Abstract
Background:
Chronic pain afflicts approximately 20% of the global adult population and is frequently undertreated, with available pharmacologic options often associated with significant long-term adverse effects. Although omega-3 fatty acids are known for their anti-inflammatory and immunomodulatory effects, current clinical evidence regarding their efficacy in pain management remains inconclusive.
Objective:
To determine how well omega-3 fatty acids reduce chronic pain, and to investigate how factors like disease type, dosage, treatment duration, and study design influence their effectiveness.
Methods:
We searched four databases (PubMed, Embase, Cochrane Library, and Web of Science) from inception to 14 February 2025 with no language restrictions. Forty-one randomised controlled trials (RCTs; n = 3,759) met predefined criteria. Risk of bias was assessed with RoB 2. Pooled standardised mean differences (SMDs) for pain intensity were obtained through random-effects meta-analyses. Subgroup, sensitivity, and publication-bias analyses were also conducted.
Results:
Omega-3 fatty acids showed a moderate, statistically and clinically significant reduction in pain intensity with a standardized mean difference (SMD) of −0.55 (95% CI –0.76 to −0.34; I2 = 87%). The relief was noticeable at 1 month (SMD = −0.27) and improved by 6 months (SMD = −0.83). Lower doses (≤1.35 g/day) were more effective (SMD = −0.60) compared to higher doses (>1.35 g; SMD = −0.53). The benefits were significant for rheumatoid arthritis, migraine, and other mixed chronic pain conditions, but not for osteoarthritis or mastalgia. There was minimal publication bias according to trim-and-fill adjustment, and leave-one-out tests confirmed robust results.
Conclusion:
Omega-3 fatty acid supplementation offers a clinically meaningful and time-dependent reduction in chronic pain, particularly at moderate doses and in certain disease contexts. Standardization of outcome measures, dose optimization, and long-term trials are needed to better define its role in pain management.
Systematic review registration:
https://www.crd.york.ac.uk/PROSPERO/view/CRD420251035960, Identifier CRD420251035960.
1 Introduction
Data from the 2023 U.S. National Health Interview Survey indicated that 24.3% of adults reported experiencing daily pain during the preceding 3 months, and 8.5% suffered from high-impact chronic pain that substantially limited their ability to participate in work and social activities (1). Globally, an estimated 1.5 billion individuals—approximately one in five of the world’s population—are affected by chronic pain (2). A 2024 systematic review including 148 studies and more than 4.3 million patients with chronic pain reported that approximately one-third exhibited signs of dependence and about 10% developed opioid use disorder with long-term therapy, further complicating management owing to reliance on traditional analgesics (3). These findings underscore the urgent need for safer and more sustainable adjuncts or alternatives to conventional analgesics.
Preclinical evidence suggests several biological pathways by which omega-3 fatty acids may exert analgesic effects. Experimental evidence indicates that eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) modulate inflammatory pathways by competing with arachidonic acid metabolism, thereby reducing the production of pro-inflammatory prostaglandin E₂ and leukotriene B₄ (4). In addition, they give rise to specialised pro-resolving mediators, including resolvins, protectins, and maresins, which actively promote the resolution of inflammation (5). Within the nervous system, omega-3 fatty acids have been shown to attenuate central sensitisation and neuroinflammation, at least in part by suppressing microglial activation through the SIRT1–HMGB1–NF-κB pathway (6). Taken together, these findings suggest that omega-3 fatty acids may alleviate pain through both peripheral and central mechanisms. Clinical evidence, however, remains inconsistent. At one end of the spectrum, the large-scale trial followed 19,611 community-dwelling older adults for 5.3 years and found that daily supplementation with 1 g of marine omega-3 fatty acids had no effect on pain prevalence or severity compared with placebo (OR = 0.99; 95% CI, 0.94–1.04) (7). In contrast, a 2024 network meta-analysis of 40 randomized controlled trials (n = 6,616) reported that high-dose EPA/DHA supplementation produced the greatest reductions in migraine frequency (SMD = −1.36; 95% CI, −2.32 to −0.39) and severity among all prophylactic interventions evaluated (8). More recent trials have provided additional evidence. A 2025 randomized controlled trial demonstrated that daily supplementation with 2,000 mg of EPA significantly reduced migraine headache days and attack frequency in patients with chronic migraine, accompanied by improvements in quality of life (9). Another randomized trial conducted in 2021 among healthy young men reported that 4 weeks of omega-3 supplementation (3 g/day) significantly reduced muscle soreness 24 h after exercise-induced muscle damage (p = 0.034) and attenuated the rise in inflammatory cytokines (10).
In this study, we will conduct a rigorous systematic review and meta-analysis of randomized controlled trials to quantitatively evaluate the efficacy of omega-3 fatty acids in managing chronic pain. This synthesis will provide high-quality evidence to inform clinical practice and guide future research into underlying mechanisms.
2 Methods
This systematic review and meta-analysis were carried out in strict accordance with the Cochrane Handbook for Systematic Reviews of Interventions. The study design and reporting were in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (11), ensuring methodological rigor and transparency. The research protocol was prospectively registered in the International Prospective Register of Systematic Reviews under the registration number CRD420251035960. The research question was formulated using the PICO framework: Population—adults with chronic pain; Intervention—omega-3 fatty acids; Comparator—any control condition; and Outcomes—subjective or objective pain measures defined as primary endpoints.
2.1 Eligibility criteria
We included only randomized controlled trials (RCTs) that evaluated the effects of omega-3 fatty acid supplementation in chronic pain conditions. Eligible participants were required to have experienced pain for at least 3 months, and trials had to compare omega-3 supplementation with placebo, usual care, sham product, or an active comparator. Studies were required to report at least one pain-related outcome at any follow-up time point. We excluded non-randomized or quasi-experimental studies, animal studies, abstracts without full data, reviews, editorials, and duplicate publications.
2.2 Information sources, search strategy, and selection process
A systematic search was performed in PubMed, Embase, the Cochrane Library, and Web of Science from database inception to February 14, 2025. The search strategy combined terms related to omega-3 fatty acids (“omega-3,” “fish oil,” “EPA,” “DHA,” “polyunsaturated fatty acid,” “Omegaven” etc.), trial filters (“randomized controlled trial,” “clinical trial”), and chronic pain descriptors (“chronic pain,” “persistent pain,” “fibromyalgia,” “headache,” “migraine disorders,” etc.). Equivalent keywords and controlled vocabulary (MeSH in PubMed, Emtree in Embase) were applied as appropriate for each database. No restrictions were imposed on language, publication year, or publication status. The full search strategy is provided in the Supplementary material.
Search results were imported into EndNote X9 (Clarivate Analytics), and duplicates were automatically removed. Two reviewers independently screened titles and abstracts, retrieved potentially eligible full texts, and assessed them against the pre-specified inclusion criteria. Discrepancies were resolved through discussion until consensus was achieved. Reasons for exclusion were documented at the full-text stage. In addition, the reference lists of all included studies, relevant reviews, and prior meta-analyses were manually screened to identify additional eligible citations.
2.3 Data collection and data items
Data extraction was independently performed by two reviewers using a standardized collection form. The extracted information was systematically organized into spreadsheets and categorized according to key study characteristics, including author and year of publication, country of origin, study design, study duration or follow-up period, sample size, participant demographics (e.g., age), type of chronic pain condition, exposure (intervention and control groups), detailed dosing regimen for the intervention, and the instruments used for pain assessment.
All studies reporting outcome data as means, mean differences, and standard deviations were eligible for inclusion in the meta-analysis. These values were either directly extracted from the original publications or derived from the available data when necessary. A random-effects model was applied to generate pooled estimates, accounting for anticipated heterogeneity across studies and enhancing the external validity of the findings. This approach yields more conservative effect size estimates, which are particularly appropriate when between-study variability is expected.
2.4 Risk of bias and study quality assessment
Two reviewers independently evaluated the methodological quality of the included studies using the Cochrane Risk of Bias tool for randomized trials (RoB 2) (12). The assessment covered the following domains: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, completeness of outcome data, selective reporting, and other potential sources of bias. Discrepancies between reviewers were resolved through consultation with a third investigator. For each domain, the risk of bias was categorized as “low,” “high,” or “unclear.”
2.5 Subgroup analyses
We also conducted a series of pre-specified subgroup analyses in addition to the primary meta-analysis examining the association between omega-3 fatty acid supplementation and chronic pain. These stratifications included disease type, type of fatty acid supplementation, pain assessment scale [e.g., Visual Analogue Scale (VAS), McMaster Universities Osteoarthritis Index (WOMAC), other tools], geographic region (e.g., United States vs. other countries), and control type (placebo vs. active comparator), as illustrated in the corresponding forest plots. Further subgroup analyses were performed based on intervention duration, categorizing studies into short-term (<3 months) and long-term (≥3 months) groups, in accordance with previous meta-analytic frameworks (13). We also stratified trials by daily omega-3 fatty acid dosage (≤1.35 g/day vs. >1.35 g/day). This threshold was selected based on prior evidence suggesting a therapeutic range between 1.35 and 2.7 g/day (14). For trials that provided dosages in mg/kg/day, the total daily intake was adjusted based on a standard adult body weight of 70 kg.
2.6 Data synthesis and statistical analysis
The meta-analysis was performed using Review Manager (RevMan, version 5.3; Cochrane Collaboration) and Stata (version 15.1; StataCorp). Standardized mean differences (SMDs) were adopted as the primary effect size metric. For each study, the SMD was weighted by the inverse of its variance, and pooled estimates with corresponding 95% confidence intervals (CIs) were subsequently calculated.
SMDs were selected because they allow aggregation of results derived from different assessment instruments across studies (e.g., various pain scales). SMDs were calculated by standardizing the mean difference between intervention and control groups using the pooled standard deviation. An SMD of zero indicates no difference between groups. In this analysis, a negative SMD favors the intervention (indicating pain reduction), whereas a positive SMD favors the control group. According to Cohen’s thresholds, an SMDs >0.8 reflects a large effect, >0.5 a moderate effect, and <0.2 a small effect (13).
For studies that did not provide complete data, a normal distribution was assumed, and the mean and standard deviation were estimated from the reported median and interquartile range (IQR) (15). When a single study reported outcomes for multiple doses of the same supplement, the corresponding SMDs were first pooled within that study to generate a single effect size for the primary analysis. These dose-specific results were subsequently examined in subgroup analyses. The present study utilized meta-regression analysis to investigate the effect of Omega-3 fatty acid intervention duration (1, 2, 3, and 6 months) on analgesic efficacy. The duration of intervention was treated as a continuous moderator to rigorously assess whether analgesic efficacy showed a significant linear trend with increasing duration of intervention.
Statistical heterogeneity was evaluated using Cochran’s Q test and the I2 statistic. Both fixed-effect and random-effects models were generated; however, results from the random-effects model were prioritized when heterogeneity was present. A two-sided p-value < 0.05 was considered statistically significant. Publication bias was assessed through the use of a funnel plot, Egger’s test (16), and Begg’s test (17).
3 Results
3.1 Study selection and characteristics
The selection process, along with excluded records and reasons for exclusion, is outlined in Figure 1. The key characteristics of the included studies are presented in Table 1. Among the 99 full-text articles evaluated, 58 were excluded for various reasons, including 4 that were reviews or meta-analyses, 20 that were conference abstracts, 9 that did not involve patients with chronic pain, 7 that did not incorporate n-3 fatty acid intervention, 8 with non-RCT, and 10 that lacked relevant outcome measures. Therefore, 41 randomized controlled trials were retained for the final analysis (18–58).
Figure 1

Flow chart of article retrieval.
Table 1
| Study | Country | Study design | Population (treatment/control) | Mean age (years) (intervention/control) | Intervention group | Control group | Disease | Duration | Daily dose | Outcome score |
|---|---|---|---|---|---|---|---|---|---|---|
| Möller et al. (18) | Spain | RCT | 23/28 | 61.2/57.3 | SPMs (derive from essential PUFAs, namely AA, EPA, and DHA | Olive oil | Symptomatic knee osteoarthritis | 12 weeks | 500 mg × 4/day (weeks 1–6), then 500 mg × 2/day (weeks 7–12) | VAS |
| Faurot et al. (19) | USA | RCT | 47/47 | 38.8/36.9 | A high n-3, an average n-6 diet | Average intakes of n-3 and n-6 fatty acids | Migraine | 16 weeks | NA | PROMIS-29 |
| 46/47 | 39.4/36.9 | A high n-3, low n-6 diet | NA | |||||||
| Pérez-Piñero et al. (20) | Spain | RCT | 31/30 | 51.1/50.2 | AvailOm® 50 High EPA | Placebo | Persistent knee pain | 8 weeks | EPA/DHA-lysine salts (25%) | WOMAC Score |
| Carlisle et al. (21) | USA | RCT | 12/13 | 55.2/ 55.1 | Calamari oil (n-3 fatty acids) | Placebo | Self-reported mixed pain (e.g., bone/muscle + back, joint + back, or cervical + joint pain) | 12 weeks | N-3 fatty acids: 230 mg (DHA 130 mg, EPA 55 mg) | NPRS-11 |
| Sasahara et al. (22) | Japan | RCT | 60/60 | 40.3/41.4 | L-Serine and EPA | Placebo | Chronic low-back and knee pain | 8 weeks | 594 mg L-Ser and 149 mg EPA | BPI |
| MacFarlane et al. (23) | Spain | RCT | 595/626 | 67.9/ 67.6 | N-3 fatty acids (Omacor®) | Placebo | Knee pain | Mean: 5.3 years | Omacor® 1 g/d (EPA + DHA 840 mg, 1.3:1 ratio) | WOMAC Score |
| Nodler et al. (24) | USA | RCT | 17/19 | 18.9/ 20.1 | Fish oil | Placebo | Endometriosis | 6 months | Fish oil 1,000 mg/d [ω-3 FAs 720 mg: EPA 488 mg, DHA 178 mg] | VAS |
| Godazandeh et al. (25) | Iran | RCT | 51/49 | NA | Flaxseed oil 1,000 mg/d (soft capsule) | Vitamin E | Fibrocystic breast: mastalgia + nodularity | 2 months | α-Linolenic acid 350 mg/d | VAS |
| Hadian et al. (26) | USA | RCT | 20/20 | 37.7/37.8 | N-3 fatty acids | Placebo | RAS | 6 months | 180 mg of EPA and 120 mg of DHA | VAS |
| Stonehouse et al. (27) | Australia | RCT | 117/118 | 55.8/ 56.0 | Krill oil | Mixed vegetable oil | Osteoarthritic knee pain | 6 months | 600 mg of EPA, 280 mg of DHA, and 0.45 mg of astaxanthin | WOMAC Score |
| Kuszewski et al. (28) | Australia | RCT | 32/31 | 65.4/65.4 | Fish oil | Placebo | Osteoarthritis | 16 weeks | 2,000 mg of DHA and 400 mg of EPA per day | VAS |
| Lustberg et al. (29) | USA | RCT | 22/22 | 59.5/ 57.8 | N-3 fatty acids | Placebo | Musculoskeletal pain | 24 weeks | 2,580 mg EPA and 1,380 mg DHA | 5-point score |
| Noguchi et al. (30) | Japan | RCT | 45/54 | 39.2/40.6 | N-3 fatty acids | Placebo | Traumatic injury | 12 weeks | 1,470 mg DHA and 147 mg EPA | SF-36 |
| Hill et al. (31) | Australia | RCT | 85/83 | 61.0/61.0 | Blend of fish oil and sunola oil | High-dose fish oil | Knee osteoarthritis | 24 months | 4.5 g omega-3 fatty acids or 0.45 g omega-3 fatty acids | WOMAC Score |
| Ramsden et al. (32) | USA | RCT | 32/32 | 41.0/42.0 | Low n-6: high n-3 ratio | Low n-6 | Headache pain | 12 weeks | Increased EPA and DHA intake | HIT-6 |
| Blommers et al. (33) | Netherlands | RCT | 30/30 | 39.6/36.8 | Fish oil | Corn oil + wheat-germ oil | Chronic mastalgia | 6 months | 1,128 mg EPA, 714 mg DHA | 4-point score |
| Ramsden et al. (34) | USA | RCT | 33/34 | NA | Low n-6: high n-3 ratio | Low n-6 | Headache pain | 12 weeks | NA | HIT-6 |
| El Khouli et al. (35) | Egypt | RCT | 25/25 | 33.7/32.5 | N-3 fatty acids | Placebo | RAS | 6 months | Capsules of 1,000 mg each/day | VAS |
| Das Gupta et al. (36) | Bangladesh | RCT | 40/41 | 49.9/44.7 | N-3 fatty acids | indomethacin | Rheumatoid arthritis | 12 weeks | Indomethacin (75 mg) along with omega-3 fatty acids (3 g) | VAS |
| Park et al. (37) | Korea | RCT | 41/40 | 49.2/47.6 | N-3 fatty acids | Placebo | Rheumatoid arthritis | 16 weeks | 2,090 mg of EPA and 1,165 mg of DHA per day | Pain scale |
| Rahbar et al. (38) | Iran | RCT | 47/48 | 20.0/19.8 | N-3 fatty acids | Placebo | Primary dysmenorrhea | 3 months | 180 mg of EPA and 120 mg of DHA per day | VAS |
| Caturla et al. (39) | Spain | RCT | 23/22 | 39.2/39.9 | Fish oil and standardized lemon verbena extract | Placebo | Joint discomfort/pain | 9 weeks | 1233.6 mg EPA + 986.4 mg DHA/day (Weeks 1–5); 616.8 mg EPA + 493.2 mg DHA/day (Weeks 6–9) | WOMAC Score |
| Galarraga et al. (40) | UK | RCT | 49/48 | 58.0/61.0 | Cod liver oil (n-3 fatty acids) | Placebo | Rheumatoid arthritis | 9 months | 1,500 mg EPA + 700 mg DHA/day | VAS |
| Harel et al. (41) | USA | RCT | 14/13 | NA | N-3 fatty acids | Placebo | Recurrent migraines | 2 months | 756 mg of EPA and 498 mg of DHA per day | Seven-point faces pain scale |
| Brunborg et al. (42) | Norway | RCT | 18/20 | 48.1/47.7 | Cod liver oil | Seal oil | Inflammatory bowel disease and joint pain | 14 days | 2.3 g EPA, 0.3 g DPA, and 3.7 g DHA per day | VAS |
| Bjørkkjaer et al. (43) | Norway | RCT | 10/9 | NA | Seal oil | Soy oil | Inflammatory bowel disease-related joint pain | 10 days | 2.0 g EPA, 0.9 g DPA and 2.2 g DHA per day | VAS |
| Hansen et al. (44) | Denmark | RCT | 36/45 | 59.0/54.0 | Fish meal | Normal diet | Rheumatoid arthritis | 6 months | 600 mg EPA, 420 mg DHA per day | VAS |
| Nordström et al. (45) | Finland | RCT | 11/11 | 51.0/53.0 | Alpha-LNA | Placebo | Rheumatoid arthritis | 3 months | 30 g of flaxseed oil (32% alpha-LNA) | VAS |
| Berbert et al. (46) | Brazil | RCT | 17/13 | 51.0/48.0 | Fish oil n-3 fatty acids + olive oil | Soy oil | Rheumatoid arthritis | 24 weeks | 3 g/d fish oil n-3 fatty acids,6.8 g oleic acid | 5-point score |
| 13/13 | 51.0/48.0 | Fish oil n-3 fatty acids | 3 g/d fish oil n-3 fatty acids | |||||||
| Remans et al. (47) | Netherlands | RCT | 26/29 | 59.5/52.9 | PUFA supplement drink | Placebo drink | Rheumatoid arthritis | 4 months | 1,400 mg EPA, 200 mg DHA- 500 mg GLA | VAS |
| Kawabata et al. (48) | Japan | RCT | 11/9 | 23.3/27.1 | Fish oil | Middle chain triglycerides (edible oil) | Asthenopia (eye-pain, low back pain, headache) | 4 weeks | 162 mg EPA, 783 mg DHA | VAS |
| Stammers et al. (49) | United Kingdom | RCT | 29/29 | 67.0/69.0 | Current NSAIDs + cod liver oil | Current NSAIDs + olive oil | Osteoarthritis | 6 months | 786 mg EPA | VAS |
| Geusens et al. (50) | Belgium | RCT | 19/20 | 56.0/59.0 | Fish oil | Olive oil | Rheumatoid arthritis | 12 months | 1,680 mg EPA, 360 mg DHA | 5-point score |
| Nielsen et al. (51) | Denmark | RCT | 27/24 | NA | Fish oil | Average diet | Rheumatoid arthritis | 12 weeks | 2000 mg EPA, 1200 mg DHA | VAS |
| Kremer et al. (52) | USA | RCT | 20/12 | 59.0/58.0 | Fish oil | Olive oil | Rheumatoid arthritis | 24 weeks | 27 and 18 mg/kg/day of EPA and DHA | 5-point score |
| 17/12 | 58.0/58.0 | 54 and 36 mg/kg/day of EPA and DHA | ||||||||
| Van der Tempel et al. (53) | Netherlands | RCT | 8/8 | NA | Fish oil | Coconut oil | Rheumatoid arthritis | 12 weeks | 12 capsules of fractionated fish oil | 4-point score |
| Sundrarjun et al. (54) | Thailand | RCT | 23/23 | 46.2 /46.0 | Low n-6 diet + fish oil | Low n-6 diet + placebo | Rheumatoid arthritis | 12 weeks | 1880 mg EPA, 1480 mg DHA | VAS |
| Kremer et al. (55) | USA | RCT | 15/14 | 58.0/57.0 | Diclofenac+ fish oil + corn oil | Diclofenac + corn oil | Rheumatoid arthritis | 48 weeks | 130 mg/kg/d of n-3 (44% EPA - 24%DHA) |
5-point score |
| Skoldstam et al. (56) | Sweden | RCT | 22/21 | 58.0/55.0 | Fish oil | Inactive oil (maize, olive and peppermint oils) | Rheumatoid arthritis | 6 months | 1800 mg EPA, 1200 mg DHA | 4-point score |
| Magarò et al. (57) | Italy | RCT | 10/10 | NA | Diclofenac + n-3 fatty acids | Diclofenac | Rheumatoid arthritis | 45 days | 1,600 mg EPA, 1100 mg DHA | VAS |
| Adam et al. (58) | Germany | RCT | 30/30 | 58.0/56.8 | Fish oil | Placebo | Rheumatoid arthritis | 3 months | 30 mg/kg/day of total n-3 fatty acids | VAS |
Characteristics of study included in meta-analysis.
RCT, randomized controlled trial; n-3 fatty acids, omega-3 fatty acids; PUFA, polyunsaturated fatty acid; NSAIDs, non-steroidal anti-inflammatory drugs; SPMs, specialized pro-resolving mediators; AA, arachidonic acid; EPA, eicosapentaenoic acid; DHA, docosahexaenoic acid; DPA, docosapentaenoic acid; PROMIS-29, Patient-Reported Outcomes Measurement Information System; WOMAC, Western Ontario and McMaster Universities Osteoarthritis Index; NPRS-11, 11-Point Numeric Pain Rating Scale; BPI, brief pain inventory; VAS, Visual Analogue Scale; SF-36, 36-Item Short Form Health Survey; HIT-6, Headache Impact Test; RAS, recurrent aphthous stomatitis; LNA, linolenic acid; GLA, gamma-linolenic acid.
Among the 41 RCTs, 26 trials (18–20, 22–25, 27–32, 34, 35, 40, 41, 43, 45–48, 51, 53, 54, 58) (63.4%) were classified as low risk of bias, 11 trials (21, 26, 37–39, 42, 44, 50, 52, 56, 57) (26.8%) had some concerns, and four trials (33, 36, 49, 55) (9.8%) were classified as high risk of bias. Refer to the Supplementary material for detailed assessments.
In the domain of bias arising from the randomization process, 37 trials (18–20, 22–25, 27–35, 37–41, 43–58) (90.2%) were classified as low risk, as they clearly described adequate random sequence generation and allocation concealment using computer-based or equivalent methods. The remaining four trials (21, 26, 36, 42) (9.8%) lacked sufficient information to confirm proper randomization procedures and were thus classified as having “some concerns.” Regarding bias due to deviations from intended interventions, the majority of studies complied with the protocol and maintained appropriate blinding. All 41 trials (18–58) (100%) were classified as low risk in this domain. In the domain of bias due to missing outcome data, 33 trials (18–32, 34, 35, 37, 38, 40–43, 45–48, 51, 53, 54, 56–58) (80.5%) had a loss-to-follow-up rate below 5% and used appropriate methods for handling missing data, and were classified as low risk. Four trials (39, 44, 50, 52) (9.8%) had >5% missing data but employed valid imputation strategies or provided transparent explanations, leading to “some concerns.” Another four trials (33, 36, 49, 55) (9.8%) had substantial missing data without adequate justification or handling, and were thus classified as high risk. For bias in the measurement of outcomes, all 41 trials (18–58) (100%) were classified as low risk, as outcome assessments were performed by blinded assessors or used objective validated instruments. In the domain of bias in the selection of the reported result, 32 trials (18–35, 39–43, 45–48, 50, 51, 53, 54, 58) (78.0%) reported prespecified outcomes consistent with protocols or registries, while nine trials (36–38, 44, 49, 52, 55–57) (22.0%) were classified as having “some concerns” due to unclear analytical plans or selective reporting.
3.2 Primary outcome: effect of omega-3 fatty acids on pain intensity
The primary analysis sought to evaluate the impact of omega-3 fatty acid supplementation on pain intensity compared to control conditions. A total of 41 randomized controlled trials (18–58) with 3,759 participants demonstrated a significant reduction in chronic pain associated with omega-3 fatty acids (SMD = −0.55; 95% CI: −0.76 to −0.34; p < 0.001; I2 = 87%). See Figure 2 and Table 2.
Figure 2

Forest plot of the effect of omega-3 fatty acid supplementation on chronic pain. Random-effects model was used to compute pooled SMDs with 95% CI based on 41 studies (n = 3,759).
Table 2
| Variables | N of studies | SMD Fixed effects (95% CI) | SMD random effects (95% CI) | Heterogeneity I2% | Q-test p-value |
|---|---|---|---|---|---|
| Main analysis | 41 | −0.34 (−0.40, −0.27) | −0.55 (−0.76, −0.34) | 87 | <0.001 |
| 1-month analysis | 9 | −0.24 (−0.39, −0.08) | −0.27 (−0.48, −0.05) | 42 | 0.01 |
| 2-month analysis | 10 | −0.36 (−0.53, −0.19) | −0.39 (−0.61, −0.18) | 37 | <0001 |
| 3-month analysis | 22 | −0.45 (−0.56, −0.35) | −0.51 (−0.87, −0.15) | 91 | 0.005 |
| 6-month analysis | 14 | −0.83 (−0.96, −0.70) | −0.83 (−1.22, −0.45) | 87 | <0.001 |
| Subgroup | |||||
| Disease | |||||
| Rheumatoid arthritis | 16 | −0.32 (−0.46, −0.17) | −0.42 (−0.76, −0.09) | 80 | 0.01 |
| Osteoarthritis | 5 | −0.88 (−1.06, −0.70) | −0.77 (−1.55, 0.00) | 94 | 0.05 |
| Migraine | 2 | −0.73 (−1.06, −0.40) | −0.84 (−1.44, −0.24) | 49 | 0.006 |
| Mastalgia | 2 | −0.04 (−0.35, 0.27) | −0.04 (−0.35, 0.27) | 0 | 0.78 |
| Others diseases | 16 | −0.21 (−0.29, −0.12) | −0.61 (−0.94, −0.29) | 87 | <0.001 |
| Fatty acid type | |||||
| Fish oil | 15 | −0.78 (−0.93, −0.64) | −0.69 (−1.09, −0.29) | 86 | <0.001 |
| N-3 fatty acids | 15 | −0.18 (−0.27, −0.09) | −0.55 (−0.91, −0.19) | 90 | 0.002 |
| Other mixed supplements | 11 | −0.36 (−0.50, −0.22) | −0.34 (−0.58, −0.09) | 59 | 0.006 |
| Country | |||||
| USA | 10 | −0.57 (−0.75, −0.39) | −0.62 (−0.98, −0.25) | 73 | 0.001 |
| Other countries | 31 | −0.30 (−0.37, −0.23) | −0.53 (−0.77, −0.28) | 89 | <0.001 |
| Intervention period (overall) | |||||
| ≥3 months | 32 | −0.34 (−0.41, −0.26) | −0.54 (−0.78, −0.29) | 90 | <0.001 |
| <3 months | 9 | −0.35 (−0.54, −0.16) | −0.53 (−0.87, −0.20) | 62 | 0.002 |
| Daily dose | |||||
| ≤1.35 g | 12 | −0.16 (−0.26, −0.07) | −0.60 (−0.99, −0.21) | 89 | 0.003 |
| >1.35 g | 29 | −0.52 (−0.62, −0.43) | −0.53 (−0.78, −0.28) | 85 | <0.001 |
| Outcome score | |||||
| VAS score | 20 | −0.48 (−0.60, −0.35) | −0.60 (−0.95, −0.26) | 86 | <0.001 |
| WOMAC score | 5 | −0.28 (−0.37, −0.18) | −0.85 (−1.63, −0.07) | 97 | 0.03 |
| Composite score | 16 | −0.29 (−0.43, −0.16) | −0.36 (−0.57, −0.14) | 59 | 0.001 |
| Control type | |||||
| Non-placebo control | 20 | −0.52 (−0.64, −0.41) | −0.50 (−0.84, −0.16) | 88 | 0.004 |
| Placebo control | 21 | −0.24 (−0.32, −0.16) | −0.59 (−0.86, −0.32) | 86 | <0.001 |
Meta-analysis of intervention studies in chronic pain.
SMD, standardized mean difference; CI, confidence interval; VAS, Visual Analogue Scale; USA, United States of America; WOMAC, Western Ontario and McMaster Universities Arthritis Index. p < 0.05 was considered statistically significant. Some studies contributed data to more than one duration category (1, 2, 3, or 6 months). Therefore, the groups are not mutually exclusive, and the total count exceeds the number of unique studies.
To further investigate the time-dependent effects of omega-3 fatty acid supplementation, an exploratory subgroup analysis was conducted, stratified by intervention duration (1, 2, 3, and 6 months). Certain trials reported pain outcomes at multiple time points, indicating that some studies contributed data to more than one subgroup. These results are intended to illustrate potential trends over time, not to be used for direct comparisons between durations. In nine studies (18, 19, 22, 25, 35, 37, 39, 48, 58) with 1-month outcomes, omega-3 fatty acid supplementation led to a significant reduction in chronic pain (n = 667, SMD = −0.27, 95% CI: −0.48 to −0.05, p = 0.01; I2 = 42%). See Figure 3 and Table 2. In 10 studies (18, 20, 25, 35, 39, 41, 46, 47, 49, 58) that reported 2-month outcomes, a similar impact was noted (n = 550, SMD = −0.39, 95% CI: −0.61 to −0.18, p < 0.001; I2 = 37%). See Figure 4 and Table 2. In 22 studies (21, 22, 24, 26, 27, 29–32, 34–36, 38, 40, 44, 45, 49–53, 56) with 3-month outcomes, pain scores were also significantly improved (n = 1,562, SMD = −0.51, 95% CI: −0.87 to −0.15, p = 0.005; I2 = 91%). See Figure 5 and Table 2. In 14 studies (26, 27, 29, 31, 33, 35, 40, 44, 46, 49, 50, 52, 54, 56) reporting 6-month outcomes, the analgesic effect became more pronounced (n = 1,053, SMD = −0.83, 95% CI: −1.22 to −0.45, p < 0.001; I2 = 87%). See Figure 6 and Table 2. The meta-regression results demonstrated a significant positive relationship between follow-up duration and analgesic efficacy. Specifically, for each additional month of intervention, the analgesic effect size increased by a factor of 10.3% [exp(b) = 1.103, 95% CI = (1.008, 1.207), p = 0.033]. This finding indicates that as the duration of omega-3 fatty acid intervention increases, analgesic efficacy improves significantly.
Figure 3

Forest plot of the effect of omega-3 fatty acid supplementation on chronic pain at 1 month. A random-effects model was used to compute pooled SMDs with 95% CI based on nine studies (n = 667).
Figure 4

Forest plot of the effect of omega-3 fatty acid supplementation on chronic pain at 2 months. A random-effects model was used to compute pooled SMDs with 95% CI based on 10 studies (n = 550).
Figure 5
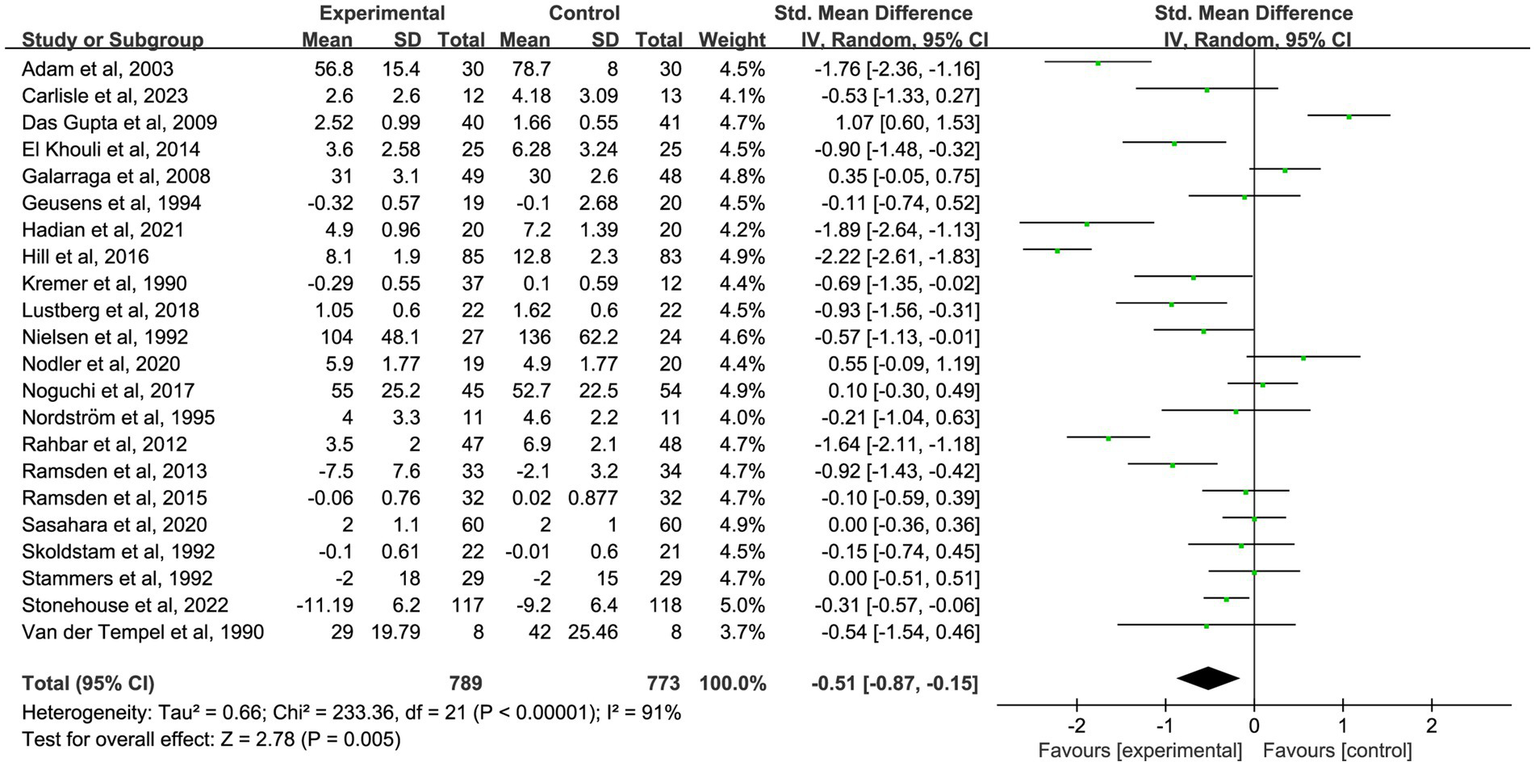
Forest plot of the effect of omega-3 fatty acid supplementation on chronic pain at 3 months. A random-effects model was used to compute pooled SMDs with 95% CI based on 22 studies (n = 1,562).
Figure 6

Forest plot of the effect of omega-3 fatty acid supplementation on chronic pain at 6 months. A random-effects model was used to compute pooled SMDs with 95% CI based on 14 studies (n = 1,053).
3.3 Subgroup analyses
Forest plot depicting the pooled and subgroup effects of omega-3 fatty acid supplementation on chronic pain intensity (random-effects model). See Figure 7.
Figure 7
![Forest plot showing the subgroup analysis of 41 studies assessing the effect size of interventions. Categories include disease type, fatty acid type, country, intervention period, daily dose, outcome score, and control type. The standardized mean difference (SMD) and 95% confidence intervals (CI) are presented for each category. Main analysis SMD is -0.55 [-0.76, -0.34]. Effects are more negative in osteoarthritis and migraine subgroups, with SMDs of -0.77 and -0.84, respectively. Positive effects are seen in placebo control with SMD of -0.59. The plot visually compares each subgroup’s SMD with a dashed line indicating no effect.](https://www.frontiersin.org/files/Articles/1654661/xml-images/fmed-12-1654661-g007.webp)
Forest plot of the pooled and subgroup effects of omega-3 fatty-acid supplementation on chronic-pain intensity (random-effects model).
3.3.1 Pain assessment tools
In 20 studies (18, 24–26, 28, 35, 36, 38, 40, 42–45, 47–49, 51, 54, 57, 58) utilizing the VAS, omega-3 fatty acid supplementation led to a significant reduction in pain intensity (n = 1,083; SMD = −0.60; 95% CI: −0.95 to −0.26; p < 0.001; I2 = 86%). A similar analgesic effect was observed in 16 studies (19, 21, 22, 29, 30, 32–34, 37, 41, 46, 50, 52, 53, 55, 56) employing other validated instruments (n = 946; SMD = −0.36; 95% CI: −0.57 to −0.14; p = 0.001; I2 = 59%). Importantly, of these, five studies (20, 23, 27, 31, 39) utilized the WOMAC, which also demonstrated a statistically significant effect (n = 1730; SMD = −0.85; 95% CI: −1.63 to −0.07; p = 0.03; I2 = 97%), despite high heterogeneity, as detailed in Supplementary material and Table 2.
3.3.2 Disease type
Omega-3 fatty acid supplementation significantly alleviated pain in patients with rheumatoid arthritis (RA), based on data from 16 studies (36, 37, 40, 44–47, 50–58) (n = 813, SMD = −0.42, 95% CI: −0.76 to −0.09, p = 0.01, I2 = 80%). A significant benefit was also observed in migraine patients, derived from pooled data from two studies (19, 41) (n = 167, SMD = −0.84, 95% CI: −1.44 to −0.24, p = 0.006, I2 = 49%). Similarly, a moderate effect size was observed in the category of “other chronic pain conditions,” derived from 16 studies (20–24, 26, 29, 30, 32, 34, 35, 38, 39, 42, 43, 48) (n = 2044, SMD = −0.61, 95% CI: −0.94 to −0.29, p < 0.001, I2 = 87%). Neither the osteoarthritis (OA) nor mastalgia subgroup showed a statistically significant benefit in the random-effects model. In OA, no significant analgesic effect was observed, derived from 5 trials (18, 27, 28, 31, 49) (n = 575, SMD = −0.77, 95% CI: −1.55 to 0.00, p = 0.05, I2 = 94%). Likewise, mastalgia showed no analgesic advantage, derived from 2 trials (25, 33) (n = 160, SMD = −0.04, 95% CI: −0.35 to 0.27, p = 0.78, I2 = 0%), as detailed in Supplementary material and Table 2.
3.3.3 Intervention duration
Subgroup analysis based on intervention duration revealed that both short-term (<3 months) and long-term (≥3 months) supplementation with omega-3 fatty acids led to significant reductions in pain. Short-term interventions (nine studies) (20, 22, 39–43, 48, 57) demonstrated a statistically significant effect (n = 450, SMD = −0.53, 95% CI: −0.87 to −0.20, p = 0.002; I2 = 62%), albeit smaller than long-term interventions (32 studies) (18, 19, 21, 23–38, 44–47, 49–56, 58) which showed a stronger analgesic effect (n = 3,309, SMD = −0.54, 95% CI: −0.78 to −0.29, p < 0.001; I2 = 90%). as shown in Supplementary material and Table 2.
3.3.4 Country
Omega-3 fatty acid supplementation was found to significantly reduce pain in both studies conducted in the United States (10 studies (19, 21, 24, 26, 29, 32, 34, 41, 52, 55); n = 521, SMD = −0.62, 95% CI: −0.98 to −0.25, p = 0.001; I2 = 73%) and those from other countries (31 studies (18, 20, 22, 23, 25, 27, 28, 30, 31, 33, 35–40, 42–51, 53, 54, 56–58); n = 3,238, SMD = −0.53, 95% CI: −0.77 to −0.28, p < 0.001; I2 = 89%). Despite moderate to high heterogeneity, the consistent effect sizes across regions suggest that omega-3’s pain-relieving benefits are applicable to diverse populations and healthcare systems as shown in Supplementary material and Table 2.
3.3.5 Type of unsaturated fatty acid supplementation
Subgroup analysis based on the type of unsaturated fatty acid supplementation showed varying analgesic efficacy. Among 15 studies (24, 28, 31, 33, 39, 44, 46, 48, 50–54, 56, 58) on fish oil, a significant reduction in pain was observed (n = 820; SMD = −0.69; 95% CI: −1.09 to −0.29; p < 0.001; I2 = 86%). Similarly, 15 studies (19, 21, 23, 26, 29, 30, 32, 34–38, 40, 41, 57) on omega-3 fatty acids also demonstrated a significant analgesic effect (n = 2,151; SMD = −0.55; 95% CI: −0.91 to −0.19; p = 0.002; I2 = 90%). Additionally, 11 studies (18, 20, 22, 25, 27, 42, 43, 45, 47, 49, 55) on mixed supplement formulations (including combined n-3 and other fatty acids) reported a comparable reduction in pain (n = 788; SMD = −0.34; 95% CI: −0.58 to −0.09; p = 0.006; I2 = 59%). as shown in Supplementary material and Table 2.
3.3.6 Omega-3 dosage
Both dosage groups showed significant reductions in pain intensity compared to control. However, the low-dose group (≤1.35 g/day) exhibited greater analgesic effects in 12 trials (21–26, 35, 38, 41, 44, 48, 49) (n = 1,873; SMD = −0.60; 95% CI: −0.99 to −0.21; p = 0.003; I2 = 89%), while the high-dose group (>1.35 g/day) showed a more modest effect in 29 trials (18–20, 27–34, 36, 37, 39, 40, 42, 43, 45–47, 50–58) (n = 1,886; SMD = −0.53; 95% CI: −0.78 to −0.28; p < 0.001; I2 = 85%). as shown in Supplementary material and Table 2.
3.3.7 Placebo-controlled vs. active-controlled trials
Omega-3 fatty acid supplementation showed a significant analgesic effect compared to placebo in 21 randomized trials (20–24, 26, 28–30, 35, 37–41, 44, 45, 47, 51, 54, 58) (n = 2,419; SMD = −0.59; 95% CI: −0.86 to −0.32; p < 0.001; I2 = 86%). Out of the 20 trials (18, 19, 25, 27, 31–34, 36, 42, 43, 46, 48–50, 52, 53, 55–57) with an active comparator, a statistically significant effect was also observed (n = 1,340; SMD = −0.50; 95% CI: −0.84 to −0.16; p = 0.004; I2 = 88%). as shown in Supplementary material and Table 2.
3.4 Sensitivity analysis and publication bias
The distribution of study weights was assessed, and no single study significantly impacted the overall pooled effect. Each study contributed relatively equally, with a weight range of 1%–5%, and no outliers were identified. Sensitivity analysis was performed by omitting one study at a time, and the results remained stable, confirming the robustness of the findings.
Publication bias was assessed using a funnel plot, as shown in Supplementary material, which exhibited slight asymmetry with a leftward skew in the distribution of effect sizes. Egger’s test provided marginal evidence of publication bias (p = 0.052), whereas Begg’s test indicated statistical significance (p = 0.009). A trim-and-fill analysis was conducted using a linear estimator under a random-effects model to further explore this possibility. Six potentially missing studies were imputed on the right side of the funnel plot, based on the trim-and-fill method. After adjustment, the pooled effect size remained statistically significant (adjusted SMD = −0.723), suggesting that the analgesic benefit of omega-3 fatty acids is robust and minimally influenced by small-study effects or selective reporting.
4 Discussion
4.1 Principal findings
In this comprehensive meta-analysis of 41 randomized controlled trials (N = 3,759), Omega-3 fatty acid supplementation demonstrated a moderate, statistically and clinically significant reduction in chronic pain intensity (random-effects SMD = −0.55). Beneficial effects emerged as early as 1 month (SMD = −0.27) and were maintained at 2 months (SMD = −0.39) and 3 months (SMD = −0.51), with the largest effect at 6 months (SMD = −0.83), indicating a time-dependent, cumulative analgesic response. A clear dose pattern was evident: low-dose regimens (≤1.35 g day−1) yielded a better effect (SMD = −0.60) than higher doses (>1.35 g day−1; SMD = −0.53). Subgroup analyses confirmed robust benefit in RA, migraine and miscellaneous chronic pain conditions, while no significant improvement was detected in OA or mastalgia. Risk-of-bias assessment indicated that 63% of trials were low risk and sensitivity analyses showed that removing any single study did not dramatically alter the pooled estimate; trim-and-fill procedures suggested minimal impact of publication bias.
4.2 Comparison with previous work
Recent systematic reviews and meta-analyses have come together to support the therapeutic benefits of omega-3 fatty acids supplementation for specific chronic pain conditions. Goldberg et al. (59), pooling 17 randomized controlled trials, demonstrated that omega-3 fatty acids significantly attenuate patient-reported pain intensity in inflammatory disorders such as RA. Concordantly, a 2025 systematic review reported a robust analgesic effect of omega-3 fatty acids in migraine, reflected by a significant reduction in standardized headache-severity scores (60). These observations closely align with the subgroup findings in this study—RA and migraine—thereby reinforcing the reliability and reproducibility of our results across studies. Mechanistically, the analgesic actions of omega-3 fatty acids stem from their multi-tiered modulation of the inflammatory cascade. First, incorporation of omega-3 fatty acids into membrane phospholipids displaces arachidonic acid, lowering biosynthesis of key pronociceptive eicosanoids such as prostaglandin E₂ and leukotriene B₄ (61). Second, omega-3 fatty acids are enzymatically converted to specialized pro-resolving mediators—for example, resolvins, protectins, and maresins—which engage receptors such as FPR2/ALX and ChemR23 to suppress NF-κB signaling and down-regulate pro-inflammatory cytokines (TNF-α, IL-1β, IL-6) (62). Third, omega-3 fatty acids promote macrophage polarization toward the anti-inflammatory M2 phenotype, thereby accelerating active resolution of inflammation (63). Collectively, these anti-inflammatory and neuro-modulatory mechanisms intercept the pathological continuum from peripheral tissue inflammation to central sensitization (64), providing a compelling molecular rationale for integrating omega-3 fatty acids into contemporary chronic-pain management paradigms.
In the present subgroup analyses, omega-3 fatty acids supplementation did not demonstrate a statistically significant analgesic effect in patients with OA or mastalgia. Several factors may account for this null finding. First, OA is characterized by a relatively low-grade inflammatory profile compared to conditions such as RA (65), thereby potentially limiting the therapeutic scope for omega-3’s anti-inflammatory mechanisms. Second, the pathophysiology of OA-related pain is largely mechanical in origin, driven by cartilage wear, subchondral bone changes, and joint loading, with central sensitization often contributing to chronic symptom persistence (66, 67). These mechanisms may be less responsive to lipid-mediated anti-inflammatory modulation. Third, outcome assessment tools may influence effect detection. Most OA trials employed the WOMAC index, which combines pain, stiffness, and physical function subdomains. The multidimensional nature of WOMAC may dilute changes in pain-specific outcomes, especially when compared to more sensitive, unidimensional measures like the VAS. Taken together, these biological and methodological factors may explain the absence of a statistically significant analgesic effect of omega-3 in OA trials. As for mastalgia, the condition is largely hormonally mediated, primarily influenced by cyclical fluctuations in estrogen and prolactin levels rather than inflammatory pathways (68).
4.3 Clinical implications
In this meta-analysis, the pooled SMD was −0.55. When back-translated to a 0–100 mm VAS using a standard deviation of 20–25 mm—commonly reported in chronic pain trials—this corresponds to an absolute pain reduction of approximately 11–14 mm. At the 6-month follow-up, an SMD of −0.83 equates to a reduction of roughly 17–21 mm on the VAS. Given that the minimal clinically important difference (MCID) for chronic pain in adults is generally considered to be ~10 mm (69), the effects observed in this analysis exceed the threshold for clinical relevance.
For comparison, the established analgesic dose of oral diclofenac (150 mg/day) yields a pooled effect size of SMD –0.56, corresponding to an approximate 14-mm reduction in VAS pain scores (70). The efficacy of omega-3 fatty acid supplementation (SMD –0.55) appears broadly comparable in magnitude; however, unlike nonsteroidal anti-inflammatory drugs (NSAIDs), omega-3 s are associated with a substantially lower risk of gastrointestinal and cardiovascular toxicities. Importantly, omega-3 fatty acids should not be regarded as equivalent to NSAIDs, which remain the first-line therapy for acute pain. Rather, omega-3 s may be best positioned as a safer adjunct or as a long-term strategy in the management of chronic pain. The analgesic effect demonstrated a clear time-dependent escalation: the SMD improved from −0.27 to −0.51 at 1–3 months and reached −0.83 at 6 months. This temporal pattern aligns with the kinetics of omega-3 fatty acids merging into cell membranes, lowering the n-6: n-3 ratio, and enhancing the synthesis of SPMs such as resolvin D1 and resolvin E1. SPMs directly down-regulate nociceptive ion channels and suppress spinal glial activation, providing a “pro-resolution” form of analgesia distinct from conventional anti-inflammatory drugs (62, 71). Our subgroup analysis yielded consistent results, reinforcing the notion that longer durations of omega-3 supplementation are necessary to achieve clinically meaningful pain relief. This finding is consistent with previous reviews suggesting that prolonged supplementation is necessary to achieve clinically meaningful analgesic effects (72). A greater effect was observed in the low-dose group (≤1.35 g day−1; SMD = −0.60), presumably due to saturation of the plasma omega-3 fatty acids curve and better adherence relative to higher doses; nevertheless, doses >1.35 g day−1 remained efficacious. These findings are consistent with prior evidence suggesting that higher doses may not confer additional benefits for chronic pain relief and could even be less effective in certain contexts (31). Accordingly, dosing can be individualized on the basis of cost-effectiveness and patient tolerability.
4.4 Strengths
The present review surpasses earlier syntheses in several critical respects. First, by pooling 41 randomized controlled trials encompassing 3,759 participants—nearly double the sample size of the largest prior meta-analysis—and spanning migraine, RA, neuropathic pain, and musculoskeletal conditions, we markedly increased both statistical power and external validity. Second, we provide the first systematic evidence that the dose–response relationship is non-linear: daily intakes ≤1.35 g of omega-3 fatty acids produced the greatest analgesic benefit, whereas higher doses yielded diminishing returns, implying a ceiling effect or reduced adherence at large dosages. Third, we delineated the full temporal trajectory of benefit, showing that pain relief emerges within 1 month and accumulates steadily through 6 months—information that refines clinical expectations and guides future trial follow-up schedules. Fourth, the review adhered to PRISMA 2020, was prospectively registered in PROSPERO, employed the RoB 2 tool, and confirmed robustness through sensitivity, leave-one-out, and trim-and-fill analyses, thereby minimizing the risk of selective-reporting bias that troubled earlier work. Finally, comprehensive subgroup analyses revealed that that placebo-controlled trials show better effect sizes than active-control trials, underscoring the importance of comparator choice.
4.5 Limitations
This study has several limitations. First, substantial heterogeneity (I2 > 50%) was observed due to pooling trials with differences in pain condition, supplement formulation, dosage regimen, intervention length, and participant characteristics. Despite using random-effects models and subgroup analyses, heterogeneity remained in most comparisons, except for the first- and second-month analyses (moderate heterogeneity) and the breast-pain and migraine subgroups (relatively low heterogeneity). Second, 36.6% of trials had “some concerns” or “high” risk of bias, mainly from attrition, selective reporting, and insufficient details on randomization or allocation concealment, which may compromise internal validity. Third, potential confounders such as concurrent NSAID use and baseline omega-3 status were not consistently reported, precluding adjustment. Finally, sex-specific differences in pain response could not be examined, as most trials did not report stratified results. Future studies should better control for these factors and provide sex-disaggregated data. Nevertheless, sensitivity analyses suggested that our overall conclusions remained robust.
5 Conclusion
This meta-analysis demonstrates that omega-3 fatty acid supplementation produces a clinically meaningful, ceiling effect for dose escalation and time-dependent reduction in chronic pain intensity. The analgesic efficacy was most evident in inflammatory pain phenotypes such as rheumatoid arthritis and migraine, whereas evidence remains inconclusive for osteoarthritis and mastalgia. These findings support the use of omega-3 fatty acids as a safe, non-pharmacological adjunct in the management of chronic pain. Future high-quality trials are warranted to clarify the phenotype-specific indications, dose–response relationships, and long-term efficacy of omega-3 supplementation, thereby informing precision strategies for chronic pain management.
Statements
Data availability statement
The original contributions presented in the study are included in the article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
LX: Funding acquisition, Validation, Resources, Writing – original draft, Formal analysis, Project administration, Data curation, Investigation, Supervision, Writing – review & editing, Conceptualization, Methodology, Visualization, Software. XiW: Validation, Conceptualization, Resources, Writing – review & editing, Funding acquisition, Supervision, Investigation, Formal analysis, Methodology, Data curation, Writing – original draft, Visualization, Software, Project administration. JC: Validation, Data curation, Writing – original draft. XH: Software, Investigation, Writing – original draft, Validation. JB: Visualization, Writing – original draft, Validation, Supervision. YX: Formal analysis, Writing – original draft, Data curation. XuW: Resources, Investigation, Supervision, Methodology, Writing – review & editing, Software. QZ: Conceptualization, Investigation, Supervision, Methodology, Writing – review & editing, Funding acquisition, Software, Writing – original draft, Formal analysis, Project administration, Resources, Data curation, Visualization, Validation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Zhejiang Medical and Health Science and Technology Plan Project (2025KY375), Zhejiang Provincial Key Clinical Specialty-Anesthesiology (2023-ZJZK-001), and Jiaxing Key Discipline of Medicine-Anesthesiology (2023-ZC-001).
Acknowledgments
We extend our sincere gratitude to the authors who kindly responded to our inquiries and provided additional data, which greatly contributed to the comprehensiveness of this review. We also thank all the participants involved in the included studies for their valuable contributions.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1654661/full#supplementary-material
References
1.
Lucas JW Sohi I . Chronic pain and high-impact chronic pain in U.S. adults, 2023. NCHS Data Brief. (2024) 518:CS355235. doi: 10.15620/cdc/169630
2.
Shetty A Delanerolle G Cavalini H Deng C Yang X Boyd A et al . A systematic review and network meta-analysis of pharmaceutical interventions used to manage chronic pain. Sci Rep. (2024) 14:1621. doi: 10.1038/s41598-023-49761-3
3.
Thomas KH Dalili MN Cheng HY Dawson S Donnelly N Higgins JPT et al . Prevalence of problematic pharmaceutical opioid use in patients with chronic non-cancer pain: a systematic review and meta-analysis. Addiction. (2024) 119:1904–22. doi: 10.1111/add.16616
4.
Calder PC . Omega-3 fatty acids and inflammatory processes. Nutrients. (2010) 2:355–74. doi: 10.3390/nu2030355
5.
Calder PC . Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. (2017) 45:1105–15. doi: 10.1042/bst20160474
6.
Chen X Chen C Fan S Wu S Yang F Fang Z et al . Omega-3 polyunsaturated fatty acid attenuates the inflammatory response by modulating microglia polarization through SIRT1-mediated deacetylation of the HMGB1/NF-κB pathway following experimental traumatic brain injury. J Neuroinflammation. (2018) 15:116. doi: 10.1186/s12974-018-1151-3
7.
Soens MA Sesso HD Manson JE Fields KG Buring JE Lee IM et al . The effect of vitamin D and omega-3 fatty acid supplementation on pain prevalence and severity in older adults: a large-scale ancillary study of the VITamin D and OmegA-3 triaL (VITAL). Pain. (2024) 1653:635–43. doi: 10.1097/j.pain.0000000000003044
8.
Tseng PT Zeng BY Chen JJ Kuo CH Zeng BS Kuo JS et al . High dosage Omega-3 fatty acids outperform existing pharmacological options for migraine prophylaxis: a network Meta-analysis. Adv Nutr. (2024) 15:100163. doi: 10.1016/j.advnut.2023.100163
9.
Mohammadnezhad G Assarzadegan F Koosha M Esmaily H . Eicosapentaenoic acid versus placebo as adjunctive therapy in chronic migraine: a randomized controlled trial. Headache. (2025) 65:153–63. doi: 10.1111/head.14808
10.
Kyriakidou Y Wood C Ferrier C Dolci A Elliott B . The effect of omega-3 polyunsaturated fatty acid supplementation on exercise-induced muscle damage. J Int Soc Sports Nutr. (2021) 18:9. doi: 10.1186/s12970-020-00405-1
11.
Page MJ McKenzie JE Bossuyt PM Boutron I Hoffmann TC Mulrow CD et al . The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. (2021) 372:n71. doi: 10.1136/bmj.n71
12.
Sterne JAC Savović J Page MJ Elbers RG Blencowe NS Boutron I et al . RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. (2019) 366:l4898. doi: 10.1136/bmj.l4898
13.
Prego-Dominguez J Hadrya F Takkouche B . Polyunsaturated fatty acids and chronic pain: a systematic review and meta-analysis. Pain Physician. (2016) 198:521–35. PMID:
14.
Rees D Miles EA Banerjee T Wells SJ Roynette CE Wahle KW et al . Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am J Clin Nutr. (2006) 83:331–42. doi: 10.1093/ajcn/83.2.331
15.
Jiang Y Fang P Shang Z Zhu W Gao S Liu X . Cognitive training in surgical patients: a systematic review and meta-analysis. Anesthesiol Perioperative Sci. (2023) 1:18. doi: 10.1007/s44254-023-00014-6
16.
Egger M Davey Smith G Schneider M Minder C . Bias in meta-analysis detected by a simple, graphical test. BMJ. (1997) 315:629–34. doi: 10.1136/bmj.315.7109.629
17.
Begg CB Mazumdar M . Operating characteristics of a rank correlation test for publication bias. Biometrics. (1994) 504:1088–101.
18.
Möller I Rodas G Villalón JM Rodas JA Angulo F Martínez N et al . Randomized, double-blind, placebo-controlled study to evaluate the effect of treatment with an SPMs-enriched oil on chronic pain and inflammation, functionality, and quality of life in patients with symptomatic knee osteoarthritis: GAUDI study. J Transl Med. (2023) 21:423. doi: 10.1186/s12967-023-04283-4
19.
Faurot KR Park J Miller V Honvoh G Domeniciello A Mann JD et al . Dietary fatty acids improve perceived sleep quality, stress, and health in migraine: a secondary analysis of a randomized controlled trial. Front Pain Res. (2023) 4:1231054. doi: 10.3389/fpain.2023.1231054
20.
Pérez-Piñero S Muñoz-Carrillo JC Victoria-Montesinos D García-Muñoz AM Andreu-Caravaca L Gómez M et al . Efficacy of Boswellia serrata extract and/or an omega-3-based product for improving pain and function in people older than 40 years with persistent knee pain: a randomized double-blind controlled clinical trial. Nutrients. (2023) 15:1517. doi: 10.3390/nu15173848
21.
Carlisle C Polley K Panda C Barron K Hamrock M Dominique A et al . Alleviation of PAIN, PAIN interference, and oxidative stress by a novel combination of hemp oil, calamari oil, and broccoli: a randomized, double-blind, placebo-controlled trial. Nutrients. (2023) 15:1512. doi: 10.3390/nu15122654
22.
Sasahara I Yamamoto A Takeshita M Suga Y Suzuki K Nishikata N et al . L-serine and EPA relieve chronic low-back and knee pain in adults: a randomized, double-blind, placebo-controlled trial. J Nutr. (2020) 150:2278–86. doi: 10.1093/jn/nxaa156
23.
MacFarlane LA Cook NR Kim E Lee IM Iversen MD Gordon D et al . The effects of vitamin D and marine omega-3 fatty acid supplementation on chronic knee pain in older US adults: results from a randomized trial. Arthritis Rheumatol. (2020) 7211:1836–44. doi: 10.1002/art.41416
24.
Nodler JL DiVasta AD Vitonis AF Karevicius S Malsch M Sarda V et al . Supplementation with vitamin D or ω-3 fatty acids in adolescent girls and young women with endometriosis (SAGE): a double-blind, randomized, placebo-controlled trial. Am J Clin Nutr. (2020) 112:229–36. doi: 10.1093/ajcn/nqaa096
25.
Godazandeh G Ala S Motlaq TM Sahebnasagh A Bazi A . The comparison of the effect of flaxseed oil and vitamin E on mastalgia and nodularity of breast fibrocystic: a randomized double-blind clinical trial. J Pharm Health Care Sci. (2021) 7:4. doi: 10.1186/s40780-020-00186-4
26.
Hadian Z Moghadamnia AA Kazemi S Shirzad A . Effect of Omega-3 on recurrent aphthous stomatitis and improvement quality of life. Int J Dent. (2021) 2021:1–9. doi: 10.1155/2021/6617575
27.
Stonehouse W Benassi-Evans B Bednarz J Vincent AD Hall S Hill CL . Krill oil improved osteoarthritic knee pain in adults with mild to moderate knee osteoarthritis: a 6-month multicenter, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. (2022) 116:672–85. doi: 10.1093/ajcn/nqac125
28.
Kuszewski JC Wong RHX Howe PRC . Fish oil supplementation reduces osteoarthritis-specific pain in older adults with overweight/obesity. Rheumatol Adv Pract. (2020) 4:rkaa036. doi: 10.1093/rap/rkaa036
29.
Lustberg MB Orchard TS Reinbolt R Andridge R Pan X Belury M et al . Randomized placebo-controlled pilot trial of omega 3 fatty acids for prevention of aromatase inhibitor-induced musculoskeletal pain. Breast Cancer Res Treat. (2018) 167:709–18. doi: 10.1007/s10549-017-4559-z
30.
Noguchi H Nishi D Matsumura K Hamazaki K Hamazaki T Matsuoka YJ . Limited effect of omega-3 fatty acids on the quality of life in survivors of traumatic injury: a randomized, placebo-controlled trial. Prostaglandins Leukot Essent Fatty Acids. (2017) 127:1–5. doi: 10.1016/j.plefa.2017.09.018
31.
Hill CL March LM Aitken D Lester SE Battersby R Hynes K et al . Fish oil in knee osteoarthritis: a randomised clinical trial of low dose versus high dose. Ann Rheum Dis. (2016) 75:23–9. doi: 10.1136/annrheumdis-2014-207169
32.
Ramsden CE Zamora D Makriyannis A Wood JT Mann JD Faurot KR et al . Diet-induced changes in n-3- and n-6-derived endocannabinoids and reductions in headache pain and psychological distress. J Pain. (2015) 16:707–16. doi: 10.1016/j.jpain.2015.04.007
33.
Blommers J de Lange-De Klerk ES Kuik DJ Bezemer PD Meijer S . Evening primrose oil and fish oil for severe chronic mastalgia: a randomized, double-blind, controlled trial. Am J Obstet Gynecol. (2002) 187:1389–94. doi: 10.1067/mob.2002.127377a
34.
Ramsden CE Faurot KR Zamora D Suchindran CM MacIntosh BA Gaylord S et al . Targeted alteration of dietary n-3 and n-6 fatty acids for the treatment of chronic headaches: a randomized trial. Pain. (2013) 154:2441–51. doi: 10.1016/j.pain.2013.07.028
35.
El Khouli AM El-Gendy EA . Efficacy of omega-3 in treatment of recurrent aphthous stomatitis and improvement of quality of life: a randomized, double-blind, placebo-controlled study. Oral Surg Oral Med Oral Pathol Oral Radiol. (2014) 117:191–6. doi: 10.1016/j.oooo.2013.09.003
36.
Das Gupta AB Hossain AK Islam MH Dey SR Khan AL . Role of omega-3 fatty acid supplementation with indomethacin in suppression of disease activity in rheumatoid arthritis. Bangladesh Med Res Counc Bull. (2009) 35:63–8. doi: 10.3329/bmrcb.v35i2.3020
37.
Park Y Lee A Shim SC Lee JH Choe JY Ahn H et al . Effect of n-3 polyunsaturated fatty acid supplementation in patients with rheumatoid arthritis: a 16-week randomized, double-blind, placebo-controlled, parallel-design multicenter study in Korea. J Nutr Biochem. (2013) 24:1367–72. doi: 10.1016/j.jnutbio.2012.11.004
38.
Rahbar N Asgharzadeh N Ghorbani R . Effect of omega-3 fatty acids on intensity of primary dysmenorrhea. Int J Gynaecol Obstet. (2012) 117:45–7. doi: 10.1016/j.ijgo.2011.11.019
39.
Caturla N Funes L Pérez-Fons L Micol V . A randomized, double-blinded, placebo-controlled study of the effect of a combination of lemon verbena extract and fish oil omega-3 fatty acid on joint management. J Altern Complement Med. (2011) 17:1051–63. doi: 10.1089/acm.2010.0410
40.
Galarraga B Ho M Youssef HM Hill A McMahon H Hall C et al . Cod liver oil (n-3 fatty acids) as an non-steroidal anti-inflammatory drug sparing agent in rheumatoid arthritis. Rheumatology (Oxford). (2008) 47:665–9. doi: 10.1093/rheumatology/ken024
41.
Harel Z Gascon G Riggs S Vaz R Brown W Exil G . Supplementation with omega-3 polyunsaturated fatty acids in the management of recurrent migraines in adolescents. J Adolesc Health. (2002) 31:154–61. doi: 10.1016/s1054-139x(02)00349-x
42.
Brunborg LA Madland TM Lind RA Arslan G Berstad A Frøyland L . Effects of short-term oral administration of dietary marine oils in patients with inflammatory bowel disease and joint pain: a pilot study comparing seal oil and cod liver oil. Clin Nutr. (2008) 27:614–22. doi: 10.1016/j.clnu.2008.01.017
43.
Bjørkkjaer T Brunborg LA Arslan G Lind RA Brun JG Valen M et al . Reduced joint pain after short-term duodenal administration of seal oil in patients with inflammatory bowel disease: comparison with soy oil. Scand J Gastroenterol. (2004) 39:1088–94. doi: 10.1080/00365520410009429
44.
Hansen GV Nielsen L Kluger E Thysen M Emmertsen H Stengaard-Pedersen K et al . Nutritional status of Danish rheumatoid arthritis patients and effects of a diet adjusted in energy intake, fish-meal, and antioxidants. Scand J Rheumatol. (1996) 25:325–30. doi: 10.3109/03009749609104066
45.
Nordström DC Honkanen VE Nasu Y Antila E Friman C Konttinen YT . Alpha-linolenic acid in the treatment of rheumatoid arthritis. A double-blind, placebo-controlled and randomized study: flaxseed vs. safflower seed. Rheumatol Int. (1995) 14:231–4. doi: 10.1007/bf00262088
46.
Berbert AA Kondo CR Almendra CL Matsuo T Dichi I . Supplementation of fish oil and olive oil in patients with rheumatoid arthritis. Nutrition. (2005) 21:131–6. doi: 10.1016/j.nut.2004.03.023
47.
Remans PH Sont JK Wagenaar LW Wouters-Wesseling W Zuijderduin WM Jongma A et al . Nutrient supplementation with polyunsaturated fatty acids and micronutrients in rheumatoid arthritis: clinical and biochemical effects. Eur J Clin Nutr. (2004) 58:839–45. doi: 10.1038/sj.ejcn.1601883
48.
Kawabata F Tsuji T . Effects of dietary supplementation with a combination of fish oil, bilberry extract, and lutein on subjective symptoms of asthenopia in humans. Biomed Res. (2011) 32:387–93. doi: 10.2220/biomedres.32.387
49.
Stammers T Sibbald B Freeling P . Efficacy of cod liver oil as an adjunct to non-steroidal anti-inflammatory drug treatment in the management of osteoarthritis in general practice. Ann Rheum Dis. (1992) 51:128–9. doi: 10.1136/ard.51.1.128
50.
Geusens P Wouters C Nijs J Jiang Y Dequeker J . Long-term effect of omega-3 fatty acid supplementation in active rheumatoid arthritis. A 12-month, double-blind, controlled study. Arthritis Rheum. (1994) 376:824–9. doi: 10.1002/art.1780370608
51.
Nielsen GL Faarvang KL Thomsen BS Teglbjaerg KL Jensen LT Hansen TM et al . The effects of dietary supplementation with n-3 polyunsaturated fatty acids in patients with rheumatoid arthritis: a randomized, double blind trial. Eur J Clin Invest. (1992) 22:687–91. doi: 10.1111/j.1365-2362.1992.tb01431.x
52.
Kremer JM Lawrence DA Jubiz W DiGiacomo R Rynes R Bartholomew LE et al . Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis. Clinical and immunologic effects. Arthritis Rheum. (1990) 33:810–20. doi: 10.1002/art.1780330607
53.
van der Tempel H Tulleken JE Limburg PC Muskiet FA van Rijswijk MH . Effects of fish oil supplementation in rheumatoid arthritis. Ann Rheum Dis. (1990) 49:76–80. doi: 10.1136/ard.49.2.76
54.
Sundrarjun T Komindr S Archararit N Dahlan W Puchaiwatananon O Angthararak S et al . Effects of n-3 fatty acids on serum interleukin-6, tumour necrosis factor-alpha and soluble tumour necrosis factor receptor p55 in active rheumatoid arthritis. J Int Med Res. (2004) 32:443–54. doi: 10.1177/147323000403200501
55.
Kremer JM Lawrence DA Petrillo GF Litts LL Mullaly PM Rynes RI et al . Effects of high-dose fish oil on rheumatoid arthritis after stopping nonsteroidal antiinflammatory drugs. Clinical and immune correlates. Arthritis Rheum. (1995) 388:1107–14. doi: 10.1002/art.1780380813
56.
Sköldstam L Börjesson O Kjällman A Seiving B Akesson B . Effect of six months of fish oil supplementation in stable rheumatoid arthritis. A double-blind, controlled study. Scand J Rheumatol. (1992) 21:178–85. doi: 10.3109/03009749209099218
57.
Magarò M Zoli A Altomonte L Mirone L De Sole P Di Mario G et al . Effect of fish oil on neutrophil chemiluminescence induced by different stimuli in patients with rheumatoid arthritis. Ann Rheum Dis. (1992) 51:877–80. doi: 10.1136/ard.51.7.877
58.
Adam O Beringer C Kless T Lemmen C Adam A Wiseman M et al . Anti-inflammatory effects of a low arachidonic acid diet and fish oil in patients with rheumatoid arthritis. Rheumatol Int. (2003) 23:27–36. doi: 10.1007/s00296-002-0234-7
59.
Goldberg RJ Katz J . A meta-analysis of the analgesic effects of omega-3 polyunsaturated fatty acid supplementation for inflammatory joint pain. Pain. (2007) 129:210–23. doi: 10.1016/j.pain.2007.01.020
60.
García-Pérez-de-Sevilla G González-de-la-Flor Á . Impact of fatty acid supplementation on migraine outcomes: a systematic review and Meta-analysis. Nutr Rev. (2025) 83:1621–30. doi: 10.1093/nutrit/nuae219
61.
Calder PC . Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology?Br J Clin Pharmacol. (2013) 75:645–62. doi: 10.1111/j.1365-2125.2012.04374.x
62.
Serhan CN Levy BD . Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. (2018) 128:2657–69. doi: 10.1172/jci97943
63.
Bodur M Yilmaz B Ağagündüz D Ozogul Y . Immunomodulatory effects of Omega-3 fatty acids: mechanistic insights and health implications. Mol Nutr Food Res. (2025) 69:e202400752. doi: 10.1002/mnfr.202400752
64.
Ji RR Xu ZZ Gao YJ . Emerging targets in neuroinflammation-driven chronic pain. Nat Rev Drug Discov. (2014) 13:533–48. doi: 10.1038/nrd4334
65.
Scanzello CR Goldring SR . The role of synovitis in osteoarthritis pathogenesis. Bone. (2012) 51:249–57. doi: 10.1016/j.bone.2012.02.012
66.
Dieppe PA Lohmander LS . Pathogenesis and management of pain in osteoarthritis. Lancet. (2005) 365:965–73. doi: 10.1016/s0140-6736(05)71086-2
67.
Wan J Qian X He Z Zhu Z Cheng P Chen A . Epidemiological trends of hand osteoarthritis from 1990 to 2019: estimates from the 2019 global burden of disease study. Front Med. (2022) 9:922321. doi: 10.3389/fmed.2022.922321
68.
Cornell LF Sandhu NP Pruthi S Mussallem DM . Current management and treatment options for breast pain. Mayo Clin Proc. (2020) 95:574–80. doi: 10.1016/j.mayocp.2019.12.014
69.
Myles PS Myles DB Galagher W Boyd D Chew C MacDonald N et al . Measuring acute postoperative pain using the visual analog scale: the minimal clinically important difference and patient acceptable symptom state. Br J Anaesth. (2017) 118:424–9. doi: 10.1093/bja/aew466
70.
da Costa BR Pereira TV Saadat P Rudnicki M Iskander SM Bodmer NS et al . Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: network meta-analysis. BMJ. (2021) 375:n2321. doi: 10.1136/bmj.n2321
71.
Ji RR . Specialized pro-resolving mediators as resolution pharmacology for the control of pain and itch. Annu Rev Pharmacol Toxicol. (2023) 63:273–93. doi: 10.1146/annurev-pharmtox-051921-084047
72.
Stamp LK James MJ Cleland LG . Diet and rheumatoid arthritis: a review of the literature. Semin Arthritis Rheum. (2005) 352:77–94. doi: 10.1016/j.semarthrit.2005.05.001
Summary
Keywords
omega-3 fatty acids, chronic pain, pain management, systematic review, meta-analysis
Citation
Xie L, Wang X, Chu J, He X, Bao J, Xi Y, Wei X and Zhou Q (2025) Effects of omega-3 fatty acids on chronic pain: a systematic review and meta-analysis. Front. Med. 12:1654661. doi: 10.3389/fmed.2025.1654661
Received
26 June 2025
Accepted
20 October 2025
Published
05 November 2025
Volume
12 - 2025
Edited by
Francisco Lopez-Munoz, Camilo José Cela University, Spain
Reviewed by
Mohsin Raza, HCA Healthcare North Florida Division, United States
Yujia Zhang, Centers for Disease Control and Prevention (CDC), United States
Updates
Copyright
© 2025 Xie, Wang, Chu, He, Bao, Xi, Wei and Zhou.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qinghe Zhou, zqh10980@zjxu.edu.cn
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.