Abstract
Bleeding and thromboembolic events (BTE) increase the mortality of COVID-19 acute respiratory distress syndrome (ARDS) treated with extracorporeal membrane oxygenation (ECMO). The current analysis aimed to assess frequency and determinants of BTE according to their location and severity in a retrospective analysis of the German ECMO COVID-19 registry. Logistic regression was applied to identify factors influencing ICU survival as well as variables associated with risks of BTE. In total, 708 of 945 patients (75%) suffered from BTE. Overall, 1,348 events were registered, including 406 (30%) major bleeding and 258 (19%) major thromboembolic events. Most common major bleeding locations were intracranial (n = 133, 10%) and pulmonary bleeding (n = 116, 9%). In-ICU survival was 35, 46% without BTE and 22% with major bleeding (p < 0.05). In summary, major bleeding was a core outcome-determinant of COVID-19 ECMO mortality with intracranial major bleeding as the most devastating complication (OR: 5.3; CI: 2.9–9.9; p < 0.001). Neither major thromboembolism nor minor BTE impacted ICU-mortality. Potentially modifiable factors associated with major bleeding included prolonged duration of ECMO >14 days (OR: 2.9; CI 1.8–4.7; p < 0.001) and platelet counts <100.000/μL ≥ 72 h (OR: 2.0; CI 1.1–3.6; p = 0.018). Hence, prevention, early recognition and treatment of major bleedings are key to increase the survival of COVID-19 ECMO. In this regard, our data indicate that the implementation of early weaning strategies to minimize duration of ECMO therapy and prevention of prolonged thrombocytopenia with platelet counts <100.000/μl ≥ 72 h could decrease the risk of devastating bleeds and could ameliorate survival.
Clinical trial registration:
Registered in the German Clinical Trials Register (study ID: DRKS00022964), retrospectively registered, September 7th 2020, https://drks.de/DRKS00022964.
Background
Veno-venous extracorporeal membrane oxygenation (V-V ECMO) serves as a bridge to recovery in severe COVID-19 induced acute respiratory distress syndrome (ARDS) (1). In-ICU mortality of COVID-19 ECMO ranges from 31 to 46%, whereas bleeding complications represent an independent risk factor of non-survival (2, 3).
Bleeding and thromboembolism are clinical manifestations of deranged hemostasis. Alterations are associated with the ARDS as well as ECMO associated coagulopathy (4, 5). ECMO associated coagulopathy is characterized by platelet dysfunction, an acquired von Willebrand syndrome (vWF) (6), consumption of coagulation factors, as well as increased prothrombin fragment 1.2, thrombin-antithrombin complex, and D-dimers (7). In addition, the large foreign surface area of the ECMO circuit requires systemic anticoagulation, which can aggravate the consumptive coagulopathy. COVID-19 itself may also worsen the situation, as the viral disease induces a coagulopathy mainly predisposing to thrombotic complications (8).
A previous study found evidence for venous thromboembolism in 31%, arterial emboli in 5% and pulmonary embolism in 19% in COVID-19 patients treated on an intensive care unit (ICU) (9). A recent meta-analysis conducted in the US, Europe and Asia described bleeding complications in 7.8% and major bleeding events in 3.9% in >18,000 hospitalized COVID-19 patients (10). Incidences of BTE in severely-ill COVID-19 ECMO patients are much higher. A cohort study from the French national ECMOSARS registry found bleeding complications in 29% associated with an approximately threefold increased risk of non-survival (11). In a preceding analysis of the German nationwide ECMO COVID-19 register, major bleeding or thromboembolic events were associated with a higher probability of non-survival during ICU stay (3). Nevertheless, BTE during COVID-19 ECMO vary in severity and minor events might not be as outcome relevant as major events such as intracerebral bleeding or pulmonary embolism.
The current analysis aimed to assess frequency and determinants of bleeding and thromboembolic events according to their location and severity as well as their impact on early outcome during ICU stay in a large multicenter register study from Germany.
Methods
Study design and patient population
The German COVID-19 ECMO registry is a nation-wide retrospective observational multi-center study (3). Consecutive patients with SARS-CoV-2 induced ARDS treated with ECMO at 29 ECMO centers across Germany between January 1st 2020 and July 31st 2021 were included.
Data collection and outcomes
The treating physicians documented anonymous data from routine clinical care in a standardized electronic case report form (RedCap®, hosted at University of Wuerzburg). Data comprise patient-related risk factors, laboratory values, ECMO runs, therapeutic regimens, complications and outcome. Patient outcomes included survival to ICU discharge, length of ECMO and ICU stay, occurrence and location of major and minor bleeding events, occurrence and location of major and minor thromboembolic events. Major bleeding events were defined according to the definition by Schulman as (1) a fatal bleeding and/or, (2) symptomatic bleeding in a critical area or organ, such as intracranial, intraspinal, intraocular, retroperitoneal, intra-articular or pericardial, or intramuscular with compartment syndrome and/or, (3) bleeding causing a fall in hemoglobin level of 20 g/L or more, or leading to transfusion of two or more units of whole blood or red cells (12). Minor bleeding events (clinical relevant non-major bleeding) were defined according to the definition by Kaatz as any sign or symptom of hemorrhage (e.g., more bleeding than would be expected for a clinical circumstance, including bleeding found by imaging alone) that does not fit the criteria for the definition of major bleeding but does meet at least one of the following criteria: (1) requiring medical intervention by a healthcare professional, (2) leading to increased level of care, (3) prompting a face to face evaluation (13). Thromboembolic events were included if diagnosed by standardized diagnostic procedures according to clinical in-house routine such as ultrasound examinations or CT scans. Documented major thromboembolic events included pulmonary thromboembolism, systemic arterial thromboembolism/ischemia and deep vein thrombosis. Diagnosis of clot formation within the ECMO circuits was at the discretion of the respective centers according to their in-house standards. According to the occurrence of BTE during ICU stay, each patient was categorized into one of the five following main groups: Major bleeding plus major thromboembolism, only major bleeding, only major thromboembolism, minor events or no BTE (serving as reference cohort). To further investigate distinct entities of BTE, each patient was categorized into the following subgroups: Major bleeding plus major thromboembolism, only major intracranial bleeding, only major pulmonary bleeding, only major intraperitoneal bleeding, only major bleeding ECMO cannula, only major bleeding other than above, only major pulmonary embolism, only major thromboembolism other than above, minor events or no BTE (serving as reference cohort).
Statistical analysis
Descriptive statistics are expressed as median (IQR) for continuous variables and as frequencies for categorical variables (including a category for missing data). Differences between groups were tested using the Kruskal-Wallis-test (continuous variables) or χ2 test (categorical variables). χ2 test with post hoc pairwise z-tests were applied to analyze survival in the subgroups (major bleeding plus major thromboembolism, only major bleeding, only major thromboembolism, only minor events or no BTE). No BTE served as reference cohort. To further investigate ICU-mortality and estimated odds ratios (ORs) with corresponding 95% confidence intervals (CIs) in these distinct entities, we used unadjusted logistic regression analyses. We performed adjusted logistic regression analyses in subgroups consisting of >40 patients and performed univariate χ2 test in subgroups consisting of ≤40 patients to study estimated odds ratios (ORs) with corresponding 95% confidence intervals (CIs). Variables were selected a priori based on clinical background knowledge: age (41–70 or >70 years vs. 18–40 years), sex (female vs. male), duration of ECMO therapy (> 14 days vs. ≤ 14 days), blood flow (>4.5 L/min ≥ 72 h vs. ≤4.5 L/min for ≥72 h or >4.5 L/min for ≤72 h), platelet count (<100.000/μL for ≥72 h vs. ≥100.000 μL for ≥72 h or <100.000/μL for <72 h), C-reactive protein (CRP) levels (>60 mg/dL for ≥72 h vs. ≤60 mg/dL for ≥72 h or >60 mg/dL for <72 h), interleukin-6 (IL-6) (>500 pg./dL for ≥72 h vs. ≤500 pg./dL for ≥72 h or >500 pg./dL for <72 h). Data analysis was performed with SPSS Statistics version 28.0.0.1 (IBM, Armonk, USA). The significance level was set to 0.05. As this is an exploratory study, p-values (two-tailed) are not corrected for multiple testing and interpreted descriptively.
Ethics
This study has been performed in accordance with the Declaration of Helsinki. The Ethics Committee of the Medical Faculty of the Julius-Maximilians-University of Wuerzburg approved the study protocol (131/20-me). Each participating ECMO centers obtained additional votes from their local ethics committee. According to German legislation, no informed consent for collecting retrospective, anonymous data is required. Hence, the Ethics Committee of the Medical Faculty of the Julius-Maximilians-University of Wuerzburg waived informed consent. This study was registered in the German Clinical Trials Register (study ID: DRKS00022964, retrospectively registered, September 7th 20201).
Results
Key results are given as graphical abstract.
Patient population
Between January 1st 2020 and December 31st 2021, 1,373 patients were entered into the database and 945 patients with complete datasets were included in the current analysis (Figure 1). Reasons for exclusion in the current analysis were: 418 datasets were incomplete (271 patients were still treated on or not discharged from the ICU after July 31st 2021, respectively; 81 patients were excluded due to ICU stays outside of the defined timeframe of the analysis; in 66 patients, data on bleeding or thromboembolic events were missing). Preexisting coagulopathies excluded ten patients.
Figure 1

Patient recruitment. Patient recruitment is depicted in this flowchart. Number and reason for exclusion are given. Nine hundred and forty-five patients were finally included and each patient was categorized into one of the five following main groups: No Bleeding and Thromboembolism, Major Bleeding and Major Thromboembolism, Only Major Bleeding or Only Major Thromboembolism.
Table 1 and Table S1 provide a detailed overview on the characteristics, co-morbidities, as well as ECMO support and adjunct therapies. Most patients (85.6%) were between 41–70 years old. 735 patients (78.4%) were male. Median BMI was 29.9 (IQR 27.1–35.2). V-V ECMO used in 914 patients (96.7%) with a median duration of 333.3 h (IQR 164–576 h). Median duration of ICU care was 26 days (IQR 16–42 days). Only 36 patients (3.8%) were on long-term anticoagulation prior to ICU admission. Anticoagulation regimen on day 1 of ECMO support was unfractionated heparin in 793 (85.7%), low-molecular-weight-heparin in 45 (4.9%) or argatroban in 81 (8.8%) (data not shown).
Table 1
| Level | Overall | No BTE | Major bleeding plus thromboembolism | Only major bleeding | Only major thromboembolism | Minor events | p-value | |
|---|---|---|---|---|---|---|---|---|
| n (%) | 945 (100) | 237 (25.1) | 86 (9.1) | 265 (28.0) | 128 (13.5) | 229 (24.2) | ||
|
||||||||
| Age group [years] | n (%) | <0.001 | ||||||
| 19–40 | 86 (9.1%) | 23 (9.7%) | 7 (8.1%) | 18 (6.8%) | 11 (8.6%) | 27 (11.8%) | ||
| 41–70 | 809 (85.6%) | 206 (86.9%) | 67 (77.9%) | 234 (88.3%) | 111 (86.7%) | 191 (83.4%) | ||
| 71–80 | 47 (5.0%) | 8 (3.4%) | 11 (12.9%) | 12 (4.5%) | 5 (3.9%) | 11 (4.8%) | ||
| Missing | 3 | 0 | 1 | 1 | 1 | 0 | ||
| Sex (by birth) | n (%) | 0.049 | ||||||
| Male | 735 (78.4%) | 181 (76.7%) | 77 (90.6%) | 209 (79.5%) | 94 (74.6%) | 174 (76.7%) | ||
| Female | 202 (21.6%) | 55 (23.3%) | 8 (9.4%) | 54 (20.5%) | 32 (25.4%) | 53 (23.3%) | ||
| Missing | 8 | 1 | 1 | 2 | 2 | 2 | ||
| BMI [kg/cm2] | Median (IQR) | 29.9 (27.1–35.2) | 31.0 (27.2–36.5) | 28.2 (26.1–31.0) | 29.6 (26.9–35.1) | 29.6 (27.6–35.0) | 30.9 (27.7–36.9) | <0.001 |
| Missing | 24 | 7 | 2 | 8 | 4 | 3 | ||
|
||||||||
| Mode of ECMO support | n (%) | 0.017 | ||||||
| V-V ECMO | 914 (96.7%) | 236 (99.6%) | 79 (91.9%) | 257 (97.0%) | 121 (94.5%) | 221 (96.5%) | ||
| V-A ECMO | 19 (2.0%) | 1 (0.4%) | 3 (3.5%) | 6 (2.3%) | 5 (3.9%) | 4 (1.7%) | ||
| V-VA ECMO | 12 (1.3%) | 0 (0.0%) | 4 (4.7%) | 2 (0.8%) | 2 (1.6%) | 4 (1.7%) | ||
| Missing | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Mean duration of ECMO therapy [h] | Median (IQR) | 333.3 (164–576) | 216 (108–426) | 356 (168–761) | 379 (175.5–648) | 355 (190–561) | 384 (192–597) | <0.001 |
| Missing | 3 | 0 | 0 | 2 | 0 | 1 | ||
|
||||||||
| Mean duration of ICU stay [d] | Median (IQR) | 26 (16–42) | 21 (14–37) | 27 (15–50) | 27 (17–41) | 28 (17.5–44.5) | 28 (19–45) | 0.004 |
| Missing | 45 | 11 | 3 | 14 | 4 | 13 | ||
| Mean duration of hospital stay [d] | Median (IQR) | 29 (18–45) | 25 (17–41.5) | 27 (15–51) | 30 (19.5–44.5) | 29.5 (20–50) | 32 (21–48) | 0.015 |
| Missing | 84 | 17 | 7 | 25 | 8 | 27 | ||
| In-ICU survival | n (%) | 325 (34.5%) | 108 (45.6%) | 19 (22.1%) | 57 (21.6%) | 55 (43.0%) | 86 (37.7) | <0.001 |
| Missing | 2 | 0 | 0 | 1 | 0 | 1 | ||
Clinical characteristics.
Clinical characteristics are presented in total population and in subgroups no BTE vs. major bleeding plus major thromboembolism vs. only major bleeding vs. only major thromboembolism vs. minor events, respectively. Descriptive statistics are expressed as frequencies for categorical variables (including a category for missing data) and as median (IQR) for continuous variables. Differences between groups were tested using the Kruskal-Wallis-test (continuous variables) or Pearson-χ2-test (categorical variables). P-values less than 0.05 were considered as statistically significant.
Significant P-values are given in bold values.
Bleeding and thromboembolic events
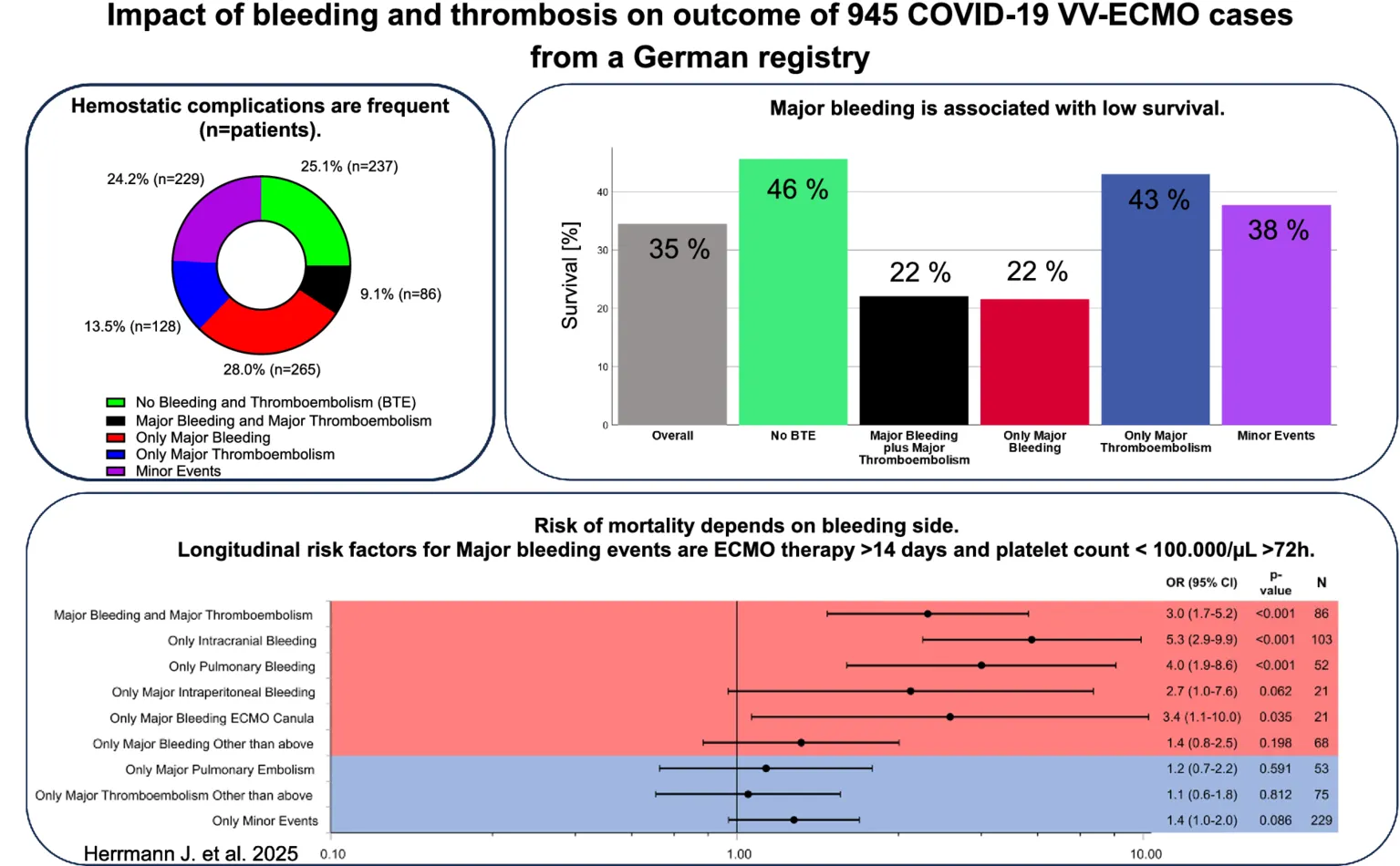
We found 1,348 BTE in 945 patients: 406 major bleeding events (1 event in 303 patients, 2 events in 42 patients, ≥3 events in 6 patients), 506 minor bleeding events (1 event in 305 patients, 2 events in 69 patients, ≥3 events in 20 patients), 258 major thromboembolic events (1 event in 180 patients, 2 events in 28 patients, ≥3 events in 6 patients) and 178 minor thromboembolic events (1 event in 106 patients, 2 events in 17 patients, ≥3 events in 10 patients) (Table 2). Two hundred and thirty-seven patients (25.1%) had neither a bleeding nor a thromboembolic event. Major bleeding as the only event occurred in 265 patients (28.0%). Eighty-six patients (9.1%) had major bleeding and major thromboembolic events. Hundred twenty-eight patients (13.5%) solely had major thromboembolic events. Two hundred twenty-nine patients (24.2%) solely had minor bleeding or minor thromboembolic events.
Table 2
| Entity and location | N events | N patients | % of total events | % of total patients (N = 945) |
|---|---|---|---|---|
| Overall | 1,348 | 708 | 100 | 74.9 |
| Major bleeding event | 406 | 346 | 30.1 | 36.6 |
| Intracranial | 133 | 133 | 9.9 | 14.1 |
| Pulmonary | 116 | 105 | 8.6 | 11.1 |
| Intraperitoneal | 42 | 38 | 3.1 | 4.0 |
| ECMO cannula | 33 | 33 | 2.4 | 3.5 |
| Surgical site or catheter | 25 | 24 | 1.9 | 2.5 |
| Mouth | 25 | 25 | 1.9 | 2.6 |
| Retroperitoneal | 10 | 10 | 0.7 | 1.1 |
| Heart | 5 | 4 | 0.4 | 0.4 |
| Bladder | 5 | 5 | 0.4 | 0.5 |
| Nose | 6 | 6 | 0.4 | 0.6 |
| Muscle (with compartment syndrome) | 4 | 4 | 0.3 | 0.4 |
| Spine | 1 | 1 | 0.1 | 0.1 |
| Eye | 0 | 0 | 0 | 0 |
| Joint | 0 | 0 | 0 | 0 |
| Missing | 1 | 1 | 0.1 | 0.1 |
| Minor bleeding event | 506 | 410 | 37.5 | 43.4 |
| ECMO cannula | 141 | 140 | 10.5 | 14.8 |
| Nose | 95 | 94 | 7.0 | 9.9 |
| Mouth | 94 | 94 | 7.0 | 9.9 |
| Surgical site or catheter | 72 | 67 | 5.3 | 7.1 |
| Intraperitoneal | 38 | 37 | 2.8 | 3.9 |
| Bladder | 30 | 30 | 2.2 | 3.2 |
| Skin | 19 | 18 | 1.4 | 1.9 |
| Muscle (without compartment syndrome) | 9 | 8 | 0.7 | 0.8 |
| Missing | 8 | 8 | 0.6 | 0.8 |
| Major thromboembolic event | 258 | 128 | 19.1 | 13.5 |
| Pulmonary embolism | 115 | 113 | 8.5 | 12.0 |
| Systemic arterial embolus or ischemia | 80 | 72 | 5.9 | 7.6 |
|
34 | 34 | 2.5 | 3.6 |
|
14 | 14 | 1.0 | 1.5 |
|
9 | 9 | 0.7 | 1.0 |
|
10 | 10 | 0.7 | 1.1 |
|
5 | 5 | 0.4 | 0.5 |
|
6 | 6 | 0.4 | 0.6 |
|
1 | 1 | 0.1 | 0.1 |
| Deep vein thrombosis | 63 | 56 | 4.7 | 5.9 |
| Minor thromboembolic event | 178 | 133 | 13.2 | 14.1 |
| Oxygenator clot | 108 | 71 | 8.0 | 7.5 |
| Other | 70 | 68 | 5.2 | 7.2 |
Total number and locations of bleeding and thromboembolic events.
Total number and locations of bleeding and thromboembolic events, as well as the respective frequencies in relation to the number of total events are shown. Multiple mentioning of events in terms of different entities or repetitive events in one single patient are possible.
Results from main groups are given in bold.
The most common major bleeding locations were intracranial hemorrhage and pulmonary bleeding. Hundred and ten patients (11.7%) exclusively suffered from intracranial hemorrhage without additional BTE. Pulmonary embolism was the most common thromboembolic event, followed by clotting of the ECMO oxygenator.
Blood products
Application and dosage of blood products are depicted in Supplementary Table S1. Patients suffering from only mayor bleeding received the highest volume with median of 4,500 mL (IQR 2150–8,000) packed red blood cells (PRBC), 800 mL (IQR 200–2,300) platelet concentrates (PC) and 880 mL (IQR 0-2200 mL) fresh frozen plasma (FFP). Patients with no BTE received the lowest volume of blood products (PRBC: 1500 mL (IQR 600–2,775), PC: 0 mL [IQR 0–400), FFP 0 mL (IQR 0-400 mL)], respectively. Prothrombin complex concentrate (PCC) was used in 109 patients (11.6%). Patients with major bleeding plus thromboembolism received PCC in 20.2% (cumulative dose: 3000 IU, IQR: 2500–6,500), patients with only major bleeding in 22.5% (cumulative dose: 3000 IU, IQR: 2000–4,000). Coagulation factor XIII was used in 342 patients (36.5%), most frequently in patients with only major bleeding (50.8%) with a cumulative dose of 3,750 IU (IQR: 3000–5,000). A quarter of patients received antithrombin supplementation. Fibrinogen was administered in 158 patients (16.8%), coagulation factor VII only in 7 patients (0.8%) (data not shown).
Outcome
Overall survival to ICU discharge was 34.5% (325/945 patients) (Figure 2). Survival significantly differed between patient groups defined by type of BTE (p < 0.001). In patients without any bleeding or thromboembolic event survival was 45.6%. Survival in patients with only a major thromboembolic event was 43.0 and 37.7% in patients with only a minor bleeding or thromboembolic event. Patients with pulmonary embolism as the sole major BTE survived in 42.2% of the cases. Only major thromboembolism (OR 1.1 [0.7–1.7; p = 0.633]) or minor events (OR 1.4 [1.0–2.0; p = 0.086]) were not significantly associated with non-survival compared to no BTE. Survival in patients with a major bleeding event was 21.6% with an odds ratio (OR) of 3.0 [2.0–4.5; p < 0.001] for non-survival versus no BTE and 22.1% in patients with a major bleeding plus major thromboembolic event with an OR of 3.0 [1.7–5.2; p < 0.001] (Figure 3A). Survival in patients with intracranial bleeding was 12% with an OR for non-survival of 5.3 [2.9–9.9; p < 0.001]. Furthermore, the OR for non-survival was 4.0 [1.9–8.6; p < 0.001] for only pulmonary bleeding and 3.4 [1.1–10.0; p = 0.035] in case of only major bleeding ECMO cannula compared to no BTE (Figure 3B). Post hoc analyses revealed significantly lower survival in patients with major bleeding and major bleeding plus major thromboembolic compared to no BTE (p < 0.05, respectively) or major thromboembolism (p < 0.05, respectively).
Figure 2
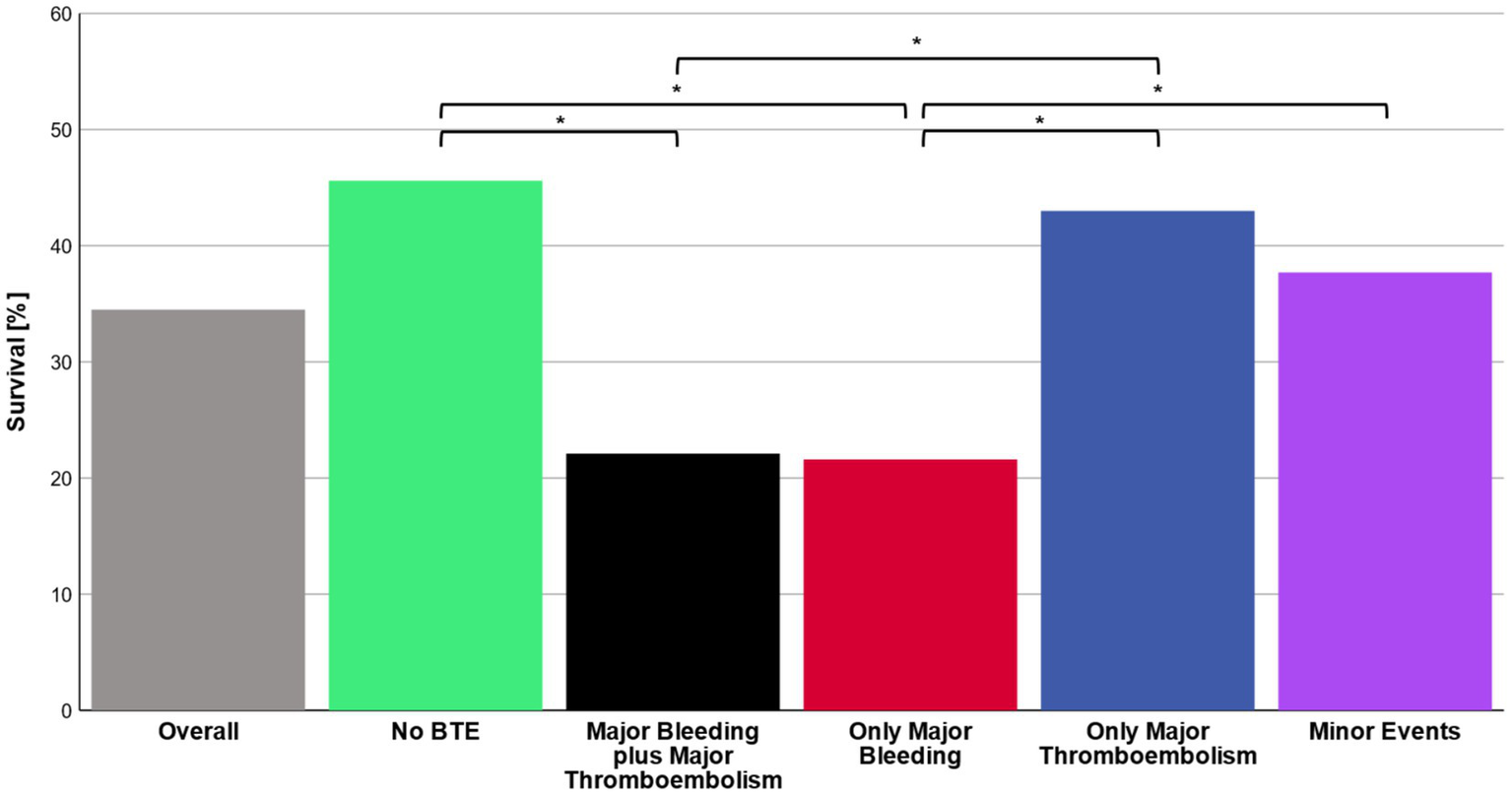
Survival. Total in-ICU survival rates are presented for the overall cohort as well as for the main groups no bleeding or thromboembolism (no BTE), major bleeding plus major thromboembolism, only major bleeding, only major thromboembolism and minor events, respectively. χ2 test with post hoc pairwise z-test was applied to analyze survival in the subgroups. p-values less than 0.05 were considered as statistically significant (*).
Figure 3

Subgroup specific risk for in-ICU mortality. Subgroup specific risk for in-ICU mortality in (a) main groups and in (b) specific subgroups are depicted. (a) Each patient from the cohort was categorized into one of the five following main groups: Major bleeding plus major Thromboembolism, only major bleeding, only major thromboembolism, minor events, no BTE (serving as reference cohort). (b) In a second step, we investigated specific risk for in-ICU mortality in distinct entities. Each patient from the cohort was categorized into one of the subgroups: Major bleeding plus major thromboembolism, only major intracranial bleeding, only major pulmonary bleeding, only major intraperitoneal bleeding, only major bleeding ECMO cannula, only major bleeding other than above, only major pulmonary embolism, only major thromboembolism other than above, minor Events, no BTE (serving as reference cohort). Analyses were performed by unadjusted logistic regression. OR, CI, p-values and number of patients in subgroups are given. p-values less than 0.05 were considered as statistically significant.
Risk factors
Factors associated with major bleeding were duration of ECMO therapy >14 days (OR: 2.9; CI 1.8–4.7; p < 0.001; reference: ≤14 days) and platelet count <100.000/μL for ≥72 h (OR: 2.0; CI 1.1–3.6; p = 0.018; reference: ≥100.000 μL for ≥72 h or <100.000/μL for <72 h). In addition, duration of ECMO therapy >14 days was associated with major bleeding and thromboembolism (OR: 2.4; CI 1.2–4.8; p = 0.01), only major thromboembolism (OR: 3.9; CI 2.1–7.2; p < 0.001) and minor events (OR: 2.4; CI 1.5–4.0; p < 0.001) (respective reference: duration of ECMO ≤14 days). In contrast, CRP level >60 mg/dL for ≥72 h was associated with lower risk for major bleeding and thromboembolism (OR: 0.2; CI 0.07–0.5; p = 0.002), and major thromboembolism (OR: 0.2; CI 0.1–0.5; p < 0.001) compared to reference CRP ≤ 60 mg/dL for ≥72 h or >60 mg/dL for <72 h (Figures 4A–D).
Figure 4
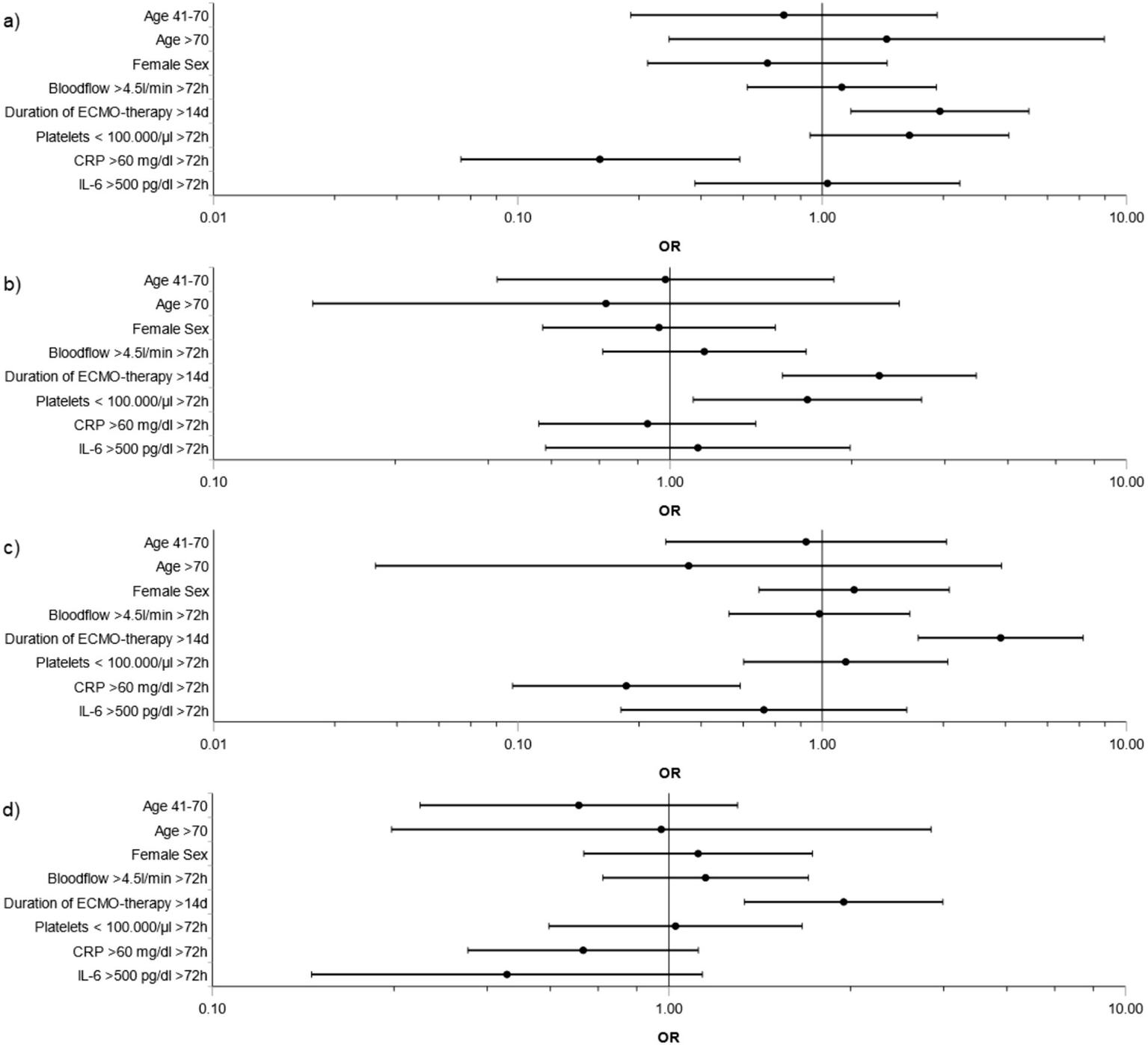
Risk factors for BTE during ECMO support in main groups. Subdivisions show risk factors during ECMO support for the main groups (a) Major bleeding plus thromboembolism, (b) Major bleeding, (c) Major thromboembolism, (d) Minor events. Variables were selected a priori based on clinical background knowledge: age (41–70 or >70 years vs. 18–40 years), sex (female vs. male), duration of ECMO therapy (>14 days vs. ≤14 days), blood flow (>4.5 L/min ≥ 72 h vs. ≤4.5 L/min for ≥72 h or >4.5 L/min for ≤72 h), platelet count (<100.000/μL for ≥72 h vs. ≥100.000 μL for ≥72 h or <100.000/μL for <72 h), C-reactive protein (CRP) levels (>60 mg/dL for ≥72 h vs. ≤60 mg/dL for ≥72 h or >60 mg/dL for <72 h), interleukin-6 (IL-6) (>500 pg./dL for ≥72 h vs. ≤500 pg./dL for ≥72 h or >500 pg./dL for <72 h). Analysis was performed by adjusted logistic regression. OR and CI are depicted.
Considering distinct bleeding and thromboembolic entities, prolonged ECMO therapy >14 days was associated with only pulmonary bleeding (OR: 4.4; CI 1.9–10.3; p < 0.001) compared to reference duration of ECMO ≤14 days. Intracranial bleeding only (OR: 2.3, CI 1.1–4.6; p = 0.021) and major intraperitoneal bleeding only (OR: 19.3, CI 1.7–217.5; p = 0.017) were associated with platelet count <100.000/μL for ≥72 h (reference: ≥100.000 μL for ≥72 h or <100.000/μL for <72 h). OR of only major pulmonary embolism was 3.2 (CI 1.3–7.7; p = 0.011) for duration of ECMO >14 days (reference: ECMO ≤14 days) (Supplementary Figures S1, S2). Sensitivity analyses for the models are given in Supplementary Figures S3–S5.
Discussion
BTE were common in COVID-19 ECMO patients. Major bleeding was the core outcome parameter associated with ICU survival. Approximately one-third of COVID-19 ECMO patients survived to ICU discharge as previously discussed in our preceding analysis of the COVID-19 ECMO database (3). One-third of the patients suffered from major bleeding, a situation in which survival halved. Odds of non-survival increased three-fold with major bleeding. The most severe complication was intracranial bleeding with five-fold increased odds of non-survival compared to patients without BTE. Thromboembolic events on the contrary did not decrease survival of COVID-19 ECMO.
Multiple studies identified bleeding complications as a factor of non-survival in COVID-19 ECMO, including our own preceding analysis (2, 3, 11). Sex was not associated with increased risk for BTE although 78% of the patients were male. In COVID-19 male sex was found to be associated with higher disease severity and case fatality (14) and multiple studies found that the majority of patients with COVID-19 ARDS and non-COVID-19 ARDS are male (15, 16). In this context, the predominance of male sex among our ECMO cohort is equal to preceding similar studies (17). A retrospective study of 620 COVID-19 ECMO patients from France found bleeding events in 29% and an odds of 2.91 for in-hospital mortality. As corroborated by our data, thrombotic events were not associated with increased in-hospital mortality (11). In our larger cohort, we were able to differentiate between major and minor bleeding, as well as major and minor thromboembolic events. We found a similar incidence for the sole occurrence of major bleeding, as well as an even higher number of minor bleeding. However, differentiation of bleeding severity and localization is critical to determine core outcomes. In this regard, a recent modified Delphi study suggested major bleeding and intracranial hemorrhage as core outcomes to assess in all ECMO research (18). Prior non-COVID-19 and COVID-19 ECMO studies often described device-related or cannulation site bleeding as the most frequent bleeding locations (11). Our data show that cannulation side bleeding is frequent, but mostly minor. In our cohort, intracranial hemorrhage most severely affected survival and was the most frequent major bleeding. We found intracranial hemorrhage in 14% of the patients and a more than five-fold increased risk of non-survival in these patients. Previous studies described incidences of 8–12% and an even higher odds ratio of non-survival (11, 18). Data on 63 ECMO autopsy cases found intracranial bleeding in 21% of the patients and classified it as the immediate or underlying cause of death in 78% (19). Besides intracranial hemorrhage, pulmonary bleeding is also frequent. Pulmonary hemorrhage is always a critical bleeding event as worsening gas exchange due to obstruction of the tracheobronchial tree, as well as continued high ECMO blood flows potentially result in a vicious cycle. The 2016 Extracorporeal Life Support Organization registry described pulmonary hemorrhage in 6.1% of non-COVID-19 ECMO (20), whereas in COVID-19 ECMO 9% of the patients had pulmonary bleeding (11). Our cohort had a similar incidence of pulmonary bleeding, which lead to a fourfold increase in odds of non-survival.
The identification of modifiable risk factors is pivotal to ameliorate core outcomes. Pathophysiological changes are complex, whereas, over-activation of the coagulation cascade and suppression of fibrinolysis (21) during COVID-19 result from hypercytokinemia, hyperinflammation, endothelial damage and platelet activation (22–24). Prior studies in non-COVID-19 ECMO already described cross-sectional data on thrombocytopenia as a risk factor of intracranial hemorrhage (25). In order to depict the dynamics of ECMO- and ICU treatment we analyzed a set of longitudinal parameters of therapy. Our granular data suggest that extended periods of low platelet counts increased the risk of major bleeding, in particular intracranial bleeding. Duration of ECMO was widely associated with major and minor bleeding and thromboembolic events, which is in line with data from the international ELSO registry in patients on ECMO previous to the COVID-19 pandemic (26). We also found that extended periods of high CRP levels were associated with lower risks for major bleeding and thromboembolism or major thromboembolism alone. CRP is a modulator of acute inflammation and processes both pro-inflammatory and anti-inflammatory properties (27). Its function in thromboinflammation appears significantly dependent upon conformational changes that can occur in response to a range of stimuli (28). Levels of CRP > 100 mg/L at initial presentation increased the risk of venous thromboemboli in COVID-19 patients, whereas severity of the thrombotic events was not specified (29). Furthermore, in prior study of 321 ECMO patients lower values of C-reactive protein associated with hemorrhage, corroborating the complex relationship between inflammation and bleeding in ECMO patients (30).
Previous reports also found a positive correlation between the incidence of venous thrombi or thrombemboli and the length of ECMO support (31, 32). Although direction of causality remains unclear, patients with any BTE had ECMO runtimes more than 1.5 times longer compared to no BTE. One third of our severely ill ECMO cohort had thrombi or thromboemboli, which is similar to the upper range of prior studies describing incidences of 13.5–31% (33, 34). Pulmonary embolism was present in 12.0% of our ECMO patients, whereas others found incidences as high as 26% in COVID-19 ICU patients (35). Concomitantly, our data suggest that major thromboembolic events have less impact onto survival compared to major bleeding. Survival in patients with sole major thromboembolic events was similar to no BTE and major thromboembolic events in conjunction with major bleeding did not worsen survival compared to only major bleeding. When considering pulmonary embolism as the sole major BTE, survival was comparable to patients without any bleeding or thromboembolic event. However, clinical features of major thromboembolism vary from intermediate to high-risk events and mechanisms of subsequent hemodynamic deterioration differ. We cannot decipher the magnitude of the pulmonary emboli and subsequent hemodynamic changes. As such, medium or high-risk events must not be underestimated.
Bleeding complications frequently cause transfusion of blood products to enhance oxygen delivery and coagulation. In the PROTECMO study, 83% of the patients received at least one unit of PRBC and a median of 1.4 liters PRBC during a median ECMO duration of 11 days (36). In our study, the amount of blood products greatly varied between groups. Patients with major bleeding received more PRBC, as well as additional blood products compared to those without any BTE. However, patients without any BTE already received a mean of 1.5 liters of PRBC and patients with only minor bleeding events more than 3 liters of PRBC. Moreover, patients with only minor bleedings received a similar amount of PCC as those with major bleeding. Factor XIII was administered to a similar amount in all groups, as previous studies described an acquired factor XIII deficiency in patients with COVID-19 (24) or non-COVID-19 caused infection and sepsis (37, 38). In particular, ARDS patients had a lower factor XIII activity, further deteriorating in a time-dependent manner after the initiation of ECMO (39). In line with this data, factor XIII activity was below normal ranges on day 1 of ECMO support across all groups in our cohort. We cannot decipher the impact of blood product administration on survival, nevertheless our data corroborate that current practice warrants a high need for blood products in any case of ECMO support.
Limitations of our study include an observation period limited to ICU treatment only without any long-term follow up. In addition, we did not document any information on the time of BTE-complication, limiting the interpretation of our findings. Hence, we can not establish a direct temporal connection between the occurrence of a bleeding complication and, e.g., PLT less than 100,000 for 72 h. Our data only allow the conclusion that PLT less than 100,000 for 72 h at any time during ICU treatment was associated with an increased risk of major bleeding. Moreover, we focused on core outcomes. We used definitions for major bleeding (“Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients“) and minor bleeding (“Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients”) by the International Society on Thrombosis and Haemostasis (12, 13), which slightly differ from ELSO definitions. They allow detection of fatal major bleedings and identification of clinically relevant non-major bleedings, gaining more detailed information in this subgroup. Results might differ from previous data according original definitions of bleeding events. Multiple BTE in a single patient were common and the study assessed only major bleeding, major thromboembolic events or major bleeding plus major thromboembolic events. These groups may include additional minor bleeding or minor thromboembolic events. Moreover, diagnosis of clot formation within the ECMO circuits was at the discretion of the respective centers. This could potentially lead to underreporting of events with consecutive and significant differences in reporting between participating centers. We did not assess anticoagulation targets and, thus, were not able to assess if differences in anticoagulation schemes contributed to our results. However, a systemic review including V-V ECMO and venoarterial (V-A) ECMO cases without continuous systemic anticoagulation still found major bleeding in 28% (40), pinpointing toward the conclusion that the ECMO circuit itself primarily causes a bleeding diathesis independent of systemic anticoagulation. We did not assess timing and thresholds for administration of blood products. Hence, it is impossible to identify a potential association between the administration of blood products and ECMO complications or survival.
Our highly granular data indicate relevant implications for clinical management of patients suffering from COVID-19 ARDS on V-V ECMO. (1) Major bleeding events are more frequent and more harmfull than major thromboembolic events in terms of mortality. Aggressive anticoagulation regimens over a long period might provoke major bleeding and should not be administered without strict indication. Continuous reevaluation of individual anticoagulation strategy seems to be paramount. However, superiority of a specific anticoagulation agent and its favorable dosage on ECMO has not yet be demonstrated. There is tremendous need for randomized controlled trials to optimize anticoagulation strategies on ECMO (41). (2) Risk of BTE is associated with duration of ECMO therapy. Regular evaluation of respiratory status and early ECMO weaning trials seem to be reasonable to minimize length of ECMO support and complications. (3) Persistent thrombocytopenia <100.000/μL were associated with major bleeding, in particularly with devastating intracranial bleeding. In this regard, periods of thrombocytopenia should be avoided and implementation of platelet transfusion protocols could be considered in these patients. Besides the role of platelet count, further studies are in dire need to decipher the impact of ECMO-induced platelet dysfunction on BTE and its impact on survival.
Conclusion
In summary, major bleeding was a core outcome-determinant of COVID-19 ECMO mortality with intracranial bleeding as the most devastating complication. Major thromboembolism or minor BTE did not alter the survival of COVID-19 ECMO. Hence, prevention, early recognition and treatment of major bleedings are key to increase the survival of COVID-19 ECMO. In this regard, our granular data indicate that the implementation of early ECMO weaning strategies could decrease the risk of devastating bleeds. Prolonged thrombocytopenia with platelet counts <100.000/μl should be avoided.
Statements
Data availability statement
The datasets presented in this article are not readily available because the datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. Requests to access the datasets should be directed to Herrmann_J4@ukw.de.
Ethics statement
The studies involving humans were approved by Ethics Committee of the Medical Faculty of the Julius-Maximilians-University of Wuerzburg. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because According to German legislation, no informed consent for collecting retrospective, anonymous data is required.
Author contributions
JH: Writing – original draft, Formal analysis, Conceptualization, Writing – review & editing, Data curation. JS: Data curation, Writing – review & editing, Formal analysis, Writing – original draft. PM: Conceptualization, Data curation, Formal analysis, Writing – review & editing, Writing – original draft, Funding acquisition. NP: Data curation, Writing – review & editing, Writing – original draft. MEH: Writing – review & editing, Writing – original draft, Data curation. QN: Writing – original draft, Writing – review & editing, Data curation. JG: Data curation, Writing – review & editing, Writing – original draft. DR: Writing – original draft, Data curation, Writing – review & editing. PK: Data curation, Writing – original draft, Writing – review & editing. MM: Data curation, Writing – review & editing, Writing – original draft. ML: Writing – review & editing, Writing – original draft, Data curation. PS: Writing – review & editing, Writing – original draft, Data curation. SKl: Writing – original draft, Data curation, Writing – review & editing. DJ: Writing – review & editing, Writing – original draft, Data curation. KR: Writing – review & editing, Writing – original draft, Data curation. MS: Writing – original draft, Writing – review & editing, Data curation. CP: Writing – original draft, Writing – review & editing, Data curation. J-CS: Writing – original draft, Data curation, Writing – review & editing. SE: Formal analysis, Writing – review & editing, Writing – original draft. SKr: Writing – original draft, Writing – review & editing, Data curation. AW: Writing – review & editing, Writing – original draft, Data curation. IC: Writing – original draft, Writing – review & editing, Data curation. KB: Writing – review & editing, Writing – original draft, Data curation. KS: Writing – review & editing, Writing – original draft, Data curation. TB: Writing – original draft, Writing – review & editing, Data curation. FH: Writing – review & editing, Writing – original draft, Data curation. FE: Writing – original draft, Writing – review & editing, Data curation. JW: Data curation, Writing – review & editing, Writing – original draft. RE: Data curation, Writing – original draft, Writing – review & editing. DO: Data curation, Writing – review & editing, Writing – original draft. BE: Writing – original draft, Data curation, Writing – review & editing. GL: Data curation, Writing – review & editing, Writing – original draft. FR: Writing – review & editing, Data curation, Writing – original draft. TW: Data curation, Writing – original draft, Writing – review & editing. DS: Writing – review & editing, Writing – original draft, Data curation. VZ: Writing – review & editing, Data curation, Writing – original draft. OM: Data curation, Writing – original draft, Writing – review & editing. CKü: Writing – original draft, Data curation, Writing – review & editing. MK: Writing – review & editing, Writing – original draft, Data curation. RM: Writing – review & editing, Writing – original draft, Data curation. PG: Writing – review & editing, Data curation, Writing – original draft. FF: Writing – review & editing, Writing – original draft, Data curation. MB: Writing – original draft, Data curation, Writing – review & editing. MF: Writing – review & editing, Data curation, Writing – original draft. VR: Writing – original draft, Data curation, Writing – review & editing. MH: Writing – original draft, Data curation, Writing – review & editing. GS: Writing – review & editing, Writing – original draft, Data curation. TL: Writing – original draft, Writing – review & editing, Data curation. J-SP: Writing – original draft, Writing – review & editing, Data curation. CH: Data curation, Writing – review & editing, Writing – original draft. PL: Writing – original draft, Data curation, Writing – review & editing. AB: Data curation, Writing – review & editing, Writing – original draft. GD: Writing – review & editing, Writing – original draft, Data curation. CM: Data curation, Writing – review & editing, Writing – original draft. PR: Data curation, Writing – review & editing, Writing – original draft. VM: Data curation, Writing – review & editing, Writing – original draft. A-MB: Writing – review & editing, Writing – original draft, Data curation. SB: Writing – original draft, Data curation, Writing – review & editing. RR: Writing – original draft, Software, Formal analysis, Writing – review & editing. AG: Writing – original draft, Formal analysis, Writing – review & editing. PH: Conceptualization, Formal analysis, Writing – original draft, Writing – review & editing. CKa: Data curation, Conceptualization, Writing – original draft, Writing – review & editing. CL: Conceptualization, Writing – review & editing, Formal analysis, Writing – original draft, Data curation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This trial was funded by institutional resources. PM’s department received research grants from the German Research Foundation (ME 3559/1-1, ME 3559/3-1, ME 6094/3-2), BMBF (01KG1815), BMG (ZMVI1-2520DAT10E) and an unrestricted research grant from csl Behring, Germany.
Acknowledgments
We very much thank the collaborators of the German ECMO COVID Study Group who contributed to this study: Department of Anaesthesiology, Intensive Care, Emergency and Pain Medicine, University Hospital Wuerzburg, Germany (Viktoria Keck, Kerstin Müller), University Medical Center Hamburg-Eppendorf, Department of Intensive Care, Hamburg Germany (Melanie Kerinn, Lisa Krebs, Andrea Conrad, Grit Ringeis), Department of Cardiac Surgery, University Hospital Erlangen, Friedrich-Alexander University, Erlangen (Michael Weyand, Christian Heim, Rene Tandler), Department of Anaesthesiology, University Hospital Erlangen, Friedrich-Alexander University, Erlangen (Stefanie Schmidt), Department of Anesthesiology and Intensive Care Medicine, University Hospital Essen, University Duisburg-Essen, Essen, Germany (Lena Garczarek), Department of Anesthesiology, Klinikum Dortmund, Dortmund, Germany (Anke Hoffmann), Medical ICU, Pulmonology and Infectiology, Klinikum Dortmund, Klinikum University Witten/Herdecke, Germany (Daniela Nickoleit-Bitzenberger, Bernhard Schaaf), Department of Cardiovascular Surgery University Heart Center Freiburg – Bad Krozingen, Medical Center – University of Freiburg, Germany (Christoph Benk, Johannes Scheumann, Michael Berchthold-Herz), Department of Cardiology and Angiology I Heart Center Freiburg - Bad Krozingen), Medical Center – University of Freiburg, Germany (Alexander Supady, Asieb Sekandarzad, Annabelle Fluegler), Department of Medicine III (Interdisciplinary Medical Intensive Care), Medical Center – University of Freiburg, Germany (Xavier Bemtgen), Department of Anesthesiology, University Medical Center Göttingen, Germany (Andrea Kernchen, Lars-Olav Harnisch), Department of Anesthesiology and Intensive Care Medicine, University of Leipzig Medical Center, Leipzig, Germany (Antje Seidel, Christian Schlegel, Klinik und Poliklinik für Innere Medizin II), Klinikum rechts der Isar der Technischen Universität München, Munich, Germany (Sebastian Rasch), Department of Cardiology I – Coronary and Peripheral Vascular Disease, Heart Failure, University Hospital Muenster, Muenster, Germany (Carsten Hullermann, Jan Padberg), Department of Anaesthesiology and Critical Care Medicine, St. Franziskus-Hospital Muenster, Germany (Ulrich Göbel), Department of Anesthesiology, University Medical Center of the Johannes Gutenberg-University Mainz, Germany (Christian Blessing, Patrick Schramm).
Conflict of interest
PM reports honoraria for scientific lectures (Biotest AG, CSL Behring, Haemonetics, Pharmacosmos GmbH, Vifor Pharma). PK reports grants or contracts (Netzwerk Universitätsmedizin, APEPTICO GmbH, Federal Ministry of Education and Research (BMBF), Eagle Pharmaceutical); consulting fees (TEVA Ratiopharm, Amicus Ltd., Senzyme); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (TEVA Ratiopharm, CSL Vifor, Sintetica, CSL Behring, Senzyme, Fresenius Kabi, Pajunk); payment for expert testimony (Kanzlei Ulsenheimer & Friedrich Law Firm (München/Berlin), Landgericht Leipzig); support for attending meetings and/or travel [ASER (Prof. Dr. TJ Gan) for Consensus Meeting PONV 2024 Houston (Texas)]; participation on a data safety monitoring board or advisory board [Pharmacosmos, TEVA Ratiopharm, University Hospital Aachen (Tele-Emergency Medicine, Prof. Rolf Rossaint)]; leadership or fiduciary role in other board society, committee or advocacy group, paid or unpaid (Chairperson/Past chairperson of guideline committee ESAIC, Speaker of the Scientific Committee on Obstetric Anaesthesia within the German Society (DGAI), Member of Obstetric Committee within WFSA). DS reports consulting fees (Medtronic); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (Getinge, AstraZeneca); support for attending meetings and/or travel (Abiomed, Getinge, Dahlhausen, Orinpharma, AstraZeneca, Medtronic). TB reports grants or contracts [Deutsche Forschungsgemeinschaft (DFG), Dietmar Hopp Stiftung, Stiftung Universitätsmedizin Essen, Heidelberger Stiftung Chirurgie, Innovationsfonds des Gemeinsamen Bundesausschusses (G-BA)]; payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (CSL Behring GmbH, Schöchl medical education GmbH, Biotest AG, Baxter Deutschland GmbH, Boehringer Ingelheim Pharma GmbH, Astellas Pharma GmbH, B. Braun Melsungen AG; MSD Sharp & Dohme GmbH, Akademie für Infektionsmedizin e. v.; Lücke Kongresse GmbH, Pfizer Deutschland GmbH, MVZ Labor Dr. Limbach & Kollegen GbR); Patents planned issued or pending (BRAHMS GmbH); participation on a data safety monitoring board or advisory board (Baxter Deutschland GmbH, Bayer AG); leadership or fiduciary role in other board society, committee or advocacy group, paid or unpaid (DGAI, DIVI, SepNet, DSG). MK reports stock or stock options (Biontech). FH reports payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (senior editor, Anesthesia and Analgesia); payment for expert testimony (in several court cases related to intensive care therapy); support for attending meetings and/or travel (various meetings, societies); leadership or fiduciary role in other board society, committee or advocacy group, paid or unpaid [senior editor, Anesthesia & Analgesia; Auditor, German Society for Anesthesia and Intensive Care (DGAI)]. VM reports grants or contracts (DFG RO 1506/4-2). Peter Rosenberger reports grants or contracts (DFG RO 3671/14-1). CK reports grants or contracts (PI of X-COPD trial sponsored by Fresenius), consulting fees (Bayer, Xenios); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (Bayer, Xenios, Getinge). Stefan Kluge reports grants or contracts (Cytosorbents, Daiichi Sankyo); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (ADVITOS, Biotest, Daiichi Sankyo, Fresenius Medical Care, Gilead, Mitsubishi Tanabe Pharma, MSD, Pfizer, Shionogi and Zoll); participation on a data safety monitoring board or advisory board (ADVITOS, Fresenius, Gilead, MSD and Pfizer). KS reports grants or contracts [Stiftung Universitätsmedizin Essen (Microcirculation/Sepsis/Covid-19), Stiftung Chirurgie Heidelberg (Microcirculation/Shock), Ernst und Berta Grimmke Stiftung (Hyperspectral imaging/shock/machine learning)]; payment for expert testimony (expert witness in court). J-SP reports payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (Abiomed Europe); support for attending meetings and/or travel (Abiomed Europe, Resuscitec). VZ reports grants or contracts (ELSO Grant for the study “Longterm outcome after COVID-ECMO-Therapy”). FF reports grants or contracts (CEOsys project funded by the Network of University Medicine [Nationales Forschungsnetzwerk der Universitätsmedizin (NUM)] by the Federal Ministry of Education and Research of Germany (Bundesministerium für Bildung und Forschung (BMBF), grant number 01KX2021). PH reports grants or contracts (German Ministry of Research and Education, European Union, German Parkinson Society, University Hospital Würzburg, German Heart Foundation, Federal Joint Committee (G-BA) within the Innovation fond, German Research Foundation, Bavarian State, German Cancer Aid, Charité – Universitätsmedizin Berlin (within Mondafis; supported by an unrestricted research grant to the Charité from Bayer), University Göttingen (within FIND-AF randomized; supported by an unrestricted research grant to the University Göttingen from Boehringer-Ingelheim), University Hospital Heidelberg (within RASUNOA-prime; supported by an unrestricted research grant to the University Hospital Heidelberg from Bayer, BMS, Boehringer-Ingelheim, Daiichi Sankyo); participation on a data safety monitoring board or advisory board [Participation on DSMB in publicly funded studies (by German Research Foundation, German Ministry of Research, Foundations)]. RM reports support for attending meetings and/or travel (Getinge). FR reports support for the present manuscript (publication fees from publication fund of the Goethe University); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (CSL Behring, King of Prussia, PA, USA, University hospital Wuerzburg, Germany, HELIOS clinics, Krefeld, Germany); support for attending meetings and/or travel (MCN congress organization, Nuernberg, Germany); patents planned issued or pending (Patent funding support from LifeSystems, Moenchengladbach, Germany). OM reports grants or contracts [research grant from Advitos for conducting experimental studies related to extracorporeal multi-organ support, member of the national CEOsys network Germany (Covid Ecosystem), and the Napkon-Tip (Therapeutic intervention platform for conducting ongoing assessments of new therapies), funded by the Federal Ministry of Education and Research (BMBF)]; royalties or licenses (honoraria from Springer, Book on neuro-monitoring in the ICU: Neuromonitoring in der Intensivmedizin 2023, ISBN: 978-3-662-65997-7); payment or honoraria for lectures, presentations, speakers, bureaus, manuscript writing or educational events (Getinge Medical, CSL Behring, OM’s Department holds courses and workshops supported by companies related to Intensive Care Medicine); leadership or fiduciary role in other board society, committee or advocacy group, paid or unpaid [German Society of Anesthesiology (DGAI)].
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1649217/full#supplementary-material
Footnotes
References
1.
Badulak J Antonini MV Stead CM Shekerdemian L Raman L Paden ML et al . Extracorporeal membrane oxygenation for COVID-19: updated 2021 guidelines from the extracorporeal life support organization. ASAIO J. (2021) 67:485–95. doi: 10.1097/MAT.0000000000001422
2.
Lebreton G Schmidt M Ponnaiah M Folliguet T Para M Guihaire J et al . Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in greater Paris, France: a multicentre cohort study. Lancet Respir Med. (2021) 9:851–62. doi: 10.1016/S2213-2600(21)00096-5
3.
Herrmann J Lotz C Karagiannidis C Weber-Carstens S Kluge S Putensen C et al . Key characteristics impacting survival of COVID-19 extracorporeal membrane oxygenation. Crit Care. (2022) 26:190. doi: 10.1186/s13054-022-04053-6
4.
Iba T Levy JH . Sepsis-induced coagulopathy and disseminated intravascular coagulation. Anesthesiology. (2020) 132:1238–45. doi: 10.1097/ALN.0000000000003122
5.
Granja T Hohenstein K Schüssel P Fischer C Prüfer T Schibilsky D et al . Multi-modal characterization of the coagulopathy associated with extracorporeal membrane oxygenation. Crit Care Med. (2020) 48:e400–8. doi: 10.1097/CCM.0000000000004286
6.
Kalbhenn J Schlagenhauf A Rosenfelder S Schmutz A Zieger B . Acquired von Willebrand syndrome and impaired platelet function during venovenous extracorporeal membrane oxygenation: rapid onset and fast recovery. J Heart Lung Transplant. (2018) 37:985–91. doi: 10.1016/j.healun.2018.03.013
7.
Malfertheiner MV Philipp A Lubnow M Zeman F Enger TB Bein T et al . Hemostatic changes during extracorporeal membrane oxygenation: a prospective randomized clinical trial comparing three different extracorporeal membrane oxygenation systems. Crit Care Med. (2016) 44:747–54. doi: 10.1097/CCM.0000000000001482
8.
Iba T Levy JH Levi M Thachil J . Coagulopathy in COVID-19. J Thromb Haemost. (2020) 18:2103–9. doi: 10.1111/jth.14975
9.
Malas MB Naazie IN Elsayed N Mathlouthi A Marmor R Clary B . Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClinicalMedicine. (2020) 29:100639. doi: 10.1016/j.eclinm.2020.100639
10.
Jiménez D García-Sanchez A Rali P Muriel A Bikdeli B Ruiz-Artacho P et al . Incidence of VTE and bleeding among hospitalized patients with coronavirus disease 2019: a systematic review and Meta-analysis. Chest. (2021) 159:1182–96. doi: 10.1016/j.chest.2020.11.005
11.
Mansour A Flecher E Schmidt M Rozec B Gouin-Thibault I Esvan M et al . Bleeding and thrombotic events in patients with severe COVID-19 supported with extracorporeal membrane oxygenation: a nationwide cohort study. Intensive Care Med. (2022) 48:1039–52. doi: 10.1007/s00134-022-06794-y
12.
Schulman S Kearon C . Definition of major bleeding in clinical investigations of antihemostatic medicinal products in non-surgical patients. J Thromb Haemost. (2005) 3:692–4. doi: 10.1111/j.1538-7836.2005.01204.x
13.
Kaatz S Ahmad D Spyropoulos AC Schulman S Subcommittee on Control of Anticoagulation . Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. (2015) 13:2119–26. doi: 10.1111/jth.13140
14.
Forsblom E Silén S Kortela E Ahava M Kreivi HR Holmberg V et al . Male predominance in disease severity and mortality in a low Covid-19 epidemic and low case-fatality area - a population-based registry study. Infect Dis (Lond). (2021) 53:789–99. doi: 10.1080/23744235.2021.1936157
15.
Bellani G Laffey JG Pham T Fan E Brochard L Esteban A et al . Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. (2016) 315:788–800. doi: 10.1001/jama.2016.0291
16.
Grasselli G Greco M Zanella A Albano G Antonelli M Bellani G et al . Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy. Italy JAMA Intern Med. (2020) 180:1345–55. doi: 10.1001/jamainternmed.2020.3539
17.
Karagiannidis C Strassmann S Merten M Bein T Windisch W Meybohm P et al . High in-hospital mortality rate in patients with COVID-19 receiving extracorporeal membrane oxygenation in Germany: a critical analysis. Am J Respir Crit Care Med. (2021) 204:991–4. doi: 10.1164/rccm.202105-1145LE
18.
Hodgson CL Burrell AJC Engeler DM Pellegrino VA Brodie D Fan E et al . Core outcome measures for research in critically ill patients receiving extracorporeal membrane oxygenation for acute respiratory or cardiac failure: an international, multidisciplinary, modified Delphi consensus study. Crit Care Med. (2019) 47:1557–63. doi: 10.1097/CCM.0000000000003954
19.
von Stillfried S Bülow RD Röhrig R Meybohm P Boor P for the German Registry of COVID-19 Autopsies (DeRegCOVID), DeRegCOVID Collaborators# et al . Intracranial hemorrhage in COVID-19 patients during extracorporeal membrane oxygenation for acute respiratory failure: a nationwide register study report. Crit Care. (2022) 26:83. doi: 10.1186/s13054-022-03945-x
20.
Thiagarajan RR Barbaro RP Rycus PT Mcmullan DM Conrad SA Fortenberry JD et al . Extracorporeal life support organization registry international report 2016. ASAIO J. (2017) 63:60–7. doi: 10.1097/MAT.0000000000000475
21.
Iba T Levy JH Levi M Connors JM Thachil J . Coagulopathy of coronavirus disease 2019. Crit Care Med. (2020) 48:1358–64. doi: 10.1097/CCM.0000000000004458
22.
Song WC FitzGerald GA . COVID-19, microangiopathy, hemostatic activation, and complement. J Clin Invest. (2020) 130:3950–3. doi: 10.1172/JCI140183
23.
Iba T Levy JH Connors JM Warkentin TE Thachil J Levi M . The unique characteristics of COVID-19 coagulopathy. Crit Care. (2020) 24:360. doi: 10.1186/s13054-020-03077-0
24.
von Meijenfeldt FA Havervall S Adelmeijer J Lundström A Magnusson M Mackman N et al . COVID-19 is associated with an acquired factor XIII deficiency. Thromb Haemost. (2021) 121:1668–9. doi: 10.1055/a-1450-8414
25.
Fletcher-Sandersjöö A Bartek J Jr Thelin EP Eriksson A Elmi-Terander A Broman M et al . Predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: an observational cohort study. J Intensive Care. (2017) 5:27. doi: 10.1186/s40560-017-0223-2
26.
Nunez JI Gosling AF O’Gara B Kennedy KF Rycus P Abrams D et al . Bleeding and thrombotic events in adults supported with venovenous extracorporeal membrane oxygenation: an ELSO registry analysis. Intensive Care Med. (2022) 48:213–24. doi: 10.1007/s00134-021-06593-x
27.
Marnell L Mold C Du Clos TW . C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. (2005) 117:104–11. doi: 10.1016/j.clim.2005.08.004
28.
McFadyen JD Kiefer J Braig D Loseff-Silver J Potempa LA Eisenhardt SU et al . Dissociation of C-reactive protein localizes and amplifies inflammation: evidence for a direct biological role of C-reactive protein and its conformational changes. Front Immunol. (2018) 9:1351. doi: 10.3389/fimmu.2018.01351
29.
Al-Samkari H Karp Leaf RS Dzik WH Carlson JCT Fogerty AE Waheed A et al . COVID-19 and coagulation: bleeding and thrombotic manifestations of SARS-CoV-2 infection. Blood. (2020) 136:489–500. doi: 10.1182/blood.2020006520
30.
Rajsic S Breitkopf R Oezpeker UC Bukumirić Z Dobesberger M Treml B . The role of excessive anticoagulation and missing hyperinflammation in ECMO-associated bleeding. J Clin Med. (2022) 11:2314. doi: 10.3390/jcm11092314
31.
Trudzinski FC Minko P Rapp D Fähndrich S Haake H Haab M et al . Runtime and aPTT predict venous thrombosis and thromboembolism in patients on extracorporeal membrane oxygenation: a retrospective analysis. Ann Intensive Care. (2016) 6:66. doi: 10.1186/s13613-016-0172-2
32.
Rajsic S Breitkopf R Rugg C Bukumiric Z Reitbauer J Treml B . Thrombotic events develop in 1 out of 5 patients receiving ECMO support: an 11-year referral Centre experience. J Clin Med. (2023) 12:1082 doi: 10.3390/jcm12031082
33.
Chang H Rockman CB Jacobowitz GR Speranza G Johnson WS Horowitz JM et al . Deep vein thrombosis in hospitalized patients with coronavirus disease 2019. J Vasc Surg Venous Lymphat Disord. (2021) 9:597–604. doi: 10.1016/j.jvsv.2020.09.010
34.
Horiuchi H Morishita E Urano T Yokoyama K the Questionnaire-survey Joint Team on The COVID-19-related thrombosis . COVID-19-related thrombosis in Japan: final report of a questionnaire-based survey in 2020. J Atheroscler Thromb. (2021) 28:406–16. doi: 10.5551/jat.RPT001
35.
Gong X Yuan B Yuan Y . Incidence and prognostic value of pulmonary embolism in COVID-19: a systematic review and meta-analysis. PLoS One. (2022) 17:e0263580. doi: 10.1371/journal.pone.0263580
36.
Martucci G Schmidt M Agerstrand C Tabatabai A Tuzzolino F Giani M et al . Transfusion practice in patients receiving VV ECMO (PROTECMO): a prospective, multicentre, observational study. Lancet Respir Med. (2023) 11:245–55. doi: 10.1016/S2213-2600(22)00353-8
37.
Zeerleder S Schroeder V Lämmle B Wuillemin WA Hack CE Kohler HP . Factor XIII in severe sepsis and septic shock. Thromb Res. (2007) 119:311–8. doi: 10.1016/j.thromres.2006.02.003
38.
Tao GZ Liu B Zhang R Liu G Abdullah F Harris MC et al . Impaired activity of blood coagulant factor XIII in patients with necrotizing enterocolitis. Sci Rep. (2015) 5:13119. doi: 10.1038/srep13119
39.
Moerer O Huber-Petersen JF Schaeper J Binder C Wand S . Factor XIII activity might already be impaired before Veno-venous ECMO in ARDS patients: a prospective, observational single-center cohort study. J Clin Med. (2021) 10:1203. doi: 10.3390/jcm10061203
40.
Olson SR Murphree CR Zonies D Meyer AD Mccarty OJT Deloughery TG et al . Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation: a systematic review. ASAIO J. (2021) 67:290–6. doi: 10.1097/MAT.0000000000001230
41.
Rajsic S Breitkopf R Jadzic D Popovic Krneta M Tauber H Treml B . Anticoagulation strategies during extracorporeal membrane oxygenation: a narrative review. J Clin Med. (2022) 11:5147. doi: 10.3390/jcm11175147
Summary
Keywords
COVID-19, acute respiraratory distress syndrome, extracorporeal membrance oxygenation, bleeding, thromboembolism
Citation
Herrmann J, Schade J, Meybohm P, Paschke N, Hübsch ME, Notz Q, Groene J, Röder D, Kranke P, Merten ML, Landoll M, Spieth P, Kluge S, Jarczak D, Roedl K, Sonntagbauer M, Putensen C, Schewe J-C, Ehrentraut SF, Kreyer S, Wehrfritz A, Castellanos I, Bihlmaier K, Schmidt K, Brenner T, Herbstreit F, Espeter F, Wiefhoff J, Ellerkmann RK, Oswald D, Ellger B, Lotz G, Raimann FJ, Wengenmayer T, Staudacher DL, Zotzmann V, Moerer O, Kühn C, Kochanek M, Muellenbach R, Glaser P, Fichtner F, Bodenstein M, Findeisen M, Rembold V, Heim M, Schneider G, Lahmer T, Padberg J-S, Hullermann C, Lepper PM, Becker AP, Danziger G, Metz C, Rosenberger P, Mirakaj V, Bernard A-M, Braune S, Roth R, Grau A, Heuschmann P, Karagiannidis C and Lotz C (2025) Impact of bleeding and thrombosis on outcome of 945 COVID-19 VV-ECMO cases from a German registry. Front. Med. 12:1649217. doi: 10.3389/fmed.2025.1649217
Received
18 June 2025
Accepted
01 August 2025
Published
20 August 2025
Volume
12 - 2025
Edited by
Zhongheng Zhang, Sir Run Run Shaw Hospital, China
Reviewed by
Sasa Dragic, University of Banja Luka, Bosnia and Herzegovina
Biljana Zlojutro, University Clinical Center of Republika Srpska, Bosnia and Herzegovina
Updates
Copyright
© 2025 Herrmann, Schade, Meybohm, Paschke, Hübsch, Notz, Groene, Röder, Kranke, Merten, Landoll, Spieth, Kluge, Jarczak, Roedl, Sonntagbauer, Putensen, Schewe, Ehrentraut, Kreyer, Wehrfritz, Castellanos, Bihlmaier, Schmidt, Brenner, Herbstreit, Espeter, Wiefhoff, Ellerkmann, Oswald, Ellger, Lotz, Raimann, Wengenmayer, Staudacher, Zotzmann, Moerer, Kühn, Kochanek, Muellenbach, Glaser, Fichtner, Bodenstein, Findeisen, Rembold, Heim, Schneider, Lahmer, Padberg, Hullermann, Lepper, Becker, Danziger, Metz, Rosenberger, Mirakaj, Bernard, Braune, Roth, Grau, Heuschmann, Karagiannidis and Lotz.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christopher Lotz, Lotz_C@ukw.de; Johannes Herrmann, Herrmann_J4@ukw.de
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.