Abstract
Background:
Kidney transplant recipients (KTRs) are at increased risk of malignancies, including lung adenocarcinoma (LUAD). The therapeutic efficacy of tyrosine kinase inhibitors (TKIs) against the rare EGFR L747P mutation remains controversial.
Case presentation:
We report a 60-years-old female renal transplant recipient who developed advanced lung adenocarcinoma harboring the EGFR L747P mutation.
Management and outcomes:
The patient was treated sequentially with three generations of EGFR TKIs–gefitinib, osimertinib, and dacomitinib–while continuing immunosuppressive therapy for graft function. Gefitinib achieved a progression-free survival (PFS) of 9 months, osimertinib 4.5 months, and dacomitinib 2.5 months, resulting in a total overall survival (OS) of 24 months. Treatment was generally well tolerated, with only mild adverse events and manageable renal function changes.
Conclusion:
To our knowledge, this is the first reported case of an EGFR L747P-mutated lung adenocarcinoma in a renal transplant recipient benefiting from sequential multi-TKI therapy. This case underscores the importance of vigilant cancer surveillance in transplant recipients and suggests that individualized TKI sequencing may offer clinical benefit, although further evidence is required.
Introduction
Kidney transplant recipients have a significantly increased risk of developing malignancies compared to the general population, partly due to long-term immunosuppression (1, 2). Among these, lung cancer is one of the most common and accounts for 3%–7% of de novo cancers after kidney transplantation (1, 3, 4).
In China, lung adenocarcinoma is the predominant histological subtype, with up to 50% of patients harboring EGFR mutations such as exon 19 deletion or exon 21 L858R substitutions (5). While EGFR tyrosine kinase inhibitors are the standard first-line therapy for EGFR-mutant LUAD, the clinical response to rare mutations remains uncertain. In particular, the exon 19 L747P mutation has shown inconsistent sensitivity to TKIs across different generations, with case reports describing variable efficacy of first-, second-, and third-generation agents (6–13).
Here, we present a rare case of a non-smoking female renal transplantation recipient with advanced LUAD harboring the EGFR L747 P mutation. She was sequentially treated with three generations of TKIs and achieved an overall survival of 24 months. This case highlights the therapeutic challenges posed by this rare mutation and provides clinical evidence for individualized treatment strategies in the transplant setting.
Case report
A 60-years-old female recipient of a living relative kidney transplant for end-stage renal disease in early July 2016 was admitted to our hospital on October 8, 2022 presenting with cough, sputum production, chest tightness, and shortness of breath persisting for over 2 months. Thoracic color Doppler ultrasound revealed massive pleural effusion. The patient underwent regular follow-up in the kidney transplant clinic of our hospital, and her renal function remained normal at the time of this admission. The immunosuppression regimen consisted of mycophenolate mofetil (250 mg, twice daily), tacrolimus (0.5 mg, twice daily), and prednisolone (5 mg, once daily). The patient denied a history of smoking and any family history of lung cancer or other malignancies. During admission, routine laboratory tests were conducted on blood, sputum, pleural fluid, urine, and stool samples. Additionally, chest computerized tomography (CT) scan (Figure 1A), medical thoracoscopy with pleural biopsy (Figure 2A) were performed. It was determined that she had type 1 respiratory failure [Arterial blood gas was analyzed (FiO2 = 0.21), and the results were as follows: pH of 7.43, partial pressure of oxygen (PO2) of 69 mmHg, partial pressure of carbon dioxide (pCO2) of 31 mmHg, standard bicarbonate (HCO3-std) of 22.7 mmol/L, oxygen saturation (SaO2) of 97%, and oxygenation index of 238] with non-infectious exudative pleural effusion. Based on the laboratory results from blood and pleural fluid (Table 1) analysis along with histological examination findings of pleural fluid cells and tissue showing cancer cells (Figure 2B), confirmed as LUAD through immunohistochemistry staining (Table 2). Subsequently, to comprehensively evaluate the lung cancer staging, the patient underwent brain magnetic resonance imaging (MRI) and 18F-FDG positron emission tomography-CT (PET-CT) scan (Figure 1B). Chest CT scan findings revealed a soft tissue density mass near the left upper lung hilum with high uptake of 18F-FDG (SUV max 9.5), accompanied by multiple lymph node metastases in the left hilus of the lung, mediastinum, and retroperitoneal para-aorta; as well as left pleural and pericardial metastases resulting in left-sided pleural effusion and pericardial effusion. No metastasis was observed in the central nervous system, liver, spleen transplanted kidney, and bone. Furthermore, mutation status of 10 driver genes in matched tumor samples was detected by a next generation sequencing (NGS) panel (Amoy Diagnostics), which can detect hotspot mutations/fusions in genes of EGFR, KRAS, BRAF, NRAS, HER2, PIK3CA, ALK, ROS1, RET, and MET in one single test. Analysis of the pleural tissues identified an EGFR exon19 c2239_2240 delinsCCp. (L747P) NM_005228.5 mutation. Considering all these results, the patient was diagnosed with stage IV LUAD (T2aN2M1c) harboring a rare EGFR L747P mutation.
FIGURE 1

(A) On admission (October 8, 2022), CT scan showed left pneumonia with massive effusion of the left lung and left lung atelectasis. (B) On October 20, 2022, 18F-FDG PET-CT scan were performed after thoracoscopic surgery and showed soft tissue density mass near the left upper lung portal. (C–F) After starting first-line treatment with gefitinib, chest CT scans on February 14, 2023, March 28, 2023, and May 28, 2023, showed that tumor size was similar to that on December 5, 2022. (G) On August 5, 2023, chest CT scan showed left hilar carcinoma with left pleural tumor metastasis and left pleural effusion progression. (H–J) From September 2023, the patient was switched to osimertinib as second-line drug, on November 2, 2023 chest CT scan indicated slow progression of the chest lesion, while January 19, 2024 chest CT scan showed that the metastatic pulmonary nodules in the right lung were found to increase significantly. (K) On February 2, 2024, we replaced the patient with dacomitinib for treatment, on March 3, 2024 chest CT scan showed that the metastatic tumors in the left pleura and the metastatic tumors in the right lung were found to shrink significantly. (L) However, on April 25, 2024, a follow-up chest enhanced CT scan showed that the lesion had progressed again.
FIGURE 2
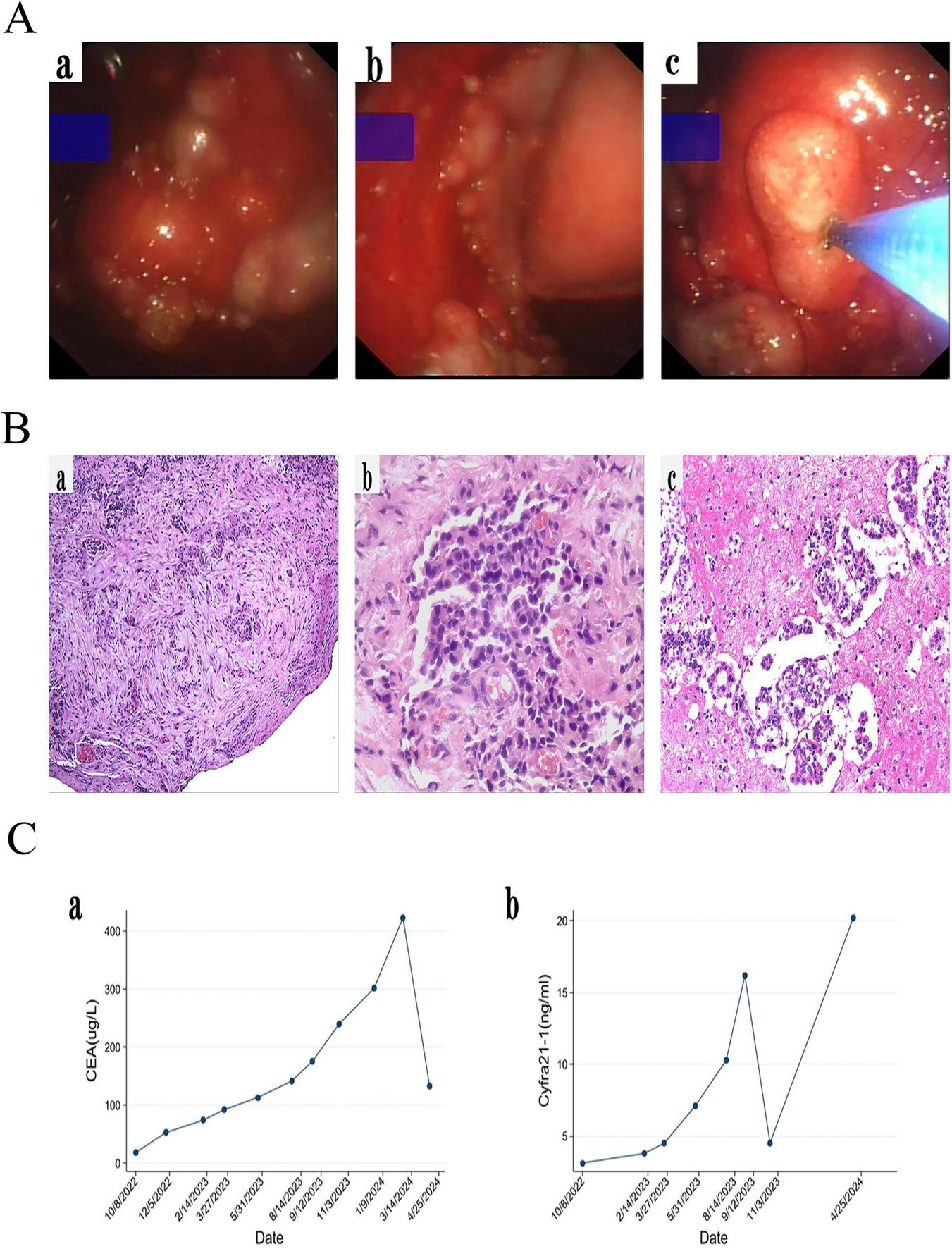
(A) Medical thoracoscopic pleural biopsy showed the visceral pleura and parietal pleura were full of new organisms of different sizes, and the new organisms were biopsied. (B) Histological examination (HE) revealed a small number of cellularly heterogeneous cells in pleural fibrous connective tissue (a,b) and in pleural effusion (c). (C) CEA and Cyfra21-1 gradually increased during follow-up of gefitinib. However, CEA and Cyfra21-1 has decreased since we replaced the patient with the second and third generation TKI for treatment.
TABLE 1
| Surveillance project | October 9,2022 | October 12,2022 | Normal reference values |
| Color | yellow | yellowish | yellowish |
| Transparency | Turbid | Slightly turbid | Clear |
| Red blood cell (RBC) | 1.9×109/L | 4.1×109/L | – |
| White blood cell (WBC) | 0.625×109/L | 0.455×109/L | – |
| Multinucleate cells (MNC) | 5% | 11% | – |
| Monocytes (Mo) | 95% | 89% | – |
| Rivalta test | ++ | ++ | negative |
| Glucose | 5.68 mmol/L | 8.04 mmol/L | 3.63∼4.78 mmol/L |
| Cl | 111.8 mmol/L | 107.0 mmol/L | 95∼106 mmol/L |
| Lactate dehydrogenase (LDH) | 175.9 U/L | 231.5 U/L | 40∼130 U/L |
| Adenosine deaminase (ADA) | 6.8 U/L | 7.6 U/L | 0∼25 U/L |
| Total protein (TP) | 39.2 g/L | 40.0 g/L | 5∼30 g/L |
| Carcinoembryonic antigen | 73.01 ng/ml | 74.08 ng/ml | Non-smokers <5 ng/ml | Smokers <5.5 ng/ml | Smokers and over 40 <65g/m |
| Sugar antigen CA12-5 | 477.8 U/ml | 419.50 U/ml | <35 U/m |
| Sugar antigen CA19-9 | / | 8.24 U/ml | <39 U/ml |
| Sugar antigen CA 15-3 | / | 62.24 U/ml | <25 U/ml |
| Ferritin | / | 557.3 ng/ml | 20-400 ng/ml for males | 13–150 ng/ml for females |
| Acid-fast-bacillus | negative | negative | negative |
| Bacteria | negative | negative | negative |
| Fungus | negative | negative | negative |
Results of the pleural fluid test.
+: positive, –: indicates no data, /: the test has not been carried out.
TABLE 2
| Specimen (date) | October 14, 2022 | October 18, 2022 | October 25, 2022 |
| Antibody type | Hydrothorax | Hydrothorax | Pleura |
| Napsin A | / | / | ++ |
| TTF-1 | ++ | ++++ | ++ |
| Ki-67 | 20% | 40% | 15% |
| CK7 | / | ++++ | / |
| P40 | − | − | − |
| Syn | − | + | − |
| CR | − | − | − |
| WT-1 | / | / | − |
Immunohistochemical results of pleural fluid cells and pleural biopsy specimens.
+: positive, −: negative, /: the test has not been carried out.
Upon admission, a closed drainage procedure for the thoracic cavity was urgently performed to treat the left-sided pleural effusion, resulting in improved hypoxia symptoms and overall condition. The patient’s Performance Status (PS) score decreased from 2 at admission to 1. Based on a comprehensive review of published case reports concerning this rare EGFR L747P mutation and taking into consideration the patient’s financial constraints alongside local medical insurance policies, gefitinib was selected as the first-line treatment regimen, gefitinib therapy was prescribed for the patient after confirmation of LUAD harboring EGFR L747P mutation on November 4, 2022 (Figures 1A, B). An unadjusted anti-rejection regimen was followed before discharge. The patient remained stable on radiography for 9 months (Figures 1C–F) until follow-up chest enhanced CT on August 5, 2023 indicated progression (Figure 1G). During the gefitinib treatment follow-up, tumor markers CEA and Cyfra 21-1 levels increased over time (Figure 2C). From September 16, 2023 onward, osimertinib (80 mg/d) was administered as a second-line drug due to disease progression (Figure 1H). At the November evaluation (Figure 1I), the target lesion diameter (sum of the longest diameters, SLD) had decreased with no new lesions observed, indicating stable disease (SD). By January 2014, however, both an increase in the target lesion diameter (SLD) and the emergence of new nodules were noted, consistent with progressive disease (PD) (Figure 1J). On February 2, 2024, dacomitinib (30 mg/d) replaced osimertinib as third-line treatment resulting in significant shrinkage of metastatic tumors in the left pleura and right lung found by chest CT on March 3, 2024 (Figure 1K). However, follow-up of a chest CT on April 25, 2024, showed that although the target lesion diameter (SLD) had decreased slightly from 51 to 50 mm, new lesions appeared, consistent with progressive disease (PD). This finding suggests mixed response to dacomitinib (Figure 1L). Urea nitrogen and creatinine remained within normal range during follow-up until March 2024; however, hematuria with a grade of +2 was initially detected on December10, 2022 progressing to +3 on August 4, 2023 with microscopy identifying glomerular hematuria while urinary proteinuria first appeared on March 4, 2024. Hematuria and proteinuria suggest endothelial injury caused by TKIs, the transplant specialist adjusted the immune transplantation regimen of patients from December 2023 to tacrolimus (0.5 mg, twice daily), prednisolone (5 mg, once daily), and sirolimus (0.5 mg and 1 mg, alternating once a day). The patient tolerated TKIs well, complicated by manageable grade 1 paronychia when using gefitinib and dacomitinib. The patient was lost to follow-up in June 2024 and subsequently passed away in November 2024, achieving an OS of 24 months. Throughout EGFR-TKI therapy, the patient maintained a strict vegetarian diet (excluding all animal protein due to Buddhist beliefs), relying on plant-based sources like soy and nuts. Physical activity was limited to light walking, consistent with his stable PS of 1 after initial improvement. No specific environmental modifications were documented. The patient was still receiving a third-generation TKI during the period of loss to follow-up, but the specific medication was unknown; the ultimate cause of death was also unclear due to the loss to follow-up.
Discussion
Lung cancer incidence is markedly higher in organ transplant recipients than in the general population (14, 15). Adenocarcinoma is the most common subtypes, consistent with patterns observed in non-transplant patients (16). In our case, risk factors included female sex, age over 35 years at transplantation, and long-term use of calcineurin inhibitors (>16 years). The patient presented with breathing difficulties due to malignant pleural effusion and was diagnosed with stage IV LUAD. As lung cancer is a leading cause of cancer-related death in KTRs, but early-stage disease remains highly curable, enhanced vigilance and early screening are warranted. Unfortunately, our patient was diagnosed at an advanced stage, missing the opportunity for early intervention. Pulmonary nodules detected on chest CT in this population should be carefully differentiated from infectious diseases such as tuberculosis or fungal infections, and the possibility of concurrent infection and malignancy must also be considered.
The main challenge in this case was managing advanced LUAD in a renal transplant recipient on long-term immunosuppressive therapy. For advanced NSCLC, first-line treatment typically involves TKIs targeting driver alterations such as EGFR mutations or ALK/ROS1 rearrangements (17). However, the EGFR L747P mutation is rare, and no consensus guidelines exist. Treatment decisions were therefore guided by published case reports. Preclinical studies suggest that the L747P mutation enhances tumorigenic potential and may confer resistance to TKIs (10). Some reports describe intrinsic resistance to first-generation [erlotinib (18), gefitinib (6)] and third-generation [osimertinib (6)] TKIs, whereas others have documented responses to agents across all three generations (7–12). Computational modeling indicates that second-generation TKIs may have stronger binding affinity to L747P than first- or third-generation agents (16), but clinical evidence remains inconsistent.
According to a previous case report, gefitinib achieved a PFS of 18 months, followed by a short period of clinical benefit from osimertinib (9). Drawing on this evidence, and considering both therapeutic efficacy and economic feasibility within the reimbursement policy, our patient adopted a similar treatment strategy. Gefitinib was initiated as first-line therapy and achieved a PFS of 8 months, which was shorter than previously reported. Upon disease progression, second-line osimertinib provided disease control for 4.5 months, consistent with outcomes described in the literature. Subsequently, dacomitinib was administered as third-line therapy, inducing an initial radiographic response; however, rapid progression occurred after 2.5 months, suggesting early resistance. The resistance mechanism could not be clarified, as no repeat biopsy was performed. The patient was later lost to follow-up, and according to family reports, she continued treatment with an unspecified third-generation TKI at another institution before succumbing to disease approximately 6 months thereafter.
During first-line gefitinib treatment, we observed biochemical progression with rising CYFRA21-1 and CEA levels despite radiographic stability. CYFRA21-1 is a recognized NSCLC biomarker, but it is more sensitive in squamous cell carcinoma than in adenocarcinoma. The unexpected increase in both markers raises important questions about tumor biology. Possible explanations include: (i) tumor heterogeneity or mixed histology (e.g., adenosquamous carcinoma) not captured by the initial biopsy; (ii) clonal evolution or phenotypic switch under TKI treatment pressure; and (iii) altered biomarker kinetics influenced by the immunocompromised state of the transplant recipient. While adenocarcinoma remained the confirmed histopathological diagnosis, this discordance highlights the complexity of tumor biology and the potential value of biomarker dynamics in monitoring therapeutic response.
Managing cancer in KTRs is particularly challenging because of the need to preserve allograft function while accounting for interactions with immunosuppressive therapy (19). TKIs have been associated with hypertension and proteinuria, likely due to reduced nitric oxide production and endothelial injury (20). Therefore, routine monitoring of blood pressure and urinalysis is essential, as demonstrated in our case. Since immunosuppression contributes to the development of post-transplant malignancies, adjustment or reduction of immunosuppressive agents remains a cornerstone of cancer management in this population. In general, tapering or discontinuation of glucocorticoids is recommended, with maintenance on calcineurin inhibitors or mammalian target of rapamycin inhibitor (mTORi) (21). At the time of LUAD diagnosis, the patient was receiving prednisone (5 mg daily), tacrolimus (0.5 mg twice daily), and mycophenolate mofetil (250 mg twice daily). This low-dose immunosuppressive regimen showed no significant drug interactions with EGFR-TKIs, so it was continued after initiation of gefitinib. During follow-up, urinalysis revealed hematuria at 2 months and progression at 7 months, likely related to endothelial injury from gefitinib, although renal function remained stable. Consequently, no adjustment was made during gefitinib or osimertinib treatment. Upon switching to dacomitinib, the dose was reduced to two-thirds of the standard (30 mg) for renal protection. Proteinuria was first detected 1 month later, again suggesting TKI-related renal toxicity. At the time of manuscript submission, serum creatinine remained within the normal range.
Proteinuria and hematuria may also result from rejection or recurrence of the primary kidney disease. However, in this case, their temporal onset following TKI initiation strongly supports a drug-related cause. Throughout follow-up, renal function (serum creatinine) remained stable, immunosuppressant levels were within the therapeutic range, and no clinical signs of acute rejection (e.g., fever, graft tenderness) or urinary tract infection were observed. Considering these factors and the well-documented endothelial and renal toxicities of TKIs, we conclude that TKI-induced injury was the most likely etiology.
Conclusion
This case highlights the importance of vigilant cancer surveillance in KTRs, who are at increased risk of malignancies. EGFR L747P mutations show inconsistent and short-lived responses to different TKIs, and treatment decisions in transplant recipients are particularly challenging due to the need to balance cancer control with immunosuppression. Our case demonstrates that sequential use of multiple TKIs can provide meaningful survival benefit, underscoring the need for individualized therapeutic strategies. Further clinical evidence is required to establish standardized treatment recommendations for this rare mutation in transplant recipients.
Statements
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Review Committee of the 900th Hospital of the Joint Logistic Support Force, People’s Liberation Army. The studies were conducted in accordance with the local legislation and institutional requirements. The human samples used in this study were acquired from a by- product of routine care or industry. Written informed consent for participation was not required from the participants or the participants’ legal guardians/next of kin in accordance with the national legislation and institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
WX: Writing – original draft. FC: Writing – original draft. LZ: Investigation, Writing – review & editing. BL: Investigation, Writing – review & editing. JY: Investigation, Writing – review & editing. ZY: Investigation, Writing – review & editing. LG: Supervision, Writing – review & editing. WL: Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Natural Science Foundation of Jiangsu Province (BK20240365), the Postdoctoral Excellence Program of Jiangsu Province (2024ZB410), and China Postdoctoral Science Foundation (2024M762314).
Acknowledgments
We would like to express our sincere gratitude to our patient and his family for their cooperation in the preparation of this report.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Au E Wong G Chapman J . Cancer in kidney transplant recipients.Nat Rev Nephrol. (2018) 14:508–20. 10.1038/s41581-018-0022-6
2.
Buxeda A Redondo-Pachon D Perez-Saez M Crespo M Pascual J . Sex differences in cancer risk and outcomes after kidney transplantation.Transplant Rev. (2021) 35:100625. 10.1016/j.trre.2021.100625
3.
Zhang S Liu Y . Primary lung cancer in Chinese renal transplant recipients: a single-center analysis.Nan Fang Yi Ke Da Xue Xue Bao. (2017) 37:715–20. 10.3969/j.issn.1673-4254.2017.06.01
4.
Jeong S Lee H Kong S Kim D Lee S Park M et al Incidence of malignancy and related mortality after kidney transplantation: a nationwide, population-based cohort study in Korea. Sci Rep. (2020) 10:21398. 10.1038/s41598-020-78283-5
5.
Wen S Dai L Wang L Wang W Wu D Wang K et al Genomic signature of driver genes identified by target next-generation sequencing in Chinese non-small cell lung cancer. Oncologist. (2019) 24:e1070–81. 10.1634/theoncologist.2018-0572
6.
Huang J Wang Y Zhai Y Wang J . Non-small cell lung cancer harboring a rare EGFR L747P mutation showing intrinsic resistance to both gefitinib and osimertinib (AZD9291): a case report.Thorac Cancer. (2018) 9:745–9. 10.1111/1759-7714.12637
7.
Zhou T Zhou X Li P Qi C Ling Y . EGFR L747P mutation in one lung adenocarcinoma patient responded to afatinib treatment: a case report.J Thorac Dis. (2018) 10:E802–5. 10.21037/jtd.2018.12.26
8.
Liang S Ko J Yang J Shih J . Afatinib is effective in the treatment of lung adenocarcinoma with uncommon EGFR p.L747P and p.L747S mutations.Lung Cancer. (2019) 133:103–9. 10.1016/j.lungcan.2019.05.019
9.
Huang X Yang Y Wang P Wang J Chen S Mao X et al rare EGFR mutation L747P conferred therapeutic efficacy to both ge fi tinib and osimertinib: a case report. Lung Cancer. (2020) 150:9–11. 10.1016/j.lungcan.2020.09.017
10.
Gerber D Mayer M Gagan J von Itzstein M . Systemic and intracranial efficacy of osimertinib in EGFR L747P-mutant NSCLC: case report.JTO Clin Res Rep. (2022) 3:100291. 10.1016/j.jtocrr.2022.100291
11.
Li J Zhu L Stebbing J Peng L . Afatinib treatment in a lung adenocarcinoma patient harboring a rare EGFR L747P mutation.J Cancer Res Ther. (2022) 18:1436–9. 10.4103/jcrt.jcrt_433_22
12.
Messeha S Nezami M Hager S Soliman KFA . A rare presentation of a non-asian female with metastatic non-small-cell lung cancer harboring EGFR L747P mutation with clinical response to multi-targeted epigenetic and EGFR inhibition.Anticancer Res. (2022) 42:441–7. 10.21873/anticanres.15502
13.
Nie L Wang Y Hsu J Hou J Chu Y Chan L et al Nuclear export signal mutation of epidermal growth factor receptor enhances malignant phenotypes of cancer cells. Am J Cancer Res. (2023) 13:1209–39.
14.
Al-Adra D Al-Qaoud T Fowler K Wong G . De novo malignancies after kidney transplantation.Clin J Am Soc Nephrol. (2022) 17:434–43. 10.2215/cjn.14570920
15.
Engels E Pfeiffer R Fraumeni J Kasiske B Israni A Snyder J et al Spectrum of cancer risk among US solid organ transplant recipients. JAMA. (2011) 306:1891–901. 10.1001/jama.2011.1592
16.
Bilek O Holanek M Jurica J Stepankova S Vasina J Selingerova I et al Drug interaction profile of TKI alectinib allows effective and safe treatment of ALK+ lung cancer in the kidney transplant recipient. Int Immunopharmacol. (2021) 99:108012. 10.1016/j.intimp.2021.108012
17.
Imyanitov E Iyevleva A Levchenko E . Molecular testing and targeted therapy for non-small cell lung cancer: current status and perspectives.Crit Rev Oncol Hematol. (2021) 157:103194. 10.1016/j.critrevonc.2020.103194
18.
Wang Y Ning W Li J Huang J . Exon 19 L747P mutation presented as a primary resistance to EGFR-TKI: a case report.J Thorac Dis. (2016) 8:E542–6. 10.21037/jtd.2016.05.95
19.
Murakami N Webber A Nair V . Transplant onconephrology in patients with kidney transplants.Adv Chronic Kidney Dis. (2022) 29:188–200.e1. 10.1053/j.ackd.2021.09.002
20.
Kandula P Agarwal R . Proteinuria and hypertension with tyrosine kinase inhibitors.Kidney Int. (2011) 80:1271–7. 10.1038/ki.2011.288
21.
Schreiber B Abdelrahim M Abudayyeh A Murakami N . Emerging concepts in managing malignancy in kidney transplant patients.Semin Nephrol. (2022) 42:63–75. 10.1016/j.semnephrol.2022.01.003
Summary
Keywords
kidney transplant recipients, lung adenocarcinoma, EGFR L747P mutation, tyrosine kinase inhibitors, sequential therapy, case report
Citation
Xie W, Chen F, Zhang L, Lin B, Ye J, Yu Z, Gu L and Liu W (2025) Sequential tyrosine kinase inhibitor therapy for EGFR L747P-mutated lung adenocarcinoma in a renal transplant recipient: a 24-month case report. Front. Med. 12:1651584. doi: 10.3389/fmed.2025.1651584
Received
10 July 2025
Accepted
15 September 2025
Published
08 October 2025
Volume
12 - 2025
Edited by
Lei Yin, Shanghai Jiao Tong University School of Medicine, China
Reviewed by
Aaron Tigor Sihombing, Universitas Padjadjaran, Indonesia
Anita La’Ah, National Cancer Institute (NIH), United States
Updates
Copyright
© 2025 Xie, Chen, Zhang, Lin, Ye, Yu, Gu and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Gu, gulei116860@163.comWei Liu, ivyliu2012@163.com
†These authors have contributed equally to this work
‡ORCID: Lei Gu, orcid.org/0000-0003-4035-3583; Wei Liu, orcid.org/0000-0001-6060-4306
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.