- Department of Thoracic Surgery, The Fourth Hospital of Hebei Medical University (Hebei Tumor Hospital), Shijiazhuang, Hebei, China
Background: Lung cancer remains the leading cause of cancer-related mortality worldwide. With advancements in molecularly targeted therapies, Epidermal growth factor receptor (EGFR) mutations have emerged as critical therapeutic targets in advanced lung adenocarcinoma. However, the EGFR p.L833V/p.H835L compound mutations are relatively uncommon, and their clinical characteristics and therapeutic response to EGFR tyrosine kinase inhibitors (TKIs) remain poorly defined.
Case presentation: This study reports two cases of advanced lung adenocarcinoma harboring the EGFR p.L833V/p.H835L compound mutation, both treated with furmonertinib. Case 1 was a 62-year-old male, and Case 2 was a 61-year-old female, both diagnosed through tissue biopsy and next-generation sequencing (NGS). Following treatment, both patients achieved partial response (PR), with progression-free survival (PFS) of 29 months and not available (NA, >12 months), respectively, demonstrating good tolerability.
Results: Furmonertinib appears to be an effective treatment for advanced lung adenocarcinoma with EGFR p.L833V/p.H835L compound mutations.
Conclusion: This study further supports the therapeutic potential of furmonertinib in EGFR-mutant lung cancer.
Introduction
Lung cancer is the leading cause of cancer-related mortality worldwide. Epidermal growth factor receptor (EGFR) is the most common genetic alteration in lung adenocarcinoma, accounting for approximately 60% of cases in Asian patients (1). Common sensitizing mutations (e.g., exon 19 deletions, L858R) typically respond well to first-to third-generation EGFR tyrosine kinase inhibitors (EGFR-TKIs). In contrast, rare EGFR mutations account for approximately 10–15% of all EGFR mutations, with an even lower prevalence observed in Asian populations (approximately 4.4%) (2). The EGFR p.L833V/p.H835L compound mutation is exceedingly rare, with an estimated incidence of less than 1%, and its biological behavior and treatment strategies lack evidence-based support. This article presents two cases of advanced lung adenocarcinoma successfully treated with furmonertinib, aiming to provide clinical insights for this rare patient population and explore the therapeutic potential of EGFR-TKIs in compound mutations.
Case reports
Case 1
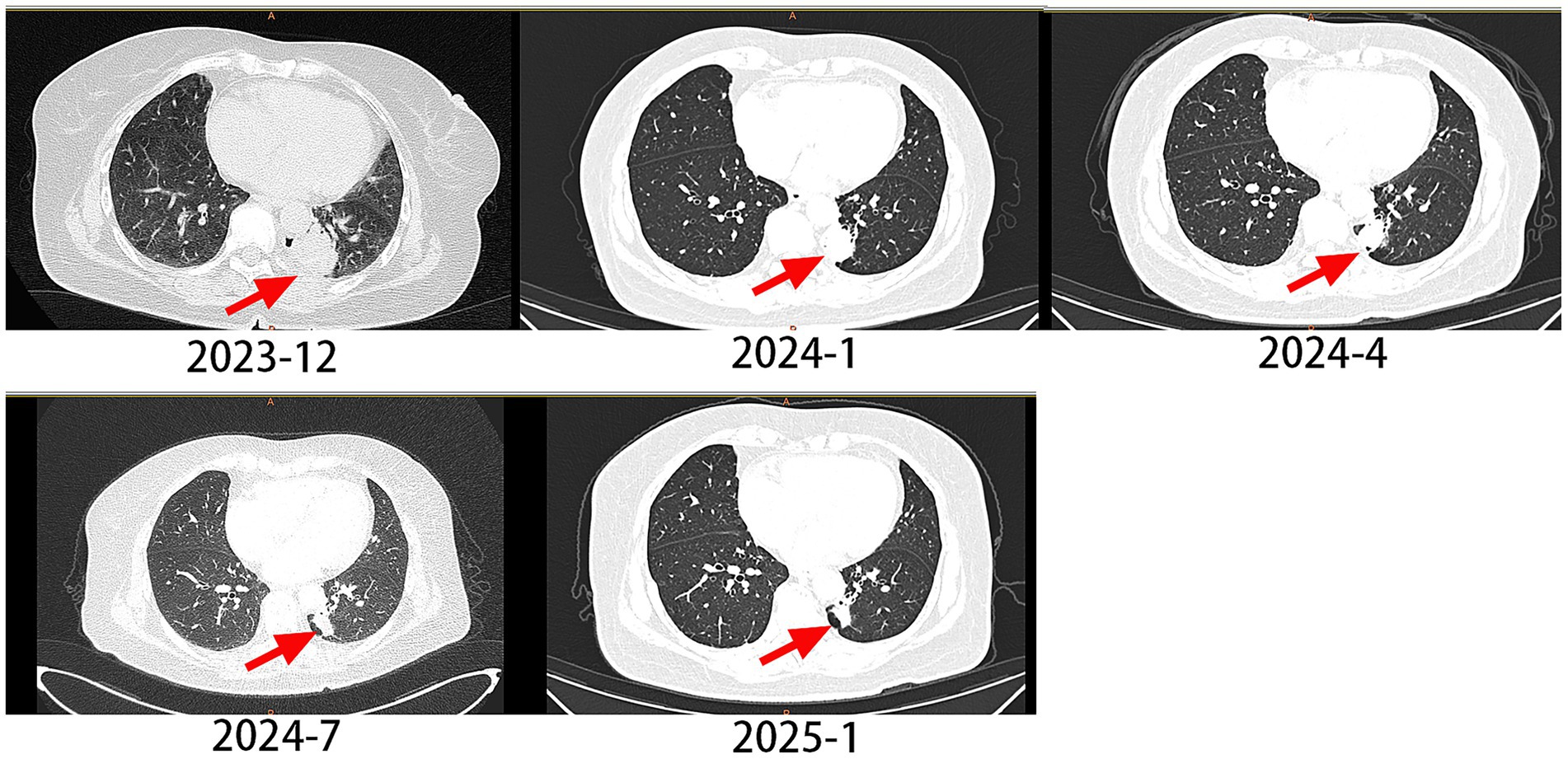
A 62-year-old male with a 20-year smoking history (quit 10 days prior to presentation) presented in December 2023 with cough and chest pain. Contrast-enhanced CT demonstrated a spiculated pulmonary mass measuring 3.9 × 3.1 cm in maximum cross-sectional diameter, exhibiting lobulated borders and pleural indentation in the left lower lobe, along with multiple bone metastases. The clinical stage was determined as cT2aN0M1c1, stage IVB. Pathology confirmed lung adenocarcinoma, and NGS testing detected an EGFR p.L833V/p.H835L compound mutation (Figure 1). The patient began oral furmonertinib (80 mg once daily) in January 2024. Follow-up CT scan at 1 month later showed the tumor size was 2.9 × 2.1 cm. Follow-up CT scans at 3, 6, and 12 months later revealed a progressive reduction in the lesion, with the size being 2.2 × 1.2 cm. According to the RECIST 1.1 criteria, the longest diameter of the tumor was reduced by ≥30%, and the efficacy was evaluated as PR (partial response) (Figure 2). The PFS exceeded 12 months, and no related adverse reactions occurred during the oral administration period. As of the time of writing, the patient remains on furmonertinib therapy.
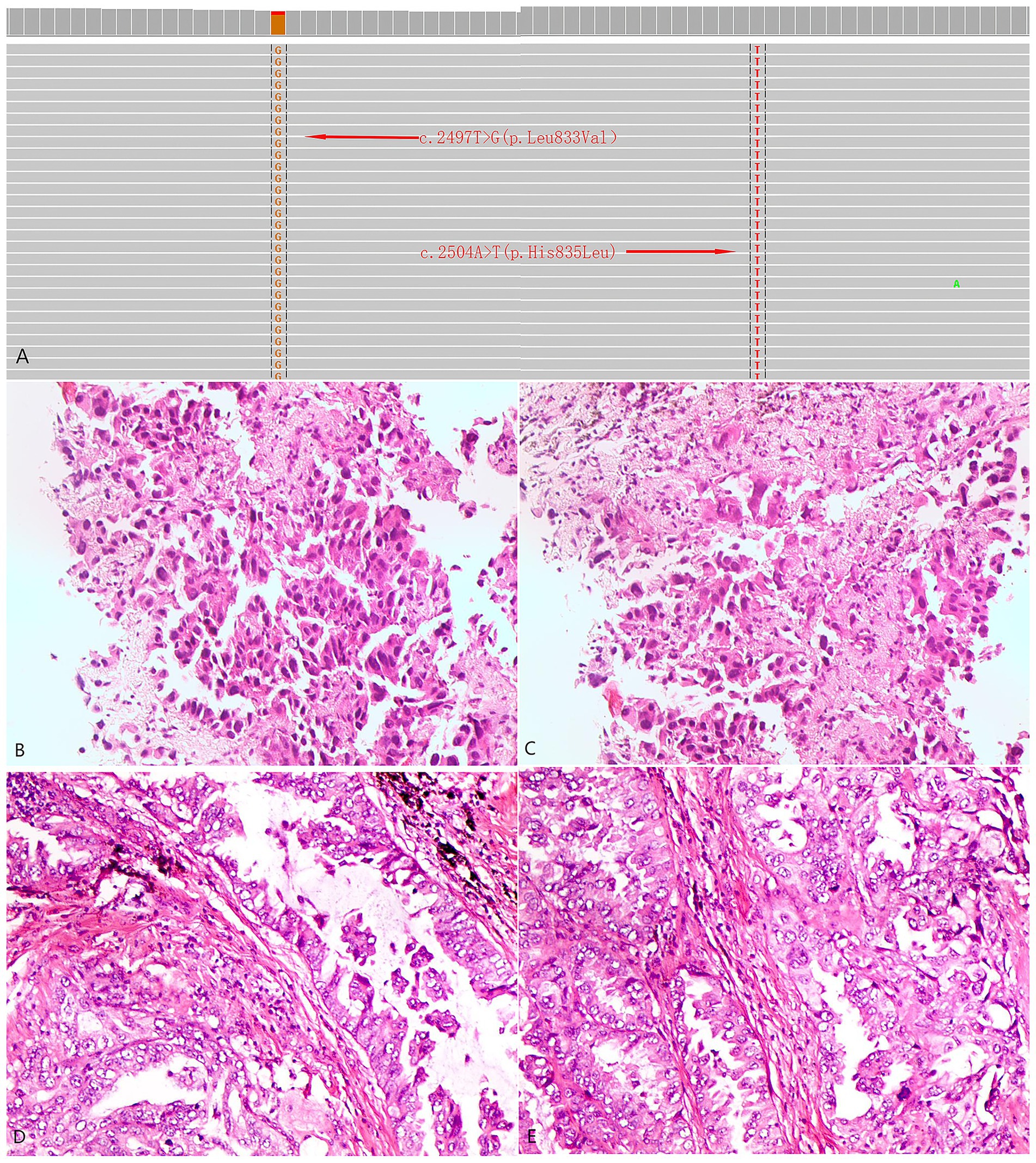
Figure 1. Next-generation genetic sequencing and histopathological profiles of the two patients. (A) Identification of L833V and H835L in EGFR exon 21 by next-generation sequencing. (B,C) Case 1: Histological sections stained with HE at a magnification of ×400 from a biopsy of a pulmonary mass. (D,E) Case 2: Histological sections stained with HE at a magnification of ×400 from a biopsy of a pulmonary mass.
Case 2
The patient was a 61-year-old female, never-smoker, who presented with dyspnea in February 2022. Histopathological analysis of a biopsy confirmed adenocarcinoma (Figure 1), with no distant metastases detected at the time of diagnosis. The patient underwent surgical exploration in February 2022, during which widespread pleural metastases were observed. As a result, only an exploratory procedure was performed, without tumor resection. The tumor was staged as cT1cN0M1a, stage IVA. NGS testing identified an EGFR p.L833V/p.H835L compound mutation. The patient began oral furmonertinib (80 mg once daily) in March 2022. During the medication period, mild skin rash occurred (CTCAE grade 2), which improved after symptomatic treatment for 1 month. Follow-up CT scan at 1 month later showed tumor shrinkage, and subsequent CT re-examinations every 3 months documented continuous reduction of the tumor to 1.1 cm × 0.7 cm. Rib metastasis was detected in the re-examination in August 2024, with a PFS (progression-free survival, the time from the start of the study to the first occurrence of disease progression or death from any cause) of 29 months (Figure 3).
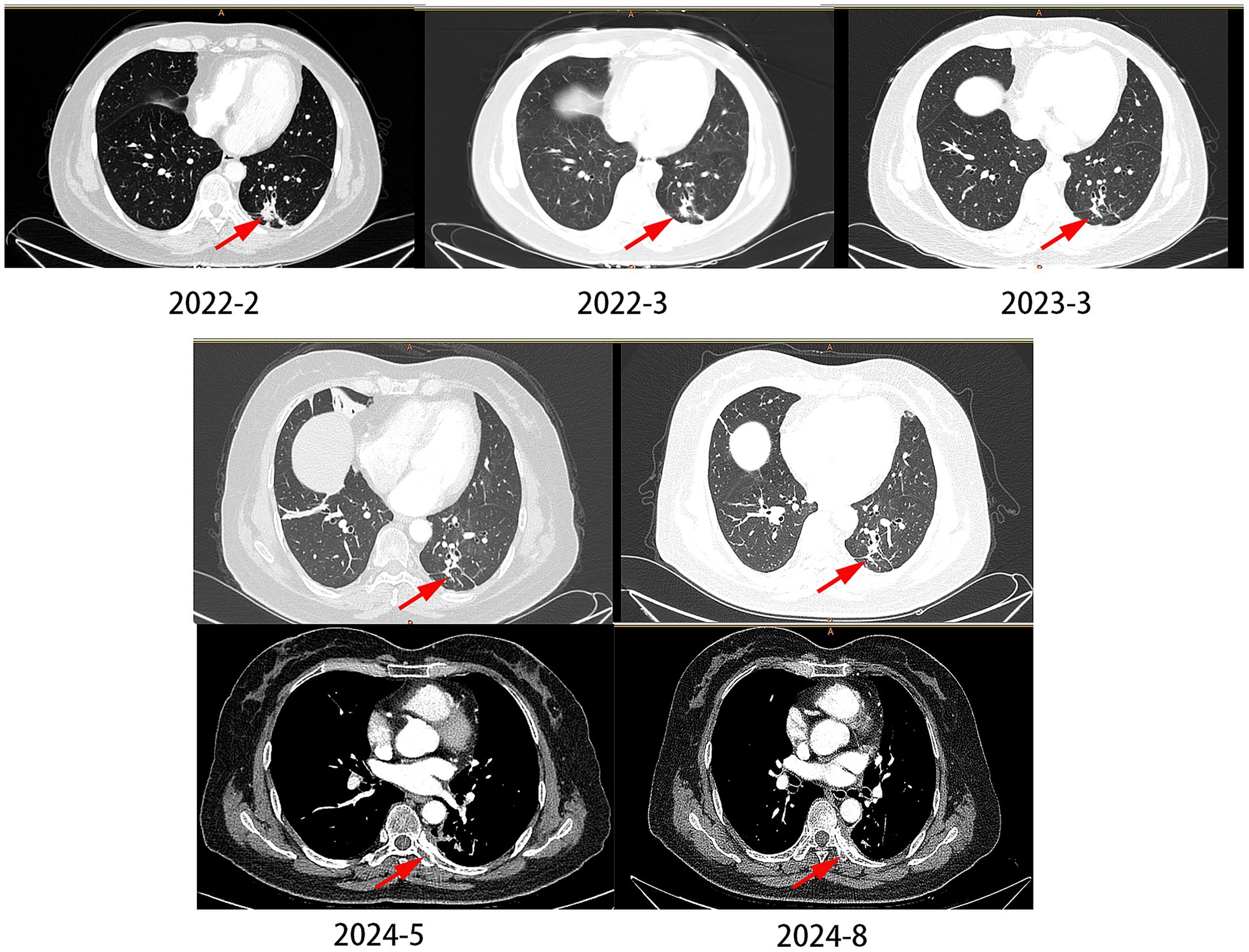
Figure 3. The changes of lung CT scan in case 2. In 2024–2028, the patient developed bone metastases, but the pulmonary nodules did not significantly enlarge.
Discussion
Lung cancer remains the leading cause of cancer-related mortality worldwide. EGFR represents the most frequent genetic alteration in lung adenocarcinoma, accounting for approximately 60% of cases in Asian populations (1). Although targeted therapy has significantly improved the prognosis of patients with advanced non-small cell lung cancer (NSCLC), the treatment of patients with rare EGFR compound mutations remains a challenge. Classic sensitizing mutations (e.g., exon 19 deletions and L858R) account for 85–90% of EGFR mutations and demonstrate favorable responses to first-and second-generation EGFR-TKIs, such as gefitinib (3) and afatinib (4) However, rare EGFR mutations account for approximately 10–15% of all EGFR alterations. These mutations may occur in isolation or coexist with common mutations or other rare variants as compound mutations, exhibiting striking heterogeneity and marked variability in sensitivity to EGFR-TKIs, with generally lower response rates compared to classic mutations (5, 6).
Third-generation EGFR-TKIs (e.g., osimertinib, furmonertinib) demonstrate therapeutic efficacy against certain rare mutations due to their broad-spectrum inhibitory activity. Floc’h N et al. (7) reported that osimertinib effectively suppressed signaling pathways and cellular proliferation in G719X-mutant cell lines in vitro. Their study further validated sustained tumor growth inhibition in patient-derived xenograft (PDX) models harboring G719X mutations either alone or in combination with L861Q and S768I. Zhang et al. (8) provided clinical evidence supporting osimertinib’s efficacy in NSCLC patients with rare EGFR mutations (including G719X, L861G, and S768I), achieving a mPFS of 8.2 months.
Furmonertinib, with its innovative trifluoroethoxypyridine structure, offers significant advantages in efficacy, safety, and coverage of uncommon mutations (9). Notably, clinical case reports have documented favorable outcomes (14 months of continuous treatment) in patients with rare G719X/S768I co-mutations treated with furmonertinib (10). This study is the first to report the significant efficacy of furmonertinib in treating EGFR p.L833V/p.H835L compound mutations. One patient achieved a PFS of 19 months, while the other had a PFS of NA (>12 months). These findings suggest that furmonertinib may overcome the efficacy limitations of traditional EGFR-TKIs in rare compound mutations, providing new evidence for clinical decision-making.
The EGFR p.L833V/p.H835L compound mutation is an extremely rare mutation, with only a few reported cases (11–25) (Table 1). We collected 15 previously reported cases with detailed clinical data, all of which involved lung adenocarcinoma patients with the EGFR p.L833V/p.H835L compound mutation, predominantly from Asian populations. The age of patients ranged from 36 to 89 years, with a male-to-female ratio of 11: 4. Most patients were in advanced stages, and all had received EGFR-TKI treatment.
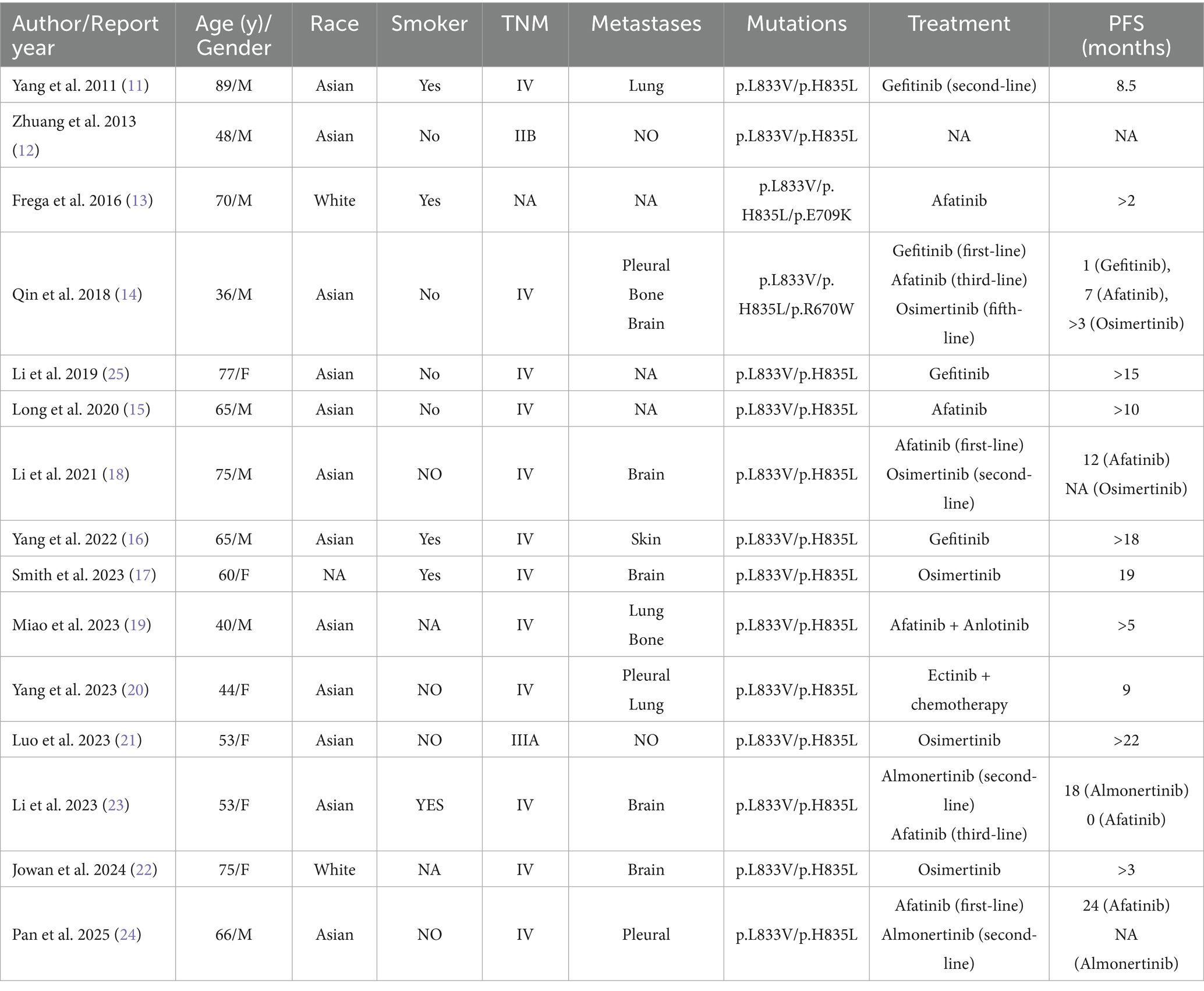
Table 1. Clinical features and prognosis of NSCLC patients with EGFR p.L833V/p.H835L compound mutations.
Yang et al. (20) reported a 44-year-old Asian female patient with pleural effusion and bilateral lung metastases at diagnosis. Tumor shrinkage was observed after first-generation EGFR-TKI treatment, with a PFS of 9 months. Li et al. (18) described a 75-year-old Asian male patient with stage IV disease (TNM classification). After 14 months of second-generation TKI (afatinib) treatment, brain metastasis developed. A repeat biopsy with next-generation sequencing (NGS) revealed an acquired EGFR T790M resistance mutation. The patient was subsequently switched to a third-generation TKI with favorable therapeutic response. Another case reported by Li et al. (23) involved a 53-year-old Asian female patient with baseline brain metastases. Tumor progression occurred after 18 months of third-generation TKI (almonertinib) treatment. Switching to a second-generation TKI failed to demonstrate therapeutic efficacy.
We summarized the currently reported cases and found that, for the rare EGFR p.L833V/p.H835L compound mutation, third-generation TKIs show better efficacy than first-and second-generation TKIs. However, in Case 2, the patient developed bone metastases at the 29th month of oral furmonertinib administration. Due to the inconvenience of obtaining samples, we did not perform re-biopsy for genetic testing nor conduct testing via blood sampling. Among the three reported cases that upgraded their TKI therapy, resistance mutations such as T790M were detected. Additionally, in one case where the TKI treatment was downgraded, tumor progression was observed after switching therapy. It is highly likely that drug-resistant mutations, such as C797S, have also emerged in this patient.
In the two cases we reported, genetic testing confirmed the presence of the EGFR p.L833V/p.H835L compound mutation, and furmonertinib was chosen as the first-line treatment. Both patients exhibited remarkable clinical responses, making this the first documented report of furmonertinib as a first-line therapy for the rare EGFR p.L833V/p.H835L compound mutation.
Conclusion
Furmonertinib appears to be an effective treatment for advanced lung adenocarcinoma with EGFR p.L833V/p.H835L compound mutations. This study further supports the therapeutic potential of furmonertinib in rare EGFR-mutant lung cancer. Additionally, we reviewed previously reported cases of non-small cell lung cancer (NSCLC) with the rare EGFR p.L833V/p.H835L compound mutation, summarizing their clinical characteristics and responses to different EGFR-TKIs. Despite the many challenges in the clinical management of rare EGFR mutations, substantial progress has been made in treatment strategies for this population in recent years. Moving forward, it is hoped that a global registry platform for rare EGFR mutations will be established, integrating multicenter clinical data with molecular characteristics to provide treatment experience for rare mutations.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving humans were approved by the Fourth Hospital of Hebei Medical University. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual (s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SW: Conceptualization, Writing – review & editing, Writing – original draft. GS: Investigation, Writing – original draft. QL: Supervision, Writing – review & editing. GL: Methodology, Supervision, Writing – review & editing. YL: Writing – review & editing, Visualization. YH: Visualization, Writing – review & editing. MG: Investigation, Writing – review & editing. KH: Writing – review & editing, Investigation. SX: Writing – original draft, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the Joint Health Commission of Hebei Province (20250744).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Xue, X, Asuquo, I, Hong, L, Gao, J, Dong, Z, Pang, L, et al. Catalog of lung cancer gene mutations among Chinese patients. Front Oncol. (2020) 10:1251. doi: 10.3389/fonc.2020.01251
2. Tavernari, D, Borgeaud, M, Liu, X, Parikh, K, Le, X, Ciriello, G, et al. Decoding the clinical and molecular signatures of EGFR common, compound, and uncommon mutations in NSCLC: a brief report. J Thorac Oncol. (2024) 20:500–6. doi: 10.1016/j.jtho.2024.12.012
3. Mok, TS, Wu, YL, Thongprasert, S, Yang, CH, Chu, DT, Saijo, N, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. (2009) 361:947–57. doi: 10.1056/NEJMoa0810699
4. Yang, JC, Wu, YL, Schuler, M, Sebastian, M, Popat, S, Yamamoto, N, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-lung 3 and LUX-lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. (2015) 16:141–51. doi: 10.1016/S1470-2045(14)71173-8
5. Robichaux, JP, Le, X, Vijayan, R, Hicks, JK, Heeke, S, Elamin, YY, et al. Structure-based classification predicts drug response in EGFR-mutant NSCLC. Nature. (2021) 597:732–7. doi: 10.1038/s41586-021-03898-1
6. Borgeaud, M, Parikh, K, Banna, GL, Kim, F, Olivier, T, Le, X, et al. Unveiling the landscape of uncommon EGFR mutations in NSCLC-A systematic review. J Thorac Oncol. (2024) 19:973–83. doi: 10.1016/j.jtho.2024.03.016
7. Floc'h, N, Lim, S, Bickerton, S, Ahmed, A, Orme, J, Urosevic, J, et al. Osimertinib, an irreversible next-generation EGFR tyrosine kinase inhibitor, exerts antitumor activity in various preclinical NSCLC models harboring the uncommon EGFR mutations G719X or L861Q or S768I. Mol Cancer Ther. (2020) 19:2298–307. doi: 10.1158/1535-7163.MCT-20-0103
8. Zhang, W, Li, F, and Gao, W. New data highlighting the efficacy and safety outcomes of third-generation EGFR-TKI in NSCLC patients with rare EGFR mutations. Thorac Cancer. (2020) 11:495–7. doi: 10.1111/1759-7714.13334
9. Shi, Y, Chen, G, Wang, X, Liu, Y, Wu, L, Hao, Y, et al. Furmonertinib (AST 2818) versus gefitinib as first-line therapy for Chinese patients with locally advanced or metastatic EGFR mutation-positive non-small-cell lung cancer (FURLONG): a multicentre, double-blind, randomised phase 3 study. Lancet Respir Med. (2022) 10:1019–28. doi: 10.1016/S2213-2600(22)00168-0
10. Pan, X, and Shi, M. Successful therapy of a critically ill non-small cell lung cancer patient with compound mutations in EGFR G719X and S768I genes using furmonertinib: a case report. Heliyon. (2024) 10:e27106. doi: 10.1016/j.heliyon.2024.e27106
11. Yang, TY, Tsai, CR, Chen, KC, Hsu, KH, Lee, HM, and Chang, GC. Good response to gefitinib in a lung adenocarcinoma harboring a heterozygous complex mutation of L833V and H835L in epidermal growth factor receptor gene. J Clin Oncol. (2011) 29:e468–9. doi: 10.1200/JCO.2010.33.5802
12. Zhuang, Y, Xu, J, Ma, H, Zhu, W, Guo, L, Kang, S, et al. A sequential method of epidermal growth factor receptor mutation detection reduces false negatives: a new case with doublet mutations of L833V and H835L in China. Clin Lung Cancer. (2013) 14:295–300. doi: 10.1016/j.cllc.2012.11.003
13. Frega, S, Conte, P, Fassan, M, Polo, V, and Pasello, G. A triple rare E709K and L833V/H835L EGFR mutation responsive to an irreversible Pan-HER inhibitor: a case report of lung adenocarcinoma treated with Afatinib. J Thorac Oncol. (2016) 11:e63-63e64. doi: 10.1016/j.jtho.2016.01.023
14. Qin, BD, Jiao, XD, Yuan, LY, Liu, K, Wang, Z, Qin, WX, et al. The effectiveness of afatinib and osimertinib in a Chinese patient with advanced lung adenocarcinoma harboring a rare triple EGFR mutation (R670W/H835L/L833V): a case report and literature review. Onco Targets Ther. (2018) 11:4739–45. doi: 10.2147/OTT.S167346
15. Long, X, Qin, T, and Lin, J. Great efficacy of Afatinib in a patient with lung adenocarcinoma harboring EGFR L833V/H835L mutations: a case report. Onco Targets Ther. (2020) 13:10689–92. doi: 10.2147/OTT.S260157
16. Yang, X, Yao, Y, and Zhu, Q. A L833V/H835L EGFR variant lung adenocarcinoma with skin metastasis: a case report and literature review. Heliyon. (2022) 8:e12080. doi: 10.1016/j.heliyon.2022.e12080
17. Smith, JT, Puri, S, and Akerley, W. Brief report: EGFR L833V/H835L duplex-mutated NSCLC with leptomeningeal Carcinomatosis responsive to Osimertinib. Clin Lung Cancer. (2023) 24:360–1. doi: 10.1016/j.cllc.2023.01.010
18. Li, T, Wang, S, Ying, J, Wang, Y, Hu, X, Hao, X, et al. Afatinib treatment response in advanced lung adenocarcinomas harboring uncommon mutations. Thorac Cancer. (2021) 12:2924–32. doi: 10.1111/1759-7714.14156
19. Miao, Y, Wang, Y, Li, P, Tan, M, Wen, T, Wang, C, et al. A rare case of lung adenocarcinoma with EGFR L833V/H835L co-mutation and literature review. Zhongguo Fei Ai Za Zhi. (2023) 26:795–800. doi: 10.3779/j.issn.1009-3419.2023.102.36
20. Zi, Y, Wei, J, Yanwu, Z, Lijia, B, and Jiemei, J. Icotinib combined with chemotherapy in the treatment of rare complex mutation of EGFR gene L833V and H835L in lung cancer: a case report[J]. Anti-tumor Pharmacy. (2023) 13:385–8. doi: 10.3969/j.issn.2095-1264.2023.03.19
21. Luo, Z, Luo, C, Zhou, R, Xiao, Y, and Wang, T. Complete response to first-line osimertinib monotherapy in a complex epidermal growth factor receptor mutant (L833V/H835L) lung adenocarcinoma patient: a case report. Anti-Cancer Drugs. (2023) 34:939–41. doi: 10.1097/CAD.0000000000001523
22. Al-Nusair, J, Bodiwala, R, Nwanwene, K, Abdallah, M, Alshal, M, and Pacioles, T. Clinical response to Osimertinib in a non-small-cell lung Cancer patient with EGFR L833V/H835L mutations: a case report. J Investig Med High Impact Case Rep. (2024) 12:23247096241300929. doi: 10.1177/23247096241300929
23. Li, L, Huang, S, Qin, L, Yan, N, Shen, S, and Li, X. Successful treatment of lung adenocarcinoma complicated with a rare compound EGFR mutation L833V/H835L using aumolertinib: a case report and literature review. Front Pharmacol. (2023) 14:1257592. doi: 10.3389/fphar.2023.1257592
24. Pan, Y, Yan, L, Gu, Y, Wang, S, Li, H, Yu, P, et al. The effectiveness of sequential afatinib and furmonertinib in an advanced lung adenocarcinoma with rare compound EGFR mutation (L833V/H835L). Anti-Cancer Drugs. (2025) 36:355–8. doi: 10.1097/CAD.0000000000001692
Keywords: lung adenocarcinoma, EGFR mutation, p.L833V/p.H835L compound mutation, furmonertinib, case report
Citation: Wang S, Shi G, Liu Q, Liu G, Liu Y, Han Y, Gao M, Han K and Xie S (2025) Treatment of advanced lung adenocarcinoma with EGFR L833V/H835L compound mutations using furmonertinib: two case reports and literature review. Front. Med. 12:1658583. doi: 10.3389/fmed.2025.1658583
Edited by:
Guangdong Wang, First Affiliated Hospital of Xi’an Jiaotong University, ChinaReviewed by:
Ye Hu, Yancheng First People's Hospital, ChinaJie Peng, The People's Hospital Medical Group of Xiangzhou, China
Copyright © 2025 Wang, Shi, Liu, Liu, Liu, Han, Gao, Han and Xie. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shaonan Xie, d2Rka2JzQDE2My5jb20=
 Shize Wang
Shize Wang Guoliang Shi
Guoliang Shi Qingyi Liu
Qingyi Liu Yaqing Han
Yaqing Han Shaonan Xie
Shaonan Xie