Abstract
Human T-lymphotropic virus type 1 (HTLV-1) is a retrovirus affecting 10–20 million people worldwide. While many carriers remain asymptomatic, HTLV-1 infection can trigger intense inflammatory responses which are defined by the sustained release of pro-inflammatory cytokines and chemokines. Central to this process is the HTLV-1 encoded Tax oncoprotein, a viral regulator that drives uncontrolled inflammation by hijacking multiple cellular signaling pathways, such as the RelA/NF-κB signal transduction pathway. CD4 T-cells are the primary targets of Tax-mediated transformation, undergoing uncontrolled proliferation and significantly contributing to chronic immune activation seen in HTLV-1-associated diseases. However, highly activated CD4 T-cells are not alone in fueling this inflammatory “wildfire.” Other immune cells, including CD8 T-cells, monocytes, macrophages, dendritic cells, and neutrophils, also play critical roles in exacerbating the inflammatory milieu. These cells, in conjunction with CD4 T-cells, release a barrage of pro-inflammatory cytokines (IL-1α/β, IL-2, IL-6, IL-12, IL-17, TNF-α/β, and IFN-γ) and chemokines (MCP-1, MIP-1α/β, RANTES, MCP-3, IL-8, CXCL9, CXCL10, and CXCL11), all of which are perpetuating the cycle of immune activation and tissue damage. This hyper stimulated immune response contributes to HTLV-1 replication/dissemination and can lead to the development of adult T-cell leukemia/lymphoma (ATLL) and HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM-TSP). Despite existing treatments aimed at controlling viral replication, the persistent inflammation in HTLV-1-infected individuals even in asymptomatic carriers (ACs) remains a major challenge, suggesting that targeting these pro-inflammatory responses may be another mandatory therapeutic strategy. In this context, this short-review focuses on the key immune responses that drive HTLV-1-associated inflammation and explores how these high pro-inflammatory responses contribute to the development of HTLV-1-related complications, including HAM-TSP, ATLL, and other associated inflammatory diseases during chronic viral infection.
1 Introduction: the need to develop new strategies for counteracting HTLV-1
The infection with the human T-cell leukemia virus type 1 (HTLV-1), the only known human oncogenic retrovirus, has been recently estimated to affect up to 20 million people worldwide. It is predominantly spreading across endemic regions in Japan, Africa, Asia, the Caribbean, Central/South America, the Middle East and includes the Australo-Melanesia area (1–3). The virus is transmitted through the bodily fluids of infected individuals, primarily breast milk, blood, and semen (4, 5). Although approximately 90% of the infected individuals remain asymptomatic carriers during their lives, chronic infection with HTLV-1 can result in multiple severe pathologies; these include the adult T-cell leukemia/lymphoma (ATLL), an aggressive neoplasm of CD25+ CD4 T-cells in about 5% of infected individuals after a prolonged latent period of 30–50 years (2, 6). HTLV-1 infection is also the causative agent of inflammatory disorders, most notably HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) asides other afflictions, such as uveitis, a chronic inflammatory interstitial lung disease called cryptogenic fibrosing alveolitis (CFA), rheumatic syndromes and a high predisposition to glaucoma, sarcopenia, atherosclerosis, helminthic and bacterial infections (7–10). Currently, there are no prospects of functional vaccines for HTLV-1, screening of blood banks and there are no universal diagnostic tools in prenatal care settings. Existing treatments for ATLL and HAM/TSP are largely ineffective, thus emphasizing the urgent need for new targeted therapies (1, 11–14). A deeper understanding of how HTLV-1 infection impacts immune responses and persist in the host is a critical step for the development of these novel antiviral strategies. In this context, this short-review aims to provide a brief overview of the uncontrolled inflammatory responses reported in HTLV-1 infections and how they actively contribute to viral dissemination and disease development.
2 HTLV-1 infection causes strong and sustained inflammatory responses
The immunopathogenesis of HTLV-1 is intriguing, since its lifelong persistence in the host determines a prolonged interaction between the virus and the immune system, which can ultimately contribute to the development of both ATLL and HAM/TSP conditions when inflammatory responses become uncontrolled. Although CD4 T-cells remain the main cell target for HTLV-1 (1, 15), the virus can also infect CD8 T-cells and immune cells of the myeloid lineage like dendritic cells (DCs), monocytes, and macrophages, altogether sustaining a strong poly-inflammatory milieu in the infected hosts due to HTLV-1 persistence (16–19). Although multiple causal factors during chronic HTLV-1 infection contribute to trigger the uncontrolled inflammatory responses, HTLV-1 Tax protein, the host innate sensing and high TNF-α release play a pivotal role in the process, mainly by constitutively inducing RelA/NF-κB signal transduction pathway in infected individuals (Figure 1 and Tables 1, 2).
FIGURE 1
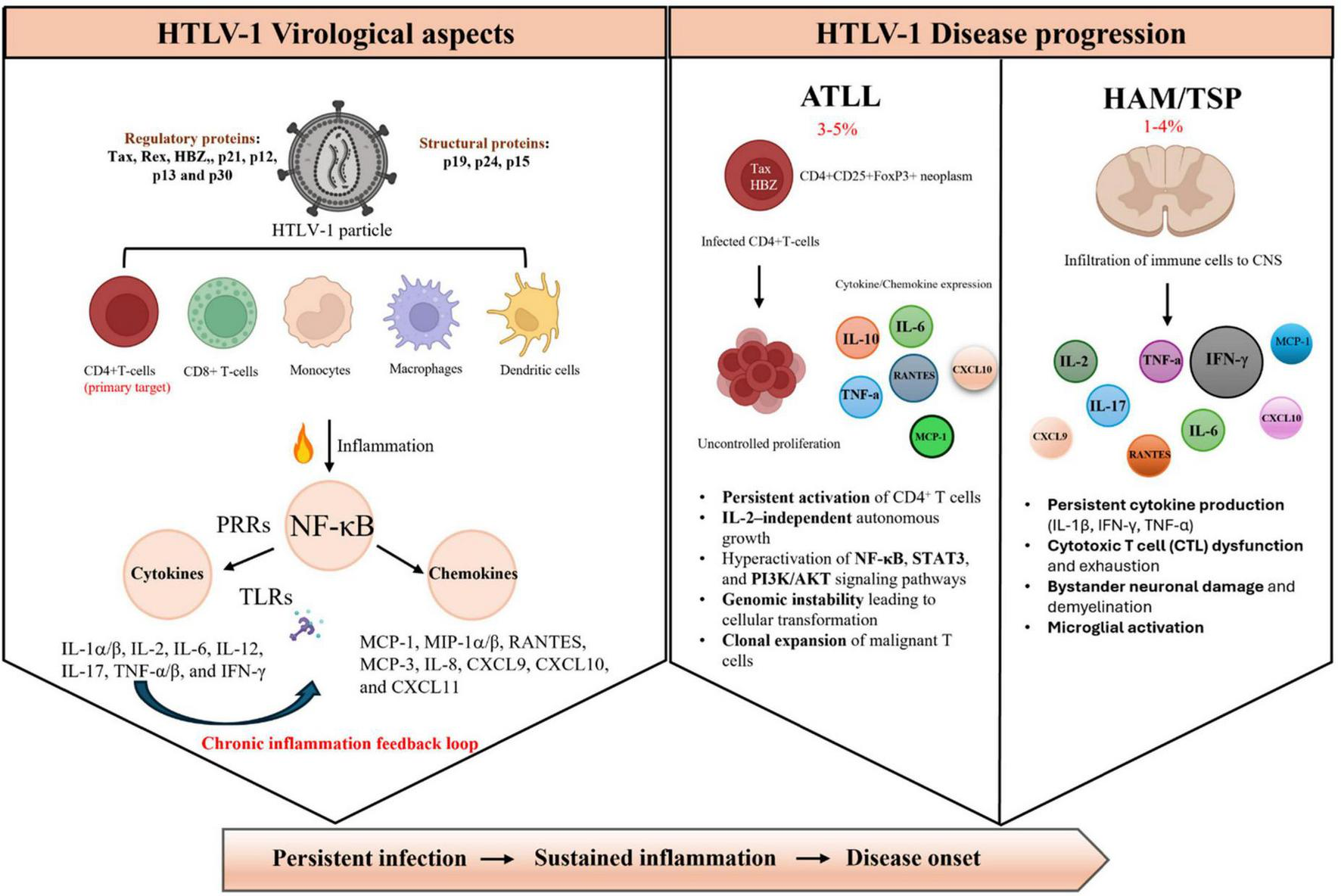
Schematic representation of HTLV-1 virological aspects and disease progression. (Left side) HTLV-1 infects multiple immune cell types, primarily CD41 T cells, but also CDS1 T cells, monocytes, macrophages, and dendritic cells. Viral proteins (regulatory: Tax, Rex, HBZ, p21, pl2, p13, p30; structural: pl9, p24, p15) activate pattern recognition receptors (PRRs) and toll-like receptors (TLRs), leading to NF-xB-mediated production of pro-inflammatory cytokines and chemokines, establishing a chronic inflammation feedback loop. (Right side) Disease outcomes include adult T-cell leukemia/lymphoma (ATLL, 3%–5% of infected individuals) characterized by uncontrolled CD4+ T-cell proliferation, persistent activation of NF-KB, STAT3, and PT3K/AKT pathways, and clonal expansion; and HTLV-1- associated myelopathy/tropical spastic paraparesis (HAM/TSP, 1%–4% of infected individuals) involving central nervous system (CNS) infiltration, persistent cytokine production, CTL dysfunction, and neuronal damage. ATLL, adult T-cell leukemia/lymphoma; CNS, central nervous system; CTL, cytotoxic T lymphocyte; CXCL, C-X-C motif chemokine ligand; HBZ, HTLV-1 basic leucine zipper factor; HAM/TSP, HTLV-1-associated myelopathy/tropical spastic paraparesis; IITLV-l, human T-cell leukemia virus type 1; IFN, interferon; IL, interleukin; MCP, monocyte chemoattractant protein; M1P, macrophage inflammatory protein; NF-kB, nuclear factor kappa-light-chain-enhancer of activated B cells; PI3K/AKT, phosphoinositide 3-kinase/protein kinase B pathway; PRRs, pattern recognition receptors; RANTES, regulated upon activation, normal T cell expressed and secreted; STAT3, signal transducer and activator of transcription 3; TLRs, toll-like receptors; TNF-a, tumor necrosis factor alpha.
TABLE 1
| Name | Aliases | Info in the context of HTLV-1 infection | Additional info | References |
| pro-lnflammatory cytokines | ||||
| IL-1 (α/β) | LAF | Tax-treated microglia cells secrete high protein levels for IL-lp | Cell supernatants (48 h of culture) | (39) |
| Higher protein release of IL-ip in HAM-TSP vs. HCs | PBMCsupernatants (24 h of unstimulated culture) | (82) | ||
| HTLV-1-infected CD4T-cells from HTLV-1+uveitis patients produced large amounts of IL-1 | Infiltrating CD4T-cells in eyes | (91) | ||
| High mRNA expression of both ILIA and IL1B in ATLLcells from Tax-transgenic mice | Expression in both Tax’ and Tax+cells | (105) | ||
| IL-2 | Lymphokine2 | Tax-treated MDDCs secrete IL-2cytokine in culture | Cell supernatants (24 h of culture) | (36) |
| Higher mRNA levels for IL2 in PBMCs from ATLL patients vs. HCs | Along with increased Nf-KB-related genes | (51) | ||
| Neutralization of IL-2 decreases IFN-y levels in PBMC culture from ACs | PBMCsupernatants (24–48 h of culture) | (62) | ||
| Higher plasma levels for IL-2 in HAM-TSP patients vs. ACs | Higher levels in HAM-TSP patients vs. ATLL | (67) | ||
| High production and cell dependency to IL-2 of HTLV-l-infected CD4T-cells for proliferation | Contribution to cell transformation (after weeks of culture stimulation) | (98) | ||
| IL-6 | Interferon beta-2 | Tax-treated microglia cells secrete high protein levels for IL-6 | Cell supernatants (48 h of culture) | (39) |
| Higher plasma levels for IL-6 in ATLLpatients with aggressive cancer form vs. “indolent” form | Correlation between high plasma IL-6levels and shorter survival rates in ATLL | (67) | ||
| Higher protein levels for IL-6 in both sera and CSF from HAM-TSP patients vs. ACs | Along with higher IL-6 activity | (69) | ||
| Higher mRNA levels of IL6 in neutrophils from HAM-TSP patients vs. ACs | Along with increased Nf-kB-related genes | (85) | ||
| HTLV-l-infected CD4T-cells from HTLV-1+uveitis patients produced large amounts of IL-6 | Infiltrating CD4T-cells in eyes | (91) | ||
| Higher sera levels for IL-6 in ATLL patients vs. ACs and HCs | Correlation between high plasma IL-6 levels and ATLL severity | (100) | ||
| High mRNA expression of both IL6 in ATLL cells from Tax-transgenic mice | Expression in both Tax’ and Tax+ cells | (105) | ||
| IL-12 | Tax-treated MDDCs secrete IL-12cytokine in culture | Cell supernatants (24/48 h of culture); induction in a Nf-KB-dependent manner | (36, 37) | |
| Higher proportion of IL-12-expressing monocytes and pDCs in HAM-TSP patients vs. ACs | PBMCs stimulated for 48 h of culture with TLR7/8 agonist (innate sensing) | (84) | ||
| Higher mRNA levels of IL17 in neutrophils from HAM-TSP patients vs. ACs | Along with increased Nf-kB-related genes | (85) | ||
| IL-17 | IL-17A; CTLA-8 | Higher mRNA levels of IL17 in PBMCsfrom ATLLpatients vs. HCs | Along with increased Nf-KB-related genes | (51) |
| Higher plasma levels for IL-17 in HAM-TSP patients vs. ACs | Higher levels in HAM-TSP patients vs. ATLL | (67) | ||
| Higher proportion of IL-17-expressing CD4T-cells in HAM-TSP patients vs. ACs and HCs | PBMC culture with 3 days of cell stimulation | (79) | ||
| Increased proportion of CD4 + CD8 + DP cells in PBMCs from HAM-TSP patients vs. ACs and HCs | DP cells are strong IL-17 producers among T-cell lineage | (80) | ||
| Higher mRNA levels of IL17 in neutrophils from HAM-TSP patients vs. ACs | Along with increased Nf-kB-related genes | (85) | ||
| TNF (α/β) | TNFSF 1,2 | Tax-treated MDDCs secrete TNF-a and -0 cytokines in culture | Cell supernatants (24/48 h of culture); induction in a Nf-KB-dependent manner | (36, 37) |
| Tax-treated microglia cells secrete high protein levels for TNF-a | Cell supernatants (48 h of culture) | (39) | ||
| Higher mRNA levels of IL17 in PBMCs from ATLL patients vs. HCs | Along with increased Nf-KB-related genes | (51) | ||
| Higher sera/plasma levels for TNF-a in HAM-TSP vs. ACs | (53) | |||
| Higher plasma levels for TNF-a in ATLLpatients with aggressive cancer form vs. “indolent” form | Correlation between high plasma TNF-a levels and shorter survival rates in ATLL | (67) | ||
| Increased proportion of CD4 + CD8 + DP cells in PBMCs from HAM-TSP patients vs. ACs and HCs | DP cells are strong TNF-a producers among T-cell lineage | (80) | ||
| Higher TNF-a expression in CD14+monocytes from HAM-TSP vs. HCs | 24 h of unstimulated PBMC culture | (82) | ||
| Maintenance of high TNF-a production by CD14+CD16+ monocytes in HAM-TSP | Maintenance despite GM-CSF and IL-4 DC-driven differentiation | (83) | ||
| Higher mRNA levels of TNFA in neutrophils from HAM-TSP patients vs. ACs | Along with increased Nf-kB-related genes | (85) | ||
| HTLV-l-infected CD4T-cells from HTLV-1+ uveitis patients produced large amounts of TNF-a | Infiltrating CD4T-cells in eyes | (91) | ||
| Increased TNF-a expression in FoxP3+ splenocytes from HBZ transgenic mice | Also higher IL-2 and IL-17 expressions in FoxP3 + cells from HBZ transgenic mice | (107) | ||
| IFN-γ | IFNG; IFG | Tax-treated MDDCs secrete IFN-y cytokine in culture | Cell supernatants (24/48 h of culture) | (36) |
| Higher sera/plasma levels for IFN-y in HAM-TSP vs. ACs | Correlation between IFN-y and IL-6 levels in HAM-TSP patients | (53) | ||
| Higher IFN-y production in PBMC supernatant of HAM-TSP patients vs. ACs (3 days if unstimulated culture) | Correlation between IFN-y and CXCL9/or CXCL10 levels in HAM-TSP patients | (66) | ||
| Higher plasma levels for IFN-y in HAM-TSP patients vs. ACs | Higher levels in HAM-TSP patients vs. ATLL | (67) | ||
| Higher IFN-y production in Tax-stimulated PBMCs in HAM-TSP patients vs. ACs and HCs | Higher IFN-y levels in ACs vs. HCs | (77) | ||
| Higher plasma and mRNA levels (within PBMC) for IFN-y in HAM-TSP patients vs. Acs and HCs | Higher IFN-y levels in ACs vs. HCs (plasma and mRNA levels in PBMCs) | (78) | ||
| Higher proportion of IFN-y-expressing CD8T-cells in HAM-TSP patients vs. ACs and HCs | PBMC culture with 3 days of cell stimulation | (79) | ||
| Increased proportion of CD4 + CD8 + DP cells in PBMCs from HAM-TSP patients vs. ACs and HCs | DP cells are strong IFN-y producers among T-cell lineage | (80) | ||
| Higher proportion of IFN-y-expressing CD56h′8hCD16′NKs in HAM-TSP patients vs. ACs | PBMCs stimulated for 48 h of culture with TLR7/8 agonist (innate sensing) | (84) | ||
| HTLV-l-infected CD4T-cells from HTLV-1+ uveitis patients produced large amounts of IFN-y | Infiltrating CD4T-cells in eyes | (91) | ||
| Increased IFN-y expression in both FoxP3+ and FoxP3’splenocytes from HBZ transgenic mice | Higher IFN-y-producing cells in both lung and PBMCs in HBZ transgenic mice | (107) | ||
Pro-inflammatory cytokines and HTLV-1 infection, including associations with virus-related HAM-TSP and ATLL conditions.
CTLA-8, cytotoxic T-lymphocyte-associated protein 8; IFN-y, interferon gamma; IL, interleukin; LAF, lymphocyte activatory factor; TNFSF, tumor necrosis factor superfamily.
TABLE 2
| Name | Aliases | Info in the context of HTLV-1 infection | Additional info | References |
| Pro-inflammatory chemokines | ||||
| MCP-1 | CCL2 | Tax-treated MDDCs secrete MCP-1 chemokinein culture | Cell supernatants (24/48 h of culture); also induction of CCLU (eotaxin) | (36) |
| ATLL cells and HTLV-l-infected CD4 T-cell lines vs. uninfected cells display higher mRN A levels for MCP1 | Tax- and Nf-KB-dependent process | (38) | ||
| Higher sera and CSF protein levels for MCP-1 in both HAM-TSP patients and ACs vs. HCs | Also increased sera/CSF levels in HAM-TSP vs ACs for CCLU, CCL17 and CXCL5 | (72) | ||
| Increased mRNA expression in lung cells for MCP1 in Tax transgenic mice vs. WT animals | Along with increased nRNA levels for pro-inflammatory cytokines (IL1B, TNFA and IFNG) | (94) | ||
| MIP-lα/β | CCL3 (MIP-lα) and CCL4 (MIP-1β) | Tax-treated MDDCs secrete both MIP-lα and -β chemokines in culture | Cell supernatants (24 h of culture); MIP-lα induction in a Nf-KB-dependent manner | (37) |
| Tax-treated PBMCs induce both MIPl-α and -β secretions in culture su pern anta nt | Nf-KB-dependent processes; 2–24 h of culture | (40, 41) | ||
| Increased CSF levels for both MIPlα/β in HAM-TSP vs. ACs | No difference in sera levels | (72) | ||
| Higher proportion of MIP-lα-expressing monocytes and pDCs in HAM-TSP patients vs. ACs | PBMCs stimulated for 48 h of culture with TLR7Z8 agonist (innate sensing) | (84) | ||
| High BALF levels for MIP-lα in HTLV-l-infected patients vs. HCs | Correlation between MIP-lα levelsand% of activated T-cells in BALFfrom HTLV-1* patients with CFA | (93) | ||
| Increased mRNA expression in lung cells for MIP1A and IB inTax transgenic mice vs. WT animals | Along with increased nRN A levels for pro-inflammatory cytokines (IL1B, TNFA and IFNG) | (94) | ||
| Increased supernatant secretion for MIP-lα in HTLV-1+CD4 T-cell lines vs. HTLV-1’ cells | Tax-dependent manner process | (103) | ||
| RANTES | CCL5 | ATLL cells and HTLV-l-infected CD4T-cell lines vs uninfected cells display higher mRNA levels for RANTES | Tax- and Nf-KB-dependent process | (38) |
| Tax-treated PBMCs induce RANTES secretion in culture supernantants | Nf-KB-dependent processes | (40, 41) | ||
| Higher sera and CSF levels for RANTES in HAM-TSP patients vs. ACs | (53, 71) | |||
| Higher RANTES release by immature MDMs from HAM-TSP patients vs. ACs and HCs | Culture supernantant (48 h of unstimulated culture) | (81) | ||
| . Increased mRNA expression in lung cells for RANTES in Tax transgenic mice vs. WT animals | Along with increased nRNA levels for pro-inflammatory cytokines (IL1B, TNFA and IFNG) | (94) | ||
| Increased supernatant secretion for RANTES in HTLV-1* CD4 T-cell lines vs. HTLV-1’cells | Tax-dependent manner process | (103) | ||
| Increased secretion for RANTES a dn intracellular mRNA levels in HTLV-1* CD4T-cell lines vs. HTLV-1 cells | Tax- and Nf-KB-dependent manner process | (104) | ||
| MCP-3 | CCL7 | Tax-treated MDDCs secrete MCP-3chemokinein culture | Cell supernatants (24/48 h of culture); also induction of CCLU (eotaxin) | (36) |
| CXCL8 | IL-8 | Higher sera and CSF protein levels for IL-8 in both HAM-TSP patients and ACs vs HCs | Also increased sera/CSF levels in HAM-TSP vs. ACs for CCL11, CCL17 and CXCL5 | (72) |
| Increased supernatant secretion for IL-8 in HTLV-1* CD4 T-cell lines vs. HTLV-1’ cells | Tax-dependent manner process | (103) | ||
| CXCL9 | MIG | Higher sera and CSF levels for CXCL9 in HAM-TSP patients vs. ACs and HCs | Similar levels between ACs and HCs; CXCL9 levels strongly correlates with disease progression | (53, 66, 71, 72) |
| Higher CXCL9 release by immature MDMs from HAM-TSP patients vs. ACs and HCs | Culture supernantant (48 h of unstimulated culture) | (81) | ||
| CXCL10 | IP-10 | Higher sera and CSF levels for CXCL10 in HAM-TSP patients vs. ACs and HCs | Increased levels in ACsva HCs; CXCL10 levels strongly correlates with disease progression | (53, 66, 68, 71, 72) |
| Higher plasma levels for CXCL10 in HAM-TSP patients vs. ACs and ATLL | Trend of higher CXCL10 levels with aggressive forms of ATLL vs. “indolent” form | (67) | ||
| Higher CSF levels for CXCL10 in HAM-TSP patients vs. ACs | Higher CXCL10 levels in deteriorating patients with loss of motor function (weelchair) | (70) | ||
| Increased proportion of CD4 + CD8 + DP cells in PBMCs from HAM-TSP patients vs. ACs and HCs | DP cells are strong CXCL10 producers among T-cell lineage | (80) | ||
| High BALF levels for CXCL10 in HTLV-l-infected patients vs. HCs | Correlation between CXCL10 levels and% of activated T-cells in BALFfrom HTLV-1* patients with CFA | (93) | ||
| Increased supernatant secretion for CXCL10 in HTLV-1* CD4T-cell lines vs. HTLV-1’ cells | Tax-dependent manner process | (103) | ||
| CXCL11 | l-TAC | Higher sera and CSF levels for CXCLllin HAM-TSP patients vs. ACs and HCs | Similar levels between ACs and HCs | (53, 66, 71, 72) |
Pro-inflammatory chemokines and HTLV-1 infection, including associations with virus-related HAM-TSP and ATLL conditions.
CCL, C-C motif chemokine ligand; CXCL, C-X-C motif chemokine ligand; IP-10, interferon-induced protein 10; l-TAC, interferon-inducible T-cell alpha chemoattractant; MCP, monocyte chemoattractant protein; MIG, monokine induced by gamma interferon; MIP, macrophage inflammatory protein; RANTES, regulated on activation, normal T-cell expressed and secreted.
2.1 Impact of HTLV-1 tax protein
Like other retroviruses, the integrated HTLV-1 proviral genome is made up of two long terminal repeat sequences, flanking structural genes gag, pol, and env. HTLV-1 also has a unique 1.6 kb accessory region, termed the pX region, which encodes a few regulatory viral proteins when cells are productively infected (14, 20, 21). These mainly include the expression of the trans-activator protein Tax, which is known to hijack multiple intracellular signaling pathways that contribute to inflammation and immune activation, thereby ultimately promoting the proliferation of HTLV-1-infected T-cells and viral dissemination (22, 23). Among those, the nuclear transcription factor NF-κB plays a central role in coordinating various cellular signals that serve as pivotal mediators of inflammatory responses in the form of multiple encoding cytokines and chemokines (IL-1β, IL-2, IL-6, IL-8, TNF-α, MIP-1α/β and RANTES among others) (22, 24–26). The prototypical NF-κB complex corresponds to a heterodimer of the NF-κB1 (p50) and RelA (p65) members of the NF-κB/Rel family of transcription factors (27). Evidence shows that HTLV-1 Tax has developed multiple hijacking strategies to activate NF-κB signaling pathway; First, it induces the phosphorylation and degradation of both IκBα and IκBβ through the activation of the IκB kinase (IKK) complex, resulting in the nuclear translocation of active NF-κB (27–29). Tax also recruits the co-activator protein p300/CBP (30, 31) whose nuclear interaction with the RelA subunit of NF-κB is vital for RelA-dependent gene transcription (32). Finally, Tax stimulates the catalytic activity of the IKK-activating kinase TAK1 and mediates the physical recruitment of IKK to TAK1
in productively infected cells, including Tax-positive HTLV-1-transformed T-cells (33–35). Evidence shows that HTLV-1 Tax induces the secretion of multiple pro-inflammatory cytokines (IL-2, IL-12, TNF-α/β, and IFN- γ) and chemokines (MCP-1, MIP-1α/β, and MCP-3) in immature monocyte-derived dendritic cells (MDDCs) in a NF-κB-dependent manner (36, 37). Jurkat CD4 T-cell line, when treated with Tax, induces transactivation of the MCP1 gene (38). Both peripheral monocyte-derived macrophages (MDMs) and microglia (specialized cells, acting as the brain’s resident macrophages), when cultivated in vitro with HTLV-1 Tax, secrete high amounts of pro-inflammatory IL-1β, and IL-6, and TNF-α cytokines (39). Similarly, HTLV-1 Tax mediates MIP-1α/β and RANTES expression in peripheral mononuclear cells (PBMCs) via the NF-κB signaling pathway (40, 41). Finally, although Tax expression in HTLV-1-infected individuals is tightly regulated and often silenced to evade immune detection, especially in ATLL patients, it can still be reactivated by multiple stressors, such as hypoxia, T-cell reactivation and oxidative stress, thereby sustaining viral persistence and long-lasting proinflammatory immune responses (42–44). So far, HTLV-1 Tax is one of the key viral proteins which has comprehensive executive function associated with developing HAM-TSP and ATLL conditions, that especially contribute in tissue inflammation/damage and T-cell hyperimmune activation (24, 45–48).
2.2 Impact of host innate sensing and autocrine regulation by TNF-α
Innate immune-mediated inflammation plays a critical role in inhibiting pathogenic viruses through the recognition of multiple viral components by the host pattern recognition receptors (PRRs) (49). In the context of HTLV-1 infection, the replication cycle yields multiple pathogen-associated molecular patterns such as viral RNA, RNA/DNA intermediates, and single- or double-stranded DNA, which are recognized by cytosolic sensors including cGAS, IFI-16, along with Ku70, which activate the STING-TBK-1 axis to induce IRF3-driven type I interferon responses. Endosomal PRRs such as toll-like receptor 3 (TLR3), TLR7/8, and TLR9 also respond to viral RNA or CpG-rich DNA via TRIF- or MyD88-dependent pathways, converging on both IRF3 and NF-κB inflammatory signaling (50). Surface TLRs such as TLR2 and TLR4 actively participate in sensing; For example, the accessory protein HTLV-1 p30 antagonizes TLR4 signaling in monocytes and dendritic cells, thereby deregulating MCP-1, TNF-α, IL-8 and IL-10 production (51). Its regulatory protein Tax robustly activates NF-κB and AP-1 transcription factors, driving expression of IL-2, IL-6, TNF-α, CCL2/MCP-1, and CXCL10 (47, 52), whereas HTLV-1 HBZ protein attenuates IRF3-mediated interferon signaling, dampening IFN-I responses (53). Additionally, HTLV-1 p12 and p8 proteins can both modulate IL-2 receptor signaling and enhance STAT5 activation even in the absence of IL-2, while facilitating immune evasion and cell-to-cell transmission (54, 55). Collectively, this complex interplay of innate sensing and viral countermeasures orchestrates a potent inflammatory and chemotactic cytokine milieu -comprising IL-1β, IL-2, IL-6, TNF-α, CCL2, CXCL10, and RANTES - that underlies chronic inflammation in HTLV-1–associated diseases such as ATLL and HAM-TSP (16, 50). Although there are intricate strategies employed by HTLV-1 to subvert the host innate IFN-I responses that contribute both to viral immune evasion and the development of HTLV-1-associated diseases (50), chronic HTLV-1 infection is still associated with unrelenting host innate sensing and immune activation accompanied by high levels of pro-inflammatory cytokines/chemokines (56–59). This outcome likely results from the host’s continuous, albeit unsuccessful, attempts to eradicate viral infection and maintain tissue homeostasis. In this context, the pro-inflammatory TNF-α cytokine, known for triggering upstream signaling events leading to the autocrine activation of NF-κB pathway (60), is likely one of the critical players of sustained HTLV-1-driven inflammation. Recent studies have shown that anti-TNF-α agents can be effective in treating inflammatory diseases related to HTLV-1 (such as arthropathy, uveitis, and other rheumatic conditions associated with the given viral infection) (61–64). In fact, the inflammatory response triggered by the increased of TNF-α along with IFN-γ and IL-2, mainly by the CD4 T-cell response, is what maintains the chronic inflammatory process in HAM-TSP patients (65–67).
3 Strong pro-inflammatory signatures in HAM-TSP and ATLL patients
Evidence shows that both aberrant expression and/or function of pro-inflammatory cytokines and chemokines actively contribute to the pathogenesis of HTLV-1-associated diseases involved in the inflammation of the central nervous system (CNS), which occurs in cases of HAM-TSP, as well as T-cell immortalization and tissue infiltration observed in ATLL patients (19, 68, 69) (Figure 1 and Tables 1, 2). In addition to immune activation, chronic HTLV-1–driven inflammation may also foster a pro-angiogenic microenvironment. Tax-mediated NF-κB activation stimulates the expression of vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF), potent mediators of angiogenesis. These factors promote endothelial proliferation, vascular remodeling, and increased permeability changes that may facilitate tumor cell migration to lymph nodes in ATLL. Thus, angiogenesis represents a complementary mechanism through which HTLV-1–induced inflammation may contribute to disease progression (70, 71).
3.1 Profile in HAM-TSP
HTLV-1-associated myelopathy/tropical spastic paraparesis is a progressive disease of the CNS that causes weakness or paralysis of the legs, lower back pain, and urinary symptoms, which occur in approximately 2%–3% of HTLV-1 carriers (72). This HTLV-1-related disease is characterized by a hyper-stimulated immune response, which includes elevated levels of inflammatory cytokines and chemokines, and the recruitment/oligonal expansion of virus-specific cytotoxic CD8 T-cells in the CNS; all of which contributes to nerve tissue damage and loss of motor functions in patients. In this context, data show that the sera/plasma and cerebrospinal fluid (CSF) from HAM-TSP patients always display higher protein levels of IL-2, IL-6, IL-17, TNF-α, IFN-γ, MCP-1, RANTES, CXCL9, CXCL10, and CXCL11 when compared to ACs (58, 73–80). Data also show increased ex vivo levels of CSF neopterin in HAM-TSP patients (77, 79–81), which is a molecule synthesized by macrophages upon stimulation with IFN-γ and is indicative of a pro-inflammatory immune status (82). In fact, CSF CXCL9, CXCL10, and neopterin are described as trustworthy prognostic biomarkers for HAM-TSP disease progression (77–79, 81, 83). Although no differences are detected between HAM-TSP patients and ACs, levels of MCP-1 and IL-8 chemokines are higher in the sera and CSF from HTLV-1-infected individuals when compared to HCs (79). Research studies have further confirmed the higher cellular ability of HAM-TSP patients to secrete pro-inflammatory cytokines and chemokines in culture. For example, peripheral blood mononuclear cells (PBMCs) and CD8 T-cells of HAM-TSP patients stimulated or not with Tax peptides display higher mRNA and protein levels of IFN-γ when compared to both ACs and HCs (84–86). PBMCs from HAM-TSP patients further show an increased proportion of IL-17-expressing CD4 T-cells (86) and of inflammatory CD4+CD8+ T-cell populations whose IFN-γ, TNF-α, IL-17, and CXCL10 productions are one of the most pronounced among stimulated T-cells (87). Immature MDMs from HAM-TSP patients, when incubated in unstimulated cultures, show higher spontaneous secretion of RANTES and CXCL9 chemokines in comparison to both ACs and HCs (88). It is worth noting that pro-inflammatory CD16+ monocytes of HAM-TSP patients, whose proportions are increased in PBMCs in comparison to both ACs and HCs (88), are unable to fully mature into dendritic cells and maintain a high production of TNF-α and IL-1β cytokines (89, 90). Similarly, higher proportions of pro-inflammatory monocytes producing IL-12 and MIP-1α, plasmacytoid DCs producing IL-12 and CD56highCD16– natural killer cells producing IFN-γ are detected in HAM-TSP blood samples in response to innate immune sensing when compared to ACs (91). The comparative gene expression profiles of polynuclear neutrophils between ACs and HAM-TSP patients have revealed higher expression of multiple genes related to the Nf-kB signaling pathway and pro-inflammatory responses including TNFA, IL6, and IL17 (92). Altogether, this evidence infers that, during HAM-TSP development model, HTLV-1-infected cells in the CNS may produce large amounts of IFN-γ that can induce resident macrophages, DCs, neutrophils and astrocytes to secrete MCP-1, MIP-1α/β, RANTES, CXCL9, and CXCL10 chemokines among others (7, 93–95). The latter recruit more infected cells, including Tax-expressing CD4 T-cells, to the aera along with cytotoxic CD8 T-cells, which constitute a T-helper type 1 (Th1)-centric feedback loop (IL-1β, IL-2, TNF-α, IL-12 and IFN-γ) that results in chronic inflammation in the CNS (95–97).
3.2 Profile in other HTLV-1-associated inflammatory conditions
In addition to the HAM-TSP disease, HTLV-1 infection can cause inflammation in other tissues than CNS. In this context, HTLV-1-infected CD4 T-cells collected from patients suffering from uveitis, which is the second-most frequent HTLV-1-associated disease in Japan after HAM-TSP, also produce large amounts of various inflammatory cytokines such as IL-1, IL-6, TNF-α, and IFN-γ (98, 99). Similarly, evidence shows that the bronchoalveolar fluids (BALFs) from HTLV-1-infected patients with CFA, a chronic inflammatory lung disease of unknown etiology, display elevated levels of MIP-1α and CXCL10 chemokines, which correlate with higher tissue infiltration of activated T-cells (100, 101). Altogether, this indicates that, similarly to HAM-TSP, HTLV-1 infection may contribute to other HTLV-1-associated inflammatory diseases via the chemokine-dependent recruitment of activated T-cells in the eyes (uveitis) and lungs (CFA), thus resulting in chronic tissue inflammation by the sustained release of Th1 pro-inflammatory cytokines.
3.3 Profile in ATLL
Adult T-cell leukemia/lymphoma is a highly aggressive mature CD4+CD25+FoxP3+ T-cell neoplasm associated with chronic HTLV-1 infection, which affects around 10 million people worldwide. Although it is obvious that HTLV-1-associated inflammatory conditions (HAM-TSP, uveitis, and CFA) are associated with elevated pro-inflammatory innate and T-cell immune responses, ATLL patients exhibit a rather immunosuppressive profile that is mainly highlighted by the abnormally high production of IL-10 cytokine (102). However, ATLL is a complex and multistep disease, which involves HTLV-1 Tax and HBZ proteins and starts with high proliferation and survival of HTLV-1-infected CD4 T-cells (21, 103). In fact, one of the major hallmarks of HTLV-1-infected CD4 T-cells in ATLL is their ability to proliferate independently of T-cell receptor stimulation, contributing to the immortalization of these infected cells overtime (6, 21, 69). Although survival of HTLV-1-infected ATLL CD4 T-cells mainly depend on IL-10 production (74, 102, 104), evidence shows that the maintenance of elevated proliferation rates is rather supported by their dependency on pro-inflammatory cytokines such as IL-2 and IL-6. In this context, in vitro data confirm that HTLV-1-infected T-cells are dependent on IL-2 for their proliferation, until they get their immortalized status after several weeks in culture (105). Levels of IL-6 in sera are higher in ATLL patients when compared to both ACs and HCs (106, 107). Interestingly, IL-6 levels in ATLL do not only correlate with T-cell proliferation but also with ATLL severity and shorter survival rate in patients (107). Recent comparative transcriptomic analyses of PBMCs between ATLL patients and HCs have revealed that ATLL pathogenesis is associated with the upregulation of many genes related to inflammatory responses such as NFKB1, RELA, IL2, IL17, and TNFA (56, 108, 109). Pro-inflammatory chemokines are also involved in ATLL pathogenesis as they recruit cancer cells into the lymph nodes, spleen, liver, skin and gastrointestinal tract, thereby contributing to cancer spreading in infected patients (63, 69). In fact, constitutive expression of various pro-inflammatory chemokines in HTLV-1-positive ATLL cells, including MCP-1, MIP-1α/β, RANTES, IL-8 and CXCL10, have been reported and involves the HTLV-1 Tax and Nf-κB signaling pathway in the process (38, 110, 111). Higher plasma levels of TNF-α and IL-6 cytokines, and CXCL10 are found in patients with aggressive ATLL when compared to those with indolent ATLL (aka. stable and slow growing form of lymphoma/leukemia usually associated with lesser fever and lesser symptoms), indicating a worsening role of strong inflammatory responses in ATLL disease severity (74, 107). Finally, Tax- and HBZ-transgenic mice, which develop HTLV-1-like impairments such as T-cell lymphoma and systemic inflammation, display higher production of TNF-α and IFN-γ in FoxP3+ splenocytes, and of IL-1α/β and IL-6 in ATLL-like cells (112–114).
4 Conclusive remarks
Overall, it is obvious that the clinical burden and lack of effective treatment options directs the need for alternative treatment strategies for HTLV-1 infection (14). In this context, a more refined understanding of how HTLV-1 infection, in the presence or absence of Tax protein, influences the sustained pro-inflammatory cytokine/chemokine host production is key for identifying new mechanisms underlying HTLV-1 persistence and development of more effective therapies against HTLV-1-associated diseases (Figure 1).
Statements
Author contributions
SS: Writing – review & editing, Writing – original draft. MA: Writing – review & editing. NG: Writing – review & editing. RT: Writing – review & editing. SH: Writing – review & editing. SI: Writing – review & editing. J-PR: Writing – review & editing. DO: Writing – review & editing. JG: Writing – review & editing, Conceptualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Canadian Institutes of Health Research (CIHR #486498).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1.
Branda F Romano C Pavia G Bilotta V Locci C Azzena I et al Human T-lymphotropic virus (HTLV): epidemiology, genetic, pathogenesis, and future challenges. Viruses. (2025) 17:664. 10.3390/v17050664
2.
Gessain A Cassar O . Epidemiological aspects and world distribution of HTLV-1 infection.Front Microbiol. (2012) 3:388. 10.3389/fmicb.2012.00388
3.
Gessain A Ramassamy J Afonso P Cassar O . Geographic distribution, clinical epidemiology and genetic diversity of the human oncogenic retrovirus HTLV-1 in Africa, the world’s largest endemic area.Front Immunol. (2023) 14:1043600. 10.3389/fimmu.2023.1043600
4.
Lairmore M Haines R Anupam R . Mechanisms of human T-lymphotropic virus type 1 transmission and disease.Curr Opin Virol. (2012) 2:474–81. 10.1016/j.coviro.2012.06.007
5.
Percher F Jeannin P Martin-Latil S Gessain A Afonso P Vidy-Roche A et al Mother-to-child transmission of HTLV-1 epidemiological aspects, mechanisms and determinants of mother-to-child transmission. Viruses. (2016) 8:40. 10.3390/v8020040
6.
Phillips A Harewood J . Adult T Cell Leukemia-Lymphoma (ATL): state of the Art.Curr Hematol Malig Rep. (2018) 13:300–7. 10.1007/s11899-018-0458-6
7.
Ahmadi Ghezeldasht S Mosavat A Rezaee S . Novel insights into human T-lymphotropic virus type-1 (HTLV-1) pathogenesis-host interactions in the manifestation of HTLV-1-associated myelopathy/tropical spastic paraparesis.Rev Med Virol. (2024) 34:e2567. 10.1002/rmv.2567
8.
Takeoka H Sagara Y Ksashiwagi S Nabeshima S . Human T-cell leukemia virus type 1 infection is a risk factor for atherosclerosis.J Clin Med Res. (2021) 13:164–9. 10.14740/jocmr4457
9.
Terada Y Miyata K Shoji N Mochizuki M . Human T-cell Leukemia Virus Type 1 (HTLV-1)-induced Uveitis.Ocul Immunol Inflamm. (2023) 31:1416–24. 10.1080/09273948.2023.2175697
10.
Yamanashi H Nobusue K Nonaka F Honda Y Shimizu Y Kawashiri S et al Human T-cell lymphotropic virus type-1 infection associated with sarcopenia: community-based cross-sectional study in Goto, Japan. Aging. (2020) 12:15504–13. 10.18632/aging.103736
11.
Das C Kundu C . Decoding the molecular complexity of viruses in human cancer: insights into host cell infection, oncogenesis, and therapeutic prospects.Crit Rev Microbiol. (2025) 10.1080/1040841X.2025.2461045. [Online ahead of print.].
12.
Letafati A Bahari M Salahi Ardekani O Nayerain Jazi N Nikzad A Norouzi F et al HTLV-1 vaccination Landscape: current developments and challenges. Vaccine X. (2024) 19:100525. 10.1016/j.jvacx.2024.100525
13.
Phillips A . Advances in the treatment of HTLV-1-associated adult T-cell leukemia lymphoma.Curr Opin Virol. (2023) 58:101289. 10.1016/j.coviro.2022.101289
14.
Wang T Hirons A Doerflinger M Morris K Ledger S Purcell D et al Current State of Therapeutics for HTLV-1. Viruses. (2024) 16:1616. 10.3390/v16101616
15.
Olagnier D Sze A Bel Hadj S Chiang C Steel C Han X et al HTLV-1 Tax-mediated inhibition of FOXO3a activity is critical for the persistence of terminally differentiated CD4+ T cells. PLoS Pathog. (2014) 10:e1004575. 10.1371/journal.ppat.1004575
16.
Sze A Belgnaoui S Olagnier D Lin R Hiscott J van Grevenynghe J . Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis.Cell Host Microbe. (2013) 14:422–34. 10.1016/j.chom.2013.09.009
17.
Shimauchi T Caucheteux S Finsterbusch K Turpin J Blanchet F Ladell K et al Dendritic cells promote the spread of human T-cell leukemia virus type 1 via bidirectional interactions with CD4+ T cells. J Invest Dermatol. (2019) 139:157–66. 10.1016/j.jid.2018.06.188
18.
Lv A Fang Y Lin X Chen J Song H Wang N et al B-cell depletion limits HTLV-1-infected T-cell expansion and ameliorate HTLV-1-associated myelopathy. Ann Clin Transl Neurol. (2024) 11:2756–68. 10.1002/acn3.52190
19.
Bangham C . Human T Cell Leukemia Virus Type 1: persistence and Pathogenesis.Annu Rev Immunol. (2018) 36:43–71. 10.1146/annurev-immunol-042617-053222
20.
Bellon M Nicot C . HTLV-1 Tax Tug-of-War: cellular senescence and death or cellular transformation.Pathogens. (2024) 13:87. 10.3390/pathogens13010087
21.
Matsuoka M Jeang K . Human T-cell leukaemia virus type 1 (HTLV-1) infectivity and cellular transformation.Nat Rev Cancer. (2007) 7:270–80. 10.1038/nrc2111
22.
Boxus M Twizere J Legros S Dewulf J Kettmann R Willems L . The HTLV-1 Tax interactome.Retrovirology. (2008) 5:76. 10.1186/1742-4690-5-76
23.
Kashanchi F Brady J . Transcriptional and post-transcriptional gene regulation of HTLV-1.Oncogene. (2005) 24:5938–51. 10.1038/sj.onc.1208973
24.
Azimi N Brown K Bamford R Tagaya Y Siebenlist U Waldmann T . Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-kappaB site.Proc Natl Acad Sci U S A. (1998) 95:2452–7. 10.1073/pnas.95.5.2452
25.
Fuggetta M Bordignon V Cottarelli A Macchi B Frezza C Cordiali-Fei P et al Downregulation of proinflammatory cytokines in HTLV-1-infected T cells by Resveratrol. J Exp Clin Cancer Res. (2016) 35:118. 10.1186/s13046-016-0398-8
26.
Liu T Zhang L Joo D Sun S . NF-κB signaling in inflammation.Signal Transduct Target Ther. (2017) 2:17023. 10.1038/sigtrans.2017.23
27.
Mitchell S Vargas J Hoffmann A . Signaling via the NFκB system.Wiley Interdiscip Rev Syst Biol Med. (2016) 8:227–41. 10.1002/wsbm.1331
28.
Geleziunas R Ferrell S Lin X Mu Y Cunningham E Grant M et al Human T-cell leukemia virus type 1 Tax induction of NF-kappaB involves activation of the IkappaB kinase alpha (IKKalpha) and IKKbeta cellular kinases. Mol Cell Biol. (1998) 18:5157–65. 10.1128/MCB.18.9.5157
29.
Kanno T Franzoso G Siebenlist U . Human T-cell leukemia virus type I Tax-protein-mediated activation of NF-kappa B from p100 (NF-kappa B2)-inhibited cytoplasmic reservoirs.Proc Natl Acad Sci U S A. (1994) 91:12634–8. 10.1073/pnas.91.26.12634
30.
Clerc I Polakowski N André-Arpin C Cook P Barbeau B Mesnard J et al An interaction between the human T cell leukemia virus type 1 basic leucine zipper factor (HBZ) and the KIX domain of p300/CBP contributes to the down-regulation of tax-dependent viral transcription by HBZ. J Biol Chem. (2008) 283:23903–13. 10.1074/jbc.M803116200
31.
Kwok R Laurance M Lundblad J Goldman P Shih H Connor L et al Control of cAMP-regulated enhancers by the viral transactivator Tax through CREB and the co-activator CBP. Nature. (1996) 380:642–6. 10.1038/380642a0
32.
Mukherjee S Behar M Birnbaum H Hoffmann A Wright P Ghosh G . Analysis of the RelA:cbp/p300 interaction reveals its involvement in NF-κB-driven transcription.PLoS Biol. (2013) 11:e1001647. 10.1371/journal.pbio.1001647
33.
Suzuki S Singhirunnusorn P Mori A Yamaoka S Kitajima I Saiki I et al Constitutive activation of TAK1 by HTLV-1 tax-dependent overexpression of TAB2 induces activation of JNK-ATF2 but not IKK-NF-kappaB. J Biol Chem. (2007) 282:25177–81. 10.1074/jbc.C700065200
34.
Wu X Sun S . Retroviral oncoprotein Tax deregulates NF-kappaB by activating Tak1 and mediating the physical association of Tak1-IKK.EMBO Rep. (2007) 8:510–5. 10.1038/sj.embor.7400931
35.
Yu Q Minoda Y Yoshida R Yoshida H Iha H Kobayashi T et al HTLV-1 Tax-mediated TAK1 activation involves TAB2 adapter protein. Biochem Biophys Res Commun. (2008) 365:189–94. 10.1016/j.bbrc.2007.10.172
36.
Ahuja J Lepoutre V Wigdahl B Khan Z Jain P . Induction of pro-inflammatory cytokines by human T-cell leukemia virus type-1 Tax protein as determined by multiplexed cytokine protein array analyses of human dendritic cells.Biomed Pharmacother. (2007) 61:201–8. 10.1016/j.biopha.2007.02.006
37.
Jain P Ahuja J Khan Z Shimizu S Meucci O Jennings S et al Modulation of dendritic cell maturation and function by the Tax protein of human T cell leukemia virus type 1. J Leukoc Biol. (2007) 82:44–56. 10.1189/jlb.1006641
38.
Mori N Ueda A Ikeda S Yamasaki Y Yamada Y Tomonaga M et al Human T-cell leukemia virus type I tax activates transcription of the human monocyte chemoattractant protein-1 gene through two nuclear factor-kappaB sites. Cancer Res. (2000) 60:4939–45.
39.
Dhib-Jalbut S Hoffman P Yamabe T Sun D Xia J Eisenberg H et al Extracellular human T-cell lymphotropic virus type I Tax protein induces cytokine production in adult human microglial cells. Ann Neurol. (1994) 36:787–90. 10.1002/ana.410360516
40.
Barrios C Castillo L Zhi H Giam C Beilke M . Human T cell leukaemia virus type 2 tax protein mediates CC-chemokine expression in peripheral blood mononuclear cells via the nuclear factor kappa B canonical pathway.Clin Exp Immunol. (2014) 175:92–103. 10.1111/cei.12213
41.
Barrios C Abuerreish M Lairmore M Castillo L Giam C Beilke M . Recombinant human T-cell leukemia virus types 1 and 2 Tax proteins induce high levels of CC-chemokines and downregulate CCR5 in human peripheral blood mononuclear cells.Viral Immunol. (2011) 24:429–39. 10.1089/vim.2011.0037
42.
Kulkarni A Bangham CRM . HTLV-1: regulating the balance between proviral latency and reactivation.Front Microbiol. (2018) 9:449. 10.3389/fmicb.2018.00449
43.
Kulkarni A Taylor G Klose R Schofield C Bangham C . Histone H2A monoubiquitylation and p38-MAPKs regulate immediate-early gene-like reactivation of latent retrovirus HTLV-1.JCI Insight. (2018) 3:e123196. 10.1172/jci.insight.123196
44.
Mahgoub M Yasunaga J Iwami S Nakaoka S Koizumi Y Shimura K et al Sporadic on/off switching of HTLV-1 Tax expression is crucial to maintain the whole population of virus-induced leukemic cells. Proc Natl Acad Sci U S A. (2018) 115:E1269–78. 10.1073/pnas.1715724115
45.
Enose-Akahata Y Vellucci A Jacobson S . Role of HTLV-1 Tax and HBZ in the pathogenesis of HAM/TSP.Front Microbiol. (2017) 8:2563. 10.3389/fmicb.2017.02563
46.
Kannagi M Hasegawa A Nagano Y Iino T Okamura J Suehiro Y . Maintenance of long remission in adult T-cell leukemia by Tax-targeted vaccine: a hope for disease-preventive therapy.Cancer Sci. (2019) 110:849–57. 10.1111/cas.13948
47.
Kannagi M Hasegawa A Nagano Y Kimpara S Suehiro Y . Impact of host immunity on HTLV-1 pathogenesis: potential of Tax-targeted immunotherapy against ATL.Retrovirology. (2019) 16:23. 10.1186/s12977-019-0484-z
48.
Matsuura E Enose-Akahata Y Yao K Oh U Tanaka Y Takashima H et al Dynamic acquisition of HTLV-1 tax protein by mononuclear phagocytes: role in neurologic disease. J Neuroimmunol. (2017) 304:43–50. 10.1016/j.jneuroim.2016.09.014
49.
Carty M Guy C Bowie A . Detection of viral infections by innate immunity.Biochem Pharmacol. (2021) 183:114316. 10.1016/j.bcp.2020.114316
50.
Mohanty S Harhaj E . Mechanisms of innate immune sensing of HTLV-1 and viral immune evasion.Pathogens. (2023) 12:735. 10.3390/pathogens12050735
51.
Fenizia C Fiocchi M Jones K Parks R Ceribelli M Chevalier S et al Human T-cell leukemia/lymphoma virus type 1 p30, but not p12/p8, counteracts toll-like receptor 3 (TLR3) and TLR4 signaling in human monocytes and dendritic cells. J Virol. (2014) 88:393–402. 10.1128/JVI.01788-13
52.
El Hajj H Bazarbachi A . Interplay between innate immunity and the viral oncoproteins Tax and HBZ in the pathogenesis and therapeutic response of HTLV-1 associated adult T cell leukemia.Front Immunol. (2022) 13:957535. 10.3389/fimmu.2022.957535
53.
Douville R Oliere S Green P Lin R Hiscott J . HTLV-1 HBZ protein inhibits IRF3-mediated innate immune responses.Retrovirology. (2011):8(Suppl 1):A99. 10.1186/1742-4690-8-S1-A99
54.
Pise-Masison C de Castro-Amarante M Enose-Akahata Y Buchmann R Fenizia C Washington Parks R et al Co-dependence of HTLV-1 p12 and p8 functions in virus persistence. PLoS Pathog. (2014) 10:e1004454. 10.1371/journal.ppat.1004454
55.
Sarkis S Galli V Moles R Yurick D Khoury G Purcell D et al Role of HTLV-1 orf-I encoded proteins in viral transmission and persistence. Retrovirology. (2019) 16:43. 10.1186/s12977-019-0502-1
56.
Forghani-Ramandi M Mostafavi B Bahavar A Dehghankar M Siami Z Mozhgani S . Illuminating (HTLV-1)-induced adult T-cell leukemia/lymphoma transcriptomic signature: a systems virology approach.Virus Res. (2023) 338:199237. 10.1016/j.virusres.2023.199237
57.
Futsch N Mahieux R Dutartre H . HTLV-1, the other pathogenic yet neglected human retrovirus: from transmission to therapeutic treatment.Viruses. (2017) 10:1. 10.3390/v10010001
58.
Neco H Teixeira V da Trindade A Magalhães P de Lorena V Castellano L et al Mediators Go Together: high Production of CXCL9, CXCL10, IFN-γ, and TNF-α in HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis. AIDS Res Hum Retroviruses. (2017) 33:1134–9. 10.1089/AID.2016.0296
59.
Vallinoto A Cayres-Vallinoto I Freitas Queiroz M Ishak M Ishak R . Influence of immunogenetic biomarkers in the clinical outcome of HTLV-1 infected persons.Viruses. (2019) 11:974. 10.3390/v11110974
60.
Hayden M Ghosh S . Regulation of NF-κB by TNF family cytokines.Semin Immunol. (2014) 26:253–66. 10.1016/j.smim.2014.05.004
61.
Bittencourt A Oliveira P Bittencourt V Carvalho E Farre L . Adult T-cell leukemia/lymphoma triggered by adalimumab.J Clin Virol. (2013) 58:494–6. 10.1016/j.jcv.2013.07.011
62.
Frenzel L Moura B Marcais A Chapdelaine H Hermine O . HTLV-1-associated arthropathy treated with anti-TNF-alpha agent.Joint Bone Spine. (2014) 81:360–1. 10.1016/j.jbspin.2013.10.006
63.
Starling A Gonçalves D Peruhype-Magalhães V Coelho-dos-Reis J Lambertucci J Labanca L et al Cytokines, chemokines and leukotrienes profile and signature analysis in HTLV-1 infection as an evidence of disease progression. Retrovirology. (2014) 11(Suppl 1):O37. 10.1186/1742-4690-11-S1-O37.
64.
Umekita K Hidaka T Miyauchi S Ueno S Kubo K Takajo I et al Treatment with anti–tumor necrosis factor biologic agents in human T lymphotropic virus type I–positive patients with rheumatoid arthritis. Arthritis Care Res. (2014) 66:788–92. 10.1002/acr.22205
65.
Hanon E Goon P Taylor G Hasegawa H Tanaka Y Weber J et al High production of interferon gamma but not interleukin-2 by human T-lymphotropic virus type I-infected peripheral blood mononuclear cells. Blood. (2001) 98:721–6. 10.1182/blood.v98.3.721
66.
Muniz A Rodrigues W Santos S de Jesus A Porto A Castro N et al Association of cytokines, neurological disability, and disease duration in HAM/TSP patients. Arq Neuropsiquiatr. (2006) 64:217–21. 10.1590/s0004-282x2006000200009
67.
Santos S Porto A Muniz A Luna T Nascimento M Guerreiro J et al Modulation of T cell responses in HTLV-1 carriers and in patients with myelopathy associated with HTLV-1. Neuroimmunomodulation. (2006) 13:145–51. 10.1159/000097259
68.
Brites C Grassi M Quaresma J Ishak R Vallinoto A . Pathogenesis of HTLV-1 infection and progression biomarkers: an overview.Braz J Infect Dis. (2021) 25:101594. 10.1016/j.bjid.2021.101594
69.
Zargari R Mahdifar M Mohammadi A Vahidi Z Hassanshahi G Rafatpanah H . The role of chemokines in the pathogenesis of HTLV-1.Front Microbiol. (2020) 11:421. 10.3389/fmicb.2020.00421
70.
El-Sabban M Merhi R Haidar H Arnulf B Khoury H Basbous J et al Human T-cell lymphotropic virus type 1-transformed cells induce angiogenesis and establish functional gap junctions with endothelial cells. Blood. (2002) 99:3383–9. 10.1182/blood.v99.9.3383
71.
Murakami M Iwai S Hiratsuka S Yamauchi M Nakamura K Iwakura Y et al Signaling of vascular endothelial growth factor receptor-1 tyrosine kinase promotes rheumatoid arthritis through activation of monocytes/macrophages. Blood. (2006) 108:1849–56. 10.1182/blood-2006-04-016030
72.
Bangham C Araujo A Yamano Y Taylor G . HTLV-1-associated myelopathy/tropical spastic paraparesis.Nat Rev Dis Primers. (2015) 1:15012. 10.1038/nrdp.2015.12
73.
Guerreiro J Santos S Morgan D Porto A Muniz A Ho J et al Levels of serum chemokines discriminate clinical myelopathy associated with human T lymphotropic virus type 1 (HTLV-1)/tropical spastic paraparesis (HAM/TSP) disease from HTLV-1 carrier state. Clin Exp Immunol. (2006) 145:296–301. 10.1111/j.1365-2249.2006.03150.x
74.
Kagdi H Demontis M Ramos J Taylor G . Switching and loss of cellular cytokine producing capacity characterize in vivo viral infection and malignant transformation in human T- lymphotropic virus type 1 infection.PLoS Pathog. (2018) 14:e1006861. 10.1371/journal.ppat.1006861
75.
Narikawa K Fujihara K Misu T Feng J Fujimori J Nakashima I et al CSF-chemokines in HTLV-I-associated myelopathy: cxcl10 up-regulation and therapeutic effect of interferon-alpha. J Neuroimmunol. (2005) 159:177–82. 10.1016/j.jneuroim.2004.10.011
76.
Nishimoto N Yoshizaki K Eiraku N Machigashira K Tagoh H Ogata A et al Elevated levels of interleukin-6 in serum and cerebrospinal fluid of HTLV-I-associated myelopathy/tropical spastic paraparesis. J Neurol Sci. (1990) 97:183–93. 10.1016/0022-510x(90)90217-b
77.
Rosadas C Zetterberg H Heslegrave A Haddow J Borisova M Taylor G . Neurofilament light in CSF and plasma is a marker of neuronal damage in HTLV-1-associated myelopathy and correlates with neuroinflammation.Neurol Neuroimmunol Neuroinflamm. (2021) 8:e1090. 10.1212/NXI.0000000000001090
78.
Sato T Coler-Reilly A Utsunomiya A Araya N Yagishita N Ando H et al CSF CXCL10, CXCL9, and neopterin as candidate prognostic biomarkers for HTLV-1-associated myelopathy/tropical spastic paraparesis. PLoS Negl Trop Dis. (2013) 7:e2479. 10.1371/journal.pntd.0002479
79.
Souza F Freitas N Gomes Y Torres R Echevarria-Lima J da Silva-Filho I et al Following the Clues: usefulness of Biomarkers of Neuroinflammation and Neurodegeneration in the Investigation of HTLV-1-Associated Myelopathy Progression. Front Immunol. (2021) 12:737941. 10.3389/fimmu.2021.737941
80.
Tamaki K Sato T Tsugawa J Fujioka S Yagishita N Araya N et al Cerebrospinal Fluid CXCL10 as a candidate surrogate marker for HTLV-1-associated myelopathy/tropical spastic paraparesis. Front Microbiol. (2019) 10:2110. 10.3389/fmicb.2019.02110
81.
Yamauchi J Sato T Yagishita N Araya N Hasegawa D Tsutsumi S et al Use of cerebrospinal fluid CXCL10 and neopterin as biomarkers in HTLV-1-associated myelopathy/tropical spastic paraparesis treated with steroids. J Neurol Neurosurg Psychiatry. (2020) 91:321–3. 10.1136/jnnp-2019-321955
82.
Höbarth K Szabo N Hallas A Aulitzky W Marberger M . Serum neopterin as a parameter for monitoring the course of renal cell carcinoma during interferon-gamma therapy.Clin Immunol Immunopathol. (1994) 70:241–4. 10.1006/clin.1994.1035
83.
Da Silva S Cabral-Castro M Faria L Rosadas C de Araújo M Dutra A et al CXCL-10 in cerebrospinal fluid detects neuroinflammation in HTLV-1-associated myelopathy with high accuracy. Viruses. (2025) 17:89. 10.3390/v17010089
84.
Best I López G Verdonck K González E Tipismana M Gotuzzo E et al IFN-gamma production in response to Tax 161-233, and frequency of CD4+ Foxp3+ and Lin HLA-DRhigh CD123+ cells, discriminate HAM/TSP patients from asymptomatic HTLV-1-carriers in a Peruvian population. Immunology. (2009) 128(1 Suppl):e777–86. 10.1111/j.1365-2567.2009.03082.x
85.
Bidkhori H Hedayati-Moghaddam M Mosavat A Valizadeh N Tadayon M Ahmadi Ghezeldasht S et al The IL-18, IL-12, and IFN-γ expression in HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) patients, HTLV-1 carriers, and healthy subjects. J Neurovirol. (2020) 26:338–46. 10.1007/s13365-020-00832-5
86.
Machado N Fagundes B Fernandes L de Oliveira A Nukui Y Casseb J et al Differential modulation of IL-4, IL-10, IL-17, and IFN-γ production mediated by IgG from Human T-lymphotropic virus-1 (HTLV-1) infected patients on healthy peripheral T (CD4+, CD8+, and γδ) and B cells. Front Med. (2023) 10:1239706. 10.3389/fmed.2023.1239706
87.
Maher A Aristodemou A Giang N Tanaka Y Bangham C Taylor G et al HTLV-1 induces an inflammatory CD4+CD8+ T cell population in HTLV-1-associated myelopathy. JCI Insight. (2024) 9:e173738. 10.1172/jci.insight.173738
88.
Amorim C Souza A Diniz A Carvalho N Santos S Carvalho E . Functional activity of monocytes and macrophages in HTLV-1 infected subjects.PLoS Negl Trop Dis. (2014) 8:e3399. 10.1371/journal.pntd.0003399
89.
Enose-Akahata Y Matsuura E Tanaka Y Oh U Jacobson S . Minocycline modulates antigen-specific CTL activity through inactivation of mononuclear phagocytes in patients with HTLV-I associated neurologic disease.Retrovirology. (2012) 9:16. 10.1186/1742-4690-9-16
90.
Nascimento C Lima M de Andrada Serpa M Espindola O Leite A Echevarria-Lima J . Monocytes from HTLV-1-infected patients are unable to fully mature into dendritic cells.Blood. (2011) 117:489–99. 10.1182/blood-2010-03-272690
91.
Rocamonde B Futsch N Orii N Allatif O Penalva de Oliveira A Mahieux R et al Immunoprofiling of fresh HAM/TSP blood samples shows altered innate cell responsiveness. PLoS Negl Trop Dis. (2021) 15:e0009940. 10.1371/journal.pntd.0009940
92.
Ghazvini K Youssefi M Keikha M . Expression changes of cytotoxicity and apoptosis genes in HTLV-1-associated myelopathy/tropical spastic paraparesis patients from the perspective of system virology.Access Microbiol. (2020) 2:acmi000088. 10.1099/acmi.0.000088
93.
Ando H Sato T Tomaru U Yoshida M Utsunomiya A Yamauchi J et al Positive feedback loop via astrocytes causes chronic inflammation in virus-associated myelopathy. Brain. (2013) 136(Pt 9):2876–87. 10.1093/brain/awt183
94.
Fuzii H da Silva Dias G de Barros R Falcão L Quaresma J . Immunopathogenesis of HTLV-1-assoaciated myelopathy/tropical spastic paraparesis (HAM/TSP).Life Sci. (2014) 104:9–14. 10.1016/j.lfs.2014.03.025
95.
Umehara F Izumo S Takeya M Takahashi K Sato E Osame M . Expression of adhesion molecules and monocyte chemoattractant protein -1 (MCP-1) in the spinal cord lesions in HTLV-I-associated myelopathy.Acta Neuropathol. (1996) 91:343–50. 10.1007/s004010050435
96.
Aye M Matsuoka E Moritoyo T Umehara F Suehara M Hokezu Y et al Histopathological analysis of four autopsy cases of HTLV-I-associated myelopathy/tropical spastic paraparesis: inflammatory changes occur simultaneously in the entire central nervous system. Acta Neuropathol. (2000) 100:245–52. 10.1007/s004019900170
97.
Tanaka Y Sato T Yagishita N Yamauchi J Araya N Aratani S et al Potential role of HTLV-1 Tax-specific cytotoxic t lymphocytes expressing a unique t-cell receptor to promote inflammation of the central nervous system in myelopathy associated with HTLV-1. Front Immunol. (2022) 13:993025. 10.3389/fimmu.2022.993025
98.
Kamoi K Mochizuki M . HTLV-1 uveitis.Front Microbiol. (2012) 3:270. 10.3389/fmicb.2012.00270
99.
Martin F Taylor G Jacobson S . Inflammatory manifestations of HTLV-1 and their therapeutic options.Expert Rev Clin Immunol. (2014) 10:1531–46. 10.1586/1744666X.2014.966690
100.
Matsuyama W Kawabata M Mizoguchi A Iwami F Wakimoto J Osame M . Influence of human T lymphotrophic virus type I on cryptogenic fibrosing alveolitis - HTLV-I associated fibrosing alveolitis: proposal of a new clinical entity.Clin Exp Immunol. (2003) 133:397–403. 10.1046/j.1365-2249.2003.02240.x
101.
Miyazato A Kawakami K Iwakura Y Saito A . Chemokine synthesis and cellular inflammatory changes in lungs of mice bearing p40tax of human T-lymphotropic virus type 1.Clin Exp Immunol. (2000) 120:113–24. 10.1046/j.1365-2249.2000.01197.x
102.
Futsch N Prates G Mahieux R Casseb J Dutartre H . Cytokine networks dysregulation during HTLV-1 infection and associated diseases.Viruses. (2018) 10:691. 10.3390/v10120691
103.
Satou Y Matsuoka M . Molecular and cellular mechanism of leukemogenesis of ATL: emergent evidence of a significant role for HBZ in HTLV-1-induced pathogenesis.Leuk Res Treatment. (2012) 2012:213653. 10.1155/2012/213653
104.
Hleihel R Skayneh H de Thé H Hermine O Bazarbachi A . Primary cells from patients with adult T cell leukemia/lymphoma depend on HTLV-1 Tax expression for NF-κB activation and survival.Blood Cancer J. (2023) 13:67. 10.1038/s41408-023-00841-7
105.
Collins N D’Souza C Albrecht B Robek M Ratner L Ding W et al Proliferation response to interleukin-2 and Jak/Stat activation of T cells immortalized by human T-cell lymphotropic virus type 1 is independent of open reading frame I expression. J Virol. (1999) 73:9642–9. 10.1128/JVI.73.11.9642-9649.1999
106.
Inagaki A Ishida T Ishii T Komatsu H Iida S Ding J et al Clinical significance of serum Th1-, Th2- and regulatory T cells-associated cytokines in adult T-cell leukemia/lymphoma: high interleukin-5 and -10 levels are significant unfavorable prognostic factors. Int J Cancer. (2006) 118:3054–61. 10.1002/ijc.21688
107.
Yamamura M Yamada Y Momita S Kamihira S Tomonaga M . Circulating interleukin-6 levels are elevated in adult T-cell leukaemia/lymphoma patients and correlate with adverse clinical features and survival.Br J Haematol. (1998) 100:129–34. 10.1046/j.1365-2141.1998.00538.x
108.
Ashrafi F Rahimzada M Parandi M Mirhosseini A Mashkani B Ahmadi Ghezeldasht S et al Molecular insight into the study of adult T-cell leukemia/lymphoma (ATLL): ten-year studies on HTLV-1 associated diseases in an endemic region. Gene. (2022) 847:146885. 10.1016/j.gene.2022.146885
109.
Mozhgani S Zarei Ghobadi M Norouzi M Rahimi H Valizadeh N Teymoori-Rad M et al Signaling factors potentially associated to the pathogenesis of Adult T-cell leukemia /lymphoma: a network-analysis and novel findings assessment. Virus Res. (2022) 319:198875. 10.1016/j.virusres.2022.198875
110.
Baba M Imai T Yoshida T Yoshie O . Constitutive expression of various chemokine genes in human T-cell lines infected with human T-cell leukemia virus type 1: role of the viral transactivator Tax.Int J Cancer. (1996) 66:124–9. 10.1002/(SICI)1097-0215(19960328)66:1<124::AID-IJC21>3.0.CO;2-C
111.
Mori N Krensky A Ohshima K Tomita M Matsuda T Ohta T et al Elevated expression of CCL5/RANTES in adult T-cell leukemia cells: possible transactivation of the CCL5 gene by human T-cell leukemia virus type I tax. Int J Cancer. (2004) 111:548–57. 10.1002/ijc.20266
112.
Lanigan L Hildreth B Dirksen W Simmons J Martin C Werbeck J et al In vivo tumorigenesis, osteolytic sarcomas, and tumorigenic cell lines from transgenic mice expressing the human T-lymphotropic virus type 1 (HTLV-1) Tax Viral Oncogene. Am J Pathol. (2021) 191:335–52. 10.1016/j.ajpath.2020.10.014
113.
Satou Y Yasunaga J Zhao T Yoshida M Miyazato P Takai K et al HTLV-1 bZIP factor induces T-cell lymphoma and systemic inflammation in vivo. PLoS Pathog. (2011) 7:e1001274. 10.1371/journal.ppat.1001274
114.
Yamamoto-Taguchi N Satou Y Miyazato P Ohshima K Nakagawa M Katagiri K et al HTLV-1 bZIP factor induces inflammation through labile Foxp3 expression. PLoS Pathog. (2013) 9:e1003630. 10.1371/journal.ppat.1003630
Summary
Keywords
HTLV-1, HTLV-1 tax protein, inflammation, NF-κB signaling pathway, cytokine/chemokine, ATLL, HAM-TSP, asymptomatic carriers
Citation
Shegefti S, Alaei M, Ghahari N, Telittchenko R, Hanafi SB, Isnard S, Routy J-P, Olagnier D and Grevenynghe Jv (2025) Sustained inflammation during human T-lymphotropic virus type 1 infection: a wildfire contributing to disease progression. Front. Med. 12:1653384. doi: 10.3389/fmed.2025.1653384
Received
24 June 2025
Accepted
26 August 2025
Published
12 September 2025
Volume
12 - 2025
Edited by
Denis Miyashiro, University of São Paulo, Brazil
Reviewed by
Juliana Echevarria Lima, Federal University of Rio de Janeiro, Brazil
Updates
Copyright
© 2025 Shegefti, Alaei, Ghahari, Telittchenko, Hanafi, Isnard, Routy, Olagnier and Grevenynghe.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julien van Grevenynghe, julien.vangrevenynghe@inrs.ca
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.