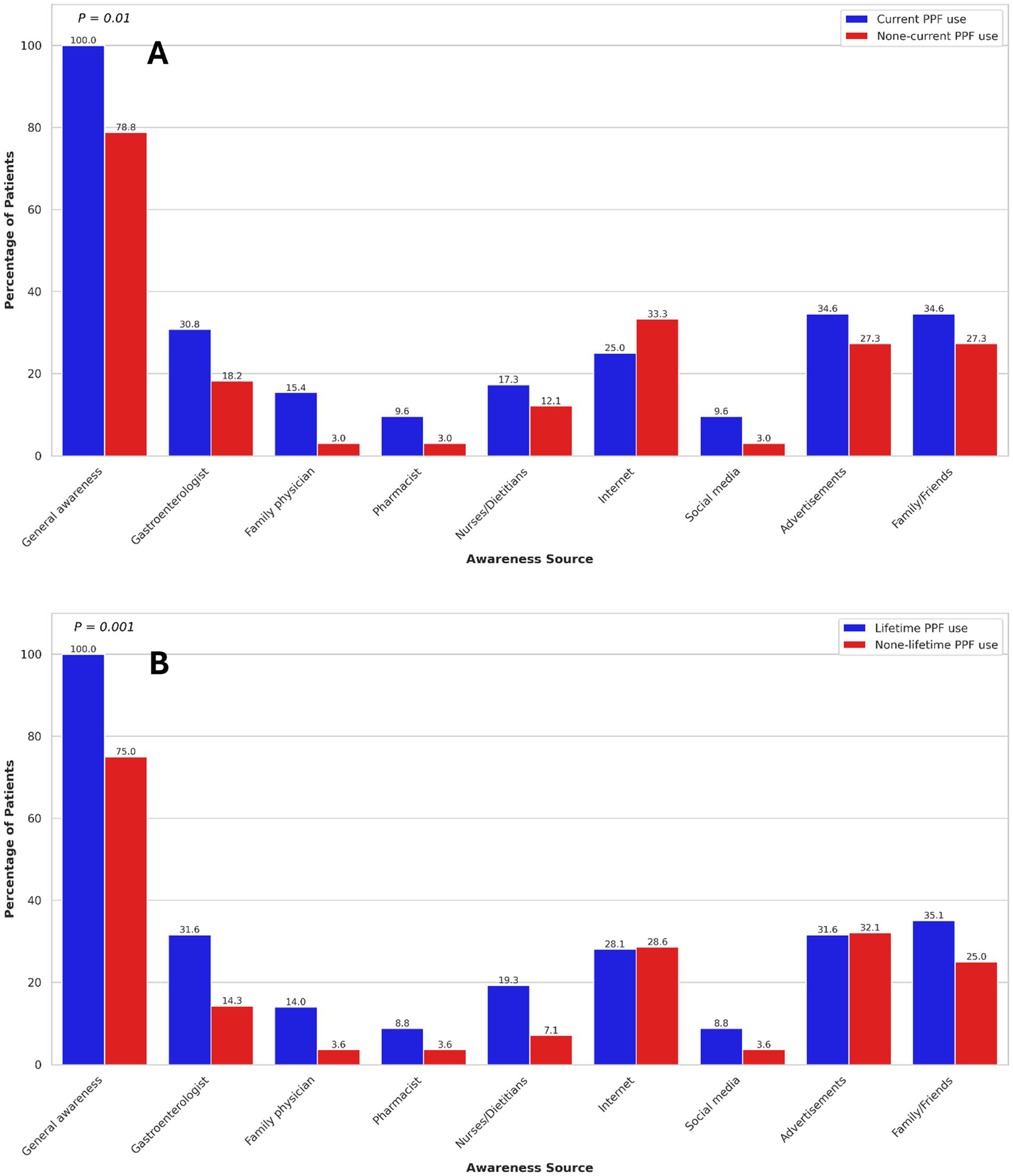
Association of current (A) and lifetime (B) probiotics, prebiotics, and fermentable dietary fibres use with sources of awareness in ulcerative colitis patients. Only p-values < 0.05 are shown.
Figure 3
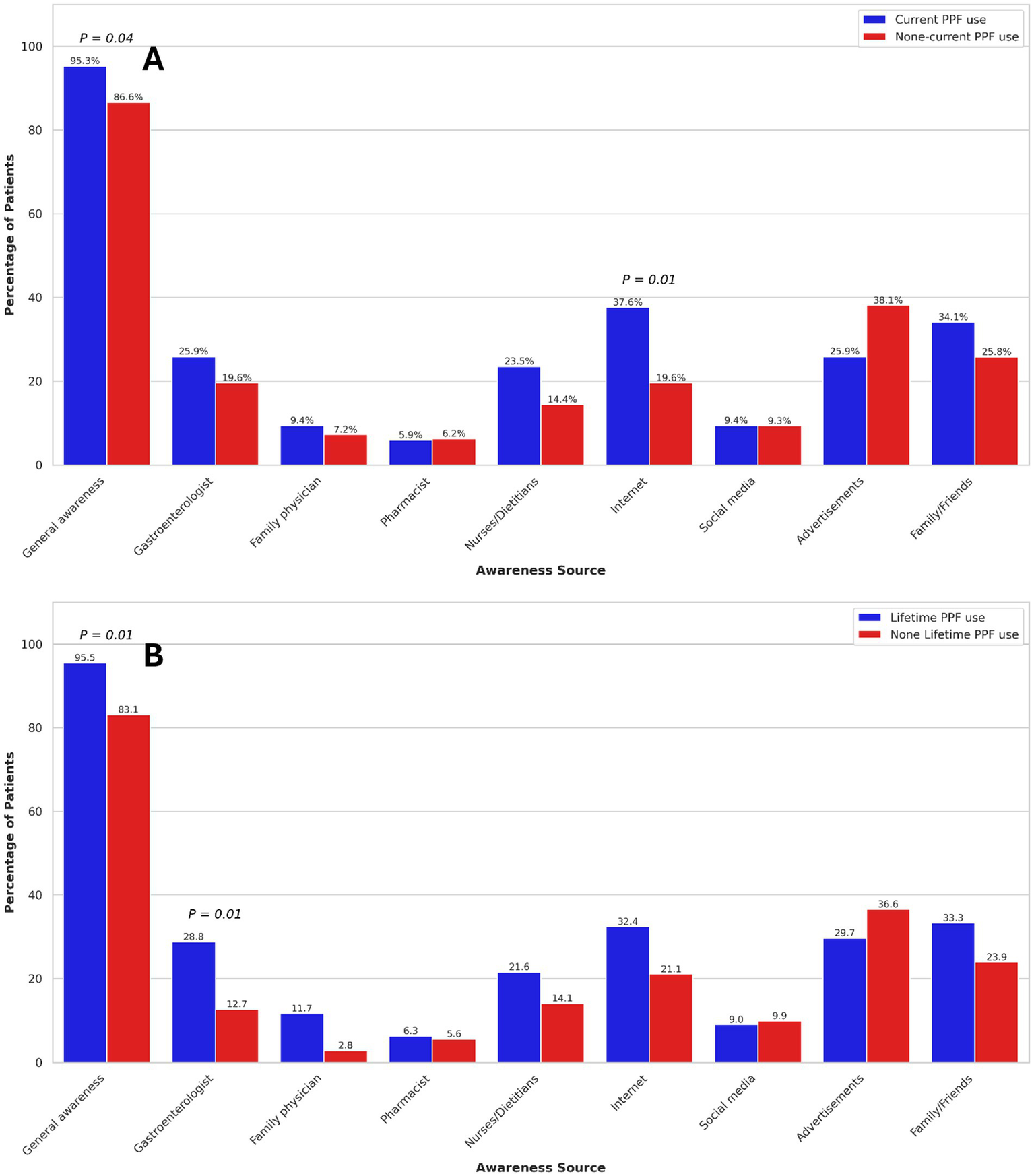
Association of current (A) and lifetime (B) probiotics, prebiotics, and fermentable dietary fibres use with sources of awareness in Crohn’s disease patients. Only p-values < 0.05 are shown.
In multivariable analysis, after adjusting for IBD subtypes and gender, sources of PPF awareness were not associated with current PPF use. However, PPF awareness through gastroenterologists (OR: 3.19, 95% CI: 1.55–6.58), family or friends (OR: 1.94, 95% CI: 1.07–3.56), family physicians (OR: 3.67, 95% CI: 1.01–13.35), and nurses or dietitians (OR: 2.16, 95% CI: 1.01–4.61) were significantly associated with increased odds of lifetime PPF use.
Discussion
In the present study, we found a high prevalence of CIM (92.1% in the past year) and PPF (63% in lifetime) use among our cohort of IBD patients. Among the various demographic and clinical factors examined, UC diagnosis and higher educational attainment were significantly associated with increased current and lifetime consumption of PPFs, respectively. Furthermore, we identified that awareness disseminated through healthcare providers, particularly gastroenterologists, as well as through family and friends, played a substantial role in influencing the utilization of PPF products.
The use of CIM is prevalent globally, particularly among patients with chronic conditions such as UC and CD. IBD patients frequently employ various CIM modalities to manage their disease, alleviate symptoms, or enhance overall well-being (6). Motivations for CIM use often include dissatisfaction with conventional therapies or a preference for more “natural” and “safer” alternatives (21). In the present study, about 90% of participants reported using at least one form of CIM within the past year. This aligns with findings from other studies, which have demonstrated high rates of CAM use among IBD patients, exceeding 80% in some studies (21, 22). A recent survey of 230 IBD patients in British Columbia, Canada, found that 84% of participants had utilized CAM over the past year (22). Variations in CAM/CIM usage across the literature may be attributed to differences in study populations and methodological approaches, including the definition of CIM. Despite this, there appears to be a strong desire among IBD patients to explore and employ CIM modalities for symptom management.
Several demographic and clinical factors, including female gender, higher education level, long-term disease progression, and prolonged steroid use, have been suggested to influence CAM utilization among IBD patients in some studies (6). Overall, no significant associations were observed between CIM use and most of the evaluated demographic and clinical characteristics in our study. Although the association between gender and CIM use did not reach statistical significance (p = 0.06), there was a trend suggesting that female patients were more likely to use CIM compared to their male counterparts. This finding is consistent with results from prior studies (23–25), highlighting the role of gender as a key determinant in CIM utilization among individuals with IBD.
This study found that the lifetime and current PPF use among the participants were 63 and 51%, respectively. Furthermore, PPFs emerged as the second most commonly utilized form of CIM, following dietary supplements. This high prevalence of PPF use, particularly probiotics, aligns with findings from previous studies, which have identified probiotics as one of the most frequently used CAM modalities among IBD patients (21, 22, 24, 26–28). For example, Klemm et al. reported that approximately 55% of IBD patients at a tertiary care referral center in Vancouver, Canada, utilized probiotics, making it the most common CAM modality in their cohort (22).
Our study showed that patients with UC were nearly twice as likely to consume PPFs within the past year compared to those with CD. This finding is consistent with a recent meta-analysis (16), which suggested that combining 5-ASA with probiotics may be beneficial for inducing remission in mild-to-moderate UC, reducing the odds of recurrence in relapsing pouchitis, and trended toward reducing clinical recurrence in inactive UC decreasing clinical recurrence in inactive UC. In contrast, probiotics did not demonstrate a significant therapeutic effect in CD (16), which may explain the observed difference in PPF use between UC and CD patients in our cohort. Notably, this meta-analysis—the largest to date evaluating probiotics in IBD—rated the certainty of evidence as low for induction of clinical and endoscopic remission in UC, and very low for prevention of clinical recurrence and other outcomes. Subgroup analyses indicated that only multi-strain probiotic formulations outperformed comparators in achieving remission and preventing recurrence in UC (16). However, evidence remains limited regarding factors such as dosage, treatment duration, specific strains or combinations, and the optimal timing throughout the disease course. These findings highlight the importance of disease-specific therapeutic strategies, in line with the principles of precision medicine, in IBD management. Future research is needed to clarify existing gaps, such as optimal dosage, probiotic formulations, and patient- and disease-related factors that influence PPF effectiveness. Furthermore, although generally considered safe for most people, excessive or inappropriate use of probiotics may lead to unwanted gastrointestinal (GI) symptoms (29). It may be helpful for patients to inform their physicians about probiotic use, and for physicians to routinely ask about such products to ensure safe and coordinated care.
Among different demographic and clinical characteristics investigated in this study, higher educational attainment was the only factor significantly associated with increased lifetime PPF use among IBD patients. The relationship between education level and probiotic use in IBD remains underexplored, with existing studies yielding mixed results. While some reports found no significant correlation (17, 27), others have indicated that IBD patients with higher education levels are more likely to use probiotics (24, 30), potentially due to more frequent access to information regarding their potential benefits and their higher economic affordability for purchasing and accessing complementary products (28). Consistent with this, our finding that participants with a university degree were more likely to be aware of PPFs through healthcare professionals suggests that educational attainment and health literacy may influence patient engagement with CIM modalities. This highlights the importance for clinicians to consider patients’ educational background and information access when discussing PPF use, to support informed and coordinated care.
The source of information significantly influences PPF utilization among IBD patients, shaping their perceptions and therapeutic decisions. Patients relying on internet-based sources or advertisements may be more inclined to use these products due to extensive marketing of their purported health benefits, however, often lacking robust scientific evidence (31). In contrast, patients informed by healthcare professionals are more likely to adopt evidence-based approaches, guided by clinical indication and potential risks. In this study, while advertisements, internet, and family or friends were major sources of PPF awareness, information provided by gastroenterologists was the primary driver of lifetime PPF use. This underscores the critical role of healthcare providers, particularly gastroenterologists, in educating patients and promoting informed decision-making. Consequently, healthcare professionals must remain well-informed and vigilant regarding PPF usage, as they represent the most trusted source of information for IBD patients (27). Nonetheless, awareness through family and friends also significantly impacted PPF consumption in our study, highlighting the need to address possible misinformation from non-medical sources to mitigate the risk of inappropriate use of PPF products.
Our study is the first to comprehensively investigate the determinants of CIM and PPF use in the Canadian province of Alberta, which has the highest prevalence of IBD (968 per 100,000) in Canada (32) and one of the highest prevalences in the world. However, its limitations should be considered while interpreting the results. This study was conducted at a tertiary center, where patients often have more complex disease and are actively treated. As such, clinical characteristics—such as disease course, medication use, steroid dependence, and history of surgery—may differ from the broader IBD population in the community. These factors should be considered when extrapolating our findings, as the patterns of CIM and PPF use observed in this cohort may not fully reflect those in the general IBD population. Additionally, we did not recruit a control group, preventing comparisons of PPF use and its contributing factors between IBD patients and healthy individuals or non-IBD patients. Furthermore, due to the cross-sectional design of the study, causal relationships cannot be inferred, the potential benefits of CIM and PPFs on disease-related outcomes cannot be assessed, and changes in their use over time or with relapsing–remitting IBD symptoms cannot be evaluated. In the present study, we did not collect detailed information regarding the frequency, consistency, or duration of use, nor did we account for the consumption of foods such as yogurt that may contribute to probiotic intake. Specific product types (e.g., probiotic strains, prebiotic structures, or sources of dietary fiber), as well as dosage and frequency of use, were not assessed in this study. This limitation may affect the generalizability and reproducibility of our findings. Another limitation of this study is that we did not assess the clinical outcomes of PPF use nor evaluate potential interactions with conventional IBD medications, and therefore cannot draw conclusions about their efficacy, safety, or potential additive or adverse effects in this patient population. Future prospective studies and clinical trials are needed to address these limitations and provide further insights.
In this study, we demonstrated a high prevalence of CIM and PPF use among IBD patients with female gender being associated with increased CIM utilization. The key determinants of PPF consumption were UC diagnosis and higher educational attainment. Awareness disseminated through formal healthcare providers like gastroenterologists, as well as informal sources such as family and friends, significantly influenced PPF consumption. Knowledge about usage of PPFs by IBD patients is valuable, as some of these products may have disease-reducing potential; however, their clinical efficacy needs to be confirmed in well-designed prospective longitudinal studies and randomized clinical trials. Future research should therefore focus on evaluating the efficacy, safety, and long-term outcomes of CIM and PPF use to optimize patient-centered care.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by University of Alberta Human Research Ethics Board. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AK: Formal analysis, Methodology, Visualization, Writing – original draft, Writing – review & editing. RC: Data curation, Investigation, Methodology, Writing – review & editing. MS: Data curation, Investigation, Methodology, Project administration, Writing – review & editing. KD: Data curation, Methodology, Writing – review & editing. RV: Data curation, Investigation, Methodology, Writing – review & editing. EW: Methodology, Writing – review & editing. LD: Conceptualization, Data curation, Funding acquisition, Investigation, Methodology, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank all participants of this study. We also extend our gratitude to Dr. Karen Goodman for her assistance with the study methodology.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Le Berre C Honap S Peyrin-Biroulet L . Ulcerative colitis. Lancet. (2023) 402:571–84. doi: 10.1016/S0140-6736(23)00966-2
2.
Dolinger M Torres J Vermeire S . Crohn's disease. Lancet. (2024) 403:1177–91. doi: 10.1016/S0140-6736(23)02586-2
3.
Langhorst J Wulfert H Lauche R Klose P Cramer H Dobos GJ et al . Systematic review of complementary and alternative medicine treatments in inflammatory bowel diseases. J Crohns Colitis. (2015) 9:86–106. doi: 10.1093/ecco-jcc/jju007
4.
National Center for Complementary and Integrative Health (NCCIH) . Complementary, alternative, or integrative health: what’s in a name? (2021) Available online at: https://www.nccih.nih.gov/health/complementary-alternative-or-integrative-health-whats-in-a-name [Accessed January 31, 2025].
5.
Cheifetz AS Gianotti R Luber R Gibson PR . Complementary and alternative medicines used by patients with inflammatory bowel diseases. Gastroenterology. (2017) 152:415–429.e15. doi: 10.1053/j.gastro.2016.10.004
6.
Lin SC Cheifetz AS . The use of complementary and alternative medicine in patients with inflammatory bowel disease. Gastroenterol Hepatol (N Y). (2018) 14:415–25.
7.
Picardo S Altuwaijri M Devlin SM Seow CH . Complementary and alternative medications in the management of inflammatory bowel disease. Ther Adv Gastroenterol. (2020) 13:1756284820927550. doi: 10.1177/1756284820927550
8.
Seminerio J . Complementary and alternative medicine in Crohn's disease. Gastroenterol Clin N Am. (2022) 51:337–51. doi: 10.1016/j.gtc.2021.12.009
9.
Torres J Ellul P Langhorst J Mikocka-Walus A Barreiro-de Acosta M Basnayake C et al . European Crohn's and colitis organisation topical review on complementary medicine and psychotherapy in inflammatory bowel disease. J Crohns Colitis. (2019) 13:673–685e. doi: 10.1093/ecco-jcc/jjz051
10.
Liu S Zhao W Lan P Mou X . The microbiome in inflammatory bowel diseases: from pathogenesis to therapy. Protein Cell. (2021) 12:331–45. doi: 10.1007/s13238-020-00745-3
11.
Shan Y Lee M Chang EB . The gut microbiome and inflammatory bowel diseases. Annu Rev Med. (2022) 73:455–68. doi: 10.1146/annurev-med-042320-021020
12.
Lee M Chang EB . Inflammatory bowel diseases (IBD) and the microbiome-searching the crime scene for clues. Gastroenterology. (2021) 160:524–37. doi: 10.1053/j.gastro.2020.09.056
13.
Foppa C Rizkala T Repici A Hassan C Spinelli A . Microbiota and IBD: current knowledge and future perspectives. Dig Liver Dis. (2024) 56:911–22. doi: 10.1016/j.dld.2023.11.015
14.
Noguera-Fernández N Candela-González J Orenes-Piñero E . Probiotics, prebiotics, fecal microbiota transplantation, and dietary patterns in inflammatory bowel disease. Mol Nutr Food Res. (2024) 68:e2400429. doi: 10.1002/mnfr.202400429
15.
Rashed R Valcheva R Dieleman LA . Manipulation of gut microbiota as a key target for Crohn's disease. Front Med (Lausanne). (2022) 9:887044. doi: 10.3389/fmed.2022.887044
16.
Estevinho MM Yuan Y Rodríguez-Lago I Sousa-Pimenta M Dias CC Barreiro-de Acosta M et al . Efficacy and safety of probiotics in IBD: an overview of systematic reviews and updated meta-analysis of randomized controlled trials. United European Gastroenterol J. (2024) 12:960–81. doi: 10.1002/ueg2.12636
17.
Hedin CR Mullard M Sharratt E Jansen C Sanderson JD Shirlaw P et al . Probiotic and prebiotic use in patients with inflammatory bowel disease: a case-control study. Inflamm Bowel Dis. (2010) 16:2099–108. doi: 10.1002/ibd.21286
18.
Lewis JD Chuai S Nessel L Lichtenstein GR Aberra FN Ellenberg JH . Use of the noninvasive components of the Mayo score to assess clinical response in ulcerative colitis. Inflamm Bowel Dis. (2008) 14:1660–6. doi: 10.1002/ibd.20520
19.
Vermeire S Schreiber S Sandborn WJ Dubois C Rutgeerts P . Correlation between the Crohn's disease activity and Harvey-Bradshaw indices in assessing Crohn's disease severity. Clin Gastroenterol Hepatol. (2010) 8:357–63. doi: 10.1016/j.cgh.2010.01.001
20.
Chang S Malter L Hudesman D . Disease monitoring in inflammatory bowel disease. World J Gastroenterol. (2015) 21:11246–59. doi: 10.3748/wjg.v21.i40.11246
21.
Zezos P Nguyen GC . Use of complementary and alternative medicine in inflammatory bowel disease around the world. Gastroenterol Clin N Am. (2017) 46:679–88. doi: 10.1016/j.gtc.2017.08.001
22.
Klemm N Trasolini R Bressler B Rosenfeld G Almasan G Leung Y . Motivations behind complementary and alternative medicine use in patients with Crohn's disease and ulcerative colitis. J Can Assoc Gastroenterol. (2024) 7:376–83. doi: 10.1093/jcag/gwae020
23.
Bertomoro P Renna S Cottone M Riegler G Bossa F Giglio L et al . Regional variations in the use of complementary and alternative medicines (CAM) for inflammatory bowel disease patients in Italy: an IG-IBD study. J Crohns Colitis. (2010) 4:291–300. doi: 10.1016/j.crohns.2009.12.009
24.
Weizman AV Ahn E Thanabalan R Leung W Croitoru K Silverberg MS et al . Characterisation of complementary and alternative medicine use and its impact on medication adherence in inflammatory bowel disease. Aliment Pharmacol Ther. (2012) 35:342–9. doi: 10.1111/j.1365-2036.2011.04956.x
25.
Opheim R Bernklev T Fagermoen MS Cvancarova M Moum B . Use of complementary and alternative medicine in patients with inflammatory bowel disease: results of a cross-sectional study in Norway. Scand J Gastroenterol. (2012) 47:1436–47. doi: 10.3109/00365521.2012.725092
26.
Bauer N Kairey L Schlee C Uecker C Öznur Ö Langhorst J . Use of complementary and alternative medicine (CAM) in patients with inflammatory bowel disease (IBD): results from a German nationwide survey of 2019 compared to a previous survey of 2002. Scand J Gastroenterol. (2022) 57:1209–15. doi: 10.1080/00365521.2022.2078667
27.
Frank L Lambert K . Use of complementary and alternative therapies in people with inflammatory bowel disease. Int J Environ Res Public Health. (2024) 21:1140. doi: 10.3390/ijerph21091140
28.
Sudhakar P Keersmaekers B Stiers R De Dycker E Geens P Paps A et al . Profiling the use of complementary alternative medicines among inflammatory bowel disease patients: results from a single center survey. GastroHep. (2022) 2022:1–10. doi: 10.1155/2022/9919542
29.
Vallejos OP Bueno SM Kalergis AM . Probiotics in inflammatory bowel disease: microbial modulation and therapeutic prospects. Trends Mol Med. (2025) 31:731–42. doi: 10.1016/j.molmed.2024.12.005
30.
Agathou CL Beales IL . Factors associated with the use of probiotics in patients with inflammatory bowel disease. F1000Res. (2013) 2:69. doi: 10.12688/f1000research.2-69.v1
31.
Neunez M Goldman M Ghezzi P . Online information on probiotics: does it match scientific evidence?Front Med (Lausanne). (2020) 6:296. doi: 10.3389/fmed.2019.00296
32.
Coward S Benchimol EI Kuenzig ME Windsor JW Bernstein CN Bitton A et al . The 2023 impact of inflammatory bowel disease in Canada: epidemiology of IBD. J Can Assoc Gastroenterol. (2023) 6:S9–S15. doi: 10.1093/jcag/gwad004
Summary
Keywords
inflammatory bowel disease, complementary and integrative medicine, complementary and alternative medicine, prebiotics, probiotics, dietary fibres
Citation
Keshteli AH, Chibbar R, Silva M, Danois K, Valcheva R, Wine E and Dieleman LA (2025) Determinants of complementary and integrative medicine use in inflammatory bowel disease: a focus on probiotics, prebiotics, and fermentable dietary fibres. Front. Med. 12:1641767. doi: 10.3389/fmed.2025.1641767
Received
05 June 2025
Accepted
17 September 2025
Published
09 October 2025
Volume
12 - 2025
Edited by
Maria Teresa Rocchetti, University of Foggia, Italy
Reviewed by
Zhipeng Gu, South China University of Technology, China
Elyse Thakur, Carolinas Healthcare System, United States
Updates
Copyright
© 2025 Keshteli, Chibbar, Silva, Danois, Valcheva, Wine and Dieleman.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Levinus A. Dieleman, l.dieleman@ualberta.ca
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.