Abstract
Nephrotic syndrome caused by mercury poisoning is rare and can often be misdiagnosed. We report a 33-years-old female patient who experienced fatigue and edema of the lower extremities for 1 month. A renal biopsy confirmed the diagnosis of membranous nephropathy (MN) for the patient. Her urinary mercury level was measured at 56.22 ug/L, with a reference range of 0–10 ug/L. A cream used to remove freckles was identified to contain about 13276 mg/kg mercury, which is the possible mercury source. The patient’s symptoms improved after chelation therapy, and their urinary mercury levels gradually decreased. After 12 months of follow-up, the patient remained in a state of complete remission. Using mercury-based cosmetics over an extended period can lead to mercury poisoning and trigger MN. Chelation therapy serves as an effective treatment, yielding positive clinical outcomes.
1 Introduction
As a toxic heavy metal, mercury silently exists in human daily life and work. Prolonged or high levels of mercury exposure can cause mercury poisoning. Common sources of mercury exposure include occupational settings, broken thermometers, mercury-containing folk remedies, and cosmetics (1). When mercury accumulates in the human body, it can cause damage to various systems, including the skin, gastrointestinal tract, kidneys, and neuromuscular system (2). Diagnosing mercury poisoning can be difficult due to its unusual clinical symptoms and often overlooked history of exposure. This report outlines the clinical characteristics, diagnosis, treatment, and prognosis of a patient with nephrotic syndrome (NS) resulting from mercury poisoning caused by a facial cream containing mercury.
2 Case presentation
A 33-years-old woman visited the outpatient clinic, presenting with a 1-month history of fatigue and edema in both legs. She had no fever, reduced urine output, gross hematuria, and flank pain. Physical examination showed moderate pitting edema in both lower extremities. She had a 3-year history of hypothyroidism, for which she regularly took levothyroxine sodium. The patient denied having hypertension, cardiovascular disorder, chronic kidney disease, or diabetes mellitus. Upon examination, urine tests showed a positive protein (+++) result. The serum total protein (TP) was measured at 44.9 g/L, and the albumin (ALB) level was 24.4 g/L. The renal function was normal.
Upon admission, further examinations were performed. Daily blood pressure and temperature monitoring were conducted, yielding normal results. Color Doppler ultrasound examination was conducted for the heart, kidneys, and veins of the lower extremities, which showed no abnormalities. Both the electrocardiograph and chest X-ray results were normal. Laboratory tests indicated: 24-h urine protein at 5.8 g, total cholesterol (TC) at 6.03 mmol/L, low-density lipoprotein cholesterol (LDL-C) at 3.29 mmol/L, serum calcium at 1.94 mmol/L, hemoglobin (Hb) at 104 g/L, and red blood cell count (RBC) at 3.71 × 10∧12/L. The patient’s fasting blood glucose, thyroid function, immunoglobulin (Ig) levels, coagulation function, and complement C3/C4 levels tests were normal. Viral tests for hepatitis B and C, as well as HIV, were negative. Serum tests for anti-M-type phospholipase A2 receptor (PLA2R), antineutrophil cytoplasmic antibody (ANCA), antidouble-stranded DNA (dsDNA), and antinuclear antibody (ANA) all returned within normal limits.
In view of NS, a renal biopsy was performed. Light microscopy revealed a stiff glomerular capillary loop, mild segmental proliferation of mesangial matrix and cells, and subepithelial fuchsinophilic deposits along the glomerular basement membrane (Figure 1). The immunofluorescence (IF) showed diffuse, granular IgG deposits in the glomerular capillary loop (Figure 2A). IF staining for PLA2R, thrombospondin type 1 domain containing 7A (THSD7A), and neural epidermal growth factor-like 1 protein (NELL-1) was negative. Electron microscopy demonstrated diffuse fusion (>80%) of podocyte foot processes, with small amounts of electron-dense deposits subepithelially (Figures 2B–D). These findings are consistent with membranous nephropathy (MN).
FIGURE 1

Renal biopsy was examined under light microscopy with HE staining (×200), PAS staining (×400), PASM staining (×400), and Masson staining (×400).
FIGURE 2
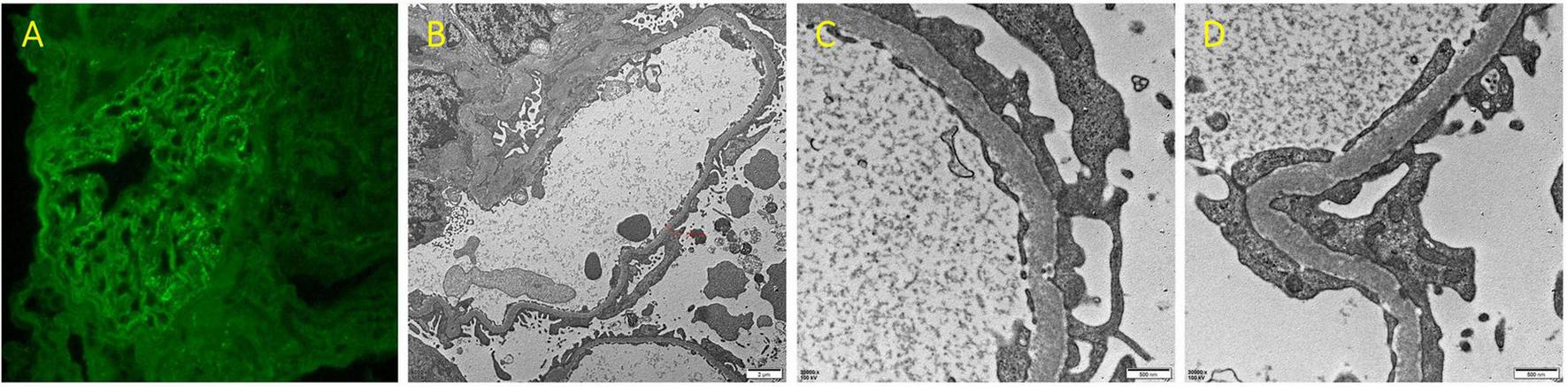
(A) Immunofluorescence staining for IgG of renal biopsy (×400). (B–D) Renal biopsy under electron microscopy (B ×6000; C,D ×30000).
No evidence was found to suggest that diabetes mellitus, systemic lupus erythematosus (SLE), or infection, which are known to lead to secondary MN, were present. Therefore, the possibility of heavy metal poisoning was considered. Urine tests revealed a urinary mercury level of 56.22 ug/L, exceeding the reference range of 0–10 ug/L. To determine the source of mercury, the patient was thoroughly questioned. She was a housewife and denied using any folk remedies. Additionally, none of her family members worked in industries related to mercury. Further investigation showed that she had been using a freckle-removing cream purchased online for 6 months, but stopped using it upon her admission. Testing revealed that the level of mercury in the cream was 13276 mg/kg, significantly exceeding the Chinese National Standards of 1 mg/kg or lower.
Given the evidence of mercury poisoning, the patient underwent chelation therapy with a 0.25 g intramuscular injection of sodium dimercaptopropane sulfonate (DMPS), once a day for 3 days, followed by intermittent treatment for 4 days. Additional treatments included irbesartan at a dosage of 75 mg daily and calcium supplements. After undergoing four courses of chelation therapy, her symptoms of fatigue disappeared, and the edema was relieved. Her urinary mercury level declined to 14.52 ug/L, and ALB increased to 31.8 g/L. During a 12-months outpatient follow-up, the levels of urinary mercury, urine protein, and ALB returned to normal, and the patient reported no discomfort. Figure 3 illustrates the changes in urinary mercury and ALB levels.
FIGURE 3
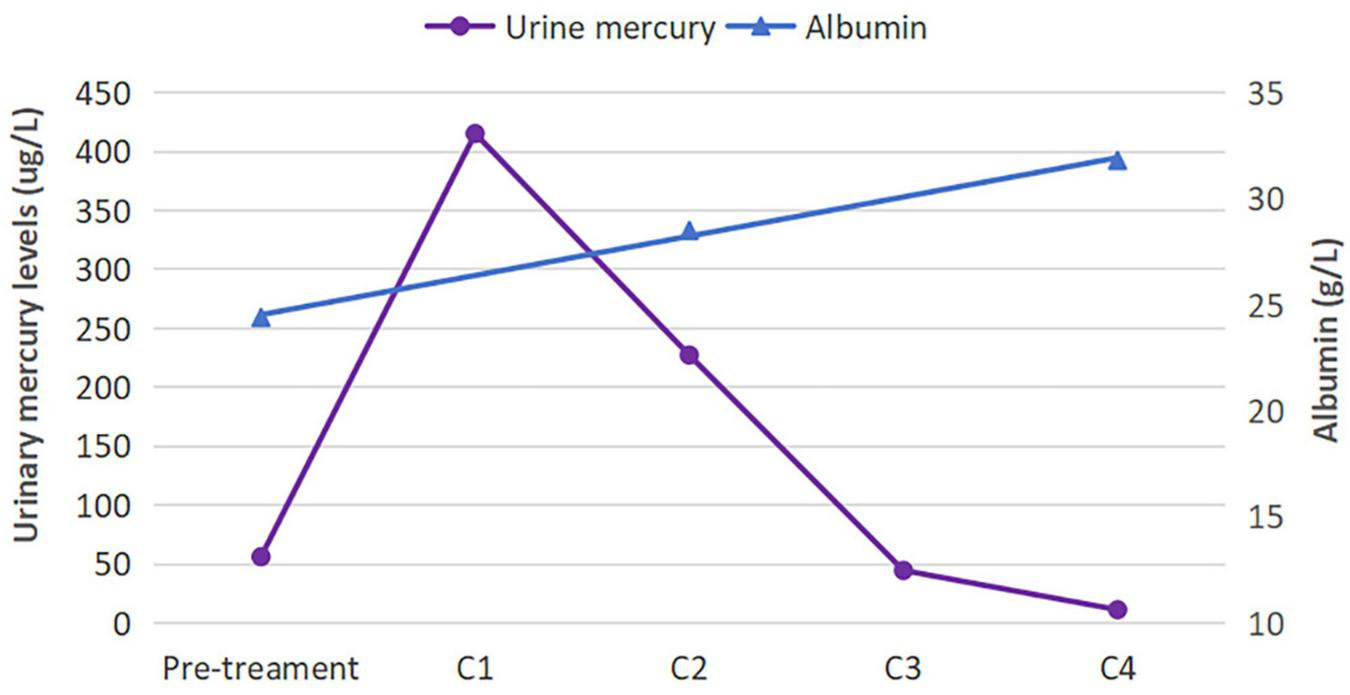
Urinary mercury and albumin levels before and after treatment. The urinary mercury level initially rose before rapidly declining, whereas the albumin level showed a steady rise. C, course of chelation therapy.
3 Discussion
Nephrotic syndrome is not challenging to diagnose clinically, as it exhibits characteristics such as significant proteinuria, hypoproteinemia, edema, and hyperlipidemia. The emphasis lies on differentiating between primary and secondary NS, which is crucial for determining subsequent treatment options. In this case, the patient was diagnosed with MN through a renal biopsy. The blood test and IF staining for PLA2R were negative, supporting the secondary MN diagnosis (3). After ruling out potential causes, such as diabetes mellitus and SLE, mercury poisoning was eventually identified as the underlying issue. Following four courses of chelation therapy, the patient’s symptoms improved, and complete remission was achieved during the 12-months follow-up period.
Mercury exists in three forms: elemental, organic, and inorganic. Common sources of human exposure to elemental mercury include thermometers, dental amalgam, and gold mining activities. The primary route of mercury entry into the human body is through inhaling mercury vapor (4). In the environment, methylmercury is the predominant form of organic mercury. Prolonged and excessive consumption of contaminated fish can lead to the accumulation of methylmercury in the body, resulting in mercury poisoning (5). Inorganic mercury compounds are used in the production of catalysts, antiseptics, and pigments. Mercury sulfide (HgS) has been incorporated into some traditional and folk remedies for medical use for thousands of years. In vivo, inorganic mercury ions can bind with sulfhydryl groups of tyrosinase, inhibiting its activity and reducing melanin production (6). Due to this property, inorganic mercury is added to some whitening cosmetics, which is illegal.
Mercury poisoning from cosmetics has become a global health concern. Despite international bans, mercury-containing cosmetics are still available online and in stores. The public uses these products for skin whitening, but often overlooks their safety. Several reports on mercury poisoning have shown that the mercury content in these cosmetics exceeds the limit significantly (7, 8), as in this case. Table 1 summarizes the characteristics of reported cases of mercury-induced NS. Notably, the most common source of mercury was whitening cosmetics (16 out of 24 cases). Even children experienced mercury poisoning through indirect contact with facial creams containing mercury (8, 9). As shown in Table 1, cosmetic products primarily affected women, especially young women, which aligns with the literature (1). In China, mercury poisoning must be diagnosed and treated at designated medical institutions like the Institute of Occupational Disease Prevention and Control. Consequently, most clinicians have limited experience with diagnosing and treating this condition, which can lead to misdiagnosis. Therefore, clinicians should consider the possibility of mercury exposure when they encounter a young female with unexplained NS.
TABLE 1
| Cases | Age/sex (number of cases) | Source of mercury | Urine mercury (reference value) | Renal pathology (number of cases) | Treatment | Outcome |
|---|---|---|---|---|---|---|
| 1. Liu et al. (20) | 55/M | Thermometer | >500 (0–2 μmol/mmol Cr) | MN | Chelation therapy with DMPS | CR |
| 2. Qin et al. (21) | 73/M | Ointment for dermatitis | 33.4 (<15 ng/ml) | NA | Chelation therapy with DMPS | PR |
| 3. Ji et al. (22) | 33/M 30/F | Skin-lightening cream | 65.4; 80 (<4 μg/g Cr) | MN (1) NA (1) | Chelation therapy with DMPS | NA |
| 4. Jawandhiya et al. (23) | 26/F 22/F | Fairness facial cream | 29.71; NA (0.14–4.2 ug/L) | MN (2) | Modified Ponticelli regimen (alternating months of steroids and cyclophosphamide) | CR |
| 5. Gao et al. (24) | 65/F | Skin lightening cream | 27.5 (<4 μg/g creatinine) | MCD with IgA deposition | Chelation therapy with DMPS | CR |
| 6. Pathak et al. (25) | 47/F | Siddha medicine | 17.7 (<10 microgram/liter) | MN | Angiotensin receptor blockers | PR |
| 7. Yawei et al. (26) | 44/F | Hair dyes + skin lightening cream | 122.5 (<8 μg/day) | MN | Chelation therapy with DMSA + prednisone + FK506 | PR |
| 8. Onwuzuligbo et al. (27) | 14/M | Mercury vapor in the environment | 42.9 (≤20 μg/d) | MN | Chelation therapy with DMSA + prednisolone | NA |
| 9. Niu et al. (28) | 39/F | Skin lightening cream | 90 (<4 μg/g creatinine) | MCD with IgA nephropathy | Chelation therapy with DMPS + medrol | CR |
| 10. Zhang et al. (29) | 28/F | Skin lightening cream | 469 (<50 μmol/L) | MCD | Chelation therapy with DMPS + prednisone | CR |
| 11. Wagrowska-Danilewicz et al. (30) | 42/M | Self-injection of elemental mercury | 830 μg/L | MCD | Chelation therapy with DMPS + steroids | CR |
| 12. Tang et al. (31) | 26–45/F (4) | Skin lightening cream | 316–2521 (<35 nmol/d) | MCD (3) MCD with IgA nephropathy (1) | 2 cases received chelation therapy with D-penicillamine; 2 cases received chelation therapy with D-penicillamine + steroids | CR |
| 13. Miller et al. (32) | 60/M | Contaminated fish | 39 (<20 nmol/d) | FSGS | Prednisone | Death |
| 14. Saleem et al. (33) | 62/M | Gold amalgam extraction | 2519 (level of concern, >5.8 nmol/mmol) | MCD | Chelation therapy with DMSA + prednisolone | CR |
| 15. Chakera et al. (34) | 26–44/F (2) | Skin lightening cream | 16.5; 77.5 (<5.5 nmol/mmol creatinine) | MN (2) | Stopping use of the cream | NA |
| 16. Campbell et al. (35) | 25/M | Lighting tubes | 127.5 (<5.5 nmol/mmol creatinine) | MCD | Chelation therapy with DMSA + prednisolone | CR |
| 17. Tang et al. (36) | 34/F | Skin lightening cream | 287 (<50 nmol/L) | MCD | Chelation therapy with D-penicillamine | CR |
| 18. This case | 33/F | Freckle-removing cream | 56.22 (0–10 ug/L) | MN | Chelation therapy with DMPS | CR |
Clinical characteristics of cases of mercury-induced nephrotic syndrome.
MN, membranous nephropathy; MCD, minimal change disease; FSGS, focal segmental glomerulosclerosis; DMPS, sodium dimercaptopropane sulfonate; DMSA, dimercaptosuccinic acid; CR, complete remission; PR, partial remission; NA, not available.
The clinical signs of mercury poisoning affect multiple systems. Most cases show neurological symptoms such as headache, dizziness, limb numbness, tremor, neuromuscular pain, fatigue, insomnia, and memory decline. Kidney damage mainly presents as edema, foamy urine, nephrotic syndrome, and hematuria. Gastrointestinal symptoms include abdominal pain, decreased appetite, nausea, vomiting, and oral ulcers. Skin lesions include rash and peeling (1, 2). Inhaling mercury vapor may cause pneumonitis, cough, chest pain, and dyspnea, potentially leading to respiratory failure (10). Since these symptoms are non-specific, patients are often misdiagnosed at their initial visit.
The pathological types of mercury-associated NS in Table 1 included MN (10/22), minimal change disease (MCD) (11/22), and focal segmental glomerulosclerosis (FSGS) (1/11). MN and MCD were the most common pathological patterns, which were consistent with the literature (11, 12). However, the exact mechanism of mercury-related kidney damage is not fully understood. High concentrations of mercury directly harm renal tubular epithelial cells, leading to tubular necrosis through the induction of oxidative stress (13). Immune mechanisms play a crucial role in mercury-related kidney damage. Mercury stimulates the production of immunoglobulin antibodies (IgG1, IgG2a, IgG3, and IgM) in autoimmune-prone mice, and granular deposits of these circulating antibodies are observed in the renal mesangium (14). IgG1 and IgG2a antibodies eluted from the glomeruli of mercury-exposed rats were pathogenic and triggered MN in unexposed rats following intravenous injection (15). In this case, renal biopsy revealed granular deposits of IgG along the glomerular capillary loop. These findings suggest that mercury-induced immune activation causes the formation of in situ immune complexes, leading to glomerular lesions.
The most important step in treating mercury poisoning is to eliminate the source of exposure. Additionally, chelation therapy, glucocorticoids, and immunosuppressants are available options (Table 1). For symptomatic patients, chelating agents such as DMPS and dimercaptosuccinic acid (DMSA) are commonly used treatments. Chelation therapy is unnecessary for asymptomatic patients. In these cases, mercury levels will gradually decrease after removing the source, without any complaints (8, 16). Therefore, the decision to initiate chelation therapy relies on a thorough clinical assessment. Another question is whether glucocorticoids are necessary for mercury-associated glomerular diseases or not. There were no significant differences in treatment outcomes for patients with mercury-associated NS between those treated with DMPS alone and those who received DMPS in combination with glucocorticoids. In severe cases, glucocorticoids may be used concurrently (12). Our patient was cured after solely receiving DMPS, which helped avoid potential side effects associated with glucocorticoids. Although hemodialysis was ineffective at removing mercury even with chelation therapy before renal function recovery, it was necessary for renal support in patients who developed acute renal failure (17). In contrast, plasma exchange can effectively eliminate mercury and enhance the patient’s condition. For acute or severe mercury poisoning cases, plasma exchange might be considered (18, 19).
In summary, mercury poisoning is uncommon but treatable. Recognizing it is crucial to prevent misdiagnosis and unsuitable treatment. This report emphasizes the significance of thoroughly investigating the patient’s history during the diagnostic process. Additionally, chelation therapy serves as an effective treatment option.
Statements
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
This study involving human participants was approved by the Ethics Committee of Anhui No. 2 Provincial People’s Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the patient for publication of this case report.
Author contributions
CW: Investigation, Writing – original draft. DL: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
We would like to express their gratitude to EditSprings (https://www.editsprings.cn) for the expert linguistic services provided.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Yawei S Jianhai L Junxiu Z Xiaobo P Zewu Q . Epidemiology, clinical presentation, treatment, and follow-up of chronic mercury poisoning in china: a retrospective analysis.BMC Pharmacol Toxicol. (2021) 22:25. 10.1186/s40360-021-00493-y
2.
Xue C Du X Zhu X Wu N Ye Q . Heavy metal poisoning caused by chinese folk remedies in psoriasis patients: a retrospective analysis.Sci Rep. (2024) 14:11777. 10.1038/s41598-024-62653-4
3.
Bobart S De Vriese A Pawar A Zand L Sethi S Giesen C et al Noninvasive diagnosis of primary membranous nephropathy using phospholipase a2 receptor antibodies. Kidney Int. (2019) 95:429–38. 10.1016/j.kint.2018.10.021
4.
Jomova K Alomar S Nepovimova E Kuca K Valko M . Heavy metals: toxicity and human health effects.Arch Toxicol. (2025) 99:153–209. 10.1007/s00204-024-03903-2
5.
Clarkson T Magos L Myers G . The toxicology of mercury–current exposures and clinical manifestations.N Engl J Med. (2003) 349:1731–7. 10.1056/NEJMra022471
6.
Sommerlad M . Skin lightening: causes and complications.Clin Exp Dermatol. (2022) 47:264–70. 10.1111/ced.14972
7.
Copan L Fowles J Barreau T McGee N . Mercury toxicity and contamination of households from the use of skin creams adulterated with mercurous chloride (calomel).Int J Environ Res Public Health. (2015) 12:10943–54. 10.3390/ijerph120910943
8.
Ori M Larsen J Shirazi F . Mercury poisoning in a toddler from home contamination due to skin-lightening cream.J Pediatr. (2018) 196:314–7. 10.1016/j.jpeds.2017.12.023
9.
Rakete S Asenbauer E Bohm S Leiz S Peters J Nowak D et al Mercury poisoning of a 4-year-old child by indirect contact to a mercury-containing facial cream: a case report. SAGE Open Med Case Rep. (2021) 9:2050313X211025227. 10.1177/2050313X211025227
10.
Nguyen-Dang K Dau-Nguyen A Tran-Ngoc N Duong-Minh N Dang-Vu T Nguyen-Ngoc S et al Long-term follow-up after acute mercury poisoning-induced pneumonitis following cinnabar heating: a rare case report. Medicine. (2024) 103:e41013. 10.1097/MD.0000000000041013
11.
Qin A Su T Wang S Zhang F Zhou F Zhao M . Mercury-associated glomerulonephritis: a retrospective study of 35 cases in a single chinese center.BMC Nephrol. (2019) 20:228. 10.1186/s12882-019-1413-z
12.
Gao Z Wu N Du X Li H Mei X Song Y . Toxic nephropathy secondary to chronic mercury poisoning: clinical characteristics and outcomes.Kidney Int Rep. (2022) 7:1189–97. 10.1016/j.ekir.2022.03.009
13.
Mehmood A Soliman M Almalki D Alotaibi K Youssef G Althobaiti S . Ameliorative impacts of sinapic acid against mercuric chloride-induced renal toxicity: role of antioxidants and inflammatory cytokines.Toxicol Res. (2024) 13:tfae066. 10.1093/toxres/tfae066
14.
Abedi-Valugerdi M Hu H Moller G . Mercury-induced renal immune complex deposits in young (NZB x NZW)f1 mice: characterization of antibodies/autoantibodies.Clin Exp Immunol. (1997) 110:86–91. 10.1046/j.1365-2249.1997.4901392.x
15.
Aten J Veninga A Bruijn J Prins F de Heer E Weening J . Antigenic specificities of glomerular-bound autoantibodies in membranous glomerulopathy induced by mercuric chloride.Clin Immunol Immunopathol. (1992) 63:89–102. 10.1016/0090-1229(92)90098-9
16.
Johnson-Arbor K Tefera E Farrell J . Characteristics and treatment of elemental mercury intoxication: a case series.Health Sci Rep. (2021) 4:e293. 10.1002/hsr2.293
17.
Worth D Davison A Lewins A Ledgerwood M Taylor A . Haemodialysis and charcoal haemoperfusion in acute inorganic mercury poisoning.Postgrad Med J. (1984) 60:636–8. 10.1136/pgmj.60.707.636
18.
Sauder P Livardjani F Jaeger A Kopferschmitt J Heimburger R Waller C et al Acute mercury chloride intoxication. Effects of hemodialysis and plasma exchange on mercury kinetic. J Toxicol Clin Toxicol. (1988) 26:189–97. 10.3109/15563658809000346
19.
Ozkale M Ozkale Y . The role of therapeutic plasma exchange in the treatment of childhood intoxication: a single-center experience.Pediatr Crit Care Med. (2020) 21:e988–95. 10.1097/PCC.0000000000002462
20.
Liu C Huang Y Wei W Hu X Yang J Zhao Y . Mercury poisoning-associated membranous nephropathy and autoimmune encephalitis.BMC Nephrol. (2025) 26:148. 10.1186/s12882-025-04082-7
21.
Qin A Liu L Gao B Su TA . case of chronic mercury poisoning associated nephrotic syndrome, abdominal pain, and neuropsychiatric symptoms.Ren Fail. (2023) 45:2261553. 10.1080/0886022X.2023.2261553
22.
Ji H Chen Y Liu D Zhou T Tang Y . Diverse clinical manifestations and prognosis in a couple’s mercury poisoning caused by skin-lightening creams: two case reports and literature review.Front Med. (2024) 11:1511493. 10.3389/fmed.2024.1511493
23.
Jawandhiya P Gupta A . Nell-1 membranous nephropathy due to mercury exposure from fairness cream: report of two cases.Nephrology. (2024) 29:996–9. 10.1111/nep.14403
24.
Gao H Liu G He Y Chen J . Nephrotic syndrome of minimal change disease following exposure to mercury-containing skin lightening cream: a case report and literature review.Clin Nephrol. (2022) 98:107–12. 10.5414/CN110751
25.
Pathak N Gunasekaran I Ambriose M Nanda SK . Nell1 as target antigen for mercury related membranous nephropathy: a case report.Indian J Nephrol. (2022) 32:502–5. 10.4103/ijn.ijn_415_21
26.
Yawei C Jing S Wenju S Yupeng L Ping Z Liping H . Mercury as a cause of membranous nephropathy and guillain-barre syndrome: case report and literature review.J Int Med Res. (2021) 49:1221799308. 10.1177/0300060521999756
27.
Onwuzuligbo O Hendricks AR Hassler J Domanski K Goto C Wolf MTF . Mercury intoxication as a rare cause of membranous nephropathy in a child.Am J Kidney Dis. (2018) 72:601–5. 10.1053/j.ajkd.2018.05.013
28.
Niu H Li S Li H Chen Y Liu W Li P et al Clinicopathological features, diagnosis, and treatment of iga nephropathy with minimal change disease related to exposure to mercury-containing cosmetics: a case report?. Clin Nephrol. (2017) 87:196–201. 10.5414/CN108967
29.
Zhang L Liu F Peng Y Sun L Chen C . Nephrotic syndrome of minimal change disease following exposure to mercury-containing skin-lightening cream.Ann Saudi Med. (2014) 34:257–61. 10.5144/0256-4947.2014.257
30.
Wagrowska-Danilewicz M Danilewicz M Zbrog Z . Mercury-induced nephrotic syndrome: a case report and review of the literature.Pol J Pathol. (2014) 65:322–6. 10.5114/pjp.2014.48194
31.
Tang HL Mak Y Chu K Lee W Fung SKS Chan TY et al Minimal change disease caused by exposure to mercury-containing skin lightening cream: a report of 4 cases. Clin Nephrol. (2013) 79:326–9.
32.
Miller S Pallan S Gangji AS Lukic D Clase CM . Mercury-associated nephrotic syndrome: a case report and systematic review of the literature.Am J Kidney Dis. (2013) 62:135–8. 10.1053/j.ajkd.2013.02.372
33.
Saleem M Alfred S Bahnisch RA Coates P Kearney DJ . Mercury poisoning from home gold amalgam extraction.Med J Aust. (2013) 199:125–7. 10.5694/mja13.10171
34.
Chakera A Lasserson D Beck LHJ Roberts ISD Winearls CG . Membranous nephropathy after use of uk-manufactured skin creams containing mercury.QJM. (2011) 104:893–6. 10.1093/qjmed/hcq209
35.
Campbell G Leitch D Lewington A Dargan PI Baker RJ . Minimal-change nephrotic syndrome due to occupational mercury vapor inhalation.Clin Nephrol. (2009) 72:216–9. 10.5414/cnp72216
36.
Tang HL Chu KH Mak YF Lee W Cheuk A Yim KF et al Minimal change disease following exposure to mercury-containing skin lightening cream. Hong Kong Med J. (2006) 12:316–8.
Summary
Keywords
membranous nephropathy, mercury poisoning, nephrotic syndrome, chelation therapy, cosmetics
Citation
Wu C and Liu D (2025) Case Report: Nephrotic syndrome caused by mercury poisoning due to freckle-removing cream. Front. Med. 12:1651441. doi: 10.3389/fmed.2025.1651441
Received
23 June 2025
Accepted
15 October 2025
Published
07 November 2025
Volume
12 - 2025
Edited by
Gian Marco Ghiggeri, IRCCS Institute Giannina Gaslini, Italy
Reviewed by
Andrea Angeletti, IRCCS Institute Giannina Gaslini, Italy
Manisha Singh, The University of Arkansas for Medical Science, United States
Updates
Copyright
© 2025 Wu and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dandan Liu, woisliu@163.com
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.