- 1Department of Biochemistry, School of Sciences, Gujarat University, Ahmedabad, India
- 2Department of Zoology, Biomedical Technology, Human Genetics & Wildlife Biology and Conservation, School of Sciences, Gujarat University, Ahmedabad, India
Extracellular vesicles (EVs) offer promising opportunities in hematology for improved diagnostics, prognostics, and therapeutics, making them valuable tools in the molecular landscape. EVs derived from red blood cells (RBCs) are the primary source of EVs in the bloodstream. They perform several critical biological and physiological functions, such as facilitating intercellular communication and transferring biomolecules like DNA, RNA, and proteins. Hence, in this review, we aim to explore RBC-derived EVs and their potential as a diagnostic tool for their clinical relevance and associated biomarkers in hematology. Furthermore, we emphasized their crucial role in both physiology and disease. RBC-EVs are found to play a role in vascular damage, inflammation, and coagulopathy in several pathophysiological conditions, potentially influencing the progression of certain diseases. They also served as indicators for numerous conditions, including hereditary hematologic abnormalities, diabetes, and cardiovascular diseases. Hence, their importance lies in their ability to reflect and influence red cell health, immune responses, and systemic disease states as accessible, non-invasive indicators. Also, their composition mirrors the physiological or pathological state of RBCs and holds promise for both diagnostics and therapeutics.
1 Introduction
Extracellular vesicles (EVs) are gaining importance in hematology as non-invasive biomarkers for early diagnosis and disease monitoring, providing insights into disease mechanisms, as they carry disease-specific proteins, lipids, and nucleic acids (1). The subtypes of EVs that are being increasingly explored in hematology include microvesicles (100–1000 nm), exosomes (30–200 nm), and apoptotic bodies (50–5000 nm) in diameter. These subtypes vary depending on their biogenesis, release pathways, size, content, and functions (2). RBCs outnumber all other blood cells, comprising ∼84% of all cells in the human body and over 99% of all blood cells by number. This sheer numerical dominance means that even if a small fraction of RBCs release vesicles, the absolute amount of RBC-EVs will be substantial, hence, RBC-derived EVs dominate the blood EV population (3). Additionally, RBCs naturally shed membranes due to aging or stress, lacking organelles for repair and protein synthesis themselves. They release damaged components through EV release, preserving flexibility and function (4, 5). RBCs circulate for ∼120 days. As they age, membrane remodeling increases, and senescent RBCs release microvesicles and exosomes to discard damaged components. This vesiculation is one of the early signals for RBC clearance by macrophages (6). RBC-EVs circulate in plasma, transporting intact biomolecules; their concentrations and properties can be employed to differentiate among distinct disease stages and phases; thus, they act as “liquid biopsy” readouts of RBC health (7).
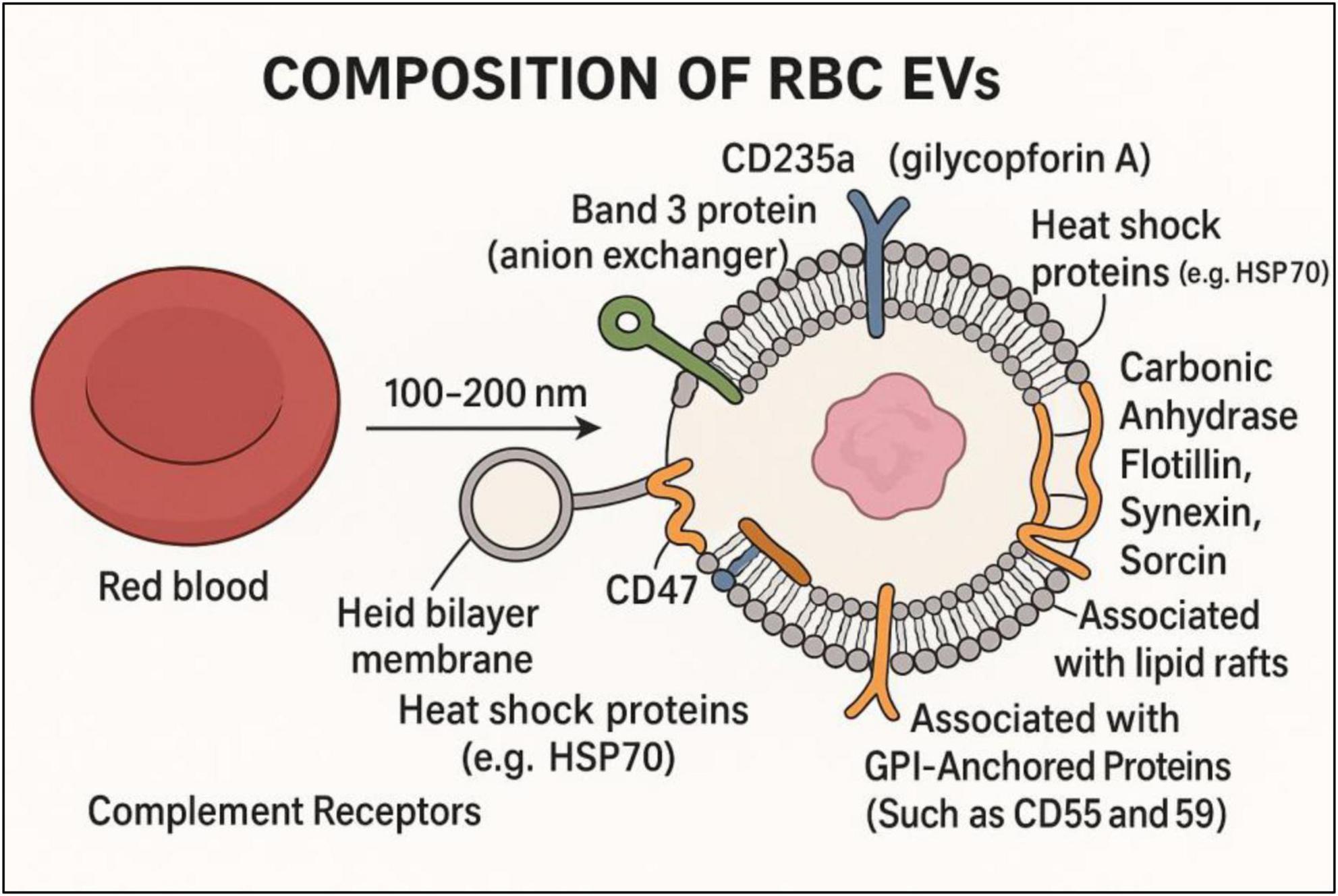
As shown in Figure 1, RBC-EVs are defined by surface molecules like tetraspanins (CD9, CD63, CD81), specific markers (e.g., glycophorins/band 3, CD235a) (8), and phosphatidylserine exposure (9). They are counted using flow cytometry (CD235a+ or Annexin V + microparticles) and profiled using proteomics or RNA assays (10), discussed in Table 1. These surface molecules on EVs aid in identifying and binding to specific recipient cells, ensuring effective delivery of EV contents (proteins, RNAs, lipids), similar to viruses or hormones (3). Integrins facilitate organotropism and direct EVs to specific tissues like the liver, brain, lungs, etc. While a few Heparan sulfate proteoglycans (HSPGs) are involved in initial EV binding to recipient cell surfaces (11). EVs also carry MHC molecules, cytokines, or ligands that activate or suppress immune responses, aiding communication between immune and non-immune cells, and can induce apoptosis or immune suppression (12).
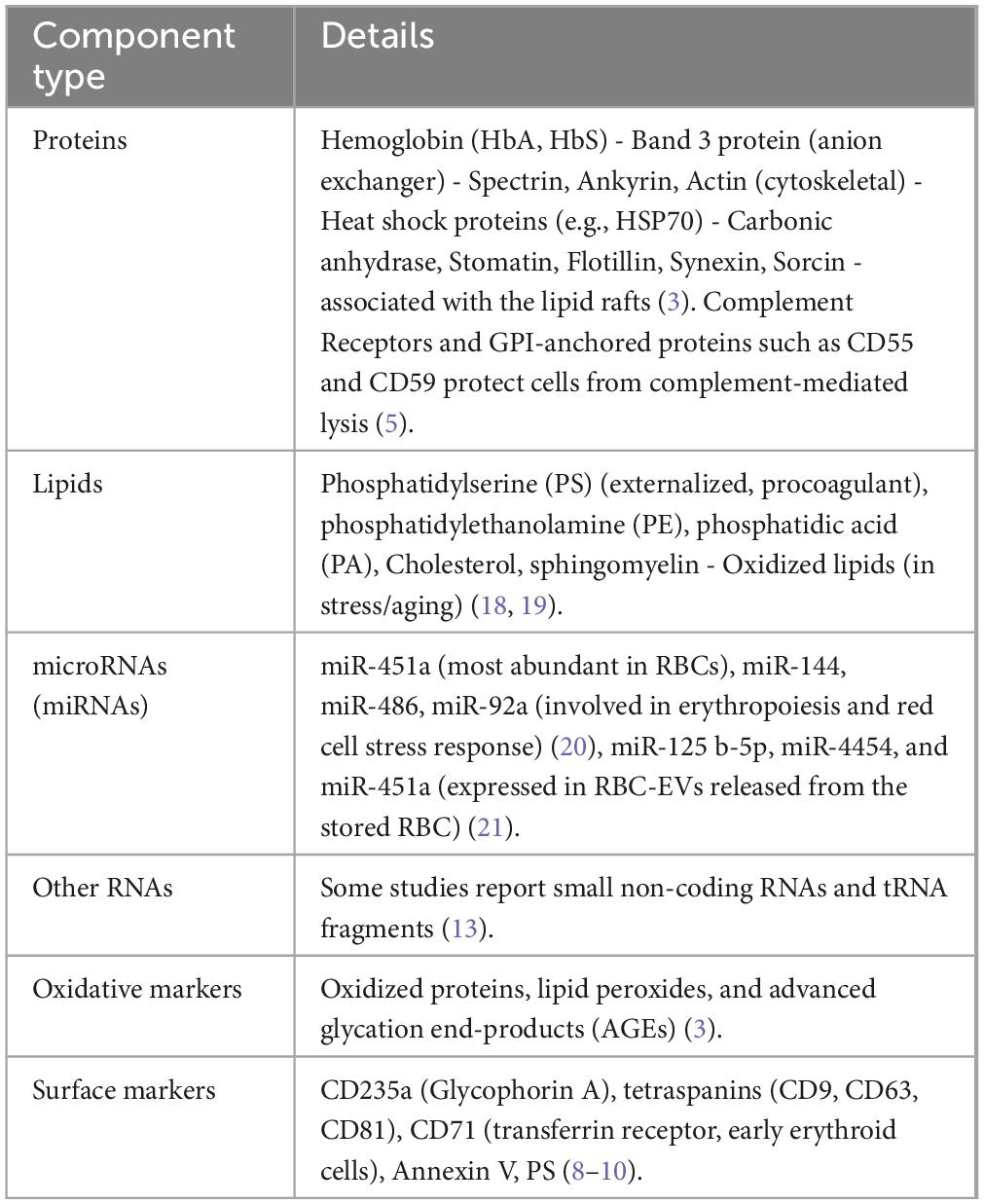
RBC-EVs contain specific biomolecules reflecting parent RBC composition and functional state, providing informative content in disease contexts due to their simpler nature without nuclei and organelles (13). Their EV production is an essential mechanism of homeostasis and is amplified in many disease states, making them a major source of circulating EVs. Studies show that RBC-EVs’ levels or contents change in disease, raising diagnostic interest (14, 15). Conditions like oxidative stress, infection, inflammation, hypoxia, hemolysis, and even exercise increase RBC-EV shedding. Diseases such as sickle cell anemia, thalassemia, malaria, and diabetes are known to cause RBCs to release more EVs than normal (16). Also, diseases such as sickle cell anemia, thalassemia, malaria, and diabetes are known to cause RBCs to release more EVs than normal (17).
2 Mechanistic insights into the pathogenicity of RBC-EVs
RBC-EVs are not inert byproducts, but bioactive mediators in both physiological processes and pathological conditions. Despite being enucleated, RBCs interact with their environment. Changes in systemic inflammation, oxidative stress, pH changes, calcium influx, or infections can lead to modifications in RBCs, and these changes get passed to their EVs (22). For instance, RBC EVs carry disease-specific cargo from patients with certain diseases, such as oxidative stress markers (e.g., 4-HNE, ROS-modified proteins), pro-coagulant factors (e.g., phosphatidylserine, tissue factor), and inflammatory signals (e.g., complement proteins in autoimmune diseases), regulatory miRNAs (e.g., miR-451, miR-144, etc.) (23). These can be transferred to endothelial cells, macrophages, and even neuronal cells, altering gene expression, inflammatory signaling, and cell survival pathways. In neurovascular or cardiovascular disease, RBC-EV cargo may exacerbate inflammation, cell death, or immune dysregulation. Thus, RBC EVs can serve as sentinels of broader pathophysiology beyond RBCs alone (23). They can also be readily extracted from biofluids like blood, urine, etc., and are considerably stable under various conditions (24). They offer a “liquid biopsy” of red blood cell functionality and overall health of the body (7). However, RBC-EVs’ heterogeneity is a considerable challenge in diagnostic applications (25). They are typically identified by the expression of CD9, CD63, and CD81, glycophorin A (CD235a) and B, displayed in Figure 1. Additional protein indicators commonly linked to exosomes include flotillin, TSG101, Alix, HSP60, HSP70, HSPA5, CCT2, and HSP90 (26). RBC-EVs are recognized as active participants, particularly in vascular and inflammatory diseases, due to their pro-inflammatory, pro-coagulant, and redox-active cargoes (22). PS is a key component of the integral membrane of RBCs, which can be exposed to the outer surface during RBC aging, stress (e.g., in sickle cell disease, malaria, sepsis), or EV formation. PS becomes externalized on the outer leaflet of EVs. This exposed PS acts as a catalytic surface for the tenase and prothrombinase complexes (factor Xa + factor Va), significantly accelerating fibrin clot formation, thrombin generation. Increased PS + RBC-EVs are observed in sickle cell illness, sepsis, and cardiovascular disease, all linked to thrombo-inflammation (18, 19, 27). Additionally, in Beta-thalassemia, chronic hemolysis increases PS + EVs, enhancing thrombotic risk (28).
RBC-EVs often contain cell-free hemoglobin or heme, which can enter endothelial cells via scavenger receptors (e.g., CD91, TLR4), activate TLR4 signaling, promoting pro-inflammatory gene expression (IL-6, VCAM-1, ICAM-1, E-selectin), and generate reactive oxygen species (ROS) via Fenton chemistry. These lead to endothelial dysfunction, leukocyte adhesion, and vascular permeability in SCD, sepsis, and transfusion-related complications (29, 30). As well, cell-free Hb in RBC-EVs scavenges and binds to nitric oxide (NO) with high affinity, Hb + NO → MetHb + nitrate. This scavenging reduces bioavailable NO, a key vasodilator and anti-platelet mediator (31). Which leads to vasoconstriction, platelet activation, and endothelial dysfunction and amplifies complications like pulmonary hypertension, especially in chronic hemolytic states (32, 33). Hence, Hb-loaded EVs contribute to vaso-occlusive crises and pulmonary hypertension, with elevated hemolysis observed in sickle cell, malaria, PNH, etc. (34). Mantel et al. also reported that in malaria, parasite-altered RBC-EVs increase endothelial activation and brain microvascular dysfunction (35). Furthermore, the CD47 signal on EVs interacts with signal regulatory protein alpha (SIRPα) on the macrophage surface, inhibiting phagocytosis and immune system activation (36). While several other RBC surface proteins restrict the interactions with the complement system are C8 binding protein (C8bp) (37), homologous restriction protein (HRP) (38), decay accelerating factor (DAF) (39), membrane cofactor protein (MCP), complement receptor 1 (CR1), and CD59 (40). Increased oxidative stress enhances Band 3 phosphorylation, clustering, and dissociation from other cytoskeletal proteins, while also initiating the elimination of aged red blood cells, phagocytosis, and complement activation (41).
Some RBC-EVs express adhesion molecules such as CD36, ICAM-4, which can bind to plasma proteins like fibrinogen, promoting EV-endothelium adhesion, EV-platelet or EV-leukocyte interactions. This is important in diseases with systemic inflammation (e.g., malaria, COVID-19, sepsis) because it facilitates leukocyte recruitment, platelet aggregation (5). Hence, an altered protein or lipid composition of RBC-EVs may indicate oxidative damage, inflammation, or disease conditions, aiding in the diagnosis and monitoring of illnesses (8), discussed in Table 2.
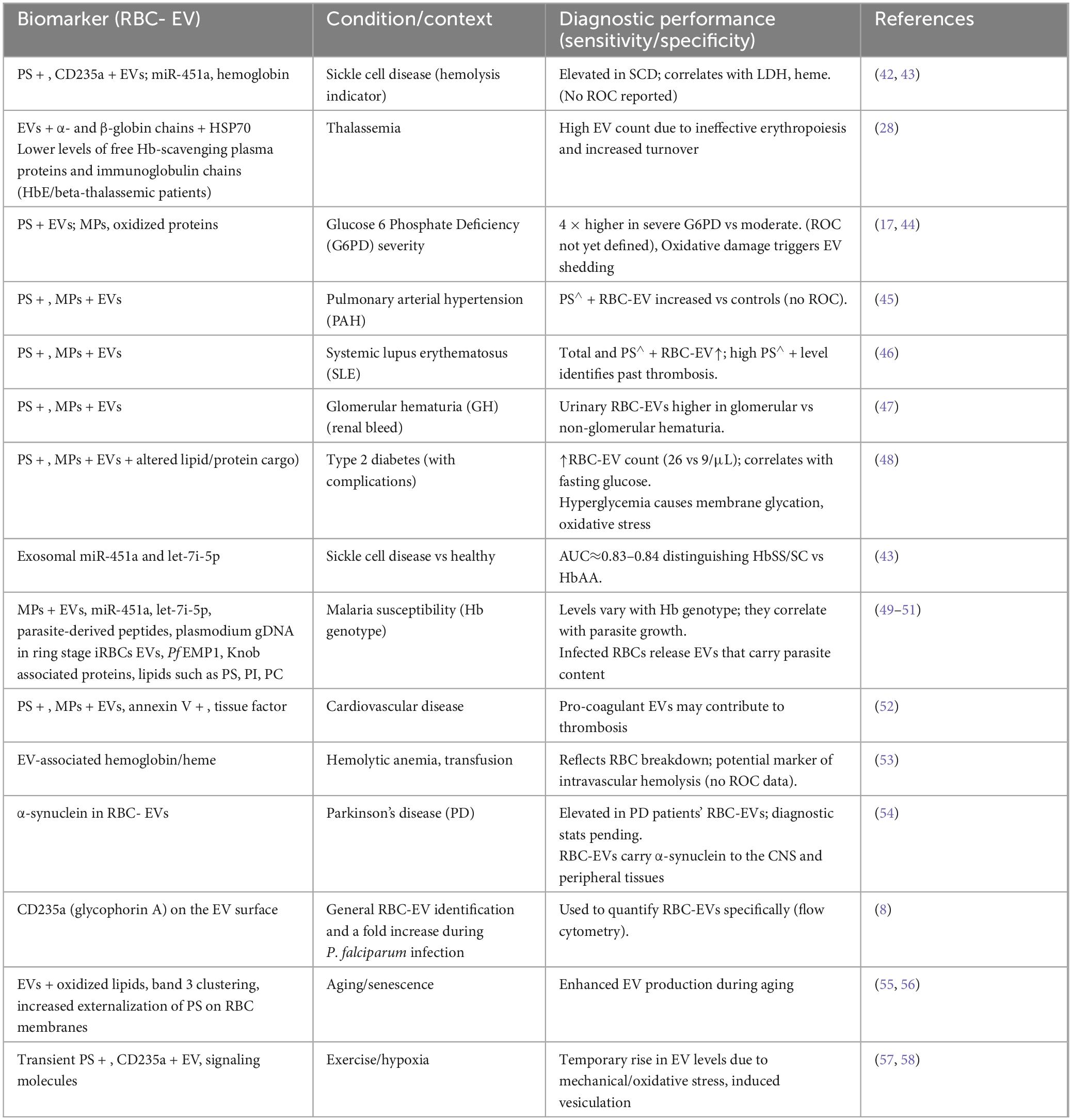
Table 2 summarizes selected red cell EV–derived biomarkers and their reported disease associations.
3 Biomarkers in inflammation, vascular damage, coagulation, and infection
RBC EVs provide a real-time snapshot of ongoing physiological or pathological processes. Their surface markers and cargo drive thrombo-inflammation through defined pathways, such as coagulation amplification, endothelial activation, NO depletion, and gene regulation (59). RBC-EVs can directly impair the function of endothelial cells, the cells via transferring the cargo molecules, changing permeability, activating immune cells, leukocyte/platelet adhesion, etc (59). For example, in conditions like type 2 diabetes, RBC-EVs can be taken up by endothelial cells and transfer pro-oxidant enzymes like Arg1, leading to impaired endothelium-dependent relaxation and promoting endothelial dysfunction (60). EVs, including those derived from RBCs, can influence vascular remodeling, a process involved in the development of various vascular diseases. They can affect oxidative stress, inflammation, calcification, and lipid plaque formation, all of which contribute to vascular remodeling (60, 61).
RBC-EVs can promote the production of pro-inflammatory cytokines like TNF-α, IL-6, and IL-1β. They can also promote the activation of immune cells like monocytes and neutrophils, leading to increased inflammation and adhesion to the endothelium (19). In sepsis, RBC-EVs can exacerbate the inflammatory response and reduce survival rates. In arthritis, RBC-EVs are involved in the inflammatory process in the inflammatory fluid of patients with arthritis, serving as a source of the lipid mediator LPA (62). Whereas, in malaria, RBCs infected with the malaria parasite Plasmodium falciparum release EVs that contribute to the local and systemic production of pro-inflammatory cytokines and chemokines, leading to vascular dysfunction and increasing the EC permeability by downregulating caveolin-1 and activating transcription factor 2 (63).
RBC-EVs also possess procoagulant properties, contributing to the activation of the coagulation cascade. This action pertains to the exposure of phosphatidylserine (PS) on their surface, which offers a negatively charged interface for the formation and activation of coagulation complexes. RBC-EVs can trigger thrombin production in an FXII-dependent way, independent of tissue factor (TF) (64). Therefore, increased levels of procoagulant RBC-EVs are observed in conditions like sudden nocturnal hemoglobinuria (PNH) and hemolytic disease, contributing to increased thrombotic and hypercoagulable states (64). It has also been found that RBC-EVs present in stored RBC products have been implicated in transfusion-related adverse effects, including a significant procoagulant effect that may worsen the condition of patients with a hypercoagulable state (65).
RBC-EVs increase in many inflammatory and infectious scenarios, indicating the immunological activation of RBCs. It has been discovered that they also harbor viral proteins, including those from respiratory viruses and SARS-CoV-2, before the production of antibodies (66). In severe dengue virus infection, dengue-infected cells provoke extensive apoptosis of red blood cells and the generation of extracellular vesicles. A clinical study found a significant correlation between higher RBC-EV levels and dengue severity, suggesting RBC-EV count can determine disease stage and be a prognostic diagnostic tool for dengue (67).
In malaria, RBC-EVs from infected cells carry parasite antigens and modulate immunity (68). Additionally, Nantakomol et al. reported that the EV secretion of P. falciparum-iRBCs was tenfold more than that of non-iRBCs and escalated with parasite maturation (69). The study found that malaria-infected RBCs release EVs with various human RNAs, including miRNAs and tRNA, Y-RNAs, vault RNAs, snoRNAs, piRNAs, and plasmodium RNAs. These RNAs can be transferred to human endothelial cells, potentially regulating gene expression and altering barrier properties. Additionally, plasmodium proteins, including ring-infected erythrocyte surface antigen, have proinflammatory activity and contribute to malaria pathology (63, 70). Malaria-infected RBC-EVs activate natural killer (NK) cells via MAD5 and monocytes, leading to immune elimination. Also, PfEMP1 and PfPTP2 are crucial proteins in efficient cell-cell communication between RBCs, reducing inflammatory cytokines and transcriptomic changes in primary human monocytes (71, 72). Recent reports show exosomal microRNAs from RBCs (notably miR-451a and let-7i-5p) differ with sickle trait and malaria status. These RBC-EV-associated miRNAs achieve good discrimination, exosomal miR-451a and let-7i-5p distinguished sickle-cell patients from controls with AUC∼0.83 and correlate inversely with parasite growth. This suggests RBC-EV miR-451a/let-7i can serve as blood biomarkers of SCD genotype and malaria severity (43).
Manakeng et al. reported that chronic inflammation also perturbs RBC-EVs. PS-positive RBC-derived EVs have demonstrated potential in detecting thrombotic events in individuals with systemic lupus erythematosus (SLE). Increased levels of these EVs correlate with previous thrombotic incidents and may signify a heightened risk of vascular problems in SLE (45). PAH patients had significantly more PS-bearing large RBC-EVs, platelets, and medium platelet-derived EVs than normal subjects. Still, they weren’t different from patients who didn’t have PAH (45). The quantity of RBC-EVs in individuals with glomerular haematuria (GH) (renal bleeding) was markedly greater than in those with non-glomerular haematuria (NGH), offering a prospective tool for the classification of GH and a predictive tool to distinguish bleeding sources (47).
Various in vitro and in vivo studies were carried out to study the role of RBC-EVs in immunomodulation and blood transfusion (73, 74). In vitro studies reported that RBC-EVs can interact mostly with monocytes and stimulate the pro-inflammatory cytokines {TNF-α, IL-1, IL-6, and MCP-1, and chemokines [macrophage-derived chemokine (MDC)], and macrophage inflammatory protein 1a, MIP-1a}, contributing to systemic inflammation (75, 76). RBC-EVs, specifically the exosome fraction, increase the ability of APCs to present antigens and stimulate T cells. The increased potency of APCs and the secretion of pro-inflammatory cytokines subsequently boost the proliferation of CD4+ and CD8+ T cells (76). Similarly, during disease conditions like malaria and dengue, RBC-EVs reflect disease burden and immune activation (22). A recent study also reported that RBC-EV treatment significantly reduced inflammation, evidenced by reduced cell death and preservation of retinal function, in a photo-oxidative damage model of retinal degeneration. Multi-omic and in vitro analyses indicated that the EVs modulate key pro-inflammatory cytokines implicated in retinal and neurodegenerative disease pathways (73).
Intriguingly, one emerging area is neurodegeneration, RBCs and their constituents exhibit potential as diagnostic instruments in neurodegenerative disorders such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). Research indicates that modifications in red blood cells may signify changes in the brain and could potentially act as indicators for these disorders (77). Studies have explored altered protein kinase C (PKC) conformation and fibril aggregation in AD patient RBCs, suggesting its potential as a diagnostic biomarker, although RBC-EVs require more attention and validation (77, 78). Additionally, reduced alpha-synuclein levels in red blood cells have been noted in Dementia with Lewy Bodies (DLB) relative to healthy individuals and those with Alzheimer’s disease (AD) or Parkinson’s disease (PD), indicating a possible diagnostic differentiator for DLB (79). Although beyond direct RBC measures, RBC-derived exosomes have drawn attention, research highlights the promise of RBC-derived exosomes as non-invasive diagnostics for neurodegenerative diseases, but they need more studies and validations. RBC-EVs in PD patients contain more α-synuclein than healthy subjects, which may play a role in overactivated immunity in monocytes. This suggests RBC-EVs may be useful for early diagnosis and treatment, but the exact link between these changes and PD progression remains unclear (79). Upregulation of miR-125a-5p and downregulation of miR-302a-5p in aged mice are likely associated with neurodegenerative pathways (80).
4 Biomarkers in hematologic disorders
RBC-EVs can serve as a potential biomarker for disease dynamics in hematological disorders like ineffective erythropoiesis, hemolysis, and associated severity like sickle cell anemia and thalassemia. For example, in SCD patients, RBC-derived microparticles (RMP) are greatly elevated, which correlates with hemolysis (81). While plasma RMP counts track free hemoglobin, LDH, and bilirubin, and are inversely correlated with Hb and haptoglobin (82). As well, SCD patients with leg ulcers or elevated pulmonary pressures have significantly higher RMP levels, suggesting RBC-EVs mark disease complications (83). Heme-enriched RBC-EVs in SCD patients induce endothelial oxidative stress, adhesion molecule expression, and pro-inflammatory vascular niche, according to a recent study by Giannaki et al. (84). The SCD microRNA study was an observational case-control analysis. Few studies focused on the exosomal miR-451a/let-7i-5p, which showed ∼80–85% sensitivity and specificity for distinguishing SCD genotypes (42, 43). Exosomal miRNAs from RBCs show high diagnostic potential, e.g., in distinguishing sickle cell genotypes (AUC∼0.83). Furthermore, miR-451a is becoming recognized as an important biomarker for red blood cell disorders (8, 68, 69, 71).
In thalassemia, RBC-EVs are similarly enhanced; additionally, β-thalassemia and other chronic anemias induce RBC vesiculation, exhibiting high amounts of RBC-EVs containing oxidized hemoglobin and procoagulant proteins (28). One pilot report also suggests that higher RBC-EV counts may predict thrombotic risk in thalassemia, although formal ROC analysis and diagnostic accuracy metrics have not been established. Additionally, HSP70, HSP90, and peroxiredoxin 6 proteins derived from RBC-EVs were identified as biomarkers for thalassemia and its subtypes (85). Glucose-6-phosphate dehydrogenase (G6PD) deficiency also shows dramatic RBC-EV release, one study found circulating EVs (≈45% RBC-derived) ∼4–10-fold higher in G6PD-deficient versus healthy blood and increasing with disease severity. Thus, elevated RBC-EVs concentration or PS∧ + RBC-EVs fraction can distinguish severe hemolytic stages in G6PD deficiency (17, 44). Conversely, alterations in RBC-EVs are less frequently reported in non-hemolytic anemias or bone marrow failure. Previous studies suggest that autoimmune hemolytic anemia or hereditary spherocytosis may enhance RBC vesiculation; however, no extensive clinical research has established their diagnostic significance (53, 86, 87). Overall, RBC-EVs count, or PS positivity, serves as a surrogate marker of ongoing intravascular hemolysis or RBC stress in human studies, even if precise sensitivity/specificity data are not yet defined (66).
5 Biomarkers in metabolic conditions
Beyond classic hemolytic/infectious diseases, RBC-EVs show promise in broader conditions. For example, in diabetic cardiovascular disease, co-morbid atrial fibrillation or heart failure is associated with higher RBC-EV levels than diabetes alone, suggesting prognostic value (17). A recent study revealed that RBC-EVs interact with cardiac cells, resulting in modifications to critical signaling pathways, such as cell cycle regulation, proliferation, and oxidative metabolism in a mouse model of ischemic heart disease (88). The concentration of RBC-EVs in patients with type 2 diabetes mellitus (26/μL) exceeded that of patients without type 2 diabetes mellitus (9/μL). Furthermore, the concentration of RBC-EVs, which may serve as a predictor for type 2 diabetes mellitus, exhibited a favorable correlation with fasting blood glucose but not glycated hemoglobin (89).
RBC-EVs also contribute to coagulopathy in hematological disorders, potentially influencing disease progression and patient outcomes. Direct RBC-EV markers in relation to cancer are not yet well established. RBC-EVs profiles may shift due to tumor-induced systemic alterations, although they are generated by bodily cells, they are more abundant in bodily fluids than circulating tumor cells and DNA (90, 91). Cancer-specific miRNAs (miR-103, miR-191, and miR-195) in RBCEVs have been identified as precise biomarkers for distinguishing healthy individuals from breast cancer patients (92). RBC-EVs can also modulate the tumor microenvironment, influence drug resistance, and are being explored as vehicles for targeted drug delivery (93). Their inherited metabolic machinery, immune and mechanical dynamics, plus customizable carrier frameworks, position them as promising diagnostic tools and therapeutic platforms. For instance, engineered RBC-EVs have been proposed as delivery systems for therapeutic agents in chronic lymphocytic leukemia and Burkitt lymphoma (94).
6 Clinical significance and limitations
Common RBC-EV markers include (1) EV count or concentration (often CD235a∧ + or PS∧ + microparticles) and (2) EV cargo molecules (proteins or miRNAs derived from RBCs). Crucially, the specificity and sensitivity of RBC-EV markers vary by context and remain to be fully established (3). However, several studies have quantified RBC-EVs in patient vs. control plasma: e.g., flow-cytometric counts of CD235a∧ + EVs or Annexin V∧ + EVs, and qPCR assays for RBC-miRNAs in exosomes (95). RBC-EV count (PS∧ + microparticles) found elevated in SCD, G6PD deficiency, PAH, SLE, glomerular hematuria, and severe diabetes (15, 17). In contrast, simple RBC-EV counts generally lack defined cutoffs or validated thresholds, but large fold-differences (e.g., 4 × in G6PD) suggest strong sensitivity for distinguishing disease states (17). RBC-EVs can deliver heme to macrophages, too (96). Elevated EV-bound hemoglobin correlates with hemolysis severity, though exact performance data are not defined (53). RBC-EVs naturally display ABO/Rh antigens. In theory, such EVs could be used for non-invasive blood typing, but diagnostics based on these are not yet reported (97). A few clinical validations are underway in specialized research centers, for example, examining RBC-EV profiles in malaria-endemic populations. Nevertheless, no RBC-EV-based test is yet in standard clinical use (25).
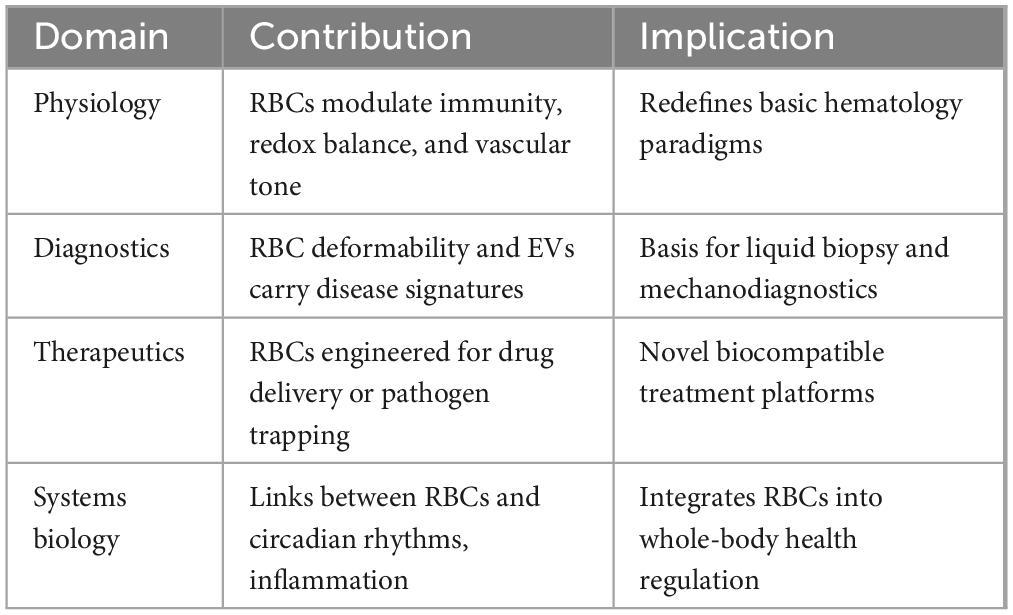
Tabular summary of cross disciplinary impact of RBC derived EVs mentioned in Table 3. Diverse methods (ultracentrifugation, size exclusion chromatography, immunoaffinity capture) for the isolation and purification of EVs with variable purity and EV subsets, impairing reproducibility and comparison across studies (98, 99). Moreover, EVs from other hematopoietic or endothelial cells coexist in blood; overlapping size, density, and surface markers (e.g., CD235a for RBCs) complicate accurate RBC-EV quantification (100). RBC-EVs vary by size (exosomes vs microvesicles), content, and trigger for release (oxidative stress, shear stress), challenging biomarker standardization (101). Pre-analytical factors, including sample handling, anticoagulant choice, storage duration, and freeze-thaw cycles, affect EV integrity and yield, leading to inconsistent results (102). Most data also derive from small cohorts or preclinical models; rigorous clinical trials are required to define sensitivity, specificity, and predictive values, hence insufficient large-scale validation (103). Hemolytic conditions, inflammation, infections, or transfusions can elevate RBC-EV levels independently, limiting specificity for malignancies or any pathophysiological conditions (104). Decisively, RBCs are viewed in health and disease and positioned as valuable tools in the future of precision medicine, nanotechnology, and immune modulation (105).
7 Conclusion
RBC-derived EVs carry unique cargo, offer a real-time snapshot of ongoing physiological or pathological processes, and can be sampled non-invasively from blood. This makes them ideal for monitoring treatment response or disease progression (e.g., in sickle cell disease flares or response to iron chelation in thalassemia). Elevated RBC-EV levels or RBC-EV-specific contents (like RBC microRNAs) have been reported in anemia, hemoglobinopathies, infections, and inflammatory disorders. Some proposed biomarkers (e.g., exosomal miR-451a) show strong diagnostic accuracy in small studies, while others (RBC-EV counts) require further validation. Their cargo (e.g., heme, Hb, ROS) and surface markers (e.g., PS, CD47) directly modulate endothelial function, trigger immune responses, and amplify thrombo-inflammation, all of which correlate with disease severity and progression. For a translational approach, in vivo validation is required for engineered RBCs, RBC-EVs in regenerative medicine, as well as biohybrid micro-devices’ navigation and release control. Overall, current human evidence – especially in plasma/serum samples – suggests RBC-EVs are a rich source of disease biomarkers. Continued clinical research is needed to define their sensitivity/specificity and to translate these findings into validated diagnostic assays.
Author contributions
UJ: Investigation, Writing – review & editing, Conceptualization, Writing – original draft. LBG: Writing – review & editing, Resources, Supervision, Conceptualization, Writing – original draft. HNH: Writing – review & editing, Validation, Supervision, Writing – original draft, Formal analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; AGEs, advanced glycation end-products; AD, Alzheimer’s disease; C8bp, C8 binding protein; CLL, chronic lymphocytic leukemia; CML, chronic myeloid leukemia; CD, cluster domain; CR1, complement receptor 1; DAF, decay accelerating factor; DLB, Dementia with Lewy Bodies; EVs, extracellular vesicles; GH, glomerular hematuria; G6PD, Glucose 6 Phosphate Deficiency; G6PD, glucose-6-phosphate dehydrogenase; HSP, heat shock proteins; Hb, hemoglobin; HSPGs, Heparan sulfate proteoglycans; HRP, homologous restriction protein; LDH, lactate dehydrogenase; MIP-la, macrophage inflammatory protein 1a; MDC, macrophage-derived chemokine; MHC, major histocompatibility complex; MCP, membrane cofactor protein; MM, multiple myeloma; PD, Parkinson’s disease; PA, phosphatidic acid; PE, phosphatidylethanolamine; PS, phosphatidylserine; PfEMP1, plasmodium falciparum erythrocyte membrane protein 1; PfPTP2, plasmodium falciparum protein 2; PKC, protein kinase C; PAH, pulmonary arterial hypertension; RBC-EVs, RBC-derived EVs; RMP, RBC-derived microparticles; ROS, reactive oxygen species; RBCs, red blood cells; SCD, sickle cell disease; SIRPα, signal regulatory protein alpha; SLE, systemic lupus erythematosus.
References
1. Kumar M, Baba S, Sadida H, Marzooqi S, Jerobin J, Altemani F, et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct Target Ther. (2024) 9:27. doi: 10.1038/s41392-024-01735-1
2. Doyle L, Wang M. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. (2019) 8:727. doi: 10.3390/cells8070727
3. Ma S, Xia H, Gong P, Yu Z. Red blood cell-derived extracellular vesicles: an overview of current research progress, challenges, and opportunities. Biomedicines. (2023) 11:2798. doi: 10.3390/biomedicines11102798
4. Díaz-Varela M, de Menezes-Neto A, Perez-Zsolt D, Gámez-Valero A, Seguí-Barber J, Izquierdo-Useros N, et al. Proteomics study of human cord blood reticulocyte-derived exosomes. Sci Rep. (2018) 8:14046. doi: 10.1038/s41598-018-32386-2
5. Chiangjong W, Netsirisawan P, Hongeng S, Chutipongtanate S. Red blood cell extracellular vesicle-based drug delivery: challenges and opportunities. Front Med. (2021) 8:761362. doi: 10.3389/fmed.2021.761362
6. Ciana A, Achilli C, Gaur A, Minetti G. Membrane remodelling and vesicle formation during ageing of human red blood cells. Cell Physiol Biochem. (2017) 42:1127–38. doi: 10.1159/000478768
7. Das S, Lyon C, Hu TA. Panorama of extracellular vesicle applications: from biomarker detection to therapeutics. ACS Nano. (2024) 18:9784–97. doi: 10.1021/acsnano.4c00666
8. Joshi U, George L, Highland H. Determination of the role of miR-451a on Plasmodium falciparum red blood cell stages, oxidative stress, and proteomic profiling. Mol Biol Rep. (2024) 51:1041. doi: 10.1007/s11033-024-09938-z
9. Avenick D, Kidd L, Istvan S, Dong F, Richter K, Edwards N, et al. Effects of storage and leukocyte reduction on the concentration and procoagulant activity of extracellular vesicles in canine packed red cells. J Vet Emerg Crit Care. (2021) 31:221–30. doi: 10.1111/vec.13050
10. Xiong Z, Oriss T, Cavaretta J, Rosengart M, Lee J. Red cell microparticle enumeration: validation of a flow cytometric approach. Vox Sang. (2012) 103:42–8. doi: 10.1111/j.1423-0410.2011.01577.x
11. Mathieu M, Martin-Jaular L, Lavieu G, Théry C. Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat Cell Biol. (2019) 21:9–17. doi: 10.1038/s41556-018-0250-9
12. van Niel G, D’Angelo G, Raposo G. Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. (2018) 19:213–28. doi: 10.1038/nrm.2017.125
13. Biagiotti S, Canonico B, Tiboni M, Abbas F, Perla E, Montanari M, et al. Efficient and highly reproducible production of red blood cell-derived extracellular vesicle mimetics for the loading and delivery of RNA molecules. Sci Rep. (2024) 14:14610. doi: 10.1038/s41598-024-65623-y
14. Olatunya O, Lanaro C, Longhini A, Penteado C, Fertrin K, Adekile A, et al. Red blood cells microparticles are associated with hemolysis markers and may contribute to clinical events among sickle cell disease patients. Ann Hematol. (2019) 98:2507–21. doi: 10.1007/s00277-019-03792-x
15. Yu Z, Liu X, Wu M, Shi S, Fu Q, Jia J, et al. Untouched isolation enables targeted functional analysis of tumour-cell-derived extracellular vesicles from tumour tissues. J Extracell Vesicles. (2022) 11:e12214. doi: 10.1002/jev2.12214
16. Bebesi T, Kitka D, Gaál A, Szigyártó I, Deák R, Beke-Somfai T, et al. Storage conditions determine the characteristics of red blood cell derived extracellular vesicles. Sci Rep. (2022) 12:977. doi: 10.1038/s41598-022-04915-7
17. Noulsri E, Lerdwana S, Palasuwan D, Palasuwan A. Cell-derived microparticles in blood products from blood donors deficient in glucose-6-phosphate dehydrogenase. Lab Med. (2021) 52:528–35. doi: 10.1093/labmed/lmab007
18. Tissot J, Rubin O, Canellini G. Analysis and clinical relevance of microparticles from red blood cells. Curr Opin Hematol. (2010) 17:571–7. doi: 10.1097/moh.0b013e32833ec217
19. Westerman M, Porter J. Red blood cell-derived microparticles: an overview. Blood Cells Mol Dis. (2016) 59:134–9. doi: 10.1016/j.bcmd.2016.04.003
20. Huang H, Zhu J, Fan L, Lin Q, Fu D, Wei B, et al. MicroRNA profiling of exosomes derived from red blood cell units: implications in transfusion-related immunomodulation. Biomed Res Int. (2019) 2019:2045915. doi: 10.1155/2019/2045915
21. Bakkour S, Acker J, Chafets D, Inglis H, Norris P, Lee T, et al. Manufacturing method affects mitochondrial DNA release and extracellular vesicle composition in stored red blood cells. Vox Sang. (2016) 111:22–32. doi: 10.1111/vox.12390
22. Thangaraju K, Neerukonda S, Katneni U, Buehler P. Extracellular vesicles from red blood cells and their evolving roles in health, coagulopathy and therapy. Int J Mol Sci. (2022) 22:153. doi: 10.3390/ijms22010153
23. Yang L, Huang S, Zhang Z, Liu Z, Zhang L. Roles and applications of red blood cell-derived extracellular vesicles in health and diseases. Int J Mol Sci. (2022) 23:5927. doi: 10.3390/ijms23115927
24. Božič D, Hočevar M, Kisovec M, Pajnič M, Pađen L, Jeran M, et al. Stability of erythrocyte-derived nanovesicles assessed by light scattering and electron microscopy. Int J Mol Sci. (2021) 22:12772. doi: 10.3390/ijms222312772
25. Blow F, Buck A. Extracellular vesicles from malaria-infected red blood cells: not all are secreted equal. EMBO Rep. (2022) 23:e55499. doi: 10.15252/embr.202255499
26. Lötvall J, Hill A, Hochberg F, Buzás E, Di Vizio D, Gardiner C, et al. Minimal experimental requirements for definition of extracellular vesicles and their functions: a position statement from the International Society for Extracellular Vesicles. J Extracell Vesicles. (2014) 3:26913. doi: 10.3402/jev.v3.26913
27. Whelihan M, Zachary V, Orfeo T, Mann K. Prothrombin activation in blood coagulation. J Thromb Haemost. (2003) 1:1535–44. doi: 10.1046/j.1538-7836.2003.00271.x
28. Levin C, Koren A, Rebibo-Sabbah A, Koifman N, Brenner B, Aharon A. Extracellular vesicle characteristics in β-thalassemia as potential biomarkers for spleen functional status and ineffective erythropoiesis. Front Physiol. (2018) 9:1214. doi: 10.3389/fphys.2018.01214
29. Belcher J, Beckman J, Balla G, Balla J, Vercellotti G. Heme degradation and vascular injury. Antioxid Redox Signal. (2010) 12:233–48. doi: 10.1089/ars.2009.2822
30. James J, Srivastava A, Varghese M, Eccles C, Zemskova M, Rafikova O, et al. Heme induces rapid endothelial barrier dysfunction via the MKK3/p38MAPK axis. Blood. (2020) 136:749–54. doi: 10.1182/blood.2019003986
31. Donadee C, Raat N, Kanias T, Tejero J, Lee J, Kelley E, et al. Nitric oxide scavenging by red blood cell microparticles and cell-free hemoglobin as a mechanism for the red cell storage lesion. Circulation. (2011) 124:465–76. doi: 10.1161/CIRCULATIONAHA.110.008698
32. Harisa G, Badran M, Alanazi F. Erythrocyte nanovesicles: biogenesis, biological roles and therapeutic approach: erythrocyte nanovesicles. Saudi Pharm J. (2017) 25:8–17. doi: 10.1016/j.jsps.2015.06.010
33. Poisson J, Tanguy M, Davy H, Camara F, El Mdawar M, Kheloufi M, et al. Erythrocyte-derived microvesicles induce arterial spasms in JAK2V617F myeloproliferative neoplasm. J Clin Invest. (2020) 130:2630–43. doi: 10.1172/JCI124566
34. Reiter C, Wang X, Tanus-Santos J, Hogg N, Cannon R, Schechter A, et al. Cell-free hemoglobin limits nitric oxide bioavailability in sickle-cell disease. Nat Med. (2002) 8:1383–9. doi: 10.1038/nm1202-799
35. Mantel P, Hoang A, Goldowitz I, Potashnikova D, Hamza B, Vorobjev I, et al. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe. (2013) 13:521–34. doi: 10.1016/j.chom.2013.04.009
36. Kaur, S, Isenberg J, Roberts D. CD47 (Cluster of Differentiation 47). Atlas Genet Cytogenet Oncol Haematol. (2021) 25:83–102.
37. Schönermark S, Rauterberg EW, Shin ML, Löke S, Roelcke D, Hänsch GM. Homologous species restriction in lysis of human erythrocytes: a membrane-derived protein with C8-binding capacity functions as an inhibitor. J Immunol. (1986) 136:1772–6. doi: 10.4049/jimmunol.136.5.1772
38. Zalman L, Wood L, Müller-Eberhard H. Isolation of a human erythrocyte membrane protein capable of inhibiting expressiof inhibiting expression of homologous complement transmembrane channels. Proc Natl Acad Sci U S A. (1986) 83:6975–9. doi: 10.1073/pnas.83.18.6975
39. Kim D, Miwa T, Kimura Y, Schwendener R, van Lookeren Campagne M, Song W. Deficiency of decay-accelerating factor and complement receptor 1-related gene/protein y on murine platelets leads to complement-dependent clearance by the macrophage phagocytic receptor CRIg. Blood. (2008) 112:1109–19. doi: 10.1182/blood-2008-01-134304
40. Fang R, Hu C, Zhang L. Nanoparticles disguised as red blood cells to evade the immune system. Expert Opin Biol Ther. (2012) 12:385–9. doi: 10.1517/14712598.2012.661710
41. Vani R, Anusha B, Christina R, Kavin P, Mohammed O, Inchara S, et al. Band 3 protein: a critical component of erythrocyte. In: Vani R editor. Red Blood Cells-Properties and Functions. London: IntechOpen (2024).
42. An R, Man Y, Cheng K, Zhang T, Chen C, Wang F, et al. Sickle red blood cell-derived extracellular vesicles activate endothelial cells and enhance sickle red cell adhesion mediated by von Willebrand factor. Br J Haematol. (2023) 201:552–63. doi: 10.1111/bjh.18616
43. Jayasinghe M, Gao C, Yap G, Yeo B, Vu L, Tay D, et al. Red blood cell-derived extracellular vesicles display endogenous antiviral effects and enhance the efficacy of antiviral oligonucleotide therapy. ACS Nano. (2023) 17:21639–61. doi: 10.1021/acsnano.3c06803
44. Zailani M, Raja Sabudin R, Ithnin A, Alauddin H, Sulaiman S, Ismail E, et al. Population screening for glucose-6-phosphate dehydrogenase deficiency using quantitative point-of-care tests: a systematic review. Front Genet. (2023) 14:1098828. doi: 10.3389/fgene.2023.1098828
45. Manakeng K, Prasertphol P, Phongpao K, Chuncharunee S, Tanyong D, Worawichawong S, et al. Elevated levels of platelet- and red cell-derived extracellular vesicles in transfusion-dependent β-thalassemia/HbE patients with pulmonary arterial hypertension. Ann Hematol. (2019) 98:281–8. doi: 10.1007/s00277-018-3518-z
46. Hasse S, Julien A, Duchez A, Zhao C, Boilard E, Fortin P, et al. Red blood cell-derived phosphatidylserine positive extracellular vesicles are associated with past thrombotic events in patients with systemic erythematous lupus. Lupus Sci Med. (2022) 9:e000605. doi: 10.1136/lupus-2021-000605
47. Charoensappakit A, Puapatanakul P, Praditpornsilpa K, Palasuwan A, Noulsri E, Palasuwan D. Urinary red blood cell-derived microparticles and phosphatidylserine-exposing red blood cells in glomerular and non-glomerular hematuria patients. Cytometry B Clin Cytom. (2022) 102:370–6. doi: 10.1002/cyto.b.22083
48. Bank I, Timmers L, Gijsberts C, Zhang Y, Mosterd A, Wang J, et al. The diagnostic and prognostic potential of plasma extracellular vesicles for cardiovascular disease. Expert Rev Mol Diagn. (2015) 15:1577–88. doi: 10.1586/14737159.2015.1109450
49. Oxendine Harp K, Bashi A, Botchway F, Dei-Adomakoh Y, Iqbal S, Wilson M, et al. MicroRNAs miR-451a and Let-7i-5p profiles in circulating exosomes vary among individuals with different sickle hemoglobin genotypes and malaria. J Clin Med. (2022) 11:500. doi: 10.3390/jcm11030500
50. Dey S, Mohapatra S, Khokhar M, Hassan S, Pandey R. Extracellular vesicles in malaria: shedding light on pathogenic depths. ACS Infect Dis. (2024) 10:827–44. doi: 10.1021/acsinfecdis.3c00649
51. Joshi U, Shah S, Gupta S, George L, Highland H. Evaluation of exosomal proteins as potential biomarkers from RBC stages of Plasmodium falciparum 3D7. ACS Infect Dis. (2025) 11:164–80. doi: 10.1021/acsinfecdis.4c00513
52. Solga I, Yogathasan V, Wischmann P, Pang T, Götzmann L, Kleimann P, et al. Large extracellular vesicles derived from red blood cells in coronary artery disease patients with anemia promote endothelial dysfunction. bioRxiv [Preprint]. (2025). doi: 10.1101/2025.03.10.642191
53. Tzounakas V, Anastasiadi A, Lekka M, Papageorgiou E, Stamoulis K, Papassideri I, et al. Deciphering the relationship between free and vesicular hemoglobin in stored red blood cell units. Front Physiol. (2022) 13:840995. doi: 10.3389/fphys.2022.840995
54. Lu Z, Yu X, Li P, Wang Y, Deng Y, Li X, et al. Correlations of erythrocytic oligomer α-synuclein levels with age, sex and clinical variables in patients with Parkinson’s disease. Front Aging Neurosci. (2024) 16:1437622. doi: 10.3389/fnagi.2024.1437622
55. Aslam R, Speck E, Kim M, Freedman J, Semple J. Transfusion-related immunomodulation by platelets is dependent on their expression of MHC Class I molecules and is independent of white cells. Transfusion. (2008) 48:1778–86. doi: 10.1111/j.1537-2995.2008.01791.x
56. Lutz H. Naturally occurring anti-band 3 antibodies in clearance of senescent and oxidatively stressed human red blood cells. Transfus Med Hemother. (2012) 39:321–7. doi: 10.1159/000342171
57. Zhou J, Liu S, Hu C, Jiang W, Xu Y, Zhang F, et al. Red blood cell-derived extracellular vesicles promote hypoxia-induced pulmonary hypertension in mice. J Clin Invest. (2022) 132:e150278. doi: 10.1172/JCI150278
58. Wilhelm E, Gonzalez-Alonso J, Pialoux V. Exercise-induced oxidative stress: friend or foe? Redox Biol. (2022) 54:102393. doi: 10.1016/j.redox.2022.102393
59. Chatterjee V, Yang X, Ma Y, Wu M, Yuan S. Extracellular vesicles: new players in regulating vascular barrier function. Am J Physiol Heart Circ Physiol. (2020) 319:H1181–96. doi: 10.1152/ajpheart.00579.2020
60. Costantino S, Mohammed S, Paneni F. Endothelial dysfunction in patients with type 2 diabetes: the truth is in the blood. J Clin Invest. (2025) 135:e193128. doi: 10.1172/JCI193128
61. Ye C, Zheng F, Wu N, Zhu G, Li X. Extracellular vesicles in vascular remodeling. Acta Pharmacol Sin. (2022) 43:2191–201. doi: 10.1038/s41401-021-00846-7
62. Fourcade O, Simon M, Viodé C, Rugani N, Leballe F, Ragab A, et al. Secretory phospholipase A2 generates the novel lipid mediator lysophosphatidic acid in membrane microvesicles shed from activated cells. Cell. (1995) 80:919–27. doi: 10.1016/0092-8674(95)90295-3
63. Mantel P, Hjelmqvist D, Walch M, Kharoubi-Hess S, Nilsson S, Ravel D, et al. Infected erythrocyte-derived extracellular vesicles alter vascular function via regulatory Ago2-miRNA complexes in malaria. Nat Commun. (2016) 7:12727. doi: 10.1038/ncomms12727
64. Noubouossie D, Key N. Red cell extracellular vesicles and coagulation activation pathways. Curr Opin Hematol. (2023) 30:194–202. doi: 10.1097/MOH.0000000000000780
65. Ye S, Li W, Li W, Xiao L, Ran F, Chen M, et al. The regulatory role of exosomes in venous thromboembolism. Front Cell Dev Biol. (2022) 10:956880. doi: 10.3389/fcell.2022.956880
66. Kawasaki T, Takeda Y, Kumanogoh A. Proteomics of blood extracellular vesicles in inflammatory respiratory diseases for biomarker discovery and new insights into pathophysiology. Inflamm Regen. (2024) 44:38. doi: 10.1186/s41232-024-00351-4
67. Punyadee N, Mairiang D, Thiemmeca S, Komoltri C, Pan-Ngum W, Chomanee N, et al. Microparticles provide a novel biomarker to predict severe clinical outcomes of dengue virus infection. J Virol. (2015) 89:1587–607. doi: 10.1128/JVI.02207-14
68. Vimonpatranon S, Roytrakul S, Phaonakrop N, Lekmanee K, Atipimonpat A, Srimark N, et al. Extracellular vesicles derived from early and late stage Plasmodium falciparum-infected red blood cells contain invasion-associated proteins. J Clin Med. (2022) 11:4250. doi: 10.3390/jcm11144250
69. Nantakomol D, Dondorp A, Krudsood S, Udomsangpetch R, Pattanapanyasat K, Combes V, et al. Circulating red cell-derived microparticles in human malaria. J Infect Dis. (2011) 203:700–6. doi: 10.1093/infdis/jiq104
70. Babatunde K, Mbagwu S, Hernández-Castañeda M, Adapa S, Walch M, Filgueira L, et al. Malaria infected red blood cells release small regulatory RNAs through extracellular vesicles. Sci Rep. (2018) 8:884. doi: 10.1038/s41598-018-19149-9
71. Regev-Rudzki N, Wilson D, Carvalho T, Sisquella X, Coleman B, Rug M, et al. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. (2013) 153:1120–33. doi: 10.1016/j.cell.2013.04.029
72. Sampaio N, Emery S, Garnham A, Tan Q, Sisquella X, Pimentel M, et al. Extracellular vesicles from early stage Plasmodium falciparum-infected red blood cells contain PfEMP1 and induce transcriptional changes in human monocytes. Cell Microbiol. (2018) 20:e12822. doi: 10.1111/cmi.12822
73. Sadallah S, Eken C, Schifferli J. Ectosomes as modulators of inflammation and immunity. Clin Exp Immunol. (2011) 163:26–32. doi: 10.1111/j.1365-2249.2010.04271.x
74. Sekar R, Cioanca A, Yang Y, Kamath K, Carroll L, Natoli R, et al. Therapeutic potential of red blood cell-derived extracellular vesicles in reducing neuroinflammation and protecting against retinal degeneration. bioRxiv [Preprint]. (2024). doi: 10.1101/2024.08.06.606930
75. Wannez A, Devalet B, Chatelain B, Chatelain C, Dogné J, Mullier F. Extracellular vesicles in red blood cell concentrates: an overview. Transfus Med Rev. (2019) 33:125–30. doi: 10.1016/j.tmrv.2019.02.002
76. Danesh A, Inglis H, Jackman R, Wu S, Deng X, Muench M, et al. Exosomes from red blood cell units bind to monocytes and induce proinflammatory cytokines, boosting T-cell responses in vitro. Blood. (2014) 123:687–96. doi: 10.1182/blood-2013-10-530469
77. Yadav S, Deepika, Moar K, Kumar A, Khola N, Pant A, et al. Reconsidering red blood cells as the diagnostic potential for neurodegenerative disorders. Biol Cell. (2024) 116:e2400019. doi: 10.1111/boc.202400019
78. Schneider T, Stöckli L, Felbecker A, Nirmalraj P. Protein fibril aggregation on red blood cells: a potential biomarker to distinguish neurodegenerative diseases from healthy aging. Brain Commun. (2024) 6:fcae180. doi: 10.1093/braincomms/fcae180
79. Liu Z, Chan R, Cai Z, Liu X, Wu Y, Yu Z, et al. α-Synuclein-containing erythrocytic extracellular vesicles: essential contributors to hyperactivation of monocytes in Parkinson’s disease. J Neuroinflammation. (2022) 19:53. doi: 10.1186/s12974-022-02413-1
80. DeCastro J, Mehta-Doshi A, Liu C, Ray A, Aran K. Red blood cell-derived exosomes as mediators of age-related neurodegeneration. Rejuvenation Res. (2025) [Online ahead of print]. doi: 10.1089/rej.2025.0013.
81. An R, Man Y, Cheng K, Zhang T, Chen C, Kucukal E, et al. Extracellular vesicles from sickle red blood cells activate endothelial cells and trigger von willebrand factor-mediated sickle red cell adhesion. Blood. (2022) 140(Suppl 1):8223–5. doi: 10.1182/blood-2022-170438
82. Liu A, Jacobs-McFarlane C, Sebastiani P, Glassberg J, McCuskee S, Curtis S. Plasma free hemoglobin is associated with LDH, AST, total bilirubin, reticulocyte count, and the hemolysis score in patients with sickle cell anemia. Res Sq. (2024) 104:2221–8. doi: 10.1007/s00277-025-06253-w
83. Kittivorapart J, Crew V, Wilson M, Heesom K, Siritanaratkul N, Toye A. Quantitative proteomics of plasma vesicles identify novel biomarkers for hemoglobin E/β-thalassemic patients. Blood Adv. (2018) 2:95–104. doi: 10.1182/bloodadvances.2017011726
84. Giannaki A, Georgatzakou H, Fortis S, Anastasiadi A, Pavlou E, Nomikou E, et al. Stratification of βSβ+ compound heterozygotes based on L-glutamine administration and RDW: focusing on disease severity. Antioxidants. (2023) 12:1982. doi: 10.3390/antiox12111982
85. Abdolalian M, Zarif M, Javan M. The role of extracellular vesicles on the occurrence of clinical complications in β-thalassemia. Exp Hematol. (2023) 127:28–39. doi: 10.1016/j.exphem.2023.08.009
86. Jiskani S. Extracellular vesicles in thalassemia: mechanisms, implications, and therapeutic potential. Asp Mol Med. (2024) 30:100061. doi: 10.1016/j.amolm.2024.100061
87. Alaarg A, Schiffelers R, van Solinge W, van Wijk R. Red blood cell vesiculation in hereditary hemolytic anemia. Front Physiol. (2013) 4:365. doi: 10.3389/fphys.2013.00365
88. Kontidou E, Collado A, Pernow J, Zhou Z. Erythrocyte-derived microRNAs: emerging players in cardiovascular and metabolic disease. Arterioscler Thromb Vasc Biol. (2023) 43:628–36. doi: 10.1161/ATVBAHA.123.319027
89. Gkaliagkousi E, Nikolaidou B, Gavriilaki E, Lazaridis A, Yiannaki E, Anyfanti P, et al. Increased erythrocyte- and platelet-derived microvesicles in newly diagnosed type 2 diabetes mellitus. Diab Vasc Dis Res. (2019) 16:458–65. doi: 10.1177/1479164119844691
90. Clos-Garcia M, Loizaga-Iriarte A, Zuñiga-Garcia P, Sánchez-Mosquera P, Rosa Cortazar A, González E, et al. Metabolic alterations in urine extracellular vesicles are associated to prostate cancer pathogenesis and progression. J Extracell Vesicles. (2018) 7:1470442. doi: 10.1080/20013078.2018.1470442
91. Boukouris S, Mathivanan S. Exosomes in bodily fluids are a highly stable resource of disease biomarkers. Proteomics Clin Appl. (2015) 9:358–67. doi: 10.1002/prca.201400114
92. Tamkovich S, Tutanov O, Efimenko A, Grigor’eva A, Ryabchikova E, Kirushina N, et al. Blood circulating exosomes contain distinguishable fractions of free and cell-surface-associated vesicles. Curr Mol Med. (2019) 19:273–85. doi: 10.2174/1566524019666190314120532
93. Van Morckhoven D, Dubois N, Bron D, Meuleman N, Lagneaux L, Stamatopoulos B. Extracellular vesicles in hematological malignancies: evidence for reshaping the tumoral microenvironment. Front Immunol. (2023) 14:1265969. doi: 10.3389/fimmu.2023.1265969
94. Gärtner K, Luckner M, Wanner G, Zeidler R. Engineering extracellular vesicles as novel treatment options: exploiting herpesviral immunity in CLL. J Extracell Vesicles. (2019) 8:1573051. doi: 10.1080/20013078.2019.1573051
95. Shao Y, Jiang Y, Yang K, Zhu Y, Liu Y, Zhang P, et al. Apoptotic vesicles derived from human red blood cells promote bone regeneration via carbonic anhydrase 1. Cell Prolif. (2024) 57:e13547. doi: 10.1111/cpr.13547
96. Nguyen D, Tran H, Kaestner L, Bernhardt I. The relation between extracellular vesicles released from red blood cells, their cargo, and the clearance by macrophages. Front Physiol. (2022) 13:783260. doi: 10.3389/fphys.2022.783260
97. Usman W, Pham T, Kwok Y, Vu L, Ma V, Peng B, et al. Efficient RNA drug delivery using red blood cell extracellular vesicles. Nat Commun. (2018) 9:2359. doi: 10.1038/s41467-018-04791-8
98. Sódar B, Kittel Á, Pálóczi K, Vukman K, Osteikoetxea X, Szabó-Taylor K, et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci Rep. (2016) 6:24316. doi: 10.1038/srep24316
99. Théry C, Witwer K, Aikawa E, Alcaraz M, Anderson J, Andriantsitohaina R, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. (2018) 7:1535750. doi: 10.1080/20013078.2018.1535750
100. Caby M, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. Int Immunol. (2005) 17:879–87. doi: 10.1093/intimm/dxh267
101. György B, Szabó T, Turiák L, Wright M, Herczeg P, Lédeczi Z, et al. Improved flow cytometric assessment reveals distinct microvesicle (cell-derived microparticle) signatures in joint diseases. PLoS One. (2012) 7:e49726. doi: 10.1371/journal.pone.0049726
102. Lannigan J, Erdbruegger U. Imaging flow cytometry for the characterization of extracellular vesicles. Methods. (2017) 112:55–67. doi: 10.1016/j.ymeth.2016.09.018
103. Minami S, Chikazu D, Ochiya T, Yoshioka Y. Extracellular vesicle-based liquid biopsies in cancer: Future biomarkers for oral cancer. Transl Oncol. (2023) 38:101786. doi: 10.1016/j.tranon.2023
104. LaRocca T, Kakarla S, Nair S. Extracellular vesicles and hemolysis: impact on biomarker identification and clinical diagnosis. Biomarkers. (2021) 26:593–602. doi: 10.1080/1354750X.2021.1887877
Keywords: extracellular vesicles (EVs), red blood cells (RBCs), hematological biomarkers, liquid biopsy, cell to cell communications
Citation: Joshi U, George LB and Highland H (2025) Red blood cell extracellular vesicles: new frontiers in hematological biomarker discovery. Front. Med. 12:1644077. doi: 10.3389/fmed.2025.1644077
Received: 09 June 2025; Accepted: 21 July 2025;
Published: 18 August 2025.
Edited by:
Tsukuru Umemura, International University of Health and Welfare, JapanReviewed by:
Alkmini Anastasiadi, University of Patras, GreeceCopyright © 2025 Joshi, George and Highland. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Urja Joshi, dXJqYWpvc2hpQGd1amFyYXR1bml2ZXJzaXR5LmFjLmlu
†ORCID: Urja Joshi, orcid.org/0000-0001-8040-6411
 Urja Joshi
Urja Joshi Linz-Buoy George2
Linz-Buoy George2