Abstract
Background:
Inflammatory pathways critically contribute to the pathogenesis of intracardiac thrombosis (ICT) following acute myocardial infarction (AMI) patients. This study evaluated the predictive value of inflammation biomarkers for ICT.
Methods:
This retrospective case–control study included 8,999 AMI patients hospitalized at the First Affiliated Hospital of Xi’an Jiaotong University from January 2018 to December 2022, among whom 88 developed ICT. To address class imbalance, 891 non-ICT patients were randomly selected as controls using a 1:10 ratio. Inflammation-related biomarkers were screened using univariate and multivariate logistic regression, and a risk heatmap was generated based on key predictors.
Results:
Multivariate logistic regression identified elevated neutrophil-to-lymphocyte ratio (NLR), N-terminal pro–B-type natriuretic peptide (NT-proBNP), low-density lipoprotein cholesterol (LDL-C), and reduced albumin-to-globulin ratio (AGR) as independent risk factors for ICT. NLR demonstrated the highest discriminatory capacity, demonstrating superior predictive performance [receiver operating characteristic (ROC) curve, area under the curve (AUC) = 0.774, 95% confidence interval (CI): 0.724–0.823, p < 0.001] that persisted after full covariate adjustment, and remained significant after full adjustment [odds ratio (OR) = 2.54, 95% CI: 1.98–3.15, p = 0.002]. Integration of NLR and LDL-C into a sex-stratified risk stratification matrix significantly enhanced diagnostic accuracy (AUC = 0.838, 95% CI: 0.799–0.878).
Conclusion:
This study established NLR as a robust indicator for ICT assessment and presents a practical, visual risk heatmap that may facilitate personalized thromboprophylaxis in AMI management.
1 Introduction
Acute myocardial infarction (AMI) remains a leading global health burden, with a steadily increasing incidence worldwide (1). AMI initiates a complex pathophysiological cascade, including inflammation, oxidative stress, platelet activation, and thrombus formation. Among these, intracardiac thrombosis (ICT) represents a clinically significant and potentially life-threatening complication, as it can compromise cardiac function and precipitate heart failure or myocardial rupture (2). Dislodged thrombi may cause major embolic events—such as ischemic stroke, acute limb ischemia, or pulmonary embolism—substantially increasing morbidity and mortality (3–5). However, ICT is often subclinical and lacks overt symptoms. Its diagnosis depends heavily on imaging, which is limited by cost and equipment availability, making widespread screening impractical (6). These limitations underscore the need for alternative, cost-effective, and readily available biomarkers for early detection and risk stratification.
Recent studies have demonstrated that persistent low-grade inflammation plays a central role in the onset, progression, and complications of cardiovascular diseases (5). In AMI patients, inflammation not only destabilizes atherosclerotic plaques but also contributes to thrombogenesis by activating neutrophils, promoting platelet aggregation, and impairing endothelial function—key mechanisms in ICT development (7). The presence and intensity of chronic inflammation can be reflected by circulating inflammatory biomarkers (8). Among these, the neutrophil-to-lymphocyte ratio (NLR), a simple marker derived from routine blood counts, has emerged as a reliable predictor of cardiovascular outcomes and thrombotic risk (8, 9). NLR reflects the dynamic balance between innate and adaptive immunity, with elevated levels indicating systemic inflammation. A prospective study by Song et al. (10) confirmed that elevated NLR is associated with cause-specific mortality—including cardiovascular, respiratory, infectious, and renal diseases—highlighting its potential as a risk stratification tool in both public health and clinical settings (11).
In addition to NLR, several other routinely assessed serum biomarkers are known to correlate with thrombosis risk and myocardia function. These include markers of myocardial injury or dysfunction [e.g., N-terminal pro–B-type natriuretic peptide (NT-proBNP), creatine kinase-MB (CK-MB), and creatine kinase (CK)], lipid metabolism indicators [e.g., high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C)], as well as composite indices such as the albumin-to-globulin ratio (AGR) and the atherogenic plasma index (API).
While inflammation is recognized as a key factor in AMI prognosis, the association between inflammatory markers and the risk of ICT remains insufficiently characterized. This study focuses on NLR and other accessible serum biomarkers to evaluate their predictive value for ICT in AMI patients and to propose a practical early risk stratification model to guide clinical management.
2 Methods
2.1 Study design and patients
This retrospective observational study was conducted at the First Affiliated Hospital of Xi’an Jiaotong University (No. 277, West Yanta Road, Xi’an, Shaanxi Province, 710061, China). Clinical data were retrospectively collected from patients diagnosed with acute myocardial infarction (AMI) between January 2018 and December 2022. AMI and ICT were diagnosed according to established criteria (12, 13), and treatment protocols adhered to the 2023 European Society of Cardiology guidelines for the management of acute coronary syndromes (14). Eligible patients included those with confirmed diagnoses of AMI, including both ST-elevation myocardial infarction and non-ST-elevation myocardial infarction. ICT was confirmed through echocardiographic imaging performed during hospitalization, encompassing thrombus detection in the left and right ventricles, atria, atrial appendages, apex, or other cardiac chambers (6, 15). All AMI patients underwent transthoracic echocardiography during admission as part of routine inpatient assessment, allowing for systematic detection of intracardiac thrombus. Standardized transthoracic echocardiography (TTE) was performed on all patients within 24 h of admission using with apical 4-chamber and subcostal views for thrombus screening.
Patients with missing data for the majority of laboratory parameters were excluded. Given the low incidence of ICT, a 1:10 random sampling strategy was employed to select non-ICT controls, thereby addressing extreme class imbalance and enhancing model robustness. To preserve the integrity of variable distribution and avoid masking the effects of potential risk factors, we did not perform matching during control selection. Instead, all relevant covariates were included in the multivariate regression models to adjust for confounding. To evaluate potential sampling bias, we additionally compared baseline characteristics between the included non-ICT controls and the excluded AMI patients without ICT; no significant differences were observed (Supplementary Table S1). All ICT cases included were first-time diagnoses, and patients with active infections within the preceding month were excluded to minimize confounding. For patients with partial laboratory data missing, multiple imputation was applied to preserve statistical power and optimize model discrimination.
The study protocol conformed to the ethical standards of the Declaration of Helsinki (1975, revised in 2024) and was approved by the Institutional Review Board of Xi’an Jiaotong University (Approval No. XJTU1AF2025LSYY-410). As this study involved retrospective analysis of de-identified data obtained from the hospital’s biobank, the requirement for written informed consent was formally waived. All data handling complied with institutional protocols governing the secondary use of biomedical data. An overview of the study design and patient selection process is shown in Figure 1.
Figure 1

A flowchart of the study.
2.2 Data collection
Comprehensive baseline data were systematically collected at the time of hospital admission, encompassing demographic characteristics, pre-existing comorbidities, and heart failure status assessed via the Killip classification. Laboratory parameters were extracted from the hospital’s electronic medical records and included cardiac injury markers, complete blood count indices, absolute neutrophil count (ANC), absolute lymphocyte count (ALC), lipid profiles, hepatic and renal function tests, as well as serum electrolyte levels.
Inflammation-related composite biomarkers were calculated as follows: the neutrophil-to-lymphocyte ratio (NLR) was derived by dividing the neutrophil count by the lymphocyte count [NLR = neutrophil count (NEUT) / ALC], and the albumin-to-globulin ratio (AGR) was computed as serum albumin divided by globulin [AGR = ALB / total protein (TP) – albumin (ALB)]. All laboratory values were obtained upon admission to ensure consistency and minimize potential confounding due to treatment interventions.
2.3 Statistical analysis
Continuous variables were evaluated for normality. Those conforming to a normal distribution were expressed as mean ± standard deviation, while skewed data were reported as medians with interquartile ranges. Categorical variables were presented as counts and percentages. Between-group differences were assessed using independent samples t-tests for normally distributed continuous variables and the Mann–Whitney U test for non-normally distributed data. Categorical comparisons were performed using Pearson’s chi-square test or Fisher’s exact test where appropriate.
To identify potential risk factors for ICT and to screen for predictive inflammation-related biomarkers among AMI patients, univariate logistic regression analyses were conducted. Variables with a p < 0.05 in univariate analysis were subsequently entered into a multivariate logistic regression model to identify independent predictors. Results were reported as odds ratios with corresponding 95% confidence intervals.
To further assess the predictive role of NLR in ICT, four hierarchical logistic regression models were constructed with progressive adjustment for potential confounders: Model 1 was unadjusted; Model 2 adjusted for age and sex; Model 3 included additional adjustments for hypertension, diabetes, chronic kidney disease, arrhythmia, and Killip class; and Model 4 further accounted for laboratory indicators including cardiac injury biomarkers, liver function enzymes, lipid profile, renal function parameters, electrolytes, and hemoglobin levels. NLR was also categorized into high and low groups based on the optimal threshold determined via ROC curve analysis, and its predictive performance was evaluated across all models.
Based on multivariate analysis, the strongest predictors—NLR and LDL-C—were used to construct overall and sex-specific risk heatmaps. Model performance was assessed using ROC curves and calibration plots. All statistical analyses were conducted using SPSS version 26.0 (IBM Corp., Armonk, NY, USA) and R version 4.5.0. A two-sided p-value of < 0.05 was considered statistically significant.
This retrospective observational study was conducted at the First Affiliated Hospital of Xi’an Jiaotong University (No. 277, West Yanta Road, Xi’an, Shaanxi Province, 710061, China). Clinical data were retrospectively collected from patients diagnosed with acute myocardial infarction (AMI) between January 2018 and December 2022. AMI and ICT were diagnosed according to established criteria (12, 13), and treatment protocols adhered to the 2023 European Society of Cardiology guidelines for the management of acute coronary syndromes (14). Eligible patients included those with confirmed diagnoses of AMI, including both ST-elevation myocardial infarction and non-ST-elevation myocardial infarction. ICT was confirmed through echocardiographic imaging performed during hospitalization, encompassing thrombus detection in the left and right ventricles, atria, atrial appendages, apex, or other cardiac chambers (6, 15).
3 Results
3.1 Clinical characteristics of study participants
A total of 9,415 patients diagnosed with AMI were admitted to the First Affiliated Hospital of Xi’an Jiaotong University. After excluding 416 patients due to incomplete laboratory data, 8,999 remained eligible for analysis, among whom 88 were diagnosed with ICT. Given the low prevalence of ICT, to address class imbalance and improve model stability and discriminatory performance, a 1:10 ratio of non-ICT controls was randomly sampled, resulting in a final cohort of 979 patients (88 ICT cases and 891 controls). For variables with missing values (<5%), multiple imputation by chained equations (MICE) was performed. The imputation model included all relevant predictors and outcome variables used in the main analysis, such as demographics, comorbidities, Killip classification, and laboratory parameters including inflammatory, cardiac, hepatic, renal, and hematologic indices. To improve transparency, we summarized the missingness for each laboratory covariate and presented their means and standard deviations in Supplementary Table S2. Given the low level of missingness, the distributions of variables before and after imputation were comparable, with no substantial shifts observed. Five imputed datasets were generated and combined using Rubin’s rules to minimize bias and optimize statistical power. No significant differences were observed between groups in baseline demographics, comorbidities, or Killip class. However, several laboratory parameters differed significantly: the ICT group had higher levels of NLR, CK-MB, CK, LDH, AST, ALT, NT-proBNP, cTnT, and LDL-C, while AGR was significantly lower (all p < 0.05). Detailed baseline characteristics are presented in Table 1.
Table 1
| Variables | Total (n = 979) | Control group (n = 891) | ICT group (n = 88) | p-value |
|---|---|---|---|---|
| Enrollment basic characteristics | ||||
| Age (years, IQR) | 64.00 (55.00, 71.00) | 64.00 (55.00, 71.00) | 62.00 (55.00, 71.00) | 0.986 |
| Female (n, %) | 253 (25.84) | 236 (26.49) | 17 (19.32) | 0.143 |
| Comorbidities | ||||
| Hypertension (n, %) | 457 (46.68) | 415 (46.58) | 42 (47.73) | 0.837 |
| Diabates (n, %) | 600 (61.29) | 543 (60.94) | 57 (64.77) | 0.482 |
| CKD (n, %) | 7 (0.72) | 5 (0.56) | 2 (2.27) | 0.125 |
| Arrhythmia (n, %) | 82 (8.38) | 72 (8.08) | 10 (11.36) | 0.289 |
| Killip classification (n, %) | ||||
| I | 815 (83.25) | 743 (83.39) | 72 (81.82) | 0.707 |
| II | 122 (12.46) | 110 (12.35) | 12 (13.64) | 0.727 |
| III | 23 (2.35) | 21 (2.36) | 2 (2.27) | 1.000 |
| IV | 19 (1.941) | 17 (1.91) | 2 (2.27) | 1.000 |
| Laboratory results | ||||
| NLR (IQR) | 2.83 (2.36–3.45) | 2.78 (2.30–3.37) | 3.63 (3.07–5.34) | <0.001 |
| AGR (IQR) | 2.15 (1.40–2.89) | 2.19 (1.55–2.90) | 1.60 (1.09–2.39) | <0.001 |
| CK-MB (U/L, IQR) | 26.00 (14.00–78.00) | 26.00 (14.00–76.00) | 34.85 (18.00–106.65) | 0.031 |
| CK (U/L, IQR) | 225.00 (96.0–763.0) | 221.0 (95.00–734.00) | 257.50 (126.00–1219.00) | 0.024 |
| LDH (U/L, IQR) | 279.0 (218.00–432.00) | 271.0(216.00–415.00) | 420.00 (266.50–705.50) | <0.001 |
| AST (U/L, IQR) | 42.00 (25.00–97.00) | 41.00 (25.00–92.00) | 59.00 (31.50–163.00) | 0.004 |
| ALT (U/L, IQR) | 31.0 (21.00–47.00) | 31.00 (21.00–46.00) | 39.00 (25.75–54.50) | 0.002 |
| NT-proBNP (pg/mL, IQR) | 749.40 (266.40–1879) | 697.00 (254.05–1715.00) | 2210.0(650.1–5071.25) | <0.001 |
| cTnT (ng/mL, IQR) | 0.46 (0.08–1.54) | 0.42 (0.07–1.46) | 0.89 (0.19–2.48) | <0.001 |
| LDL-C (mmol/L, IQR) | 2.13 (1.69–2.70) | 2.09 (1.63–2.60) | 2.70 (2.27–3.17) | <0.001 |
| HDL-C (mmol/L, IQR) | 0.95 (0.81–1.09) | 0.95 (0.82–1.10) | 0.91 (0.76–1.06) | 0.104 |
| BUN (mmol/L, IQR) | 5.57 (4.48–6.92) | 5.54 (4.46–6.91) | 5.72 (4.57–6.93) | 0.465 |
| Creatinine (μmol/L, IQR) | 64.00 (54.00–77.50) | 64.00 (54.00–78.00) | 62.50 (53.00–77.00) | 0.758 |
| ANC (×109/L, IQR) | 6.54 (4.76–9.06) | 6.61 (4.79–9.03) | 6.05 (4.34–9.12) | 0.443 |
| ALC (×109/L, IQR) | 1.42 (1.04–1.89) | 1.42 (1.04–1.89) | 1.43 (1.09–1.90) | 0.697 |
| WBC (×109/L, IQR) | 8.62 (6.96–11.20) | 8.68 (6.99–11.16) | 8.22 (6.75–11.38) | 0.481 |
| NEUT (100%, IQR) | 76.40 (68.40–83.20) | 76.40 (68.55–83.30) | 76.65 (66.90–81.03) | 0.269 |
| Hb (g/L, IQR) | 142.00 (129.00–153.50) | 142.00 (129.50–154.00) | 139.00 (123.25–151.00) | 0.123 |
| K+ (mmol/L, IQR) | 3.95 (3.67–4.23) | 3.95 (3.67–4.23) | 3.99 (3.66–4.25) | 0.707 |
| Na+ (mmol/L, IQR) | 140.00 (138.00–142.00) | 140.00 (138.00–142.00) | 139.93 (137.45–142.00) | 0.331 |
| Cl− (mmol/L, IQR) | 102.00 (99.40–104.90) | 102.10 (99.40–104.80) | 101.80 (100.00–105.40) | 0.618 |
| Ca2+ (mmol/L, IQR) | 2.26 (2.15–2.35) | 2.26 (2.15–2.35) | 2.24 (2.11–2.36) | 0.320 |
| Mg2+ (mmol/L, IQR) | 0.98 (0.92–1.06) | 0.98 (0.92–1.06) | 0.99 (0.91–1.08) | 0.870 |
Baseline characteristics of patients with AMI.
AMI, acute myocardial infarction; ICT, intracardiac thrombosis; IQR, interquartile range; CKD, chronic kidney disease; NLR, neutrophil-to-lymphocyte ratio; AGR, albumin-to-globulin ratio; CK-MB, creatine kinase–MB isoenzyme; CK, creatine kinase; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; ALT, alanine aminotransferase; NT-proBNP, N-terminal pro–B-type natriuretic peptide; cTnT, cardiac troponin T; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; BUN, blood urea nitrogen; ANC, absolute neutrophil count; ALC, absolute lymphocyte count; WBC, white blood cell count; NEUT, neutrophil percentage; Hb, hemoglobin; K+, serum potassium; Na+, serum sodium; Cl−, serum chloride; Ca2+, serum calcium; Mg2+, serum magnesium. All p-values presented are unadjusted due to the hypothesis-driven nature of the analysis; multivariable models were used to confirm independent associations.
3.2 Identification of inflammation-related predictors of ICT
Baseline demographic and clinical variables were compared between the ICT and non-ICT groups. NLR, CK-MB, CK, LDH, ALT, NT-proBNP, cTnT, LDL-C, and AGR were significantly associated with ICT (p < 0.05). These variables were entered into multivariate logistic regression (Table 2), identifying NLR [odds ratio (OR) = 2.25, 95% confidence interval (CI): 1.79–2.83, p < 0.001], NT-proBNP (OR = 1.01, 95% CI: 1.00–1.02, p = 0.012), and LDL-C (OR = 2.86, 95% CI: 2.03–4.02, p < 0.001) as independent predictors, while AGR was protective (OR = 0.79, 95% CI: 0.64–0.97, p = 0.026).
Table 2
| Variables | Univariate regression (p < 0.05) | Multivariate analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| NLR | 2.35 (1.90–2.90) | <0.001 | 2.25 (1.79–2.83) | <0.001 |
| AGR | 0.67 (0.53–0.84) | <0.001 | 0.79 (0.64–0.97) | 0.026 |
| CK-MB (U/L) | 1.01 (1.00–1.03) | <0.001 | 1.00 (1.00–1.01) | 0.709 |
| CK (U/L) | 1.05 (1.03–1.07) | <0.001 | 1.00 (1.00–1.00) | 0.871 |
| LDH (U/L) | 1.01 (1.01–1.01) | <0.001 | 1.00 (1.00–1.00) | 0.131 |
| AST (U/L) | 1.01 (1.01–1.01) | <0.001 | 1.00 (0.99–1.00) | 0.162 |
| ALT (U/L) | 1.00 (1.00–1.00) | 0.078 | ||
| NT-proBNP (pg/mL) | 1.02 (1.00–1.04) | <0.001 | 1.01 (1.00–1.02) | 0.012 |
| cTnT (ng/mL) | 1.15 (1.06–1.24) | <0.001 | 1.07 (0.92–1.24) | 0.371 |
| LDL-C (mmol/L) | 2.75 (2.06–3.67) | <0.001 | 2.86 (2.03–4.02) | <0.001 |
Results of multivariate logistic regression model.
NLR, neutrophil-to-lymphocyte ratio; AGR, albumin-to-globulin ratio; CK-MB, creatine kinase–MB isoenzyme; CK, creatine kinase; LDH, lactate dehydrogenase; AST, aspartate aminotransferase; NT-proBNP, N-terminal pro–B-type natriuretic peptide; cTnT, cardiac troponin T; LDL-C, low-density lipoprotein cholesterol. Bold values indicate statistical significance (p < 0.05) in the multivariate logistic regression analysis.
Receiver operating characteristic (ROC) curve analysis was performed to evaluate the predictive performance of these markers (Figure 2). NLR [area under the curve (AUC) = 0.774, 95% CI: 0.724–0.823, p < 0.001] and LDL-C (AUC = 0.728, 95% CI: 0.677–0.778, p < 0.001) demonstrated favorable discriminative ability. These findings highlight the potential clinical utility of NLR and LDL-C as inflammation-related biomarkers for predicting ICT in AMI patients.
Figure 2
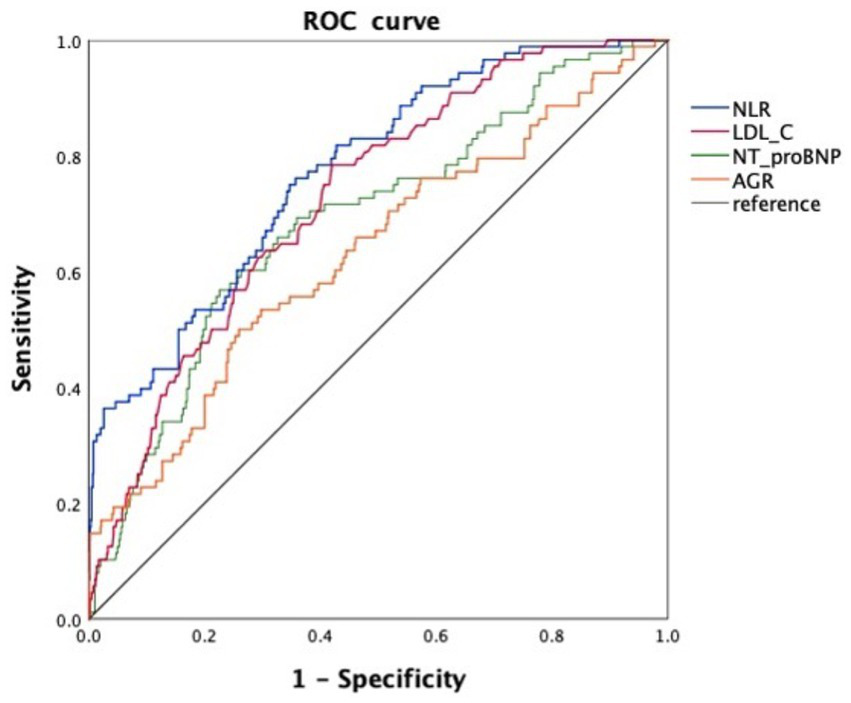
ROC curves of four inflammation-related biomarkers for predicting ICT in patients with AMI. NLR, neutrophil-to-lymphocyte ratio; LDL-C, low-density lipoprotein cholesterol; NT-proBNP, N-terminal pro–B-type natriuretic peptide; AGR, albumin-to-globulin ratio.
3.3 Predictive value of NLR after adjustment and stratification
To evaluate the independent predictive value of NLR for ICT, we constructed four logistic regression models with progressive adjustment for potential confounders.
When included as a continuous variable, NLR remained significantly associated with ICT across all four models (all p < 0.001), demonstrating consistent independent predictive value. In the fully adjusted Model 4, the OR for NLR was 2.54 (95% CI: 1.98–3.15) (Figure 3A). We then stratified NLR using the ROC-derived cut-off value of 3.894 and analyzed it as a categorical variable. NLR remained significantly predictive of ICT in all models, with an OR of 18.9 (95% CI: 10.8–33.4) in Model 4 (Figure 3B), further supporting its role as a stable and reliable inflammation-related biomarker for predicting ICT in AMI patients.
Figure 3
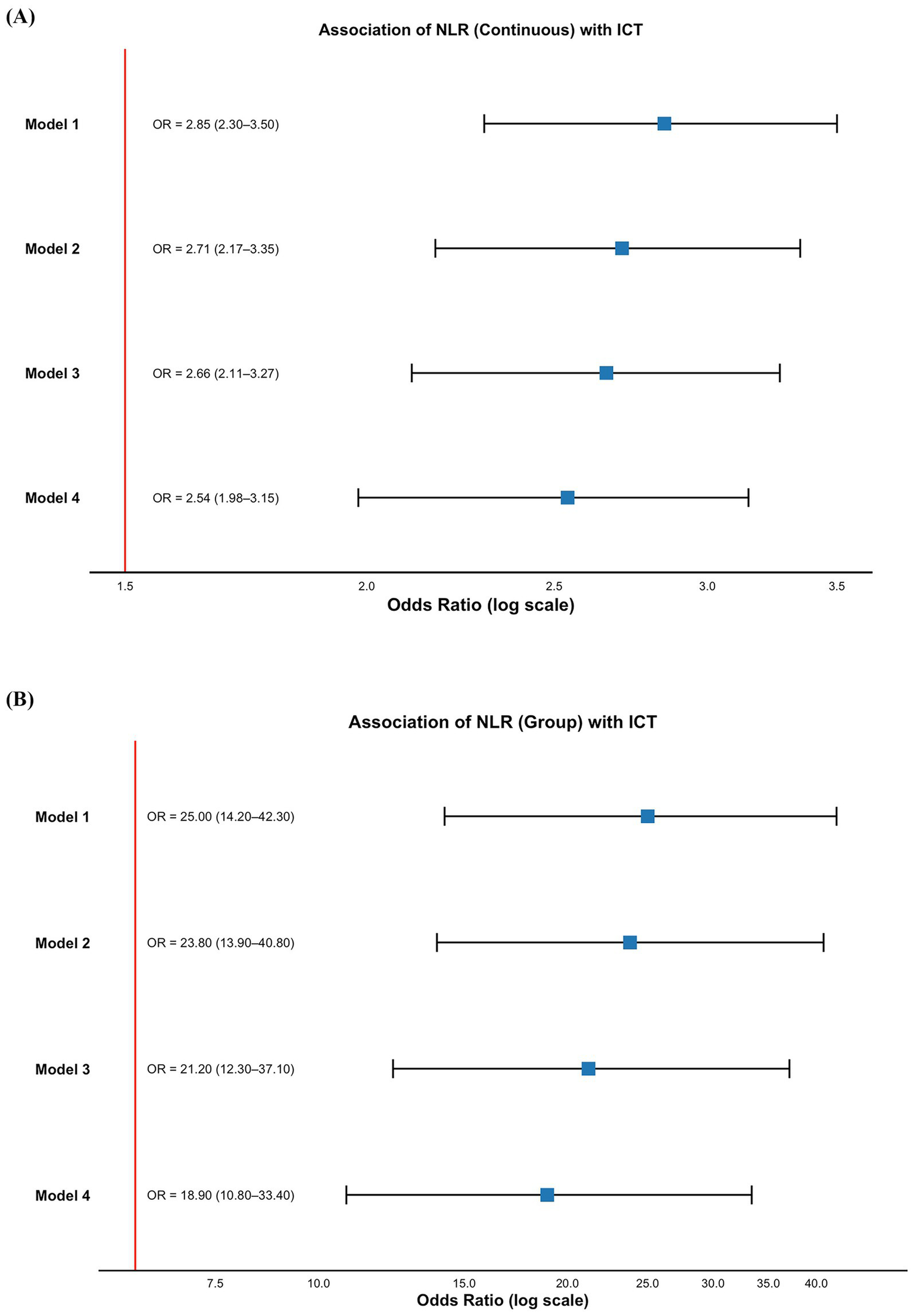
Association between NLR and ICT in patients with AMI. (A) NLR as a continuous variable; (B) NLR as a categorical variable. Model 1: unadjusted; Model 2: adjusted for age and sex; Model 3: adjusted for age, sex, comorbidities (hypertension, diabetes, CKD, arrhythmia), and Killip classification; Model 4: additionally adjusted for laboratory parameters including liver function, renal function, serum electrolytes, and cardiac injury biomarkers. AMI, acute myocardial infarction; ICT, intracardiac thrombosis; NLR, neutrophil-to-lymphocyte ratio; CKD, chronic kidney disease; OR, odds ratio; CI, confidence interval.
3.4 Visual heatmap model for risk prediction
To enhance the clinical applicability of our findings, we developed a visual risk heatmap model based on the two top-performing inflammation-related biomarkers—NLR and LDL-C. Both markers were divided into sextiles, and a combined risk heatmap was generated for the entire cohort (Figure 4A), followed by sex-stratified heatmaps to visualize differential risk distribution (Figure 4B). This model intuitively illustrated the probability of ICT occurrence across varying levels of NLR and LDL-C.
Figure 4
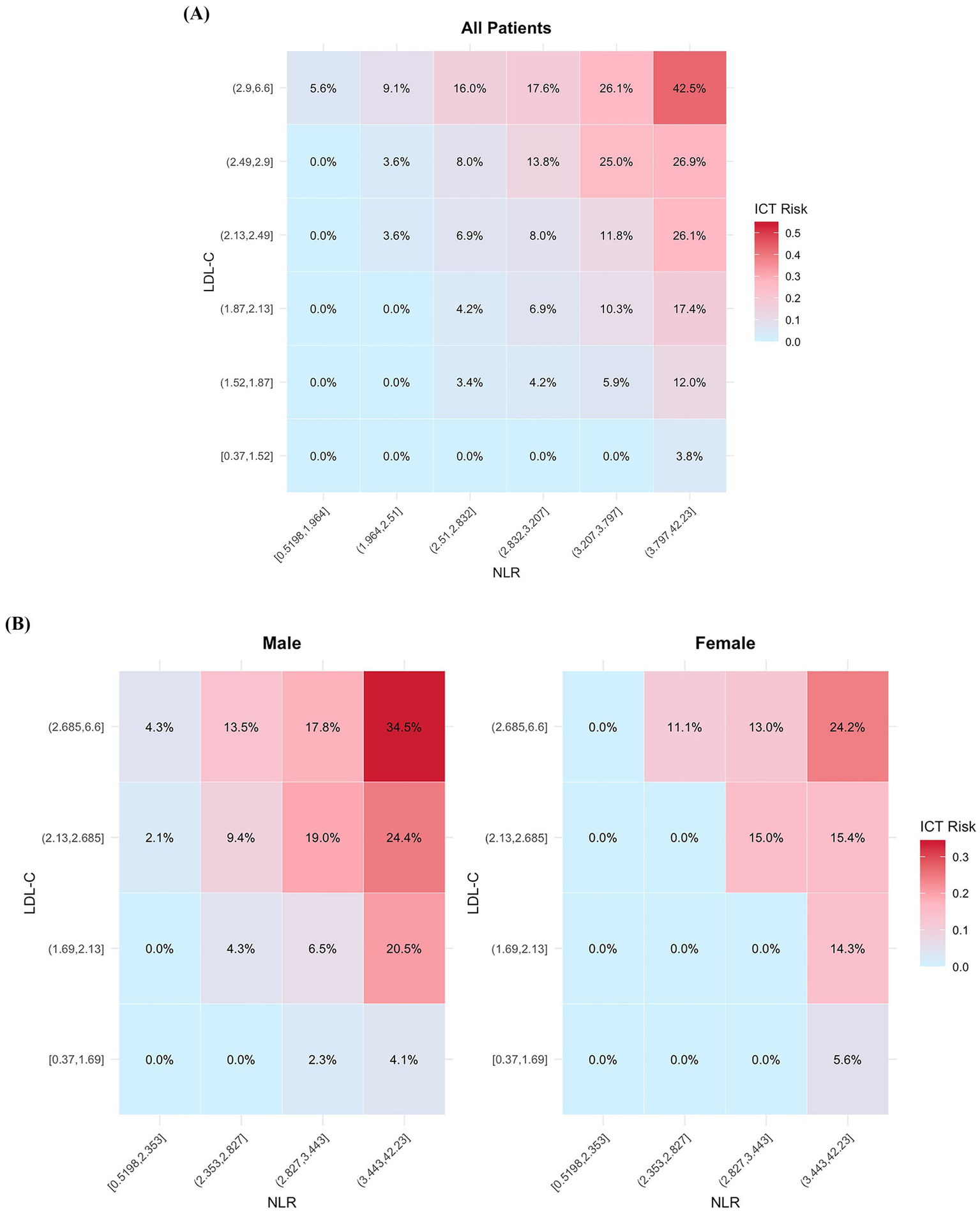
Heatmap visualization of ICT risk based on the combined distribution of NLR and LDL-C. (A) Risk heatmap for the overall cohort. (B) Sex-stratified risk heatmaps. ICT, intracardiac thrombosis; NLR, neutrophil-to-lymphocyte ratio; LDL-C, low-density lipoprotein cholesterol.
The model achieved strong discriminatory performance with an AUC of 0.838 (95% CI: 0.799–0.878), outperforming either marker alone (Figure 5A). The calibration curve showed close agreement between predicted and observed probabilities, and the Hosmer–Lemeshow test (χ2 = 8.872, p = 0.357) confirmed the model’s goodness of fit and reliability (Figure 5B).
Figure 5
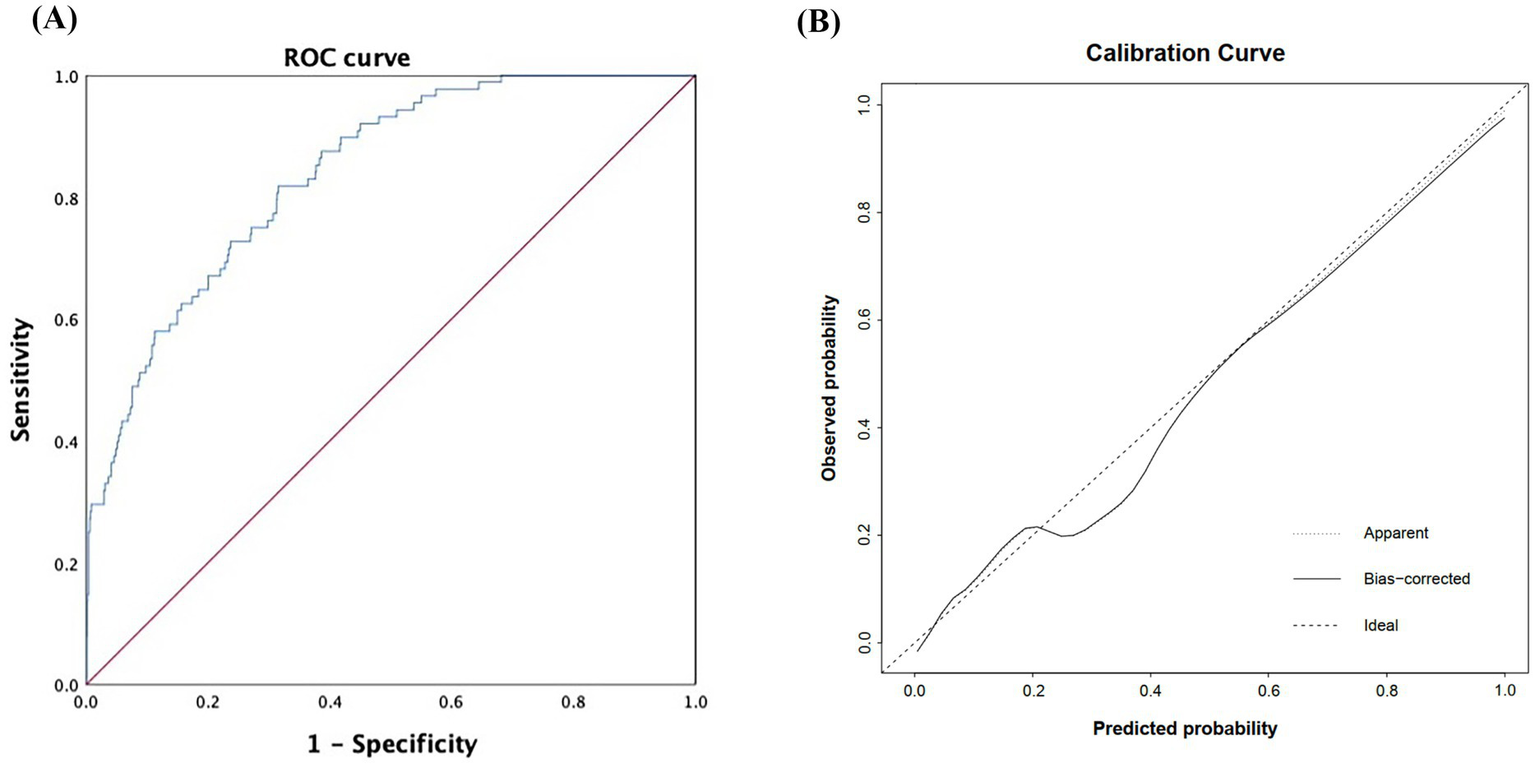
Discrimination and calibration performance of the risk heatmap model based on NLR and LDL-C. (A) ROC curve of the model (B) calibration curve assessing the agreement between predicted and observed probabilities. NLR, neutrophil-to-lymphocyte ratio; LDL-C, low-density lipoprotein cholesterol; ROC, receiver operating characteristic; AUC, area under the curve.
4 Discussion
This study investigated the predictive value of inflammation-related biomarkers for ICT in patients with AMI. The main findings are as follows: (1) four biomarkers—elevated NLR, LDL-C, NT-proBNP, and decreased AGR—were independently associated with ICT occurrence in multivariable logistic regression (all p < 0.05); (2) among them, NLR demonstrated the strongest predictive power, remaining statistically significant across all adjusted models (fully adjusted OR = 2.54; subgroup OR = 18.9); (3) a risk heatmap combining NLR and LDL-C exhibited good discriminatory performance (AUC = 0.838), suggesting its potential clinical utility in ICT risk stratification in AMI patients.
Inflammation plays a central role in AMI, not only as a response to cardiomyocyte necrosis but also as a driver of post-infarction complications. Necrotic myocardium releases cytokines such as IL-6, TNF-α, and IL-1β, which recruit neutrophils and monocytes to the infarcted area (16). This inflammatory cascade may aggravate injury, trigger oxidative stress, and activate coagulation, fostering a prothrombotic state (17, 18). Persistent inflammation following AMI contributes to endothelial dysfunction, tissue factor expression, and platelet activation, all of which promote ICT (19). As noted by Frangogiannis, inflammation orchestrates both early tissue damage and late-stage thrombus deposition (20). Structural substrates, including anterior wall infarction, reduced LVEF, ventricular aneurysm, and high Killip class, are established risk factors for left ventricular thrombus (LVT) (21–23), and elevated CK-MB levels are also associated with thrombosis (24). Although Sia et al. linked elevated NLR with LVT (25), data on inflammation-based predictors for other ICT subtypes remain limited. Additionally, neutrophil extracellular traps (NETs) contribute to thrombogenesis by interacting with TF and platelets within infarcted myocardium (25, 26), suggesting inflammation may directly participate in ICT pathogenesis.
The NLR is a simple and accessible inflammation-related biomarker derived from routine peripheral blood counts, reflecting the balance between innate immune activation and adaptive immune suppression (27). In our study, NLR was the strongest independent predictor of intracardiac thrombosis (ICT) among all evaluated biomarkers, with its predictive value remaining robust across fully adjusted and subgroup models. Previous studies have linked elevated NLR to adverse cardiovascular outcomes, including acute coronary syndrome, heart failure, and stroke (28–30). While Zazula et al. demonstrated its association with left ventricular thrombus (LVT) in AMI patients (31), more recent findings suggest that elevated NLR is also associated with left atrial thrombus and spontaneous echocardiographic contrast in patients with atrial fibrillation or dilated ventricles (32, 33), supporting its broader role in identifying prothrombotic conditions across cardiac chambers. Mechanistically, an elevated NLR reflects a shift toward a pro-inflammatory and pro-thrombotic state. Activated neutrophils release reactive oxygen species, proteolytic enzymes, and myeloperoxidase, which contribute to endothelial damage and upregulate tissue factor expression (34). In addition, neutrophil extracellular traps (NETs)—web-like DNA structures enriched with tissue factor and platelets—facilitate thrombus propagation (35–37). Concurrently, reduced lymphocyte levels indicate impaired immunoregulation, promoting sustained neutrophil activation and thrombin generation (38). These immuno-thrombotic mechanisms provide a biological rationale for the association between elevated NLR and ICT development (26, 39). In our cohort, an NLR threshold of 3.894 was derived using receiver operating characteristic (ROC) analysis, offering an optimal trade-off between sensitivity and specificity for ICT risk stratification. Although cut-off values may vary across populations, similar thresholds have been reported—for example, 4.25 for predicting ventricular remodeling following anterior STEMI (40), and 5.509 for in-hospital mortality in NSTEMI patients (41). Accordingly, we applied this ROC-derived threshold to facilitate stratified analysis and improve clinical interpretability, while acknowledging that it is context-specific and requires external validation in independent cohorts.
Our study identified elevated low-density lipoprotein cholesterol (LDL-C) as an independent predictor of ICT in patients with AMI, with significant predictive value (OR = 2.86, 95% CI: 2.03–4.02, p < 0.001). Mechanistically, LDL-C contributes to post-AMI thrombogenesis through multiple pathways. Oxidized LDL promotes endothelial expression of tissue factor and adhesion molecules, thereby activating the factor VII–dependent extrinsic coagulation cascade and facilitating thrombus formation (42). It also upregulates inflammatory mediators such as MCP-1 and IL-6, enhancing local inflammation and plaque instability (43). In parallel, LDL-C binds to the platelet surface receptor CD36, promoting platelet adhesion and aggregation (44), which in the prothrombotic milieu of AMI further amplifies clot formation. Moreover, elevated LDL-C is closely associated with endothelial dysfunction. It reduces nitric oxide (NO) bioavailability and impairs anti-inflammatory and antiplatelet responses, while abnormal vascular tone promotes local flow disturbances that favor thrombus development (44, 45). Collectively, these findings suggest that beyond its role in atherogenesis, LDL-C may actively drive ICT via a cascade involving inflammation, endothelial injury, and coagulation activation. As such, LDL-C represents a clinically relevant inflammation-related biomarker for ICT risk stratification in AMI patients (46).
To improve bedside applicability, we further incorporated NLR and LDL-C into a visual risk heatmap, enabling intuitive stratification of ICT risk using admission laboratory data. This tool may assist clinicians in identifying AMI patients at heightened thrombotic risk who warrant early or repeat echocardiographic screening—even in the absence of overt clinical signs. In scenarios with limited imaging availability or diagnostic uncertainty, the heatmap may also support individualized decisions regarding closer surveillance or early initiation of prophylactic anticoagulation, in accordance with bleeding risk profiles. By translating biomarker data into a user-friendly format, this approach provides a practical aid for timely and personalized decision-making in routine AMI care.
In addition to NLR and LDL-C, our analysis identified elevated NT-proBNP and decreased AGR as independent predictors of ICT in AMI patients. NT-proBNP, a marker of myocardial stress and volume overload, may reflect left ventricular dilation and intracardiac stasis—conditions that favor thrombus formation. Prior studies have linked NT-proBNP levels to increased risk of left ventricular thrombus in anterior STEMI patients (47–49). AGR, an index reflecting systemic inflammation and nutritional status, has been associated with adverse cardiovascular outcomes and in-hospital mortality (50, 51). Mechanistically, a reduced AGR may indicate persistent inflammation and impaired immune regulation, thereby promoting endothelial dysfunction and coagulation activation (52). Together, these markers may provide supplementary predictive value beyond traditional inflammatory and lipid indicators.
We also observed that male patients had a higher risk of ICT than females at similar levels of inflammatory biomarkers, suggesting a possible sex-based vulnerability. While the underlying mechanisms remain unclear, prior studies have reported sex-specific differences in thrombotic susceptibility, vascular response, and diagnostic patterns in AMI patients (53–55). These findings warrant further investigation in sex-stratified prospective cohorts.
We acknowledge several limitations in this study. Firstly, the retrospective nature of data collection introduces a potential risk of information bias, such as incomplete clinical documentation and variability in diagnostic testing. These factors may compromise the accuracy of outcome ascertainment and the consistency of biomarker evaluation. Moreover, the retrospective design inherently limits causal inference between inflammation-related biomarkers and clinical outcomes.
Second, there is a risk of residual confounding due to unmeasured or incompletely captured clinical variables, such as medication use (e.g., anticoagulants) and in-hospital treatments (e.g., thrombolysis, percutaneous interventions). These factors may influence both systemic inflammation and thrombosis risk but were not adequately adjusted for due to dataset limitations. Third, the incidence of ICT observed in our study was 1%, which is notably lower than the 5–8% reported in earlier literature (56, 57). This discrepancy may be attributed to several factors, including the absence of recent large-scale epidemiological data, improvements in clinical management that have reduced thrombotic complications, and the early and effective interventions available at our center, a high-volume tertiary hospital in Northwestern China. Lastly, while we applied a 1:10 random sampling strategy to address class imbalance, this approach may have introduced information loss or sampling variability, potentially affecting model robustness. Despite these limitations, our study provides important evidence on the role of inflammation-related biomarkers in predicting ICT among patients with acute myocardial infarction. It helps to address a current gap in the literature and lays the groundwork for improved risk stratification and individualized management in clinical practice.
5 Conclusion
In this large retrospective analysis of 8,999 patients with AMI, we employed a 1:10 case–control matching strategy to systematically evaluate the predictive value of inflammation-related biomarkers for ICT. NLR, NT-proBNP, and LDL-C were identified as independent risk factors, while AGR appeared protective. Multivariable logistic regression and sensitivity analyses consistently confirmed the robust predictive power of NLR, which remained significant even after full adjustment (OR = 18.9). Building on these findings, we developed a visual risk heatmap model based on NLR and LDL-C, which demonstrated strong discrimination (AUC = 0.838) and good calibration, thereby enhancing clinical applicability. Our findings provide a practical tool for early ICT risk stratification in AMI patients, underscore the pivotal role of inflammation in post-AMI thrombogenesis, and offer new perspectives on the application of inflammatory biomarkers in cardiovascular disease management.
Statements
Data availability statement
The datasets presented in this article are not readily available because raw data cannot be provided due to laboratory policies and confidentiality agreements. However, we are available for any inquiries or further clarification if needed. Requests to access the datasets should be directed to NM, mn19761221@mail.xjtu.edu.cn.
Ethics statement
The studies involving humans were approved by the Ethics Committee of the First Affiliated Hospital of Xi’an Jiaotong University. The studies were conducted in accordance with the local legislation and institutional requirements. The Ethics Committee/Institutional Review Board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because this study is a single-center retrospective study.
Author contributions
SZ: Writing – original draft, Software, Methodology, Writing – review & editing, Conceptualization, Investigation, Validation. ZL: Writing – original draft, Conceptualization, Methodology, Validation, Software. XW: Investigation, Validation, Writing – review & editing. NM: Writing – review & editing, Funding acquisition, Investigation, Project administration, Validation, Methodology, Supervision, Visualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Key Research and Development Program of Shaanxi (2023-YBGY-145).
Acknowledgments
We thank all participants of this study, and are grateful to The Biobank of the First Affiliated Hospital of Xi’an Jiao Tong University for providing clinical data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1643933/full#supplementary-material
References
1.
Anderson JL Morrow DA . Acute myocardial infarction. N Engl J Med. (2017) 376:2053–64. doi: 10.1056/NEJMra1606915
2.
Patel M Wei X Weigel K Gertz ZM Kron J Robinson AA et al . Diagnosis and treatment of intracardiac thrombus. J Cardiovasc Pharmacol. (2021) 78:361–71. doi: 10.1097/FJC.0000000000001064
3.
Ghaffarpasand E Tehrani MD Marszalek J Chi G . Non-vitamin K antagonist oral anticoagulants for the treatment of intracardiac thrombosis. J Thromb Thrombolysis. (2018) 46:332–8. doi: 10.1007/s11239-018-1693-3
4.
Branigan B Brown S Zavala R Merritt H . Contemporary management of intracardiac thrombi: a tale of two clots. J ExtraCorporeal Technol. (2023) 55:82–5. doi: 10.1051/ject/2023010
5.
Manabe I . Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J. (2011) 75:2739–48. doi: 10.1253/circj.CJ-11-1184
6.
Strom J Manning W . Identification of Intracardiac Thrombus. Essential echocardiography: A companion to Braunwald’s heart disease. St. Louis, NY: Elsevier Health Sciences (2017).
7.
Libby P Loscalzo J Ridker PM Farkouh ME Hsue PY Fuster V et al . Inflammation, immunity, and infection in atherothrombosis: JACC review topic of the week. J Am Coll Cardiol. (2018) 72:2071–81. doi: 10.1016/j.jacc.2018.08.1043
8.
Tudurachi B-S Anghel L Tudurachi A Sascău RA Stătescu C . Assessment of inflammatory hematological ratios (NLR, PLR, MLR, LMR and monocyte/HDL–cholesterol ratio) in acute myocardial infarction and particularities in young patients. Int J Mol Sci. (2023) 24:14378. doi: 10.3390/ijms241814378
9.
Lattanzi S Brigo F Trinka E Cagnetti C Di Napoli M Silvestrini M . Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl Stroke Res. (2019) 10:137–45. doi: 10.1007/s12975-018-0649-4
10.
Song M Graubard BI Rabkin CS Engels EA . Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci Rep. (2021) 11:464. doi: 10.1038/s41598-020-79431-7
11.
Yilmaz H Cakmak M Inan O Darcin T Akcay A . Can neutrophil–lymphocyte ratio be independent risk factor for predicting acute kidney injury in patients with severe sepsis?Ren Fail. (2015) 37:225–9. doi: 10.3109/0886022X.2014.982477
12.
Levine GN Bates ER Bittl JA Brindis RG Fihn SD Fleisher LA et al . 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non–ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. (2016) 134:e123–55. doi: 10.1161/CIR.0000000000000404
13.
Bhatt DL Lopes RD Harrington RA . Diagnosis and treatment of acute coronary syndromes: a review. JAMA. (2022) 327:662–75. doi: 10.1001/jama.2022.0358
14.
Bittl JA Baber U Bradley SM Wijeysundera DN . Duration of dual antiplatelet therapy: a systematic review for the 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. J Am Coll Cardiol. (2016) 68:1116–39. doi: 10.1016/j.jacc.2016.03.512
15.
Byrne RA Rossello X Coughlan J Barbato E Berry C Chieffo A et al . 2023 ESC guidelines for the management of acute coronary syndromes: developed by the task force on the management of acute coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J Acute Cardiovasc Care. (2024) 13:55–161. doi: 10.1093/ehjacc/zuad156
16.
Ridker PM Cushman M Stampfer MJ Tracy RP Hennekens CH . Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. (1997) 336:973–9. doi: 10.1056/NEJM199704033361401
17.
Libby P Ridker PM Maseri A . Inflammation and atherosclerosis. Circulation. (2002) 105:1135–43. doi: 10.1161/hc0902.104353
18.
Hansson GK Hermansson A . The immune system in atherosclerosis. Nat Immunol. (2011) 12:204–12. doi: 10.1038/ni.2001
19.
Golia E Limongelli G Natale F Fimiani F Maddaloni V Pariggiano I et al . Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep. (2014) 16:1–7. doi: 10.1007/s11883-014-0435-z
20.
Frangogiannis NG . The inflammatory response in myocardial injury, repair, and remodelling. Nat Rev Cardiol. (2014) 11:255–65. doi: 10.1038/nrcardio.2014.28
21.
Delewi R Zijlstra F Piek JJ . Left ventricular thrombus formation after acute myocardial infarction. Heart. (2012) 98:1743–9. doi: 10.1136/heartjnl-2012-301962
22.
Lattuca B Bouziri N Kerneis M Portal J-J Zhou J Hauguel-Moreau M et al . Antithrombotic therapy for patients with left ventricular mural thrombus. J Am Coll Cardiol. (2020) 75:1676–85. doi: 10.1016/j.jacc.2020.01.057
23.
McCarthy CP Vaduganathan M McCarthy KJ Januzzi JL Bhatt DL McEvoy JW . Left ventricular thrombus after acute myocardial infarction: screening, prevention, and treatment. JAMA Cardiol. (2018) 3:642–9. doi: 10.1001/jamacardio.2018.1086
24.
Stratton JR Lighty JG Pearlman AS Ritchie J . Detection of left ventricular thrombus by two-dimensional echocardiography: sensitivity, specificity, and causes of uncertainty. Circulation. (1982) 66:156–66.
25.
Sia CH Leow AS Tan BY Low CJ Kaur R Yeo TC et al . The neutrophil-lymphocyte ratio and platelet-lymphocyte ratio predict left ventricular thrombus resolution in acute myocardial infarction without percutaneous coronary intervention. Thromb Res. (2020) 194:16–20. doi: 10.1016/j.thromres.2020.06.003
26.
Brill A Fuchs T Savchenko A Thomas G Martinod K De Meyer S et al . Neutrophil extracellular traps promote deep vein thrombosis in mice. J Thromb Haemost. (2012) 10:136–44. doi: 10.1111/j.1538-7836.2011.04544.x
27.
Bhat T Teli S Rijal J Bhat H Raza M Khoueiry G et al . Neutrophil to lymphocyte ratio and cardiovascular diseases: a review. Expert Rev Cardiovasc Ther. (2013) 11:55–9. doi: 10.1586/erc.12.159
28.
Li X Liu M Wang G . The neutrophil–lymphocyte ratio is associated with all-cause and cardiovascular mortality in cardiovascular patients. Sci Rep. (2024) 14:26692. doi: 10.1038/s41598-024-76836-6
29.
Zhou Y Tao W Shen F Du W Xu Z Liu Z . The emerging role of neutrophil extracellular traps in arterial, venous and cancer-associated thrombosis. Front Cardiovas Med. (2021) 8:786387. doi: 10.3389/fcvm.2021.786387
30.
Tamhane UU Aneja S Montgomery D Rogers E-K Eagle KA Gurm HS . Association between admission neutrophil to lymphocyte ratio and outcomes in patients with acute coronary syndrome. Am J Cardiol. (2008) 102:653–7. doi: 10.1016/j.amjcard.2008.05.006
31.
Fan W Zhang Y Gao X Liu Y Shi F Liu J et al . The prognostic value of a derived neutrophil-lymphocyte ratio in patients with acute coronary syndrome undergoing percutaneous coronary intervention. Clin Appl Thromb Hemost. (2021) 27:10760296211034579. doi: 10.1177/10760296211034579
32.
Yalcin M Aparci M Uz O Isilak Z Balta S Dogan M et al . Neutrophil-lymphocyte ratio may predict left atrial thrombus in patients with nonvalvular atrial fibrillation. Clin Appl Thromb Hemost. (2015) 21:166–71. doi: 10.1177/1076029613503398
33.
Deng Y Zhou F Li Q Guo J Cai B Li G et al . Associations between neutrophil-lymphocyte ratio and monocyte to high-density lipoprotein ratio with left atrial spontaneous echo contrast or thrombus in patients with non-valvular atrial fibrillation. BMC Cardiovasc Disord. (2023) 23:234. doi: 10.1186/s12872-023-03270-3
34.
Soehnlein O Steffens S Hidalgo A Weber C . Neutrophils as protagonists and targets in chronic inflammation. Nat Rev Immunol. (2017) 17:248–61. doi: 10.1038/nri.2017.10
35.
Gould TJ Vu TT Swystun LL Dwivedi DJ Mai SH Weitz JI et al . Neutrophil extracellular traps promote thrombin generation through platelet-dependent and platelet-independent mechanisms. Arterioscler Thromb Vasc Biol. (2014) 34:1977–84. doi: 10.1161/ATVBAHA.114.304114
36.
Wang X Lin Y Wang F . Development of a risk score model for the prediction of patients needing percutaneous coronary intervention. J Clin Lab Anal. (2023) 37:e24849. doi: 10.1002/jcla.24849
37.
Natorska J Ząbczyk M Undas A . Neutrophil extracellular traps (NETs) in cardiovascular diseases: from molecular mechanisms to therapeutic interventions. Polish Heart J. (2023) 81:1205–16. doi: 10.33963/v.kp.98520
38.
Fuchs TA Brill A Duerschmied D Schatzberg D Monestier M Myers DD Jr et al . Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci. (2010) 107:15880–5. doi: 10.1073/pnas.1005743107
39.
Libby P Simon DI . Inflammation and thrombosis: the clot thickens. Philadelphia, PA: Lippincott Williams & Wilkins; (2001) 1718–1720. doi: 10.1161/01.cir.103.13.1718
40.
Börekçi A Gür M Türkoğlu C Baykan AO Şeker T Şahin DY et al . Neutrophil to lymphocyte ratio predicts left ventricular remodeling in patients with ST elevation myocardial infarction after primary percutaneous coronary intervention. Korean Circulation J. (2016) 46:15–22. doi: 10.4070/kcj.2016.46.1.15
41.
Ji Z Liu G Guo J Zhang R Su Y Carvalho A et al . The neutrophil-to-lymphocyte ratio is an important indicator predicting in-hospital death in AMI patients. Front Cardiovascular Med. (2021) 8:706852. doi: 10.3389/fcvm.2021.706852
42.
Bochkov VN Mechtcheriakova D Lucerna M Huber J Malli R Graier WF et al . Oxidized phospholipids stimulate tissue factor expression in human endothelial cells via activation of ERK/EGR-1 and ca++/NFAT. Blood. (2002) 99:199–206. doi: 10.1182/blood.v99.1.199
43.
Zhong Y Liu C Feng J Li J-F Fan Z-C . Curcumin affects ox-LDL-induced IL-6, TNF-α, MCP-1 secretion and cholesterol efflux in THP-1 cells by suppressing the TLR4/NF-κB/miR33a signaling pathway. Exp Ther Med. (2020) 20:1856–70. doi: 10.3892/etm.2020.8915
44.
Ghosh A Murugesan G Chen K Zhang L Wang Q Febbraio M et al . Platelet CD36 surface expression levels affect functional responses to oxidized LDL and are associated with inheritance of specific genetic polymorphisms. Blood. (2011) 117:6355–66. doi: 10.1182/blood-2011-02-338582
45.
Jiang H Zhou Y Nabavi SM Sahebkar A Little PJ Xu S et al . Mechanisms of oxidized LDL-mediated endothelial dysfunction and its consequences for the development of atherosclerosis. Front Cardiovas Med. (2022) 9:925923. doi: 10.3389/fcvm.2022.925923
46.
Obermayer G Afonyushkin T Binder CJ . Oxidized low-density lipoprotein in inflammation-driven thrombosis. J Thromb Haemost. (2018) 16:418–28. doi: 10.1111/jth.13925
47.
Katsioupa M Kourampi I Oikonomou E Tsigkou V Theofilis P Charalambous G et al . Novel biomarkers and their role in the diagnosis and prognosis of acute coronary syndrome. Life. (2023) 13:1992. doi: 10.3390/life13101992
48.
Clerico A Passino C . Predictive value of NT-proBNP in patients with acute myocardial infarction. Clin Chem. (2017) 63:1045–6. doi: 10.1373/clinchem.2016.269522
49.
Azab B Bibawy J Harris K Khoueiry G Akerman M Selim J et al . Value of albumin–globulin ratio as a predictor of all-cause mortality after non-ST elevation myocardial infarction. Angiology. (2013) 64:137–45. doi: 10.1177/0003319712436577
50.
Li K Fu W Bo Y Zhu Y . Effect of albumin-globulin score and albumin to globulin ratio on survival in patients with heart failure: a retrospective cohort study in China. BMJ Open. (2018) 8:e022960. doi: 10.1136/bmjopen-2018-022960
51.
Wang X Guo R Huang M Li Z Lai Z Yang R et al . Fibrinogen-to-albumin ratio and glucose metabolic states in patients with coronary heart disease. Angiology. (2025) 76:271–80. doi: 10.1177/00033197231206235
52.
Haass M Kitzman DW Anand IS Miller A Zile MR Massie BM et al . Body mass index and adverse cardiovascular outcomes in heart failure patients with preserved ejection fraction: results from the Irbesartan in heart failure with preserved ejection fraction (I-PRESERVE) trial. Circ Heart Fail. (2011) 4:324–31. doi: 10.1161/CIRCHEARTFAILURE.110.959890
53.
Stehli J Martin C Brennan A Dinh DT Lefkovits J Zaman S . Sex differences persist in time to presentation, revascularization, and mortality in myocardial infarction treated with percutaneous coronary intervention. J Am Heart Assoc. (2019) 8:e012161. doi: 10.1161/JAHA.119.012161
54.
Lansky AJ Ng VG Maehara A Weisz G Lerman A Mintz GS et al . Gender and the extent of coronary atherosclerosis, plaque composition, and clinical outcomes in acute coronary syndromes. Cardiovascular Imaging. (2012) 5:S62–72. doi: 10.1016/j.jcmg.2012.02.003
55.
Mehta LS Beckie TM DeVon HA Grines CL Krumholz HM Johnson MN et al . Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. (2016) 133:916–47. doi: 10.1161/CIR.0000000000000351
56.
Gianstefani S Douiri A Delithanasis I Rogers T Sen A Kalra S et al . Incidence and predictors of early left ventricular thrombus after ST-elevation myocardial infarction in the contemporary era of primary percutaneous coronary intervention. Am J Cardiol. (2014) 113:1111–6. doi: 10.1016/j.amjcard.2013.12.015
57.
Osherov AB Borovik-Raz M Aronson D Agmon Y Kapeliovich M Kerner A et al . Incidence of early left ventricular thrombus after acute anterior wall myocardial infarction in the primary coronary intervention era. Am Heart J. (2009) 157:1074–80. doi: 10.1016/j.ahj.2009.03.020
Summary
Keywords
acute myocardial infarction, intracardiac thrombosis, neutrophil-to-lymphocyte ratio, low-density lipoprotein cholesterol, inflammatory biomarkers, risk prediction
Citation
Zhao S, Lian Z, Wang X and Mei N (2025) Inflammation-related biomarkers for intracardiac thrombosis in acute myocardial infarction: predictive value and mechanistic implications of NLR and LDL-C. Front. Med. 12:1643933. doi: 10.3389/fmed.2025.1643933
Received
09 June 2025
Accepted
21 July 2025
Published
05 August 2025
Volume
12 - 2025
Edited by
Jin Lei, Huazhong University of Science and Technology, China
Reviewed by
Songfeng Zhao, Central South University, China
Veysel Ozan Tanik, Ankara Etlik City Hospital, Türkiye
Updates
Copyright
© 2025 Zhao, Lian, Wang and Mei.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoning Wang, wangxiaoning@xjtufh.edu.cnNa Mei, mn19761221@mail.xjtu.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.