Abstract
Objective:
To evaluate and compare the effectiveness and safety of latanoprost, bimatoprost, travoprost, and tafluprost in lowering intraocular pressure (IOP) in individuals with glaucoma or ocular hypertension.
Methods:
We searched PubMed, Embase, Web of Science, and the Cochrane Library for randomized controlled trials (RCTs) published up to April 2025 comparing latanoprost, bimatoprost, travoprost, and tafluprost in adults with glaucoma or ocular hypertension. Primary outcomes were IOP reduction and conjunctival hyperemia. We assessed study quality using the Cochrane Risk of Bias 2.0 tool. Evidence certainty was evaluated with the CINeMA framework. A Bayesian network meta-analysis was conducted in RStudio. This review is registered with PROSPERO (CRD420251034803).
Results:
25 RCTs published between 2001 and 2024, involving 4,045 participants, were included. All studies compared monotherapy with latanoprost, bimatoprost, travoprost, or tafluprost. Among these, bimatoprost showed the most effective reduction in intraocular pressure compared to latanoprost [mean difference (MD) 0.69; 95%confidence interval (CI) 0.28–1.1; SUCRA 95.6%; moderate confidence]. It also performed significantly better than travoprost (MD 0.64; 0.14–1.09; 39.2%; low confidence). No other comparisons showed statistically significant differences. Overall, the quality of evidence for this outcome ranged from low to moderate. In terms of safety, 16 trials, including 3,119 participants, reported on conjunctival hyperemia. Both bimatoprost [odds ratio (OR) 3.3; 2.5–4.5; 18.4%, high confidence] and travoprost (0.46; 0.33–0.63; 55%, high confidence) were associated with a higher risk of hyperemia compared to latanoprost. Bimatoprost also posed a significantly greater risk than travoprost (1.51; 1.06–2.16, high confidence).
Conclusion:
Bimatoprost provided the greatest IOP reduction but carried a higher risk of conjunctival hyperemia. Latanoprost and tafluprost offered balanced efficacy with better tolerability, making them suitable for patients with mild disease.
Systematic review registration:
Introduction
Elevated intraocular pressure (IOP) is a key factor in ocular health and the most significant modifiable risk factor for glaucoma and optic nerve damage (1). Glaucoma is a progressive condition that can lead to permanent vision loss if untreated, and elevated IOP is a primary driver of its progression (2). Effective IOP management is, therefore, crucial to slowing disease progression and protecting vision (3).
Medication, laser treatments, and surgeries are therapies for elevated IOP (4). Prostaglandin analogs are the first choice for pharmacological treatment due to their high efficacy and convenient dosing schedule. These drugs lower IOP by increasing uveoscleral outflow, a vital pathway for aqueous humor drainage (5). Common agents include latanoprost (LAT), bimatoprost (BIM), travoprost (TRA), and tafluprost (TAF). These agents are valued for their reliable results and minimal systemic side effects, making them essential in managing elevated IOP (6).
Although prostaglandin drugs are widely used in clinical practice, comprehensive comparisons of all available options are limited. Most studies focus on pairwise comparisons or use inconsistent methods to evaluate efficacy and safety (7–10). This fragmented approach has resulted in conflicting findings, creating uncertainty about these drugs’ relative benefits and risks. A Bayesian network meta-analysis (NMA) offers a powerful solution to these gaps. Combining data from multiple studies enables indirect comparisons of drugs that have not been directly compared in trials (11). This study aims to use Bayesian NMA to evaluate and compare the efficacy and safety of prostaglandin drugs for reducing IOP. This study used Bayesian NMA to evaluate and compare the efficacy and safety of prostaglandin drugs, aiming to refine treatment guidelines for glaucoma and elevated IOP.
Methods
This NMA was carried out in accordance with the PRISMA Extension guidelines, explicitly designed for systematic reviews involving network meta-analyses of healthcare interventions (12). A comprehensive PRISMA checklist can be found in Appendix 1. The review protocol has also been officially registered with the International Prospective Register of Systematic Reviews (PROSPERO: CRD420251034803).
Inclusion criteria
Participants
The study population comprised patients diagnosed with glaucoma or ocular hypertension, aged 18 years or older. There were no restrictions based on gender, race, or ethnicity.
Interventions
The control group consisted of patients receiving LAT monotherapy. The experimental groups included those treated with BIM monotherapy, TRA monotherapy, or TAF monotherapy. Only relevant groups were selected for analysis in clinical trials involving multiple treatment arms to maintain coherence and consistency.
Outcome measures
The primary efficacy outcome was intraocular pressure reduction (IOPR), defined as the difference between baseline IOP and endpoint IOP, with a study duration of 3 months. The IOPR and its standard deviation (SDIOPR) were calculated as follows: IOPR = IOPbaseline - IOPendpoint; SDIOPR = √(SDbaseline2 + SDendpoint2-2*r*SDbaseline*SDendpoint) (13). Since none of the included studies reported the standard deviation of change, the correlation coefficient (r) was assumed rather than derived. We set r = 0.5, reflecting a moderate level of measurement repeatability commonly accepted in previous literature. This assumption was made to balance the potential variability between baseline and endpoint measurements and to support the robustness and reliability of the results (13). The safety outcome was the incidence of conjunctival hyperemia.
Study design
Only randomized controlled trials (RCTs) were included to ensure robust evidence quality.
Exclusion criteria
Studies were excluded if they used non-RCT study designs, involved combination therapy with non-prostaglandin IOP-lowering agents, lacked primary outcome measures, or presented insufficient or non-extractable data.
Search strategy
The databases PubMed, Embase, Web of science and Cochrane Library were systematically searched using a range of terms, including “latanoprost,” “PhXA34,” “PHXA41,” “Xalatan,” “bimatoprost,” “Latisse,” “Lumigan,” “AGN192024,” “AGNA,” “travoprost,” “Travatan Z,” “Travatan,” “tafluprost,” “AFP-168,” “glaucoma,” “ocular hypertension,” “randomized controlled trial,” and “RCT.” To ensure thoroughness, the references of all included studies were also manually reviewed. The search encompassed all relevant literature available from the inception of each database through April 2025. Detailed search strategies are available in Appendix 2.
Study selection and data extraction
The study selection process involved a structured approach, including initial screening, re-screening, and detailed evaluation. Two independent researchers systematically reviewed the literature using EndNote, employing automated and manual methods to remove duplicates. Studies were assessed based on predefined inclusion and exclusion criteria, and those not meeting the criteria were excluded. Full-text reviews were conducted to eliminate studies lacking relevant outcomes or not adhering to the specified interventions. Discrepancies during the review process were resolved through discussion or, if necessary, consultation with a third reviewer.
Data extraction was performed using a standardized form, organizing data into the following categories: ①general study information (authors, publication year, sample size, participant characteristics); ②methodological details (randomization, allocation concealment, blinding, and completeness of outcome data); ③intervention specifics (details of prostaglandin treatments in experimental and control groups, duration of therapy); and ④outcome measures (IOPR and incidence of conjunctival hyperemia).
Risk of bias and quality assessment
The risk of bias in the included studies was evaluated using the Cochrane Risk of Bias tool (RoB 2.0) (14). This method assesses five critical domains: issues with the randomization process, deviations from the intended interventions, missing outcome data, measurement of outcomes, and selection of reported results. Two independent reviewers conducted the assessment, resolving any disagreements through discussion or consultation with a third reviewer when necessary. To evaluate the strength of evidence from the network meta-analysis, the Confidence in Network Meta-Analysis (CINeMA) framework was employed. CINeMA assesses evidence quality across six domains: within-study bias, indirectness, imprecision, heterogeneity, inconsistency, and reporting bias. Each domain was carefully examined to provide a comprehensive appraisal of the evidence (15, 16). This rigorous process ensured a nuanced understanding of the certainty of evidence supporting the findings.
Data synthesis and analysis
The NMA was conducted using Stata SE 17 to generate the network plot, illustrating the relationships among treatments. Bayesian network analysis was performed in RStudio, utilizing the Gemtc and Ggplot2 packages. The analysis employed Monte Carlo Markov Chain (MCMC) methods within a consistency model (17). Four MCMC chains were run with 30,000 burn-in iterations and 50,000 sampling iterations. Convergence was assessed through trace plots of posterior sample values over iterations, with overlapping and stable plots indicating successful convergence. A league table was then constructed to present the results quantitatively. For dichotomous outcomes, results were expressed as odds ratios (OR) with 95% confidence intervals (CI), highlighting the relative likelihood of an event occurring between groups. Continuous outcomes were presented as mean differences (MD) with 95% CI, representing the average difference between groups. Heterogeneity was assessed using τ2 values, which were classified into four levels: low (<0.04), low-to-moderate (0.04–0.16), moderate-to-high (0.16–0.36), and high (>0.36), following established guidelines (18–20). The ranking of treatments was visualized using cumulative ranking curves and Surface Under the Cumulative Ranking (SUCRA) graphs, created in RStudio. SUCRA values ranged from 0 (indicating a minimal effect) to 1 (indicating a robust effect), providing a clear visualization of the comparative performance of each treatment (21).
To evaluate small-study effects, funnel plot symmetry was assessed in Stata based on direct comparisons. Publication bias was examined for each pairwise comparison individually using estimates from direct evidence.
Results
Literature search results
A total of 1990 records were identified through database searches, including 225 from PubMed, 353 from Embase, 503 from the Cochrane Library, and 909 from the Web of Science. No additional records were identified through other sources. After removing 782 duplicates, 1,208 unique records were retained for screening. 1,123 records were excluded during the screening phase based on title and abstract screening. This left 85 records for full-text eligibility assessment. Of these, 60 records were excluded for the following reasons: 32 contained duplicate data, 5 had incomplete data, 9 lacked relevant outcome measures, 6 involved interventions that did not meet the study criteria, 3 lacked full-text availability, and 5 were subgroup analyses that were not eligible. Ultimately, 25 RCTs (22–46) met the inclusion criteria and were included in the final analysis. This process is summarized in Figure 1.
Figure 1

Flowchart of literature search and selection process.
Included study characteristics
The included trials involved 4,439 participants. The interventions included BIM, TRA and TAF for the treatment group and LAT for the control group. The mean age of patients varied from 22 to 94 years, except in 2 RCTs where the age was not specified. Sample sizes ranged from 60 to 661 participants. The treatment duration spanned from 3 to 12 months. Detailed information about the included studies is provided in Appendix 3 and Table S3.1. Further details on the four prostaglandin analogues evaluated in these trials are provided in Appendix 3 and Table S3.2.
Risk of bias, certainty of evidence, and consistency
The risk of bias for each trial is outlined in Appendix 4. A major drawback was the insufficient details on blinding methods for participants and researchers. Among the 25 trials reviewed, 25 studies had a low risk of bias in random sequence generation, measurement of the outcome, and selection reporting. Additionally, 14 studies had a low risk of bias in deviations from intended interventions, and 22 had a low risk of missing outcome data. Overall, two studies had a high risk of bias, 6 raised concerns about potential bias, and 15 had a low risk. Our evaluation of the alignment between direct and indirect evidence showed strong consistency across all comparisons.
Furthermore, two outcomes did not reveal significant statistical evidence of global inconsistency. The τ2 results showed no significant heterogeneity within the network, with most comparisons displaying low to moderate heterogeneity levels (Appendix 5). The density, trace, and convergence diagnostic plots all showed strong convergence, confirming the robustness of the results (Appendices 6, 7). We evaluated the evidence quality with CINeMA and found that most pairwise comparisons had low to moderate confidence (Appendix 8). All networks adhered to the transitivity principle, ensuring the validity of indirect comparisons (Appendix 8; Table S8.1). Furthermore, we found no evidence of asymmetry in the funnel plots (Appendix 11).
Intraocular pressure reduction
Our NMA assessed the improvement in IOPR, encompassing 24 trials with 4,045 participants. As depicted in Figure 2A, the analysis included direct comparisons between prostaglandins. The thickness of the connecting lines indicates that prostaglandins were compared with each other. The forest plot displayed the direct comparison results among BIM, TAF, and TRA (Figure 2A). Specifically, BIM (MD 0.69, 95% CI 0.28 to 1.1, SUCRA 95.6%, moderate confidence of evidence) was associated with a significantly greater reduction in IOPR compared to LAT (Figure 2B; Appendix 9). Further comparisons of various prostaglandins revealed that BIM significantly outperformed TRA in improving the IOPR (MD 0.64, 95% CI 0.14 to 1.09, low confidence of evidence) (Appendix 10; Table S10.1). No statistically significant differences were observed in the comparisons among the other prostaglandins. According to CINeMA, the overall quality of evidence for IOPR was mainly rated as low to moderate (Appendix 8; Table S8.2).
Figure 2
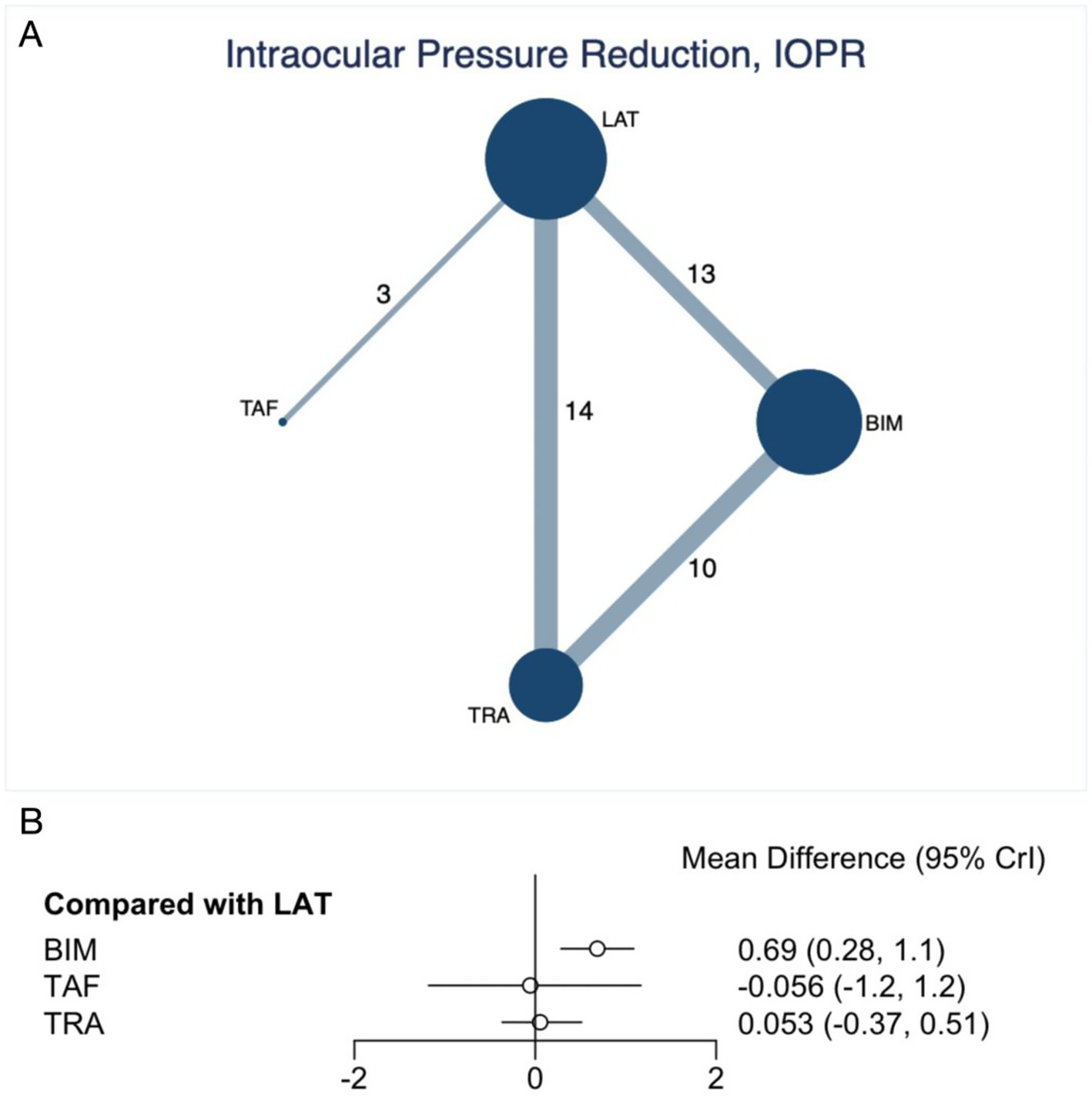
Network plot and forest plot of direct comparisons for IOPR. (A) Network of available comparisons of prostaglandins. The size of the nodes is proportional to the number of trial participants, and the thickness of the line connecting the nodes is proportional to the randomised number of trial participants directly comparing the two treatments. (B) Forest plot of network effect sizes between prostaglandins for IOPR.
Incidence of conjunctival hyperemia
The NMA on the incidence of conjunctival hyperemia included 16 RCTs involving 3,119 patients. Figure 3A presents the network plot, which directly compares prostaglandins. The thickness of the connecting lines highlights the more frequent comparisons between BIM and LAT. Figure 3 forest plot shows that BIM and TRA significantly increased the risk of conjunctival hyperemia. BIM, compared to LAT (OR 3.3, 95% CI 2.5 to 4.5, SUCRA 18.4%, high confidence of evidence), resulted in a significantly higher risk of conjunctival hyperemia. Similarly, TRA also showed a significantly increased risk of conjunctival hyperemia (OR 0.46, 95% CI 0.33 to 0.63, SUCRA 55%, high confidence of evidence) (Figure 3B; Appendix 9). Indirect comparisons indicated that BIM had a more significant risk on the incidence of conjunctival hyperemia than TRA (OR 1.51, 95% CI 1.06 to 2.16, high confidence of evidence) (Appendix 10; Table S10.2). Further comparisons of the incidence of conjunctival hyperemia are detailed in the SUCRA data (Appendix 9; Figure S9.2) and a table (Appendix 10; Table S10.2).
Figure 3

Network plot and forest plot of direct comparisons for incidence of conjunctival hyperemia. (A) Network of available comparisons of prostaglandins. The size of the nodes is proportional to the number of trial participants, and the thickness of the line connecting the nodes is proportional to the randomised number of trial participants directly comparing the two treatments. (B) Forest plot of network effect sizes between prostaglandins for incidence of conjunctival hyperemia.
Sensitivity analysis
To further examine the robustness of our findings, we performed sensitivity analyses by excluding studies at high risk of bias. As shown in Appendix 12, the results remained consistent with the primary analyses, supporting the robustness of our conclusions.
Discussion
In this Bayesian network meta-analysis of 25 randomised controlled trials (4,045 participants) we found that, among the four licensed prostaglandin analogues for glaucoma or ocular hypertension, BIM produced the greatest additional reduction in intraocular pressure relative to LAT and was superior to TRA, whereas no other efficacy differences reached statistical significance. However, BIM and TRA were both associated with considerably higher odds of conjunctival hyperaemia than LAT, and indirect comparison showed BIM carried a higher risk than TRA. Overall, the certainty of evidence ranged from low to moderate for efficacy outcomes and was predominantly high for safety outcomes. These findings underscore the clinical trade-off between maximising pressure lowering and minimising ocular side effects when selecting a first-line prostaglandin analogue.
LAT, TRA, and TRA are PGF2α analogs that lower IOP by increasing aqueous humor outflow (47). In contrast, BIM has unique pharmacological properties. It is a synthetic prostamide that reduces IOP by stimulating FP prostaglandin receptors and is synthesized through cyclooxygenase-2 activity (48). This distinct mechanism may account for BIM’s superior IOP-lowering efficacy compared to other prostaglandin analogs.
Conjunctival hyperemia is the most common adverse effect associated with prostaglandin analogs (49). In this study, the incidence of conjunctival hyperemia was highest in the BIM group; however, no evidence from the included studies indicated an association with ocular surface inflammation. Preclinical data suggest that BIM, like other prostaglandin analogs, activates nitric oxide synthase, releasing nitric oxide and causing vasodilation (50). Moreover, multi-dose safety evaluations in rabbits, dogs, and non-human primates have shown that BIM does not induce or exacerbate conjunctival inflammation, suggesting that the hyperemia it causes is a non-inflammatory vasodilatory response (50).
Although local adverse reactions such as conjunctival hyperemia may negatively affect treatment adherence, evidence from two long-term open-label clinical studies indicates that hyperemia symptoms diminish significantly over time (51, 52). Therefore, discontinuing prostaglandin analogs solely due to conjunctival hyperemia is not recommended once IOP is effectively controlled.
In summary, effective clinical decision-making for patients with glaucoma and ocular hypertension requires a careful balance between therapeutic efficacy and tolerability. Based on our findings, BIM may be preferred in patients with advanced disease who require greater IOP reduction, while LAT may be more suitable for patients prioritizing tolerability. Treatment strategies should be individualized, taking into account the patient’s disease severity, risk tolerance, and preferences. Although prostaglandin analogues remain the first-line therapy, our analysis highlights the need for clearer guidance in choosing among them to optimize both efficacy and safety.
Study limitations
This study has several limitations. First, although we conducted a comprehensive literature search, the possibility of publication bias cannot be fully excluded. Second, more than two-thirds of the included RCTs were conducted in high-income countries, with limited representation from low- and middle-income settings. This geographic imbalance may limit the generalisability of our findings to healthcare systems with different clinical practices, drug availability, and infrastructure. Third, estimates of intraocular pressure reduction were based exclusively on outcomes at 3 months, leaving the long-term efficacy of treatments uncertain. Finally, while this network meta-analysis offers robust comparative evidence by integrating data from multiple RCTs, its real-world applicability remains unclear. Differences in patient adherence, comorbidities, and treatment contexts are often underrepresented in trial populations. Real-world studies are needed to assess long-term effectiveness and tolerability across diverse clinical settings.
Conclusion
This network meta-analysis provides comparative evidence on four prostaglandin analogues for glaucoma and ocular hypertension. BIM was associated with the greatest reduction in intraocular pressure, but also with a significantly higher risk of conjunctival hyperaemia. TRA and LAT showed more favourable safety profiles, albeit with modest efficacy. These results suggest a trade-off between pressure-lowering potency and tolerability.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
JP: Data curation, Writing – review & editing, Conceptualization, Writing – original draft. WH: Conceptualization, Software, Writing – review & editing, Formal analysis, Methodology. JD: Writing – review & editing, Supervision, Visualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We thank all authors of previous studies who kindly provided additional information and data of their studies for this meta-analysis.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1642986/full#supplementary-material
References
1.
Asrani SG McGlumphy EJ Al-Aswad LA Chaya CJ Lin S Musch DC et al . The relationship between intraocular pressure and glaucoma: an evolving concept. Prog Retin Eye Res. (2024) 103:101303. doi: 10.1016/j.preteyeres.2024.101303
2.
Weinreb RN Aung T Medeiros FA . The pathophysiology and treatment of glaucoma: a review. JAMA. (2014) 311:1901–11. doi: 10.1001/jama.2014.3192
3.
Baghban R Talebnejad MR Meshksar A Heydari M Khalili MR . Recent advancements in nanomaterial-laden contact lenses for diagnosis and treatment of glaucoma, review and update. J Nanobiotechnology. (2023) 21:402. doi: 10.1186/s12951-023-02166-w
4.
Marquis RE Whitson JT . Management of glaucoma: focus on pharmacological therapy. Drugs Aging. (2005) 22:1–21. doi: 10.2165/00002512-200522010-00001
5.
Toris CB Gabelt BT Kaufman PL . Update on the mechanism of action of topical prostaglandins for intraocular pressure reduction. Surv Ophthalmol. (2008) 53:S107–20. doi: 10.1016/j.survophthal.2008.08.010
6.
Islam S Spry C . Prostaglandin analogues for ophthalmic use: a review of comparative clinical effectiveness, cost-effectiveness, and guidelines. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; (2020). Available online at: https://www.ncbi.nlm.nih.gov/books/NBK562924/
7.
Dai HY Wang JY Li YQ Diao HJ Zhang L . Therapeutic efficacy of latanoprost on primary open angle glaucoma: a protocol for a systematic review of randomized controlled trial. Medicine (Baltimore). (2018) 97:e13833. doi: 10.1097/MD.0000000000013833
8.
Tang W Zhang F Liu K Duan X . Efficacy and safety of prostaglandin analogues in primary open-angle glaucoma or ocular hypertension patients: a meta-analysis. Medicine (Baltimore). (2019) 98:e16597. doi: 10.1097/MD.0000000000016597
9.
Liu HW Lu YT Ren YB Meng Y . Efficacy of bimatoprost for the treatment of primary open-angle glaucoma: a protocol of systematic review and meta-analysis. Medicine (Baltimore). (2020) 99:e20356. doi: 10.1097/MD.0000000000020356
10.
Xing Y Zhu L Zhang K Huang S . The efficacy of the fixed combination of latanoprost and timolol versus other fixed combinations for primary open-angle glaucoma and ocular hypertension: a systematic review and meta-analysis. PLoS One. (2020) 15:e0229682. doi: 10.1371/journal.pone.0229682
11.
Tonin FS Steimbach LM Mendes AM Borba HH Pontarolo R Fernandez-Llimos F . Mapping the characteristics of network meta-analyses on drug therapy: a systematic review. PLoS One. (2018) 13:e0196644. doi: 10.1371/journal.pone.0196644
12.
Hutton B Salanti G Caldwell DM Chaimani A Schmid CH Cameron C et al . The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
13.
Higgins J. P. Green S. (eds.) Cochrane handbook for systematic reviews of interventions. Available online at: http://www.cochrane-handbook.org (2008).
14.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
15.
Nikolakopoulou A Higgins JPT Papakonstantinou T Chaimani A del Giovane C Egger M et al . CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. (2020) 17:e1003082. doi: 10.1371/journal.pmed.1003082
16.
Papakonstantinou T Nikolakopoulou A Higgins JPT Egger M Salanti G . CINeMA: software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst Rev. (2020) 16:e1080. doi: 10.1002/cl2.1080
17.
Gelman A Rubin DB . Markov chain Monte Carlo methods in biostatistics. Stat Methods Med Res. (1996) 5:339–55. doi: 10.1177/096228029600500402
18.
Turner RM Davey J Clarke MJ Thompson SG Higgins JP . Predicting the extent of heterogeneity in meta-analysis, using empirical data from the Cochrane database of systematic reviews. Int J Epidemiol. (2012) 41:818–27. doi: 10.1093/ije/dys041
19.
Chawla N Anothaisintawee T Charoenrungrueangchai K Thaipisuttikul P McKay GJ Attia J et al . Drug treatment for panic disorder with or without agoraphobia: systematic review and network meta-analysis of randomised controlled trials. BMJ. (2022) 376:e066084. doi: 10.1136/bmj-2021-066084
20.
da Costa BR Juni P . Systematic reviews and meta-analyses of randomized trials: principles and pitfalls. Eur Heart J. (2014) 35:3336–45. doi: 10.1093/eurheartj/ehu424
21.
Salanti G Ades AE Ioannidis JP . Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J Clin Epidemiol. (2011) 64:163–71. doi: 10.1016/j.jclinepi.2010.03.016
22.
Arcieri ES Santana A Rocha FN Guapo GL Costa VP . Blood-aqueous barrier changes after the use of prostaglandin analogues in patients with pseudophakia and aphakia: a 6-month randomized trial. Arch Ophthalmol. (2005) 123:186–92. doi: 10.1001/archopht.123.2.186
23.
Birt CM Buys YM Ahmed II Trope GE Toronto Area Glaucoma Society . Prostaglandin efficacy and safety study undertaken by race (the PRESSURE study). J Glaucoma. (2010) 19:460–7. doi: 10.1097/IJG.0b013e3181c4aeac
24.
Cantor LB Hoop J Morgan L Wudunn D Catoira Y Bimatoprost-Travoprost Study Group . Intraocular pressure-lowering efficacy of bimatoprost 0.03% and travoprost 0.004% in patients with glaucoma or ocular hypertension. Br J Ophthalmol. (2006) 90:1370–3. doi: 10.1136/bjo.2006.094326
25.
Cardascia N Vetrugno M Trabucco T Cantatore F Sborgia C . Effects of travoprost eye drops on intraocular pressure and pulsatile ocular blood flow: a 180-day, randomized, double-masked comparison with latanoprost eye drops in patients with open-angle glaucoma. Curr Ther Res Clin Exp. (2003) 64:389–400. doi: 10.1016/S0011-393X(03)00112-7
26.
Cellini M Caramazza R Bonsanto D Bernabini B Campos EC . Prostaglandin analogs and blood-aqueous barrier integrity: a flare cell meter study. Ophthalmologica. (2004) 218:312–7. doi: 10.1159/000079472
27.
Chiseliţă D Antohi I Medvichi R Danielescu C . Comparative analysis of the efficacy and safety of latanoprost, travoprost and the fixed combination timolol-dorzolamide; a prospective, randomized, masked, cross-over design study. Oftalmologia. (2005) 49:39–45.
28.
Faridi UA Saleh TA Ewings P Venkateswaran M Cadman DH Samarasinghe RA et al . Comparative study of three prostaglandin analogues in the treatment of newly diagnosed cases of ocular hypertension, open-angle and normal tension glaucoma. Clin Experiment Ophthalmol. (2010) 38:678–82. doi: 10.1111/j.1442-9071.2010.02305.x
29.
Fogagnolo P Dipinto A Vanzulli E Maggiolo E de Cilla’ S Autelitano A et al . A 1-year randomized study of the clinical and confocal effects of tafluprost and latanoprost in newly diagnosed glaucoma patients. Adv Ther. (2015) 32:356–69. doi: 10.1007/s12325-015-0205-5
30.
Gandolfi S Simmons ST Sturm R Chen K VanDenburgh AM Bimatoprost Study Group 3 . Three-month comparison of bimatoprost and latanoprost in patients with glaucoma and ocular hypertension. Adv Ther. (2001) 18:110–21. doi: 10.1007/BF02850299
31.
Hepsen IF Ozkaya E . 24-h IOP control with latanoprost, travoprost, and bimatoprost in subjects with exfoliation syndrome and ocular hypertension. Eye (Lond). (2007) 21:453–8. doi: 10.1038/sj.eye.6702243
32.
Kim JM Park SW Seong M Ha SJ Lee JW Rho S et al . Comparison of the safety and efficacy between preserved and preservative-free latanoprost and preservative-free tafluprost. Pharmaceuticals (Basel). (2021) 14:501. doi: 10.3390/ph14060501
33.
Konstas AG Holló G Irkec M Konstas AGP Tsironi S Durukan I et al . Diurnal IOP control with bimatoprost versus latanoprost in exfoliative glaucoma: a crossover, observer-masked, three-Centre study. Br J Ophthalmol. (2007) 91:757–60. doi: 10.1136/bjo.2006.106690
34.
Mishra D Sinha BP Kumar MS . Comparing the efficacy of latanoprost (0.005%), bimatoprost (0.03%), travoprost (0.004%), and timolol (0.5%) in the treatment of primary open angle glaucoma. Korean J Ophthalmol. (2014) 28:399–407. doi: 10.3341/kjo.2014.28.5.399
35.
Muz OE Dagdelen K Pirdal T Guler M . Comparison of BAK-preserved latanoprost and polyquad-preserved travoprost on ocular surface parameters in patients with glaucoma and ocular hypertension. Int Ophthalmol. (2021) 41:3825–35. doi: 10.1007/s10792-021-01947-2
36.
ClinicalTrails.gov . Safety and efficacy of bimatoprost compared with latanoprost in patients with glaucoma or ocular hypertension [internet]. (2007). Available online at: https://clinicaltrials.gov/show/NCT00541242 (Accessed August 15, 2022).
37.
Netland PA Landry T Sullivan EK Andrew R Silver L Weiner A et al . Travoprost compared with latanoprost and timolol in patients with open-angle glaucoma or ocular hypertension. Am J Ophthalmol. (2001) 132:472–84. doi: 10.1016/s0002-9394(01)01177-1
38.
Noecker RJ Earl ML Mundorf T Peace J Williams RD . Bimatoprost 0.03% versus travoprost 0.004% in black Americans with glaucoma or ocular hypertension. Adv Ther. (2003) 20:121–8. doi: 10.1007/BF02850259
39.
Park SH Yoo SH Ha SJ . Comparison of ocular pulse amplitude-lowering effects of Tafluprost and Latanoprost by dynamic contour tonometry. J Ocul Pharmacol Ther. (2015) 31:617–22. doi: 10.1089/jop.2014.0122
40.
Parmaksiz S Yuksel N Karabas VL Ozkan B Demirci G Caglar Y . A comparison of travoprost, latanoprost, and the fixed combination of dorzolamide and timolol in patients with pseudoexfoliation glaucoma. Eur J Ophthalmol. (2006) 16:73–80. doi: 10.5301/EJO.2008.5155
41.
Parrish RK Palmberg P Sheu WP XLT Study Group . A comparison of latanoprost, bimatoprost, and travoprost in patients with elevated intraocular pressure: a 12-week, randomized, masked-evaluator multicenter study. Am J Ophthalmol. (2003) 135:688–703. doi: 10.1016/s0002-9394(03)00098-9
42.
Stalmans I Oddone F Cordeiro MF Hommer A Montesano G Ribeiro L et al . Comparison of preservative-free latanoprost and preservative-free bimatoprost in a multicenter, randomized, investigator-masked cross-over clinical trial, the SPORT trial. Graefes Arch Clin Exp Ophthalmol. (2016) 254:1151–8. doi: 10.1007/s00417-016-3299-9
43.
Varma R Hwang LJ Grunden JW Bean GW . Inter-visit intraocular pressure range: an alternative parameter for assessing intraocular pressure control in clinical trials. Am J Ophthalmol. (2008) 145:336–42. doi: 10.1016/j.ajo.2007.10.002
44.
Whitson JT Trattler WB Matossian C Williams J Hollander DA . Ocular surface tolerability of prostaglandin analogs in patients with glaucoma or ocular hypertension. J Ocul Pharmacol Ther. (2010) 26:287–92. doi: 10.1089/jop.2009.0134
45.
Walters TR Kothe AC Boyer JL Usner DW Lopez K Duquesroix B et al . A randomized, controlled comparison of NCX 470 (0.021, 0.042, and 0.065%) and Latanoprost 0.005% in patients with open-angle Glaucoma or ocular hypertension: the Dolomites study. J Glaucoma. (2022) 31:382–91. doi: 10.1097/IJG.0000000000002030
46.
Fechtner R Mansberger S Branch J Mulaney J Ziebell S Lopez K et al . A randomized, controlled comparison of NCX 470, a nitric oxide-donating Bimatoprost, and Latanoprost in subjects with open-angle Glaucoma or ocular hypertension: the MONT BLANC study. Am J Ophthalmol. (2024) 264:66–74. doi: 10.1016/j.ajo.2024.03.002
47.
Sharif NA Odani-Kawabata N Lu F Pinchuk L . FP and EP2 prostanoid receptor agonist drugs and aqueous humor outflow devices for treating ocular hypertension and glaucoma. Exp Eye Res. (2023) 229:109415. doi: 10.1016/j.exer.2023.109415
48.
Kong XM Sun XH Meng FR Qian SH Jin XH Liu CS et al . Comparison of intraocular pressure-lowering effects of three prostaglandin analogues. Chin J Optom Ophthalmol. (2006) 8:228–30. doi: 10.3760/cma.j.issn.1674-845X.2006.04.007
49.
Chen XL Fu YJ Qu B Wang YW Tang X Wang YH et al . Safety profile of 0.0015% tafluprost eye drops in China: a post-marketing observational study. Int J Ophthalmol. (2023) 16:108–14. doi: 10.18240/ijo.2023.01.16
50.
Chen J Dinh T Woodward DF Holland JM Yuan YD Lin TH et al . Bimatoprost: mechanism of ocular surface hyperemia associated with topical therapy. Cardiovasc Drug Rev. (2005) 23:231–46. doi: 10.1111/j.1527-3466.2005.tb00168.x
51.
Abelson MB Mroz M Rosner SA Dirks MS Hirabayashi D . Multicenter, open-label evaluation of hyperemia associated with use of bimatoprost in adults with open-angle glaucoma or ocular hypertension. Adv Ther. (2003) 20:1–13. doi: 10.1007/BF02850114
52.
Watson PG . Latanoprost. Two years’ experience of its use in the United Kingdom. Ophthalmology. (1998) 105:82–7. doi: 10.1016/s0161-6420(98)91372-0
Summary
Keywords
intraocular pressure, glaucoma, prostaglandin analogs, network meta-analysis, Bayesian
Citation
Peng J, Huang W and Duan J (2025) Efficacy and safety of prostaglandin drugs for elevated intraocular pressure: a Bayesian network meta-analysis. Front. Med. 12:1642986. doi: 10.3389/fmed.2025.1642986
Received
07 June 2025
Accepted
28 July 2025
Published
11 August 2025
Volume
12 - 2025
Edited by
Michele Lanza, University of Campania Luigi Vanvitelli, Italy
Reviewed by
Matheus Chavez, Federal University of Santa Catarina, Brazil
Ivana Senese, Federico II University Hospital, Italy
Updates
Copyright
© 2025 Peng, Huang and Duan.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Junguo Duan, duanjg@cdutcm.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.