Abstract
Objective:
To introduce the classification and focus on retrospectively investigating clinical factors associated with uterine rupture.
Materials and methods:
We retrospectively analyzed 222 cases of angular pregnancies from January 2010 and December 2021. The selected cases were classified into two types, type I (n = 19) and type II (n = 199). Additionally, type II cases were further subdivided into the ruptured group (n = 25) and the unruptured group (n = 174). Clinical data were collected, and univariate and multivariate analyses were performed to identify significant indicators.
Results:
The mean maternal age was 31.5 ± 5.8 years, with a mean BMI (body mass index) of 22.0 ± 3.2 kg/m2 in 199 type II patients. Spontaneous uterine rupture occurred in 25 (12.6%) patients, while 174 (87.4%) remained unruptured. Univariate analysis revealed that abdominal pain (P < 0.001), a history of previous ipsilateral salpingectomy (P = 0.002), vaginal bleeding (P = 0.005), and gestational age (GA) ≥ 7 weeks (P = 0.044) were significant factors of rupture in type II angular pregnancy. Multivariate analysis identified abdominal pain (OR = 10.410, 95% CI: 3.286–32.977, P < 0.000) and ipsilateral salpingectomy (OR = 3.270, 95% CI: 1.209–8.847, P = 0.020) as statistically significant independent risk factors. The ruptured group had clinically and statistically significant lower hemoglobin and higher transfusion rates.
Conclusion:
The classification system of angular pregnancy (AP) is a valuable tool that facilitates appropriate management and good prognostic outcomes. Type I angular pregnancy can be followed up till term. Type II angular pregnancy is a high-risk form, and clinicians must carefully assess and investigate other factors such as the history of ipsilateral salpingectomy and abdominal pain and high alert for uterine rupture.
Introduction
Angular pregnancy is characterized as an eccentric intrauterine pregnancy (1–3). Kelly in the 19th century, defined it as an embryo implant located at a lateral angle of the uterine cavity, medial to the utero-tubal junction (4). Even though the first mention of angular pregnancy is over a century ago, there remains a paucity of literature on the subject. The prognosis reported in different literature is indeed quite divergent. Some studies have shown that patients with angular pregnancy can achieve live births during close-interval follow-up (5–7). However, uterine rupture, one of the major complications of angular pregnancy, which can lead to severe intra-abdominal hemorrhage and consequent maternal death, has been reported in several studies (8–11).
A classification system was proposed by Chinese experts in 2000 (12). Based on the gestational sac’s growth pattern, angular pregnancy is divided into type I and type II (12). The gestational sac of type I angular pregnancy is situated at the mediolateral junction of the uterine cavity, enveloped by two circular decidual bands and partially surrounded by a myometrial layer (Figure 1). The risk of uterine rupture is low, and the pregnancy usually progresses to the mid or late trimester. In contrast, type II angular pregnancy is characterized by the outward growth of the gestational sac toward the cornual region, resulting in significant bulging, associated with an elevated risk of uterine rupture and life-threatening hemorrhage (Figure 2). Utilizing this classification system enables clinicians to enhance their comprehension of diverse pathological processes, thus facilitating the selection of the most appropriate management options to ensure optimal patient prognoses. A diagnosis of angular pregnancy can be made by two-dimensional transvaginal/transabdominal ultrasound (Figure 2) and confirmed with a laparoscopy or laparotomy. The Figure 3 shows a schematic diagram of angular pregnancy.
FIGURE 1

Type I angular pregnancy: (A) A gestational sac of about 24 mm × 14 mm × 24 mm in size was seen near the right uterine horn in the endometrium by transvaginal two-dimensional ultrasonography. (B) The echogenic embryo was seen inside the sac, with a long diameter of about 6 mm and a visible heart tube.
FIGURE 2

Type II angular pregnancy: (A) The two-dimensional ultrasound showed a mixed echo pattern mass measuring 21 mm × 14 mm × 18 mm in the left uterine angle, expanding toward the cornual region of the uterus. (B) A mixed echo identified a gestational sac measuring 7 mm × 5 mm × 6 mm. (C) Image of blood flow of AP in the left uterine angle. (D) The 3-dimensional ultrasound showed a mass adjacent to the endometrium, expanding toward the cornual region of the uterus. The arrow indicates a thin myometrial wall.
FIGURE 3

Angular pregnancy schematic diagram (12).
In this study, we first introduce the classification of angular pregnancy to identify the type II patients requiring clinical intervention. Then we focus on retrospectively investigating clinical factors associated with uterine rupture in type II angular pregnancy. We hope our study will help clinicians develop appropriate treatment strategies and avoid adverse outcomes.
Materials and methods
Population and data collection
This study was approved by the Ethical Committee of the First Affiliated Hospital of Wenzhou Medical University with the number KY2022-R213, and written informed consent was obtained. A cohort of inpatients diagnosed with angular pregnancy who underwent surgery between January 2010 and December 2021 was collected. All patients were offered ultrasound scans during the first trimester of pregnancy. There were 222 patients with a confirmed diagnosis of angular pregnancy. Among them, 4 patients had incomplete data, 19 patients were diagnosed with type I, and 199 patients were diagnosed with type II. Additionally, according to the intraoperative status of the uterus, type II was further subdivided into ruptured group with 25 patients and unruptured group with 174 patients (Figure 4). The collected data included age (years), BMI (body mass index, calculated as weight in kilograms divided by height in meters squared), abortion history, gravidity, parity, IVF-ET (in vitro fertilization–embryo transfer), previous ectopic pregnancy, salpingectomy history, uterine fibroids or adenomyoma, and intraoperative uterine status. Postoperative histological examination confirmed chorionic villus tissue of the excised tissue in all patients.
FIGURE 4

Flow chart of patient selection.
Statistical analysis
The SPSS software version 25 (IBM, Chicago, IL, USA) was used. For continuous variables, distributed data were presented as mean ± standard deviation (SD) and median (range) or median [interquartile range (IQR)]. Univariate and multivariate analyses were used to preliminarily screen out potentially significant indicators, and a 95% confidence interval (95% CI) of risk factors for the ruptured and unruptured groups, respectively. The difference in overall mean was explored using a two-sample Student’s t-test or Wilcoxon rank-sum test. Categorical variables were expressed as raw numbers or percentages using Fisher’s exact test or the Chi-square test. It is imperative to note that a two-sided test was used to carry out all statistical analyses. The statistical significance level was set at Q = 0.05, and P < 0.050 was considered statistically significant. Forest plot was drawn by GraphPad Prism 8.
Results
Clinical characteristics
The clinical characteristics of 199 type II angular pregnancies are shown in Table 1. The mean age was 31.5 ± 5.8 years, with a mean body mass index (BMI) of 22.0 ± 3.2 kg/m2. Previous salpingectomy surgery was documented in 70 (35.2%) patients. Of all these patients, 49 had abdominal pain, 54 had vaginal bleeding, and 66 had no symptoms. A mixed echogenic mass was detected in 39 (19.6%) patients, a gestational sac in 160 (80.4%) patients, and a heartbeat in 69 (34.7%) patients by ultrasound examination. The median preoperative serum HCG was 11,897 IU/L (838–22,500 IU/L). After surgery, uterine rupture was confirmed in 25 (12.6%) patients.
TABLE 1
| Characteristics | Patients (N = 199) |
| Age, years, mean ± SD | 31.50 ± 5.665 |
| BMI, kg/m2, mean ± SD | 22.0659 ± 3.26091 |
| Gravidity ≥ 1, n (%) | 167 (83.9) |
| Parity ≥ 1, n (%) | 125 (62.8) |
| Abortion ≥ 1, n (%) | 140 (70.3) |
| IVF-ET, n (%) | 34 (17.1) |
| Previous ectopic pregnancy, n (%) | 46 (23.1) |
| Salpingectomy history, n (%) | 70 (35.2) |
| Symptoms, n (%) | |
| Abdominal pain | 49 (24.6) |
| Vaginal bleeding | 54 (27.1) |
| Abdominal pain + vaginal bleeding | 30 (15.1) |
| No symptoms | 66 (33.2) |
| Ultrasound, n (%) | |
| Mixed echo mass | 39 (19.6) |
| Gestational sac | 160 (80.4) |
| Heartbeat | 69 (34.7) |
| Uterine fibroids or adenomyoma, n (%) | 37 (18.6) |
| Preoperative HCG, IU/L, median (range) | 11,897 (838−22,500) |
| Intraoperative status | |
| Ruptured | 25 (12.6) |
| Unruptured | 174 (87.4) |
Basic clinical characteristics of 199 type II angular pregnancies.
BMI, body mass index; IVF-ET, in vitro fertilization and embryo transfer; HCG, human chorionic gonadotrophin.
Univariate analysis of clinical symptoms and specific clinical parameters between the two groups is shown in Table 2. The results showed that abdominal pain (P < 0.001), a history of previous ipsilateral salpingectomy (P = 0.002), vaginal bleeding (P = 0.005), and gestational age (GA) ≥ 7 weeks (P = 0.044) were significant factors. There were no statistically significant differences including age (P = 0.727), BMI (P = 0.236), abortion ≥ 1 (P = 0.093), gravidity ≥ 1 (P = 0.149), parity ≥ 1 (P = 0.896), IVF-ET (P = 1.000), history of ectopic pregnancy (P = 0.230), the presence of heartbeat (P = 0.099), combined with uterine myoma or adenomyoma (P = 0.388), and preoperative serum HCG levels (P = 0.601).
TABLE 2
| Parameter | Ruptured group (N = 25) | Unruptured group (N = 174) | T/χ2 value | P-value |
| Age, years, mean ± SD | 31.1 ± 5.4 | 31.6 ± 5.8 | −0.356 | 0.727 |
| BMI, kg/m2, mean ± SD | 21.3 ± 2.4 | 22.3 ± 3.3 | −1.221 | 0.236 |
| Abortion ≥ 1, n (%) | 14 (56.0) | 126 (72.4) | 2.823 | 0.093 |
| Gravidity ≥ 1, n (%) | 18 (72.0) | 149 (85.6) | 3.010 | 0.149 |
| Parity ≥ 1, n (%) | 16 (64.0) | 109 (62.6) | 0.017 | 0.896 |
| IVF-ET, n (%) | 4 (16.0) | 30 (17.2) | 0.024 | 1.000 |
| History of ectopic pregnancy, n (%) | 8 (32.0) | 37 (21.3) | 1.270 | 0.230 |
| Ipsilateral salpingectomy, n (%) | 14 (56.0) | 45 (25.7) | 10.974 | 0.002* |
| Abdominal pain, n (%) | 21 (84.0) | 58 (33.3) | 23.441 | < 0.001* |
| Vaginal bleeding, n (%) | 4 (16.0) | 79 (45.4) | 7.78 | 0.005* |
| Gestational age ≥ 7 w, n (%) | 9 (36.0) | 100 (57.5) | 0.024 | 0.044* |
| Heartbeat, n (%) | 5 (20.0) | 64 (36.8) | 3.063 | 0.099 |
| Combined with uterine fibroids or adenomyoma, n (%) | 7 (28.0) | 32 (18.4) | 1.672 | 0.388 |
| Preoperative HCG, IU/L, median (IQR) | 11,381 (3,349–31,314) | 11,936.5 (4,502–32,068) | 0.285 | 0.601 |
Univariate analysis of factors related to rupture in type II angular pregnancy.
SD, standard deviation; BMI, body mass index; IVF-ET, in vitro fertilization and embryo transfer; HCG, human chorionic gonadotrophin.
*Represents p < 0.05.
All the related variables were entered into the multivariate logistic regression model. The analysis identified abdominal pain (OR = 10.410, 95% CI: 3.286–32.977, P < 0.000) and ipsilateral salpingectomy (OR = 3.270, 95% CI: 1.209–8.847, P = 0.020) as statistically significant independent risk factors. Results are detailed in Table 3 and Figure 5.
TABLE 3
| Parameter | β | SE | OR | 95% CI | P-value |
| Abdominal pain | 2.343 | 0.588 | 10.410 | 3.286–32.977 | 0.000* |
| Vaginal bleeding | −0.913 | 0.569 | 0.401 | 0.132–1.223 | 0.108 |
| Ipsilateral salpingectomy | 1.185 | 0.508 | 3.270 | 1.209–8.847 | 0.020* |
| Gestational age ≥ 7 w | −0.357 | 0.513 | 0.700 | 0.256–1.913 | 0.487 |
Multivariate analysis of factors related to rupture in type II angular pregnancy.
*Represents p < 0.05.
FIGURE 5
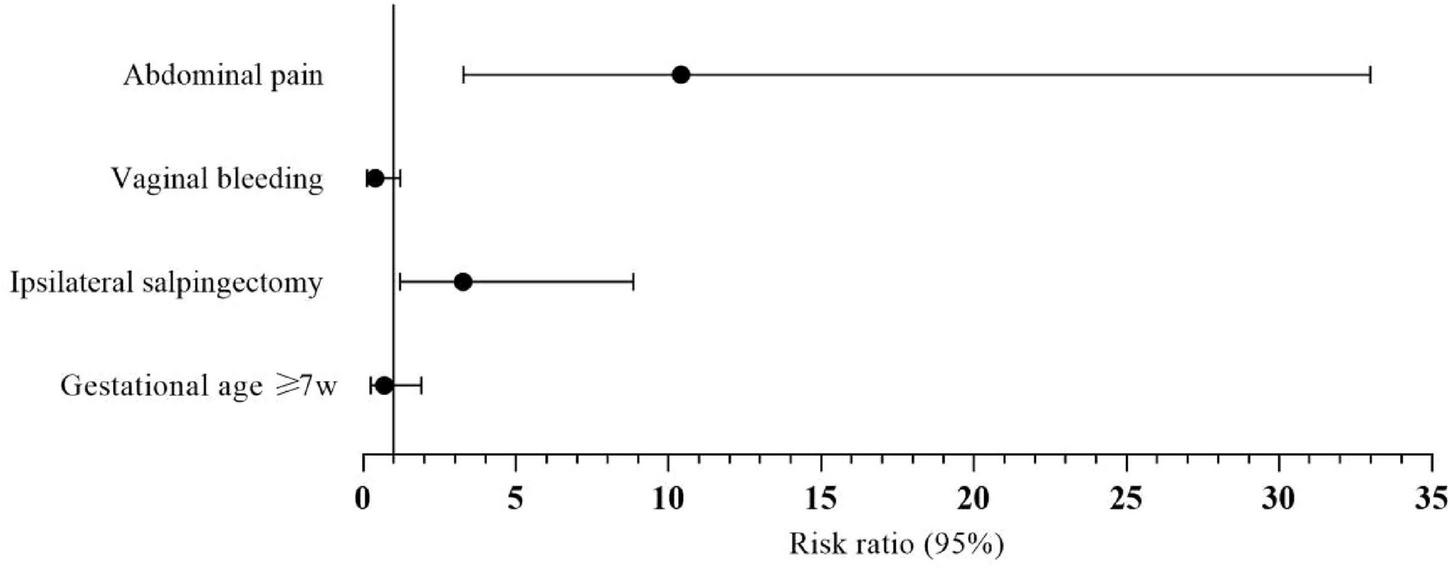
Forest plot showing the results of multivariate logistic regression analysis and visualizing the risk ratios of the characteristics for type II angular pregnancy.
The results in Table 4 indicated that the median preoperative hemoglobin is 80 g/L, and 52% (13/25) of patients had a blood transfusion with hemorrhagic shock in the ruptured group, while the median preoperative hemoglobin is 127 g/L and no blood transfusion in the unruptured group. The ruptured group had clinically and statistically significantly lower hemoglobin and higher transfusion rates (P < 0.001).
TABLE 4
| Parameter | Ruptured group (N = 25) | Unruptured group (N = 174) | P-value |
| Preoperative hemoglobin, g/L, median (IQR) | 80 (71.0–96.5) | 127.0 (117.3–133.0) | < 0.001* |
| Blood transfusion | 13 (52.0) | 0 (0) | < 0.001* |
Significant differences in preoperative hemoglobin levels and blood transfusion rates between the ruptured and unruptured groups.
*Represents p < 0.05.
Discussion
Finlinson et al. (13) conducted the first review on the specific signs or diagnostic criteria for angular pregnancy located at the utero-tubal junction; however, there was no detailed description of it about its categories and the factors associated with uterine rupture. Uterine rupture is a rare, life-threatening complication in 52% of blood transfusions in our study. It was reported that the incidence of uterine rupture is 5.1 per 10,000 deliveries in a scarred uterus, approximately 0.45 to 0.7 per 10,000 deliveries in an unscarred uterus, and the overall incidence of uterine rupture is approximately 0.04% to 0.09% in the general population (14–17). Our decade-long retrospective cohort analysis of 222 angular pregnancies provides critical insights into risk stratification and predictors of uterine rupture in this high-risk obstetric condition. As far as I know, it is the first to propose a novel classification system for angular pregnancy in English literature, to facilitate appropriate management and favorable prognoses. The implementation of a novel classification system (type I vs. type II) allowed for nuanced risk assessment, with type II angular pregnancy demonstrating a 12.6% rupture rate, lower than historical reports of 13.6%–28% rupture in angular pregnancies (18, 19). We reinforce the heterogeneity of angular pregnancy outcomes and emphasize the clinical utility of sub-classification in tailoring management strategies.
The multivariate analysis identified abdominal pain and prior ipsilateral salpingectomy as independent predictors of rupture in type II angular pregnancy. Notably, the absence of vaginal bleeding in ruptured cases suggests rupture may occur before significant intrauterine detachment, highlighting the limitations of relying solely on bleeding as a warning sign. These findings align with existing literature regarding the spontaneous rupture of inter-tubal pregnancies following salpingectomy but also expand its scope. Some studies, conducted on factors associated with uterine rupture for intrauterine pregnancy, revealed that salpingectomy-associated uterine rupture caused 67% fetal death (20, 21). Studies have reported angular pregnancy-associated uterine ruptures as early and as late as the 10th and 21st gestational weeks, respectively (22). In our study, the earliest and latest gestational ages reported for uterine rupture were 6th and 14.7th weeks, respectively.
Acute abdominal pain is a classical sign of uterine rupture; however, for most first and second-trimester gravidas presenting in the outpatient department, the differential diagnosis of uterine rupture may be overlooked. One study identified 61 cases of first-trimester uterine rupture with a median gestation of 11 weeks, 97% of which had abdominal pain as a presenting symptom (23). The symptom of asymmetrical pain may or may not subside during a diagnosed abnormal pregnancy, according to Jansen and associates (18). To proceed, they observed that the closer the implantation site is to the fallopian tube, the more intense the abdominal pain. Moreover, abdominal pain was one of the persistent symptoms presented by angular pregnancies in the emergency room (24). In addition, abdominal pain was identified as a hallmark of impending rupture due to tension at the uterine cornu (9), making it the most common symptom amongst the other indicators. As the gestational sac enlarges, it grows toward the tubal ostium, causing a thin myometrial layer at the uterine cornu, leading to a significantly asymmetrical and tender uterus.
Prior ipsilateral salpingectomy emerged as a significant risk factor for uterine rupture in our study, a finding not previously emphasized in other literature on angular pregnancy. Salpingectomy (partial or complete) continues to be the main treatment for ectopic pregnancies or hydrosalpinx (21). However, numerous studies have made a direct link between salpingectomy with or without cornual resection and early gestational uterine rupture (20). A previous history of salpingectomy via laparoscopy could be a risk factor for uterine rupture in pregnant women (25). We hypothesize that inflammation and fibrosis occurring in the angular region and the tissues surrounding it in patients with prior ipsilateral salpingectomy could result in reduced blood supply, decreased tissue elasticity, and restricted blood volume to the myometrium at the uterine cornual region. As the gestational sac grows and enlarges, it bulges outwards, resulting in the myometrial layer at the uterine angle becoming thinner and tense. This may reflect postsurgical anatomical changes, such as altered cornual vascularity or myometrial weakness, which could predispose to asymmetric gestational sac growth and uterine rupture. We propose that for a patient to be considered a candidate for observational management, factors such as the type presented, history of salpingectomy, and preliminary test results should be analyzed carefully.
Strengths and limitations
To date, most of the literature on angular pregnancy consists of small case reports and review studies. Currently, there is a paucity of literature on type II angular pregnancy and the risk of rupture. The retrospective nature of our study constitutes a limitation in selection bias. Moreover, although our study was conducted by reviewing decade-long records, a type II angular pregnancy is not a common phenomenon, resulting in a relatively smaller sample size. Consequently, there is a necessity for additional large-scale prospective cohort studies to be conducted on type II angular pregnancy and the risk factors for rupture in the future.
Conclusion
The clinical diagnosis and treatment of angular pregnancy (AP) can be challenging because of its distinct anatomic implantation. The results of APs can vary depending on the type that is present. Although type 1 AP is typically permitted for term and vaginal birth, there is a higher chance of placenta accreta at the uterine horn, necessitating the placenta’s manual removal after delivery. Pregnancy rupture is more likely in type II angular pregnancies, which are high-risk eccentric pregnancies. Thus, by classifying APs (type I and II) according to severity, physicians will be able to choose the appropriate course of treatment for their patients. Furthermore, because AP patients with a history of ipsilateral salpingectomy and abdominal pain are more likely to experience uterine rupture, we suggest timely intervention and close monitoring of such patients to avoid catastrophic outcomes.
Statements
Data availability statement
The original contributions presented in this study are included in this article/supplementary material, further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving humans were approved by the Ethics Committee Approval for Clinical Research in accordance with the First Affiliated Hospital of Wenzhou Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
B-YH: Writing – original draft, Investigation, Writing – review & editing. PC: Writing – original draft, Writing – review & editing. H-RH: Writing – review & editing, Methodology. Y-SZ: Validation, Writing – review & editing. M-QY: Resources, Writing – review & editing. J-YD: Data curation, Writing – review & editing. X-xX: Validation, Writing – review & editing. H-jZ: Supervision, Writing – review & editing. BY: Supervision, Writing – review & editing. L-FH: Funding acquisition, Supervision, Writing – review & editing.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study received funding from the Basic Scientific Research Program of Wenzhou Science and Technology Bureau (Y20220051).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
BMI, body mass index; HCG, human chorionic gonadotropin; SD, standard deviation; IVF-ET, in vitro fertilization and embryo transfer; IQR, interquartile range; OR, odds ratio; CI, confidence interval.
References
1.
Grant A Murji A Atri M . Can the presence of a surrounding endometrium differentiate eccentrically located intrauterine pregnancy from interstitial ectopic pregnancy?J Obstet Gynaecol Can. (2017) 39:627–34. 10.1016/j.jogc.2017.03.087
2.
Goh J Ng Z Shahul Hameed M . Hysteroscopy is a useful diagnostic and therapeutic tool for the treatment of angular pregnancy.Gynecol Minim Invasive Ther. (2022) 11:78–9. 10.4103/GMIT.GMIT_120_20
3.
Meichen Y Jing F Lingyun Z Jianwei Z . Two cases of angular pregnancy with incomplete abortion treated with hysteroscopy: a case report and review of literature.BMC Surg. (2021) 21:76. 10.1186/s12893-021-01077-7
4.
Kelly H. Operative Gynecology. New York, NY: D Appleton (1918).
5.
Yang P Shen L Ai J Zhao Y . Expectant treatment for angular pregnancy after assisted reproduction technology: a safe and patient-friendly treatment strategy.Front Med. (2023) 10:1234425. 10.3389/fmed.2023.1234425
6.
Bollig K Schust D . Refining angular pregnancy diagnosis in the first trimester: a case series of expectant management.Obstet Gynecol. (2020) 135:175–84. 10.1097/AOG.0000000000003595
7.
Alves J Alves N Alencar Júnior C Feitosa F da Silva Costa F . Term angular pregnancy: successful expectant management.J Obstet Gynaecol Res. (2011) 37:641–4. 10.1111/j.1447-0756.2010.01405.x
8.
Chiung H Pai A Yen C . Hysteroscopic removal of a first-trimester angular pregnancy.Gynecol Minim Invasive Ther. (2024) 13:200–1. 10.4103/gmit.gmit_27_24
9.
Chen P Liu X Fang C Zhao W . Angular pregnancy after in-vitro fertilization with timely termination to avoid uterine rupture: a case report.Asian J Surg. (2023) 46:2454–6. 10.1016/j.asjsur.2022.12.059
10.
Yao F Fan Y Shao L Miao P Ding H Yang M . The dilemmas in the diagnosis and management of angular pregnancy.Taiwan J Obstet Gynecol. (2021) 60:582–3. 10.1016/j.tjog.2021.03.040
11.
To G Kodama K Onoyama I Yahata H Kato K . Ipsilateral right angular pregnancy after a laparoscopic right salpingo-oophorectomy: a case report.Cureus. (2023) 15:e46171. 10.7759/cureus.46171
12.
Ren C Gu X Liu X Yang Q . Expert consensus on the diagnosis and treatment of uterine angular pregnancy.Chinese J Pract Gynecol Obstetr. (2020) 36:329–32.
13.
Finlinson A Bollig K Schust D . Differentiating pregnancies near the uterotubal junction (angular, cornual, and interstitial): a review and recommendations.Fertil Res Pract. (2020) 6:8. 10.1186/s40738-020-00077-0
14.
Ofir K Sheiner E Levy A Katz M Mazor M . Uterine rupture: risk factors and pregnancy outcome.Am J Obstet Gynecol. (2003) 189:1042–6. 10.1067/s0002-9378(03)01052-4
15.
Ronel D Wiznitzer A Sergienko R Zlotnik A Sheiner E . Trends, risk factors and pregnancy outcome in women with uterine rupture.Arch Gynecol Obstet. (2012) 285:317–21. 10.1007/s00404-011-1977-8
16.
Zwart J Richters J Ory F de Vries J Bloemenkamp K van Roosmalen J . Uterine rupture in The Netherlands: a nationwide population-based cohort study.BJOG. (2009) 116:1069–78. 10.1111/j.1471-0528.2009.02136.x
17.
Gibbins K Weber T Holmgren C Porter T Varner M Manuck T . Maternal and fetal morbidity associated with uterine rupture of the unscarred uterus.Am J Obstet Gynecol. (2015) 213: 382.e1-6. 10.1016/j.ajog.2015.05.048
18.
Jansen R Elliott P . Angular intrauterine pregnancy.Obstet Gynecol. (1981) 58:167–75.
19.
Rankin M . Angular pregnancy: a review of cases reported in the past 80 years.Obstet Gynecol Cases Rev. (2014) 1:3.
20.
Stanirowski P Trojanowski S Słomka A Cendrowski K Sawicki W . Spontaneous rupture of the pregnant uterus following salpingectomy: a literature review.Gynecol Obstet Invest. (2015) 80:73–7. 10.1159/000398795
21.
Hua Z Wu M . Spontaneous rupture of the uterus following salpingectomy: a case report and literature review.J Int Med Res. (2019) 47:5328–36. 10.1177/0300060519874903
22.
Marfori C Kotzen M . Angular vs. interstitial pregnancy: a case report highlighting diagnostic nuances with stark management differences.Case Rep Womens Health. (2018) 19:e00068. 10.1016/j.crwh.2018.e00068
23.
Perdue M Felder L Berghella V . First-trimester uterine rupture: a case report and systematic review of the literature.Am J Obstet Gynecol. (2022) 227:209–17. 10.1016/j.ajog.2022.04.035
24.
Hasanzadeh M Dadgar S Arian Y Yousefi Y . Angular ectopic pregnancy presenting as rupture of lateral wall of the uterus: late presentation in gestation week 20.Iran J Med Sci. (2017) 42:314–7.
25.
Dong J Cao Y Ma Q Xue L Zhu W . Misdiagnosis of a twin pregnancy with double-corner uterine rupture following salpingectomy and protrusion of the amniotic sac as an adnexal cyst: a case report.BMC Pregnancy Childbirth. (2020) 20:71. 10.1186/s12884-020-2773-x
Summary
Keywords
angular pregnancy, salpingectomy, uterine rupture, diagnostic ultrasonography, eccentric intrauterine pregnancy
Citation
Huang B-Y, Cobbinah P, Hu H-R, Zhu Y-S, Yang M-Q, Ding J-Y, Xu X-x, Zhou H-j, Yin B and Han L-F (2025) Clinical factors associated with uterine rupture in type II angular pregnancy: a 10-year single-institution retrospective study. Front. Med. 12:1656273. doi: 10.3389/fmed.2025.1656273
Received
29 June 2025
Accepted
15 September 2025
Published
24 September 2025
Volume
12 - 2025
Edited by
Ferdinando Antonio Gulino, University of Messina, Italy
Reviewed by
Zhongxian Yang, Southern Medical University, China
Chen Cheng, Washington University in St. Louis, United States
Updates
Copyright
© 2025 Huang, Cobbinah, Hu, Zhu, Yang, Ding, Xu, Zhou, Yin and Han.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Bo Yin, 2031237@tongji.edu.cnLing-Fei Han, lingfeihan@tongji.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.