Abstract
Background:
Brucellosis with atypical presentations, such as submandibular abscess without fever, is frequently misdiagnosed.
Methods:
Metagenomic next-generation sequencing (mNGS) was applied to pus samples from a 47-year-old female with a treatment-refractory submandibular abscess and a history of livestock exposure; results were confirmed serologically.
Results:
Within 48 h, mNGS identified Brucella suis—representing, to our knowledge, the first reported afebrile submandibular infection caused by this pathogen. Targeted therapy with doxycycline and rifamycin led to symptom resolution within 6 days.
Conclusion:
This case highlights that mNGS, combined with a thorough epidemiological history, can resolve diagnostic dilemmas in atypical brucellosis, guide precise treatment, and mitigate antibiotic misuse.
Background
Brucellosis is a global zoonotic disease, with more than 500,000 cases reported worldwide each year (the actual incidence rate is even higher) (1). In China, more than 95% of the disease burden is in northern regions such as Inner Mongolia, and the peak incidence is closely related to the livestock breeding season (2, 3). The Brucella genus includes Brucella melitensis (65–70%), Brucella abortus (20–25%), Brucella suis (<5%). There are significant differences in their host adaptability and virulence factors (4). In China, brucellosis is mainly prevalent in sheep. Due to host specificity and geographical distribution limitations, Brucellosis infections in pigs are reported less frequently at present. The pathogen can be transmitted through skin and mucous membrane contact, ingestion of contaminated dairy products or inhalation of aerosols. The typical symptoms are wave fever, excessive sweating and joint pain. However, about 15–20% of cases present with atypical site infections (such as central nervous system infections and soft tissue abscesses) as the initial symptom (5).
Introduction
Here we report a case of brucellosis misdiagnosed as submandibular space infection. The correct diagnosis was attributed to metagenomic next-generation sequencing (mNGS) technology, which provides detailed information on the microbial genome sequence (6). This unbiased high-throughput sequencing method can accurately diagnose by simultaneously and independently sequencing thousands to billions of DNA fragments to identify the total DNA or RNA content of all known pathogenic microorganisms (7).
Case description
The patient was a 47-year-old female with a right maxillofacial abscess accompanied by distension and pain for 14 days. On November 22, 2024, the patient visited the local hospital’s Department of stomatology and was initially diagnosed with submandibular space infection. After local puncture and pus drainage, intravenous infusion of drugs such as clindamycin hydrochloride, levofloxacin and metronidazole for 3 days, the local symptoms worsened. On November 25, 2024, the patient visited the Oral and maxillofacial surgery outpatient Department of our hospital. The blood routine test did not show a positive result. The ultrasound examination indicated that a hypoechoic mass could be seen in the superficial part of the right masseter muscle of the patient, approximately 1.7*1.1 cm in size, with a clear boundary, regular shape, and uniform internal echo. No obvious blood flow signal was observed within it. Multiple lymph nod-like echoes could be seen in the right submandibular gland area, approximately 2.0*1.0 cm in size, with a clear structure and no obvious blood flow signal. Specialized physical examination indicated that the swelling range in the right submandibular area was approximately 2.0*1.5 cm (as shown in Figure 1), with local skin redness and swelling, elevated local skin temperature, tenderness (+), local fluctuation sensation, and slightly limited occlusal function. The re-diagnosis was submandibular space infection. Intravenous infusion of clindamycin, levofloxacin, ornidazole and other drugs for 3 days, but the therapeutic effect was not good. On November 29, 2024, the patient was referred to the Department of Infectious Diseases of our hospital. Based on her long-term epidemiological history of contact (including feeding and delivering) with livestock such as cattle and sheep, brucellosis is highly suspected. The suspension was sent to Jilin Jinyu Medical Laboratory for sequencing of the next generation of the metagenome (mNGS) the results showed that the relative abundance of Brucella with sequence number of 9133191 was 99.97%, and the coverage of Brucella suis with sequence number of 8525228 was 98.94%. Supplementary Brucella test tube agglutination method test suggests Brucella test tube agglutination 1:200++++. The blood culture and drug sensitivity tests were completed, indicating that the pathogenic bacterium was Brucella. Combined with the epidemiological history, metagenomic next-generation sequencing (mNGS) (as shown in Figure 2) and other auxiliary examination results, the diagnosis was modified to brucellosis. Surgical dressing changes were performed daily, and intravenous infusion of doxycycline 0.1 g q12h and rifamycin 0.5 g q12h was carried out. After 6 days of anti-brucellosis treatment, the patient’s symptoms were relieved and the patient was discharged. Blood cultures were negative at the first, third and sixth month after discharge (see Table 1).
Figure 1
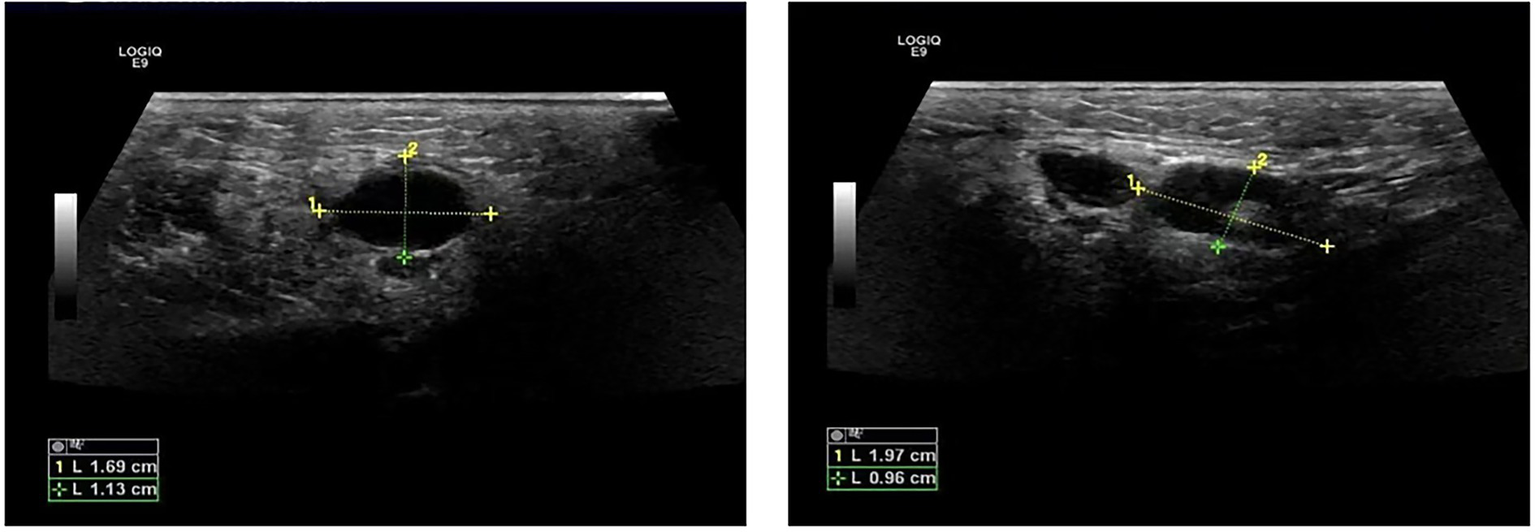
Ultrasound images of abscess.
Figure 2
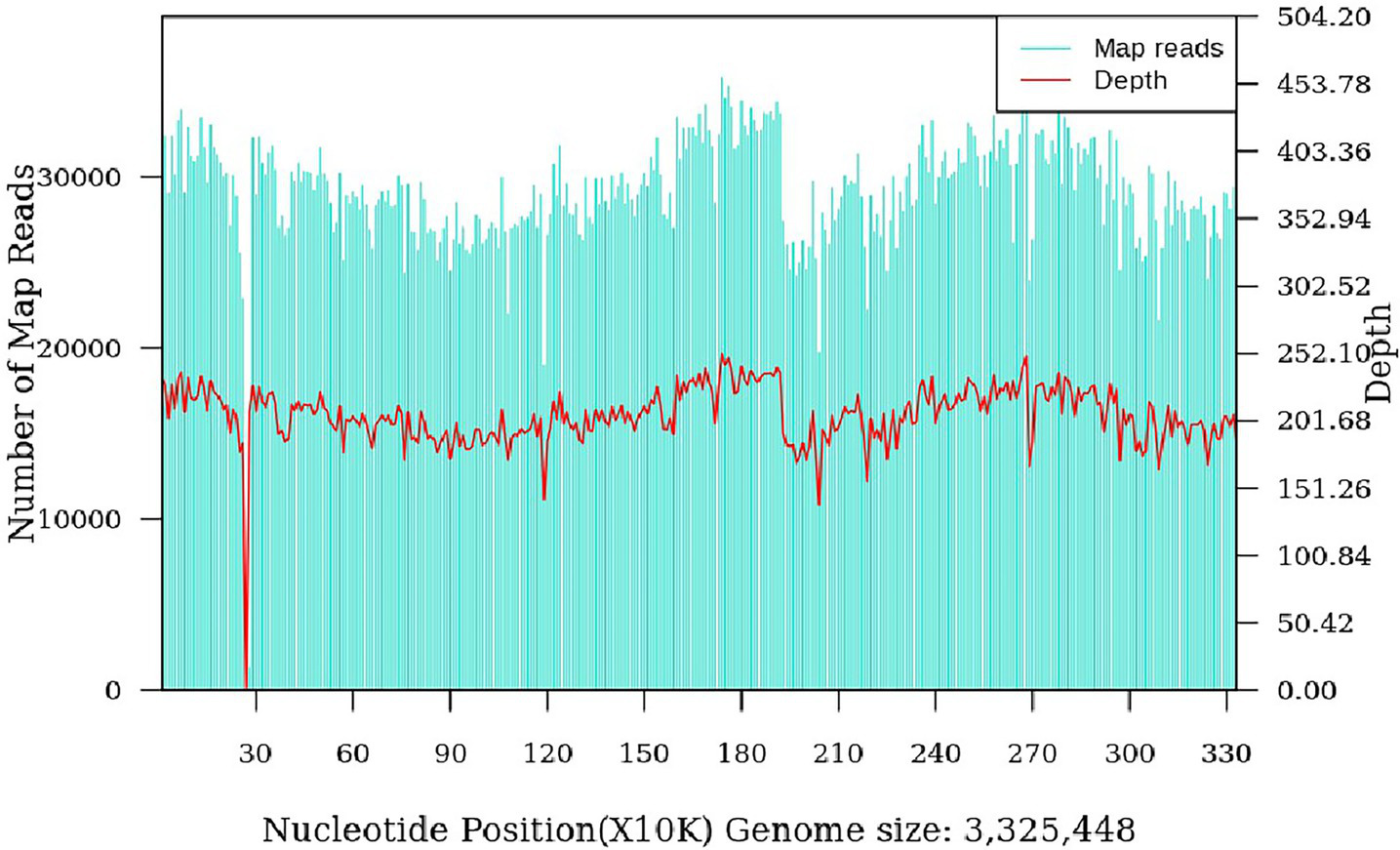
Brucella suis genome coverage map (coverage: 98.94%).
Table 1
| Test item | Day1 | Day5 | Day8 | Day13 |
|---|---|---|---|---|
| WBC (3.5–9.5/L) | 9.36*109 | 8.41*109 | 5.83*109 | 3.88*109 |
| NEUT% (40.0–75.0%) | 55.3 | 76.8 | 60.1 | 42.8 |
| NEUT# (1.8–6.3/L) | 5.17*109 | 6.46*109 | 3.50*109 | 1.66*109 |
| LYMPH% (20.0–50.0%) | 31.6 | 15.3 | 23.3 | 39.4 |
| LYMPH# (1.1–3.2/L) | 2.96*109 | 1.29*109 | 1.36*109 | 1.53*109 |
| MONO% (3.0–10.0%) | 10.9 | 6.9 | 14.6 | 13.7 |
| MONO# (0.10–0.60/L) | 1.02*109 | 0.58*109 | 0.85*109 | 0.53*109 |
| HGB (115–150 g/L) | 124 | 123 | 108 | 105 |
| HCT (0.35–0.45 L/L) | 0.401 | 0.390 | 0.346 | 0.337 |
| MCHC (316-354 g/L) | 309 | 315 | 312 | 312 |
| RDW-CV (12.0–14.3%) | 15.3 | 15.6 | 15.5 | 14.9 |
| RDW-SD (37.1–49.2 fL) | 50.5 | 51.1 | 50.4 | 48.6 |
| IG% (0.16–0.61%) | 1.2 | 0.6 | 0.3 | 0.5 |
| IG# (0.01–0.03/L) | 0.11*109 | 0.05*109 | 0.02*109 | 0.02*109 |
| K (3.5–5.3 mmol/L) | – | 3.94 | 3.94 | 4.64 |
| Hs-CRP (0–10.0 mg/L) | – | 4.65 | 8.15 | 5.34 |
Laboratory test.
Discussion
This case reports a case of brucellosis with infection in a rare site as the initial symptom and without fever, presenting as a submandibular space abscess without typical fever symptoms. Although brucellosis is relatively common in epidemic areas, its clinical manifestations are atypical brucellosis and diagnostic delays caused by traditional culture methods, highlighting the diagnostic challenges of zoonotic brucellosis. However, metagenomic next-generation sequencing (mNGS) can identify pathogens as early as possible and prompt the treatment plan to be rapidly adjusted from broad-spectrum antibiotics to targeted therapy with doxycycline combined with rifamycin, which aligns with the standard recommended regimen outlined in the World Health Organization’s guidelines for the treatment of human brucellosis and is in line with the strategic goal of the World Health Organization to curb antibiotic resistance (8, 9).
The pathogenic mechanism of Brucella suis in this case is consistent with the results of molecular studies in recent years. Two studies in 2024 revealed that the T4SS secretion system of Brucella suis promotes soft tissue colonization by regulating host cell apoptosis, providing a molecular mechanism explanation for the formation of local abscesses (10, 11). Furthermore, the patient’s long-term occupational exposure to cattle and sheep—including feeding and assisting with deliveries—supports the hypothesis of pathogen invasion through skin breaches or aerosols, with subsequent macrophage-mediated lymphatic spread to the submandibular space, particularly in the context of potential predisposing factors, such as the patient’s known hypertension, which may be associated with altered immune function.
However, the traditional culture methods have a misdiagnosis rate as high as 25–40% (12, 13) due to the slow growth of Brucella (requiring 5–7 days) and interspecific phenotypic overlap. This limitation is consistent with the conclusion of a 2022 study, which confirmed that mNGS can significantly reduce medical costs through rapid detection (specificity >99%) and early optimized treatment (6, 14).
This study is the first to report brucellosis with the main clinical manifestation of submandibular space infection, which expands the clinical pedigree of brucellosis involving rare anatomic sites and fills the literature gap of brucellosis involving rare sites of head and neck. This case suggests that even in non-epidemic areas, there should be diagnostic vigilance for unexplained soft tissue infections (especially those with a history of contact with livestock), and brucellosis should also be included in the differential diagnosis (15); this finding underscores the pivotal role of technological innovation in clinical practice. By enabling rapid pathogen identification and guiding optimal treatment, mNGS plays a crucial role in addressing the global challenge of antimicrobial resistance. However, the need to further explore the cost-effectiveness of their application in low- and middle-income countries has led to mNGS reasons for not being widely used (6, 16); Moreover, this case exemplifies the necessity of a multidisciplinary approach for complex infection management, highlighting the urgent need to establish standardized guidelines that enhance collaboration and improve patient outcomes. Finally, a standardized multidisciplinary diagnosis and treatment framework needs to be constructed, and clinical education on atypical brucellosis manifestations should be strengthened to optimize diagnosis, treatment and follow-up, and ultimately reduce the global burden caused by misdiagnosis of zoonoses and the abuse of antibiotics (8).
Limitations
This study has several limitations. First, the findings are based on a single case report, which limits the generalizability of our conclusions. Second, while mNGS proved decisive in this instance, its higher cost and limited accessibility in low-resource settings may restrict its widespread adoption in areas where brucellosis is endemic. Future multi-center studies with larger sample sizes are needed to validate our findings and further evaluate the cost-effectiveness of mNGS in the management of atypical brucellosis.
Patient perspective evaluation
Following an accurate diagnosis and targeted treatment, the patient’s symptoms resolved rapidly, thereby avoiding a prolonged period of misdiagnosis and ineffective therapy. This experience, as a livestock farmer, heightened her awareness of occupational exposure risks, prompting a commitment to implement stricter protective measures and undergo regular physical examinations in the future.
Statements
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Medical ethics committee of Affiliated Hospital of Inner Mongolia University for the Nationalities. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article. Written informed consent was obtained from the participant/patient(s) for the publication of this case report.
Author contributions
DA: Writing – original draft. XL: Formal analysis, Data curation, Writing – original draft, Methodology, Investigation, Writing – review & editing. GZ: Writing – original draft. HM: Writing – original draft. LY: Writing – original draft. HT: Writing – original draft. SA: Writing – original draft. JF: Writing – original draft. WG: Writing – review & editing, Methodology, Project administration, Writing – original draft, Investigation, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Supported by Natural Science Foundation of Inner Mongolia Autonomous Region (Grant No.: 2024MS08084).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Jiang H O'Callaghan D Ding JB . Brucellosis in China: history, progress and challenge. Infect Dis Poverty. (2020) 9:55. doi: 10.1186/s40249-020-00673-8
2.
Liu Z Gao L Wang M Yuan M Li Z . Long ignored but making a comeback: a worldwide epidemiological evolution of human brucellosis. Emerg Microbes Infect. (2024) 13:2290839. doi: 10.1080/22221751.2023.2290839
3.
Pappas G Papadimitriou P Akritidis N Christou L Tsianos EV . The new global map of human brucellosis. Lancet Infect Dis. (2006) 6:91–9. doi: 10.1016/S1473-3099(06)70382-6
4.
Roop RM Barton IS Hopersberger D Martin DW . Uncovering the hidden credentials of Brucella virulence. Microbiol Mol Biol Rev. (2021) 85:e00021-19. doi: 10.1128/MMBR.00021-19
5.
Gu Z Yang Z Fei L Wei D Ma L Liu Q et al . Quantifying research hotspots and trends in Brucella spondylitis: a bibliometric analysis. Front Surg. (2024) 11:1465319. doi: 10.3389/fsurg.2024.1465319
6.
Gu W Miller S Chiu CY . Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. (2019) 14:319–38. doi: 10.1146/annurev-pathmechdis-012418-012751
7.
Chiu CY Miller SA . Clinical metagenomics. Nat Rev Genet. (2019) 20:341–55. doi: 10.1038/s41576-019-0113-7
8.
Qureshi KA Parvez A Fahmy NA Abdel Hady BH Kumar S Ganguly A et al . Brucellosis: epidemiology, pathogenesis, diagnosis and treatment-a comprehensive review. Ann Med. (2023) 55:2295398. doi: 10.1080/07853890.2023.2295398
9.
Okeke IN de Kraker MEA Van Boeckel TP Kumar CK Schmitt H Gales AC et al . The scope of the antimicrobial resistance challenge. Lancet. (2024) 403:2426–38. doi: 10.1016/S0140-6736(24)00876-6
10.
Zheng M Lin R Zhu J Dong Q Chen J Jiang P et al . Effector proteins of type IV secretion system: weapons of Brucella used to fight against host immunity. Curr Stem Cell Res Ther. (2024) 19:145–53. doi: 10.2174/1574888X18666230222124529
11.
He J Yin S Deng X Ma Z Zhang H Miao Y et al . The effector protein BspE affects Brucella survival by regulating the inflammatory response and apoptosis. Int Immunopharmacol. (2025) 144:113576. doi: 10.1016/j.intimp.2024.113576
12.
Al Dahouk S Tomaso H Nöckler K Neubauer H Frangoulidis D . Laboratory-based diagnosis of brucellosis—a review of the literature. Part II: serological tests for brucellosis. Clin Lab. (2003) 49:577–89.
13.
Godfroid J Al Dahouk S Pappas G Roth F Matope G Muma J et al . A "one health" surveillance and control of brucellosis in developing countries: moving away from improvisation. Comp Immunol Microbiol Infect Dis. (2013) 36:241–8. doi: 10.1016/j.cimid.2012.09.001
14.
Xi H Zhang L Xu B Liu H Li S . Metagenomic next-generation sequencing to investigate infectious endophthalmitis of Brucella: a case report. Front Med (Lausanne). (2022) 9:847143. doi: 10.3389/fmed.2022.847143
15.
Pereira CR Cotrim de Almeida JVF Cardoso de Oliveira IR Faria de Oliveira L Pereira LJ Zangerônimo MG et al . Occupational exposure to Brucella spp.: a systematic review and meta-analysis. PLoS Negl Trop Dis. (2020) 14:e0008164. doi: 10.1371/journal.pntd.0008164
16.
Purushothaman S Meola M Egli A . Combination of whole genome sequencing and metagenomics for microbiological diagnostics. Int J Mol Sci. (2022) 23:9834. doi: 10.3390/ijms23179834
Summary
Keywords
brucellosis, submandibular space infection, metagenomic next-generation sequencing, Brucella suis , high-throughput sequencing
Citation
Ao D, Li X, Zhang G, Ma H, Yang L, Tian H, Ao S, Feng J and Geng W (2025) Diagnostic value of metagenomic next-generation sequencing in atypical brucellosis: a case report. Front. Med. 12:1652671. doi: 10.3389/fmed.2025.1652671
Received
24 June 2025
Accepted
25 September 2025
Published
10 October 2025
Volume
12 - 2025
Edited by
Zhangnv Yang, Zhejiang Center for Disease Control and Prevention (Zhejiang CDC), China
Reviewed by
Ali Sobhy Dawood, Mississippi State University, United States
Lv Jun, First Affiliated Hospital of Zhengzhou University, China
Updates
Copyright
© 2025 Ao, Li, Zhang, Ma, Yang, Tian, Ao, Feng and Geng.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wanru Geng, gengwanru@imun.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.