- 1Heilongjiang University of Chinese Medicine, Harbin, China
- 2Department of Epidemiology and Public Health, University College London, London, United Kingdom
- 3Ordos Hospital of Traditional Chinese Medicine, Ordos, China
- 4Department of Critical Care Medicine, The First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, China
Objective: This study seeks to examine the dynamics of endogenous interleukin-7 (IL-7) and its associated regulatory factors in sepsis, and to elucidate the mechanisms by which exogenous IL-7 may confer therapeutic benefits. The ultimate objective is to evaluate its dual potential as a clinical biomarker and as a novel therapeutic agent.
Method: We searched PubMed, Embase, and Web of Science from inception to April 24, 2025, using terms such as Interleukin-7, CD127, CYT107, interleukin-7 receptor, sepsis, septic shock, and lymphopenia.
Results: In sepsis, endogenous IL-7 levels rise from a low baseline and may remain elevated for a prolonged period. Exogenous IL-7 can enhance immune function, regulate inflammation, and exert anti-apoptotic effects. Endogenous IL-7 levels may represent a potential prognostic indicator in sepsis. Exogenous IL-7 may modulate immune function in patients with clinical sepsis but does not reduce mortality.
Conclusion: Endogenous IL-7 is closely associated with sepsis, whereas exogenous IL-7 shows promise for aiding the recovery of patients with sepsis, although further research is required.
1 Introduction
Sepsis is a serious disease associated with high morbidity, characterized by severe organ dysfunction induced by infection (1–4). Sepsis troubled 48.9 million people globally, causing 11.0 million deaths and a major socioeconomic burden (5). The prevalence was 22.4%, with 20.9% in low- and lower-middle-income countries. Kidney injury is a common complication, occurring in approximately 18% of cases (6, 7). Sepsis typically arises from infections, sterile inflammation, autoimmune diseases, and cancers (8). Smoking, alcohol consumption, and vitamin D deficiency are common risk factors for sepsis (8). Recently, studies have comprehensively examined the rehospitalization of sepsis patients, identifying aging and male gender as high-risk factors (9). Sepsis can be divided into adult and pediatric forms based on age (3, 4). Additionally, it can be classified by complications, such as respiratory, brain, and kidney sepsis, among others (10). In the affected organs, the primary pathological features include endothelial changes, high glycolysis, microcirculatory dysfunction, barrier damage, and immune system dysfunction. At the molecular level, the pathological mechanisms involve inflammation, oxidative stress, complement activation, and metabolic dysfunction (8, 11–14). Among these changes, dysfunction of the immune system represents a key pathological alteration during the course of sepsis.
Early screening for sepsis is crucial for reducing mortality. Recommended methods include using SOFA in combination with SIRS or NEWS as a single screening tool (15). However, the prognosis of sepsis is difficult to predict owing to the heterogeneity of the condition at the individual patient level (16). In the management of sepsis, fluid resuscitation and antibiotics remain the cornerstone of therapy, with corticosteroids administered as an adjunct in selected cases (17, 18). However, the bacterial drug resistance is a significant challenge. Fluid therapy is merely a basic approach to alleviate symptoms and reduce pathogen loads. Moreover, corticosteroids may exacerbate immune dysfunction (19, 20). Therefore, additional prognostic markers and therapeutic agents need to be explored.
Interleukin-7 (IL-7), a 25 kDa protein, could be expressed in a general tissue but the lymph nodes, liver, lung, and skin are high. In the cellular sources, the epithelial and endothelial cells are identified as sources (21). The IL-7 receptor (IL-7R) consists of α-subunit (CD127) and γ-chain, and is expressed in CD4+ and CD8+ T cells (21–23).
Several studies have demonstrated a strong association between IL-7, IL-7R, and immune function. HIV patients typically exhibit decreased IL-7R levels and impaired endogenous IL-7 function. In healthy individuals, endogenous IL-7 is present at significant concentrations and can increase following antiretroviral therapy in HIV patients (24, 25). These results suggest that endogenous IL-7 and IL-7R may hold value as prognostic markers in sepsis. In recent years, immunotherapy has attracted growing attention (26, 27) and exogenous IL-7 has shown potential as an immunotherapeutic agent. Exogenous IL-7 can enhance lymphocyte function through various pathways (24, 25, 28–35). COVID-19 has recently become a well-known virus. The administration of exogenous IL-7 could potentially benefit patients suffering from COVID-19 and other viral infections (36–41). Sepsis patients often have compromised immune function, and several studies have shown that IL-7 can regulate immune by enhancing T cell activity (42–44). Moreover, IL-7R appears to be associated with sepsis mortality (45). The potential of IL-7 in treating sepsis is promising.
2 Methods
We searched PubMed, Embase, and WOS from inception to April 24, 2025, for a comprehensive review. We used a combination of search terms, including “Interleukin-7,” “CD127,” “CYT107,” “Interleukin-7 receptor,” “sepsis,” “septic shock,” and “lymphopenia.” These terms were carefully selected to capture a wide range of relevant studies and articles related to IL-7 and its role in sepsis. We included animal experiments, ex vivo preclinical trials, and clinical trials, without language restrictions, to explore changes in endogenous IL-7, IL-7R, and related factors in sepsis, as well as the role of exogenous IL-7 in this condition, with the aim of providing greater reliability for the clinical application of IL-7 and IL-7R in sepsis. Articles excluded from the analysis comprised case reports, reviews, and studies in which sepsis was not the primary disease. No methods were employed to assess either the robustness of the data synthesis or the certainty of the evidence.
3 Results
3.1 The change of IL-7 and relevant factors in sepsis
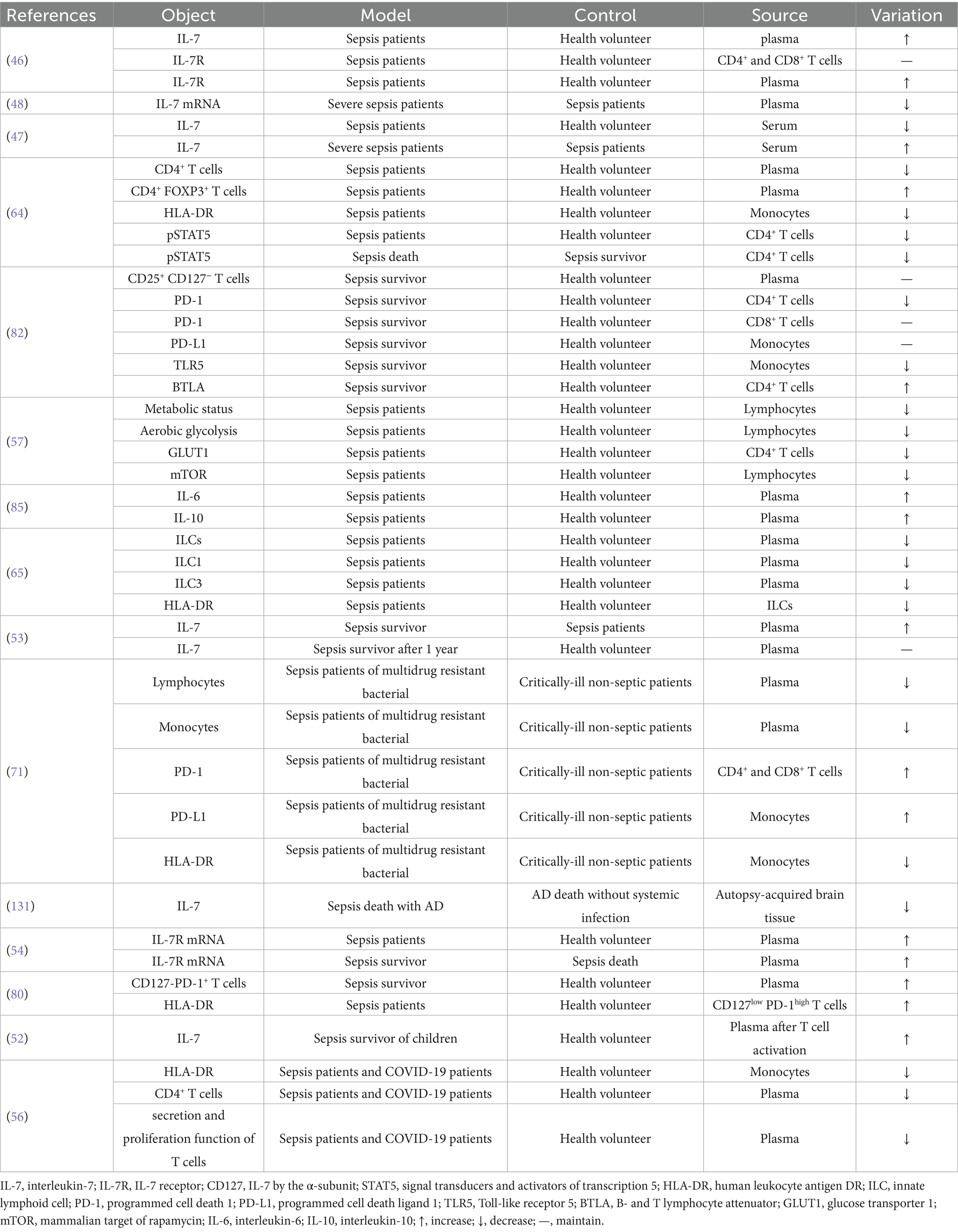
We included 16 studies examining changes in endogenous IL-7 and related factors (Table 1). When sepsis occurs, the immune system is often suppressed. Sepsis patients exhibit substantial alterations in IL-7, IL-7R, and associated factors (Figure 1).
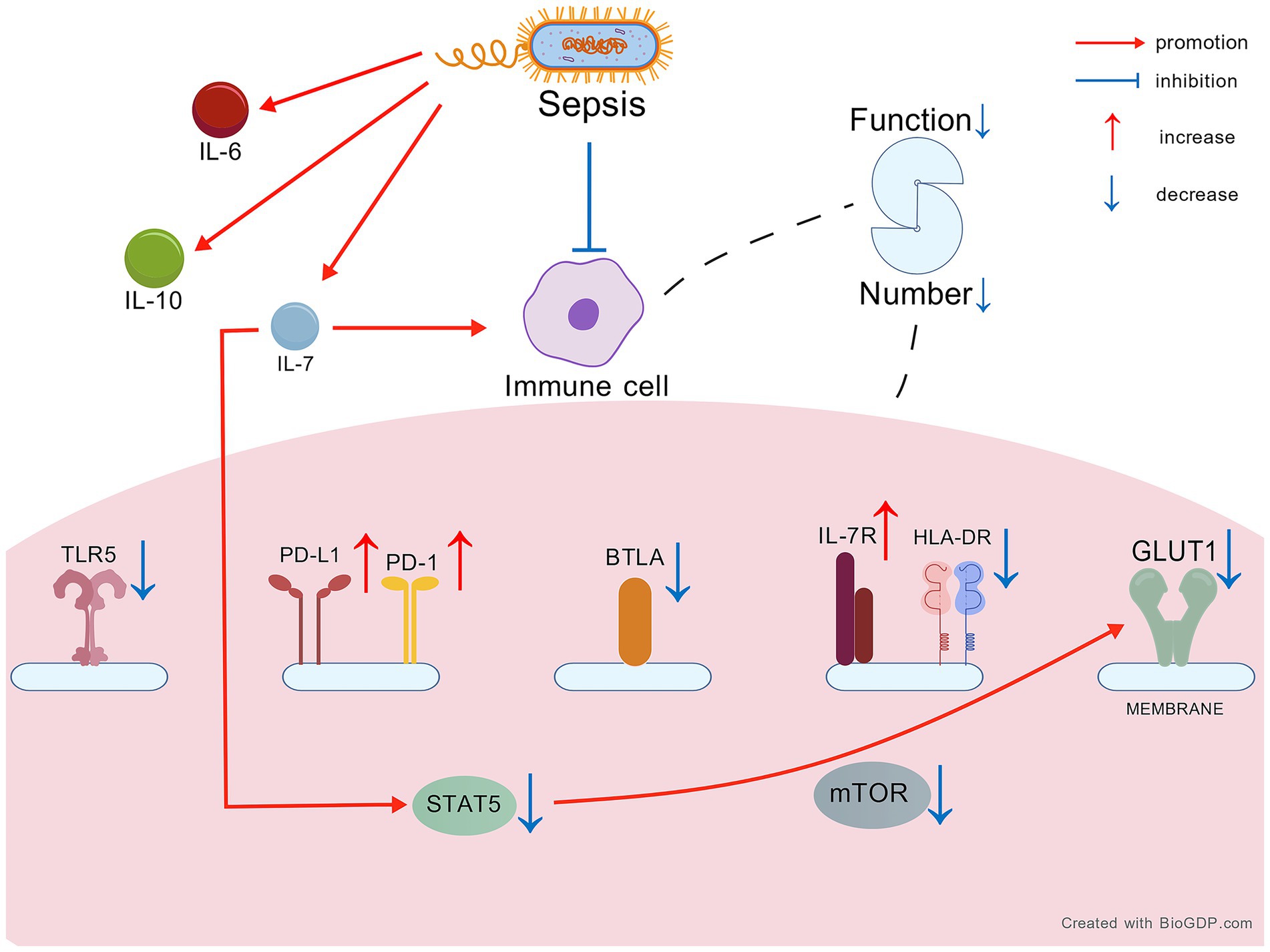
Figure 1. The change of IL-7 and relevant factors in sepsis. Created with BioGDP.com (130). IL-7, interleukin-7; IL-7R, IL-7 receptor; STAT5, signal transducers and activators of transcription 5; HLA-DR, human leukocyte antigen DR; PD-1, programmed cell death 1; PD-L1, programmed cell death ligand 1; TLR5, Toll-like receptor 5; BTLA, B- and T lymphocyte attenuator; GLUT1, glucose transporter 1; mTOR, mammalian target of rapamycin; IL-6, interleukin-6; IL-10, interleukin-10.
3.1.1 Elevated IL-7 and IL-7R in the plasma of sepsis patients from a low baseline
Compared to healthy individuals, IL-7 in the plasma of sepsis patients slightly increase within 1–4 days (46). However, IL-7 levels are relatively low compared with those in healthy individuals prior to any therapeutic intervention. Patients with severe sepsis exhibit higher IL-7 concentrations than those with uncomplicated sepsis, yet these levels remain lower than in healthy controls before treatment. Interestingly, patients with severe sepsis display lower blood IL-7 mRNA expression than individuals with less severe disease (47, 48). Moreover, individuals with Alzheimer’s disease who die from sepsis have lower IL-7 levels in the brain (49–51). During sepsis, IL-7R levels increase, particularly on CD4+ and CD8+ T cells, and remain elevated for 1–4 days (46). In patients with sepsis, IL-7R mRNA levels are higher than in healthy individuals. Overall, IL-7 exhibits marked fluctuations during sepsis. Its plasma concentration rises sharply within the first 1–4 days and remains elevated from a low baseline, compared with healthy controls, at the initiation of conventional treatment; a similar pattern is observed for IL-7R.
3.1.2 The higher levels of plasma IL-7 and IL-7R in sepsis survivors than health
In sepsis survivors, IL-7 levels can rise and remain comparable to those of healthy individuals for up to one year. Similarly, pediatric sepsis survivors often have higher IL-7 levels compared to healthy children (52, 53). Additionally, plasma IL-7R concentrations are higher in sepsis survivors than in those who succumb to the condition, at least 2 days after onset (54). This suggests that, although patients with sepsis have impaired IL-7 function, they are still capable of secreting higher amounts of IL-7, likely as a result of epithelial and endothelial cell activation (54, 55). Additionally, immune dysfunction in sepsis may impair the clearance of IL-7, thereby contributing to its sustained levels. Survival in patients with sepsis may be associated with higher numbers of active lymphocytes and elevated IL-7 concentrations compared with healthy individuals.
3.1.3 The change of relevant factors in sepsis
3.1.3.1 The metabolic and proliferation disorder of immune system
Aerobic glycolysis is a crucial pathway for the growth and differentiation of lymphocytes (55). In sepsis patients, lymphocyte metabolism and aerobic glycolysis are impaired, and T cell secretion and proliferation functions are diminished (56, 57), indicating lymphocyte dysfunction. IL-7 can activate signal transducers and activators of transcription 5 (STAT5) to promote lymphocyte function (58–63). In sepsis patients, p-STAT5 are reduced in CD4+ T cells and lower in those who die from sepsis compared to survivors (64). The STAT5 is also the key of aerobic glycolysis for naïve CD4+ T cells (65, 66) and the higher expression of glycolysis gene could be activated by STAT5 (67, 68). Glucose transporter 1 (GLUT1), a key protein for aerobic glycolysis, is decreased in CD4+ T cells of sepsis (69). The low levels of GLUT1 and aerobic glycolysis could be caused by the low STAT5 in sepsis (64). Mammalian target of rapamycin (mTOR) is also decreased in lymphocytes of sepsis patients (59, 70). Additionally, sepsis patients with multidrug-resistant bacterial infections exhibit lower counts of lymphocytes and monocytes than critically ill non-septic patients (71). CD4+ T cells are the key and IL-7 is essential for their differentiation (72), suggesting that higher IL-7 levels could promote more CD4+ T cells. However, the number of CD4+ T cells of sepsis or COVID-19 patients is often lower at 3–4 days after onset (56, 64). IL-7 can promote the function of naive CD4+FOXP3+ T cells, a subset of CD4+ T cells (73). The proportion of CD4+ FOXP3+ T cells is elevated in sepsis patients. An early rise in these cells among ICU patients is believed to have an adverse effect on sepsis outcomes (64, 74). CD25+ CD127− T cells are detrimental to sepsis patients. In HIV patients, these cells can be reduced and restored by IL-7, whereas in sepsis patients, their levels tend to remain elevated (75–77). Toll-like receptor 5 (TLR5) can exacerbate sepsis and is decreased in monocytes of sepsis survivors (78, 79). In conclusion, the metabolism and proliferation of immune cells may be impaired, even in the presence of elevated IL-7 concentrations.
3.1.3.2 The immune cells dysfunction
Human leukocyte antigen-DR (HLA-DR) serves as an indicator of immune system function. Its expression is reduced in monocytes and innate lymphoid cells (ILCs) of patients with sepsis and COVID-19, but increased in CD127low PD-1high T cells in sepsis (56, 64–66, 71, 80). Low programmed cell death 1 (PD-1) can mitigate sepsis-related damage. PD-1 is decreased in CD4+ T cells but remains elevated in CD8+ T cells. Meanwhile, programmed cell death ligand 1 (PD-L1) levels are maintained in monocytes of sepsis survivors (67, 68, 78, 79, 81). However, in sepsis patients with drug-resistant bacterial infections, PD-1 levels are increased in CD4+ and CD8+ T cells compared to critically ill non-septic patients. Similarly, PD-L1 levels are elevated in monocytes of these sepsis patients (71).
3.1.3.3 Decompensated protective response
B- and T-lymphocyte attenuator (BTLA) plays a role in suppressing cytokine storms. Its expression is increased in CD4⁺ T cells of sepsis survivors (70, 82–84). Low interleukin-6 (IL-6) and high interleukin-10 (IL-10) levels are associated with improved outcomes in sepsis. However, both cytokines are frequently elevated in patients with sepsis (85–90), suggesting that the increase in IL-10 represents a compensatory response that is insufficient to prevent disease progression (65, 91, 92).
3.2 The mechanisms of exogenous IL-7 in sepsis
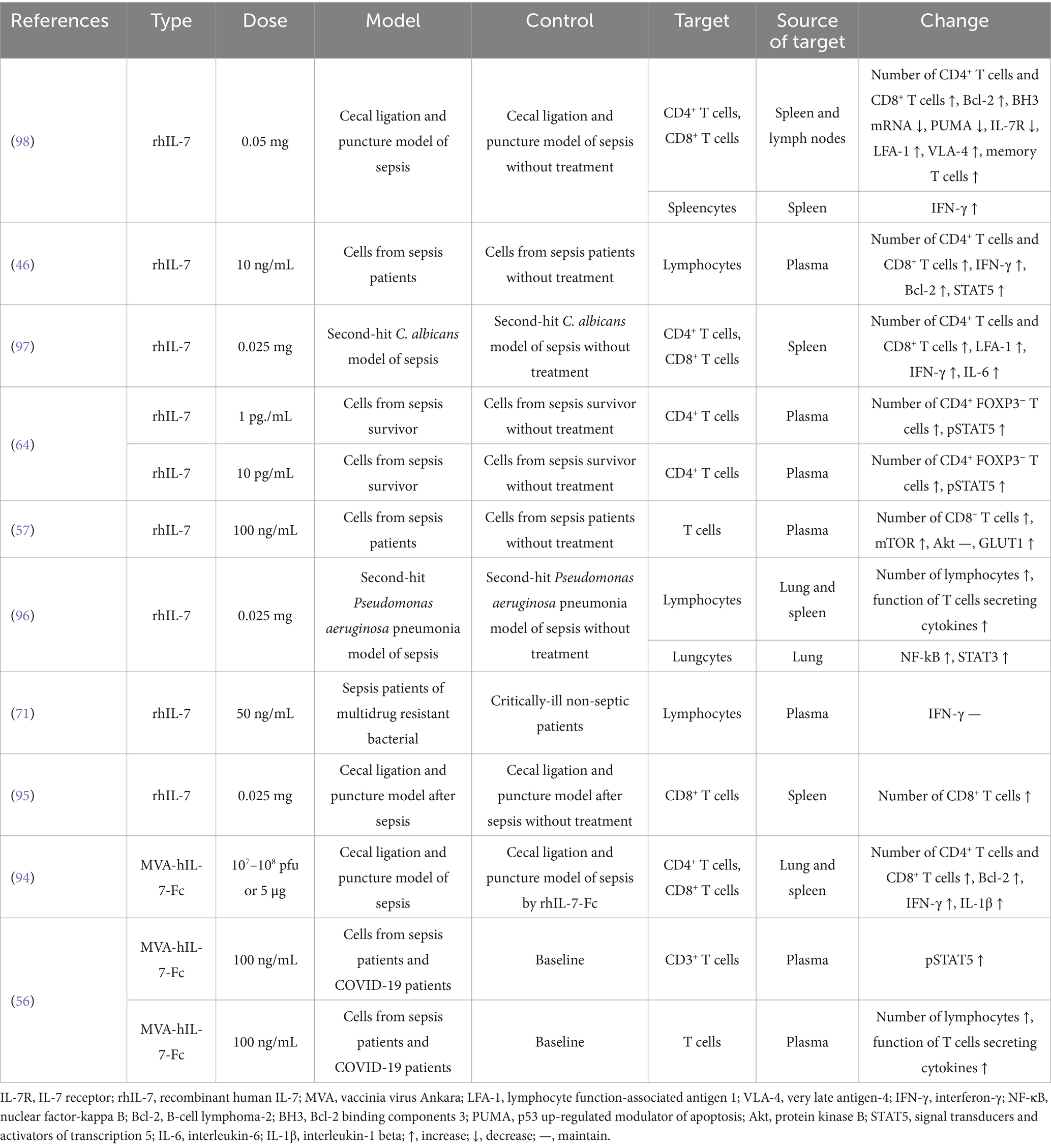
We included 10 studies investigating the potential therapeutic mechanisms of exogenous IL-7 in sepsis (Table 2). Recombinant human IL-7 (rhIL-7) is widely used as a research agent. A newer type is the vaccinia virus Ankara (MVA)-human IL-7 (hIL-7)-Fc protein. This study builds on a 2021 systematic review that detailed the functions of IL-7 in sepsis and identified key molecular changes (93). The exogenous IL-7 to the bloodstream can help identify potential cures for immune system dysfunction and regulate inflammatory factors as well as inhibit apoptosis (Figure 2).
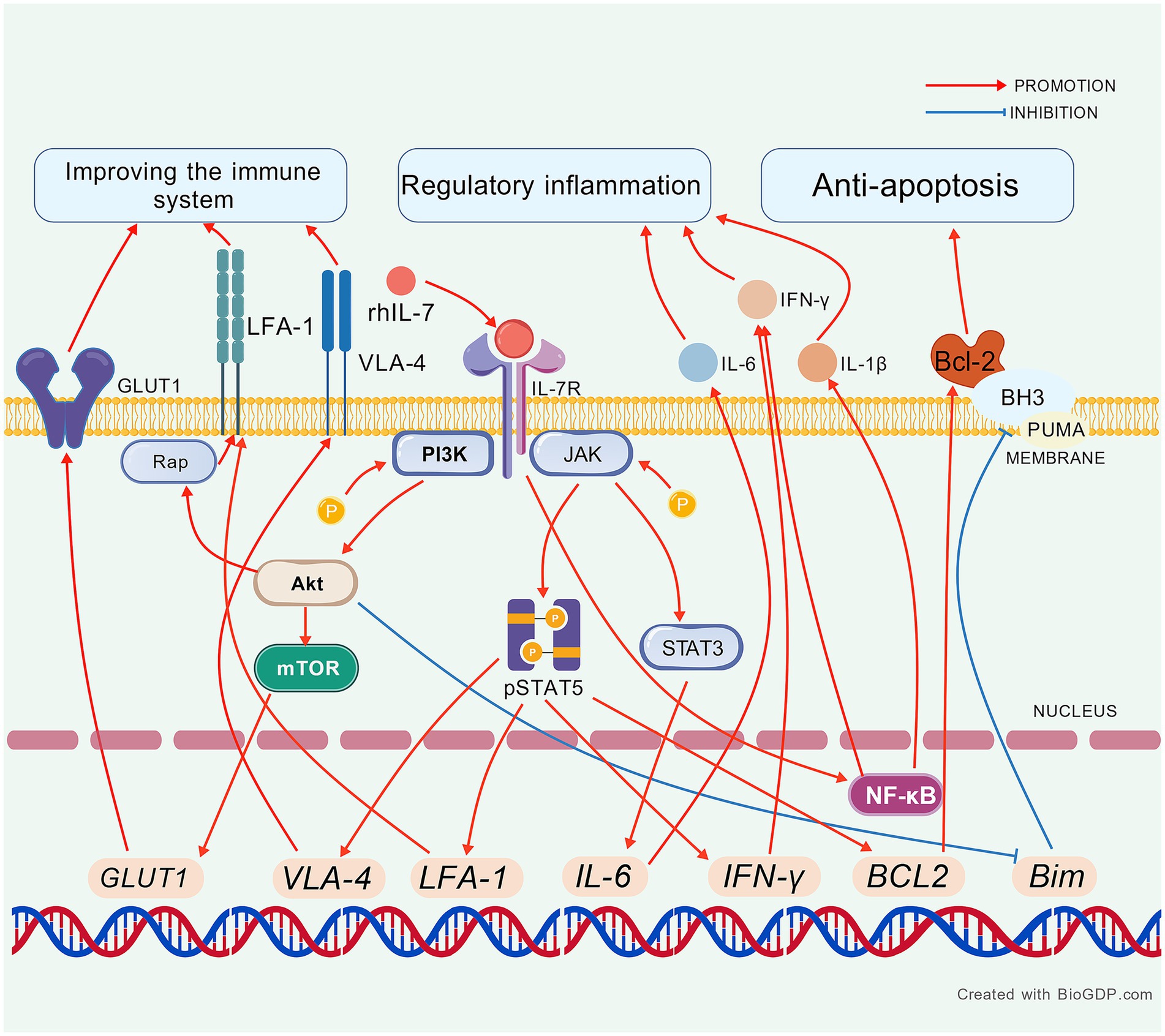
Figure 2. The mechanism of exogenous IL-7 in sepsis. Created with BioGDP.com (130). rhIL-7, recombinant human IL-7; LFA-1, lymphocyte function-associated antigen 1; VLA-4, very late antigen-4; IFN-γ, interferon-γ; NF-κB, nuclear factor-kappa B; Bcl-2, B-cell lymphoma-2; BH3, Bcl-2 binding components 3; PUMA, p53 up-regulated modulator of apoptosis; STAT5/3, signal transducers and activators of transcription 5/3; IL-6, interleukin-6; IL-1β, interleukin-1 beta; Rap-1, Ras-related protein 1.
3.2.1 Improving the immune system
Several studies have reported increased lymphocyte counts in the spleen and lymph nodes of mouse models (94–98), with corresponding increases in CD4⁺ and CD8⁺ T-cell subsets. In vitro studies using plasma from patients with sepsis have demonstrated an increase in lymphocytes following treatment with rhIL-7 (46, 56, 57, 64). Memory T cells are vital T cell subsets, and rhIL-7 can aid in their preservation in sepsis (98). Lymphocyte function-associated antigen 1 (LFA-1) is leukocyte adhesion marker involved in cell migration also very late antigen-4 (VLA-4). In mice, these markers can be enhanced by rhIL-7 via protein kinase B (Akt)/Ras-related protein 1 (Rap-1) and STAT5 (97–100). The rhIL-7 not only increases the number and migratory capacity of lymphocytes but also enhances their aerobic glycolysis and secretory functions, in both animal models and human blood (46, 56, 57, 96, 97). Overall, the therapeutic use of rhIL-7 shows great promise in combating sepsis-induced lymphopenia and immune dysfunction.
3.2.2 Regulatory inflammation
Interferon-γ (IFN-γ) has an important function for activating the cellular immunity and IL-7 could induce it to regulate the immune system (101–104). IFN-γ was increased in spleen of sepsis mice also in blood of sepsis patients, survivors, and COVID-19 patients by rhIL-7 and MVA-hIL-7-Fc. However, the increased IFN-γ in sepsis is same compared the critically-ill non-septic patients (46, 71, 94, 96, 97). The nuclear factor-kappa B (NF-κB) interacts with IL-7 and promote the inflammation, could be increased in sepsis mice by rhIL-7 (96, 105–108). IL-6 is the dependent cytokine for the viral clearance of IL-7, which could be increased in spleen of sepsis mice by rhIL-7 (97, 109). The increased interleukin-1 beta (IL-1β) and IL-6 could be found in spleen and lung of sepsis mice and the higher levels could be seen by MVA-hIL-7-Fc versus by rhIL-7, which could regulate the function of IL-7 (94, 110, 111).
3.2.3 Anti apoptosis
IL-7 promotes thymocyte proliferation via B-cell lymphoma-2 (Bcl-2) in T cells derived from septic animals and human sources. MVA-hIL-7-Fc can induce higher Bcl-2 levels than rhIL-7 in the lungs and spleens of sepsis mice (46, 94, 98). Bcl-2 binding components 3 (BH3) and p53 up-regulated modulator of apoptosis (PUMA) are pro-apoptotic factors in sepsis. These factors can be decreased by rhIL-7 in mouse models (98, 112, 113). STAT3 and STAT5 are key pathways involved in apoptosis. Both rhIL-7 and MVA-hIL-7-Fc can increase these pathways in sepsis mice or the blood of sepsis patients (46, 56, 96). According to the above text, the STAT5 also is the key of glycolysis and histone lactylation (63, 114–116) and the change of immune function could attribute to the higher STAT5. The mTOR is another pathway involved in apoptosis via IL-7. It is increased in the blood of sepsis patients, while Akt levels remain unchanged (57, 117). In short, IL-7 therapies combat sepsis-induced lymphocyte apoptosis and metabolic dysfunction by modulating key survival and signaling pathways.
3.3 The clinical application of IL-7 in sepsis
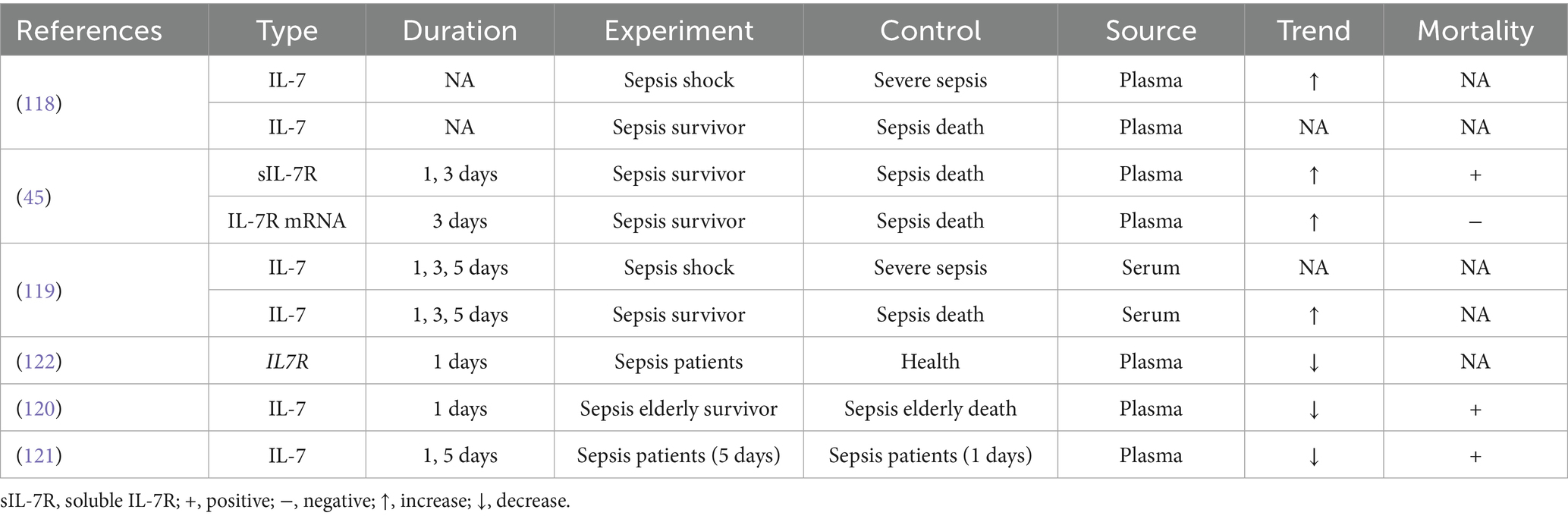
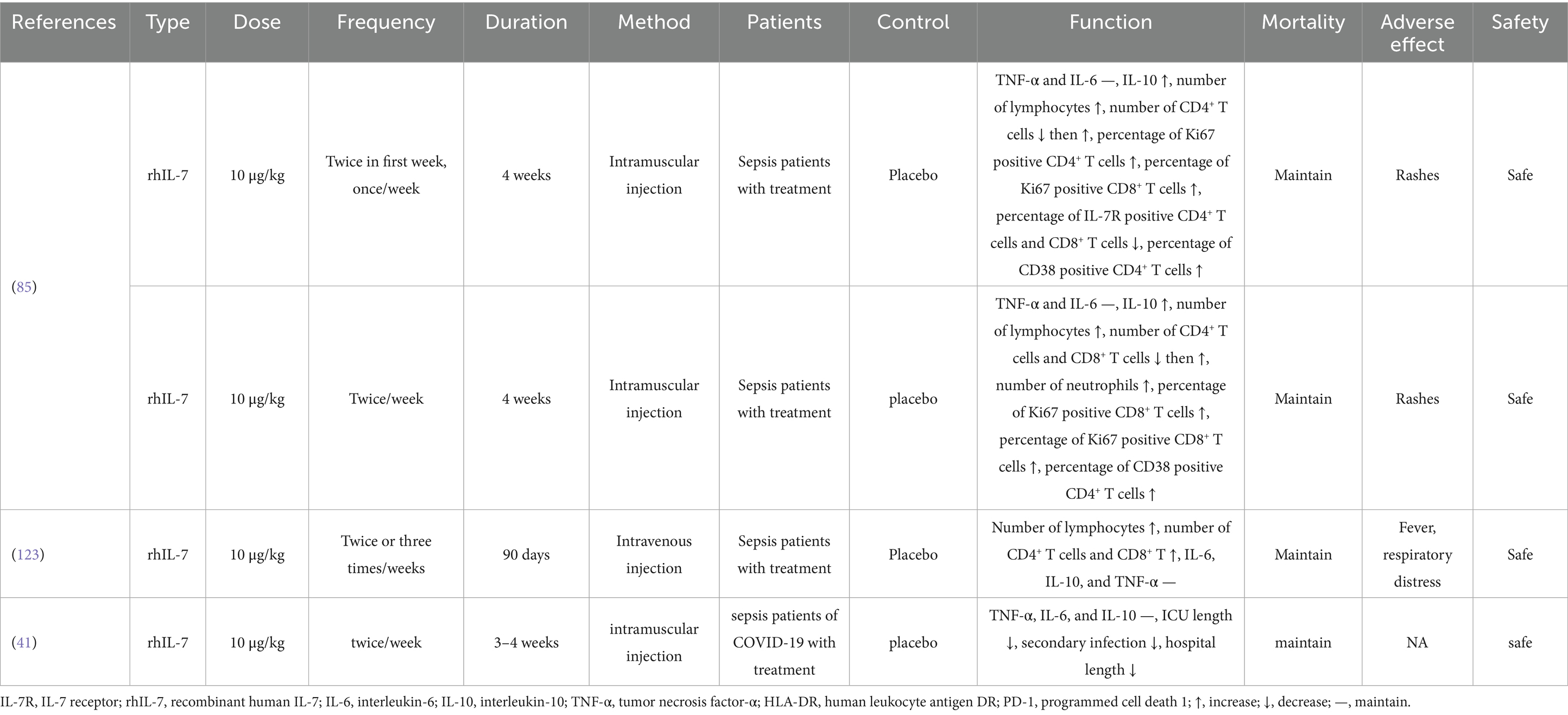
We included nine studies investigating the clinical application of IL-7 in sepsis (Tables 3, 4). We found that endogenous IL-7 in the blood may serve as a marker for assessing disease severity and prognosis in elderly patients with sepsis. Exogenous IL-7, such as rhIL-7, may represent a potential immunotherapeutic agent.
3.3.1 As the prognosis marker of sepsis
Endogenous IL-7 levels vary between septic shock and severe sepsis patients, yet no significant difference is observed between sepsis survivors and non-survivors in plasma (118). One study revealed a change in serum IL-7 between sepsis survivors and non-survivors, but it cannot be regarded as a mortality marker (119). Interestingly, we found that endogenous IL-7 levels differ between elderly sepsis survivors and elderly sepsis deaths, with higher endogenous IL-7 associated with higher mortality (120). Among patients with sepsis, lower plasma levels of endogenous IL-7 may be associated with increased mortality over time (121). Bioinformatics analysis suggests that IL-7R may also serve as an underlying marker for sepsis (122). Soluble IL-7R (sIL-7R) levels differ between sepsis survivors and sepsis deaths in plasma, with higher sIL-7R associated with higher mortality. In contrast, IL-7 mRNA levels in plasma have an inverse relationship with mortality (45). Overall, endogenous IL-7 may serve as a prognostic marker in elderly patients with sepsis, whereas sIL-7R may be used to evaluate prognosis in the general sepsis population. Reduced IL-7 mRNA levels inversely correlate with survival, hinting at transcriptional suppression in fatal cases.
3.3.2 As the immunotherapy agent in sepsis
The typical dose of rhIL-7 is 10 μg/kg, administered once or twice weekly. Francois et al. (85) reported that intramuscular administration of rhIL-7 in patients with sepsis increased the number of lymphocytes and their subsets, as well as IL-10 levels; however, mortality remained unchanged, suggesting that immune recovery alone may be insufficient to reverse severe infection and organ damage. The only adverse effect observed was rashes, which resolved after discontinuing rhIL-7, indicating that intramuscular injection of rhIL-7 is safe. Intravenous administration of rhIL-7 results in higher blood concentrations compared to intramuscular injection. Patients receiving intravenous rhIL-7 experienced increased lymphocyte counts without cytokine storms. However, fever and respiratory distress may occur as potential side effects (123). Shankar-Hari et al. (41) reported that COVID-19 sepsis patients treated with rhIL-7 had shorter ICU and hospital stays and a lower risk of secondary infections, though mortality rates were unchanged. Administered at standard doses (10 μg/kg, weekly or biweekly) via intramuscular or intravenous routes, rhIL-7 safely increases lymphocyte subsets including CD4+/8+ T cells and modulates cytokines like IL-10, with manageable side effects like transient rashes and fever. However, clinical trials, including those involving COVID-19-associated sepsis, have not demonstrated a reduction in mortality, despite shorter ICU and hospital stays and a lower incidence of secondary infections.
4 Discussion
Sepsis is a major contributor to patient mortality and has diverse causes. Its hallmark is infection-induced organ dysfunction, and it imposes a substantial socioeconomic burden. Current treatments are limited, primarily targeting bacteria or viruses, which can lead to drug resistance and the emergence of superbugs. Recently, immunotherapy has gained attention, with PD-L1, IL-7, and others emerging as key therapeutic candidates (124). This article reviews the changes and effects of both endogenous and exogenous IL-7 in sepsis.
We try to demonstrate the continuous progression of changes in endogenous IL-7 and related factors based on the above results. In sepsis, blood levels of endogenous IL-7 are typically elevated initially as a result of immune cell activation and endothelial damage caused by the infection. This initial response is part of immune system. However, the subsequent cytokine storm and pathogen-induced damage rapidly deplete normal lymphocytes, leading to a decline in immune function. In response to this overwhelming immune activation, regulatory mechanisms are triggered. The percentage of CD4+ FOXP3+ and CD25+ CD127− T cells increased to suppress cytokine storm, as evidenced by elevated BTLA levels. However, this regulatory response is often insufficient to control the pathogen load, leading to further lymphocyte depletion. Elevated IL-6 from the cytokine storm exacerbates endothelial damage, resulting in increased IL-7 release. With the release of IL-7, expression of the IL-7R increases. Meanwhile, damage to endothelial cells results in reduced IL-7 mRNA levels. Despite elevated IL-7 levels, the capacity of immune cells to perform essential functions is impaired. The rapid turnover of immune cells results in defective glycolysis, as evidenced by reduced STAT5, GLUT1, and HLA-DR expression. Proliferation is further inhibited by increased PD-L1 and diminished mTOR activity. Activation of PD-L1 may be initiated by STAT5 through the facilitation of histone lactylation during immune suppression (78), suggesting that the elevated PD-L1 observed is pathogen-induced, in line with the above findings. While IL-10 may offer some support to IL-7, its overall impact is limited by the severely compromised immune system. Additionally, low TLR5 levels indicate reduced pathogen-killing capacity. Ultimately, the combination of immune dysfunction and endothelial changes leads to a persistent and stable state of immune suppression. Elevated IL-7 levels are maintained due to reduced clearance mechanisms, further complicating the recovery process. Collectively, dysregulated IL-7 is closely linked to sepsis pathology, immune compensation, and clinical outcomes. Its sustained elevation may reflect the body’s attempt to restore immune homeostasis, and targeted modulation of this pathway could offer therapeutic opportunities to improve sepsis management and survival.
The primary mechanisms of IL-7 in vitro for sepsis animal and human blood include immune system recovery, inflammation regulation, and anti-apoptosis. A network can be illustrated where rhIL-7 enters the bloodstream and upregulates STAT5/3 and GLUT1 to enhance glycolysis. rhIL-7 also modulates apoptosis-related factors: it increases Bcl-2 while decreasing BH3 and PUMA, and it elevates mTOR levels, all of which contribute to reducing immune system depletion. Additionally, rhIL-7 upregulates LFA-1 and VLA-4, promoting the migration of white cells and thereby replenishing the immune’s resources. In terms of inflammation, rhIL-7 increases the levels of inflammatory factors like IFN-γ, IL-6, NF-κB, and IL-1β to combat pathogens. Notably, NF-κB can promote IL-7 production, thereby enhancing the efficacy of rhIL-7. Ultimately, these mechanisms work together to restore the immune system and reduce pathogen load.
In clinical settings, endogenous IL-7 and IL-7R have shown promise as prognostic markers in sepsis. These markers may offer valuable insights into the mortality risk in sepsis. The above findings highlight the complexity of IL-7 biology in sepsis, with its prognostic value influenced by age, anatomical compartment, and molecular form. Clinically, integrating IL-7, sIL-7R, and IL-7 mRNA measurements could enhance risk stratification; however, their interpretation requires careful contextualization to avoid paradoxical conclusions. Future studies should validate these markers in stratified cohorts and investigate the mechanisms underlying their discordant associations with clinical outcomes.
Moreover, rhIL-7 has demonstrated considerable potential in boosting the immune system of sepsis patients. This enhancement can lead to shorter hospital stays and a reduced likelihood of developing secondary infections, both of which are critical factors in improving patient outcomes. The high safety profile of rhIL-7 is another major advantage, making it an attractive therapeutic option for managing sepsis. Given the complexity and severity of sepsis, a safe and effective treatment that can enhance the immune system and reduce complication risks represents a significant advancement in managing this condition. However, exogenous IL-7 has the potential to reverse immune dysfunction in patients with sepsis, although mortality rates remain unchanged. This suggests that, while IL-7 may enhance immune function, it does not address the broader pathophysiology of sepsis. The discrepancy between improved immune parameters and unaltered survival underscores the need for precision medicine approaches to optimize IL-7 therapy. Precision medicine, characterized by tailoring treatment to the individual through artificial intelligence and large-scale modelling, has already been applied in oncology (124). Genomic, microbic, and radiomic analyses are key methods for detecting differences (76, 125–127). The principles of precision medicine may be applied to the clinical use of IL-7, given the variability in IL-7 mRNA and IL-7R mRNA expression. Precision medicine strategies should be considered in future clinical trials of IL-7. The discrepancy between improved immune parameters and unchanged survival underscores the need to optimize IL-7 therapy through tailored approaches. Artificial intelligence-driven multi-omics methods, including genomics, microbiome profiling, and radiomics, could facilitate the stratification of patients with sepsis according to IL-7/IL-7R mRNA variability, microbial ecology, or metabolic–epigenetic states. For example, selecting patients with low endogenous IL-7, specific IL-7R polymorphisms, or suppressed STAT5-glycolytic pathways may enhance therapeutic efficacy. Likewise, biomarker-guided dosing based on sIL-7R levels or lymphocyte recovery kinetics could mitigate the risk of hyperinflammation or futile immune activation. Combining IL-7 with adjunctive therapies and employing precision biomarkers could unlock its full potential to transform sepsis management beyond immune reconstitution.
However, several limitations should be noted: variability in patient responses, lack of FDA approval, and small trial sizes, all of which reduce the reliability of the findings. Employing endogenous IL-7 and IL-7R as prognostic markers in sepsis currently lacks a well-defined standard. Given the intricate and variable nature of sepsis, depending solely on IL-7 or IL-7R may not adequately reflect the disease’s multifactorial nature. Integrating IL-7 with additional markers, such as PD-L1, into a multi-marker strategy could yield a more robust and nuanced assessment of patient conditions. This comprehensive approach may enhance diagnostic accuracy, refine risk stratification, and offer deeper insights into disease trajectories and therapeutic efficacy (76, 118, 119, 121, 122, 125, 126). A comprehensive and systematic evaluation plan that integrates IL-7 with other relevant markers is valuable for advancing our idea and clinical management of sepsis. Most existing studies have focused on in vitro experiments, which, though valuable, do not fully translate to clinical settings. As a result, determining the optimal clinical dosing regimen for rhIL-7 remains a critical area for further investigation. Additionally, the potential synergistic effects of combining rhIL-7 with conventional treatments such as antibiotics or antivirals need to be rigorously tested through experimental studies to validate their efficacy and safety in clinical practice. Moreover, the choice of administration method is pivotal. Both intravenous and intramuscular injection routes need to be thoroughly evaluated to determine which method offers the best balance of efficacy, safety, and patient tolerance. The formulation of the drug itself is another crucial factor that can significantly impact its therapeutic effectiveness. For instance, the MVA-hIL-7-Fc fusion protein has demonstrated superior outcomes in preclinical mouse models compared to other forms of IL-7, highlighting the importance of exploring innovative dosage forms to enhance drug performance (56, 94, 127, 128). In addition to the points discussed earlier, precision medicine could be leveraged based on the specific genetic profiles of patients. Endothelial function and immune cell counts are crucial in sepsis pathophysiology and could guide personalized treatments. Therefore, collecting individual data on blood and vascular health could guide the targeted use of rhIL-7. Regarding the dosing of rhIL-7, it would be rational to administer it at a concentration sufficient to elevate IL-7 levels above the median observed in sepsis survivors. This approach aligns with the underlying mechanisms of IL-7’s action. Additionally, considering the complex interactions among cytokines, such as the upregulation of IL-7 by NF-κB, could further refine treatment protocols. It should be noted that endothelial damage is a major factor in sepsis. Although rhIL-7 may not reverse this damage, it could potentially worsen it. Therefore, adjunctive therapies aimed at promoting endothelial repair, such as PCSK9 inhibitors, should be considered to mitigate these effects (129). Additionally, it is important to consider that STAT5 may promote the PD-L1 in white cells through histone lactylation. This suggests that PD-1 inhibitors could be beneficial in modulating this pathway and enhancing the therapeutic effects of IL-7. Further research is essential to explore these areas more thoroughly.
5 Conclusion
In summary, endogenous IL-7 and IL-7R levels can be elevated in sepsis; however, this does not reverse the dysfunction and depletion of immune cells, even when accompanied by other decompensatory changes. These findings indicate that endogenous IL-7 and IL-7R have potential as prognostic markers in sepsis. Exogenous IL-7 can enhance immune function, regulate inflammation, and exert anti-apoptotic effects, but it does not reduce sepsis-related mortality, suggesting that its therapeutic potential may require combination with other interventions. Further research is needed to elucidate the complex interactions between IL-7 and other immune components, as well as its role in modulating inflammation and promoting immune recovery in sepsis. More comprehensive and rigorous clinical trials will be essential to optimize IL-7-based therapy and improve outcomes in this condition.
Author contributions
TZ: Conceptualization, Formal analysis, Investigation, Methodology, Writing – review & editing. HL: Formal analysis, Writing – original draft. Y-fT: Writing – review & editing, Resources. T-wM: Validation, Visualization, Writing – original draft. QL: Funding acquisition, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by National Natural Science Foundation Project (82374400) and Heilongjiang Province “Double First-Class” New Round of Construction Disciplines Collaborative Innovation Achievements Construction Project (LJGXCG2022-097).
Acknowledgments
The author is profoundly appreciative of the peerless mentorship and unwavering support received from colleagues and academic advisors alike, whose contributions were invaluable throughout the conception and progression of this scholarly work. Thanks to BioGDP.com for their help in drawing the illustrations very much.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. van der Poll, T, Shankar-Hari, M, and Wiersinga, WJ. The immunology of sepsis. Immunity. (2021) 54:2450–64. doi: 10.1016/j.immuni.2021.10.012
2. Sanchez-Pinto, LN, Bennett, TD, DeWitt, PE, Russell, S, Rebull, MN, Martin, B, et al. Development and validation of the phoenix criteria for pediatric sepsis and septic shock. JAMA. (2024) 331:675–86. doi: 10.1001/jama.2024.0196
3. Schlapbach, LJ, Watson, RS, Sorce, LR, Argent, AC, Menon, K, Hall, MW, et al. International consensus criteria for pediatric sepsis and septic shock. JAMA. (2024) 331:665–74. doi: 10.1001/jama.2024.0179
4. Strunk, T, Molloy, EJ, Mishra, A, and Bhutta, ZA. Neonatal bacterial sepsis. Lancet. (2024) 404:277–93. doi: 10.1016/S0140-6736(24)00495-1
5. Rudd, KE, Johnson, SC, Agesa, KM, Shackelford, KA, Tsoi, D, Kievlan, DR, et al. Global, regional, and national sepsis incidence and mortality, 1990–2017: analysis for the Global Burden of Disease Study. Lancet. (2020) 395:200–11. doi: 10.1016/S0140-6736(19)32989-7
6. White, KC, Serpa-Neto, A, Hurford, R, Clement, P, Laupland, KB, See, E, et al. Sepsis-associated acute kidney injury in the intensive care unit: incidence, patient characteristics, timing, trajectory, treatment, and associated outcomes. A multicenter, observational study. Intensive Care Med. (2023) 49:1079–89. doi: 10.1007/s00134-023-07138-0
7. Tirupakuzhi Vijayaraghavan, BK, and Adhikari, NKJ. Sepsis epidemiology and outcomes in Asia: advancing the needle. Am J Respir Crit Care Med. (2022) 206:1059–60. doi: 10.1164/rccm.202207-1257ED
8. Gotts, JE, and Matthay, MA. Sepsis: pathophysiology and clinical management. BMJ. (2016) 353:i1585. doi: 10.1136/bmj.i1585
9. Shankar-Hari, M, Saha, R, Wilson, J, Prescott, HC, Harrison, D, Rowan, K, et al. Rate and risk factors for rehospitalisation in sepsis survivors: systematic review and meta-analysis. Intensive Care Med. (2020) 46:619–36. doi: 10.1007/s00134-019-05908-3
10. Cecconi, M, Evans, L, Levy, M, and Rhodes, A. Sepsis and septic shock. Lancet. (2018) 392:75–87. doi: 10.1016/S0140-6736(18)30696-2
11. Kuwabara, S, Goggins, E, and Okusa, MD. The pathophysiology of sepsis-associated AKI. Clin J Am Soc Nephrol. (2022) 17:1050–69. doi: 10.2215/CJN.00850122
12. Giamarellos-Bourboulis, EJ, Aschenbrenner, AC, Bauer, M, Bock, C, Calandra, T, Gat-Viks, I, et al. The pathophysiology of sepsis and precision-medicine-based immunotherapy. Nat Immunol. (2024) 25:19–28. doi: 10.1038/s41590-023-01660-5
13. Weiss, SL, and Fitzgerald, JC. Pediatric sepsis diagnosis, management, and sub-phenotypes. Pediatrics. (2024) 153:e2023062967. doi: 10.1542/peds.2023-062967
14. Tang, F, Zhao, XL, Xu, LY, Zhang, JN, Ao, H, and Peng, C. Endothelial dysfunction: pathophysiology and therapeutic targets for sepsis-induced multiple organ dysfunction syndrome. Biomed Pharmacother. (2024) 178:117180. doi: 10.1016/j.biopha.2024.117180
15. Evans, L, Rhodes, A, Alhazzani, W, Antonelli, M, Coopersmith, CM, French, C, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. (2021) 47:1181–247. doi: 10.1007/s00134-021-06506-y
16. Stanski, NL, and Wong, HR. Prognostic and predictive enrichment in sepsis. Nat Rev Nephrol. (2020) 16:20–31. doi: 10.1038/s41581-019-0199-3
17. Chaudhuri, D, Nei, AM, Rochwerg, B, Balk, RA, Asehnoune, K, Cadena, R, et al. 2024 focused update: guidelines on use of corticosteroids in Sepsis, acute respiratory distress syndrome, and community-acquired pneumonia. Crit Care Med. (2024) 52:e219–33. doi: 10.1097/CCM.0000000000006172
18. Zampieri, FG, Bagshaw, SM, and Semler, MW. Fluid therapy for critically ill adults with sepsis: a review. JAMA. (2023) 329:1967–80. doi: 10.1001/jama.2023.7560
19. Yao, TC, Chang, SM, Wu, CS, Tsai, YF, Sheen, KH, Hong, X, et al. Association between antenatal corticosteroids and risk of serious infection in children: nationwide cohort study. BMJ. (2023) 382:e075835. doi: 10.1136/bmj-2023-075835
20. Venkatesh, B, Finfer, S, Cohen, J, Rajbhandari, D, Arabi, Y, Bellomo, R, et al. Adjunctive glucocorticoid therapy in patients with septic shock. N Engl J Med. (2018) 378:797–808. doi: 10.1056/NEJMoa1705835
21. Barata, JT, Durum, SK, and Seddon, B. Flip the coin: IL-7 and IL-7R in health and disease. Nat Immunol. (2019) 20:1584–93. doi: 10.1038/s41590-019-0479-x
22. Meyer, A, Parmar, PJ, and Shahrara, S. Significance of IL-7 and IL-7R in RA and autoimmunity. Autoimmun Rev. (2022) 21:103120. doi: 10.1016/j.autrev.2022.103120
23. Ponda, P, Schuval, SJ, Kaplan, B, Logalbo, P, Roberts, JL, and Bonagura, VR. Interleukin 7 receptor alpha-chain-mutation severe combined immunodeficiency without lymphopenia: correction with haploidentical T-cell-depleted bone marrow transplantation. Ann Allergy Asthma Immunol. (2006) 97:755–8. doi: 10.1016/S1081-1206(10)60965-9
24. Lum, JJ, Schnepple, DJ, Nie, Z, Sanchez-Dardon, J, Mbisa, GL, Mihowich, J, et al. Differential effects of interleukin-7 and interleukin-15 on NK cell anti-human immunodeficiency virus activity. J Virol. (2004) 78:6033–42. doi: 10.1128/JVI.78.11.6033-6042.2004
25. Lécuroux, C, Girault, I, Boutboul, F, Urrutia, A, Goujard, C, Meyer, L, et al. Antiretroviral therapy initiation during primary HIV infection enhances both CD127 expression and the proliferative capacity of HIV-specific CD8+ T cells. AIDS. (2009) 23:1649–58. doi: 10.1097/QAD.0b013e32832e6634
26. Liu, D, Huang, SY, Sun, JH, Zhang, HC, Cai, QL, Gao, C, et al. Sepsis-induced immunosuppression: mechanisms, diagnosis and current treatment options. Mil Med Res. (2022) 9:56. doi: 10.1186/s40779-022-00422-y
27. Zhang, YY, and Ning, BT. Signaling pathways and intervention therapies in sepsis. Signal Transduct Target Ther. (2021) 6:407. doi: 10.1038/s41392-021-00816-9
28. Darcissac, EC, Vidal, V, De La Tribonniere, X, Mouton, Y, and Bahr, GM. Variations in serum IL-7 and 90K/mac-2 binding protein (mac-2 BP) levels analysed in cohorts of HIV-1 patients and correlated with clinical changes following antiretroviral therapy. Clin Exp Immunol. (2001) 126:287–94. doi: 10.1046/j.1365-2249.2001.01670.x
29. Imamichi, H, Degray, G, Asmuth, DM, Fischl, MA, Landay, AL, Lederman, MM, et al. HIV-1 viruses detected during episodic blips following interleukin-7 administration are similar to the viruses present before and after interleukin-7 therapy. AIDS. (2011) 25:159–64. doi: 10.1097/QAD.0b013e328340a270
30. Landires, I, Bugault, F, Lambotte, O, de Truchis, P, Slama, L, Danckaert, A, et al. HIV infection perturbs interleukin-7 signaling at the step of STAT5 nuclear relocalization. AIDS. (2011) 25:1843–53. doi: 10.1097/QAD.0b013e32834a3678
31. Saison, J, Demaret, J, Venet, F, Chidiac, C, Malcus, C, Poitevin-Later, F, et al. CD4+ CD25+ CD127− assessment as a surrogate phenotype for FOXP3+ regulatory T cells in HIV-1 infected viremic and aviremic subjects. Cytometry B Clin Cytom. (2013) 84:50–4. doi: 10.1002/cyto.b.21047
32. Guaraldi, G, Luzi, K, Bellistrì, GM, Zona, S, Domingues da Silva, AR, Bai, F, et al. CD8 T-cell activation is associated with lipodystrophy and visceral fat accumulation in antiretroviral therapy-treated virologically suppressed HIV-infected patients. J Acquir Immune Defic Syndr. (1999) 64:360–6. doi: 10.1097/QAI.0000000000000001
33. Castro, P, Torres, B, López, A, González, R, Vilella, A, Nicolas, JM, et al. Effects of different antigenic stimuli on thymic function and interleukin-7/CD127 system in patients with chronic HIV infection. J Acquir Immune Defic Syndr. (2014) 66:466–72. doi: 10.1097/QAI.0000000000000207
34. Kerzerho, J, McIlvaine, EJ, Anthony, P, Mack, WJ, Wang, CH, Frederick, T, et al. Impact of hepatitis C virus on the circulating levels of IL-7 in HIV-1 coinfected women. J Acquir Immune Defic Syndr. (2016) 71:172–80. doi: 10.1097/QAI.0000000000000832
35. Shin, MS, Park, HJ, Salahuddin, S, Montgomery, RR, Emu, B, Shaw, AC, et al. Alterations in high-dimensional T-cell profile and gene signature of immune aging in HIV-infected older adults without viremia. Aging Cell. (2022) 21:e13702. doi: 10.1111/acel.13702
36. de Sousa Barros, JB, da Silva, PAN, de Carvalho Rocha Koga, R, Gonzalez-Dias, P, Filho, JRC, Nagib, PRA, et al. Acute Zika virus infection in an endemic area shows modest proinflammatory systemic immunoactivation and cytokine-symptom associations. Front Immunol. (2018) 9:821. doi: 10.3389/fimmu.2018.00821
37. Laterre, PF, François, B, Collienne, C, Hantson, P, Jeannet, R, Remy, KE, et al. Association of interleukin 7 immunotherapy with lymphocyte counts among patients with severe coronavirus disease 2019 (COVID-19). JAMA Netw Open. (2020) 3:e2016485. doi: 10.1001/jamanetworkopen.2020.16485
38. Remy, KE, Mazer, M, Striker, DA, Ellebedy, AH, Walton, AH, Unsinger, J, et al. Severe immunosuppression and not a cytokine storm characterizes COVID-19 infections. JCI Insight. (2020) 5:e140329. doi: 10.1172/jci.insight.140329
39. Bekele, Y, Sui, Y, and Berzofsky, JA. IL-7 in SARS-CoV-2 infection and as a potential vaccine adjuvant. Front Immunol. (2021) 12:737406. doi: 10.3389/fimmu.2021.737406
40. Kalinina, O, Golovkin, A, Zaikova, E, Aquino, A, Bezrukikh, V, Melnik, O, et al. Cytokine storm signature in patients with moderate and severe COVID-19. Int J Mol Sci. (2022) 23:8879. doi: 10.3390/ijms23168879
41. Shankar-Hari, M, Francois, B, Remy, KE, Gutierrez, C, Pastores, S, Daix, T, et al. A randomized, double-blind, placebo-controlled trial of IL-7 in critically ill patients with COVID-19. JCI Insight. (2025) 10:e189150. doi: 10.1172/jci.insight.189150
42. Kaiser, FMP, Janowska, I, Menafra, R, de Gier, M, Korzhenevich, J, Pico-Knijnenburg, I, et al. IL-7 receptor signaling drives human B-cell progenitor differentiation and expansion. Blood. (2023) 142:1113–30. doi: 10.1182/blood.2023019721
43. Lee, KJ, Choi, D, Tae, N, Song, HW, Kang, YW, Lee, M, et al. IL-7-primed bystander CD8 tumor-infiltrating lymphocytes optimize the antitumor efficacy of T cell engager immunotherapy. Cell Rep Med. (2024) 5:101567. doi: 10.1016/j.xcrm.2024.101567
44. Wang, L, Wan, J, He, W, Wang, Z, Wu, Q, Zhou, M, et al. IL-7 promotes mRNA vaccine-induced long-term immunity. J Nanobiotechnology. (2024) 22:716. doi: 10.1186/s12951-024-02993-5
45. Demaret, J, Villars-Méchin, A, Lepape, A, Plassais, J, Vallin, H, Malcus, C, et al. Elevated plasmatic level of soluble IL-7 receptor is associated with increased mortality in septic shock patients. Intensive Care Med. (2014) 40:1089–96. doi: 10.1007/s00134-014-3346-0
46. Venet, F, Foray, AP, Villars-Méchin, A, Malcus, C, Poitevin-Later, F, Lepape, A, et al. IL-7 restores lymphocyte functions in septic patients. J Immunol. (2012) 189:5073–81. doi: 10.4049/jimmunol.1202062
47. Andreu-Ballester, JC, Cuellar, C, Garcia-Ballesteros, C, Pérez-Griera, J, Amigó, V, Peiró-Gómez, A, et al. Deficit of interleukin 7 in septic patients. Int Immunopharmacol. (2014) 23:73–6. doi: 10.1016/j.intimp.2014.08.015
48. Grealy, R, White, M, Stordeur, P, Kelleher, D, Doherty, DG, McManus, R, et al. Characterising cytokine gene expression signatures in patients with severe sepsis. Mediat Inflamm. (2013) 2013:164246. doi: 10.1155/2013/164246
49. Smith, JR, Galie, PA, Slochower, DR, Weisshaar, CL, Janmey, PA, and Winkelstein, BA. Salmon-derived thrombin inhibits development of chronic pain through an endothelial barrier protective mechanism dependent on APC. Biomaterials. (2016) 80:96–105. doi: 10.1016/j.biomaterials.2015.11.062
50. Ma, S, Sun, S, Li, J, Fan, Y, Qu, J, Sun, L, et al. Single-cell transcriptomic atlas of primate cardiopulmonary aging. Cell Res. (2021) 31:415–32. doi: 10.1038/s41422-020-00412-6
51. Velloso, FJ, Zaritsky, R, Houbeika, RY, Rios, N, and Levison, SW. Interleukin-6 produces behavioral deficits in pre-pubescent mice independent of neuroinflammation. Brain Behav Immun. (2025) 126:275–88. doi: 10.1016/j.bbi.2025.02.009
52. Uebelhoer, LS, Gwela, A, Thiel, B, Nalukwago, S, Mukisa, J, Lwanga, C, et al. Toll-like receptor-induced immune responses during early childhood and their associations with clinical outcomes following acute illness among infants in sub-Saharan Africa. Front Immunol. (2021) 12:748996. doi: 10.3389/fimmu.2021.748996
53. Riché, F, Chousterman, BG, Valleur, P, Mebazaa, A, Launay, JM, and Gayat, E. Protracted immune disorders at one year after ICU discharge in patients with septic shock. Crit Care. (2018) 22:42. doi: 10.1186/s13054-017-1934-4
54. Delwarde, B, Peronnet, E, Venet, F, Cerrato, E, Meunier, B, Mouillaux, J, et al. Low interleukin-7 receptor messenger RNA expression is independently associated with day 28 mortality in septic shock patients. Crit Care Med. (2018) 46:1739–46. doi: 10.1097/CCM.0000000000003281
55. MacIver, NJ, Michalek, RD, and Rathmell, JC. Metabolic regulation of T lymphocytes. Annu Rev Immunol. (2013) 31:259–83. doi: 10.1146/annurev-immunol-032712-095956
56. Crausaz, M, Monneret, G, Conti, F, Lukaszewicz, AC, Marchand, JB, Martin, P, et al. A novel virotherapy encoding human interleukin-7 improves ex vivo T lymphocyte functions in immunosuppressed patients with septic shock and critically ill COVID-19. Front Immunol. (2022) 13:939899. doi: 10.3389/fimmu.2022.939899
57. Venet, F, Demaret, J, Blaise, BJ, Rouget, C, Girardot, T, Idealisoa, E, et al. IL-7 restores T lymphocyte immunometabolic failure in septic shock patients through mTOR activation. J Immunol. (2017) 199:1606–15. doi: 10.4049/jimmunol.1700127
58. Li, HB, Tong, J, Zhu, S, Batista, PJ, Duffy, EE, Zhao, J, et al. m6A mRNA methylation controls T cell homeostasis by targeting the IL-7/STAT5/SOCS pathways. Nature. (2017) 548:338–42. doi: 10.1038/nature23450
59. Kabata, H, Moro, K, and Koyasu, S. The group 2 innate lymphoid cell (ILC2) regulatory network and its underlying mechanisms. Immunol Rev. (2018) 286:37–52. doi: 10.1111/imr.12706
60. Kato, A. Group 2 innate lymphoid cells in airway diseases. Chest. (2019) 156:141–9. doi: 10.1016/j.chest.2019.04.101
61. Zhang, B, Zhang, Y, Xiong, L, Li, Y, Zhang, Y, Zhao, J, et al. CD127 imprints functional heterogeneity to diversify monocyte responses in inflammatory diseases. J Exp Med. (2022) 219:e20211191. doi: 10.1084/jem.20211191
62. Fernandes, MB, Gomes, AM, Oliveira, ML, Caldas, J, Lúcio, P, Kim, R, et al. Differential activation of basal and IL-7-induced PI3K/akt/mTOR and JAK/STAT5 signaling distinguishes pediatric from adult acute lymphoblastic leukemia. Haematologica. (2024) 109:2671–5. doi: 10.3324/haematol.2023.284102
63. Tremblay, CS, Saw, J, Boyle, JA, Haigh, K, Litalien, V, McCalmont, H, et al. STAT5 activation promotes progression and chemotherapy resistance in early T-cell precursor acute lymphoblastic leukemia. Blood. (2023) 142:274–89. doi: 10.1182/blood.2022016322
64. Demaret, J, Dupont, G, Venet, F, Friggeri, A, Lepape, A, Rimmelé, T, et al. STAT5 phosphorylation in T cell subsets from septic patients in response to recombinant human interleukin-7: a pilot study. J Leukoc Biol. (2015) 97:791–6. doi: 10.1189/jlb.5AB1114-545R
65. Cruz-Zárate, D, Cabrera-Rivera, GL, Ruiz-Sánchez, BP, Serafín-López, J, Chacón-Salinas, R, López-Macías, C, et al. Innate lymphoid cells have decreased HLA-DR expression but retain their responsiveness to TLR ligands during sepsis. J Immunol. (2018) 201:3401–10. doi: 10.4049/jimmunol.1800735
66. Yao, RQ, Zhao, PY, Li, ZX, Liu, YY, Zheng, LY, Duan, Y, et al. Single-cell transcriptome profiling of sepsis identifies HLA-DRlowS100Ahigh monocytes with immunosuppressive function. Mil Med Res. (2023) 10:27. doi: 10.1186/s40779-023-00462-y
67. Triantafyllou, E, Gudd, CL, Mawhin, MA, Husbyn, HC, Trovato, FM, Siggins, MK, et al. PD-1 blockade improves Kupffer cell bacterial clearance in acute liver injury. J Clin Invest. (2021) 131:e140196. doi: 10.1172/JCI140196
68. Wang, JF, Wang, YP, Xie, J, Zhao, ZZ, Gupta, S, Guo, Y, et al. Upregulated PD-L1 delays human neutrophil apoptosis and promotes lung injury in an experimental mouse model of sepsis. Blood. (2021) 138:806–10. doi: 10.1182/blood.2020009417
69. Huang, L, Zhang, X, Fan, J, Liu, X, Luo, S, Cao, D, et al. EGFR promotes the apoptosis of CD4+ T lymphocytes through TBK1/Glut1 induced Warburg effect in sepsis. J Adv Res. (2023) 44:39–51. doi: 10.1016/j.jare.2022.04.010
70. Shao, R, Li, CS, Fang, Y, Zhao, L, and Hang, C. Low B and T lymphocyte attenuator expression on CD4+ T cells in the early stage of sepsis is associated with the severity and mortality of septic patients: a prospective cohort study. Crit Care. (2015) 19:308. doi: 10.1186/s13054-015-1024-4
71. Thampy, LK, Remy, KE, Walton, AH, Hong, Z, Liu, K, Liu, R, et al. Restoration of T cell function in multi-drug resistant bacterial sepsis after interleukin-7, anti-PD-L1, and OX-40 administration. PLoS One. (2018) 13:e0199497. doi: 10.1371/journal.pone.0199497
72. Rochman, Y, Spolski, R, and Leonard, WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. (2009) 9:480–90. doi: 10.1038/nri2580
73. Heninger, AK, Theil, A, Wilhelm, C, Petzold, C, Huebel, N, Kretschmer, K, et al. IL-7 abrogates suppressive activity of human CD4+ CD25+ FOXP3+ regulatory T cells and allows expansion of alloreactive and autoreactive T cells. J Immunol. (2012) 189:5649–58. doi: 10.4049/jimmunol.1201286
74. Leng, FY, Liu, JL, Liu, ZJ, Yin, JY, and Qu, HP. Increased proportion of CD4+ CD25+ Foxp3+ regulatory T cells during early-stage sepsis in ICU patients. J Microbiol Immunol Infect. (2013) 46:338–44. doi: 10.1016/j.jmii.2012.06.012
75. Venet, F, Chung, CS, Kherouf, H, Geeraert, A, Malcus, C, Poitevin, F, et al. Increased circulating regulatory T cells (CD4+ CD25+ CD127−) contribute to lymphocyte anergy in septic shock patients. Intensive Care Med. (2009) 35:678–86. doi: 10.1007/s00134-008-1337-8
76. Hein, F, Massin, F, Cravoisy-Popovic, A, Barraud, D, Levy, B, Bollaert, PE, et al. The relationship between CD4+ CD25+ CD127− regulatory T cells and inflammatory response and outcome during shock states. Crit Care. (2010) 14:R19. doi: 10.1186/cc8876
77. Sereti, I, Dunham, RM, Spritzler, J, Aga, E, Proschan, MA, Medvik, K, et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood. (2009) 113:6304–14. doi: 10.1182/blood-2008-10-186601
78. Sun, X, Wu, J, Liu, L, Chen, Y, Tang, Y, Liu, S, et al. Transcriptional switch of hepatocytes initiates macrophage recruitment and T-cell suppression in endotoxemia. J Hepatol. (2022) 77:436–52. doi: 10.1016/j.jhep.2022.02.028
79. Lin, Z, Meng, F, Ma, Y, Zhang, C, Zhang, Z, Yang, Z, et al. In situ immunomodulation of tumors with biosynthetic bacteria promote anti-tumor immunity. Bioact Mater. (2024) 32:12–27. doi: 10.1016/j.bioactmat.2023.09.007
80. Mouillaux, J, Allam, C, Gossez, M, Uberti, T, Delwarde, B, Hayman, J, et al. TCR activation mimics CD127(low)PD-1(high) phenotype and functional alterations of T lymphocytes from septic shock patients. Crit Care. (2019) 23:131. doi: 10.1186/s13054-018-2305-5
81. Di Nitto, C, Ravazza, D, Gilardoni, E, Look, T, Sun, M, Prodi, E, et al. An IL-7 fusion protein targeting EDA fibronectin upregulates TCF1 on CD8+ T-cells, preferentially accumulates to neoplastic lesions, and boosts PD-1 blockade. J Immunother Cancer. (2024) 12:e008504. doi: 10.1136/jitc-2023-008504
82. Arens, C, Bajwa, SA, Koch, C, Siegler, BH, Schneck, E, Hecker, A, et al. Sepsis-induced long-term immune paralysis—results of a descriptive, explorative study. Crit Care. (2016) 20:93. doi: 10.1186/s13054-016-1233-5
83. Sherwood, ER, and Hotchkiss, RS. BTLA as a biomarker and mediator of sepsis-induced immunosuppression. Crit Care. (2013) 17:1022. doi: 10.1186/cc13143
84. Wang, Q, Deng, J, Sun, J, Zhang, H, Liu, D, Gao, C, et al. PDGFR kinase inhibitor protects against septic death via regulation of BTLA. Sci China Life Sci. (2022) 65:1917–28. doi: 10.1007/s11427-021-2136-y
85. Francois, B, Jeannet, R, Daix, T, Walton, AH, Shotwell, MS, Unsinger, J, et al. Interleukin-7 restores lymphocytes in septic shock: the IRIS-7 randomized clinical trial. JCI Insight. (2018) 3:e98960. doi: 10.1172/jci.insight.98960
86. Kang, S, and Kishimoto, T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp Mol Med. (2021) 53:1116–23. doi: 10.1038/s12276-021-00649-0
87. Hamilton, FW, Thomas, M, Arnold, D, Palmer, T, Moran, E, Mentzer, AJ, et al. Therapeutic potential of IL6R blockade for the treatment of sepsis and sepsis-related death: a Mendelian randomisation study. PLoS Med. (2023) 20:e1004174. doi: 10.1371/journal.pmed.1004174
88. Drost, CC, Rovas, A, Osiaevi, I, Schughart, K, Lukasz, A, Linke, WA, et al. Interleukin-6 drives endothelial glycocalyx damage in COVID-19 and bacterial sepsis. Angiogenesis. (2024) 27:411–22. doi: 10.1007/s10456-024-09916-w
89. Yeung, ST, Ovando, LJ, Russo, AJ, Rathinam, VA, and Khanna, KM. CD169+ macrophage intrinsic IL-10 production regulates immune homeostasis during sepsis. Cell Rep. (2023) 42:112171. doi: 10.1016/j.celrep.2023.112171
90. Vergadi, E, Kolliniati, O, Lapi, I, Ieronymaki, E, Lyroni, K, Alexaki, VI, et al. An IL-10/DEL-1 axis supports granulopoiesis and survival from sepsis in early life. Nat Commun. (2024) 15:680. doi: 10.1038/s41467-023-44178-y
91. Lai, D, Tang, J, Chen, L, Fan, EK, Scott, MJ, Li, Y, et al. Group 2 innate lymphoid cells protect lung endothelial cells from pyroptosis in sepsis. Cell Death Dis. (2018) 9:369. doi: 10.1038/s41419-018-0412-5
92. Shao, R, Liu, W, Feng, Y, Guo, X, Ren, Z, Hou, X, et al. LAMP2-FLOT2 interaction enhances autophagosome-lysosome fusion to protect the septic heart in response to ILC2. Autophagy. (2025) 21:1888–910. doi: 10.1080/15548627.2025.2469207
93. de Moraes, FOC, Numakura, GTY, Hokama, LT, and Soriano, FG. Effects of IL-7 on sepsis: a systematic review. Rev Med. (2021) 100:254–68. doi: 10.11606/issn.1679-9836.v100i3p254-268
94. Lélu, K, Dubois, C, Evlachev, A, Crausaz, M, Baldazza, M, Kehrer, N, et al. Viral delivery of IL-7 is a potent immunotherapy stimulating innate and adaptive immunity and confers survival in sepsis models. J Immunol. (2022) 209:99–117. doi: 10.4049/jimmunol.2101145
95. Ammer-Herrmenau, C, Kulkarni, U, Andreas, N, Ungelenk, M, Ravens, S, Hübner, C, et al. Sepsis induces long-lasting impairments in CD4+ T-cell responses despite rapid numerical recovery of T-lymphocyte populations. PLoS One. (2019) 14:e0211716. doi: 10.1371/journal.pone.0211716
96. Shindo, Y, Fuchs, AG, Davis, CG, Eitas, T, Unsinger, J, Burnham, CAD, et al. Interleukin 7 immunotherapy improves host immunity and survival in a two-hit model of pseudomonas aeruginosa pneumonia. J Leukoc Biol. (2017) 101:543–54. doi: 10.1189/jlb.4A1215-581R
97. Unsinger, J, Burnham, CAD, McDonough, J, Morre, M, Prakash, PS, Caldwell, CC, et al. Interleukin-7 ameliorates immune dysfunction and improves survival in a 2-hit model of fungal sepsis. J Infect Dis. (2012) 206:606–16. doi: 10.1093/infdis/jis383
98. Unsinger, J, McGlynn, M, Kasten, KR, Hoekzema, AS, Watanabe, E, Muenzer, JT, et al. IL-7 promotes T cell viability, trafficking, and functionality and improves survival in sepsis. J Immunol. (2010) 184:3768–79. doi: 10.4049/jimmunol.0903151
99. Katayama, Y, Sekai, M, Hattori, M, Miyoshi, I, Hamazaki, Y, and Minato, N. Rap signaling is crucial for the competence of IL-7 response and the development of B-lineage cells. Blood. (2009) 114:1768–75. doi: 10.1182/blood-2009-03-213371
100. Shan, J, Jing, W, Ping, Y, Shen, C, Han, D, Liu, F, et al. LFA-1 regulated by IL-2/STAT5 pathway boosts antitumor function of intratumoral CD8+ T cells for improving anti-PD-1 antibody therapy. Onco Targets Ther. (2024) 13:2293511. doi: 10.1080/2162402X.2023.2293511
101. Gerace, E, Pasquali, P, Oesch, B, Falduto, M, Mandanici, F, Fiasconaro, M, et al. Stimulation of bovine whole-blood samples cultured in media supplemented with recombinant interleukin-7 (IL-7) and IL-12 extends the life span of the gamma interferon assay to detect Mycobacterium bovis-infected cattle. J Clin Microbiol. (2016) 54:2315–20. doi: 10.1128/JCM.00629-16
102. Belarif, L, Mary, C, Jacquemont, L, Mai, HL, Danger, R, Hervouet, J, et al. IL-7 receptor blockade blunts antigen-specific memory T cell responses and chronic inflammation in primates. Nat Commun. (2018) 9:4483. doi: 10.1038/s41467-018-06804-y
103. Jorgovanovic, D, Song, M, Wang, L, and Zhang, Y. Roles of IFN-γ in tumor progression and regression: a review. Biomark Res. (2020) 8:49. doi: 10.1186/s40364-020-00228-x
104. Azizi, G, Van den Broek, B, Ishikawa, LLW, Naziri, H, Yazdani, R, Zhang, GX, et al. IL-7Rα on CD4+ T cells is required for their survival and the pathogenesis of experimental autoimmune encephalomyelitis. J Neuroinflammation. (2024) 21:253. doi: 10.1186/s12974-024-03224-2
105. Bauer, A, Villunger, A, Labi, V, Fischer, SF, Strasser, A, Wagner, H, et al. The NF-kappaB regulator Bcl-3 and the BH3-only proteins Bim and Puma control the death of activated T cells. Proc Natl Acad Sci USA. (2006) 103:10979–84. doi: 10.1073/pnas.0603625103
106. Miller, ML, Mashayekhi, M, Chen, L, Zhou, P, Liu, X, Michelotti, M, et al. Basal NF-κB controls IL-7 responsiveness of quiescent naïve T cells. Proc Natl Acad Sci USA. (2014) 111:7397–402. doi: 10.1073/pnas.1315398111
107. Kong, F, Hu, W, Zhou, K, Wei, X, Kou, Y, You, H, et al. Hepatitis B virus X protein promotes interleukin-7 receptor expression via NF-κB and Notch1 pathway to facilitate proliferation and migration of hepatitis B virus-related hepatoma cells. J Exp Clin Cancer Res. (2016) 35:172. doi: 10.1186/s13046-016-0448-2
108. Wang, Y, Quan, Y, He, J, Chen, S, and Dong, Z. Slam-family receptors promote resolution of ILC2-mediated inflammation. Nat Commun. (2024) 15:5056. doi: 10.1038/s41467-024-49466-9
109. Pellegrini, M, Calzascia, T, Toe, JG, Preston, SP, Lin, AE, Elford, AR, et al. IL-7 engages multiple mechanisms to overcome chronic viral infection and limit organ pathology. Cell. (2011) 144:601–13. doi: 10.1016/j.cell.2011.01.011
110. Reading, JL, Vaes, B, Hull, C, Sabbah, S, Hayday, T, Wang, NS, et al. Suppression of IL-7-dependent effector T-cell expansion by multipotent adult progenitor cells and PGE2. Mol Ther. (2015) 23:1783–93. doi: 10.1038/mt.2015.131
111. Shireman, JM, Gonugunta, N, Zhao, L, Pattnaik, A, Distler, E, Her, S, et al. GM-CSF and IL-7 fusion cytokine engineered tumor vaccine generates long-term th-17 memory cells and increases overall survival in aged syngeneic mouse models of glioblastoma. Aging Cell. (2023) 22:e13864. doi: 10.1111/acel.13864
112. Chang, KC, Unsinger, J, Davis, CG, Schwulst, SJ, Muenzer, JT, Strasser, A, et al. Multiple triggers of cell death in sepsis: death receptor and mitochondrial-mediated apoptosis. FASEB J. (2007) 21:708–19. doi: 10.1096/fj.06-6805com
113. Brahmamdam, P, Watanabe, E, Unsinger, J, Chang, KC, Schierding, W, Hoekzema, AS, et al. Targeted delivery of siRNA to cell death proteins in sepsis. Shock. (2009) 32:131–9. doi: 10.1097/SHK.0b013e318194bcee
114. Zhang, L, Zhang, J, Liu, Y, Zhang, P, Nie, J, Zhao, R, et al. Mitochondrial STAT5A promotes metabolic remodeling and the Warburg effect by inactivating the pyruvate dehydrogenase complex. Cell Death Dis. (2021) 12:634. doi: 10.1038/s41419-021-03908-0
115. Huang, ZW, Zhang, XN, Zhang, L, Liu, LL, Zhang, JW, Sun, YX, et al. STAT5 promotes PD-L1 expression by facilitating histone lactylation to drive immunosuppression in acute myeloid leukemia. Signal Transduct Target Ther. (2023) 8:391. doi: 10.1038/s41392-023-01605-2
116. Robb, CT, Zhou, Y, Felton, JM, Zhang, B, Goepp, M, Jheeta, P, et al. Metabolic regulation by prostaglandin E2 impairs lung group 2 innate lymphoid cell responses. Allergy. (2023) 78:714–30. doi: 10.1111/all.15541
117. Yu, M, Chen, Y, Zeng, H, Zheng, Y, Fu, G, Zhu, W, et al. PLCγ-dependent mTOR signalling controls IL-7-mediated early B cell development. Nat Commun. (2017) 8:1457. doi: 10.1038/s41467-017-01388-5
118. Bozza, FA, Salluh, JI, Japiassu, AM, Soares, M, Assis, EF, Gomes, RN, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. (2007) 11:R49. doi: 10.1186/cc5783
119. Leśnik, P, Janc, J, Mierzchala-Pasierb, M, Tański, W, Wierciński, J, and Łysenko, L. Interleukin-7 and interleukin-15 as prognostic biomarkers in sepsis and septic shock: correlation with inflammatory markers and mortality. Cytokine. (2023) 169:156277. doi: 10.1016/j.cyto.2023.156277
120. Zhao, J, Zhang, Y, Wang, JY, Wei, B, and Liu, YG. Expression pattern of serum interleukin-7 in elderly septic patients and its prognostic value for predicting short-term mortality. Cytokine. (2024) 180:156664. doi: 10.1016/j.cyto.2024.156664
121. Coman, O, Grigorescu, BL, Huțanu, A, Bacârea, A, Văsieșiu, AM, Ștefania, FR, et al. The role of PD-1/PD-L1 and IL-7 in lymphocyte dynamics and sepsis progression: a biomarker study in critically ill patients. Int J Mol Sci. (2024) 25:12612. doi: 10.3390/ijms252312612
122. Li, J, Wang, L, Yu, B, Su, J, and Dong, S. IL7R, GZMA and CD8A serve as potential molecular biomarkers for sepsis based on bioinformatics analysis. Front Immunol. (2024) 15:1445858. doi: 10.3389/fimmu.2024.1445858
123. Daix, T, Mathonnet, A, Brakenridge, S, Dequin, PF, Mira, JP, Berbille, F, et al. Intravenously administered interleukin-7 to reverse lymphopenia in patients with septic shock: a double-blind, randomized, placebo-controlled trial. Ann Intensive Care. (2023) 13:17. doi: 10.1186/s13613-023-01109-w
124. Winer, H, Rodrigues, GOL, Hixon, JA, Aiello, FB, Hsu, TC, Wachter, BT, et al. IL-7: comprehensive review. Cytokine. (2022):156049. doi: 10.1016/j.cyto.2022.156049
125. Venet, F, Guignant, C, and Monneret, G. Flow cytometry developments and perspectives in clinical studies: examples in ICU patients. Methods Mol Biol. (2011) 761:261–75. doi: 10.1007/978-1-61779-182-6_18
126. Bhavani, SV, Spicer, A, Sinha, P, Malik, A, Lopez-Espina, C, Schmalz, L, et al. Distinct immune profiles and clinical outcomes in sepsis subphenotypes based on temperature trajectories. Intensive Care Med. (2024) 50:2094–104. doi: 10.1007/s00134-024-07669-0
127. Coupet, CA, Dubois, C, Evlachev, A, Kehrer, N, Baldazza, M, Hofman, S, et al. Intravenous injection of a novel viral immunotherapy encoding human interleukin-7 in nonhuman primates is safe and increases absolute lymphocyte count. Hum Vaccin Immunother. (2022) 18:2133914. doi: 10.1080/21645515.2022.2133914
128. Marton, C, Minaud, A, Coupet, CA, Chauvin, M, Dhiab, J, Vallet, H, et al. IL-7 producing immunotherapy improves ex vivo T cell functions of immunosenescent patients, especially post hip fracture. Hum Vaccin Immunother. (2023) 19:2232247. doi: 10.1080/21645515.2023.2232247
129. Xu, S, Ilyas, I, Little, PJ, Li, H, Kamato, D, Zheng, X, et al. Endothelial dysfunction in atherosclerotic cardiovascular diseases and beyond: from mechanism to pharmacotherapies. Pharmacol Rev. (2021) 73:924–67. doi: 10.1124/pharmrev.120.000096
130. Jiang, S, Li, H, Zhang, L, Mu, W, Zhang, Y, Chen, T, et al. Generic diagramming platform (GDP): a comprehensive database of high-quality biomedical graphics. Nucleic Acids Res. (2025) 53:D1670–6. doi: 10.1093/nar/gkae973
Keywords: sepsis, IL-7, septic shock, immune dysfunction, interleukin-7
Citation: Zhang T, Liu H, Tie Y-f, Meng T-w and Liang Q (2025) Potential of interleukin-7 in sepsis as a biomarker and therapeutic agent: a narrative review. Front. Med. 12:1649049. doi: 10.3389/fmed.2025.1649049
Edited by:
Ennio Polilli, Azienda USL di Pescara, ItalyReviewed by:
Maria Danielma dos Santos Reis, Universidade Federal de Alagoas, BrazilEdward Kurnia Setiawan Limijadi, Diponegoro University, Indonesia
Copyright © 2025 Zhang, Liu, Tie, Meng and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qun Liang, bGlhbmdxdW4xQHNpbmEuY29t
 Tong Zhang1
Tong Zhang1 Han Liu
Han Liu Yi-fu Tie
Yi-fu Tie Tian-wei Meng
Tian-wei Meng Qun Liang
Qun Liang