Abstract
Background:
Androgenetic alopecia is a common form of hair loss primarily mediated by dihydrotestosterone (DHT), which induces apoptosis and inhibits proliferation in dermal papilla cells (DPCs). Current treatments, such as minoxidil and finasteride, often show limited efficacy and can cause adverse effects, underscoring the need for safer and more targeted therapies.
Methods:
This study investigated the protective and proliferative effects of angiopoietin-1 (Ang1) on human follicle dermal papilla cells (HFDPCs) under DHT-induced stress. Apoptosis and proliferation were assessed using flow cytometry and BrdU assays. Western blotting was used to examine intracellular signaling pathways. The expression and functional relevance of Tie and integrin receptors were evaluated using gene expression analysis and blocking antibodies.
Results:
Ang1 significantly reduced DHT-induced apoptosis and restored proliferation in HFDPCs. These effects were mediated via activation of the PI3K/AKT and MAPK/ERK1/2 pathways through integrin α5β1. Neither Tie-1 nor Tie-2 receptors were detected in HFDPCs, indicating that Ang1 acts through a Tie-2-independent mechanism. Given the well-established role of Ang1 in promoting vascular stability via the Tie-1–Tie-2 axis in endothelial cells, it is plausible that Ang1 may also support follicular health indirectly by enhancing perifollicular vascularization.
Conclusion:
Ang1 enhances HFDPC survival and proliferation through integrin α5β1-mediated signaling. In addition to its direct protective effects on DPCs, Ang1 may promote angiogenic support in the hair follicle microenvironment. These findings position Ang1 as a potential dual-action therapeutic candidate for androgenetic alopecia.
1 Introduction
Androgenetic alopecia, often referred to as male pattern baldness, is characterized by the miniaturization of hair follicles owing to the influence of dihydrotestosterone (DHT), a potent derivative of testosterone that transforms thick terminal hairs into thin vellus hairs (1). Without treatment, the condition results in gradual and progressive hair thinning (2). This is the leading cause of hair loss, and its incidence tends to increases with age (3–5). Male pattern baldness can negatively impact mental well-being, contributing to issues like persistent self-consciousness, age-related anxiety, and a lack of energy, affecting individuals of both sexes (6–8). Currently, the U. S. Food and Drug Administration (FDA) has approved minoxidil (MNX) and finasteride as treatments for hair loss. Nevertheless, even with the use of these treatments, many individuals continue to struggle with hair loss. Moreover, MNX may cause adverse reactions such as itching and contact dermatitis (9), while the side effects of finasteride include headaches, dizziness, skin irritation, and sexual dysfunction (10–13). Accordingly, there is a growing need for alternative hair loss therapies that offer both effectiveness and a reduced risk of side effects.
The hair follicle, an epidermal appendage, consists of both epidermal and dermal sections. At the base of the follicle lies the dermal papilla, a crucial dermal component essential for hair follicle development and growth cycles (14–17). Notably, the DP has the unique capacity to initiate new hair follicle formation and regulate the number of matrix cells, influencing hair size and density (18–23). Thus, it is proposed that enhancing the number of DP cells or inhibiting their apoptosis may contribute to preventing hair loss by promoting hair thickness and density. Vascular endothelial growth factor (VEGF), a key angiogenic factor primarily recognized for stimulating blood vessel formation, has also been shown to directly increase DP cell proliferation in hair follicles (24). However, the effects of other angiogenic factors on DP cells remain largely unexplored, warranting further investigation.
Angiopoietin-1 (Ang1) is a protein that plays an important role in vascular development and angiogenesis along with VEGF. Both Ang1 and VEGF are angiogenic factors that are involved in survival, proliferation, migration, and tube formation by activating AKT or ERK1/2 in various types of endothelial cells (25–29). Ang1 and VEGF bind to Tie-2 receptor and integrin or VEGF receptors, respectively, transmit downstream signaling, and perform various actions including angiogenesis (26–28, 30–33). Although Ang1 and VEGF act specifically on endothelial cells, VEGF is known to be involved in the survival or proliferation of various cells including DP cells (24, 34–36). However, the effect of Ang1 on DP cells is completely unknown. Therefore, we aimed to investigate the effect of Ang1 on DP cells and the related mechanism.
In this study, it was demonstrated that Ang1 prevented the survival and proliferation of human follicle dermal papilla cells (HFDPCs) decreased by DHT. In addition, Ang1 induced the survival of HFDPCs through the AKT pathway and the proliferation through the ERK1/2 pathway. In addition, Ang1 was found to be involved in the survival and proliferation of HFDPCs through integrin α5β1, not Tie-2. These results suggest that the Ang1/integrin α5β1 axis can be a potential treatment for androgenetic alopecia by preventing the survival and proliferation of HFDPCs decreased by DHT.
2 Materials and methods
2.1 Cell cultures
Human follicle dermal papilla cells (PromoCell, Heidelberg, Germany) and human dermal microvascular endothelial cells (PromoCell) were cultured in a follicle dermal papilla cell growth medium and endothelial cell growth medium (both from PromoCell), respectively. The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
2.2 Reagents and antibodies
Recombinant human Ang1, Ang2, and VEGF were purchased from R&D Systems (Minneapolis, MA, USA). MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide), Wortmannin, PD98059, SB202190, anti-Tie-1, anti-Tie-2, Gly-Arg-Gly-Asp-Ser (GRGDS) peptide, and functional blocking antibodies against integrins α1–6, αv, β1, and α5β1 were purchased from Millipore-Sigma (St. Louis, MO, USA). Other reagents and antibodies used were: anti-cleaved caspase-3, anti-Bax, anti-Bcl-2, anti-Bcl-xL, anti-phospho-AKT, anti-AKT, anti-phospho-ERK1/2, anti-ERK1/2, anti-phospho-p38, anti-p38, anti-integrin α4, anti-integrin α5, anti-integrin αv, anti-integrin β1, anti-integrin β3, anti-integrin β4, and anti-integrin β5 (Cell signaling Technology, Danvers, MA, USA), anti-β-tubulin and peroxidase-conjugated secondary antibodies (Santa Cruz Biotechnology, Dallas, TX, USA), Muse® Annexin V & Dead Cell Assay Kit (FITC) (Luminex Corporation, Austin, TX, USA), and 5′-bromodeoxy-2′-uridine (BrdU) cell proliferation ELISAs (Roche, Indianapolis, IN, USA).
2.3 Cell viability assay
Cell viability was measured using the MTT assay kit (Millipore-Sigma). About 5 × 103 cells were plated in 96-well plates for 24 h and treated with indicated reagents for 48 h. Thereafter, the cells were treated with 100 μL of MTT (5 mg/mL) for 3 h. The formazan levels were measured using the absorbance at 570 nm.
2.4 Apoptosis assay
The apoptotic effect was assessed using the Annexin-V-FITC/PI double-staining assay, following the instructions provided by the manufacturer (Muse® Annexin V & Dead Cell Assay Kit). Cells (3 × 105) were treated with the indicated agents for 48 h. Post incubation, cells were collected in 1 mL of medium containing 1% fetal bovine serum (FBS). Subsequently, the cell suspension (100 μL) was mixed with Muse® Annexin V & Dead Cell reagent and vortexed for 5 s. The mixture was then incubated for 20 min at room temperature and analyzed using the Muse™ Cell Analyzer. Data were processed using the Muse Analysis Software, and cells positive for annexin-V only or annexin-V/PI double staining were considered apoptotic. Each experiment was conducted in triplicate.
2.5 Western blot analysis
Cells were harvested and lysed in radioimmunoprecipitation assay (RIPA) buffer (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein concentration was determined using the bicinchoninic acid (BCA) assay (Thermo Fisher Scientific), and equal amounts of protein (30 μg per lane) were resolved on 10–12% SDS–polyacrylamide gels. Following electrophoresis, proteins were transferred onto nitrocellulose membranes (0.45 μm pore size; GE Healthcare, Chicago, USA) using a semi-dry blotting system (Bio-Rad, Hercules, CA, USA). Membranes were blocked in 5% non-fat dry milk diluted in TBST buffer (Tris-buffered saline with 0.1% Tween-20) for 1 h at room temperature. Primary antibodies were diluted (1: 1000 in 5% BSA in TBST) and incubated overnight at 4 °C. Following three washes with TBST (10 min each), membranes were incubated with HRP-conjugated anti-rabbit or anti-mouse secondary antibodies (Santa Cruz Biotechnology, diluted 1: 5,000 in 5% non-fat dry milk/TBST) for 1 h at room temperature. Bands were visualized using an enhanced chemiluminescence (ECL) detection reagent (Thermo Fisher Scientific) and imaged using the ImageQuant LAS 500 system (GE Healthcare).
2.6 BrdU ELISA proliferation assay
To measure cell proliferation, a Cell Proliferation BrdU ELISA kit (Roche) was used according to the manufacturer’s protocol. Cells treated with the indicated agents for 48 h were labeled with 10 μM BrdU for 1 h. The anti-BrdU peroxidase conjugated antibody was then incubated for 90 min. After washing, the bound peroxidase was detected based on the substrate reaction, which was measured at 450 nm.
2.7 Real-time quantitative PCR
All RNA was extracted from cells and tissues using the RNeasy Plus Mini kit (Qiagen). cDNAs were generated from RNAs (1 μg) using 2.5 μM oligo-dT primers, 1 mM dNTPs, and MuLV reverse transcriptase. qRT-PCR assays were performed in the qPCR Master Mix for SYBR Green PCR Master Mix (Applied Biosystems). qRT-PCR was performed using the following primers: TIE2 (forward: 5’-GCTTGCTCCTTTCTGGAACTGT-3′ and reverse: 5′- CGCCACCCAGAGGCAAT-3′); TIE1 (forward: 5’-AGAACCTAGCCTCCAAGATT-3′ and reverse: 5’-ACTGTAGTTCAGGGACTCAA-3′); ITGA4 (forward: 5’-GCTTCTCAGATCTGCTCGTG-3′ and reverse: 5’-GTCACTTCCAACGAGGTTTG-3′); ITGA5 (forward: 5’-TGCAGTGTGAGGCTGTGTACA-3′ and reverse: 5’-GTGGCCACCTGACGCTCT-3′); ITGAV (forward: 5’-AATCTTCCAATTGAGGATATCAC-3′ and reverse: 5’-AAAACAGCCAGTAGCAACAAT-3′); ITGB1 (forward: 5’-GAAGGGTTGCCCTCCAGA-3′ and reverse: 5’-GCTTGAGCTTCTCTGCTGTT-3′); ITGB3 (forward: 5’-CCGTGACGAGATTGAGTCA-3′ and reverse: 5’-AGGATGGACTTTCCACTAGAA-3′); ITGB4 (forward: 5’-AGACGAGATGTTCAGGGACC-3′ and reverse: 5’-GGTCTCCTCTGTGATTTGGAA-3′); ITGB5 (forward: 5’-GGAGCCAGAGTGTGGAAACA-3′ and reverse: 5’-GAAACTTTGCAAACTCCCTC-3′); and ACTB (forward: 5’-GGGAAATCGTGCGTGACATT-3′ and reverse: 5’-AGTTTCGTGGATGCCACAGG-3′). A mean quantity was estimated from triplicate qRT-PCR reactions following normalization to the control gene.
2.8 Statistical analysis
Statistical analyses were performed using the GraphPad Prism software (GraphPad. Inc., La Jolla, CA, USA). Depending on the experimental design, unpaired two-tailed Student’s t-test (assuming unequal variances), one-way analysis of variance, or two-way analysis of variance followed by Tukey’s post-hoc test was used. A p-value of less than 0.05 was considered statistically significant. All quantitative data are presented as the mean ± standard deviation.
3 Results
3.1 Ang1 increases survival and proliferation in HFDPCs
Initially, an MTT assay was conducted to assess whether angiogenic factors, including Ang1 and Ang2, influence the viability of HFDPCs. When HFDPCs were treated with Ang1 for 48 h, similar to VEGF, it enhanced cell viability, whereas Ang2 had no effect (Figure 1A).
Figure 1

Ang1 induces survival and proliferation in HFDPCs. (A) HFDPCs were treated with Ang1 (300 ng/mL), Ang2 (300 ng/mL), and VEGF (10 ng/mL) for 48 h. The cell viability was analyzed via MTT assay. Bar graph represents mean ± SD (n = 5). *p < 0.05 by one-way ANOVA. (B–E) HFDPCs were treated with Ang1 (300 ng/mL) and/or DHT (2 μM) for 48 h. (B) Apoptosis of HFDPCs was analyzed using annexin-V/PI staining and flow cytometry. Bar graph represents mean ± SD (n = 3). *p < 0.05 by two-way ANOVA. (C) Western blot analysis was performed on lysates from HFDPCs to detect cleaved caspase-3, Bax, Bcl-2, and Bcl-xL. β-tubulin was used as a loading control. (D) Quantitative densitometric analysis in (C) to calculate the ratio of each protein to β-tubulin (n = 3). n.s, not significant. *p < 0.05 by two-way ANOVA. (E) Cell proliferation of HFDPCs was determined by 5′-bromodeoxy-2′-uridine (BrdU) proliferation ELISA. Bar graph represents mean ± SD (n = 5). *p < 0.05 by two-way ANOVA.
Next, to accurately assess whether the enhancement in cell viability induced by Ang1 was associated with cell survival or proliferation, Muse® Annexin V & Dead Cell Assay Kit-based cytometric analysis, western blot analysis, and the BrdU cell proliferation ELISA assay were conducted. Ang1 inhibited DHT-induced apoptosis in HFDPCs (Figure 1B). Similarly, Ang1 prevented DHT-induced increases in cleaved caspase-3 and proapoptotic Bax levels, as well as DHT-induced decreases in antiapoptotic Bcl-2 levels in HFDPCs (Figures 1C,D). However, neither ang1 nor DHT affected antiapoptotic Bcl-xL levels in HFDPCs (Figures 1C,D). In addition, Ang1 increased the proliferation of HFDPCs even when treated alone and prevented the decrease in HFDPCs proliferation caused by DHT (Figure 1E). These results demonstrate that Ang1 prevents DHT-induced increase in apoptosis and decrease in proliferation of HFDPCs.
3.2 Ang1 induces survival through the AKT pathway and proliferation through the ERK1/2 pathway in HFDPCs
Next, the mechanisms through which Ang1 promotes survival and proliferation in HFDPCs were explored. Ang1 is widely recognized for activating AKT, ERK1/2, and p38 in endothelial cells (37, 38), with these signaling pathways playing a key role in survival or proliferation (37, 39). Based on this, it was hypothesized that Ang1 may similarly regulate survival or proliferation in HFDPCs through these pathways.
Ang1 enhanced the phosphorylation of AKT, ERK1/2, and p38 in HFDPCs following treatment for 15, 30, and 60 min (Figure 2A). When treated with DHT, HFDPCs exhibited reduced phosphorylation of AKT and ERK1/2, while p38 phosphorylation remained unchanged (Figures 2B,C). Furthermore, Ang1 inhibited the DHT-induced reduction in AKT and ERK1/2 phosphorylation in HFDPCs (Figures 2B,C). To investigate whether Ang1-induced activation of AKT, ERK1/2, and p38 plays a role in apoptosis or proliferation, the AKT inhibitor Wortmannin, the ERK1/2 inhibitor PD98059, and the p38 inhibitor SB202190 were utilized. Wortmannin blocked Ang1-induced AKT phosphorylation in HFDPCs with no impact on the phosphorylation of ERK1/2 or p38 (Supplementary Figure 1A). PD98059 blocked Ang1-induced ERK1/2 phosphorylation with no impact on the phosphorylation of AKT or p38 (Supplementary Figure 1A), while SB202190 blocked Ang1-induced p38 phosphorylation with no impact on the phosphorylation of AKT or ERK1/2 (Supplementary Figure 1B). Interestingly, Wortmannin fully blocked Ang1-mediated survival under DHT treatment in HFDPCs, whereas PD98059 and SB202190 had no effect (Figure 2D). Furthermore, Wortmannin fully prevented the Ang1-induced reduction in cleaved caspase-3 and pro-apoptotic Bax levels, as well as the increase in anti-apoptotic Bcl-2 levels under DHT treatment in HFDPCs (Figures 2E,F). In contrast, PD98059 fully inhibited Ang1-driven proliferation in HFDPCs, while neither Wortmannin nor SB202190 contributed to proliferation. (Figure 2G). These findings indicate that Ang1 promotes survival via the AKT pathway and drives proliferation through the ERK1/2 pathway in HFDPCs.
Figure 2
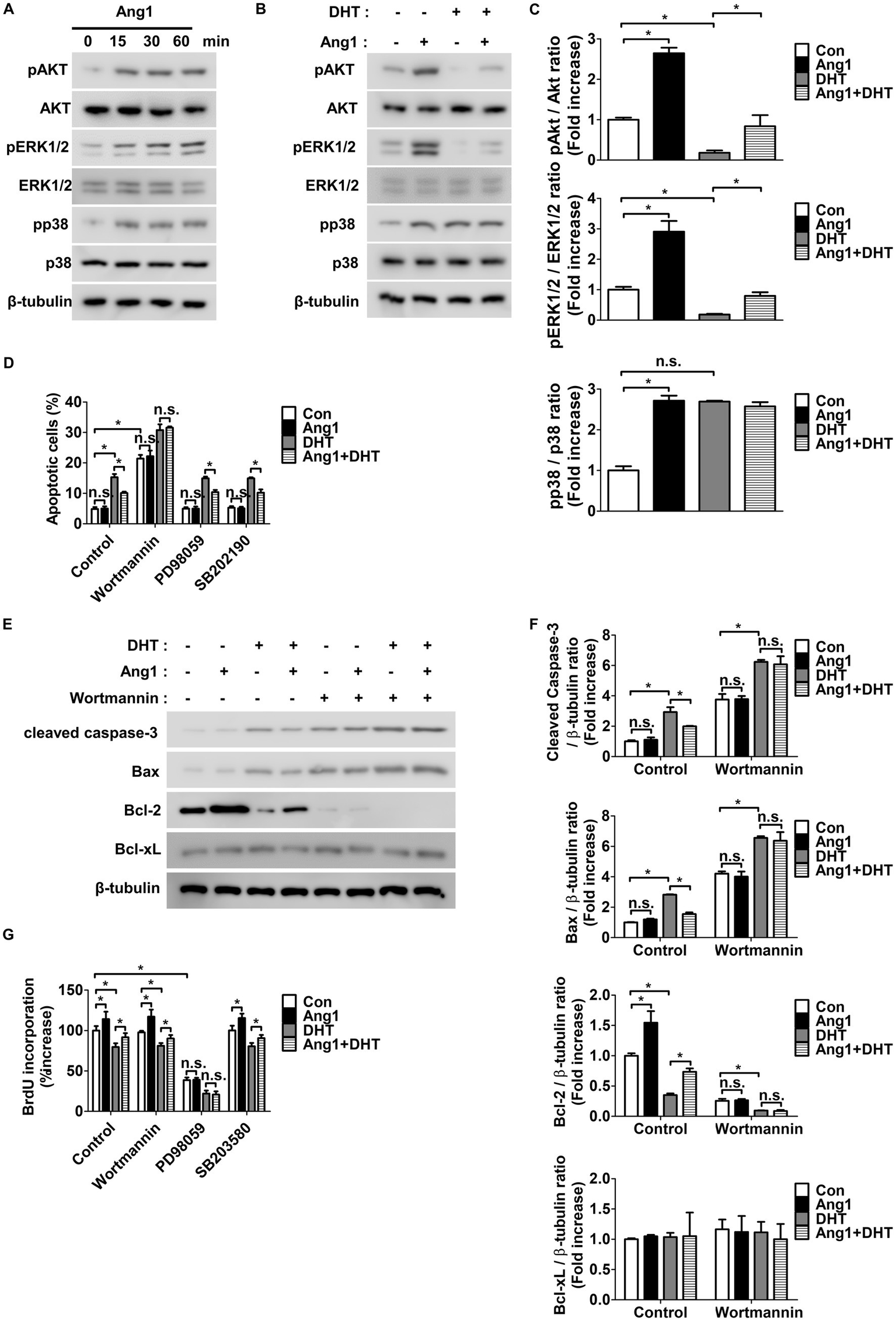
Ang1 induces survival and proliferation of HFDPCs by activating AKT and ERK1/2, respectively. (A) Western blot analysis for pAKT, AKT, pERK1/2, ERK1/2, pp38, and p38 were performed on lysates obtained from HFDPCs treated with Ang1 (300 ng/mL) for the indicated times. β-tubulin was used as a loading control. (B) Western blot analysis for pAKT, AKT, pERK1/2, ERK1/2, pp38, and p38 were performed on lysates obtained from HFDPCs treated with Ang1 (300 ng/mL) and/or DHT (2 μM) for 30 min. β-tubulin was used as a loading control. (C) Quantitative densitometric analysis in (B) to calculate the ratio of each protein to β-tubulin (n = 3). n.s, not significant. *p < 0.05 by two-way ANOVA. (D–G) HFDPCs preincubated with Wortmannin (1 μM), PD98059 (25 μM), or SB202190 (10 μM) for 1 h and then treated with Ang1 (300 ng/mL) and/or DHT (2 μM) for 48 h. (D) Apoptosis of HFDPCs was analyzed using annexin-V/PI staining and flow cytometry. Bar graph represents mean ± SD (n = 3). n.s, not significant. *p < 0.05 by two-way ANOVA. (E) Western blot analysis was performed on lysates from HFDPCs to detect cleaved caspase-3, Bax, Bcl-2, and Bcl-xL. β-tubulin was used as a loading control. (F) Quantitative densitometric analysis in (E) to calculate the ratio of each protein to β-tubulin (n = 3). n.s, not significant. *p < 0.05 by two-way ANOVA. (G) Cell proliferation of HFDPCs was determined by 5′-bromodeoxy-2′-uridine (BrdU) proliferation ELISA. Bar graph represents mean ± SD (n = 5). n.s, not significant. *p < 0.05 by two-way ANOVA.
3.3 HFDPCs lack Tie-2 receptors but express a variety of integrins
Western blot and qRT-PCR were subsequently performed to examine the presence of Tie-2 and integrins, which serve as receptors for Ang1, in HFDPCs. Notably, since Tie-2 is specifically and highly expressed in endothelial cells (40), HDMECs, a type of endothelial cell, were utilized. Interestingly, neither Tie-1 nor Tie-2, both members of the Tie receptor family, were expressed in HFDPCs (Figures 3A,B). Furthermore, the mRNA expression of Tie-2 and Tie-1 was nearly undetectable in HFDPCs compared to HDMECs (Supplementary Figure 2A). In contrast, integrin α4, α5, αv, β1, β3, β4, and β5 were prominently expressed in HFDPCs, with their mRNA levels also being significantly detectable (Figures 3C,D and Supplementary Figure 2B). These findings indicate that the Tie-2 receptor is absent in HFDPCs, while integrins are present.
Figure 3

HFDPCs do not express Tie-2 receptors, but they exhibit a variety of integrin receptors. (A) Western blot analysis for Tie-2 and Tie-1 were performed on lysates obtained from HFDPCs and HDMECs. β-tubulin was used as a loading control. (B) Quantitative densitometric analysis in (A) to calculate the ratio of each protein to β-tubulin (n = 3). *p < 0.05 by Student t test. (C) Western blot analysis for Integrin α4, α5, αv, β1, β3, β4, and β5 were performed on lysates obtained from HFDPCs and HDMECs. β-tubulin was used as a loading control. (D) Quantitative densitometric analysis in (C) to calculate the ratio of each protein to β-tubulin (n = 3). *p < 0.05 by Student t test.
3.4 Ang1 promotes survival and proliferation in HFDPCs via the integrin α5β1 receptor
Since Tie-2 is absent in HFDPCs and multiple integrins are expressed, it was hypothesized that Ang1 contributes to survival and proliferation via these integrins, with an aim to identify the specific integrin involved. To determine whether Ang1 promotes the survival and proliferation of HFDPCs through integrins, the Gly-Arg-Gly-Asp-Ser (GRGDS) peptide was utilized to inhibit integrins that recognize the Arg-Gly-Asp (RGD) sequence. GRGDS inhibited the activation of Akt and ERK1/2, which contribute to Ang1-induced survival and proliferation in HFDPCs, while also reducing p38 activation. (Figure 4A). Next, various α integrin neutralizing antibodies were used to identify the specific integrin involved in Ang1-induced survival and proliferation in HFDPCs. Among the tested neutralizing antibodies, only integrin α5 inhibited Ang1-induced phosphorylation of AKT, ERK1/2, and p38 (Figure 4B). Since integrin α5 can form a heterodimer with integrin β1, a neutralizing antibody for integrin β1 and an integrin α5β1 neutralizing antibody were used to investigate whether Ang1 promotes survival and proliferation through integrin α5β1 in HFDPCs. The neutralizing antibodies for integrin β1 and integrin α5β1 inhibited Ang1-induced phosphorylation of AKT, ERK1/2, and p38 in HFDPCs (Figure 4C). Furthermore, the integrin α5β1 neutralizing antibody completely blocked Ang1-mediated survival under DHT treatment in HFDPCs (Figure 4D). Additionally, it fully prevented the Ang1-induced decrease in cleaved caspase-3 and pro-apoptotic Bax levels, while also inhibiting the increase in anti-apoptotic Bcl-2 levels under DHT treatment in HFPDCs (Figure 4E and Supplementary Figure 3A). The integrin α5β1 neutralizing antibody also fully inhibited Ang1-mediated proliferation under DHT treatment in HFPDCs (Figure 4F). These results indicate that Ang1 mediates survival and proliferation in HFDPCs through integrin α5β1.
Figure 4
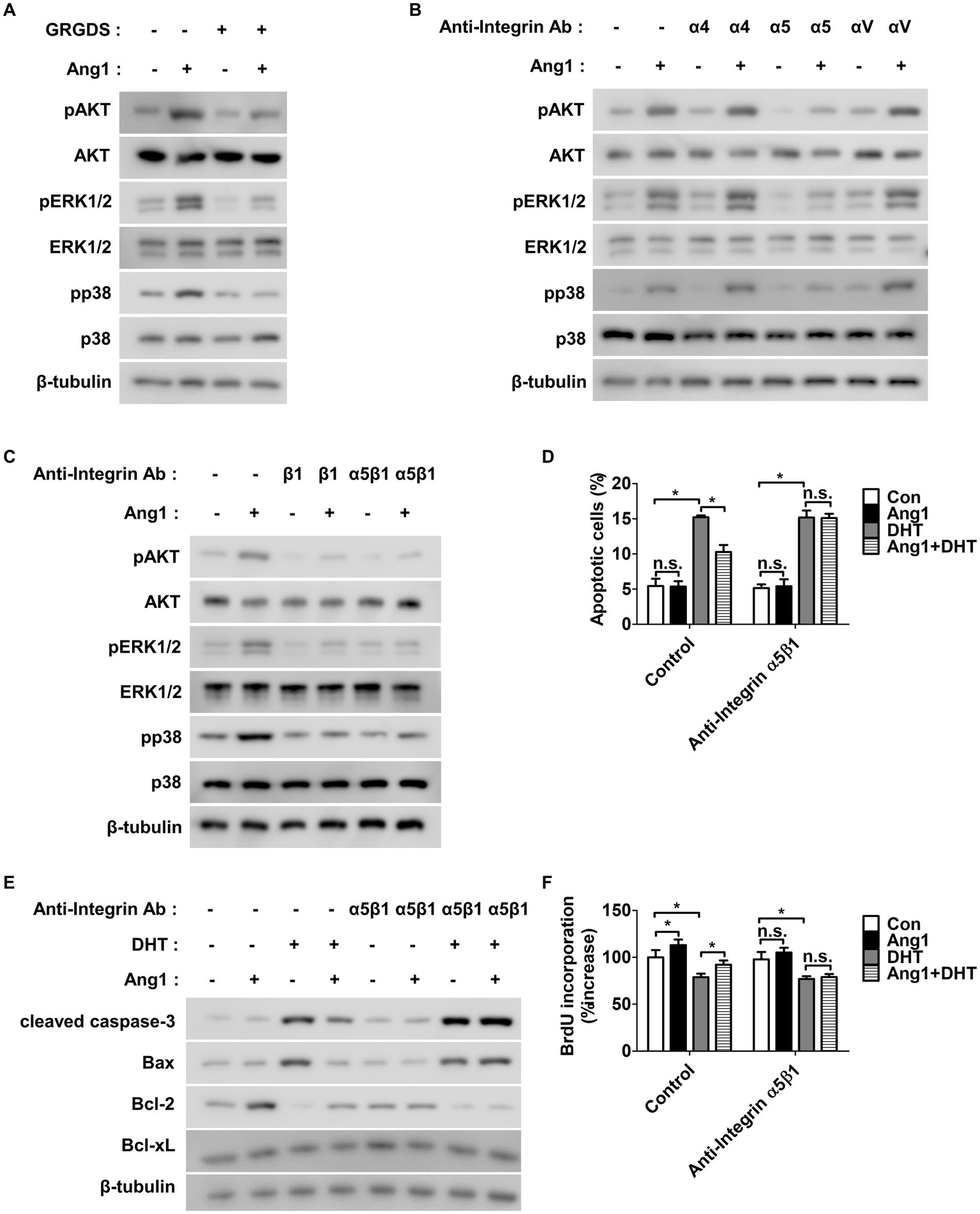
Ang1 promotes survival and proliferation of HFDPCs via integrin α5β1 receptor. (A) HFDPCs preincubated with GRGDS (0.5 mg/mL) for 1 h and then treated with Ang1 (300 ng/mL) for 30 min. Western blot analysis for pAKT, AKT, pERK1/2, ERK1/2, pp38, and p38 were performed on lysates obtained from HFDPCs. β-tubulin was used as a loading control. (B,C) HFDPCs preincubated with various integrin-blocking antibodies (5 μg/mL, α4, α5, αv, β1, α5β1) for 1 h and then treated with Ang1 (300 ng/mL) for 30 min. Western blot analysis for pAKT, AKT, pERK1/2, ERK1/2, pp38, and p38 were performed on lysates obtained from HFDPCs. β-tubulin was used as a loading control. (D–F) HFDPCs preincubated with integrin α5β1-blocking antibody (5 μg/mL) for 1 h and then treated with Ang1 (300 ng/mL) and/or DHT (2 μM) for 48 h. (D) Apoptosis of HFDPCs was analyzed using annexin-V/PI staining and flow cytometry. Bar graph represents mean ± SD (n = 3). n.s, not significant. *p < 0.05 by two-way ANOVA. (E) Western blot analysis was performed on lysates from HFDPCs to detect cleaved caspase-3, Bax, Bcl-2, and Bcl-xL. β-tubulin was used as a loading control. (F) Cell proliferation of HFDPCs was determined by 5′-bromodeoxy-2′-uridine (BrdU) proliferation ELISA. Bar graph represents mean ± SD (n = 5). n.s, not significant. *p < 0.05 by two-way ANOVA.
4 Discussion
Androgenetic alopecia is the most common form of hair loss worldwide, characterized by the progressive miniaturization of terminal hairs after puberty. It affects over 80% of men and nearly 50% of women by the age of 70, with prevalence varying among ethnic groups—White individuals being the most affected, followed by Asians and Black individuals (41). Androgenetic alopecia is primarily driven by DHT, a potent androgen derived from testosterone through the action of 5α-reductase. Upon binding to androgen receptors in hair follicles, DHT promotes miniaturization by damaging DPCs, which are essential for hair follicle maintenance and cycling (42–44). This leads to the transformation of thick, pigmented terminal hairs into fine, vellus hairs and eventually results in follicular atrophy and visible hair loss.
Previous studies have demonstrated that DHT induces apoptosis in DPCs by upregulating pro-apoptotic factors, including cleaved caspase-3 and Bax, while downregulating anti-apoptotic proteins such as Bcl-2 (45). In line with these findings, our study showed that DHT increased apoptosis and suppressed proliferation in HFDPCs. Importantly, Ang1 significantly reversed these effects by restoring cell viability, inhibiting apoptosis, and promoting proliferation (Figures 1B–E), highlighting its potential as a protective agent against androgen-induced cellular damage.
Ang1, a well-characterized member of the angiopoietin family, plays a crucial role in angiogenesis, vascular stabilization, and endothelial cell survival. It typically signals through the Tie-2 receptor and integrins such as α5β1, αvβ3, and αvβ5 (27). In endothelial cells, Ang1-Tie-2 interaction activates downstream pathways, including PI3K/AKT and MAPK/ERK, which mediate cell survival and anti-inflammatory responses. However, our data revealed that Tie-2 is not expressed in HFDPCs (Figures 3A,B and Supplementary Figure 2A), suggesting that Ang1 acts through an alternative mechanism in these cells. Instead, we observed that HFDPCs express a variety of integrin subunits, including α4, α5, αv, β1, β3, β4, and β5 (Figures 3C,D and Supplementary Figure 2B), consistent with previous reports on DPCs (46, 47). Functional assays identified integrin α5β1 as the key mediator through which Ang1 enhances both survival and proliferation under DHT-induced stress (Figures 4D–F). These results establish integrin α5β1 as a novel conduit for Ang1 signaling in HFDPCs, independent of the Tie-2 receptor.
To further contextualize these findings, it is noteworthy that hair follicle growth is also regulated by additional survival and metabolic pathways such as autophagy. Recent studies have demonstrated that small molecules capable of activating autophagy stimulate hair regeneration (48), and isoquercitrin has been shown to promote hair growth through autophagy and angiogenesis via the AMPK–IGF-1R axis (49). While our study primarily focused on the PI3K/AKT and MAPK/ERK cascades downstream of integrin α5β1, it is plausible that Ang1-mediated signaling may interface with autophagy-related mechanisms, thereby further contributing to the protection and regeneration of dermal papilla cells.
While our findings emphasize the integrin-mediated effects of Ang1 in DPCs, it is important to consider the broader biological context of angiopoietin signaling. Although Tie-1 and Tie-2 were not expressed in HFDPCs, these receptors are critical regulators of vascular stability and remodeling, particularly in endothelial cells. Tie-1, unlike Tie-2, does not bind angiopoietins directly but modulates Tie-2 activity via heterodimerization and conformational regulation (27). Tie-1 is predominantly expressed under basal vascular conditions and may act as a negative regulator of Tie-2 activation (50). However, under pathological conditions such as hypoxia or inflammation, Tie-1 becomes phosphorylated and can either enhance or inhibit Tie-2 signaling depending on the context. Studies have shown that Tie-1 deficiency leads to increased vascular permeability and compromised endothelial integrity (28, 40). Our previous work demonstrated that hypoxia-induced phosphorylation of Tie-1 attenuates Ang1–Tie-2 signaling, underscoring its role as a dynamic, context-dependent modulator of vascular function (28).
Given that the dermal papilla is located within a highly vascularized microenvironment, Ang1 may also indirectly contribute to hair follicle health by promoting angiogenesis and vascular stabilization in adjacent endothelial cells via the Tie-1–Tie-2 axis. Vascular supply is essential for initiating and maintaining the anagen phase of the hair growth cycle (51–53), and reduced perifollicular vascularization has been implicated in androgenetic alopecia pathogenesis (54). Therefore, Ang1 may serve dual functions: (1) directly protecting DPCs through integrin α5β1-mediated signaling and (2) indirectly supporting follicular viability by enhancing the surrounding vascular network.
For comparison, minoxidil—the most widely used FDA-approved treatment for androgenetic alopecia—was originally developed as an antihypertensive agent. Its hair growth–promoting effect is attributed to increased perifollicular blood flow and VEGF induction, thereby enhancing angiogenesis (55, 56). Similarly, Ang1 is a potent angiogenic factor capable of promoting microvascular remodeling and endothelial stabilization (27, 57). Although our study did not directly assess Ang1-induced angiogenesis, it is plausible that Ang1 contributes to hair regeneration not only by protecting DPCs but also by facilitating vascular support within the follicular niche. This dual action may offer a mechanistic advantage over current therapies.
In conclusion, our study demonstrates that Ang1 counteracts the detrimental effects of DHT on HFDPCs by suppressing apoptosis and restoring proliferation via PI3K/AKT and MAPK/ERK1/2 signaling pathways. These effects are mediated through integrin α5β1, independently of the classical Tie-2 receptor. While Tie-1 and Tie-2 are not expressed in HFDPCs, their roles in regulating endothelial stability remain essential to the in vivo follicular microenvironment. Future in vivo studies incorporating endothelial–mesenchymal interactions will be important to fully elucidate whether Ang1’s direct cellular effects are complemented by its vascular functions, potentially offering a comprehensive strategy for the treatment of androgenetic alopecia.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
Ethical approval was not required for the studies on humans in accordance with the local legislation and institutional requirements because only commercially available established cell lines were used.
Author contributions
J-HY: Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the Specialized Graduate Program for Training Wildlife Professionals through the National Institute of Wildlife Disease Control and Prevention, Ministry of Environment (20241104000003125600).
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1649763/full#supplementary-material
References
1.
Imperato-McGinley J Guerrero L Gautier T Peterson RE . Steroid 5alpha-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. (1974) 186:1213–5. doi: 10.1126/science.186.4170.1213
2.
Kaufman KD Girman CJ Round EM Johnson-Levonas AO Shah AK Rotonda J . Progression of hair loss in men with androgenetic alopecia (male pattern hair loss): long-term (5-year) controlled observational data in placebo-treated patients. Eur J Dermatol. (2008) 18:407–11. doi: 10.1684/ejd.2008.0435
3.
Hamilton JB . Patterned loss of hair in man; types and incidence. Ann N Y Acad Sci. (1951) 53:708–28. doi: 10.1111/j.1749-6632.1951.tb31971.x
4.
Gan DC Sinclair RD . Prevalence of male and female pattern hair loss in Maryborough. J Investig Dermatol Symp Proc. (2005) 10:184–9. doi: 10.1111/j.1087-0024.2005.10102.x
5.
Krupa Shankar D Chakravarthi M Shilpakar R . Male androgenetic alopecia: population-based study in 1,005 subjects. Int J Trichology. (2009) 1:131–3. doi: 10.4103/0974-7753.58556
6.
Cash TF . The psychological effects of androgenetic alopecia in men. J Am Acad Dermatol. (1992) 26:926–31. doi: 10.1016/0190-9622(92)70134-2
7.
Cash TF Price VH Savin RC . Psychological effects of androgenetic alopecia on women: comparisons with balding men and with female control subjects. J Am Acad Dermatol. (1993) 29:568–75. doi: 10.1016/0190-9622(93)70223-G
8.
Ludwig E . Classification of the types of androgenetic alopecia (common baldness) occurring in the female sex. Br J Dermatol. (1977) 97:247–54. doi: 10.1111/j.1365-2133.1977.tb15179.x
9.
Friedman ES Friedman PM Cohen DE Washenik K . Allergic contact dermatitis to topical minoxidil solution: etiology and treatment. J Am Acad Dermatol. (2002) 46:309–12. doi: 10.1067/mjd.2002.119104
10.
Irwig MS Kolukula S . Persistent sexual side effects of finasteride for male pattern hair loss. J Sex Med. (2011) 8:1747–53. doi: 10.1111/j.1743-6109.2011.02255.x
11.
Samplaski MK Lo K Grober E Jarvi K . Finasteride use in the male infertility population: effects on semen and hormone parameters. Fertil Steril. (2013) 100:1542–6. doi: 10.1016/j.fertnstert.2013.07.2000
12.
Amory JK Wang C Swerdloff RS Anawalt BD Matsumoto AM Bremner WJ et al . The effect of 5alpha-reductase inhibition with dutasteride and finasteride on semen parameters and serum hormones in healthy men. J Clin Endocrinol Metab. (2007) 92:1659–65. doi: 10.1210/jc.2006-2203
13.
Gupta AK Sharma N Shukla P . Atypical post-finasteride syndrome: a pharmacological riddle. Indian J Pharmacol. (2016) 48:316–7. doi: 10.4103/0253-7613.182898
14.
Paus R Cotsarelis G . The biology of hair follicles. N Engl J Med. (1999) 341:491–7. doi: 10.1056/NEJM199908123410706
15.
Alonso L Fuchs E . The hair cycle. J Cell Sci. (2006) 119:391–3. doi: 10.1242/jcs.02793
16.
Paus R Foitzik K . In search of the "hair cycle clock": a guided tour. Differentiation. (2004) 72:489–511. doi: 10.1111/j.1432-0436.2004.07209004.x
17.
Stenn KS Paus R . Controls of hair follicle cycling. Physiol Rev. (2001) 81:449–94. doi: 10.1152/physrev.2001.81.1.449
18.
Huntzicker EG Oro AE . Controlling hair follicle signaling pathways through polyubiquitination. J Invest Dermatol. (2008) 128:1081–7. doi: 10.1038/sj.jid.5700957
19.
Ehama R Ishimatsu-Tsuji Y Iriyama S Ideta R Soma T Yano K et al . Hair follicle regeneration using grafted rodent and human cells. J Invest Dermatol. (2007) 127:2106–15. doi: 10.1038/sj.jid.5700823
20.
Lu ZF Cai SQ Wu JJ Zheng M . Biological characterization of cultured dermal papilla cells and hair follicle regeneration in vitro and in vivo. Chin Med J. (2006) 119:275–81. doi: 10.1097/00029330-200602020-00002
21.
Schmidt-Ullrich R Paus R . Molecular principles of hair follicle induction and morphogenesis. BioEssays. (2005) 27:247–61. doi: 10.1002/bies.20184
22.
Reynolds AJ Jahoda CA . Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development. (1992) 115:587–93. doi: 10.1242/dev.115.2.587
23.
Reynolds AJ Lawrence C Cserhalmi-Friedman PB Christiano AM Jahoda CA . Trans-gender induction of hair follicles. Nature. (1999) 402:33–4. doi: 10.1038/46938
24.
Li W Man XY Li CM Chen JQ Zhou J Cai SQ et al . VEGF induces proliferation of human hair follicle dermal papilla cells through VEGFR-2-mediated activation of ERK. Exp Cell Res. (2012) 318:1633–40. doi: 10.1016/j.yexcr.2012.05.003
25.
Karar J Maity A . PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. (2011) 4:51. doi: 10.3389/fnmol.2011.00051
26.
Ferrara N Gerber HP LeCouter J . The biology of VEGF and its receptors. Nat Med. (2003) 9:669–76. doi: 10.1038/nm0603-669
27.
Brindle NP Saharinen P Alitalo K . Signaling and functions of angiopoietin-1 in vascular protection. Circ Res. (2006) 98:1014–23. doi: 10.1161/01.RES.0000218275.54089.12
28.
Yun JH Lee HM Lee EH Park JW Cho CH . Hypoxia reduces endothelial Ang1-induced Tie2 activity in a Tie1-dependent manner. Biochem Biophys Res Commun. (2013) 436:691–7. doi: 10.1016/j.bbrc.2013.06.018
29.
Park J Kim HO Park KH Wie MB Choi SE Yun JH . A 60% edible Ethanolic extract of Ulmus davidiana inhibits vascular endothelial growth factor-induced angiogenesis. Molecules. (2021) 26:781. doi: 10.3390/molecules26040781
30.
Lee J Kim KE Choi DK Jang JY Jung JJ Kiyonari H et al . Angiopoietin-1 guides directional angiogenesis through integrin alphavbeta5 signaling for recovery of ischemic retinopathy. Sci Transl Med. (2013) 5:203ra127. doi: 10.1126/scitranslmed.3006666
31.
Dallabrida SM Ismail NS Pravda EA Parodi EM Dickie R Durand EM et al . Integrin binding angiopoietin-1 monomers reduce cardiac hypertrophy. FASEB J. (2008) 22:3010–23. doi: 10.1096/fj.07-100966
32.
Eklund L Kangas J Saharinen P . Angiopoietin-tie signalling in the cardiovascular and lymphatic systems. Clin Sci (Lond). (2017) 131:87–103. doi: 10.1042/CS20160129
33.
Somanath PR Ciocea A Byzova TV . Integrin and growth factor receptor alliance in angiogenesis. Cell Biochem Biophys. (2009) 53:53–64. doi: 10.1007/s12013-008-9040-5
34.
Lichtenberger BM Tan PK Niederleithner H Ferrara N Petzelbauer P Sibilia M . Autocrine VEGF signaling synergizes with EGFR in tumor cells to promote epithelial cancer development. Cell. (2010) 140:268–79. doi: 10.1016/j.cell.2009.12.046
35.
Pons J Huang Y Arakawa-Hoyt J Washko D Takagawa J Ye J et al . VEGF improves survival of mesenchymal stem cells in infarcted hearts. Biochem Biophys Res Commun. (2008) 376:419–22. doi: 10.1016/j.bbrc.2008.09.003
36.
Chen G Shi X Sun C Li M Zhou Q Zhang C et al . VEGF-mediated proliferation of human adipose tissue-derived stem cells. PLoS One. (2013) 8:e73673. doi: 10.1371/journal.pone.0073673
37.
Papapetropoulos A Fulton D Mahboubi K Kalb RG O'Connor DS Li F et al . Angiopoietin-1 inhibits endothelial cell apoptosis via the Akt/survivin pathway. J Biol Chem. (2000) 275:9102–5. doi: 10.1074/jbc.275.13.9102
38.
Harfouche R Gratton JP Yancopoulos GD Noseda M Karsan A Hussain SN . Angiopoietin-1 activates both anti- and proapoptotic mitogen-activated protein kinases. FASEB J. (2003) 17:1523–5. doi: 10.1096/fj.02-0698fje
39.
Roux PP Blenis J . ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev. (2004) 68:320–44. doi: 10.1128/MMBR.68.2.320-344.2004
40.
Peters KG Kontos CD Lin PC Wong AL Rao P Huang L et al . Functional significance of Tie2 signaling in the adult vasculature. Recent Prog Horm Res. (2004) 59:51–71. doi: 10.1210/rp.59.1.51
41.
Ho CH Sood T Zito PM . Androgenetic Alopecia. Treasure Island, FL: StatPearls (2025).
42.
Trueb RM . Molecular mechanisms of androgenetic alopecia. Exp Gerontol. (2002) 37:981–90. doi: 10.1016/S0531-5565(02)00093-1
43.
Liang A Fang Y Ye L Meng J Wang X Chen J et al . Signaling pathways in hair aging. Front Cell Dev Biol. (2023) 11:1278278. doi: 10.3389/fcell.2023.1278278
44.
Winiarska A Mandt N Kamp H Hossini A Seltmann H Zouboulis CC et al . Effect of 5alpha-dihydrotestosterone and testosterone on apoptosis in human dermal papilla cells. Skin Pharmacol Physiol. (2006) 19:311–21. doi: 10.1159/000095251
45.
Nam GH Jo KJ Park YS Kawk HW Yoo JG Jang JD et al . The peptide AC 2 isolated from Bacillus-treated Trapa japonica fruit extract rescues DHT (dihydrotestosterone)-treated human dermal papilla cells and mediates mTORC1 signaling for autophagy and apoptosis suppression. Sci Rep. (2019) 9:16903. doi: 10.1038/s41598-019-53347-3
46.
Fujiwara H Ferreira M Donati G Marciano DK Linton JM Sato Y et al . The basement membrane of hair follicle stem cells is a muscle cell niche. Cell. (2011) 144:577–89. doi: 10.1016/j.cell.2011.01.014
47.
Kim DS Cho HJ Yang SK Shin JW Huh CH Park KC . Insulin-like growth factor-binding protein contributes to the proliferation of less proliferative cells in forming skin equivalents. Tissue Eng Part A. (2009) 15:1075–80. doi: 10.1089/ten.tea.2008.0236
48.
Chai M Jiang M Vergnes L Fu X de Barros SC Doan NB et al . Stimulation of hair growth by small molecules that activate autophagy. Cell Rep. (2019) 27:3413–3421.e3. doi: 10.1016/j.celrep.2019.05.070
49.
Manzoor M Chen D Lin J Wang Y Xiang L Qi J . Isoquercitrin promotes hair growth through induction of autophagy and angiogenesis by targeting AMPK and IGF-1R. Phytomedicine. (2025) 136:156289. doi: 10.1016/j.phymed.2024.156289
50.
Savant S La Porta S Budnik A Busch K Hu J Tisch N et al . The orphan receptor Tie1 controls angiogenesis and vascular remodeling by differentially regulating Tie2 in tip and stalk cells. Cell Rep. (2015) 12:1761–73. doi: 10.1016/j.celrep.2015.08.024
51.
Headington JT . Hair follicle biology and topical minoxidil: possible mechanisms of action. Dermatologica. (1987) 175:19–22.
52.
Ellis RA Moretti G . Vascular patterns associated with categen hair follicles in the human scalp. Ann N Y Acad Sci. (1959) 83:448–57. doi: 10.1111/j.1749-6632.1960.tb40918.x
53.
Yano K Brown LF Detmar M . Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest. (2001) 107:409–17. doi: 10.1172/JCI11317
54.
Goldman CK Tsai JC Soroceanu L Gillespie GY . Loss of vascular endothelial growth factor in human alopecia hair follicles. J Invest Dermatol. (1995) 104:18S–20S.
55.
Messenger AG Rundegren J . Minoxidil: mechanisms of action on hair growth. Br J Dermatol. (2004) 150:186–94. doi: 10.1111/j.1365-2133.2004.05785.x
56.
Suchonwanit P Thammarucha S Leerunyakul K . Minoxidil and its use in hair disorders: a review. Drug Des Devel Ther. (2019) 13:2777–86. doi: 10.2147/DDDT.S214907
57.
Koh GY . Orchestral actions of angiopoietin-1 in vascular regeneration. Trends Mol Med. (2013) 19:31–9. doi: 10.1016/j.molmed.2012.10.010
Summary
Keywords
angiopoietin-1, human follicle dermal papilla cells, integrin α5β1, survival, proliferation, alopecia
Citation
Yun J-H (2025) Angiopoietin-1 induces survival and proliferation of hair follicle dermal papilla cells through integrin α5β1 signaling. Front. Med. 12:1649763. doi: 10.3389/fmed.2025.1649763
Received
19 June 2025
Accepted
15 September 2025
Published
24 September 2025
Volume
12 - 2025
Edited by
Elisa Zavattaro, University of Eastern Piedmont, Italy
Reviewed by
Saurat Jean Hilaire, University of Geneva, Switzerland
Majid Manzoor, Zhejiang University, China
Updates
Copyright
© 2025 Yun.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jang-Hyuk Yun, yunjh@kangwon.ac.kr
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.