Abstract
Objective:
The purpose of this study is to explore the value of cough sounds and forced exhalation sounds monitored by smartwatches with audio collection capabilities for screening obstructive sleep apnea (OSA) in patients with chronic obstructive pulmonary disease (COPD).
Methods:
Stable COPD patients were recruited from an outpatient clinic. All participants completed questionnaires and underwent pulmonary function testing and overnight polysomnography (PSG). A novel smartwatch capable of collecting audio signals was worn to continuously monitor peripheral oxygen saturation (SpO₂), heart rate (HR), heart rate variability (HRV), and respiratory rate (RR). Additionally, voluntary cough and forced exhalation sounds were recorded twice daily. Audio data were denoised, segmented, and analyzed using time- and frequency-domain features. Correlations between audio features and OSA diagnosis/severity were assessed and a predicting model were developed based on these data.
Results:
Among the 29 participants with stable COPD, 26 underwent PSG, and 17 were diagnosed with comorbid OSA. Multiple cough and forced exhalation subfeatures correlated significantly with OSA diagnosis and apnea and hypopnea index (AHI). Cough sounds showed the highest correlation with OSA diagnosis (r = −0.6629, p < 0.001). A logistic regression model using a cough sound subfeature (the median of MFCC_35) achieved 92% accuracy with a Cohen’s kappa value of 0.8276 in predicting OSA in COPD patients.
Conclusion:
This study demonstrates a strong association between cough sounds and OSA risk in COPD patients. Cough sounds recorded by smartwatches may serve as a valuable tool for screening OSA in COPD patients, contributing to the management of patients with overlap syndrome.
1 Introduction
Chronic obstructive pulmonary disease (COPD) and obstructive sleep apnea (OSA) are both common chronic respiratory diseases with widespread impact. COPD, characterized by irreversible airflow limitation and progressive decline in lung function, most commonly presents with symptoms such as shortness of breath, chronic cough (sometimes with sputum), and fatigue (1, 2). It is one of the leading causes of disease burden, mortality, and healthcare resource consumption worldwide (2). OSA is characterized by recurrent upper airway collapse during sleep, leading to intermittent hypoxia, sleep fragmentation, and sympathetic activation (3). It is closely associated with multiple systemic conditions, including cardiovascular and metabolic diseases, and is often regarded as an upstream risk factor for these disorders (4).
With factors such as population aging, rising obesity rates, air pollution, and persistently high smoking prevalence, the prevalence of both diseases continues to increase (5–8). In 2019, approximately 391.9 million people worldwide were affected by COPD (2, 9), and around 1 billion people were affected by OSA (10). The coexistence of COPD and OSA is referred to as “overlap syndrome (OS)” (11). Overlap syndrome is relatively uncommon in the general population (prevalence: 1.0–3.6%) (12), but its prevalence significantly increases among patients diagnosed with obstructive sleep apnea (prevalence of OS: 7.6–55.7%) or chronic obstructive pulmonary disease (prevalence of OS: 2.9–65.9%) (12, 13).
Patients with OSA-COPD overlap syndrome (OS) typically experience more severe respiratory symptoms and poorer quality of life, with a higher relative risk of exacerbations, hospitalization, and mortality compared to patients with either disease alone (11, 14). Raising awareness, prompt screening, and timely initiating treatment can improve the overall prognosis of OS patients (11, 14). The American Thoracic Society recommends a screening strategy to identify OSA in COPD patients with chronic stable hypercapnia (13).
Therefore, screening and early detection of OSA in COPD patients is of importance. Currently, smart wearable devices are gaining increasing significance in health monitoring and chronic disease management (15). Many studies have also explored the use of smart wearable devices for screening COPD and OSA separately (16, 17). These studies primarily rely on classic physiological parameters, including peripheral oxygen saturation (SpO2), respiratory rate (RR), heart rate (HR), heart rate variability (HRV), and activity levels (18–23).
In terms of using audio data for screening COPD, some studies have utilized microphones, smartphones, or dedicated sensors to monitor audio data such as cough sounds for COPD diagnosis and lung function prediction (24–26). Our research team has been using a new smart watch with audio collection capabilities, which collects audio data from cough sounds and breath sounds of COPD patients to assess the severity of COPD (27). When it comes to using audio signals for screening OSA, the most commonly used audio signal is snoring during sleep (28). Until now, there is no research on using cough sounds for screening OSA. If monitoring cough sounds and breath sounds can be used to predict COPD severity while screening for OSA, it would undoubtedly be beneficial for the early identification of OSA in COPD patients, facilitating the management of overlap syndrome (OS).
Thus, we conducted the present study based on our previous research (27), aiming to preliminarily explore the potential value of monitoring daytime cough and forced exhalation sounds for screening OSA.
2 Methods
2.1 Study population
Patients with suspected chronic obstructive pulmonary disease (COPD) were recruited from the outpatient clinic of the Department of Respiratory and Critical Care Medicine at Peking University First Hospital between June and August 2022. Inclusion criteria required participants to be over 18 years old, in a stable COPD condition, and capable of independently operating a mobile phone. Exclusion criteria included patients with other chronic respiratory conditions, a history of lobectomy or lung transplantation, pleural diseases, severe comorbidities, malnutrition (body mass index, BMI <18 kg/m2), bilateral wrist and hand edema or injury, unable to wear a smartwatch. The detailed inclusion and exclusion criteria are provided in our previously published article (27).
All participants provided written informed consent before the study commenced. The study adhered to the ethical guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Peking University First Hospital (Approval Number: 2022083). It was registered on www.clinicaltrials.gov (NCT05551169).
2.2 Clinical examinations
Demographic information such as age, gender, height, and weight was collected, and participants completed questionnaires including the COPD Assessment Test (CAT) and the modified Medical Research Council (mMRC) dyspnea scale. Pulmonary function tests, the 6-min walk test (6MWT), electrocardiogram (ECG), and arterial blood gas analysis were performed. The BODE index was assessed. Overnight polysomnography (PSG) study was conducted.
Based on the results of the pulmonary function tests, COPD patients were classified according to the GOLD criteria: GOLD 1: FEV₁ ≥ 80% of predicted value, GOLD 2: 50% ≤ FEV₁ < 80% of predicted value, GOLD 3: 30% ≤ FEV₁ < 50% of predicted value, and GOLD 4: FEV₁ < 30% of predicted value. Based on the PSG study results, OSA was diagnosed and classified by severity using the apnea-hypopnea index (AHI): AHI ≥ 5 events/h: diagnosis of OSA, AHI ≥ 5 and < 15 events/h: mild OSA, AHI ≥ 15 and <30 events/h: moderate OSA, AHI ≥ 30 events/h: severe OSA.
2.3 Wearing the smartwatch and signal recording
Each participant was provided with a smartwatch (Watch GT3/Watch 3, Huawei, China) and was required to wear it continuously for 7–14 days. The device automatically collected photoplethysmography (PPG) and acceleration (ACC) signal data to calculate RR, SpO2, HR, and HRV.
Participants were also instructed to record cough and forced exhalation sounds twice daily.
The recordings were conducted in a quiet environment with the participants in a resting state. Subjects maintained a normal seated posture, with their left hand placed flat on the table approximately 30 cm directly in front of the face. For cough sound recording, participants were instructed to slightly open their mouths, take a deep breath, and then cough forcefully two to three times. This procedure was repeated three to five times. For exhalation sound recording, participants took a deep breath and then exhaled as quickly and forcefully as possible through pursed lips, continuing for more than 6 s until no further airflow was expelled. After a 1-min interval, the procedure was repeated at least three times. A schematic diagram is shown in Figure 1. The standardized procedures for data collection are detailed in our previously published article (27).
Figure 1

Schematic diagram of the sound recording procedure.
2.4 Signal extraction and data analysis of smartwatch data
2.4.1 Physiological parameters
The photoplethysmography (PPG) and acceleration (ACC) signals collected by the smartwatch were processed using digital signal processing techniques such as filtering to remove noise and extract relevant features. Key physiological parameters including HR, RR, SpO2, and HRV were derived.
Data collected during the monitoring period were summarized using statistical indicators such as mean, standard deviation, median, 25th percentile, 75th percentile, and variability measures (including daily and weekly variability). The detailed methods of calculation are described in our previously published article (27).
2.4.2 Audio parameters
The collected audio data were first denoised using speech signal processing techniques. Then, audio segmentation algorithms were applied to extract individual audio segments. For each segment, multidimensional feature extraction methods were used to obtain both time-domain and frequency-domain characteristics. These included commonly used speech signal features such as Mel Frequency Cepstral Coefficients (MFCC), spectral features, chroma (pitch-related) features, time-domain features, and higher-order features. Each type of feature yielded multiple sub-features across different spectrums and audio frames. These sub-features were then aggregated using statistical measures such as mean and variance.
A total of 772 features were extracted from the cough sounds and 1,295 from the forced exhalation sounds. For each participant, all extracted features during the monitoring period were further aggregated using statistical functions including mean, standard deviation, median, 25th percentile, and 75th percentile. As a result, the statistically aggregated cough sound features comprised 3,860 sub-features (772 × 5), and the forced exhalation sound features comprised 6,475 sub-features (1,295 × 5). Detailed values can be found in our previously published article and Supplementary material (27).
2.5 Statistical analysis and data processing methods
Count variables were presented as frequencies and percentages. Continuous variables with a normal distribution were expressed as means and standard deviations (SD), while those with a non-normal distribution were expressed as medians and interquartile ranges (IQR). For group comparisons, chi-square tests, t-tests and Mann–Whitney U tests were used for two-group comparisons. For comparisons among three groups, chi-square tests, analysis of variance (ANOVA), and Kruskal–Wallis H tests were applied.
To explore the correlation between smartwatch-derived physiological and audio parameters and clinical data, univariate analysis was first performed. Pearson correlation analysis was used for normally distributed continuous variables, while Spearman correlation analysis was applied to non-normally distributed continuous variables and categorical variables.
The subfeature of smartwatch-derived physiological and audio parameters showing the strongest correlation with OSA indicators in the univariate analysis was selected as the representative feature and included in the regression model and predictive models. A logistic regression model was used for the prediction of OSA diagnosis, while a linear regression model was employed for the prediction of AHI. Variable selection was performed using bidirectional stepwise regression combined with the Akaike information criterion (AIC). Multicollinearity was assessed for the variables included in the AIC-selected optimal models, and features with high collinearity were excluded accordingly. Multiple models were compared, and the best-performing model was selected for further analysis. The data processing workflow is illustrated in Figure 2.
Figure 2

Workflow of data processing.
All statistical analyses were performed using R version 4.3.2 (R Foundation for Statistical Computing, Vienna, Austria). A p-value less than 0.05 was considered statistically significant for all tests.
3 Results
3.1 Baseline demographics and clinical characteristics
A total of 31 patients with suspected COPD were screened, of whom 29 met the inclusion and exclusion criteria and were enrolled in the study. Of these, 26 participants underwent overnight polysomnography (PSG). Based on the PSG results, patients were categorized into two groups: those with comorbid obstructive sleep apnea (COPD-OSA overlap syndrome group, n = 17) and those without OSA (COPD group, n = 9) (Figure 3).
Figure 3

Participant enrollment flow diagram.
The COPD-OSA group had significantly higher proportions of male participants, smokers, and higher BMI compared to the COPD-only group (100% vs. 66.7%, 100% vs. 55.6%; 25.8 ± 3.7 vs. 22.5 ± 1.6, p < 0.05, respectively). There were no significant differences between the two groups in terms of age, CAT score, mMRC score, BODE index, 6-min walk distance (6MWD), or GOLD classification (p > 0.05) (Table 1).
Table 1
| Characteristic | All (N = 26) | COPD (N = 9) | COPD-OSA (N = 17) | p-value |
|---|---|---|---|---|
| Age (years) | 67.2 ± 6.1 | 68.0 ± 6.0 | 66.7 ± 6.3 | 0.617 |
| Sex (male) | 23 (88.5) | 6 (66.7) | 17 (100) | 0.032 |
| BMI (kg/m2) | 24.6 ± 3.5 | 22.5 ± 1.6 | 25.8 ± 3.7 | 0.004 |
| Smoking history | 0.008 | |||
| Never smoked | 4 (15.4) | 4 (44.4) | 0 (0) | |
| Former/current smoker | 22 (84.6) | 5 (55.6) | 17 (100.0) | |
| Hypertension | 12 (46.2) | 3 (33.3) | 9 (52.9) | 0.429 |
| Cardiovascular disease | 3 (11.5) | 0 (0) | 3 (17.6) | 0.529 |
| Diabetes | 4 (15.4) | 0 (0) | 4 (23.5) | 0.263 |
| FEV1% (%) | 72.6 (50.0, 84.7) | 79.7 (34.4, 84.1) | 72.3 (59.5, 87.3)* | 0.419 |
| FVC% (%) | 99.2 ± 15.9 | 100.3 ± 17.9 | 98.6 ± 15.4 | 0.795 |
| FEV1/FVC | 0.6 (0.5, 0.6) | 0.6 (0.3, 0.6) | 0.6 (0.5, 0.6) | 0.235 |
| Blood gas analysis | ||||
| PaO2 (mmHg) | 84.2 ± 7.8 | 91.1 ± 7.8 | 80.8 ± 5.2 | 0.001 |
| PaCO2 (mmHg) | 39.8 ± 3.9 | 38.9 ± 2.5 | 40.3 ± 4.5 | 0.411 |
| GOLD grade | 0.063 | |||
| 1 | 10 (38.5) | 4 (44.4) | 6 (35.3) | |
| 2 | 10 (38.5) | 1 (11.1) | 9 (52.9) | |
| 3 + 4 | 6 (23.1) | 4 (44.4) | 2 (11.8) | |
| CAT score | 3.0 (2.0, 4.3) | 3.0 (1.0, 10.0) | 3.0 (2.0, 4.0) | 0.847 |
| mMRC score | 0.220 | |||
| 0 | 18 (69.2) | 6 (66.7) | 12 (70.6) | |
| 1 | 5 (19.2) | 3 (33.3) | 2 (11.8) | |
| ≥2 | 3 (11.5) | 0 (0) | 3 (17.6) | |
| BODE index | 1.0 (0, 2.0) | 1.0 (0, 3.0) | 1.0 (0, 1.5) | 0.610 |
| BODE group | 0.302 | |||
| 0–2 | 21 (80.8) | 6 (66.7) | 15 (88.2) | |
| ≥2 | 5 (19.2) | 3 (33.3) | 2 (11.8) | |
| 6MWD (m) | 448.0 ± 96.5 | 467.3 ± 82.0 | 437.6 ± 104.2 | 0.468 |
| 6MWD group | 0.628 | |||
| ≥350 | 21 (80.8) | 8 (88.9) | 13 (76.5) | |
| ≤349 | 5 (19.2) | 1 (11.1) | 4 (23.5) | |
| SpO2 mean | 93.5 (92.5, 95.0) | 94.3 (93.0, 96.0) | 93.2 (92.2, 94.5) | 0.099 |
| SpO2 lowest | 88.0 (83.0, 91.0) | 91.0 (88.5, 92.5) | 87.0 (80.5, 88.0) | 0.001 |
| ODI | 8.6 (3.6, 24.8) | 1.3 (0.6, 4.1) | 19.1 (8.6, 32.5) | <0.001 |
| AHI | 8.3 (1.8, 20.6) | 1.3 (0.5, 2.6) | 17.0 (8.3, 31.1) | <0.001 |
Characteristics of participants in COPD group and COPD-OSA group.
Data were presented as numbers and percentages [n (%)], mean and standard deviation (mean ± SD), median with upper and lower quartiles [median (Q1, Q3)]. BMI, body mass index; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; FEV1/FVC, the ratio of FEV1 to FVC; ODI, oxygen desaturation index (≥3% desaturations); AHI, apnea-hypopnea index.
3.2 Correlation analysis and predictive modeling of smartwatch physiological parameters and audio data with OSA-related indicators
Physiological parameters collected by the smartwatch, including SpO2, RR, HR, and HRV, as well as audio data such as cough and exhalation sounds, were analyzed in relation to OSA diagnosis and AHI. From these six categories of smartwatch data, the most strongly correlated subfeatures are selected as predictive variables to construct predictive models.
3.2.1 Correlation analysis between physiological and audio data with OSA diagnosis, and predictive model development
3.2.1.1 Correlation analysis between physiological and audio data with OSA diagnosis
Using AHI ≥ 5 as the diagnostic criterion, smartwatch-monitored SpO₂, HRV, cough sounds, and exhalation sounds showed significant correlations with OSA diagnosis. RR and HR did not demonstrate significant correlations with OSA diagnosis.
Among these, the subfeatures most strongly associated with OSA diagnosis were as follows: for SpO₂, the upper quartile value during monitoring (all_SpO2_75%); for HRV, the median power of the very low frequency band in 0–0.04 Hz band (psdVlf_median); for cough sounds, the median value of the Mel-frequency cepstral coefficient (MFCC_35_median) during monitoring; and for exhalation sounds, the median value of the polynomial fitting coefficient (Poly Coef_1292_median) during monitoring. The abbreviations of the subfeatures and their corresponding explanations can be found in Supplementary Table S1. Cough sounds showed the highest correlation (r = −0.6629, p < 0.001), followed by exhalation sounds (r = 0.6090, p < 0.001) (Figure 4). Comparisons of these four features between the two groups are presented in Table 2.
Figure 4

Correlation between smartwatch-derived SpO₂, HRV, exhalation sounds, and cough sounds, as illustrated by boxplots and density plots. In the boxplots, the three lines of the box represent the median, 25th percentile, and 75th percentile, respectively. The black dots indicate outliers. In the density plots, the x-axis represents the range of data values, while the y-axis indicates the density.
Table 2
| Parameters | ALL (N = 26) | COPD (N = 9) | COPD-OSA (N = 17) | p |
|---|---|---|---|---|
| HRV (psdVlf_median) | 44.7 (27.4, 78.3) | 31.1 (20.0, 40.6) | 57.5 (29.5, 132.9) | 0.036 |
| SpO2 (all_SpO2_75%) | 97.0 (97.0, 98.0) | 98.0 (97.0, 98.0) | 97.0 (97.0,97.0) | 0.013 |
| Exhalation sounds (Poly Coef_1292_median) | 0.0083 ± 0.0035 | 0.0058 ± 0.0015 | 0.0096 ± 0.0036 | 0.006 |
| Cough sounds (MFCC_35_median) | 2.51 ± 2.68 | 4.74 ± 1.24 | 1.33 ± 2.48 | 0.001 |
Comparison of smartwatch data between the COPD group and the COPD-OSA group.
Values are presented as mean ± standard deviation, frequency (percentage), or median (lower quartile, upper quartile).
Poly Coef_1292_median is defined as the median value of the polynomial fitting coefficient of forced exhalation sounds during the monitoring period.
MFCC_35_median refers to the median value of the Mel-frequency cepstral coefficient of cough sounds recorded during the monitoring period.
psdVlf_median refers to the median of psdVlf during the monitoring period. psdVlf is the very low frequency power in the 0–0.04 Hz band.
all_SpO2_75% refers to the 75th percentile of SpO2 during the monitoring period.
3.2.1.2 Predictive model for OSA diagnosis
The above four subfeatures most strongly correlated with OSA diagnosis, HRV (psdVlf_median), exhalation sound (Poly_Coef_1292_median), cough sound (MFCC_35_median), and SpO₂ (all_SpO2_75%) were initially included as predictor variables in a logistic regression model. After variable selection using the Akaike information criterion (AIC), HRV was excluded, resulting in a refined model that included subfeatures from SpO₂, cough sound, and exhalation sound.
Multicollinearity analysis revealed strong collinearity between these cough and exhalation sound sub-features. To address this, two separate models were constructed: SpO₂ + cough sound, and SpO₂ + exhalation sound. The SpO₂ + cough sound model outperformed the other, with an overall accuracy of 0.88 (95% CI: 0.6878–0.9745), Cohen’s kappa of 0.7492, specificity of 100%, sensitivity of 82.4%, and AUC of 0.897. In comparison, the SpO₂ + exhalation sound model achieved an accuracy of 0.84 (95% CI: 0.6392–0.9546), Cohen’s kappa of 0.6552, specificity of 87.5%, sensitivity of 82.4%, and AUC of 0.890.
Thus, the SpO₂ + cough sound model was selected for further analysis. The SpO₂ subfeature (all_SpO2_75%) was found to be statistically insignificant and was removed. Only the cough sound subfeature (MFCC_35_median) was preserved in the final optimized model. This logistic regression model achieved an accuracy of 92.0%, a sensitivity of 88.2%, a specificity of 100%, an AUC of 0.890, and a Cohen’s kappa value of 0.8276 in diagnosing OSA among COPD patients (Figure 5).
Figure 5
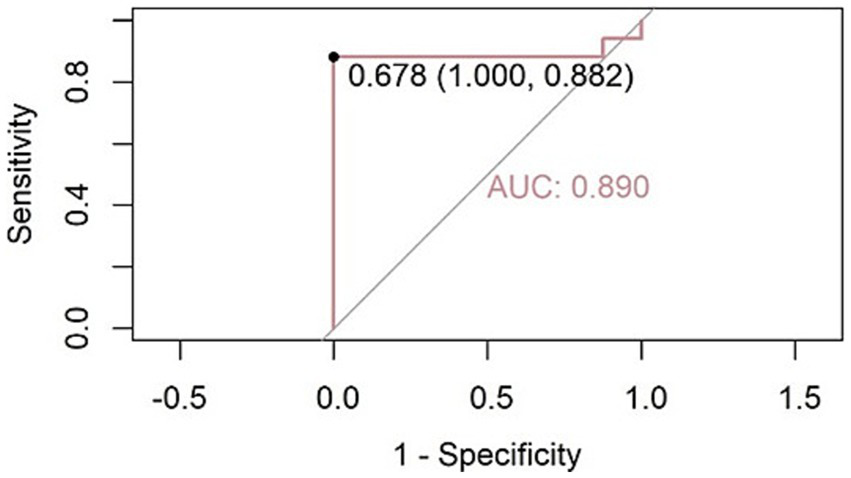
Receiver operating characteristic (ROC) curve for OSA diagnosis using the cough sound feature (MFCC_35_median) as the predictor. The model achieved an AUC of 0.890. At the optimal cutoff threshold of 0.678, the model reached a sensitivity of 88.2% and specificity of 100%.
3.2.2 Correlation analysis between physiological and audio data with AHI, and predictive model development
3.2.2.1 Selection of the strongest subfeatures from smartwatch parameters correlated with AHI
Following the above method, the subfeatures of SpO₂, RR, HR, HRV, exhalation sound, and cough sound that showed the strongest correlations with AHI were analyzed. RR and HR showed no significant correlation with AHI (p > 0.05, respectively). In contrast, SpO₂, HRV, cough sound, and exhalation sound were significantly correlated with AHI. The subfeatures exhibiting the strongest correlations were as follows: SpO₂ subfeature all_SpO2_75% (r = −0.440, p = 0.02), HRV subfeature validRriNum_sd (r = −0.541, p = 0.01), cough sound subfeature Spectral Contrast_556_sd (r = −0.652, p < 0.001), and exhalation sound subfeature Poly Coef_1292_median (r = 0.664, p < 0.001).
3.2.2.2 Predictive model for AHI
The above subfeatures most strongly correlated with AHI were used as predictor variables to construct the predictive model. After performing bidirectional stepwise regression and variable selection based on the Akaike information criterion (AIC), the optimal predictive model for AHI was determined. The final model retained HRV (validRriNum_sd), cough sound feature (Spectral_Contrast_556_sd), and exhalation sound feature (Poly_Coef_1292_median). The multiple linear regression equation for predicting AHI is as follows (Figure 6):
Figure 6
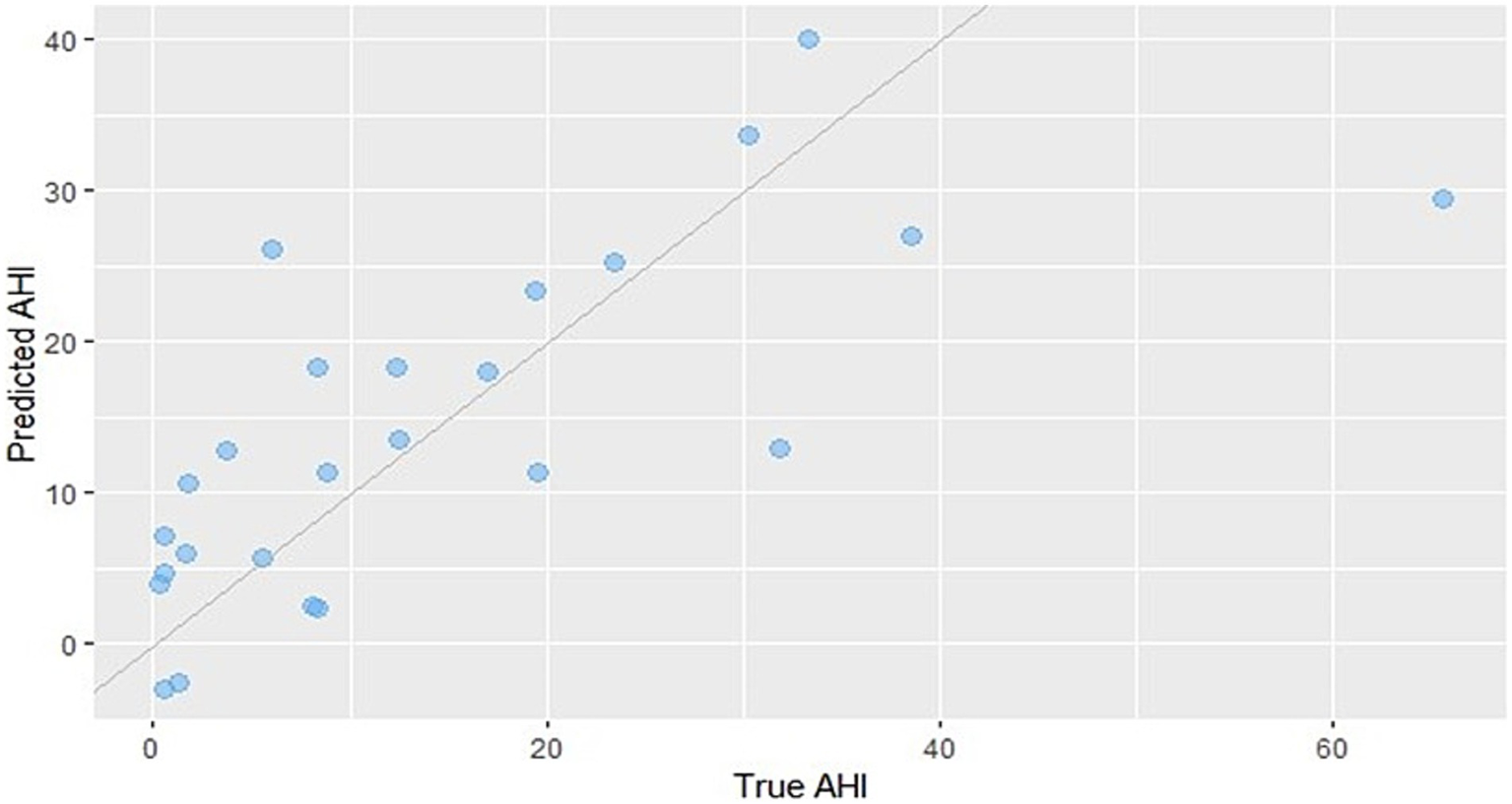
Scatter plot comparing predicted AHI values from the regression model with the actual measured values. The predictive formula is as follows: AHI = 14.364 − 4.017 × HRV + 5.728 × exhalation sound − 4.516 × cough sound.
The predictive model achieved a root mean square error (RMSE) of 10.64 events/h, a mean absolute error (MAE) of 7.48 events/h, and an R2 (coefficient of determination) of 0.5236.
4 Discussion
This study demonstrates that cough and forced exhalation sounds in patients with COPD contain signal parameters associated with OSA, indicating potential value for screening and assessment of overlap syndrome. Multiple parameters derived from cough and forced exhalation sounds were found to be related to the diagnosis and AHI. A regression model based on a cough sound subfeature (MFCC_35_median) showed high accuracy in predicting OSA. These findings offer new insights for the early detection of OSA and the management of overlap syndrome in COPD patients.
Identifying coexisting obstructive sleep apnea (OSA) in patients with chronic obstructive pulmonary disease (COPD) holds significant clinical value. The management of patients with overlap syndrome (OS) differs from that of patients with COPD alone. OS patients often present with multiple comorbidities and have a higher risk of developing cardiovascular diseases (CVD) and experiencing acute exacerbations of COPD (29, 30). Moreover, the survival rate of OS patients receiving nocturnal positive airway pressure therapy is significantly lower if they do not receive appropriate treatment (29, 30). The American Thoracic Society recommends a screening strategy to identify OSA in COPD patients with chronic stable hypercapnia (31). Despite its impact, OSA-COPD overlap syndrome has not yet received sufficient attention in clinical practice (11). Early screening and recognition of OSA in COPD patients are of great importance.
As an emerging data source, audio signals have also attracted growing attention in recent years. Compared to traditional physiological signals, audio signals may offer greater specificity and can exhibit stable patterns even in complex environments. Much research has been carried out in this field, and sound signals have come to play an increasingly important role in the monitoring and management of chronic diseases (27, 32, 33).
In recent years, the application of intelligent wearable devices in chronic disease management has become increasingly widespread and rapidly developed (15). With advances in monitoring technology and algorithms, their capabilities have expanded from tracking basic physiological parameters (such as HR, SpO2 and physical activity) to new parameters and more complex analyses including sound signals. Previous studies have frequently utilized the built-in microphones of smartphones to collect audio data. For instance, some research has used forced exhalation sounds recorded via smartphone microphones to screen for COPD (34). Similarly, other studies have employed smartphone-based cough sound monitoring for predicting COPD diagnosis, lung function, and airway obstruction (25, 35, 36). Smartwatches, as the most common type of wearable smart device, offer a new avenue for such applications. In our previous study, we initially used a novel smartwatch capable of audio monitoring to collect cough and forced exhalation sounds, demonstrating its potential to assess the severity of COPD (27). However, no studies have yet explored the use of cough and forced exhalation sounds for OSA screening.
In the context of OSA, although direct evidence is currently lacking, several indirect findings and theoretical considerations (37) support the possibility that OSA may influence cough and forced exhalation sounds. Obesity, a major risk factor for OSA, can lead to fat accumulation in the pharyngeal region, which may cause upper airway narrowing and affect the aerodynamic characteristics of airflow—potentially altering the acoustic properties of respiratory sound (38).
In addition, patients with OSA often experience laryngopharyngeal reflux (LPR), which can result in pharyngeal wall edema (39, 40). OSA is also closely associated with chronic cough, and continuous positive airway pressure (CPAP) therapy has been shown to improve chronic cough in OSA patients (40, 41). Chronic cough itself may further contribute to changes in acoustic features such as those of the cough sounds.
In this study, we found that multiple subfeatures of both cough and forced exhalation sounds were associated with OSA diagnosis. Specifically, around 100–200 subfeatures from each sound type showed significant correlations with the diagnosis of OSA. Through regression analysis and the development of the optimal predictive model, we found that the regression model based on cough sound subfeatures demonstrated high accuracy in identifying OSA among COPD patients. These findings highlight the significant screening potential of cough sounds for detecting OSA in patients with COPD.
Given the increasing role of cough sounds monitored by wearable smart devices in the early screening and disease monitoring of COPD patients, utilizing the same signals for both COPD screening and OSA risk assessment would undoubtedly be of significant importance for the detection and management of the overlap syndrome (OS).
In the present study, we used daytime cough sounds to screen for OSA, a condition characterized by respiratory events that primarily occur during sleep. This raises an important question about whether daytime acoustic signals can predict nighttime respiratory events. Previous studies have explored the relationship between daytime speech and the risk of OSA (42–45). These findings suggest the potential utility of wakeful vocal signals as convenient screening tools for assessing the risk and severity of OSA (42–45). However, it is important to note that these methods are currently limited to screening purposes only and cannot replace clinical diagnosis. The use of daytime acoustic signals may offer a more accessible and non-invasive approach to OSA screening.
This study is still in its preliminary exploration stage and has several limitations that need to be addressed in future research. First, the sample size of this study is small, which may introduce sampling bias and affect the stability and representativeness of the results. Therefore, a larger sample size is needed to validate our findings. Second, due to the lack of a normal control group or a pure OSA group, it remains unclear whether the cough sound features identified are specific to COPD-OSA overlap or are common to OSA in general. Therefore, our conclusions are limited to the COPD population and cannot be extrapolated beyond this group. Third, differences in microphone sensitivity, sampling rate, and built-in noise reduction algorithms may exist across devices. The current results are primarily based on the devices used in this study. Future research should validate the model’s generalizability and robustness in various smart devices. Last but not least, due to the exploratory nature of this study and in order to record audio data more clearly and accurately, we chose voluntary coughs. However, spontaneous coughs may better reflect the patient’s actual pathological state.
In summary, this study innovatively proposes using a smartwatch to collect cough and forced exhalation sounds to assess the risk of OSA in COPD patients. This method provides a new perspective on traditional sleep apnea monitoring with wearable devices, which usually involves the use of pulse oximetry and snoring sounds.
Given the widespread use of wearable devices in home-based chronic disease management and the common occurrence of cough as a symptom in chronic respiratory conditions, this method can help in the early identification of OSA in patients with existing chronic airway inflammatory diseases. In future studies, in addition to expanding the sample size and including healthy controls and general OSA patients as comparisons, it would be valuable to explore the use of spontaneous cough audio features for OSA screening, in addition to voluntary cough. Furthermore, the mechanisms linking OSA and cough sounds remain to be explored in greater depth through basic research.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the Ethics Committee of Peking University First Hospital (Approval Number: 2022083). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
CheZ: Formal analysis, Writing – review & editing, Investigation, Writing – original draft. ChuZ: Writing – original draft, Writing – review & editing, Investigation, Formal analysis, Data curation. ZheJ: Investigation, Writing – review & editing. KY: Formal analysis, Writing – review & editing. SW: Writing – review & editing, Investigation. MZ: Investigation, Writing – review & editing. ZhoJ: Investigation, Writing – review & editing. JL: Writing – review & editing, Supervision, Conceptualization, Funding acquisition. GW: Supervision, Writing – review & editing, Funding acquisition, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the National High Level Hospital Clinical Research Funding (High Quality Clinical Research Project of Peking University First Hospital) (No. 2023HQ01), National High Level Hospital Clinical Research Funding (Interdepartmental Research Project of Peking University First Hospital) (No. 2023IR49).
Acknowledgments
The authors would like to acknowledge Huawei Technologies Co., Ltd. for the development and optimization of the device and Huawei Research platform to this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. To improve the clarity and readability of the manuscript, the authors used ChatGPT for language refinement. All AI-assisted edits were thoroughly reviewed and adjusted where necessary. The authors take full responsibility for the accuracy and integrity of the scientific content.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1650014/full#supplementary-material
References
1.
Lareau SC Fahy B Meek P Wang A . Chronic obstructive pulmonary disease (COPD). Am J Respir Crit Care Med. (2019) 199:P1–p2. doi: 10.1164/rccm.1991P1
2.
Christenson SA Smith BM Bafadhel M Putcha N . Chronic obstructive pulmonary disease. Lancet. (2022) 399:2227–42. doi: 10.1016/S0140-6736(22)00470-6
3.
Jordan AS McSharry DG Malhotra A . Adult obstructive sleep apnoea. Lancet. (2014) 383:736–47. doi: 10.1016/S0140-6736(13)60734-5
4.
Mitra AK Bhuiyan AR Jones EA . Association and risk factors for obstructive sleep apnea and cardiovascular diseases: a systematic review. Diseases. (2021) 9:88. doi: 10.3390/diseases9040088
5.
Wang C Xu J Yang L Xu Y Zhang X Bai C et al . Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China pulmonary health [CPH] study): a national cross-sectional study. Lancet. (2018) 391:1706–17. doi: 10.1016/S0140-6736(18)30841-9
6.
Zhong N Wang C Yao W Chen P Kang J Huang S et al . Prevalence of chronic obstructive pulmonary disease in China: a large, population-based survey. Am J Respir Crit Care Med. (2007) 176:753–60. doi: 10.1164/rccm.200612-1749OC
7.
Peppard PE Young T Barnet JH Palta M Hagen EW Hla KM . Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. (2013) 177:1006–14. doi: 10.1093/aje/kws342
8.
Young T Palta M Dempsey J Peppard PE Nieto FJ Hla KM . Burden of sleep apnea: rationale, design, and major findings of the Wisconsin sleep cohort study. WMJ. (2009) 108:246–9.
9.
GBD Chronic Respiratory Disease Collaborators . Prevalence and attributable health burden of chronic respiratory diseases, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet Respir Med. (2020) 8:585–96. doi: 10.1016/S2213-2600(20)30105-3
10.
Benjafield AV Ayas NT Eastwood PR Heinzer R Ip MSM Morrell MJ et al . Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med. (2019) 7:687–98. doi: 10.1016/S2213-2600(19)30198-5
11.
Brennan M McDonnell MJ Walsh SM Gargoum F Rutherford R . Review of the prevalence, pathogenesis and management of OSA-COPD overlap. Sleep Breath. (2022) 26:1551–60. doi: 10.1007/s11325-021-02540-8
12.
Shawon MS Perret JL Senaratna CV Lodge C Hamilton GS Dharmage SC . Current evidence on prevalence and clinical outcomes of co-morbid obstructive sleep apnea and chronic obstructive pulmonary disease: a systematic review. Sleep Med Rev. (2017) 32:58–68. doi: 10.1016/j.smrv.2016.02.007
13.
Lurie A Roche N . Obstructive sleep apnea in patients with chronic obstructive pulmonary disease: facts and perspectives. COPD. (2021) 18:700–12. doi: 10.1080/15412555.2021.1950663
14.
van Zeller M McNicholas WT . Sleep disordered breathing: OSA-COPD overlap. Expert Rev Respir Med. (2024) 18:369–79. doi: 10.1080/17476348.2024.2373790
15.
MacKinnon GE Brittain EL . Mobile health technologies in cardiopulmonary disease. Chest. (2020) 157:654–64. doi: 10.1016/j.chest.2019.10.015
16.
Wu R Calligan M Son T Rakhra H de Lara E Mariakakis A et al . Impressions and perceptions of a smartphone and smartwatch self-management tool for patients with COPD: a qualitative study. COPD. (2024) 21:2277158. doi: 10.1080/15412555.2023.2277158
17.
Wu CT Li GH Huang CT Cheng YC Chen CH Chien JY et al . Acute exacerbation of a chronic obstructive pulmonary disease prediction system using wearable device data, machine learning, and deep learning: development and cohort study. JMIR Mhealth Uhealth. (2021) 9:e22591. doi: 10.2196/22591
18.
Lukas H Xu C Yu Y Gao W . Emerging telemedicine tools for remote COVID-19 diagnosis, monitoring, and management. ACS Nano. (2020) 14:16180–93. doi: 10.1021/acsnano.0c08494
19.
Cretikos MA Bellomo R Hillman K Chen J Finfer S Flabouris A . Respiratory rate: the neglected vital sign. Med J Aust. (2008) 188:657–9. doi: 10.5694/j.1326-5377.2008.tb01825.x
20.
Miller DJ Sargent C Roach GD . A validation of six wearable devices for estimating sleep, heart rate and heart rate variability in healthy adults. Sensors. (2022) 22:6317. doi: 10.3390/s22166317
21.
Li K Cardoso C Moctezuma-Ramirez A Elgalad A Perin E . Heart rate variability measurement through a smart wearable device: another breakthrough for personal health monitoring?Int J Environ Res Public Health. (2023) 20:7146. doi: 10.3390/ijerph20247146
22.
Debeij SM Aardoom JJ Haaksma ML Stoop WAM van Dam van Isselt EF Kasteleyn MJ . The potential use and value of a wearable monitoring bracelet for patients with chronic obstructive pulmonary disease: qualitative study investigating the patient and health care professional perspectives. JMIR Form Res. (2024) 8:e57108. doi: 10.2196/57108
23.
Hermans F Arents E Blondeel A Janssens W Cardinaels N Calders P et al . Validity of a consumer-based wearable to measure clinical parameters in patients with chronic obstructive pulmonary disease and healthy controls: observational study. JMIR Mhealth Uhealth. (2024) 12:e56027. doi: 10.2196/56027
24.
Porter P Claxton S Brisbane J Bear N Wood J Peltonen V et al . Diagnosing chronic obstructive airway disease on a smartphone using patient-reported symptoms and cough analysis: diagnostic accuracy study. JMIR Form Res. (2020) 4:e24587. doi: 10.2196/24587
25.
Xu W He G Pan C Shen D Zhang N Jiang P et al . A forced cough sound based pulmonary function assessment method by using machine learning. Front Public Health. (2022) 10:1015876. doi: 10.3389/fpubh.2022.1015876
26.
Serrurier A Neuschaefer-Rube C Röhrig R . Past and trends in cough sound acquisition, automatic detection and automatic classification: a comparative review. Sensors. (2022) 22:2896. doi: 10.3390/s22082896
27.
Zhang C Yu K Jin Z Bao Y Liao J Wang G . Intelligent wearable devices with audio collection capabilities to assess chronic obstructive pulmonary disease severity. Digit Health. (2025) 11:20552076251320730. doi: 10.1177/20552076251320730
28.
Cho SW Jung SJ Shin JH Won TB Rhee CS Kim JW . Evaluating prediction models of sleep apnea from smartphone-recorded sleep breathing sounds. JAMA Otolaryngol Head Neck Surg. (2022) 148:515–21. doi: 10.1001/jamaoto.2022.0244
29.
Voulgaris A Kalkanis A Steiropoulos P . Overlap syndrome (COPD and OSA): a treatable trait for triple treatment?Pulm Ther. (2025) 11:1–5. doi: 10.1007/s41030-024-00282-y
30.
McNicholas WT . COPD-OSA overlap syndrome: evolving evidence regarding epidemiology, clinical consequences, and management. Chest. (2017) 152:1318–26. doi: 10.1016/j.chest.2017.04.160
31.
Macrea M Oczkowski S Rochwerg B Branson RD Celli B Coleman JM 3rd et al . Long-term noninvasive ventilation in chronic stable Hypercapnic chronic obstructive pulmonary disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med. (2020) 202:e74–87. doi: 10.1164/rccm.202006-2382ST
32.
Lozano-Garcia M Paredes FA Jolley CJ Jane R . Respiratory sound intensity as a noninvasive acoustic biomarker in COPD. Annu Int Conf IEEE Eng Med Biol Soc. (2024) 2024:1–4. doi: 10.1109/EMBC53108.2024.10782895
33.
Kulnik ST Williams NM Kalra L Moxham J Birring SS . Cough frequency monitors: can they discriminate patient from environmental coughs?J Thorac Dis. (2016) 8:3152–9. doi: 10.21037/jtd.2016.11.02
34.
Windmon A Minakshi M Chellappan S Athilingam P Johansson M Jenkins BA . (2018). On detecting chronic obstructive pulmonary disease (COPD) cough using audio signals recorded from smart-phones. HEALTHINF.
35.
Infante C Chamberlain D Fletcher R Thorat Y Kodgule R . (2017). Use of cough sounds for diagnosis and screening of pulmonary disease. 2017 IEEE Global Humanitarian Technology Conference (GHTC)
36.
Sharan RV Abeyratne UR Swarnkar VR Claxton S Hukins C Porter P . Predicting spirometry readings using cough sound features and regression. Physiol Meas. (2018) 39:095001. doi: 10.1088/1361-6579/aad948
37.
McGarvey L Rubin BK Ebihara S Hegland K Rivet A Irwin RS et al . Global physiology and pathophysiology of cough: part 2. Demographic and clinical considerations: CHEST expert panel report. Chest. (2021) 160:1413–23. doi: 10.1016/j.chest.2021.04.039
38.
Jung SY Park DC Kim SH Yeo SG . Role of obesity in Otorhinolaryngologic diseases. Curr Allergy Asthma Rep. (2019) 19:34. doi: 10.1007/s11882-019-0865-3
39.
He J Wang C Li W . Laryngopharyngeal reflux in obstructive sleep apnea-hypopnea syndrome: an updated meta-analysis. Nat Sci Sleep. (2022) 14:2189–201. doi: 10.2147/NSS.S390272
40.
Faruqi S Fahim A Morice AH . Chronic cough and obstructive sleep apnoea: reflux-associated cough hypersensitivity?Eur Respir J. (2012) 40:1049–50. doi: 10.1183/09031936.00025012
41.
Sundar KM Stark AC Dicpinigaitis P . Chronic cough and obstructive sleep apnea. Sleep Med Clin. (2024) 19:239–51. doi: 10.1016/j.jsmc.2024.02.004
42.
Ding Y Sun Y Li Y Wang H Fang Q Xu W et al . Selection of OSA-specific pronunciations and assessment of disease severity assisted by machine learning. J Clin Sleep Med. (2022) 18:2663–72. doi: 10.5664/jcsm.9798
43.
TaghiBeyglou B Čuljak I Bagheri F Suntharalingam H Yadollahi A . Estimating the severity of obstructive sleep apnea during wakefulness using speech: a review. Comput Biol Med. (2024) 181:109020. doi: 10.1016/j.compbiomed.2024.109020
44.
Goldshtein E Tarasiuk A Zigel Y . Automatic detection of obstructive sleep apnea using speech signals. IEEE Trans Biomed Eng. (2011) 58:1373–82. doi: 10.1109/TBME.2010.2100096
45.
Espinoza-Cuadros F Fernández-Pozo R Toledano DT Alcázar-Ramírez JD López-Gonzalo E Hernández-Gómez LA . Reviewing the connection between speech and obstructive sleep apnea. Biomed Eng Online. (2016) 15:20. doi: 10.1186/s12938-016-0138-5
Summary
Keywords
cough sound, smartwatch, COPD, OSA, audio
Citation
Zhang C, Zhang C, Jin Z, Yu K, Wei S, Zhang M, Jin Z, Liao J and Wang G (2025) The value of cough sound monitoring via an audio-enabled smartwatch for OSA screening in COPD patients: a cross-sectional exploratory study. Front. Med. 12:1650014. doi: 10.3389/fmed.2025.1650014
Received
20 June 2025
Accepted
09 September 2025
Published
08 October 2025
Volume
12 - 2025
Edited by
Christina Maria Pabelick, Mayo Clinic, United States
Reviewed by
Ximing Liao, Shanghai East Hospital, China
Xia Linglin, Nanchang University—Qingshanhu Campus North, China
Yogendra Bharadwaj, Indian Institute of Technology Patna, India
Updates
Copyright
© 2025 Zhang, Zhang, Jin, Yu, Wei, Zhang, Jin, Liao and Wang.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiping Liao, jipingliao@hotmail.com; Guangfa Wang, wangguangfa@hotmail.com
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.