- 1Department of Cardiovascular Internal Medicine, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2National Resource Center for Chinese Materia Medica, China Academy of Chinese Medical Sciences, Beijing, China
- 3National Clinical Research Center for Chinese Medicine Cardiology, Xiyuan Hospital, China Academy of Chinese Medical Sciences, Beijing, China
Background: Heart failure (HF) is a leading cause of global disease burden and mortality. Accurate prognosis assessment is critical for reducing the risk of adverse events. In recent years, numerous predictive models have been developed for different HF subtypes. However, the quality of existing models varies considerably, and there remains a lack of consensus on models suitable for widespread clinical application. This study systematically reviews the current landscape of HF prediction models, analyzes their strengths and limitations, and provides guidance for future research.
Methods: This review systematically retrieved studies on prognostic prediction models for HF from databases including PubMed and Embase, with a search period spanning from the inception of each database to 19 September 2025. The risk of bias of the included studies was assessed using the prediction model risk of bias assessment tool, and the performance of the prediction models was evaluated through metrics such as the C-index and calibration.
Results: A total of 46 prediction models from 38 studies were included. According to target population classification, 14 models were developed for predicting outcomes in HF patients with reduced ejection fraction, nine models were applicable to HF patients with preserved ejection fraction, one model targeted HF patients with mildly reduced ejection fraction, and the remaining 22 were designed for all HF patients regardless of subtype. The risk of bias assessment showed that 10 models had a high risk of bias, 21 models demonstrated an unclear risk of bias, and 15 models exhibited a low risk of bias. The study systematically summarized each model's study cohort, modeling methodology, predictors, outcomes, prediction performance, presentation format, as well as strengths and limitations.
Conclusion: Refining the methodological processes of model construction—including optimizing study cohort selection, updating predictor screening (such as incorporating novel biomarkers, imaging indicators, and multi-omics data), improving modeling strategies, and enhancing model presentation—will contribute to the development of more accurate and clinically applicable prediction models. Such advancements hold significant potential for improving clinical outcomes in patients across all types of HF. This review provides a substantive reference for future research in this field.
Introduction
Heart failure (HF) represents one of the major global public health challenges. According to the Global Burden of Disease (GBD) study, approximately 64.3 million individuals worldwide suffer from HF, with the disease burden continuing to escalate (1). Despite advances in therapeutic strategies, patient prognosis remains suboptimal, with annual mortality rates in high-risk patients ranging from 15 to 30% and 5-year mortality rates reaching 50%−75% (1, 2). Readmission rates also persist at elevated levels (1, 2).
Early risk assessment is crucial for improving patient outcomes and optimizing healthcare resource allocation. High-risk patients require enhanced monitoring and intervention, with consideration given to ventricular assist device implantation or heart transplantation where necessary; extremely high-risk patients should focus on symptom relief and end-of-life care; while low-risk patients may undergo appropriately simplified follow-up to conserve healthcare resources (3, 4). Notably, the 2021 European Society of Cardiology (ESC) guidelines classify HF into three subtypes based on left ventricular ejection fraction (LVEF): HF with reduced ejection fraction (HFrEF), HF with mildly reduced ejection fraction (HFmrEF), and HF with preserved ejection fraction (HFpEF), advancing HF management into a stage of precision medicine (5). The baseline characteristics, incidence, and prognosis of different types of HF differ significantly. Consequently, prognostic prediction models tailored to distinct HF subtypes have emerged as a research priority in recent years (6).
However, existing prediction models exhibit substantial heterogeneity in predictive performance, generalizability, and clinical utility, necessitating systematic evaluation and comparison. This study undertakes a review of prediction models for different HF subtypes, summarizing their strengths and limitations to provide direction for future research, thereby advancing the implementation of precision medicine in HF management.
Methods
Search strategy
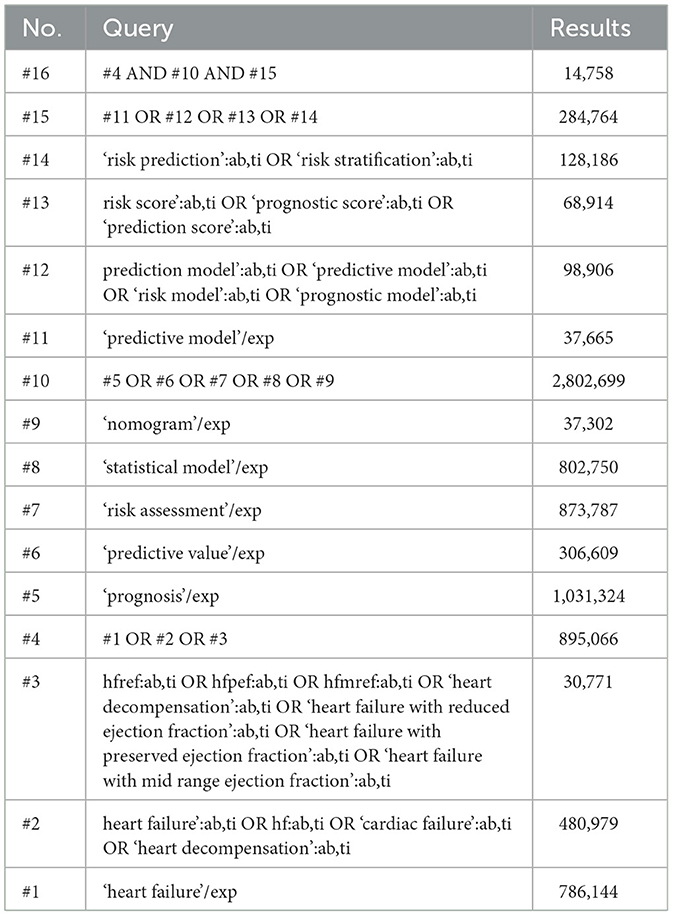
Studies on HF prediction models were systematically searched in PubMed and Embase from inception to September 19, 2025. Search terms included “heart failure,” “heart failure with preserved ejection fraction,” “heart failure with mildly reduced ejection fraction,” “heart failure with reduced ejection fraction,” “prognosis,” “prediction,” “risk score,” and their synonyms. The detailed search strategies were provided in Tables 1, 2. Additionally, the reference lists of relevant literature were screened to avoid omissions (7).
Inclusion and exclusion criteria
Studies on multivariable prediction models for predicting the prognosis of HF in adults (age ≥18 years) were included. “Prognosis” was defined as the occurrence of major long-term endpoints, such as all-cause mortality, all-cause hospitalization/rehospitalization, and cardiovascular/cerebrovascular events. The exclusion criteria for the study were as follows: (1) studies of diagnostic models predicting the occurrence of HF (rather than its prognosis); (2) studies that only validated existing models without developing new ones; (3) studies that merely explored the association between risk predictors and HF prognosis without constructing a complete prediction model; (4) studies restricted to specific subgroups of HF patients (such as those with specific comorbidities or caused by specific etiologies); (5) reviews, systematic reviews, meta-analyses, or similar studies; (6) other studies unrelated to this research topic; (7) studies of predictive models lacking external validation (a model developed without external validation could still be considered externally validated if it was validated independently by other studies).
Literature screening and management were conducted using the Covidence platform and EndNote 21. Two investigators (Yue Wei, Siyu Liu) independently screened studies for eligibility based on titles, abstracts, and full texts (including Supplementary materials). Any discrepancies were resolved through discussion or by arbitration from a third investigator (Yunying Mu) to reach consensus.
Data extraction and risk of bias assessment
Two investigators (Yue Wei, Siyu Liu) independently extracted information from each eligible study using a standardized data extraction sheet. We extracted the following information: first author name, publication date, participant cohort and regions, number of participants, methods, outcomes, predictors, predictive performance [Concordance statistic (C-statistic), validation, calibration, decision curve analysis (DCA), net reclassification improvement (NRI)], and model presentation.
Two investigators (Yue Wei, Siyu Liu) assessed the risk of bias for each eligible model using the Prediction Model Bias Risk Assessment Tool (PROBAST) (8). Any discrepancies were resolved through discussion and consensus with a third investigator (Yunying Mu).
Results
Study selection
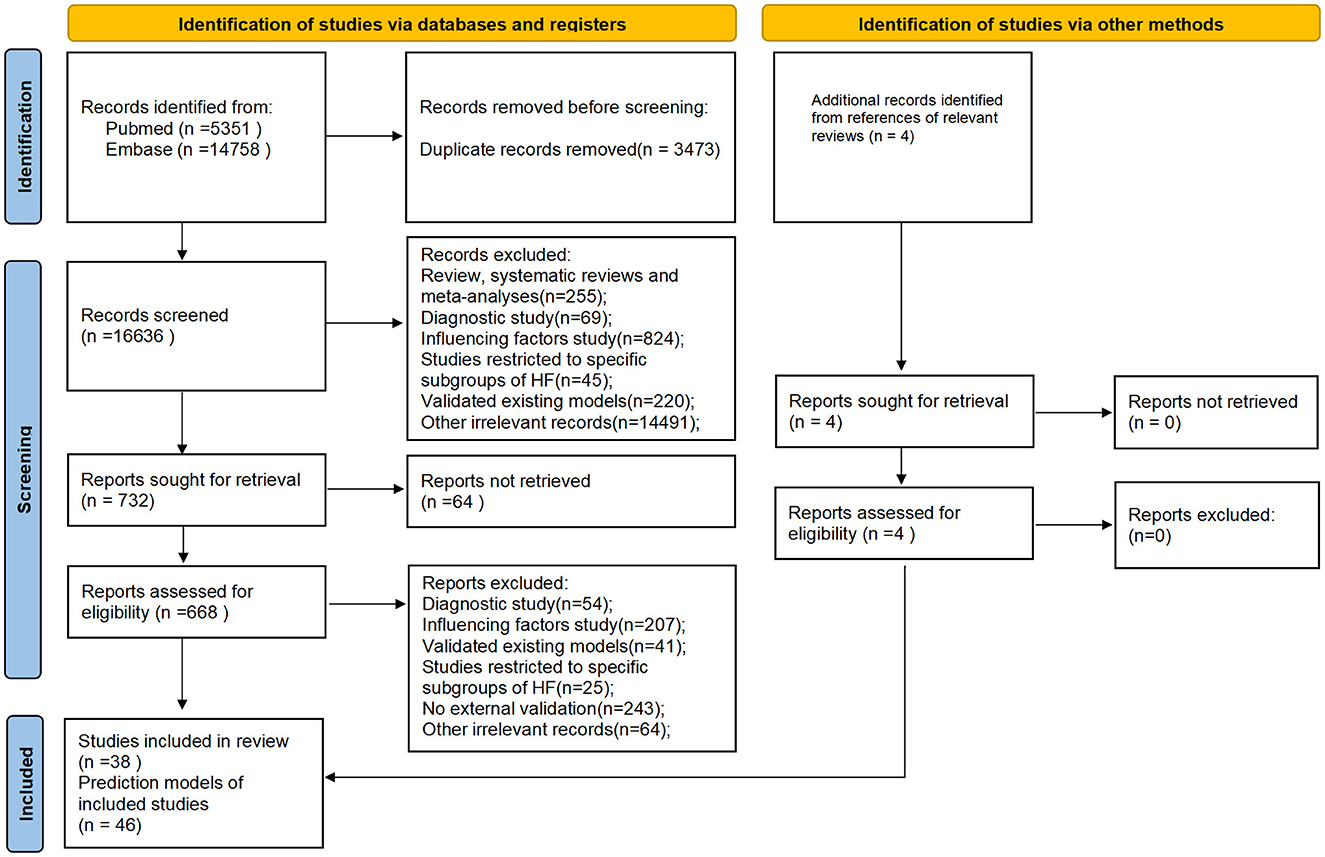
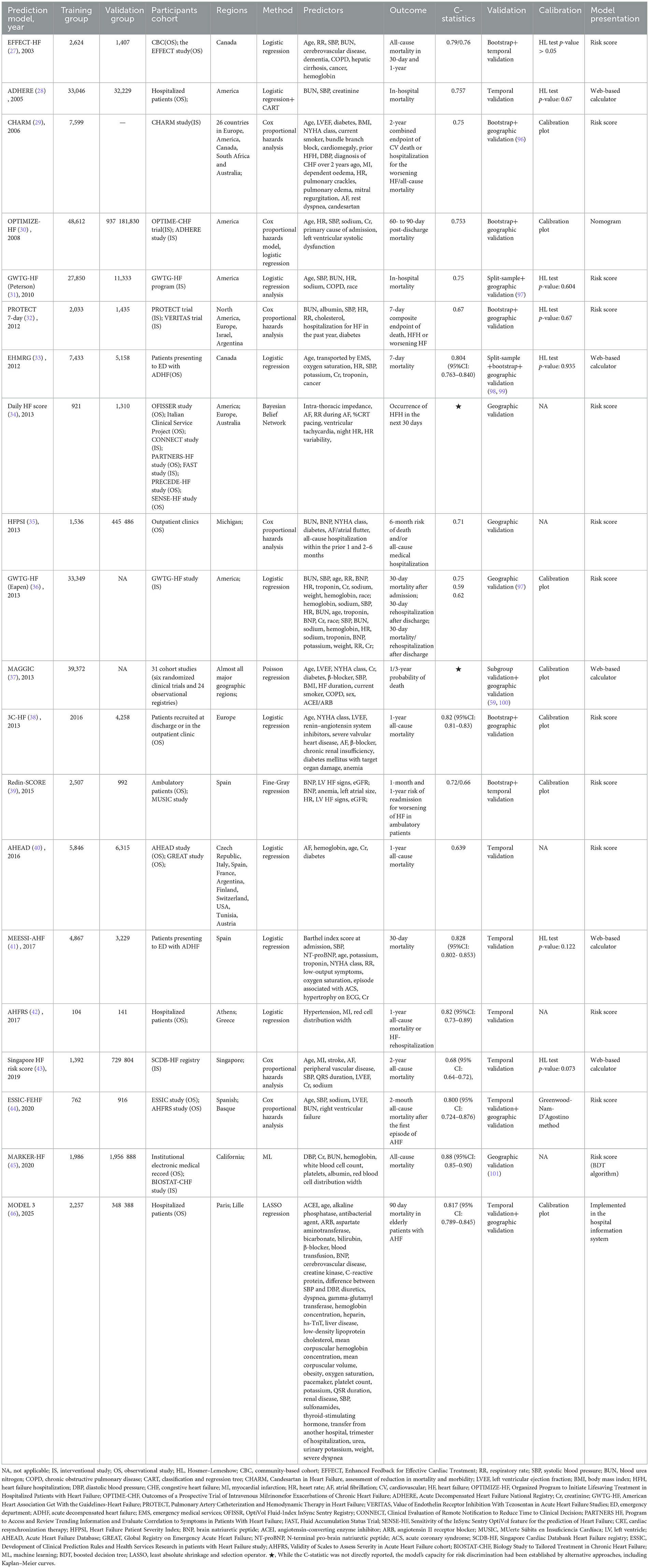
A total of 20,113 studies were initially identified. After removing 3,473 duplicates, 38 studies comprising 46 prediction models met the eligibility criteria following a review of titles, abstracts, and full-text articles (Figure 1). Notably, five of these studies reported on two or more prediction models. Among the included prediction models, 14 models from nine studies (9–17) were developed to predict outcomes in HFrEF patients, nine models from eight studies focused on HFpEF patients (18–25), and one model from a single study (26) targeted HFmrEF patients. The remaining 22 prediction models, derived from 20 studies (27–46), were designed for all HF patients regardless of subtype.
Characteristics of included prediction models
As shown in Tables 3–5, among the 46 included prediction models, 31 (67%) were developed based on published cohort studies, while the remaining 15 (33%) utilized data from outpatient or inpatient recruitment, original electronic health records (EHR), or registry data. Only 11 (24%) prediction models were derived from study populations with broad geographical representation, covering most regions globally; the populations of the remaining prediction models were primarily concentrated in North America and Europe, with only 4 models specifically developed for Asian populations.
Regarding modeling approaches, Cox proportional hazards regression was the most frequently employed (52%), followed by logistic regression (28%). A minority of studies used methods such as Fine-Gray competing risks regression, Bayesian belief networks, classification and regression trees, least absolute shrinkage and selection operator (LASSO) regression, and machine learning (ML). The predictors incorporated in the models spanned multiple categories including demographic information, signs/symptoms, comorbidities, medical history related to HF, laboratory data, electrocardiogram data, echocardiographic data, devices, and medications, ranging from a minimum of 3 to a maximum of 41. In terms of predicted outcomes, 30 (65%) models predicted all-cause mortality, 5 (11%) predicted HF rehospitalization, 1 (2%) predicted cardiovascular death, and 10 (22%) predicted composite outcomes.
Model discrimination was predominantly assessed using the concordance statistic (C-statistic), with values ranging from 0.62 to 0.88. Only two prediction models evaluated their discrimination ability using other methods, such as Kaplan–Meier curves. Regarding prediction model calibration, 37 (80%) prediction models underwent calibration assessment. Among these, 24 (65%) were evaluated through calibration plots, 12 (32%) were assessed through Hosmer–Lemeshow test p-values, and 1 (3%) was evaluated through the Greenwood–Nam–D'Agostino method. Only three models further provided DIC or NRI analysis (20, 24, 26). All models underwent external validation, either temporal or geographical, while 20 (20/46) also underwent internal validation. In terms of model presentation, 14 (30%) prediction models were presented as web-based calculators, 26 (57%) as risk scores, 5 (11%) as nomograms, and 1 (2%) prediction model was implemented in the hospital information system for application.
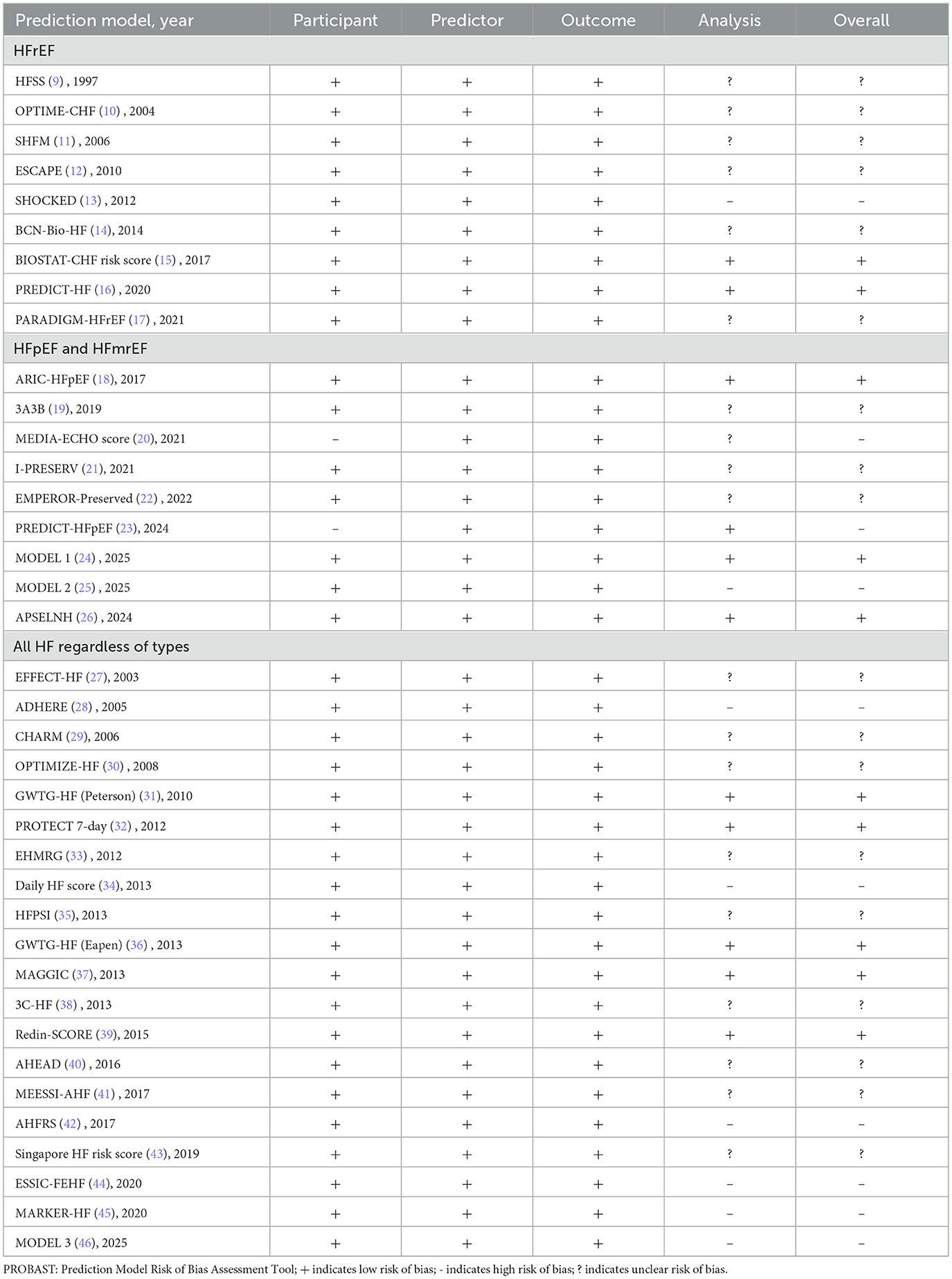
Risk of bias assessment of included prediction models
The risk of bias for 46 prediction models from 38 included studies was assessed using the PROBAST tool. Results showed that 10 (22%) models from 10 studies had a high risk of bias, 21 (45%) from 18 studies had an unclear risk of bias, and 15 (33%) from 10 studies had a low risk of bias (Table 6). Regarding study populations, bias primarily stemmed from certain cohorts excluding high-risk patients through exclusion criteria, thereby limiting model representativeness and hindering generalizability to broader populations. Furthermore, among prediction models for HFpEF, inconsistencies in diagnostic criteria across studies led to heterogeneous populations, further compromising model applicability. Within the predictor domain, all studies explicitly reported predictor definitions and assessment time points, resulting in generally low bias risk. Regarding the outcome domain, all studies clearly described outcome definitions, measurement methods, and evaluation intervals, yielding similarly low bias risk in this domain. The analysis domain presented the highest concentration of bias risks. Potential sources of bias included: failure to meet the requirement of at least 20 events per variable; inappropriate handling of missing data; reliance solely on univariate analysis for predictor selection; and lack of consideration for model overfitting.
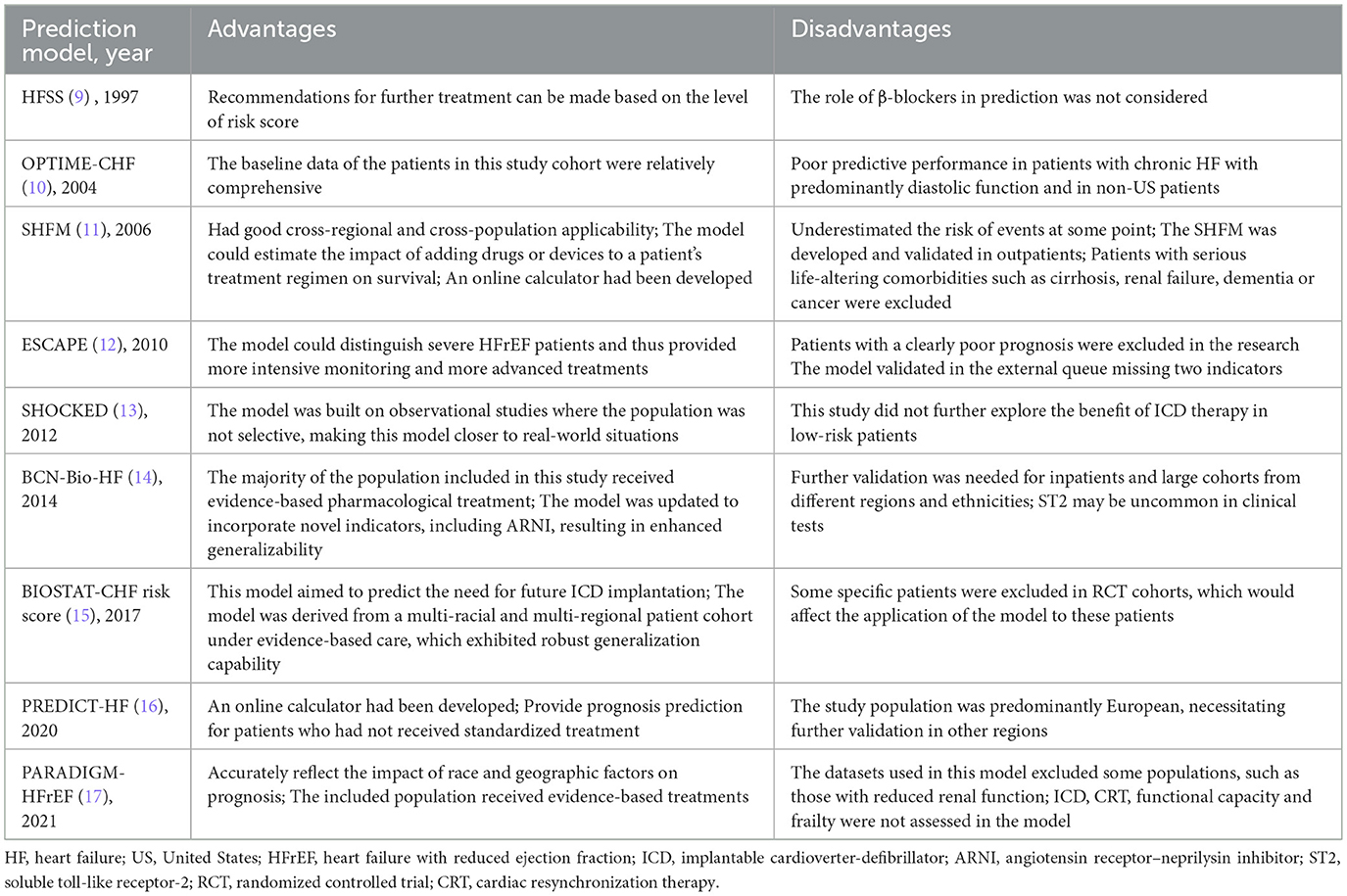
Prediction models for HFrEF
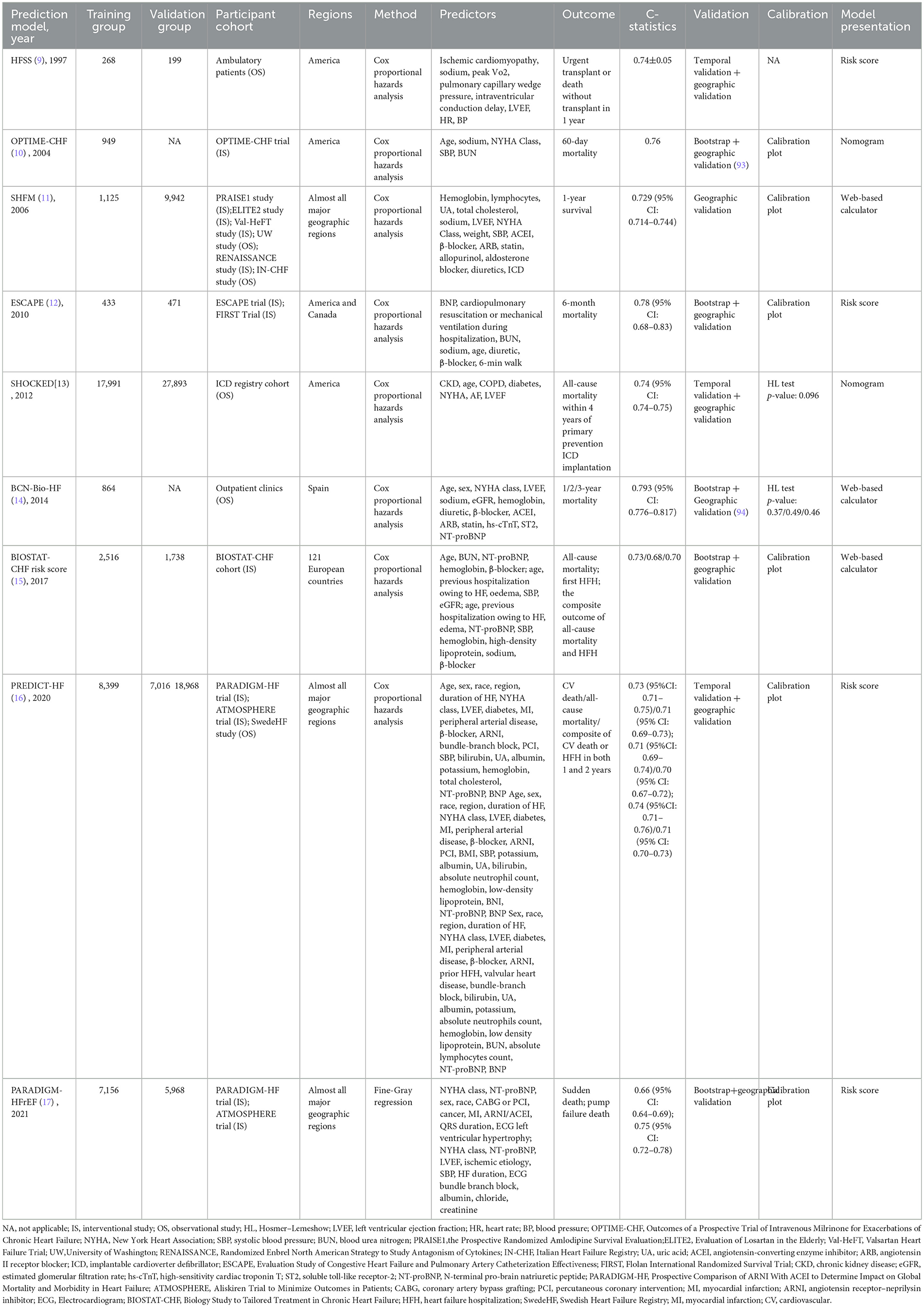
As shown in Table 3, multiple prediction models were available for assessing all-cause mortality in patients with HFrEF, including the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic HF (OPTIME-CHF) prediction model (10), the Seattle HF Model (SHFM) (11), the Evaluation Study of Congestive HF and Pulmonary Artery Catheterization Effectiveness (ESCAPE) prediction model (12), the Barcelona Bio-HF (BCN-Bio-HF) prediction model (14), Biology Study to Tailored Treatment in Chronic HF (BIOSTAT-CHF) risk score (15), and the PREDICT-HF prediction model (16).
The OPTIME-CHF prediction model (10) was employed to predict short-term (60-day) mortality. However, this model was primarily developed in American patients with systolic dysfunction, limiting its applicability to those with diastolic dysfunction and non-American populations. External validation in a Chinese patient cohort demonstrated its poor predictive performance (47). Similarly, the ESCAPE model (12) predicted 60-month all-cause mortality, but it was derived from an interventional cohort that excluded some high-risk patients, thereby limiting its broader applicability.
In terms of long-term mortality prediction (1-year, 2-year, and 3-year), the SHFM, BCN-Bio-HF, BIOSTAT-CHF risk score, and PREDICT-HF all demonstrated predictive capability. Both the SHFM (11) and the PREDICT-HF (16) prediction models were developed using multi-regional global cohorts, affording them relatively wide applicability. The SHFM, in particular, was one of the most widely used models in clinical practice, partly due to the availability of a web-based calculator. Nevertheless, as an earlier model, the SHFM did not include key predictors such as B-type natriuretic peptide (BNP), and the absence of certain demographic variables may have compromised its predictive accuracy (48). Studies have also indicated that the SHFM may underestimate mortality in Black people individuals, those aged ≥65 years, and patients with implantable cardioverter defibrillators (ICDs) (49, 50). In contrast, the PREDICT-HF prediction model was developed in 2020 based on patients receiving guideline-directed medical therapy. It incorporated a comprehensive set of predictors spanning demographics, comorbidities, laboratory parameters, and medication use, contributing to its robust predictive performance. Notably, it included both BNP and angiotensin receptor–neprilysin inhibitor (ARNI) as predictive variables, making it particularly suitable for HFrEF patients receiving contemporary standard treatment. However, since the derivation cohort excluded ICD recipients, the model's applicability in such patients remained uncertain. Additionally, the model provided only a risk score without an online calculation tool, which limited its clinical convenience. Using the PREDICT-HF prediction model, the BIOSTAT-CHF risk score (15) was specifically designed for patients not receiving standard therapy, predicting all-cause mortality using just five simple variables. However, as this model was developed in European patients, further validation in broader regions remained necessary. Both the PREDICT-HF prediction model and BIOSTAT-CHF prediction model could also predict HF hospitalization (HFH) and composite endpoints. The BCN-Bio-HF prediction model (14) enhanced predictive performance by incorporating three emerging biomarkers—BNP, high-sensitivity cardiac troponin T (hs-cTnT), and soluble toll-like receptor-2 (ST2)—in addition to conventional predictors. This model was also derived from a guideline-treated cohort and was supported by an online calculator, facilitating its clinical dissemination. In recent years, researchers have integrated updated biomarker and treatment data to develop a refined version, BCN-Bio-HF prediction model 2.0, which has been widely applied (51).
A head-to-head comparative study (52) of contemporary HF prediction models evaluated the performance of BCN-Bio-HF 2.0, SHFM, and PREDICT-HF in predicting mortality among 1,166 HF outpatients. Results indicated that no single model demonstrated superior performance across all metrics. Among them, BCN-Bio-HF showed the best discriminative ability and overall performance, albeit with a tendency to overestimate mortality risk. In contrast, both SHFM and PREDICT-HF were observed to underestimate mortality risk.
The HF survival score (HFSS) (9) was employed to assess whether patients with end-stage HF require cardiac transplantation. This model categorized patients into three risk tiers through risk scoring: moderate-to-high risk patients, with a higher probability of death within 1 year, were recommended for cardiac transplantation within that timeframe, whereas low-risk patients could defer transplantation. However, BNP, a crucial prognostic predictor for HF, was not incorporated into the model. Subsequent studies reported that the addition of BNP to the HFSS enhanced the predictive capacity of the model (53, 54). With advances in medical care, guideline-recommended therapies including β-blockers, ICD, and cardiac resynchronization therapy (CRT) were widely applied after the creation of the model; although there were small sample sizes of studies showing that HFSS performed well in those populations, further validation was still required (55, 56).
The Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in HF (PARADIGM-HFrEF) prediction model (17) was employed to assess patients' risk of sudden cardiac death or pump failure, thereby guiding decisions regarding ICD implantation. Developed using a cohort spanning multiple countries worldwide, this model demonstrated considerable universality. However, as its modeling data primarily originated from interventional studies subject to stringent exclusion criteria, certain high-risk patients remained excluded. Consequently, the model exhibited limitations in its applicability. The SHOCKED prediction model (13) was specifically designed to predict 4-year all-cause mortality in HF patients with implanted ICDs, addressing the shortcomings of most previous models that did not cover this population. Unlike the PARADIGM-HFrEF model, the SHOCKED model was constructed from observational study cohorts with fewer excluded cases, thereby offering greater clinical applicability. However, this model still required further validation in populations outside the United States.
Prediction models for HFmrEF
APSELNH prediction model
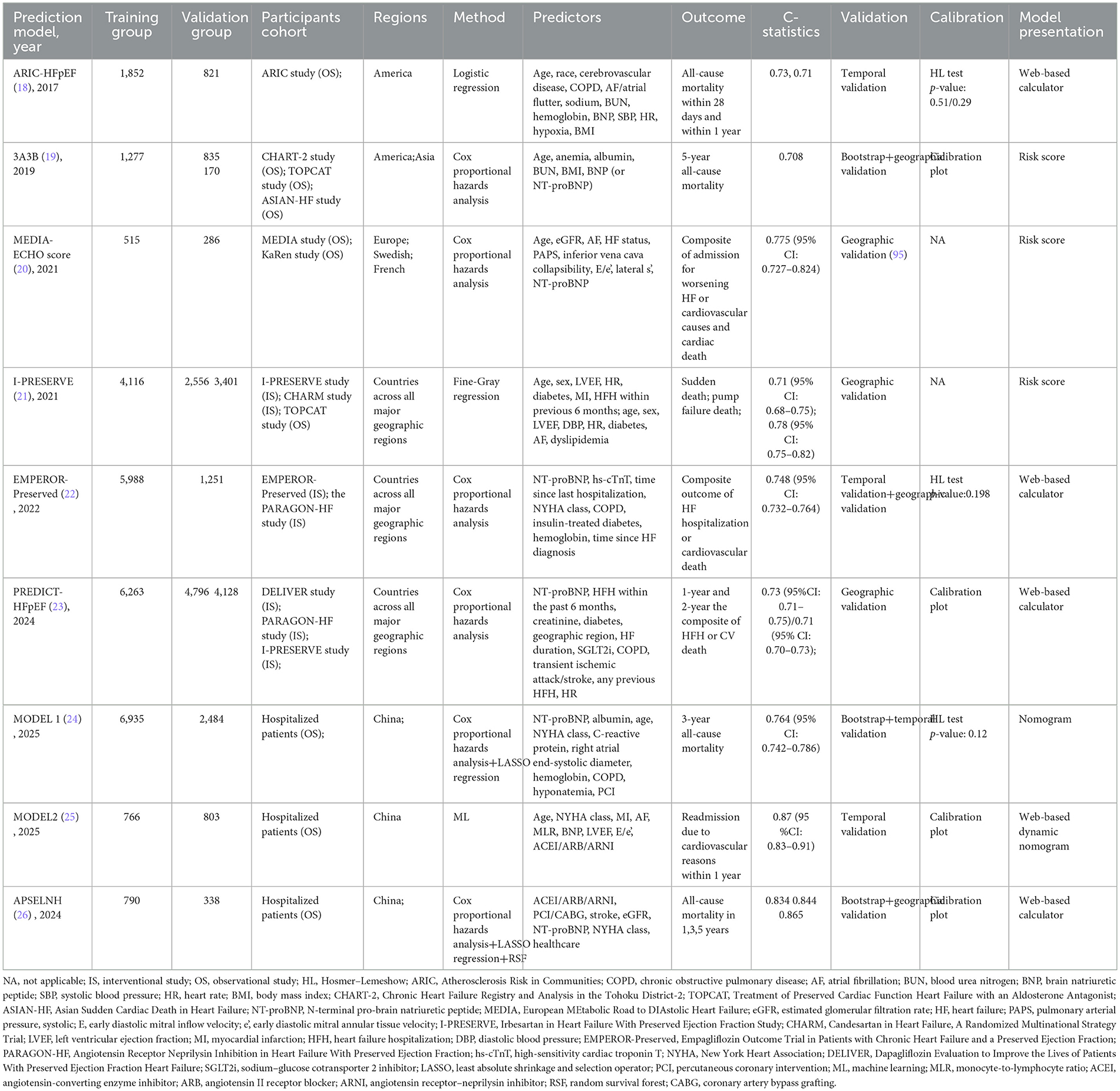
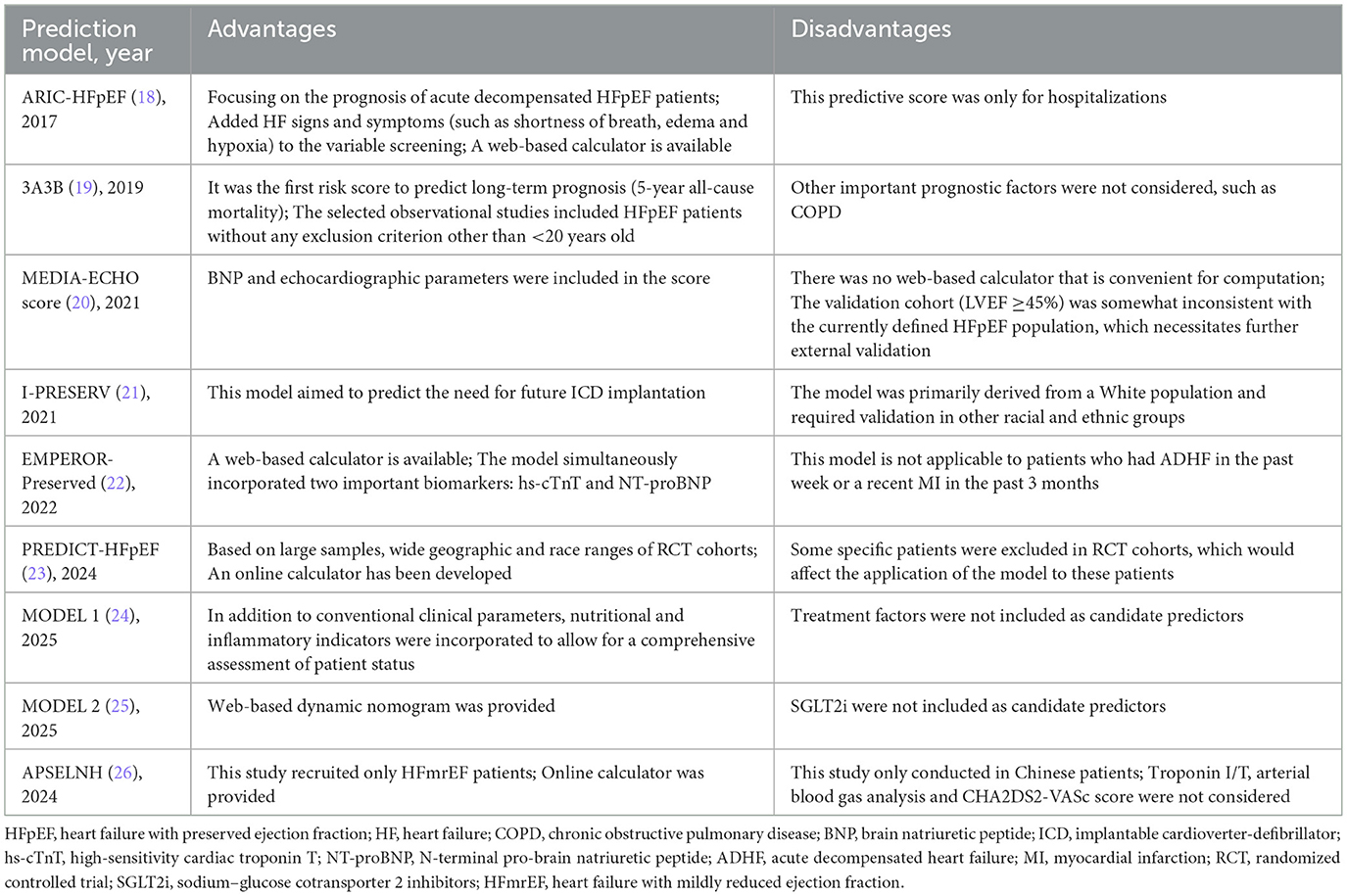
As shown in Table 4, the APSELNH prediction model (26) predicted the 1-, 2-, and 3-year all-cause mortality risk in HFmrEF patients. The model achieved C-statistics above 0.8 at all time points, indicating good predictive performance. The model was ultimately presented as a nomogram, and the risk score could be calculated on the online platform. Patients could be categorized into three classes based on the risk score: low risk (point < 219.5), moderate risk (219.5 ≤ point ≤ 304.2), and high risk (point >304.2). Individualized risk stratification could guide treatment intensity and follow-up frequency, thereby enhancing the management of high-risk patients while appropriately reducing unnecessary interventions in low-risk patients. However, as the model was derived exclusively from Chinese patients, external validation in more diverse regions and ethnic groups was warranted. Furthermore, key biomarkers such as troponin I/T (TnI/T) were not included during model development, representing a potential direction for future refinement.
Prediction models for HFpEF
As shown in Table 4, Atherosclerosis Risk in Communities (ARIC-HFpEF) (18), MODEL 1 (24), and the 3A3B risk score (19) were three prediction models that could be used to predict all-cause mortality in patients with HFpEF. Among them, the ARIC-HFpEF prediction model (18) was capable of predicting both short- (28-day) and long-term (1-year) mortality risk. Notably, it was the first prediction model specifically developed for patients with acute decompensated HFpEF (LVEF ≥50%), who typically presented with poorer prognoses. During its development, the model innovatively incorporated variables related to signs/symptoms, such as shortness of breath, edema, and hypoxia, with hypoxia ultimately being established as a formal predictor. Additionally, the model was presented as a web-based calculator, greatly facilitating its clinical application. MODEL 1 (24) was used to predict 3-year mortality in HFpEF patients (LVEF ≥50%) and included a series of novel predictors related to inflammation and nutritional status, though it did not incorporate treatment-related variables. The 3A3B risk score (19) aimed to predict 5-year mortality risk in HFpEF patients (LVEF ≥50%). Constructed based on an observational cohort with few exclusions, this model demonstrated good applicability. However, all three models were developed using cohorts from single regions, and their generalizability required further external validation in other populations. Similar to the PARADIGM-HFrEF prediction model, the Irbesartan in HF with Preserved Ejection Fraction Study (I-PRESERVE) prediction model was employed to predict sudden death and pump failure in patients with HFpEF (LVEF >40%, LVEF ≥45%). However, this model did not incorporate BNP as a predictor and also exhibited shortcomings in calibration.
On the other hand, the European MEtabolic Road to DIAstolic HF (MEDIA) echo score (20), Empagliflozin Outcome Trial in Patients with Chronic HF and a Preserved Ejection Fraction (EMPEROR-Preserved) (22), PREDICT-HFpEF (23), and MODEL 2 (25) were models used to predict either HFH or a composite outcome of HFH and cardiovascular death. Echocardiography played a crucial role in the diagnosis and prognosis assessment of HFpEF. The MEDIA echo score (20) could predict the composite outcomes in HFpEF patients (LVEF >50%, LVEF ≥45%) by incorporating echocardiographic parameters [pulmonary artery systolic pressure, inferior vena cava collapsibility, early diastolic mitral inflow velocity/early diastolic mitral annular tissue velocity (E/e′), and lateral s′] into traditional clinical predictors. Subsequent studies had confirmed that this model demonstrated good predictive performance in both outpatient and inpatient settings. The EMPEROR-Preserved (22) and PREDICT-HFpEF models (23) also assessed the risk of composite outcomes in HFpEF patients (LVEF >45%) via online web-based calculators. Since both models were developed using cohort data from multiple regions worldwide, they provided high applicability. However, as both models were based on interventional study cohorts that excluded some high-risk populations, their predictive performance in high-risk groups still required further validation. It was worth noting that the EMPEROR-Preserved model included key predictors such as N-terminal pro-brain natriuretic peptide (NT-proBNP) and hs-cTnT, whereas the newly developed PREDICT-HFpEF model was the first to incorporate sodium–glucose cotransporter 2 inhibitor (SGLT2i) as a predictive variable. The MODEL 2 (25) predicted the risk of HFH within 1 year in HFpEF patients (LVEF ≥50%) through a web-based dynamic nomogram. It included important variables such as BNP, LVEF, E/e′, and the use of angiotensin-converting enzyme inhibitor (ACEI)/angiotensin II receptor blocker (ARB)/ARNI, but did not include SGLT2i, indicating room for further refinement in the future.
It was worth noting that the LVEF thresholds for defining HFpEF in the selected data cohorts of various prediction models were not consistent, ranging from 40 to 45 to 50. This may be due to the fact that some of the studies were earlier, and at that time, there was not yet a uniform and clear delineation of HFpEF. Studies have shown significant differences in baseline characteristics, incidence, and prognosis between HFpEF and HFrEF populations (48). It is suggested that future predictive modeling studies should pinpoint LVEF at 50% when selecting study populations. In addition, previous prediction models (I-PRESERVE, the MEDIA echo score, EMPEROR-Preserved, PREDICT-HFpEF) need to be validated in populations with LVEF ≥50% to further assess their predictive efficacy. Furthermore, as HFpEF patients have been further subdivided into stages A/B/C/D, more researchers have focused on developing predictive models to prevent patients' progression from stage A/B (where HF symptoms have not developed) to stage C/D (where HF has occurred), advancing the front line of prevention and treatment to improve patients' prognosis (57, 58).
Prediction models for all HF regardless of types
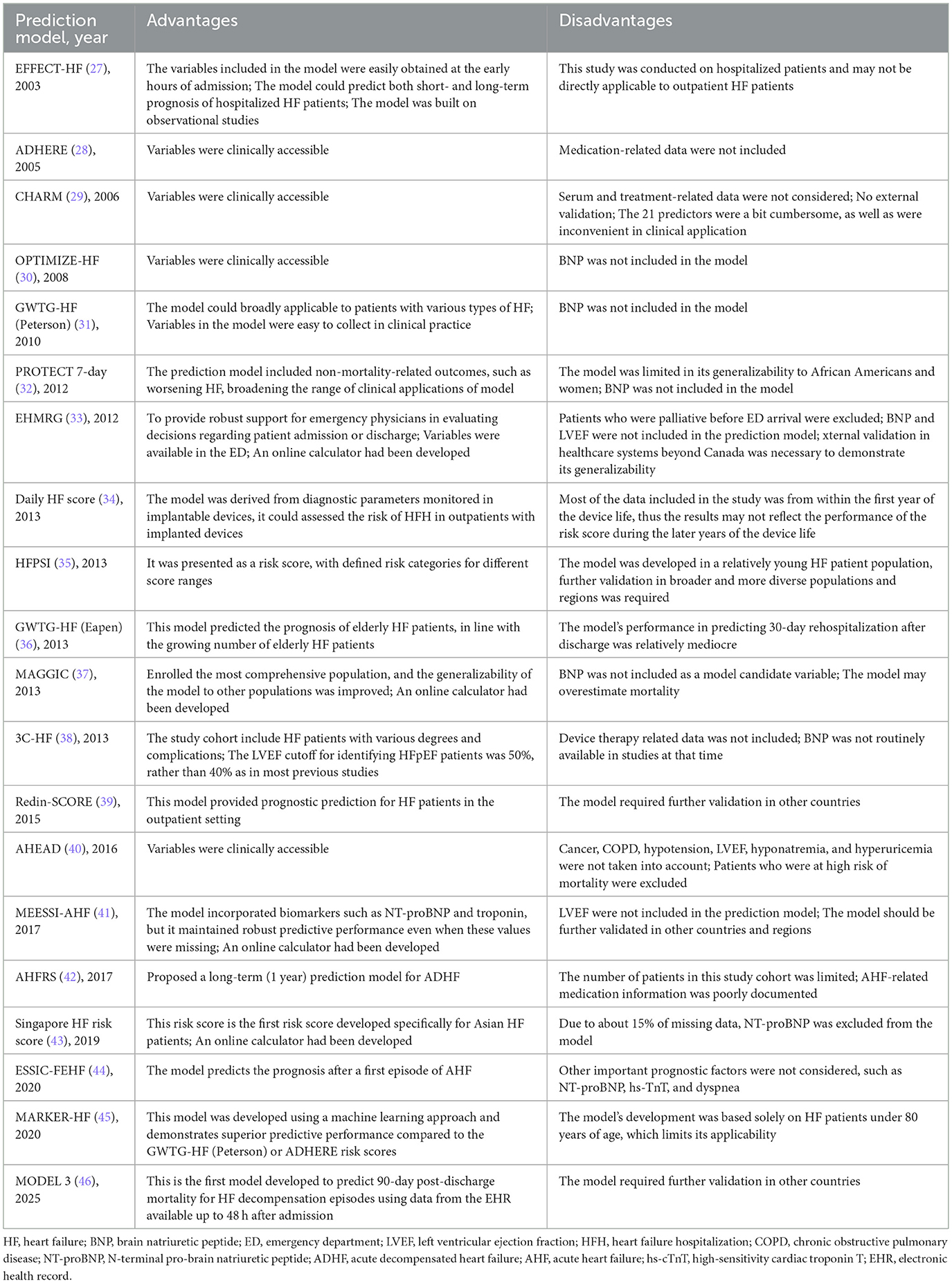
As shown in Table 5, a total of 22 prediction models are currently available for assessing the prognosis of all HF patients regardless of subtype, with prediction timeframes ranging from the inpatient period up to 3 years. The American Heart Association Get With the Guidelines-HF (GWTG-HF) (Peterson) model (31) is suitable for predicting in-hospital mortality. GWTG-HF (Eapen) (36), Enhanced Feedback for Effective Cardiac Treatment (EFFECT-HF) (27), and Daily HF score (34) were employed to forecast 30-day mortality, readmission, and their composite endpoints. Specifically, GWTG-HF (Eapen) (36) is more applicable to HF patients over 65 years of age, EFFECT-HF (27) is more suitable for hospitalized HF patients, and the Daily HF score (34) is better suited for prognostic assessment in outpatients with implanted pacemakers. None of these models incorporated BNP, and they still require further external validation in broader geographical regions. Additionally, as the Daily HF score was derived from pacemaker data, it did not include demographic or medication-related variables.
The Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients with HF (OPTIMIZE-HF) (30), Redin-SCORE (39), the Development of Clinical Prediction Rules and Health Services Research in Patients with HF study (ESSIC-FEHF) (44), MODEL 3 (46) and HF patient severity index (HFPSI) (35) prediction models were employed to forecast all-cause mortality or HFH over periods ranging from 1 to 6 months. The OPTIMIZE-HF prediction model (30) was developed to predict mortality within 60–90 days post-discharge, while the ESSIC-FEHF prediction model (44) was employed to forecast mortality within 2 months; however, neither of these models incorporated BNP. Both Redin-SCORE and HFPSI prediction models were developed using outpatient cohorts: Redin-SCORE (39) predicted 1-month mortality, while the HFPSI prediction model (35) forecasts 6-month all-cause mortality or HFH. MODEL 3 (46) was a predictive model developed using LASSO regression on EHR data to forecast 90-day mortality in elderly HF patients. This model could be directly implemented in the hospital information system for intelligent prediction of HF patients. All three models incorporated BNP but required further external validation in other countries and regions. Additionally, the EFFECT-HF and Redin-SCORE prediction models could predict 1-year all-cause mortality.
For long-term prognosis prediction, the Meta-Analysis Global Group In Chronic HF (MAGGIC) (37), 3C-HF (38), Singapore HF risk score (43), MARKER-HF (45), and Candesartan in HF: A Randomized Multinational Strategy Trial (CHARM) (29) prediction models were employed to forecast all-cause mortality over 1–3 years. The MAGGIC prediction model (37) remained the most widely employed model for predicting 1- or 3-year mortality. Its strengths lie in its construction from cohorts across multiple global regions and the provision of an online web calculator, demonstrating outstanding clinical applicability and convenience. Consequently, it has become the most extensively utilized model in clinical practice and research. However, this model excluded BNP, potentially leading to an overestimation of mortality risk (52). Research indicated that MAGGIC demonstrated poor predictive performance in Japanese cohorts; however, incorporating BNP into the model enhanced its predictive performance (59). The Singapore HF risk score (43) was a 2-year mortality prediction model specifically developed for East Asian HF patients; the CHARM prediction model (29) could predict a composite endpoint of cardiovascular death, all-cause mortality, and HFH within 2 years; the MARKER-HF prediction model (45) was an all-cause mortality prediction model built using ML methods, suitable for individuals under 80 years of age.
In addition to the prediction models mentioned above, several prediction models were specifically designed for patients with acute decompensated HF (ADHF). The Emergency HF Mortality Risk Grade (EHMRG) (33) and the Multiple Estimation of Risk based on the Emergency Department Spanish Score In Patients with AHF (MESSI-AHF) risk score (41) were models that could be rapidly applied in the emergency department (ED) to assist in determining whether an ADHF patient required hospitalization. The EHMRG prediction model (33) primarily predicted 7-day mortality, while the MESSI-AHF risk score (41) predicted 30-day mortality. In terms of predictor selection, both models utilized clinical variables readily available in the ED. The MESSI-AHF risk score incorporated NT-proBNP and troponin as predictors. Validation results demonstrated that even without NT-proBNP, these two models could maintain retain a certain level of predictive performance; however, the inclusion of NT-proBNP further enhanced their predictive accuracy. Considering the comprehensiveness of predictors, the rigor of validation methodologies, and the representativeness of study populations, MESSI-AHF demonstrated superior predictive performance and greater potential for clinical applicability.
The Acute HF Risk Score (AHFRS) (42) was also a prediction model established for patients presenting to the ED. In contrast to the two prediction models mentioned above, it was designed to predict the risk of 1-year all-cause mortality or HFH for patients who received guideline-directed medical therapy during hospitalization and after discharge. This model provided a long-term prognostic assessment for ADHF patients admitted to the ED.
Furthermore, several prediction models were available to assess the prognosis of ADHF patients who had already been hospitalized. The Acute Decompensated HF National Registry (ADHERE) risk tree (28) was constructed to predict in-hospital mortality. By judging blood urea nitrogen (BUN) levels (threshold: 43 mg/dl), systolic blood pressure (SBP; threshold: 115 mm Hg), and serum creatinine levels (threshold: 2.75 mg/dl) sequentially, ADHF patients were readily categorized into low-, intermediate-, and high-risk groups for in-hospital death, with the risk of death ranging from 2.1 to 21.9%. The Pulmonary Artery Catheterization and Hemodynamic Therapy in HF (PROTECT) 7-day model (32) predicted a 7-day composite outcome of death, HFH, or worsening HF in ADHF patients with renal dysfunction and elevated BNP levels. The AHEAD prediction model (40) also constructed risk scores to predict 1-year all-cause mortality. However, BNP was not included in any of the above models, probably because these studies were limited by the research context at the time and the records related to serum markers were incomplete. However, serum markers played an increasingly important role in the treatment and diagnosis of HF. A predictive model based on ML had screened a biomarker cluster to predict the prognosis of HF patients, and its predictive performance was even better than MAGGIC prediction model (60). This phenomenon was worthy of consideration.
Discussion
Due to the heterogeneity of HF, researchers have developed different prediction models for different types of HF. Prediction models for HFrEF and all HF types are more mature, while the number of studies on HFpEF and HFmrEF are fewer due to the newer concepts and definitions, although related studies are gradually growing (61). This study comprehensively summarizes the characteristics and shortcomings of each prediction model and visually presents them in the form of tables (Tables 7–9). Among them, the prediction models in bold are considered to have better predictive performance than the others.
In earlier studies, HF types were relatively simple, mainly dominated by HFrEF, so the earliest prediction models focused mainly om HFrEF patients. With advances in research, it was found that the LVEF in some HF patients was not significantly reduced, which put forward the concept of diastolic HF (62, 63). However, the definition of LVEF for diastolic HF has long been controversial (>40%, >45%, or ≥50%) (20–23), posing significant challenges for prognostic prediction. In this context, general prediction models that do not distinguish HF types emerged, the most representative of which was the MAGGIC-HF prediction model. In recent years, with the clarification of HF classification criteria, diastolic HF has been formally defined as HFpEF by the guidelines (64, 65), and a specific LVEF cutoff (≥50%) has been established. Additionally, the guidelines introduced a new classification called HFmrEF. This refinement in classification has driven the development of prediction models for specific HF subtypes. Several studies (23, 66) have confirmed that prediction models based on specific HF subtypes are significantly better than MAGGIC-HF models (which do not distinguish types), suggesting the inherent limitations of unified prediction methods. At present, constructing prediction models for different HF subtypes has become the mainstream strategy to improve prediction accuracy and reduce the occurrence of adverse events.
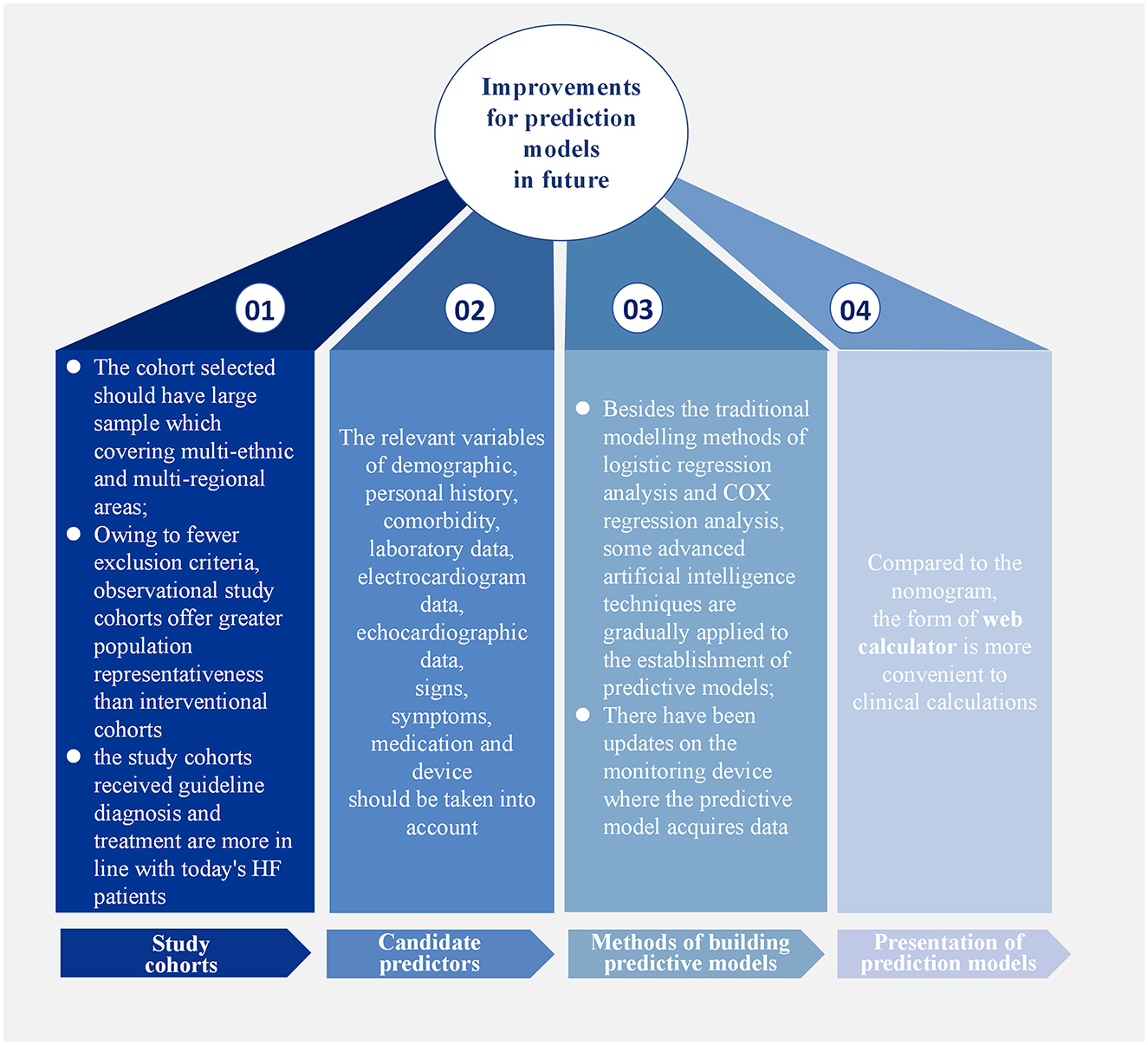
Among the predictive models for different HF subtypes, those for HFrEF have been the most extensively studied. However, due to limitations in research conditions at that time, early models did not include certain predictors now regarded as essential, which restricted their applicability in contemporary clinical practice. For HFpEF prediction models, a major issue is that the LVEF criteria used in early studies do not align with current guidelines, making it difficult to directly apply these models to today's patient populations. In contrast, research on HFmrEF prediction models is relatively scarce; only one externally validated model was included in this review, underscoring the need for further exploration in this area. Notably, no single model currently dominates in terms of predictive efficacy or clinical applicability. This may be attributed to several factors, such as incomplete selection of study cohorts, insufficient candidate predictors, and inconvenient presentation of prediction models (Figure 2).
The study cohort was both source of research data and the basis for model construction, making the selection an appropriate study cohort crucial. Most of the study cohorts used in developing prediction models were small to medium-sized single-center cohorts. As these cohorts covered insufficient populations, representative predictors, such as race, region, and gender, could not be screened out. Prediction models established in this way also needed to be externally verified by multi-center studies and larger cohorts. In contrast, widely used prediction models, such as MAGGIC-HF and SHFM-HFrEF, selected large sample cohorts that included multi-ethnic and multi-regional areas to ensure their universal applicability to a broader patient population. Additionally, prediction models based on observational study cohorts are applicable to a wider range of populations than those based on intervention studies. Interventional studies inevitably excluded patients with a high mortality risk, such as those with hypoalbuminemia or cancer, or those with a recent history of myocardial infarction or HFH within the past 3 months. This not only limited the application of prediction models in these populations but also introduces bias into the models. While observational studies have fewer exclusion criteria and can include patients more comprehensively, the larger the number of intervention studies in the real world has led most of prediction models to rely on intervention studies as their modeling cohorts, thus limiting their applicability. Finally, study cohorts that have received guideline-recommended diagnostics and treatments are more suitable as target populations for developing prediction models. In recent years, significant progress has been made in the diagnosis and treatment of HF, and guideline recommendations for clinical practice have been established. Although earlier prediction models helped predict the prognosis of HF patients, biomarkers such as BNP/NT-proBNP and echocardiography-related indices were not regarded as important for HF diagnosis at that time, nor were β-blockers, ACEI, ARB, ARNI, and SGLT2i used as routine treatments. Subsequent studies have proven the predictive incremental effect of BNP/NT-proBNP on early prediction models (48, 59), indicating that the applicability of earlier prediction models in contemporary HF patients receiving guideline-based therapy remains limited. In contrast, the PREDICT-HFpEF and APSELNH-HFmrEF prediction models created in recent years, selected study populations that received standardized evidence-based treatment, and the final models included the novel diagnostic and therapeutic indices mentioned above, confirming the important role of these indices in HF prognosis.
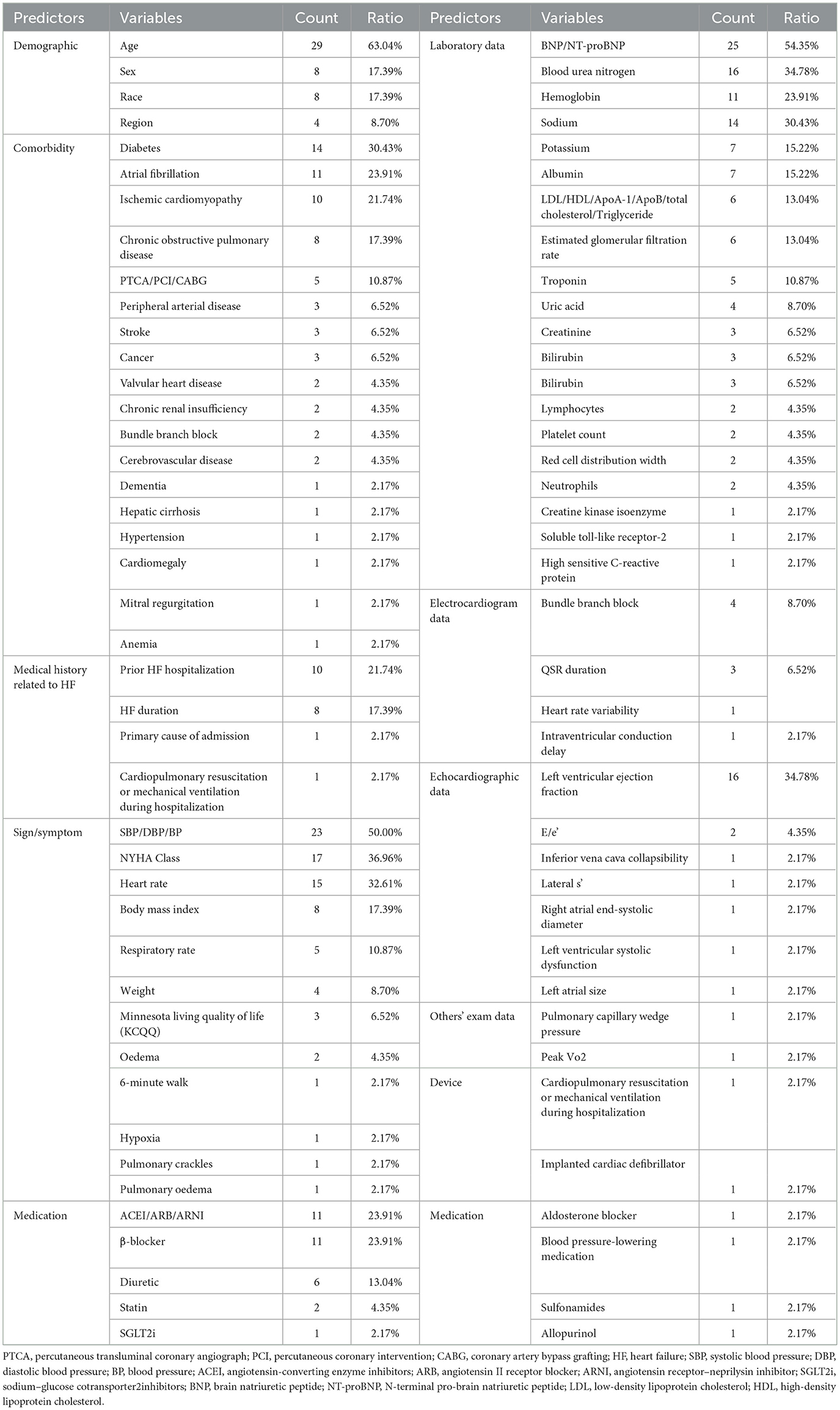
Predictors play an essential role in the model, so their selection should be more comprehensive, taking into account relevant variables such as demographic information, personal history, comorbidities, laboratory data, electrocardiogram data, echocardiographic data, signs, symptoms, medication, and devices. All variables mentioned in the above prediction models are shown in Table 10. Prediction models such as I-PRESERVE, ADHERE, and 3C-HF illustrate that predictive performance can be limited by incomplete predictor selection. In recent years, numerous biomarkers for predicting HF prognosis have emerged, such as ST2, growth differentiation factor-15 (GDF-15), and microRNAs (67–69). Several studies have demonstrated that incorporating novel predictors into existing models enhances predictive performance (54, 70, 71). Furthermore, several studies (72, 73) have employed ML approaches to develop prognostic prediction models by integrating inflammatory biomarkers with clinical variables. These models have demonstrated good discriminatory performance, showing potential not only to assist in clinical decision-making but also to improve patient outcomes. Emerging detection technologies, including proteomics (74, 75), metabolomics (76, 77), genomics (78), and artificial intelligence-based imaging data analysis (79, 80) are progressively being applied to the construction of HF prognosis prediction models. It should be noted that most of these models have currently undergone only internal validation and have yet to be externally validated in independent cohorts. Their predictive efficacy and clinical applicability require further confirmation. Concurrently, the correlation between established clinical scoring tools—such as the CHA2DS2-VASc score (81) and H2FPEF score (82)—and HF prognosis has been substantiated, providing additional rationale for selecting potential predictive factors within forecasting models. Echocardiographic indicators have become increasingly important references in the diagnosis of HF (83, 84), especially HFpEF, but they are less frequently included in current prediction models, warranting more attention in the future. In addition, there have been updates on the monitoring devices from which predictive models acquire data. Recent studies have found a way to monitor the physiological information of HF patients remotely by minimally inserting a cardiac monitor under the skin, subsequently developing an HF risk score to predict the occurrence of worsening HF events, thus providing a multi-parameter and comprehensive prediction method (85, 86). However, the sample size of this study was small, and further verification is needed. The application of novel technologies has undoubtedly enhanced the real-time capability and accuracy of predictions. In developing future predictive models, the selection of predictive factors must take full account of the aforementioned considerations.
Moreover, the presentation of the model is equally important. Most models are presented in the form of computational formulas or nomograms, but the web calculator format is more conducive to clinical calculations, facilitating the wider dissemination of models. The bold models in the table are all in the form of web calculators. With advances in technology, predictive models using ML for EHR data have been gradually developed. These models could be implemented in the hospital information systems to intelligently identify the prognosis of in-hospital HF patients (46).
In addition to the traditional modeling methods of logistic regression analysis and COX regression analysis, some advanced artificial intelligence techniques have gradually been applied to the establishment of predictive models. For example, a growing number of researchers are leveraging ML algorithms (such as LASSO regression, light gradient-boosting machine, and extreme gradient boosting) for model development. The advantage of ML lies in its ability to train multiple candidate models in parallel and, through performance comparison, ultimately identify the model with the highest predictive accuracy (73, 87–89). The study by Jawadi et al. (90) reported that an ML-based model developed to predict in-hospital mortality in HF patients demonstrated superior discrimination, sensitivity, and specificity compared to traditional ADHERE and GWTG-HF prediction models (90). The study by Abdul-Samad et al. also indicated that the ML model exhibited better calibration capability than conventional statistical models (91). As discussed in the preceding section, ML's powerful computing capabilities provide a natural advantage for processing high-throughput, multi-modal data like omics and medical imaging. Given this capability, it is positioned as an ideal tool for developing such high-performance predictive models. However, most ML-based prediction models have only undergone internal validation and have not yet been translated into risk scoring systems ready for clinical application. This critical aspect requires improvement in future model development.
Last but not least, how clinical prediction models and risk scores can be better integrated with clinical treatment remains a worthy question for consideration. Although HF patients can be assessed as low, intermediate, or high risk through prediction models, clinical HF guidelines have not provided specific therapeutic recommendations for patients at different risk levels. Clinicians often make treatment decisions directly based on examination results, while risk classification often plays an indirect role. For risk scores to play a more effective role in the management of HF, clinical trials are needed to explore the safety and effectiveness of individualized treatment strategies based on risk assessment, thereby forming a higher level of evidence. It is expected that in the future, the risk score can directly guide clinical medication and treatment in the same way as the atrial fibrillation CHA2DS2-VASc score (92).
Limitations and strengths
This study has the following strengths: we systematically retrieved all well-performing prediction models for HF prognosis that have undergone external validation since the databases were established and assessed the risk of bias in the included models using the PROBAST tool. Furthermore, the study categorized the retrieved models based on their target populations, encompassing models with HFrEF, HFmrEF, and HFpEF, as well as models for all types of HF, with each category discussed separately. By comparing the similarities and differences among models within the same category and providing a detailed evaluation of their strengths and limitations, this review provides clear guidance for researchers selecting appropriate models. Concurrently, this study summarized the essential characteristics of high-quality prediction models across multiple dimensions, including cohort selection, predictive factor screening, and model presentation formats. The review also outlined the latest advances in current prediction model development, offering valuable reference points for future research.
However, this study also has several limitations. Given the large number of HF prognosis prediction models, to ensure the robustness and clinical applicability of the included models, we excluded those without external validation (typically indicating insufficient evidence of predictive efficacy and generalizability). This approach may have resulted in incomplete coverage of models. Nevertheless, we included models that, although not externally validated during their original development, subsequently underwent external validation in other studies. Furthermore, novel biomarkers and modeling methods reported in studies without external validation were also discussed in our study, thereby partially mitigating this limitation.
Conclusion
In summary, this systematic review included 38 studies and provided a detailed discussion of 46 prediction models for prognostic risk stratification across different types of HF. Analysis revealed that most models exhibited some degree of bias risk, suggesting that future research should fully adhere to PROBAST criteria when developing models, employing standardized methodologies to construct models with better predictive performance. Furthermore, the latest and most comprehensive strategies should be adopted in aspects such as cohort selection, predictor screening, model construction methods, and presentation formats. This approach will facilitate the development of high-quality prediction models that demonstrate robust predictive performance, strong generalizability, and clinical utility. In addition, prediction models that have consistently demonstrated strong performance through multiple external validations should be incorporated into clinical guidelines to enhance their practical application.
Author contributions
YW: Writing – original draft. SL: Writing – original draft. YM: Data curation, Investigation, Writing – review & editing. XL: Writing – original draft. ZC: Writing – original draft. YL: Writing – original draft. GD: Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (82574852); Research Project on Enhancing the Level of Clinical Evidence-Based Research in Traditional Chinese Medicine at Xiyuan Hospital of the China Academy of Chinese Medical Sciences (XYZX0201-02).
Acknowledgments
We would like to express our gratitude to the friends who provided help when writing this manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Savarese G, Becher PM, Lund LH, Seferovic P, Rosano GMC, Coats AJS. Global burden of heart failure: a comprehensive and updated review of epidemiology. Cardiovasc Res. (2023) 118:3272–87. doi: 10.1093/cvr/cvac013
2. Tsao CW, Aday AW, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, et al. Heart disease and stroke statistics-2023 update: a report from the American heart association. Circulation. (2023) 147:e93–e621. doi: 10.1161/CIR.0000000000001137
3. Wehbe RM, Khan SS, Shah SJ, Ahmad FS. Predicting high-risk patients and high-risk outcomes in heart failure. Heart Fail Clin. (2020) 16:387–407. doi: 10.1016/j.hfc.2020.05.002
4. Taha MB, Patel KV, Nasir K. Team-based strategies to prevent heart failure. Curr Opin Cardiol. (2022) 37:294–301. doi: 10.1097/HCO.0000000000000959
5. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. (2021) 42:3599–726. doi: 10.1093/eurheartj/ehab368
6. Meta-analysis Global Group in Chronic Heart Failure (MAGGIC). The survival of patients with heart failure with preserved or reduced left ventricular ejection fraction: an individual patient data meta-analysis. Eur Heart J. (2012) 33:1750–57. doi: 10.1093/eurheartj/ehr254
7. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. (2015) 162:777–84. doi: 10.7326/M14-2385
8. Moons KGM, Wolff RF, Riley RD, Whiting PF, Westwood M, Collins GS, et al. PROBAST: a tool to assess risk of bias and applicability of prediction model studies: explanation and elaboration. Ann Intern Med. (2019) 170:W1–W33. doi: 10.7326/M18-1377
9. Aaronson KD, Schwartz JS, Chen TM, Wong KL, Goin JE, Mancini DM. Development and prospective validation of a clinical index to predict survival in ambulatory patients referred for cardiac transplant evaluation. Circulation. (1997) 95:2660–7. doi: 10.1161/01.CIR.95.12.2660
10. Felker GM, Leimberger JD, Califf RM, Cuffe MS, Massie BM, Adams KF, et al. Risk stratification after hospitalization for decompensated heart failure. J Card Fail. (2004) 10:460–6. doi: 10.1016/j.cardfail.2004.02.011
11. Levy WC, Mozaffarian D, Linker DT, Sutradhar SC, Anker SD, Cropp AB, et al. The Seattle heart failure model: prediction of survival in heart failure. Circulation. (2006) 113:1424–33. doi: 10.1161/CIRCULATIONAHA.105.584102
12. O'Connor CM, Hasselblad V, Mehta RH, Tasissa G, Califf RM, Fiuzat M, et al. Triage after hospitalization with advanced heart failure: the ESCAPE (evaluation study of congestive heart failure and pulmonary artery catheterization effectiveness) risk model and discharge score. J Am Coll Cardiol. (2010) 55:872–8. doi: 10.1016/j.jacc.2009.08.083
13. Bilchick KC, Stukenborg GJ, Kamath S, Cheng A. Prediction of mortality in clinical practice for medicare patients undergoing defibrillator implantation for primary prevention of sudden cardiac death. J Am Coll Cardiol. (2012) 60:1647–55. doi: 10.1016/j.jacc.2012.07.028
14. Lupón J, de Antonio M, Vila J, Peñafiel J, Galán A, Zamora E, et al. Development of a novel heart failure risk tool: the Barcelona bio-heart failure risk calculator (BCN bio-HF calculator). PLoS ONE. (2014) 9:e85466. doi: 10.1371/journal.pone.0085466
15. Voors AA, Ouwerkerk W, Zannad F, van Veldhuisen DJ, Samani NJ, Ponikowski P, et al. Development and validation of multivariable models to predict mortality and hospitalization in patients with heart failure. Eur J Heart Fail. (2017) 19:627–34. doi: 10.1002/ejhf.785
16. Simpson J, Jhund PS, Lund LH, Padmanabhan S, Claggett BL, Shen L, et al. Prognostic models derived in PARADIGM-HF and validated in ATMOSPHERE and the Swedish heart failure registry to predict mortality and morbidity in chronic heart failure. JAMA Cardiol. (2020) 5:432–41. doi: 10.1001/jamacardio.2019.5850
17. Shen L, Claggett BL, Jhund PS, Abraham WT, Desai AS, Dickstein K, et al. Development and external validation of prognostic models to predict sudden and pump-failure death in patients with HFrEF from PARADIGM-HF and ATMOSPHERE. Clin Res Cardiol. (2021) 110:1334–49. doi: 10.1007/s00392-021-01888-x
18. Thorvaldsen T, Claggett BL, Shah A, Cheng S, Agarwal SK, Wruck LM, et al. Predicting risk in patients hospitalized for acute decompensated heart failure and preserved ejection fraction: the atherosclerosis risk in communities study heart failure community surveillance. Circ Heart Fail. (2017) 10:e003992. doi: 10.1161/CIRCHEARTFAILURE.117.003992
19. Kasahara S, Sakata Y, Nochioka K, Tay WT, Claggett BL, Abe R, et al. The 3A3B score: the simple risk score for heart failure with preserved ejection fraction - a report from the CHART-2 study. Int J Cardiol. (2019) 284:42–9. doi: 10.1016/j.ijcard.2018.10.076
20. Huttin O, Fraser AG, Lund LH, Donal E, Linde C, Kobayashi M, et al. Risk stratification with echocardiographic biomarkers in heart failure with preserved ejection fraction: the media echo score. ESC Heart Fail. (2021) 8:1827–39. doi: 10.1002/ehf2.13251
21. Shen L, Jhund PS, Anand IS, Carson PE, Desai AS, Granger CB, et al. Developing and validating models to predict sudden death and pump failure death in patients with heart failure and preserved ejection fraction. Clin Res Cardiol. (2021) 110:1234–48. doi: 10.1007/s00392-020-01786-8
22. Pocock SJ, Ferreira JP, Packer M, Zannad F, Filippatos G, Kondo T, et al. Biomarker-driven prognostic models in chronic heart failure with preserved ejection fraction: the EMPEROR-preserved trial. Eur J Heart Fail. (2022) 24:1869–78. doi: 10.1002/ejhf.2607
23. McDowell K, Kondo T, Talebi A, Teh K, Bachus E, de Boer RA, et al. Prognostic models for mortality and morbidity in heart failure with preserved ejection fraction. JAMA Cardiol. (2024) 9:457–65. doi: 10.1001/jamacardio.2024.0284
24. Zhu Y, Zhao W, Liu Z, Tan D, Tao L, He W, et al. Development and validation of a prediction model for all-cause death in heart failure patients with preserved ejection fraction: a single-centre cohort study in China. BMJ Open. (2025) 15:e094909. doi: 10.1136/bmjopen-2024-094909
25. Hu Y, Ma F, Hu M, Shi B, Pan D, Ren J. Development and validation of a machine learning model to predict the risk of readmission within one year in HFpEF patients: short title: prediction of HFpEF readmission. Int J Med Inf. (2025) 194:105703. doi: 10.1016/j.ijmedinf.2024.105703
26. Guo W, Tian J, Wang Y, Zhang Y, Yan J, Du Y, et al. Web-based dynamic nomogram for predicting risk of mortality in heart failure with mildly reduced ejection fraction. Risk Manag Healthc Policy. (2024) 17:1959–72. doi: 10.2147/RMHP.S474862
27. Lee DS, Austin PC, Rouleau JL, Liu PP, Naimark D, Tu JV. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. (2003) 290:2581–7. doi: 10.1001/jama.290.19.2581
28. Fonarow GC Adams KF Abraham WT Yancy CW Boscardin WJ ADHERE Scientific Advisory Committee Study Group and Investigators. Risk stratification for in-hospital mortality in acutely decompensated heart failure: classification and regression tree analysis. JAMA. (2005) 293:572–80. doi: 10.1001/jama.293.5.572
29. Pocock SJ, Wang D, Pfeffer MA, Yusuf S, McMurray JJV, Swedberg KB, et al. Predictors of mortality and morbidity in patients with chronic heart failure. Eur Heart J. (2006) 27:65–75. doi: 10.1093/eurheartj/ehi555
30. O'Connor CM, Abraham WT, Albert NM, Clare R, Gattis Stough W, Gheorghiade M, et al. Predictors of mortality after discharge in patients hospitalized with heart failure: An analysis from the organized program to initiate lifesaving treatment in hospitalized patients with heart failure (OPTIMIZE-HF). Am Heart J. (2008) 156:662–73. doi: 10.1016/j.ahj.2008.04.030
31. Peterson PN, Rumsfeld JS, Liang L, Albert NM, Hernandez AF, Peterson ED, et al. A validated risk score for in-hospital mortality in patients with heart failure from the American heart association get with the guidelines program. Circ Cardiovasc Qual Outcomes. (2010) 3:25–32. doi: 10.1161/CIRCOUTCOMES.109.854877
32. O'Connor CM, Mentz RJ, Cotter G, Metra M, Cleland JG, Davison BA, et al. The PROTECT in-hospital risk model: 7-day outcome in patients hospitalized with acute heart failure and renal dysfunction. Eur J Heart Fail. (2012) 14:605–12. doi: 10.1093/eurjhf/hfs029
33. Lee DS, Stitt A, Austin PC, Stukel TA, Schull MJ, Chong A, et al. Prediction of heart failure mortality in emergent care: a cohort study. Ann Intern Med. (2012) 156:767–75, W-261, W-262. doi: 10.7326/0003-4819-156-11-201206050-00003
34. Cowie MR, Sarkar S, Koehler J, Whellan DJ, Crossley GH, Tang WH, et al. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur Heart J. (2013) 34:2472–80. doi: 10.1093/eurheartj/eht083
35. Hummel SL, Ghalib HH, Ratz D, Koelling TM. Risk stratification for death and all-cause hospitalization in heart failure clinic outpatients. Am Heart J. (2013) 166:895–903.e1. doi: 10.1016/j.ahj.2013.09.002
36. Eapen ZJ, Liang L, Fonarow GC, Heidenreich PA, Curtis LH, Peterson ED, et al. Validated, electronic health record deployable prediction models for assessing patient risk of 30-day rehospitalization and mortality in older heart failure patients. JACC Heart Fail. (2013) 1:245–51. doi: 10.1016/j.jchf.2013.01.008
37. Pocock SJ, Ariti CA, McMurray JJV, Maggioni A, Køber L, Squire IB, et al. Predicting survival in heart failure: a risk score based on 39 372 patients from 30 studies. Eur Heart J. (2013) 34:1404–13. doi: 10.1093/eurheartj/ehs337
38. Senni M, Parrella P, De Maria R, Cottini C, Böhm M, Ponikowski P, et al. Predicting heart failure outcome from cardiac and comorbid conditions: the 3C-HF score. Int J Cardiol. (2013) 163:206–11. doi: 10.1016/j.ijcard.2011.10.071
39. Álvarez-García J, Ferrero-Gregori A, Puig T, Vázquez R, Delgado J, Pascual-Figal D, et al. A simple validated method for predicting the risk of hospitalization for worsening of heart failure in ambulatory patients: the redin-SCORE. Eur J Heart Fail. (2015) 17:818–27. doi: 10.1002/ejhf.287
40. Spinar J, Jarkovsky J, Spinarova L, Mebazaa A, Gayat E, Vitovec J, et al. AHEAD score–long-term risk classification in acute heart failure. Int J Cardiol. (2016) 202:21–6. doi: 10.1016/j.ijcard.2015.08.187
41. Miró Ò, Rossello X, Gil V, Martín-Sánchez FJ, Llorens P, Herrero-Puente P, et al. Predicting 30-day mortality for patients with acute heart failure in the emergency department: a cohort study. Ann Intern Med. (2017) 167:698–705. doi: 10.7326/M16-2726
42. Xanthopoulos A, Giamouzis G, Tryposkiadis K, Paraskevopoulou E, Paraskevopoulou P, Karagiannis G, et al. A simple score for early risk stratification in acute heart failure. Int J Cardiol. (2017) 230:248–54. doi: 10.1016/j.ijcard.2016.12.131
43. Yap J, Chia SY, Lim FY, Allen JC, Teo L, Sim D, et al. The Singapore heart failure risk score: prediction of survival in Southeast Asian patients. Ann Acad Med Singap. (2019) 48:86–94. doi: 10.47102/annals-acadmedsg.V48N3p86
44. García-Gutiérrez S, Antón-Ladislao A, Quiros R, Lara A, Rilo I, Morillas M, et al. Short-term mortality risk score for de novo acute heart failure (ESSIC-FEHF). Eur J Intern Med. (2020) 77:52–8. doi: 10.1016/j.ejim.2020.02.012
45. Adler ED, Voors AA, Klein L, Macheret F, Braun OO, Urey MA, et al. Improving risk prediction in heart failure using machine learning. Eur J Heart Fail. (2020) 22:139–47. doi: 10.1002/ejhf.1628
46. Weller J, Gutton J, Hocquet G, Pellet L, Aroulanda MJ, Bruandet A, et al. Prediction of 90 day mortality in elderly patients with acute HF from e-health records using artificial intelligence. ESC Heart Fail. (2025) 12:2200–9. doi: 10.1002/ehf2.15244
47. Zhao H-L, Gao X-L, Liu Y-H, Li S-L, Zhang Q, Shan W-C, et al. Validation and derivation of short-term prognostic risk score in acute decompensated heart failure in China. BMC Cardiovasc Disord. (2022) 22:307. doi: 10.1186/s12872-022-02743-1
48. AbouEzzeddine OF, French B, Mirzoyev SA, Jaffe AS, Levy WC, Fang JC, et al. From statistical significance to clinical relevance: a simple algorithm to integrate brain natriuretic peptide and the seattle heart failure model for risk stratification in heart failure. J Heart Lung Transplant. (2016) 35:714–21. doi: 10.1016/j.healun.2016.01.016
49. Kalogeropoulos AP, Georgiopoulou VV, Giamouzis G, Smith AL, Agha SA, Waheed S, et al. Utility of the Seattle heart failure model in patients with advanced heart failure. J Am Coll Cardiol. (2009) 53:334–42. doi: 10.1016/j.jacc.2008.10.023
50. Li S, Marcus P, Núñez J, Núñez E, Sanchis J, Levy WC. Validity of the Seattle heart failure model after heart failure hospitalization. ESC Heart Fail. (2019) 6:509–15. doi: 10.1002/ehf2.12427
51. Lupón J, Simpson J, McMurray JJV, de Antonio M, Vila J, Subirana I., et al. Barcelona bio-HF calculator version 20: Incorporation of angiotensin II receptor blocker neprilysin inhibitor (ARNI) and risk for heart failure hospitalization. Eur J Heart Fail. (2018) 20:938–40. doi: 10.1002/ejhf.949
52. Codina P, Lupón J, Borrellas A, Spitaleri G, Cediel G, Domingo M, et al. Head-to-head comparison of contemporary heart failure risk scores. Eur J Heart Fail. (2021) 23:2035–44. doi: 10.1002/ejhf.2352
53. Gardner RS, Ozalp F, Murday AJ, Robb SD, McDonagh TA, N-terminal pro-brain natriuretic peptide. A new gold standard in predicting mortality in patients with advanced heart failure. Eur Heart J. (2003) 24:1735–43. doi: 10.1016/j.ehj.2003.07.005
54. Szczurek W, Gasior M, Skrzypek M, Szyguła-Jurkiewicz B. Apelin improves prognostic value of HFSS (heart failure survival score) and MAGGIC (meta-analysis global group in chronic heart failure) scales in ambulatory patients with end-stage heart failure. J Clin Med. (2020) 9:2300. doi: 10.3390/jcm9072300
55. Goda A, Lund LH, Mancini D. The heart failure survival score outperforms the peak oxygen consumption for heart transplantation selection in the era of device therapy. J Heart Lung Transplant. (2011) 30:315–25. doi: 10.1016/j.healun.2010.09.007
56. Koelling TM, Joseph S, Aaronson KD. Heart failure survival score continues to predict clinical outcomes in patients with heart failure receiving beta-blockers. J Heart Lung Transplant. (2004) 23:1414–22. doi: 10.1016/j.healun.2003.10.002
57. Odajima S, Fujimoto W, Takegami M, Nishimura K, Iwasaki M, Okuda M, et al. BEEAF2 score: a new risk stratification score for patients with stage B heart failure from the KUNIUMI registry chronic cohort. J Am Heart Assoc. (2024) 13:e034793. doi: 10.1161/JAHA.124.034793
58. Potter E, Huynh Q, Haji K, Wong C, Yang H, Wright L, et al. Use of clinical and echocardiographic evaluation to assess the risk of heart failure. JACC Heart Fail. (2024) 12:275–86. doi: 10.1016/j.jchf.2023.06.014
59. Sawano M, Shiraishi Y, Kohsaka S, Nagai T, Goda A, Mizuno A, et al. Performance of the MAGGIC heart failure risk score and its modification with the addition of discharge natriuretic peptides. ESC Heart Fail. (2018) 5:610–9. doi: 10.1002/ehf2.12278
60. Chirinos JA, Orlenko A, Zhao L, Basso MD, Cvijic ME Li Z, et al. Multiple plasma biomarkers for risk stratification in patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. (2020) 75:1281–95. doi: 10.1016/j.jacc.2019.12.069
61. Nagai T, Nakao M, Anzai T. Risk stratification towards precision medicine in heart failure- current progress and future perspectives. Circ J. (2021) 85:576–83. doi: 10.1253/circj.CJ-20-1299
62. Oh JK, Hatle L, Tajik AJ, Little WC. Diastolic heart failure can be diagnosed by comprehensive two-dimensional and Doppler echocardiography. J Am Coll Cardiol. (2006) 47:500–6. doi: 10.1016/j.jacc.2005.09.032
63. Chopra HK. Diastolic heart failure: a clinical challenge early recognition & timely intervention is the need of the hour. Indian Heart J. (2009) 61:138–45.
64. McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European society of cardiology. Developed in collaboration with the heart failure association (HFA) of the ESC. Eur Heart J. (2012) 33:1787–847. doi: 10.1093/eurheartj/ehs104
65. Writing Committee Members, Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American college of cardiology foundation/American heart association task force on practice guidelines. Circulation. (2013) 128:e240–327. doi: 10.1161/CIR.0b013e31829e8776
66. Valsaraj A, Kalmady SV, Sharma V, Frost M, Sun W, Sepehrvand N, et al. Development and validation of echocardiography-based machine-learning models to predict mortality. Ebiomedicine. (2023) 90:104479. doi: 10.1016/j.ebiom.2023.104479
67. Singh G, Bamba H, Inban P, Chandrasekaran SH, Priyatha V, John J, et al. The role of biomarkers in the prognosis and risk stratification in heart failure: a systematic review. Dis-a-mon: DM. (2024) 70:101782. doi: 10.1016/j.disamonth.2024.101782
68. Ferreira JP, Packer M, Butler J, Filippatos G, Pocock SJ, Januzzi JL, et al. Growth differentiation factor-15 and the effect of Empagliflozin in heart failure: Findings from the EMPEROR program. Eur J Heart Fail. (2024) 26:155–64. doi: 10.1002/ejhf.3078
69. Masson S, Batkai S, Beermann J, Bär C, Pfanne A, Thum S, et al. Circulating microRNA-132 levels improve risk prediction for heart failure hospitalization in patients with chronic heart failure. Eur J Heart Fail. (2018) 20:78–85. doi: 10.1002/ejhf.961
70. Ky B, French B, Levy WC, Sweitzer NK, Fang JC, Wu AHB, et al. Multiple biomarkers for risk prediction in chronic heart failure. Circ, Heart Fail. (2012) 5:183–90. doi: 10.1161/CIRCHEARTFAILURE.111.965020
71. Pérez-Sanz TM, Gómez-Salvador I, Codina P, Calvo Antón B., de la Torre Carpente MM, Redondo Bermejo B, et al. Soluble ST2, BCN-bio-HF calculator and MAGGIC-HF score in long-term risk prediction after an urgent visit for heart failure. Heart Vessels. (2024) 39:216–25. doi: 10.1007/s00380-023-02327-9
72. Ma F, Hu Y, Han P, Qiu Y, Liu Y, Ren J. Machine learning-driven prediction of one-year readmission in HFrEF patients: the key role of inflammation. Clin Interv Aging. (2025) 20:1071–84. doi: 10.2147/CIA.S528442
73. Zhou M, Du X. Inflammation-driven prognosis in advanced heart failure: a machine learning-based risk prediction model for one-year mortality. J Inflamm Res. (2025) 18:5047–60. doi: 10.2147/JIR.S514192
74. Kuku KO, Shearer JJ, Hashemian M, Oyetoro R, Park H, Dulek B, et al. Development and validation of a protein risk score for mortality in heart failure : a community cohort study. Ann Intern Med. (2024) 177:39–49. doi: 10.7326/M23-2328
75. Harris E. Protein score may reclassify death risk in patients with heart failure. JAMA. (2024) 331:467. doi: 10.1001/jama.2023.27971
76. Oexner RR, Ahn H, Theofilatos K, Shah RA, Schmitt R, Chowienczyk P, et al. Serum metabolomics improves risk stratification for incident heart failure. Eur J Heart Fail. (2024) 26:829–40. doi: 10.1002/ejhf.3226
77. Joo J, Shearer JJ, Wolska A, Remaley AT, Otvos JD, Connelly MA, et al. Incremental value of a metabolic risk score for heart failure mortality: a population-based study. Circ, Genom Precis Med. (2024) 17:e004312. doi: 10.1161/CIRCGEN.123.004312
78. Gao Y, Chen B, Han Y, Lu J, Li X, Tian A, et al. Prognostic value of a multi-mRNA signature for 1-year all-cause death in hospitalized patients with heart failure with a preserved ejection fraction. Circ, Heart Fail. (2024) 17:e011118. doi: 10.1161/CIRCHEARTFAILURE.123.011118
79. Predictive Modeling of Heart Failure Outcomes Using ECG Monitoring Indicators and Machine Learning - PubMed. Available online at: https://pubmed.ncbi.nlm.nih.gov/40579382/ (Accessed October 11, 2025).
80. Jia H, Liao S, Zhu X, Liu W, Xu Y, Ge R, et al. Deep learning prediction of survival in patients with heart failure using chest radiographs. Int J Cardiovasc Imaging. (2024) 40:1891–901. doi: 10.1007/s10554-024-03177-w
81. The CHA2DS2-VASc Score for Risk Stratification of Stroke in Heart Failure with-vs-Without Atrial Fibrillation - PubMed. Available online at: https://pubmed.ncbi.nlm.nih.gov/34274114/ (Accessed October 11, 2025).
82. Sun Y, Wang N, Li X, Zhang Y, Yang J, Tse G, et al. Predictive value of H2 FPEF score in patients with heart failure with preserved ejection fraction. ESC Heart Fail. (2021) 8:1244–52. doi: 10.1002/ehf2.13187
83. Li T, Li Z, Guo S, Jiang S, Sun Q, Wu Y, et al. The value of using left ventricular pressure-strain loops to evaluate myocardial work in predicting heart failure with improved ejection fraction. Int J Cardiol. (2024) 394:131366. doi: 10.1016/j.ijcard.2023.131366
84. Cao L, Guo X, Liao K, Qin J, Zheng Y. A comprehensive nomogram integrating phonocardiogram and echocardiogram features for the diagnosis of heart failure with preserved ejection fraction. Clin Cardiol. (2024) 47:e70022. doi: 10.1002/clc.70022
85. Zile MR, Kahwash R, Sarkar S, Koehler J, Zielinski T, Mehra MR, et al. A novel heart failure diagnostic risk score using a minimally invasive subcutaneous insertable cardiac monitor. JACC Heart Fail. (2024) 12:182–96. doi: 10.1016/j.jchf.2023.09.014
86. Kahwash R, Zile MR, Chalasani P, Bertolet B, Gravelin L, Khan MS, et al. Personalized intervention strategy based on a risk score generated from subcutaneous insertable cardiac monitor: results from phase 1 of ALLEVIATE-HF. J Am Heart Assoc. (2024) 13:e035501. doi: 10.1161/JAHA.124.035501
87. Xu Q, Yu R, Cai X, Chen G, Zheng Y, Xu C, et al. Machine learning-based risk factor analysis and prediction model construction for mortality in chronic heart failure. J Glob Health. (2025) 15:4242. doi: 10.7189/jogh.15.04242
88. Sun Z, Wang Z, Yun Z, Sun X, Lin J, Zhang X, et al. Machine learning-based model for worsening heart failure risk in chinese chronic heart failure patients. ESC Heart Fail. (2025) 12:211–28. doi: 10.1002/ehf2.15066
89. Shang S, Wei M, Lv H, Liang X, Lu Y, Tang B. Construction and validation of a hospital mortality risk model for advanced elderly patients with heart failure based on machine learning. Int J Gen Med. (2025) 18:3277–88. doi: 10.2147/IJGM.S514972
90. Jawadi Z, He R, Srivastava PK, Fonarow GC, Khalil SO, Krishnan S, et al. Predicting in-hospital mortality among patients admitted with a diagnosis of heart failure: a machine learning approach. ESC Heart Fail. (2024) 11:2490–8. doi: 10.1002/ehf2.14796
91. Abdul-Samad K, Ma S, Austin DE, Chong A, Wang CX, Wang X, et al. Comparison of machine learning and conventional statistical modeling for predicting readmission following acute heart failure hospitalization. Am Heart J. (2024) 277:93–103. doi: 10.1016/j.ahj.2024.07.017
92. Abou Alaiwi S, Samsky MD, Ahmad T. Is there a role for risk scores in the management of heart failure? JACC Heart Fail. (2024) 12:197–8. doi: 10.1016/j.jchf.2023.10.007
93. Wessler BS, Ruthazer R, Udelson JE, Gheorghiade M, Zannad F, Maggioni A, et al. Regional validation and recalibration of clinical predictive models for patients with acute heart failure. J Am Heart Assoc. (2017) 6:e006121. doi: 10.1161/JAHA.117.006121
94. Lupón J, Januzzi JL, de Antonio M, Vila J, Peñafilel J, Bayes-Genis A. Validation of the Barcelona bio-heart failure risk calculator in a cohort from boston. Rev Esp Cardiol. (2015) 68:80–1. doi: 10.1016/j.rec.2014.08.009
95. Coiro S, Huttin O, Kobayashi M, Lamiral Z, Simonovic D, Zannad F, et al. Validation of the MEDIA echo score for the prognosis of heart failure with preserved ejection fraction. Heart Fail Rev. (2023) 28:453–64. doi: 10.1007/s10741-022-10266-2
96. Canepa M, Fonseca C, Chioncel O, Laroche C, Crespo-Leiro MG, Coats AJS, et al. Performance of prognostic risk scores in chronic heart failure patients enrolled in the European Society of Cardiology Heart Failure Long-Term Registry. JACC Heart Fail. (2018) 6:452–62.
97. Lagu T, Pekow PS, Shieh M-S, Stefan M, Pack QR, Kashef MA, et al. Validation and comparison of seven mortality prediction models for hospitalized patients with acute decompensated heart failure. Circ Heart Fail. (2016) 9:e002912. doi: 10.1161/CIRCHEARTFAILURE.115.002912
98. Garg N, Pekmezaris R, Stevens G, Becerra AZ, Kozikowski A, Patel V, et al. Performance of emergency heart failure mortality risk grade in the emergency department. West J Emerg Med. (2021) 22:672–7. doi: 10.5811/westjem.2021.1.48978
99. Sepehrvand N, Youngson E, Bakal JA, McAlister FA, Rowe BH, Ezekowitz JA. External validation and refinement of emergency heart failure mortality risk grade risk model in patients with heart failure in the emergency department. CJC Open. (2019) 1:123–30. doi: 10.1016/j.cjco.2019.03.003
100. Cheng Y, Chai K, Zhu W, Wan Y, Liang Y, Du M, et al. Performance of prognostic risk scores in elderly Chinese patients with heart failure. Clin Interv Aging. (2021) 16:1669–77. doi: 10.2147/CIA.S323979
Keywords: heart failure, heart failure with mildly reduced ejection fraction, heart failure with preserved ejection fraction, heart failure with reduced ejection fraction, prediction model
Citation: Wei Y, Liu S, Mu Y, Liang X, Chen Z, Liu Y and Dong G (2025) Prediction models and risk scores in different types of heart failure: a review. Front. Med. 12:1652307. doi: 10.3389/fmed.2025.1652307
Received: 10 July 2025; Accepted: 24 October 2025;
Published: 21 November 2025.
Edited by:
Zhengwu Sun, Dalian Municipal Central Hospital, ChinaReviewed by:
Wu Guanlin, Dalian Municipal Central Hospital, ChinaKun Lu, LMU Munich University Hospital, Germany
Copyright © 2025 Wei, Liu, Mu, Liang, Chen, Liu and Dong. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guoju Dong, MTM2OTEzOTM1ODlAMTYzLmNvbQ==
 Yue Wei1
Yue Wei1 Xiaoyu Liang
Xiaoyu Liang Ziyi Chen
Ziyi Chen Guoju Dong
Guoju Dong