Abstract
Introduction:
Ixekizumab is a biologic agent primarily indicated for the treatment of moderate to-severe plaque psoriasis. This study aimed to evaluate the post-marketing safety profile of ixekizumab by analyzing adverse event (AE) reports retrieved from the Food and Drug Administration Adverse Event Reporting System (FAERS) database and VigiAccess databases.
Methods:
Four disproportionality analysis methods were employed in this study to detect positive signals associated with ixekizumab, including Reporting Odds Ratio (ROR), Proportional Reporting Ratio (PRR), Bayesian Confidence Propagation Neural Network (BCPNN), and Multi-item Gamma Poisson Shrinker (MGPS). Sensitivity analyses were conducted to ensure the robustness of the findings. Additionally, the time to onset of AEs was further analyzed.
Results:
In the FAERS databases and VigiAccess databases, 72,847 AE reports in total. Commonly reported AEs included injection site reactions, hypersensitivity reactions, fungal infections, upper respiratory tract infections, and inflammatory bowel disease. In addition, several unexpected AEs were identified, such as cellulitis, ear infection, bronchitis, herpes zoster, tooth infection, diverticulitis, kidney infection, and gastroenteritis viral. Sensitivity analysis further confirmed the robustness of these findings. Notably, 41.1% of the AEs occurred within the first month after treatment initiation.
Discussion:
This study confirmed several known AEs and identified some unexpected AEs, providing preliminary safety insights to guide clinicians in the safe use of ixekizumab in clinical practice. It is important to note that findings from spontaneous adverse event reporting systems are hypothesis-generating and may be limited by underreporting, variable reporting quality, and confounding factors.
1 Introduction
Psoriasis, a chronic inflammatory dermatological condition, impacts approximately 125 million people globally (1). It is characterized by extensive erythematous plaques and scales on the skin, frequently accompanied by other chronic disorders such as arthritis, hypertension, diabetes, and obesity (2). Psoriatic arthritis is a heterogeneous, chronic inflammatory musculoskeletal disorder affecting approximately 30% of individuals with psoriasis. Notably, more than half of psoriatic arthritis patients develop a progressive and erosive form of the disease, which ultimately leads to functional impairment (3, 4). Previous research has already linked psoriasis and psoriatic arthritis with significant psychological burdens, including depression, unhealthy risk behaviors, and negative body image (5). Research indicates that psoriasis and psoriatic arthritis share a common underlying pathophysiological mechanism involving the disorder of the immune system, particularly the impact of cytokines such as IL-17 (2, 3, 6). Psoriatic lesions are characterized by the infiltration of a substantial number of inflammatory cells, and the IL-17 signaling pathway is pivotal in the disease’s pathogenesis (6).
Ixekizumab (IXE) is a monoclonal antibody that selectively targets IL-17A, an inflammatory cytokine that plays a critical role in the pathogenesis of psoriasis. The drug received approval from the FDA for the treatment of psoriasis in 2016, followed by its approval for psoriatic arthritis in 2017 (7). Various clinical trials have confirmed the efficacy and safety of IXE for the treatment of psoriasis (8, 9). Data from multiple clinical trials indicate that IXE treatment demonstrates good long-term tolerability in adult patients with plaque psoriasis, psoriatic arthritis, and axial spondyloarthritis. Numerous randomized clinical trials and real-world studies have consistently demonstrated that ixekizumab (IXE) is both effective and safe for the treatment of psoriasis, with the majority of associated adverse events being mild to moderate in severity. Additionally, the incidences of serious AE and discontinuations due to AEs were low, with these rates remaining consistently low throughout the five-year period (9–11). However, as the use of IXE increases across diverse patient populations, with greater variability in dosing frequency and duration, and considering that psoriasis typically requires long-term treatment, identifying unexpected rare or serious AEs, late complications, and previously unassessed occurrences is crucial.
FAERS is a powerful database that collects and monitors AE reports related to drugs and biologics. FAERS provides a valuable resource for assessing the safety of medications in a broader patient population, especially those who were not included in clinical trials. Due to its extensive and accessible real-world data, an increasing number of researchers have used the FAERS database to assess the safety of drugs (12, 13). VigiAccess, a global pharmacovigilance database managed by the World Health Organization, compiles AE reports from a wide range of countries and regions, offering a comprehensive, worldwide view of drug safety data (14). This study aimed to evaluate the real-world safety profile of IXE by mining drug AEs reported in the VigiAccess and FAERS databases, with a focus on identifying unexpected safety signals and providing preliminary safety data for the long-term use of this drug.
2 Methods
2.1 Data source and study design
The data for this study were sourced from the VigiAccess database, encompassing its entire history up until 8 September 2025, as well as the FAERS database, covering the period from the first quarter of 2016 to the third quarter of 2024.
FAERS database aggregates spontaneous AE reports from healthcare providers, patients, and manufacturers. The specific information categories are classified as follows: demographic and administrative information (DEMO), drug information (DRUG), adverse drug reaction information (REAC), patient outcome information (OUTC), reporting source information (RPSR), date of treatment initiation and end date of reported medication (THER), and medication administration indications (INDI). The seven datasets were merged using the primary parameter to facilitate further analysis.
We removed duplicate reports from the data using Case Identifiers (CASEIDs), FDA Receipt Date (FDA_DT), and Primary Identifiers (PRIMARYID). In accordance with FDA recommended practices, when two reports share the same CASEID, the report with the later FDA_DT is retained; when both CASEID and FDA_DT match, the report with the highest PRIMARYID is retained. Deduplication of AE reports prior to disproportionality analysis is crucial for ensuring the accuracy of the analysis. Additionally, we excluded irrelevant AEs, such as those unrelated to the drug (e.g., drug ineffectiveness and treatment discontinuation) or events inherently associated with the disease itself (e.g., psoriasis, psoriatic arthritis, erythema). The details of data identification, extraction, and processing are depicted in Figure 1.
Figure 1
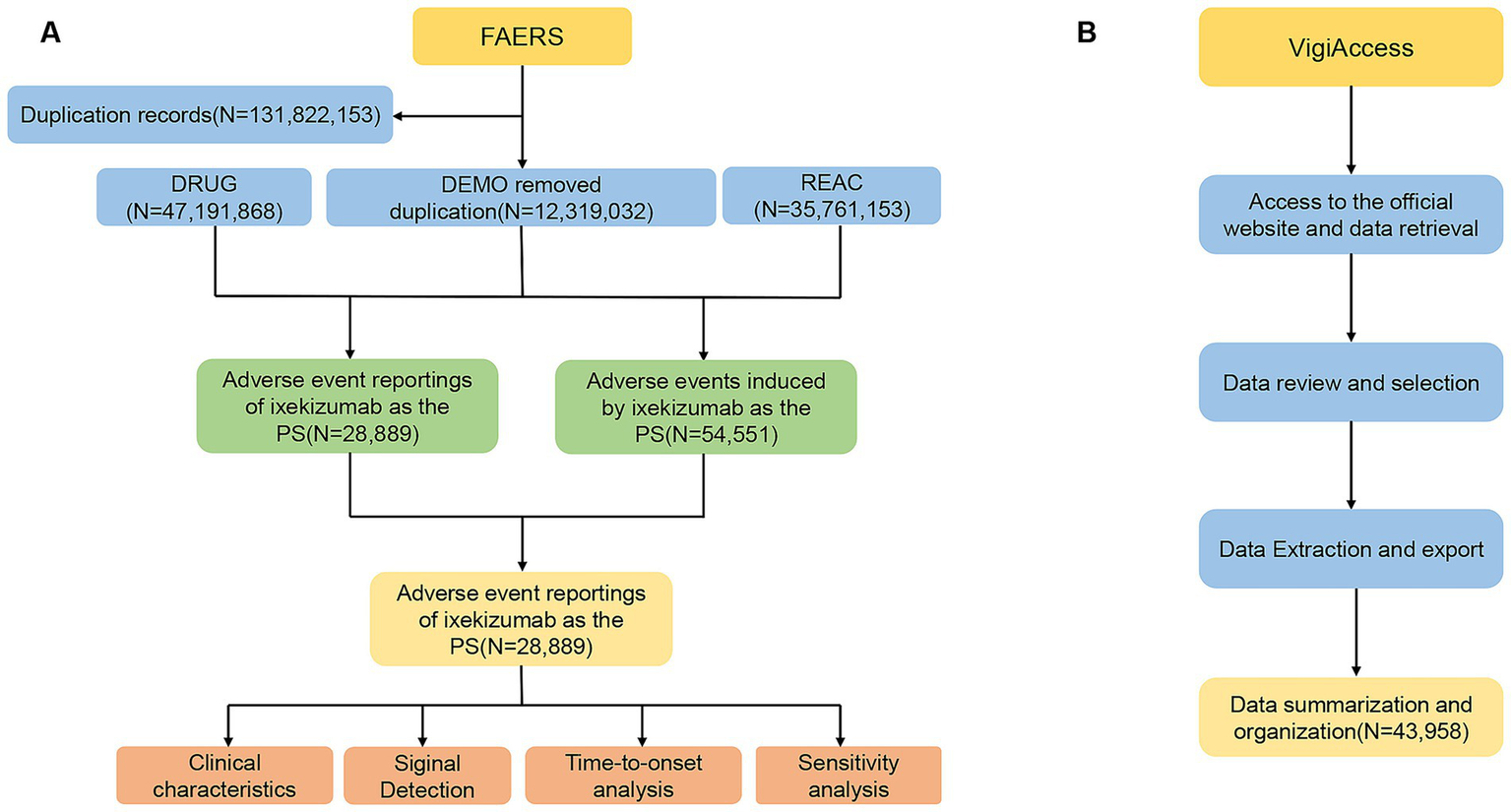
Flow diagram of the selection process for ixekizumab-related adverse events from FAERS and VigiAccess. (A) Data identification, extraction, and processing in FAERS. (B) Data identification, extraction, and processing in VigiAccess.
Furthermore, VigiAccess data were collected from https://www.vigiaccess.org, encompassing various age groups, genders, reporting years, and geographic regions. Searches were conducted using generic names, and AEs were described according to the System Organ Class (SOC) and Preferred Term (PT) of the Medical Dictionary for Regulatory Activities (MedDRA, version 27.1). This approach aims to enhance the credibility of the association between IXE and AEs.
2.2 Statistical analysis
We employed disproportionality analysis methods to quantify unexpected signals of IXE-associated AEs. The specific algorithms included ROR, PRR, BCPNN, and MGPS (15–17). We define a positive signal as meeting the criteria for positive according to all four algorithms. Supplementary Tables 1, 2 provide the criteria for positive signals and equations used for the aforementioned algorithms. We also conducted a sensitivity analysis by excluding the drugs most commonly co-administered with IXE. Additionally, we used the Weibull distribution to analyze changes in AE incidence over time and evaluated the time-to-event onset comprehensively using median, quartiles, and Weibull testing. The Weibull distribution include shape parameter (β) and scale parameter (α), which can further explain the trend of AEs and describe the characteristic time of occurrence. All analyses were performed using R software version 4.2.2.
3 Results
3.1 Baseline characteristics
In this study, the FAERS and VigiAccess databases reported 28,889 and 43,958 AE reports, respectively, associated with IXE, for which IXE was considered the primary suspected drug responsible for the adverse reactions. The demographic characteristics are shown in Table 1. Among the AE reports, females accounted for a higher proportion than males in both databases. With respect to age distribution, the 18–65 years age group represented the largest demographic, comprising 41.6% of reported cases in the FAERS database and 48.7% in the VigiAccess database. In both databases, the majority of reports originated from the Americas.
Table 1
| Characteristics | FAERS | VigiAccess |
|---|---|---|
| Number of event reports | 28,889 | 43,958 |
| n (%) | ||
| Gender | ||
| Male | 10,400(36%) | 16,527(37.6%) |
| Female | 16,050(55.6%) | 24,279(55.2%) |
| Miss | 2,439(8.4%) | 3,152(7.2%) |
| Age(years) | ||
| Median (IQR) | 53(42,62) | |
| <18 | 255(0.9%) | 364(0.8%) |
| 18–64 | 12,004(41.6%) | 21,416(48.7%) |
| ≥65 | 2,522(8.7%) | 4,338(9.7%) |
| Miss | 14,108(48.8%) | 17,920(40.8%) |
| Reporting year | ||
| 2016 | 360(1.2%) | 19(0.04%) |
| 2017 | 2020(7.0%) | 1,601(3.64%) |
| 2018 | 3,583(12.4%) | 3,678(8.37) |
| 2019 | 4,487(15.5%) | 7,373(16.77%) |
| 2020 | 3,138(10.9%) | 4,149(9.44%) |
| 2021 | 3,598(12.5%) | 5,156(11.73%) |
| 2022 | 4,529(15.7%) | 6,713(15.27%) |
| 2023 | 4,036(14%) | 6,322(14.38%) |
| 2024 | 3,138(10.9%) | 6,082(13.84%) |
| 2025 | 2,865(6.52%) | |
| Geographical distribution | ||
| Americas | 27,081(93.7%) | 28,652(65.2%) |
| Europe | 439(1.5%) | 12,375(28.1%) |
| Asia | 181(0.6%) | 2,509(5.7%) |
| Africa | 255(0.6%) | |
| Oceania | 167(0.4%) | |
| Miss | 1,188(4.2%) | |
| Reporter | ||
| Consumer | 15,673(54.3) | |
| Healthcare professional | 3,434(11.9%) | |
| Other health-professional | 3,112(10.8%) | |
| Physician | 1822(6.3%) | |
| Pharmacist | 1,559(5.4%) | |
| Lawyer | 1 | |
| Missing | 3,288(11.4%) | |
Clinical characteristics of IXE adverse event reports from the FAERS and VigiAccess database.
IQR, interquartile range.
3.2 Distribution of AEs at the system organ class level
Table 2 shows the distribution and signal values of IXE-related AEs at the SOC level. Among the IXE-induced AEs, “infections and infestations,” “general disorders and administration site conditions,” and “surgical and medical procedures” met the criteria of all four algorithms. “Skin and subcutaneous tissue disorders” and “immune system disorders” met the criteria of the ROR and BCPNN algorithms. The distribution of AEs at the SOC level is shown in Figure 2. In the VigiAccess database, the top 5 SOCs for IXE are General disorders and administration site conditions (31.0%), Infections and infestations (13.4%), Skin and subcutaneous tissue disorders (13.2%), Injury, poisoning and procedural complications (8.9%), Gastrointestinal disorder (5.9%), with detailed results presented in Supplementary Table 3. In summary, when AE signals for IXE are categorized by SOC, General disorders and administration site conditions, Infections and infestations, and Skin and subcutaneous tissue disorders consistently emerge as the top three SOC categories for the drug in both the FAERS and VigiAccess databases.
Table 2
| SOC | Case numbers | ROR(95%CI) | PRR(χ2) | EBGM(EBGM05) | IC(IC025) |
|---|---|---|---|---|---|
| Infections and infestations* | 6,712 | 2.42 (2.36–2.48) | 2.24 (4882.58) | 2.24 (2.19) | 1.16 (1.13) |
| General disorders and administration site conditions* | 19,940 | 2.66 (2.61–2.7) | 2.05 (13022.97) | 2.05 (2.02) | 1.03 (1.01) |
| Investigations | 1,188 | 0.36 (0.34–0.38) | 0.38 (1307.97) | 0.38 (0.36) | −1.41 (−1.5) |
| Respiratory, thoracic and mediastinal disorders | 1,379 | 0.53 (0.51–0.56) | 0.55 (543.34) | 0.55 (0.52) | −0.87 (−0.95) |
| Skin and subcutaneous tissue disorders* | 5,777 | 1.95 (1.89–2) | 1.85 (2367.2) | 1.84 (1.8) | 0.88 (0.84) |
| Gastrointestinal disorders | 3,419 | 0.74 (0.72–0.77) | 0.76 (288.82) | 0.76 (0.74) | −0.4 (−0.45) |
| Immune system disorders* | 818 | 1.23 (1.15–1.32) | 1.23 (34.21) | 1.22 (1.16) | 0.29 (0.19) |
| Blood and lymphatic system disorders | 206 | 0.22 (0.19–0.26) | 0.23 (554.33) | 0.23 (0.2) | −2.14 (−2.34) |
| Nervous system disorders | 1,696 | 0.38 (0.36–0.4) | 0.4 (1673.1) | 0.4 (0.38) | −1.33 (−1.4) |
| Musculoskeletal and connective tissue disorders | 2,341 | 0.83 (0.79–0.86) | 0.83 (81.44) | 0.83 (0.81) | −0.26 (−0.32) |
| Injury, poisoning and procedural complications | 4,771 | 0.75 (0.73–0.77) | 0.77 (371.3) | 0.77 (0.75) | −0.38 (−0.42) |
| Psychiatric disorders | 803 | 0.26 (0.25–0.28) | 0.27 (1623.52) | 0.28 (0.26) | −1.86 (−1.96) |
| Social circumstances | 85 | 0.34 (0.28–0.43) | 0.35 (105.63) | 0.35 (0.29) | −1.53(−1.84) |
| Eye disorders | 462 | 0.42 (0.38–0.46) | 0.43 (363.42) | 0.43 (0.4) | −1.23(−1.36) |
| Hepatobiliary disorders | 178 | 0.39 (0.34–0.45) | 0.39 (169.36) | 0.39 (0.35) | −1.35(−1.57) |
| Metabolism and nutrition disorders | 326 | 0.29 (0.26–0.32) | 0.29 (562.35) | 0.29 (0.27) | −1.76(−1.92) |
| Cardiac disorders | 437 | 0.38 (0.35–0.42) | 0.39 (430.78) | 0.39 (0.36) | −1.36 (−1.5) |
| Vascular disorders | 382 | 0.36 (0.32–0.4) | 0.36 (434.77) | 0.36 (0.33) | −1.46(−1.61) |
| Product issues | 303 | 0.3 (0.27–0.34) | 0.3 (489.36) | 0.31 (0.28) | −1.71(−1.88) |
| Surgical and medical procedures* | 1,834 | 2.39 (2.28–2.5) | 2.34 (1424.74) | 2.34 (2.25) | 1.22 (1.16) |
| Renal and urinary disorders | 342 | 0.31 (0.28–0.35) | 0.32 (510.66) | 0.32 (0.29) | −1.65(−1.81) |
| Reproductive system and breast disorders | 128 | 0.33 (0.28–0.39) | 0.33 (175.04) | 0.33 (0.29) | −1.6 (−1.85) |
| Neoplasms benign, malignant and unspecified (incl cysts and polyps) | 753 | 0.44 (0.41–0.47) | 0.45 (531.47) | 0.45 (0.42) | −1.16(−1.27) |
| Ear and labyrinth disorders | 184 | 0.77 (0.67–0.89) | 0.77 (12.6) | 0.77 (0.68) | −0.38(−0.59) |
| Endocrine disorders | 25 | 0.17 (0.12–0.26) | 0.17 (98.06) | 0.17 (0.13) | −2.52(−3.09) |
| Congenital, familial and genetic disorders | 14 | 0.09 (0.06–0.16) | 0.09 (122.33) | 0.09 (0.06) | −3.41(−4.15) |
| Pregnancy, puerperium and perinatal conditions | 48 | 0.23 (0.17–0.3) | 0.23 (126.78) | 0.23 (0.18) | −2.14 (−2.55) |
Signal strength of ixekizumab AEs across system organ classes (SOC) in the FAERS database.
Asterisks (*) indicate statistically significant signals in algorithm; ROR, reporting odds ratio; PRR, proportional reporting ratio; EBGM, empirical Bayesian geometric mean; EBGM05, the lower limit of the 95% CI of EBGM; IC, information component; IC025, the lower limit of the 95% CI of the IC; CI, confidence interval; AEs, adverse events.
Figure 2
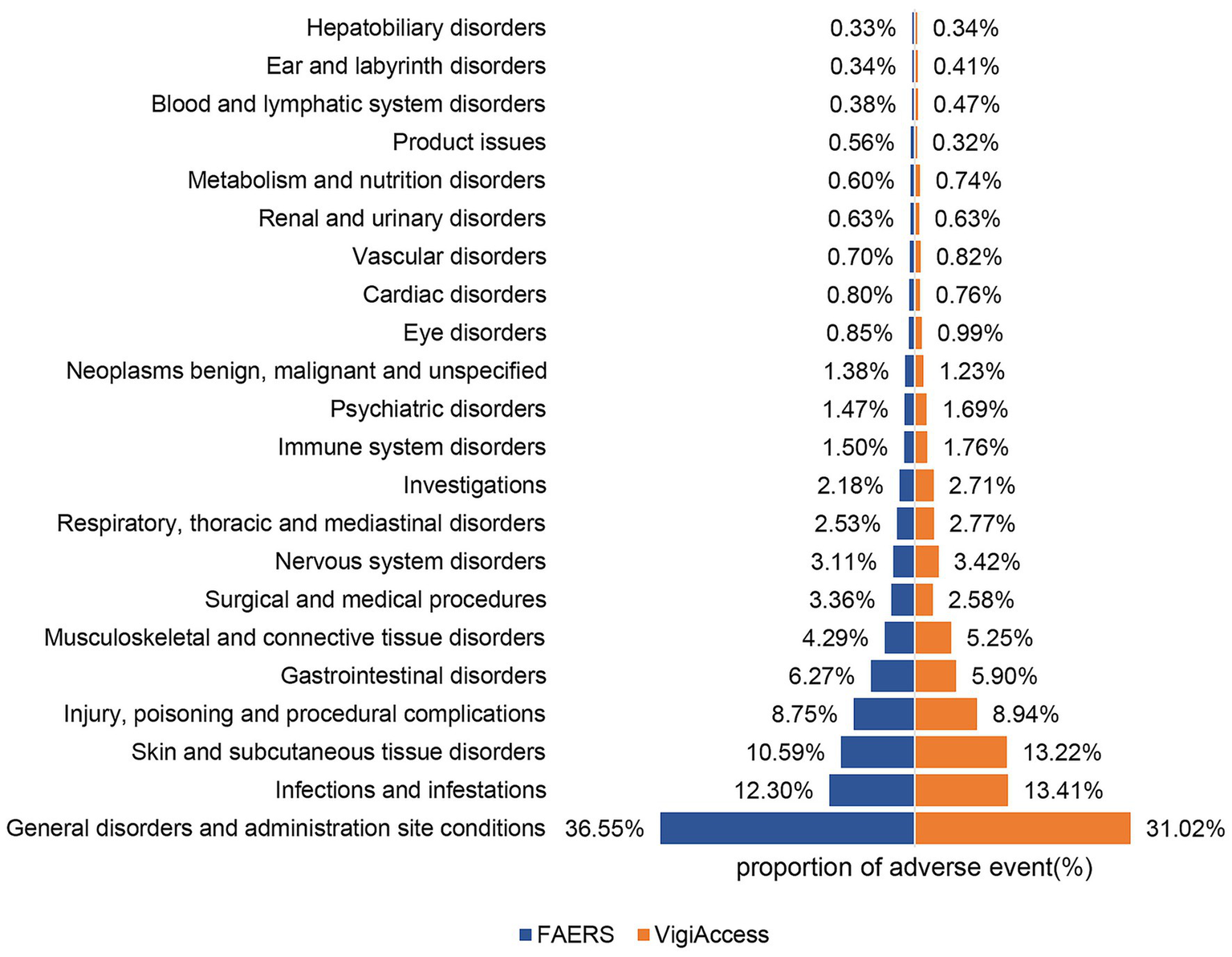
Proportions of AEs by system organ class for ixekizumab based on data from FAERS and VigiAccess.
3.3 Distribution of AEs at the PT level
We further analyzed all signals at the PT level, focusing on PTs with a count of ≥45 with the highest signal strength and the top 70 most frequent PTs detected in both the FAERS and VigiAccess databases, as listed in Table 3 and Supplementary Table 4.
Table 3
| PT | Case numbers | ROR(95%Cl) | PRR(χ2) | EBGM(EBGM05) | IC(IC025) |
|---|---|---|---|---|---|
| Injection site pain | 3,581 | 16.05(15.51–16.61) | 15.06 (46163.17) | 14.75 (14.33) | 3.88 (3.83) |
| Injection site erythema | 2,014 | 26.19(25.03–27.41) | 25.26 (45249.64) | 24.36 (23.45) | 4.61 (4.54) |
| Injection site swelling | 1,519 | 28.29 (26.86–29.8) | 27.53 (37306.84) | 26.46 (25.33) | 4.73 (4.65) |
| Injection site reaction | 1,459 | 30.4 (28.83–32.06) | 29.62 (38626.91) | 28.37 (27.14) | 4.83 (4.75) |
| Injection site pruritus | 695 | 14.49(13.44–15.63) | 14.32 (8434.19) | 14.03 (13.17) | 3.81 (3.7) |
| Injection site urticaria | 606 | 32.49(29.93–35.27) | 32.14 (17432.15) | 30.68 (28.64) | 4.94 (4.82) |
| Injection site mass | 549 | 15.18(13.94–16.52) | 15.03 (7034.93) | 14.72 (13.71) | 3.88 (3.75) |
| Injection site hemorrhage | 512 | 7.79 (7.13–8.5) | 7.72 (2964.55) | 7.64 (7.1) | 2.93 (2.81) |
| Nasopharyngitis | 475 | 2.81 (2.56–3.07) | 2.79 (545.06) | 2.78 (2.58) | 1.48 (1.34) |
| Injection site rash | 468 | 20.43(18.63–22.41) | 20.27 (8317.37) | 19.69 (18.22) | 4.3 (4.16) |
| Injection site warmth | 451 | 38.5 (35–42.35) | 38.19 (15,434) | 36.13 (33.36) | 5.18 (5.04) |
| Urticaria | 436 | 3.12 (2.84–3.43) | 3.1 (618.94) | 3.09 (2.86) | 1.63 (1.49) |
| Injection site bruising | 415 | 6.66 (6.05–7.34) | 6.62 (1962.04) | 6.56 (6.05) | 2.71 (2.57) |
| Sinusitis | 397 | 4.34 (3.93–4.79) | 4.31 (1005.2) | 4.29 (3.95) | 2.1 (1.96) |
| Influenza | 293 | 2.81 (2.51–3.16) | 2.81 (339.55) | 2.8 (2.54) | 1.48 (1.32) |
| Cellulitis | 261 | 6.08 (5.38–6.87) | 6.06 (1093.16) | 6.01 (5.43) | 2.59 (2.41) |
| Ear infection | 213 | 8.66 (7.56–9.91) | 8.63 (1417.93) | 8.53 (7.61) | 3.09 (2.89) |
| Upper respiratory tract infection | 185 | 4.51 (3.9–5.21) | 4.5 (500.14) | 4.47 (3.96) | 2.16 (1.95) |
| Bronchitis | 175 | 2.76 (2.38–3.21) | 2.76 (195.63) | 2.75 (2.43) | 1.46 (1.24) |
| Fungal infection | 172 | 5.84 (5.02–6.78) | 5.82 (681.08) | 5.78 (5.1) | 2.53 (2.31) |
| Herpes zoster | 135 | 2.53 (2.14–3) | 2.53 (124.68) | 2.53 (2.19) | 1.34 (1.09) |
| Oral candidiasis | 134 | 12.94 (10.9–15.35) | 12.91 (1443.68) | 12.68 (10.98) | 3.66 (3.41) |
| Candida infection | 125 | 7.24 (6.07–8.63) | 7.22 (663.12) | 7.16 (6.17) | 2.84 (2.58) |
| Pharyngitis streptococcal | 111 | 11.46 (9.49–13.82) | 11.43 (1038.89) | 11.25 (9.62) | 3.49 (3.22) |
| Inflammatory bowel disease | 98 | 20.41(16.69–24.97) | 20.38 (1751.5) | 19.79 (16.73) | 4.31 (4.01) |
| Colitis | 94 | 2.72 (2.22–3.33) | 2.72 (101.63) | 2.71 (2.29) | 1.44 (1.14) |
| Staphylococcal infection | 93 | 3.83 (3.12–4.69) | 3.82 (192.71) | 3.81 (3.21) | 1.93 (1.63) |
| Irritable bowel syndrome | 83 | 4.65 (3.74–5.77) | 4.64 (235.56) | 4.62 (3.85) | 2.21 (1.89) |
| Tooth infection | 79 | 6.62 (5.3–8.26) | 6.61 (372.37) | 6.55 (5.44) | 2.71 (2.39) |
| Respiratory tract infection | 66 | 2.74 (2.15–3.49) | 2.74 (72.59) | 2.73 (2.23) | 1.45 (1.1) |
| Diverticulitis | 64 | 2.54 (1.99–3.25) | 2.54 (59.41) | 2.53 (2.06) | 1.34 (0.98) |
| Immune system disorder | 60 | 4.84 (3.75–6.24) | 4.83 (181.03) | 4.8 (3.88) | 2.26 (1.89) |
| Kidney infection | 53 | 2.87 (2.19–3.76) | 2.87 (64.13) | 2.86 (2.28) | 1.51 (1.12) |
| Conjunctivitis | 52 | 2.95 (2.25–3.88) | 2.95 (66.69) | 2.94 (2.34) | 1.56 (1.16) |
| Tuberculosis | 52 | 4.54 (3.45–5.96) | 4.54 (142.36) | 4.51 (3.59) | 2.17 (1.78) |
| Gastroenteritis viral | 47 | 2.95 (2.22–3.93) | 2.95 (60.38) | 2.94 (2.31) | 1.56 (1.14) |
| Malignant melanoma | 46 | 3.55 (2.66–4.74) | 3.55 (83.71) | 3.53 (2.77) | 1.82 (1.4) |
Signal strength of reports of IXE with preferred terms (PTs) ≥ 45 from FAERS.
In the FAERS database, a total of 192 positive signals were identified that met the criteria for positivity across all four algorithms. Among them, injection site warmth and injection site urticaria have the strongest signals. The top 70 positive signals of IXE are listed in Supplementary Table 5. In our data analysis, injection site reactions, hypersensitivity reactions, fungal infections, upper respiratory tract infections, and inflammatory bowel disease were consistent with the instructions and medication warnings. In addition, unexpected AEs not listed on the label have been identified, such as cellulitis, ear infection, bronchitis, herpes zoster, tooth infection, diverticulitis, kidney infection, gastroenteritis viral and malignant melanoma. In the VigiAccess database, injection site pain (7.17%), injection site erythema (4.19%), and injection site swelling (2.93%) were the three most frequently reported AEs. The ranking of PTs differed slightly between the FAERS and Vigiaccess databases, but the overall reported PTs were similar.
3.4 Subgroup analysis
Further subgroup analysis was conducted on the 50 most common AEs associated with IXE that met the criteria for all four algorithms. AEs occurring exclusively in males were serum sickness, aphthous ulcer, necrotising fasciitis, miliaria, and large intestine perforation while those occurring exclusively in females included immune system disorder. The specific details are provided in Supplementary Tables 6, 7. In adolescent patients under the age of 18, common AEs in addition to those mentioned on the drug label include alopecia, drug hypersensitivity, and vitiligo. In the patient population aged 18–64 years, additional AEs identified included herpes zoster, Spinal operation, immune system disorder, antibiotic therapy, cardiac surgery, and multiple allergies. For patients over the age of 64, common AEs included coronary arterial stent insertion, and bursitis (Supplementary Tables 8–10).
3.5 Sensitivity analysis
IXE is commonly used in combination with other medications such as clobetasol, prednisone, triamcinolone, betamethasone calcipotriene, and vitamin D3. After excluding reports of concomitant use of other therapeutic agents, we identified 28,633 reports involving 53,765 AEs. Cellulitis, herpes zoster and malignant melanoma were identified as unexpected AEs (Supplementary Table 11).
3.6 Onset time of events
The time-to-event onset represents the time interval between the date of drug administration and the occurrence of the AE. As shown in Figure 3, the majority of AEs associated with IXE occurred primarily within the first month of treatment. Additionally, the cumulative incidence curve of AEs is illustrated in Figure 4. The Weibull distribution analysis exhibits a shape parameter less than 1, indicating a higher probability of failure in the early stage, referred to as the early failure mode. Table 4 provides the detailed parameters of this analysis.
Figure 3
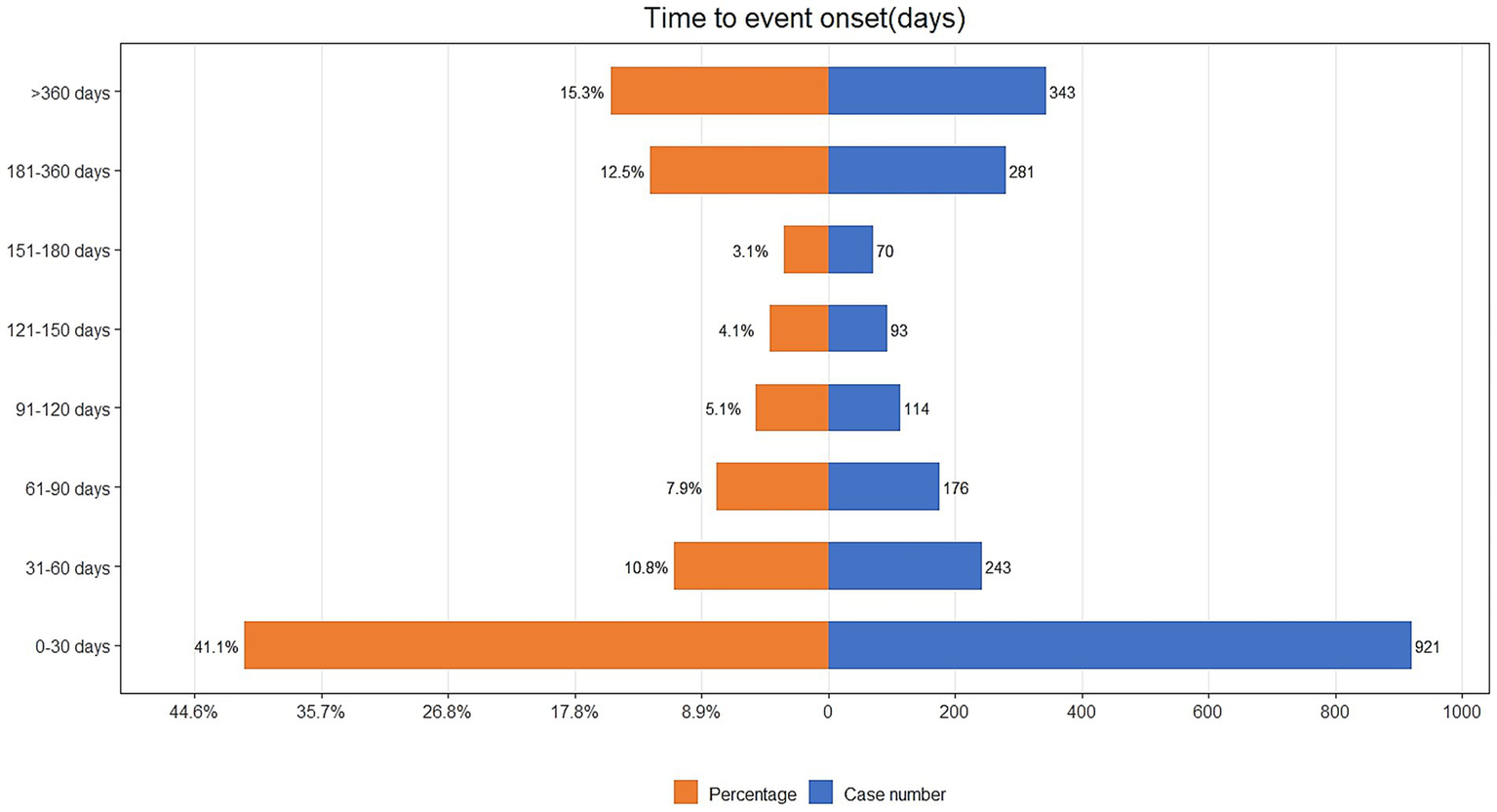
Time to onset of IXE-related AEs.
Figure 4
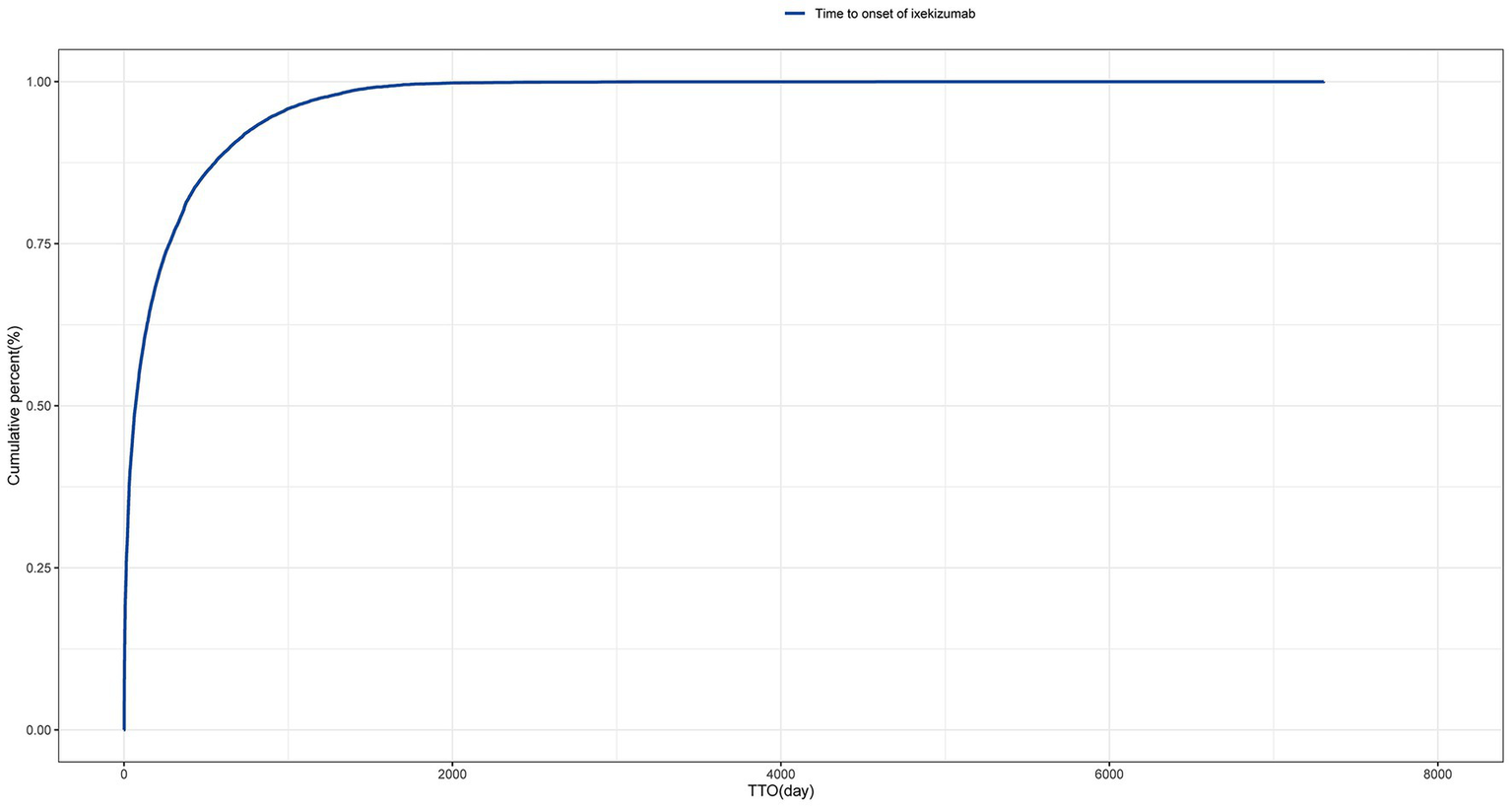
Cumulative incidence of adverse events related to tralokinumab over time.
Table 4
| Drug | TTO(days) | Weibull distribution | |||
|---|---|---|---|---|---|
| Case reports | Median(d)(IQR) | Scale parameter: α(95%CI) | Shape parameter: β(95%CI) | Type | |
| Ixekizumab | 28,889 | 70(14,276) | 143.6(140.0,147.2) | 0.60(0.59,0.61) | Early failure |
Time to onset of ixekizumab-associated adverse events and Weibull distribution analysis.
TTO, time to onset; CI, confidence interval; IQR, interquartile range.
4 Discussion
Due to the chronic nature of psoriasis, long-term treatment and continuous safety monitoring are essential. This study represents the first extensive and systematic pharmacovigilance analysis of AEs associated with IXE using the FAERS and VigiAccess databases following its market approval. By comparing and analyzing IXE-related adverse events reported in the VigiAccess and FAERS databases, this study provides an in-depth understanding of the common, novel, and rare AEs associated with this drug. Through data mining, we confirmed previously identified AEs listed on the drug labeling, such as injection site reactions, nasopharyngitis, infections, urticaria, and inflammatory bowel disease. Additionally, we identified unexpected AEs, including cellulitis, bronchitis, and herpes zoster.
Multiple clinical trials have demonstrated that the common AEs associated with IXE include nasopharyngitis, upper respiratory tract infections, and injection-site reactions. Other AEs include bronchitis, sinusitis, inflammatory bowel disease, among others (18, 19). These findings are consistent with our conclusions.
In our study, injection site reactions were the most commonly observed AEs. Research indicates that injection-site reactions are associated with the components of IXE. The new citrate-free formulation was developed to enhance the overall patient experience, indicating a lower reported frequency of injection site reactions and reduced injection site pain, thereby enhancing tolerability and patient satisfaction (20).
Another notable AEs is infection. Previously, concerns have been raised regarding the safety profile of immunomodulators, primarily due to the potential for these agents to increase the risk of infections (21, 22). The infection issues caused by biological agents, represented by IXE, also deserve attention. IL-17 is involved in mucocutaneous defense. Inhibition of IL-17 may compromise the immune system and increase the risk of infections, particularly enhancing susceptibility to staphylococcal infections and mucocutaneous candidiasis (23). Across multiple clinical trials, the most common infections reported among all AEs were nasopharyngitis, upper respiratory tract infections and bronchitis. Infections were reported among 4,307 patients with PSO (62.5%, IR 23.9 per 100 PY), 759 patients with PSA (54.2%, IR 33.8 per 100 PY) (17, 23). The study also reported that the opportunistic infections observed after the use of IXE were primarily oral candidiasis, esophageal candidiasis, and oral fungal infections (18, 19, 24, 25). Our study indicates that the most commonly reported infections include nasopharyngitis and sinusitis. Additionally, influenza, cellulitis, ear infection, upper respiratory tract infection, bronchitis, fungal infection, oral candidiasis, and candida infection also appeared among the positive signals. Severe infections, such as mucosal candidiasis, often necessitate the discontinuation of IXE and prolonged systemic antimicrobial therapy, which may lead to the exacerbation of the underlying condition and an increased disease burden. Therefore, strictly monitoring and preventing the occurrence of common AEs during the treatment process is crucial. This approach can effectively prevent the progression of serious AEs, thereby improving drug retention and patient adherence.
Our study identified a positive signal for inflammatory bowel disease (IBD). IBD, which includes ulcerative colitis (UC) and Crohn’s disease (CD), is primarily characterized by inflammation of the gastrointestinal tract, weight loss, and diarrhea. Although UC and Crohn’s disease CD do not manifest identically, both conditions have significant long-term impacts on patients.
At present, this condition is incurable and necessitates lifelong treatment to manage symptoms, prevent complications, and ultimately enhance the quality of life (26). The Phase 3 trials UNCOVER-2 and UNCOVER-3 explored the efficacy and safety of IXE in patients with moderate-to-severe psoriasis. In these studies, 4 cases of CD and 7 cases of ulcerative colitis UC were reported in the IXE treatment groups, compared to zero cases in the placebo groups (27). In 2023, Deng Z et al. published a comprehensive paper on the prevalence, clinical characteristics, and management of IBD events associated with anti-IL-17 therapies. Their study also involved a retrospective analysis of case reports and case series of anti-IL-17 drug-induced IBD cases from 2015 to 2022. A total of 388 cases of gastrointestinal inflammatory events associated with IL-17 inhibitors were reported, including 268 cases of IBD induced by secukinumab and IXE. The main presenting symptoms were diarrhea (90.9%), abdominal pain (57.6%), and bloody diarrhea (51.5%), with a total of 120 cases diagnosed as colitis (28). Furthermore, multiple case reports have highlighted the development of steroid-refractory ulcerative colitis with a superimposed cytomegalovirus infection, Crohn’s-like colitis, CD, and other gastrointestinal disorders in patients treated with IXE (29–32).
In a nationwide Danish cohort study, nearly one-quarter of IBD patients with psoriasis had a family history of psoriasis. Interestingly, the risk of developing IBD—including Crohn’s disease and ulcerative colitis—increases with the severity of psoriasis (33, 34). Limited data suggest an association between IL-17 inhibitor therapy and the development of IBD.
Researchers have proposed a gut-skin-joint axis, which illustrates how the gut microbiota, disturbances in immune balance, and increased gut permeability can influence the skin and joints, affecting their homeostasis and leading to inflammation (35). The IL-23/Th17 axis plays a critical role in the pathogenesis of both diseases. Although IL-23 inhibition has been proven to be an effective mechanism in the treatment of IBD in clinical trials, there is no evidence that IL-17 inhibition provides any benefits; instead, it is associated with an increase in AEs. Researchers hypothesize that these results may reflect the heterogeneous functions of various IL-17 subtypes. Some of these subtypes may act as pro-inflammatory mediators, while others appear to have a protective role against IBD (36, 37).
Studies have indicated that Th17 cells infiltrate the intestinal mucosa of IBD patients more extensively than in healthy controls. Furthermore, the levels of IL-17, a cytokine secreted by Th17 cells, are elevated in these patients (38). Clinical trials utilizing IL-17 inhibitors for the treatment of IBD have not shown any therapeutic benefits, and in certain cases, have been associated with disease worsening. This indicates that while IL-17 serves a pro-inflammatory function in psoriasis, it may play a protective role in maintaining gut integrity in IBD (39, 40).
However, disproportionality analysis only suggests a statistical association, not causality. The causal relationship among psoriasis, IL-17 inhibitors, and IBD remains incompletely understood. Future research should prioritize elucidating the potential pathophysiological mechanisms linking psoriasis and IL-17 blockade to the development of IBD, rather than focusing primarily on statistical associations.
In addition, our study revealed several unexpected AEs. Notably, our study also identified herpes zoster as an unexpected AE. Herpes zoster is a disease caused by the reactivation of the Varicella-Zoster, which can lead to severe pain, rash, and postherpetic neuralgia, affecting sleep, mood, and daily activities, significantly reducing the quality of life (41). In the COAST-W study, one case of herpes zoster was reported in the group receiving IXE every 4 weeks (42). Additionally, two cases of herpes zoster were reported in the same dosing group in the COAST-X study. The study found that herpes zoster is one of the most common opportunistic infections associated with IXE treatment. Herpes zoster was reported among 120 patients with PSO (1.7%, IR 0.6 per 100 PY), 16 patients with PSA (1.1%, IR 0.7 per 100 PY) (43). The exact mechanism by which IXE leads to herpes zoster remains to be elucidated. During IXE treatment, preventive measures, including vaccination, should be implemented for individuals with compromised immune status. Close monitoring and follow-up are crucial for further reducing this risk.
In our analysis, an unexpected condition showed a positive signal—malignant melanoma—which warrants clinical attention. Cutaneous malignancies are a significant concern for patients with psoriasis due to their association with sun exposure (44). Malignant melanoma results from the malignant transformation of melanocytes, which are melanin-producing cells derived from the neural crest. It is characterized by local invasion, recurrence, early metastasis, drug resistance, and high mortality rates (45). Immune checkpoint inhibitors ICIs, particularly those targeting programmed cell death 1 (PD-1), have significantly transformed the landscape of cancer treatment.
A retrospective study including a total of 61,692 patients with psoriasis compared the incidence of malignant melanoma with that in a matched control group, showing that patients with psoriasis, particularly those with severe disease, had a higher risk of developing malignant melanoma, especially melanoma in situ (46). In contrast, other studies have reported similar incidence rates of these tumors compared to the general population. Additional evidence suggests that these patients might have a higher risk of cutaneous malignancies, especially for non-melanoma skin cancer, compared with psoriasis-free patients., relative to individuals without psoriasis (47). In addition, evidence has shown that psoralen plus ultraviolet A therapy is associated with an increased risk of non-melanoma skin cancer, but not with melanoma (48). Limited studies suggest that patients treated with methotrexate or tumor necrosis factor-alpha inhibitors may have a slightly increased risk of melanoma (49, 50). In contrast, studies have evaluated the risk of developing melanoma with conventional therapies and newer agents for the treatment of psoriasis. No significant increase in the risk of melanoma was found associated with the use of anti-psoriatic treatments (51). Shamarke et al. reported an increased risk of melanoma in patients receiving biologic agents compared to those receiving conventional systemic therapies, although the difference was not statistically significant (44). The development and progression of melanoma is a highly complex process, and the chronic inflammatory state associated with long-standing psoriasis, as well as its various treatment modalities, may exert differential influences on the development and progression of melanoma.
Currently, there is limited real-world evidence regarding the association between IL-17 inhibitors and malignant melanoma. A 2024 systematic review assessed melanoma and non-melanoma skin cancer risks in psoriasis and psoriatic arthritis patients on targeted therapies. The pooled melanoma incidence rates were: 0.06 (95% CI, 0.02–0.18) per 100 patient-years for IL-17 inhibitors, 0.10 (95% CI, 0.05–0.21) for IL-23 inhibitors, and 0.09 (95% CI, 0.03–0.28) for JAK inhibitors (52). Although the pathophysiological mechanisms by which IXE contributes to melanoma development remain unclear, studies in melanoma animal models have demonstrated that IL-17A-deficient mice are susceptible to developing spontaneous melanoma (53). Moreover, studies have shown that increased IL-17 signaling and elevated serum IL-17 levels are positively correlated with better therapeutic responses in melanoma patients treated with dual cytotoxic T-lymphocyte antigen 4 and PD-1 immune checkpoint inhibitors, and are linked to prolonged overall survival for these patients (54). These findings suggest a potential association between IL-17 and melanoma, but cannot be directly attributed to the effect of IL-17 inhibition. The development and progression of melanoma is a highly complex process likely influenced by the interplay of multiple factors. Further research is needed to better understand the potential link between IXE treatment for psoriasis and melanoma, particularly long-term observational studies with matched control groups and adjustment for known risk factors. Such studies will be essential to elucidate the true nature of this potential association.
Subgroup analysis revealed that and serum sickness in males require additional attention, while females should be vigilant against the risks of immune system disorders. For patients under 18 years of age, it is important to monitor for alopecia and vitiligo. For patients aged 18 to 65, attention should be given to immune-related issues such as herpes zoster. Addressing these concerns can help improve medication adherence.
In addition, sensitivity analysis revealed persistent unexpected AEs associated with IXE monotherapy, such as cellulitis, herpes zoster and malignant melanoma. These AEs may exert negative effects on treatment satisfaction, adherence, and efficacy.
This study also performed a temporal analysis of AEs and utilized the Weibull distribution to model and predict the timing of these events. This approach provides a robust temporal framework for monitoring drug-related AEs, enabling more informed and timely clinical interventions. The results highlight the importance of monitoring for specific AEs, especially during the first month of IXE treatment. Additionally, a gradual reduction in AEs was observed as time progressed. This underscores the importance of closely monitoring AEs during the initial phase of drug administration while also maintaining continuous attention to the long-term safety profile of the medication. Monitoring and preventing at specific stages are crucial for managing unexpected AEs, which helps to improve the satisfaction, efficacy, and safety of medication use.
This study has some limitations. First, due to the spontaneous reporting nature of the FAERS and VigiAccess databases, they may inherently contain missing or inaccurate data. Additionally, there is a potential risk of over-reporting for serious adverse events, which may be more likely to be reported due to frequent follow-up visits or medical consultations. Second, while the sensitivity analysis partially accounted for the influence of certain medications on the results, other confounding factors may still exist due to the nature of real-world data. Therefore, the results should be interpreted with caution. Finally, although disproportionality analysis effectively identified positive signals for AEs and detected unexpected AEs, it is not capable of establishing a causal relationship between the events. Therefore, further prospective studies are essential to validate the unexpected AEs identified in this study.
5 Conclusion
This study explored the overall real-world safety profile of IXE. Our analysis confirmed several known adverse events. Additionally, several potential unexpected adverse events were identified, including herpes zoster, and cellulitis. Furthermore, this study highlights the importance of monitoring during the first month following IXE initiation. Prospective studies are warranted to validate the findings of this study.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding authors.
Author contributions
KZ: Writing – review & editing. XT: Writing – review & editing, Supervision. MQ: Writing – review & editing, Data curation. QL: Writing – review & editing, Formal analysis. QM: Writing – review & editing, Investigation. YL: Writing – review & editing, Methodology. JLi: Writing – review & editing, Supervision. JLiu: Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by grants from the National Natural Science Foundation of China (82404112), the Natural Science Foundation of Shaanxi Province (2024JC-YBQN-0824).
Acknowledgments
The authors express their gratitude to the Dermatology Department at Xi′an Children′s Hospital. Zhao expresses heartfelt thanks to all the staff and leadership of the Department of Dermatology at the Second Affiliated Hospital of Xi′an Jiaotong University for their invaluable support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Correction note
This article has been corrected with minor changes. These changes do not impact the scientific content of the article.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1652401/full#supplementary-material
References
1.
Li L Zhang L Li Y Cai Y Wen X Zheng C et al . Overview of current research on traditional Chinese medicine in skin disease treatment: a bibliometric analysis from 2014 to 2024. Pharm Biol. (2025) 63:27–41. doi: 10.1080/13880209.2024.2443415
2.
Mrowietz U Lauffer F Sondermann W Gerdes S Sewerin P . Psoriasis as a systemic disease. Dtsch Arztebl Int. (2024) 121:467–72. doi: 10.3238/arztebl.m2024.0064
3.
Lembke S Macfarlane GJ Jones GT . The worldwide prevalence of psoriatic arthritis-a systematic review and meta-analysis. Rheumatology. (2024) 63:3211–20. doi: 10.1093/rheumatology/keae198
4.
Kane D Stafford L Bresnihan B FitzGerald O . A prospective, clinical and radiological study of early psoriatic arthritis: an early synovitis clinic experience. Rheumatology. (2003) 42:1460–8. doi: 10.1093/rheumatology/keg384
5.
Husni ME Merola JF Davin S . The psychosocial burden of psoriatic arthritis. Semin Arthritis Rheum. (2017) 47:351–60. doi: 10.1016/j.semarthrit.2017.05.010
6.
Hawkes JE Chan TC Krueger JG . Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. (2017) 140:645–53. doi: 10.1016/j.jaci.2017.07.004
7.
Berman J Furer V Berman M Isakov O Zisman D Haddad A et al . Treatment with Ixekizumab following Secukinumab failure in patients with psoriatic arthritis: real-life experience from a resistant population. Biologics. (2021) 15:463–70. doi: 10.2147/BTT.S326792
8.
Kirkham BW Egeberg A Behrens F Pinter A Merola JF Holzkämper T et al . A comprehensive review of Ixekizumab efficacy in nail psoriasis from clinical trials for moderate-to-severe psoriasis and psoriatic arthritis. Rheumatol Ther. (2023) 10:1127–46. doi: 10.1007/s40744-023-00553-1
9.
Griffiths CEM Gooderham M Colombel JF Terui T Accioly AP Gallo G et al . Safety of Ixekizumab in adult patients with moderate-to-severe psoriasis: data from 17 clinical trials with over 18,000 patient-years of exposure. Dermatol Ther. (2022) 12:1431–46. doi: 10.1007/s13555-022-00743-9
10.
Blauvelt A Lebwohl MG Mabuchi T Leung A Garrelts A Crane H et al . Long-term efficacy and safety of ixekizumab: a 5-year analysis of the UNCOVER-3 randomized controlled trial. J Am Acad Dermatol. (2021) 85:360–8. doi: 10.1016/j.jaad.2020.11.022
11.
Mastorino L Dapavo P Burzi L Rosset F Giunipero di Corteranzo I Leo F et al . Drug survival, effectiveness and safety of ixekizumab for moderate-to-severe psoriasis up to 5 years. J Eur Acad Dermatol Venereol. (2024) 38:568–75. doi: 10.1111/jdv.19682
12.
Zhao K Zhao Y Xiao S Tu C . Assessing the real-world safety of tralokinumab for atopic dermatitis: insights from a comprehensive analysis of FAERS data. Front Pharmacol. (2024) 15:1458438. doi: 10.3389/fphar.2024.1458438
13.
He K Zhao K Yin T Liu M Liu J Du W et al . A real-world pharmacovigilance study of brodalumab based on the FDA adverse event reporting system. Sci Rep. (2025) 15:2346. doi: 10.1038/s41598-025-86976-y
14.
Sultana J Scondotto G Cutroneo PM Morgante F Trifirò G . Intravitreal anti-VEGF drugs and signals of dementia and Parkinson-like events: analysis of the VigiBase database of spontaneous reports. Front Pharmacol. (2020) 11:315. doi: 10.3389/fphar.2020.00315
15.
Slattery J Alvarez Y Hidalgo A . Choosing thresholds for statistical signal detection with the proportional reporting ratio. Drug Saf. (2013) 36:687–92. doi: 10.1007/s40264-013-0075-1
16.
Raschi E Poluzzi E Salvo F Pariente A De Ponti F Marchesini G et al . Pharmacovigilance of sodium-glucose co-transporter-2 inhibitors: what a clinician should know on disproportionality analysis of spontaneous reporting systems. Nutr Metab Cardiovasc Dis. (2018) 28:533–42. doi: 10.1016/j.numecd.2018.02.014
17.
Noguchi Y Tachi T Teramachi H . Detection algorithms and attentive points of safety signal using spontaneous reporting systems as a clinical data source. Brief Bioinform. (2021) 22:22. doi: 10.1093/bib/bbab347
18.
Genovese MC Mysler E Tomita T Papp KA Salvarani C Schwartzman S et al . Safety of ixekizumab in adult patients with plaque psoriasis, psoriatic arthritis and axial spondyloarthritis: data from 21 clinical trials. Rheumatology. (2020) 59:3834–44. doi: 10.1093/rheumatology/keaa189
19.
Deodhar A Blauvelt A Lebwohl M Feely M Kronbergs A Eberhart N et al . Long-term safety of Ixekizumab in adults with psoriasis, psoriatic arthritis, or axial spondyloarthritis: a post-hoc analysis of final safety data from 25 randomized clinical trials. Arthritis Res Ther. (2024) 26:49. doi: 10.1186/s13075-023-03257-7
20.
Chabra S Gill BJ Gallo G Zhu D Pitou C Payne CD et al . Ixekizumab citrate-free formulation: results from two clinical trials. Adv Ther. (2022) 39:2862–72. doi: 10.1007/s12325-022-02126-0
21.
Murdaca G Spanò F Contatore M Guastalla A Penza E Magnani O et al . Infection risk associated with anti-TNF-α agents: a review. Expert Opin Drug Saf. (2015) 14:571–82. doi: 10.1517/14740338.2015.1009036
22.
Selmi C Ceribelli A Naguwa SM Cantarini L Shoenfeld Y . Safety issues and concerns of new immunomodulators in rheumatology. Expert Opin Drug Saf. (2015) 14:389–99. doi: 10.1517/14740338.2015.993605
23.
Burshtein J Shah M Zakria D Lockshin B Crowley J Merola JF et al . Candida infections in patients with psoriasis and psoriatic arthritis treated with interleukin-17 inhibitors and their practical management. Ital J Dermatol Venerol. (2021) 156:545–57. doi: 10.23736/S2784-8671.20.06580-3
24.
Combe B Rahman P Kameda H Cañete JD Gallo G Agada N et al . Safety results of ixekizumab with 1822.2 patient-years of exposure: an integrated analysis of 3 clinical trials in adult patients with psoriatic arthritis. Arthritis Res Ther. (2020) 22:14. doi: 10.1186/s13075-020-2099-0
25.
Deodhar AA Combe B Accioly AP Bolce R Zhu D Gellett AM et al . Safety of ixekizumab in patients with psoriatic arthritis: data from four clinical trials with over 2000 patient-years of exposure. Ann Rheum Dis. (2022) 81:944–50. doi: 10.1136/annrheumdis-2021-222027
26.
Rosen MJ Dhawan A Saeed SA . Inflammatory bowel disease in children and adolescents. JAMA Pediatr. (2015) 169:1053–60. doi: 10.1001/jamapediatrics.2015.1982
27.
Gordon KB Blauvelt A Papp KA Langley RG Luger T Ohtsuki M et al . Phase 3 trials of Ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. (2016) 375:345–56. doi: 10.1056/NEJMoa1512711
28.
Deng Z Wang S Wu C Wang C . IL-17 inhibitor-associated inflammatory bowel disease: a study based on literature and database analysis. Front Pharmacol. (2023) 14:1124628. doi: 10.3389/fphar.2023.1124628
29.
Philipose J Ahmed M Idiculla PS Mulrooney SM Gumaste VV . Severe de novo ulcerative colitis following ixekizumab therapy. Case Rep Gastroenterol. (2018) 12:617–21. doi: 10.1159/000493922
30.
Smith MK Pai J Panaccione R Beck P Ferraz JG Jijon H . Crohn's-like disease in a patient exposed to anti-Interleukin-17 blockade (Ixekizumab) for the treatment of chronic plaque psoriasis: a case report. BMC Gastroenterol. (2019) 19:162. doi: 10.1186/s12876-019-1067-0
31.
Merino GE Gómez TK Martínez AE . Debut of inflammatory bowel disease associated to Ixekizumab in patient with moderate, difficult -to-manage psoriasis. Gastroenterol Hepatol. (2020) 43:622–3. doi: 10.1016/j.gastrohep.2020.04.009
32.
Nazarian A Grin A Wijeratne DT . Ixekizumab associated new-onset inflammatory bowel disease. ACG Case Rep J. (2020) 7:e00316. doi: 10.14309/crj.0000000000000316
33.
Egeberg A Mallbris L Warren RB Bachelez H Gislason GH Hansen PR et al . Association between psoriasis and inflammatory bowel disease: a Danish nationwide cohort study. Br J Dermatol. (2016) 175:487–92. doi: 10.1111/bjd.14528
34.
Alinaghi F Tekin HG Burisch J Wu JJ Thyssen JP Egeberg A . Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease-a systematic review and Meta-analysis. J Crohns Colitis. (2020) 14:351–60. doi: 10.1093/ecco-jcc/jjz152
35.
Sun Y Li Y Zhang J . The causal relationship between psoriasis, psoriatic arthritis, and inflammatory bowel diseases. Sci Rep. (2022) 12:20526. doi: 10.1038/s41598-022-24872-5
36.
Moschen AR Tilg H Raine T . IL-12, IL-23 and IL-17 in IBD: immunobiology and therapeutic targeting. Nat Rev Gastroenterol Hepatol. (2019) 16:185–96. doi: 10.1038/s41575-018-0084-8
37.
Hueber W Sands BE Lewitzky S Vandemeulebroecke M Reinisch W Higgins PD et al . Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. (2012) 61:1693–700. doi: 10.1136/gutjnl-2011-301668
38.
Braga-Neto MB Gaballa JM Bamidele AO Sarmento OF Svingen P Gonzalez M et al . Deregulation of long intergenic non-coding RNAs in CD4+ T cells of lamina propria in Crohn's disease through transcriptome profiling. J Crohns Colitis. (2020) 14:96–109. doi: 10.1093/ecco-jcc/jjz109
39.
Hedin CRH Sonkoly E Eberhardson M Ståhle M . Inflammatory bowel disease and psoriasis: modernizing the multidisciplinary approach. J Intern Med. (2021) 290:257–78. doi: 10.1111/joim.13282
40.
Hohenberger M Cardwell LA Oussedik E Feldman SR . Interleukin-17 inhibition: role in psoriasis and inflammatory bowel disease. J Dermatolog Treat. (2018) 29:13–8. doi: 10.1080/09546634.2017.1329511
41.
Yamaoka-Tojo M Tojo T . Herpes zoster and cardiovascular disease: exploring associations and preventive measures through vaccination. Vaccine. (2024) 12:252. doi: 10.3390/vaccines12030252
42.
Deodhar A Poddubnyy D Pacheco-Tena C Salvarani C Lespessailles E Rahman P et al . Efficacy and safety of Ixekizumab in the treatment of radiographic axial Spondyloarthritis: sixteen-week results from a phase III randomized, double-blind, placebo-controlled trial in patients with prior inadequate response to or intolerance of tumor necrosis factor inhibitors. Arthritis Rheum. (2019) 71:599–611. doi: 10.1002/art.40753
43.
Deodhar A Mease P Marzo-Ortega H Hunter T Sandoval D Kronbergs A et al . Ixekizumab improves sleep and work productivity in patients with non-radiographic axial spondyloarthritis: results from the COAST-X trial at 52 weeks. BMC Rheumatol. (2021) 5:50. doi: 10.1186/s41927-021-00218-y
44.
Esse S Mason KJ Green AC Warren RB . Melanoma risk in patients treated with biologic therapy for common inflammatory diseases: a systematic review and Meta-analysis. JAMA Dermatol. (2020) 156:787–94. doi: 10.1001/jamadermatol.2020.1300
45.
Slominski RM Kim TK Janjetovic Z Brożyna AA Podgorska E Dixon KM et al . Malignant melanoma: an overview, new perspectives, and vitamin D signaling. Cancer. (2024) 16:2262. doi: 10.3390/cancers16122262
46.
Polesie S Gillstedt M . Psoriasis and risk of cutaneous melanoma: a retrospective, comparative, registry-based cohort study. J Am Acad Dermatol. (2022) 86:215–7. doi: 10.1016/j.jaad.2021.01.084
47.
Butrón-Bris B Daudén E Rodríguez-Jiménez P . Psoriasis therapy and skin Cancer: a review. Life (Basel). (2021) 11:1109. doi: 10.3390/life11101109
48.
Ko MJ Tsai WC Tsai PH Hsu LY Chien KL Wu HY . Risk of nonmelanoma skin cancers and cutaneous melanoma after psoralen and ultraviolet a therapy in psoriasis patients: a nationwide Taiwanese cohort study. Clin Exp Dermatol. (2025):8114739. doi: 10.1093/ced/llaf169
49.
Yan MK Wang C Wolfe R Mar VJ Wluka AE . Association between low-dose methotrexate exposure and melanoma: a systematic review and meta-analysis. JAMA Dermatol. (2022) 158:1157–66. doi: 10.1001/jamadermatol.2022.3337
50.
Kreher MA Konda S Noland MMB Longo MI Valdes-Rodriguez R . Risk of melanoma and nonmelanoma skin cancer with immunosuppressants, part II: methotrexate, alkylating agents, biologics, and small molecule inhibitors. J Am Acad Dermatol. (2023) 88:534–42. doi: 10.1016/j.jaad.2022.11.043
51.
Semaka A Salopek TG . Risk of developing melanoma with systemic agents used to treat psoriasis: a review of the literature. J Cutan Med Surg. (2022) 26:87–92. doi: 10.1177/12034754211038509
52.
Krzysztofik M Brzewski P Cuber P Kacprzyk A Kulbat A Richter K et al . Risk of melanoma and non-melanoma skin cancer in patients with psoriasis and psoriatic arthritis treated with targeted therapies: a systematic review and meta-analysis. Pharmaceuticals. (2023) 17:14. doi: 10.3390/ph17010014
53.
Martin-Orozco N Muranski P Chung Y Yang XO Yamazaki T Lu S et al . T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity. (2009) 31:787–98. doi: 10.1016/j.immuni.2009.09.014
54.
Váraljai R Zimmer L Al-Matary Y Kaptein P Albrecht LJ Shannan B et al . Interleukin 17 signaling supports clinical benefit of dual CTLA-4 and PD-1 checkpoint inhibition in melanoma. Nat Can. (2023) 4:1292–308. doi: 10.1038/s43018-023-00610-2
Summary
Keywords
ixekizumab, biologics, psoriasis, FAERS, disproportionality analysis, adverse events
Citation
Zhao K, Tan X, Qin M, Li Q, Meng Q, Liu Y, Li J and Liu J (2025) Real-world safety of ixekizumab: a disproportionality analysis using the FDA adverse event reporting system and the VigiAccess databases. Front. Med. 12:1652401. doi: 10.3389/fmed.2025.1652401
Received
23 June 2025
Accepted
29 September 2025
Published
13 October 2025
Corrected
19 October 2025
Volume
12 - 2025
Edited by
Chiara Moltrasio, Fondazione IRRCS Ca' Granda Ospedale Maggiore Policlinico, Italy
Reviewed by
Luca Mastorino, University of Turin, Italy
Xihui Yu, Second Affiliated Hospital of Shantou University Medical College, China
Updates
Copyright
© 2025 Zhao, Tan, Qin, Li, Meng, Liu, Li and Liu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiashu Liu, dermatol_liu@163.com; Jing Li, lijing428@sina.com
†These authors share last authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.