Abstract
Background:
Fractures are increasing due to ageing populations. Physical agent modalities, a non-invasive treatment, enhances healing and reduces non-union risk.
Objective:
This meta-analysis evaluates the effectiveness of physical agent modalities in patients with fractures and compares the outcomes of different interventions on healing and pain relief.
Methods:
Articles published up to April 2025 were retrieved from PubMed, Embase, and Web of Science. Two authors independently reviewed and extracted data from randomized controlled trials assessing seven types of physical agent modalities: Electrical Stimulation (ES), Pulsed Electromagnetic Fields Stimulation (PEMFS), Ultrasound Therapy (UST), Low-Level Laser Therapy (LLLT), Magnetic Stimulation (MS), Extracorporeal Shock Wave Therapy (ESWT), and Capacitively Coupled Electric Field Stimulation (CCEFS). Standard meta-analysis and network meta-analysis (NMA) were performed for three outcomes: Pain Relief Difference, Time to Complete Fracture Healing (days), and Number of Cases Achieving Complete Fracture Healing. Cumulative ranking curves (SUCRA) scores were calculated for each therapy, with data presented as mean differences (MD) and 95% confidence intervals (CI).
Results:
This meta-analysis includes 39 studies with 2,379 participants. The standard meta-analysis results show that physical agent modalities can markedly enhance fracture healing, with significant pain relief (MD = 1.30, 95% CI: 0.61, 1.99), P = 0.0002, shorter time to complete fracture healing (days) (MD = −21.58, 95% CI: −31.05, −12.11), P < 0.0001, and more number of cases achieving complete fracture healing (RR = 1.37, 95% CI: 1.17, 1.60), P < 0.0001. However, the NMA findings indicate that most direct or indirect comparisons between different physical agent therapies yield pooled effect sizes whose 95% confidence intervals include the null value (0 or 1), showing no significant differences between groups. SUCRA rankings revealed that LLLT (87.5%) and ES (80.8%) were more effective in pain relief, while UST (82.9%) and CCEFS (99.9%) excelled in promoting fracture healing.
Conclusion:
LLLT, ES, UST, and CCEFS may yield improved outcomes for fracture patients; however, further high-quality, large-scale randomized controlled trials are required to validate these findings.
1 Introduction
Bone fractures, a prevalent condition, are currently witnessing an upward trend in global incidence (1), predominantly linked to population ageing and increased life expectancy. According to United Nations research, the global population aged ≥65 years is projected to reach 1.5 billion by 2050 (2, 3). As the ageing process accelerates, the incidence of fractures continues to rise, imposing substantial socioeconomic burdens on individuals, families, and societies (4). Fracture patients often experience major symptoms such as restricted mobility and acute pain (5), which typically ease over a two-month period, with most individuals reaching optimal recovery within 3–6 months (6). Depending on the severity of the fracture, treatment may involve either conservative management or surgery. For non-displaced fractures, conservative treatment generally leads to good outcomes; according to the BMJ Clinical Practice Guidelines1, recovery usually takes 3–4 weeks with relatively rapid pain relief. Displaced fractures, on the other hand, often require surgical intervention, which can support earlier functional recovery (around 10–14 weeks) (7), but may also carry about a 20% risk of non-union and persistent pain, potentially affecting quality of life (8–11). It is important to note that the duration of pain relief following surgery varies considerably among individuals (12).
Bone healing following a fracture is a complex physiological process that is typically divided into four stages: the fracture and inflammatory phase, the angio-mesenchymal phase, the bone formation phase, and the bone remodeling phase. Although each phase possesses distinct characteristics, they often occur alternately and exhibit a degree of overlap (13). The process of bone healing is influenced by a variety of factors, including the nature and extent of the injury, the damage to the surrounding soft tissues, blood supply, the differentiation capacity of osteoblasts, and the cellular microenvironment as internal factors. Additionally, external factors such as the stability of fracture fixation, the gap between fracture ends, the inflammatory response, and external physical stimuli also play a significant role in the healing process (14). In the final stage of bone healing, known as the bone remodeling phase, approximately 5–10% of long bone fractures may experience non-union (15). In cases of delayed healing or non-union during the fracture healing process, surgical intervention is often required. Autologous bone grafting, regarded as the gold standard for the treatment of fractures and bone defects, has been widely employed in clinical practice (16). In addition to surgical treatment, non-invasive physical agent modalities such as electrical stimulation, electromagnetic stimulation, low-intensity pulsed ultrasound, and low-level laser therapy have been shown to facilitate the acceleration of fracture healing and have gained widespread recognition in clinical practice. These adjunctive therapies provide effective supplementary strategies for optimizing the healing of fractures (17–20).
Most randomized controlled trials (RCTs) use standard care as a control, while few directly compare distinct physical agent modalities modalities. Traditional meta-analyses typically allow for the comparison of only two treatment methods at a time, failing to provide comprehensive evidence regarding the relative efficacy of various interventions for fracture healing. Network meta-analysis (NMA) addresses this limitation by facilitating simultaneous comparisons of multiple treatment options and enabling the ranking of each intervention based on various outcomes. This approach offers clinicians a clear, evidence-based framework for treatment decisions, thereby assisting in making informed and scientifically sound clinical choices when addressing complex cases of delayed healing or non-union (21).
Consequently, we conducted a NMA aimed at synthesizing the existing evidence to compare the efficacy of different physical agent modalities in promoting fracture healing and alleviating pain. The specific objective is to identify the most effective physical agent modalities approach, thereby providing robust support for clinical decision-making and assisting in the optimization of treatment strategies for fracture healing.
2 Methods
The protocol was registered with PROSPERO under registration number CRD420251030229.
2.1 Search strategy
This NMA adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (22). As of April 2025, we conducted a comprehensive search for relevant literature in the PubMed, Embase, and Web of Science databases. Search strategies were developed for each of the three databases (See Supplementary Table 1 for details). Two authors (LL and SFH) independently conducted literature searches and screenings, with any discrepancies resolved through mutual discussion. To augment potential relevant studies, the authors also examined the references of the included literature. The language was restricted to English, with no date limitations applied.
2.2 Exclusion and inclusion criteria
Studies that met the following criteria were included: (1) Patients with fractures or delayed healing following a fracture, regardless of fracture location and severity. (2) Physical agent modalities involving one or more of the following: Electrical Stimulation (ES), Pulsed Electromagnetic Fields Stimulation (PEMFS), Ultrasound Therapy (UST), Low-Level Laser Therapy (LLLT), Magnetic Stimulation (MS), Extracorporeal Shock Wave Therapy (ESWT), and Capacitively Coupled Electric Field Stimulation (CCEFS). (3) Control groups receiving either placebo stimulation or standard treatment alone. (4) studies reporting at least one outcome of interest, including pain, time to complete fracture healing, and the number of cases of complete fracture healing. (5) Randomized controlled trial (RCT) design. We excluded: ➀ Non-human studies; ➁ Studies lacking quantifiable outcome measures; ➂ Studies not involving disease models; ➃ Case reports, reviews, editorials, commentaries, conference abstracts, and articles not in English.
2.3 Data extraction
Two authors independently conducted eligibility assessments on the retrieved articles, initially excluding irrelevant studies based on their titles and abstracts. The remaining articles were then downloaded for a comprehensive review of the full texts, from which data were extracted for the eligible studies, including the first author’s name, publication year, country/region, participant characteristics (sample size, mean age, fracture location), interventions, follow-up duration, and outcomes of interest (Table 1). When extracted data were presented as medians and interquartile ranges, we applied Hozo’s formula to convert them into means and standard deviations (23). In cases of discrepancies, discussions were held to reach consensus. If any required information was missing, the corresponding author of the article was contacted via email.
TABLE 1
| References | Country/ Regions |
Mean age (years) (SD) Intervention/Comparator |
Intervention/ Comparator |
Sample size (n) (intervention/comparator) Intervention/Comparator |
Fracture site | Follow-up | Outcomes | ||
| Acosta-Olivo et al. (36) | Mexico | 54.8 (13.07) | Laser acupuncture/acupuncture | 13 | 13 | Wrist Bone | 1 month | Pain relief difference | |
| Barker et al. (37) | UK | >18 | >18 | Magnetic field/placebo stimulation | 9 | 7 | Tibia | 12 month | Number of cases achieving complete fracture healing |
| Beck et al. (38) | Australia | 28.33 (7.68) | 26.09 (7.99) | Capacitively coupled electric field/placebo stimulation | 22 | 21 | Tibia | 2 month | Time to complete fracture healing |
| Busse et al. (39) | Canada | 37.1 (13.2) | 39.1 (14.6) | LIPUS/placebo stimulation | 214 | 201 | Tibia | 1 year + | Number of cases achieving complete fracture healing |
| Chang et al. (40) | Taiwan | 33.64 (7.82) | 30.56 (9.61) | Laser/placebo stimulation | 25 | 25 | Wrist and Hand | 2 week | Pain relief difference |
| Cheing et al. (41) | China | 63.8 (12.6) | 60.3 (20.2) | Electromagnetic field/placebo stimulation | 23 | 22 | Distal radius | 5 day | Pain relief difference |
| Duran et al. (42) | Istanbul | 58.9 (10.7) | 62.0 (9.5) | IFC/placebo stimulation | 18 | 17 | Proximal humeral | 4 month + | Pain relief difference |
| Elboim-Gabyzon et al. (43) | Israel | 80.26 (9.83) | 78.06 (8.45) | TENS/placebo stimulation | 23 | 18 | Hip | 5 day | Pain relief difference |
| Elsebahy et al. (44) | Egypt | 5 ∼ 8 | LIPUS/none | 15 | 15 | supracondylar | 1 month + | Time to complete fracture healing | |
| Factor et al. (45) | Israel | 58 (13.25) | 59 (16.75) | Electromagnetic field/placebo stimulation | 14 | 13 | Distal radius | 3 month | Number of cases achieving complete fracture healing; time to complete fracture healing |
| Factor et al. (46) | Israel | 49 | 59 | Electromagnetic field/placebo stimulation | 11 | 14 | Distal radius | 6 month | Number of cases achieving complete fracture healing |
| Fourie et al. (47) | South Africa | 35 (11) | 31 (13.25) | IFC/placebo stimulation | 41 | 35 | Tibial shaft | 2 years + | Time to complete fracture healing |
| Gopalan et al. (48) | India | 28 (7.291) | 26.75 (8.723) | LIPUS/none | 20 | 20 | Mandibular | 3 month | Number of cases achieving complete fracture healing |
| Gorodetskyi et al. (49) | Russia | 71.5 (2) | 70.8 (3) | Non-invasive interactive neurostimulation/placebo stimulation | 30 | 30 | Trochanteric of the femur | 10 day | Pain relief differences |
| Gorodetskyi et al. (50) | Russia | 35.3 (9) | 38.4 (9) | Non-invasive interactive neurostimulation/placebo stimulation | 30 | 30 | Ankle | 11 day | Pain relief difference |
| Hannemann et al. (51) | Netherlands | 35 (13) | 34 (14.75) | Electromagnetic field/placebo stimulation | 51 | 51 | Scaphoid | 1 year + | Time to complete fracture healing |
| Hannemann et al. (52) | Netherlands | 44.3 (17) | 37.7 (13.25) | Electromagnetic field/placebo stimulation | 24 | 29 | Scaphoid | 1 year + | Time to complete fracture healing |
| Heckman et al. (53) | USA | 36 (2.3) | 31 (1.8) | LIPUS/placebo stimulation | 33 | 34 | Tibia | 9 month + | Number of cases achieving complete fracture healing; time to complete fracture healing |
| Kristiansen et al. (54) | USA | 54 (3) | 28 (2) | LIPUS/placebo stimulation | 30 | 31 | Distal radial | 3 month + | Number of cases achieving complete fracture healing; time to complete fracture healing |
| Liu et al. (55) | China | 61.5 (2.1) | 63.5 (1.2) | Electromagnetic field/placebo stimulation | 40 | 42 | Vertebral | 3 month + | Pain relief difference |
| Liu et al. (56) | China | 67.9 (5.58) | 65.7 (6.09) | LIPUS/none | 41 | 40 | Distal radius | 1 month + | Time to complete fracture healing |
| Martinez-Rondanelli et al. (57) | Colombia | 31 (10) | 29 (9) | Electromagnetic field/placebo stimulation | 32 | 31 | Diaphyseal femoral | 6 month | Number of cases achieving complete fracture healing |
| Mohajerani et al. (58) | Iran | 37.06 (10.6) | 37 (10.7) | Electromagnetic field/none | 16 | 16 | Mandibular | 2 week | Pain relief difference |
| Moncada et al. (59) | Colombia | 30.2 | Magnetic field/placebo stimulation | 32 | 32 | Femoral shaft | 6 month | Number of cases achieving complete fracture healing | |
| Oncel et al. (60) | Turke | 44 (15) | 40 (16) | TENS/placebo stimulation | 25 | 25 | rib | 3 day | Pain relief difference |
| Patel et al. (61) | India | 15 ∼ 35 | LIPUS/none | 14 | 14 | Mandibular | 1 month + | Pain relief difference | |
| Piazzolla et al. (62) | Italy | 73.6 (7.82) | 72.88 (6.09) | Capacitively coupled electric field/none | 33 | 33 | Vertebral | 6 month | Pain relief difference |
| Ricardo et al. (63) | Cuba | 26.7 | LIPUS/placebo stimulation | 10 | 11 | Scaphoid | 2.3 years | Time to complete fracture healing | |
| Santana-Rodríguez et al. (64) | Saudi Arabia | 64 (13.1) | 58.9 (17.3) | PUS/placebo stimulation | 24 | 23 | Rib | 6 month | Pain relief difference |
| Schofer et al. (65) | Germany | 42.6 (14.6) | 45.1 (11.9) | LIPUS/placebo stimulation | 51 | 50 | Tibia | 4 month | Number of cases achieving complete fracture healing |
| Scott et al. (66) | UK | 40 (9.05) | 46 (20.09) | Capacitively coupled electric field/placebo stimulation | 10 | 11 | Long bones | 9 month | Number of cases achieving complete fracture healing |
| Sharrard et al. (67) | UK | 34.7 (17.66) | 45.4 (14.76) | Electromagnetic field/placebo stimulation | 20 | 25 | Tibia | 3 month | Number of cases achieving complete fracture healing |
| Shi et al. (68) | China | 41.1 (14.5) | 38.4 (11.6) | Electromagnetic field/placebo stimulation | 31 | 27 | Long bones | 4 month + | Number of cases achieving complete fracture healing |
| Simonis et al. (69) | UK | 31.7 (14.6) | 32.3 (16.3) | Electrical stimulation/placebo stimulation | 18 | 16 | Tibia | 6 month | Number of cases achieving complete fracture healing |
| Streit et al. (70) | USA | 47 (9.75) | Electrical stimulation/placebo stimulation | 5 | 3 | Metatarsal | 5 month + | Time to complete fracture healing | |
| Wang et al. (71) | Taiwan | 35.5 (16.0) | 35.4 (19.2) | Shock wave/none | 27 | 30 | Long bones | 12 month | Pain relief difference; number of cases achieving complete fracture healing |
| White et al. (72) | Canada | 27.1 (9.4) | 26.5 (12.1) | LIPUS/placebo stimulation | 69 | 73 | Scaphoid | 2.4 years | Number of cases achieving complete fracture healing |
| Wu et al. (73) | China | 43.1 (9.6) | 42.5 (8.2) | ST + PNF + TEAS/ST + PNF | 20 | 20 | Tibial plateau | 1 month + | Pain relief difference |
| Yadav et al. (74) | India | Unclear | Unclear | Ultrasound/placebo stimulation | 39 | 28 | Tibia | 1 month + | Number of cases achieving complete fracture healing |
Main characteristics of included studies.
2.4 Risk of bias
The risk of bias in the included studies was assessed using the Cochrane Risk of Bias Tool (RoB 2.0)2 (24) across six domains: the randomization process, deviations from intended interventions, missing outcome data, measurement of the outcome, selection of the reported results, and other sources of bias. The two authors independently rated each study as “low risk,” “high risk,” or “some concerns” for each of the aforementioned domains. Any discrepancies that arose during the review process were resolved through discussion or negotiation between the two authors.
2.5 Statistical analysis
In this study, for dichotomous outcomes, we reported risk ratios (RR) with 95% credible intervals, while for continuous outcome variables, we reported mean differences (MD) with 95% confidence intervals (CIs). Traditional meta-subgroup analyses were conducted using Review Manager 5.4.1, while calculations and visualizations were carried out using R 4.4.3 (R Foundation for Statistical Computing) and Stata SE 15.1 (StataCorp, College Station, TX). Given the heterogeneity between trials, we employed a Bayesian hierarchical random effects model for multiple comparisons (25, 26). Based on the theory of the likelihood function and certain initial assumptions, we performed Markov Chain Monte Carlo (MCMC) simulations using R 4.4.3, with 500,000 iterations and 20,000 for annealing to investigate posterior distributions (27–29). We assessed model goodness-of-fit by calculating the deviance information criterion (DIC) and employed the node splitting method to compare the consistency of direct and indirect evidence for each comparison (30). To address heterogeneity in the study, a random effects model was employed, and the degree of heterogeneity was quantified using the I2 statistic. To rank the interventions, we calculated the Surface Under the Cumulative Ranking Curve (SUCRA) probability values, which range from 0 to 1, with higher values indicating that the intervention is more likely to be the most effective (31, 32). A network diagram was created to analyse the geometrical structure of the intervention network and to identify potential biases, with the size of the nodes representing the number of participants in each group and the thickness of the lines reflecting the number of studies. A conjugate prior distribution was used for the Bayesian network meta-analysis (NMA), and a ranking table was generated to illustrate the comparisons of each pair of interventions for each outcome. Pairwise meta-analyses were conducted using the DerSimonian-Laird random effects model to estimate the variance of heterogeneity and obtain direct evidence (33). Finally, we utilized a comparison-adjusted funnel plot to assess potential publication bias (34, 35). Furthermore, sensitivity analyses were performed to explore their potential impact on the conclusions.
3 Results
3.1 Description of the included studies
We searched the PubMed, Embase, and Web of Science databases, identifying 341, 797, and 404 articles, respectively. After removing 410 duplicate articles, a total of 1,132 articles were identified. Based on title and abstract screening, 1,042 articles were excluded, leaving 90 articles that underwent full-text review, of which 39 articles met the eligibility criteria for our systematic review and NMA (36–74). The detailed PRISMA flow chart is presented in Figure 1.
FIGURE 1

Flow diagram of the preferred reporting items for systematic reviews and meta-analyses (PRISMA) criteria. RCTs, randomized controlled trials.
Table 1 summarizes the characteristics of the 39 studies. Among these, 15 studies assessed pain improvement before and after physical agent modalities, 13 studies reported the time to complete fracture healing, and 17 studies documented the number of patients with fully healed fractures. Three studies evaluated capacitively coupled electric field stimulation, nine studies assessed electrical stimulation (ES), two studies investigated magnetic stimulation (MS), ten studies examined pulsed electromagnetic field stimulation (PEMFS), two studies focused on low-level laser therapy (LLLT), one study assessed extracorporeal shockwave therapy (ESWT), and twelve studies investigated ultrasound therapy (UST). This research was conducted across multiple countries, including China, the United States, Russia, Israel, Colombia, and India, and included 2,379 participants, with ages ranging from 5 to 72 years. The fracture sites were diverse, comprising 2.90% for humeral fractures (reported in two articles), 8.34% for femoral fractures (reported in three articles), 2.67% for ankle fractures (reported in one article), 14.18% for scapular fractures (reported in four articles), 10.66% for radial fractures (reported in five articles), 40.30% for tibial fractures (reported in ten articles), 1.82% for hip fractures (reported in one article), 4.32% for rib fractures (reported in two articles), 2.23% for carpal fractures (reported in two articles), 4.46% for mandibular fractures (reported in three articles), 0.4% for metatarsal fractures (reported in one article), and 6.60% for vertebral fractures (reported in two articles). Additionally, three studies reported on long bone fractures involving a total of 136 participants. Finally, the follow-up durations for the three outcome measures varied: the pain relief difference ranged from 5 days to 6 months; the time to complete fracture healing ranged from 1 month to 2.3 years; and the number of cases achieving complete fracture healing ranged from 1 month to 2.4 years.
3.2 Risk of bias
We conducted an assessment of the risk of bias, the results of which are illustrated in Figure 2. Among the studies, 26 indicated a low risk of bias, 11 reported a moderate risk, and 2 demonstrated a high risk. All studies reported randomization. However, J. D. Heckman et al. (53), M. Oncel et al. (60) and G. Scott et al. (66) conducted unplanned treatments during the intervention phase, leading to their classification as moderate risk. W. J. Sharrard et al. (67) and N. J. White et al. (72) were deemed high risk due to individual participants withdrawing from the trial as a result of the intervention. Furthermore, B. R. Beck et al. (38), J. W. Busse et al. (39), E. Duran et al. (42), A. Piazzolla et al. (62), N. Santana-Rodríguez et al. (64) and H. F. Shi et al. (68) were classified as moderate risk due to dropout or loss to follow-up for personal reasons, whereas all other studies reported no loss of outcome data. B. R. Beck et al. (38) was also rated as moderate risk for failing to assess whether fractures had fully healed based on imaging reports; all other studies were classified as low risk. Additionally, all studies, except for S. Y. Elsebahy et al. (44) and M. E. Moncada et al. (59), did not show any potential risk of selective reporting bias.
FIGURE 2
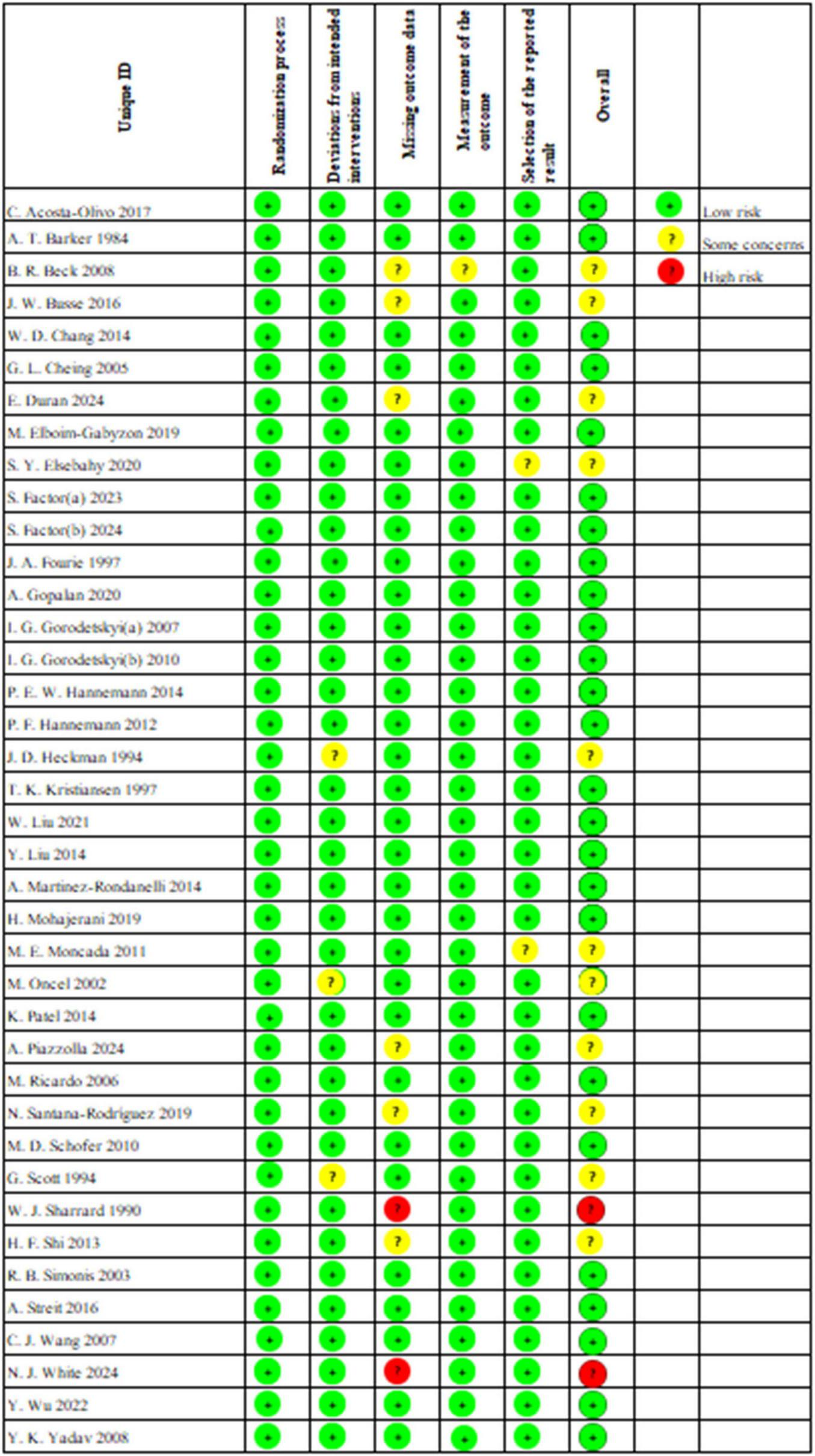
Traffic light plot for the risk-of-bias assessment of included trials.
3.3 Standard meta-analysis
3.3.1 Pain relief difference
This study included 14 investigations that assessed pain intensity using the Visual Analogue Scale (VAS). To illustrate the extent of pain relief, we used the difference between the VAS score recorded before the application of physical agent modalities and the score obtained after the final treatment session as the primary analytical measure. Due to significant overall heterogeneity (I2 = 90.8%, P < 0.00001), a random-effects model was employed for the meta-analysis of pain scores. The analyses were further divided into six subgroups based on different physical interventions (LLLT, CCEFS, UST, ESWT, ES, PEMFS) (Figure 3A). For ES, I2 was found to be 92% (P < 0.00001), while LLLT and PEMFS reported I2 values of 0% and 4%, respectively. Due to the limited number of studies, I2 could not be calculated for the remaining three interventions. Hence, the variation in different physical agent modalities may have contributed to the high heterogeneity observed. The analysis results demonstrated that LLLT significantly reduced pain in fracture patients: MD = 2.22, 95% CI (1.50, 2.94), P < 0.00001. This was followed by ES: MD = 1.86, 95% CI (0.90, 2.82), P = 0.0002; UST: MD = 1.30, 95% CI (0.39, 2.21), P = 0.005; PEMFS: MD = 0.52, 95% CI (0.24, 0.81), P = 0.0003; ESWT: MD = 0.52, 95% CI (0.16, 0.88), P = 0.004. Lastly, CCEFS showed an MD of −0.56, 95% CI (−1.01, −1.11), P = 0.01, indicating that CCEFS did not alleviate pain, this may be largely attributed to two factors: First, the number of studies included in the analysis was limited, with only one investigation evaluating the analgesic effect of CCEFS, resulting in insufficient statistical power. Second, that study adopted a follow-up period of up to 6 months; although pain levels showed marked improvement over time, the dominant role of the body’s own repair mechanisms during the natural fracture healing process may have substantially diluted the additional effects of physical agent therapy in the later stages. This could be a key reason for the negative effect observed. Future high-quality studies with shorter follow-up periods, particularly during the acute phase, are recommended to clarify the true effectiveness of CCEFS in pain management. In the sensitivity analysis, excluding individual studies did not lead to significant changes in the overall results, suggesting that the findings are robust (Supplementary Figure 1A).
FIGURE 3

Forest plot of comparison: physical agent modalities group versus control group. (A) Difference between post-treatment and pre-treatment pain scores; (B) time to complete fracture healing (days); (C) number of patients with fully healed fractures.CI, confidence interval; MD, mean difference.
3.3.2 Time to complete fracture healing (days)
In total, 9 studies were included, reporting on the fracture healing times of patients with different fracture locations following physical agent modalities. Due to the high overall heterogeneity of the included studies (I2 = 82.7%, P = 0.0006), a random-effects model was employed for the meta-analysis. Furthermore, subgroup analyses were conducted for different physical agent modalities (CCEFS, ES, PEMFS, UST) (Figure 3B). The I2 values for PEMFS and UST were 29% and 99%, respectively, while I2 could not be calculated for CCEFS and ES due to an insufficient number of studies. Thus, the high heterogeneity may be attributed to the different physical intervention methods employed. The results indicated that the treatment group showed a significant improvement in fracture healing time compared to the control group, UST: MD = −28.09, 95% CI (−40.27, −15.91), P < 0.00001; PEMFS: MD = −11.26, 95% CI (−22.39, −0.13), P = 0.05; CCEFS: MD = 3.10, 95% CI (−5.67, 11.87), P = 0.49; ES: MD = −19.49, 95% CI (−51.23, 12.25), P = 0.23. Although CCEFS and ES did not show statistical significance, the overall results indicated that physical agent modalities effectively reduced fracture healing time: MD = −21.58, 95% CI (−31.05, −12.11), P < 0.00001. In the sensitivity analysis, the summary results remained stable after the exclusion of individual studies, suggesting that the findings are robust (Supplementary Figure 1B).
3.3.3 Number of cases achieving complete fracture healing
16 included studies reported on the number of cases achieving complete fracture healing. Due to moderately high overall heterogeneity (I2 = 46.9%, P = 0.09), a random-effects model was still employed. The results indicated that, overall, physical agent modalities significantly increased the number of cases achieving complete fracture healing compared to the control group, with a risk ratio (RR) of 1.37, 95% confidence interval (CI) (1.17, 1.60), P < 0.0001. Further subgroup analyses were conducted based on different physical agent modalities (MS, CCEFS, PEMFS, ES, ESWT, UST) (Figure 3C), revealing that PEMFS and UST exhibited statistically significant effects and played a positive role in promoting fracture healing, PEMFS: RR = 1.92, 95% CI (1.01, 3.65), P = 0.05; UST: RR = 1.44, 95% CI (1.13, 1.84), P = 0.003. In addition, the I2 values for MS, PEMFS, and UST were 6%, 75%, and 77%, respectively, indicating that the high heterogeneity may still be attributed to the different physical intervention methods. In the sensitivity analysis, after excluding individual studies, the summary results did not show significant changes, suggesting that the findings are robust (Supplementary Figure 1C).
3.4 Network meta-analysis
3.4.1 Network map
We generated three network node diagrams (Figures 4A–C), three primary outcomes, each involving different physical agent modalities. Analysing these study data, we assessed the relative efficacy of seven types of physical agent modalities (Figures 4D–F). Further details are provided in the ranking table (Supplementary Table 2). We conducted pairwise comparative analyses of all treatment approaches using MD and RR with 95% confidence intervals. The results showed that, for most comparisons between physical agent modalities, the 95% confidence intervals included the null value (MD = 0 or RR = 1), indicating that the differences in effectiveness between these interventions did not reach statistical significance. This suggests that the relative efficacy of different physical agent modalities remains uncertain. However, a trend suggesting that Low-Level Laser Therapy (LLLT) may be more effective in alleviating post-fracture pain compared with the other five groups. The following data supports this assertion: ESWT vs LLLT: MD = −1.74, 95% CI (−3.94, 0.45); CCEFS vs LLLT: MD = −2.82, 95% CI (−5.03, –0.61); PEMFS vs LLLT: MD = −1.68, 95% CI (−3.41, 0.04); UST vs LLLT: MD = −0.96, 95% CI (−3.31, 1.39), and ES vs LLLT: MD = −0.37, 95% CI (−1.97, 1.22) (Figure 4D). Similarly, when considering the time to complete fracture healing (days), although no statistically significant differences were observed, UST showed a notable trend toward reducing the time to complete fracture healing (days) compared to the other groups. This trend is supported by the data: UST vs CCEFS: MD = −31.22, 95% CI (−69.42, 6.97); UST vs PEMFS: MD = −12.36, 95% CI (−38.40, 13.69); ES vs UST: MD = 8.63, 95% CI (−40.25, 57.51) (Figure 4E). Moreover, when considering the number of patients achieving complete fracture healing, CCEFS demonstrated a significant advantage over the other intervention groups, CCEFS vs MS: RR = 13.79, 95% CI(0.78, 244.89); CCEFS vs ESWT: RR = 12.61, 95% CI (0.70, 228.37); PEMFS vs CCEFS: RR = 0.12, 95% CI (0.01, 2.03); UST vs CCEFS: RR = 0.10, 95% CI (0.01, 1.80); ES vs CCEFS: RR = 0.13, 95% CI (0.01, 2.36) (Figure 4F).
FIGURE 4
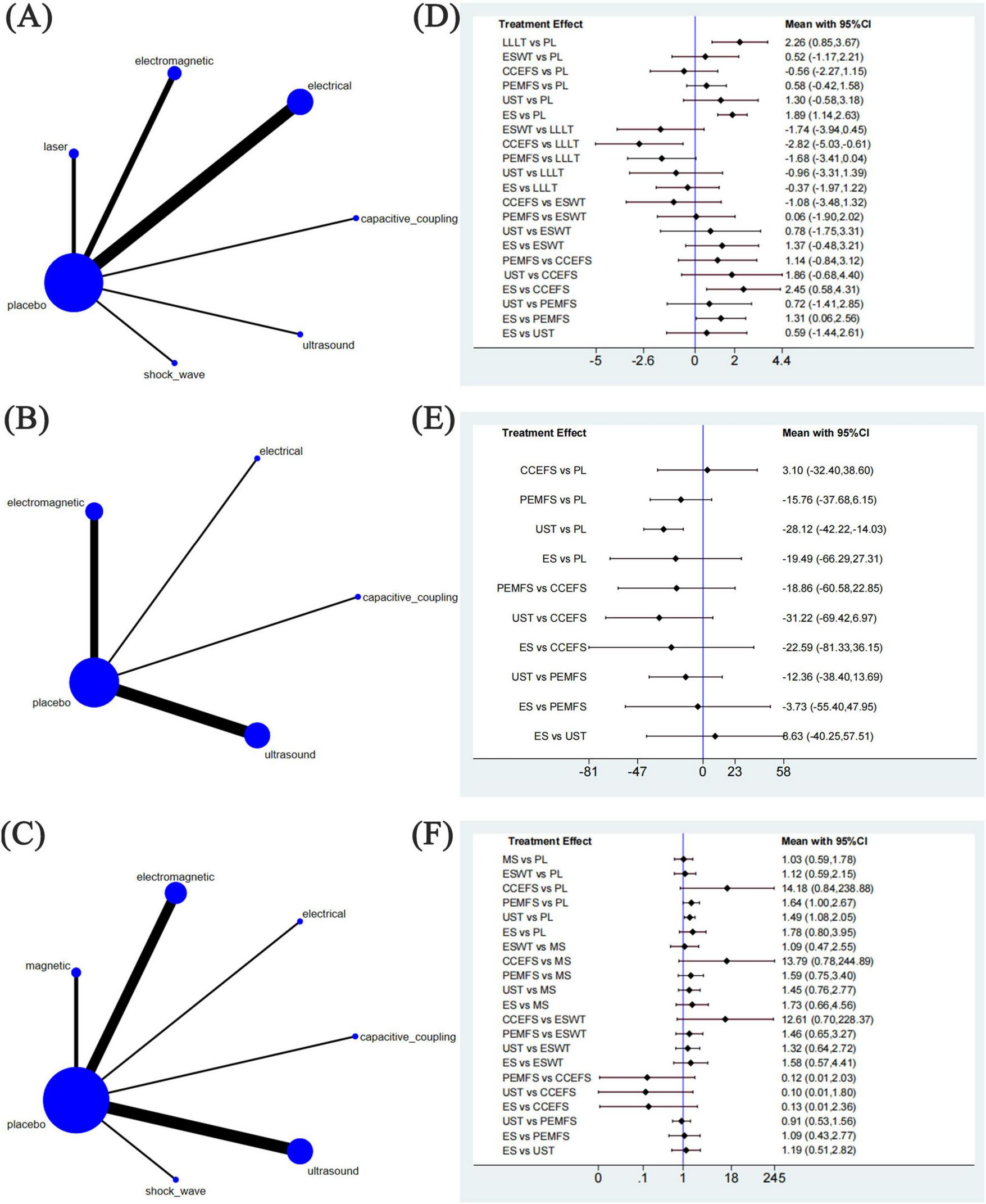
Network meta-analysis of physical agent modalities for fractures. (A–C) Network evidence plots for fractures. (D–F) Forest plot represents the direct and indirect comparison. PL, placebo; LLLT, low-level laser therapy; MS, magnetic stimulation; ESWT, extracorporeal shock wave therapy; CCEFS, capacitively coupled electric field stimulation; PEMFS, pulsed electromagnetic fields stimulation; UST, ultrasound therapy; ES, electrical stimulation.
3.4.2 Ranking of treatments
Figure 5 illustrates the cumulative probabilities of each intervention across various potential rankings, represented by SUCRA values, which indicate the ranking of treatments; a higher SUCRA value signifies a more favorable ranking among all available treatments. A SUCRA value of 100% denotes the best treatment effect, while a SUCRA value of 0% indicates the poorest treatment effect. According to the ranking results shown in Table 2, the two highest-ranked interventions for pain relief are LLLT (SUCRA 87.5%) and ES (SUCRA 80.8%), followed by UST (SUCRA 62.5%), PEMFS (SUCRA 42.9%), ESWT (SUCRA 41.0%), and CCEFS (SUCRA 13.7%). In terms of time to complete fracture healing (days), UST (SUCRA 82.9%) and ES (SUCRA 61.3%) are ranked highest, followed by PEMFS (SUCRA 58.7%) and CCEFS (SUCRA 24.7%). Finally, with respect to the number of patients achieving complete fracture healing, CCEFS (SUCRA 99.9%) and PEMFS (SUCRA 67.6%) ranked highest, followed by ES (SUCRA 58.4%), UST (SUCRA 53.6%), ESWT (SUCRA 30.2%), and MS (SUCRA 23.2%).
FIGURE 5
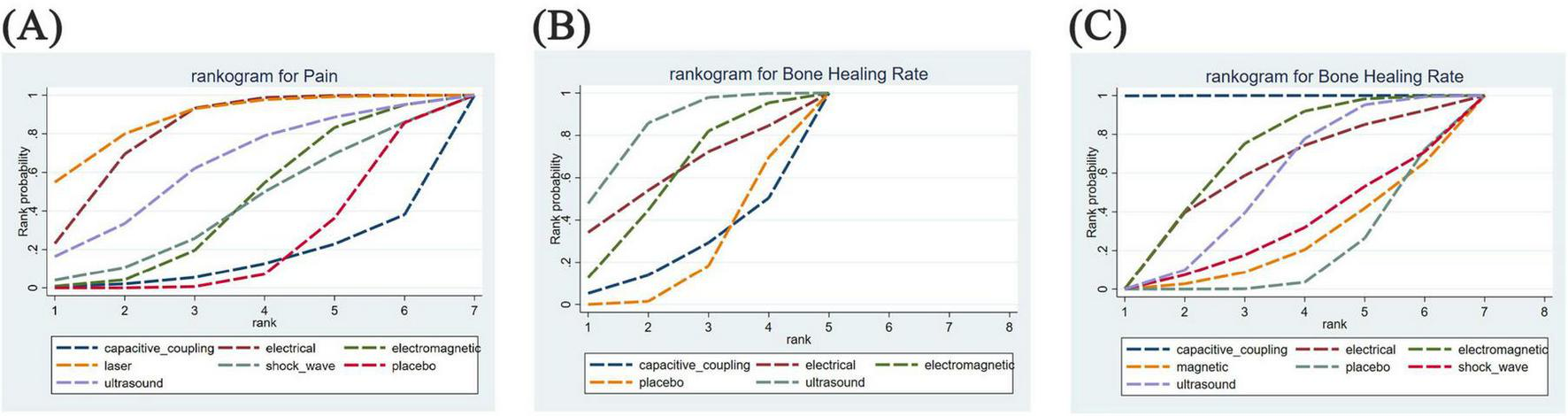
Rankogram for all outcomes. Each line segment represents a treatment. The area enclosed by the line segment and the coordinate axis represents the cumulative probability of treatment. (A) Difference between post-treatment and pre-treatment pain scores; (B) time to complete fracture healing (days); (C) number of patients with fully healed fractures.
TABLE 2
| Physical agent modalities | Pain relief difference | Time to complete fracture healing (days) | Number of cases achieving complete fracture healing |
| Capacitive_coupling | 13.7% | 24.7% | 99.90% |
| Electrical | 80.8% | 61.3% | 58.40% |
| Electromagnetic | 42.9% | 58.7% | 67.60% |
| Laser | 87.5% | − | − |
| Placebo | 21.7% | 22.4% | 17.10% |
| Shock_wave | 41.0% | − | 30.20% |
| Ultrasound | 62.5% | 82.9% | 53.60% |
| magnetic | − | − | 23.20% |
SUCRA ranking of different outcome indicators.
3.5 Publication of bias
Funnel plots were employed to assess publication bias for all outcome indicators. The funnel plots for Pain Relief Difference, Time to Complete Fracture Healing (days), and Number of Cases Achieving Complete Fracture Healing exhibited a symmetrical and even distribution, suggesting the absence of significant publication bias (Figure 6).
FIGURE 6
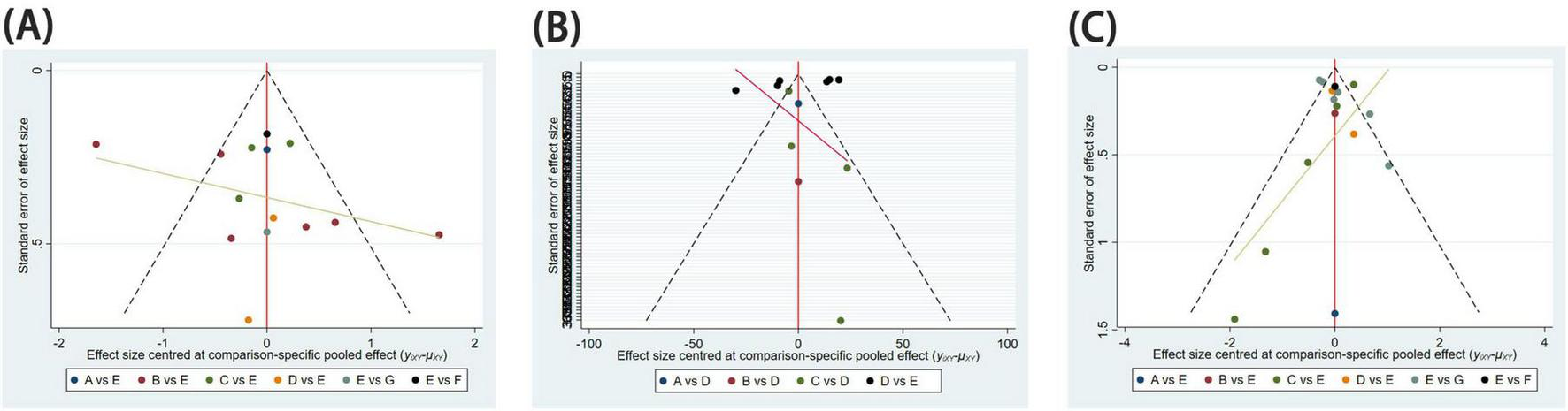
Funnel diagram. Publication bias for assessing study results. (A) Difference between post-treatment and pre-treatment pain scores; (B) time to complete fracture healing (days); (C) number of patients with fully healed fractures.
4 Discussion
To the best of our knowledge, this study represents the first NMA comparing the efficacy of different physical agent modalities for treating patients with fractures. This NMA meticulously reviewed the most recent data from 39 eligible randomized controlled trials, evaluating the effectiveness of physical agent modalities in fracture patients. This study confirms that physical agent modalities significantly promotes fracture healing and alleviates pain. To compare the effects of different physical agent modalities, we sought indirect evidence through pairwise comparisons. However, indirect treatment comparisons revealed no statistically significant differences in efficacy among the seven modalities. Subsequently, by calculating the SUCRA values for these therapies and conducting a ranking analysis, we found that both LLLT and ES significantly relieve pain, with the reduction in pain scores likely reaching or even exceeding the minimal clinically important difference. This indicates that their effects go beyond statistical significance and provide pain relief that is genuinely meaningful to patients. Such a degree of improvement can help enhance sleep quality and daily functioning, thereby improving overall quality of life and potentially reducing dependence on analgesic medications. Consequently, these two physical agent modalities demonstrate substantial clinical value as complementary approaches to pain management. UST markedly shortens fracture healing time, while CCEFS significantly increases the healing rate in patients with fractures, followed by PEMFS. These effects enable patients to regain physical function earlier, fundamentally reducing the risk of complications associated with prolonged immobilization or delayed healing, and ultimately leading to substantial savings in healthcare resources. In this context, these physical agent modalities serve not only as effective clinical tools for preventing fracture non-union but also as strategies that enhance the overall quality of treatment. They therefore provide strong evidence supporting the clinical prioritization of efficient physical therapy modalities. However, since only a few studies have examined the application of LLLT and CCEFS in patients with fractures, this result warrants cautious interpretation.
Patients with fractures often experience acute postoperative pain, which is typically managed with analgesic medications. However, such treatments frequently come with adverse side effects, including nausea, vomiting, delirium, constipation, and gastrointestinal dysfunction. The incidence of these side effects is particularly higher in the elderly population (75), and postoperative analgesic efficacy is often poorer compared to younger individuals (76). Consequently, the application of non-pharmacological and non-invasive analgesic methods in the management of acute postoperative pain has garnered increasing attention. Our research found that low-level laser therapy (LLLT) exhibits the best analgesic effects. However, due to the low certainty of evidence, further studies are required to validate this conclusion. LLLT is based on a specialized technical device capable of emitting light beams with precise characteristics for medical applications. It stimulates the mitochondria to produce ATP, enhances mitochondrial electron transport rates, regulates reactive oxygen species to reduce oxidative stress, and induces the activation of transcription factors such as AP-1, p53, NF-kB, and HIF, thereby promoting extracellular matrix deposition and activating anti-inflammatory and anti-apoptotic pathways. Clinically, these effects manifest as pain and inflammation relief, as well as facilitation of tissue repair (77–79). Literature indicates that in vitro, LLLT modulates the inflammatory response by activating the WNT pathway and inhibiting the NF-kB signaling pathway (80). In vivo, it regulates the levels of inflammatory precursor factors such as IL-1, IL-6, IL-8, and IL-18 to control the inflammatory response (81). Furthermore, LLLT positively influences bone tissue metabolism and fracture healing (82, 83) by stimulating microcirculation and increasing the activity of osteoblasts, thus enhancing the osteogenic effect (84). However, therapeutic benefits appear restricted to early healing phases (< 21 days post-fracture), with diminished efficacy in chronic non-union models.(85).
Beyond LLLT, electrical stimulation (ES) encompasses established analgesic approaches such as transcutaneous electrical nerve stimulation (TENS), non-invasive interactive neurostimulation (NIN), and interferential current (IFC). TENS delivers pulsed electrical currents transcutaneously, making it a prevalent non-pharmacological intervention for pain management (86, 87). It is capable of generating a sensation similar to acupuncture at frequencies of 2–4 Hz by stimulating Aδ and C fibers in the afferent nerves, thereby activating downstream pain inhibition pathways and producing a spatially diffuse analgesic effect (88). Studies by Gorodetskyi I et al. (49) and Lord SR et al. (89) have investigated the role of TENS in reducing acute postoperative pain in elderly patients following hip fractures, both reporting significant pain relief, which is consistent with our findings. Moreover, NIN has been shown to have a positive effect in the postoperative care of patients with femoral neck fractures (49), with its pain relief mechanism thought to involve segmental and descending neural inhibition (90). IFC operates on the principle of a low-frequency-modulated medium-frequency current created by the superposition of two medium-frequency currents with slight phase differences (91). IFC therapy is believed to alleviate pain through gate control mechanisms and the release of endogenous opioids (92). Although this therapy has been in use for decades, its physiological effects have not been fully substantiated, making it challenging to completely elucidate its analgesic action (93).
Fracture patients frequently endure acute postoperative pain, conventionally managed with analgesics. However, these pharmacological interventions often induce adverse effects such as nausea, constipation, delirium, and gastrointestinal dysfunction. According to our study, physical agent modalities significantly shortens the time to fracture healing and improves the complete healing rate in fracture patients. SUCRA ranking in this study indicates that ultrasound therapy (UST) can markedly reduce the time to complete fracture healing (days) compared to other physical agent modalities, which aligns with the findings of Kristiansen et al. (54) who reported that treatment with low-intensity pulsed ultrasound (LIPUS) shortened radiological healing times by 38%. When ultrasound propagates through biological tissues, it generates micro-mechanical strain, which stimulates biochemical responses at the cellular level and promotes bone formation (94). Fracture patients often experience prolonged immobilization, leading to a deficiency of mechanical load at the injury site. However, ultrasound can produce mechanical forces that improve the mechanical environment of the affected area, potentially facilitating endochondral ossification, a key mechanism in fracture healing (95, 96). Additionally, the mechanical stress generated by ultrasound further promotes osteogenesis, protein synthesis, calcium uptake, and DNA synthesis in various cell types (97). As ultrasound transmits through the tissue to the bone, cells adjacent to the fracture site convert biomechanical stimuli into biochemical responses via integrins, which serve as crucial molecular mediators of mechanotransduction (98). Furthermore, ultrasound stimulation increases the expression of integrins, enhancing the adhesion of osteoblasts at the fracture site, thereby aiding in fracture healing (99, 100).
Early clinical studies suggest that high-intensity ultrasound stimulation in the range of 5000 to 25000 mW/cm2 may induce adverse effects including necrosis, cessation of bone healing, and fibrous tissue formation (101, 102). Consequently, low-intensity pulsed ultrasound (LIPUS) has consequently become the clinical standard (103). LIPUS has been shown to positively influence fracture healing regardless of the patient’s age, smoking status, the presence of a fracture gap, fibular fractures, or the location of distal fractures (104). Animal studies indicate that LIPUS not only accelerates the formation of bone callus but also enhances the mechanical strength at the fracture site (105, 106). During the fracture healing process, The periosteum serves as a primary reservoir for osteoprogenitor cells during bone regeneration, playing a central role in callus formation. Tam et al. (107) demonstrated that LIPUS interventions positively stimulate osteogenesis and the activation of cell differentiation in human periosteal cells. Additionally, cyclooxygenase-2 (COX-2) and prostaglandin E2 (PGE2) are key biological processes involved in the mineralization and remodeling phases of bone healing (108), COX-2 promotes fracture healing by upregulating genes associated with endochondral ossification and angiogenesis (98, 109), whereas PGE2 enhances collagen synthesis in cultured bone and further stimulates osteoblast proliferation (110). Tang et al. (99) and Kokubu T et al. (111) found that expression of COX-2 and PGE2 in osteoblasts was significantly increased when cells were subjected to ultrasound stimulation.
Pulsed Electromagnetic Fields Stimulation (PEMFS) has emerged as a clinical mainstay (2), demonstrating particular efficacy during the angiogenic-osteogenic coupling phase of bone repair and remodeling (112). Multiple studies have demonstrated that PEMFS actively promotes bone healing by regulating voltage-gated ion channels, increasing cytosolic calcium ion concentrations, enhancing early angiogenesis, and facilitating the differentiation and maturation of osteoblasts (113). Research indicates that PEMFS upregulates TGF-β expression and promotes the proliferation and osteogenic differentiation of stem cells via coordinated signaling through BMP, ERK/MAPK, and Notch pathways (114–117). The effectiveness of PEMFS is closely related to exposure duration, to significantly enhance fracture healing, PEMFS should be applied for at least 8 h per day over a period of 45–60 days (118). Furthermore, studies have found that PEMFS can upregulate the expression of placental growth factor (PIGF) and brain-derived neurotrophic factor (BDNF). PIGF, a member of the vascular endothelial growth factor (VEGF) subfamily, is a key regulator of angiogenesis and vasculogenesis (119). BDNF promotes angiogenesis through two mechanisms: Firstly, by locally activating subsets of endothelial cells, and secondly, by recruiting bone marrow-derived cells. These both mechanisms contribute to the formation of new blood vessels, thereby facilitating bone formation (120). Additionally, Parhampour et al. (121) discovered that PEMFS can improve bone metabolic disorders and restore joint function.
In our study, CCEFS demonstrated significant effects on fracture healing and was ranked first in the third outcome measure based on the SUCRA rankings. As a non-invasive bone growth stimulation method, CCEFS has the potential to enhance osteoblast function and increase new bone formation (122). An in vitro study revealed the mechanism of action of CCEFS, which involves the activation of voltage-gated calcium channels in the plasma membrane, leading to increased cytosolic calcium concentration and phospholipase A2 (PLA2) activity (123). The rise in cytosolic calcium activates the calmodulin pathway, further upregulating the expression of osteogenic-related genes, including fibroblast growth factor (FGF) 2, osteocalcin (OCN), TGF-β, BMP, and alkaline phosphatase (ALP) (124, 125). PLA2 promotes the synthesis of PGE2, thereby further facilitating the osteogenic process (126). Additionally, a study (127) have reported that CCEFS has a positive impact on alleviating chronic pain. However, our NMA did not demonstrate a significant effect of CCEFS on post-fracture pain relief. This may be attributed to the inclusion of only one relevant study (62), which, although indicating that CCEFS could more rapidly relieve pain, found no significant difference in the overall level of pain relief compared to the control group.
In the included studies, the number of investigations on LLLT, MS, ESWT, and CCEFS was relatively limited, largely reflecting the characteristics of each technique and their current clinical use. ESWT carries a potential risk of secondary injury due to possible adverse effects such as hematoma formation and increased pain (128). Research on MS has focused mainly on neurological rehabilitation (129), with comparatively less application in fracture treatment. CCEFS already has a well-established therapeutic protocol for fracture healing (122), and its technical stability has resulted in fewer novel research directions. For LLLT, progress in fracture pain management has been slow, partly due to the lack of standardization in pain assessment tools (such as VAS, NRS, and the McGill Pain Questionnaire) and partly because current clinical practice still relies heavily on pharmacological analgesia (130). These factors may together contribute to the current relative scarcity of research on these therapies in the field of fracture rehabilitation.
5 Limitation
First, restricted study availability and underpowered sample sizes compromised generalizability while reducing statistical precision. Second, there was an imbalance in the number of comparisons and sample sizes among the physical agent modalities. Among the three different outcome measures, studies on UST constituted the largest proportion, while those on CCEFS and MS comprised the smallest, which may have impacted the research findings. Third, we acknowledge the substantial heterogeneity observed in this study, which may largely stem from clinical differences among the included trials, such as variations in fracture type, duration of intervention, and device parameters. Because the original studies provided insufficient data, we were unable to perform subgroup analyses to further explore the specific influence of these factors. Therefore, the findings of this network meta-analysis should be interpreted as representing an overall effect across diverse clinical contexts. Future studies should adopt more standardized designs and provide more detailed reporting to better account for these key variables. Fourth, the lack of direct comparisons between physical agent modalities, relying instead on indirect evidence, may limit the reliability and comprehensiveness of the conclusions. Finally, the use of SUCRA scores does not account for differences in study quality and relies solely on relative ranking to evaluate treatment efficacy. Including low-quality studies may introduce bias and lead to systematic errors in effect size estimation. When such biased estimates are incorporated into a network meta-analysis model, they can distort the true comparative effectiveness between treatments, causing the SUCRA rankings to deviate from reality and reducing their overall reliability. Given that the design of the SUCRA scoring system focuses on relative efficacy while neglecting effect size, it may inadvertently undermine the clinical significance of the treatment effects.
6 Conclusion
Physical agent modalities demonstrate therapeutic potential in fracture management, effectively reducing pain and enhancing osseous regeneration. In indirect head-to-head comparisons, although different physical agent modalities did not show clear advantages or disadvantages in pain relief and fracture healing, this study provides valuable insights for clinical decision-making. Notably, LLLT and ES displayed potential advantages in pain alleviation, while UST and CCEFS exhibited superior effectiveness in promoting fracture healing. However, these preliminary conclusions require validation through high-quality, large-sample randomized controlled trials, and further clinical research is necessary to confirm the efficacy of these interventions.
Statements
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
LL: Writing – original draft, Visualization, Writing – review & editing, Formal analysis, Conceptualization. LY: Formal analysis, Writing – original draft, Writing – review & editing, Conceptualization, Visualization. YY: Writing – original draft, Writing – review & editing. JZ: Software, Writing – review & editing, Data curation, Methodology. RS: Validation, Methodology, Writing – review & editing, Data curation. QD: Data curation, Writing – review & editing, Validation, Methodology. JW: Software, Data curation, Validation, Writing – review & editing. FS: Writing – review & editing, Funding acquisition.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was supported by the fund of Southwest Medical University under Grant 2023ZD003, and the Luzhou City Science and Technology Bureau under Grant 2020LZXNYDJ44.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1646903/full#supplementary-material
Abbreviations
RCTs, randomized controlled trials; NMA, network meta-analysis; ES, electrical stimulation; PEMFS, pulsed electromagnetic fields stimulation; UST, ultrasound therapy; LLLT, low-level Laser therapy; MS, magnetic stimulation; ESWT, extracorporeal shock wave therapy; CCEFS, capacitively coupled electric field stimulation; TENS, transcutaneous electrical nerve stimulation; NIN, non-invasive interactive neurostimulation; LIPUS, low-intensity pulsed ultrasound; RR, risk ratios; MD, mean differences; CIs, confidence intervals; MCMC, Markov Chain Monte Carlo; DIC, deviance information criterion; SUCRA, surface under the cumulative ranking curve; COX-2, cyclooxygenase-2; PGE2, prostaglandin E2; PIGF, placental growth factor; BDNF, brain-derived neurotrophic factor; VEGF, vascular endothelial growth factor; PLA2, phospholipase A2; FGF, fibroblast growth factor; OCN, osteocalcin; ALP, alkaline phosphatase.
References
1.
Wu J Che Y Zhang Y Wang J Chen M Jiang J et al Global, regional, national trends of femur fracture and machine learning prediction: comprehensive findings and questions from global burden of disease 1990-2019. J Orthop Translat. (2024) 46:46–52. 10.1016/j.jot.2024.03.002
2.
Massari L Benazzo F Falez F Perugia D Pietrogrande L Setti S et al Biophysical stimulation of bone and cartilage: state of the art and future perspectives. Int Orthop. (2019) 43:539–51. 10.1007/s00264-018-4274-3
3.
Vasconcelos PAB Rocha AJ Fonseca RJS Teixeira TRG Mattos ESR Guedes A . Femoral fractures in the elderly in Brasil - incidence, lethality, and costs (2008-2018).Rev Assoc Med Bras. (2020) 66:1702–6. 10.1590/1806-9282.66.12.1702
4.
Cauley JA . The global burden of fractures.Lancet Healthy Longev. (2021) 2:e535–6. 10.1016/s2666-7568(21)00183-5
5.
Platts-Mills TF Flannigan SA Bortsov AV Smith S Domeier RM Swor RA et al Persistent pain among older adults discharged home from the emergency department after motor vehicle crash: a prospective cohort study. Ann Emerg Med. (2016) 67:166–76.e1. 10.1016/j.annemergmed.2015.05.003
6.
MacDermid JC Roth JH Richards RS . Pain and disability reported in the year following a distal radius fracture: a cohort study.BMC Musculoskelet Disord. (2003) 4:24. 10.1186/1471-2474-4-24
7.
Ma XZ Zhang BS Wang ZD Wang MY . [Comparisons of clinical effects among three kinds of treatments of 1/3 displaced midshaft clavicular fractures in adults].Zhonghua Yi Xue Za Zhi. (2016) 96:25–9. 10.3760/cma.j.issn.0376-2491.2016.01.006
8.
Brinker MR Hanus BD Sen M O’Connor DP . The devastating effects of tibial nonunion on health-related quality of life.J Bone Joint Surg Am. (2013) 95:2170–6. 10.2106/jbjs.L.00803
9.
Ekegren CL Edwards ER Gabbe BJ . Incidence, costs and predictors of non-union, delayed union and mal-union following long bone fracture.Int J Environ Res Public Health. (2018) 15:2845. 10.3390/ijerph15122845
10.
Mills LA Aitken SA Simpson A . The risk of non-union per fracture: current myths and revised figures from a population of over 4 million adults.Acta Orthop. (2017) 88:434–9. 10.1080/17453674.2017.1321351
11.
Moghaddam A Zimmermann G Hammer K Bruckner T Grützner PA von Recum J . Cigarette smoking influences the clinical and occupational outcome of patients with tibial shaft fractures.Injury. (2011) 42:1435–42. 10.1016/j.injury.2011.05.011
12.
Xiao Y Guangrong Y . 余霄译, and 俞光荣, 桡骨远端骨折的 AAOS 治疗指南 [AAOS guidelines for the treatment of distal radius fractures].中国矫形外科杂志 [Chinese J Orthopedics]. (2010) 18:1887–91. Chinese.
13.
Bahney CS Zondervan RL Allison P Theologis A Ashley JW Ahn J et al Cellular biology of fracture healing. J Orthop Res. (2019) 37:35–50. 10.1002/jor.24170
14.
Zura R Xiong Z Einhorn T Watson JT Ostrum RF Prayson MJ et al Epidemiology of fracture nonunion in 18 human bones. JAMA Surg. (2016) 151:e162775. 10.1001/jamasurg.2016.2775
15.
Griffin XL Costa ML Parsons N Smith N . Electromagnetic field stimulation for treating delayed union or non-union of long bone fractures in adults.Cochrane Database Syst Rev. (2011) 4:Cd008471. 10.1002/14651858.CD008471.pub2
16.
Winocour SJ Schultz KP Davis MJ Abu-Ghname A Bohl M Ropper AE et al Vascularized posterior iliac crest bone grafting: indications, techniques, clinical outcomes, and alternatives. Semin Plast Surg. (2021) 35:37–40. 10.1055/s-0041-1725986
17.
Berni M Brancato AM Torriani C Bina V Annunziata S Cornella E et al The role of low-level laser therapy in bone healing: systematic review. Int J Mol Sci. (2023) 24:7094. 10.3390/ijms24087094
18.
Chalidis B Sachinis N Assiotis A Maccauro G . Stimulation of bone formation and fracture healing with pulsed electromagnetic fields: biologic responses and clinical implications.Int J Immunopathol Pharmacol. (2011) 24:17–20. 10.1177/03946320110241s204
19.
Khalifeh JM Zohny Z MacEwan M Stephen M Johnston W Gamble P et al Electrical stimulation and bone healing: a review of current technology and clinical applications. IEEE Rev Biomed Eng. (2018) 11:217–32. 10.1109/rbme.2018.2799189
20.
Schandelmaier S Kaushal A Lytvyn L Heels-Ansdell D Siemieniuk RA Agoritsas T et al Low intensity pulsed ultrasound for bone healing: systematic review of randomized controlled trials. Bmj. (2017) 356:j656. 10.1136/bmj.j656
21.
Hutton B Salanti G Caldwell DM Chaimani A Schmid CH Cameron C et al The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. 10.7326/m14-2385
22.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Bmj. (2021) 372:n71. 10.1136/bmj.n71
23.
Hozo SP Djulbegovic B Hozo I . Estimating the mean and variance from the median, range, and the size of a sample.BMC Med Res Methodol. (2005) 5:13. 10.1186/1471-2288-5-13
24.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al RoB 2: a revised tool for assessing risk of bias in randomised trials. Bmj. (2019) 366:l4898. 10.1136/bmj.l4898
25.
Dias S Welton NJ Sutton AJ Caldwell DM Lu G Ades AE . Evidence synthesis for decision making 4: inconsistency in networks of evidence based on randomized controlled trials.Med Decis Making. (2013) 33:641–56. 10.1177/0272989x12455847
26.
Mills EJ Thorlund K Ioannidis JP . Demystifying trial networks and network meta-analysis.Bmj. (2013) 346:f2914. 10.1136/bmj.f2914
27.
Bois FY . Bayesian inference.Methods Mol Biol. (2013) 930:597–636. 10.1007/978-1-62703-059-5_25
28.
Dias S Sutton AJ Welton NJ Ades AE. NICE Decision Support Unit Technical Support Documents, Heterogeneity: Subgroups, Meta-Regression, Bias And Bias-Adjustment. London: National Institute for Health and Care Excellence (2012).
29.
Hamra G MacLehose R Richardson D . Markov chain monte carlo: an introduction for epidemiologists.Int J Epidemiol. (2013) 42:627–34. 10.1093/ije/dyt043
30.
Veroniki AA Vasiliadis HS Higgins JP Salanti G . Evaluation of inconsistency in networks of interventions.Int J Epidemiol. (2013) 42:332–45. 10.1093/ije/dys222
31.
Rücker G Schwarzer G . Ranking treatments in frequentist network meta-analysis works without resampling methods.BMC Med Res Methodol. (2015) 15:58. 10.1186/s12874-015-0060-8
32.
Trinquart L Attiche N Bafeta A Porcher R Ravaud P . Uncertainty in treatment rankings: reanalysis of network meta-analyses of randomized trials.Ann Intern Med. (2016) 164:666–73. 10.7326/m15-2521
33.
Jackson D Bujkiewicz S Law M Riley RD White IRA . matrix-based method of moments for fitting multivariate network meta-analysis models with multiple outcomes and random inconsistency effects.Biometrics. (2018) 74:548–56. 10.1111/biom.12762
34.
Chaimani A Higgins JP Mavridis D Spyridonos P Salanti G . Graphical tools for network meta-analysis in STATA.PLoS One. (2013) 8:e76654. 10.1371/journal.pone.0076654
35.
Whegang Youdom S Tahar R Basco LK . Comparison of anti-malarial drugs efficacy in the treatment of uncomplicated malaria in African children and adults using network meta-analysis.Malar J. (2017) 16:311. 10.1186/s12936-017-1963-0
36.
Acosta-Olivo C Siller-Adame A Tamez-Mata A Vilchez-Cavazos F Peña-Martinez V Acosta-Olivo C . Laser treatment on acupuncture points improves pain and wrist functionality in patients undergoing rehabilitation therapy after wrist bone fracture. a randomized, controlled, blinded study.Acupunct Electrother Res. (2017) 42:11–25. 10.3727/036012917x14908026365007
37.
Barker AT Dixon RA Sharrard WJ Sutcliffe ML . Pulsed magnetic field therapy for tibial non-union. Interim results of a double-blind trial.Lancet. (1984) 1:994–6. 10.1016/s0140-6736(84)92329-8
38.
Beck BR Matheson GO Bergman G Norling T Fredericson M Hoffman AR et al Do capacitively coupled electric fields accelerate tibial stress fracture healing? A randomized controlled trial. Am J Sports Med. (2008) 36:545–53. 10.1177/0363546507310076
39.
Busse JW Bhandari M Einhorn TA Schemitsch E Heckman JD Tornetta P et al Re-evaluation of low intensity pulsed ultrasound in treatment of tibial fractures (TRUST): randomized clinical trial. Bmj. (2016) 355:i5351. 10.1136/bmj.i5351
40.
Chang WD Wu JH Wang HJ Jiang JA . Therapeutic outcomes of low-level laser therapy for closed bone fracture in the human wrist and hand.Photomed Laser Surg. (2014) 32:212–8. 10.1089/pho.2012.3398
41.
Cheing GL Wan JW Kai Lo S . Ice and pulsed electromagnetic field to reduce pain and swelling after distal radius fractures.J Rehabil Med. (2005) 37:372–7. 10.1080/16501970510041055
42.
Duran E Durmaz B Atamaz F Kad MR Küçük L . Does interferential current provide additional benefit to orthopedic rehabilitation for the patients with proximal humeral fractures? A randomized controlled study.BMC Musculoskelet Disord. (2024) 25:114. 10.1186/s12891-024-07232-4
43.
Elboim-Gabyzon M Andrawus Najjar M Shtarker H . Effects of transcutaneous electrical nerve stimulation (TENS) on acute postoperative pain intensity and mobility after hip fracture: a double-blinded, randomized trial.Clin Interv Aging. (2019) 14:1841–50. 10.2147/cia.S203658
44.
Elsebahy SY Olama KA Elsayed MM . Low-intensity pulsed ultrasound for healing supracondylar fracture.Physiotherapy Quarterly. (2020) 28:15–9. 10.5114/PQ.2020.92473
45.
Factor S Druckmann I Atlan F Rosenblatt Y Tordjman D Krespi R et al The effects of novel pulsed electromagnetic field therapy device on acute distal radius fractures: a prospective, double-blind, sham-controlled, randomized pilot study. J Clin Med. (2023) 12:1866. 10.3390/jcm12051866
46.
Factor S Druckmann I Kazum E Atlan F Tordjman D Rosenblatt Y et al A novel pulsed electromagnetic field device as an adjunct therapy to surgical treatment of distal radius fractures: a prospective, double-blind, sham-controlled, randomized pilot study. Arch Orthop Trauma Surg. (2024) 144:543–50. 10.1007/s00402-023-05117-0
47.
Fourie JA Bowerbank P . Stimulation of bone healing in new fractures of the tibial shaft using interferential currents.Physiother Res Int. (1997) 2:255–68. 10.1002/pri.110
48.
Gopalan A Panneerselvam E Doss GT Ponvel K Raja K . Evaluation of efficacy of low intensity pulsed ultrasound in facilitating mandibular fracture healing-A blinded randomized controlled clinical trial.J Oral Maxillofac Surg. (2020) 78:997.e1–e7. 10.1016/j.joms.2020.01.036
49.
Gorodetskyi IG Gorodnichenko AI Tursin PS Reshetnyak VK Uskov ON . Non-invasive interactive neurostimulation in the post-operative recovery of patients with a trochanteric fracture of the femur. A randomised, controlled trial.J Bone Joint Surg Br. (2007) 89:1488–94. 10.1302/0301-620x.89b11.19352
50.
Gorodetskyi IG Gorodnichenko AI Tursin PS Reshetnyak VK Uskov ON . Use of noninvasive interactive neurostimulation to improve short-term recovery in patients with surgically repaired bimalleolar ankle fractures: a prospective, randomized clinical trial.J Foot Ankle Surg. (2010) 49:432–7. 10.1053/j.jfas.2010.05.007
51.
Hannemann PF Göttgens KW van Wely BJ Kolkman KA Werre AJ Poeze M et al The clinical and radiological outcome of pulsed electromagnetic field treatment for acute scaphoid fractures: a randomised double-blind placebo-controlled multicentre trial. J Bone Joint Surg Br. (2012) 94:1403–8. 10.1302/0301-620x.94b10.28844
52.
Hannemann PF van Wezenbeek MR Kolkman KA Twiss EL Berghmans CH Dirven PA et al CT scan-evaluated outcome of pulsed electromagnetic fields in the treatment of acute scaphoid fractures: a randomised, multicentre, double-blind, placebo-controlled trial. Bone Joint J. (2014) 96-B:1070–6. 10.1302/0301-620x.96b8.33767
53.
Heckman JD Ryaby JP McCabe J Frey JJ Kilcoyne RF . Acceleration of tibial fracture-healing by non-invasive, low-intensity pulsed ultrasound.J Bone Joint Surg Am. (1994) 76:26–34. 10.2106/00004623-199401000-00004
54.
Kristiansen TK Ryaby JP McCabe J Frey JJ Roe LR . Accelerated healing of distal radial fractures with the use of specific, low-intensity ultrasound. A multicenter, prospective, randomized, double-blind, placebo-controlled study.J Bone Joint Surg Am. (1997) 79:961–73. 10.2106/00004623-199707000-00002
55.
Liu W Jin X Guan Z Zhou Q . Pulsed electromagnetic field affects the development of postmenopausal osteoporotic women with vertebral fractures.Biomed Res Int. (2021) 2021:4650057. 10.1155/2021/4650057
56.
Liu Y Wei X Kuang Y Zheng Y Gu X Zhan H et al Ultrasound treatment for accelerating fracture healing of the distal radius. A control study. Acta Cir Bras. (2014) 29:765–70. 10.1590/s0102-86502014001800012
57.
Martinez-Rondanelli A Martinez JP Moncada ME Manzi E Pinedo CR Cadavid H . Electromagnetic stimulation as coadjuvant in the healing of diaphyseal femoral fractures: a randomized controlled trial.Colomb Med. (2014) 45:67–71. 10.25100/cm.v45i2.1479
58.
Mohajerani H Tabeie F Vossoughi F Jafari E Assadi M . Effect of pulsed electromagnetic field on mandibular fracture healing: a randomized control trial, (RCT).J Stomatol Oral Maxillofac Surg. (2019) 120:390–6. 10.1016/j.jormas.2019.02.022
59.
Moncada ME Sarmiento C Martinez C Martinez A . Magnetic stimulation for fracture consolidation–clinical study.Annu Int Conf IEEE Eng Med Biol Soc. (2011) 2011:1141–4. 10.1109/iembs.2011.6090267
60.
Oncel M Sencan S Yildiz H Kurt N . Transcutaneous electrical nerve stimulation for pain management in patients with uncomplicated minor rib fractures.Eur J Cardiothorac Surg. (2002) 22:13–7. 10.1016/s1010-7940(02)00206-3
61.
Patel K Kumar S Kathiriya N Madan S Shah A Venkataraghavan K et al An evaluation of the effect of therapeutic ultrasound on healing of mandibular fracture. Craniomaxillofac Trauma Reconstr. (2015) 8:299–306. 10.1055/s-0034-1544104
62.
Piazzolla A Bizzoca D Barbanti-Brodano G Formica M Pietrogrande L Tarantino U et al Capacitive biophysical stimulation improves the healing of vertebral fragility fractures: a prospective multicentre randomized controlled trial. J Orthop Traumatol. (2024) 25:17. 10.1186/s10195-024-00758-2
63.
Ricardo M . The effect of ultrasound on the healing of muscle-pediculated bone graft in scaphoid non-union.Int Orthop. (2006) 30:123–7. 10.1007/s00264-005-0034-2
64.
Santana-Rodríguez N Clavo B Llontop P Fiuza MD Calatayud-Gastardi J López DD et al Pulsed ultrasounds reduce pain and disability, increasing rib fracture healing, in a randomized controlled trial. Pain Med. (2019) 20:1980–8. 10.1093/pm/pny224
65.
Schofer MD Block JE Aigner J Schmelz A . Improved healing response in delayed unions of the tibia with low-intensity pulsed ultrasound: results of a randomized sham-controlled trial.BMC Musculoskelet Disord. (2010) 11:229. 10.1186/1471-2474-11-229
66.
Scott G King JB . A prospective, double-blind trial of electrical capacitive coupling in the treatment of non-union of long bones.J Bone Joint Surg Am. (1994) 76:820–6. 10.2106/00004623-199406000-00005
67.
Sharrard WJ . A double-blind trial of pulsed electromagnetic fields for delayed union of tibial fractures.J Bone Joint Surg Br. (1990) 72:347–55. 10.1302/0301-620x.72b3.2187877
68.
Shi HF Xiong J Chen YX Wang JF Qiu XS Wang YH et al Early application of pulsed electromagnetic field in the treatment of postoperative delayed union of long-bone fractures: a prospective randomized controlled study. BMC Musculoskelet Disord. (2013) 14:35. 10.1186/1471-2474-14-35
69.
Simonis RB Parnell EJ Ray PS Peacock JL . Electrical treatment of tibial non-union: a prospective, randomised, double-blind trial.Injury. (2003) 34:357–62. 10.1016/s0020-1383(02)00209-7
70.
Streit A Watson BC Granata JD Philbin TM Lin HN O’Connor JP et al Effect on clinical outcome and growth factor synthesis with adjunctive use of pulsed electromagnetic fields for fifth metatarsal nonunion fracture: a double-blind randomized study. Foot Ankle Int. (2016) 37:919–23. 10.1177/1071100716652621
71.
Wang CJ Liu HC Fu TH . The effects of extracorporeal shockwave on acute high-energy long bone fractures of the lower extremity.Arch Orthop Trauma Surg. (2007) 127:137–42. 10.1007/s00402-006-0236-0
72.
White NJ Patterson ED Dhaliwal GS Hildebrand KA . Low-intensity pulsed ultrasound versus sham in the treatment of operatively managed scaphoid nonunions: the SNAPU randomized controlled trial.J Bone Joint Surg Am. (2024) 106:1573–82. 10.2106/jbjs.23.00783
73.
Wu Y Zhou J Zhu F Zhang M Chen W . The effects of pain relief on proprioception and muscle strength for tibial plateau fractures: a randomized controlled trial.Musculoskelet Sci Pract. (2022) 62:102658. 10.1016/j.msksp.2022.102658
74.
Yadav YK Salgotra KR Banerjee A . Role of ultrasound therapy in the healing of tibial stress fractures.Med J Armed Forces India. (2008) 64:234–6. 10.1016/s0377-1237(08)80101-3
75.
Pasero CL McCaffery M . Overcoming obstacles to pain assessment in elders.Am J Nurs. (1997) 97:20. 10.1097/00000446-199709000-00017
76.
Morrison SR Magaziner J McLaughlin MA Orosz G Silberzweig SB Koval KJ et al The impact of post-operative pain on outcomes following hip fracture. Pain. (2003) 103:303–11. 10.1016/s0304-3959(02)00458-x
77.
Avci P Gupta A Sadasivam M Vecchio D Pam Z Pam N et al Low-level laser (light) therapy (LLLT) in skin: stimulating, healing, restoring. Semin Cutan Med Surg. (2013) 32:41–52.
78.
Cardoso MV do Vale Placa R Sant’Ana ACP Greghi SLA Zangrando MS Rezende LR et al Laser and LED photobiomodulation effects in osteogenic or regular medium on rat calvaria osteoblasts obtained by newly forming bone technique. Lasers Med Sci. (2021) 36:541–53. 10.1007/s10103-020-03056-5
79.
Nampo FK Cavalheri V Ramos S Camargo E . A. Effect of low-level phototherapy on delayed onset muscle soreness: a systematic review and meta-analysis.Lasers Med Sci. (2016) 31:165–77. 10.1007/s10103-015-1832-4
80.
Sakata S Kunimatsu R Tsuka Y Nakatani A Gunji H Yanoshita M et al High-frequency near-infrared diode laser irradiation suppresses IL-1β-induced inflammatory cytokine expression and NF-κB signaling pathways in human primary chondrocytes. Lasers Med Sci. (2022) 37:1193–201. 10.1007/s10103-021-03371-5
81.
Rodríguez-Merchán EC . A review of recent developments in the molecular mechanisms of bone healing.Int J Mol Sci. (2021) 22:767. 10.3390/ijms22020767
82.
Kazem Shakouri S Soleimanpour J Salekzamani Y Oskuie MR . Effect of low-level laser therapy on the fracture healing process.Lasers Med Sci. (2010) 25:73–7. 10.1007/s10103-009-0670-7
83.
Renno AC McDonnell PA Parizotto NA Laakso EL . The effects of laser irradiation on osteoblast and osteosarcoma cell proliferation and differentiation in vitro.Photomed Laser Surg. (2007) 25:275–80. 10.1089/pho.2007.2055
84.
Schmidt-Bleek K Schell H Schulz N Hoff P Perka C Buttgereit F et al Inflammatory phase of bone healing initiates the regenerative healing cascade. Cell Tissue Res. (2012) 347:567–73. 10.1007/s00441-011-1205-7
85.
Fekrazad R Sadeghi Ghuchani M Eslaminejad MB Taghiyar L Kalhori KA Pedram MS et al The effects of combined low level laser therapy and mesenchymal stem cells on bone regeneration in rabbit calvarial defects. J Photochem Photobiol B. (2015) 151:180–5. 10.1016/j.jphotobiol.2015.08.002
86.
Breit R Van der Wall H . Transcutaneous electrical nerve stimulation for postoperative pain relief after total knee arthroplasty.J Arthroplasty. (2004) 19:45–8. 10.1016/s0883-5403(03)00458-3
87.
Sluka KA Bjordal JM Marchand S Rakel BA . What makes transcutaneous electrical nerve stimulation work? Making sense of the mixed results in the clinical literature.Phys Ther. (2013) 93:1397–402. 10.2522/ptj.20120281
88.
Kalra A Urban MO Sluka KA . Blockade of opioid receptors in rostral ventral medulla prevents antihyperalgesia produced by transcutaneous electrical nerve stimulation (TENS).J Pharmacol Exp Ther. (2001) 298:257–63. 10.1016/s0022-3565(24)29376-9
89.
Lord SR Murray SM Chapman K Munro B Tiedemann A . Sit-to-stand performance depends on sensation, speed, balance, and psychological status in addition to strength in older people.J Gerontol A Biol Sci Med Sci. (2002) 57:M539–43. 10.1093/gerona/57.8.m539
90.
Sluka KA Walsh D . Transcutaneous electrical nerve stimulation: basic science mechanisms and clinical effectiveness.J Pain. (2003) 4:109–21. 10.1054/jpai.2003.434
91.
Hussein HM Alshammari RS Al-Barak SS Alshammari ND Alajlan SN Althomali OWA . Systematic review and meta-analysis investigating the pain-relieving effect of interferential current on musculoskeletal pain.Am J Phys Med Rehabil. (2022) 101:624–33. 10.1097/phm.0000000000001870
92.
Fuentes JP Armijo Olivo S Magee DJ Gross DP . Effectiveness of interferential current therapy in the management of musculoskeletal pain: a systematic review and meta-analysis.Phys Ther. (2010) 90:1219–38. 10.2522/ptj.20090335
93.
Rampazo PÉ Liebano RE . Analgesic effects of interferential current therapy: a narrative review.Medicina. (2022) 58:141. 10.3390/medicina58010141
94.
Urita A Iwasaki N Kondo M Nishio Y Kamishima T Minami A . Effect of low-intensity pulsed ultrasound on bone healing at osteotomy sites after forearm bone shortening.J Hand Surg Am. (2013) 38:498–503. 10.1016/j.jhsa.2012.11.032
95.
Nolte PA Klein-Nulend J Albers GH Marti RK Semeins CM Goei SW et al Low-intensity ultrasound stimulates endochondral ossification in vitro. J Orthop Res. (2001) 19:301–7. 10.1016/s0736-0266(00)00027-9
96.
Thompson Z Miclau T Hu D Helms JAA . model for intramembranous ossification during fracture healing.J Orthop Res. (2002) 20:1091–8. 10.1016/s0736-0266(02)00017-7
97.
Chen YJ Wang CJ Yang KD Chang PR Huang HC Huang YT et al Pertussis toxin-sensitive Galphai protein and ERK-dependent pathways mediate ultrasound promotion of osteogenic transcription in human osteoblasts. FEBS Lett. (2003) 554:154–8. 10.1016/s0014-5793(03)01157-8
98.
Harrison A Lin S Pounder N Mikuni-Takagaki Y . Mode & mechanism of low intensity pulsed ultrasound (LIPUS) in fracture repair.Ultrasonics. (2016) 70:45–52. 10.1016/j.ultras.2016.03.016
99.
Tang CH Yang RS Huang TH Lu DY Chuang WJ Huang TF et al Ultrasound stimulates cyclooxygenase-2 expression and increases bone formation through integrin, focal adhesion kinase, phosphatidylinositol 3-kinase, and Akt pathway in osteoblasts. Mol Pharmacol. (2006) 69:2047–57. 10.1124/mol.105.022160
100.
Yang RS Lin WL Chen YZ Tang CH Huang TH Lu BY et al Regulation by ultrasound treatment on the integrin expression and differentiation of osteoblasts. Bone. (2005) 36:276–83. 10.1016/j.bone.2004.10.009
101.
Ardan NI Janes JM Herrick JF . Ultrasonic energy and surgically produced defects in bone.J Bone Joint Surg Am. (1957) 39-A:394–402. 10.2106/00004623-195739020-00013
102.
Bender LF Janes JM Herrick JF . Histologic studies following exposure of bone to ultrasound.Arch Phys Med Rehabil. (1954) 35:555–9.
103.
Warden SJ Fuchs RK Kessler CK Avin KG Cardinal RE Stewart RL . Ultrasound produced by a conventional therapeutic ultrasound unit accelerates fracture repair.Phys Ther. (2006) 86:1118–27. 10.1093/ptj/86.8.1118
104.
Watanabe Y Matsushita T Bhandari M Zdero R Schemitsch EH . Ultrasound for fracture healing: current evidence.J Orthop Trauma. (2010) 24:S56–61. 10.1097/BOT.0b013e3181d2efaf
105.
Lai CH Chuang CC Li JK Chen SC Chang WH . Effects of ultrasound on osteotomy healing in a rabbit fracture model.Ultrasound Med Biol. (2011) 37:1635–43. 10.1016/j.ultrasmedbio.2011.06.014
106.
Yang KH Parvizi J Wang SJ Lewallen DG Kinnick RR Greenleaf JF et al Exposure to low-intensity ultrasound increases aggrecan gene expression in a rat femur fracture model. J Orthop Res. (1996) 14:802–9. 10.1002/jor.1100140518
107.
Tam KF Cheung WH Lee KM Qin L Leung KS . Osteogenic effects of low-intensity pulsed ultrasound, extracorporeal shockwaves and their combination - an in vitro comparative study on human periosteal cells.Ultrasound Med Biol. (2008) 34:1957–65. 10.1016/j.ultrasmedbio.2008.06.005
108.
Pounder NM Harrison AJ . Low intensity pulsed ultrasound for fracture healing: a review of the clinical evidence and the associated biological mechanism of action.Ultrasonics. (2008) 48:330–8. 10.1016/j.ultras.2008.02.005
109.
Rawool NM Goldberg BB Forsberg F Winder AA Hume E . Power Doppler assessment of vascular changes during fracture treatment with low-intensity ultrasound.J Ultrasound Med. (2003) 22:145–53. 10.7863/jum.2003.22.2.145
110.
Palanisamy P Alam M Li S Chow SKH Zheng YP . Low-intensity pulsed ultrasound stimulation for bone fractures healing: a review.J Ultrasound Med. (2022) 41:547–63. 10.1002/jum.15738
111.
Kokubu T Matsui N Fujioka H Tsunoda M Mizuno K . Low intensity pulsed ultrasound exposure increases prostaglandin E2 production via the induction of cyclooxygenase-2 mRNA in mouse osteoblasts.Biochem Biophys Res Commun. (1999) 256:284–7. 10.1006/bbrc.1999.0318
112.
Yuan J Xin F Jiang W . Underlying signaling pathways and therapeutic applications of pulsed electromagnetic fields in bone repair.Cell Physiol Biochem. (2018) 46:1581–94. 10.1159/000489206
113.
Peng L Fu C Xiong F Zhang Q Liang Z Chen L et al Effectiveness of pulsed electromagnetic fields on bone healing: a systematic review and meta-analysis of randomized controlled trials. Bioelectromagnetics. (2020) 41:323–37. 10.1002/bem.22271
114.
Bagheri L Pellati A Rizzo P Aquila G Massari L De Mattei M et al Notch pathway is active during osteogenic differentiation of human bone marrow mesenchymal stem cells induced by pulsed electromagnetic fields. J Tissue Eng Regen Med. (2018) 12:304–15. 10.1002/term.2455
115.
Ehnert S Falldorf K Fentz AK Ziegler P Schröter S Freude T et al Primary human osteoblasts with reduced alkaline phosphatase and matrix mineralization baseline capacity are responsive to extremely low frequency pulsed electromagnetic field exposure - Clinical implication possible. Bone Rep. (2015) 3:48–56. 10.1016/j.bonr.2015.08.002
116.
Kuzyk PR Schemitsch EH . The science of electrical stimulation therapy for fracture healing.Indian J Orthop. (2009) 43:127–31. 10.4103/0019-5413.50846
117.
Selvamurugan N He Z Rifkin D Dabovic B Partridge NC . Pulsed electromagnetic field regulates MicroRNA 21 expression to activate TGF-β signaling in human bone marrow stromal cells to enhance osteoblast differentiation.Stem Cells Int. (2017) 2017:2450327. 10.1155/2017/2450327
118.
Cadossi R Massari L Racine-Avila J Aaron RK . Pulsed electromagnetic field stimulation of bone healing and joint preservation: cellular mechanisms of skeletal Response.J Am Acad Orthop Surg Glob Res Rev. (2020) 4:e1900155. 10.5435/JAAOSGlobal-D-19-00155
119.
Ribatti D . The discovery of the placental growth factor and its role in angiogenesis: a historical review.Angiogenesis. (2008) 11:215–21. 10.1007/s10456-008-9114-4
120.
Kermani P Hempstead B . Brain-derived neurotrophic factor: a newly described mediator of angiogenesis.Trends Cardiovasc Med. (2007) 17:140–3. 10.1016/j.tcm.2007.03.002
121.
Parhampour B Torkaman G Hoorfar H Hedayati M Ravanbod R . Effects of short-term resistance training and pulsed electromagnetic fields on bone metabolism and joint function in severe haemophilia A patients with osteoporosis: a randomized controlled trial.Clin Rehabil. (2014) 28:440–50. 10.1177/0269215513505299
122.
Rossini M Viapiana O Gatti D de Terlizzi F Adami S . Capacitively coupled electric field for pain relief in patients with vertebral fractures and chronic pain.Clin Orthop Relat Res. (2010) 468:735–40. 10.1007/s11999-009-1088-z
123.
Brighton CT Wang W Seldes R Zhang G Pollack SR . Signal transduction in electrically stimulated bone cells.J Bone Joint Surg Am. (2001) 83:1514–23. 10.2106/00004623-200110000-00009
124.
Clark CC Wang W Brighton CT . Up-regulation of expression of selected genes in human bone cells with specific capacitively coupled electric fields.J Orthop Res. (2014) 32:894–903. 10.1002/jor.22595
125.
Zhuang H Wang W Seldes RM Tahernia AD Fan H Brighton CT . Electrical stimulation induces the level of TGF-beta1 mRNA in osteoblastic cells by a mechanism involving calcium/calmodulin pathway.Biochem Biophys Res Commun. (1997) 237:225–9. 10.1006/bbrc.1997.7118
126.
Wu X Zeng LH Taniguchi T Xie QM . Activation of PKA and phosphorylation of sodium-dependent vitamin C transporter 2 by prostaglandin E2 promote osteoblast-like differentiation in MC3T3-E1 cells.Cell Death Differ. (2007) 14:1792–801. 10.1038/sj.cdd.4402190
127.
Brighton CT Hozack WJ Brager MD Windsor RE Pollack SR Vreslovic EJ et al Fracture healing in the rabbit fibula when subjected to various capacitively coupled electrical fields. J Orthop Res. (1985) 3:331–40. 10.1002/jor.1100030310
128.
Zhang L Fu XB Chen S Zhao ZB Schmitz C Weng CS . Efficacy and safety of extracorporeal shock wave therapy for acute and chronic soft tissue wounds: a systematic review and meta-analysis.Int Wound J. (2018) 15:590–9. 10.1111/iwj.12902
129.
Ahmed Z Wieraszko A . Combined effects of acrobatic exercise and magnetic stimulation on the functional recovery after spinal cord lesions.J Neurotrauma. (2008) 25:1257–69. 10.1089/neu.2008.0626
130.
Pain Medicine Branch of Chinese Medical Doctor Association, National Key Clinical Specialty, China-Japan Friendship Hospital Pain Specialist Medical Alliance, Beijing Pain Treatment Quality Control and Improvement Center. 慢性肌肉骨骼疼痛的药物治疗专家共识 [Expert consensus on drug treatment of chronic musculoskeletal pain]. 中国疼痛医学杂志 [Chinese J Pain Med]. (2018) 24: 881–7. Chinese.
Summary
Keywords
physical agent modalities, fracture, bone healing, pain relief, network meta-analysis
Citation
Li L, Yang L, Yang Y, Zhu J, Shi R, Deng Q, Wang J and Sun F (2025) Evaluation of the efficacy of physical agent modalities in patients with fractures: a systematic review and network meta-analysis. Front. Med. 12:1646903. doi: 10.3389/fmed.2025.1646903
Received
14 June 2025
Accepted
16 October 2025
Published
29 October 2025
Volume
12 - 2025
Edited by
Pedro Morouço, Clínica Espregueira - FIFA Medical Centre of Excellence, Portugal
Reviewed by
Federica Buccino, Fondazione Politecnico di Milano, Italy
Jonathan Koa, Thomas Jefferson University, United States
Updates
Copyright
© 2025 Li, Yang, Yang, Zhu, Shi, Deng, Wang and Sun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jianxiong Wang, jianxiongwang_swmu@126.comFuhua Sun, sunfuhua330@163.com
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.