Abstract
Introduction:
Extensive lower limb arterial calcification complicates revascularization and is linked to poor outcomes, including limb loss and cardiovascular events. Standardized scoring systems are lacking, particularly in aortoiliac TASC II D lesions. This study evaluated the prognostic value of a CT-based Iliac Calcium Score (ICS) in predicting major adverse limb events (MALE), cardiovascular events (MACE), and all-cause mortality in patients with severe aortoiliac disease.
Methods:
In this prospective cohort (2013–2024), 109 patients with TASC II D aortoiliac occlusive disease underwent elective revascularization and preoperative CT angiography. Iliac artery calcification was scored semiquantitatively by morphology, circumference, and lesion length. Patients were stratified into low (≤ 36) and high (≥ 37) ICS groups. Outcomes included MALE, MACE, and mortality, analyzed using Kaplan-Meier and Cox regression.
Results:
The study included 109 patients (95.4% male) with a median follow-up of 67 months. Baseline characteristics were similar across ICS groups, though ICS ≥ 37 was associated with more advanced Rutherford stages (p = 0.035). At 30 days, both groups improved clinically, but Rutherford class improvement was greater in the ICS ≤ 36 group (p = 0.013), with no other significant differences. At 1 year, MALE was more frequent in patients with ICS ≥ 37 (48.1% vs. 27.3%; p = 0.024). At 60 months, this group showed significantly lower amputation-free (74.5% vs. 97.8%; p = 0.002), MACE-free (47.3% vs. 73.4%; p = 0.005), and overall survival (54.6% vs. 77.0%; p = 0.013). Acute heart failure occurred only in the high ICS group (p = 0.015), while patency rates were similar. ICS ≥ 37 remained an independent predictor of MACE (aHR 2.30; p = 0.008) and major amputation (aHR 7.52; p = 0.008) in multivariable analysis.
Conclusion:
In patients with extensive TASC II D aortoiliac occlusive disease, an ICS ≥ 37 was independently associated with increased long-term risk of MACE, MALE, and reduced overall survival, despite similar short-term outcomes. These findings support the integration of preoperative calcium scoring as a simple, lesion-specific tool for risk stratification, procedural planning, and personalized postoperative surveillance in complex peripheral arterial disease.
Highlights
-
Calcium score ≥ 37 independently predicted limb loss and cardiovascular mortality, with a 7.5-fold higher amputation risk and 2.3-fold higher major adverse cardiovascular event risk (p < 0.01).
-
Late divergence in survival curves (e.g., acute heart failure and mortality after 24 months) suggests calcification may contribute to progressive systemic vascular dysfunction.
-
Primary/secondary patency pattern similarities suggest that calcification impacts long-term clinical outcomes more than procedural success, highlighting the need for post-intervention monitoring.
Introduction
Coronary artery calcium (CAC) scoring has become a cornerstone in coronary heart disease risk stratification, with robust evidence supporting its incremental prognostic value beyond traditional risk factors and also for cardiovascular risk stratification (1, 2). Moreover, a systematic review and meta-analysis demonstrated that CAC scoring improves discrimination for cardiovascular events [pooled C-statistic gain: 0.036 (95% CI 0.020–0.0520)] while highlighting limitations in cost-benefit ratios for routine use (3). Coronary artery calcium (CAC) scores were significantly associated with biological aging, with scores > 400 adding up to 30 years to predicted age in younger individuals, while scores < 10 reduced biological age by up to 10 years in older patients; calcium-adjusted age reclassified 55% of low-risk and 45% of intermediate-risk individuals (by Framingham score) into higher-risk categories, improving mortality prediction beyond observed age (p < 0.0001) (4).
In peripheral artery disease (PAD), extensive aortoiliac occlusive disease, classically classified as TransAtlantic Inter-Society Consensus (TASC) II type D aortoiliac lesions, presents unique challenges. While open surgery remains the gold standard, endovascular and hybrid approaches show comparable mid-term patency rates (85% at 5 years) with lower perioperative risks in high-comorbidity patients (5–7). In this complex aortoiliac disease, characterized by extensive occlusions or calcifications involving the distal aorta and bilateral iliac arteries, vascular calcification exacerbates technical challenges during revascularization, especially with endovascular techniques.
The pathophysiology of lower limb arterial calcification (LLAC) in aortoiliac disease involves both intima and media layers, with distinct clinical implications. While intimal calcification is classically linked to atherosclerosis and inflammatory processes, predominating in patients with traditional risk factors (e.g., smoking, hyperlipidemia), medial calcification is systematically regulated and linked to diabetes, chronic kidney disease (CKD), and aging-related reduction in arterial compliance, exacerbating hemodynamic impairment and limb ischemia (8–11).
In the iliac arteries, calcification predominantly localizes to the intima in atherosclerotic plaques, but medial calcification coexists in patients with metabolic comorbidities, amplifying cardiovascular risk (9, 10). This dual calcification pattern mirrors findings in femoral and tibial arteries, where medial calcification correlates strongly with advanced ischemia severity and limb-related morbidity (11–13).
Emerging evidence suggests that scoring LLAC could refine PAD management. Studies in PAD populations associate elevated calcium scores with limb-related events, including major amputations (12, 14), but also with cardiovascular events, including all-cause mortality (15).
Despite its clinical relevance to revascularization outcomes and amputation risk, LLAC lacks standardized quantification. Current methods range from qualitative visual scores like Peripheral Arterial Calcium Scoring System (PACSS) to adapted Agatston protocols, creating heterogeneity in research and clinical applications (9, 14, 16, 17). Furthermore, despite their complexity and clinical relevance, no validated calcium scoring system currently exists for TASC II type D aortoiliac lesions.
This study aims to evaluate the prognostic value of a standardized lower limb calcium scoring system in predicting outcomes such as major adverse limb events (MALE), MACE, and all-cause mortality, helping to optimize risk stratification and guide therapeutic decisions in this high-risk population.
Materials and methods
Patient selection
A total of 180 consecutive patients who underwent elective aortoiliac revascularization between January 2013 and January 2024 were included in a prospective cohort. All patients were selected from a tertiary and a community hospital and had aortoiliac TASC II type D lesions, excluding those with aortoiliac aneurysmatic disease (18). The choice between open surgery and an endovascular procedure was made by a shared decision between the patient and the surgeon, considering the surgeon and the institution’s experience and preferences.
A post hoc analysis was performed, including only patients who had undergone preoperative computed tomography (CT). Of the initial 180 patients, only 109 had preoperative CT and were included in the final analysis (Figure 1). Among baseline comorbidities, the only statistically significant difference between included and excluded patients was a higher prevalence of smoking in the included cohort (95.4% vs. 85.9%, p = 0.024) (Supplementary Table 1).
FIGURE 1
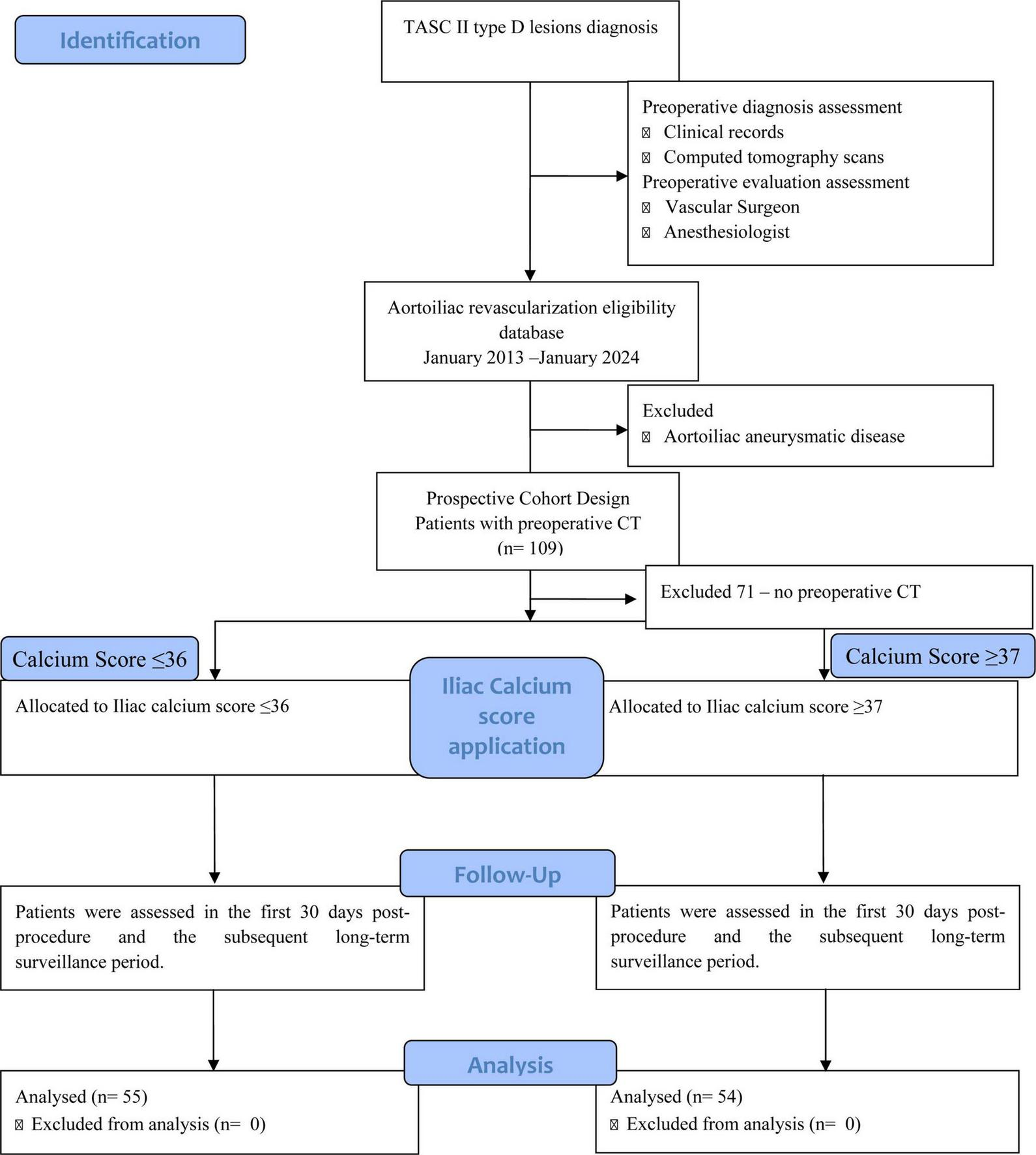
Flowchart of patient selection and cohort allocation.
The patients’ demographic and clinical profiles — encompassing cardiovascular risk factors as well as procedural and lesion-specific information — were obtained through a review of their medical records, including data on whether a CT scan had been performed (7). Further information regarding the type of lesion is described elsewhere (18). Patients underwent evaluation during the initial 30 days following the procedure and throughout the subsequent long-term follow-up period, which included regular clinical and hemodynamic assessments in an outpatient setting. This study is under the framework of the Strengthening the Reporting of Cohort Studies in Surgery (STROCSS) 2019 Guideline (19).
The local Ethics Committee approved the study protocol and respects the Declaration of Helsinki. Patient informed consent was handled accordingly, and all data processing was anonymous (Protocol Number 246-18). It respects the European Union General Data Protection Regulation (GDPR).
Definitions and outcomes
Retrieved data was registered in agreement with the Reporting Standards of the Society for Vascular Surgery for lower extremity ischemia (20). The Rutherford chronic ischemia classification was used to classify the symptoms and severity of chronic lower extremity ischemia (21).
The primary outcome was long-term MACE, defined as a composite outcome, including myocardial infarction (MI), acute heart failure (AHF), and all-cause mortality (22). MALE was described as a composite of reintervention, including reintervention due to primary assisted patency, secondary patency, major amputation of the revascularized artery segment, and occlusion without intervention (23).
Imagiological parameters
Iliac artery calcification was quantified by two independent operators using a validated CT-based semiquantitative scoring system, which evaluates three distinct features: morphology, circumferential involvement, and length of calcification. Axial CT images with 5 mm slice thickness and bone window settings (width 3077 HU; level 570 HU) were analyzed by experienced vascular surgeons and non-contrast CT slices acquired before contrast injection were used for quantification. Each iliac arterial segment (common and external, bilaterally) was scored separately. Morphology was graded from 0 to 3, with 0 indicating no calcification, 1 representing thin linear calcifications ≤ 1 mm (“eggshell” type), two corresponding to thick linear calcifications > 1 mm with convex margins, and 3 indicating bulky calcifications > 2 mm with convex luminal margins. Circumferential and length involvement were each scored from 0 to 4, based on percentage involvement (0 = none; 1 = 1%–25%; 2 = 26%–50%; 3 = 51%–75%; 4 = 76%–100%). The highest value in each segment was recorded (24). The final score by patient was calculated by adding the scores from each of the four iliac segments.
Statistical analysis
The sample size for a survival test was calculated using online Sample Size Calculators for Designing Clinical Research,1 with a statistical power (β) of 80% and a significance level of 0.05. The sample size (95 patients) was estimated based on an expected hazard ratio of 2, for cardiovascular events, between groups and a projected 80% event-free survival rate at the end of follow-up (7, 25, 26).
Statistical analyses were performed using SPSS (IBM Corp., released 2023. IBM SPSS Statistics for Windows, version 29.0.2.0, Armonk, NY, United States). Continuous variables were assessed for normality using histograms and skewness. Normally distributed variables were reported as mean ± standard deviation (SD), and comparisons between groups were performed using the independent samples t-test. Non-normally distributed continuous variables were reported as median and interquartile range (IQR), and compared using the Mann-Whitney U test. When appropriate, categorical variables were presented as frequencies and percentages and compared using the chi-square or Fisher’s exact test.
To facilitate the interpretation and clinical applicability of the Iliac Calcium Score (ICS), dichotomization was performed using visual binning rather than Receiver Operating Characteristic (ROC) analysis. A threshold of 36.5 was selected based on observed data distribution and clinical reasoning, corresponding to the point of greatest contrast in event rates (MACE) between groups. This cut-off was then used to stratify patients into low (≤ 36) and high (≥ 37) ICS groups for further analysis.
Kaplan-Meier survival analyses were conducted to evaluate time-to-event outcomes, including MACE, MALE, and all-cause mortality. Log-rank tests were used to compare survival curves between groups.
The Log Rank estimator was utilized to test the effect of the ICS on time-dependent variables. Multivariate Cox regression analysis was performed for independent predictors of long-term MACE and all-cause mortality, using the backward stepwise regression method. The backward stepwise regression method was applied, and variables with p < 0.20 were included.
All statistical tests were two-sided; a p-value < 0.05 was considered statistically significant.
Results
Population data
The cohort included 109 patients with a median follow-up of 67 (IQR 56.2–77.8) months. Table 1 presents the baseline characteristics of the patients included, stratified by ICS (≤ 36 vs. ≥ 37), with 55 patients and 54 patients, respectively. Most patients were male (95.4%), with similar distribution between groups (92.7% vs. 98.1%; p = 0.363). Although the two groups did not differ significantly in most comorbidities, patients with higher calcium scores (≥ 37) presented a trend for hypertension (77.8% vs. 61.8%; p = 0.070) and had a higher prevalence of chronic kidney disease (13.0% vs. 5.5%; p = 0.175) and chronic heart failure (CHF) (13.0% vs. 5.5%; p = 0.175), albeit without reaching statistical significance.
TABLE 1
| Characteristics | Total n = 109 (%) |
Iliac Calcium Score ≤ 36 n = 55 |
Iliac Calcium Score ≥ 37 n = 54 |
P-value |
| Age, years (mean ± SD) | 62.0 ± 8.70 | 59.8 ± 8.55 | 64.2 ± 8.33 | 0.989 |
| Gender, male | 104 (95.4) | 51 (92.7) | 53 (98.1) | 0.363 |
| Smoking history | 104 (95.4) | 54 (98.2) | 50 (92.6) | 0.206 |
| Hypertension | 76 (69.7) | 34 (61.8) | 42 (77.8) | 0.070 |
| Dyslipidemia | 79 (72.5) | 39 (70.9) | 40 (74.1) | 0.711 |
| Diabetes | 29 (26.6) | 12 (21.8) | 17 (31.5) | 0.254 |
| CKD | 10 (9.2) | 3 (5.5) | 7 (13.0) | 0.175 |
| CAD | 29 (26.6) | 12 (21.8) | 17 (31.5) | 0.254 |
| CVD | 16 (14.7) | 8 (14.5) | 8 (14.8) | 0.968 |
| COPD | 12 (11.0) | 7 (12.5) | 5 (9.3) | 0.563 |
| CHF | 10 (9.2) | 3 (5.5) | 7 (13.0) | 0.175 |
| ASA | ||||
| III | 46 (42.2) | 23 (41.8) | 23 (42.6) | 0.356 |
| IV | 58 (53.2) | 31 (56.4) | 27 (50.0) | |
| V | 5 (4.6) | 1 (1.8) | 4 (7.4) | |
| Rutherford classification | ||||
| 3 | 28 (25.7) | 18 (32.7) | 10 (18.5) | 0.035 |
| 4 | 45 (41.3) | 24 (43.6) | 21 (38.9) | |
| 5 | 29 (26.6) | 13 (23.6) | 16 (29.6) | |
| 6 | 7 (6.4) | 0 (0.0) | 7 (13.0) | |
| Open surgery | 70 (64.2) | 39 (70.9) | 31 (57.4) | 0.103 |
| Endovascular technique | 29 (26.6) | 14 (25.5) | 15 (27.8) | |
| Hybrid surgery | 10 (9.2) | 2 (3.6) | 8 (14.8) | |
| ABI, (mean ± SD) | 0.30 ± 0.120 | 0.31 ± 0.133 | 0.29 ± 0.107 | 0.065 |
Patient’s demographics and comorbidities.
Variables are presented as N (%) unless otherwise specified. ABI, Ankle-brachial index (preoperative); ASA, American Society of Anesthesiologists Physical Status Classification System; CAD, coronary artery disease; CHF, chronic heart failure; CKD, chronic kidney disease (creatinine = 1.5 mg/dl); COPD, chronic obstructive pulmonary disease; CVD, cerebrovascular disease; SD, standard deviation. Bold value represents statistically significant results (p < 0.05).
Notably, a significant difference was observed in the distribution of Rutherford classification (p = 0.035), with advanced stages (V and VI) being more frequent in the higher calcium score group. Overall, approximately 75% of the cohort presented with chronic limb-threatening ischemia.
The majority of procedures were open surgery (64.2%), followed by endovascular (26.6%) and hybrid (9.2%), with no significant differences between ICS groups (p = 0.103). The mean preoperative ABI was 0.30 ± 0.120, slightly higher in the ICS ≤ 36 group (0.31 ± 0.133) than in the ICS ≥ 37 group (0.29 ± 0.107; p = 0.065).
Thirty-day outcomes according to ICS
Table 2 summarizes the 30 days outcomes. The mean change in ABI was 0.43 ± 0.235 (0.48 ± 0.241 in ICS ≤ 36 vs. 0.37 ± 0.218 in ICS ≥ 37; p = 0.167). Although both groups showed clinical improvement in the Rutherford chronic ischemia classification (Δ = −2.6 ± 1.41), patients with lower calcium scores exhibited a significantly greater mean improvement (Δ = −2.9 ± 1.10 vs. −2.4 ± 1.61, p = 0.013). Median intensive care unit (ICU) stay was 2 days (IQR 0–3), with no significant difference between groups (p = 0.710). Median ward stay was seven days (IQR 4–17.75), also similar between groups (p = 0.131).
TABLE 2
| Total n = 109 (%) |
Iliac Calcium Score ≤ 36 n = 55 |
Iliac Calcium Score ≥ 37 n = 54 |
P-value | |
| AKI | 13 (11.9) | 6 (10.9) | 7 (13.0) | 0.765 |
| ABI Δ, (mean ± SD) | 0.43 ± 0.235 | 0.48 ± 0.241 | 0.37 ± 0.218 | 0.167 |
| Rutherford Δ, (mean ± SD) | 2.6 ± 1.41 | −2.9 ± 1.10 | −2.4 ± 1.61 | 0.013 |
| ICU (days), (median–IQR) | 2 (0–3) | 2 (0.5–3) | 1 (0–3) | 0.710* |
| Ward (days), (median–IQR) | 7 (4–17.75) | 6 (3–11.5) | 10 (4–21) | 0.131* |
| MINS | 19 (17.4) | 9 (16.4) | 10 (18.5) | 0.645 |
| MALE | 24 (22.0) | 9 (16.4) | 15 (27.8) | 0.150 |
| MACE | 11 (10.1) | 7 (12.7) | 4 (7.4) | 0.357 |
| All cause-mortality | 6 (5.5) | 4 (7.3) | 2 (3.7) | 0.679 |
Patient’s 30 days outcomes according to iliac calcium score.
*Non-parametric test. Variables are presented as N (%) unless otherwise specified. ABI, Ankle-brachial index Δ - postoperative minus preoperative; AKI, Acute kidney injury; Rutherford chronic ischemia Δ, preoperative minus postoperative; MACE, major adverse cardiovascular event; MALE, major adverse limb event; MINS, myocardial injury after non-cardiac surgery;ICU, in stay on intensive care unit; IQR, interquartile range. Bold value represents statistically significant results (p < 0.05).
The incidence of acute kidney injury (AKI) was 11.9% overall (10.9% vs. 13.0%; p = 0.765). The incidence of myocardial injury after non-cardiac surgery (MINS) was 17.4% (16.4% vs. 18.5%; p = 0.645), MALE 22.0% (16.4% vs. 27.8%; p = 0.150), MACE 10.1% (12.7% vs. 7.4%; p = 0.357), and all cause-mortality 5.5% (7.3% vs. 3.7%; p = 0.679).
One-year outcomes according to ICS
Table 3 shows the 1 year outcomes. Prosthetic infection occurred in 5.5% of patients, equally distributed between groups (5.5% vs. 5.5%; p = 0.981). Patients with higher ICS (≥ 37) experienced a significantly greater incidence of MALE compared to those with lower scores (48.1% vs. 27.3%, p = 0.024). Although higher rates of MACE and all-cause mortality were observed in the high ICS group (24.1% vs. 16.4% and 20.4% vs. 12.7%, respectively), these differences were not statistically significant (p = 0.316 and p = 0.283, respectively).
TABLE 3
| Total n = 109 (%) |
Iliac Calcium Score ≤ 36 n = 55 |
Iliac Calcium Score ≥ 37 n = 54 |
P-value | |
| Prosthetic infection | 6 (5.5) | 3 (5.5) | 3 (5.5) | 0.981 |
| MALE | 41 (37.6) | 15 (27.3) | 26 (48.1) | 0.024 |
| MACE | 22 (20.2) | 9 (16.4) | 13 (24.1) | 0.316 |
| All cause-mortality | 18 (16.5) | 7 (12.7) | 11 (20.4) | 0.283 |
Patient’s 1 year outcomes according to iliac calcium score.
Variables are presented as N (%) unless otherwise specified. MACE, major adverse cardiovascular event; MALE, major adverse limb event. Bold value represents statistically significant results (p < 0.05).
Multivariable analysis of predictors for MACE and major lower limb amputation
Table 4 presents the Cox proportional hazards regression analysis results for MACE and major lower limb amputation (MLLA).
TABLE 4
| Non-adjusted hazard ratios | 95% confidence interval | P-value | Adjusted hazard ratios | 95% confidence interval | P-value | |
| MACE | ||||||
| Hypertension | 1.325 | 0.768–2.286 | 0.312 | NC | – | – |
| CKD | 2.708 | 1.478–4.962 | < 0.001 | NC | – | – |
| CHF | 3.485 | 1.954–6.215 | < 0.001 | 2.604 | 1.147–5.908 | 0.022 |
| ICS ≥ 37 | 2.310 | 1.256–4.250 | 0.007 | 2.296 | 1.246–4.229 | 0.008 |
| ICS (cont) | 1.035 | 1.005–1.066 | 0.021 | 1.036 | 1.006–1.068 | 0.020 |
| MLLA | ||||||
| Hypertension | 2.424 | 0.714–8.242 | 0.156 | NC | – | – |
| CKD | 2.521 | 0.841–7.560 | 0.099 | NC | – | – |
| CHF | 1.693 | 0.494–5.803 | 0.402 | NC | – | – |
| ICS ≥ 37 | 7.521 | 1.693–33.409 | 0.008 | 7.521 | 1.693–33.409 | 0.008 |
| ICS (cont) | 1.142 | 1.030–1.26 | 0.011 | 1.142 | 1.030–1.26 | 0.011 |
Cox multivariable regression proportional hazard ratio for major adverse cardiovascular events and major lower limb amputation.
CHF chronic heart failure; CKD, chronic kidney disease (creatinine = 1.5 mg/dl); ILC, Iliac Calcium Score; MACE, major adverse cardiovascular event; MLLA, major lower limb amputation; NC, not confirmed; cont, continuous variable.
For MACE, unadjusted analysis revealed that CKD (HR 2.708, 95% CI 1.478–4.962, p < 0.001), CHF (HR 3.485, 95% CI 1.954–6.215, p < 0.001), and an ICS ≥ 37 (HR 2.310, 95% CI 1.256–4.250, p = 0.007) were significant predictors. After adjustment, CHF (adjusted HR 2.604, 95% CI 1.147–5.908, p = 0.022) and calcium score ≥ 37 (adjusted HR 2.296, 95% CI 1.246–4.229, p = 0.008) remained independently associated with increased MACE risk.
For MALE, CHF was a significant predictor (adjusted HR 2.116, 95% CI 1.105–4.052, p = 0.024), while calcium score did not reach statistical significance in the adjusted model.
For MLLA, ICS ≥ 37 was strongly associated with increased risk both in unadjusted (HR 7.521, 95% CI 1.693–33.409; p = 0.008) and adjusted models (aHR 7.521, 95% CI 1.693–33.409; p = 0.008).
The continuous calcium score variable was also significant for MACE (aHR 1.035, 95% CI 1.005–1.066; p = 0.021) and MLLA (aHR 1.142, 95% CI 1.030–1.260; p = 0.011).
Survival analysis
Limb-related outcomes
Kaplan-Meier analysis revealed significantly lower amputation-free survival in patients with calcium scores ≥ 37 vs. ≤ 36 (log-rank p = 0.002), with 60 months rates of 74.5% vs. 97.8% (Figure 2A). MALE trended higher in the ≥ 37 group (50.8% vs. 62.5% survival; p = 0.139) (Figure 2D), while primary/secondary patency rates showed no significant differences (Figures 2B, C).
FIGURE 2
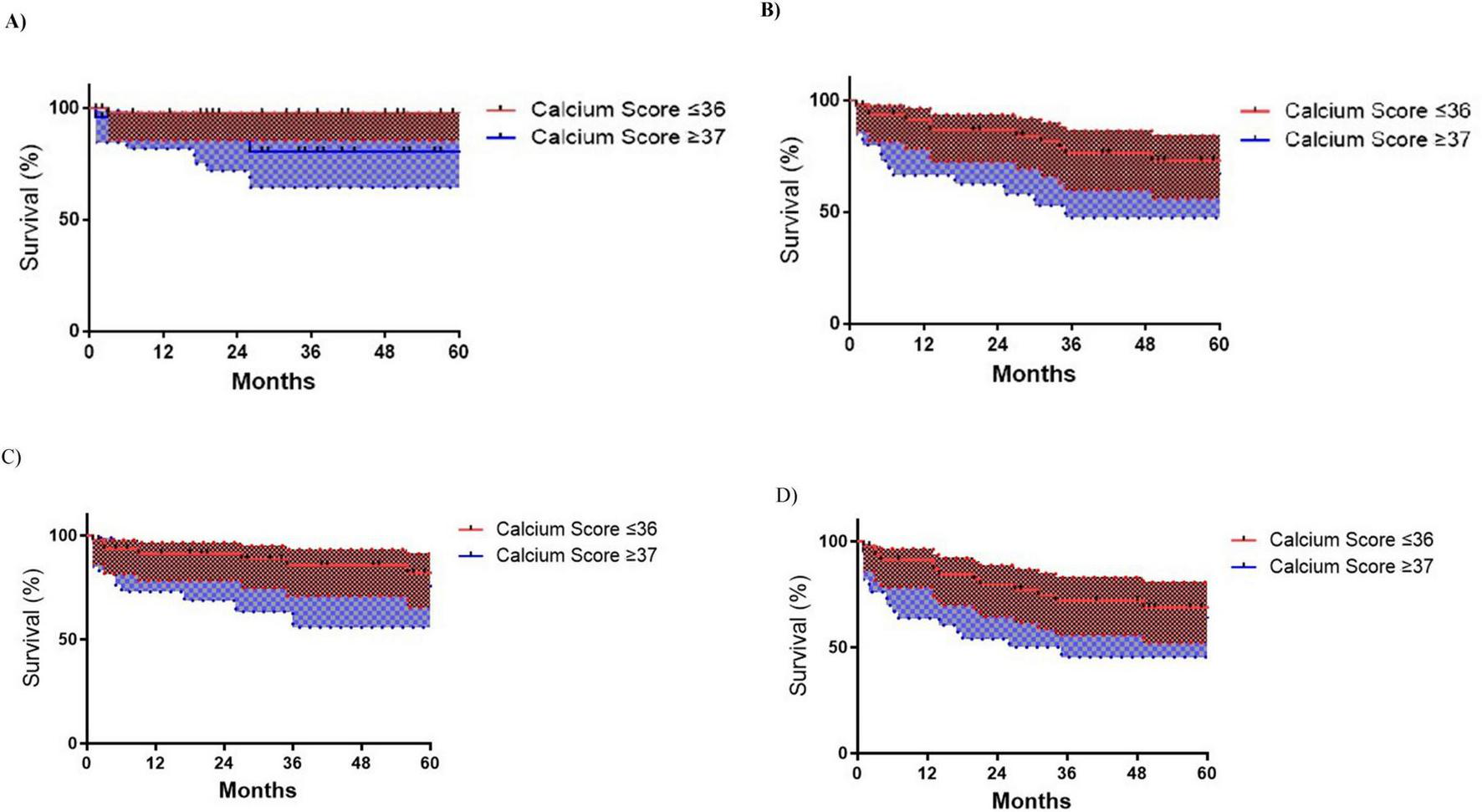
Kaplan-Meyer limb outcomes survival curves according to Iliac Calcium Score cut-off ≥ 37 status. (A) Major Lower limb amputation (MLLA) (p = 0.002); (B) Primary patency (p = 0.193); (C) Secondary patency (p = 0.233); (D) Major adverse limb events (MALE) (p = 0.139).
Cardiovascular outcomes
Patients with ICS ≥ 37 exhibited significantly worse cardiovascular outcomes, including a higher risk of MACE (log-rank p = 0.005), with 60-month MACE-free survival rates of 47.3% compared to 73.4% in the ≤ 36 group (Figure 3D) and higher risk of all-cause mortality (log-rank, p = 0.013), with 60-month survival rates of 54.6% versus 77.0% (Figure 3C). Notably, AHF occurred exclusively in the ≥ 37 group, where AHF-free survival at 60 months was 87.7% compared to 100% in the ≤ 36 group (p = 0.015) (Figure 3B). Acute myocardial injury (AMI) rates showed no significant differences (Figure 3A).
FIGURE 3
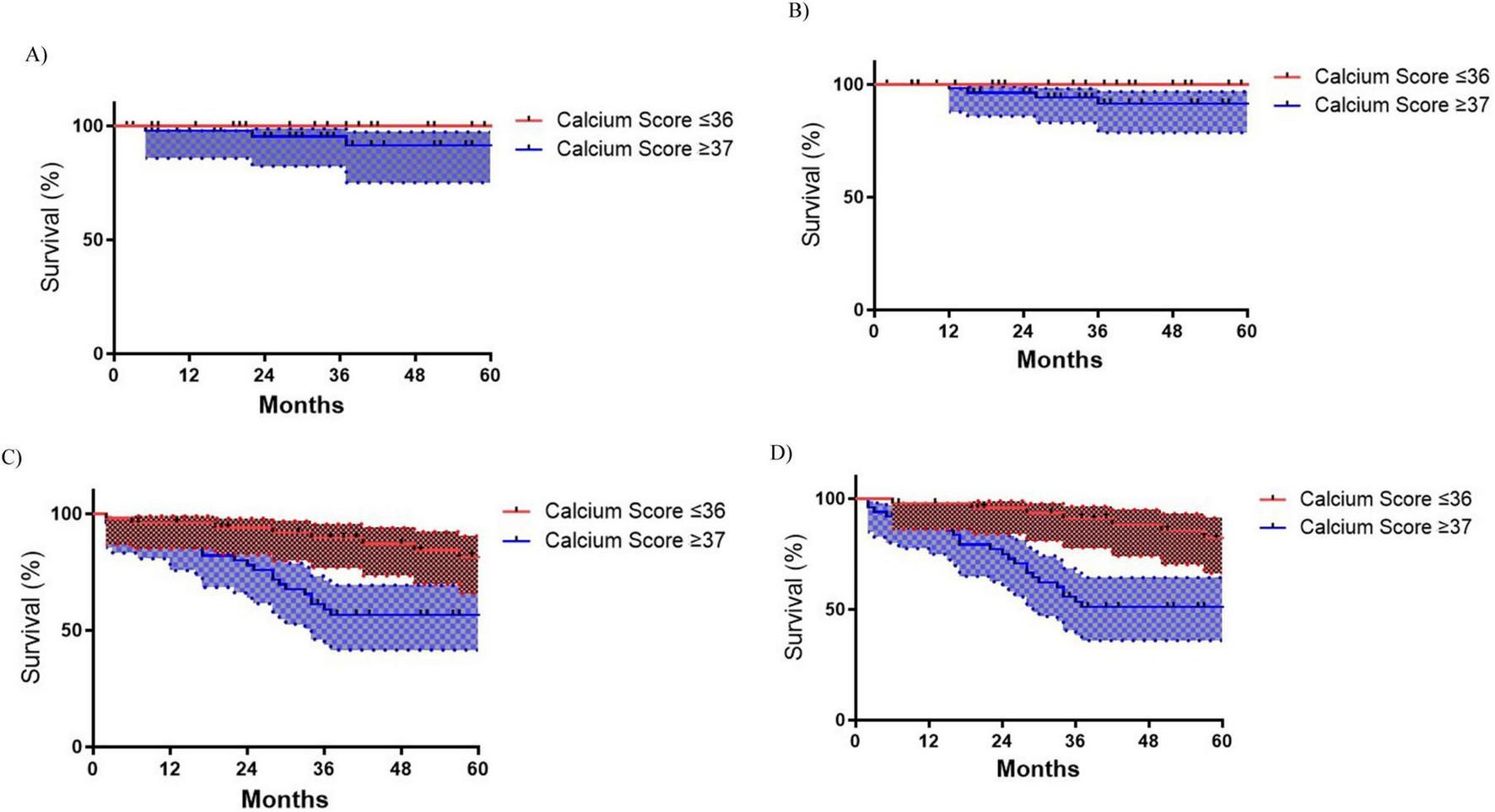
Kaplan-Meyer cardiovascular outcomes survival curves according to Iliac Calcium Score cut-off ≥ 37 status. (A) Acute myocardial infarction (AMI) (p = 0.407); (B) Acute heart failure (AHF) (p = 0.015); (C) All-cause mortality (p = 0.013); (D) Major adverse cardiac events (MACE) (p = 0.005).
Discussion
This study demonstrates that a standardized ICS ≥ 37 could be considered a prognostic marker for 1 year and midterm adverse limb and cardiovascular outcomes in patients with TASC D aortoiliac disease undergoing revascularization. However, calcium burden does not seem to significantly impact short-term survival and perioperative complications. Elevated calcium burden was independently associated with a 2.3-fold increased risk of MACE and a striking 7.5-fold higher risk of major lower limb amputation over time. The threshold effect was particularly pronounced in longitudinal outcomes, with patients scoring ≥ 37 in the ICS exhibiting significantly reduced amputation-free survival (74.5% vs. 97.8% at 60 months) and cardiovascular event-free survival (47.3% vs. 73.4%). Notably, calcium burden correlated with advanced baseline ischemia severity and attenuated clinical improvement in Rutherford classification, underscoring its dual role as both a marker of anatomical complexity and a predictor of functional recovery.
The findings that higher calcium scores are associated with more advanced Rutherford stages (5 and 6) are consistent with previous studies that have identified a link between arterial calcification and the severity of limb ischemia. In a study of 116 patients, increasing Rutherford categories were independently associated with higher calcification scores, even after adjusting for cardiovascular risk factors and the extent of occlusive disease (11). Similarly, another study demonstrated that patients with Rutherford stages 5–6 had significantly higher iliac calcification scores compared to those with milder ischemia (stages 1–2) (13). Huang et al. (8) also reported that patients with more advanced Fontaine stages tended to have significantly higher calcification scores, further supporting the association between calcification burden and clinical severity. These findings reinforce the prognostic significance of calcium scoring in assessing lower limb ischemia.
At 30 days follow-up, the cohort with higher calcification score demonstrated attenuated improvement in Rutherford classification post-revascularization, with no significant differences in other limb-related outcomes or adverse cardiovascular events. These results are partially consistent with those of Megale et al. (12), who also reported no significant association between aortic calcium scores and 30 days mortality (p = 0.679), patency (p = 0.167), or technical success (p = 0.103) across 14 interventions in 11 patients. However, in contrast to our findings, Megale et al. observed a significantly higher calcium score in patients who required amputation (5,767.6 vs. 805.3; p = 0.02) or underwent subsequent revascularization (3,686.8 vs. 645.2; p = 0.008) within 30 days. These comparisons suggest that while calcification burden may influence early clinical recovery, its association with short-term major events remains inconsistent.
While no procedural preference differences were found (p = 0.103), this may reflect center-specific strategies rather than calcium’s technical irrelevance. Our cohort’s high open surgery rate (64.2%) might mask this relationship, as bypass grafting may circumvent calcification-related challenges. While Megale et al. (15) also did not find any statistical difference, Kang et al. on the other hand, in a cohort with 124 patients, reported that technical success of infra-popliteal angioplasty was significantly lower in patients with extensive tibial artery calcification (71.1%) compared to those with minimal (95.3%) or intermediate calcification (91.7%) (p = 0.001), with extensive calcification emerging as an independent predictor of technical failure (p = 0.014) (10).
In this study, no significant differences were observed in primary or secondary patency rates between patients with high versus low calcium scores, both in short-term (30 days) and long-term follow-up (up to 60 months), which may reflect our high open surgery rate (64.2%) but aligning with the findings of Megale et al., which included patients submitted to both open and endovascular techniques, who also reported no significant association between calcium score and patency at any time point (12, 15). However, a more anatomically focused study which included 733 limbs submitted to drug-coated balloon (DCB) angioplasty for de novo femoropopliteal lesions, demonstrated that femoropopliteal lesions with bilateral calcification ≥ 5 cm [peripheral artery calcification scoring system (PACSS) 4] had significantly lower 1 year primary patency (82.6%; p < 0.001), with PACSS 4 emerging as an independent predictor of restenosis (HR 1.82; 95% CI 1.15–2.87; p = 0.010), suggesting that the pattern and extent of calcification may play a relevant role in long-term durability of revascularization with DCB (27).
Regarding amputation, this study data showed robust association with a significantly lower major amputation-free survival in patients with calcium scores ≥ 37 (74.5% vs. 97.8% at 60 months; log-rank p = 0.002), in agreement with previous studies linking extensive calcification to worse limb outcomes, albeit some exceeding rates likely reflecting our cohort’s critical ischemia severity. For instance, Huang et al. (8) reported a 2.88-fold increased risk of amputation in patients in the highest calcium score quartile (95% CI 1.18–12.72; p = 0.03), and Kang et al. (10) found that patients with extensive tibial calcification had significantly lower 2 years amputation-free survival (Extensive Calcification: 58.9% vs. Minimal Calcification: 79.0% vs. Intermediate Calcification: 95.3%; p < 0.001), with extensive calcification being an independent predictor (HR 9.90; 95% CI 2.05–47.75; p = 0.004). Similarly, Dong et al. (28) identified tibial artery annular calcification as an independent predictor of unplanned amputation (HR 3.74; 95% CI 1.71–8.19; p = 0.001), reinforcing the importance of the calcification pattern.
In terms of MALE, a significantly higher incidence was observed at 1 year in patients with higher calcium scores (48.1% vs. 27.3%; p = 0.024), although this association did not remain significant in adjusted analysis. These results likely reflect the persistent significance of major amputations over time, while the lack of sustained differences in primary/secondary patency rates may have diluted the composite MALE outcome’s statistical significance in adjusted models. This suggests that calcification’s strongest prognostic impact, measured through ICS, lies in irreversible limb loss rather than revascularization-dependent endpoints in the aortoiliac population, and since distal vessel disease were not systematically included in the analysis, ICS may serve as a reflection of the overall systemic arterial calcification burden, including in the lower limbs. This trend is supported by findings from Megale et al., where higher below-the-knee calcium scores were associated with MALE both at 30 days (679.4 vs. 158.9; p = 0.019) and 1 year (910.1 vs. 12.3; p = 0.002) (12).
Patients with higher ICS (≥ 37) experienced significantly worse cardiovascular outcomes over time in this cohort. At 5 years, MACE-free survival was markedly lower in the high calcium group compared to those with lower scores (47.3% vs. 73.4%; log-rank p = 0.005), and all-cause mortality was also higher (54.6% vs. 77.0%; log-rank p = 0.013). Notably, acute heart failure (AHF) occurred exclusively in the high calcium group, with 60 months heart failure-free survival rates of 87.7% versus 100% in the lower calcium group (p = 0.015). Although rates of acute myocardial injury did not differ significantly between groups, as opposite to AHF and all-cause mortality, multivariable analysis confirmed that a calcium score ≥ 37 remained an independent predictor of MACE (adjusted HR 2.30, 95% CI 1.25–4.23; p = 0.008), even after adjusting for comorbidities. These findings underscore the prognostic value of arterial calcification for long-term cardiovascular morbidity and mortality in this population. In the study published by Huang et al. (8), patients in the highest calcium score quartile had a 5.16-fold (95% CI 1.13–21.61, p = 0.04) increased risk of death compared to those in the lowest quartile. Similarly, annular tibial artery calcification has been identified as a robust predictor of all-cause mortality (HR = 3.19) (28), and complete annular calcification in crural or femoropopliteal arteries independently predicted 10 years mortality (29). Kang et al. (10) also reported that survival free of MACE was significantly higher in the lowest calcification score group (p = 0.028). More recently, Megale et al. (15) reported among 72 patients and 88 lower limb revascularizations that the calcium score of the operated limb was higher in patients who died within 30 days and 6 months (6571 vs. 2590.6; p = 0.026) and (5227.8 vs. 2335.3; p = 0.036). Additionally, in a cohort of 84 patients, higher ICS had significantly lower left ventricular ejection fraction, with a moderate inverse correlation between the two (r = −0.54; p < 0.0001) (13). Collectively, these data reinforce the prognostic value of arterial calcification as a marker of systemic cardiovascular risk in this patient population.
As above described, several studies have demonstrated that peripheral arterial calcium scores, including those of the iliac and femoral arteries, are associated with increased severity of PAD and worse cardiovascular outcomes in symptomatic patients (30) and additionally in this study ICS demonstrated to be an independent prognostic value by stratifying long-term cardiovascular and limb-related risk in patients undergoing revascularization for complex aortoiliac disease, suggesting it may serve as a global marker of systemic atherosclerosis. may serve as a reflection of the overall systemic arterial calcification burden. However, the use of these calcium scores as a stratification and prevention tool in asymptomatic and claudicant populations remains underexplored. Future prospective research could focus on the potential value of iliac calcium scoring for early risk stratification and cardiovascular prevention in these patient groups, similar to the role already established for coronary artery calcium scoring in primary prevention settings (31, 32).
This study has several important limitations. Although it was conducted across two centers — a large academic institution and a community referral hospital, which enhances external validity — the sample size remains relatively small. Additionally, the marked predominance of male patients (95.4%) significantly limits the generalizability of findings to the broader population, particularly to women. To mitigate potential selection bias arising from the inclusion of only patients with available CT imaging, a sensitivity analysis was performed (Supplementary Table 1). Second, the post hoc design introduces inherent limitations, including the possibility of selection bias: patients with significant comorbidities or unfavorable clinical status may have been more likely to undergo less invasive procedures or to be excluded from revascularization altogether. This is particularly relevant given that current guidelines recommend open surgery for healthier patients with extensive aortoiliac disease. At the same time, an endovascular-first approach is often favored in those with higher operative risk (33). Third, although significant associations were found between calcium scores and clinical outcomes, causality cannot be established due to the study’s observational nature. In addition, variability in the measurement of calcium scores may have influenced patient classification into high-score vs. low-score groups, and absolute score values are known to be affected by age, sex, and ethnicity—factors not fully controlled for in this cohort, despite the limited ethnic diversity in the region. Finally, the pattern of arterial calcification was not evaluated (intima layer vs. media layer) or specific morphological features such as circumferential characteristics or thickness of the artery, which recent studies have identified as potentially relevant predictors of outcomes (28, 29).
Despite these limitations, the study’s strengths include its prospective data collection, extended follow-up period, and the relative homogeneity of aortoiliac lesions, all of which support the robustness of the observed associations. Further prospective cohort studies with larger and more diverse populations are needed to validate these findings.
Conclusion
This study introduces the ICS as a lesion-specific tool capable of stratifying long-term cardiovascular and limb-related risk in patients undergoing revascularization for complex aortoiliac disease. The ICS demonstrated independent prognostic value beyond perioperative outcomes, highlighting its relevance for long-term clinical decision-making. By quantifying arterial calcification in a standardized manner, the score enhances preoperative risk assessment, informs procedural planning, and supports individualized surveillance strategies. Further prospective cohort studies are needed to confirm the reproducibility and generalizability of these findings across broader populations and to explore the impact of ICS-guided interventions on clinical outcomes.
Statements
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by Unidade Local de Saúde de São João. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants’ legal guardians/next of kin because observational study without change in therapeutic options.
Author contributions
AP-N: Supervision, Writing – original draft, Formal analysis, Writing – review & editing, Investigation, Methodology, Conceptualization. JR-N: Methodology, Writing – review & editing, Funding acquisition, Supervision, Investigation, Writing – original draft, Conceptualization, Formal analysis. TC-P: Writing – review & editing. HR: Writing – review & editing. LD: Writing – review & editing. LD-G: Writing – review & editing. JA: Writing – review & editing, Funding acquisition, Supervision.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This article was supported by National Funds through FCT - Fundação para a Ciência e a Tecnologia, I.P., within CINTESIS, R&D Unit (reference UIDB/4255/2020).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) verify and take full responsibility for the use of generative AI in the preparation of this manuscript. Generative AI was used Generative AI was applied to improve the English of the manuscript but not for writing original data or research purposes.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2025.1655229/full#supplementary-material
References
1.
Khan S Post W Guo X Tan J Zhu F Bos D et al Coronary artery calcium score and polygenic risk score for the prediction of coronary heart disease events. JAMA. (2023) 329:1768–77. 10.1001/jama.2023.7575
2.
Nguyen H Liu J Del Castillo M Shah T . Role of coronary calcium score to identify candidates for ASCVD prevention.Curr Atheroscler Rep. (2019) 21:53. 10.1007/s11883-019-0812-8
3.
Bell K White S Hassan O Zhu L Scott A Clark J et al Evaluation of the incremental value of a coronary artery calcium score beyond traditional cardiovascular risk assessment: a systematic review and meta-analysis. JAMA Intern Med. (2022) 182:634–42. 10.1001/jamainternmed.2022.1262
4.
Shaw L Raggi P Berman D Callister T . Coronary artery calcium as a measure of biologic age.Atherosclerosis. (2006) 188:112–9. 10.1016/j.atherosclerosis.2005.10.010
5.
Freyermuth M Roisin S Saidak Z Matray L Sevestre M Reix T et al Contemporary minimally invasive surgery for TASC-D aorto-iliac lesions: analysis of outcomes and risk factors for primary and secondary patency. Ann Vasc Surg. (2023) 97:367–74. 10.1016/j.avsg.2023.05.018
6.
Gabel J Kiang S Abou-Zamzam A Oyoyo U Teruya T Tomihama R . Trans-atlantic inter-society consensus class D aortoiliac lesions: a comparison of endovascular and open surgical outcomes.AJR Am J Roentgenol. (2019) 213:696–701. 10.2214/AJR.18.20918
7.
Rocha-Neves J Ferreira A Sousa J Pereira-Neves A Vidoedo J Alves H et al Endovascular approach versus aortobifemoral bypass grafting: outcomes in extensive aortoiliac occlusive disease. Vasc Endovascular Surg. (2020) 54:102–10. 10.1177/1538574419888815
8.
Huang C Wu I Wu Y Hwang J Wang S Chen W et al Association of lower extremity arterial calcification with amputation and mortality in patients with symptomatic peripheral artery disease. PLoS One. (2014) 9:e90201. 10.1371/journal.pone.0090201
9.
Dong Y Liu Y Cheng P Liao H Jiang C Li Y et al Lower limb arterial calcification and its clinical relevance with peripheral arterial disease. Front Cardiovasc Med. (2023) 10:1271100. 10.3389/fcvm.2023.1271100
10.
Kang I Lee W Choi B Choi D Hong M Jang Y et al Semiquantitative assessment of tibial artery calcification by computed tomography angiography and its ability to predict infrapopliteal angioplasty outcomes. J Vasc Surg. (2016) 64:1335–43. 10.1016/j.jvs.2016.04.047
11.
Zettervall S Marshall A Fleser P Guzman R . Association of arterial calcification with chronic limb ischemia in patients with peripheral artery disease.J Vasc Surg. (2018) 67:507–13. 10.1016/j.jvs.2017.06.086
12.
Megale A Wolosker N Kalil V Nigro J Wakisaka C Dias B et al Aortic calcium score predicts early outcomes in aortoiliac revascularization. Einstein. (2025) 23:eAO0527. 10.31744/einstein_journal/2025AO0527
13.
Jeremias Z Rat N Benedek I Rapolti E Ratiu M Muresan A et al High iliac calcium score is associated with increased severity and complexity of peripheral arterial disease and predicts global atherosclerotic burden. Vasa. (2018) 47:377–86. 10.1024/0301-1526/a000718
14.
Lee S Kalra K Kashikar A Redpath B Bernheim A Brewster L et al Evaluation of lower extremity calcium score as a measure of peripheral arterial disease burden and amputation risk. Ann Vasc Surg. (2023) 95:154–61. 10.1016/j.avsg.2023.02.009
15.
Megale A Wolosker N Kalil V Nigro J Wakisaka C Dias B et al Calcium score predicts mortality after revascularization in critical limb ischemia. J Endovasc Ther. (2022) 29:438–43. 10.1177/15266028211059911
16.
Okuno S Iida O Shiraki T Fujita M Masuda M Okamoto S et al Impact of calcification on clinical outcomes after endovascular therapy for superficial femoral artery disease: assessment using the peripheral artery calcification scoring system. J Endovasc Ther. (2016) 23:731–7. 10.1177/1526602816656612
17.
Rocha-Singh K Zeller T Jaff M . Peripheral arterial calcification: prevalence, mechanism, detection, and clinical implications.Catheter Cardiovasc Interv. (2014) 83:E212–20. 10.1002/ccd.25387
18.
Norgren L Hiatt W Dormandy J Nehler M Harris K Fowkes F et al Inter-society consensus for the management of peripheral arterial disease (TASC II). J Vasc Surg. (2007) 45(Suppl S):S5–67. 10.1016/j.jvs.2006.12.037
19.
Agha R Abdall-Razak A Crossley E Dowlut N Iosifidis C Mathew G et al STROCSS 2019 Guideline: strengthening the reporting of cohort studies in surgery. Int J Surg. (2019) 72:156–65. 10.1016/j.ijsu.2019.11.002
20.
Stoner M Calligaro K Chaer R Dietzek A Farber A Guzman R et al Reporting standards of the Society for Vascular Surgery for endovascular treatment of chronic lower extremity peripheral artery disease. J Vasc Surg. (2016) 64:e1–21. 10.1016/j.jvs.2016.03.420
21.
Conte M Bradbury A Kolh P White J Dick F Fitridge R et al Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. (2019) 58:S1–S109.e33. 10.1016/j.ejvs.2019.05.006
22.
Thygesen K Alpert J Jaffe A Chaitman B Bax J Morrow D et al Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol. (2018) 72:2231–64. 10.1016/j.jacc.2018.08.1038
23.
Fashandi A Mehaffey J Hawkins R Kron I Upchurch G Robinson W . Major adverse limb events and major adverse cardiac events after contemporary lower extremity bypass and infrainguinal endovascular intervention in patients with claudication.J Vasc Surg. (2018) 68:1817–23. 10.1016/j.jvs.2018.06.193
24.
Davis B Marin D Hurwitz L Ronald J Ellis M Ravindra K et al Application of a Novel CT-based iliac artery calcification scoring system for predicting renal transplant outcomes. AJR Am J Roentgenol. (2016) 206:436–41. 10.2214/AJR.15.14794
25.
Werlin E Braun H Walker J Freise J Amara D Liu I et al Utility of a simplified iliac artery calcium scoring system to guide perioperative management for renal transplantation. Front Med. (2021) 8:606835. 10.3389/fmed.2021.606835
26.
TerBush M Rasheed K Young Z Ellis J Glocker R Doyle A et al Aortoiliac calcification correlates with 5-year survival after abdominal aortic aneurysm repair. J Vasc Surg. (2019) 69:774–82. 10.1016/j.jvs.2018.05.242
27.
Mori S Takahara M Nakama T Tobita K Hayakawa N Iwata Y et al Impact of calcification on clinical outcomes after drug-coated balloon angioplasty for superficial femoral artery disease: assessment using the peripheral artery calcification scoring system. Catheter Cardiovasc Interv. (2023) 101:892–9. 10.1002/ccd.30622
28.
Dong Y Liu Y Liao H Cheng P Liu X Huang W et al Circumferential degree of tibial artery calcification is associated with infrapopliteal endovascular revascularization outcomes in patients with chronic limb-threatening ischemia. Int Angiol. (2023) 42:528–36. 10.23736/S0392-9590.23.05130-1
29.
Konijn L Takx R de Jong P Spreen M Veger H Mali W et al Arterial calcification and long-term outcome in chronic limb-threatening ischemia patients. Eur J Radiol. (2020) 132:109305. 10.1016/j.ejrad.2020.109305
30.
Devia-Rodriguez R Derksen M El Moumni M de Groot K Vedder I Zeebregts C et al Association of Iliofemoral calcium score and major vascular complications within the first year after lower limb endovascular revascularization. Ann Vasc Surg. (2025) 111:290–8. 10.1016/j.avsg.2024.11.009
31.
Hussain B Mahmood A Flynn M Alexander T . Coronary artery calcium scoring in asymptomatic patients.HCA Healthc J Med. (2023) 4:341–52. 10.36518/2689-0216.1565
32.
Gupta A Bera K Kikano E Pierce J Gan J Rajdev M et al Coronary artery calcium scoring: current status and future directions. Radiographics. (2022) 42:947–67. 10.1148/rg.210122
33.
Aboyans V Ricco J Bartelink M Björck M Brodmann M Cohnert T et al Editor’s Choice - 2017 ESC guidelines on the diagnosis and treatment of peripheral arterial diseases, in collaboration with the European Society for Vascular Surgery (ESVS). Eur J Vasc Endovasc Surg. (2018) 55:305–68. 10.1016/j.ejvs.2017.07.018
Summary
Keywords
peripheral artery disease, arterial calcification, TASC II, revascularization, amputation, cardiovascular outcomes
Citation
Pereira-Neves A, Rocha-Neves J, Costa-Pereira T, Ribeiro H, Dias LR, Duarte-Gamas L and Andrade JP (2025) Iliac Calcium Score thresholds predict cardiovascular and limb-related outcomes in TASC D aortoiliac disease. Front. Med. 12:1655229. doi: 10.3389/fmed.2025.1655229
Received
27 June 2025
Accepted
05 September 2025
Published
17 September 2025
Volume
12 - 2025
Edited by
Ofir Koren, Cedars-Sinai Medical Center, United States
Reviewed by
Gregoire Detriche, Hôpital Européen Georges-Pompidou, France
Bingchen Hou, Heidelberg University, Germany
Updates
Copyright
© 2025 Pereira-Neves, Rocha-Neves, Costa-Pereira, Ribeiro, Dias, Duarte-Gamas and Andrade.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: António Pereira-Neves, antonio.hpneves@gmail.com
†These authors have contributed equally to this work and share first authorship
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.