- 1Department of Respiratory and Critical Care Medicine, Tianjin Medical University General Hospital, Tianjin Medical University, Tianjin, China
- 2Department of Pulmonary and Critical Care Medicine, Linfen Central Hospital, Linfen, China
- 3Linfen Clinical Medicine Research Center, LinFen Central Hospital, Linfen, China
- 4Shanxi Provincial Clinical Medical Research Center for Respiratory Diseases (COPD), Linfen, China
- 5Joint Research Center for Thoracic and Pulmonary Diseases, Shanxi Medical University, Linfen, China
- 6Department of Respiratory and Critical Care Medicine, Peking University People’s Hospital, Beijing, China
Background: Lung function impairment, a hallmark of chronic airway diseases like chronic obstructive pulmonary disease (COPD), is often underdiagnosed in China. Preserved Ratio Impaired Spirometry (PRISm) may represent an early, subclinical stage of this process. However, a comprehensive understanding of their clinical phenotypes, effective predictive strategies for early identification in large populations, and the role of systemic inflammation remains underexplored, particularly in the Chinese context. This study aimed to describe the clinical phenotypes of lung function impairment, identify predictive factors using machine learning, and explore associated systemic inflammation in a large-scale population screening.
Methods: A prospective cross-sectional study was conducted in Hongtong County, China (2021–2024). Participants were classified into airflow obstruction, PRISm, and normal groups via portable spirometry. Using demographic, clinical, and laboratory data, we developed and validated several machine learning (ML) models to predict lung function impairment. Model performance was evaluated by the area under the receiver operating characteristic curve (AUC). Serum cytokines were measured by ELISA in matched sub-cohorts to assess systemic inflammation.
Results: Among 9,284 enrolled adults, 51.0% had airflow obstruction, 6.7% had PRISm, and 42.3% were normal. We identified distinct phenotypes: the PRISm group was predominantly female with lower smoking rates but a higher risk of coronary heart disease. The airflow obstruction group was characterized by classical risk factors (older age, male sex, lower BMI, smoking) and specific renal and cerebrovascular comorbidities. The ML models identified older age, male sex, lower BMI, respiratory symptoms (cough, dyspnea), and higher creatinine and hemoglobin as key predictors, demonstrating modest performance with an AUC of 0.635 in the validation set. Immunologically, individuals with airflow obstruction or PRISm showed significantly lower serum IL-2 and higher IL-5 and IL-17A levels compared to controls.
Conclusion: In a large-scale screening, individuals with airflow obstruction and PRISm present with distinct clinical phenotypes. A predictive model using simple clinical variables can help identify individuals at higher risk for lung function impairment, despite modest performance. Serum IL-2, IL-5, and IL-17A are potential biomarkers for the early recognition and understanding of airflow limitation.
1 Introduction
Lung function impairment represents a fundamental physiological aberration common to numerous chronic airway diseases, such as chronic obstructive pulmonary disease (COPD), asthma, bronchiectasis, and tuberculosis-ravaged lung. COPD remains a critical global health burden, ranking as the third leading cause of mortality worldwide (WHO, 2020) (1, 2). Updated 2023 data indicate COPD accounts for 6.4% of global deaths (1), with prevalence among Chinese adults ≥40 years reaching 13.7% (2012–2015) (3). Alarmingly, due to relatively low socioeconomic status and insufficient public awareness, especially in rural regions of China, less than 30% of cases are diagnosed early, and approximately 65% exhibit irreversible lung function decline at initial diagnosis (4). This high burden and low diagnosis rate highlight an urgent need for effective early identification and risk stratification strategies tailored for this vulnerable population.
In recent years, increased focus has been directed towards individuals who do not meet the spirometric criteria for persistent airflow obstruction yet display respiratory symptoms or diminished lung function. A noteworthy subgroup in this regard is Preserved Ratio Impaired Spirometry (PRISm), characterized by a preserved FEV1/FVC ratio (≥0.70) alongside a reduced FEV1 (<80% predicted) (5). PRISm is relatively common and has been linked to increased respiratory symptoms, exacerbations, cardiovascular comorbidities, and mortality compared to individuals with normal spirometry; however, its long-term progression and optimal management remain poorly understood (6–8). Several studies suggest that PRISm may signify an early stage of COPD or represent a distinct clinical phenotype (9–13).
Despite these advancements, a comprehensive understanding of the clinical heterogeneity of lung function impairment across its spectrum (from normal to PRISm and established obstruction), and the development of effective, practical predictive strategies for early identification in large populations, remains largely underexplored. Particularly, in high-prevalence, under-diagnosed rural populations like those in China, a systematic “comprehensive atlas” detailing distinct clinical phenotypes, associated predictive factors, and underlying biological mechanisms is still urgently needed. While conventional spirometry is the gold standard for diagnosis, its logistical impracticality and time-consuming nature present a significant barrier for large-scale population screening. To overcome this, the advent and validation of portable spirometers offer a crucial solution, enabling robust and feasible data collection in community settings (14).
Clinically, distinguishing between individuals with normal lung function, PRISm (subclinical), and advanced airflow obstruction is vital for implementing targeted prevention and management strategies. Although spirometry is fundamental, its results can be influenced by subjective factors. For instance, elderly individuals may have difficulty cooperating with lung function testing, while younger individuals are often unwilling to complete this time-consuming procedure. This variability underscores the need to find simpler, non-invasive factors correlated with lung function impairment. Integrating readily available blood test indicators with comorbidity metrics represents a crucial step toward developing effective predictive models. However, a more comprehensive understanding and robust risk stratification require moving beyond these isolated factors to incorporate multidimensional data, including demographic information, clinical history, laboratory markers, and environmental exposures (15–17). Analyzing such intricate and high-dimensional datasets presents a significant challenge, making machine learning techniques particularly well-suited for identifying complex patterns and constructing generalizable predictive models (18, 19).
Furthermore, the pathophysiology of lung function decline is deeply rooted in chronic inflammation (20). Neutrophilic inflammation was regarded as the key process in the pathogenesis of COPD. Systemic and airway-localized Th2 inflammation is characterized in a subgroup of COPD, usually with eosinophilia, served as a indicator for the usage of inhaled corticosteroid in the long-term management of COPD (21). While systemic inflammation is a known feature of COPD, its specific signature in the PRISm state, which may represent a transitional or distinct biological entity, remains poorly characterized (22). Investigating systemic inflammatory markers could therefore provide crucial insights into the processes that differentiate these early phenotypes from both normal lung function and established disease, thereby contributing to a more complete understanding of disease progression and guiding potential therapeutic targets.
Therefore, leveraging the feasibility of large-scale population screening using portable spirometry in a high-burden region of Shanxi Province, China, this study aimed to (1) comprehensively characterize the distinct clinical phenotypes of airflow obstruction and PRISm, (2) identify key clinical and laboratory predictive factors for lung function impairment using machine learning, and (3) explore associated systemic inflammatory profiles. By doing so, we seek to contribute to the early recognition and improved management strategies for chronic airway diseases in high-risk populations.
2 Methods and materials
2.1 Study design and population
This cross-sectional study was conducted between January 2021 and July 2024 as part of a large-scale epidemiological survey in the rural regions of Hongtong County, Linfen City, Shanxi Province, China. All adult residents aged 18 years and older were invited to participate. Participants were excluded if they met any of the following criteria: (1) inability to perform spirometry or failure to produce acceptable and repeatable results (quality grade below ‘A’ or ‘B’); (2) presence of an acute respiratory infection at the time of assessment; (3) current pregnancy; or (4) incomplete data, defined as >20% missing values for key variables.
Clinical data were systematically collected using an electronic questionnaire. This included demographic information [age, sex, Body Mass Index (BMI)], lifestyle factors (smoking status, biofuel and dust exposure), self-reported symptoms (chronic cough, sputum production, dyspnea, and chest pain), and the history of self-reported respiratory diseases and comorbidities (hypertension, chronic bronchitis, emphysema, COPD, asthma, lung cancer, other malignancies, tuberculosis, coronary heart disease, arrhythmia, diabetes mellitus, hyperthyroidism, and cerebrovascular disease). The study was approved by the ethics committee of Linfen Central Hospital (Ethics Approval No. 2021-1-1) and adhered to the principles of the Declaration of Helsinki. Informed consent was obtained from all participants prior to their involvement.
2.2 Spirometry and group classification
Given the nature of this large-scale population screening, all classifications were based on pre-bronchodilator spirometry. Pre-bronchodilator spirometry was performed on all participants using portable spirometry devices (BreathHome, Inc., China). To ensure quality control, all spirometry results were independently reviewed by two experienced pulmonologists. The following parameters were recorded: FEV1/FVC, FEV1, FVC, FEV1% predicted, FVC% predicted, forced expiratory flow at 25, 50, and 75% of FVC (FEF25, FEF50, FEF75), Forced Expiratory Time (FET), and FEF25-75. Quality grade of ‘A’ or ‘B’ was regarded to be eligible, which were defined as acceptable data in at least 2 repeated tests with repeatability difference less than 15%. Optimal results in repeated tests were utilized for further analysis. Based on these pre-bronchodilator results, participants were classified into three distinct groups:
• Airflow Obstruction Group: FEV1/FVC < 0.70
• PRISm Group: FEV1/FVC ≥ 0.70 and FEV1 < 80% predicted
• Normal Group: FEV1/FVC ≥ 0.70 and FEV1 ≥ 80% predicted
2.3 Laboratory test parameters
Routine assays of complete blood count, liver function, renal function, lipid profiles, and fasting glucose were performed for all the participants. Additionally, serum cytokine levels, including interleukin (IL)-2 (cat. no. 10353), IL-4 (cat. no. 10375), IL-5 (cat. no. 10376), IL-10 (cat. no. 13626), IL-17A (cat. no. 10344), and IL-22 (cat. no. 10356), were quantified by ELISA (LunChangShuoBiotech Inc., China) in matched cohorts. In consideration of IL-2 representing for Th1 inflammation, IL-4 and IL-5 for Th2 inflammation, IL-17A and IL-22 for Th17 pathway and neutrophil inflammation, and IL-10 for regulatory T cells, a comprehensive immunologic status can be evaluated. From the complete blood count data, several composite inflammatory indices were calculated: the neutrophil-to-lymphocyte ratio (NLR), neutrophil-to-eosinophil ratio (NER), systemic immune-inflammation index (SII = neutrophil × platelet/lymphocyte), systemic inflammation response index (SIRI = neutrophil × monocyte/lymphocyte), and the platelet-to-lymphocyte ratio (PLR).
2.4 Statistical analysis
All statistical analyses were performed using R software (version 4.4.1). Continuous variables with a normal distribution were presented as mean ± standard deviation (SD), while nonparametric variables were presented as median and interquartile range (IQR, 25th and 75th percentiles). Student’s t-test and analysis of variance with a post hoc Tukey HSD test were used for continuous parametric data, while continuous nonparametric data were analyzed using the Kruskal–Wallis test. Categorical variables were presented as counts (percentages) and were compared using the chi-square test or Fisher’s exact test, as appropriate.
In the training cohort, various multivariate modeling approaches were initially employed to identify predictive factors for airflow obstruction and PRISm. Specifically, LASSO regression with 10-fold cross-validation for optimal lambda selection, random forest (500 trees, max_depth = 15, min_samples_leaf = 5), and gradient boosting machine (1,000 boosting stages, learning_rate = 0.01, max_depth = 3) were used. Concurrently, adjusted odds ratios (ORs) with 95% confidence intervals (CIs) were provided by conventional logistic regression. Subsequently, using the selected features, four supervised learning algorithms were implemented to classify individuals with impaired versus normal lung function. These included logistic regression (L2 regularization, C = 1.0), random forest (1,000 trees, min_samples_split = 10), gradient boosting (2000 estimators, learning_rate = 0.005, with early stopping), and XGBoost (max_depth = 6, eta = 0.1, gamma = 0.5). Model performance was evaluated through stratified 5-fold cross-validation to ensure robustness.
A two-sided p value <0.05 was considered statistically significant.
3 Results
3.1 Comparison of clinical characteristics among individuals with airflow obstruction, PRISm and normal control
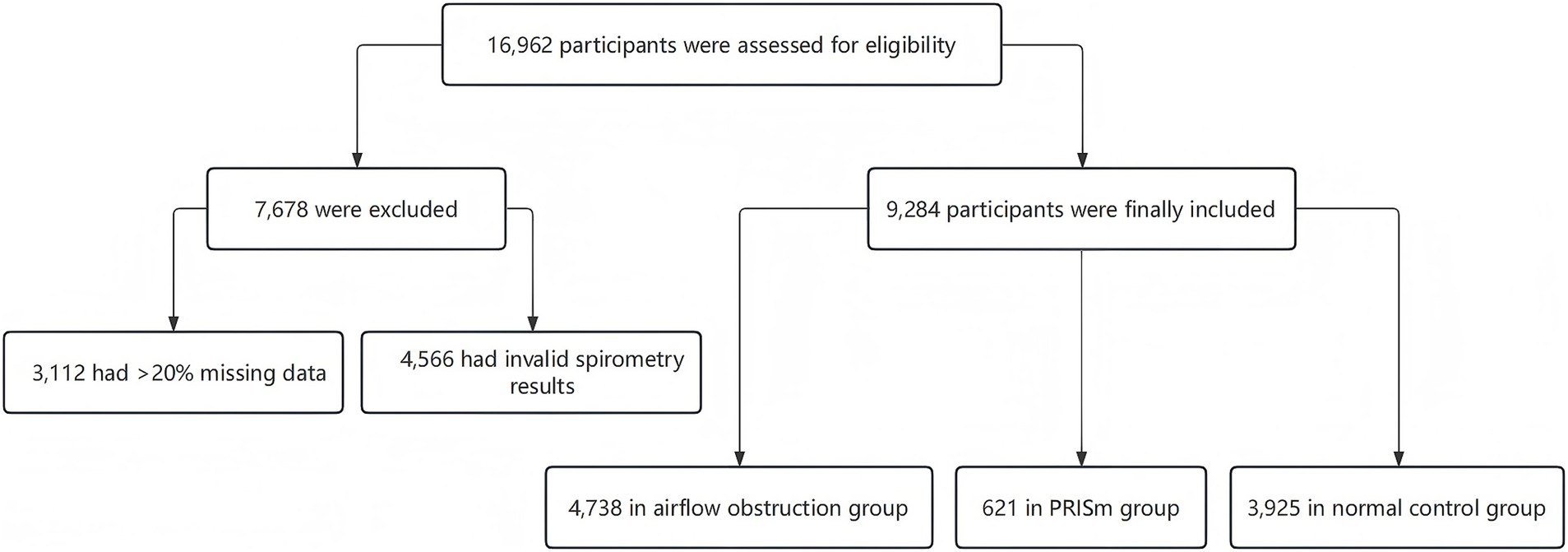
A total of 16,962 participants were enrolled. After checking for missing data, a total of 9,284 participants were included in the final analysis, comprising 4,738 (51.0%) in the airflow obstruction group, 621 (6.7%) in the PRISm group, and 3,925 (42.3%) in the normal spirometry group (Figure 1).
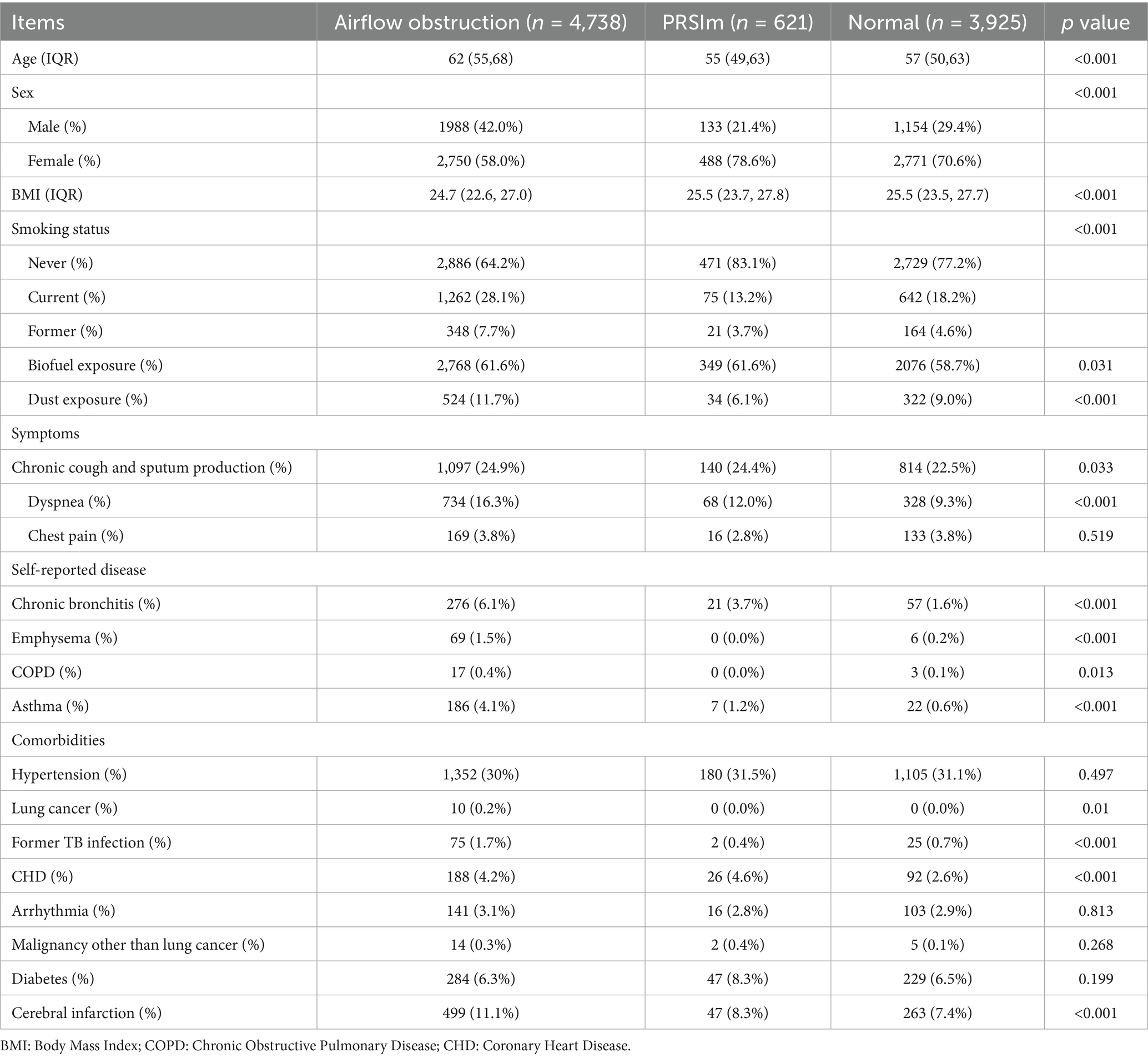
Table 1 summarizes the important demographic and clinical characteristics of the study participants across the three groups. Participants with airflow obstruction were strikingly older [median (IQR): 62 (55–68) years] than both PRISm [55 (49–63) years] and normal groups [57 (50–63) years] (both p < 0.001). Furthermore, a significantly higher proportion of males characterized the airflow obstruction group compared to the other two groups. Participants in the airflow obstruction group exhibited a lower BMI than both the PRISm and normal groups.
Regarding lifestyle factors, the prevalence of current and former smoking was highest in the airflow obstruction group. Notably, the current smoking rate in airflow obstruction group (28.1%) was double that of the PRISm group (13.2%, p < 0.001). Dust exposure was also most prevalent in airflow obstruction group (11.7%), significantly high than in the PRISm (6.1%) and normal (9.0%) groups (p < 0.001).
Dyspnea was the predominant differentiating symptom, showing a clear gradient in prevalence: highest in the airflow obstruction group, followed by the PRISm group, and lowest in the normal group.
A significant discrepancy was observed between objective spirometry findings and self-reported diagnoses of respiratory diseases. Strikingly, only 0.4% of participants in the airflow obstruction group reported a prior diagnosis of COPD. The prevalence of self-reported asthma was highest in the airflow obstruction group compared to the other two groups.
Individuals with airflow obstruction exhibited a higher prevalence of self-reported prior tuberculosis and cerebrovascular diseases compared to the other groups. Coronary heart disease rates were also elevated in both the airflow obstruction (4.2%) and PRISm (4.6%) groups compared to the normal group (2.6%) (p < 0.001).
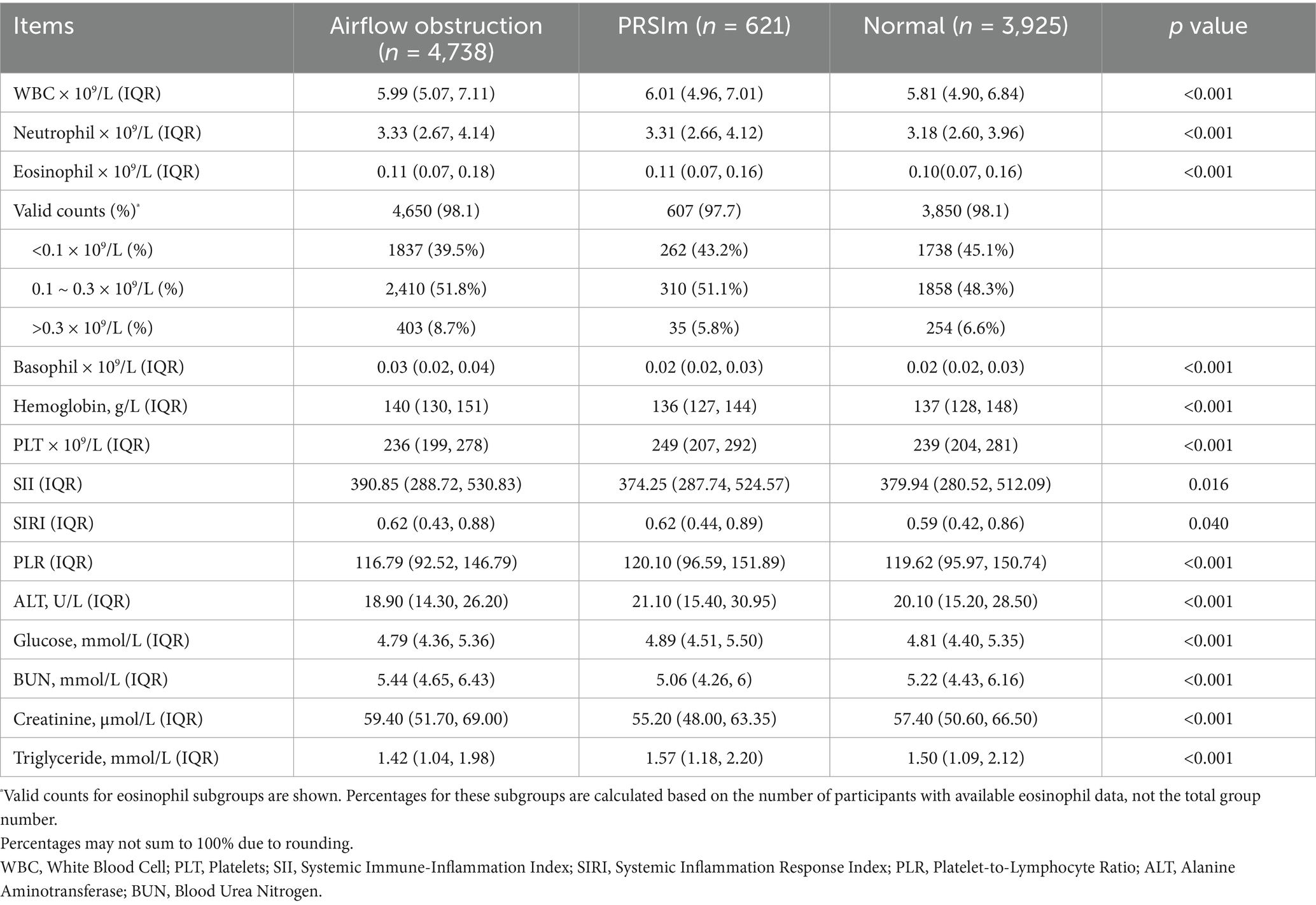
Higher counts of WBC and neutrophils were observed in both the airflow obstruction and PRISm group compared to the normal group. The highest levels of eosinophils and hemoglobin was shown in the airflow obstruction group. While the calculated inflammatory indices were less effective in discriminating between the impaired lung function groups, systemic inflammatory markers, including the NLR and SII, were significantly elevated in the airflow limitation group compared to the normal spirometry group (both p < 0.001). Furthermore, the airflow obstruction group exhibited higher levels of Blood Urea Nitrogen (BUN) and creatinine, and lower levels of Alanine Aminotransferase (ALT) and triglycerides than the other two groups. (Table 2).
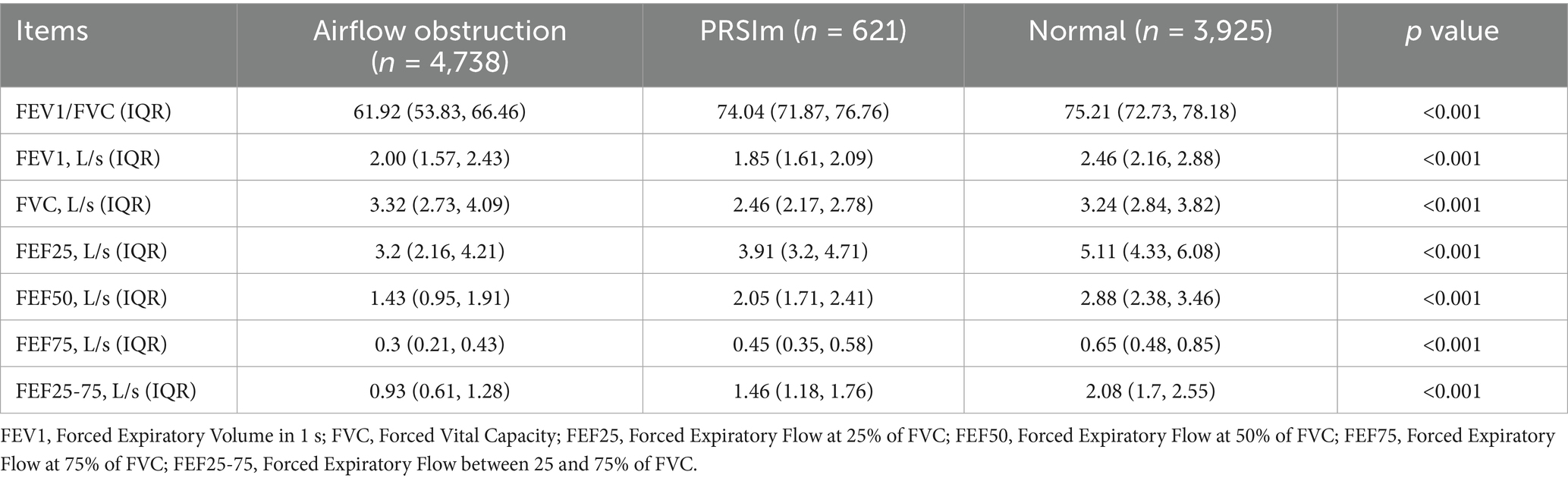
As expected from the group definitions, spirometric parameters showed a gradient of decline from the normal group to the PRISm and airflow obstruction groups. This trend was particularly evident in parameters of small airway function (FEF25, FEF50, and FEF75), which demonstrated a more pronounced stepwise deterioration across the groups. These small airway parameters also exhibited better sensitivity for identifying lung function impairment compared to conventional parameters such as FEV1/FVC, FEV1, and FVC (Table 3).
3.2 Predictive model for subclinical and advanced lung function impairment
To identify meaningful predictive factors for the early and precise recognition of lung function impairment, we combined the airflow obstruction group (representing advanced impairment) and the PRISm group (representing subclinical impairment) into a single “lung function impairment” entity (Group 1). This combined group was then compared against the normal control group (Group 2). The entire cohort was randomly divided into a training set and a validation set at a 70:30 ratio.
Univariate analysis of the training set was shown in Table 4. Group 1 exhibited a higher prevalence of tobacco and dust exposure, increased rates of chronic cough, sputum production, and dyspnea, and a greater proportion of individuals reporting a history of chronic respiratory diseases. Spirometry results revealed FEV1 and small airway functions parameters were distinguishable between Group 1 and Group 2, whereas FVC showed less discriminatory power.
To identify robust predictive factors for lung function impairment, several machine learning algorithms for modeling were employed, including logistic regression, Random Forest (RF), Gradient Boosting Machine (GBM) and XGBoost. The validation set was served as an independent external validation for model performance.
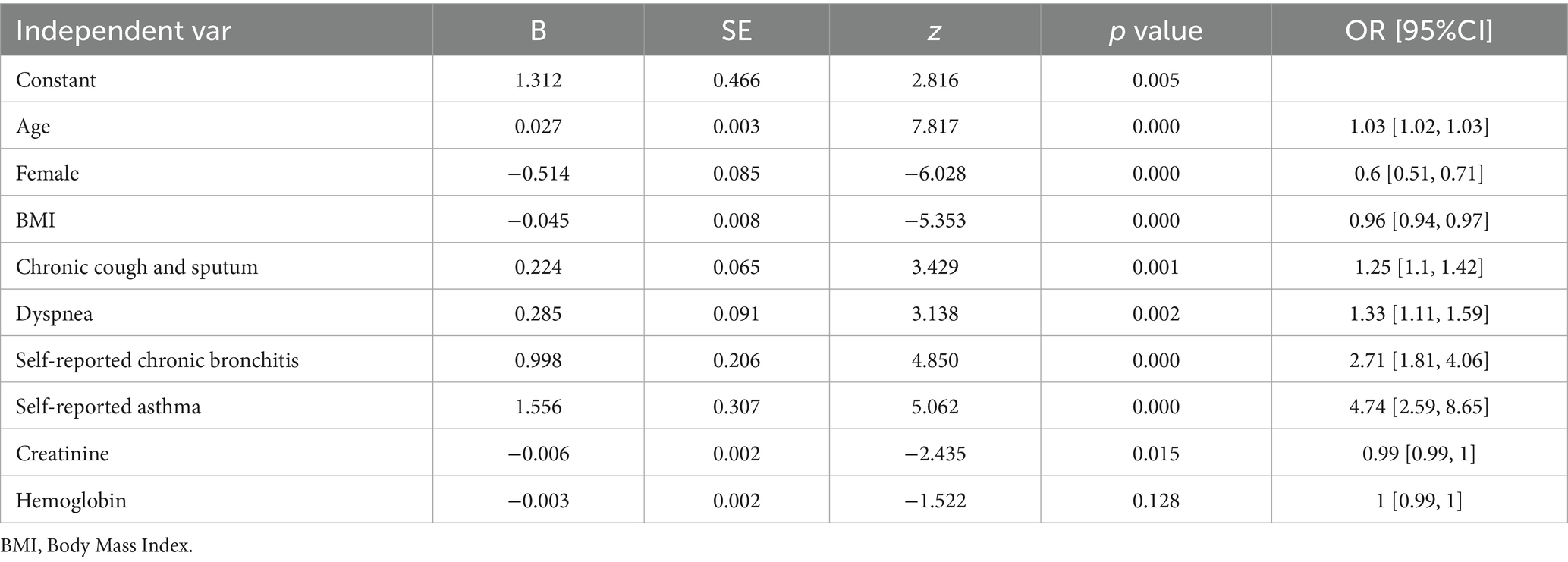
Multivariable logistic regression analysis identified multiple predictive factors, including older age, male sex, lower BMI, chronic cough and sputum, dyspnea, a history of chronic bronchitis and asthma, higher creatinine, and higher hemoglobin level (Table 5; Figure 2). The logistic model was evaluated to be the optimal model rather than RF, GBM and xgb models, with an area under the curve (AUC) of 0.615 in the training set and 0.635 in the validation set (Figure 3).

Figure 2. Predictive factors of lung function impairment by multivariable logistic regression analysis.
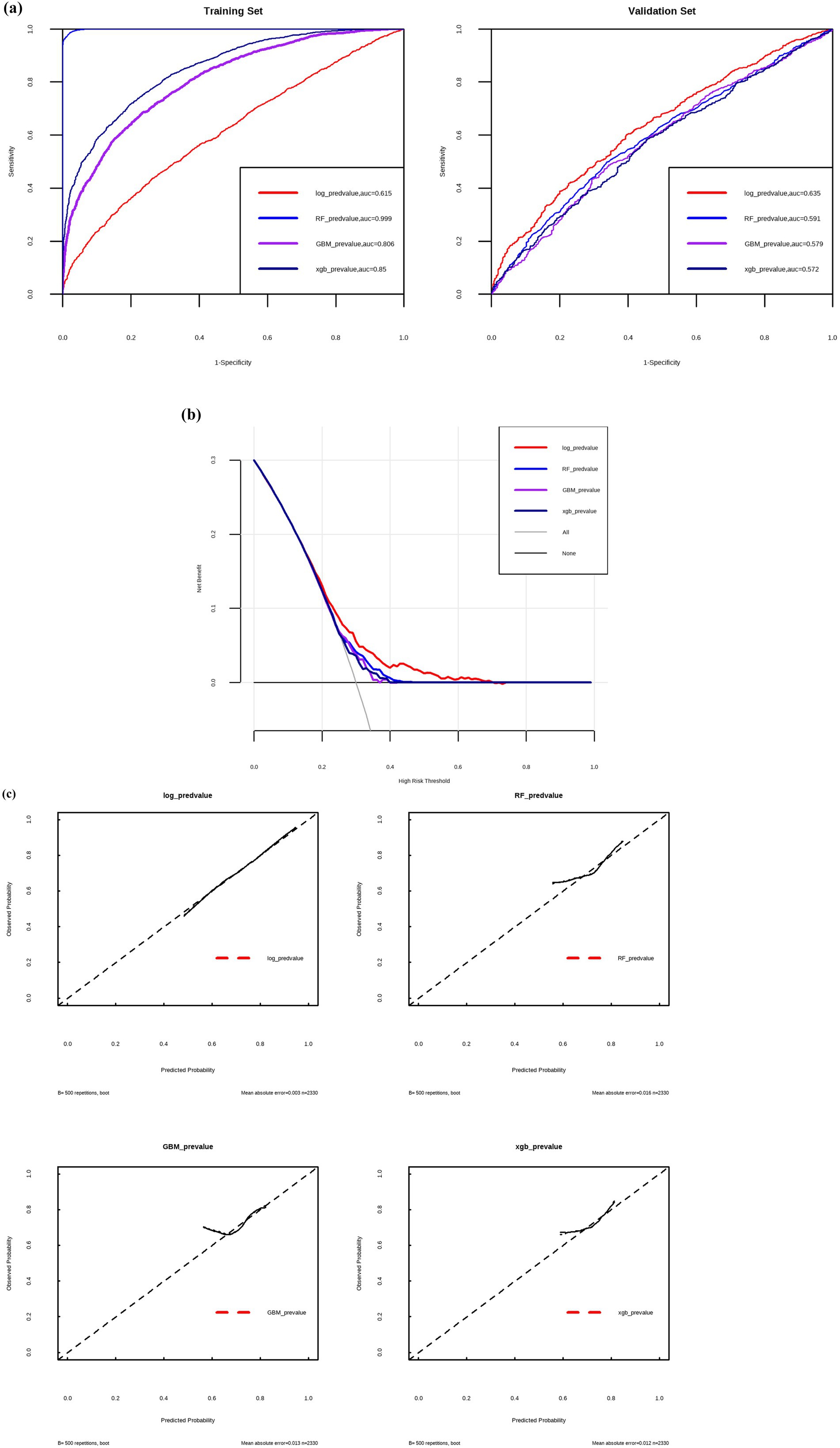
Figure 3. ROC-AUC curves (a), clinical decision curves (b) and calibration curves (c) to evaluate the predictive efficiency by different models. Log, logistic regression; RF, random forest; GBM, gradient boosting machine; xgb, XGBoost.
3.3 Comparison of systemic inflammation among lung function groups
After matching for age, sex and smoking status, we quantified six key cytokines in a sub-cohort consisting of 202 participants with airflow obstruction, 202 participants in PRISm, and 209 participants in normal group (Table 6).

Table 6. Serum cytokine Levels among airflow obstruction, PRISm and normal groups in matched sub-cohorts.
While statistically significant differences were observed for all measured cytokines across the three groups, some did not demonstrate a clear or clinically relevant gradient. Upon pairwise comparison, IL-2 levels showed a significant gradient, with the lowest levels in the airflow obstruction group, intermediate levels in the PRISm group, and the highest levels in the normal group (airflow obstruction < PRISm < Normal). Notably, the levels of IL-5 and IL-17A were significantly elevated in the combined lung function impairment group (Group 1: airflow obstruction and PRISm) compared to the normal control group (Group 2). These findings suggest that IL-2, IL-5 and IL-17A could serve as potential biomarkers for the identification of lung function impairment.
4 Discussion
This large-scale, real-world population screening characterized the distinct clinical, metabolic, and inflammatory phenotypes of airflow obstruction and PRISm in rural China, a region with a significant COPD burden (3). We identified a high prevalence of undiagnosed lung function impairment (51.0% airflow obstruction; 6.7% PRISm), reflecting a profound discrepancy between objective spirometric abnormalities and self-reported disease. Only 0.4% of participants with airflow obstruction reported a prior COPD diagnosis, highlighting the critical need for population-based screening initiatives. The diagnosis of chronic bronchitis, COPD, asthma and bronchiectasis was widely confounded in the underdeveloped regions of China. Therefore, simplified predictive model in our study which could be easily obtained from questionnaire and regular blood tests can prompt the identification of population with airflow limitation. Recommendations for these high-risk or undiagnosed individuals can be made for further spirometry, CT scan and other clinical examinations to define the accurate diagnosis of chronic airway diseases.
Our study provides an in-depth characterization of PRISm as a distinct clinical entity. Unlike the airflow obstruction group, PRISm was more prevalent in females, associated with lower smoking and dust exposure rates, and exhibited symptom burden intermediate to normal and obstructed groups. Notably, PRISm showed a unique comorbidity pattern, including an elevated risk of coronary heart disease and a characteristic atherogenic lipid profile (higher triglycerides), suggesting intrinsic metabolic dysregulation as a potential underlying mechanism that warrants further investigation. In contrast, the airflow obstruction group presented with a classical risk profile tied to smoking and dust exposure, a higher burden of respiratory symptoms, and comorbidities like tuberculosis and cerebrovascular disease.
We identified several readily available clinical and laboratory markers with predictive value for lung function impairment, consistent with some previous literature (11, 23–25). The predictive associations with metabolic (e.g., lower ALT, higher creatinine) and hematological indices (e.g., higher hemoglobin) reinforce the systemic nature of these conditions, suggesting that metabolic dysfunction and chronic hypoxia may be key pathophysiological drivers. Immunologically, individuals with lung function impairment (airflow obstruction or PRISm) exhibited elevated serum IL-5 and IL-17A, alongside lower IL-2 levels. This cytokine profile, coupled with cellular findings (elevated neutrophils and eosinophils in airflow obstruction), suggests the predominance of Th2 and Th17-driven inflammation over Th1 pathways in the pathogenesis of airflow limitation. These findings contribute to the complex and heterogeneous landscape of inflammatory pathways in chronic airway diseases (22, 26–29), highlighting specific targets for future mechanistic studies.
Our study has several limitations. Its cross-sectional design allows for identification of associations and predictive factors, but not causality; longitudinal follow-up is required to confirm true predictors of disease progression. Second, our reliance on pre-bronchodilator spirometry, while pragmatic for large-scale screening and supported by its utility for risk stratification (30), limits our ability to definitively differentiate between reversible (e.g., asthma) and persistent (e.g., COPD) airflow limitation. Third, cytokine measurements were performed on a smaller, matched sub-cohort, which, while controlled for confounders, limited statistical power for inclusion in the overall predictive model.
Future research should focus on validating these distinct phenotypes and the predictive model in diverse independent cohorts, particularly through prospective longitudinal studies. Further investigation is also warranted to elucidate the precise mechanistic roles of serum IL-2, IL-5, and IL-17A in the early pathogenesis of lung function impairment and their potential as prognostic biomarkers or therapeutic targets. Finally, exploring the integration of additional, readily available clinical or biological parameters, and potentially more advanced machine learning approaches, could further enhance the predictive power of such models.
5 Conclusion
In summary, this large-scale, real-world screening study reveals the distinct clinical, metabolic, and inflammatory landscapes of airflow obstruction and PRISm, highlighting a substantial burden of undiagnosed lung function impairment. We established a potential predictive model using simple clinical history and routine laboratory assays that could help identify high-risk individuals for targeted, early spirometric screening. Furthermore, our findings suggest that serum IL-2, IL-5 and IL-17A may serve as biomarkers for airflow limitation, providing a deeper understanding of the immunological mechanisms driving the gradual impairment of lung function. These insights can inform public health strategies aimed at the early detection and management of chronic airway diseases.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
The studies involving humans were approved by Ethical approval was obtained from the Human Research Ethics Committee of Linfen Central Hospital (Approval No. 2021-1-1) on January 18, 2021. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
XM: Data curation, Funding acquisition, Project administration, Writing – original draft, Writing – review & editing, Formal analysis. YY: Validation, Methodology, Data curation, Writing – review & editing. WG: Data curation, Writing – review & editing, Formal Analysis. SG: Writing – review & editing, Supervision, Funding acquisition. ZG: Writing – review & editing, Methodology, Supervision. MJ: Writing – review & editing, Data curation. PL: Data curation, Writing – review & editing. LC: Writing – review & editing, Data curation. CC: Data curation, Writing – review & editing. KM: Data curation, Writing – review & editing. YZ: Writing – review & editing, Data curation. RL: Project administration, Data curation, Methodology, Writing – review & editing. QW: Writing – review & editing, Supervision, Project administration, Methodology.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by Tianjin Key Medical Discipline (Specialty) Construction Project (TJYXZDXK008A). Funded by Shanxi Provincial Clinical Medical Research Center for Respiratory Diseases (COPD) Project (20240410501006). Funded by Linfen Key Research and Development Program (2414).
Acknowledgments
The authors would like to extend their heartfelt gratitude to all individuals and institutions whose contributions were instrumental to the success of this study. First and foremost, we sincerely appreciate the invaluable dedication of our colleagues at Linfen Central Hospital. Their exceptional expertise and unwavering support, particularly during the critical phase of epidemiological investigation, were pivotal to the smooth progression of this research. We deeply acknowledge the trust placed in our work by these institutions, as well as their essential funding that enabled the realization of this study. We are truly indebted to all those who have contributed to this endeavor.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Wang, Z, Lin, J, Liang, L, Huang, F, Yao, X, Peng, K, et al. Global, regional, and national burden of chronic obstructive pulmonary disease and its attributable risk factors from 1990 to 2021: an analysis for the Global Burden of Disease Study 2021. Respir Res. (2025) 26:2. doi: 10.1186/s12931-024-03051-2
2. Singh, D, Han, MK, Hawkins, NM, Hurst, JR, Kocks, JWH, Skolnik, N, et al. Implications of cardiopulmonary risk for the management of COPD: a narrative review. Adv Ther. (2024) 41:2151–67. doi: 10.1007/s12325-024-02855-4
3. Wang, C, Xu, J, Yang, L, Xu, Y, Zhang, X, Bai, C, et al. Prevalence and risk factors of chronic obstructive pulmonary disease in China (the China Pulmonary Health [CPH] study): a national cross-sectional study. Lancet. (2018) 391:1706–17. doi: 10.1016/S0140-6736(18)30841-9
4. Gao, J, and Prasad, N. Chronic obstructive pulmonary disease in China: the potential role of indacaterol. J Thorac Dis. (2013) 5:548–558. doi: 10.3978/j.issn.2072-1439.2013.08.04
5. Miura, S, Iwamoto, H, Omori, K, Yamaguchi, K, Sakamoto, S, Horimasu, Y, et al. Preserved ratio impaired spirometry with or without restrictive spirometric abnormality. Sci Rep. (2023) 13:2988. doi: 10.1038/s41598-023-29922-0
6. Zhang, Y, Li, X, Wang, J, and Chen, L. Associated Factors and Pulmonary Function Outcomes of Preserved Ratio Impaired Spirometry: A Scoping Review. Int J Chron Obstruct Pulmon Dis. (2025) 20:135–148. doi: 10.2147/COPD.S506115
7. Li, M, Chen, M, Li, Y, Liu, Z, Li, X, Lang, X, et al. Association of preserved ratio impaired spirometry with mortality and cardiovascular diseases: a systematic review and meta-analysis. Syst Rev. (2024) 13:147. doi: 10.1186/s13643-024-02549-6
8. Huang, J, Li, W, Sun, Y, Huang, Z, Cong, R, Yu, C, et al. Preserved Ratio Impaired Spirometry (PRISm): A Global Epidemiological Overview, Radiographic Characteristics, Comorbid Associations, and Differentiation from Chronic Obstructive Pulmonary Disease. Int J Chron Obstruct Pulmon Dis. (2024) 19:753–764. doi: 10.2147/COPD.S453086
9. Zhao, NN, and Zhou, YM. Progress in preserved ratio impaired spirometry. Zhonghua Jie He He Hu Xi Za Zhi. (2022) 45:1046–50. doi: 10.3760/cma.j.cn112147-20220518-00426
10. Lai, Y, Yang, T, Zhang, X, and Li, M. Associations between life's essential 8 and preserved ratio impaired spirometry. Sci Rep. (2025) 15:8166. doi: 10.1038/s41598-025-90381-w
11. Wu, J, Wang, G, Gan, J, Yang, L, Zhang, H, Xian, J, et al. Nomogram to predict progression from preserved ratio impaired spirometry to chronic obstructive pulmonary disease. Sci Rep. (2025) 15:10447. doi: 10.1038/s41598-025-93359-w
12. Cadham, CJ, Oh, H, Han, MK, Mannino, D, Cook, S, Meza, R, et al. The prevalence and mortality risks of PRISm and COPD in the United States from NHANES 2007-2012. Respir Res. (2024) 25:208. doi: 10.1186/s12931-024-02841-y
13. Yang, S, Liao, G, and Tse, LA. Association of preserved ratio impaired spirometry with mortality: a systematic review and meta-analysis. Eur Respir Rev. (2023) 32:230135. doi: 10.1183/16000617.0135-2023
14. Jankowski, P, Mycroft, K, Górska, K, Korczyński, P, and Krenke, R. How to enhance the diagnosis of early stages of chronic obstructive pulmonary disease (COPD)? The role of Mobile spirometry in COPD screening and diagnosis-a systematic review. Adv Respir Med. (2024) 92:158–74. doi: 10.3390/arm92020018
15. Wan, ES, Castaldi, PJ, Cho, MH, Hokanson, JE, Regan, EA, Make, BJ, et al. Epidemiology, genetics, and subtyping of preserved ratio impaired spirometry (PRISm) in COPDGene. Respir Res. (2014) 15:89. doi: 10.1186/s12931-014-0089-y
16. Georgopoulos, PG, Brinkerhoff, CJ, Isukapalli, S, Dellarco, M, Landrigan, PJ, and Lioy, PJ. A tiered framework for risk-relevant characterization and ranking of chemical exposures: applications to the National Children's study (NCS). Risk Anal. (2014) 34:1299–316. doi: 10.1111/risa.12165
17. Marrie, RA. Demographic, genetic, and environmental factors that modify disease course. Neurol Clin. (2011) 29:323–41. doi: 10.1016/j.ncl.2010.12.004
18. Amirthalingam, P, Alatawi, Y, Chellamani, N, Shanmuganathan, M, Ali, MAS, Alqifari, SF, et al. Sea horse optimization-deep neural network: a medication adherence monitoring system based on hand gesture recognition. Sensors (Basel). (2024) 24:5224. doi: 10.3390/s24165224
19. Ramasundaram, M, Sohn, H, and Madhavan, T. A bird's-eye view of the biological mechanism and machine learning prediction approaches for cell-penetrating peptides. Front Artif Intell. (2024) 7:1497307. doi: 10.3389/frai.2024.1497307
20. Norris, KA, Morris, A, Patil, S, and Fernandes, E. Pneumocystis colonization, airway inflammation, and pulmonary function decline in acquired immunodeficiency syndrome. Immunol Res. (2006) 36:175–88. doi: 10.1385/IR:36:1:175
21. Tashkin, DP, and Wechsler, ME. Role of eosinophils in airway inflammation of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. (2018) 13:335–49. doi: 10.2147/COPD.S152291
22. Jesus, FR, Moraes, ACS, da Silva, ILN, Passos, FC, Salles, C, Neves, MCLC, et al. Analysis of endocrine and inflammatory markers in preserved ratio impaired spirometry. Med Sci (Basel). (2024) 12:18. doi: 10.3390/medsci12020018
23. Divo, MJ, Liu, C, Polverino, F, Castaldi, PJ, Celli, BR, and Tesfaigzi, Y. From pre-COPD to COPD: a simple, low cost and easy to IMplement (SLIM) risk calculator. Eur Respir J. (2023) 62:2300806. doi: 10.1183/13993003.00806-2023
24. Hou, X, and Ran, P. Development and validation a nomogram to predict long-term mortality risks of PRISm and mild-to-moderate COPD based on NHANES 2007-2012. Sci Rep. (2025) 15:16000. doi: 10.1038/s41598-025-94399-y
25. Du, W, Guan, H, Wan, X, et al. Circulating liver function markers and the risk of COPD in the UK biobank. Front Endocrinol (Lausanne). (2023) 14:1121900. doi: 10.3389/fendo.2023.1121900
26. Agustí, AG, Noguera, A, Sauleda, J, Sala, E, Pons, J, and Busquets, X. Systemic effects of chronic obstructive pulmonary disease. Eur Respir J. (2003) 21:347–60. doi: 10.1183/09031936.03.00405703
27. Bożek, A, and Rogala, B. IgE-dependent sensitization in patients with COPD. Ann Agric Environ Med. (2018) 25:417–20. doi: 10.26444/aaem/83413
28. Starkey, MR, Plank, MW, Casolari, P, Papi, A, Pavlidis, S, Guo, Y, et al. IL-22 and its receptors are increased in human and experimental COPD and contribute to pathogenesis. Eur Respir J. (2019) 54:1800174. doi: 10.1183/13993003.00174-2018
29. Zou, Y, Chen, X, Liu, J, Zhou, D, Kuang, X, Xiao, J, et al. Serum IL-1β and IL-17 levels in patients with COPD: associations with clinical parameters. Int J Chron Obstruct Pulmon Dis. (2017) 12:1247–54. doi: 10.2147/COPD.S131877
30. Çolak, Y, Lange, P, Vestbo, J, Nordestgaard, BG, and Afzal, S. Susceptible young adults and development of chronic obstructive pulmonary disease later in life. Am J Respir Crit Care Med. (2024) 210:607–17. doi: 10.1164/rccm.202308-1452OC
Glossary
ALT - Alanine Aminotransferase
BMI - Body Mass Index
BUN - Blood Urea Nitrogen
CHD - Coronary heart disease
COPD - Chronic Obstructive Pulmonary Disease
FEF25 - Forced Expiratory Flow at 25% of FVC
FEF50 - Forced Expiratory Flow at 50% of FVC
FEF75 - Forced Expiratory Flow at 75% of FVC
FEF25–75 - Forced Expiratory Flow between 25 and 75% of FVC
FET - Forced expiratory time
FEV1 - Forced expiratory volume in the first 1.0 s
FVC - Forced vital capacity
IL-2 - Interleukin-2
IL-4 - Interleukin-4
IL-5 - Interleukin-5
IL-10 - Interleukin-10
IL-17A - Interleukin-17A
IL-22 - Interleukin-22
ML - Machine Learning
NER - Neutrophil-to-eosinophil ratio
NLR - Neutrophil-to-lymphocyte ratio
PLR - Platelet-to-lymphocyte ratio
PLT - Platelets
PRISm - Preserved Ratio Impaired Spirometry
SIRI - Systemic inflammation response index
SII - Systemic immune-inflammation index
TB - Tuberculosis
WBC - White blood cells
Keywords: lung function impairment, preserved ratio impaired spirometry (PRISm), predictive model, population screening, early diagnosis, inflammatory biomarkers
Citation: Ma X, Yu Y, Guan W, Guo S, Gao Z, Jin M, Liu P, Cheng L, Chen C, Ma K, Zhou Y, Li R and Wu Q (2025) Relationship between lung function impairment, clinical characteristics and systemic inflammation based on a large-scale population screening. Front. Med. 12:1657151. doi: 10.3389/fmed.2025.1657151
Edited by:
Liang Zhao, Dalian University of Technology, ChinaReviewed by:
Hiroya Ohta, Hokkaido University of Science, JapanDa-Wei Wu, Kaohsiung Medical University, Taiwan
Hitesh Singh Chaouhan, National Institute of Neurological Disorders and Stroke (NIH), United States
Copyright © 2025 Ma, Yu, Guan, Guo, Gao, Jin, Liu, Cheng, Chen, Ma, Zhou, Li and Wu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ran Li, bGlyYW4yMDA0QGJqbXUuZWR1LmNu; Qi Wu, d3E1NzIwMDRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Xiaojun Ma
Xiaojun Ma Yan Yu
Yan Yu Wenxia Guan2†
Wenxia Guan2† Shuming Guo
Shuming Guo Zhancheng Gao
Zhancheng Gao Ran Li
Ran Li Qi Wu
Qi Wu