Abstract
Introduction:
Complex multimorbid patients often experience uncoordinated care transitions, increasing the risk of poor adherence, fragmented care, and adverse outcomes. Multicomponent, patient-centered interventions may improve transitional care, but evidence remains limited and heterogeneous.
Methods:
This pre-post intervention study evaluated the impact of SPICA, a multicomponent, high-intensity, patient-centered transitional care program implemented in Tenerife, Spain. Eligible adult patients with multimorbidity and complexity were consecutively enrolled between September 2023 and June 2024. Primary outcome was adherence to pharmacological treatment (Morisky Medication Adherence Scale-4). Secondary outcomes included patient satisfaction (Baker’s Questionnaire), health-related quality of life (HRQoL, EQ-5D-5L), disease (Disease Burden Morbidity Assessment), and treatment burden (Treatment Burden Questionnaire). Outcomes were assessed at baseline and one-month post-discharge. Multivariate linear regression was used for the satisfaction outcome, and bivariate models were conducted to explore predictors of the remaining intervention outcomes. McNemar’s Chi-squared test was used to evaluate changes in adherence rates, and ANCOVA models for other outcomes measured at both pre- and post-intervention.
Results:
Among the 112 patients, adherence improved from 53.4 to 84.9% (p < 0.001). Satisfaction with care was high (median 71; IQR 67–81). Significant improvements were observed in HRQoL (mobility [β − 0.56], pain/discomfort [β − 0.55], anxiety/depression [β − 0.37], EQ-5D Index [β 0.14], EQ-VAS [β 7.08]), and treatment burden (β − 12.24). Baseline scores were the most consistent predictors of improvement; age, sex, and comorbidity were not significant factors.
Discussion:
A multicomponent, high-intensity, patient-centered intervention such as SPICA appears to be associated with improvements in adherence and health outcomes in complex multimorbid patients transitioning from hospital to primary care, and may also be linked to high levels of patient satisfaction. Effects were more pronounced in those with worse baseline scores, suggesting a positive impact among those most in need. Nevertheless, further studies with more robust methodological designs are required to confirm these associations.
1 Introduction
Multimorbidity, defined as the coexistence of two or more chronic health problems in the same individual (1), has become a predominant challenge for health systems worldwide. The global prevalence of multimorbidity has been estimated at 42.4% (2). In Europe, the prevalence of multimorbidity among adults over 50 years was 28.2% in men and 34.5% in women (3). This condition has important consequences on mortality, quality of life (4), and health costs (5). In addition, the construct of complexity is related to multimorbidity, although it is not a necessary or sufficient condition on its own. Chronic diseases coexist with social and environmental conditions that have an impact on self-care and hinder access to resources, worsening health outcomes and increasing hospital admission and readmission rates (6). From this perspective, complexity not only depends on health-related characteristics, but also on socioeconomic, cultural, environmental and care ones (7). Thus, some of the factors that have been identified in the literature as influencing complexity are polypharmacy (8, 9), functional or mobility limitations (10), difficulties in understanding (9, 10), cognitive impairment (11), limited access to resources (9, 12), as well as disease and treatment burden (10), among other factors. Complexity could be understood as a dynamic state in which personal, social and biological aspects of patients operate as factors that add difficulty (13). As such, complex multimorbid patients represent a vulnerable group within the population.
In this context, the transition of care from primary care to the hospital and subsequently from the hospital to primary care is a critical point, which is a concern for health systems around the world (14, 15). This point is frequently associated with uncoordinated continuity of care, errors in treatment and follow-up of care plans (16), and difficulties in follow-up, which increases the risk of hospital readmissions, low adherence to treatments and decreased patient satisfaction (17–19).
Although it is clear that therapeutic adherence is essential to achieve positive clinical results, especially after hospital discharge, recent studies show that up to 50% of patients with multimorbidity do not adequately comply with the prescribed treatments (20). This situation has been associated with various factors such as the complexity of the regimens, including treatment with multiple medications (polypharmacy) (21), the presence of adverse effects (22) and the lack of coordination and adequate follow-up (23).
To address these challenges, different types of interventions have been designed and implemented. Among them, multicomponent interventions, that is, those that combine strategies such as personalized health education, interprofessional coordination and the use of technologies for remote monitoring, have proven to be effective (10, 24). These interventions seek not only to improve adherence to treatment but also to reduce the burden perceived by the patient and increase their satisfaction with the care received (25). Evidence suggests that the effective integration of these strategies could facilitate safer and more efficient care transitions, reducing the gaps between levels of care. However, the heterogeneity in the design and implementation of these interventions makes it difficult to comparatively evaluate their effectiveness, highlighting the need for more robust and specific studies.
Among these interventions, the SPICA program (Subprograma de Integración y Coordinación Asistencial - Subprogram for Care Integration and Coordination) incorporates the components identified in the literature as essential to effective transitional care. It stands out as a multicomponent and high-intensity care initiative carried out at the Hospital Universitario de Canarias, Spain. This program is led by a multidisciplinary team, including family doctors, nurses, and social workers, who provide specialized care for complex multimorbid patients during care transitions. SPICA program aims to ensure social and family reintegration; enhance primary healthcare continuity for discharged patients; improve clinical outcomes through a structured, patient-centered approach (26); and incorporating and developing the core elements of the Chronic Care Model (27–30).
The present study evaluates the impact of a multicomponent, high-intensity, and patient-centeredness intervention (SPICA program) on complex multimorbid patients during the transition from primary care to hospital and subsequent return to primary care.
2 Materials and methods
2.1 Study design
A pre-post multicomponent, high-intensity, and patient-centered intervention study was conducted and reported in accordance with the Transparent Reporting of Evaluations with Nonrandomized Designs (TREND) statement (31).
2.2 Participants
Patients routinely accessed the SPICA program, where their eligibility for the study was verified based on specific selection criteria.
Patients are recruited and included in the SPICA program via two main pathways (
26):
Hospital inpatient screening: The SPICA team’s professionals identify hospitalized patients as a high-risk population, particularly in terms of continuity of care after discharge, selecting those who may benefit most from specialized care.
Opportunistic recruitment: Patients can be referred by the service responsible for the hospital admission, primary care professionals, social workers, family members or through self-request.
For study inclusion, patients had to:
Be actively enrolled in the SPICA program.
Meet the following specific eligibility criteria, in line with those established by the SPICA program:
Adults aged 18 years and older.
Classified as complex patients due to multimorbidity, defined by:
≥2 chronic health problems; and
Cognitive impairment (Pfeiffer Questionnaire > 4) (32); or
Dependency in activities of daily living (Katz Index of Independence in Activities of Daily Living [Katz ADL] > 1 or Lawton-Brody Instrumental ADL Scale < 6) (33, 34); or
Living alone; or
At least three of the following minor criteria:
Age over 74
Severe visual impairment
Severe hearing impairment
Malnutrition
Poor self-perceived health
Provide informed consent to participate in the study.
2.3 Setting and recruitment
The study was conducted within the Primary Care Management of Tenerife under the Canary Islands Health Service (SCS), specifically at the Teaching Unit of Family and Community Care “La Laguna-Tenerife Norte,” located in Hospital Universitario de Canarias.
Recruitment was consecutive and conducted at the same hospital using the same mechanisms as the SPICA program. The enrolment in the SPICA program was a prerequisite for study inclusion. All patients who met both the SPICA program criteria (26) and the study’s eligibility requirements, and provided informed consent within the specified timeframe, were included. The recruitment period was between September 2023 and June 2024. The recruitment ended earlier than expected due to the closure of the program.
Accepted patients signed an informed consent form and completed the baseline questionnaires. In cases where the patient presented cognitive impairment, consent was provided by their legal representative.
2.4 Intervention
The SPICA program provided a multicomponent, high-intensity, and patient-centered care intervention aimed at ensuring continuity of care for hospitalized complex patients (
26). It incorporated comprehensive biopsychosocial assessment, multidisciplinary coordination, and shared decision-making to improve patient outcomes. The intervention included:
A comprehensive biopsychosocial evaluation covering medical, functional, psychological, and contextual and social aspects.
The design and implementation of individualized care plans based on patient needs, preferences, and clinical evidence.
Regular case conferences with hospital and primary care professionals, occasionally including patients and their families.
Patient and caregiver support, with a focus on promoting self-care training, empowering the patient, providing emotional support, and enhancing the patient-clinician relationship.
A structured discharge plan to ensure proper transition to outpatient or home care.
The intervention was delivered face-to-face during hospital admission and continued after discharge through primary care follow-ups. It involved:
Direct patient-professional interactions (bedside visits, structured interviews, and shared decision-making sessions).
Care coordination meetings among hospital specialists, primary care professionals, and social workers.
Ongoing communication between SPICA professionals and primary care professionals to ensure follow-up and adherence to the care plan.
The intervention was performed by a multidisciplinary team composed of SPICA professionals, including family doctors and primary care nurses, in functional alliance with other medical specialists from both medical and surgical specialties, social workers, and nurses specialized in various areas depending on the case they attend to, as well as other healthcare and administrative professionals involved in patient care.
The intervention was delivered in two phases, beginning with an in-hospital phase that involved regular bedside visits and assessments throughout hospitalization, followed by a post-discharge phase focused on follow-up and coordination with primary care services, including communication with primary care professionals and adjustments to the care plan based on each patient’s needs, ensuring continuity of care.
In order to enhance adherence to the care plan and ensure treatment continuity, the intervention involved frequent patient-caregiver interactions, personalized self-care education, family involvement in decision-making, and close follow-up with primary care professionals. Further details on the design, implementation, and evaluation of the SPICA intervention are available in García Hernández et al. (26).
2.5 Objectives
The objectives of the present study are:
To evaluate the impact of SPICA intervention—a multicomponent, high-intensity, and patient-centered care approach designed to ensure continuity of care for hospitalized complex multimorbid patients—by assessing its effects on adherence, patient satisfaction, and health outcomes, including health-related quality of life (HRQoL), disease burden, and treatment burden.
To identify protective and risk factors influencing adherence, patient satisfaction, and HRQoL in this population.
The hypothesis is that improvements in adherence, satisfaction, and HRQoL levels following SPICA intervention are influenced by patients’ social, clinical, and personal factors.
2.6 Outcomes
2.6.1 Primary outcome
Patient adherence to pharmacological treatment assessed by the Spanish validated version of the Morisky Medication Adherence Scale-4 (MMAS-4™). This self-reported measure consists of four dichotomous (“yes” or “no”) questions designed to identify barriers to proper therapeutic adherence across a variety of chronic medical conditions. The scale has been shown to have moderate reliability (α = 0.62) (35–38). Permission to use the MMAS-4™ was obtained through a formal license agreement. All conditions for the authorized use of the instrument were fulfilled in accordance with the licensing agreement.
For the analyses, adherence levels were categorized into two groups: ‘adherent’ (all responses indicate adherence) versus ‘non-adherent’ (at least one response does not reflect adherence). Patient classification as adherent or non-adherent was determined based on their adherence to all medications within their treatment regimen. Adherence was assessed at baseline, and one-month post-discharge.
2.6.2 Secondary outcomes
Satisfaction with general practice consultations was measured one-month post-discharge using the Spanish version of Baker’s questionnaire (39).
Baker’s questionnaire is a valid and reliable self-reporting scale (39), consisting of 18 items, each answered on a 5-point Likert scale (ranging from ‘totally disagree’ to ‘totally agree’). It consists of three dimensions: care provided by the professional, time spent during the consultation, and the depth of the relationship with the professional. This scale helps identify areas of patient-perceived strengths and weaknesses in general practice.
Other secondary outcomes were assessed at baseline and one-month post-discharge, including: HRQoL, measured with the Spanish version of EuroQol-5-Dimension-5-Level (EQ-5D-5L); disease burden, assessed using the Spanish version of Disease Burden Morbidity Assessment (DBMA) (40); Treatment burden and its impact on patient well-being, evaluated through the Spanish version of Treatment Burden Questionnaire (TBQ) (41).
EQ-5D-5L is a reliable and validate self-administered instrument, divided into two sections (42): the descriptive system and the Visual Analogue Scale (VAS). The first section assesses an individual’s current health status across five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Each dimension includes five response levels, representing increasing severity: no problems (1), slight problems (2), moderate problems (3), severe problems (4), and extreme problems or inability (5). Each respondent selects the level that best reflects their status for each of the five dimensions. The second section asks respondents to rate their current health status using a 20-cm vertical scale, resembling a thermometer. The top of the scale, marked as ‘the best health state you can imagine,’ is assigned a value of 100, while the bottom, labeled ‘the worst health state you can imagine,’ is assigned a value of 0. Additionally, the five-digit health states from the descriptive system can be converted into a single utility index score (EQ-5D Index) using country-specific value sets. This index, which typically ranges from values below 0 (indicating health states perceived as worse than death) to 1 (full health), reflects societal preferences for different health states (43).
DBMA has demonstrated satisfactory feasibility and acceptability, with a Cronbach’s alpha of 0.72 (40). This self-reported instrument includes 21 common medical conditions. Patients first indicate whether they have any of the listed conditions and, if applicable, rate the extent to which each condition limits their daily activities using a Likert scale ranging from ‘none’ (1) to ‘a lot’ (5). The total score is calculated as the sum of limitation scores across all conditions, with higher scores reflecting greater limitations in activities of daily living.
TBQ is a self-administered reliable instrument (α > 0.8) (41). The scale has 16 items, each rated on a Likert scale from 0 (‘no effort’) to 10 (‘extreme effort’). The scores for all items are summed to generate a total score ranging from 0 to 160, with higher scores indicating a greater treatment burden.
2.6.3 Additional sociodemographic and clinical variables
In addition, the following measures were collected at baseline from the patients: Functional status, specifically the ability to perform activities of daily living independently, was assessed using the Spanish version of Katz ADL Index (33, 34) and Lawton-Brody Instrumental ADL Scale; comorbid disease burden, used to predict the risk of one-year mortality in hospitalized patients based on the presence of specific chronic health conditions, was assessed using the Charlson Comorbidity Index (44, 45); cognitive function, specifically that indicative of cognitive impairment, was evaluated using the Spanish version of Pfeiffer test (32, 46); and sociodemographic data (age, sex, education level, marital status, number of children, family type, and cohabitants) were collected through an ad hoc questionnaire.
Finally, clinical data, including number of prescribed drugs—categorized based on the presence or absence of polypharmacy, defined as the routine use of five or more medications (47)—number of chronic health problems and medical specialties involved in patient care, were collected from the electronic health records and defined based on the information available at the time of the patient’s hospitalization.
2.6.4 Data collection
The data was obtained from two different sources: the patients themselves, as well as information obtained from the electronic health records from primary care (Drago AP) and specialized care (SAP), and continuous electronic prescription of the Canary Islands Health Service.
All questionnaires, including those on sociodemographic characteristics, MMAS-4™, Baker’s questionnaire, EQ-5D-5L, DBMA, TBQ, Katz ADL Index, Charlson Comorbidity Index, and the Pfeiffer test, were administered face-to-face by a SPICA professional during the patient’s hospital admission interview. Follow-up assessments were conducted via a telephone interview, for measures also collected post-intervention, between the SPICA family doctor/nurse and the patient. The clinical data of all patients were collected from electronic health records by SPICA professionals.
All the information was stored in a protected Excel document that met the required confidentiality criteria.
2.7 Sample size
A two-tailed test at a 5% significance level and 80% power, accounting for a 20% loss to follow-up, determined that 264 patients were required to detect a 12.5% difference in treatment adherence, using the MMAS-4™, according to previous literature (48).
2.8 Assignment method
Since this study followed a pre-post intervention design with a single-arm approach, no randomization or group assignment was performed. Instead, all eligible participants were consecutively included in the study upon meeting the inclusion criteria.
2.9 Blinding
No blinding was employed in this study, as it followed an open-label pre-post intervention design.
2.10 Unit of analysis
The individual was the unit of analysis in this single-arm study.
2.11 Statistical analyses
Continuous variables were summarized using means and standard deviations (SD) or medians and interquartile ranges (IQR), depending on their distribution. Categorical variables were presented as frequencies and percentages.
Differential adherence rates for the main variable were analyzed with McNemar’s Chi-squared test with continuity correction. Changes in each of the five EQ-5D-5L domains (mobility, self-care, usual activities, pain/discomfort, and anxiety/depression) were assessed using the Wilcoxon signed-rank test.
Bivariate regression analyses were conducted to explore associations between sociodemographic factors (e.g., sex and age) and clinical characteristics (e.g., number of chronic health problems, number of prescribed drugs, number of dependencies, Katz Index score, and number of medical specialties involved), and the change scores of the outcome measures (i.e., the difference between post- and pre- intervention scores) for adherence, health-related quality of life (HRQoL), disease burden, and treatment burden. Univariate linear regression analyses were performed for satisfaction, which was assessed only at post-intervention. Variables yielding a p-value ≤ 0.10 in the previous analyses were considered for inclusion in subsequent models as covariates. If the variables were not significant, the models were adjusted for age, sex, and baseline outcome measure score.
Finally, for outcomes with both pre- and post-intervention measurements (e.g., adherence, HRQoL, disease burden, and treatment burden), analyses of covariance (ANCOVA) models were applied to assess changes over time. A linear link function was used for continuous dependent variables. In contrast, satisfaction—assessed only once at the post-intervention time point—was analyzed using multivariate linear regression.
All analyses followed an intention-to-treat approach. Statistical analyses were conducted using R version 4.3. (49).
2.12 Ethical consideration
The study was conducted according to the guidelines of the Declaration of Helsinki, applicable local legislation and institutional requirements, and was approved by the Clinical Research Ethics Committee with Medicines of Hospital Universitario de Canarias (CHUNSC_2023_97 [PIFIISC22/25]).
3 Results
3.1 Participant flow and recruitment
Out of the 785 patients attended to in the SPICA program between September 2023 and July 2024, 112 individuals were enrolled in the study (see Figure 1). Eleven participants (9.82%) died before the one-month follow-up, and 100 participants completed the intervention and had evaluable pre- and post-intervention data. These were included in the final per-protocol analysis. Participants who withdrew consent, were lost to follow-up, or had incomplete data on primary outcomes were excluded from the analysis. No imputation methods were applied for missing data.
Figure 1
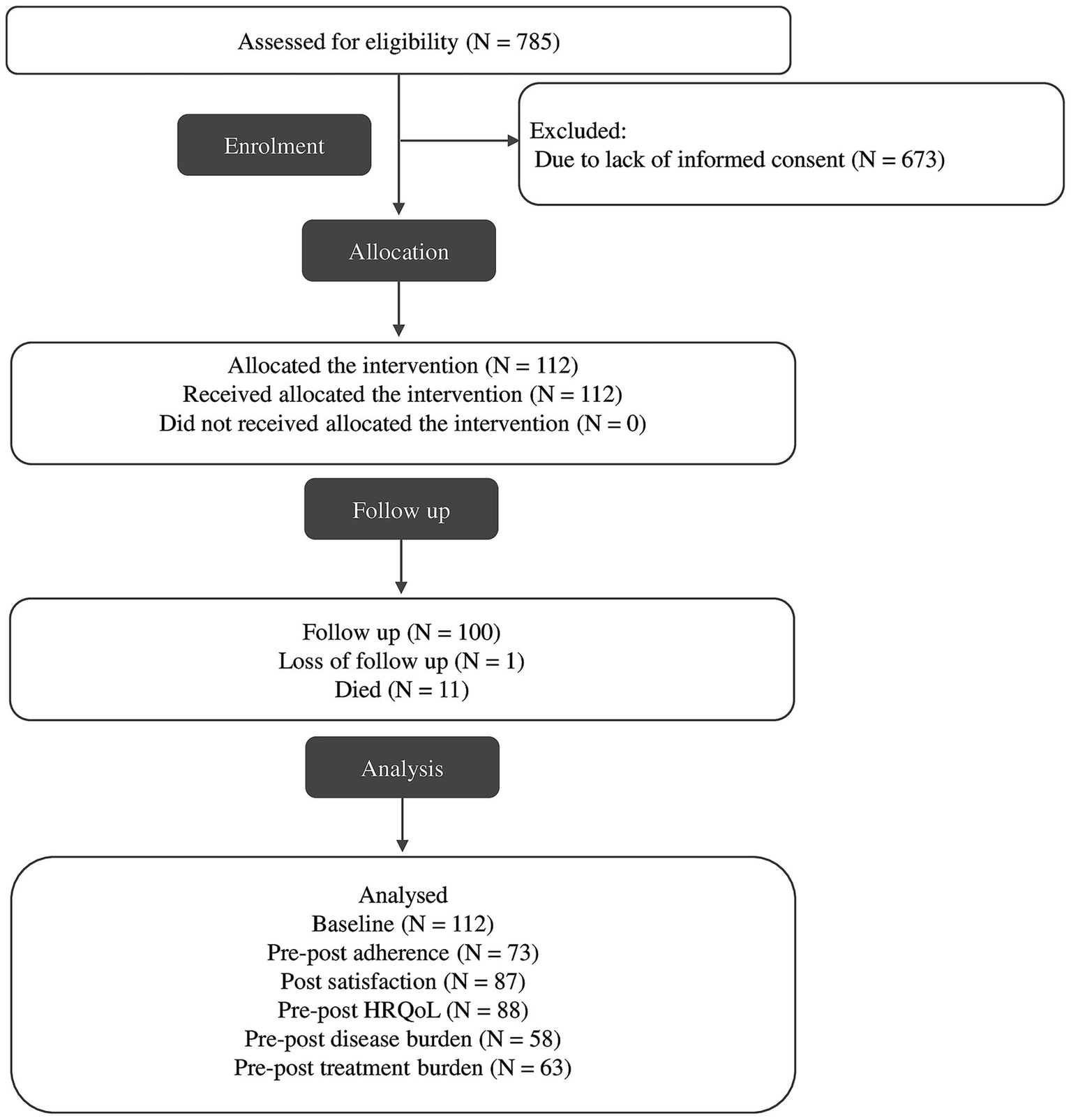
Flowchart of selection process.
The recruitment process was prematurely halted due to the external discontinuation of the SPICA program, a system-level decision unrelated to the study protocol.
3.2 Baseline sample characteristics
At baseline, the mean age was 76.13 years (SD ± 10.73), ranging from 40 to 94 years. Approximately half of the participants were men, and the other half were women. Only about half were married or in a relationship, although more than 80% lived with a partner, family, or friends. On average, participants had 9.65 (SD ± 5.37) health conditions and over 80% of participants presented with polypharmacy.
A total of 19 participants (16.97%) had moderate to severe cognitive impairment (Pfeiffer Questionnaire > 4), while 74 (66.07%) had significant dependency in ADL (Katz ADL Index score ≠ A). Regarding healthcare resource utilization, participants had an average of one hospital admission in the previous year (SD ± 0.15) and 20.64 primary care visits (SD ± 1.73). Table 1 summarizes the participants’ baseline characteristics.
Table 1
| Variables | Total (N = 112) |
|---|---|
| Age, N (%) | |
| <54 yrs. | 3 (2.68) |
| 55–64 yrs. | 13 (11.61) |
| 65–79 yrs. | 49 (43.75) |
| >80 yrs. | 47 (41.96) |
| Sex, N (%) | |
| Female | 54 (48.21) |
| Male | 58 (51.79) |
| Education level, N (%) | |
| No formal education | 31 (27.68) |
| Primary education | 58 (51.79) |
| Secondary education | 12 (10.71) |
| University education | 10 (8.93) |
| Marital status, N (%) | |
| Single | 10 (8.93) |
| Married/partnered | 53 (47.32) |
| Separated/divorced | 10 (8.93) |
| Widowed | 37 (33.04) |
| Other | 1 (0.89) |
| Children, N (%) | |
| Yes | 100 (89.29) |
| No | 11 (9.82) |
| Type of family, N (%) | |
| Nuclear | 73 (65.18) |
| Binuclear | 1 (0.89) |
| Single-parent | 2 (1.79) |
| Extended | 7 (6.25) |
| Family equivalent | 9 (8.04) |
| Lives alone | 20 (17.86) |
| Cohabitants, N (%) | |
| Alone | 28 (25.00) |
| Partner | 32 (28.57) |
| Family/friends | 52 (46.43) |
| Number of prescribed drugs, M (SD) | 9.67 (5.12) |
| No polypharmacy (0–4), N (%) | 20 (17.86) |
| Polypharmacy (≥5), N (%) | 92 (82.14) |
| Number of Chronic Health Problems, M (SD) | 9.65 (5.37) |
| Charlson Comorbidity Index, M (SD) | 6.66 (2.50) |
| Katz ADL Index, M (SD) | 3.28 (2.39) |
| Dependent on at least in one activity, N (%) | 74 (66.07) |
| Independent for all activities, N (%) | 38 (33.93) |
| Lawton-Brody instrumental ADL scale, N (%) | |
| Total dependence (0–1) | 24 (21.43) |
| Severe dependence (2–3) | 10 (8.93) |
| Moderate dependence (4–5) | 9 (8.04) |
| Mild dependence (6–7) | 13 (11.61) |
| Independent (8) | 55 (49.11) |
| Missing | 1 (0.89) |
| Pfeiffer questionnaire, N (%) | |
| Normal (0–2) | 78 (69.64) |
| Mild impairment (3–4) | 13 (11.61) |
| Moderate impairment (5–7) | 10 (8.93) |
| Severe impairment (8–10) | 9 (8.04) |
| Missing | 2 (1.79) |
| Psychoaffective problem, N (%) | |
| Yes | 50 (44.64) |
Baseline demographic and clinical characteristics of patients.
ADL, Activities of Daily Living; IQR, Interquartile range; M, mean; N, number of patients; SD, standard deviation; yrs., Years.
3.3 Outcomes and estimation
3.3.1 Primary outcome: adherence
Among the 73 patients evaluated at both time points, adherence improved significantly from 39 patients (53.42%) at baseline to 62 patients (84.93%) post-intervention (p < 0.001). Of the 39 patients initially classified as adherent, 34 remained adherent, while five became non-adherent. Conversely, among the 34 patients who were non-adherent at baseline, 28 became adherent, while six remained non-adherent.
Table 2 shows the bivariate regression model examining the association between patients’ change in adherence score and baseline sociodemographic and clinical characteristics. No association was found between differences in adherence score and any of the variables analyzed, including sex, age, Charlson Comorbidity Index, number of chronic health problems, number of prescribed drugs, functional status (Katz ADL Index), or the number of medical specialties involved.
Table 2
| Predictors | β | 95% CI | p |
|---|---|---|---|
| Sex (Women; Reference: Men) | −0.03 | −0.57, 0.50 | 0.90 |
| Age (years) | −0.02 | −0.04, 0.00 | 0.12 |
| Charlson Comorbidity Index | −0.05 | −0.15, 0.06 | 0.35 |
| Number of Chronic Health Problems | 0.01 | −0.04, 0.06 | 0.82 |
| Number of prescribed drugs | −0.03 | −0.07, 0.02 | 0.31 |
| Katz ADL Index | 0.09 | −0.02, 0.21 | 0.12 |
| Medical specialties involved | 0.01 | −0.10, 0.13 | 0.83 |
Association between baseline sociodemographic and clinical characteristics and patients’ change in adherence score (bivariate regression).
ADL, Activities of Daily Living; β, Regression coefficient; CI, Confidence Interval; p, p-value.
The SPICA intervention resulted in a statistically significant improvement in medication adherence, as evidenced by a reduction in MMAS-4™ scores from pre- to post-intervention. The regression model confirmed that higher baseline MMAS-4™ scores were predictive of greater improvement post-intervention (β − 0.95). Notably, neither age nor sex were significant predictors, suggesting that the observed improvements in adherence were not associated with demographic factors (Table 3).
Table 3
| Outcomes | Crude change (Post vs Pre) | Adjusted models for change scores | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Intercept | Baseline score | Age | Sex | Other variables3 | Model fit R2; adj R2 | |||||||||
| Pre | Post | p | β | p | β | p | β | p | β | p | β | p | ||
| Non-Adherence (MMAS-4™ Score), M (SD) | 0.8 (1.0) | 0.2 (1.0) | <0.001 1 | −0.59 | <0.001 | −0.95 | <0.001 | −0.01 | 0.30 | 0.14 | 0.39 | 0.70; 0.69 | ||
| EQ-5D-5L Mobility, Median (IQR) | 3.0 (2.0–4.0) | 2.5 (2.0–4.0) | <0.001 1 | −0.56 | <0.001 | −0.46 | <0.001 | 0.01 | 0.59 | 0.05 | 0.83 | 0.23; 0.21 | ||
| EQ-5D-5L Self-care, Median (IQR) | 3.0 (2.0–4.0) | 3.0 (1.0–5.0) | 0.591 | −0.15 | 0.52 | −0.50 | <0.001 | 0.01 | 0.38 | 0.04 | 0.89 | 0.21 | <0.01 | 0.22; 0.18 |
| EQ-5D-5L Usual activities, Median (IQR) | 3.5 (2.0–5.0) | 3.0 (1.0–5.0) | 0.511 | −0.16 | 0.50 | −0.49 | <0.001 | 0.03 | 0.05 | 0.05 | 0.88 | 0.05 | 0.09 | 0.29; 0.25 |
| EQ-5D-5L Pain/ Discomfort, Median (IQR) | 2.0 (1.0–3.0) | 1.0 (1.0–2.0) | 0.009 1 | −0.55 | <0.01 | −0.80 | <0.001 | −0.03 | 0.80 | 0.03 | 0.92 | 0.46; 0.43 | ||
| EQ-5D-5L Anxiety/ Depression, Median (IQR) | 2.0 (1.0–3.0) | 1.0 (1.0–3.0) | 0.02 1 | −0.37 | <0.05 | −0.84 | <0.001 | −0.01 | 0.44 | −0.07 | 0.78 | 0.51; 0.49 | ||
| EQ-VAS, M (SD) | 58.34 (27.68) | 63.96 (22.95) | 0.082 | 7.08 | 0.05 | 0.83 | <0.001 | 0.17 | 0.44 | −2.25 | 0.66 | 0.58; 0.56 | ||
| EQ-5D Index, M (SD) | 0.47 (0.32) | 0.58 (0.29) | 0.001 2 | 0.14 | <0.001 | −0.63 | <0.001 | −0.00 | 0.83 | −0.03 | 0.59 | 0.37; 0.35 | ||
| Disease burden (IQR) | 8.0 (5.0–13.0) | 6.0 (3.0–9.75) | 0.007 1 | −1.27 | 0.21 | −0.68 | <0.001 | −0.04 | 0.48 | −2.19 | 0.12 | 0.67; 0.64 | ||
| Treatment burden (IQR) | 21.0 (6.0–48.5) | 9.0 (0.0–21.0) | <0.001 1 | −12.24 | <0.001 | −0.79 | <0.001 | 0.09 | 0.42 | −10.92 | <0.001 | 0.84; 0.83 | ||
Change scores and associated predictors for adherence, HRQoL, and disease and treatment burden scores.
1Wilcoxon test; 2T-test; 3Katz ADL Index (for EQ-5D-5L Self-care) and Prescribed drugs (for EQ-5D-5L Usual activities); Adjusted R2 = Adjusted coefficient of determination (accounts for the number of predictors in the model); β = Regression coefficient; IQR = Interquartile range; M = Mean; p: p-value; Pre: Pre-intervention; Post: Post-intervention; R2 = Coefficient of determination; SD = standard deviation; SE = Standard error.
The quantitative variables have been included in the models centered on the mean.
The numbers in bold represent statistically significant differences.
3.3.2 Secondary outcomes
3.3.2.1 Satisfaction
Among participants (N = 87), the satisfaction score with general practice consultations had a median of 71 (IQR 67–81). Regression analyses revealed no statistically significant associations between post-intervention satisfaction scores and any of the sociodemographic or clinical variables analyzed, indicating a relatively uniform perception of satisfaction across demographic and clinical subgroups (Table 4).
Table 4
| Predictors | Univariate | Multivariate | ||||
|---|---|---|---|---|---|---|
| β | SE | p | β | SE | p | |
| Sex (Women; Reference: Men) | 1.76 | 2.31 | 0.76 | 0.62 | 2.43 | 0.80 |
| Age | 0.11 | 0.10 | 0.27 | 0.11 | 0.11 | 0.32 |
| Charlson Comorbidity Index | −0.55 | 0.46 | 0.23 | – | – | – |
| Number of Chronic Health Problems | −0.41 | 0.20 | 0.046 | −0.41 | 0.21 | 0.051 |
| Number of prescribed drugs | −0.18 | 0.22 | 0.41 | – | – | – |
| Katz ADL Index | −0.86 | 0.49 | 0.08 | −0.85 | 0.50 | 0.09 |
| Medical specialties involved | −0.23 | 0.52 | 0.66 | – | – | – |
Univariate and multivariate linear regression models predicting satisfaction.
ADL, Activities of Daily Living; β, Regression coefficient; Katz Index, functional status scale assessing independence in basic ADLs; p, p-value; SE, Standard error.
The quantitative variables have been included in the models centered on the mean.
3.3.2.2 HRQoL
Among the 88 patients evaluated at both time points, significant improvements were observed in three EQ-5D-5L dimensions: mobility (p < 0.001), pain/discomfort (p = 0.009), and anxiety/depression (p = 0.017). For mobility, the proportion of patients reporting “no problems” increased from 11 (9.82%) at baseline to 18 (16.07%) post-intervention, while those reporting “unable/extreme” problems decreased from 21 (18.75%) to 9 (8.04%). Similarly, for pain/discomfort, 42 patients (37.50%) initially reported “no problems,” increasing to 53 (47.32%) after the intervention. In anxiety/depression, 47 patients (41.96%) reported “no problems” at baseline, rising to 53 (47.32%) post-intervention, with a reduction in those reporting severe or extreme issues. In contrast, no significant changes were observed in the self-care and usual activities dimensions, with the distribution of responses remaining relatively stable (Table 5).
Table 5
| EQ-5D-5L Dimension/Scores | No problems | Slight problems | Moderate problems | Severe problems | Unable/extreme | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre (n = 109) | Post (n = 88) | Pre (n = 109) | Post (n = 88) | Pre (n = 109) | Post (n = 88) | Pre (n = 109) | Post (n = 88) | Pre (n = 109) | Post (n = 88) | ||
| Mobility | 11 (9.82) | 18 (16.07) | 25 (22.32) | 26 (23.21) | 27 (24.11) | 21 (18.75) | 25 (22.32) | 14 (12.50) | 21 (18.75) | 9 (8.04) | <0.0011 |
| Self-care | 22 (19.64) | 32 (28.57) | 24 (21.43) | 8 (7.14) | 23 (20.54) | 10 (8.93) | 14 (12.50) | 12 (10.71) | 26 (23.21) | 26 (23.21) | 0.591 |
| Usual activities | 20 (17.86) | 23 (20.54) | 15 (13.39) | 12 (10.71) | 19 (16.96) | 10 (8.93) | 13 (11.61) | 7 (6.25) | 42 (37.50) | 36 (32.14) | 0.501 |
| Pain/discomfort | 42 (37.50) | 53 (47.32) | 23 (20.54) | 14 (12.50) | 20 (17.86) | 11 (9.82) | 19 (16.96) | 7 (6.25) | 5 (4.46) | 3 (2.68) | 0.0091 |
| Anxiety/depression | 47 (41.96) | 53 (47.32) | 21 (18.75) | 11 (9.82) | 22 (19.64) | 17 (15.18) | 11 (9.82) | 3 (2.68) | 8 (7.14) | 4 (3.57) | 0.0171 |
Frequencies (%) and patient’s change in severity levels of EQ-5D-5L domains.
1Wilcoxon test; EQ-5D-5L, EuroQol 5-Dimension 5-Level scale; p, p-value.
Table 6 presents the results of the bivariate regression model examining the associations between patients’ changes in EQ-5D-5L domain scores and baseline sociodemographic and clinical characteristics. No statistically significant associations were found for sex, age, Charlson Comorbidity Index, number of chronic health problems, poorer functional status (measured by the Katz Index), or number of medical specialties involved. However, a higher number of prescribed drugs was marginally significantly associated with greater limitations in performing usual activities (β = 0.08, p = 0.02). Similarly, although age was not significantly related to HRQoL domains, a borderline relationship was observed between older age and increased difficulty in usual activities (p = 0.07).
Table 6
| Predictors | Mobility | Self-care | Usual activities | Pain/discomfort | Anxiety/depression | EQ-VAS | EQ-5D Index | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | β | 95% CI | p | |
| Sex (Women; Reference: Men) | 0.07 | −0.45, 0.59 | 0.78 | 0.21 | −0.45, 0.88 | 0.53 | 0.24 | −0.48, 0.96 | 0.51 | −0.14 | −0.78, 0.50 | 0.66 | −0.00 | −0.68, 0.67 | 0.99 | 8.93 | −3.5, 21 | 0.16 | −0.03 | −0.17, 0.11 | 0.69 |
| Age | 0.00 | −0.02, 0.03 | 0.87 | 0.01 | −0.02, 0.04 | 0.65 | 0.03 | 0.00, 0.06 | 0.07 | −0.01 | −0.03, 0.02 | 0.71 | −0.00 | −0.03, 0.03 | 0.88 | −0.08 | −0.66, 0.48 | 0.76 | 0.00 | −0.01, 0.01 | 0.97 |
| Charlson Comorbidity Index | 0.06 | −0.05, 0.16 | 0.27 | −0.08 | −0.21, 0.05 | 0.23 | 0.01 | −0.13, 0.16 | 0.87 | 0.09 | −0.04, 0.22 | 0.19 | −0.02 | −0.16, 0.12 | 0.76 | −0.57 | −3.1, 1.9 | 0.65 | −0.00 | −0.03, 0.03 | 0.98 |
| Number of Chronic Health Problems | 0.03 | −0.02, 0.08 | 0.21 | 0.01 | −0.06, 0.07 | 0.87 | 0.03 | −0.04, 0.10 | 0.38 | −0.01 | −0.07, 0.05 | 0.75 | −0.03 | −0.10, 0.03 | 0.30 | 0.17 | −1.1, 1.4 | 0.79 | −0.00 | −0.01, 0.01 | 0.78 |
| Number of prescribed drugs | 0.01 | −0.04, 0.06 | 0.68 | 0.03 | −0.03, 0.09 | 0.33 | 0.08 | 0.02, 0.15 | 0.02 | 0.00 | −0.06, 0.06 | 0.91 | 0.00 | −0.06, 0.06 | 0.97 | 0.25 | −0.93, 1.4 | 0.68 | −0.01 | −0.02, 0.01 | 0.33 |
| Katz ADL Index | −0.03 | −0.14, 0.09 | 0.65 | 0.12 | −0.02, 0.26 | 0.10 | −0.01 | −0.16, 0.15 | 0.95 | 0.01 | −0.13, 0.15 | 0.91 | −0.00 | −0.15, 0.14 | 0.99 | −1.90 | −4.5, 0.75 | 0.16 | −0.00 | −0.03, 0.03 | 0.76 |
| Medical specialties involved | −0.04 | −0.15, 0.08 | 0.53 | −0.02 | −0.17, 0.12 | 0.76 | 0.10 | −0.05, 0.26 | 0.19 | −0.05 | −0.19, 0.09 | 0.46 | −0.05 | −0.20, 0.10 | 0.49 | 1.59 | −1.2, 4.4 | 0.26 | 0.01 | −0.02, 0.04 | 0.52 |
Association between baseline sociodemographic and clinical characteristics changes in EQ-5D-5L domains scores (bivariate regression).
ADL, Activities of Daily Living; β, Regression coefficient; EQ-5D-5L, EuroQol 5-Dimension 5-Level scale; EQ-VAS, EuroQol Visual Analogue Scale (self-rated health from 0 = worst to 100 = best imaginable); Katz Index, functional status scale assessing independence in basic ADLs; p, p-value.
The quantitative variables have been included in the models centered on the mean.
The SPICA intervention was associated with statistically significant improvements in the mobility, pain/discomfort, and anxiety/depression dimensions of HRQoL. Additionally, improvements were observed in both overall health status (EQ-5D Index) and self-perceived health status (EQ-VAS). The regression model predicting changes in outcomes identified baseline scores as the strongest and most consistent predictors of improvement across most domains. Specifically, participants with worse initial scores experienced more pronounced improvements, particularly in the domains of mobility, pain/discomfort, and anxiety/depression, as well as in the overall EQ-5D score. Neither age nor sex emerged as significant predictors in most models, suggesting that the observed improvements were broadly consistent across demographic groups (Table 3).
3.3.2.3 Disease and treatment burden
As shown in Table 7, neither sex, age, Charlson Comorbidity Index, number of chronic health problems, number of prescribed drugs, Katz Index, nor the number of medical specialties involved were significantly associated with either changes in disease burden or treatment burden.
Table 7
| Predictors | Disease burden | Treatment burden | ||||
|---|---|---|---|---|---|---|
| β | 95% CI | p | β | 95% CI | p | |
| Sex (Women; Reference: Men) | −3.67 | −7.80, 0.45 | 0.08 | −11.62 | −24, 0.63 | 0.06 |
| Age | −0.09 | −0.26, 0.08 | 0.29 | 0.37 | −0.14, 0.89 | 0.15 |
| Charlson Comorbidity Index | 0.48 | −0.32, 1.3 | 0.24 | 1.80 | −0.66, 4.3 | 0.15 |
| Number of Chronic Health Problems | 0.08 | −0.33, 0.49 | 0.70 | −0.12 | −1.2, 1.0 | 0.84 |
| Number of prescribed drugs | −0.17 | −0.55, 0.22 | 0.39 | −0.09 | −1.3, 1.1 | 0.89 |
| Katz ADL Index | −0.29 | −1.2, 0.65 | 0.55 | 1.33 | −1.5, 4.1 | 0.35 |
| Medical specialties involved | −0.08 | −1.0, 0.84 | 0.86 | −0.13 | −2.9, 2.7 | 0.93 |
Association between baseline sociodemographic and clinical characteristics and pre-post changes in disease/treatment burden (bivariate regression).
ADL, Activities of Daily Living; β, Regression coefficient; CI, Confidence Interval; Katz Index, functional status scale assessing independence in basic ADLs; p, p-value.
The quantitative variables have been included in the models centered on the mean.
Both disease burden and treatment burden showed statistically significant improvements between pre- and post-intervention scores (Table 3).
The improvement in disease burden score was no longer statistically significant in the multivariate model (p = 0.21), whereas treatment burden continued to show a significant reduction. The multivariate regression model confirmed that these improvements were primarily predicted by participants’ baseline burden levels—those with higher initial scores experienced the greatest benefit. Although age was not significantly associated with changes, sex was a significant predictor of treatment burden improvement, with women showing greater reductions in burden compared to men.
4 Discussion
The main objective of this pre-post study was to assess the effects of the SPICA intervention on patients with complex multimorbidity, focusing on adherence, patient satisfaction, and health outcomes, such as HRQoL, disease burden, and treatment burden. Moreover, the study aimed to explore which patient characteristics were associated with greater response to the intervention in terms of satisfaction, adherence, HRQoL, and perceived burden.
The results suggest a potential improvement in medication adherence post-intervention. This is consistent with previous literature (50, 51). Notably, the patients who benefited the most were those with lower baseline adherence levels, highlighting the intervention could be particular useful in individuals with greater intervention needs in this regard. Furthermore, no significant associations were found between adherence outcomes and sociodemographic variables such as age or sex, suggesting that the observed improvements were consistent across demographic groups.
Previous studies have shown that patient satisfaction with transitional care interventions is associated with improved medication adherence, especially when these interventions include educational components, structured follow-up, and ongoing support (52). In our study, we found a high level of overall satisfaction, compared to other studies (53, 54), regardless of the patients’ demographic and clinical characteristics. These beneficial results on satisfaction in the care transition were expected, taking into account previous studies (55, 56). Several factors have been identified that potentially influence satisfaction in these situations, such as care coordination and continuity (55, 57), self-management education and ongoing support (55, 58), improved quality of life (57), multidisciplinary interventions (57, 59), and structured follow-up after discharge (60). In fact, single-component interventions are generally ineffective for patients with multimorbidity, and an integrated, multifaceted approach is recommended to optimize the care of these patients (60–62). In the case of the SPICA intervention, all of these factors are combined.
As we have already seen, HRQoL is a factor that directly influences patient satisfaction. In this study, HRQoL scores indicated that the SPICA intervention could have produced significant improvements in the mobility, pain/discomfort, and anxiety/depression domains. Overall health status also improved, and a positive change was observed in self-perceived health status. Specifically, those with more severe baseline problems—both in individual domains and in overall HRQoL—showed the greatest improvements. Once again, these results are consistent with previous literature. Transitional care interventions, especially those that are multidisciplinary and complex, have been shown to have a positive impact on the quality of life of these patients (57, 63). However, these results should be interpreted with caution, as baseline HRQoL and other clinical variables may have been temporarily affected by the recent hospitalization. In this context, part of the observed improvement could reflect a natural post-acute recovery process, rather than the sole effect of the intervention.
Regarding disease and treatment burden, both domains showed significant improvements following the intervention, which is consistent with previous literature (50). These improvements were primarily influenced by baseline burden levels, with participants experiencing higher initial burden reporting greater post-intervention reductions. Additionally, sex appeared to play a role, as women demonstrated more pronounced improvements in treatment burden.
The hypothesis that improvements in adherence, satisfaction, and HRQoL levels following the SPICA intervention would be influenced by patients’ social, clinical, and personal factors was only partially confirmed. Although improvements were consistently observed, most outcomes were not significantly associated with variables such as sex, age, or number of health problems. In fact, patient-centered interventions were found to improve discharge readiness, quality of transition, and HRQoL in both women and men, with no significant gender differences in most outcomes. However, women reported lower quality of life at discharge and experienced greater treatment benefit at this point compared to men (64). Instead, baseline status emerged as the most consistent predictor of change, with greater improvements observed among patients who started with poorer adherence, lower HRQoL, or higher burden levels. These findings suggest that this kind of intervention, which is multicomponent, high-intensity and patient-centered in transitional care of complex multimorbid patients may be particularly effective in reaching those most in need, regardless of other individual characteristics. These results are to be expected given that scientific evidence suggests that more complex patients benefit more from transitional care interventions (55–57).
4.1 Strengths and limitations
The present study has several limitations that should be acknowledged. Firstly, the pre-post design without a control group limits the ability to attribute observed changes—both in primary and secondary outcomes—exclusively to the SPICA intervention, as other external factors may have contributed. In this regard, improvements may partly reflect natural recovery following hospital discharge, as patients often present their lowest scores for quality of life and functional capacity during hospitalization. Secondly, the interpretation of improvements in adherence and other outcomes is limited by methodological constraints. On the one hand, regression to the mean may have influenced the results, as patients with complex conditions and low baseline scores are statistically more likely to show some improvement at follow-up regardless of the intervention. On the other hand, outcomes were assessed only at baseline and one-month post-discharge, which may not adequately capture longer-term patterns or the sustainability of these changes. This short follow-up period may also help explain why improvements were observed in mobility, pain/discomfort, and anxiety/depression, but not in self-care and usual activities, as significant functional recovery in a complex, multimorbid population is unlikely to be detectable within just 1 month. Thirdly, although the recruitment was prematurely halted due to the discontinuation of the program, and the final sample did not reach the initially calculated target of 264 patients, the achieved sample size was sufficient to detect significant changes in the primary outcome of adherence. Nonetheless, the reduced sample may have limited the statistical power for secondary outcomes and subgroup analyses, particularly where effect sizes were smaller, and results from these analyses should therefore be interpreted with caution. Fourthly, all outcomes were based on self-reports, and the lack of blinding may have introduced response and detection bias. Although validated instruments were used, there remains the potential for social desirability bias, particularly in the context of an intensive intervention in which patients may have wished to please the research team. In addition, the increased attention participants received as part of the study (visits, questionnaires, and follow-ups) could, in itself, have promoted better adherence and well-being—a potential Hawthorne effect—regardless of the specific content of the intervention. Fifthly, the study experienced a high rate of refusal among potential participants, which could have influenced the results and introduced selection bias. The authors consider that this high refusal rate may be partly explained by the characteristics of the included patients. Not only are they generally older than the typical study population and less accustomed to participating in this kind of research, but their high clinical complexity places them in a situation of increased clinical and social vulnerability. This may limit their willingness to participate, as they often feel overwhelmed by their own health problems and psychosocial circumstances at the time of recruitment. Finally, as this is evaluative research in health services, it is highly influenced by the healthcare and organizational context and may not be directly extrapolated to other contexts.
Despite these limitations, the study also has notable strengths. A major strength is the comprehensive evaluation of multiple dimensions relevant to patients with complex needs, including adherence, satisfaction, quality of life, and burden of disease and treatment. Additionally, the study employed validated instruments and a mixed analytical strategy, combining pre-post comparisons with multivariate models adjusted for relevant clinical and sociodemographic variables. Another strength lies in its pragmatic approach, reflecting real-world clinical practice and enhancing ecological validity. Furthermore, although the design was not randomized, the significant improvements observed and the robustness of the analyses contribute valuable preliminary evidence for the potential effectiveness in this patient population.
4.2 Conclusion
This study suggests an association between a multicomponent, patient-centered transitional care intervention and improvements in adherence, patient satisfaction, and health outcomes in complex multimorbid patients transitioning from hospital to primary care. Nevertheless, further research using more robust methodological designs is needed to confirm and strengthen these findings.
Statements
Data availability statement
The datasets presented in this article are not readily available because of concerns regarding patient anonymity. Requests to access the datasets should be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Clinical Research Ethics Committee with Medicines of Hospital Universitario de Canarias. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants, and when necessary, by their legal guardians or next of kin.
Author contributions
TP-S: Project administration, Data curation, Conceptualization, Supervision, Methodology, Writing – original draft, Funding acquisition, Formal analysis. BG: Methodology, Investigation, Conceptualization, Writing – original draft, Funding acquisition. MG: Writing – review & editing, Conceptualization, Investigation. PC: Conceptualization, Writing – review & editing, Investigation. YR: Conceptualization, Investigation, Writing – review & editing. VM: Writing – review & editing, Investigation, Conceptualization. EG: Investigation, Writing – review & editing, Conceptualization. EB: Conceptualization, Writing – review & editing, Investigation. SB: Conceptualization, Writing – review & editing, Investigation. AA-L: Formal analysis, Data curation, Writing – original draft, Methodology. MG-B: Methodology, Data curation, Writing – original draft, Formal analysis. YG-H: Writing – original draft, Conceptualization. JL-R: Writing – original draft, Conceptualization. JV-D: Methodology, Conceptualization, Writing – review & editing.
Funding
The author(s) declared that financial support was received for this work and/or its publication. This work was supported by the Fundación Canaria Instituto de Investigación Sanitaria de Canarias in the Call for Research, Development and Innovation Projects to be carried out in the fields of Hospital Care and Public Health, aimed at meeting the health needs of the population [grant number PIFIISC22/25]. The project also received a research grant from the Carlos III Institute of Health, Ministry of Economy and Competitiveness (Spain), awarded on the call for the creation of Health Outcomes-Oriented Cooperative Research Networks (RICORS), with reference RD21/0016/0001 and RD21/0016/0013, co-funded by the European Union – NextGenerationEU. The funders had no role in the design of the study, data collection, analysis, interpretation, or writing of the manuscript.
Acknowledgments
The authors wish to acknowledge Patrick Dennis for his assistance with the English language editing of the final manuscript. MMAS® 2006 used with permission (www.adherence.cc).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Gen AI was used in the creation of this manuscript. Generative AI (ChatGPT-4o) was used to assist with language editing, and improving clarity and coherence of the text. All content was reviewed and validated by the authors.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fmed.2025.1756368.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
- ADL
Activities of Daily Living
- ANCOVA
Analysis of Covariance
- DBMA
Disease Burden Morbidity Assessment
- EQ-5D-5L
EuroQol 5-Dimension 5-Level
- EQ-VAS
EuroQol Visual Analogue Scale
- HRQoL
Health-Related Quality of Life
- IQR
Interquartile Range
- Katz ADL
Katz Index of Independence in Activities of Daily Living
- M
Mean
- MMAS-4™
Morisky Medication Adherence Scale – 4 item version
- N
number of patients
- p
p-value
- Post
Post-intervention
- Pre
Pre-intervention
- R2
Coefficient of determination
- SCS
Canary Islands Health Service
- SD
Standard Deviation
- SE
Standard error
- SPICA
Subprogram for Care Integration and Coordination
- TBQ
Treatment Burden Questionnaire
- TREND
Transparent Reporting of Evaluations with Nonrandomized Designs
- Yrs
Years
- β
Regression coefficient
Glossary
References
1.
Le Reste JY Nabbe P Manceau B Lygidakis C Doerr C Lingner H et al . The European general practice research network presents a comprehensive definition of multimorbidity in family medicine and long term care, following a systematic review of relevant literature. J Am Med Dir Assoc. (2013) 14:319–25. doi: 10.1016/j.jamda.2013.01.001,
2.
Ho IS-S Azcoaga-Lorenzo A Akbari A Davies J Hodgins P Khunti K et al . Variation in the estimated prevalence of multimorbidity: systematic review and meta-analysis of 193 international studies. BMJ Open. (2022) 12:e057017. doi: 10.1136/bmjopen-2021-057017,
3.
Bezerra de Souza DL Oliveras-Fabregas A Espelt A Bosque-Prous M de Camargo CM Teixidó-Compañó E et al . Multimorbidity and its associated factors among adults aged 50 and over: a cross-sectional study in 17 European countries. PLoS One. (2021) 16:e0246623. doi: 10.1371/journal.pone.0246623,
4.
Williams JS Egede LE . The association between multimorbidity and quality of life, health status and functional disability. Am J Med Sci. (2016) 352:45–52. doi: 10.1016/j.amjms.2016.03.004,
5.
Picco L Achilla E Abdin E Chong SA Vaingankar JA McCrone P et al . Economic burden of multimorbidity among older adults: impact on healthcare and societal costs. BMC Health Serv Res. (2016) 16:173. doi: 10.1186/s12913-016-1421-7,
6.
Wallace E Stuart E Vaughan N Bennett K Fahey T Smith SM . Risk prediction models to predict emergency hospital admission in community-dwelling adults: a systematic review. Med Care. (2014) 52:751–65. doi: 10.1097/MLR.0000000000000171,
7.
Safford MM Allison JJ Kiefe CI . Patient complexity: more than comorbidity. The vector model of complexity. J Gen Intern Med. (2007) 22:382–90. doi: 10.1007/s11606-007-0307-0,
8.
Grant RW Ashburner JM Hong CS Chang Y Barry MJ Atlas SJ . Defining patient complexity from the primary care physician’s perspective: a cohort study. Ann Intern Med. (2011) 155:797–804. doi: 10.7326/0003-4819-155-12-201112200-00001,
9.
Quek JS Lew JK Lee ES Smith HE Wong SKW . Prevalence of complexity in primary care and its associated factors: a Singapore experience. Ann Acad Med Singap. (2025) 54:87–100. doi: 10.47102/annals-acadmedsg.2024312,
10.
Shippee ND Shah ND May CR Mair FS Montori VM . Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. (2012) 65:1041–51. doi: 10.1016/j.jclinepi.2012.05.005,
11.
Koroukian SM Schiltz NK Warner DF Stange KC Smyth KA . Increasing burden of complex multimorbidity across gradients of cognitive impairment. Am J Alzheimers Dis Other Dement. (2017) 32:408–17. doi: 10.1177/1533317517726388,
12.
Webster F Rice K Bhattacharyya O Katz J Oosenbrug E Upshur R . The mismeasurement of complexity: provider narratives of patients with complex needs in primary care settings. Int J Equity Health. (2019) 18:107. doi: 10.1186/s12939-019-1010-6,
13.
de Jonge P Huyse FJ Stiefel FC . Case and care complexity in the medically ill. Med Clin North Am. (2006) 90:679–92. doi: 10.1016/j.mcna.2006.04.005,
14.
Shepperd S Lannin NA Clemson LM McCluskey A Cameron ID Barras SL . Discharge planning from hospital to home. Cochrane Database Syst Rev. (2013) 1:CD000313. doi: 10.1002/14651858.CD000313.pub4
15.
Willis CD Best A Riley B Herbert CP Millar J Howland D . Systems thinking for transformational change in health. Evid Policy. (2014) 10:113–26. doi: 10.1332/174426413X662815
16.
Dinh TS Hanf M Klein A-A Brueckle M-S Rietschel L Petermann J et al . Informational continuity of medication management in transitions of care: qualitative interviews with stakeholders from the HYPERION-TransCare study. PLoS One. (2024) 19:e0300047. doi: 10.1371/journal.pone.0300047,
17.
Fernández E Estévez M . La valoración geriátrica integral en el anciano frágil hospitalizado: revisión sistemática. Gerokomos. (2013) 24:8–13. doi: 10.4321/S1134-928X2013000100002
18.
Heidenreich PA Bozkurt B Aguilar D Allen LA Byun JJ Colvin MM et al . 2022 AHA/ACC/HFSA guideline for the Management of Heart Failure: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. J Am Coll Cardiol. (2022) 79:e263–421. doi: 10.1016/j.jacc.2021.12.012,
19.
Kern LM Riffin C Phongtankuel V Aucapina JE Banerjee S Ringel JB et al . Gaps in the coordination of care for people living with dementia. J Am Geriatr Soc. (2024) 72:3119–28. doi: 10.1111/jgs.19105,
20.
Foley L Larkin J Lombard-Vance R Murphy AW Hynes L Galvin E et al . Prevalence and predictors of medication non-adherence among people living with multimorbidity: a systematic review and meta-analysis. BMJ Open. (2021) 11:e044987. doi: 10.1136/bmjopen-2020-044987,
21.
Kassaw AT Sendekie AK Minyihun A Gebresillassie BM . Medication regimen complexity and its impact on medication adherence in patients with multimorbidity at a comprehensive specialized hospital in Ethiopia. Front Med (Lausanne). (2024) 11:1369569. doi: 10.3389/fmed.2024.1369569,
22.
Committee W Birtcher KK Allen LA Anderson JL Bonaca MP Gluckman TJ et al . ACC expert consensus decision pathway for integrating atherosclerotic cardiovascular disease and multimorbidity treatment: a framework for pragmatic, patient-centered care: a report of the American College of Cardiology Solution set Oversight Committee. J Am Coll Cardiol. (2022) 2023:292–317. doi: 10.1016/j.jacc.2022.08.754
23.
Maddox TM Januzzi JL Allen LA Breathett K Brouse S Butler J et al . 2024 ACC expert consensus decision pathway for treatment of heart failure with reduced ejection fraction. JACC. (2024) 83:1444–88. doi: 10.1016/j.jacc.2023.12.024,
24.
Morkisch N Upegui-Arango LD Cardona MI van den Heuvel D Rimmele M Sieber CC et al . Components of the transitional care model (TCM) to reduce readmission in geriatric patients: a systematic review. BMC Geriatr. (2020) 20:345. doi: 10.1186/s12877-020-01747-w,
25.
Pepping RMC Vos RC Numans ME Kroon I Rappard K Labots G et al . An emergency department transitional care team prevents unnecessary hospitalization of older adults: a mixed methods study. BMC Geriatr. (2024) 24:668. doi: 10.1186/s12877-024-05260-2,
26.
García-Hernández M González de León B Barreto-Cruz S Vázquez-Díaz JR . Multicomponent, high-intensity, and patient-centered care intervention for complex patients in transitional care: SPICA program. Front Med (Lausanne). (2022) 9:1033689. doi: 10.3389/fmed.2022.1033689,
27.
Wagner EH . Chronic disease care. BMJ. (2004) 328:177–8. doi: 10.1136/bmj.328.7433.177,
28.
Wagner EH . Organizing care for patients with chronic illness revisited. Milbank Q. (2019) 97:659–64. doi: 10.1111/1468-0009.12416,
29.
Wagner EH Austin BT Von Korff M . Organizing care for patients with chronic illness. Milbank Q. (1996) 74:511–44. doi: 10.2307/3350391,
30.
Wagner EH Glasgow RE Davis C Bonomi AE Provost L McCulloch D et al . Quality improvement in chronic illness care: a collaborative approach. Jt Comm J Qual Improv. (2001) 27:63–80. doi: 10.1016/s1070-3241(01)27007-2,
31.
Des Jarlais DC Lyles C Crepaz N TREND Group . Improving the reporting quality of nonrandomized evaluations of behavioral and public health interventions: the TREND statement. Am J Public Health. (2004) 94:361–6. doi: 10.2105/ajph.94.3.361
32.
Martínez de la Iglesia J Dueñas Herrero R Onís Vilches MC Aguado Taberné C Albert Colomer C Luque R . Spanish language adaptation and validation of the Pfeiffer’s questionnaire (SPMSQ) to detect cognitive deterioration in people over 65 years of age. Med Clin (Barc). (2001) 117:129–34. doi: 10.1016/s0025-7753(01)72040-4
33.
Alvarez Solar M de Alaiz Rojo AT Brun Gurpegui E Cabañeros Vicente JJ Calzón Frechoso M Cosío Rodríguez I et al . Functional capacity of patients over 65 according to the Katz index. Reliability of the method. Aten Primaria. (1992) 10:812–6.
34.
Brorsson B Asberg KH . Katz index of independence in ADL. Reliability and validity in short-term care. Scand J Rehabil Med. (1984) 16:125–32. doi: 10.2340/165019778416125132,
35.
Morisky DE Green LW Levine DM . Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. (1986) 24:67–74. doi: 10.1097/00005650-198601000-00007,
36.
Nguyen T-M-U La Caze A Cottrell N . What are validated self-report adherence scales really measuring?: a systematic review. Br J Clin Pharmacol. (2014) 77:427–45. doi: 10.1111/bcp.12194,
37.
Val Jiménez A Amorós Ballestero G Martínez Visa P Fernández Ferré ML León SM . Descriptive study of patient compliance in pharmacologic antihypertensive treatment and validation of the Morisky and Green test. Aten Primaria. (1992) 10:767–70.
38.
Morisky DE DiMatteo MR . Improving the measurement of self-reported medication nonadherence: final response. J Clin Epidemiol. (2011) 64:262–3. doi: 10.1016/j.jclinepi.2010.09.010
39.
Fernández San Martín MI Rebagliato Nadal O de Gispert Uriach B Roig Carrera H Artigas Guix J Bonay Valls B et al . Adaptación de un cuestionario de satisfacción del paciente con la consulta médica y de enfermería. Atenc Primaria. (2008) 40:611–6. doi: 10.1016/S0212-6567(08)75694-4,
40.
Wijers IGM Ayala A Rodriguez-Blazquez C Rodriguez-Laso A Rodriguez-Rodriguez V Forjaz MJ . Disease burden morbidity assessment by self-report: psychometric properties in older adults in Spain. Geriatr Gerontol Int. (2017) 17:1102–8. doi: 10.1111/ggi.12835,
41.
Ysrraelit MC Fiol MP Peña FV Vanotti S Terrasa SA Tran V-T et al . Adaptation and validation of a Spanish version of the treatment burden questionnaire in patients with multiple sclerosis. BMC Neurol. (2019) 19:209. doi: 10.1186/s12883-019-1441-0,
42.
Ministerio de Sanidad, Servicios Sociales e Igualdad . Encuesta Nacional de Salud. España 2011/12. Calidad de vida relacionada con la salud en adultos: EQ-5D-5L. [Serie Informes monográficos no 3]. Madrid: Ministerio de Sanidad, Servicios Sociales e Igualdad (2014).
43.
Ministry of Health, Social Services, and Equality . National Health Survey. Spain 708 2011/12. Health-related quality of life in adults: EQ-5D-5L. [Monographic Report Series 709 no. 3]. Madrid: Ministry of Health, Social Services, and Equality. (2014) 710. Available online at: https://www.sanidad.gob.es/estadEstudios/estadisticas/encuestaNacional/encuestaNac2011/infor711mesMonograficos/CVRSadultosEQ5D5L.pdf (Accessed January 01, 2023).
44.
Charlson ME Pompei P Ales KL MacKenzie CR . A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. (1987) 40:373–83. doi: 10.1016/0021-9681(87)90171-8,
45.
Charlson ME Charlson RE Peterson JC Marinopoulos SS Briggs WM Hollenberg JP . The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol. (2008) 61:1234–40. doi: 10.1016/j.jclinepi.2008.01.006,
46.
Pfeiffer E . A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J Am Geriatr Soc. (1975) 23:433–41. doi: 10.1111/j.1532-5415.1975.tb00927.x,
47.
World Health Organization . Medication safety in polypharmacy. Geneva: World Health Organization. (WHO/UHC/SDS/2019.11). Licence: CC BY-NC-SA 3.0 IGO (2019). (Accessed January 01, 2023).
48.
Hedegaard U Kjeldsen LJ Pottegård A Henriksen JE Lambrechtsen J Hangaard J et al . Improving medication adherence in patients with hypertension: a randomized trial. Am J Med. (2015) 128:1351–61. doi: 10.1016/j.amjmed.2015.08.011,
49.
R Core Team . R: a language and environment for statistical computing. (2022). Available online at: https://www.R-project.org/
50.
Tomlinson J Cheong V-L Fylan B Silcock J Smith H Karban K et al . Successful care transitions for older people: a systematic review and meta-analysis of the effects of interventions that support medication continuity. Age Ageing. (2020) 49:558–69. doi: 10.1093/ageing/afaa002,
51.
Yang C Zhu S Lee DTF Chair SY . Interventions for improving medication adherence in community-dwelling older people with multimorbidity: a systematic review and meta-analysis. Int J Nurs Stud. (2022) 126:104154. doi: 10.1016/j.ijnurstu.2021.104154,
52.
Tyler N Hodkinson A Planner C Angelakis I Keyworth C Hall A et al . Transitional care interventions from hospital to community to reduce health care use and improve patient outcomes: a systematic review and network meta-analysis. JAMA Netw Open. (2023) 6:e2344825. doi: 10.1001/jamanetworkopen.2023.44825,
53.
Heilat HB Gharaibeh MK Shtaiyat WM Gharaibeh RS Ananbeh AM Baniyaseen MJ et al . Patients’ satisfaction with services provided by family physicians at the comprehensive healthcare centers in northern Jordan. J Family Community Med. (2025) 32:28–35. doi: 10.4103/jfcm.jfcm_185_24,
54.
Weiss MC Platt J Riley R Chewning B Taylor G Horrocks S et al . Medication decision making and patient outcomes in GP, nurse and pharmacist prescriber consultations. Prim Health Care Res Dev. (2015) 16:513–27. doi: 10.1017/S146342361400053X,
55.
Berthelsen C Møller N Bunkenborg G . Transitional care model for older adults with multiple chronic conditions: an evaluation of benefits utilising an umbrella review. J Clin Nurs. (2024) 33:481–96. doi: 10.1111/jocn.16913,
56.
Le Berre M Maimon G Sourial N Guériton M Vedel I . Impact of transitional care services for chronically ill older patients: a systematic evidence review. J Am Geriatr Soc. (2017) 65:1597–608. doi: 10.1111/jgs.14828,
57.
Collet R van Grootel J Dongen J Wiertsema S Ostelo R van der Schaaf M et al . The impact of multidisciplinary transitional care interventions for complex care needs: a systematic review and meta-analysis. Gerontologist. (2025) 65:gnaf088. doi: 10.1093/geront/gnaf088,
58.
Naylor MD Shaid EC Carpenter D Gass B Levine C Li J et al . Components of comprehensive and effective transitional care. J Am Geriatr Soc. (2017) 65:1119–25. doi: 10.1111/jgs.14782,
59.
McParland C Johnston B Cooper M . A mixed-methods systematic review of nurse-led interventions for people with multimorbidity. J Adv Nurs. (2022) 78:3930–51. doi: 10.1111/jan.15427,
60.
Markle-Reid M McAiney C Fisher K Ganann R Gauthier AP Heald-Taylor G et al . Effectiveness of a nurse-led hospital-to-home transitional care intervention for older adults with multimorbidity and depressive symptoms: a pragmatic randomized controlled trial. PLoS One. (2021) 16:e0254573. doi: 10.1371/journal.pone.0254573,
61.
Smith SM Wallace E Clyne B Boland F Fortin M . Interventions for improving outcomes in patients with multimorbidity in primary care and community setting: a systematic review. Syst Rev. (2021) 10:271. doi: 10.1186/s13643-021-01817-z,
62.
Zhou Y Dai X Ni Y Zeng Q Cheng Y Carrillo-Larco RM et al . Interventions and management on multimorbidity: an overview of systematic reviews. Ageing Res Rev. (2023) 87:101901. doi: 10.1016/j.arr.2023.101901,
63.
Bernocchi P Vitacca M La Rovere MT Volterrani M Galli T Baratti D et al . Home-based telerehabilitation in older patients with chronic obstructive pulmonary disease and heart failure: a randomised controlled trial. Age Ageing. (2018) 47:82–8. doi: 10.1093/ageing/afx146,
64.
Blumer V Gayowsky A Xie F Greene SJ Graham MM Ezekowitz JA et al . Effect of patient-centered transitional care services on patient-reported outcomes in heart failure: sex-specific analysis of the PACT-HF randomized controlled trial. Eur J Heart Fail. (2021) 23:1488–98. doi: 10.1002/ejhf.2312,
Summary
Keywords
multimorbidity, transitional care, patient-centered care, adherence, quality of life, treatment burden, integrated care, SPICA program
Citation
del Pino-Sedeño T, González de León B, García Hernández M, Coronil Olmedo P, Reyes Melián YS, Martínez Hernández V, García Bautista E, Arraez EB, Barreto Cruz S, Abrante-Luis A, García-Bello MA, González-Hernández Y, López-Rodríguez JA and Vázquez-Díaz JR (2025) A multicomponent, high-intensity, patient-centered care intervention to optimize transitional care coordination for complex multimorbid people: a pre-post design. Front. Med. 12:1650973. doi: 10.3389/fmed.2025.1650973
Received
20 June 2025
Accepted
29 September 2025
Published
24 October 2025
Corrected
06 January 2026
Volume
12 - 2025
Edited by
Yiu Wing Kam, Duke Kunshan University, China
Reviewed by
Manuel Lopes, University of Evora, Portugal
Kagiso Seakamela, University of Limpopo, South Africa
Updates
Copyright
© 2025 del Pino-Sedeño, González de León, García Hernández, Coronil Olmedo, Reyes Melián, Martínez Hernández, García Bautista, Barrios Arraez, Barreto Cruz, Abrante-Luis, García-Bello, González-Hernández, López-Rodríguez and Vázquez-Díaz.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tasmania del Pino-Sedeño, tasmania.delpino@sescs.es
†These authors share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.