- 1School of Medicine, RCSI – Medical University of Bahrain, Al Sayh, Bahrain
- 2Department of Surgery, Yale, CT, United States
- 3Department of Biochemistry, School of Medicine, RCSI – Medical University of Bahrain, Al Sayh, Bahrain
Background: Vitamin D deficiency, colorectal cancer, and tumor progression are increasingly linked in recent research. Beyond its well-established roles in bone metabolism and immune regulation, vitamin D has emerged as a potential modulator of cancer prevention and prognosis, particularly in colorectal cancer, where deficiency may worsen outcomes.
Purpose: Vitamin D is critical in the prevention and prognosis of colorectal cancer, such as colorectal adenocarcinoma. This review aims to explore the impact of Vitamin D deficiency on colorectal cancer progression and assess the role of vitamin D supplementation in improving outcomes.
Methods: A narrative review was conducted, utilizing five databases: PubMed, Medline Plus, ScienceDirect, Scopus, and Google Scholar, focusing on human studies published in the last 15 years (from 2012 to 2025). Priority was given to primary studies like randomized controlled trials and cohort studies, while systematic reviews were included for broader context. Exclusion criteria included animal studies, non-English papers, and non-peer-reviewed content.
Results: The review synthesizes evidence from 33 primary studies and 16 high-quality reviews. Findings indicate that vitamin D supplementation may enhance prognosis by influencing serum levels, immune modulation, and gut microbiota. However, clinical trials results are mixed, particularly concerning optimal dosing, genetic variability, and factors like obesity.
Discussion: Vitamin D supplementation shows promise in improving colorectal cancer prognosis, but further research is necessary to refine dosing strategies and develop personalized therapies tailored to individual patient needs.
1 Introduction
Vitamin D is a fat-soluble vitamin known for its roles in calcium homeostasis, immune function, and cellular regulation. Deficiency in vitamin D is a widespread global issue affecting approximately 1 billion people globally and has been linked to various chronic diseases (1). Up to 90% of active vitamin D (1,25-(OH)₂D₃) is synthesized endogenously through cutaneous exposure to ultraviolet B (UVB) radiation, while dietary sources and supplements contribute a much smaller share (2). In recent years, the role of Vitamin D in the pathophysiology and prognosis of colorectal cancer (CRC) has garnered increasing attention, particularly due to its involvement in processes such as inflammation, cell proliferation, and apoptosis.
While early detection and therapeutic interventions have improved outcomes, prognosis in advanced CRC disease remains poor. To tackle that, there has been a growing interest in understanding how micronutrients like vitamin D might potentially influence cancer progression and therapeutic response.
This review aims to explore the current body of primary evidence and reviews regarding vitamin D supplementation and its effects on CRC outcomes. We examine proposed biological mechanisms, clinical trial results, and potential confounders such as obesity and genetic factors. The objective is to analyze data presented in original research studies and some reviews to clarify the prognostic significance of vitamin D in CRC.
2 Methods
This review is a narrative synthesis of existing literature examining the relationships between vitamin D supplementation, obesity, and colorectal cancer (CRC) outcomes. We searched five databases—PubMed, MedlinePlus, ScienceDirect, Scopus, and Google Scholar—for human studies published in English over the past 15 years (from 2012 to 2025). Keywords included combinations of “vitamin D,” “colorectal cancer,” “supplementation,” “obesity,” and “VDR polymorphisms.” Priority was given to clinical studies (e.g., randomized controlled trials, cohort studies, case–control) and high-quality systematic reviews or meta-analyses.
We predominantly excluded non-peer-reviewed articles, non-English publications, animal studies, and studies lacking relevance to the clinical aspects of vitamin D in CRC. Based on thematic relevance rather than formal screening, a total of 41 studies were included: 18 primary studies and 23 systematic reviews/meta-analyses. See Table 1 for a systematic summary of the primary studies in our review.
Two included articles—Reference (3) (animal study) and Reference (4) (commentary)—were retained despite not meeting inclusion criteria due to their conceptual and mechanistic relevance. Additionally, eight AI-related papers were removed after a corresponding section was excluded from the manuscript during revision.
The selection process is illustrated in a PRISMA-style flow diagram to enhance transparency, even though the review did not follow a formal systematic protocol (Figure 1).
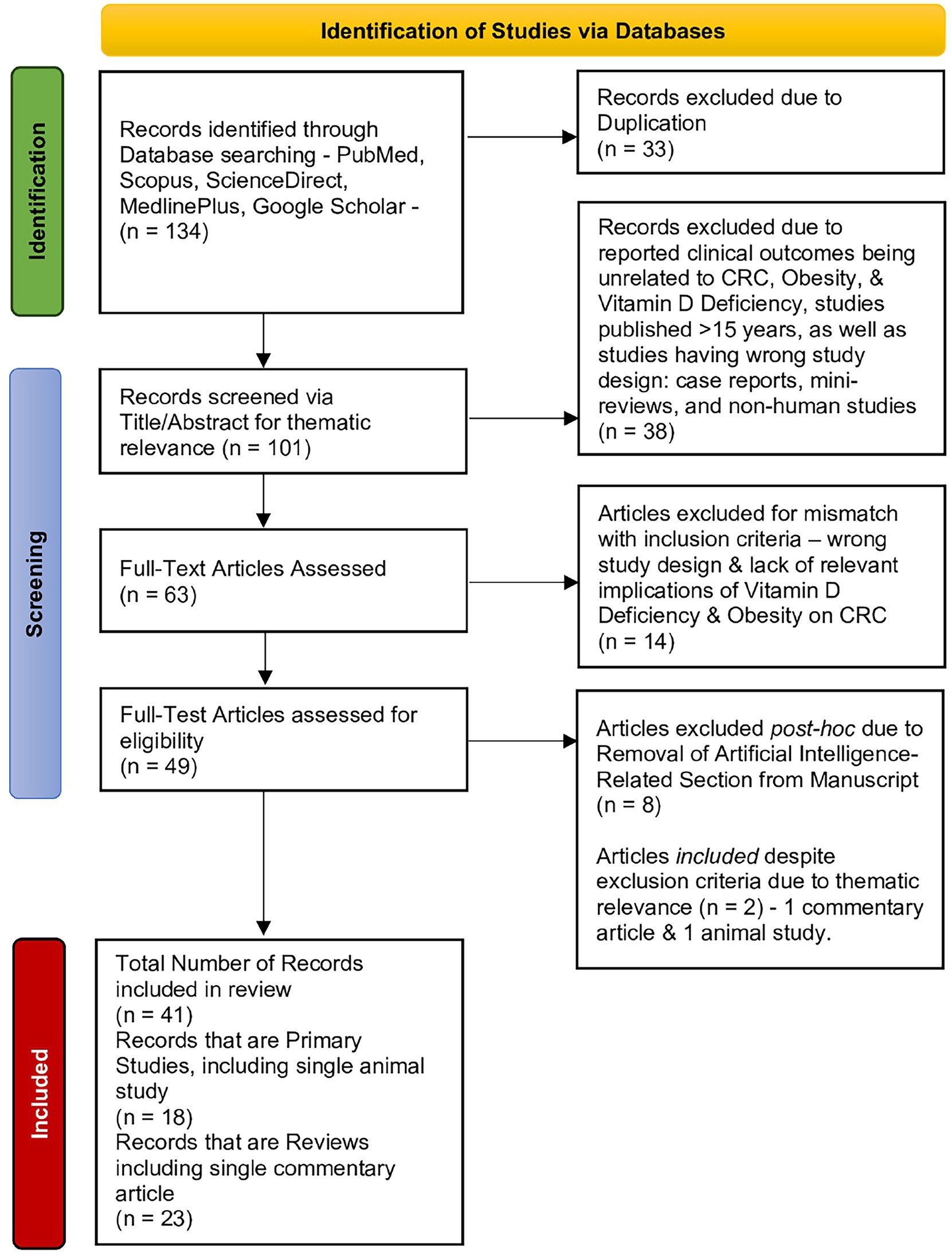
Figure 1. PRISMA flowchart illustrating the literature screening and inclusion process for studies addressing the interrelations among vitamin D deficiency, colorectal cancer outcomes, and obesity. Out of 134 initial records, 41 were included in the final review (18 primary studies and 23 reviews).
3 Mechanisms of vitamin D in colorectal carcinogenesis and progression
Upon entering the bloodstream, vitamin D is hydroxylated in the liver into 25-hydroxyvitamin D (calcidiol), the primary circulating form of vitamin D. This process is initiated primarily by cholecalciferol (vitamin D₃), which is generated in the skin upon exposure to UVB radiation. As shown in Figure 2, the 1α-hydroxylase enzyme in the kidney is responsible for further hydroxylation of 25-(OH) D3 into its active form 1,25-(OH)2D3 (calcitriol), which exerts its effects by binding to vitamin D receptors (VDR). As depicted in Figure 3, the VDR induces heterodimer complex formation with retinoid X receptor (RXR), the complex subsequently binds to vitamin D response elements (VDREs) in the promoter regions of target genes, regulating its transcription (5).
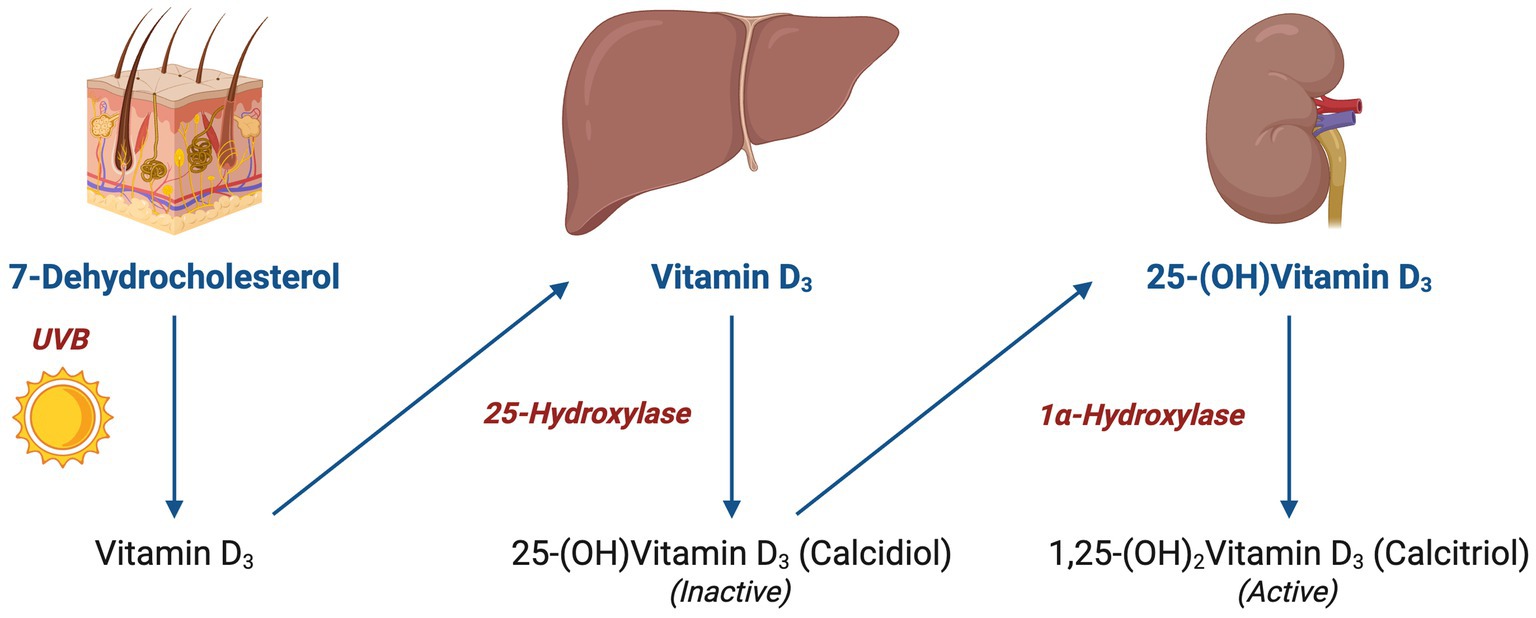
Figure 2. Vitamin D hydroxylation and conversion: illustration of vitamin D hydroxylation pathway, starting from 7-dehydrocholesterol in the skin to the formation of active 1,25-(OH)₂Vitamin D₃ via hepatic and renal conversion.
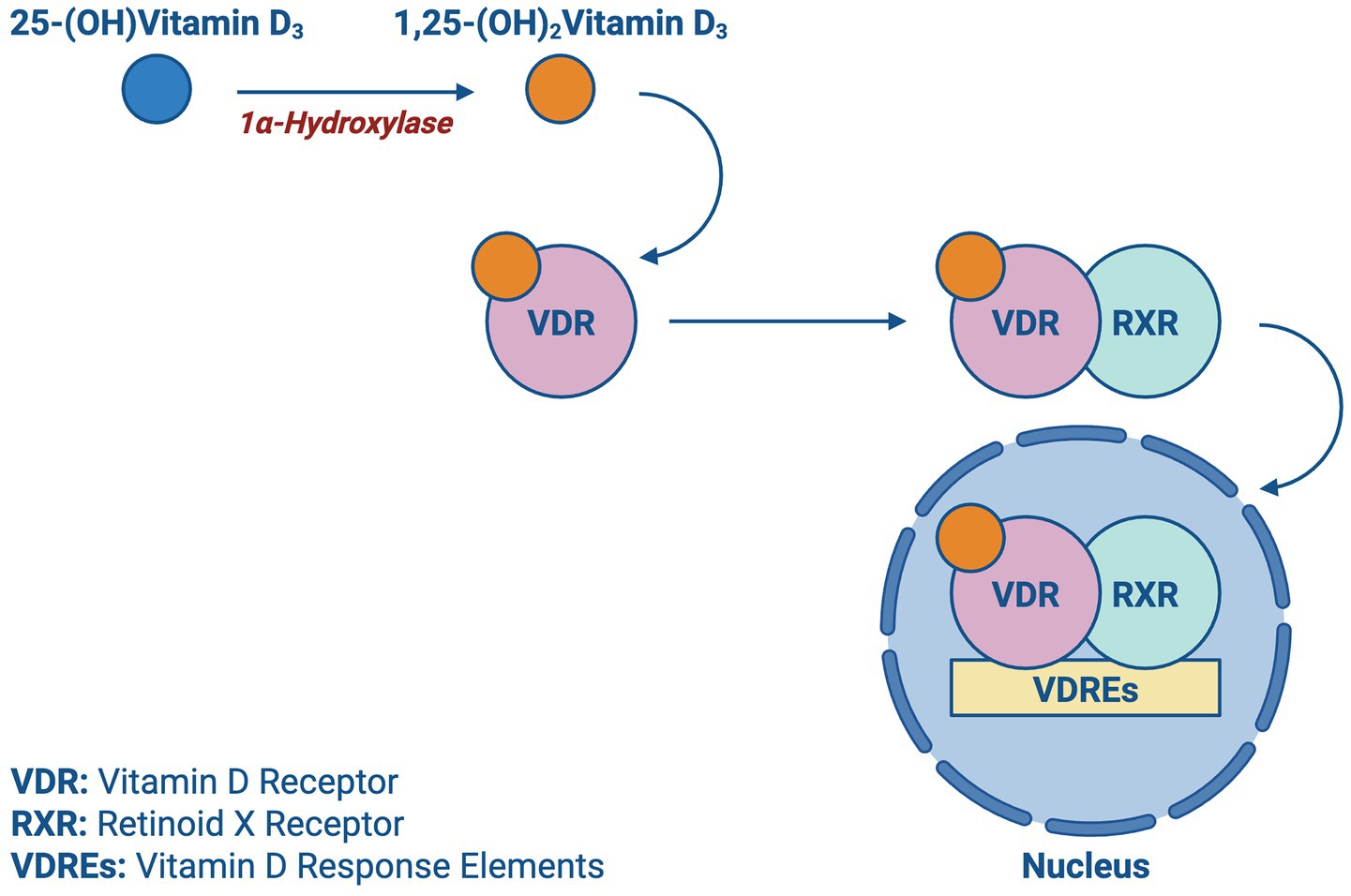
Figure 3. Vitamin D transcription: a schematic showing the activation of vitamin D receptor (VDR) by calcitriol and its subsequent nuclear translocation and interaction with vitamin D response elements (VDREs).
As shown in Figure 4, vitamin D inhibits cell proliferation while promoting differentiation as it influences the gene expression of various proteins involved in cell cycle regulation, such as cyclins and cyclin-dependent kinases, and CDK inhibitors, causing cell cycle arrest in the G1/S phase. Furthermore, vitamin D has been shown to induce apoptotic responses in cancer through the upregulation of pro-apoptotic factors like BAX and the downregulation of anti-apoptotic proteins such as BCL-2 (5).
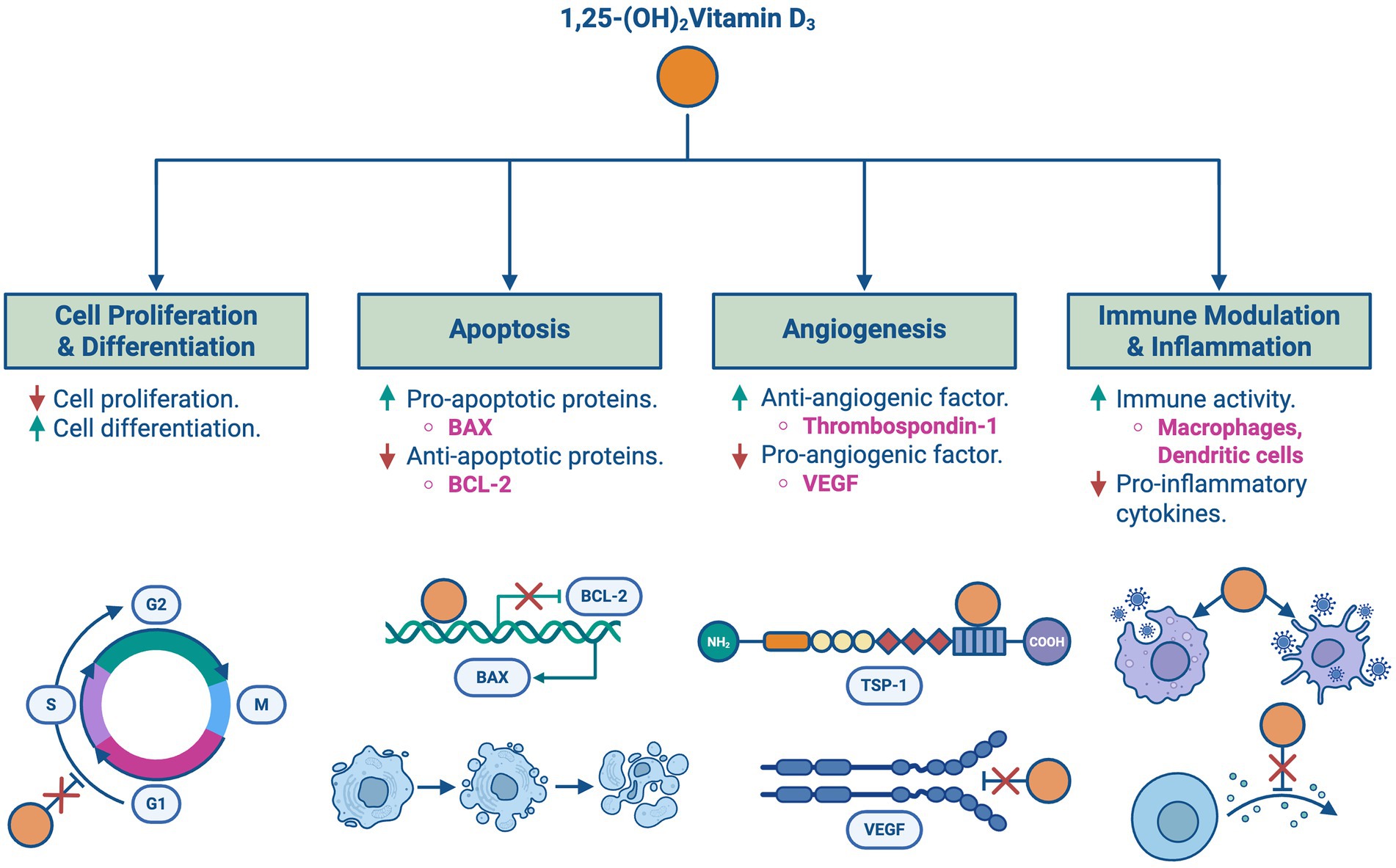
Figure 4. Vitamin D’s roles in cancer regulation: an overview of the key roles of 1,25-(OH)₂Vitamin D₃ in cancer regulation, including effects on cell proliferation, apoptosis, angiogenesis, and immune modulation. (A) Inhibits cell proliferation and promotes differentiation via regulation of cyclins and CDK inhibitors. (B) Induces apoptosis by upregulating pro-apoptotic factors such as BAX and downregulating anti-apoptotic proteins like BCL-2. (C) Inhibits angiogenesis by downregulating VEGF and increasing anti-angiogenic factors such as thrombospondin-1. (D) Modulates immune responses by enhancing macrophage and dendritic cell activity while suppressing pro-inflammatory cytokines.
Angiogenesis is an essential process for tumor growth and spread. Vitamin D downregulates the expression of pro-angiogenic factors such as vascular endothelial growth factor (VEGF) and upregulates the expression of anti-angiogenic factors including thrombospondin-1, a tissue inhibitor of metalloproteinases. Additionally, vitamin D is involved in modulating immune responses and inflammation, which is crucial in controlling cancer progression through upregulating the activity of immune cells such as macrophages and dendritic cells and suppressing pro-inflammatory cytokines (5).
The pathophysiological role of vitamin D in colorectal cancer (CRC) is supported by substantial evidence. Several epidemiological studies have demonstrated that lower serum levels of 25(OH)D₃ are associated with an increased risk of CRC, with a reported hazard ratio (HR) of 1.62 (95% CI: 1.04–2.53) (6). This inverse relationship is thought to be mediated through the mechanisms of cell differentiation, apoptosis, and proliferation.
Increased risk of colorectal neoplasms, including adenomatous polyps, has been linked to vitamin D deficiency. A cross-sectional study conducted by Zamora-Ros et al. (2023) highlighted that low serum 25(OH)D₃ levels were significantly associated with the presence of colorectal polyps (CRPs), particularly in individuals aged 50 to 64 years (OR = 1.81, 95% CI: 1.12–2.92, p = 0.016) (7). Vitamin D deficiency, combined with metabolic disorders such as hyperglycemia and elevated triglycerides, further increases the risk of developing colorectal polyps (CRPs). The overall odds ratio (OR) of metabolic syndrome (MetS) for CRPs was 2.50 (95% confidence interval [CI] = 1.95–3.21) (7).
Genetic variations in the VDR gene have been associated with a higher risk of developing CRC as well. A study conducted by Lv et al. (2021) demonstrated that polymorphisms in the VDR gene affect its functional ability to regulate target genes involved in cell growth and differentiation, as certain VDR gene polymorphisms were associated with an increased susceptibility to CRC, emphasizing the significance of vitamin D signaling pathway in colorectal carcinogenesis (8).
Emerging evidence also suggests that vitamin D may exert its protective effects through modulation of the gut microbiome. Gut microbiotas play a critical role in colorectal homeostasis by regulating immune tolerance, epithelial integrity, and inflammatory signaling functions that overlap with vitamin D’s known actions. Vitamin D influences microbiota composition via its receptor (VDR), bolstering the growth of beneficial microbial taxa and suppressing dysbiotic profiles associated with tumorigenesis. These effects are particularly relevant in CRC, where dysbiosis contributes to barrier dysfunction and chronic mucosal inflammation, thereby facilitating carcinogenic progression (4).
Moreover, vitamin D enhances the structural integrity of the gut barrier by upregulating the expression of tight junction proteins such as claudins and occludins, thus reducing intestinal permeability and bacterial translocation. It also regulates the local immune environment by modulating cytokine production and promoting regulatory T cell (Treg) responses, which limit chronic inflammation in the colonic mucosa. This interplay between vitamin D, microbiota, and mucosal immunity has shown promising results in preclinical and translational studies, highlighting a potential adjunctive role of vitamin D in microbiota-targeted cancer therapy (9).
4 Serum vitamin D status and prognostic outcomes in colorectal cancer
Vitamin D deficiency may influence the progression and prognosis of CRC as adequate serum 25-(OH)D3 levels have been associated with improved outcomes. For instance, in a prospective cohort study by Wesselink et al. (2020), CRC patients with both sufficient serum 25-(OH)D3 concentrations (≥50 nmol/L) and high dietary magnesium intake (≥322 mg/d or ≥383 mg/d depending on cohort) had a 47% lower risk of all-cause mortality (Hazard Ratio: 0.53; 95% CI: 0.31–0.89) compared to those with deficient vitamin D and low magnesium intake. Furthermore, within the subgroup of patients with sufficient vitamin D, high magnesium intake was associated with a 57% reduction in mortality (HR: 0.43; 95% CI: 0.25–0.76). These findings suggest two key points: reduced vitamin D concentrations seem to be associated with a higher risk of all-cause mortality in CRC patients, and the data points at a possible synergistic role between Vitamin D status and magnesium intake in CRC prognosis (given that magnesium is essential in the conversion of 25-(OH)D3 to the active form of vitamin D).
These findings align with a large-scale international pooling project by Weinstein et al. (2018), which analyzed data from 17 cohorts that tested 5,706 CRC case participants and 7,107 control participants with a wide range of circulating 25-(OH)D3 concentrations and confirmed an inverse association between vitamin D levels and CRC risk, as individuals that had 25-(OH)D3 deficiency had a 31% higher risk of CRC, particularly among women and those with initially low vitamin D levels (10). This extensive study underscores the importance of maintaining sufficient vitamin D levels as a potential preventive measure against CRC. Other studies, such as the population-based study by Keum et al. (2019), further demonstrate that individuals with vitamin D deficiency have a significantly increased risk of developing CRC, even after accounting for various confounders such as age, sex, lifestyle, and other health conditions. Based on this, the association between low vitamin D levels and increased CRC risk is significant, therefore suggesting that vitamin D deficiency is an independent risk factor for CRC (11).
Geographic location, skin pigmentation, and dietary habits are also significant determinants of vitamin D status. Individuals residing at higher altitudes with less sunlight exposure are more likely to experience vitamin D deficiency due to reduced opportunities for skin synthesis of vitamin D. Similarly, people with darker skin pigmentation have higher melanin levels, which can reduce the skin’s ability to produce vitamin D in response to sunlight. Dietary habits also play a crucial role, as diets low in vitamin D-rich foods, such as fatty fish, fortified dairy products, and egg yolks can contribute to deficiency (12).
Collectively, these studies provide compelling evidence for the multifaceted role of vitamin D in reducing the risk of CRC while also highlighting the importance of environmental, genetic, and lifestyle factors when assessing vitamin D status.
5 Obesity, vitamin D, and colorectal cancer prognosis
As shown in Figure 5, obesity has been shown to affect serum 25-(OH)D3 levels and CRC prognosis, with some studies reporting a direct relationship between the three. Obesity affects vitamin D metabolism by increasing its storage in subcutaneous fat, leading to circulating deficiency. One case–control study by Väyrynen, J. P. et al. (2016) reviewing 25-(OH)D3 deficiency and prognosis in CRC patients concluded CRC patients with BMI > 30 have serum 25-(OH)D3 deficiency compared to those with a BMI ≤ 30. This implies that patients with a greater BMI are likely to have lower serum 25-(OH)D3 levels (13).
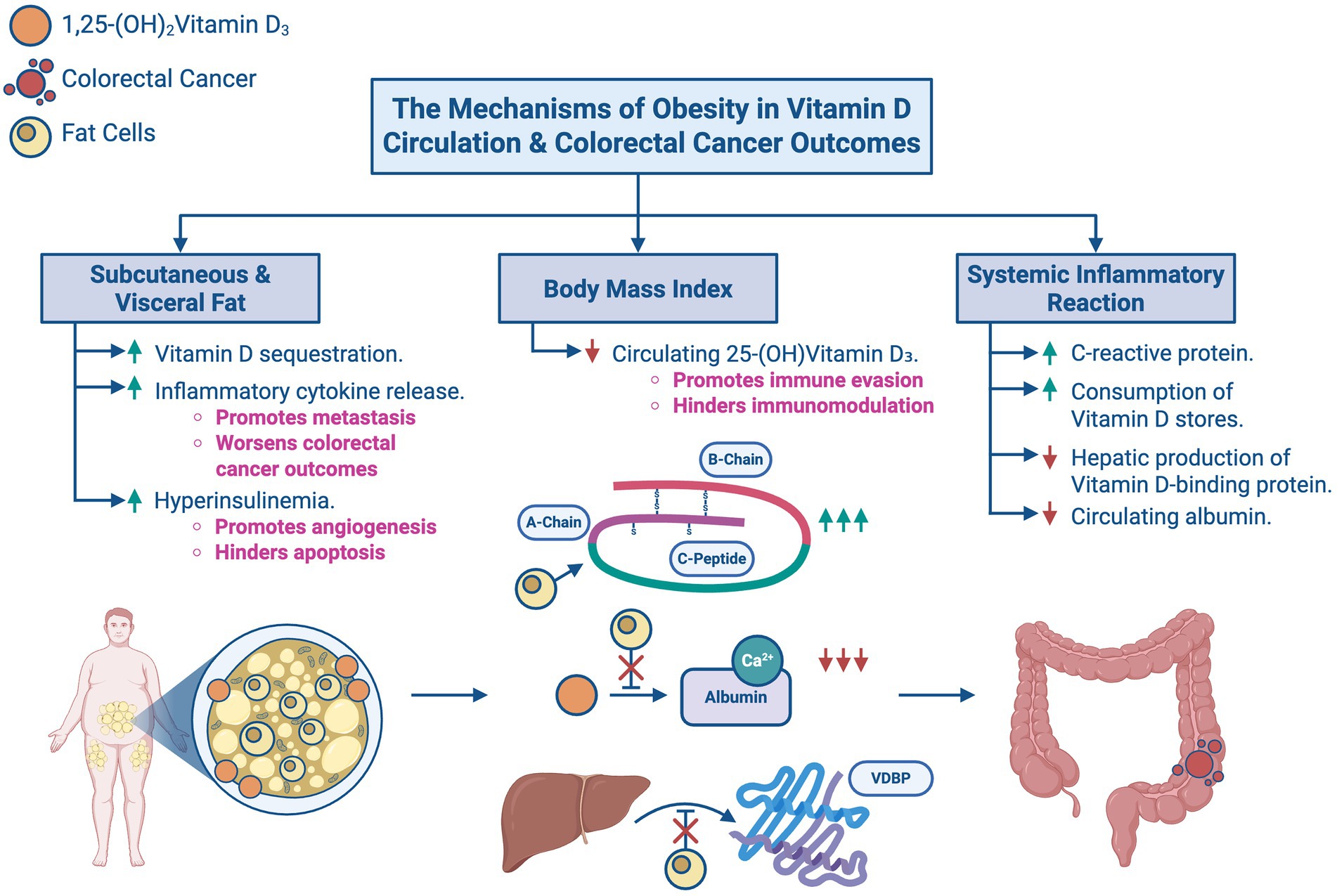
Figure 5. The mechanisms of obesity in Vitamin D circulation and colorectal cancer outcomes: an overview of how obesity modulates Vitamin D levels and influences colorectal cancer outcomes. (A) Subcutaneous and visceral fat promotes Vitamin D sequestration, inflammatory cytokine release, and hyperinsulinemia. (B) Increased body mass index reduces circulating 25-(OH)Vitamin D3, thereby promoting immune evasion and hindering immunomodulation. (C) Systemic inflammatory reaction causes an increase in CRP and consumes Vitamin D stores while reducing hepatic production of Vitamin D-binding protein (VDBP) and circulating albumin.
However, it is worthwhile to note that the same case–control study hinted that the effects of BMI on serum 25-(OH)D3 levels may have been confounded by systemic inflammatory responses. These responses control metastasis and thereby influence the association between 25-(OH)D3 concentrations and CRC via the immunomodulatory and immunosuppressive functions of vitamin D19 and proinflammatory cytokines released due to cancer progression. These suppress the hepatic production of vitamin D carrier proteins resulting in the redistribution or consumption of vitamin D storages. In other words, high systemic inflammatory responses to CRC do not only reduce serum albumin levels and increase serum CRP levels, but they can also potentiate vitamin D deficiency, eliciting a worse prognosis. Furthermore, a multivariate analysis by Conway and McMillan perceived that systemic inflammatory responses had a higher association with low serum 25-(OH)D3 levels than high BMI (13).
A meta-analysis by Pereira-Santos, M. et al. (2015) focused on the relationship between 25-(OH)D3 deficiency and body measurements and discovered that obese individuals have prevalence ratio (PR) of 35% for vitamin D deficiency compared to normal-weight individuals. In the subgroup analysis, obese children and adolescents had a 37% PR, while obese adults had a 33% PR of vitamin D deficiency (14). These findings conclude that obesity is associated with a higher prevalence of vitamin D deficiency, therefore implying that obesity has an indirect effect on CRC prevalence.
Regarding the combined impact of BMI and Obesity, a study by Budny A. et al. (2019) found that increased visceral adipose tissue levels are associated with hyperinsulinemia, another risk factor for CRC (15). Further emphasizing the effects of hyperinsulinemia, a study by Zhang A. M. Y. et al. (2021) found that the percentage of cancer cases associated with obesity and diabetes increased by 20 and 30%, respectively, between 1980 and 2002. Notably, the same study stated that while obesity and diabetes contribute to hyperinsulinemia, hyperinsulinemia itself can also act as an independent factor for cancer risk. These findings underscore the complexity of the relationship and highlight the importance of identifying the underlying mechanisms that link cancer with obesity and diabetes (16).
Further investigating the potential benefits of weight management and vitamin D supplementation in improving CRC outcomes, a study by Wesselink, E. et al. (2020) explaining vitamin D’s role in CRC outcomes, suggested improved survival rates with increased 25-(OH)D3 levels that can be adjusted through dietary and lifestyle modifications, including moderate-to-vigorous outdoor activity during solar noon, vitamin D supplementation, and calcium intake. However, 25-(OH)D3 levels can change over time due to dietary adjustments after diagnosis or treatment. Median 25-(OH)D3 levels decreased over time in CRC patients undergoing surgery or chemotherapy. To counter that, vitamin D supplements could be administered, whereas supplementation at 6 months post-diagnosis results in a 4 nmol/L less reduction in vitamin D levels compared to non-users. Alcohol consumption should be taken into consideration as a confounder as it may also affect serum levels (17).
In addition, a meta-analysis by Boughanem, H. et al. (2021) evaluated 47,540 cases and 70,567 controls in case–control studies and discovered a 25% reduced risk of CRC when comparing the highest to the lowest dietary vitamin D consumption. The meta-analysis also examined 14,676 CRC-incident cases in prospective cohorts from 16 countries, but they did not show any significant associations thus further analytic confirmation is required (18).
6 Therapeutic implications of vitamin D supplementation
Several randomized controlled trials (RCTs) that examined the effectiveness of vitamin D supplementation in CRC exhibited no significant reduction of ≥15% in relative risk of cancer or ≥10% in cancer mortality with the provision of vitamin D supplementation. These analyses also did not differentiate between the effects of calcium and vitamin D on CRC prognosis (19).
Furthermore, the Keum et al. (2019) study, which evaluated RCTs on vitamin D supplementation and total cancer rates, supports the prior meta-analyses by Beatriz, G. et al. However, this study has limitations—such as low dosing, mixed populations, and limited follow-up (20). Therefore, instead of relying solely on these secondary analyses, we synthesized CRC-specific data: Vaughan-Shaw et al. (2020) observed a 30% improvement in survival, while Bellerba et al. (2022) found reduced recurrence with daily supplementation. These findings suggest potential therapeutic benefit in CRC when higher or consistent dosing is applied.
Conversely, a meta-analysis by Keum et al. (2014) reported no statistically significant association between vitamin D supplementation and overall cancer incidence. Supporting this, a 2022 meta-analysis of RCTs by Emmanouilidou G. et al. found that vitamin D supplementation did not appear to play a chemopreventive role in colorectal neoplasms. Nonetheless, both reviews acknowledged the limitations in existing studies—particularly the use of low vitamin D doses (≤1,100 IU/day) and small sample sizes—highlighting the need for further investigation in this area (19). Interestingly, while these studies failed to establish a role for vitamin D in reducing cancer incidence, some observed modest but notable reductions in cancer-related mortality (see Table 2 for summarized findings from major studies). For instance, more recent data suggest that daily vitamin D supplementation may reduce cancer mortality by approximately 13%, with a relative risk (RR) of 0.87 (95% CI: 0.78–0.96), although this effect did not vary significantly across different subgroups (20). Moreover, the initial Keum meta-analysis also reported a 7% reduction in overall mortality, of which one-third of deaths were attributed to cancer. While these findings are not definitive, they suggest a potential survival benefit, particularly when consistent, higher-dose regimens are utilized—underscoring the necessity for larger, well-designed trials to clarify vitamin D’s therapeutic role in CRC prevention and prognosis (20).
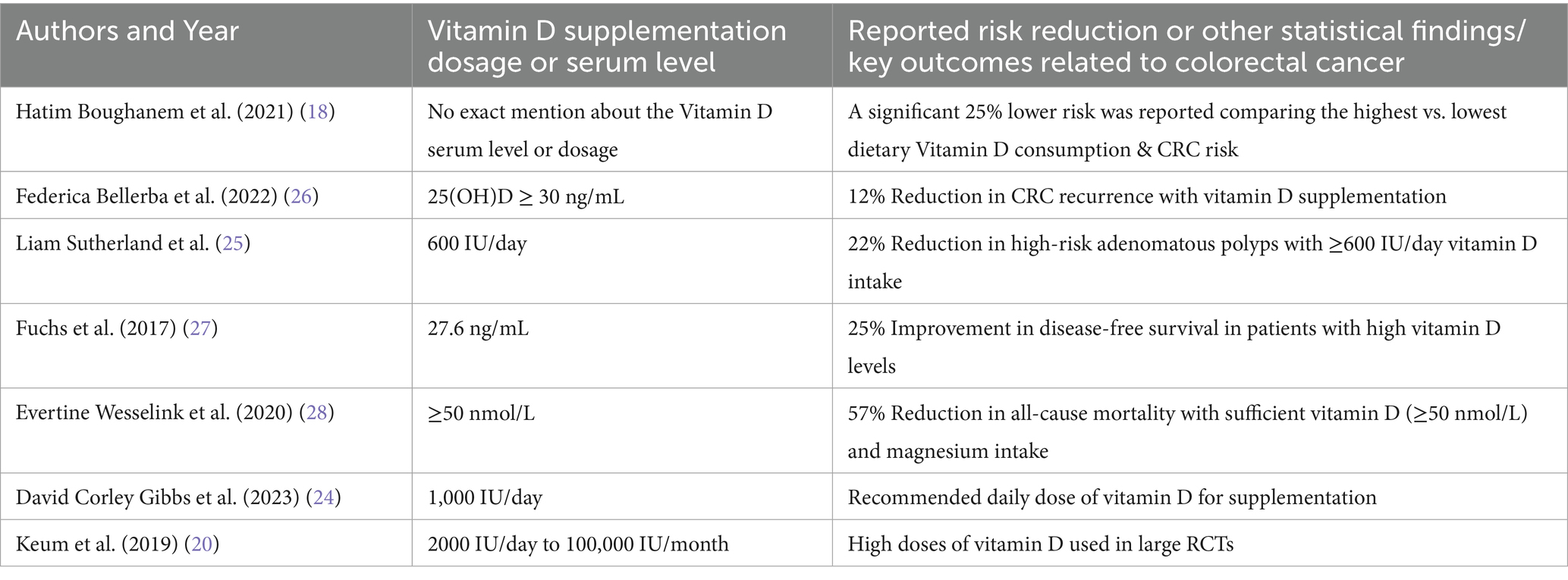
Table 2. Summary of statistical findings on the impact of Vitamin D supplementation on colorectal cancer prognosis and therapeutic dosages of Vitamin D.
Another study assessing the effect of vitamin D supplementation on survival rates showed a 30% reduction in adverse CRC outcomes (21). Additionally, a systematic review by Ajebli et al. found that while vitamin D alone may not prevent precancerous growth, its combination with omega-3 fatty acids not only improved inflammatory markers but also showed potential, indirect prognostic benefits by reducing tumor-promoting inflammation and enhancing immune response (22).
One study tested the effect of a personalized vitamin D₃ loading dose to optimize 25-(OH)D3 levels. While the intervention safely increased serum levels with low cost and minimal adverse effects, the reported 12% reduction in CRC mortality was based on modeled projections rather than direct clinical outcome data (23). Thus, while promising, the mortality impact remains suggestive and warrants further validation.
Vitamin D doses are usually recommended within the range of 600 to 1,000 IU per day. The typical clinical approach is a daily combination of 1,000 IU of vitamin D3 with 1,200 mg of calcium, which has been shown to reduce adenoma recurrence, especially in patients with a genetic predisposition to low vitamin D levels (24). Gigic et al. (2022) emphasized the importance of consistency in taking supplements during the first 6 months following diagnoses when patients are undergoing chemotherapy, which may decrease levels of vitamin D. It is taken orally specifically through cholecalciferol (vitamin D3) due to its easy absorption and high bioavailability (17). In areas with limited sunlight such as high-latitude regions, McGregor et al. (2020) suggested that a daily intake of ≥600 IU can successfully cut down on high-risk adenomatous polyps by 22% (25). It is taken orally specifically through cholecalciferol (vitamin D3) due to its easy absorption and high bioavailability (17). In areas with limited sunlight such as high-latitude regions, McGregor et al. (2020) suggested that a daily intake of ≥600 IU can successfully cut down on high-risk adenomatous polyps by 22% (25). These levels are maintained using the daily dosing protocol as confirmed by blood test results, checking optimal serum concentration of 25-(OH)D3, and patient-specific adjustments.
Building upon these dosing strategies, recent high-quality evidence reinforces the therapeutic benefits of maintaining optimal vitamin D levels in CRC patients. The Bellerba et al. (2022) study demonstrated that patients receiving vitamin D supplementation exhibited a 12% reduced risk of recurrence compared to controls, with a hazard ratio (HR) of 0.88; 95% CI: 0.78–0.99 (26). This protective effect aligns with the findings of Fuchs et al. (2017), who reported a 25% improvement in disease-free survival (HR: 0.75; 95% CI: 0.60–0.93) among CRC patients with higher predicted vitamin D status (27). This is also complemented by the results from Wesselink et al. (2020) study discussed earlier that mentioned the 57% reduction in all-cause mortality derived from the COLON study when there are sufficient serum 25-(OH)D3 levels (≥50 nmol/L) supported by adequate magnesium intake (28). In parallel, emerging research on genetic determinants such as VDR polymorphisms, as reviewed by Pereira et al. (2024), suggests that individual genomic profiles may further modulate responsiveness to vitamin D therapy. Together, these findings not only corroborate the physiological rationale for supplementation but also highlight its real-world clinical impact across multiple endpoints in CRC care (29).
7 Genetic predispositions and the interaction with vitamin D in colorectal Cancer
The expression of VDR in epithelial and stromal colon cells plays a crucial role in tumor progression. While some CRC cell lines retain VDR expression, others lose it, becoming resistant to the antitumor effects of 1,25-(OH)2D3. VDR expression typically increases in precancerous lesions but decreases in later, advanced stages, limiting the potential effectiveness of VDR agonists (8, 29, 30). Key polymorphisms such as ApaI, TaqI, BsmI, and FokI, which are essential for vitamin D’s biological functions, affect mRNA stability and gene expression regulation, with their prognostic associations summarized in Figure 6 and Table 2. These variants can disrupt the vitamin D pathway, potentially impacting CRC development (3, 31, 32).
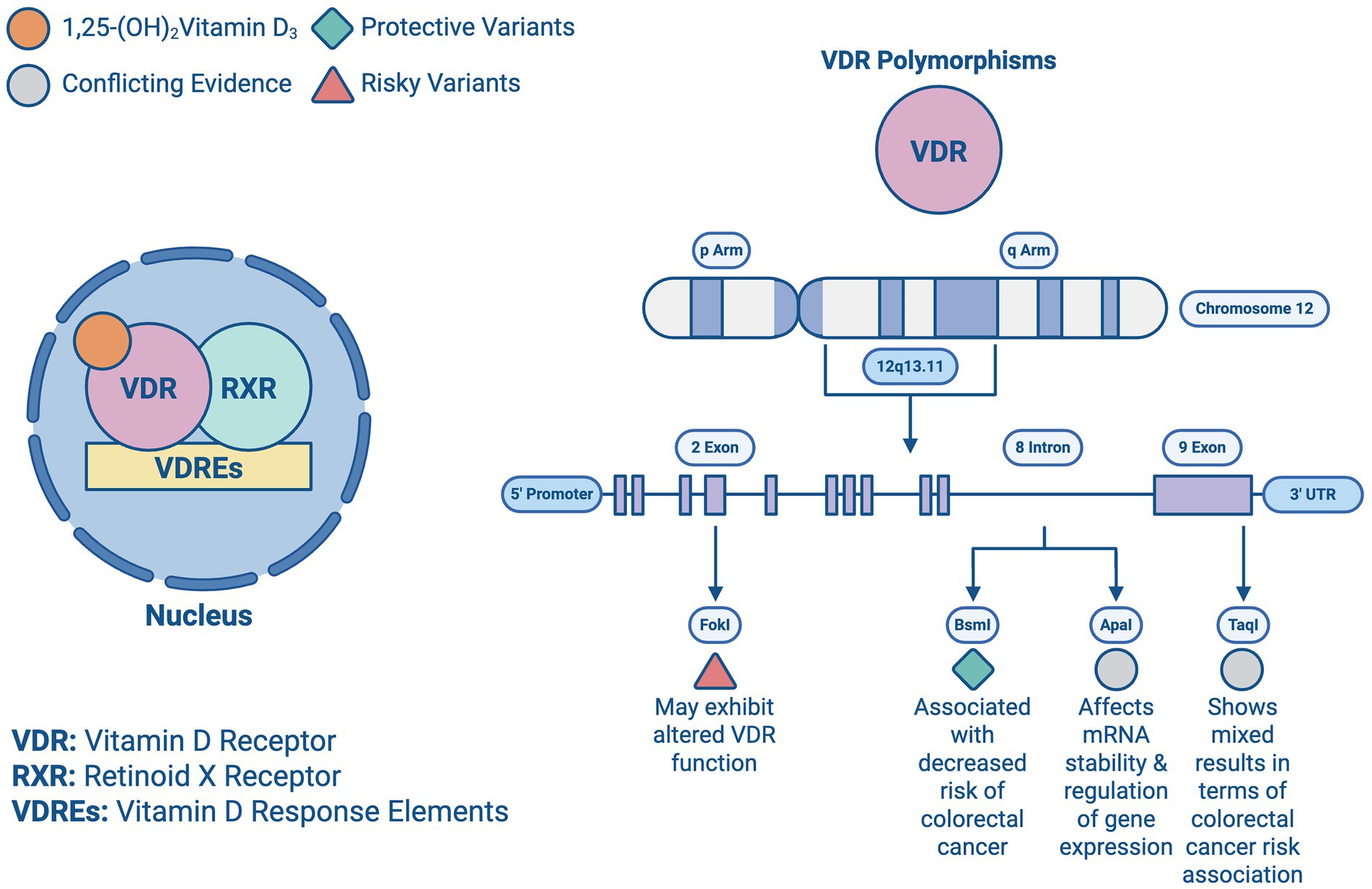
Figure 6. Vitamin D receptor polymorphisms: illustration of the Vitamin D transcription from a previous figure and all the vitamin D receptors (VDR) present in chromosome 12 and their beneficial or risky outcomes.
Heritable factors account for about 35% of the disease risk, despite less than 5% of cases being directly attributed to genetic predisposition. Variations in genes like VDR are important, as they modulate both the risk and progression of CRC. The BsmI polymorphism, for instance, has been associated with a decreased risk of CRC, particularly among Caucasians, while the TaqI polymorphism has shown mixed results in terms of risk association. The complex interplay between these genetic factors and vitamin D levels suggests a multifaceted influence on CRC prognosis (33–35).
Studies highlight the interaction between genetic predispositions and vitamin D levels in CRC. High VDR expression in stromal fibroblasts generally correlates with better outcomes, suggesting that even patients with low VDR-expressing tumor cells might benefit from VDR agonists if their stromal cells are adequately expressing VDR. Additionally, microRNAs such as miR-27b and miR-372/373 can downregulate VDR, influencing CRC resistance to vitamin D (36, 37). Studies investigating gene-vitamin D interactions have provided insights into how genetic predispositions modulate the effects of vitamin D on CRC. Meta-analyses have demonstrated that certain VDR polymorphisms, such as BsmI and Cdx-2, correspond with an altered CRC risk, like the BsmI variant showing protective effects in specific populations. These interactions highlight the importance of considering genetic background when assessing vitamin D’s role in CRC progression and prognosis (38–40).
Based on this information, the potential for personalized medicine utilizing genetic testing to tailor vitamin D supplementation in CRC patients, enhance therapeutic efficacy, and improve patient outcomes seems promising. Recent studies have shown that specific polymorphisms and mutations in the VDR gene can significantly influence the effectiveness of vitamin D treatment. Individuals with the FokI polymorphism may exhibit altered VDR function, necessitating personalized dosing strategies to achieve optimal therapeutic levels (40). Similarly, while the BsmI and TaqI polymorphisms have been widely studied, current evidence shows inconsistent associations with treatment outcomes; however, their potential role in modulating vitamin D responsiveness in specific subpopulations (like IBD patients) continues to be explored (3). Understanding a patient’s VDR genotype can help healthcare providers design customized supplementation plans that maximize the antitumor effects of vitamin D, potentially reducing CRC risk and improving prognosis (32, 33). Additionally, the integration of microRNA profiling with genetic testing could further refine personalized treatment approaches. MicroRNAs such as miR-27b and miR-372/373 could serve as biomarkers for resistance to vitamin D therapy, enabling clinicians to identify patients who would benefit most from alternative or adjunctive treatments (34). This precision medicine approach aligns with the broader trends in oncology, aiming to tailor interventions based on individual genetic and molecular profiles.
The interplay between genetic predispositions and vitamin D in CRC is complex and ever-changing. Understanding the specific genetic variants affecting vitamin D metabolism, alongside broader genetic susceptibility to CRC, is crucial for developing targeted interventions. Future research should focus on large-scale, multi-ethnic studies to validate these findings and optimize personalized vitamin D supplementation strategies in clinical practice (35). By integrating genetic information with nutritional interventions, there is potential to significantly impact the management and prognosis of CRC (40) (Table 3).
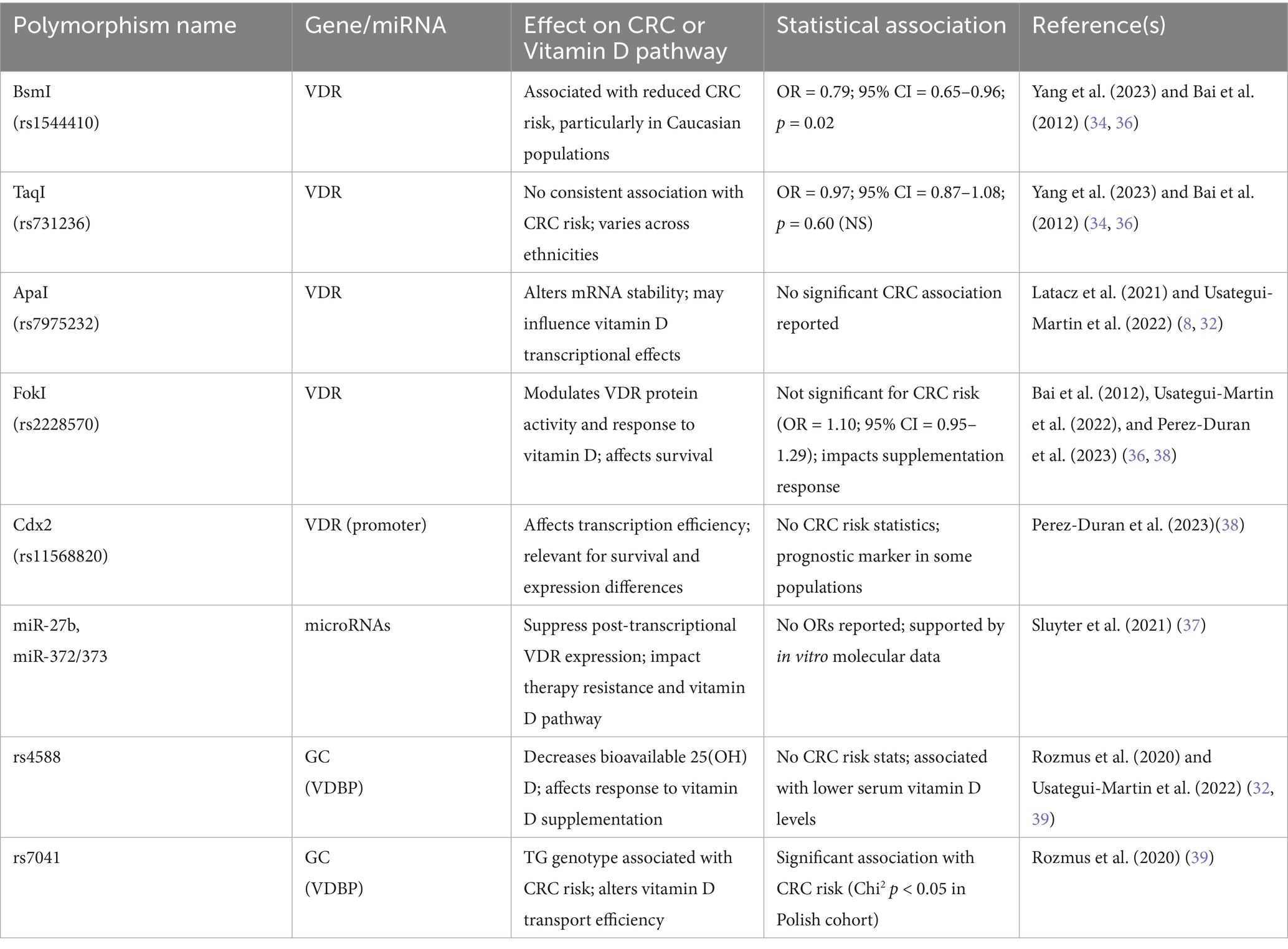
Table 3. Genetic polymorphisms and their associations with colorectal cancer risk and Vitamin D responsiveness.
8 Discussion
The impact of vitamin D supplementation on the prognosis of CRC presents a critical intersection of nutritional science and oncology, reflecting both the therapeutic potential and the complexities of clinical applications. This article consolidates the insights summarized from numerous studies, emphasizing the role of vitamin D in CRC prognosis and potential therapy.
Current literature consistently demonstrates an inverse relationship between serum vitamin D levels and the incidence of CRC. For instance, Peixoto and de Carvalho Oliveira (2022) highlighted that higher serum levels of vitamin D are associated with a reduced risk of CRC, aligning with the findings of Hernández-Alonso and Boughanem (2023), who confirmed that adequate vitamin D levels significantly lower CRC risk. These studies underscore the preventive role of vitamin D, suggesting that maintaining sufficient levels could potentially diminish the onset of CRC, particularly in high-risk populations.
However, the conversation shifts when considering the influence of vitamin D supplementation on CRC prognosis. While epidemiological evidence firmly supports the protective role of vitamin D, the impact of supplementation in influencing disease outcomes, particularly in those already diagnosed with CRC, remains less clear. Santorsola et al. (2024) noted low serum vitamin D levels pre-chemotherapy can negatively impact outcomes in metastatic CRC patients, implying that supplementation might improve prognosis. Yet, this remains a complex area of study, where the interplay of dosage, timing, pathological staging, and individual patient factors, such as baseline vitamin D levels, genetic predispositions, and social factors, requires further exploration.
Clinical trials investigating vitamin D supplementation have yielded mixed results. Vaughan-Shaw et al. (2020) demonstrated a 30% reduction in adverse outcomes among CRC patients receiving vitamin D supplementation. Despite this promising figure, the variation in study designs, dosage regimens, clinical staging, and patient populations across different trials complicates the generalizability of these findings. For instance, the study by Ng et al. (2019) involving high-dose vitamin D3 supplementation in metastatic CRC patients showed a modest improvement in progression-free survival. However, the overall survival benefit was not statistically significant. These outcomes highlight the need for more standardized, large-scale trials accounting for confounding variables to clarify the potential therapeutic benefits of vitamin D in CRC management.
Moreover, it is important to note that most RCTs focus exclusively on oral supplementation without addressing UV exposure, the primary physiological source of vitamin D. Given that supplementation may only marginally impact serum calcitriol levels, future research should also consider therapeutic sunlight exposure, particularly in regions or patient populations where deficiency is driven by limited UV access rather than dietary insufficiency.
Also, in the context of inter-individual variability, the role of genetic factors cannot be overlooked. Genetic polymorphisms in the VDR gene, as discussed by Lv et al. (2021), can significantly influence the efficacy of vitamin D supplementation. For example, certain VDR gene variants may enhance or diminish the body’s response to vitamin D, potentially altering the therapeutic outcomes in CRC patients. This genetic variability underscores the importance of personalized medicine approaches in optimizing vitamin D therapy, where genetic testing could guide individualized supplementation strategies.
Furthermore, the relationship between obesity, vitamin D deficiency, and CRC prognosis introduces additional complexity. Obesity, a known risk factor for CRC, is often associated with lower serum vitamin D levels due to sequestration within adipose tissue. Väyrynen et al. (2016) indicated that CRC patients with a higher BMI tend to have lower serum vitamin D levels, which may partially explain the poorer outcomes observed in this patient group. This interplay suggests that concurrently addressing obesity and vitamin D deficiency could be a critical component of CRC management.
In conclusion, while the protective role of vitamin D against CRC development is well-supported, its impact on CRC prognosis through supplementation remains a controversial and evolving field. Current literature suggests potential benefits, particularly in specific subgroups of patients, but also highlights the deficits in current trials and the need for more targeted research. Future studies should focus on elucidating the optimal dosing strategies, understanding the role of genetic factors, and exploring the combined effects of vitamin D supplementation and weight management in improving CRC outcomes. Through these avenues, vitamin D may become a cornerstone of a more personalized and effective approach to CRC treatment and potential prevention.
The relationship between obesity, vitamin D deficiency, and CRC prognosis introduces additional complexity. Obesity, a known risk factor for CRC, is associated with lower serum 25-(OH) D3 levels due to sequestration in adipose tissue and may confound the impact of supplementation. Väyrynen et al. (2016) showed that higher BMI in CRC patients correlated with lower vitamin D levels, possibly contributing to poorer outcomes. However, it is critical to recognize that 80–90% of circulating calcitriol is derived from UV exposure, not diet. Thus, dietary supplementation—though practical—may only modestly influence systemic levels in CRC patients, many of whom also face reduced intake and absorption due to illness or treatment. This underscores a neglected therapeutic consideration: controlled sunlight or UV exposure may offer greater physiological impact than oral supplementation alone. Despite observational links between vitamin D and CRC prognosis, there is significant heterogeneity in trial results. For example, closer reading of the COLON study shows that survival benefits were notably influenced by magnesium intake, and not solely by vitamin D status (Wessellink et. Al - 2020). Therefore, while the literature supports a role for vitamin D, publication bias and selective reporting of positive results remain concerns. A more balanced synthesis—considering confounders, non-supplemental sources of vitamin D, and methodological quality—is essential to clarify vitamin D’s true prognostic value in CRC (Tables 4, 5).
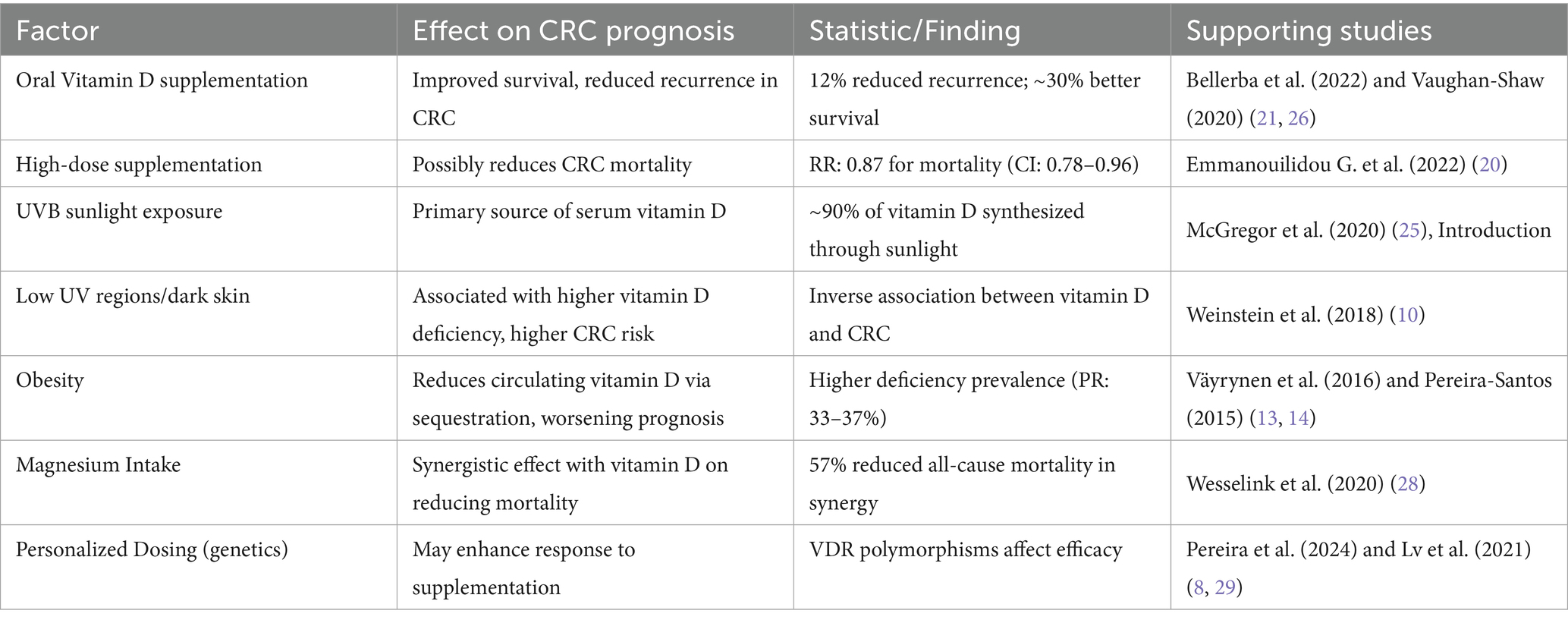
Table 5. Summary of statistical associations between Vitamin D supplementation, sunlight exposure, and CRC prognosis.
9 Conclusion
While the protective role of vitamin D against CRC development is well-supported, its impact on CRC prognosis through supplementation remains a controversial and evolving field. Current literature suggests potential benefits, particularly in specific subgroups of patients, but highlights the need for more targeted research to address deficits in existing trials. Furthermore, while supplementation may provide marginal benefit in deficient individuals, its impact is modest compared to endogenous synthesis via UV exposure, which accounts for the majority of circulating calcitriol. The relationship between CRC, vitamin D deficiency, and obesity presents an additional layer of complexity, as changes in vitamin D metabolism secondary to obesity may affect CRC prognosis. Although conflicting data exist regarding cancer rates and mortality reduction, vitamin D supplementation has shown clinically significant improvement in survival rates, reduced recurrence risk, and enhanced quality of life in certain cases. Genetic factors also play a crucial role, with variations in genes like VDR influencing CRC risk and progression by affecting mRNA stability and gene expression regulation. Future studies should focus on optimal dosing strategies, understanding the role of genetic predispositions, and exploring the combined effects of vitamin D supplementation and weight management in improving CRC outcomes. Through these avenues, a more personalized and effective approach to CRC treatment and prevention may be achieved.
Author contributions
BN: Writing – review & editing, Investigation, Conceptualization, Writing – original draft, Visualization, Data curation, Formal analysis, Methodology. ME: Methodology, Investigation, Writing – review & editing, Data curation, Writing – original draft, Conceptualization, Visualization, Formal analysis. NN: Writing – review & editing, Data curation, Visualization, Investigation, Writing – original draft, Conceptualization, Formal analysis, Methodology. OA: Visualization, Formal analysis, Conceptualization, Data curation, Methodology, Writing – review & editing, Writing – original draft, Investigation. JP: Methodology, Project administration, Supervision, Writing – review & editing, Validation. SF: Funding acquisition, Validation, Writing – review & editing, Project administration, Methodology, Supervision.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Correction note
A correction has been made to this article. Details can be found at: 10.3389/fmed.2025.1706937.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
CRC, Colorectal Cancer; VDR, Vitamin D Receptor; VDRE, Vitamin D Response Elements; RXR, Retinoid X Receptor; CDK, Cyclin-Dependent Kinase; RCT, Randomized Controlled Trial; BMI, Body Mass Index; IU, International Units; UVB, Ultraviolet B Radiation; 25-(OH)D3, 25-hydroxyvitamin D (Calcidiol); 1,25-(OH)2D3, 1,25-dihydroxyvitamin D (Calcitriol); AI, Artificial Intelligence; Treg, Regulatory T Cells; CI, Confidence Interval; HR, Hazard Ratio; SNP, Single Nucleotide Polymorphism; miR, microRNA; COLON Study, Colorectal cancer cohort study analyzing diet, lifestyle, and cancer outcomes.
References
1. Amrein, K, Scherkl, M, Hoffmann, M, Neuwersch-Sommeregger, S, Köstenberger, M, Tmava Berisha, A, et al. Vitamin D deficiency 2.0: an update on the current status worldwide. Eur J Clin Nutr. (2020) 74:1498–513. doi: 10.1038/s41430-020-0558-y
3. Zhu, Y, Mahon, BD, Froicu, M, and Cantorna, MT. Calcium and 1 alpha,25-dihydroxyvitamin D3 target the TNF-alpha pathway to suppress experimental inflammatory bowel disease. Eur J Immunol. (2005) 35:217–24. doi: 10.1002/eji.200425491
4. Izquierdo, JM. Vitamin D-dependent microbiota-enhancing tumor immunotherapy. Cell Mol Immunol. (2024) 21:1083–6. doi: 10.1038/s41423-024-01184-4
5. Negri, M, Gentile, A, de Angelis, C, Monto, T, Patalano, R, Colao, A, et al. Vitamin D-induced molecular mechanisms to potentiate Cancer therapy and to reverse drug-resistance in Cancer cells. Nutrients. (2020) 12, 1197–1206. doi: 10.3390/nu12061798
6. Zhu, K, Knuiman, M, Divitini, M, Hung, J, Lim, EM, Cooke, BR, et al. Lower serum 25-hydroxyvitamin D is associated with colorectal and breast cancer, but not overall cancer risk: a 20-year cohort study. Nutr Res. (2019) 67:100–7. doi: 10.1016/j.nutres.2019.03.010
7. Chiang, CH, Chang, YJ, He, SR, Chao, JN, Yang, CH, and Liu, YT. Association of 25(OH)-vitamin D and metabolic factors with colorectal polyps. PLoS One. (2023) 18:e0286654. doi: 10.1371/journal.pone.0286654
8. Latacz, M, Rozmus, D, Fiedorowicz, E, Snarska, J, Jarmolowska, B, Kordulewska, N, et al. Vitamin D receptor (VDR) gene polymorphism in patients diagnosed with colorectal Cancer. Nutrients. (2021) 13, 2829–2837. doi: 10.3390/nu13010200
9. Onali, T, Slaba, H, Jian, C, Koivumaki, T, Paivarinta, E, Marttinen, M, et al. Berry supplementation in healthy volunteers modulates gut microbiota, increases fecal polyphenol metabolites and reduces viability of colon cancer cells exposed to fecal water- a randomized controlled trial. J Nutr Biochem. (2025) 141:109906. doi: 10.1016/j.jnutbio.2025.109906
10. McCullough, ML, Zoltick, ES, Weinstein, SJ, Fedirko, V, Wang, M, Cook, NR, et al. Circulating vitamin D and colorectal cancer risk: an international pooling project of 17 cohorts. J Natl Cancer Inst. (2019) 111:158–69. doi: 10.1093/jnci/djy087
11. Keum, N, and Giovannucci, E. Global burden of colorectal cancer: emerging trends, risk factors and prevention strategies. Nat Rev Gastroenterol Hepatol. (2019) 16:713–32. doi: 10.1038/s41575-019-0189-8
12. Na, SY, Kim, KB, Lim, YJ, and Song, HJ. Vitamin D and colorectal Cancer: current perspectives and future directions. J Cancer Prev. (2022) 27:147–56. doi: 10.15430/JCP.2022.27.3.147
13. Vayrynen, JP, Mutt, SJ, Herzig, KH, Vayrynen, SA, Kantola, T, Karhu, T, et al. Decreased preoperative serum 25-Hydroxyvitamin D levels in colorectal cancer are associated with systemic inflammation and serrated morphology. Sci Rep. (2016) 6:36519. doi: 10.1038/srep36519
14. Pereira-Santos, M, Costa, PR, Assis, AM, Santos, CA, and Santos, DB. Obesity and vitamin D deficiency: a systematic review and meta-analysis. Obes Rev. (2015) 16:341–9. doi: 10.1111/obr.12239
15. Budny, A, Grochowski, C, Kozlowski, P, Kolak, A, Kaminska, M, Budny, B, et al. Obesity as a tumour development triggering factor. Ann Agric Environ Med. (2019) 26:13–23. doi: 10.26444/aaem/100664
16. Zhang, AMY, Wellberg, EA, Kopp, JL, and Johnson, JD. Hyperinsulinemia in obesity, inflammation, and Cancer. Diabetes Metab J. (2021) 45:622. doi: 10.4093/dmj.2021.0131
17. Wesselink, E, Bours, MJL, de Wilt, JHW, Aquarius, M, Breukink, SO, Hansson, B, et al. Chemotherapy and vitamin D supplement use are determinants of serum 25-hydroxyvitamin D levels during the first six months after colorectal cancer diagnosis. J Steroid Biochem Mol Biol. (2020):199. 1007–1017. doi: 10.1016/j.jsbmb.2020.105577
18. Boughanem, H, Canudas, S, Hernandez-Alonso, P, Becerra-Tomas, N, Babio, N, Salas-Salvado, J, et al. Vitamin D intake and the risk of colorectal Cancer: an updated Meta-analysis and systematic review of case-control and prospective cohort studies. Cancers (Basel). (2021) 13. doi: 10.3390/cancers13112814
19. Beatriz, G, Fiona, S, Ford, JA, MacLennan, G, and Avenell, A. Cancer and vitamin D supplementation: a systematic review and meta-analysis. Am J Clin Nutr. (2018) 107:15. doi: 10.1093/ajcn/nqx047
20. Keum, N, Lee, DH, Greenwood, DC, Manson, JE, and Giovannucci, E. Vitamin D supplementation and total cancer incidence and mortality: a meta-analysis of randomized controlled trials. Ann Oncol. (2019) 30:733–43. doi: 10.1093/annonc/mdz059
21. Vaughan-Shaw, PG, Buijs, LF, Blackmur, JP, Theodoratou, E, Zgaga, L, Din, FVN, et al. The effect of vitamin D supplementation on survival in patients with colorectal cancer: systematic review and meta-analysis of randomised controlled trials. Br J Cancer. (2020) 123:1705–12. doi: 10.1038/s41416-020-01060-8
22. Ajebli, M, Meretsky, CR, Akdad, M, Amssayef, A, and Hebi, M. The role of dietary vitamins and antioxidants in preventing colorectal cancer: a systematic review. Cureus J Med Sci. (2024) 16:e64277. doi: 10.7759/cureus.64277
23. Kuznia, S. (2023) Test of efficacy of a personalized vitamin D supplementation to treat vitamin D deficiency in colorectal cancer patients and the potential implications on cancer prognosis, 1003–1016.
24. Gibbs, DC, Barry, EL, Fedirko, V, Baron, JA, and Bostick, RM. Impact of common vitamin D-binding protein isoforms on supplemental vitamin D3 and/or calcium effects on colorectal adenoma recurrence risk: A secondary analysis of a randomized clinical trial. JAMA Oncol. (2023) 9:546–51. doi: 10.1001/jamaoncol.2022.6924
25. Sutherland, RL, Ormsbee, J, Pader, J, Forbes, N, Town, S, Hilsden, RJ, et al. Vitamin D supplementation reduces the occurrence of colorectal polyps in high-latitude locations. Prev Med. (2020) 135:106072. doi: 10.1016/j.ypmed.2020.106072
26. Bellerba, F, Serrano, D, Harriet, J, Pozzi, C, Segata, N, NabiNejad, A, et al. Colorectal cancer, vitamin D and microbiota: A double-blind phase II randomized trial (ColoViD) in colorectal cancer patients. Neoplasia. (2022) 34:100842. doi: 10.1016/j.neo.2022.100842
27. Fuchs, MA, Yuan, C, Sato, K, Niedzwiecki, D, Ye, X, Saltz, LB, et al. Predicted vitamin D status and colon cancer recurrence and mortality in CALGB 89803 (Alliance). Ann Oncol. (2017) 28:1359–67. doi: 10.1093/annonc/mdx109
28. Wesselink, E, Kok, DE, Bours, MJL, de Wilt, JHW, van Baar, H, van Zutphen, M, et al. Vitamin D, magnesium, calcium, and their interaction in relation to colorectal cancer recurrence and all-cause mortality. Am J Clin Nutr. (2020) 111:1007–17. doi: 10.1093/ajcn/nqaa049
29. Pereira, F, Fernández-Barral, A, Larriba, MJ, Barbáchano, A, and González-Sancho, JM. From molecular basis to clinical insights: a challenging future for the vitamin D endocrine system in colorectal cancer. FEBS J. (2023) 291:2485–2518. doi: 10.1111/febs.16955
30. Messaritakis, I, Koulouridi, A, Sfakianaki, M, Vogiatzoglou, K, Gouvas, N, Athanasakis, E, et al. The role of vitamin D receptor gene polymorphisms in colorectal Cancer risk. Cancers (Basel). (2020) 12. doi: 10.3390/cancers12061379
31. Serrano, D, Gnagnarella, P, Raimondi, S, and Gandini, S. Meta-analysis on vitamin D receptor and cancer risk: focus on the role of TaqI, ApaI, and Cdx2 polymorphisms. Eur J Cancer Prev. (2016) 25:85–96. doi: 10.1097/CEJ.0000000000000132
32. Usategui-Martin, R, De Luis-Roman, DA, Fernandez-Gomez, JM, Ruiz-Mambrilla, M, and Perez-Castrillon, JL. Vitamin D receptor (VDR) gene polymorphisms modify the response to vitamin D supplementation: A systematic review and Meta-analysis. Nutrients. (2022) 14, 2232–2242. doi: 10.3390/nu14020360
33. Rong, K, He, Q, Chen, S, Yu, Y, Mei, L, Mi, Y, et al. The mechanism of vitamin D3 in preventing colorectal cancer through network pharmacology. Front Pharmacol. (2023) 14:1192210. doi: 10.3389/fphar.2023.1192210
34. Yang, M, Ji, W, Xu, N, Zong, C, Gu, J, Guo, X, et al. Association of vitamin D receptor polymorphisms with colorectal cancer susceptibility: A systematic meta-analysis. Medicine (Baltimore). (2023) 102:e32575. doi: 10.1097/MD.0000000000032575
35. Kang, S, Zhao, Y, Wang, L, Liu, J, Chen, X, Liu, X, et al. Vitamin D receptor Taq I polymorphism and the risk of prostate cancer: a meta-analysis. Oncotarget. (2018) 9:7136–47. doi: 10.18632/oncotarget.23606
36. Bai, YH, Lu, H, Hong, D, Lin, CC, Yu, Z, and Chen, BC. Vitamin D receptor gene polymorphisms and colorectal cancer risk: a systematic meta-analysis. World J Gastroenterol. (2012) 18:1672–9. doi: 10.3748/wjg.v18.i14.1672
37. Sluyter, JD, Manson, JE, and Scragg, R. Vitamin D and clinical cancer outcomes: a review of meta-analyses. JBMR Plus. (2021) 5:e10420. doi: 10.1002/jbm4.10420
38. Perez-Duran, C, Marquez-Pete, N, Galvez-Navas, JM, Cura, Y, Rojo-Tolosa, S, Pineda-Lancheros, LE, et al. Single nucleotide polymorphisms in the vitamin D metabolic pathway as survival biomarkers in colorectal Cancer. Cancers (Basel). (2023) 15, 386S–393S. doi: 10.3390/cancers15164077
39. Rozmus, D, Ciesielska, A, Plominski, J, Grzybowski, R, Fiedorowicz, E, Kordulewska, N, et al. Vitamin D binding protein (VDBP) and its gene polymorphisms—the risk of malignant tumors and other diseases. Int J Mol Sci. (2020) 21, 691–706. doi: 10.3390/ijms21217822
40. Chen, QY, Kim, S, Lee, B, Jeong, G, Lee, DH, Keum, N, et al. Post-diagnosis vitamin D supplement use and survival among Cancer patients: A Meta-analysis. Nutrients. (2022) 14, 17–39. doi: 10.3390/nu14163418
Keywords: vitamin D, colorectal cancer, VDR polymorphisms, vitamin D supplementation, obesity, cancer prognosis
Citation: Naji B, Eltawil M, Nemer N, Abdelazim O, Patil JD and Fredericks S (2025) Vitamin D deficiency, supplementation, and colorectal cancer outcomes: interactions with obesity and risk profiles. Front. Med. 12:1657534. doi: 10.3389/fmed.2025.1657534
Edited by:
Huan Tong, Sichuan University, ChinaReviewed by:
Hojat Dehghanbanadaki, Tehran University of Medical Sciences, IranAli Faryabi, Tehran University of Medical Sciences, Iran
Copyright © 2025 Naji, Eltawil, Nemer, Abdelazim, Patil and Fredericks. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Najla Nemer, bmVtZXJuYWpsYUBob3RtYWlsLmNvbQ==
 Basmalah Naji1
Basmalah Naji1 Najla Nemer
Najla Nemer Omer Abdelazim
Omer Abdelazim Jayaditya D. Patil
Jayaditya D. Patil Salim Fredericks
Salim Fredericks